Chapter 2.9 Dairy Hygiene Inspection
This chapter outlines FSA dairy hygiene inspection controls on farms
Sections
1. Introduction
In this section
1.1 Overview
The FSA is responsible for the inspection and enforcement of hygiene regulations on registered cow’s milk production holdings in England and Wales and for inspections at registered milk production holdings for other species.
1.2 Legislation
1.2.1 Regulations
The following list is not intended to be exhaustive, but details the main legislation under which official hygiene controls are conducted on dairy production holdings:
- (EC) 852/2004
- (EC) 853/2004
- (EU) 2017/625
- (EU) 2019/627
- The Food Safety and Hygiene (England) Regulations 2013 (as amended)
- The Food Hygiene (Wales) Regulations 2006 (as amended)
- (EC) 178/2002
- (EC) 2073/2005
- (EC) 2074/2005
1.3 Background
1.3.1 Registration requirement
Food Business Operators (FBOs) are required to register details of their milk production business and premises.
Reference: The Food Safety and Hygiene (England) Regulations 2013 (as amended)
The Food Hygiene (Wales) Regulations 2006 (as amended)
Regulation (EC) No 852/2004, Article 6
1.3.2 Advisory visits
Any FBO who intends to start milking for the first time or who intends to put in new milking equipment should contact the Approvals and Registrations Team, where the FBO can be put in touch with their local Dairy Hygiene Inspector (DHI) for an advisory visit to take place at the planning stage. This should prevent any issues developing with poor structural design or inappropriate equipment being purchased. These visits are voluntary and are at the discretion of the FBO.
1.3.3 Registration contact details
The FBO should complete an ‘Application for registration of a milk production holding’. Application forms are available from the FSA website at Food Standards Agency - Dairy hygiene inspections or contact the Approvals and Registrations Team.
1.3.4 Registered Milk production premises database
Following receipt and processing of a completed Application for registration, details of the premises will be entered on to the database of registered milk production premises.
The Approvals and Registrations Team will issue a letter to the FBO, confirming registration of the premises, and will confirm the registration details to other interested parties (Rural Payments Agency (RPA)), (local authorities (LA)).
Following confirmed registration of the premises they will be inspected within three months and thereafter according to the frequency as detailed in sub-topic 1.4.1 on ‘Inspection frequency’.
Important Note: If the application for registration indicates an intention to sell raw drinking milk (RDM) direct to the consumer or a business that is already registered for wholesale production, the same letter is sent to the producer and an immediate inspection visit will be scheduled. This visit should be completed as a priority and undertaken as soon as possible (but definitely no later than 2 weeks after the notification has been given). The intention of the inspection will be to ensure that all legal obligations are being met and to collect a verification sample to be sent for examination.
1.3.5 Who conducts dairy hygiene inspections?
Dairy hygiene inspections on registered milk production holdings in England and Wales will be conducted by FSA Operations Group Dairy Hygiene Inspectors (DHIs), following a schedule of visits provided to them. The majority of inspections are carried out without an appointment being arranged (unannounced).
Reference: See section 2 on ‘Inspection Procedures’ in this chapter.
1.4 Inspection frequency
1.4.1 Frequency of inspection
The routine inspection frequency for registered milk production premises is based upon:
- the type of product
- species producing the milk
- the date of the last inspection
- membership of the third-party accreditation scheme (Red Tractor Farm Assurance Dairy Scheme (RTFADS)
- the compliance history of the farm
- any other local intelligence, complaints or investigations that might grant an inspection
Frequency of inspection
| Production status | Species | Inspection interval | Inspection by |
|---|---|---|---|
| Raw milk sales (for drinking) | Cows | 6 months | FSA |
| Raw milk sales (for drinking) | All other species | 6 months | FSA (LA remains the enforcement authority) |
| Raw milk production | Sheep, goats, buffalo | 2 years | FSA (LA remains the enforcement authority) |
| Raw milk production non-assured dairy farm | Cows | 2 years | FSA |
| Raw milk production- assured dairy farm | Cows | 10 years | FSA |
| Any establishment following enforcement action (for example, warning letter, HIN, court action) | All | According to specified deadline, until issue resolved. Following satisfactory resolution, an inspection after 6 months. If satisfactory at that point, return to default intervals as specified above. | FSA |
2. Inspection procedures
In this section
2.3 Preparing for an inspection
2.1 Types of inspection
2.1.1 Pre-production inspection
This type of visit will be an advisory visit undertaken before production commences, when the FBO of a newly registered premises is proposing to sell either untreated (raw) milk direct for human consumption or raw milk for pasteurisation. These visits are not compulsory and are at the discretion of the FBO.
2.1.2 Primary inspections
This is a routine, scheduled inspection, determined through a risk based process. The primary inspection will identify the level of FBO compliance with legislative requirements. The result of the primary inspection will dictate any follow up action required. Primary inspections are conducted without prior notice to the FBO.
Other circumstances which will lead to a primary inspection being conducted are detailed in the following paragraphs.
2.1.3 Inspection in response to receipt of a complaint
In the event that a complaint is received about a milk production premises, an inspection should be carried out within five working days to investigate the issue. The only exceptions to this are if the complaint is in relation to RDM (in which case the visit should take priority and be arranged for as soon as possible), or if enforcement action is in progress and a follow up inspection is already planned.
2.1.4 Inspection in response to a report from a third party
If information is received from a third party body (such as RTFADS, RPA, a laboratory or a milk buyer) which suggests that an inspection is required, an inspection will be scheduled to investigate the issue within five working days of receiving and understanding the complaint, unless enforcement action is in progress and a follow up inspection is already planned.
2.1.5 Follow up inspections
Follow up inspection visits are carried out to check that improvements identified at a previous inspection have been carried out. It may be necessary to undertake more than one follow-up visit, with appropriate enforcement action being taken as necessary, until compliance is achieved.
2.2 Scheduling of inspections
2.2.1 Scheduling of inspections
Visits are to be scheduled in an order of priority based on risk and premises location.
The DHI should create a schedule of visits using the following order of priority:
- inspection of premises that have indicated an intention to sell RDM direct to the final consumer either as part of an initial registration or as an additional activity for a business already registered (visit as soon as possible)
- inspections in response to a report of a raw drinking milk (RDM) failed sample (within 5 working days)
- inspections in response to a complaint (visited within 5 working days)
- inspections in response to information received from a third party (visited within 5 working days)
- inspections in response to a report of an antibiotic failure (within 10 working days)
- follow up visits / inspections (as appropriate, depending on the issue)
- primary inspections generated under routine inspection frequency arrangements
The DHI will be provided with details of the type of inspection to be carried out and the compliance history of the establishment to be inspected.
2.3 Preparing for an inspection
2.3.1 Equipment
DHIs have been provided with, and must carry the following equipment when undertaking dairy hygiene inspections:
- mobile phone (including access to GPS software)
- FSA ID card and warrants
- official notebook
- hard copies of enforcement notices
- tablet for recording inspection details
- torch
- FSA lairage coat & spares
- GPS equipment
- green safety wellingtons
- waterproof leggings and jacket
- FSA lairage coat
- hard hat
- infra-red calibrated thermometer
- disposable nitrile gloves
- bucket and brush
- 5 litre water container filled with tap water or disinfectant already mixed to recommended dilution
- approved disinfectant
- hand sanitiser
- hand wipes
- first aid kit
- storage box to contain all Personal Protective Equipment (hard case suitable for cleansing and disinfection)
- tamper-proof evidence bags
2.3.2 Preliminary actions on arrival
The DHI must:
- identify themselves, showing their FSA Authorisation card; the DHI must always carry the FSA warrants with them during inspections
- establish to whom they are talking and their position in the business
- if that person is not the FBO, determine whether the FBO is available; if not, consider whether the person has been designated as a duly authorised representative by the FBO and is an appropriate individual to accompany the inspector and to speak on behalf of the business
- if the DHI is satisfied that they are dealing with the FBO or their appropriate representative, they should conduct a pre-inspection meeting
Note: If there is no appropriate person available (FBO or their representative) the inspection should not proceed and must be rescheduled.
2.3.3 Pre-inspection meeting
At the pre-inspection meeting, the DHI must:
- explain the purpose of the inspection and the approach being taken
- check that the business details which FSA hold are current, namely:
- holding number (county parish holding (CPH) number)
- legal name of the business
- names of business partners, if relevant
- contact telephone / mobile numbers / email address
- LA area in which the business is located
- check farm assurance status if appropriate
- herd size
- types of milk use, whether milk is sold untreated direct for human consumption or whether the FBO has knowledge that the milk is being used to produce unpasteurised products such as cheese or cream
- milking times
- water supply details and testing results
- milk purchaser details (destination for pasteurisation)
- current TB status
- change into PPE and disinfect boots/leggings as soon as is practical to limit the chance of contamination
2.4 Primary inspections
2.4.1 Overview
The inspection should cover the entire process of milk production, including the housing of the animals, storage and dispatch of the raw milk, with particular emphasis on the milking process, but not to extend to any bottling, wrapping and packaging processes.
Ancillary processes such as cleaning and maintenance schedules, waste management or laboratory results should be inspected.
During the course of the inspection, the DHI should discuss issues relating to the effectiveness of control systems in ensuring safe food production with the FBO, management representative and other relevant personnel.
2.4.2 Key areas for review
The inspection will concentrate on the key areas as listed below, together with an overall assessment of the hygiene conditions and management practices at the premises:
- animal cleanliness and health
- veterinary medicines, usage/records
- milking operations
- operator hygiene and cleaning routine
- general hygiene and management
- equipment cleanliness and cleaning methods
Each of these areas is covered on the digital enabled ‘Hygiene inspection report: Dairy’ form (DH2) on the tablet, and guidance on what to review in each of these key areas is provided in the following paragraphs.
2.4.3 Animal cleanliness and health
Depending on the time the inspection is carried out, the milking process will either be observed by the DHI or will be discussed with the FBO. If inspections are carried out during milking operations, the DHI should ensure that any disruption to milking is minimised.
Animals presented for milking must have clean teats, udders and adjacent parts before milking takes place.
The DHI must establish:
- that the FBO is aware and complies with the requirement that milk from heavily soiled animals should not be sold for human consumption, due to the high risk of contamination
- whether the FBO is aware and complies with the requirement that milk must only come from animals that present no sign of disease that might result in the contamination of milk and colostrum; this includes:
- animals suffering from any infection of the genital tract with discharge
- those suffering from enteritis with diarrhoea
- animals with a recognisable inflammation of the udder or udder wound likely to affect the milk or colostrum
- the tuberculosis (TB) and brucellosis (BR) status of the individual animal and herd; raw cow’s milk for direct human consumption or for use in the production of unpasteurised dairy products must only come from animals and herds that are free from TB and BR.
The DHI should ask questions about procedures for milking animals that are observed to have problems of this type and how milk from these animals is isolated and discarded as Animal By-Product (ABP).
The details of any non-compliance must be recorded on the inspection form and the DHI must ensure that the FBO is made aware of the non-compliances.
2.4.4 Veterinary medicines
The DHI should inspect the FBOs veterinary records. Medicine usage records should be kept up to date with treatments (within 72 hours of drug administration). There is no set form or medium in which the records must be kept, but there should be an overview of the medicine storage undertaken and the records should show the following as a minimum:
- the name of the veterinary medicine used
- the date of administration
- the name of the person who administered the medication
- the quantity of veterinary medicine used
- the identity of the animal / group of animals treated
- the date on which any withdrawal period for milk, meat or any other product ended / ends
- any necessary batch numbers for medicines
2.4.5 Milking operations: teat preparation
Teats, udders and adjacent parts must be clean before milking. The DHI should check that:
- appropriate facilities are available to enable the washing and drying of soiled teats and udders
- that foremilk is taken from each animal at each milking and examined for abnormality or that an equivalent method is used (conductivity tests)
- the milking routine demonstrates that adequate procedures to avoid contamination of the milk are applied
- teat cup liners are free from faecal contamination
- teat dips and sprays are being used in accordance with manufacturer’s instructions.
2.4.6 Milking operations: detection and rejection of abnormal milk and milking of TB reactors
The DHI should establish what procedures are in place for isolating TB reactors and animals being treated with veterinary medicines and/or unhealthy animals.
The DHI should check that the FBO is aware and is taking appropriate actions to detect and ensure that milk for human consumption derives only from individual animals that:
- do not show symptoms of disease communicable to humans through milk, or any signs of disease that may contaminate the milk, are free from enteritis with diarrhoea, inflammation of the udder or infectious discharge from the genital tract
- are free from udder wounds likely to affect the milk
- do not show a positive reaction to tests for TB or BR or are not deemed to show a positive reaction
- have not been subjected to unauthorised or illegal treatments and that the correct withdrawal periods have been observed for legal treatments
- that foremilk is taken from each animal at each milking and examined for abnormality or that an equivalent method is used (conductivity tests)
- that milk unfit for human consumption is rejected at the time of detection
- that milk obtained from animals undergoing medical treatment, likely to transfer residues to the milk, or from animals with infections which can be passed on through milk, is kept out of the human food chain
- that treated animals are effectively identified and that there is a readily available way of verifying which animals must have their milk kept out of the food chain and when it can be put back into the food chain; what procedures are acceptable will depend on the size and nature of the business
- that the FBO is aware and takes appropriate action to ensure that milk from TB reactors, direct contacts or inconclusive reactors (IRs) must be kept out of the food chain
- what method is, or would be, used to keep milk from TB or BR reactors out of the human food chain
- that the FBO is aware that if a herd loses its TB free status, milk produced on the holding from animals in the herd which are not reactors must be heat treated and the milk buyer must be notified.
2.4.7 Operator hygiene and cleaning routines
To judge compliance in this area, the DHI should determine:
- the clothing worn during milking
- that protective clothing is clean and is kept clean or changed as needed
- the availability of facilities for washing hands and arms and, if at an observed milking, that they are kept clean
- the availability of facilities for cleaning the structure of the milking area
- how the milking area is kept clean during milking
- how the milking area is cleaned at the end of milking
- that scheduled cleansing and disinfection procedures are carried out
2.4.8 General hygiene and management
The DHI should assess the milking area, food storage room and dairy wash-room for cleanliness, construction and location:
- walls, floors, roof windows and doors and any fixtures should be inspected, they should be tight fitting and discourage any vermin entry
- floors should be free draining
- all surfaces should be in a sufficiently good state of repair to enable them to be kept clean by the methods being used and should not themselves represent a hazard
- there is adequate protection against vermin; the DHI should question the FBO on their control procedures and may request the FBO to provide evidence if this is considered necessary
- discuss milk hygiene test results from milk buyers such as Total Viable Count (TVC), bactoscans and somatic cell counts with FBO and note them on the Dairy Hygiene Inspection report (DH2 – remember that the results need to be assessed in rolling geometrical average); also record if test results were not seen
Note: If not seen due to the FBO not being able to find them, ask the FBO to send them to you before the visit report (DH2) is completed and sent (a follow up phone conversation with the FBO could be had if needed). If the FBO has not got the sample results or they are not acting on them, this needs to be enforced (please see Section 4- Enforcement).
- there is effective separation from areas used to house animals
- it is constructed and maintained to limit the risk of contamination
- if there are separate food storage rooms and wash-rooms, both of these areas need to be inspected and assessed to the same standards.
Any areas of non-compliance should be discussed with the FBO and recorded on the tablet.
2.4.9 Equipment and cleaning methods
The location and condition of the milking and cooling equipment, the method of cleaning and cleanliness of the equipment must ensure that the milk is not subjected to avoidable contamination. These aspects should be assessed in accordance with the following guidelines, and any non-compliance recorded on the tablet.
2.4.10 Cleanliness of milk tanks and equipment
The DHIs assessment of the cleanliness of the milk tanks and equipment should cover:
- all surfaces intended to come into contact with milk sold for human consumption
- any other internal surfaces where air movement within the plant could contaminate the milk
- external surfaces close to inlets where air entering the equipment could carry contamination into the milk
- condition of milk tanks and equipment
If the tank contains milk, the DHI should check the temperature shown on its thermometer and record this on the tablet, this temperature can also be compared to previous recorded temperatures for comparisons. When this is not possible, milk collection tickets should be checked for the temperature at the time of collection. The cooling process must begin immediately after milking. The DHI must ascertain the FBOs method for taking milk temperatures and make sure they are in compliance with legislation. If any doubt exists over effective operation, the DHI needs to request servicing / calibration of FBOs equipment to confirm whether or not it is in good working condition and reliable readings are being obtained from it.
Prior to collection, the milk temperature must not exceed 8°C in the case of daily collection or 6°C if collection is less frequent than daily.
If the tank is fitted with serviceable filters, the servicing of these should be checked.
2.4.11 Inappropriate articles and processes
The milk storage area and any separate dairy wash-rooms should be assessed for the presence of any inappropriate or hazardous articles and processes. Only items directly related to milking and cleaning of milking equipment should be stored in these rooms. Check that:
- the milk storage area and rooms connected with milk storage areas are free from any articles that should not be there or any poisons and items likely to cause contamination
- clutter is not preventing effective cleaning
- that the use of rooms connected to milk storage areas does not result in potential contamination of the milk storage area.
2.4.12 Cleaning process and records
This covers the processes for the milking machine and the milk cooling/storage equipment. Depending on the time of the inspection, the cleaning and disinfection routines should be observed, monitored and/or discussed. Consideration should be given to the suitability of the cleaning process, including:
- frequency of application
- method of application
- detergents / disinfectants used, concentration, contact time
- water temperature
- evidence of residues / contamination within the milking equipment
- FBOs validation and verification of the method
The FBO or their representative may be requested to provide evidence that the temperature of the water and concentration of the chemical is regularly monitored.
2.4.13 Water supply
Check that the FBO has arranged with the LA for the monitoring of all private water supplies used for cleaning and disinfection of milking equipment and milk storage tanks, hand washing and washing of teats and udders. The FBO should, on request, provide evidence of the last test date and results. The FBO would need to communicate the frequency of this test.
The type of water supply (mains, or private supply) should be noted.
Where a private supply is in use, the DHI must check:
- the purposes it is used for
- the treatment is applied to the private water supply for each form of use
If a private water supply is being used and not tested by the LA, the inspector should inform the FBO or their representative, that FSA will inform the LA of the situation.
The DHI should seek immediate advice from the LA regarding testing, and from LDHI / FVL (Dairy) to determine what further action should be taken.
Where a private water supply is in use but the LA have reported that it is unsatisfactory due to contamination (for example, bacterial), the FBO should be informed that they must change to mains water or ensure that all water used for dairy purposes is treated. This requirement should be recorded on the DH2.
2.5 RDM premises only
Where a premise is producing RDM for direct supply to the final consumer, they are required to have a Food Safety Management System (FSMS). They must also demonstrate that they can verify the controls they have in place through this system is effective and being applied correctly, there is no other way to demonstrate this than by having a programme of sampling, the results of which can be used as evidence. For these premises at least one of the 6 monthly inspections each year will require the DHI to undertake an audit of the FSMS checking that it covers the elements required and that records are accurate and up to date as required, this audit will take place alongside the usual routine hygiene inspection. They will also be required to audit the FBO’s own sampling results, this will be aimed at gaining assurance that the controls applied are effective whilst also checking to see that all results have been satisfactory. Any unsatisfactory results should have been reported the FSA at the time that they were received. If this is not the case, then enforcement action may be appropriate. The audit checklist on the tablet will assist the DHI with prompts regarding the areas that should be covered as part of the audit. It is important to ensure that checks are made to verify that activities recorded within the FSMS are actually being applied correctly in practice. Checks of the operations of the processes recorded in the FSMS should be undertaken as far as practical. Where possible, reviewing previous records can be used to provide an overall picture of historic activity.
2.6 Follow up inspections
2.6.1 Follow up inspections
A follow up inspection should concentrate on the items that contravened the regulations at the previous inspection.
The DHI must assess each contravention as
- satisfactorily corrected
- not rectified
2.6.2 Follow up inspection: steps
The DHI must:
- record findings on the DH2 and dependent upon the action taken at the previous inspection, follow up with a warning letter, a HIN or in Wales only, a Remedial Action Notice (RAN)
- record those contraventions that are now satisfactory on DH2 and if there are no further outstanding contraventions update the DH1 with the date of compliance achieved, copies of all completed reports will be emailed to the FBO when they are submitted
- record any contraventions that are outstanding on the DH1, remedial actions required and update DH2
- record any significant new contraventions that were observed on the DH1 and remedial actions required
- record any recommendations of good practice you have suggested that may be appropriate
- summarise the contraventions with the FBO, giving sufficient technical advice on how to comply with the Regulations
- where a further secondary inspection is required, specify that a clear timescale for completion of the required works and must be recorded on the DH1
- when a HIN has been complied with to the satisfaction of the DHI, send a letter to the FBO informing them that the formal notice has been complied with
- when a HIN has not been complied with the next stage of enforcement is a Referral for Investigation.
2.7 Guidance on applying final compliance ratings
The below guidance provides a framework for DHIs to reference when deciding on the final compliance rating to be applied following their inspections. These are just meant for guidance and it will be up to the DHI to have the final decision and this can be based on factors not mentioned in the guidance below. Explanation on the decision for the final rating applied should always be provided to the FBO as part of the closing meeting.
| Rating | Guidelines |
|---|---|
| Good |
|
| Generally Satisfactory |
|
| Improvement Necessary |
|
| Urgent Improvement Necessary |
|
2.8 Post inspection procedures
2.8.1 Concluding the inspection
Before leaving the farm the DHI should ensure that all required information has been obtained and conclude the inspection visit with a post-inspection meeting. They should ensure all PPE is cleaned and disinfected thoroughly.
2.8.2 Post inspection meeting
The FBO of the establishment or management representative, together with other relevant managers / supervisors should be present at this meeting.
- The DHI should summarise the inspection, highlighting significant findings and ensuring that a clear distinction is made between contraventions of the legislation and recommendations of good practice.
- The DHI should explain what enforcement action (if any) will be taken.
- Solutions to problems arising and timescales for required actions should be discussed at the post-inspection meeting.
- Whether virtual evidence of compliance would be acceptable.
- Any issues raised at this meeting, which have not already been recorded, should be noted.
2.8.3 Notification of Inspection form
At the conclusion of the post-inspection meeting, and following successful completion of digital forms, the FBO should be informed that copies of all completed forms will be sent to their nominated email address.
2.8.4 Procedures after leaving the farm: primary inspection
Following a primary inspection, DHI should ensure that any completed forms are submitted via the tablet, they should allow any time needed to undertake recording of any enforcement of any non-compliances found during the inspection. Where remedial actions are required and a secondary inspection will take place, the time allowed for completion of the required works should be clearly stated in any correspondence.
2.8.5 Enforcement action
Any warning letter, HIN or RAN (Wales only) resulting from the inspection, should be drafted and sent electronically to the Dairy Operations mailbox on the same day that the inspection took place. It is important that inspectors allow time at the end of each dairy day in case of this. Following the appropriate quality checks, the Dairy Hygiene Data Team will post warning letters to the FBO. If a HIN or RAN is issued these must be either handed to the FBO directly or the DHI should post them recorded delivery. The cost of the postage can be claimed as part of routine expense claims. Copies of the HINs/RANs should be emailed to the dairy hygiene data team for storage.
3. Raw cows’ drinking milk sampling
In this section
3.1 Overview
The FSA’s DHIs have the responsibility for the collection and dispatch of Raw Cow’s Drinking Milk (RCDM) samples from registered milk production holdings in England and Wales.
3.2 Introduction
3.2.1 Legislation
Regulation 34, Schedule 6 of The Food Safety and Hygiene (England) Regulations 2013 (as amended) / Regulation 32, Schedule 6 of the Food Hygiene (Wales) Regulations 2006 (as amended) places restriction on the sale of raw milk intended for human consumption.
3.2.2 Standards applicable to RCDM
RCDM must meet the following standards:
- plate count at 300C (cfu/ml) ≤ 20,000
- coliforms (cfu/ml) < 100
3.2.3 Background
Under the consolidated EU hygiene rules, which took effect from 1 January 2006, Member States are able to introduce or maintain national rules prohibiting or restricting the placing on the market, within its territory, of raw milk or raw cream intended for direct human consumption.
RCDM must be labelled on the container or on a notice which must be displayed prominently at a farm catering establishment stating,
In England 'This milk has not been heat treated and may therefore contain organisms harmful to health’.
In Wales “This milk has not been heat treated and may therefore contain organisms harmful to health. The Food Standards Agency strongly advises that it should not be consumed by children, pregnant women, older people or those who are unwell or have chronic illness”.
A sample of raw milk will usually be procured for microbiological analysis at least twice per year. Whenever possible this sample will be taken in its final product container (FPC), if this is not possible then a sample will be collected from the bulk tank.
Samples must be delivered to the designated laboratory within 24 hours of the time at which the sample was collected (not 24 hours from the time at which the courier collected the sample from the DHI) to maintain the integrity of the sample.
3.3 Sampling frequency
3.3.1 Sampling frequency
Establishments wishing to sell RCDM must have the milk sampled and tested at least twice per year by FSA, to verify compliance with microbiological standards for total bacterial count, coliforms and pathogens that can commonly be found in raw milk
3.3.2 Routine sample
Routine sampling (RT) is conducted twice yearly. To take account of resource deployment, the next routine sample can be taken 6 months after the previous compliant sample.
3.3.3 Sample failure: FBO ceases sale of RCDM for direct human consumption
Should a routine sample of untreated milk fail to achieve the standards required by the regulations:
- the milk producer will be advised by telephone to cease selling raw milk directly to the public this will be recorded on the DH1 as verbal advice provided the failure occurred due to unsatisfactory results for indicator bacteria (TVC/Coliforms)
- If the failure is a result of a pathogen failure then sales MUST stop and the DHI should inform the FBO of this and record on the DH1 as above, if it is felt necessary enforcement at this stage could move straight to a warning letter, advice on this can be sought from the LDHI
- The producer should be advised to undertake an investigation into the root cause of the problem and consider their own sampling regime to test the effectiveness of any corrective actions taken
- follow up visits and further sampling will be undertaken where necessary but not before the FBO has provided some evidence that they have applied corrective action that has been effective
- this will continue until such time that satisfactory samples have been achieved and the DHI is content with the improvement in hygienic conditions at the premises; these follow up samples are taken by the DHI as per normal procedures
3.3.4 Sample failure: FBO continues to sell RCDM for direct human consumption
It is an offence to place raw milk on the market that has failed to meet the requirements of the regulations. Should a routine sample of untreated milk fail to achieve the standards required by the Regulations and the milk producer continues to sell untreated milk after having been advised to cease sales:
- escalation of enforcement should be applied. The enforcement approach and timescales applied could vary depending on whether the failure was due to indicator bacteria or pathogens, advice should be sought from the LDHI
- first failure: verbal advice
- second failure: inspection followed by formal warning in writing (Warning Letter)
- third failure: issue of a HIN or RAN (Wales)
- fourth or subsequent failure: referral to the Lead DHI for consideration of further action including issuing of a HEPN/HEPO in England and referral for investigation.
- in all cases it will be important to get advice from the LDHI on the correct course of action to take.
3.4 Sampling follow up
Upon receipt of a non-compliant test result, either for indicator bacteria or detection of a pathogen, the establishment will move to follow up (FU). The DHI must arrange to conduct an inspection of the establishment once they have been notified by the FBO that an investigation into the root cause of the issue has been done, that corrective action has been taken and their own sampling is indicating that this has been effective.
If the DHI is satisfied conditions upon the farm (such as cleanliness, maintenance, animal cleanliness and hygienic operations) are favourable, the FSMS is being implemented effectively and the FBO has sampling evidence suggesting this then a verification sample will be taken by the DHI and delivered to the nearest nominated laboratory.
If the DHI is dissatisfied with conditions on the farm (such as unsatisfactory cleanliness and maintenance, dirty animals and unhygienic operations) and the FSMS is not being implemented effectively, appropriate action must be taken to achieve compliance (see section 3) and recorded on the Digital Corrective Action Report form (DH1). A further sample will be taken only when the DHI is satisfied that the FBO has achieved compliance and conditions at the farm and its operations are satisfactory and evidence as such is provided.
If there is any doubt in the inspector’s mind which tests should be completed at the follow up stage then advice should be sought from the LDHI.
Should there be any dispute or disagreement on sampling results between FSA and FBO advice should be sought from the LDHI on the steps to be taken.
3.5 Sampling procedure
3.5.1 General considerations
This procedure describes how work is to be planned, lists the materials required to undertake sampling and inspection duties and details how records are to be kept.
In the interest of economy and efficiency, sampling should be planned as far as possible to farms / processing establishments in close proximity. The DHI must ensure that they have all the relevant details of the sampling schedule including the names, addresses and registration numbers of RCDM production holdings and laboratory reference (sampling point reference) for each premises.
All records MUST be completed at the time of sampling and / or inspection. Under no circumstances should records be completed after sampling / inspection nor should records be made in a notebook and then be transcribed onto the official record after sampling / inspection.
Where records are to be despatched in the secure insulated box these must always be placed in a sealed plastic bag to prevent damage.
Where the sampling DHI observes irregularities or instances where hygiene regulations are being contravened, these should be noted on the DH1 on the tablet in accordance with usual inspection procedures.
3.5.2 Pre-sampling checks
Before commencing routine or follow up RCDM sampling, the DHI must ensure that they have considered the following:
- courier service (if used) should be booked at least 24 hours in advance of collection using the Topspeed website
- sampling times should be considered with regard to which courier service is being used
- for same day service samples must be collected before 9am
- for overnight service samples must not be collected until after 10pm
They should also ensure that they have the following equipment:
- an adequate supply of pre-prepared disinfectant solution prepared according to the manufacturer’s instructions; FAM 30 should be mixed in the following ratio:
- 22ml of FAM30 to every 4 litres of clean water
- clean protective garment either:
- FSA green lairage coat
- white disposable lab coat
- clean wellington boots
- hairnet or other suitable covering for the head
- single use nitrile gloves
- container and brush for the disinfection of wellington boots
- disinfectant hand wipes
- hand sanitising gel
- data logger (supplied by PHE)
- sterile sampling container(s)
- tamper evident sample bags
- pens (always ensure that you have at least 2)
- supply of RCDM sampling sheets for dispatch to the lab
- calibrated temperature probe / thermometer
- sample dispatch cool box (supplied by PHE and containing all supplies needed as indicated by the packing instructions sheet PHE have supplied to the DHI’s)
- sufficient supply of refrigerant material (supplied by PHE). These should be frozen for at least 24 hours at <-18 degrees celsius before using
- security seals to seal cool box (supplied by PHE)
- courier contact details to arrange collection
- fully charged tablet to record any findings.
3.5.3 On farm procedure
When entering the farm, the sampling DHI must clean their boots using the pre-prepared FAM30 solution, using the provided container and brush. If gloves are used whilst cleaning boots, these must be removed and discarded before entering the milk storage area.
Before entering the milk storage area, the sampling DHI must put on their protective outer garment, hair covering and clean single use nitrile gloves. The external surface of the gloves should be sanitised using the hand gel provided.
3.5.4 Milk sampling
Whenever possible, routine samples should be taken from the FPC. If an FPC is not available, then the sample should be collected using a sterile single use sample pot. The source of any sample should be clearly recorded on the RCDM sampling sheet.
The PHE sampling record documentation must be completed and placed in a plastic bag to be sent with the sample.
3.5.5 Bulk storage sampling
A definition of a bulk storage container will vary depending on the scale of operation and could be:
- bulk tank
- milk churns
- storage jug in refrigerated store
The type of bulk storage vessel must be clearly indicated on the RCDM sampling sheet.
If a bulk tank is sampled the temperature reading must be checked. If the milk is <6°C then the milk agitator must be activated for at least 2 minutes prior to sampling. If a mobile bulk storage vessel is used then the contents must be dipped at least 15 times to achieve a sufficient agitation.
If the milk is >6°C (because milking has recently taken place) then time should be allowed for it to cool to <6°C before sampling should take place.
Remove the sterile sample pot / dipper from the packaging. Do not handle the inner surfaces of lids or necks of sampling vessels and dippers. You must discard any equipment or samples which come in contact with non-sterile surfaces. Dip the sample pot below the surface level of the bulk storage vessel and collect at least 30ml of liquid milk. This milk should then be decanted into another sterile pot taking care not to spill any milk onto the screw mechanism of the pot, the lid should be added and tightened adequately enough to prevent any leakage.
Place the collected milk sample into a tamper evident bag and seal. Ensure that the details of the establishment are clearly recorded on the space provided on the tamper evident bag and ask the FBO to sign the bag to confirm the details are correct.
3.5.6 Finished product container sampling
If the temperature is to be taken from the FPC, extra care should be taken not to cross contaminate the sample. The supplied non-invasive laser probe must be used to take temperature. The milk temperature should be no more than 6°C in order to be able to take the sample.
Clearly record the details of the establishment in the space provided on the tamper evident bag. Place the FPC into the bag and seal. Record the serial number of the tamper evident bag in the space provided on the RCDM sampling sheet.
3.5.7 Sample storage during collection
All samples must be transported in the PHE supplied cool storage box containing coolant materials. The container will ensure that the ambient internal temperature is maintained, provided that the lid is securely fitted.
The sampling DHI must take care to ensure that all samples are transferred to the cool storage box as soon as is practicable to ensure that the sample is cooled to and maintained at a temperature of no greater than 6°C.
When the first sample is transferred to the storage box the temperature logger provided must be activated according to the instructions provided by PHE.
3.6 Sample despatch
3.6.1 Sample delivery options
The method used to deliver the samples to the nominated laboratory will largely depend upon location. However, each DHI will have the option to use the nominated courier service if required.
It is essential that whenever possible the most efficient means of sample delivery are employed.
All milk samples must be delivered to the nominated laboratory within 24 hours of sample collection by the DHI. Please note the clock starts from the time that the DHI collects the sample and not from the time the courier picks the sample up.
No samples are to be delivered to the labs on a Friday each week. The only exception to this would be if sampling was being undertaken as part of the incident/outbreak protocol, if this is the case the DHI must contact the lab prior to sending the samples on a Friday.
3.6.2 Direct DHI drop off
In instances where the nominated laboratory is close to the home address of the DHI, close to the RCDM establishment or along the route of the sampling DHIs journey, it is acceptable for the DHI to drop the samples off at the laboratory directly.
When all samples are collected, contact must be made with the nominated laboratory to inform them that the samples are to be delivered. The laboratory must be made aware of how many samples are to be consigned.
3.6.3 Courier delivery option
Any samples that require a courier to facilitate delivery to the nominated laboratory must be notified before 4pm on the previous day. Book a collection at Topspeed. See Annex 10 for further information on the booking process.
The collected samples must be packaged into the PHE cool box following the packaging instructions supplied by PHE and making sure there is sufficient supply of refrigerant material to maintain the samples refrigerated throughout transportation. The temperature logger must be activated and hung /clipped into the sample holding frame taking care that it does not come into contact with the refrigerant blocks.
RCDM samples taken and booked for collection via Topspeed before 9am in areas north of Birmingham will go on a same day run to PHE - FERA York.
RCDM samples taken and booked for collection after 10am will go on the over-night run to PHE – FERA York.
RCDM samples taken and booked for collection before 9am in areas south of Birmingham will go on a same day run to PHE Porton Down.
There is no over-night run to PHE Porton Down.
3.7 Raw drinking milk from horses
Specific legislation on horse identification considers the fact that, horses are food producing animals and understands the specific situation of horses which are born as animals of a food producing species, but which are not in all cases primarily bred for that purpose and are in most cases not kept throughout their lives by food business operators.
Horse passports contain certain pages where, if the horse is eligible for slaughter for human consumption, veterinarians are required to record the administration of vaccines and any of the “essential substances”.
Where the passport has been signed to indicated that the horse is non-eligible for slaughter for human consumption, the horse loses the status as food producing animal and veterinarians can prescribe active substances from either the allowed or the prohibited substances lists.
Horses live long lives compared to more traditional food producing animals and can change ownership frequently during their life which makes tracing the use of medicinal products extremely difficult. Veterinary records are not always found in the passport, but records must be kept for at least five years even if the animal has been sold or slaughtered during that time and the FBO no longer has the passport.
The identification document (passport) is the target of significant fraud. The main risk represents the illegal reintroduction into the food chain of horses previously excluded from slaughter for human consumption and treated with medicinal products not authorised for food producing animals.
Regulation (EU) No. 37/2010 regulates the pharmacologically active substances that can be administered to food producing animals and also those which are prohibited because no MRL (maximum residue limits of veterinary medicinal products in foodstuffs of animal origin) have been established.
Food producing animals can only be treated with substances from the “Allowed Substances” and should never be treated with any of the “Prohibited Substances”. Phenylbutazone (BUTE) is not included in the list of prohibited substances, but it is not included in the list of allowed substances either. BUTE cannot be administered to horses unless their passports are marked as non-eligible for slaughter for human consumption.
Because of the peculiarities around the veterinary medicines administered, horses signed as non-eligible, should be considered as horses removed from the human food chain altogether and that their milk should not be allowed into the human food chain.
4. Enforcement
In this section
4.2 Relevant references and definitions
4.3 Legislation and enforcement provisions
4.5 Division of enforcement responsibilities
4.7 Recording and monitoring enforcement action
4.8 Guidance on completion of the corrective action log (CAL)
4.9 Gathering and preserving evidence
4.12 Detention under the Food Safety Act 1990
4.13 Certification procedure for non-compliant food
4.15 Hierarchy of enforcement: Introduction
4.16 Informal enforcement action: Verbal
4.17 Informal enforcement action: Written
4.18 Formal enforcement action: Statutory notice
4.19 Statutory notices for hygiene contraventions
4.20 Remedial action notice (RAN): Wales only
4.21 Hygiene improvement notice (HIN)
4.22 Hygiene emergency prohibition notices (HEPN)
4.23 Hygiene emergency prohibition orders (HEPO)
4.24 Referral for investigation
4.25 Change of FBO during enforcement action
4.26 Warrant to enter premises
4.27 Process for obtaining a warrant to enter premises, in England and Wales
4.1 Purpose
4.1.1 FSA enforcement role
These enforcement arrangements apply to all primary production dairy establishments registered in England and Wales and under supervision by FSA DHIs.
Enforcement action is taken in accordance with the FSA Dairy Hygiene procedures
4.2 Relevant references and definitions
4.2.1 Authorised Officers (AOs)
AOs involved in enforcement activities must bear in mind the definitions contained within the various pieces of legislation.
4.2.2 Food business operator (FBO)
FBO means the natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control.
Reference: (EC) No 178/2002, Article 3, Paragraph 6.
4.2.3 Court
References to the ‘Court’ in England and Wales should be taken to mean the Magistrates’ Court.
4.2.4 Justice of the Peace
References to the ‘Justice of the Peace’ (JP) should be taken to mean the Magistrate.
4.2.5 Duly authorised representative
Duly authorised representative is a responsible person who has the authority to act on behalf of the FBO.
4.2.6 Legal definitions
Most legislation includes a definition section that provides guidance on many of the phrases contained within it.
The table below identifies where this guidance can be found in the main pieces of legislation that we enforce.
Legal definitions
| Legislation | Location of definition |
|---|---|
| (EC) 178/2002 | Articles 2 and 3 |
| (EU) 2019/627 | Article 2 |
| (EC) 852/2004 | Article 2 |
| (EC) 853/2004 | Article 2 and Annexes I, II, III |
| The Food Safety Act 1990 (as amended) | Sections 1,2 and 53 |
| The Food Safety and Hygiene (England) Regulations 2013, as amended/ The Food Hygiene (Wales) Regulations 2006, as amended | Regulation 2 |
4.2.7 Guidance documents
European Commission Guidance document on the implementation of certain provisions of (EC) No 852/2004
European Commission Guidance document on the implementation of certain provisions of Regulation (EC) No 853/2004
Guidance Notes for FBOs on Food Safety, Traceability, Product Withdrawal and Recall – A guide to compliance with Articles 14, 16, 18 and 19 of General Food Law Regulation (EC) 178/2002
Reference: These documents can be viewed using the web links quoted above, and are also reproduced in Volume 2 of the MOC.
4.3 Legislation and enforcement provisions
4.3.1 Requirement to enforce
Each Member State (MS) must enforce food law by monitoring and verifying that relevant legislative requirements are met through a system of official controls and other activities. It is for each member state to lay down the rules on measures and penalties to be applied when infringements of food law are detected.
Reference: (EC) 178/2002, Article 17, Paragraph 2.
Food law includes all statutes, regulations and administrative provisions governing food in general, and food safety in particular. It covers all stages of production, processing and distribution of food, and also of feed produced for, or fed to, food-producing animals.
4.3.2 Enforcement provisions
EC Regulations are directly applicable in all MS. They detail both the legal requirements the FBO must comply with, as well as official controls that must be applied by the competent authority.
They do not, however, specify the powers of AOs, powers of entry, time limits to bring a prosecution, or the split in enforcement responsibilities between different agencies that enforce the same legislation.
Each MS is required to separately introduce national implementing legislation providing enforcement powers, setting out offences for failure to comply with the European Regulations and to establish the administrative system under which non-compliances by FBOs can be brought before the courts.
4.3.3 General principles
(EC) No. 178/2002 sets out the general principles and requirements of food law, establishes the European Food Safety Authority (EFSA) and lays down procedures in matters of food safety. It contains:
- definitions (such as food, food business operator)
- basic principles: FBO responsibility for food safety
- traceability requirements
4.3.4 EC Hygiene Regulations
The expression ‘Hygiene Regulations’ is defined in Regulation 2 of the domestic hygiene regulations to include:
(EC) 852/2004 dealing with the hygiene of foodstuffs. It applies to all food businesses, encourages good hygiene practices and introduces the concept of industry guides.
(EC) 853/2004 laying down specific hygiene rules for food of animal origin. It sets out additional requirements beyond 852/2004 for specific food of animal origin; such as milk, eggs, meat, fish.
(EU) 2019/627 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. It covers the nature of official controls, inspection, verification, auditing and the role of AOs.
4.3.5 Domestic Regulations
In addition to the EU legislation listed above, ‘Hygiene Regulations’ also includes the domestic national hygiene regulations introduced in the respective devolved countries to allow for the enforcement and administration of EU hygiene legislation.
4.3.6 Amendments
Periodically EU and domestic legislation is amended and such changes must be read in conjunction with the original published versions. Whilst the MOC is updated with consolidated versions of the EU Regulations, it is suggested that if you are unsure whether the most up to date version of the legislation exists, you should check the latest version of the EC Regulations.
4.3.7 Domestic regulations
The domestic regulations that apply to dairy establishments include:
- the Food Safety and Hygiene (England) Regulations 2013 (as amended) / The Food Hygiene (Wales) Regulations 2006 (as amended), which provide enforcement powers in respect of the obligations that apply in Regulations (EC) No. 852/2004, (EC) No. 853/2004 and (EU) No. 2019/627
- the General Food Regulations 2004 (Wales only); these provide enforcement powers in respect of the obligations that apply in (EC) 178/2002, for example:
- Article 14 ‘the food safety requirements’
- Article 19 ‘recall, withdrawal and notification requirements’
Note: Specific provisions contained in the Food Safety Act 1990 and its amendments still apply to these Regulations; for example, powers and definitions.
4.3.8 Enforcement Concordat
In addition to the legal requirements imposed by the EC legislation, FSA Operations Group has been a signatory to the Enforcement Concordat since June 2001 and is required to adhere to its main principles, which include proportionality and consistency of enforcement.
The DTI Enforcement Concordat: Good Practice Guide for England and Wales states of proportionality that apart from taking a progressive approach, enforcement will mean applying the principles of risk assessment to enforcement activity and enforcement bodies should focus their attention on the most serious risks, or where potential hazards are least well controlled. Compliance in lower risk business activities should be encouraged by being open and helpful (paragraph 44).
In respect of consistency, the Enforcement Concordat states that ‘it is important to ensure, and demonstrate, that enforcement activities are consistent both within a single enforcement body and between enforcers regionally and nationally. Whilst consistency of approach does not mean uniformity, it does mean taking a similar approach in similar circumstances to achieve similar ends’ (paragraph 50).
4.4 Premises database
4.4.1 Database contents
An electronic record will be maintained for all dairy establishments inspected by the FSA. This will be in the form of a database which will include details of the farm registration, the FBO responsible for potential offences, all correspondence in chronological order, copies of all formal notices and inspection reports.
Prior to an inspection, DHIs will extract the relevant information required to inform their inspection.
4.4.2 Security
The inspection reports and all enforcement literature must be kept securely at all times and will be incorporated on to the database upon completion of the visit. Contents of the database may contain evidence that is required for formal court action at a later date, together with additional unused material that the FSA may have to disclose should a case go to trial.
4.5 Division of enforcement responsibilities
4.5.1 FSA enforcement responsibilities
- Inspection and enforcement of Regulation (EC) No. 852 and (EC) No. 853/2004 at an establishment where one or more farmed animals are kept to produce milk with a view to placing it on the market as food.
- Sampling of raw cow’s milk supplied direct for human consumption for compliance with the microbiological requirements of Schedule 6 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) / Food Safety and Hygiene (Wales) Regulations 2006 (as amended).
- Enforcement of the national marketing requirements for the sale of raw cow’s milk intended for direct human consumption under Schedule 6 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) / Food Hygiene (Wales) Regulations 2006 (as amended).
- Inspection and Enforcement of the labelling requirements in schedule 6 of the domestic hygiene regulations with respect to the health warnings required for RCDM sales.
- Enforcement of Regulation (EC) No. 178/2002 with respect to Articles 14, 18 and 19 at primary production establishments producing raw cow’s milk for direct supply for human consumption.
4.5.2 LA enforcement responsibilities
- Inspection and enforcement of Regulation (EC) No. 852 and Regulation (EC) No. 853/2004 at establishments undertaking heat treatment of raw milk and further processing of milk and milk products at milk establishments subject to approval under Regulation (EC) No. 853/2004.
- Sampling of raw milk (other than cow’s milk) supplied direct for human consumption for compliance with the microbiological requirements of Schedule 6 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) / Food Hygiene (Wales) Regulations 2006 (as amended).
- Inspection and enforcement of the safety labelling requirements in schedule 6 of the domestic hygiene regulations with respect to the health warning required for all raw milk sales (except cows and buffalo).
- Inspection and enforcement of Regulations (EC) No. 852 and 853/2004 at establishments undertaking bottling operations.
- Enforcement of Regulation (EC) No. 178/2002 with respect to Articles 14, 16, 18 and 19 at primary production establishments producing raw milk other than cow’s milk for direct supply for human consumption.
4.6 Communication with FBOs
4.6.1 Communication channels
The key to a successful working relationship is communication. There is nowhere that this is more important than in relation to guiding the FBO on compliance with legal requirements, as well as best practice.
The majority of day-to-day compliance can be achieved through verbal discussion.
It is important that contingency arrangements exist to avoid difficulties when the FBOs normal contact person is unavailable.
Note: Establish details of any duly authorised representative that has authority to act on behalf of the FBO. This is especially important for the service of RAN (Wales only).
4.6.2 FBO contact details
The AO must have available all registration details for the FBO, including:
- full name(s) of owner / partners
- address(es)
- telephone number(s)
- limited company name and registered office address
- any duly authorised representative
Regulation (EC) No. 852/2004, Article 6, Paragraph 2, requires FBOs to ensure that the Competent Authority always has up to date information on establishments, including the notification of any significant change in activities and any closure of an existing establishment.
However, this requirement is rarely complied with and therefore DHIs should always check that the registration details of the establishment have not changed to ensure information held by the FSA is accurate.
Updates on registration details must be provided to the Approvals and Registrations team. This will ensure that the AO is always aware of the legal entity responsible for any potential offences within the establishment, whether they are a sole trader, partnership or limited company and whether the FBO has started to sell raw drinking milk for direct consumption without notifying the FSA.
4.6.3 Key communication functions
The AO is responsible for:
- advising the FBO on compliance with legal requirements
- advising the FBO when infringements of legal requirements have been detected
4.7 Recording and monitoring enforcement action
4.7.1 FSA corrective action log
Enforcement action taken must be recorded accurately on the corrective action log of the Hygiene Inspection Report.
The purpose of this document is to help the AO in their:
- assessment and prioritisation of enforcement action
- communication of enforcement action to other AOs
- tracking or monitoring of enforcement action through to compliance or a referral for investigation
The document:
- acts as an aide memoire and provides a record of enforcement action taken in the establishment
- enables the FSA to assess the FBOs past record as regards compliance with food law
- contributes to any changes in the inspection frequency as a result of formal enforcement action that has been taken
- provides an outline of the non-compliances to ITLs, FVCs, management and internal audit
4.7.2 Ongoing enforcement action
Prior to visiting any milk production holding, the AO must:
- familiarise themselves with all ongoing enforcement action
- maintain the momentum of existing enforcement action; only where they are able to support this enforcement, should they escalate it
4.7.3 Completing the corrective action report
The corrective action report should be a ‘live’ form, updated as necessary every time enforcement action is taken.
Non-compliances of a recurring nature (that are not solved when corrective action is requested) should be entered under the same reference number in the corrective action log, in order to demonstrate continuity of enforcement action and if necessary, prove repeated non-compliance in cases referred for investigation. In electronic forms, the additional entries can be made on subsequent lines of the same box, underneath the original entry.
Where a non-compliance is corrected but reoccurs some time later, details may be entered under a different reference number, which you may cross-reference to the previous entry where details of the offence were recorded.
4.8 Guidance on completion of the corrective action log (CAL)
4.8.1 Reference number
The AO should enter the milk production holding’s CPH number, followed by the month, then the last two digits of the year, and lastly a sequential number for each deficiency (for example, HN/07/12/001, HN/07/12/002/, HN/07/12/003/) on the CAL.
These numbers should correlate with the reference number for any written enforcement. In letters, the reference number should be provided in the format of, producer ID, Letter type, DHI initials, date of issue, (for example 1234-WL-CB-01.04.19-01). This reference number should also be entered into the subject bar of any email communication linked to it; this will aid with storing of these documents.
4.8.2 Regulation reference and deficiency
The AO should:
- state the year and Regulation reference number
- give a short description of the deficiency, for example, failure to clean equipment
More than one line may be used if required.
4.8.3 Action required
The AO must detail any action the FBO must take to comply with the requirements of the legislation.
4.8.4 Agreed completion dates
The AO must insert the date agreed with the FBO for the correction of the deficiency, or the date for compliance specified in any formal notice.
If the FBO does not agree to a completion date, the AO must still insert the date they consider appropriate and indicate that it was ‘not agreed’. Any letters should also include this date.
A revisit should be made to the milk production holding after the date on which compliance was required to be achieved. Where compliance has not been achieved by the due date, the FBO should be reminded of the issue and enforcement action should be escalated to the next stage of the hierarchy.
When agreeing or setting completion dates, a reasonable deadline for the rectification of each deficiency should be agreed. The deadline should be realistic to allow the FBO to rectify the deficiency, whilst still considering the risk to public health.
4.8.5 Detention notice served and withdrawn
The AO should specify the date on which a formal detention notice (DH ENF 11/1) has been served on the FBO. It is essential that any investigation to determine the fitness of the milk for human consumption is undertaken in a timely manner, paying particular attention to the 21 day limit.
Where the AO is satisfied that the detained milk can enter the food chain, they should insert the date on which the detention notice is withdrawn, below the date on which it was served.
If, as a result of investigation, the AO decides not to release the milk for human consumption, they must insert ‘Not Released’ under the date the notice was served. If the milk is not voluntarily surrendered, it must be formally seized prior to the expiry of the 21 day period.
4.8.6 Date compliance achieved
Record the actual date on which compliance is achieved, even if it is the same day that enforcement action was taken.
4.8.7 Structural work
Where structural work must be undertaken, the ‘corrective action’ section of an advisory letter or HIN should be specific enough to explain the legal requirement and the outcome to be achieved, without being too prescriptive about the exact way in which this must be achieved.
There may be many ways that the FBO can achieve compliance, but provided they comply with the legal requirement, they have the option to do the work in the way they see fit, or carry out works to an equivalent effect.
4.8.8 Monitoring progress
The AO should regularly monitor progress towards compliance to identify whether the deficiency is likely to be rectified within the agreed time scale. If necessary, they should ask to see evidence of how corrective action is progressing, such as planning permission application / copies of quotes for work / structural plans.
Where the work does not progress at the agreed rate, the AO should consider escalating the issue, such as serving a HIN, which will formalise the agreed time scale and thereby maintain the momentum in enforcement.
It is important that agreed action plans are set out at the start and that the AO takes a reasonable approach where certain issues arise that are outside the FBOs control, provided that the risk to public health permits this approach.
4.9 Gathering and preserving evidence
4.9.1 Introduction
The AO must gather evidence at the time the offence is witnessed, making detailed contemporaneous notes, in their pocketbook, which at a later stage could be relied upon in Court. (See guidance below or further guidance in this section). It may be impossible to gather evidence retrospectively as it may no longer exist. Evidence may come in a variety of forms and must supplement a witness statement as an exhibit in order that it may be admissible in court. Where possible, or if problems are envisaged, it is always useful to obtain corroboration and assistance from other colleagues.
Detailed evidence gathering at the time of the offence will provide the AO with as much material as possible to support their witness statement and prove the elements of the offence.
Note: look after evidence - keep it secure. It is fundamental to proving the offence should formal action be pursued.
4.9.2 Best evidence rule
The AO should also have regard to the ‘best evidence’ rule. Whenever possible, any original items of evidence should be preserved, (the original form of a document, rather than a photocopy). If the evidence is a milk sample, this should be submitted for testing as soon as possible and a Certificate of Analysis retained. Where practical, the FBO should also be given the opportunity to have the evidence examined by an expert before destruction.
The AO may also wish to consider taking photographs and / or sample evidence before perishable goods are destroyed. If there is doubt about what evidence should be retained, the AO can obtain further advice from FSA Legal.
4.9.3 Note taking
When gathering evidence, remember to record the details of any other persons present. This will enable the FSA Investigation Officers (IO) to identify all potential witnesses in the case and will enable witness statements to be taken.
The AO must make full use of their pocketbook to make factual contemporaneous notes. These may be referred to in court to help recollect facts and figures that it is impossible to recall in detail after the event.
In court, a witness is able to refer to contemporaneous notes recorded in their pocketbook, that were made either at the time of the incident or straight afterwards. Where an officer refers to their notebook when giving evidence in court, the defence is entitled to see that notebook.
Note: However, witnesses are not permitted to read from their witness statement when giving evidence, except in certain limited circumstances.
4.9.4 Important points
Pocketbooks may be inspected in court, therefore the following guidance must be followed to maintain validity:
- record name on front cover, designation and date started
- make all entries with ink or ballpoint pen
- include only original entries and do not copy notes from elsewhere
- record the date and time at commencement, and upon completion
- enter the notes at the time ‘the offence’ is witnessed or as soon as possible afterwards (contemporaneously), whilst the facts are fresh in the memory
- to make alterations, strike a pen through the error and write the correction; then initial in the left hand column
- notes must not be erased
- do not remove pages from the notebook
- sign and date each entry at the bottom of each page
Entries must be relevant, factual, legible, concise and written in plain English.
If accompanied by a colleague whilst witnessing a contravention, one AO may record the details in their pocketbook. The other may read through the notes made and where they agree with what has been recorded, they may countersign at the end of the entry to acknowledge that it is a true and accurate account of events
4.9.5 Security
The AO is responsible for ensuring the security of their notebook and for producing it in court. Further notebooks are available from CSU York on return of the completed notebook.
4.9.6 Return of all notebooks
Notebooks remain the property of the FSA and must be returned to York prior to leaving the FSA.
4.9.7 Disclosure of unused material
The Criminal Procedures and Investigations Act 1996 (CPIA) places an obligation on the prosecuting authority to retain and record all relevant information relating to any formal action.
The prosecuting authority, a term which includes the AO, the IO and the FSA itself, has a duty to disclose to the defence all relevant unused material which:
- might undermine the case for the prosecution, or
- might reasonably be expected to assist the defence case
This material may include:
- informal and formal memos
- email traffic
- previously unreported offences and / or warnings recorded on operational paperwork
- daybook entries
- contemporaneous notebook entries
- minutes of meetings
- draft witness statements
- photographs as part of unused material
- instructions to expert witnesses or analysts
4.9.8 Storage and availability
Anything that is relevant to the case and which is not used by the prosecution is unused material and can be potentially disclosed. This fact makes it important that when notes are taken, emails written or drafts prepared, they should be made on the understanding that the defence may be entitled to see them and refer to them in open court. Even if there are good reasons for arguing that they are so sensitive that the defence should not see them, it is for the court to decide.
The AO and FSA team should ensure that:
- all material should be recorded and retained
- all material should be safely stored
The IO must be made aware of its existence as soon as possible after a recommendation is made.
4.9.9 Photographic evidence
Taking photographs for the purposes of evidence gathering will often be a fundamental part of the evidence gathering process.
The AO may inform the FBO of their intention to take photographs as a matter of courtesy, however, the FBO cannot stop an AO from taking photographs for the purposes of evidence gathering and it could be an offence of obstruction for them to prevent the AO carrying out their duties.
- when photographs are taken, details should be recorded in a contemporaneous notebook, including the photograph number, the subject, location and date / time. Colleagues should assist one another in this process, if available.
- photographs should be taken with a suitable digital camera; however, a record must be kept of how the digital information was downloaded and on to what medium it was stored, together with the ‘Supporting evidence photographic report’ for recording full details of digital images taken (DH ENF 11/14).
Reference: See sub-topic 4.9.11 on ‘Digital camera protocol’ in this section for additional information.
- always add details to the reverse of the photograph, clearly indicating the subject matter, location and other relevant details.
- record details of when and where any films are processed (if relevant).
- video filming may be very useful to demonstrate a particular operation. However, it is advisable that where the AO is not familiar with the equipment, that they receive some instruction and / or practice with the equipment prior to gathering the evidence that may be required for court.
Note: Any verbal comment recorded whilst any filming is being undertaken must later be transcribed word for word and will constitute part of the evidence.
Tip: Give the camera lens time to adjust to the temperature / humidity before taking pictures in order to prevent fogging.
4.9.10 Digital camera protocol
When the AO captures images using a digital camera, they must ensure the following:
- the memory card is clear of previous images
- any poor quality images must not be deleted
- full particulars of images are recorded using the ‘Supporting evidence photographic report’ available at Annex 6
- images, along with the corresponding photographic evidence report, are downloaded onto the hard-drive of a computer
- the images and supporting photographic evidence report are copied onto two separate non-reusable CD-ROMs
- one CD is marked as the ‘Master copy’; this must be bagged / tagged / its details recorded in the AOs contemporaneous note book and stored somewhere secure
- the other CD is marked as the ‘Working copy’; it should also be tagged and its details recorded in the AOs contemporaneous note book and stored in a secure place for the IO.
4.9.11 Supporting evidence photographic report
The Supporting Evidence Photographic Report provides a contemporaneous record of images taken whilst gathering evidence.
In ideal circumstances, the report should be completed at the time the evidence is gathered. However, when this is not feasible, it should be completed as soon as possible thereafter.
The report should be stored electronically in the same file as the images to which it relates.
A new report should be prepared to accompany images of each separate incident.
The Supporting Evidence Photographic Report is available at Annex 6 to this chapter. (This will open as a separate document when accessed using the chapter bookmark on the left hand side of this page.)
4.9.12 Retention of unused photographic images
All unused photographs, images and negatives must be retained. These may be disclosed to the defence as ‘unused material’ in line with the provisions of the Criminal Procedures and Investigations Act 1996 in England and Wales.
4.9.13 Powers to photograph
Although all AOs have powers to take photographs for the purpose of evidence gathering, they must always seek the permission of the FBO, if they are taking photographs for any other reason than evidence gathering.
4.9.14 Samples: physical confirmation of the failure
A variety of different types of sample may be used as evidence, for example:
- dirt from soiled equipment
- caked milk residues on equipment
- samples of milk
The AO should inform the operator of their intentions. Enlist the services of a colleague (if available) to witness the collection of the sample and also to record details of what, when, where and how, it was obtained, putting the date and time in their pocket notebook. The samples should be effectively bagged and labelled with all relevant details. It should then be sealed with a talisman security tag.
All samples must be kept under secure conditions in an environment where they will not deteriorate. Details of storage location and transportation should also be recorded to maintain continuity of evidence. Temperature logs and relevant calibration records of chillers and freezers, where evidence samples are stored, should be accurately maintained, as they may be required as legal evidence in court.
4.9.15 Temperature readings: factual figures
The AO should ensure that where thermometers are used for evidential purposes: the thermometer used is correctly calibrated, and prior to a court hearing, ensure it is recalibrated. The calibration certificate will be required as an exhibit. All relevant temperatures must be recorded where necessary (ambient, surface).
4.10 Information obtained from unauthorised sources (RIPA)
4.10.1 Introduction
This topic covers instruction on dealing with information which may be provided under the Regulation of Investigatory Powers Act 2000 (known as RIPA).
4.10.2 Information received
Under the law, AOs should take extreme care when dealing with a case where farm staff, consumers, residents, or other contacts have provided information about possible offences or misconduct.
Where this sort of information is provided, the AO must always inform their line manager, who must in turn notify FSA Legal Services and the Investigations Branch.
4.10.3 Questioning contacts
Any person passing on information must never be tasked to obtain or pass on information about possible offences or misconduct. If they are asked to pass on information, it almost certainly will not be possible to conduct a successful investigation into the allegations since it will not be possible to use the evidence obtained.
4.10.4 Use of informers
It is essential that AOs remember not to ask contacts to obtain or pass on information about possible offences or misconduct even where they have first come forward of their own free will and given information about such matters.
AOs must not try to get someone to act as an informer or obtain information in an undercover way.
4.10.5 Example
A disgruntled employee contacts you to inform you that the FBO of a farm is secretly selling raw cow’s milk direct for human consumption at a local fitness club, without FSA sampling and contrary to the marketing provisions. He is in a position to know when this is happening and to contact you at the time it is taking place.
4.11 Surrender, detention, seizure and condemnation voluntary surrender
4.11.1 Means of voluntary surrender
Where milk has not been produced / processed or distributed in accordance with the hygiene regulations or fails to meet the food safety requirements, the FSA should seek voluntary surrender of the milk.
An ‘Agreement to Destroy Food’ (DH ENF 11/7) notice should be completed where any dispute arises, or where issues are more complex. For example:
- there are large quantities of milk
- the FBO is selling raw milk to third parties for further processing to satisfy contracts
This agreement should be completed before the milk is consigned to the slurry tank.
Reference: See chapter 9 on ‘Forms’ for DH ENF 11/7.
4.11.2 Legal powers
The authorised AO has powers to detain food under:
- Section 9 of The Food Safety Act 1990 (as amended), via Regulation 23 of the Food Hygiene (Wales) Regulations 2006 / Regulation 25 of the Food Safety and Hygiene (England) Regulations 2013 (as amended).
4.11.3 Labelling detained milk
Milk detained for further inspection should be stored securely in a container that allows for its individual identification. Containers can be tagged using individually numbered talisman seal(s). The individual seal numbers should be recorded with any other relevant details on the Detention of Food Notice.
The seals must remain in place until the milk has been sampled, or a decision made on whether or not the milk complies with the food safety requirements.
4.11.4 Detention tape
Detention tape may be used to help identify large quantities of raw milk in conjunction with a Detention of Food Notice – for example, the contents of a bulk tank.
4.11.5 When to formally detain
There may be occasions where milk cannot be dealt with immediately because an investigation into:
- the fitness and suitability of any milk under the FBOs control is required
- the suitability of milk coming from a specific animal due to its TB /BR or animal welfare status
- any antibiotic residues contained in the milk or the administration of any banned substances to the food producing animals under the FBOs control
For example, in such circumstances, the AO will require the FBO to store the milk in an appropriate container. The product and / or animal must be sampled / tested and any non-compliant milk must be disposed of as an ABP. In the event that the FBO refuses to dispose of non-compliant milk, further action will be required through the LA or APHA, who have relevant enforcement powers to require disposal under the respective legislation.
4.12 Detention of food under the Food Safety Act 1990
4.12.1 Relevant legislation
Regulation 23 of The Food Hygiene (Wales) Regulations 2006 and / or Regulation 25 of The Food Safety and Hygiene (England) Regulations 2013 (as amended) allow the AO to detain suspect food for further investigation. This is achieved via the detention provisions contained in Section 9(3)(a) of the Food Safety Act 1990, which provides powers for the AO to detain, inspect and seize any food that is thought may not comply with the food safety requirements and is intended for human consumption. The Detention of Food Notice to use in these circumstances is the DH ENF 11/1.
4.12.2 When to serve a Detention of Food Notice (DH ENF 11/1)
When FBOs are unwilling to either surrender milk that the AO has judged unfit, or to detain milk for further sampling to enable its fitness or compliance with food safety requirements to be properly assessed, the AO must detain or seize (as appropriate) the food in accordance with Food Safety Act Section 9.
Note: The AO shall as soon as is reasonably practicable, and in any event within 21 days, determine whether or not he is satisfied that the food complies with the food safety requirement.
4.12.3 Reasons for service
Milk which fails to comply with food safety requirements under Article 14, (EC) No. 178/2002, includes:
- milk that is unsafe
- milk that is unfit for human consumption
- milk that is injurious to health
4.12.4 Service of notice
Prior to serving a notice, the AO must have in their possession all the evidence to justify its service. The Detention of Food Notice should be served by hand on the person in possession of the milk who is deemed to be ‘the owner’.
4.12.5 Content of notice
The notice must specify:
- description
- quantity
- identification marks if any (detained tags, numbers or labels)
- any location the food may be moved to (if applicable)
4.12.6 Number of notices
Where a quantity of milk of different types or batches is being detained, the AO should issue a separate Detention of Food Notice for each type or batch.
Where the milk that fails to comply with the hygiene requirements is part of a batch of the same class or description, it shall be presumed unless the contrary is shown that the whole batch fails to comply and the AO should detain all of it. Part of the food may subsequently be seized if necessary and an Order for Condemnation of Food applied for. The Detention Notice must be withdrawn in respect of the remainder if the AO is satisfied that the problem affects only part of the batch.
Reference: The Food Hygiene (Wales) Regulations 2006 (as amended), Regulation 27 (3) / Food Safety and Hygiene (England) Regulations 2013 (as amended), Regulation 29 (3) and Food Safety Act 1990 Section 8 (3).
4.12.7 Right of appeal
No right of appeal exists for a Detention of Food Notice under the Food Safety Act 1990. However, if not voluntarily surrendered the food must be seized and taken before a Justice of the Peace for them to decide whether it should be condemned or not.
4.12.8 Time limit
The AO shall, as soon as is reasonably practicable, and in any event within 21 days, determine whether or not they are satisfied that the milk complies with the food safety requirement.
If they are satisfied that the food complies with food safety requirements, the AO must immediately withdraw the notice.
Or, if the AO is not satisfied that the food complies, they must seize the food and have it dealt with by a Justice of the Peace.
4.12.9 Withdrawal
If the notice is to be withdrawn, the AO must immediately serve a Withdrawal of Detention of Food Notice upon the recipient of the original Detention of Food Notice.
If a Detention of Food Notice is withdrawn, or condemnation order is refused, compensation is payable to the owner of the food for any depreciation in its value which can be shown to result from the AOs actions.
The AO must ensure that all detained food is suitably and securely stored to minimise any deterioration.
4.12.10 AO checklist
Where the detained food is not released, specify in the AO checklist on the reverse of the Detention of Food Notice:
- the nature of disposal
- whether an Agreement to Destroy Food Notice was signed by the FBO and the Notice reference number
- whether the detention led to the food being certified, seized and taken before a court to have it condemned
4.13 Certification procedure for non-compliant food
4.13.1 Legislation
Regulation 27 of the Food Hygiene (Wales) Regulations 2006 / Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) specifies that where food has not been ‘produced, processed, or distributed’ in accordance with the ‘Hygiene Regulations’ it shall be treated for the purposes of Section 9 of the Food Safety Act, as failing to comply with the food safety requirements.
Art 14 (EC) No. 178/2002 defines the expression ‘food safety requirements’ to include:
- food which is unsafe, injurious to health or unfit for human consumption, having regard to the normal conditions of use of the food by the consumer and to the information provided to the consumer, including information on the label, or other information generally available to the consumer concerning the avoidance of specific adverse health effects from a particular food or category of foods.
- in determining whether any food is injurious to health, regard shall be had to the probable immediate short and long term effects of that food on the health of a person consuming it, but also on subsequent generations; to the probable cumulative toxic effects; to the particular health sensitivities of a specific category of consumers where the food is intended for that category of consumers.
- in determining whether any food is unfit for human consumption, regard shall be had to whether the food is unacceptable for human consumption according to its intended use, for reasons of contamination, whether by extraneous matter or otherwise, or through putrefaction, deterioration or decay.
Where evidence can be tendered to prove that the milk has not been produced, processed or distributed in accordance with the Hygiene Regulations, certification of the non- compliant milk will deem the milk to fail to comply with the food safety requirements of Regulation (EC) No. 178/2002 through the provisions of Regulation 27(2) (Wales) or Regulation 29(2) (England), and the Magistrate will be required to condemn the milk.
4.14 Condemnation procedure
4.14.1 When to apply for a condemnation order from the court
Only where milk has failed to be produced, processed or distributed in accordance with the hygiene regulations, or breaches the ‘food safety requirements’ should the AO:
- formally detain the food (DH ENF 11/1)
- certify the food as non-compliant (DH ENF 11/25)
- formally seize the food (DH ENF 11/ 27)
and apply to a Magistrate to issue a Condemnation Order.
Note: It is not a legal requirement to formally detain the milk prior to its formal seizure.
4.14.2 England and Wales
In England and Wales a Condemnation Order may be obtained from a Justice of the Peace at the Magistrates’ Court.
4.14.3 Action to take
The AO is to follow the steps in the table below.
Regulation: The Food Safety Act 1990 Section 9 (3) (b), Section 9(4) (b).
Reference: See Code of Practice.
Action to take
| Action | Detail |
|---|---|
| Detain the food (ENF 11/1) |
Where the milk you suspect does not comply with the food safety requirements and needs to be secured to prevent it from being used for human consumption, is should be formally detained using a Food Safety Act Detention of Food Notice (DH ENF 11/1). Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/1. |
| Advise FSA Legal | FSA York will arrange legal representation. A summary of events and copy of all legal notices must be sent to FSA Legal. |
| Contact local police | Establish which court covers the area for the establishment where the detained milk is held. |
| Contact the Court |
Speak to the Clerk of the Court to establish local procedures. Explain:
In England and Wales, establish with the clerk a date, time and location for the court hearing. The location can be either the local courtroom or the holding depending upon circumstances. Note: the court date must coincide with the availability of the FSA legal representative, so liaise with FSA York prior to serving the Food Condemnation Warning Notice (DH ENF 11-3). |
| Complete and serve Certification of Food Notice (DH ENF 11/25) |
Once the AO has determined that the food has not been produced, processed or distributed in accordance with the provisions of the Hygiene Regulations they must serve notice on the FBO with the reasons why it fails to comply. Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/25. |
| Complete and serve a Seizure of Food Notice (DH ENF 11/27) |
If after certifying the food, the FBO refuses to voluntarily surrender it, complete a Seizure of Food Notice (DH ENF 11/27) and serve it on the FBO and a copy on the owner of the food where relevant. Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/27. |
| Complete and serve a Food Condemnation Warning Notice (DH ENF 11/3) |
DH ENF 11/3 should be served on the owner / person in charge of the food (FBO). Ensure that the notice is served by the most appropriate method available in the circumstances to ensure that all relevant parties are informed of the time and place of the hearing in good time. Document and retain records of service to show the court. Retain copies of the Food Condemnation Warning Notice, the Certification of Food Notice and the Seizure of Food Notice to produce to the Justice of the Peace, the Clerk to the Court and the FSA legal representative. Always have a copy of the Food Law Code of Practice and Practice Guidance. Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/3. |
| Attend the hearing |
Prepare three copies of the Complaint for Condemnation of Food Order (DH ENF 11/15) and of the Order for Condemnation of Food (DH ENF 11/16) itself for the Justice of the Peace to sign. Read the papers again before going to court. Attend court early to meet the FSA advocate. On attending the hearing, the AO should take:
Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/15 and 11/16. Explain clearly when presenting the evidence in court:
|
| If successful: maintain supervision to ensure milk is disposed of | If the Justice of the Peace issues an ‘Order for Condemnation of Food’, upon receipt of the Order ensure that the person in charge of the food (and the owner if notified) receives a copy. Ensure that the details of disposal have been recorded and that a copy of the waste transfer note has been scanned into the dairy hygiene database. |
| If unsuccessful: milk must be restored to owner | Where any issue of compensation arises, the AO must not discuss or negotiate any compensation for depreciation in value of the food. The AO should ask the FBO / owner of the food to put any complaint in writing to the HOD. |
4.15 Hierarchy of enforcement: Introduction
4.15.1 The hierarchy of enforcement
Updated [The flow diagram below outlines the stages that comprise the hierarchy of enforcement and explains that the process begins with informal action (verbal advice or letter) and progresses to formal action (formal statutory notice or referral for investigation).]
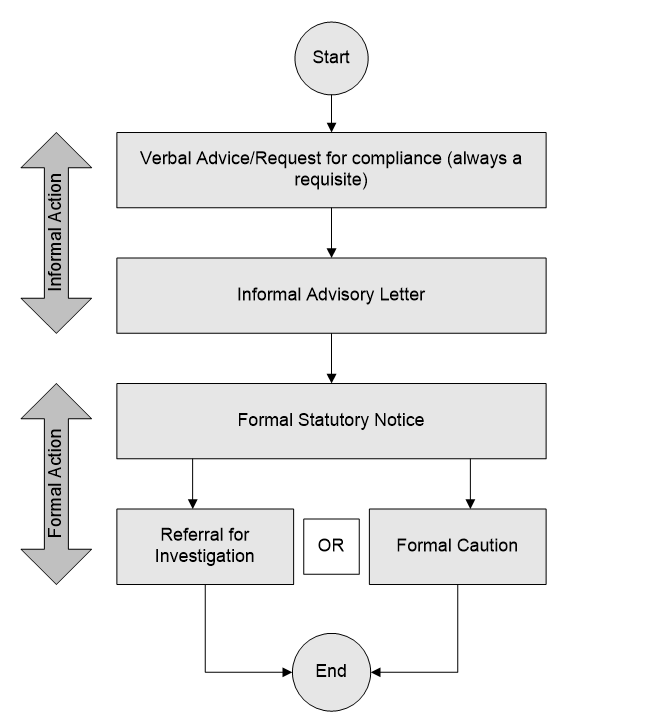
4.15.2 Approach to the hierarchy
The approach to the hierarchy of enforcement and level at which the AO commences enforcement action will be dependent upon:
- the urgency / severity of the situation
- the most appropriate course of action that will control the risk
- the enforcement tools available under that piece of legislation
- the history of the FBO and their willingness to comply
4.15.3 Enforcement
The term ‘Enforcement’ includes advisory visits, assisting the FBO with compliance and formal enforcement action (see Enforcement Concordat: Good Practice Guide for England and Wales Paragraph 88).
Verbal advice, written advice and written warnings all constitutes informal enforcement action.
Formal enforcement action includes official detention of food, the service of formal notices, and referrals for investigation and prosecutions.
4.15.4 Subject of enforcement action
Any FBO who is the subject of enforcement action should be kept fully informed of any intended or actual enforcement action by the AO.
4.16 Informal enforcement action: verbal
4.16.1 When to give verbal advice
The first stage of enforcement action should be education and advice. Whilst it is the FBOs job to know the legal provisions relating to their business, the AO should ensure that, where necessary, they clarify and update the FBO on any relevant legal requirements, so that they understand any all legal outcomes that must be achieved.
Verbal advice should go hand in hand with all stages in the enforcement process to help the FBO achieve compliance and understand the enforcement action being taken. For example, AOs must always try to explain to the FBO why immediate action may be required, why a statutory notice is being served, or why the matter is being referred for investigation, if appropriate.
Where verbal advice relates to a structural issue or is of a technical nature, it is helpful to follow up such discussions with a letter confirming the issues.
It is important that the AO does not continue to give verbal advice where this is being ignored, without escalating enforcement action in the appropriate way.
Note: Where immediate action is required on public health grounds, verbal advice should be given, but if ignored it would be appropriate to move straight to formal enforcement action to secure compliance as soon as possible (for example, Public Health – RAN (in Wales) or hygiene emergency prohibition notice (HEPN) for matters where there is an imminent risk of injury to health).
4.16.2 Records
If it appears likely that the enforcement may be escalated through the enforcement hierarchy or the FBO has a history of non-compliance, verbal advice should be recorded on the CAL.
4.17 Informal enforcement action: written
4.17.1 Advisory letters
Advisory letters are considered ‘informal’ enforcement action and failure by the FBO to comply with a letter of advice will not necessarily constitute an offence. However, an advisory letter produced later in court will help to demonstrate fairness and proportionality in the enforcement approach and that the FBO may have ignored previous advice.
Advisory letters should be sent by the AO to the FBO when:
- the FBO or a staff member has failed to take appropriate corrective action following verbal advice
- there is a contravention of the Regulations which does not have an immediate impact on public health
The AO should inform the FBO of their intention to write an advisory letter. Ideally, the AO should meet with the FBO or their representative before issuing an advisory letter to discuss all the issues including the timescale for completion. It is good practice to ask the FBO to confirm in writing their agreement to any timescale. Accurate minutes of any meetings in respect of compliance should be taken.
In advisory letters, the AO must not warn of prosecution action in the event of future contraventions, as this could prejudice any future formal investigation.
All advisory letters must be sent by the AO on official letterhead paper and be typed. In the case of advisory letters sent to limited companies, these should be addressed to the Company Secretary at the Registered Office address and a copy handed to the person appearing to be in charge at the farm.
4.17.2 Checklist for warning letters
The table below lists the points that an AO should follow when drafting a warning letter. Updated [It explains what does and does not need to be included within the warning letter, how the letter should be structured and who needs to be informed that the letter has been drafted.]
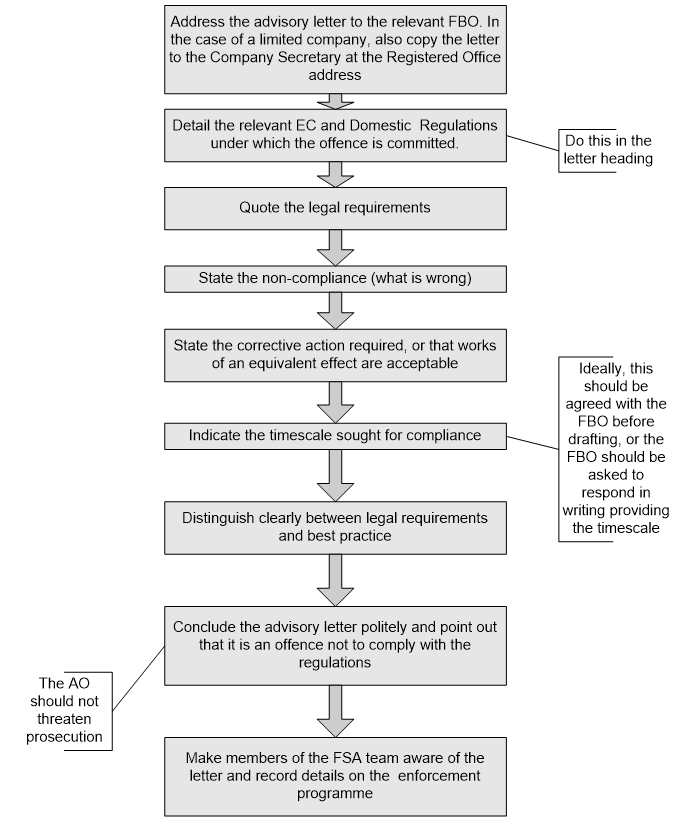
4.18 Formal enforcement action: Statutory Notice
4.18.1 Preparation for formal action
Before taking formal enforcement action, the AO should:
- aim to advise the FBO verbally of this intention
- be fully aware of any ongoing enforcement action by reviewing the corrective action report
- ensure that evidence has been secured to demonstrate that the contravention still exists that will warrant the escalation of enforcement action
4.18.2 Statutory Notices
Statutory Notices are legal documents and care must be taken to ensure they are completed correctly and used appropriately. They should only be served by FSA AOs authorised to do so.
4.18.3 Checklist prior to serving Statutory Notices
The diagram below lists the points that an AO should follow before serving a Statutory Notice. Updated [it includes a decision tree that can be followed once verbal advice has been issued.]
The AO should:

4.18.4 Checklist when serving Statutory Notices
At the time of serving a formal Statutory Notice, the AO should ensure that all the following checks are complied with:
- the formal notice is addressed to and served on the correct person / legal entity
- the local manager or duly authorised representative has received a copy of the formal notice where the original was served on the limited company and sent c/o ‘The Company Secretary’ to the Registered Office address
- the action required is clearly stated with details of any time limits within which compliance must be achieved
- the notice is clearly worded, concise and easily understood; it is typed, dated and signed by the AO
- the notice accurately reflects the non-compliance and refers to the relevant legislation breached
- an official hard copy notice should be used and not a photocopied, sample or draft
- all sections have been completed correctly and any irrelevant areas deleted as necessary
- the notice includes any relevant information on rights of appeal and on the applicable procedure and time limits
- a copy has been retained for scanning into the premises file
If any of the above checks are not complied with then the AO must ensure that action is taken to secure compliance before proceeding to serve a formal Statutory Notice.
4.19 Statutory Notices for hygiene contraventions
4.19.1 Food Hygiene Regulations
The Food Hygiene (Wales) Regulations 2006 / Food Safety and Hygiene (England) Regulations 2013 (as amended), provide 3 types of notice for hygiene non-compliances:
- Remedial Action Notice (Regulation 9(1)) (Wales only)
- Hygiene Improvement Notice (Regulation 6)
- Hygiene Emergency Prohibition Notice and Order (Regulation 8)
Reference: See section 4.11 on ‘Surrender, detention, seizure and condemnation’ in this chapter for details of detention for further investigation under Section 9 of the Food Safety Act 1990, via the provisions of Regulation 23 (Wales)/Regulation 25 (England) of these Regulations.
4.19.2 Service details
The Food Safety Act Detention Notice should be served on the person in charge of the food.
4.19.3 Formal service and delivery of notices
When drafting formal notices, it is very important to ensure they are directed at the correct legal entity responsible for any potential offences that can be committed.
4.19.4 Finding company addresses
Checks on a company’s registered office details may be done through Companies House website. Click on to the free company details link under the ‘find company information’ heading.
The organisation can also be contacted on 0870 33 33 636, or by email at 1HH enquiries@companies-house.gov.uk between 08:30 and 18:00, Monday to Friday.
4.19.5 Statutory Notices for hygiene contraventions
| Notice Type | Legislation | Purpose | Serve upon |
|---|---|---|---|
| Hygiene Improvement Notice | Regulation 6 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 6 Food Safety and Hygiene (England) Regulations 2013 (as amended) | To seek compliance with non-urgent hygiene deficiencies | FBO |
| Detention of Food Notice | Section 9 Food Safety Act 1990 via [Regulation 23 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 25 Food Safety and Hygiene (England) Regulations 2013 (as amended)] | To detain food while further investigation is carried out. | The person in charge of the food (the FBO) |
| Certification of Food Notice | Regulation 27 of The Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 29 Food Safety and Hygiene (England) Regulations 2013 (as amended) | To certify food that has not been produced, processed or distributed in accordance with the Hygiene Regulations to deem it to fail to comply with the Food Safety Requirements and facilitate condemnation. | The person in charge of the food (the FBO) |
| Seizure of Food Notice | Section 9 Food Safety Act 1990 via [Regulation 23 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 25 Food Safety and Hygiene (England) Regulations 2013 (as amended)] | To formally seize food in order that it may be taken before the court to be condemned | The person in charge of the food (the FBO) |
| Remedial Action Notices [Wales only] | Regulation 9 Food Hygiene (Wales) Regulations 2006 (as amended) | To seek compliance with hygiene matters that require immediate rectification | FBO or duly authorised represent-ative |
| Hygiene Prohibition Order | Regulation 7 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 7 Food Safety and Hygiene (England) Regulations 2013 (as amended) | Prohibition of a food business proprietor or manager from participating in the management of any food business | FBO |
| Hygiene Emergency Prohibition Notices and Orders | Regulation 8 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 8 Food Safety and Hygiene (England) Regulations 2013 (as amended) | To obtain the backing of the court to deal with circumstances that pose an imminent risk of injury to health | FBO |
4.20 Remedial Action Notice (Wales only)
4.20.1 When to use a Remedial Action Notice (DH ENF 11-24 (W))
The RAN may be used:
- when any of the requirements of the Hygiene Regulations are being breached
- when inspection under the Hygiene Regulations is being hampered
They are a versatile tool that may be used to ensure the FBO takes immediate action to achieve compliance, for example, poor operational practices, person hygiene, failure to adequately clean.
The AO must verbally request that the FBO rectifies the situation and only serve the notice if compliance is not met. It is essential to gather the necessary evidence at the time the contravention is identified to justify its service in case an appeal is lodged by the FBO.
The AO must verbally inform the FBO of the intention to serve the notice and record the information in the corrective action report.
Note: Currently RANs are only available for use in registered premises in Wales. See Annex 7 to this chapter for examples of situations when it may be appropriate to serve a RAN.
4.20.2 Purpose of a RAN
RAN’s place a legal requirement on a FBO to take immediate action to achieve compliance with the hygiene regulations. This may require:
- prohibiting the use of any equipment or any part of the establishment specified in the notice
- imposing conditions upon or stopping a process
- requiring the rate of operation to be reduced to such extent as specified in the notice, or to be stopped completely
The notice must clearly specify the non-compliance identified by the AO and the remedy the FBO must achieve.
RAN’s can be used to direct the FBO to rectify both hygiene and structural / maintenance deficiencies, which fall under Regulation (EC) 852 and 853/2004 that require immediate action.
In the case of maintenance and structural problems, that do not pose an immediate risk and can be rectified in the longer term, a HIN served under Regulation 6 of The Food Hygiene (Wales) Regulations 2006 should be used.
4.20.3 Service and withdrawal
A separate RAN should be served on the FBO in respect of each specific legal deficiency. In limited circumstances, the effect of serving the notice may be to stop the entire operation.
RANs will not normally be served on the premises as a whole, but may be in certain circumstances, for example a pest infestation, e-coli in the water supply or inadequate cleaning.
Note: Where a notice has the effect of stopping the operation completely, the AO must inform their line manager to ensure the action is proportionate to the risk.
4.20.4 Tagging
Any equipment that has been the subject of a RAN must be identified and tagged using a numbered security seal, and accurately cross-referenced to a RAN.
4.20.5 Alternative service methods
Where it is not possible to identify the name and address of the person on whom the notice should be served, it can be served by addressing it to the FBO in their capacity as ‘occupier’ of establishment at which corrective action is required (name the establishment). The notice may then either be handed to someone else at the establishment who appears to be in charge, or by attaching the notice or a copy of it to some conspicuous part of the establishment.
The provisions relating to the service of notices are Regulation 28 of The Food Hygiene (Wales) Regulations. They correspond with the provisions of Section 50 of the Food Safety Act 1990.
4.20.6 Information for notices
The following information is to be included on the reverse of the AO copy:
- the name of the farm representative to whom any copy notices have been handed, where the original has been served at the registered office address of a limited company
- any comments made by the farm representative when handed the notice
- details of any food detained at the same time as the service of the RAN
- the reference number of the Detention Notice served
- details of any appeal that is lodged by the FBO in respect of the service of the RAN
4.20.7 Rights of appeal
The FBO has the right of appeal (Regulation 20) to a Justice of the Peace regarding the decision of the AO to serve a RAN.
If the FBO appeals, the AO must notify the Lead DHI and the FSA Legal team to arrange legal representation for the appeal hearing.
The provisions of RAN remain in force until such time as the appeal is upheld.
4.20.8 If removed or defaced or destroyed
The notice is the property of the FSA. If the AO discovers that any notice affixed to an establishment has been removed, defaced, or destroyed, the notice should be replaced as soon as possible and the events recorded in the AOs contemporaneous notebook.
4.20.9 Failure to comply
Failure to comply with a RAN is an offence (Food Hygiene (Wales) Regulations 2006, Regulation 9(7)). If the FBO has failed to comply with such a notice, complete a Referral for Investigation report – DH ENF 11/6.
4.20.10 Corroborative evidence rules
Where possible, service of a notice should be evidenced or corroborated in some way. If a notice is served by hand, then a witness should corroborate this fact. Both, the AO who served the original notice and any corroborating witness should sign a copy of the notice and indicate the date and time of service.
When posting a notice, the AO should obtain a proof of postage certificate and retain this as evidence. Where this is not possible, they should record the details of where the notice is posted and the postage address in their contemporaneous pocket book and if possible have a colleague corroborate the postage and countersign the entry.
4.20.11 Multiple offences
Where different offences have been identified, a different notice must be served for each and every contravention. These must never be recorded on the same notice.
This will prevent situations where an FBO complies with one of the issues detailed on the notice, but not the others.
In these circumstances, a notice containing multiple offences:
- cannot be withdrawn as there will be certain issues still outstanding
- cannot be referred for investigation as certain aspects of the notice have been complied with
- if appealed, will result in all of the issues being the subject of the appeal, even where the FBO may have been happy to comply with some of the issues
4.21 Hygiene Improvement Notices
4.21.1 When to use a HIN (DH ENF 11-23 (E/W))
The HIN should be used:
- where there is a record of non-compliance with breaches of the regulations
- where the history of compliance by the FBO is such that the AO has reason to believe that an informal approach will not be successful
- where formal action is proportionate to the risk to public health
Both verbal and written advice should be given to a FBO prior to a HIN being served. However, there may be circumstances where the AO believes this informal approach will be unsuccessful. If these informal stages are to be bypassed, the AO must have suitable evidence to demonstrate that the FBO has ignored previous informal advice in this area, prior to circumventing the informal approach.
4.21.2 Purpose of a HIN
They place a legal requirement on a FBO to take action to achieve compliance with the EU Food Hygiene Regulations. They can be used to require the FBO to:
- address any hygiene deficiency that does not require immediate action
- repair a structural defect with the building
- to build or construct additional facilities to cope with an increased throughput
The HIN must clearly specify the non-compliance identified by the AO and set out the remedy the FBO must achieve.
Note: As RANs are not available in England, HINs are used to achieve compliance with most breaches of the Regulations. Where a matter is clearly causing an imminent risk of injury to health, a Hygiene Emergency Prohibition Notice should be considered.
4.21.3 When not to issue a HIN
A HIN cannot be used to impose a continuing burden, and should not be used in the following circumstances:
- where breaches exist which require immediate rectification; in these circumstances it is more appropriate to use a RAN (in England, a HIN will have to be used)
- where the contravention might be continuing, for example, personal cleanliness of staff, and a HIN would only secure an improvement at one point in time; in these circumstances it is more appropriate to use a RAN (in England, a HIN will have to be used).
- where there is a breach of good hygiene practice but no failure to comply with an appropriate regulation
- where there is evidence of an imminent risk of injury to health; in these cases it is more appropriate to use an Hygiene Emergency Prohibition Notice (subject to HOD / FSA legal approval)
Note: A HIN cannot be issued unless a contravention of the appropriate regulations is identified.
4.21.4 Service
HINs must be served on the FBO.
Note: Where the FBO is a limited company, the envelope but not the notice itself should be addressed to the Company Secretary at the registered office address.
Details of how the notice was served should be recorded on the back of the HIN and in the AOs contemporaneous pocket book.
4.21.5 Service checklist
When serving a HIN the AO must:
- have in their possession all the evidence to justify its service
- verbally inform the FBO of the intention to serve the notice
- state why it is served and the action needed to remedy the breach
- sign, date and type the notice
4.21.6 Drafting and serving a notice to a sole trader
Ensure that the name on the formal notice indicating the individual, who acts as the FBO, is sufficient to identify that individual beyond doubt, and will include both their forename(s) and surname.
Where family members have the same names, try to include any additional names that the person may have, to avoid confusion. The notice may be served by hand on the sole trader at the farm, or served by mail addressed to them personally at the farm address.
4.21.7 Drafting and serving a notice to a partnership
Where a number of individuals act as the FBO under a partnership type arrangement, a copy of the notice must be served on each and every partner. The box identifying the FBO must include the full name of all partners.
The notices may be served by hand on each partner at the farm, if they are present, or served by mail addressed to each of them personally at the farm address. A covering letter should be included explaining that the same notice has been served on all partners in the business.
4.21.8 Drafting a notice to an FBO with limited liability status
Where the FBO has limited liability status, the name of the FBO will be the full name of the limited company, for example, ‘ABC Dairy Ltd’. The notice must be sent to the registered office or principal address of the company, with a copy of the notice handed to the relevant person in charge at the farm. The envelope and any covering letter must be addressed to ‘The Company Secretary’.
Note: The Company Secretary is the person responsible within a limited company structure, who receives such notices. They are not the FBO and therefore should not be referred to on the formal notice.
4.21.9 Content of notice
The notice must specify the:
- grounds for believing the FBO is failing to comply with the regulations
- precise nature of the alleged breach
- measures needed to be taken to secure compliance
- timescale for compliance
- appeal provisions, including the name and address of the relevant local court
Note: Alternative works of equivalent effect may be acceptable.
4.21.10 Time limits
HINs place the FBO under a legal obligation to take specified action within a set time period, during which operations may continue.
The time period given as the date of compliance must not be less than 14 clear days. When calculating this, the AO must not include the day on which the notice is served. They should begin counting from the day after the day on which they intend to serve the notice, count 14 clear days and then put the date for compliance as the day after this.
Example:
- Offence is identified on 1 January
- Notice to be served on 1 January
- The AO must count 14 days starting from 2 January; this will be the close of operations on 15 January
- Mark the date for compliance as 16 January
Note: The period specified for compliance by the AO must be reasonable, given the measures required, and should, if possible, be agreed with the recipient.
Where the AO is unsure what may constitute a reasonable timeframe to specify in a HIN, it is important that they seek advice from FSA Legal to avoid appeals being lodged for unrealistic timescales.
4.21.11 Posting
Ideally, all HINs should be posted at a Post Office and proof of posting obtained. Where it is impossible to gain access to a Post Office the notice should be posted in a post box, with corroboration obtained if possible and a record made in the AOs pocketbook which should be countersigned.
4.21.12 Right of appeal
Recipients have a right of appeal against Hygiene Improvement Notices to the magistrates’ court. During the appeal period, the requirements of the notice are suspended.
In the event of an appeal by someone who is aggrieved by the service of the HIN, the AO is to inform the Lead DHI and the FSA Legal team to arrange legal representation for the appeal hearing.
4.21.13 Requests for notice extension
If the FBO were to request an extension to a HIN, this must be in writing prior to the expiry of the HIN.
Where there is a genuine reason for such an extension, the AO should withdraw the existing notice. A template letter is available in Annex 4 of this chapter.
The AO must issue a new HIN with a revised time frame that:
- provides a minimum 14 clear days to comply
- concludes on an agreed date that the FBO / AO believes that compliance may be achieved
The AO must consider again whether the conditions prevailing at the plant still warrant the issuing of a new notice. Where this is the case, new evidence must be gathered to justify its service.
The AO must retain the written request for the extension as well as the original notice. This will ensure that where any complaints or appeals are lodged against the timescales in the second notice, the AO can demonstrate the overall timeframe provided in both notices and the proportionality of their actions.
4.21.14 Failure to comply
Failure to comply with an HIN is an offence. If the FBO has failed to comply with a notice, complete a Referral for Investigation report for the breach of the formal notice as well as a breach of the substantive offence that led to the notice being served in the first place.
Reference: See the topic 4.24 on ‘Referral for investigation’ in this section for additional information.
4.21.15 Compliance and withdrawal
After the service of a HIN, the AO must check that it is complied with by the stated date.
Where compliance is achieved, the AO must confirm formally in writing that they are satisfied with the works carried out.
Measures that achieve the same outcome as those specified in the notice must be accepted as achieving compliance.
Where the AO is satisfied that the action required in the HIN has been carried out and compliance has been achieved to their satisfaction, a template is available in Annex 2 to this chapter that can be used as the basis of a letter to the FBO.
Where the AO has served the HIN in error and / or it has to be withdrawn due to a technicality, a template is available in Annex 3 to this chapter which can be used as the basis of a letter to the FBO.
4.22 Hygiene Emergency Prohibition Notices (HEPN)
4.22.1 Caution
HEPN can only be issued after authorisation from FSA Legal.
4.22.2 When to use
Issuing a HEPN should only be considered after discussion with the FVC, and where there is an imminent risk of injury to health that requires the backing of the court, for example, contamination of the potable water supply.
Reference: Specific examples and further guidance are given in the Code of Practice made under Regulation 24 (Wales) / Regulation 26 (England) of the Regulations.
The HEPN must be served on the FBO or using the same procedures as outlined in the topic ‘Hygiene Improvement Notices’.
Note: The limited timescales are set out in the subsequent topics.
4.23 Hygiene Emergency Prohibition Orders (HEPO)
4.23.1 Application process
The section below provides an overview of the application process for a HEPO.
| Stage | Description |
|---|---|
| 1 |
The AO must give the proprietor at least U1 day’s notice of their intention to apply for a HEPO by serving a HEPN on the FBO. A HEPN has an immediate prohibitory effect and once served the AO should contact the local court to immediately arrange for a hearing. Note: A copy of the HEPN must be affixed in a conspicuous position to the premises at which the notice relates. |
| 2 | The AO applies for an HEPO from the magistrates’ court within 3 days of the service of the notice. The day of the service of the notice being day one. There is no legal requirement for the application to be heard in 3 days, although the court should be asked to list the hearing at the earliest opportunity. Once made the HEPO supersedes the HEPN. The AO must also affix a copy of the HEPO in a conspicuous position to the premises at which the Order relates. |
| 3 |
Once the FBO applies, in writing, for the HEPO to be lifted, the application must be determined as soon as practicable and within 14 days. Once the AO is satisfied that the proprietor has taken significant steps to remove the health risk(s) specified in the notice, the AO should sign the withdrawal certificate at part 5 of the HEPN. Reference: The Food Hygiene (Wales) Regulations 2006 (as amended)/ Food Safety and Hygiene (England) Regulations 2013 (as amended), Regulation 8. |
4.23.2 Sources of advice
Advice should be sought from FSA Legal, who will, assist in the preparation of the case prior to the court's hearing of a HEPO.
4.23.3 Evidence
Monitoring of the prohibition and any action taken by the proprietor must be recorded. Suitable evidence should be gathered prior to serving the HEPN for production in court.
4.23.4 Procedure
The table below shows the steps for an AO to follow when applying for a HEPO.
| Stage | Description |
|---|---|
| Contact local court to arrange hearing | The application of the HEPO must be lodged with the court within 3 days of service of the HEPN. On establishing dates and times, the AO must notify the FBO by serving a Notice of Intention to Apply for a HEPO. |
| Prepare for hearing | Prior to the hearing the AO should:
|
| At the hearing | It is crucial that the AO has gathered significant evidence at the time the HEPN was served and that this evidence is presented to the court. |
| Court will decide whether to issue the HEPO or not |
If the order is made the AO should produce the completed order for signing by the magistrate. The order must then be served on the FBO as soon as possible and a copy affixed to the premises in a conspicuous place. Any breaches of the order whist in force should be recorded and evidence collected. The matter should then be referred for investigation. |
| Risk is removed | The AO must then formally cancel the HEPO by writing to the FBO. The withdrawal of such a HEPO must not be unreasonably withheld. Once the order has been complied with, the business can recommence its operation. |
4.24 Referral for investigation
4.24.1 Appropriate uses
A referral for investigation is required in the following circumstances:
- breaches of the European Regulations and / or the Food Safety Act leading to an imminent risk to public health
- continual failure to observe requirements of Regulations
- obstruction of FSA personnel engaged in official duties
- failure to comply with a HIN or RAN (RAN - Wales only)
- breaches of an official detention notice
4.24.2 Evidence
The AO must collect adequate evidence at the time of the offence before referring the matter for investigation. The AO must identify the contravention and complete the corrective action report.
4.24.3 Referral to FSA Legal
Where the AO considers that an incident requires investigation, the matter will be referred to FSA Legal for an investigation to be undertaken.
Note: The process to follow when making a referral for investigation is detailed in the table in sub-topic 4.24.8 on ‘Protocol for referral for investigation’.
4.24.4 Decision to prosecute
In England and Wales, the decision whether or not to prosecute for contraventions of hygiene legislation is made by FSA Legal, after being investigated by an FSA Investigation Officer.
4.24.5 Rules of evidence
The AOs main task will be in the gathering of facts and evidence at the time of the offence, which may be used in court at a later stage. An AO must not attempt to conduct a full investigation as specific training is needed to ensure that the investigation is carried out in compliance with the Police and Criminal Evidence Act 1984 (PACE) (or equivalent) requirements. Only specially trained FSA Investigation Officers conduct investigations. AOs must not attempt to caution suspects or take statements.
4.24.6 Statements
Statements will be taken by an FSA Investigation Officer. They are a record of specific events an individual witnessed in a chronological order. They must refer to all relevant evidence and produce these as exhibits for the case. For example:
- photographs
- samples
- copies of notices
- copies of notebook entries
Exhibits are usually identified by the initials of the AO and then consecutively numbered. The IO will assist with numbering when preparing the final statement.
Note: Where the AO is satisfied that the action required or work specified in a formal notice has been completed, the date that it was completed should be specified in a witness statement and on the back of the copy notice.
4.24.7 Protocol for a referral for investigation
The table below outlines the process and rationale for a referral by the AO for formal investigation.
| Process | Rationale |
|---|---|
| The AO is to discuss the issue with the HOD for Dairy Hygiene when the contravention occurs, and prior to completing the DH ENF 11/6 ‘Referral For Investigation’. | This will ensure that the HOD is aware of all formal enforcement action taking place at the farm. |
| If advice is needed on the correct enforcement approach, the AO should consult with the Lead DHI in the first instance and further advice can be obtained from FSA Legal if necessary. | Early advice will provide the necessary support to quickly address any queries regarding the enforcement approach. |
| The AO is to collect all relevant evidence relating to the referral. | All relevant evidence must be supplied to prove the elements of the offence. Photographs must be taken to assist the court, especially where the nature of the offence may be difficult to visualise. |
| The AO must send all evidence together with the DH ENF 11/6 to CSU at FSA York within 10 working days of the offence being identified and the AOs decision to refer for investigation. | Hygiene offences carry a 12-month time limit within which charges should be laid at the court. The time limit between identifying obstruction contraventions and laying charges at the court is 6 months. The IO must be afforded enough time to investigate the offences identified. |
| Once received, CSU will acknowledge receipt of the recommendation, paperwork and allocate a unique number to the referral. This number will be notified to the AO in the confirmation email. (If confirmation is not received within 5 working days the AO should contact CSU. | Confirmation will be sent to provide assurance that the documentation has been received. |
| If compliance is achieved after a referral for investigation has been made, the AO must record this. | This will demonstrate the effectiveness of operators ‘Due diligence’ systems and identify any defences or mitigation that the operator may wish to put forward. |
| Where additional information is required, FSA Legal will request further details, to gain a better understanding of the issues involved. | Where; evidence is lacking, the issue is complex, the approach taken by the AO requires further explanation. FSA Legal may contact relevant colleagues so that a comprehensive and informed picture can be gained of the issues surrounding the referral. |
| This may include checking that: | |
|
To prove the elements of the offence beyond all reasonable doubt. |
|
To stand up to legal scrutiny. |
|
To make sure that all the procedural requirements relating to enforcement have been followed. |
|
To ensure that the notice is legally compliant and defendable in case of an appeal. |
|
To ensure that all policies and procedures have been complied with. |
|
When the investigation is complete FSA solicitors will review all case files relating to hygiene contraventions and make a decision on the appropriate course of action. This could be:
|
When a decision is made NOT to take the case forward, the AO and Lead DHI will be advised of the reason by FSA legal. |
| OR | - |
|
When a decision is made to take the case forward to court, the IO must inform the AO and HOD before informing the farmer of this intention. |
| Where the FBO pleads not guilty and the case goes forward to trial, all witnesses must be made aware of where and when their presence will be required at court. | Any AO who is unfamiliar with court procedure may benefit from some discussion with FSA Legal before any court appearance. Witnesses will have the opportunity to review their statements before appearing at court. |
| If a case goes to court and the FBO pleads guilty. Once the sentence has been determined, this information will be cascaded back to the AO, Lead DHI and the HOD. | If the witnesses are not required to attend a guilty plea, the outcome of the case will be cascaded to all witnesses. |
4.25 Change of FBO during ongoing enforcement action
4.25.1 New FBO
From the moment a new FBO takes over a food establishment, they are responsible for its condition and operation. Any enforcement action initiated prior to the change of ownership should be reassessed. Where the new FBO fails to immediately address any outstanding enforcement issues, these should be pursued by the AO, through the hierarchy in the normal way.
4.25.2 Re-issue of notices
In the event of the premises changing ownership when a notice is still in force, the existing notice should be withdrawn. If the new FBO fails to immediately address the outstanding issues a similar notice should be issued on the new FBO with an explanation of why the notice is being issued. Evidence must be gathered again to justify the service of the notice.
The situation should always be reconsidered prior to re-issuing the notice. The AO may have to justify to a court on some future occasion why they (re-)issued the notice.
Note: Where the FBO has changed, a new registration must be completed by the FBO and submitted to Approvals and Registrations Team so that the establishment’s registration details and ownership records can be updated.
4.26 Warrant to enter premises
4.26.1 Access refused
An AO who has been refused entry to premises should contact the Lead DHI and seek further advice from FSA Legal.
In the event that access to farm premises is refused, it may be necessary for an AO to apply to a Justice of the Peace for a Warrant to Enter Premises, to gain access to carry out their duties.
FSA Legal should be contacted for advice on any refusal by the FBO to grant entry to an AO. Where there is a threat of violence, reference should be made to the Bullying and Harassment Policy UH for guidance. A report must also be made to the local police force.
Examples of when it is necessary to apply for a Warrant to Enter Premises include:
- circumstances where the AO needs to enter to find out if there has been a contravention of the Hygiene Regulations
- entry is required to find out if there is evidence of any such contravention and
- reasonable access has been refused or the AO believes it will be refused and the AO has given the occupier notice of intention to apply for a warrant
- the premises are unoccupied
- asking for permission, or giving notice of asking for permission would defeat the object of the entry
- where urgent access is needed
4.26.2 Execution of the warrant
The warrant must be executed within one month and is no longer valid once the AO has used it to gain access. It cannot be used twice. When executing a Warrant to Enter Premises in England or Wales, Code B of the Codes of Practice, made under the Police and Criminal Evidence Act 1984 (PACE), should be complied with. Legal advice on this and other aspects of the Warrant should be obtained before entry is attempted. FSA Legal will advise further.
4.26.3 Access
Advise the local police of the intention to execute the Warrant at a certain time and date. The establishment must be visited as soon as possible and, on production of the Warrant to Enter Premises, the occupier should grant access. If the occupier fails to grant access, he or she will be in breach of the warrant. Record the events in the contemporaneous notebook and inform the Lead DHI and FSA Legal.
4.26.4 Forced entry
The Warrant to Enter Premises allows the use of force to gain entry when necessary. However, the AOs should never attempt a forced entry themselves, but contact the Police for assistance.
4.27 Process for obtaining a warrant to enter premises, in England and Wales
Updated [The diagram below explains how warrants of entry can be obtained and deals with two scenarios, entry with warning and entry without warning. It explains the steps that would need to be taken and the process to follow.]

4.27.1 Hampered or obstructed inspection
Regulation 14 of The Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 16 of The Food Safety and Hygiene (England) Regulations 2013 (as amended), give authorised inspectors a right of entry in both England and Wales and the inspector is entitled to receive any assistance that they may require from the FBO. If the inspection is refused, obstructed or is hampered the inspector should consider if any grounds given for hampering the inspection are reasonable (for example, a farm or family emergency such as a bereavement, TB testing or RPA inspection taking place). If so, agree to come back after at a later date.
If the grounds are not reasonable and it is considered safe to do so, the inspector should inform the FBO that they are hampering the inspection. If cooperation is not obtained a letter will be sent to the FBO and further legal action will be considered.
The DHI should report any instance of obstruction to the ITL for further action. The DHI should make a comprehensive note of the circumstances surrounding the visit, who they spoke to and the reasons they were not allowed to carry out the inspection. This note should be made in the DHIs note book and in the Dairy Hygiene Inspection Report (DH2).
The DHI should send the standard letter ‘Hampered / Obstructed Inspection’ DH ENF 11/12 (E&W)) indicating to the FBO their legal powers of entry and potential offences for obstructing the authorised officer. The DHI will undertake a further visit accompanied by a colleague.
Until a visit is completed the premises will remain on the outstanding visits schedule.
5. Milk safety incidents
In this section
5.1 Introduction
5.1.1 Background
Within England and Wales FSA, DHIs have responsibility for controls up to and including any bulk storage vessel, after which enforcement responsibilities pass to the LA Environmental Health Team. FSA Northern Ireland subcontracts dairy official controls across the food chain to DAERA.
A key part of the FSA’s work is investigating and managing incidents to ensure that food safety is protected. The procedures to be followed when managing an incident are included in the FSA’s Incident Response Protocol (Revised May 2012).
5.1.2 Section objective
Describe the DHIs role in the event of a milk incident including how this relates to the FSA’s Incident Response Protocol.
Describe the procedures to be followed in the event of a reported milk incident to ensure that it is managed consistently and promptly in order to minimise the risk to public health.
Clearly identify the roles and responsibilities of those involved during a milk incident.
5.1.3 Regulations
The procedures in this section apply when dealing with reported milk incidents in accordance with:
- (EU) 2019/627
- (EC) 852/2004
- (EC) 853/2004
- (EU) 2017/625
- The Food Hygiene (Wales) Regulations 2006 (as amended) / the Food Safety and Hygiene (England) Regulations 2013 (as amended)
- (EC) 178/2002
- The General Food Regulations 2004
5.2 Incidents
5.2.1 Types of milk incident
Milk incidents may include:
- milk from TB / BR reactor animals entering the food chain / milk storage vessels
- incidents of physical or chemical contamination including taints, the presence of unauthorized substances or substances present above legal limits
- food poisoning outbreaks
- sabotage of product (malicious tampering)
5.2.2 Notification
A DHI may be alerted to a potential milk incident in a number of ways including:
- outcome of an inspection
- notification by FBO
- routine laboratory analysis
- complaint / laboratory analytical results from LA Environmental Health Department
- notification by other government departments (Defra, AHPA, RPA)
- notification by a member of the public or media
As notifications may be received from different sources, it is vital that this information be passed to the relevant DHI and the Lead DHI immediately. If the DHI is unavailable or cannot be contacted within 1 hour of receipt, another DHI within close proximity must be informed.
The DHI receiving the notification will fulfil the role of preliminary investigating officer and must carry out an assessment of the potential incident.
The DHI is required to notify FSA Incidents Unit and relevant LAs upon identification of a potential milk safety incident. Login details for the electronic FSA Incidents report have been issued to DHIs.
Notifications must be recorded on the summary section of the Dairy Hygiene Report form (DH2).
5.2.3 Assessment of potential incident
Within the FSA Incident Response Protocol, an incident is defined as:
‘Any event where, based on the information available, there are concerns about actual or suspected threats to the safety or quality of food and /or feed that could require intervention to protect consumers’ interests’.
To determine whether an actual or suspected threat to the safety of milk exists, the DHI must immediately assess the information available to determine the probability that an incident has occurred.
5.2.4 Classification of incident
All potential incidents will be classified as high, medium or low as follows:
- High probability – evidence is available suggesting a systematic breakdown of controls within the milk supply and / or multiple potential incidents have been reported / identified.
- Medium probability – inconclusive evidence available to determine if probability is ‘High’ or ‘Low’, more information needed to determine action.
- Low probability – no evidence of breakdown of control systems, only an isolated potential incident identified.
5.3 Action in case of Incident
5.3.1 Key actions
As soon as a potential incident has been classified, the DHI must complete the actions for each classification as outlined below.
Upon receipt of a potential milk incident notification an assessment must be initiated in accordance with the protocols of this section.
5.3.2 High probability
High probability action must be completed on the same day as receipt of notification of the incident.
- Report incident to Lead DHI / FVL to agree classification.
- Report incident to FSA Incidents Unit by completing the on-line incidents notification.
(Login details have been issued to DHIs). Confirmation of receipt of the report will be sent by Incidents Unit to the dairy hygiene inbox.
- Complete a preliminary investigation at affected milk production holding and record all details on the DH2 on the tablet.
- Arrange for samples to be tested (if required).
- Re-assess risk following investigation and / or upon receipt of testing results, liaising with the Lead DHI.
- Lead DHI will provide a preliminary report to the Head of Operational Delivery.
5.3.3 Medium probability
Action to be completed on the same day as receipt of notification of the incident.
Please note medium probability classification is to be used only where there is insufficient information to classify the incident as either high or low. All incidents must eventually be classified as either high or low once sufficient evidence is available.
- Report incident to Lead DHI to agree classification.
- Report incident to FSA incidents team by completing the on-line incidents notification.
(Login details have been issued to DHIs). Confirmation of receipt of the report will be sent by the Incidents Unit to the dairy hygiene inbox.
- Complete a preliminary investigation at affected milk production holding and record all details on the DH2 on the tablet.
- Arrange for samples to be tested (if required).
- Re-assess risk following investigation and / or upon receipt of testing.
- Once probability is changed to either high or low, then actions outlined in those classifications must be taken.
Lead DHI will provide a preliminary report to the Head of Operational Delivery.
5.3.4 Low probability
First 3 points to be completed on the same day as receipt of notification of the incident. Remaining action to be completed within 3 days of receipt of the notification of the incident.
- Report incident to Lead DHI to agree classification.
- Report incident to FSA Incidents Unit by completing the on-line incidents notification. (login details have been issued to DHIs). Confirmation of the report will be sent by the Incidents Unit to the dairy hygiene inbox.
- Complete a routine inspection following the guidance found at section 2 on ‘Inspection procedures’ of this chapter.
5.3.5 Escalation
Once the FSA Incidents Unit have been notified of a milk incident, they will decide if they should invoke their incident response protocol and establish an Operational Incident Management Team (OIMT) or a Strategic Operational Incident Management Team if the incident was of a sufficiently high level.
Where the FSA OIMT has been established, the DHI will take direction from the OIMT for the remainder of the investigation, continuing to provide technical support and participate in the OIMT as requested.
5.3.6 Enforcement
In the event that the DHI is required to undertake enforcement duties, the relevant enforcement procedure in Section 4 of this chapter must be followed.
Enforcement actions may include detention, seizure, Warning Letters, HIN,RAN - Wales only and HEPN.
If the DHI is made aware that milk from animals that are not officially TB free has been consigned for wholesale sales, or that milk from herds that have lost their official TB free status has been sold as RCDM then appropriate action must be taken to ensure that the FBO is aware of the seriousness of the offence.
In such incidents, enforcement starting no lower down the enforcement hierarchy than a Warning Letter must be issued, indicating clearly the legislative requirements relating to the breach.
5.3.7 Interpretation of laboratory results and guidance on follow up action on RDM incidents
The Dairy Hygiene team, in collaboration with Food Hygiene Policy and Food Incidents teams, has developed guidance in case of incidents in case of incidents related to RDM. This guidance can be found at Annex 9.
The guidance outlines how to interpret laboratory results and what action, investigations and sampling should be taken by DHIs regarding unsatisfactory results from samples of milk to be sold as RDM.
The guidance covers:
- interpretation of results for both pathogenic and indicator criteria
- actions and further investigations
- resuming RDM sales
- questions to consider for information gathering purposes
- additional action subject to the outcome of on-farm investigations
5.4 Roles and responsibilities
5.4.1 The Dairy Operational Lead
The Dairy Operational Lead (DOL) is responsible for providing strategic support in the management of the incident including updates to Senior FSA management.
5.4.2 Lead DHI / FVC
The lead DHI / FVC:
- has responsibility for the co-ordination of the incident, if necessary identify and assign DHI
- provides support to the investigating officer and guidance where enforcement action is taken
- provides DOL and FVL with regular updates.
5.4.3 DHI
Up to and including bulk storage vessel:
- carries out preliminary assessments and take action as necessary to protect public health
- report incident to LA, LDHI, ITL and FSA Incidents team as required
- implements formal / informal enforcement actions as necessary
- identifies the source of the incident (in liaison with the FBO) and ensure that the appropriate action is taken to rectify the problem
- completes DH2/DH1 as appropriate.
5.4.4 Local Authority
The LA is responsible for all controls after the bulk storage vessel, including following up where necessary with milk purchasers, processors and consumers.
6. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Dairy hygiene enforcement policy This document has been withdrawn and is currently subject to review
Annex 2: HIN withdrawal letter: Improvements complete
Annex 3: HIN withdrawal letter: Issued in error
Annex 4: HIN withdrawal letter: Period extension
Annex 5: Hampered / Obstruction inspection letter- This document has been removed and can be found within chapter 9 ‘Forms’ as DH ENF 11/12 (E&W)
Annex 6: Supporting evidence photographic report template- This document has been removed and can be found within chapter 9 ‘Forms’ as DH ENF 11/14
Annex 7: Use of RANs in Wales: examples
Annex 8: New RCDM labelling enforcement provisions / responsibilities
Annex 9: Guidance on RDM incidents
Annex 10: Sample despatch process
Hanes diwygio
Published: 13 Ionawr 2022
Diweddarwyd ddiwethaf: 10 Medi 2024
Hanes diwygio
Published: 13 Ionawr 2022
Diweddarwyd ddiwethaf: 10 Medi 2024