Salmonella risk profile of UK-produced hen shell eggs: Hazard characterisation
The symptoms of Salmonella infection can range from asymptomatic carriage to severe diarrhoea and septicaemia.
4.1 Disease characterisation
The symptoms of Salmonella infection can range from asymptomatic carriage to severe diarrhoea and septicaemia. The incubation period of non-typhoidal Salmonella infection is usually from 12 to 96 h, although periods of 96 to 144 h (4 to 6 days) are not unusual and incubations of 7 to 9 days and occasionally longer also occur (Eikmeier, Medus and Smith, 2018). The principal symptoms of mild fever, nausea and vomiting, abdominal pain, and diarrhoea last for a few days but can persist for a week or more.
Whilst the illness is usually self-limiting, it can be more severe in vulnerable groups, including the elderly, pregnant women, the young and immunocompromised, leading to systemic infection and death. Currently there is no human vaccine available for non-typhoidal Salmonella. After symptoms have subsided, carriage and shedding of the organism can occur for a few weeks, up to months.
The median infective dose required to infect 50% of the population (ID50) for non-typhoidal Salmonella is generally high, estimated from volunteer feeding studies to be 104 CFU, but varies between serovar and food vehicle, for instance the ID50 in eggs is suggested to be as low as 1.09 x 101 CFU (Teunis et al., 2010).
4.2 Survival and growth
Salmonella growth has been observed between 5 and 47 °C with an optimum
growth temperature of 37 °C. Salmonella are readily destroyed by pasteurisation temperatures and the standard 70 °C for two minutes cooking advice is normally sufficient (FSA, 2018). This is affected by the food composition, however, for example in low water activity foods, such as peanut butter, the survival of Salmonella at 70 °C
is increased (Beuchat et al., 2013). Curing and fermentation are also generally effective at reducing bacterial loads (Mandal and Kwon, 2017), but are unlikely to be utilised in the production of eggs or egg based products or recipes (uncertainty).
The minimum water activity that permits growth of Salmonella is 0.94, however, cells are able to survive in dried foods for extended periods of time (Beuchat et al., 2013). Cells exposed to desiccation are also more tolerant to heat, UV, and chemical treatments. It has been reported that Salmonella can grow at pH 3.8-9.5 although the optimal pH for growth is 7.
The age of the egg also has an impact on the viability of Salmonella, as the pH of an egg white increases, from neutral (pH 7) to alkaline (pH 9 – 9.5), through the loss of carbon dioxide via the eggshell pores. The optimum pH for Salmonella growth is pH 7, although it can grow between pH 3.8-9.5. Therefore, a pH above 9 would reduce the viability of Salmonella. An additional confounding factor in raw egg-based sources and dressings is the fat content. High fat content decreases the water activity which increases the survival of Salmonella during thermal treatment (Szpinak, Ganz and Yaron, 2022) and lowers the dose required for infection. Fat free mayonnaise was found to have a faster decrease in Salmonella levels compared to full-fat mayonnaise at the same pH (Keerthirathne et al., 2019). The addition of other flavouring compounds such as garlic and salt may also have an impact of the survivability of Salmonella as these compounds are often used as antibacterial agents.
Chlorine and ozone-based treatments have been shown to reduce Salmonella counts on a variety of foods, although are unlikely to be utilised in the egg supply chain (uncertainty). In addition, UV treatment has been shown to reduce bacterial counts in foods (Mandal and Kwon, 2017), and is often utilised in the egg supply chain (British Lion Eggs, 2021), but it is unknown how effective this process is (uncertainty) and will not be effective for contamination internalised within the egg.
Cold temperatures have been shown to decrease the growth of Salmonella in the egg yolk and albumen but also increase the survival of Salmonella on the egg surface (Khan et al., 2021). A study of eggs contaminated with Salmonella from New Zealand found that the viability of Salmonella declined over time on the shell surface of the egg at 15C and at 22C. They also found that the bacterium survived better on visibly clean eggshells at 15C than at 22C. Survival on the eggshell was enhanced by the presence of faecal contamination and the study found no contamination within the egg contents at either temperature (Kingsbury, Thom and Soboleva, 2019).
4.3 Antimicrobial resistance
The ACMSF report, (2016), observed that resistance profiles of Salmonella strains can reflect the country of origin – for instance nalidixic acid resistance is typically found in isolates from countries where fluoroquinolones are inexpensive and routinely used. An FSA survey of Salmonella contamination of non-UK eggs on retail sale between March 2005 and July 2006 found that the majority of isolates (83.2%) were resistant to one or more antimicrobial drugs of which most were resistant to nalidixic acid with reduced susceptibility to ciprofloxacin (78.6%) (ACMSF, 2016). Ten Salmonella strains found in UK eggs were fully sensitive to 10 antimicrobial compounds (Food Standards Agency, 2004).
The Salmonella in animals and feed report from APHA, for 2021, found that most isolates from chickens were fully susceptible to a panel of 16 antimicrobials (74.3%).
Resistance to sulphonamide compounds (15.7% of isolates), tetracycline (10.8%) and streptomycin (10.5%) were the most common in chickens. Cefotaxime, ceftazidime or ciprofloxacin resistance was not detected in S. Enteritidis in 2021 (APHA, 2022). A study on Salmonella isolates received at Public Health England’s Gastrointestinal Bacteria Reference Unit between 2014 and 2015 found full susceptibility in 63% of isolates. Resistance to tetracycline was most commonly observed (26%), followed by sulphonamide resistance (24%) and ampicillin resistance (21%) (Neuert et al., 2018)
4.4 Salmonella cases in the UK
Prior to the 1980s, S. Enteritidis was rarely responsible for disease. During the late 1980s, it was the cause of a foodborne epidemic infecting more than 500,000 people in England and Wales, traced back to contaminated chicken but especially shell eggs (ACMSF, 2016). Infections started to decline with the introduction of risk management actions such as vaccination and flock hygiene programmes (see Figure 1).
Levels of Salmonella over the last 10 years have been relatively steady (Figure 3 and Figure 4). In 2020 and 2021, the COVID-19 pandemic has resulted in a decrease of reported gastrointestinal illness, including Salmonella levels (Love et al., 2022). This is likely due to a combination of factors, including increased underreporting of gastrointestinal illness, reduced foreign travel, UK lockdowns and implementation of enhanced hygiene measures. It is uncertain whether this downward trend in Salmonella cases will persist.
Figure 3: Rate of reported non-typhoidal Salmonella infections by country per 100,000 population for 2012-2021.
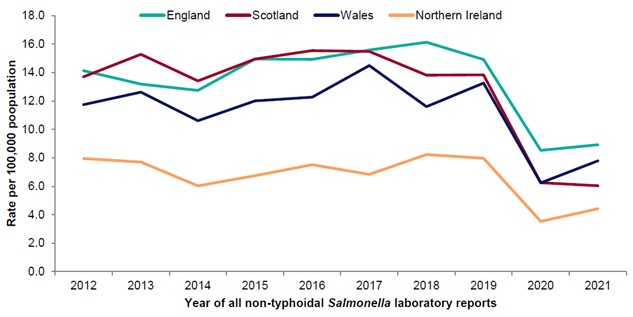
Source: (EFIG 2022 (UKHSA, PHS, PHW, PHA , 2021)) The number of reported cases is shown for the four nations, England, Scotland, Wales and N. Ireland, with year of laboratory report against rate per 100,000 of population. Please note that data for 2020 onwards is provisional.
Figure 4: Rate of reported Salmonella Enteritidis infections in the United Kingdom and by nation per 100,000 population, 2012-2021.
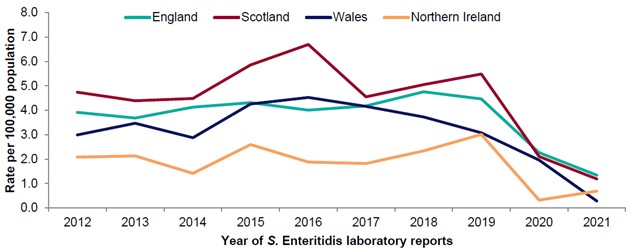
Source: (EFIG 2022 (UKHSA, PHS, PHW, PHA , 2021)). Please note that data for 2020 onwards is provisional.
4.5 Summary of foodborne outbreaks associated with Salmonella and eggs
The 2016 ACMSF report includes descriptions of 26 salmonellosis outbreaks with confirmed or putative links to eggs or egg products over the period 2009 – 2014, with a total of 1176 cases. Most of these outbreaks (22/26) reported fewer than 40 cases. The largest outbreaks, in 2009 (489 cases), 2011 (263 cases) and 2014 (100 cases), were linked to imported eggs (ACMSF, 2016). A further outbreak of S. Typhimurium in 2010 with 81 cases was linked to consumption of UK-produced duck eggs. Four small general outbreaks were linked to UK produced eggs, of which only one outbreak of 33 cases in 2009 was linked to Lion Code eggs.
The data collected from UKHSA non-typhoidal Salmonella outbreaks is shown in Table 8. This data is provisional and incomplete and does not indicate whether the eggs associated with the outbreaks were Lion Code or imported and which of the outbreaks are recurring with the data only available up to 2019. Additional data has been requested from UKHSA. Therefore, this presents a key uncertainty. Between 2015 and 2019, a total of 954 confirmed cases of salmonellosis were investigated as part of outbreak investigations and determined to be associated with consumption of eggs and/or egg products, in a total of 15 reported investigations. The majority of outbreaks (10/15) reported fewer than 45 cases. The largest outbreaks occurred in 2016 (158 cases), 2017 (162 cases) and 2018 (259 cases).
Table 8: Foodborne outbreaks of non-typhoidal Salmonella reported in England and Wales associated with eggs and/or egg products (UKHSA, 2016, 2018, 2021a, 2021b, 2021c.)
| Year | Agent | Total affected | Laboratory confirmed | Hospitalised | Deaths | Setting | Food description |
|---|---|---|---|---|---|---|---|
| 2015 | Salmonella Enteritidis | 3 | 3 | 0 | 0 | Hotel | eggs and egg products |
| 2015 | Salmonella Enteritidis | 2 | 2 | 1 | 0 | Residential institution | eggs and egg products |
| 2016 | Salmonella Enteritidis | 90 | 116 | 2 | 0 | National: multiple exposure settings | eggs |
| 2016 | Salmonella Enteritidis | 95 | 95 | 0 | 0 | National: multiple exposure settings | eggs |
| 2016 | Salmonella Enteritidis | 116 | 158 | 14 | 0 | National: multiple exposure settings | eggs |
| 2016 | Salmonella Enteritidis | 21 | 13 | 1 | 0 | Household | Tiramisu |
| 2017 | Salmonella Enteritidis | 162 | 162 | 0 | 0 | National: multiple exposure settings | eggs |
| 2017 | Salmonella Enteritidis | 27 | 27 | 1 | 0 | National: multiple exposure settings | eggs |
| 2018 | Salmonella Enteritidis | 26 | 26 | 0 | 0 | Multiple places of exposure | eggs |
| 2018 | Salmonella Enteritidis | 259 | 259 | 0 | 0 | Multiple places of exposure | eggs |
| 2019 | Salmonella Enteritidis | 44 | 44 | 11 | 0 | Multiple places of exposure | eggs |
| 2019 | Salmonella Enteritidis | 2 | 2 | 1 | 1 | Multiple places of exposure | eggs |
| 2019 | Salmonella Enteritidis | 22 | 22 | 0 | 0 | Multiple places of exposure | eggs |
| 2019 | Salmonella Enteritidis | 22 | 22 | 0 | 0 | Multiple places of exposure | eggs |
| 2019 | Salmonella Enteritidis | 3 | 3 | 1 | 0 | Multiple places of exposure | eggs |
*this data is provisional a full dataset has been requested from UKHSA.
Not all outbreaks are microbiologically linked to an implicated food vehicle as food vehicles are not always identified or available for microbiological testing, and the level of evidence derived through epidemiological and microbiological investigations varies with some outbreaks having stronger epidemiological evidence in support of a link between the implicated food product and the outbreak than other outbreaks (uncertainty).
Additionally, for some outbreaks, not all individuals linked to the outbreak will have laboratory confirmation of illness. The number of hospitalisations reported is only known for cases which received public health follow-up, for example via interviews. Where individuals are reported to have died, it is usually not known whether the cause of death was directly related to the outbreak (uncertainty).
Some outbreaks highlighted in Table 8 were recurring, intermittently persistent, cluster events, which occurred across multiple years with epidemiological evidence for some of these explained below. While published UKHSA data is not available for 2020- 2022, FSA records show no new outbreaks have occurred. Additional cases were recorded for ongoing outbreaks, as described below.
A recurring cluster of S. Enteritidis t5:175 and t5:360 was re-opened and investigated after first being identified in 2016. In 2016 the outbreak was linked to eggs imported from Poland. There have been 533 cases in this outbreak since June 2014; 251 cases in 2018 to date, with 77 alone in September 2018 (the highest number of cases reported in any month in this outbreak). A cluster of these cases was linked to liquid egg product which was produced in a factory linked to contamination with the outbreak strain t5:360. WGS was carried out on the liquid egg product with the result indistinguishable from that of an isolate found in an egg from Poland during the 2016 outbreak.
A recurring cluster of S. Enteritidis t5:2669 has had 117 cases to date from April 2016 – March 2020. Epidemiological investigations indicated that four farms associated with this cluster in Nottinghamshire, Northamptonshire, Dorset and Kent were all part of the BEIC Lion Code egg assurance scheme. All farms used the same packing centre. There is the potential that cross contamination occurred at the packing centre with eggs from non-positive farms as the outbreak isolate was found in the packing centre environment. It was not clear if infection was moved from laying farms to packing centre or vice versa (uncertainty) (Internal FSA communication, 2022).
A recurring cluster in 2019 identified samples of S. Enteritidis from two farms and environmental samples from an egg packing centre. It linked to a t5:180 UK lineage sub-cluster which was first identified in early 2019 and has been intermittently persistent in 2019, 2020 and early 2021. To date isolates have been linked to four farms and one packing centre. Epidemiological investigations indicated most cases with onset of illness between June and October 2020 had purchased and consumed Lion Code eggs predominantly from two retailers. As of February 2022, 109 cases of S. Enteritidis t5:180 UK lineage cases have been identified using whole genome sequencing (WGS)
4.6 Detection
Since the ACMSF report in 2016 whole genome sequencing has been implemented as a routine method for further characterisation of Salmonella. WGS can be used to perform genome characterisations of isolates as well as identify antimicrobial resistance genes.
Across the EU WGS is already in use in different sectors including public health and food safety (EFSA et al., 2018). The UKHSA have been routinely sequencing all presumptive Salmonella isolates since 2014 (Chattaway et al., 2019). The epidemiological precision offered by WGS for outbreak investigation and attribution is much more valuable than previous systems that were used (ACMSF, 2016) and can link cases of illness to outbreaks that would have been deemed unrelated by previous methods such as PCR, gene probes and enzyme-linked immunosorbent assay (ELISA)(ACMSF, 2016).
The high resolution WGS typing of isolates for pathogen strain discrimination has enhanced the detection of outbreaks and enables ‘sensitive and specific’ case definitions to be applied, improving case ascertainment, focussing outbreak investigations and increasing the strength of association in analytical studies to identify the implicated food vehicles. Where possible, integration of the microbiological, genomic and epidemiological data derived from analysis of the human disease data with that from animal samples, environmental sampling or the food chain, has significantly improved the ability to identify the source of the outbreak and better understand transmission of contamination through food supply chains. The use of WGS has also resulted in an enhanced ability to detect re-emergence of outbreaks and trace them back to the same source of contamination as previously identified when control measures have not been fully effective in eliminating contamination (Kathie Grant et al., 2018).
Implementation of WGS has enabled the consolidation of multiple local/regional outbreaks into single national level outbreaks based on the WGS and epidemiological information obtained during the investigations. This has resulted in a higher proportion of outbreaks being identified to be national rather than local/regional outbreaks with an associated increase in case numbers. Therefore, while consideration of total numbers of outbreaks reported is useful, these data are affected by whether WGS is used or not.
Both the re-emergence of cases associated with outbreak clusters and the consolidation of multiple outbreaks into large national outbreaks of long duration has meant that comparison of number of foodborne outbreaks and number of associated cases pre and post the implementation of WGS should be undertaken with caution, and the foodborne outbreak surveillance data reported for the years prior to implementation of WGS (pre-2014 for Salmonella) is not directly comparable to the data held for subsequent years. Therefore, the size of the outbreak and number of individuals affected should be considered together with the information given on the overall numbers of outbreaks.
Revision log
Published: 29 June 2023
Last updated: 5 July 2024