Annual FSA Science Update
FSA 23-12-09 This paper gives an annual update on the FSA’s science's work.
1. Summary
1.1 This paper gives an annual update on the FSA’s science, covering:
a) A summary of progress made, and impact delivered since the last update.
b) An overview of our plans for the future.
1.2 The Board is asked to:
a) Review the progress and impact made in 2023.
b) Comment on our future plans and agree the focus for 2024 and beyond.
2. Introduction
2.1 This paper builds on the 2022 FSA Science Update. It should be considered alongside other relevant papers from this year, including:
a) The annual report of the CSA
b) The annual report of the Science Council
c) The annual report of the Advisory Committee for Social Science
d) Risk Analysis and Regulated Products Service: Regular update to FSA Board
2.2 The FSA’s science capability is delivered by a team of over 160 scientists and analysts, who sit within the Science, Evidence and Research Division (SERD). More detail on how we deliver our science can be found in Annex 1.
2.3 Aligned to the FSA Strategy, the primary role of SERD is as an Evidence Generator, delivering and supporting five core functions for the FSA:
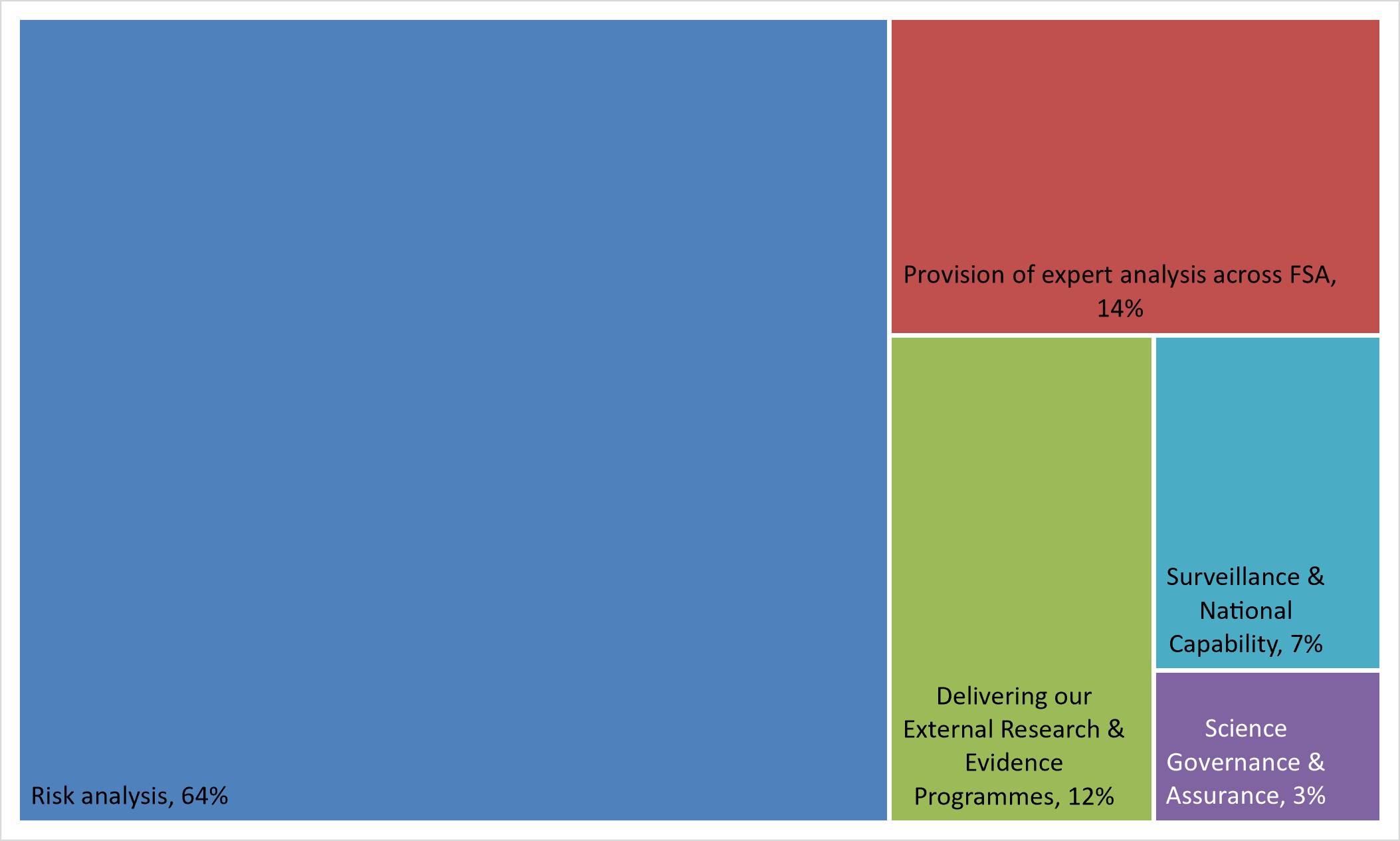
a) Risk analysis including risk assessment and evidence on the other legitimate factors pertaining to risk management and risk communication.
This function provides secretariat and admin support to our 100 plus experts within our scientific advisory committees (SACs) who provide independent scientific advice on food-related risks.
This is the biggest function within SERD and accounts for over 100 FTE (64%) our total staff resource.
b) Provision of expert analysis including economics, statistics, strategic insight, operational and social research to directly support the work of the FSA and its transformation programmes.
This function is required to be responsive to the needs of the FSA. As an internal capability, it can respond more rapidly than through other evidence-generating routes such as external commissioning.
This function accounts for around 14% of our total SERD staff effort.
c) Delivering our External Research & Evidence Programmes co-ordinating and managing our portfolio of funded research and evidence projects, worth ca. £9M per annum.
In addition to funding research projects to fill knowledge gaps, this function also maintains core FSA science capabilities and statutory functions such as our SACs and National Reference Laboratories (NRLs).
The staff effort dedicated to this is 12% of the total and includes a small internal and external coordination function and science project contract managers from across SERD.
d) Surveillance & National Capability, leading and co-ordinating on sampling, laboratories, and new method development (this includes the PATH-SAFE Programme)
This includes our network of NRLs, which provide expert advice and training on sampling/testing, develop new methods, and provide contingency support for major incidents.
The total effort dedicated to this is 7% of total SERD staff input.
e) Science Governance & Assurance, including supporting the work of the FSA’s Chief Scientific Advisor (CSA), providing secretariat support to the FSA’s Science Council and working across the government science network.
This includes ensuring the FSA is delivering science obligations that all government departments are required to attain/maintain, such as publishing our Areas of Research Interest (ARIs). It also includes input into the Government Science & Engineering (GSE) profession.
This is our smallest function at 3% of staff effort.
3. Delivery Progress & Impact during 2023
3.1 In the 2022 FSA Science Update, we laid out our priorities and plans for 2023. In accordance with the FSA Corporate Plan and our science capability plan, we have delivered a wide range of outputs and Annex 2 provides a list of the science publications (papers and reports) published since December 2022.
3.2 While over the last 12 months, we have made progress against our listed priorities and capability plans, pressures on our science budget and headcount have required us to prioritise some activities over others.
3.3 This has included protecting resources to deliver the regulated products service and maintaining our support for risk analysis and incidents.
3.4 We have reduced efforts in other areas such as some of our exploratory analysis programmes. This has included finding efficiencies in our consumer tracker and Food and You 2, slowing down our research programme on the cost of illness, and reducing spend on our foresight programme.
3.5 Despite resource challenges, we have made progress across all our main functions and delivered impact for the FSA, as summarised below:
Risk Analysis
3.6 In 2023, we continued to support the risk analysis process and regulated products service, through the provision of risk and safety assessments, and expert technical advice. Annex 2 provides a list of 47 published risk and safety assessments published.
3.7 Key examples of our risk assessment activity in 2023 include:
3.8 Risk assessment to support UK import controls: As part of the development of the cross–government Border Target Operating Model, scientific advice was needed to adopt a more flexible and adaptive approach that focuses controls on the commodities and countries that pose the greatest risk to public or animal health, whilst maintaining proportional controls on SPS commodities.
3.9 The FSA developed a tool that provides robust, evidence-based risk categorisations that are used to set the initial checking rates for all GB imported food products of animal origin due to start in April 2024. Key to the tool is the dynamic estimation of risk, so that a reassessment of the categorisation can be undertaken quickly, to target to target resources on commodities and countries that pose the greatest risk to public health. Our risk assessment, operational and economic analyses have underpinned and improved cross-Government work, allowing for implementation of risk based, consistent controls on food and feed, and optimising border resources.
3.10 Science supporting policy work on Precision Bred Organisms (PBOs): to develop the regulatory scientific understanding on PBOs, we have continued to work with the Advisory Committee on Novel Foods and Processes (ACNFP) and its newly established Products of Genetic Technologies sub-committee.
3.11 Our scientific advice from ACNFP, our consumer research and the economic analysis underpinned the proposed regulatory framework for PBOs We are now developing the guidance for applicants to use in triaging their future products and applications to FSA and will continue to monitor public understanding through the consumer tracker.
3.12 Incidents: We have contributed to around 150 incidents. For example, in the case of Glycerol in slushed drinks Following a report of an unresponsive toddler being hospitalised with glycerol toxicity after consuming three slushed drinks in one hour, a risk assessment was conducted. Slushed drinks contain glycerol to prevent them fully freezing. An EFSA opinion on the medicinal use of glycerol notes that side effects such as headache, nausea and vomiting can occur and advised that consumption should not exceed 125 mg/kg body weight/hour. This value would be exceeded by consumption of many slushed products by young children. We worked with industry to develop voluntary guidance on age and consumption limits and would apply to all products. We established a threshold which recommended that products were not consumed by children aged four or below and that a free refill model was not appropriate for children aged 10 or below. This is still protective, but better represents the degree of risk associated with the product than a limit based on body weight.
3.13 Building risk assessment: we continued to build our capability in 2023 by launching a new in-house training programme focused on areas of risk assessment, toxicology, and microbiology, which is providing a more structured and accessible resource for our staff. We also collaborated with other government departments for the successful addition of the role ‘risk assessor’ to the UK Government Analysis Function. This raises the profile of risk assessors, and we hope will provide an improved career pathway, and better retention.
Expert analysis
3.14 We have reduced non-statutory economic and social research, as outlined above at paragraph 3.4, and focused on providing s support for FSA programme boards, the FSA Board and others in Government For example:
a) We provide Regulatory Impact Assessments (IAs), Post Implementation Reviews (PIRs) and Business Case development, so that Policy and Operational teams can assess the impacts of any proposed regulatory/policy change and make better risk management decisions.
b) The evidence we provided to the Board and Defra on consumer views and economic impacts of precision breeding and on the wider consumer interest in food framed complex policy decisions and the principles of our regulated products reform programme.
c) Our analyses of the standards of what we eat as a country underpins Our Food, the FSA’s flagship annual report. The report for 2022 focused on the impacts of inflation on society, consumer behaviours as well as trade and food safety resource pressures within the system.
d) Our work on food inflation and household food insecurity has shone a light on the issues that consumers consider as important. Since 2020 we have built a bank of evidence on household food insecurity, including statistics which are now used by stakeholders across Government and civil society. This year we also convened government departments to map the current data and research landscape, and published evidence on models, risk and needs in community food provision (such as food banks, food sharing apps) , which resulted in new policy guidance for community food providers to support food redistribution.
e) We pushed successfully for a more accurate assessment of the impact of the new Borders Target Operating Model on traders and consumers, and worked with Defra to provide economic analyses as part of the Windsor Framework to enable and extend public health, marketing, and organics regulations in GB to ensure they also apply to goods moving to NI via the Northern Ireland Retail Movement Scheme
f) Our analysis of animal welfare breaches in slaughterhouses supports the FSA’s and Defra’s reporting and evaluation of interventions, and our analysis of how inspectors focus their time in slaughterhouses, helped the operations team get more accurate data with which to manage charging.
3.15 Underpinning this, we have been improving our established research vehicles and exploring new and emerging methods. For example:
a) We have developed new survey instruments which are more cost effective while maintaining speed and sample size, relaunching our consumer tracker which supports our ability to call out issues in the consumer interest. For example, in previous years this has supported our response to household food insecurity (see above) and is now highlighting the growth in concern about ultra-processed foods in recent months. Taking a longer term and deeper approach, our relaunched flagship survey, Food and You 2, continues, with the first trends report from the new web-push survey published this month. We have brought most of the analysis in house which is less expensive. We have also built partnerships with behavioural scientists around healthy sustainable diets, through participation in the cross-cutting SALIENT programme of trials, co-funded by FSA, Defra, UKRI and HMT and in partnership with Nesta and a leading academic consortium.
b) Our Kitchen Life 2 project, which this year won the Analysis in Government Award for Innovation, uses motion sensitive cameras in households and food business kitchens, to give quantitative evidence of the frequency of risky behaviours (over 650 hours of footage has been coded into a database of almost 300,000 data labels, each corresponding to a food safety behaviour and its context) and what influences specific risky behaviours. This unique observed data set will inform future risk assessments, interventions, and guidance. For example, it identified that improvements could be made to consumer guidance on reheating, whilst food businesses could be better supported on the accuracy and recording of fridge and freezer temperatures. Policy and communications teams are considering the best way to approach these gaps.
c) To understand more citizen science approaches, we co-funded a programme with UKRI supporting short term projects trialling various approaches. The projects showed varying levels of success, but we now know more about the usefulness of such methods. One of the successful projects found a previously unknown risk: that that formula preparation machines did not consistently produce water at the minimum temperature stated in NHS guidance. In response we pushed successfully for new consumer-facing advice to be added to NHS guidance pages and shared by DHSC, the Royal College of Midwives and the Institute of Health Visiting, and for the Office of Products Standards and Safety to commit to a risk assessment and testing.
d) Working as part of a wider horizon scanning network which includes Cabinet Office, GO-Science, FAO/WHO and others, our analysts monitor the known drivers of change in the food system, and report these via our Strategic Assessment. We work across the FSA to interpret the impact and identify risk and opportunities. How we have responded to these as an organisation was set out in a paper taken to the June Board - this analysis is now helping us frame the future organisation in the spending review as well as giving our regulated product reform programme sight of the future food technologies coming down the line.
Research and Evidence Programmes
3.16 Our Research & Evidence Programmes (REPs) are prioritised and co-ordinated portfolios of projects across different areas of research interest (such as foodborne disease). Details about how our spend has been distributed across the different programmes can be found in Annex 1 and the outputs from these programmes are listed in Annex 2.
3.17 Despite budget limitations, we have been able to protect and progress some of our key evidence generation priorities, which will allow us to provide better evidence for risk analysis in future. Key research project milestones include:
a) Starting the third study of infectious intestinal disease in the UK (IID3) project, which will track the incidence of different foodborne pathogens (such as through testing and reporting cases from GPs) and give us more accurate foodborne disease estimates in the future.
b) Concluding the current phase of our work on the unseen costs in the food system, publishing new insights on the cost of food crime (which found that the total impact of food crime on the UK is estimated between £409 million and £1.96 billion per year) and the cost of food hypersensitivities (which found that on average an FHS household spends an additional 12-27% more on weekly food purchases compared to households without FHS).
c) Completing the Kitchen Life 2 project (see 3.15 above)
3.18 We have continued to strengthen our cross-FSA steering groups to ensure we are asking the right research questions, whilst also prioritising new asks. This has allowed us to commission new work in year including:
a) A new literature review on BPA (supporting our risk assessment work on food contact materials)
b) Tracking consumer opinions on PBOs (supporting our policy work on regulated products)
c) Additional module to FHRS audit to explore displays of FHRS ratings on websites (supporting our work on better regulation)
d) Assessing how to maximise consumer awareness and understanding of allergen risks (supporting our policy work on food hypersensitivity).
e) Economic evidence to support the FSA’s work for the next government spending review.
Surveillance and National Capability
3.19 Our approach to building a resilient laboratory system was presented in the September Board Paper 2022 (footnote 1). It outlined the challenges faced by Public Analyst (PA)-led Official Laboratories (OLs) and concerns around the sustainability of the official laboratory system (there have been multiple lab closures over the last decade, so that by 2019, there were only five PA OLs left in England and Wales).
3.20 Through the FSA’s immediate intervention, by delivering targeted sampling and supporting laboratory capability, no PA OLs have closed since 2019. However, there is continued financial pressures on Local Authorities that may impact the ongoing sustainability of the PA OLs. As a result, ongoing proactive intervention is required to protect capacity, build capability, and ensure future resilience.
3.21 Phase 2 of the laboratory plan (approved by the Board in September 2022, being delivered between 2022 and 2024) has helped maintain core laboratory capability by providing a flow-through of samples; improved UK capacity by decreasing turnaround time for analysis and expanding the range of available testing; and provided investment to improve analytical methods and deliver new testing approaches to better prepare the UK food system for the future.
3.22 In 2023, we delivered across multiple Phase 2 workstreams including:
a) Developing PA OLs capability and capacity by providing approximately £500k in targeted grants focused on the following areas: metals analysis in food and feed; DNA testing, including Genetically Modified Organisms (GMOs) and meat speciation, allergens; food additives; and alcohol adulteration.
b) Improving incident response by facilitating the development of analytical methods by NRLs enabling them to undertake non-routine testing. For example, this has enabled shellfish samples to be tested for chemical contaminants during the Poole Harbour oil spill incident supporting the FSA’s risk assessment and advice to local authorities.
c) Ensuring GB PA OLs have the support they need, we have initiated a workstream for NRLs to provide an affordable service in difficult and novel testing areas. The list of agreed targets is currently being finalised.
d) To ensure the future of the Public Analyst profession, we have provided MChemA candidates with additional support during their studies.
e) To test hypotheses on potential food safety and standards issues, and to enable the testing of a wider range of areas than those routinely looked at by local authorities, we continue to deliver an annual, intelligence-led retail sampling surveillance programme, with ‘at-risk’ products are identified through use of intelligence and other surveillance activities. A summary of the overall findings has been published in the Our Food 2022 Report and the full survey results are due to be published early next year.
f) Supported innovation and improve efficiencies through funding the development of new analytical methods, such as identifying alternative methods for herbs and spices authenticity and a review of allergen testing methods.
3.23 PATH-SAFE (Pathogen Surveillance in Agriculture, Food and the Environment) is an FSA-led, £19M programme funded through HM Treasury’s Shared Outcomes Fund, designed to improve surveillance systems for foodborne pathogens and antimicrobial resistance (AMR) (footnote 2)
3.24 There are four workstreams and over 25 projects underway and an established collaboration of over 50 cross-government, academic and industry partners, across all four nations (footnote 3). The programme is due to conclude March 2024.
3.25 While it is too early to establish impact now, key outputs will include:
a) A new data system for sharing whole genome sequence data for foodborne pathogens between different departments to better support outbreaks and disease control.
b) Novel testing methods for pathogens including the use of wastewater and portable diagnostics.
c) New knowledge of foodborne pathogens and AMR in the UK agri-food system
d) A model system for AMR surveillance in the wider environment.
3.26 The interim evaluation (to be published in 2024) has concluded that there is evidence to indicate many positive contributions of the programme and this progress will be used to develop a continuation bid (see 4.17 to 4.19 below).
Governance and Assurance
3.27 We continued to support and develop our Science Advisory Committees (SACs; see Annex 3 for more details on our SACs). Notable activities were:
a) An internal review of the SACs and independent review of the Advisory Committee on Social Science (ACSS) and the Science Council; both of which were positive in their assessments and showed they were operating effectively and efficiently, but with suggestions on improvements.
b) A recruitment campaign for new members including better promotion across all four UK nations to ensure the committees are inclusive.
3.28 The Concordat to Support Research Integrity provides a national framework for good research conduct and its governance. The FSA was one of the first departments to publish our annual statement of compliance with the Concordat. This statement describes how we meet the Concordat’s principles, indicating our commitment to upholding the highest standards in research and having the right environment and processes in place to support this.
3.29 As part of our commitment for continuous improvement, we commissioned an independent assessment of the Social Science team (people) and outputs (products) against the Government Social Research (GSR) code of professional standards. The independent review identified areas where we were doing well and areas for improvement, and we published this alongside an FSA response in August 2023.
Science Key Performance Indicators (KPIs)
3.30 In last year’s paper, we presented a new set of 16 science KPIs, designed to measure our progress against a set of desired outcomes. These are presented in Annex 4, including the updated results against targets for this year. This shows that there only two KPIs where the status is not green; 1 amber and 1 red. For the red KPI, which relates to staff learning & development, action has been taken within the SERD People Plan 2023 to address this.
4. Our Future Focus
4.1 Looking to the future, we have identified four strategic areas of focus for 2024 and beyond, and under each of these there are specific delivery priorities. Below we present the four strategic areas and some of the key priorities identified for progressing in 2024-25.
Improving evidence to allow us to better understand and control foodborne illness, including those caused by pathogens but also food hypersensitivity, chemical contaminants, and AMR.
4.2 New UK AMR National Action Plan (NAP): UK Government is developing a new NAP to covering 2024-29. This will provide detail on how different departments will contribute to a cross-government effort to mitigate the against the risk of AMR and the chronic impact that would have on human health.
4.3 The FSA leads the foodborne AMR workstream of the NAP and we are working with partners to develop a set of relevant but realistic future commitments and will focus on surveillance to understand the incidence of AMR in food and the potential for food as a roue of spread.
4.4 Influenced by the work we have already done and synergies with the work of other departments (such as Defra), our plans have been guided and shaped by the 2023 review of the FSA’s AMR research and surveillance programme, the results of which were published in the peer review literature.
Transforming our science capabilities (and associated service provision) to support regulatory reforms being prioritised by the FSA, especially those around our future regulated products service and the risk analysis process.
4.5 Analysis in support of core and change priorities: looking ahead, given continuing budget cuts, we will maintain (but not expand) our key research tools such as Food and You 2, and focus our efforts on providing high quality analysis to support the FSA’s core business priorities as set out in the three-year plan.
4.6 The new Better Regulation Framework has been reformed to increase the consideration and use of alternatives to regulation and a consistent and proportionate approach to economic impact assessment. We will continue to support the delivery of the FSA Strategy through the delivery of options appraisals, risk assessments, impact assessments and evidence on the consumer interest across a range of FSA priorities including:
a) Official vet (OV) resourcing – helping teams develop and evaluate potential interventions to increase recruitment and retention of Ovs.
b) Supporting risk management decisions with evidence on economic impact and consumer attitudes and behaviours.
c) Regulated products review (and other areas of reform under the Smarter Regulation programme, formerly known as REUL), supporting policy decisions with evidence around the impact of potential legislative changes.
d) FSA regulatory and operational reform programmes – supporting teams with technical advice, business cases, options appraisals and impact assessments to help leadership make the right decisions.
4.7 We will support the delivery of the borders target operating model and the classification of risks from imported food, alongside the evaluation of our trusted trader pilots. We will support the implementation of the Windsor Framework, giving monthly updates on border notifications to assess how it has been implemented and updating our modelling of requirements at borders.
4.8 We will support analysis of animal welfare incidents, and review of how time allocated to animal welfare breaks down to ensure accurate recovery of FSA costs from other Departments. We will gather and synthesise the data behind and the quality assurance of the Annual Report on Food Standards, ensuring that robust and trustworthy evidence underpins this key product for the FSA.
4.9 Where we can, we will draw on others’ expertise. We are working with academic colleagues to conduct further secondary analysis of our consumer data, including, for example, on food safety behaviours among older adults, which will be published over the course of next year.
4.10 Foresight is a shared priority across Government, and following a successful FSA-Defra joint bid, GO-Science will be undertaking an analysis of the food system to identify longer-term system vulnerabilities. We have put forward ideas for further GO-Science joint projects including mapping the future impact of Artificial Intelligence on the food system and its regulation.
4.11 Science Council work programme: following the review in 2023, we are working support the development of a future work programme to ensure we can fully utilise the expertise and capacity of the Science Council to address strategic science questions.
4.12 The first planned activity will be convening and coordinating a pilot study using an Expert Working Group to consider how we might approach evidencing the wider impacts of novel foods/regulated products (selected as case studies), extending beyond the question of safety as considered by a risk assessment.
4.13 The plan for future projects for delivery in 2024 is still being developed and this will be presented to the Board in March 2024 (when the Science Council is due to present its annual report).
4.14 Advisory Committee for Social Sciences (ACSS): following the review of the committee in 2023 and the conclusion of work reviewing our consumer tracker, ACSS will have capacity to contribute to other FSA priorities in 2024-5. We are proposing that we direct the work of the (existing) Wider Consumer Interests Working Group towards supporting our evidence needs on ultra-processed foods and regulated product reform. Research questions and methods will be discussed with the ACSS in the New Year.
Sustaining and building our national surveillance and science capabilities, focusing on improving our OL and NRL laboratory networks, investing in new methods, better data sharing (such as pathogen sequence data) and skills (such as regulatory toxicology)
4.15 Phase 3 Future Laboratories Plan: Phase 3 will be our final step to deliver the laboratory system we require, building on the intervention and support delivered in Phase 2. The Phase 3 plan will be developed in Q4 2023-24, and subject to Board approval and availability of funding from the spending review, we will aim to start delivering in the following financial year. We will use the outcomes of Phase 2 to identify what works effectively within the system and what gaps still exist. This, along with a survey of the capability of PA laboratories to run in 23-24, will be the evidence base for designing the long-term solution to build a resilient national capability.
4.16 We will also develop further options to improve coordination and efficiencies between laboratories, bolster relevant research and method development, and deliver capability and capacity, in conjunction with other government departments, including FSS, Defra and HSE. One option being explored is the development of a ‘hub and spoke’ model with an overarching, ‘centre of excellence for food safety and standards’ hub operating with existing NRLs and OLs as the spokes. FSA officials and the CSA are continuing to engage across government to identify and deliver cross-departmental solutions to lab capacity.
4.17 PATH-SAFE continuation: with the PATH-SAFE programme due to conclude on 31 March 2024, the consortia will be bidding into HMT Shared Outcomes Fund Round 2 Continuation funding in January 2024 for funding to continue work in FY 2024/25.
4.18 We aim to build on the progress made via the programme’s successful pilots, focusing on four key themes: data, foodborne disease, AMR and remote diagnostics. An example would include expanding the sequence data platform to include pathogens beyond salmonella. We also aim to maintain the community PATH-SAFE has established, seeking other external opportunities (such as new UKRI AMR Network call) and maintaining activities like our Communities of Interest.
4.19 The continuation funding would allow us to align with ongoing/new cross-government/external biosurveillance opportunities (such as Biological Security Strategy, National Biosurveillance Network, etc.).
4.20 Enhancing our research partnerships: a key strategy has been to build stronger partnerships with UKRI to develop programmes which are better focused on our areas of interest and hence leverage research funding. The co-funding of the £1.45M Food Safety Research Network (FSRN) with BBSRC is an example of this (FSA contribution £250k).
4.21 Ongoing work with UKRI is identifying mutual future priorities for research and evidence, with a view to develop new programmes in 2024-25 and beyond. As well as investigating the continuation of established networks (such as PATH-SAFE and FSRN), further ideas include addressing skills gaps (such as toxicology), developing new capabilities (such as novel approach methodologies) and research to fill knowledge gaps (such as the role of microbiome in food safety).
4.22 In addition to focusing on foodborne disease illness (led by BBSRC), we are engaging with Innovate UK, who are the lead on funding research and innovation within the novel foods space innovation (such as development of alternative proteins). This will allow us to leverage research to develop better regulator science and facilitate engagement with innovators, giving us early insight into new innovations and supporting knowledge exchange (such as better information on the approvals process supporting better applications).
Improving our understanding of consumer interests in relation to the food system.
4.23 Given resource limits we will have to be very focused in our evidence gathering in this area. Areas of specific interest include consumer attitudes to novel foods/regulated products (such as alternative protein sources), the standards of imported food, supporting behavioural interventions around healthy sustainable diet and building the evidence base around ultra processed foods (UPF).
4.24 We will:
a) continue to monitor food affordability and other consumer concerns through Food and You 2 and the consumer tracker,
b) explore the drivers and enablers of consumer confidence in novel food products and processes (s alternative proteins) to support the development of our regulated products processes,
c) maximise the impact of the SALIENT programme working with ESRC, Nesta and partners across Government to deliver new evidence from in situ trials on evaluating potential behavioural interventions to encourage healthy and sustainable food choices,
d) develop proposals on Ultra Processed Food (UPF) focusing on the scientific questions that we need to answer to inform FSA and wider work. Potential areas of focus include the impact that different combinations of additives have on longer term health, and the impact of UPF on appetite and microbiome, working through our CSA, DHSC and the CMO, to influence wider policy.
e) Support the delivery of the Science Council Expert Working Group on the wider impacts of novel foods/regulated products (see 4.13 above)
Future Risks & Challenges
4.25 However, in developing our plans against these priority areas, we need to consider a range of significant risks and challenges over the next 12 months. These are:
a) Staff resources: with reduced budgets forecast for next year, we are increasingly constrained for resource to deliver both internal and external science. We have continued to rigorously reprioritise, but this is becoming increasingly challenging and without additional resource we are likely to need to stop work that is vital to ensuring we are an evidence-led organisation.
b) Research & evidence budgets: a large portion of our research and evidence budget is pre-committed to maintaining core capability functions (such as NRLs, supporting our SACs) or existing, multi-year projects. As a result, without additional resources our spend on new projects in 2024-25 is likely to be negligible.
c) Single-year budget cycles: tied to the previous comment, to maximise the use of resources, and leverage external funding, we need to plan further ahead than a single budget year and commit to multi-year activities putting additional pressure on future budgets.
4.26 As a result of these challenges, we will be increasingly seeking to work in partnership with others outside of the FSA (such as UKRI and other government departments; see 4.20 to 4.22 below) to access additional resources.
4.27 We will also be seeking to build a strong and compelling multi-year bid for the government’s spending review in 2024, focusing on the four priority areas outlined in 4.1. A more detailed workplan will be developed as part of business planning (to be presented in Q4 of this financial year).
5. Conclusions
5.1 In the last 12 months, we have been able to progress against the priorities outlined in 2022 update and in doing so, deliver impact and support work across the FSA. We have also been able to formulate plans for future work, to both build new science capabilities but also support the FSA’s major policy and delivery priorities.
5.2 The Board is asked to note the progress over the last year and comment on our future focus, including our areas of strategic focus (section 4.1) and our plans for the coming year (sections 4.5 to 4.31).
Annex 1 Business Analysis, Finance and Performance Data
1.FSA Science delivers 5 main functions (as described in the introduction)
2. We deliver our science through a combination of internal expertise and the coordination of external spend. The current distribution of FTE across each function within SERD is:
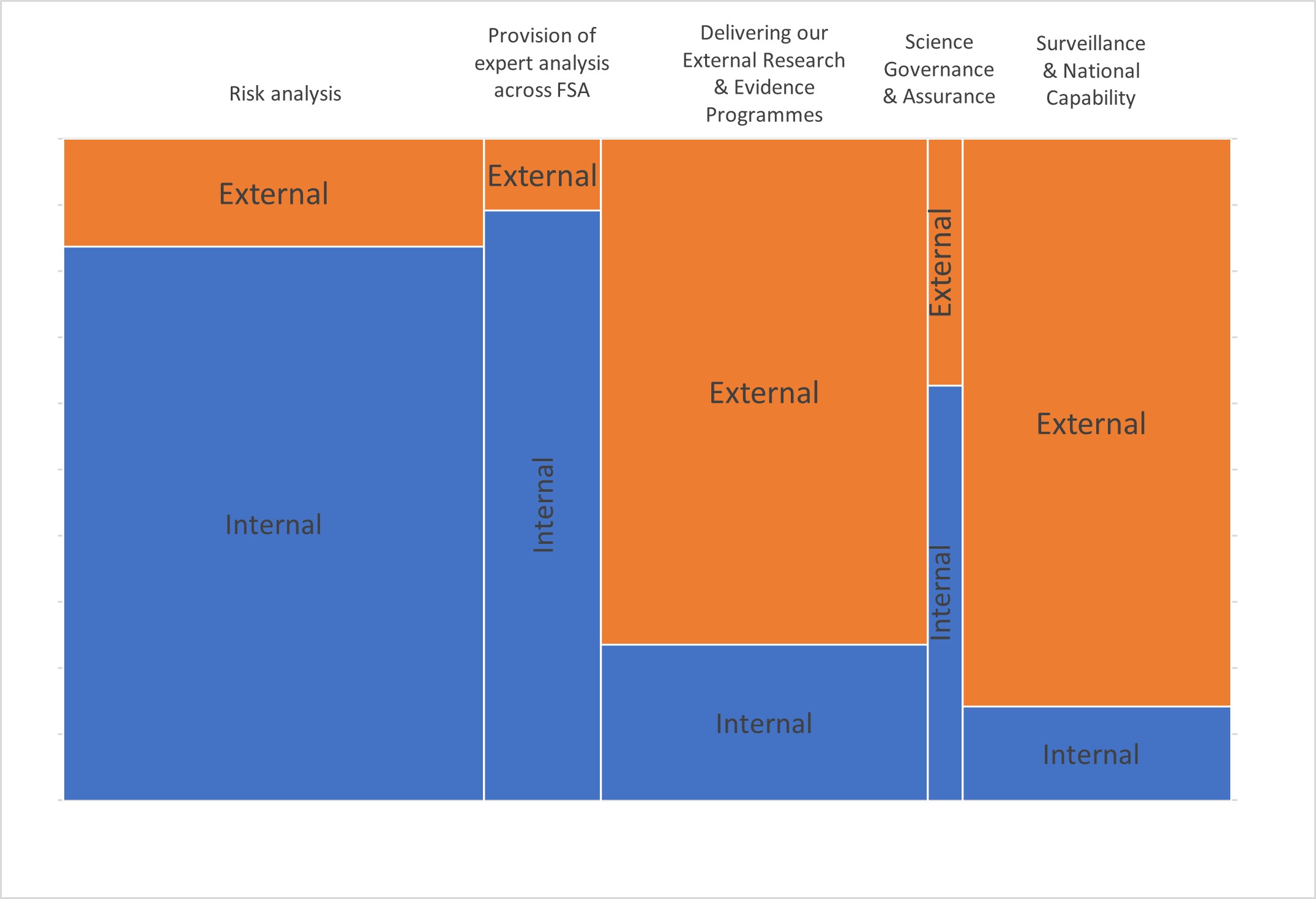
3. Each function is delivered through a combination of spend on in-house resources and external commissioning as follows which for the 5 main functions is as follows.
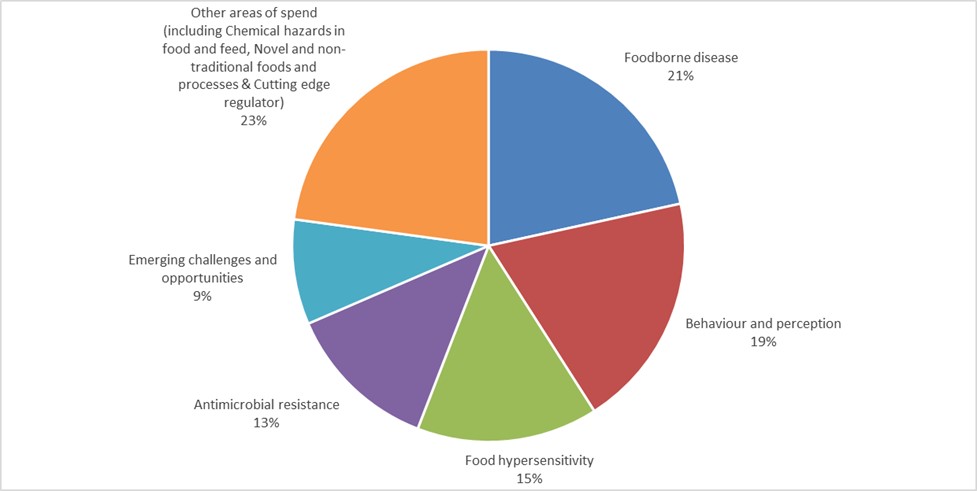
4. As discussed in previous board papers, we are now delivering our research through a series of Research and Evidence Programmes and the distribution of our £5.1 million R&D spend in 2022/23 across the different programmes is as follows.
5. Total science spending in 2022/23 was £17.7 million, compared to £16.6 million in 2021/22. This is both external spend and on FSA staff costs. This means science spending accounts for 12.9% of the Annual FSA Net Expenditure which is a small increase over previous years. This can be seen as follows.

Annex 2 FSA Science Publications
As part of our impact workstream, we monitor access to FSA research projects published on food.gov.
The top 5 research projects which have seen the most page visitors from November 2022 – October 2023 were:
1. The effects of consumer freezing of food on its use-by date - 4,144 page visitors
2. UK-wide Survey of Salmonella and Campylobacter Contamination of Fresh and Frozen Chicken on Retail Sale - 1,792 page visitors
3. Kitchen Life 2: Literature Review - 1,730 page visitors
4. EAT Study: early introduction of allergenic foods to induce tolerance - 1,477 page visitors
5. Survival of SARS-CoV-2 on the surfaces of food and food packaging materials - 1,442 page visitors
The top 5 science landing pages[32] which have seen the most page visitors from November 2022 – October 2023 were:
1. Food and You 2 - 4,172 page visitors
2. Food hypersensitivity – 2,928 page visitors
3. National Diet and Nutrition Survey (NDNS) - 2,284 page visitors
4. Foodborne pathogens - 1,461 page visitors
5. Food and You - 1,001 page visitors
The top 5 risk assessments which have seen the most page visitors from November 2022 – October 2023 were:
1. Rapid Risk Assessment: What is the long-term risk of erucic acid to UK consumers if sunflower oil in food is substituted with refined rapeseed oil? - 13,647 page visitors
2. Rapid risk assessment on the risk of allergic reactions in UK consumers if sunflower oil is substituted with refined rapeseed oil - 1,222 page visitors
3. Rapid risk assessment on the risk of allergic reactions in UK consumers if sunflower oil is substituted with certain vegetable oils (June 2022) – 586 page visitors
4. Salmonella risk profile of UK-produced hen shell eggs: Exposure assessment – 274 page visitors
5. Rapid risk assessment on the risk of allergic reactions in UK consumers if sunflower oil is substituted with certain vegetable oils (April 2022) – 234 page visitors
Published risk assessments between November 2022 – November 2023
4. RP1058 Outcome of assessment of 3-Nitrooxypropanol “3-NOP” as a feed additive for all ruminants for milk production and reproduction
9. Safety assessments of twelve feed additive applications
10. RP270 Assessment of the application for the feed additive Pediococcus pentosaceus IMI507025 for all animal species
17. Risk of campylobacteriosis from low-throughput poultry slaughterhouses
18. Salmonella risk profile of UK-produced hen shell eggs
20. Safety Assessment RP200: Outcome of assessment of Cetylated Fatty Acids as a Novel Food
21. Safety Assessment RP1202: Outcome of the assessment of 3-fucosyllactose (3-FL) as a novel food
27. Safety Assessment RP1259 Muramidase
28. Safety Assessment RP1199 L-Lysine
29. Safety Assessment RP1105 L-Histidine
30. Safety Assessment RP1125 L-Tryptophan
31. Safety Assessment RP1126 L-Lysine Sulfate
32. Safety Assessment RP1198 Butylated Hydroxyanisole
33. Safety Assessment RP1200 Disodium 5’-Guanylate
34. Safety Assessment RP1349 Vitamin K1
35. Safety Assessment RP1591 Fumonisin Esterase
36. Safety Assessment RP140-141-142-284 Monensin Sodium
37. Safety Assessment RP641 Bacillus Velezensis DSM 15544
38. Safety Assessment RP1388 Zinc Chelate of Hydroxy Analogue of Methionine
39. Safety Assessment RP24-25-26 Saccharomyces Cerevisiae MUCL39885 - 4B1710
40. Safety Assessment RP1386 Copper Chelate of Hydroxy Analogue of Methionine
41. Safety Assessment RP1387 Manganese Chelate of Hydroxy Analogue of Methionine
43. Safety Assessment RP16 Chromium Chelate of DL-Methionine
44. Safety Assessment RP29 Pediococcus Acidilactici CNCM I-4622
45. Safety Assessment RP185 6-Phytase from Komagataella Phaffii DSM 23036
46. Safety Assessment RP222 Selenised Yeast Saccharomyces Cerevisiae CNCM I-3060 Inactivated
47. Risk assessment to support guidance for norovirus outbreaks in oysters
FSA reports published since last update:
1. Consumer Handwashing Research: Handwashing in a Pandemic
2. Qualitative research to explore consumer attitudes to food sold online
3. Radioactivity in Food and the Environment (RIFE) report 2021
4. Transmission of AMR bacteria during the processing of chicken meat
5. FSA and Official Controls: Research with Food Business Operators
6. Promoting healthy and sustainable diets: How to effectively generate and translate evidence
7. Survival of SARS-CoV-2 on the surfaces of food and food packaging materials
8. Food Hygiene Rating Scheme (FHRS) Food and You 2: Wave 4
9. Guiding Principles for translating evidence on diet shift for people in the real world
10. Shifting toward healthy and sustainable diets: How to optimise evidence use for policy and practice
11. Food and You 2: Northern Ireland Wave 3-4 Key Findings
12. Nitrate Surveillance Monitoring Program (Annual Report May 2021 - March 2022)
13. Food Hygiene Rating Scheme Audit of Display and Business Survey 2021: Technical report
15. Exploring food behaviours in the UK student population: Interim findings
16. Honey authenticity: collaborative data sharing feasibility study
17. Food and You 2: Wave 5 Key Findings
18. Developing rapid and effective communications testing
20. Final report for the efficacy of withdrawals and recalls evaluation
24. 3D printing technologies in the food system for food production and packaging
25. The Evolution of Personalised Nutrition
27. Consumer perceptions of precision breeding
30. The value of the Food Hygiene Rating Scheme: Business research
31. The value of the Food Hygiene Rating Scheme: Local authority research
32. Identification of hazards in meat products manufactured from cultured animal cells
33. Good Practice Regulatory Change
34. Eating Well Choosing Better Tracker Survey Wave 8 2022
36. Inter-laboratory collaborative trial of real-time PCR method phase 2
37. Survey of knowledge of, and behaviours towards, smoked fish consumption
38. The risk to vulnerable consumers from Listeria monocytogenes in ready to eat smoked fish
40. What works to prevent food fraud
41. Risk from Listeria in smoked fish and survey of knowledge of smoked fish
44. Exploring methods of measuring and collecting data relating to imported food production standards
45. List of commodity codes and categories
46. Food Hygiene Rating Scheme (FHRS) Audit of Display and Business Survey 2022: Technical report
47. Online display of food hygiene ratings by food businesses in Wales
48. Evaluation of the implementation of prepacked for direct sale (PPDS) allergen labelling requirements
49. What is the cost of a healthy food basket in Northern Ireland in 2022?
52. SME allergen provision in the non-prepacked sector
53. Development of Reference Materials for food allergen analysis
54. Local Authority Recovery Plan Assurance Assessment (tranche 2): summary report (England)
55. Consumer Insights Tracker Report March 2022 - March 2023
56. Novel Foods Regulatory Framework Review: Executive Summary
57. International review of the literature and guidance on food allergen cleaning
58. Food System Strategic Assessment 2023
59. Household food insecurity in the UK: data and research landscape
60. Food Hygiene Rating Scheme Online Display in Wales: Research report
61. Evaluation of the Food Standards pilot
62. Retail Surveillance Sampling Programme during Covid-19 pandemic
63. Surveillance Sampling Programme
64. Identifying online display of Food Hygiene Rating Scheme ratings
65. Food Hygiene Rating Scheme (FHRS) Audit of Display and Business Survey 2022
66. Food and You 2: Wave 6 Key Findings
67. Evaluation of the use of remote assessments for FHRS requested re-inspections in England
68. Evaluation of the PATH-SAFE programme
69. Food and You 2: Wave 6 Technical report
73. Review of FSA Social Science
75. Alternatives to single-use plastics in food packaging and production
76. The third study of infectious intestinal disease in the UK (IID3)
78. Literature review on analytical methods for the detection of precision bred products
79. Exploring the safety of at home powdered formula preparation
80. Acrylamide and Furans UK Retail Survey 2020-21
81. The Cost of Food Crime Phase 2
83. Consumer Insights Tracker September 2023
84. Consumer insights tracker (April 2022 to June 2023)
86. Kitchen Life 2
87. Vulnerabilities in the Animal By-Products Food System
88. Radioactivity in Food and the Environment (RIFE) report 2022
Scientific peer-reviewed papers published with FSA co-authors in since the last update:
Scientific peer-reviewed papers published by FSA funded research or using FSA data in 2023:
15. The unknowing expert: Talking to a man about supply chains, Brice J., (2023), Area
Annex 3 Science Advisory Committees
1. The FSA is advised by independent Scientific Advisory Committees (SACs), which provide independent advice and challenge on risk assessment and our use of science.
2. The FSA’s SACs gather scientific information and evaluate its relevance. This is to make sure that the FSA’s advice is based on the best and most recent scientific evidence. SAC members are appointed from a wide range of disciplines and include specialist academics, experienced practitioners and consumer representatives. The independent advice and support that SAC’s and the Joint Expert Groups provide is crucial to protecting public health and the interests of consumers.
3. The FSA SACs are independent expert committees made up of over 100 technical experts and are as follows:
a) Science Council, comprising a Chair and seven members, it provides high-level, expert strategic insight, challenge and advice to the FSA's Chief Scientific Adviser, the Board and executive on the FSA's use of science to deliver its objectives.
b) Advisory Committee for Social Science (ACSS) provides expert strategic advice to the FSA on its use of the social sciences including new and emerging methods, processes and systems to interrogate data, to deliver the FSA's objectives. Its purpose is to help FSA utilise these sciences and approaches to shape and deliver its strategic objectives and understand its impact.
c) Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) provides advice to the Food Standards Agency, the Department of Health and Social Care, and other Government Departments and Agencies on matters concerning the toxicity of chemicals.
d) Advisory Committee on the Microbiological Safety of Food (ACMSF) provides expert advice to Government on questions relating to microbiological issues and food.
e) Advisory Committee on Novel Foods and Processes (ACNFP) provides advise on matters pertaining to novel foods, traditional novel foods, genetically modified foods and feed and novel food processes including food irradiation.
f) Advisory Committee on Animal Feeding stuffs (ACAF) advises on the safety and use of animal feeds and feeding practices, with particular emphasis on protecting human health, and with reference to new technical developments.
4. The SACs are supported by Joint Expert Groups (JEGs) for regulated products, that work to the same principles as our SACs, which will tackle most work required for regulated products with SAC oversight.
5. Details about each of the SACs, including membership, recent work and forwards loom can be found on the dedicated websites (footnote 4).
6. In August 2022, the FSA set out our approach to undertake the required periodic review of the SACs which was an internal review of three of its SACs, and one review encompassing all three Joint Expert Groups and an independent review of the Science Council and Advisory Committee for Social Sciences. The purpose of these reviews was to provide assurance to the FSA, and its stakeholders, that the SACs and JEGs roles and purposes are appropriate in addressing the future needs of the FSA, consumers and wider government, and that the bodies are operating effectively and efficiently. We published the outcomes of both the internal and independent reviews and he FSA response to them. The reviews were generally positive, and we are currently addressing any outstanding recommendations.
7. Members are recruited to the SACs based on their expertise and skill set and not on nationality/place of residence. However, we are aware that the distribution is not as representatives of the four nations as we would like and therefore, we have taken additional measures in the most recent recruitment campaign to disseminate the vacant committee posts as widely as possible to our target audiences, including stakeholders in the four nations.
8. Each of the SACs are bound by their Code of Conduct which includes declaration of conflicts of interest. All SACs have a published list of their members’ registered interests that can be found on the relevant SAC website.
Annex 4 Science Key Performance Indicators (KPI)
1. The development of science KPIs has been an ongoing process involving input from Science Council.
2. While seeking to develop truly outcome focused KPIs in the future, we recognise that this is challenging whilst we are still in the early stages of delivering our strategic plan, and hence lack the long-term data to identify if outcomes are being/have been achieved. Outcome-focused KPIs are also challenging to measure and required significant resources to implement.
3. To address this, the current KPIs are output-related measures which act as leading indicators towards achieving our desired goals and outcomes. These will allow us to benchmark our position in terms of outputs, which will ultimately feed into delivering outcomes in the future. They are also more resource effective to implement, in most cases building on data that is already being collected or which is easily obtainable. Therefore, we have developed a series of output focussed KPIs that are indicative of 4 key outcomes:
a. We are a trusted provider of independent evidence.
b. Science will continue to sit at the heart of the FSA.
c. We have a motivated and inspired team of experts.
d. We have a culture of openness and collaboration.
4. Under each outcome we have 4 KPIs, creating a total of 16 against which we can measure our progress.
5. We see this current set of KPIs as an initial approach, which can evolve and develop over time, as measuring outcomes becomes more viable. The current interim status of the KPI dashboard showing targets and current status is overleaf:
Trusted provider of independent evidence ('Delivery)
A1 - Risk Analysis Process is followed, evidence is assured and outputs fit for purpose
How measured - combined score of all evidence packages delivered
Updated Quarterly
| FY 23/24 Target | 100% |
|---|---|
| Previous measure | N/A (New measure) |
| Current measure | 95% (FY 22/23 data) |
| RAG | Amber |
| Trend on previous | Improving |
A2 - Maintain and improve the use of research and evidence across the FSA
How measured - SERD satisfaction as measured through internal stakeholder survey
Updated annually when internal survey complete (November?)
| FY 23/24 Target | 70% |
|---|---|
| Previous measure | 47% (FY 21/22) |
| Current measure | 70% (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Improving |
A3 - Optimise SERD R&D spending
How measured - Variance in R&D budget
Updated monthly
| FY 23/24 Target | ±10% |
|---|---|
| Previous measure | -10.5% (FY 22/23) |
| Current measure | -7.9% |
| RAG | Green |
| Trend on previous | Improving |
A4 - Maintain or increase the total science investment
How measured - % of FSA spend on science
Updated at end of Financial year
| FY 23/24 Target | 10% |
|---|---|
| Previous measure | 12.7% (FY21/22) |
| Current measure | 13.4% (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Improving |
Science sits at the heaty of the FSA ('Capability')
B1 - Safeguard national science capacity through the network of NRLs
How measured - FSA spend on NRLs
Updated monthly
| FY 23/24 Target | £1,000,000 |
|---|---|
| Previous measure | £1,306,070 (FY 22/23) |
| Current measure | £562,182 |
| RAG | Green |
| Trend on previous | To be confirmed |
B2 - Sustain or improve horizon scanning programme
How measured - Number of HS reports
Updated quarterly
| FY 23/24 Target | 3 |
|---|---|
| Previous measure | 3 (FY21/22) |
| Current measure | 4 (Quarter 2 FY 23/24 data) |
| RAG | Green |
| Trend on previous | Improving |
B3 - Retain access to external expertise through Science Advisory Committees
How measured - Number of experts on SACs
Updated annually at end of recruitment process (March?)
| FY 23/24 Target | 100 |
|---|---|
| Previous measure | 104 (FY21/22) |
| Current measure | 105 (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Improving |
B4 - Levelling up of SERD investments across the UK
How measured % of FSA third party spend outside of London/South East
Updated at end of financial year
| FY 23/24 Target | 50% |
|---|---|
| Previous measure | 43% (FY 21/22) |
| Current measure | 56% (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Improving |
Motivated and inspired team of experts ('People')
C1 - Sustain or improve SERD engagement index
How measured - Engagement score from staff survey
Updated annually from Civil Service staff survey
| FY 23/24 Target | 70% |
|---|---|
| Previous measure | 76% (FY 21/22) |
| Current measure | 72% (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Declining |
C2 - Support staff participation in learning and development activities
How measured - L&D score from staff survey
Updated annually from Civil Service staff survey
| FY 23/24 Target | 70% |
|---|---|
| Previous measure | 64% (FY 21/22) |
| Current measure | 59% (FY 22/23 data) |
| RAG | Red |
| Trend on previous | Declining |
C3 - Effectively manage SERD staff turnover
How measured - Rate of SERD churn
Updated monthly
| FY 23/24 Target | 10% |
|---|---|
| Previous measure | 13% (FY 22/23) |
| Current measure | 9% |
| RAG | Not applicable |
| Trend on previous | Improving |
C4 - Maintain or increase SERD staffing
How measured - % of FSA staff in SERD
Updated Quarterly
| FY 23/24 Target | 15 |
|---|---|
| Previous measure | 19 (FY 22/23) |
| Current measure | 15 |
| RAG | Green |
| Trend on previous | Declining |
Culture of openness and collaboration ('Partnership')
D1 - Maintain or increase the use of SERD science
How measured - Number of SERD reports and peer reviewed papers published as a result of SERD funding
Updated quarterly
| FY 23/24 Target | 75 |
|---|---|
| Previous measure | 129 (FY 23/24) |
| Current measure | 73 (Q2 FY 23/24) |
| RAG | Green |
| Trend on previous | Improving |
D2 - Maintain or increase research collaboration by co-funding join projects
How measured - Leverage of external funds
Updated at end of financial year
| FY 23/24 Target | 10% |
|---|---|
| Previous measure | N/A (New measure) |
| Current measure | 56% (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Not applicable |
D3 - Retain and enhance support for fellows and PhD students working with the FSA
How measured - Number of fellows and students
Updated Quarterly
| FY 23/24 Target | 15 |
|---|---|
| Previous measure | 19 (FY 22/23) |
| Current measure | 15 |
| RAG | Green |
| Trend on previous | Declining |
D4 - Retain and enhance SERD internal science outreach
How measured - Number of internal engagements
Updated at end of Financial year
| FY 23/24 Target | 20 |
|---|---|
| Previous measure | 44 (FY 21/22) |
| Current measure | 57 (FY 22/23 data) |
| RAG | Green |
| Trend on previous | Improving |