Risk Characterisation - Microcystins in Fish
This risk assessment is presented in a qualitative form with the scales for the frequency of occurrence, severity of foodborne risks, and the level of associated uncertainty described in a multidimensional risk assessment framework.
The estimated intakes in Tables 3 and 6 on Exposure Assessment - Microcystins in Fish, are within the provisional TDI in all age groups for high consumers of eel or of roach, pollan, perch and/or bream, even taking the upper bound approach. There are not expected to be other sources of dietary exposure to microcystins. Therefore, no health concern is identified for consuming the flesh of eel, roach, pollan, perch and/or bream.
The estimated intakes in Table 7 on Exposure Assessment - Microcystins in Fish, based on an approximate exposure scenario for the inadvertent consumption of small amounts of viscera together with the edible flesh, marginally exceed the provisional TDI for 4-10 year old high consumers. However, this is only a marginal exceedance in the context of the uncertainty factors used to establish the provisional TDI, which is unlikely to be of toxicological significance. It is not clear that there is truly any exceedance since this exposure assessment used an upper bound approach to the concentration in flesh. Furthermore, the highest concentration in any viscera sample was used for this exposure assessment. Overall, it appears unlikely that consumers will substantially exceed the provisional TDI on a long-term basis due to incomplete evisceration of fish.
To present this risk assessment in a qualitative form, the scales for the frequency of occurrence and severity of foodborne risks and level of associated uncertainty that is described in the multidimensional risk assessment framework outlined by the Advisory Committee on the Microbiological Safety of Food (ACMSF 2020) was used.
This is described in Figure 1.
Figure 1: The probability of an adverse event occurring per serving.

Exposure to microcystins from eating the edible flesh of the tested fish species would not be expected to cause adverse effects in consumers, including if the fish is inadequately eviscerated. Therefore, we consider the frequency of adverse reactions in the general population to be negligible, so rare that it does not merit to be included.
Figure 2: Severity of detriment
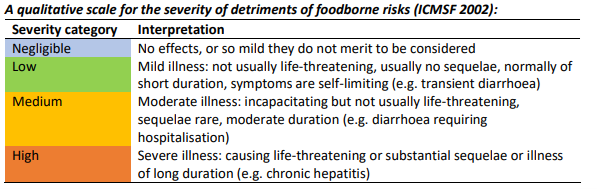
The severity of any adverse effect on the liver would depend on the level of exposure. An outbreak of acute liver injuries occurred in a dialysis clinic in which the dialysis water was contaminated with microcystins and possibly cylindrospermopsin in Brazil in 1996. However, levels of internal exposure to microcystins were very high.
In a second case of dialysis water contaminated with microcystins, increases in biomarkers of hepatic cellular injury and cholestasis exceeding the normal range were frequently observed and were concluded to be consistent with mild-moderate liver injury. Based on the possible very low levels of exposure to microcystins from fish from Lough Neagh, it is considered that any liver injury in consumers of fish, were it to occur, would result from long term exposure and be mild.
Overall, we consider the severity of illness that could potentially occur as a result of exposure to microcystins from consuming edible fish flesh from Lough Neagh to be medium (i.e. moderate illness, incapacitating but not usually life-threatening and of moderate duration).
Figure 3: An assessment of quality of data
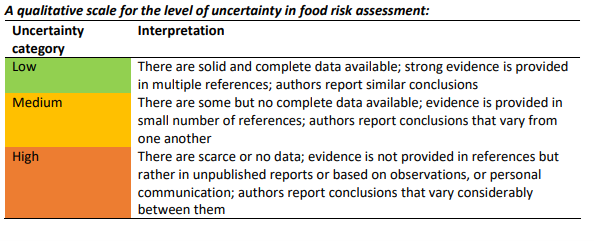
Hepatotoxicity has been reported both in experimental animals (rats and mice) and exposed humans. There are limitations to the toxicological data in experimental animals, which include limited data investigating reproductive toxicity and neurological, immunological and haematological effects, and a lack of chronic toxicity studies in laboratory animals. However, these data limitations were addressed in the provisional TDI used in this risk assessment by the application of an additional uncertainty factor of 10.
While a combined risk assessment is indicated for the microcystins since they are expected to cause the same adverse effects by the same mode of action, there is uncertainty in comparing estimated intakes of total microcystins to the provisional TDI set for MC-LR. There are likely to be significant differences in the toxicological potencies of different microcystins following oral intake due to differences in their inherent potencies and in their toxicokinetics. However, much of the residues detected in viscera were of MC-LR and MC-YR, and the latter has a similar toxic potency following intraperitoneal dosing to MC-LR (WHO, 2020), so the combined approach appears to be reasonable based on the limited data currently available.
Uncertainties in the exposure assessment include the limited consumption data for eels, the use of consumption data for trout as a surrogate for the consumption of for roach, pollan, perch and bream, and the validity of the assumed exposure scenario to address the risks of inadequate evisceration. However, it is exposure averaged over time, rather than on any individual day, which is of relevance to the chronic toxicity of microcystins and the provisional TDI applied in the risk assessment, and considering the consumption data used for adults aged 19-64 years of 89 g/day on a per person basis in the assessment of roach, pollan, perch and/or bream, this is equivalent to 620 g consumption per week, which is equivalent to about 4 portions per week consumption if assuming a portion size of 150 g. This is considered unlikely to underestimate long term consumption of roach, pollan, perch and/or bream by the majority of consumers.
Overall, we consider the level of uncertainty to be medium (i.e. there are some but no complete data available), but that this does not affect the conclusion of the risk assessment that adverse effects are unlikely. The key remaining sources of uncertainty are listed in the next section.
Revision log
Published: 7 March 2024
Last updated: 7 March 2024