Chapter 6 Notifiable Diseases
This chapter details the controls required by the FSA on Notifiable Diseases
Sections
2. Action on suspicion of notifiable diseases
5. Enzootic Bovine Leukosis (EBL)
6. Transmissible spongiform encephalopathies (TSE)
8. Outbreak of Avian Influenza
9. Outbreak of Foot and Mouth Disease
10. Outbreak of Bluetongue Virus Disease (BTV)
11. Outbreak of African Swine Fever (ASF)
12. Updated: Pre-designation & Designations of meat establishments
13. Updated: Enforcement during a Notifiable disease outbreak
1. Introduction
In this section
1.1 Purpose
1.1.1 Background
The prompt identification and notification of certain animal diseases allows the FSA, APHA, Defra and Welsh Government to take action to prevent the spread of the disease. This chapter covers day to day procedures in notifiable disease monitoring and passive surveillance (for example, cases when the disease is suspected) at FSA approved establishments.
Active surveillance procedures for notifiable diseases are included in other chapters of the MOC (for example, chapter 2.4 ‘Post-mortem inspection’ and chapter 2.6 ‘TSE testing’).
When an outbreak is declared, emergency instructions will be issued at the time, since different rules may apply depending on the specifics of the case. Outbreak instructions for Avian Influenza and Foot and Mouth Disease, African Swine Fever and Bluetongue are included within this chapter to facilitate the familiarisation of FSA staff with the expected procedures in place in case of outbreak in Great Britain and the pre-designation of slaughterhouses in “peace-time”.
1.1.2 Legislation
Powers to control notifiable diseases are derived from the Animal Health Act 1981 (as amended) and specific Orders made under the Act or Regulations made under the European Communities Act 1972.
1.1.3 Enforcement
The legislative powers are usually enacted by APHA staff or Local Authority (LA) inspectors. Some FSA staff are authorised under the legislation to undertake certain functions. The legislation is enforced by LAs.
1.1.4 Introduction to FSA duties
FSA staff have a duty to notify the Secretary of State or APHA Vet (Defra Rural Services Helpline on 0300 020 0301 in England or Wales Field Services on 0300 303 8268 in Wales) of any suspect case of a notifiable disease that they may encounter during the course of their work. In practice, they will deal with the APHA Vet.
The decision whether to take further action or not rests with the Vet and it is the responsibility of the Official Veterinarian (OV) to report suspect cases for the decision to be made by APHA.
Also, the FSA participates in monitoring and surveillance schemes aimed at the detection of certain notifiable diseases.
Note: ‘Suspect animal’ includes any animal in which disease is suspected and any animal which came from the same premises of origin.
2. Action on suspicion of notifiable diseases
In this section
2.1 Current notifiable diseases
2.1 Current notifiable diseases
2.1.1 Reporting notifiable diseases
Any person who suspects a notifiable disease has a duty to report it to the APHA Vet.
Official information of notifiable diseases in the UK and further guidance.
The list of notifiable diseases in England and Wales includes:
African Horse Sickness, African Swine Fever, Anthrax, Aujeszky’s Disease, Avian Influenza, BSE, Bluetongue, Brucellosis, Chronic wasting disease, Classical Swine Fever, Contagious Agalactia, Contagious Bovine/Caprine Pleuro-pneumonia, Contagious Epididymitis, Contagious Equine Metritis, Dourine, Echinococcus multilocularis, Enzootic Bovine Leukosis, Epizootic Haemorrhagic Virus Disease, Epizootic Lymphangitis, Equine Viral Arteritis, Equine Viral Encephalomyelitis, Equine Infectious Anaemia, Foot and Mouth Disease, Glanders and Farcy, Goat Pox, Lumpy Skin Disease, Newcastle Disease, Paramyxovirus in pigeons, Peste des Petits Ruminants, Porcine Epidemic Diarrhoea, Rabies, Rift Valley Fever, Rinderpest, Scrapie, Sheep Pox, Surra, Swine Vesicular Disease, Teschen Disease, Tuberculosis (Bovine), Vesicular Stomatitis, West Nile Virus.
Notifiable diseases can be:
- endemic – already present in the UK (for example, bovine TB)
- exotic – not normally present in the UK (for example, FMD, ASF, BT, AI).
2.1.2 Clinical signs of notifiable diseases
Foot and Mouth Disease in ruminants
Bluetongue in cattle and sheep
A collection of guides to notifiable diseases in animals can be found in the following link: Notifiable diseases in animals
2.2 FSA responsibilities and action
2.2.1 When to report
The OV must immediately report to APHA suspicious signs of notifiable disease in:
- live animals or birds
- carcases and offal
If the OV is not present the MHI must consult an OV before reporting a notifiable disease, provided that such consultation will not cause undue delay.
Reports of notifiable disease are to APHA (Defra Rural Services Helpline on 03000 200 301 in England or Wales Field Services on 03000 303 8268 in Wales) and to the FVL for onward reporting to the Portfolio Lead for notifiable diseases.
The OV (or MHI where applicable) MUST keep a written record in the daybook of the time when the suspect cases were reported and the name of the person making the report.
The OV (or MHI where applicable) must follow precisely the instructions given by the APHA Vet. The period between when the OV (or MHI where applicable) reports suspicion of disease and arrival of the VO into the establishment may be critical in controlling the spread of disease.
2.2.2 Reporting details
Provide the following information to the APHA Vet:
- the plant name, address and contact telephone number
- the animal’s breed, age, sex and identification mark(s) (eartag number or slapmark)
- details of any clinical signs and history in the suspect cases such as, time of arrival, size of the consignment, origin of the animals in the consignment and any in-contact animal from the same establishment
- details of the lesions found during meat inspection
- the name, address and the holding County Parish Holding (CPH) number of the establishment where the suspect animal or carcase(s) came from and details about when the animal arrived in the lairage and what other animals arrived in the same consignment. This will allow APHA to arrange an investigation at this establishment if needed
2.2.3 Instructions from APHA
Instructions given by the APHA Vet could include:
- isolating the animal until an investigation has been completed
- restricting movement of all animals, birds, products, vehicles or people into or out of the slaughterhouse until an investigation has been completed
- stopping slaughter
2.2.4 Record keeping
The OV must keep a contemporaneous record in the daybook of all instructions received from the APHA Vet and confirm that they have been followed.
The OV must ensure that the ante-mortem and post-mortem inspection records input in IRIS are consistent with the findings and the suspicion of the notifiable disease.
When an exotic notifiable disease is suspected, and reported to APHA, regardless of the outcome or response from APHA, the OV must send an e-mail to the FSA Incident Team describing the situation and including relevant information (for example, animal identification and origin, disease suspected, response received from APHA). The FVL for the area in which the slaughterhouse is located must be copied into this e-mail.
2.2.5 C and D
No disinfectant should be used on or near animals, birds or carcases suspected of disease, while waiting for the APHA Vet to attend, as this may adversely affect the likelihood of correct laboratory diagnosis.
2.2.6 Consultation cases
Providing that the OV is in the establishment and remains there, APHA may decide to deal with the investigation as a ‘consultation case’.
A consultation case takes place between two or more veterinary surgeons when one of them considers that a notifiable disease may be included in the differential diagnosis for a specific case, but the probability of it being that disease is very low.
The OV should discuss the report of disease with the APHA Vet on arrival at the establishment.
The APHA Vet will place restrictions only if the result of the consultation is that a notifiable disease is suspected.
2.2.7 Report case
In other cases, APHA may call the case a ‘Report Case’ and place specific restrictions on the establishment pending veterinary enquiry. These restrictions may affect the movement of animals, products, people and vehicles from the establishment.
2.2.8 Legislative responsibilities
The OV remains responsible for:
- ensuring that all public health legislation is complied with while the establishment is under APHA restrictions
- monitoring hygiene and animal welfare
- following APHA instructions and informing them immediately if any of them cannot be implemented
2.2.9 Procedure for suspect notifiable disease
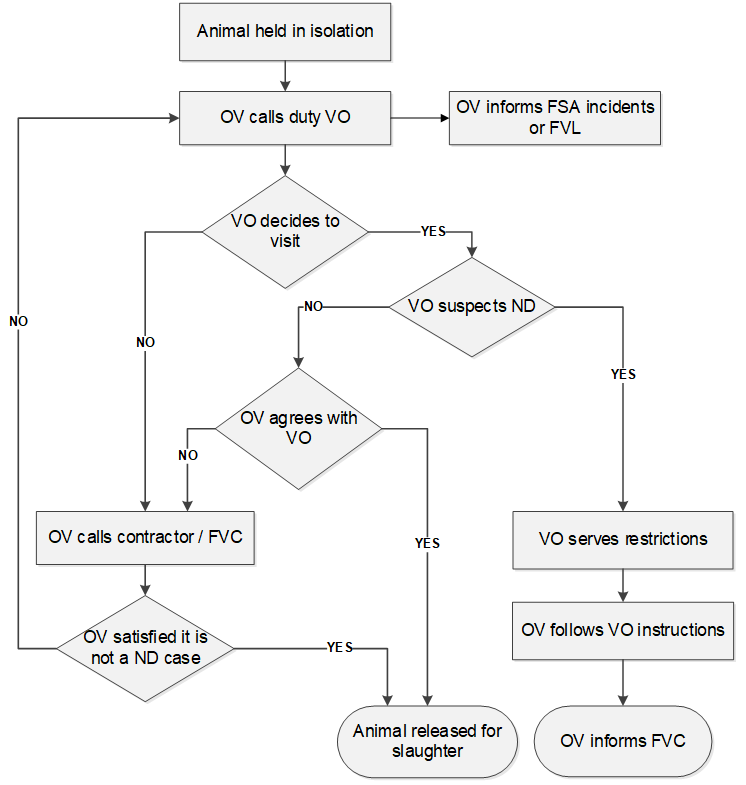
If the APHA Vet agrees the possibility of a Notifiable Disease, the premises should be treated as contaminated, until proven otherwise. The Food Business Operator (FBO) should:
- not bring more susceptible animals on to the premises
- not slaughter live suspect animals (so the VO can sample them)
- isolate suspect / potentially contaminated carcases
The chart below outlines the procedure to follow if the OV suspects a notifiable disease.
2.3 Responsibilities of APHA
2.3.1 Main duties
APHA has responsibility for:
- applying animal health disease control measures to minimise the spread of notifiable disease
- fully investigating the OV (or other FSA AO) report
2.3.2 APHA Vet investigation
An APHA Vet will visit the slaughterhouse to carry out an investigation. Other VOs may be sent to the farm of origin to undertake a simultaneous veterinary enquiry.
Once at the establishment, the APHA Vet will discuss the report with the OV / MHI / FBO and examine the suspect animals / carcases / offal. The APHA Vet may also consult with other APHA Vets who may have gone to the farm of origin to gain a full clinical picture, and with APHA Veterinary Exotic Notifiable Diseases Unit (VENDU).
2.3.3 After investigations
If the presence of a notifiable disease is ruled out, restrictions will be lifted. The OV should follow ante-mortem procedures as per 2.1.16 Ante-mortem inspection summary in MOC Chapter 2.2.
If the presence of notifiable disease cannot be ruled out, the APHA Vet will:
- serve a restriction notice closing establishments (or parts), or
- amend any restriction notice that has already been served, and / or
- collect whatever samples are necessary for diagnostic purposes
If the initial investigation began as a consultation case, it will now become a report case.
2.3.4 Restrictions
APHA will regularly review the extent of the restrictions bearing in mind quick recommence of the operations but it will be subjected to some conditions such as thorough C and D. The APHA Vet will undertake this assessment taking advice from the OV and FBO.
2.4 Other responsibilities
2.4.1 Compliance
All persons at the establishment, including FSA staff, must comply with any restrictions in any notices served on the establishment.
2.4.2 Local authority
The LA is responsible for taking enforcement action under disease control legislation.
2.5 Detained meat storage
2.5.1 Storage sites
Any meat detained at the slaughterhouse will be kept locked in a ‘storage site’ under control of the OV and APHA. Access to this storage site will be facilitated through the OV or APHA Vet. The FBO is responsible for the way the meat is stored, in compliance with updated: [assimilated regulations] (EC) 852/2004 and 853/2004.
The storage site is likely to be kept under restrictions until the final results are known and disease is confirmed or not.
2.5.2 Preparation for storage
The FBO may discuss procedures for preparing the meat for storage with APHA and FSA.
2.5.3 Test results
Negative results take longer to reach completion. APHA will provide information on how long it could take before the results are known.
2.5.4 Public health
FSA are fully responsible for ensuring that public health legislation is complied with at all times the meat is at the establishment.
Meat is to be declared unfit for human consumption if it derives from animals affected by animal diseases for which animal health rules are laid down in Directive 2002/99/EC except if it is obtained in conformity with the specific requirements provided for in that Directive. This exemption does not apply if otherwise provided for in the requirements on the official controls of tuberculosis and brucellosis provided for in Articles 33 and 34 of Regulation (EU) 2019/627.
The diseases listed in the Directive are: Classical and African Swine Fever, Foot and Mouth Disease, Avian Influenza, Newcastle Disease, Rinderpest, Sheep and Goat Plague (Peste des Petits Ruminants) and Swine Vesicular Disease.
Reference: Regulation (EU) 2019/627, Article 45 (e)
Meat shall also be declared unfit for human consumption if in the opinion of the OV, after examination of all the relevant information, may constitute a risk to human or animal health or is for any other reason not suitable for human consumption.
Reference: Regulation 2019/627 Article 45(t)
2.5.5 Clearance
Meat detained on suspicion of disease will usually be released once all the tests are negative. The OV must seek clearance from APHA and keep a written record before opening any sealed container.
2.6 C and D of the establishment
2.6.1 Requirement to Cleanse and Disinfect
When certain diseases cannot be ruled out, APHA may require the FBO to cleanse and disinfect specified parts of their establishment. FBOs are responsible for doing this at their own expense. APHA may request FSA assistance in supervising the C and D of the establishment.
When carrying out C and D activities in the event of an outbreak (or during the investigation of a suspected outbreak) of a Notifiable Disease, FBOs are requested to use the relevant disinfectant as listed on the Defra website.
These C and D activities need to be documented by protocols where the FBO should describe how to C and D the relevant equipment, utensils and vehicles. This should at least be in line with the manufacturers’ instructions for the chemical in use.
2.6.2 After C and D
The APHA Vet will be able to confirm when the operations can re-commence after the C and D - in some cases the establishment may have to be rested for a specified period. The aim will always be to allow resumption of operations as soon as possible.
3. Anthrax
In this section
3.1 Introduction
3.1.1 Background
The OV (or MHI where applicable) may consider the possibility of anthrax in the course of normal duties. In reaching a decision, the OV must take into account factors such as history or clinical signs.
Official information about Anthrax in UK can be found online.
3.1.2 Anthrax: clinical and pathological signs
Suspicion of anthrax should be considered:
- if the cause of death is unexplained, particularly sudden death, in apparently healthy animals
- when potential signs of anthrax are observed in the dead animal (for example, dark, tarry uncoagulated bloody discharges from natural orifices, rapid bloating of the carcass, incomplete rigor mortis)
- if indications in the Food Chain Information (FCI) or any other information indicate higher risk of the farm / area of origin
- if clinical signs at ante-mortem inspection indicate that the disease might be present, for example, high temperature, bloody diarrhoea or a discharge of dark tarry uncoagulated blood from the nose, mouth and anus
- if post-mortem evidence suggests that the animal might have been suffering from anthrax (for example, swollen spleen with bloodstained fluid in all body cavities).
Note: If the OV suspects anthrax, the carcase should not be opened as this can result in the formation of highly resistant Anthrax spores.
3.1.3 Suspect live animals
Suspect animals and animals in direct contact must be detained, isolated and reported to the APHA Duty Vet immediately.
The APHA Vet will place restrictions upon the animal, but it will not be slaughtered. It may be treated in situ, but for as long as the animal shows signs of disease the restrictions will remain in place.
3.1.4 Suspect carcases
In some cases, suspicion of disease will not be raised until the carcase has been opened. The whole of the suspect carcase, offal, hide and blood must be detained (including any parts already removed) and people kept away from the carcase, its parts and the area where the carcase is held.
All other carcases and offal at the establishment should be detained pending completion of enquiries. No other animals should be allowed to enter the slaughterhall until the results of the enquiry are known.
Holding pens should not be cleaned, and no other product or waste should be allowed to leave the site until authorised by APHA staff.
3.1.5 Details to report
The OV (or MHI where applicable) must report suspect cases to the APHA Vet immediately, giving details as instructed in section 2 of this chapter. The decision whether to take further action or not rests with the APHA Vet and it is the duty of the OV to report suspect cases for the decision to be made by APHA.
3.1.6 APHA action
The APHA Vet will inform that restrictions apply and will also arrange for an immediate enquiry to be carried out by APHA Vet. OVs authorised through the Official Controls Qualification (Veterinary) – Statutory Surveillance (OCQ(V)-SS) can carry out an enquiry into anthrax.
If the OV is a designated OV with an OCQ(V)-SS, APHA Vet may instruct the OV to undertake the enquiry providing suitable facilities are available for testing.
OVs cannot carry out enquiries in anticipation of authorisation from APHA.
3.1.7 C and D
Holding pens should not be cleaned and no other product or waste allowed to leave the site until authorised by APHA staff.
It is likely that APHA requires the FBO to carry out the C and D of any place associated with any animal notified as a suspect case pending the veterinary inquiry. If the results of the veterinary inquiry are positive or inconclusive, the FBO will be required to carry out a more thorough C and D procedure.
3.2 Investigation and diagnostic sampling
3.2.1 Anthrax bacilli suspected: initial investigation
Under no circumstances must the OV attempt to collect and examine samples for anthrax without having informed the APHA Vet and being authorised to do so.
If the OV is a designated OV with an OCQ(V)-SS and facilities are available, APHA Vet may request them to make the initial investigation.
3.2.2 TSE testing
If a bovine or ovine animal is found dead in the lairage or dead on arrival and the OV suspects anthrax, then the animal must be tested for anthrax and this disease rules out before being subjected to Transmissible Spongiform Encephalopathies (TSE) testing in eligible cattle or, in the case of adult sheep in selected slaughterhouses.
3.2.3 Suspect anthrax out of hours
If it is necessary for an examination for suspected anthrax to be carried out at a slaughterhouse outside normal OV hours of attendance, the APHA Vet will request an APHA vet or authorised veterinarian designated as OV with an OCQ(V)-SS to attend the establishment to conduct such an examination. If the OV is a designated OV with an OCQ(V)-SS, the APHA Vet may ask them to do this.
3.2.4 Anthrax suspected
If disease is suspected, the attending vet with an OCQ(V)-SS will report this to the APHA Vet who will make arrangements for the submission of further samples for testing.
3.2.5 Detention of suspect carcases
Where anthrax is suspected, the carcase should be detained until the results are received.
The FBO may dispose of the carcase as Category 2 Animal by-products (ABP) only if suspicion of anthrax has been ruled out.
3.2.6 Anthrax ruled out
Where the APHA vet with an OCQ(V)-SS is satisfied that anthrax does not exist in the live animal, they will notify the APHA Vet and FBO by completing form AN2 (Certificate – Non-existence of Disease in a Carcase).
Reference: See Annex 3 on ‘AN2 – Certificate’ for a sample.
If the animal has died and requires TSE testing, the procedure for testing fallen stock must be followed once the presence of anthrax has been ruled out.
If an owner requests an investigation into the cause of death, this is a private matter which must be arranged between the owner and private veterinary surgeon.
4. Bovine Brucellosis
In this section
4.1 Overview
4.1.1 Introduction
The UK achieved official brucellosis free status in 1985.
Official information about Brucellosis in the UK is available online.
There are measures in place to prevent the disease being re-introduced and subsequently spreading, such as:
- post import testing of imported cattle
- compulsory reporting of all bovine abortions and premature calvings with investigation of all outside a specified low risk category
- quarterly testing of bulk milk samples from all dairy herds, including those of producer retailers
4.1.2 Responsibilities
APHA will inform the FSA about proposed slaughter of reactors. Collection and packaging of samples from brucellosis cases consigned for slaughter is the FSA responsibility, and will include:
- reactors and inconclusive reactors to the brucellosis tests, and
- contacts with confirmed cases
The despatch of the samples to the laboratory is the responsibility of APHA who will collect the samples from the slaughterhouse.
Note: The OV must report any abortions and premature births to APHA and follow any additional instructions. All FSA staff should be aware of the potential danger of infection primarily from the uterus and udder.
4.1.3 Movement licences
Cattle from restricted premises will be consigned directly to slaughterhouses accompanied by a BS112 (Licence authorising the movement of cattle on to or off premises under restriction or authorising the movement of specified cattle which are under restriction awaiting the completion of tests for brucellosis).
APHA will send a copy of the BS112 licence, to the OV as advanced warning.
Reference: See Annex 4 on ‘BS112 – Licence’ for a sample of the form.
In addition, where the owner has opted to slaughter the animal at their own expense (private slaughter) the animal will be accompanied by form BS15B. These are handed to the FBO on arrival.
Reference: See Annex 5 on ‘BS15B – Notice’ for a sample of the form.
4.2 Slaughter and sampling
4.2.1 Slaughter procedure
The OV / MHI must collect the following samples from the carcase:
| All animals |
|---|
Paired lymph nodes
|
| In addition for bulls |
|---|
|
4.2.2 Sampling packaging
Samples must be taken as cleanly as possible using sterilised knives, and placed in a labelled polythene bag (each pair of nodes or organs should be placed in a separate bag), which is then sealed.
All specimens from each animal sampled should then be placed together in a further single outer polythene bag and this bag then sealed and labelled.
Polythene bags should be self-sealable or tightly knotted and of sufficient strength to prevent leakage and potential cross-contamination.
4.2.3 Labelling
Label all sample bags with the ear tag number plus the details of any reactor tag.
4.2.4 Storage
All samples should be placed in a refrigerator (not freezer) until collected by APHA staff. FSA staff should inform APHA when the samples are ready for collection.
5. Enzootic Bovine Leukosis (EBL)
In this section
5.2 Investigation of tumours in cattle carcases or offal
5.1 Introduction
5.1.1 Enzootic Bovine Leukosis (EBL)
The OV must notify the APHA Vet of:
- any live animal affected with, or suspected of being affected with, EBL, and
- any carcase or offal showing certain tumorous changes
Detain any suspect live animal or any suspect carcase with its offal until the APHA Vet issues instructions. Retain the passport and FCI until any investigations have been carried out.
Official information about EBL in UK can be found online.
5.1.2 Signs to report
The OV should report suspect cases in live animals or carcases when there is evidence of tumours (other than papillomata or haemangiomata) or of swollen lymph nodes (LN). Tumours in young animals normally arise from sporadic leukosis and not EBL; the latter being associated with tumours in animals aged three years or more.
Note: Swollen lymph glands identified in a live animal suffering from EBL will be painless.
5.1.3 Documentation
Animals from establishments under movement restrictions because of EBL may be moved to slaughter under licence from APHA (Form EBL9).
Reference: See Annex 6 on ‘EBL9 – Licence’ for a sample of the form.
Other animals licensed for slaughter from restricted establishments will not usually need to be inspected by an APHA Vet and the FSA should subject such carcases and their offal to normal meat inspection procedures, paying particular attention for evidence of tumorous change.
5.1.4 Dentition check
Whenever suspect disease is reported in a live animal, the APHA Vet will ask for the date of birth of the animal recorded in the cattle passport and whether either of the animal's second pair of permanent incisors has erupted – that is, whether there are more than two ‘broad teeth’.
If the answer is no, then in most cases no further action will be required other than the provision of outline data (APHA is required to keep a record of such cases for reporting to the EU), and the animal can be slaughtered and subjected to normal post-mortem inspection procedures and judgement.
5.1.5 Three or more permanent incisors
If either of the second pair of permanent incisors has erupted (there are three or more ‘broad teeth’), then APHA will instruct an APHA Vet to carry out an investigation, and the OV must ensure the animal is detained in the lairage pending this investigation.
5.1.6 After the investigation
Following the completion of the APHA Vet investigation, the animal may be slaughtered and subjected to normal post-mortem inspection procedures and judgement.
Appropriate samples of tumorous swollen lymph nodes should be taken from the carcase or offals at the request of the APHA Vet, where EBL has not been ruled out. As per instructions in section 5.2.2
The carcase and offal need not be detained pending the results of the tests on any collected samples.
5.2 Investigation of tumours in cattle carcases or offal
5.2.1 Tumours in cattle
All cattle tumours seen at post-mortem inspection are notifiable, with the exception of papillomata or haemangiomata and should therefore be reported IMMEDIATELY to the APHA Vet, who will note the details of all cases and instruct when sampling by the FSA is to be carried out.
A large proportion of tumour notifications concern animals aged less than two years. Although collection of tumour specimens from cattle with fewer than three permanent incisors is not normally required, APHA retains discretion to require sampling or to instruct an APHA Vet to carry out an investigation.
5.2.2 Sampling of tumours
When asked to do so, the FSA is responsible for collecting the appropriate samples from carcases and / or offal and retaining these along with details of the tumour site and the FCI. Cattle passports and FCI should always be retained by the FSA to assist APHA in the process of tracing.
The FSA will arrange for collection of the samples and complete all relevant details on the EBL7 submission form. Details to be included in the from include the WAS number provided by APHA. The FSA will prepare, pack and send the samples along with the completed submission forms to the laboratory.
FSA staff must positively differentiate between lesions which are tumorous (EBL) and those which are tuberculosis (TB) as different sampling and diagnostic testing is required.
The FSA will sample a tumorous carcase and / or its offal, the following 2 sets of samples should be collected:
- tissue samples for Polymerase Chain Reaction Test (PCRT)
- tissue samples for histology
5.3 Sampling of tumour carcases
5.3.1 Samples of PCRT
A PCRT has been developed to detect the presence of Bovine Leukosis Virus (BLV – the agent responsible for EBL infection) in cattle tissues and LN.
The PCRT requires fresh refrigerated samples.
5.3.2 Samples of histology
Samples for histological analysis are also needed as a backup should the fresh samples prove unsatisfactory for PCRT.
These samples should consist of a specimen from each of the grossly affected organs and representative enlarged LNs.
5.3.3 Collection of samples
Follow the steps in the tables below to collect the samples.
Note: Remove samples within 24 hours of slaughter.
Sample for PCR test:
| Step | Action |
|---|---|
| 1 | Use sterilised knives and gloves for each carcase |
| 2 | Take tissue sample from undisturbed part of tumour and from one accessible non-lesioned lymph node of 5-10g |
| 3 | Transfer sample to individual sterile 60 ml pot |
| 4 | Write ‘PCR Test’, ear tag number, Work Schedule Activity (WSA) and organ tissue sampled on label and stick on pot |
| 5 | Store chilled until dispatch by courier |
Sample for histology:
| Step | Action |
|---|---|
| 1 | Take sample from affected organs and representative enlarged LNs |
| 2 | Cut specimens about 1cm thick; a slice of organ should show both normal and diseased tissue |
| 3 | LNs should be transverse across the long axis of the node and should include the capsule |
| 4 | Transfer sample to individual sterile 60ml pot |
| 5 | Write ‘Histo Test’, ear tag number, case reference number and organ tissue sampled on label and stick on pot |
| 6 | Store chilled until dispatch by courier |
5.3.4 Post-mortem inspection
Once the required samples have been removed, the carcase may be subjected to normal post-mortem inspection procedures and judgement – it need not be detained pending the results of the tests for EBL.
5.3.5 Recording of post-mortem findings
Details of the tumour site should be recorded on the form EBL7, together with all available identification information. Complete only those parts of the form for which you have information; the remainder will be completed by APHA staff.
Reference: See Annex 7 on ‘EBL7 – Submission form’ for a sample of the form.
5.3.6 Notifying FSA
The OV should notify the Service Level Agreement (SLA) and Contracts team by email of the following details of the sample:
- Plant number
- Plant name
- Date case found
- passport number of the sampled animal, date of birth and breed
- name of owner and premises of origin or market lot number if applicable
- CPH number
- name of APHA office contacted
- case reference number assigned by APHA after initial notification
- date despatched via Topspeed
5.4 Packaging and despatch
5.4.1 Packing
- All samples must be submitted in a 60ml pot.
- Outside of pot must be kept clean.
- Remember to tighten lids. Give an extra turn before packing.
- Avoid cross threading the lids as they will cause the pots to leak.
- Place each individual pot in a plastic bag which is knotted tightly. Trim off excess bag.
- Place all bagged pots into a biobox / biobottle along with the absorbent pad / material and seal the box. The process for sending forms is as follows:
- Signed original EBL7 forms must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the original forms internally to the relevant APHA regional office
- Copies of the EBL7 forms should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. Copies of these forms should be faxed or emailed to the relevant APHA office. The OV should retain a further copy in the plant files for future reference (retention period 12 months).
- Place biobottle into the outer box.
- Attach address label.
- Attach security seal
- Store the package in the chiller until the time of collection. Ideally place in a waterproof bag / container to avoid contamination.
5.4.2 Despatch
The current courier for the new sampling process is Topspeed Couriers. The courier process is as follows:
As soon as you receive the sampling request information from APHA, email: ebl@topspeedcouriers.co.uk with the following information:
- establishment name and approval number
- slaughter date of the samples (this information will allow Topspeed Couriers to plan the collections to include multiple pickups where possible)
- destination laboratory:
BLV – PCR Virology Department
APHA Weybridge
New Haw
Addlestone
Surrey
KT15 3NB
- name and telephone number for the FSA contact at the plant
On detection of a tumour that needs samples submitting, notify the courier that samples are required to be collected. The courier will organise a collection which meets the two working days delivery requirement (for example, a tumour found on Monday; samples are required to be with APHA by 5pm Wednesday. However, collection could take place on Monday, Tuesday or Wednesday, as the couriers are required to consolidate their delivery runs to be cost effective.)
5.4.3 Ordering consumables
The OV at each abattoir is responsible for ensuring that there are sufficient supplies of consumables for packing samples. It is important that only the specified packaging materials (such as pots and labels) are used as failure to do so may result in the sample being un-assayable at the lab.
The consumables must be ordered directly from APHA Weybridge by using the following procedure:
- Fill in the requisition form (Annex 8a/b) specifying the type of materials required and the number of units.
- Make sure that you complete all the boxes (establishment name, address, FSA contact name and telephone number, and any others).
- The requisition form should be emailed to: StoresStockOrders@apha.gov.uk or faxed to APHA Weybridge: 01932 357497.
APHA will endeavour to complete delivery of consumables orders within 7 working days of receipt. If you have any queries regarding an order that you have placed you should telephone the APHA stores in Weybridge on 01932 359451.
6. Transmissible spongiform encephalopathies (TSE)
In this section
6.1 TSE overview
6.1.1 Introduction
This section outlines action to be taken when a TSE is suspected in an animal.
Instructions regarding sampling of animals when TSEs are not suspected can be found in the chapter 2.6 on ‘Transmissible Spongiform Encephalopathy’.
6.1.2 Information about TSEs
Information about TSEs is carried on Defra’s website.
Information about the clinical signs of the most relevant TSEs:
Updated: [clinical signs in goats]
6.1.3 Reporting requirements
TSEs are notifiable diseases and their suspicion must be reported immediately to APHA.
6.1.4 Records
For all reported cases, the OV should ensure accurate details are recorded in the daybook.
6.2 Reporting suspicions
6.2.1 Suspect live animals
If FSA or plant staff suspect that live cattle, sheep, goats or deer are affected with Bovine Spongiform Encephalopathy (BSE), Scrapie or other TSE, they must take action as detailed in this topic.
The requirement of an OV to carry out the ante-mortem inspection of every animal is key for the identification of clinical suspects of TSE. OVs working in ruminant slaughterhouses must be aware of the clinical signs of TSE and take them into consideration during the ante-mortem inspection. Links with information about clinical signs of TSE including videos are available at point 6.1.2 of this section.
Caution: The OV, especially in the case of BSE, should be aware that an affected animal may, because of behavioural changes associated with the disease, be likely to cause injury to itself, other livestock or staff.
| Step | Action |
|---|---|
| 1 | Suspect animal is held in isolation in the lairage. On no account should a suspect animal be allowed to enter the main slaughterhall unless and until the OV is satisfied that it should no longer be considered a suspect. |
| 2 |
The OV telephones the APHA Vet to notify the suspicion of a TSE. There are two possible outcomes to the telephone conversation:
If 1 occurs then the OV should follow Option 1 below. If 2 occurs the OV should follow steps at Option 2 below. |
Option 1 The table below details the action to take if the APHA Vet cannot rule out the disease over the phone.
| Step | Action |
|---|---|
| 1 |
The APHA Vet answering the call makes arrangements for a APHA Vet to visit the slaughterhouse as soon as possible to carry out an investigation. APHA may request the following details:
|
| 2 | The OV obtains FCI and cattle passport before the APHA vet arrives |
| 3 | The FBO informs the owner of the animal |
| 4 | The APHA vet examines the animal and determines whether the disease can be ruled out or not on clinical grounds |
| 5 | The OV report by e-mail to the FSA incidents team and the FVL for the establishment, the suspected case including details of the animal (including eartag number, date of birth), scanned copies of relevant documents (for example, cattle passport, ARAMS document, FCI) and an update about the referral to APHA. |
Option 2 The table below details the action to take if the VO considers they are able to negate the disease over the phone.
| Step | Action |
|---|---|
| 1 | Once the case is negated, the OV shall carry out the ante-mortem inspection following the procedures as per 2.1.16 (Ante-mortem inspection summary in MOC Chapter 2.2) |
| 2 | The OV report by e-mail to the FSA incidents team and the FVL for the establishment the suspected case including details of the animal (including eartag number, date of birth), scanned copies of relevant documents (for example, cattle passport, FCI) and an update about the referral to APHA |
6.3 At visit: APHA Vet does not suspect TSE
The APHA Vet considers that the suspect is not affected by BSE, Scrapie or other TSE.
Notifiable disease would then be ruled out and the OV should follow ante-mortem procedures as per 2.1.16 Ante-mortem inspection summary in MOC Chapter 2.2.
Note: Certain bovine animals which are not considered to be BSE suspects require TSE testing (see chapter 2.6 ‘TSE testing’).
6.4 At visit: APHA Vet suspects TSE
6.4.1 Restrictions on animals
If the APHA Vet cannot rule out the suspicion of the disease on clinical grounds, they will serve restrictions on the animal. Once restricted, the FBO must not allow the animal to be slaughtered.
6.4.2 Slaughter and destruction
The APHA Vet will euthanize the animal by injection of barbiturate and arrange for the dead animal to be transported either to an incineration plant or a veterinary laboratory where the head will be sampled.
In the case of sheep or goats, if the suspect animal is considered fit to travel, the APHA Vet may make arrangements to transport it live under licence to the nearest available veterinary laboratory.
6.4.3 Restrictions on premises
No restrictions will be imposed on the slaughterhouse premises in the case of a TSE suspect, provided the animal was not slaughtered, although the APHA Vet may give advice on cleaning and disinfection in clinically positive cases.
6.4.4 Informing the FVL and the FSA Incident Team
The OV should inform their FVL and the FSA Incidents Team that a TSE suspect animal has been killed at or removed from an approved establishment by APHA staff.
7. Tuberculosis (TB)
In this section
7.4 Reactor animals: notifications and responsibilities
7.5 Reactor animals: inspection requirements
7.6 Reactor animals: actions when rejected at ante-mortem due to being dirty
7.7 Reactor animals: post-mortem decision
7.11 The slaughterhouse case: additional detailed inspection
7.12 The slaughterhouse case: sampling
7.13 Packing and despatch of samples
7.14 Private Slaughter of TB Reactors (Rs), Direct Contacts (DCs) and Inconclusive Reactors (IRs)
7.1 Introduction
7.1.1 Introduction
Bovine TB is an infectious and contagious disease of cattle and one of the biggest challenges for the cattle farming industry. It is caused by the bacterium Mycobacterium bovis (M. bovis), which can also infect and cause TB in many other mammals.
APHA is responsible for the control of TB in farms. The FSA, through an SLA, deals with sampling of tuberculin tested animals at APHA’s request and suspect TB lesions identified at slaughterhouses.
If TB is suspected in the carcase of any bovine, deer or farmed mammal, APHA must be notified immediately.
Reference: The Tuberculosis in Animals (England) Order 2021 and the Tuberculosis (Wales) Order 2010 (as amended).
Note: Health and safety procedures must be adhered to when handling suspect TB lesions. See FSA’s Health and safety manual.
7.1.2 Definitions
TB reactor plants are red meat slaughterhouses where reactor (and Direct Contacts (DC)) animals that have undergone a tuberculin test are sent for slaughter. Slaughterhouses access this status through a contract with APHA.
In some instances, where an agreement has been reached between APHA/FBO/ OV, the keepers can send Reactors, Direct Contacts, Inconclusive Reactors for a private slaughter at a slaughterhouse which is not a TB reactor plant. These are defined, in this context, as Rs / DCs/ IRs animals sent for a private slaughter.
Reference: See topic 7.14 on: Private Slaughter of TB Reactors (Rs), Direct Contacts (DCs) and Inconclusive Reactors (IRs)
Depending on the result of the tuberculin test, animals can be classed as reactors (R), inconclusive reactors (IR) and direct contacts (DCs). These animals can be compulsorily (R and DC) or voluntarily (IR) slaughtered.
Restricted premises are those farms where APHA has established cattle movement restrictions.
A full list of the movement licences for these animals and the relevant TB forms is given in the Annex list.
7.1.3 Timesheet coding
All work undertaken by the FSA on behalf of APHA (such as additional inspection requirements, Reactor tag checking, collection and submission of samples and record keeping) must be coded to GNTB.
7.1.4 Scope of the instructions
This section details instructions to FSA staff for dealing with reactors and other cattle from restricted premises, including:
- forms accompanying animals from restricted premises
- inspection of R, IRs and DCs
- death of R/IRs/DCs before reaching the slaughterhouse
- collection and submission of samples
- form completion
- carcases and offal from cattle with suspicious lesions encountered in the course of normal production, also known as ‘The Slaughterhouse Case’
- carcases and offal from other species with suspicious TB lesions
The instructions apply to:
- R and DCs compulsorily slaughtered by APHA
- IRs voluntarily slaughtered but for which APHA require samples, that is stock accompanied by a TB24 and where advance warning has been given by APHA by means of entering information on TB110 (reactor abattoirs) or via SLA and Contract team (elsewhere), whether alive or dead
- cattle and any other mammals that have been slaughtered in the course of normal production, where lesions consistent with TB are found during post-mortem inspection, also known as slaughterhouse cases.
They do not apply to other cattle from TB restricted herds.
Note: The OV must be aware that animals with clinical TB must not be slaughtered for human consumption.
Reference: Regulation (EU) 2019/627, Article 45(f).
7.2 Slaughter
7.2.1 Where or when to slaughter
Where animals have reacted positively or inconclusively to the tuberculin test, or there are other grounds for suspecting infection, they are to be slaughtered separately, taking precautions to avoid the risk of contamination of other carcases, the slaughter line and staff present in the slaughterhouse.
This applies to:
- cattle that require a TB24 movement licence and have been entered on a TB110 by APHA
- cattle that have a TB24 marked ‘Inconclusive Reactor’
- deer that require a TB24a movement licence and APHA has advised of intended slaughter by means of a TB55a form
- sheep or other mammals that were tuberculin tested
It does not apply to animals moved under any other licences, or with a TB24 where the animal is not included on a TB110.
To reduce cross-contamination, the slaughter line must be cleansed and disinfected after processing reactor cattle, IRs and DCs. All such cattle should either be slaughtered:
- last in the day, before full C and D of the slaughter line
- at any other time provided that the slaughter line is cleaned and disinfected before the slaughter of non-suspect animals resumes
- in a separate slaughterhall used for diseased animals or those suspected of being diseased
Reference: Regulation (EU) 2019/627, Article 33.
Any species with TB suspect lesions found during the course of post mortem inspection, particularly where there are no suitable facilities for detailed inspection and sampling in the dressing line, should immediately be placed in the detained area.
7.2.2 Transfer of carcases and offal to the detained facilities
When transferring offal / carcases to a detained area for further inspection or sampling, care must be taken to prevent cross-contamination of other meat / equipment / fittings in the slaughterhall. In the event of suspected contamination, C and D of the affected area / equipment must take place before production recommences.
Note: Failure by the plant operator to co-operate with this procedure would constitute a contravention of the operator’s responsibility to prevent cross-contamination and must be dealt with accordingly.
Reference: Regulation (EU) 2019/627, Article 33
7.3 Reactor animals
7.3.1 Types of animals
The table below shows the animals that may be despatched from TB-restricted premises.
| Consigned to slaughter | By | Examples |
|---|---|---|
| Compulsorily | APHA | Test reactors, DCs |
| Voluntarily | Herd owner | Fat stock, surplus calves, culled cows/which the herd owner chooses to slaughter |
7.3.2 Forms
In addition to the official identification documents and the FCI, animals from TB-restricted establishments may also be accompanied by one or more of the following forms:
- Emergency Slaughter Certificate
- TB24, TB24b, TB24c, TB24g, TB16b, TB24a, TB55a
- electronic notification by APHA via a TB110 sent to the OV by noon the day before the kill
Reference: See Annexes 9 to 14 for sample movement licences and FCI forms.
7.3.3 C and D of transport vehicles
All the cattle from bovine Tuberculosis restricted farms moved to slaughter, including animals with negative test results, are covered by a general of specific movement licence requiring the transport vehicle to be cleansed and disinfected with a disinfectant and concentration approved under Tuberculosis Orders. The OV must verify availability of the approved disinfectant during the routine attendance and verify its adequate use during the established C and D verification checks (see MOC chapter 2,2. Section 5). The list of approved disinfectants and concentrations.
7.3.4 Food chain information
All animals sent for slaughter must be provided with FCI.
Since some TB restricted animals are compulsorily slaughtered, the OV should verify that withdrawal periods have been observed for veterinary medicines and other treatments administered to the animals, this includes substances used for diagnosis purposes such as tuberculin.
Keepers submitting cattle from a farm with movement restrictions due to TB must declare this as part of the FCI. APHA requires all cattle moving for slaughter from TB-restricted herds to be marked with an orange stripe along the back. This is irrespective of test results so applies to animals moving under general licence as well as with movement licences.
The OV must be present on site during the processing of animals from a TB restricted farm.
Reference: Regulation 853/2004, Annex II, Section II and Regulation (EU) 2019/627, Article 10, 1
7.3.5 TB110 electronic TB sampling and submission form
APHA will submit electronically a TB110 form providing details of the reactor and DC cattle sent for compulsory slaughter and the sampling code that applies to each herd. This code determines the level of sampling that is required.
Note: In most of the cases, these animals will only be sent to selected slaughterhouses contracted by APHA for processing TB suspect cattle, excepting the animals subject to private slaughter. Contact the SLA and Contract Team for the current list of those slaughterhouses and the associated APHA TB diagnostic laboratory.
A number of Reactors/ DCs and IRs may be privately slaughtered by the owner. The owner can choose any non-APHA contracted cattle plant to slaughter them (as long as there are adequate inspection facilities and capability of the plant to process TB animals), but similar arrangements to those above apply.
APHA will e-mail a TB110 to the OV and other agreed FSA officers by noon the day before the kill date.
The TB110 must be completed after post-mortem inspection, recording the findings. The process for sending the forms is as follows:
- signed hard copy TB110 must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle; APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office
- copies of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox; a copy of the forms should be faxed or emailed to the relevant APHA office; the OV should retain a further copy in the plant files for future reference (retention period 12 months)
Reference: See Annex 15 on ‘Sample: TB110 Reactor Sampling and submission form’ for a sample of the form.
7.3.6 TB55a movement licences
Form TB55a is the proposal to slaughter deer. It will inform the OV of the arrival of deer from a restricted TB premises.
A copy of the TB55a will be sent by fax to the OV in advance.
Reference: See Annex 19 on ‘Sample: TB55a’ for a sample copy of the form. See The Tuberculosis in Animals (England) Order 2021.
Note: Reactor deer moved for slaughter under movement licence must have a broad arrow 15 cm long clipped on the left hind quarter.
7.3.7 TB24 movement licences
Form TB24 is a movement licence issued by APHA authorising transport of cattle (reactors, IRs, DCs and any cattle from TB restricted herds that have not been tested for TB) to a slaughterhouse. It must accompany animals during transport. Most animals accompanied by a TB24 need to be slaughtered separately, and if they appear on the TB110, inspected in detail.
Some cattle that are not reactors, IRs or DCs may travel to slaughter under a TB24. These cattle do not in principle have a higher risk of infection with TB than other cattle from restricted herds.
Since the EC regulations require that animals that have reacted inconclusively to the tuberculin are to be slaughtered separately, APHA will mark the TB24 of these animals with the words ‘Inconclusive Reactor’.
When animals that should have arrived with a TB24 are found not to have one, this should be reported to APHA and the relevant Trading Standards department.
Reference: See topic 7.2.1 on ‘Where or when to slaughter’ onwards in section 7.
Reference: See Annex 9 on ‘Sample: TB24’.
7.3.8 TB24a movement licences
Form 24a is a licence issued by APHA authorising movement of deer to a slaughterhouse. It must be given to the FSA representative on arrival to the slaughterhouse.
A copy of the TB24a will be sent by fax to the OV in advance.
Note: For welfare reasons the deer should be slaughtered within 3 hours of arrival at the slaughterhouse and shall not be removed from there alive.
Reference: See Annex 18 on ‘Sample: TB24a’ for a sample copy of the form.
7.3.9 TB24b/g/h movement licences
Form TB24b is a movement licence issued by APHA authorising transport of cattle, listed by ear tag, from TB restricted herds to a slaughterhouse via an approved TB slaughter gathering (collection centre / market).
Form TB24g is a licence authorising movement of cattle from approved finishing units under restrictions to a licensed slaughterhouse via an approved TB slaughter gathering (collection centre / market).
Form TB24h is a licence authorising movement of cattle from approved finishing units or licensing finishing units under restrictions to a licensed slaughterhouse via an approved TB slaughter gathering (collection centre / market.)
Animals eligible for a TB24b/g/h are not considered reactors, IRs or DCs. They need only be subject to normal inspection procedures.
Reference: See Annex 12 on ‘TB24b’ for a sample copy of the form and Annex 13 on ‘Sample TB24g’ for a sample copy of the form and Annex 32 for sample copy of TB24h form.
Note: Bovine animals moved to slaughter under the authority of TB24b are permitted to travel on the same vehicles with bovine animals from other restricted premises transported under a similar licence, as stated in TB24b.
7.3.10 TB24c movement licences
Most clear testing cattle and calves under 8 weeks of age travelling direct to slaughter from holdings under TB restrictions, no longer require a specific TB24/TB24b licence. These animals can be consigned to slaughter by their owners under the terms of a general movement licence (TB24c), issued by the APHA at the time the herd is placed under restrictions.
Herd owners who are granted a general TB24c licence will not be required to forward a copy to the slaughterhouse, nor will it be necessary for a copy of the general TB24c licence to travel with the animals.
These animals, as with all cattle from a TB restricted herd, should be identified by means of an orange stripe along the back and FCI should indicate the herd is under restriction, but they will be subject to the normal inspection procedures.
General TB24c licences will automatically expire on lifting of TB restrictions. APHA retains the power to rescind a general movement licence at any time.
Reference: See Annex 10 on ‘Sample: TB24c’ for a sample copy of the form.
7.3.11 Exclusions from general licence (TB24c)
Reactors, IRs, DCs and any untested cattle aged 8 weeks or more are explicitly excluded from the general licence and will continue to be licensed to slaughter by APHA, under a specific TB24 travelling with the animal.
Animals may arrive at the slaughterhouse accompanied by TB24s prior to the OV receiving notification from APHA. In these circumstances, FSA staff should inform APHA of the arrival of such animals and wait for instructions.
7.3.12 TB16b movement licence
TB16b movement licences are issued to authorise movement of ear tag listed cattle from restricted premises to Approved Finishing Units, Approved Quarantine Unit or to a slaughterhouse through a Dedicated Sale for TB Restricted Cattle. These animals have passed a tuberculin test in the 90 days before movement and are not reactors, IR or DC. The licences should accompany the animals to the abattoir but, as with animals moved under a TB24b/g, they need only be subject to normal post-mortem inspection procedures.
Reference: see Annex 11 on ‘Sample: TB16b’ for a sample copy of the form.
7.3.13 FSA copy of licences
The person transporting the animals, on arrival at the slaughterhouse, must give a copy of the TB24, TB24b, the TB24g, TB24h TB16b, TB24a or the TB55a licences to the FSA representative.
The table below shows which forms, licences and certificates accompany which animals to the slaughterhouse.
| Form / licence | Reactors | DCs | IRs | Cattle not tested for TB | Clear-testing cattle and calves under 8 weeks | On-farm slaughter |
|---|---|---|---|---|---|---|
| FCI | Yes | Yes | Yes | Yes | Yes | Yes |
| TB110 | Yes | Yes | Yes | No | No | Yes |
| TB24 | Yes | Yes | Yes | Yes | May happen | No |
| TB24b | No | No | No | No | Yes | No |
| TB24c | No | No | No | No | Yes | No |
| TB24g/h | No | No | No | Yes | No | No |
| TB16b | No | No | No | No | Yes | No |
| TB24a (deer) | Yes | Yes | Yes | No | No | No |
| TB55a (deer only) | Yes | No | No | No | No | No |
7.3.14 Irregularities
APHA will contact the OV if, after submission of the TB110, there is any change to the number of cattle sent for slaughter or to the sampling code.
Note: in some cases fewer cattle may be delivered than expected, but never more than pre-arranged.
If the OV believes that animals from a TB restricted establishment have been presented for slaughter without all the necessary documentation, they should inform APHA and the LA.
APHA should also be contacted if, due to missing paperwork, conflicting information, or any other circumstances, the OV is not sure if an animal from a TB restricted establishment requires detailed post-mortem examination and sampling.
7.4 Reactor animals: notification and responsibilities
7.4.1 Overview of responsibilities
Type: Reactors, IRs and DCs
| Responsibility | Duty |
|---|---|
| APHA |
|
| FSA |
|
7.5 Reactor animals, inconclusive reactors and direct contact cattle: inspection requirements
7.5.1 Additional detailed inspection
A detailed inspection must be carried out on animals included in the following categories:
- Reactor or direct contact cattle compulsorily purchased and slaughtered by APHA at contracted slaughterhouses. (These animals must arrive at the slaughterhouse with FCI advising they originate from a restricted herd, a movement licence (TB24), and be listed on the TB110.)
- Reactors, DCs or IR cattle privately slaughtered (these will be accompanied by the same documents as above but they may be sent to any slaughterhouse). When samples are required for animals in this category, APHA will submit the TB110 form to the FSA staff at the selected slaughterhouse.
- Deer compulsorily purchased and slaughtered by APHA.
- Other farmed non-bovine animals (pigs, sheep or goats) compulsorily purchased and slaughtered by APHA.
In the case of reactor animals, IRs and DCs the following LNs and organs must be examined in detail (visual inspection, palpation and incision) if they have not been examined already:
- Retropharyngeal LN*
- Parotid LN
- Submandibular / Submaxillary LN
- Bronchial* and Mediastinal* LN
- Lungs*
- Pleura
- Hepatic LN
- Liver
- Mesenteric LN (representative sample)
- Supramammary LN
- Udder**
- Prescapular LN
- Superficial inguinal LN
* Tissues where tuberculosis lesions are most commonly found
** See subtopic below
Note: Additional examinations of any other lymph nodes, such as those enlarged and / or haemorrhagic, may take place whenever considered necessary.
Reference: Regulation (EU) 2019/627, Article 14.
7.5.2 Udder inspection
The inspection of udders from reactor cattle is particularly important as they are not routinely incised unless they are for human consumption. In addition to the visual inspection and incision of the supra-mammary LNs, the udder of cows must be visually inspected and palpated. If abnormalities are found during these, or when the udder is intended for human consumption, then deep incisions must be done into each quarter of the udder as far as the lactiferous sinuses.
Reference: Regulation (EU) 2019/627, Article 19, 2 (g).
7.5.3 Incision method
Cuts into the LNs should be made across the node in at least two directions (criss-cross pattern) to reveal as much as possible of the core of the node. Care should be taken to examine the tips of the node. This method will reveal most TB lesions or reveal an area which appears abnormal which can be further incised.
Lesions in the lungs, liver and udder are most commonly found on inspection or palpation. Where abnormalities are felt on palpation the abnormal areas should be incised for further investigation. Careful small incisions at the border of the lesions should be made to reduce exposure to infective material. If the lesion is found to be typical of TB, no further incision is required into that lesion.
7.5.4 Hygiene precautions
Any equipment used to incise or examine the LNs must be cleansed and sterilised before undertaking post-mortem procedures on subsequent carcases, including changing of gloves in between different carcases/ sets of offal, when lesions are identified.
7.6 Reactor animals: actions when rejected at ante-mortem due to being dirty
Whenever a TB Reactor animal is rejected at ante-mortem inspection because it was dirty and it could not be processed hygienically, the OV must inform APHA by reporting through the ‘LA notification form: welfare breaches’ found in Chapter 2.3 on Animal Welfare, Annex 4, as an animal welfare concern, including a picture(s) of the rejected animal(s) and its ear tag.
The LA notification form is to be submitted to the CSC one health welfare mailbox CSCOneHealthWelfare@apha.gov.uk like other animal welfare referrals.
The OV will also complete the relevant details of this event in the comments box of the TB110 form, describing the reason why the animal was rejected, for example: when presented for slaughter the animal was not clean and it could not be processed hygienically, adding details of the nature of the contamination.
7.7 Reactor animals: post-mortem decision
7.7.1 Judgement of meat
Decision on whether meat is fit for human consumption is based on the findings during post-mortem inspection.
Where there are indications of generalised TB or TB lesions with emaciation the entire carcase and all the blood and offal should be rejected as unfit for human consumption.
All meat from animals in which post-mortem inspection has revealed localised TB in a number of organs or a number of areas of the carcase are to be declared unfit for human consumption. However, when a TB lesion has been found in the LNs of only one organ or part of the carcase, only the affected organ or part of the carcase and the associated LNs need to be declared unfit for human consumption.
Reference: Regulation (EU) 2019/627, Article 33
7.8 Reactor animals: sampling
7.8.1 Relevant animals
In general, the collection of diagnostic samples by the FSA is limited to reactors and DCs compulsorily slaughtered and some reactors, DCs or IRs which have been privately slaughtered (cattle entered on a TB110 as requiring detailed post-mortem inspection).
When reactors, DCs or IRs arrive to a non-contracted plant (considering that farmers do have the option of private slaughter), APHA will issue a TB110 and advice on the sampling protocol. These animals cannot be considered / treated as slaughterhouse cases.
7.8.2 Responsibility for collecting samples
APHA, before sending animals to the abattoir, will provide the OV with the details of likely numbers and sampling protocol 48 hours in advance and will then submit electronically to the OV a copy of the TB110 (see Annex 15) by noon the day before the kill date. The form will include:
- the number of animals to be sent from each holding
- the reason for submission (reactor, IR, DC)
- the sampling code for each batch
Once the required samples have been collected the carcases and offal can be released if they have been found fit for human consumption.
7.8.3 Death of reactors / DC / IR on arrival or in lairage
In the event of a Reactor being found dead on arrival (DOA), or dead in the lairage (DIL), the OV must contact APHA and explain the circumstances. APHA will inform the OV if any diagnostic samples for TB are to be collected.
Reference: The OV must be aware of the requirement to test for TSEs in O48M/O24M DOA or DIL bovines as per instructions in chapter 2.6 on ‘TSE Testing’ and also consider the possibility of anthrax.
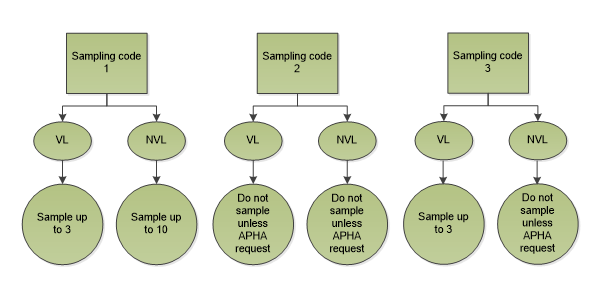
7.8.4 Sampling codes
APHA will request a sampling protocol for suspect animals from each farm using three sampling codes (SC1, SC2 and SC3). The sampling codes are allocated by APHA depending on the herd history and its current status. In addition, APHA will indicate whether additional or exceptional sampling is required.
| Sampling code 1 | Sampling code 2 | Sampling code 3 |
|---|---|---|
| Visible lesions (VL) | Visible lesions (VL) | Visible lesions (VL) |
|
For animals from herds in England or Scotland, collect samples from maximum of 3 VL animals per herd. No NVL samples required For animals from herds in Wales (CPH starts 52-60), collect samples from maximum of 3 VL animals per herd. No NVL samples required |
Do not collect samples unless APHA request |
Collect samples from maximum of 3 VL animals per herd. Do not collect samples unless APHA request |
| No visible lesions (NVL) | No visible lesions (NVL) | |
|
For animals from herds in England or Scotland, submit samples from 10 animals per herd (or from all if less than 10 animals) * For animals from herds in Wales (CPH starts 52-60), submit samples from 3 animals per herd (or from all if less than 3 animals) ** |
Do not collect samples unless APHA request |
*APHA will indicate which 10 need to be sampled where all are NVL and more than 10 cattle are submitted from each farm
**APHA will indicate in the Specific Info box of TB110 which 3 animals need to be sampled where all from the same farm are NVL.
7.8.5 Sampling code 1: typical lesions identified (VL)
All lesions typical of TB should be collected when required (sampling code 1 and 3 or sampling code 2 with specific request from APHA).
A typical lesion is where infection with M bovis is suspected and common colours (cream / yellow) and common consistency (caseous / calcified / purulent) are identified.
TB lesions in pigs are generally whitish-yellow granulomatous lesions which may contain areas of calcification. When collecting these suspected TB lesions, those with the most characteristic TB lesions should be chosen and only tissue samples from a maximum of three VL from pigs from the same herd should be collected.
APHA has defined a VL as a lesion that is visible to the naked eye and typical of infection with M bovis.
Lesions due to skin TB should not be collected and will not be classed as VL.
All the lesions from each carcase should be pooled and placed in a single sealed 60 ml plastic pot to give one submission per animal. The samples should be two-thirds of the pot and should include the lesion plus some normal tissue from the border of the lesion, where possible. However, this may result in a large amount of tissue if a carcase presents multiple TB lesions. In this situation, sample only the two most characteristic lesions; however, if the lesion in its entirety does not fill two-thirds of the pot, please include comments to that effect in the relevant comments box of the form.
Note: Unaffected LNs must never be submitted when typical TB lesions have already been found in the same carcase.
7.8.6 Sampling code 1: typical lesions not identified (NVL)
NVL are those where no lesions typical of infection with M bovis are visible to the naked eye.
While this is not part of the APHA definition of NVL, for practical purposes this includes both where no lesions are found and where there are lesions that can be seen but infection with M bovis has been ruled out.
Where no lesions are found it is necessary to collect samples from all the following LNs:
- all bronchial and mediastinal LNs
- paired retropharyngeal LNs
- any other LN if enlarged, abnormal and / or haemorrhagic
Section 7.8.6 will also apply to non-bovines if a skin testing regime is in place.
Note: As of 1st September 2023, the MHI and OVs would no longer be required to collect samples from NVL TB reactors from Welsh holdings (CPH 52-60) in two separate pots. These samples can be collected in a single pot by pooling broncho-mediastinal and retropharyngeal LN, as per the procedure for samples from NVL TB reactors originating from English or Scottish holdings.
7.8.7 Sampling code 1: atypical lesions identified
An atypical lesion is a lesion where infection cannot be definitely attributed to M bovis and where common colours (cream / yellow) or common consistency (caseous / calcified / purulent) are not identified, but where infection with M bovis cannot be ruled out.
Please note that an atypical lesion is neither a VL nor NVL for reporting purposes.
In terms of case management, APHA treats such lesions as NVL. Therefore, an atypical lesion should be recorded as NVL in the TB110, and ‘atypical lesions’ note entered in the TB110 comments box. The lesion descriptions should clearly reflect that it is atypical having ‘A’ at the end (e.g. M3YMxA).
If both typical and atypical lesions are found on the same carcase, submit samples from the typical lesion only. The only exception to this is when suspect udder / supra-mammary lesions are found; these should be submitted in addition to the typical lesion and in a separate pot (one per holding).
Where only atypical lesions are found, sample a pool of LNs and record as NVL but also collect and send the atypical lesion in a separate pot.
This should only be used where a decision cannot be made and the possibility of infection with TB cannot be ruled out.
When lesions are found in mesenteric lymph nodes in reactors from herds in Scotland, these should be treated as atypical, recorded as NVL in the TB110, and ‘atypical lesions’ note entered in the TB110 comments box.
7.8.8 Sampling code 2
Where APHA has allocated a sampling code 2 to a batch of animals there is no need to collect any samples, with only two exceptions:
- APHA may specifically request samples in certain cases.
- Where atypical lesions are found and there are no typical lesions in any animal from the same herd, sample the atypical lesion only and send for polymerase chain reaction (PCR) testing, making remarks to that effect on the ‘specific information’ section of the TB110.
7.8.9 Method
Each animal from which samples are needed must be individually sampled. Samples from more than one animal must never be pooled in the same pot. Care must be taken to prevent cross-contamination.
The following method should be used to collect samples for TB diagnosis.
| Stage | Description |
|---|---|
| 1 | Collect samples cleanly to limit contamination. Ensure the equipment used for inspection and sampling of carcases is disinfected between carcases to prevent the possibility of cross-contamination. |
| 2 | Dissect samples free of surrounding tissues to limit the volume of tissue submitted. Samples should be as fat and muscle free as possible. |
| 3 |
Where the carcase had VL or NVL samples are to be treated as follows: VL: Remove suspicious node or lesion in its entirety if small or a sample the size of 2/3 of a pot if large and pool up to two of the lesions from the same area of the carcase in a pot. If the lesion in its entirety does not fill 2/3 of the pot please include comments to that effect in the relevant comments box of the form. NVL: Pool LNs collected from the same carcase and place in a pot. The 60ml pot should be 2/3 full. If there are any atypical lesions, collect separately from pool. |
| 4a | Mesenteric chain LNs should only be collected when no other lesions are present. They must not be included in the pooled sample and must be collected separately from other LNs from the same carcase. This is to minimise contamination of the pooled sample with bacteria that could inhibit the growth of M. bovis in the laboratory. |
| 4b | Suspicious lesions in the supramammary nodes should always be submitted from any carcase (max. 1 per CPH). As for mesenteric nodes they should not be included in any pool of samples they need to be submitted in a separate pot. |
| 5 | The OV must be present in the slaughterhall during the post-mortem inspection to ensure that the correlation is maintained and that findings are accurately recorded for each carcase. The OV must also ensure that the samples are secured prior to despatch. |
| 6 |
APHA requires complete and accurate records of all findings from each animal, including those from which no samples have been taken, in the electronic form (TB110). This information will be used in deciding the future management of the herd. The completed form must be e-mailed to APHA (at the email address from which the TB110 originated) before despatch of samples (by 3pm if samples sent to the lab on the same day, or by noon next day when the samples are despatched the following day). If samples are collected, the TB110 must also be emailed to the APHA laboratory (TBDiagnosticTeam@apha.gov.uk). A hard copy of the TB110 must be signed by the OV and should be faxed without delay to the relevant APHA office. The signed hard copy must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office. A copy of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. The OV should retain a further copy in the plant files for future reference. |
| 7 | Each sample pot must have a unique traceability label stuck on the outside of the pot. The outside of the pot must be kept clean and the lids must be tightly closed to prevent leakage. In the event of the pot getting wet, it must be dried to ensure that the traceability label can be affixed when the sample is placed inside the pot. To maintain traceability, pots must be labelled before being moved from the slaughterhall. Each pot must then be placed inside a bag which is knotted tightly and excess bag trimmed off. |
| 8 | If more than one pot is submitted for a single animal (i.e. pool in one pot and atypical lesion in a separate pot) place all the individual sample pots, each in its own bag. |
| 9 | All bagged pots must then be placed in a biobox or biobottle (depending on number of pots) which is sealed. A copy of the completed forms must then be placed in a ziplock bag which is taped to the outside of the biobox. |
| 10 | Further packaging (box / bag) is then applied in line with courier instructions (see topic 7.12 on ‘Packing and despatch of samples’). |
| 11 | Retain chilled, pending their collection by a courier for transfer to the APHA laboratory. They must not be frozen unless instructed to do so by APHA. If frozen the sample and the packaging must be marked: ‘frozen sample’. |
7.8.10 Sampling code 3
Where APHA has allocated a sampling code 3 to a batch of animals, only VL samples need to be taken up to a maximum of 3 animals per specific holding.
Check the ‘specific information’ column of the TB 110 form because in some cases only 1 or 2 samples per holding may be required by APHA.
Please ensure that at least 2/3 of the pot is full when collecting the sample.
NVL lesions do not need to be submitted with this sampling code.
7.8.11 Completion of sampling and submission from (TB110)
The TB110 has two parts.
- The first will be completed by APHA with details of the holding, CPH number, ear tags, any other relevant information and the sampling code that applies to each batch.
- The second part must be completed by the FSA and be signed by the OV. The findings in each carcase, including those for which samples are not required, must be recorded using codes to identify the LNs / tissues and the description of the lesions where applicable (see below).
Where lesions are found in the lungs and / or udder suggestive of possible discharge of bacilli to the exterior (open tuberculosis) this has epidemiological importance and should be recorded in the comments box of the TB110.
The form must be sent electronically on completion to the originating email address. A hard copy of the TB110 must be signed by the OV and should be faxed without delay to the relevant APHA office. The signed hard copy must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office.
A copy of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. The OV should retain a further copy in the plant files for future reference
The TB110 must also be sent electronically to the APHA laboratory (TBDiagnosticTeam@apha.gov.uk) and a signed hard copy must accompany the samples.
7.8.12 Completion of TB50/TN50/TN60
The TB50 form is used to record post-mortem findings on suspect TB carcases of bovine animals (cattle, buffalo and bison).
The TN50 form is used to record post-mortem findings in all farmed non-bovine species such as deer, pigs, goats and sheep.
The TN60 form is used to record post-mortem findings in wild deer carcases only.
These three forms are to be used for ‘The slaughterhouse case’ only (see section 7.10).
Note: There is no need to complete TB50/ TN50 forms for bovine/ non-bovine reactors slaughtered at APHA contracted abattoirs, as the post-mortem findings are collated on the TB110.
Reference: For copies of these three forms see Annex 16a ‘Sample: TB50’, Annex 16c ‘Sample: TN50’ and Annex 16d ‘Sample: TN60’.
Bovine species:
The details of each case need to be recorded on a TB50 form signed by the OV and emailed to the APHA mailbox specified on the form, so that TB Officers can make a decision as to whether samples must be sent to the laboratory. Please note, each slaughterhouse case detected requires a separate sample submission form (TB50) to be completed.
Non-bovine species:
In England and Wales, for cases reported in non-bovines, APHA will not issue a WSA ID number, therefore this does not need to be recorded at the top of the TN50 form. In Scotland, a WSA number will be supplied by Scottish Government to the OV once the suspicion has been reported. Please note, each slaughterhouse case detected requires a separate sample submission form (TN50) to be completed.
A copy of the TN50 form is to be emailed ASAP to the relevant email address: see 7.10.5 for details for the different countries. In addition, a copy of the TN50 should be emailed to sla.contracts@food.gov.uk. No samples should be submitted or discarded without authorisation from APHA.
Wild deer:
For wild deer carcasses brought to FSA establishments for post-mortem inspection, the detection of suspect TB lesions must be reported to APHA. The completed TN60 form must be emailed to the APHA mailbox specified on the form, so that TB Officers can make a decision as to whether samples must be sent to the laboratory.
7.8.13 Codes used to complete the TB forms
Codes will be used to describe the lesions, with six criteria used: location, number, size, colour, consistency / texture and presentation.
- Location: Retropharyngeal (RP); Parotid (PA); Submandibular / Submaxilary (SM); Bronchial and Mediastinal (BM); Lungs (Lu); Pleura (Pl); Hepatic (HEP); Liver (Li); Prescapular (PSc); Superficial Inguinal (SI); Mesenteric (MES); Supramammary (SMA); Udder (U); Other (O)
- Number:
- Single (S) – a distinct single lesion in the LN / organ
- Multiple (M) – up to 6 distinct lesions in the LN / organ
- Diffuse (D) – multiple lesions throughout the LN / organ that may or may not coalesce / lesion spreading over a large area, not concentrated
- Size:<2mm – (1); 2-10mm – (2); 11-50mm-(3); >50mm- (4)
- Colour: Cream (C); Yellow (Y); White (W); Other (O)
- Consistency / texture: Caseous (Ca); Calcified (Cf); Purulent (P); Granulomatous (Gr); Mixed [Ca and CF] or [Ca and P] (Mx)
- Presentation: Typical (T); Atypical (A)
For atypical lesions if the description cannot be provided from the above options a description can be entered in the comments box.
Reference: a template for recording findings on the line during post-mortem inspection is available at Annex 17 on ‘Description of lesion template’.
Note: For packing and despatch of samples, please see topic 7.12 on ‘Packing and despatch of samples’ later in this section.
7.9 Reactor tag sampling
7.9.1 Overview
The aim of this programme is to compare the ears collected from TB reactors in order to audit fraudulent procedures in relation to reactor removal. This will be audited by cross matching 2 tissue samples:
- tissue collected in the DNA capsule when tagging TB reactors at the time of the TB test
- tissue taken from the ear of TB reactors at the point of slaughter
The Reactor Ear testing programme will comprise of 3 elements:
- targeted collection where FSA have identified at point of slaughter possible tampering with tags, either official or reactor tags, or missing reactor tags
- targeted collection where APHA identify a risk and request FSA to collect both whole ears (which do not have to be connected), from specifically identified animals
- random collection of the required number of ears selected by FSA at each slaughterhouse on a monthly basis
The OV at contracted TB Reactor slaughterhouses will prepare a protocol (already known also as authorised method of operation) to summarise and ensure that the previously described instructions for the processing of TB Reactor animals are followed. Guidance for the preparation of such protocol can be found in Annex 15b.
7.9.2 Notification to slaughterhouse / FSA of reactor details
Animals submitted for slaughter for TB control will either be R or DCs and will be sent for slaughter in one of the following ways:
- submitted as part of haulage and salvage to one of the slaughterhouses contracted by APHA to process TB reactors
- private slaughter organised by the owner but moved under licence issued by APHA
DCs will not have reactor tags and are excluded from this programme, however any other suspicion of fraud should be investigated as described in the MOC.
Most TB reactors will have a reactor tag applied. However, there are a few exceptions to that rule where reactors may not be tagged and are considered ineligible categories:
- reactors identified following skin test re-interpretation (standard to severe) after PM/ PCR (or culture) results
- animals have not been tagged at Tuberculin Test day 2 (test reading day) for operational reasons
- gamma positive reactors
The assumption is therefore that apart from those ineligible for this programme all reactors disclosed at a skin test and entering the slaughterhouse will be marked with a reactor tag. In the comments box of the TB110, the following reasons will be given to indicate that an animal will not have a reactor tag and is ineligible:
- ‘tag not applied’ where APHA are aware that an animal has not been tagged for any reason
- ‘re-interpretation’ where an animal became a reactor after the skin test due to re-interpretation of the skin measurements
- ‘gamma’ where an animal has failed the gamma interferon test
7.9.3 Action when animal arrives at slaughterhouse
Apart from those specifically requested by APHA, the level of reactor animal identity checking by FSA should be as per existing instructions in the MOC.
Where FSA undertake an identity check, the following details should be compared with the information submitted to them by APHA:
- ear tags match the cattle passport
- reactor tag present if not reported as ‘tag not applied’ or one of the categories not eligible for tagging (re-interpretations or gammas)
The following action should be taken:
- record findings, on ID checklist or FBO sheets where applicable
- check if any evidence of tampering or other fraud
- if evidence found, notify LA Trading Standards as per existing processes and retain relevant part of the animal
7.9.4 When is an ear sample required?
The reactor tag scheme requires a sample (comprising both whole ears and all tags present in those ears) to be collected from any animal which comply with one of the criteria described below.
A sample will be required in the following circumstances unless otherwise instructed. The FSA Targeted and the APHA Targeted may be required in slaughterhouses in England and Wales whilst the FSA Random samples are required in slaughterhouses in England and Wales:
- FSA targeted – Whenever a FSA Operations Group officer finds evidence of fraud, the tag has been tampered with or other ID non-compliance (NC).
- For example, reactor tag missing when expected to be present (TB110 will state if ‘not applied’ or one of the other ineligible categories), ear tags tampered with, indecipherable documentation, animal does not appear to match that expected (age, breed, sex). Guidance is being produced, that gives details of what constitutes ear tag tampering.
- APHA targeted - When requested by APHA, Intelligence led targeted examination of animal ID and sampling.
- APHA will state ‘COLLECT EARS’ in the TB110 comments box when ears are required to be collected.
In exceptional cases APHA may contact the FSA representative at a slaughterhouse (by phone) to request an urgent identify check and request ear samples to be taken.
- FSA random – random testing process.
- Slaughterhouses in England, 3 samples should be collected every month at each of the slaughterhouses which regularly receive reactors. The random samples should only be taken from reactors which originate from England and which have reactor tags.
- Slaughterhouses in Wales, 3 samples should be collected every month at each of the slaughterhouses which regularly receive reactors. The samples should be taken at different times in the month and not together and only from animals with reactor tags.
- Random samples should not be collected on a Friday although targeted samples may have to be taken.
For all other animals, that is TB reactors that have not had a reactor tag applied or Direct Contacts, any suspicion of fraud should be investigated as described in the chapter 6 on ‘notifiable diseases’, section 7.
7.9.5 Collection of sample, packing and despatch of ear samples from FSA
For continuity of evidence all processes should be completed by the same person (removal of the ears, completion of sample submission form, labelling and bagging in tamperproof / evidence bag and packaging of samples packed for dispatch).
The following protocol should be followed:
A Preparation of packing systems:
| Step | Action |
|---|---|
| 1 | The packing materials consist of the following:
|
| 2 | Biotherm 5 boxes have been issued for routine sampling and only one pair of ears should be packed in this system. In the event multiple sample collection is required (targeted sampling) the Biotherm 10 and 15 systems should be used and will be supplied by APHA. |
| 3 |
All Biotherm systems will be supplied by APHA and need to be prepared for first initial use; once preparation has been completed, using the protocol below, the systems can be re-used and will be returned by APHA. Nett Qty: One sample Dry Ice: less than 1 kilogram. Name and telephone number of responsible person: FSA contact name and number Ice Brix must be ‘hard’ frozen before use, x2 Ice Brix should be sufficient for the Biotherm 5 system. |
| 4 |
On the lid of the box complete legibly and accurately. 
a) Consignee details with: APHA TB DNA Testing b) Consignor details with: Full Address of the abattoir |
| 5 | Open the box and remove the labels supplied, place to one side. |
| 6 |
On the front panel stick the UN3373 label in one of the pre-marked diamonds and place the Biological Substance Category B label adjacent to the UN3373 diamond (see photographs). 

|
| 7 |
Discard the Infectious Substance label; this must not be used. |
| 8 |
Complete legibly and accurately the front panel: Proper shipping name: Biological Substance Category B UN Number: UN3373 Nett Qty: One sample Dry Ice: less than 1 kilogram. Name and telephone number of responsible person: FSA contact name and number Ice Brix must be ‘hard’ frozen before use, x2 Ice Brix should be sufficient for the Biotherm 5 system. |
B Notify APHA that ear samples have been taken:
| Step | Action |
|---|---|
| 1 | Whenever ear samples are taken, FSA abattoir staff must notify APHA Central Tagging Team that a sample has been taken and submitted to APHA Lab at Weybridge. |
| 2 |
A copy of the signed sample submission form (Annex 22 on ‘Material for DNA analysis’) should be scanned and emailed to the APHA central tagging team at: CSC.TBDNATagging@apha.gov.uk |
C Collection and preparation of ears (x1 pair):
| Step | Action |
|---|---|
| 1 | Place the pair of ears (from the same animal) into a grip seal bag (8” x 11”); remove any excess air from the bag and seal. |
| 2 | Place the bagged sample (x1 pair of ears) inside another grip seal bag (8” x 11”), add an absorbent pad, remove excess air and seal. |
| 3 | Place the ‘double bagged’ sample (x1 pair of ears) into the tamperproof / evidence bag and seal to meet continuity of evidence requirements. |
| 4 | Complete legibly and accurately the tamperproof / evidence bag in the section marked ‘FSA Use Only’. |
| 5 | Put in the refrigerator or freezer for chilling. This will reduce excessive moisture collecting in the bag. |
| 6 | Complete the sample submission form legibly and accurately. If samples have been taken due to evidence of tampering, ensure the tampering suspected box is ticked on the sample submissions form. |
| 7 | Send a copy by fax to APHA Central Tagging Team (as above at B step 2) and place in a grip seal bag (8” x 11”) remove excess air and seal. |
| 8 | Add the hard frozen Ice Brix to the Biotherm system and place the sample next to the Ice Brix (x2 Ice Brix per biotherm system). |
| 9 | Place the sample submission form on top of the sample (inside a plastic bag), close the polystyrene lid (expanded polystyrene), close outer flaps and seal with security label or brown tape. Where samples from more than one animal are in the box, ensure the bag containing the sample submission form is attached to the corresponding tamperproof bag. |
| 10 | As soon as you receive the sampling request information from APHA, email the APHA Preferred Courier (currently Topspeed Couriers at tb@topspeedcouriers.co.uk) with the following information:
|
D Preparation of biotherm replacement of outer box:
| Step | Action |
|---|---|
| 1 |
If the outer carton becomes damaged a replacement carton should be obtained and prepared for use, using the protocol below: N.B. A replacement outer carton is not supplied with UN3373 label and this will need to be obtained when ordering replacement carton
‘BIOLOGICAL SUBSTANCE CATEGORY B’ |
| 2 |
Complete legibly and accurately the front panel: Proper shipping name: Biological Substance Category B UN Number: UN3373 Nett Qty: One Sample Dry Ice: less than 1 kilogram. Name and telephone number of responsible person: FSA contact name and number |
| 3 | Insert the polystyrene box |
| 4 | Ice Brix must be ‘hard’ frozen before use, x2 Ice Brix should be sufficient for the Biotherm 5 system. |
| 5 | Follow Collection and Preparation of Ears (x1 pair) protocol |
| 6 | Resupply of packaging and dispatch equipment should be ordered by completing and submitting the CS115 form (Annex 21) |
7.10 The slaughterhouse case
7.10.1 Definition
Carcases and offal with suspicious TB lesions found during routine meat inspection are called ‘slaughterhouse cases’. The animals may or may not have come from a TB restricted premises.
7.10.2 Responsibilities
The table below outlines the responsibilities.
| Responsibility | Duty |
|---|---|
| APHA |
|
| FSA |
|
7.10.3 Skin tuberculosis
Animals presenting skin lesions only should not be treated as a slaughterhouse case, surveillance is not required and samples do not need to be collected. M bovis is rarely isolated from skin lesions, as usually these lesions are caused by environmental mycobacteria.
7.10.4 Differentiate between lesions
Because different sampling and diagnostic testing is required in each situation, FSA staff must positively differentiate between lesions which are:
- tuberculous (TB) Action – Inform APHA and collect samples for analysis
- tumorous (EBL) Action – Reference: See section 5 on ‘Enzootic Bovine Leukosis’ for additional information
7.10.5 Notifying APHA
Where the OV cannot positively rule out TB as the possible cause of the lesion(s) the suspect case must be reported to APHA without delay. The OV must inform APHA by telephone, to allow trace back to the farm of origin, giving details of the case such as:
- the nature of lesions found with their location
- the name and address of the person submitting the animal with ear tag number, lot number, CPH number and kill number in the additional remarks box of the TB50
- a description of the animal
- when the sample can be despatched
7.10.5.1 Contact details for slaughterhouse cases in bovine animals:
If the animal is detected in a slaughterhouse in England:
- Contact the administrative team dealing with bovine slaughterhouse cases at 0208 026 0178 or,
- E-mail the administrative team dealing with bovine slaughterhouse cases at: CSC.TBSlaughterhouseCases@apha.gov.uk.
If the animal is detected in a slaughterhouse in Wales:
- E-mail APHA Wales administrative team dealing with slaughterhouse cases at: APHAWalesTBSlaughterNon-Bovine@apha.gov.uk or,
- Contact APHA Wales at 03000 303 8268 and ask to be put through the administrative team dealing with slaughterhouse cases.
In all cases, following to the initial notification of a suspect slaughterhouse case to APHA, email the completed TB50 form to the respective team mailbox as prescribed above.
7.10.5.2 Contact details for slaughterhouse cases in non-bovine species:
If the animal originates from England:
- E-mail the non-bovine administrative team at CSC.TBOS@apha.gov.uk or,
- contact the non-bovine administrative team at 0208 720 0992.
If the animal originates from Scotland:
- E-mail ScotlandDutyVet@apha.gov.uk and copy in ScotlandEndemics@apha.gov.uk or,
- contact APHA Scotland on 03000 600708 and ask to be put through to the Duty Vet.
If the animal originates from Wales:
In both cases the email should include in the subject the species (for example “PIG SLAUGHTERHOUSE CASE”) and marked as ‘High importance’. The following information should be included in the email:
- Completed TN50/TN60
- CPH of originating farm
- Name of the owner
- Name of the farm
- Slaughter Date
- Slaughterhouse name and approval number
- Number of animals
- Contact mobile number OV
The OV will be contacted as soon as possible, to discuss if samples need to be submitted for PCR testing. Where an APHA Vet is not available to review the slaughterhouse case notification immediately, samples should not be sent, nor discarded (advice from APHA can be sought the next day). It may be that samples will need to be submitted from more than three carcasses with Visible Lesions, from the same holding.
More about the completion of TB50/TN50/TN60 can be found in sections 7.8.12 and 7.12.2. Details on the codes to be used for the completion of the forms are described in section 7.8.13.
The OV must retain legible copies of the animal’s identification (for example, cattle passport), kill sheet, FCI and other records that can be necessary for future investigations.
Reference: The Tuberculosis in Animals (England) Order 2021 and the Tuberculosis (Wales) Order 2010 (as amended).
7.10.6 APHA action
On notification from the FSA of the finding of suspect TB lesions, APHA must provide the sample WSA ID number that must be recorded in the box at the top of the TB50 form.
As of 1st September 2023, APHA will no longer apply triage to the reported bovine slaughterhouse cases for animals originating from English and Scottish holdings, i.e. from this date onwards all bovine SLH cases would be subject to laboratory investigation. All actions taken by the OVs regarding SLH cases would remain unchanged apart from the need to await APHA’s decision on sampling requirement. This would allow OVs to proceed directly to sample packing/labelling and dispatch once they have obtained the required WSA number from APHA.
For non-bovine cases, APHA will continue to triage and advise whether samples should be submitted for laboratory investigation. In England and Wales, the WSA ID number is not required.
7.10.7 Movement to detained area
After dressing, carcases and offal suspected of being affected with tuberculosis should be placed immediately in the detained area before additional detailed inspection is carried out and before being sampled, if required by APHA.
7.10.8 Transfer of carcases and offal
When transferring offal / carcases to a detained area for further inspection or sampling, care must be taken to prevent cross-contamination of other meat / equipment / fittings in the slaughterhall. In the event of suspected contamination, C and D of the affected area / equipment must take place before production recommences.
The PCR will detect DNA. Immersing the knives in water at 82°C or above will not inactivate the DNA. Adequate cleaning of the knives used to take the samples prior to disinfection will be essential to remove residues and to avoid potential false positive results.
Note: Failure by the plant operator to co-operate with this procedure would constitute a contravention of the operator’s responsibility to prevent cross-contamination and must be dealt with accordingly.
Reference: Regulation (EU) 2019/627, Article 33
7.11 The slaughterhouse case: additional detailed inspection
7.11.1 Detailed inspection
In the case of animals in which there are grounds for suspecting TB the following LNs and organs must be examined in detail (visual inspection, palpation and incision) if they have not been examined already:
- Retropharyngeal LN*
- Parotid LN
- Submandibular / Submaxillary LN
- Bronchial* and Mediastinal* LN
- Lungs*
- Pleura
- Hepatic LN
- Liver
- Mesenteric LN (representative sample)
- Supramammary LN
- Udder**
- Prescapular LN
- Superficial inguinal LN
* Tissues where tuberculosis lesions are most commonly found
** See subtopic below
Note: Additional examinations of any other LNs, such as those enlarged and / or haemorrhagic, may take place whenever considered necessary.
Reference: Regulation (EU) 2019/627, Article 14
7.11.2 Udder inspection
The inspection of udders in ‘slaughterhouse case’ is particularly important as they are not routinely incised unless they are for human consumption. In addition to the visual inspection and incision of the supra-mammary LNs, the udder of cows must be visually inspected and palpated. If abnormalities are found during these, or when the udder is intended for human consumption, then deep incisions must be done into each quarter of the udder as far as the lactiferous sinuses.
Reference: Regulation (EU) 2019/627, Article 19, 2(g)
7.11.3 Incision method
Cuts into the LNs should be made across the node in at least two directions (criss-cross pattern) to reveal as much as possible of the core of the node. Care should be taken to examine the tips of the node. This method will reveal most TB lesions or reveal an area which appears abnormal which can be further incised.
Lesions in the lungs, liver and udder are most commonly found on inspection or palpation. Where abnormalities are felt on palpation the abnormal areas should be incised for further investigation. Careful small incisions at the border of the lesions should be made to reduce exposure to infective material. If the lesion is found to be typical of TB, no further incision is required into that lesion.
7.11.4 Hygiene precautions
Any equipment used to incise or examine the LNs must be cleansed and sterilised before undertaking post-mortem procedures on subsequent carcases.
7.11.5 Correlation of TB suspect carcases and offal
The OV at any red meat slaughterhouse, where a TB suspect carcase and offal might be identified, will prepare a protocol to ensure the proper identification and correlation of TB suspect carcasses and offal. The protocol will be tailored to each plant so that any issues related to identifying and correlating the TB suspect carcase and offal are addressed. It must state that ‘each TB suspect carcase and offal are identified by a detained grey tag’. Guidance for the preparation of such protocol can be found in Annex 16b.
The detained grey tags will be ordered by the Inspection Team Leader (ITL) from CSU@food.gov.uk to ensure that each red meat slaughterhouse holds a stock of these tags on the premises.
7.11.6 Judgement of meat
Decision on whether meat is fit for human consumption is based on the findings during post-mortem inspection.
Where there are indications of generalised TB or TB lesions with emaciation. the entire carcase and all the blood and offal should be rejected as unfit for human consumption.
All meat from animals in which post-mortem inspection has revealed localised TB in a number of organs or a number of areas of the carcase are to be declared unfit for human consumption. However, when a TB lesion has been found in the LNs of only one organ or part of the carcase, only the affected organ or part of the carcase and the associated LNs need to be declared unfit for human consumption.
Reference: Regulation 2019/627, Article 33.
7.12 The slaughterhouse case: sampling
7.12.1 Collection of samples
When VLs found during post-mortem inspection cause suspicion of TB, samples need to be collected and sent for analysis, when authorised by APHA. The sampling procedures are the same as previously described for reactors, where VL are found and Sampling Code 1 applies. Please note that NVL samples are NOT to be sent for slaughterhouse cases.
Remove suspicious node or lesion in its entirety if small or a sample the size of two-thirds of the pot if large and pool up to two of the suspected lesion tissues from the same carcase.
If the size of the affected tissue and / or lesions from slaughterhouse cases is too small to make up two-thirds of the pot, then comments must be included on the TB50/ TN50/TN60 form to that effect. If the lesion identified is small, but there are multiple lesions, the multiple lesions must be included to make up the maximum required volume. However, mesenteric LN and supramammary / udder tissue are exceptions and should be submitted separately, as they are generally more heavily contaminated with other bacteria and could interfere with the TB culture process conducted on PCR positive samples to enable Whole Genome Sequencing (WGS) analysis.
Please note that fat interferes with the mycobacterial detection diagnostic methods for TB (both PCR and culture) and there is a specific requirement from APHA to trim the sample of fat tissue as much as possible.
Samples are not required from clear testing cattle/ farmed non-bovine species from TB restricted establishments (arriving at the slaughterhouse without a TB24), unless lesions suggestive of TB are found during post-mortem inspection. In this case, the ‘slaughterhouse case’ procedures apply.
7.12.2 Completion of TB50 / TN50 / TN60 form
In addition to the telephone report, fill in a separate sample submission form (TB50/ TN50/TN60) for each slaughterhouse case detected. The OV must give a detailed description of the location and nature of the suspect lesions on the TB50 / TN50 / TN60, including comments where the sample is smaller than the required volume. A properly completed TB50/ TN50/TN60 form (including the WSA number, unless specifically this number is not required) will enable APHA to quickly trace back the slaughterhouse case to its herd of origin. Based on this information APHA will put in place the appropriate TB control measures.
In this type of scenario, the OV is expected to either confirm the lesion as being characteristic of TB or, alternatively, be able to rule it out. If the OV has any doubts and / or difficulties are found when completing the TB50/ TN50/TN60 form, the OV can contact APHA and discuss any concerns with the APHA Vet to obtain the necessary advice.
Reference: See Annex 16a for sample of TB50 form, Annex 16c for sample of TN50 form an Annex 16d for a sample of TN60.
7.12.3 Distribution of the TB50/ TN50/ TN60 form
- The properly completed and signed TB50/ TN50/TN60 form must initially be emailed to the local relevant APHA administrative team (see 7.10.5.1 and 7.10.5.2) as soon as possible. Subsequently: signed hard copy of original TB50/ TN50/TN60 form must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle; APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office
- a copy of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox; copy of the forms should be emailed to the relevant APHA administrative team
OV should retain a further copy in the plant files for future reference (retention period 12 months).
Where a non-bovine is being sampled, the OV must also send a copy of the completed TN50 / TN60 to SLA and Contracts.
7.12.4 Packing and despatch of all TB samples
Samples must be sent to the APHA laboratory with the forms. They should be sent as soon as possible and by the next working day at the latest.
If APHA advises that the samples do not need to be sent to the laboratory then they must be disposed of as ABP. These discarded samples are classed as category 2 ABP and can also be disposed of as category 1 ABP.
Reference: See topic 7.12 on ‘Packing and despatch of samples’ at the end of this section.
7.13 Packing and despatch of samples
7.13.1 Packing
- All samples must be submitted in a 60ml pot.
- Outside of pot must be kept clean.
- Remember to tighten lids. Give an extra turn before packing.
- Avoid cross threading the lids as they will cause the pots to leak.
- Stick label on outside of pot: ear tag / CPH printed on label.
- Place each individual pot in a plastic bag which is knotted tightly. Trim off excess bag.
- If submitting more than one pot for a single animal (pool in one pot) atypical lesion in a separate pot:
- Label each pot and write on label what is in each pot, for example, pool / mesenteric.
- Place each pot in a separate bag and tie as previously.
- Place both bagged pots in a third bag and tie the bag.
- Make note in comments section on the TB110 or TB50/ TN50 / TN60 detailing how many pots submitted and what is in each pot.
- Place all bagged pots into a biobox / biobottle along with the absorbent pad / material and seal the box. The person introducing samples inside the biobox / biobottle must wipe their hands with 70% ethanol wipes before introducing the samples. The outside of the biobox / biobottle must also be wiped. The process for sending forms is as follows:
- Signed hard copy TB110 and original TB50/ TN50/ TN60 forms must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office.
- Copies of those forms should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. Copies of these forms should be faxed or emailed to the relevant APHA office. The OV should retain a further copy in the plant files for future reference. (Retention period 12 months)
- Place biobottle into the outer box. Before use the biobox / biobottle must be stored in a separate clean area to avoid possible cross contamination.
- Attach address label.
- Attach security seal.
Store the package in the chiller until the time of collection. Ideally, place in a waterproof bag / container to avoid contamination. The outer box needs to clearly read: ‘TB samples open only in CL3’. If the box has not been pre-stamped, please write or use the sticker provided.
Despatch: The current courier for the sampling process is Topspeed Couriers. The courier process is as follows:
| Step | Action |
|---|---|
| 1 | As soon as you receive the required information from APHA, book a collection with the following information:
|
| 2 | The APHA preferred courier will confirm the date that the samples will be collected. If samples need to be kept at the establishment overnight, please ensure that they are sealed in the packaging requested from APHA and store in a chiller or cold room. |
| 3 | The APHA preferred courier is required to deliver the samples within 2 working days. For example, if samples are taken on Tuesday, samples are required to be with APHA by 5pm on Thursday. Samples can be delivered up to 3pm only on a Friday. |
| 4 | On detection of a slaughterhouse case, notify the courier that samples are required to be collected and they will organise a collection which meets the 2 working days delivery requirement; for example, a SH case found on a Monday, samples are required to be with APHA laboratory by 5pm Wednesday but collection could either take place on Monday, Tuesday or Wednesday, as the couriers are required to consolidate their delivery runs to be cost effective. |
7.13.2 Ordering consumables
The OV at each abattoir is responsible for ensuring that there are sufficient supplies of consumables for packing samples. It is important that only the specified packaging materials (such as pots and labels) are used as failure to do so may result in the sample being un-assayable at the lab.
The consumables must be ordered directly from APHA Weybridge by using the following procedure:
- Fill in the requisition form (Annex 8a and 8b)
- Make sure that you complete all the boxes (establishment name, address, FSA contact name and telephone number, and any others).
- The requisition form should be emailed to: StoresStockOrders@apha.gov.uk.
APHA will endeavour to complete delivery of consumables orders within 7 working days of receipt. If you have any queries regarding an order that you have placed you should telephone the APHA stores in Weybridge on 01932 359451.
7.14 Private Slaughter of TB Reactors (Rs), Direct Contacts (DCs) and Inconclusive Reactors (IRs)
7.14.1 Background
On 1st of November 2018, with the implementation of The Cattle Compensation (England) (Amendment) Order 2018 (SI 2018/754), Defra introduced a change to the existing private slaughter arrangements for cattle compulsorily removed for TB control purposes in England.
Note: more details about compensation payments could be found at the TBHub website.
As a result of this change, APHA and FSA agreed a process to follow for the private slaughter of Rs, DCs and IRs at non APHA contracted slaughterhouses. The aim is that FSA staff at the non-contracted abattoirs are given enough notice to ensure the required enhanced post-mortem and sampling procedures can be followed according to the agreed instructions.
7.14.2 Notification process
It involves the completion of an agreement form (TR558) which will display the proposed kill date and the last date by which a R/DC can be slaughtered as a private kill.
Reference: See Annex 31b for sample TR558 - Private Slaughter of TB Reactors/Direct Contacts/Inconclusive Reactors Arrangements Agreement Form.
APHA will provide the TR558 form to cattle keepers interested in the private slaughter of Rs, DCs and IRs on their request.
For the private slaughter of TB Rs, DCs or IRs from herds in England, the cattle keepers must initially discuss their intentions with the FBO of the slaughterhouse where the cattle will be processed, signing the TR558 form. Where the FBO has shown an interest in processing these private Rs, DCs or IRs, the agreement will be updated with the proposed kill dates and passed to the OV. The OV will assess the feasibility of the request and completion of form (see 7.14.3). The agreement form will be then sent back to APHA with no less than 24 hours before the scheduled/agreed kill date.
For the private slaughter of Rs, DCs or IRs from herds in Wales, APHA will complete the keeper/FBO sections of the agreement form on their behalf and return it to both the FBO and FSA for the confirmation of the kill date. If both the FBO and FSA agreed (see 7.14.3), the OV must return the form to APHA by 5.00pm the day after receiving the form from APHA.
Note: the notification process depends on the location where the TB Rs, IRs or DCs have been disclosed.
If the deadline date cannot be met or will be missed, then the R/DCs may need to be removed and killed at another abattoir privately or through the APHA haulage and salvage contract.
Once the completed TR558 agreement form is received, APHA will produce the relevant movement licence for the animal/s and TB110 form, identifying any samples required.
Note: The same process will be followed for the private slaughter of IRs with the difference that there will not be a latest date by which they will need to be slaughtered by.
7.14.3 OV assessment
Where the FBO intends to process private Rs, DCs, or IRs cattle, the OV should carry out a technical assessment to decide whether the premises are/are not adequate for processing Rs, DCs or IRs prior to signing the TR558 form.
Note: Annex 31a- FSA TB Ready Check list can be used for this purpose.
Any concerns that the OV might have about the lack of adequate inspection facilities and capability of the plant to process these cattle (e.g. deficiencies related to animal identification, correlation, provision of relevant PPE, segregation, availability of sampling kit and most importantly sample correlation with the animal’s identification, cleaning and disinfection) should be discussed with the FVC/FVL of the area. APHA should also be informed of the outcome as soon as possible.
Note: If a TB R, DC or IR animal is over 48 months old and there are not agreed procedures in place for BSE testing (e.g. RMOP) in the proposed slaughterhouse, the OV should refuse a private kill.
8. Outbreak of Avian Influenza
In this section
8.3 Movement licences for poultry to slaughter
8.4 Movement of meat during outbreak in domestic poultry
8.7 FBO duties within AI free zones
8.8 FSA duties in establishments within the protection, surveillance, restricted and free zones
8.1 Introduction
8.1.1 Avian Influenza outbreak
This section describes the actions when an Avian Influenza outbreak is declared.
For suspected cases of Avian Influenza found at slaughterhouses, section 2 (Action on suspicion of notifiable diseases) of this chapter must be followed.
In preparation for a potential outbreak of Avian Influenza (AI), the FBO and the OV should have contingency plans in place, including, if the FBO considers it necessary, the pre-designation of the slaughterhouse for processing birds from controlled zones.
Upon confirmation that AI has been found in poultry, captive and/or wild birds in England and Wales, animal health protection measures are imposed to prevent the spread of disease. Scotland and Northern Ireland might follow similar approach.
The latest information about the AI situation in UK, including the “Rules on meat produced from poultry and farmed game birds originating in Protection Zone(s)” and applicable general licences for meat can be found in the above link.
Pictures of clinical signs of avian influenza are available in this link.
8.1.2 Domestic legislation
Domestic legislation that applies in the case of a domestic poultry outbreak:
- the Avian Influenza and Influenza of Avian Origin in Mammals (England) / (Wales) (No. 2) Order 2006
- the Avian Influenza and Influenza of Avian Origin in Mammals (Scotland) / (Wales) Order 2006
- the Avian Influenza (Vaccination) (England / Wales) Regulations 2006
- the Avian Influenza (Slaughter and Vaccination) (Scotland) Regulations 2006
- the Avian Influenza (H5N1 in Poultry) (England / Wales) Order 2006
Domestic legislation that applies in the case of a wild bird outbreak:
- the Avian Influenza (H5N1 in Wild Birds) (England / Wales) Order 2006
- the Avian Influenza (H5N1 in Wild Birds) (Scotland) Order 2006 as amended
- the Avian Influenza and Influenza of Avian Origin in Mammals (England) / (Wales) (No.2) Order 2006
8.2 Controlled zones
8.2.1 Controlled zones and AI free zones
Controlled zones may be declared around the place(s) where birds have been found to be infected with notifiable AI virus. Those zones are classified as follows:
- Low Pathogenic Restricted Zone (RZ): minimum size of 1km around the premises where low pathogenic notifiable AI has been confirmed.
- Protection Zone (PZ) (or wild bird control area or wild bird protection zone, under the Wild Bird Order): a ring with a radius of at least 3 km around the point where high pathogenic AI virus has been confirmed.
- Surveillance Zone (SZ) (or wild bird monitoring area or wild bird surveillance zone, under the wild bird Order): a ring with a radius of at least 10 km around the point where high pathogenic AI virus has been confirmed and including the PZ.
- Restricted Zone (RZ): an area beyond the SZ that separates the SZ from the Free Zone normally when H5N1 is present, but not exclusively. To note that RZ are not always declared, but when they are they must be adjacent to SZ or other RZ and centred around the IP, and there may be more than one declared.
- Temporary movement restriction zones (TMRZ) and temporary control zones (TCZ): temporary zone with a radius of at least 10 km around the point where AI of the subtype H5 or H7 has been confirmed but not the virus N-type and pathogenicity. These zones are normally used prior to confirmation of pathogenicity. TCZ can also have two separate areas within – TCZ A and TCZ B – which replicate PZ and SZ in terms of size and control measures applicable within.
- Avian influenza prevention zone (AIPZ): zone declared by the secretary of state in England or the Welsh Ministers in Wales when considered necessary to reduce the risk of transmission of NAD from wild birds to poultry (including game birds and poultry kept as pets) or other captive birds (e.g., requirement for poultry and other captive birds to be housed within a define area; ban on hunting, etc).
- Controlled Zones (CZ): means a protection zone, a surveillance zone, a restricted zone, a low pathogenic avian influenza restricted zone, a temporary movement restriction zone, a temporary control zone, an avian influenza prevention zone, or an avian influenza (restrictions on mammals) zone.
An APHA Interactive Avian Influenza Disease Map is available here.
Moreover, there are a few other zones of importance in an outbreak of Avian influenza:
- Free Zone (FZ): area outside the PZ, SZ and RZ that is free of notifiable AI. The transport of live birds and meat within this area is allowed without restriction.
- Vaccination Zone (VZ) (or emergency vaccination zone or preventive vaccination zone): The Secretary of State or the Welsh Ministers considers that poultry or other captive birds in this zone should be vaccinated under a preventive vaccination plan or an emergency vaccination notice.
8.3 Movement licences for poultry to slaughter
Biosecurity and veterinary surveillance measures are imposed within the controlled zones to prevent the introduction of the virus into healthy poultry flocks. These include licensing birds to slaughter, under a specific movement license issued by a Veterinary Inspector (VI) or by an inspector under the direction of a VI.
Whether these licences are available or not will depend on an assessment by APHA/Defra/Welsh Government. These may not be available for premises located in certain zones or for certain species of birds. The requirement for a movement licence applies even where poultry sheds are co-located on the same premises as the abattoir.
Part of the movement licence requires completion by the OV/MHI confirming the slaughtering of all the birds and requires that the FBO returns the completed licence to the APHA licensing team. The OV should monitor that FBO returns the movement licences to APHA without delay.
It is to be noted that there is the potential that different movement licences operate in England and Wales, respectively, and the correct licence must be applied for.
8.3.1 Types of movement licences
Movements involving poultry for slaughter from and/or to premises situated within a PZ, SZ or RZ are only allowed under a movement licence issued by an APHA VI or Inspector under the direction of a VI (check licence requirements for details):
There are three types of movement licence:
- Specific Movement Licence (LS)
- Multiple Movement Licence (LM)
- General Movement Licence (LG)
Full details of movement licences can be found online APHA licensing team Bird flu (avian influenza) movement licences - GOV.UK (www.gov.uk).
Note: Movements of poultry to an abattoir located within the same farm complex also need to be licensed.
Note: Welsh Government may also issue movement licences for premises located in Wales
8.3.2 Specific movement licences (LS)
The movement of poultry from a PZ or SZ to any abattoir, or the movement of any poultry to an abattoir located within a control zone, is subject to LS.
A VI or Inspector under the direction of a VI must examine the birds within 24 hours before they leave the premises of origin. This licence is issued as a single document for each flock (not lorry load) of birds and can only be used once.
The person transporting the birds must comply with the conditions attached to the licence; carry the original with them and produce it on request.
Conditions for movement of live poultry are detailed in the licence and are specific to that individual move.
8.3.3 Multiple movement licences (LM)
For regular movement of poultry from a PZ or SZ to any abattoir, or the movement of any poultry to an abattoir located within a control zone a Multiple Movement Licence (LM) may be used. This licence is issued as a unique document that can be used between the same premises of origin and destination for several times and for a limited period as instructed by APHA when issuing the licence.
The person transporting the birds must comply with the conditions attached to the licence; carry the original document with them and produce it on request. The licence and the consignment note must be produced on request to an inspector or other officer of the Secretary of State / Welsh Ministers on demand and allow a copy or extract to be taken; and on such demand, furnish his name and address.
Conditions for movement of live poultry are detailed in the licence and are specific to the individual licence.
8.3.4 General licence (LG)
These licenses allow on certain instances for the movement of poultry to take place without applying for a specific or multiple licence. The person moving live birds and poultry products under this licence must at all times carry a consignment note.
This licence is not issued as a document, but is published on the Defra or on the Welsh Government website. It is for the person responsible for the transport of the poultry to ensure this movement is covered by the LG and that its conditions are met.
8.3.5 Conditions of the LG
Conditions of the LG are listed in the licence.
8.3.6 No licence required
The movement of live poultry between premises situated in the FZ to a slaughterhouse in the FZ does not require a licence.
8.3.7 Summary
The tables below summarise the movement licences required for poultry to slaughter during AI outbreaks:
Movement licences for live poultry to slaughter in a highly pathogenic AI outbreak:
|
Birds from |
To slaughterhouse situated in |
|||
|
PZ (3km) |
SZ (10km) |
RZ |
FZ |
|
|
PZ (3km) |
Specific Licence (LS) |
|||
|
SZ (10km) |
||||
|
RZ |
General Licence (LG)*/Specific License (LS) [* A specific or multiple licence will be required if a LG is not issued by Defra and/or Welsh Government] |
|||
|
FZ |
|
No licence required |
||
Movement Licences for live poultry to slaughter in a low pathogenic AI outbreak:
|
Birds from |
To slaughterhouse situated in |
|
|
LPRZ (1km) |
FZ |
|
|
LPRZ (1km) |
Direct movement under a General Licence (LG) by VI / AHI to designated slaughterhouse |
|
|
FZ |
|
No licence required |
Movement Licences for live poultry to slaughter in an H5N1 in a wild bird AI outbreak:
|
Birds from |
To slaughterhouses situated in |
||
|
PZ (3km) |
SZ (10km) |
FZ |
|
|
PZ (3km) |
Specific Licence (LS) |
||
|
SZ (10km) |
|||
|
FZ |
*General Licence (LG) Specific License (LS) [* A specific or multiple licence will be required if a LG is not issued by Defra and/or Welsh Government] |
No licence required |
|
8.4 Movement of meat during outbreak in domestic poultry
The movement of poultry and wild bird meat, minced meat, meat preparations and meat products from poultry or wild bird meat originating from within a PZ will be subjected to restrictions as follows:
- meat from poultry originating in a PZ must be restricted meat; the restricted meat or its packaging must bear a special mark which will replace the ID mark and be produced in accordance with the domestic legislation
- the categorisation as restricted meat and special mark may also apply to poultry meat from a PZ produced within the 21 days prior to the estimated earliest infection date
- When applied to retail packaging, the size of the mark may vary according to the size of the packaging; however, it must be legible to the naked eye

Only designated slaughterhouses can slaughter poultry meat from birds originating in the PZ, SZ and RZ.
Also, cutting plants, cold stores and establishments preparing minced meat, meat products and meat preparations situated within the PZ can dispatch their products into GB providing the applicable general licence/s are complied with, the raw material has been obtained in accordance with the domestic legislation and is not intended for supply to international trade, including NI.
8.4.1 Hunting ban
Where there is a confirmed outbreak in wild birds the hunting of wild birds may be prohibited in the wild bird PZ or wild bird control area and the wild bird SZ or wild bird monitoring area.
Where the confirmed outbreak is in domestic poultry no person shall release game birds in the PZ or SZ.
A shooting ban might also be introduced.
8.5 FBO responsibilities
8.5.1 Designation of slaughterhouses under a domestic poultry outbreak
Slaughterhouses will need to be designated by the FSA on behalf of Defra or Welsh Government before receiving and processing poultry from premises within a:
- highly pathogenic AI protection zone
- highly pathogenic AI surveillance zone
- highly pathogenic AI restricted zone
- low pathogenic AI restricted zone
- vaccination zone
In addition, slaughterhouses situated within a zone subject to movement restrictions must also be designated by Defra in case of slaughterhouses located in England or by the Welsh Government, in the case of being located in Wales, to receive and process poultry including where this originates from an area free from disease restrictions.
In both England and Wales, the applications will be assessed and pre-designated by FSA with recommendation given to Defra or Welsh Government, who will then sign off/grant the designation.
Designations are only valid during the outbreak period. Once the outbreak officially ends, the designations for the slaughterhouses will be removed. In the case of a new outbreak, the establishment will have to apply for the activation of the pre-designation. This can be done by approaching the approvals team on approvals@food.gov.uk
There are two types of designation for slaughterhouses:
- Level 1. To receive and process poultry from premises within:
- a highly pathogenic AI surveillance zone,
- a highly pathogenic AI restricted zone,
- a low pathogenic AI restricted zone (or vaccination zone in Scotland)
- or being a slaughterhouse within a zone subject to movement restrictions, to receive and process poultry from premises within an area free from disease control restrictions.
- Level 2. To receive and process poultry from premises within a highly pathogenic AI protection zone (Protection Zone) and, therefore, producing restricted meat.
Only approved establishments can be designated. Establishments, registered with the local authorities and slaughtering on farm fewer than 10,000 birds per year do not follow this designation process, although, if they are located in the protection or surveillance zone, will have to obtain a licence from APHA and meet some specific criteria if they want to operate during an outbreak.
Designations only apply to slaughterhouses processing farmed poultry, including farmed game birds. There are no controls on meat produced from wild game birds, including those shot within PZ or SZ, except for when H5N1 High Path AI is isolated. In that case, controls on wild game meat from Approved Game Handling Establishments (AGHE) might be implemented.
Activation of a designation does not mean the establishment will be able to receive poultry for slaughter automatically as the movements of poultry from controlled zones will still need to be accompanied by a specific or multiple licence (LS or LM).
8.5.2 Designation and activation process
Updated: [The FBO is required to submit Annex 25. Refer to section 12 of this chapter for details.]
8.5.2.1 Designations of slaughterhouses located nearby to active poultry farms.
Abattoirs, adjacent to an active commercial poultry premises, for example a broiler or laying farm, should not be designated, unless enhanced biosecurity measures are implemented as below to prevent the spread of Avian Influenza to nearby farms. Where they achieve designation, these abattoirs will remain designated until the NAD outbreak finishes. At that point their pre-designated status will be removed from Establishments and People Data base.
To evaluate the specific circumstances of each case, it is recommended that a joint visit is carried out by an FSA and an APHA veterinarian to assess the risks on site and provide advice to the FBO on the key areas where biosecurity needs to be enhanced.
The FBO is then responsible for preparing an SOP to manage these risks. Once the FSA is satisfied with the proposed measures, the designation can be signed off in accordance with the set of conditions/restrictions imposed and detailed in Part 4 of the designation approval document (Annex 25).
In essence, this means that there is scope for each situation to be assessed throughout the duration of the outbreak and treated accordingly on a case-by-case basis, in consultation with the Notifiable Disease Portfolio Team.
Associated risk factors to be taken into consideration during the assessment of abattoirs with active nearby farms can include (not exclusively):
- Physical separation between the farm and the abattoirs (for example fence, different entries, use of different tools and equipment)
- Location/extent/species of the on-farm poultry populations
- Outdoor/free-range/indoor units
- Staff movements
- Pest control (e.g. birds, feral cats, rodents, insects...)
- Geographical factors (e.g. nearby water reservoirs or streams)
- Animal welfare implications on farms associated to the abattoir (e.g. integrated systems)
- Airborne cross-contamination
- C&D procedures
- Handling and Storage of ABPs.
Please note that the risks associated to Level 2 designations will be higher and a more thorough assessment and SOP might be required.
8.5.3 Pre-designations and annual review
Updated: [Refer to section 12 of this chapter for further details.]
FBOs can apply at any time for their establishment to be pre-designated. The process is the same as detailed in 8.5.1, except for the completion of part 4 of Annex 25 (activation of the pre-designation), which will not be required at this stage. The same procedure as above should be followed.
Establishments with a pre-designated status can ask for their procedures to be reviewed on a yearly basis. This review will be carried out by the local FSA veterinary team before the start of the AI season (end of September each year). For that purpose, Annex 28 has been created, which is a simplified version of the initial pre-designation document.
The FSA approvals team in liaison with the Notifiable Disease Portfolio will send a reminder to each poultry slaughterhouse OV, FVC and FVL of the reminding them of the importance to review the AI pre-designated status prior to the AI season starts. The OV, in liaison with the FSA local team will review the FBO SOPs and procedures on site to ensure these are still valid and up to date using the Annex 28 check list.
Once the AI annual pre-designation checklist (Annex 28) is completed and signed by the OV/FVC/FVL, and upon submission to approvals@food.gov.uk, the approvals team will send to the FBO, cc'ed to ND lead, FVL, FVC, AM, TM and OV via email confirmation of the pre-designation being renewed and will update the information in Establishments and People database.
Establishments that do not review their pre-designations every year, will have to go through the review of their pre-designation before they can apply for activation of designation to operate during an outbreak.
8.5.4 Production of poultry meat from a PZ
Updated: [Meat produced from birds originating in the PZ and slaughtered within the 21 days from the earliest date of infection at the IP of that zone might become restricted meat once the PZ is declared. APHA tracing team might, pending a risk assessment, request the tracing and detention of this meat.
Defra/Welsh Government may request the disposal of the meat from birds that were sent to slaughter during the unregulated period of infection. This means the date when, in the opinion of a veterinary inspector, avian influeza virus may first have been introduced into the infected premises.
Following the declaration of protection and surveillance zones to control Highly Pathogenic Avian Influenza (HPAI), FBOs (excluding distributors and retailers) must follow the rules on meat produced from poultry and farmed game birds originating in Disease Control Zones. Bird flu (avian influenza) movement licences.]
Production of poultry meat, minced meat, meat products and meat preparations from healthy birds originating in the PZ can take place in slaughterhouses, cutting plants and other establishments within the PZ, SZ, RZ and / or FZ if:
- the slaughterhouse is approved and designated and complies with all the designation requirements
- the birds have been transported under a Licence
- the meat is produced in accordance with EU Regulations 852/2004, 853/2004 and the subject to official controls under Regulation (EU) 2017/625 and the associated Commission Delegated and Implementing Regulations
- the special mark is applied to the final product, indicating that the meat is restricted
- the conditions of the applicable general licence(s) are complied with
Restricted poultry meat and products from healthy birds originating from the PZ can be marketed within GB with no further treatment.
Meat from the PZ can only be exported (including NI) if ALL of the following conditions are complied with:
- the product undergoes heat treatment to inactivate any AI virus present (e.g. minimum of 70C reached through the entire meat or product)
- the oval ID Mark is applied after the treatment has been completed
- the establishment applying the heat treatment and the oval ID mark follows the conditions as detailed in general licences
- requirements of the Export Health Certificate are met
Where birds from a PZ are slaughtered and processed updated: [separated from any other birds], the parts of the slaughterhouse and the equipment used for the slaughter and processing of those birds must be cleansed and disinfected before other poultry is slaughtered or processed.
See 8.10.2 below for further details
8.5.5 Production of poultry meat from SZ and RZ
These restrictions only apply for a highly pathogenic outbreak in domestic poultry.
Production of poultry meat, minced meat, meat products and meat preparations from healthy birds originating in the SZ (or RZ when applicable) can take place in slaughterhouses, cutting plants and other establishments within the PZ, SZ, RZ or FZ provided
- the birds have been transported under a licence, and
- the slaughterhouse is approved, designated and complies with all the designation requirements
- The meat is handled and transported in compliance with the applicable general licence(s)
It is important to note that meat from poultry originating from the SZ or from abattoirs located within the PZ, SZ and RZ, despite being “unrestricted” might still be subject to international trading restrictions (see 8.5.1 for details). In most instances this meat will be only suitable for trade within GB.
8.5.6 Production of wild feathered game meat from PZ and SZ
Defra/Welsh Government may establish restrictions and controls on wild feathered game meat depending on the epidemiology and risk of the outbreak.
8.6 Traceability and Commercial documents
8.6.1 Commercial documents for poultry meat and wild feathered game meat
For details of commercial and export documentation see the Defra website. It is important to note that during AI outbreaks, regionalisation measures around the Infected Premises (i.e. PZ, SZ, RZ) are normally applied, limiting the export capacity of the premises in the area (for example farms and abattoirs). Each receiving country might apply different criteria and any individual requirement must be assessed before signing any EHC. To facilitate traceability and the completion of the EHC for the EU and NI, Support Health Attestations (SHA) have been made available.
8.6.2 Traceability of poultry meat before the PZ
Traceability of the meat is a legal requirement in all circumstances. The FBO should be aware that robust internal traceability systems will help to minimise the costs of the required tracing of the meat produced from the PZ before the declaration of the PZ.
8.6.3 Reporting requirements for the traceability of restricted meat
For FBOs of slaughterhouses which have been designated as Level 2, every time they process poultry originating from the PZ, must complete and submit approvals@food.gov.uk the traceability information for this meat using the form provided at the time of the designation (annex 27). This information will be used to verify the compliance with the general licence for restricted meat, through risk-based unannounced inspections at cutting plants receiving that meat.
The OV of a slaughterhouse which has been designated as Level 2 must verify that FBO completes and provides the traceability information requested in the form for all the dispatched restricted meat to the FSA Approvals team without delay.
8.7 FBO duties within the AI free zone in not designated slaughterhouses
8.7.1 Restriction applicable to establishments
Slaughterhouses within the FZ must be designated by Defra in England/by the Welsh Government in Wales to receive birds that originate within the PZ, SZ, RZ or VZ.
Reference: See sub-topic: ‘Designation of slaughterhouses under a domestic poultry outbreak’.
Any slaughterhouse situated within the FZ can receive birds that originate within the FZ without any designation
8.7.2 Production of poultry meat from the FZ
There are no restrictions to the production of poultry meat from birds originating in the AI FZ in establishments situated within the AI FZ, providing it is produced in accordance with Commission Regulations 852/2004, 853/2004 and subjected to official controls and inspection in accordance with Regulations (EU) 2017/625, 2019/624, 2019/625 and particularly 2019/627, and that adequate biosecurity measures are implemented.
The oval ID Mark is to be applied.
8.7.3 Production of wild feathered game meat from the FZ
There are no restrictions to the production of wild feathered game meat from birds originating in the AI FZ in establishments situated within the AI FZ, providing it is produced in accordance with Commission Regulations 852/2004, 853/2004 and subjected to official controls and inspection in accordance with Regulations (EU) 2017/625, 2019/624, 2019/625 and particularly 2019/627.
Where the establishment is approved, the oval ID Mark is to be applied.
8.8 FSA duties in establishments within the PZ, SZ, RZ and in designated slaughterhouses in the FZ
8.8.1 FSA presence
The FSA must be present at all times when slaughtering takes place until all birds have passed post-mortem inspection in designated slaughterhouses.
If there is an outbreak of a notifiable disease in poultry (such as Avian Influenza or Newcastle Disease), batch AMI is not permitted when poultry is moved to the slaughterhouse under a movement licence (e.g. from a restricted zone). In those circumstances, the OV must undertake immediate full veterinary Ante-Mortem Inspection (AMI) upon unloading from the vehicle.
Poultry which have come from premises in the PZ are deemed to be under official control, and must be lairaged, slaughtered, chilled and stored separately from product which is not under official control until such time as it is wrapped and packaged.
The OV must verify that the FBO complies with the requirements to keep those birds and meat separated and the required Cleaning and Disinfection takes place before slaughter of other birds commences.
8.8.2 Confirmation of designation
OVs must obtain confirmation from the FSA that the slaughterhouse is designated before receiving birds from PZ, SZ or RZ for slaughter. Up to date designation status can be found in Establishments and People, under slaughterhouse activities, The latest designation application, including specific plant procedures to meet the designation requirements, can be found in the plant folder. The OV must verify that the FBO is in compliance with the procedures set out in the designation. A summary of the main OV/FSA team checks required during an AI outbreak can be found in Annex 33
OVs are to encourage FBOs to apply for pre-designation to the FSA so they can minimise disruption should they require designation at a later stage.
8.8.3 Movement of live birds
Live birds originating from the FZ can be accepted by a slaughterhouse situated within the FZ without a movement licence.
The OV or MHI must obtain from the FBO a list of all expected farms delivering live birds from PZ, SZ and / or RZ for slaughter 24 hours in advance.
On arrival to the slaughterhouse the OV or MHI must inspect the movement licence or consignment note to verify:
- the origin of the birds
- that the consignment is intended for that slaughterhouse
- that where the birds originate from a PZ, they are kept separated from other birds, by means agreed with in the application for designation
Reference: See topic ‘Movement licences for poultry to slaughter’.
8.8.4 Cleansing and Disinfection
Additional FSA checks are required to verify compliance with the Cleansing and Disinfection (C&D) conditions attached to the licences:
After unloading at the premises of destination, the parts of the vehicle used to transport (including crates and equipment) anything which might be contaminated with mud, slurry, animal faeces, excretions, feathers or any other similar matter must be cleansed and disinfected on site.
FSA staff must carry out 100% checks of C&D of crates, modules and vehicles used to:
- transport birds from a PZ or SZ
- transport birds to a slaughterhouse located within a PZ or SZ.
Note: AI C&D related checks, including any breaches/actions encountered by the OV, should be recorded daily using the Cleansing and Disinfection of Livestock Vehicles K2 form.
Additional FSA staff may be required to perform those checks.
A reminder of some important areas to be considered:
- Birds in the sheds / crates / modules must have no access to the abattoir area. There should be no poultry freely roaming within the curtilage of the abattoir. Welfare standards must be maintained.
- The hard-standing area used for the Cleansing and Disinfection of the livestock transport must be maintained clean and free of animals / vermin / pets. This area must be cleaned and disinfected before commencing operations if necessary.
- All transport vehicles and crates must be thoroughly cleansed and disinfected after unloading the birds. Special care must be taken to avoid any recontamination of vehicles, modules and crates after C&D, particularly through soil and dirt adhering to the wheel arches and surrounding parts (as this is not controlled by the wheel mats at the exit). This may necessitate spraying the exterior of the vehicle at the boundary of the site.
- The abattoir must be clean prior to commencing killing. Naturally vermin, wildlife (including wild birds) and poultry should not have access to the abattoir to avoid transmission of undetected disease.
No additional FSA C&D checks are required for the transport of birds within FZ. However, the FBO must maintain high standards of Cleansing and Disinfection of all crates, modules and vehicles.
8.8.5 Confirmation of slaughter
The OV or an MHI under the OV supervision must confirm that the birds arriving under a Specific Licence have been slaughtered by endorsing the licence presented by the FBO.
Where any problems arise relating to live bird movements the OV must contact the local Trading Standards Office and the local APHA office.
The OV should verify that the FBO is returning the completed Specific Licence to the issuing APHA office.
8.8.6 Use of Plant Inspection Assistants (PIAs)
PIAs are not authorised to carry out Animal Health Official Controls. Only OVs or MHIs are authorised to carry out animal health tasks (e.g. C&D verification procedures). However, PIA can still carry out enhanced post-mortem, under the supervision of the OV.
8.8.7 Application of special mark
The special mark must be applied to poultry meat from birds originating from the Protection Zone or its packaging under the direction and control of the FSA.
8.8.8 Restricted meat
The term “restricted meat” is used to mean:
- meat from poultry originating from a Protection Zone (PZ)
- when instructed by Defra/Welsh Government, meat from poultry originating from an area that subsequently became a protection zone and was slaughtered within 21 days of the estimated earliest date of infection in the relevant zone,
- meat that has not been kept separate from the previous 2 categories
- any meat, processed meat or meat products derived from any of the above that has not been heat treated to a temperature of at least 70°C reached through the entire meat or product
The term “unrestricted meat” is used to mean:
- meat from poultry originating outside of a PZ
- meat from poultry originating in an area that subsequently became a PZ but was slaughtered at least 21 days before the date estimated by a veterinary inspector as being the earliest date of infection at a premises in the relevant PZ
- any meat falling into the restricted meat category that has been heat treated to at least 70°C throughout by an approved establishment (in accordance with Article 4 of Regulations (EC) 853/2004)
Restricted meat bearing the special mark can be dispatched from designated slaughterhouses to approved establishments under a general licence issued by Defra or the Welsh Government and marketed within GB.
When dispatching any restricted meat, the FBO of level 2 designated slaughterhouses are required to notify their clients to make sure they are aware of the requirement of maintaining the special mark after the meat is cut or processed in the cutting plants and the need of keeping the segregation of the restricted meat from other meat (annex 26).
The primary purpose of this special mark is to prevent the meat or resultant products from being exported.
Establishments that intend to further process meat bearing the special mark don’t need to be designated. Further process in this context means any activity that removes the special mark from the meat / wrapping / packing. When removed, the special mark will need to be replaced by the new establishment’s own special (“beer mat”) mark all the way down to retail level.
FSA staff will carry out risk-based unannounced inspections (UAIs) in establishments receiving meat bearing the special mark to ensure that it is processed separate from meat bearing the oval ID mark and that the special mark is maintained.
FSA officials are to check, in particular:
- correct marking (special mark) of the meat obtained from birds originating from the PZ
- separation of special marked meat from oval ID mark meat in cutting establishments
- special marked meat is processed separately from oval ID marked meat
- if special marked meat is mixed with oval ID mark meat, the resulting meat / products must bear the special mark
- special marked meat is wrapped / packed with the special mark of the new establishment where it has been unwrapped / unpacked; the requirements for the correct application of the special mark are equivalent to those for the oval ID mark
When restricted meat is heat treated to at least 70°C throughout in an approved establishment (in accordance with Article 4 of Regulations (EC) 853/2004) to produce unrestricted meat, the HACCP based procedures, document and records – particularly the monitoring records for the heat treatment process and the prevention of cross-contamination must be strictly adhered to.
8.9 Waste disposal
8.9.1 Waste disposal
Lv2 Designated slaughterhouses slaughtering birds from the PZ:
Specific ABP categorisation and disposal rules apply for ABPs originating from birds from the PZ as follows:
- No category 3 ABP is allowed to go into raw pet food production.
- FBO will need a written confirmation from the rendering company that Category 3 ABP material will be subjected to a minimum heat treatment of 70°C.
- If the FBO does not have this confirmation, Category 3 ABPs from birds originating from a PZ must be disposed as Category 2 ABP or above.
- If Category 3 ABPs from birds originating in the PZ get mixed with Category 3 ABP from birds outside the PZ, the above controls apply to the entirety.
Meat processing establishments:
Meat processing establishments handling meat from birds from PZ must dispose Cat 3 ABP through a route that involves heat treatment to a minimum of 70°C.
8.10 Disinfection procedures (level 2 designations only)
8.10.1 Disinfection of contaminated litter, manure and slurry
Manure and used bedding must be treated by one of the following methods:
- be steam treated at a temperature of at least 70°C
- be destroyed by burning
- be buried deep enough to prevent access by wild birds and animals
- be stacked to heat, sprayed with disinfectant and left for at least 42 days
Slurry must be stored for at least 60 days after the last addition of infectious material unless (in the case of slurry which has been treated in accordance with a VIs instructions) a VI authorises a shorter storage period.
Manure, litter and bedding which may be contaminated may, if licensed by a VI, be moved to:
- a treatment plant carrying out procedures for the destruction of AI virus
- storage prior to destruction
- such other place as the VI may license.
The transport of such manure, litter or bedding must be in closed, leak-proof vehicles or containers and in accordance with an APHA’s VIs instructions. Standard land spreading is not permitted from material that originates from poultry in the PZ
8.10.2 General procedures for cleansing, disinfection and treatment
Defra/Welsh Government approved disinfectants must be used in live animal areas in accordance with this list.
FBOs using a detergent and/or disinfectant agent must ensure that they are used as effectively as possible, to the correct concentration as stipulated in the approved disinfectants list and must, in particular, give consideration to the following in deciding which products to use and how to use them:
- the nature of the premises to be cleansed or disinfected
- the type of vehicle or other thing to be cleansed or disinfected
- any instructions from the manufacturer of the product (or of a veterinary inspector) as to pressure, concentration, minimum temperature and required contact time
FBOs must ensure during the Cleansing and Disinfection processes that:
- bedding, litter and faecal matter from transport vehicles are thoroughly soaked with disinfectant if not disposed as required in 8.10.1
- equipment and installations are effectively Cleansed and Disinfected
- the ground, any floors, ramps and walls are washed and Cleansed and Disinfected by thorough brushing and scrubbing
When washing with liquids applied under pressure, recontamination of areas or parts previously cleansed must be avoided.
FBOs carrying out a Cleansing and Disinfection procedure must ensure that a written record of that procedure is made, showing the date and time the procedure took place. Such record must be kept at the premises for a period of 12 months.
8.11 Enforcement
8.11.1 Enforcement of licence requirements
Updated: [Refer to section 13 of this chapter for further details.]
8.11.2 Enforcement of Cleansing and Disinfection requirements
Additional FSA checks are required to verify compliance with the Cleansing and Disinfection conditions attached to the licences.
In accordance with the Transport of Animals (Cleansing and Disinfection) (No 3) (England) (Wales) (2003) Order, Defra/WG can ask FSA staff to carry out 100% checks of C and D of crates, modules and vehicles used to:
- transport birds from a PZ or SZ
- transport birds to a slaughterhouse situated within a PZ or SZ
Where Cleansing and Disinfection is unsatisfactory, FSA officers must serve a notice on the person in charge of the vehicle and report the incident to the LA.
Reference: See Chapter 2.2 Section 5 on ‘Cleansing and Disinfection’.
8.11.3 Designated slaughterhouses - suspension
Updated: [Refer to section 13 of this chapter for further details.]
8.12 Timesheet codes
8.12.1 GAVI code
Any time spent by FSA officials on additional controls related to AI must be coded as GAVI in the timesheet.
In particular, FSA Veterinarians and OVs who undertake work to support the designation process should code any additional time spent to GAVI.
The additional official controls required due to the slaughterhouse being within the PZ, SZ or RZ, or receiving birds from a PZ, SZ or RZ, including additional Cleansing & Disinfection checks, potentially increased PM inspection and paperwork verification checks, should be coded to GAVI. The scheduled C&D checks should continue to be coded to GAWG (please refer to C&D section under chapter 2.2).
9. Outbreak of Foot and Mouth Disease
In this section
9.1 Introduction
9.1.1 Purpose of section
This section describes how an outbreak of Foot and Mouth Disease (FMD) would be managed in Great Britain focussing on the controls applicable in slaughterhouses, cutting plants and meat processing plants.
For suspected cases of FMD found at slaughterhouses, section 2 (Action on suspicion of notifiable diseases) of this chapter must be followed.
Responsibility for managing outbreaks in the different countries of Great Britain falls to the respective Governments; Defra, Welsh Government and Scottish Government. Northern Ireland is recognised as a separate epidemiological unit and would expect to operate separate but similar controls in the event of an outbreak in accordance with EU and national law.
FSA Operations is a key operational partner of Defra and the Welsh Government in managing the outbreak in relation to slaughterhouses, approved meat establishments and registered dairy production holdings. Food Standards Scotland (FSS) is the key operational partner of Scottish Government for the same issues.
These instructions reflect the FMD Control Strategy for Great Britain 2011.
9.1.2 Background
FMD is a highly infectious, notifiable vesicular disease of domestic ruminants (cattle, sheep, goats), pigs, other farmed or wild cloven-hoofed mammals.
The economic significance of this disease is very high due to its ability to spread very rapidly and its profound effect on productivity. A very small quantity of the virus is capable of infecting an animal, and the disease could spread rapidly throughout the country if it is not controlled quickly.
FMD is not considered a public health threat. The FSA’s advice is that FMD is not transmitted to humans through the food chain.
9.1.3 Clinical signs and effects
Seven distinct serotypes of the FMD virus have been identified. The clinical signs of FMD are similar to other vesicular diseases and confirmation of diagnosis can only currently be made following laboratory tests. Affected animals have a high fever, which is followed by the development of blisters mainly in the mouth and on the feet. In some species however (notably sheep and goats), the disease is less severe or occurs as a sub-clinical infection.
Some strains can give rise to high levels of mortality in young animals. In adult animals the disease is not usually fatal, however it causes severe pain and distress, especially in cattle, and animals may be left permanently lame with reduced productivity following recovery.
Further information about the clinical signs of FMD is available here:
The virus is present in great quantity in the fluid from the vesicles, and it can also occur in the saliva, milk and dung. Contamination of any objects with any of these secretions or excretions is a danger to other susceptible animals. Heat and some disinfectants will destroy the virus, whereas cold and darkness tend to keep it alive. Survival of the virus in the environment depends on a range of factors and is highly variable. Under field conditions, this can range from days to months.
The virus can be transmitted on fomites (an inanimate object capable of transmitting infectious organisms from one individual to another, for example, vehicles and farm equipment), as well as mechanically by animals and other living vectors. Susceptible animals can pick up the virus either by direct contact with an infected animal, or by contact with foodstuffs or other things which have been contaminated by an infected animal, or by eating or coming into contact with some part of an infected carcase.
Airborne spread of the virus can also occur and, under favourable climatic conditions, the disease could spread several miles by this route.
9.1.4 Legislation
Primary domestic legislation:
- The Animal Health Act 1981 and the European Communities Act 1972.
- The Animal Health Act 2002 (England and Wales) amending the AHA 1981.
- Animal Health and Welfare (Scotland) Act 2006.
Council Directive 2003/85/EC implemented by the following domestic legislation:
- The Animal Health Act 1981 (Amendment) Regulations 2005
- The Foot and Mouth Disease (England) Order 2006,
- The Foot and Mouth Disease (Control of Vaccination) (England) Regulations 2006
- The Foot and Mouth Disease (Wales) Order 2006
- The Foot and Mouth Disease (Control of Vaccination) (Wales) Regulations 2006
- The Foot and Mouth Disease (Scotland) Order 2006
- The Foot and Mouth Disease (Slaughter and Vaccination) (Scotland) Regulations 2006
- The Foot-and-Mouth Disease (Scotland) Amendment Order 2007
- The Foot-and-Mouth Disease (Scotland) Amendment (No. 2) Order 2007
Further information regarding the legislation is available on the Defra website.
During an outbreak additional legislation may come into force imposing new or varying existing measures.
9.1.5 Controlled zones
According to the OIE, “zone” means a part of a country defined by the Veterinary Authority, containing an animal population or subpopulation with a specific animal health status with respect to an infection or infestation for the purposes of international trade or disease prevention or control.
Zones will be put in place on suspicion or confirmation of disease at an IP to limit spread of disease. The zones have associated restrictions on the movement of animals, animal products and anything else which can spread disease. The restrictions are stricter close to IP.
A Protection Zone (PZ) - mandatory on confirmation of disease and will cover a minimum of 3km radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
A Surveillance Zone (SZ) - mandatory on confirmation of disease and will cover a minimum of 10km in radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
A Restricted Zone (RZ) - will be declared to implement a national movement ban across GB by each Administration at the beginning of any FMD outbreak.
Other zones may be established at the time of suspicion or if vaccination is implemented.
9.1.6 Designation
There are two types of FMD designation for establishments:
- Level 1. Establishments producing unregulated meat during an outbreak of FMD.
- Level 2. Establishments producing regulated meat during an outbreak of FMD.
‘Regulated meat’ means, for the purposes of this document, fresh meat etc. referred to in article 21(1) and article 28(1) of Schedule 5 of the Foot-and-Mouth Disease (England) Order 2006 or Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006.
‘Regulated meat’ is meat from susceptible animals originated in PZ or SZ produced within the 21 days prior to the earliest infection date and any other meat which may not have been stored and transported separately from it.
Regulated meat does require specific treatment for inactivating the virus and must be ‘regulated meat’ at all times until it is subjected to a specific treatment in a designated treatment establishment.
Approved meat establishments will need to be designated by the FSA on behalf of Defra or by the Welsh Government (FSS will do so on behalf of Scottish Ministers) before receiving and processing susceptible animals and meat from premises within a PZ or SZ.
Establishments operating and situated within a PZ or SZ must be designated to be able to operate during the outbreak regardless of the origin of the animals or meat.
Additionally, all the slaughterhouses operating during an FMD outbreak within the Restricted Zone (RZ) (likely the whole of Great Britain) need to be designated even if they are not receiving animals from a PZ or SZ.
In order to facilitate preparedness for a potential outbreak, FBOs may apply for a pre-designation anytime. On request of the FBO, the pre-designation may be subsequently activated during an outbreak of FMD. Regardless of pre-designation, plants are not designated until the FSA or Welsh Government activates the designation during an outbreak. Movement of animals or product to the plant may additionally require a movement licence issued by APHA.
Designations are only valid during the outbreak period and to process products produced during the outbreak period. Once the outbreak officially ends, the establishment will remain pre-designated providing that there are no relevant changes. If there are any changes affecting the legal entity, the management, the biosecurity facilities or the control procedures required for the designation, the FBO must inform both the OV and FSA Approvals and, if applicable, re-apply for a pre-designation. In the case of a new outbreak, the establishments will have to apply for the activation of the pre-designation.
Only approved establishments demonstrating robust compliance with all the designation requirements will be designated to operate during an FMD outbreak.
For any establishment handling ‘regulated meat’ (level 2 designation) they must have satisfactory chiller capacity for maintaining the separation between different categories of meat. This will imply separate chillers in the case of exposed meat or clear physical separation in case of fully wrapped and packed meat and satisfactory handling and disposal of ABP generated from ‘regulated meat’.
For slaughterhouses applying for any designation (i.e. level 1 or level 2), the following requirements must be met:
- Satisfactory C and D facilities for ensuring the 100% C and D of livestock lorries on site. This may be achieved by limiting throughput.
- Satisfactory capacity and arrangement for the handling, treatment and disposal of manure.
- Satisfactory presentation of heads and feet for post-mortem inspection.
- Satisfactory biosecurity measures in place covering all visitors, vehicles and laundry.
- Satisfactory arrangements with the FSA for ensuring full OV attendance and the post-mortem inspection of all the heads and feet of susceptible species.
For treatment establishments, satisfactory implementation of HACCP-based procedures guaranteeing and demonstrating the effective treatment of the ‘regulated meat’.
Activation of a designation does not mean the establishment will be able to receive animals for slaughter or meat as such movements will additionally require a licence.
9.1.7 FMD suspected in a slaughterhouse
The actions to be taken by the OV in cases of suspected vesicular diseases found at the ante-mortem or post-mortem inspection are explained in Section 2 (Action on notifiable diseases) of this chapter.
Where the APHA veterinary inspector is unable to rule out disease during the investigation of a suspected slaughterhouse case, all animals present will be slaughtered quickly and the meat isolated whilst investigations are undertaken.
No meat is allowed to be removed from the premises until the VI is satisfied that meat to be moved is not at risk of spreading FMD virus. Meat, other products and by-products of animal origin that have come from suspected animals, or may have come into contact with such products or by-products, will be isolated within the slaughterhouse pending the outcome of the investigation.
The place of origin of all animals suspected of being affected by FMD will be investigated. The FBO is advised to detain the meat in suitable conditions to ensure that the meat remains fit for human consumption if disease is negated and the meat is released for sale.
If FMD is confirmed, this meat and any other product or by-product from the animal will be disposed of.
9.2 FMD Outbreak
9.2.1 Outbreak – Early Stages
On confirmation of FMD, a total nationwide ban on the movement of animals is likely to be implemented (National Movement Ban). During this time, the animals already present in the lairages of the slaughterhouses must be slaughtered as soon as possible and without delay.
Only animals completing journeys that started before the national movement ban should arrive at slaughterhouses after the national movement ban is put in force. No other animals should arrive at the slaughterhouse during the full movement ban.
9.2.2 Outbreak – FSA Action
Any consignment of livestock breaching the national movement ban must be reported to Trading Standards immediately and the FVL must be informed as soon as possible.
Any undue delay in the slaughtering beyond 48 hours of arrivals must be reported to APHA and the FVL.
FSA staff must carry out a thorough ante-mortem and post-mortem inspection of those animals to ensure that they are not showing any sign of FMD. In addition to the inspection requirements, this will imply the post-mortem inspection of all the heads and feet of susceptible animals even if they are not harvested for human consumption.
Flexibilities in the deployment and completion of the official inspections are cancelled during the outbreak so cold inspection and OV-flexibility arrangements are cancelled.
9.2.3 Outbreak – Designation
During this stage, the OV should discuss with the FBO the potential FMD designation of the slaughterhouse in preparation for the national movement ban being relaxed. For this, the FBO must provide assurance to the OV about the compliance with all designation requirements. FBOs wishing to be designated will need to submit the application fully completed and countersigned by the OV to the FSA approvals team.
FBOs should prepare the slaughterhouse for the potential FMD designation by cleansing and disinfecting the lairage leaving it ready for the required daily cleansing and disinfection required by the designation.
Slaughterhouses located outside of the SZ or PZ can dispatch the meat without restrictions providing that the meat is not coming from animals originated in those areas.
Slaughterhouses, cutting plants and meat processing establishments located in a PZ or SZ would require FMD designation for allowing the marketing of meat, regardless of the course of the animal. The OV must inform the FBO of this requirement and record that in the daybook. Any failure to comply with this requirement must be reported to APHA, Defra and / or Welsh Government.
Any ‘regulated meat’ found in the establishment must be detained using a Detention Notice (ENF 11-26) waiting for a Defra or Welsh Government decision for that meat.
9.2.4 Controlled zones during the suspicion phase
When an APHA VI is unable to rule out disease during the investigation of a suspected case, samples will be taken. The APHA veterinary inspector will also serve a notice on the occupier of the premises designating it a Suspect Premises. No movements of any person or thing is permitted on or off the premises unless licensed by a VI.
If samples are submitted because FMD cannot be ruled out, a Temporary Control Zone (TCZ) may be put in place around the suspect premises with a default size of 10km in radius. The zone can be larger or smaller if considered more appropriate for controlling the spread of disease. Within the TCZ, movements of susceptible animals to and from premises (including into or out of the zone) are not allowed except under licence.
A Supplementary Movement Control Zone (SMCZ) may also be established at suspicion stage, restricting the movement of animals in a wider area.
If laboratory tests and veterinary investigations do not indicate the presence of FMD any longer (or the virus of any other notifiable vesicular disease), restrictions on the premises will be lifted.
9.2.5 Controlled zones after confirmation of FMD
The following zones will be put in place on confirmation of disease at an IP to limit spread of disease. The restrictions are stricter close to IP:
- A Protection Zone (PZ) - mandatory on confirmation of disease and will cover a minimum of 3km radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
- A Surveillance Zone (SZ) - mandatory on confirmation of disease and will cover a minimum of 10km in radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
- A Restricted Zone (RZ) will be declared to implement a national movement ban across GB by each Administration at the beginning of any FMD outbreak.
9.2.6 Controlled zones if vaccination is implemented
Routine, preventative vaccination is banned under EU law, allowing the EU to maintain the highest FMD status under international trade rules of “countries free from FMD without vaccination”. However, from the outset of an outbreak the Government is legally obliged to consider whether vaccination would assist disease control and as appropriate activate arrangements to implement vaccination.
If vaccination is implemented, it will normally be carried out within Vaccination Zone(s) (VZ).
A Vaccination Surveillance Zone (VSZ) extending at least 10 km beyond the edge of the vaccination zone will be put in place. This zone and its restrictions remain until FMD-free status is achieved.
Once vaccinated, live animals cannot be traded either within the EU or Internationally. EU safeguard measures (for example, special certification or special marking) will be in place restricting non-heat-treated meat and meat products to the domestic market for most of the duration of an outbreak. The nature of the restrictions may depend on the slaughter date of the animal.
9.2.7 Movement of susceptible animals
At the start of any outbreak, there will be a high degree of uncertainty about where in the country FMD may exist. The position will start to become clearer as tracings, surveillance and the epidemiological investigation progress. Decisions onto change control measures will only be taken when the epidemiological position for any particular outbreak indicates that the risk of spread can be adequately mitigated by biosecurity conditions. It is essential that restrictions remain in place as long as necessary to ensure the disease can be controlled and eradicated as quickly as possible.
Changes in movement restrictions can be expected to be phased. The first phase will be limited to those activities which need to happen at the beginning of any outbreak to address immediate animal welfare needs, for example, movement of dairy cows for milking, transport of feed to animals within zones or very low risk activities, collection and processing of milk.
Restrictions can be expected to be eased incrementally as certainty about the outbreak increases. Low risk movements will be considered, for example, movements direct to slaughter to a designated slaughterhouse within a short distance, before higher risk movements to live.
Government will address issues relating to ensuring what operations industry can reasonably continue to carry out during an outbreak through discussion with the FMD core group in England and industry stakeholders in Scotland and Wales.
9.3 Controls on meat
9.3.1 Meat controls in the Temporary Control Zone (TCZ) and Supplementary Movement Control Zone (SMCZ)
There is no specific control requirement for meat and milk from TCZ and SMCZ, unless premises are also within another zone, in which case the conditions for that zone apply.
However, the controls for PZ and SZ will be applied retrospectively and therefore, some of the meat will subsequently need to be traced, marked and treated if FMD is confirmed (see below sub topics).
9.3.2 Tracing of potentially infected material from an IP
Meat and meat products, carcases, milk and milk products, hides and skins derived from susceptible animals from the IP will need to be traced. Once traced the owner will be required to either dispose of them, or treat them as directed to kill any virus that may be present. This includes meat, milk or other products at the IP that were produced from susceptible animals originating from the IP or in some cases originating from other farms where the IP product has been in contact with such products. Compensation is not paid.
The FSA Incidents team will coordinate the tracings of meat and other products of animal origin intended for human consumption from animals originated in the IP. Once found, FSA staff should detain them for ensuring their adequate disposal.
9.3.3 Tracing of ‘regulated meat’ produced before the confirmation of the IP and the establishing of the PZ and SZ
Meat produced from animals originating from an area that subsequently became a PZ which was produced in the 21 days before the earliest infection date in that PZ and any other meat which was not stored and transported separately from it becomes ‘regulated meat’. Such must be traced for ensuring its marking and treatment.
If Defra / Welsh Government require the tracing, the FSA Incidents team will coordinate the tracings of meat and other products of animal origin intended for human consumption. Once found, FSA staff should detain them for ensuring their adequate disposal or over-marking followed by treatment.
9.3.4 Meat controls in a Protection Zone (PZ)
Fresh meat from animals originating from a PZ can be marketed if either:
- it was produced more than 21 days before the earliest infection date and stored and transported separately from meat produced 21 days or fewer before the earliest infection date; or
- a treatment is applied before being marketed. This meat is ‘regulated meat’ until it is treated for inactivating the FMD virus.
The production of ‘regulated meat’ requires:
- separation of animals and product in abattoirs, transport and storage and subsequent plants until treatment complete,
- meat to be health marked or identification marked and that mark to be over stamped until treated, and
- meat to be treated for inactivating the FMD virus in an FMD designated establishment. The main treatment allowed for meat and offal is heat treatment (cooking). (See 9.3.9)
Slaughterhouses handling animals originating from farms in the PZ updated: [and SZ must have a level 2 designation for FMD.].
Any commercial premises located in the PZ which handles meat must be designated for operating under the FMD outbreak.
9.3.5 Requirements for fresh meat, minced meat, mechanically separated meat and meat preparations
Where this meat is from susceptible animals and produced on approved establishments in a protection zone, the establishment updated: [might be designated by Defra or Welsh Government] for operating during the FMD outbreak.
The establishment must process only meat which was either:
- produced in the protection zone more than 21 days before the earliest infection date there, or
- produced from animals reared and slaughtered outside a protection zone, or
- produced from animals transported to the establishment under the authority of a licence granted under paragraph 12(2)(e) of Schedule 5 of The Foot and Mouth Disease (England) Order 2006 or paragraph 12(2)(e) of Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006 and slaughtered there.
The establishment must at all times during the production process stores, identifies and transports restricted meat separately from other meat.
9.3.6 Meat controls in a Surveillance Zone (SZ)
Fresh meat from animals from a SZ can be marketed if either:
- the animals were on the same premises for at least 21 days before slaughter and were identified so as to allow tracing of the premises; and the meat has been detained under supervision for at least 7 days and until any suspicion of infection on the premises of origin has been ruled out; or
- the animals were on the same premises for at least 21 days before slaughter during which no susceptible animals were brought onto the premises; samples taken within the 48 hours before loading have tested negative; and meat has been detained under supervision for 24 hours and not released until after a repeat inspection of animals on the premises of origin has ruled out on clinical grounds the presence of infected or suspect animals.
Treatments required for meat before being marketed:
- Separation required in abattoirs, transport and storage and subsequent premises until treatment complete.
- Beef and sheep carcases (excluding heads, viscera and offal) must be health marked or identification marked, those marks over-stamped and subsequently heat treated (cooked) or matured and deboned to specific standards (see 9.3.9 and 9.3.10).
- Pig meat (excluding heads, viscera and offal) must be health marked or identification marked, those marks over-stamped and subsequently heat treated (cooked) to specific standards (see 9.3.10).
- Offal must be trimmed to specific standards (see 9.3.9), packaged, identified by an over-stamped ID Mark and subsequently treated by specific heat treatment or by specific fermentation and maturation for destroying the FMD virus (see 9.3.9).
9.3.7 Requirements for fresh meat, minced meat, mechanically separated meat and meat preparations from susceptible animals and produced on approved establishments in a SZ.
The establishment must be designated for operating during the FMD outbreak.
The establishment must process only meat which was either:
- produced from animals transported to the slaughterhouse from the surveillance zone and it falls within paragraph 28(4), 28(5) or 28(6) of Schedule 5 of The Foot and Mouth Disease (England) Order 2006 or Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006
- produced from animals reared and slaughtered outside the surveillance zone and its associated protection zone, or
- produced from animals transported to the slaughterhouse from the protection zone under the authority of a licence granted under paragraph 12(2)(e) of Schedule 5 of The Foot and Mouth Disease (England) Order 2006 or Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006.
The establishment must at all times during the production process; store, identify and transport products intended to be eligible for despatch outside the protection zone separately from those which are not eligible for that movement, and in accordance with the conditions of the authorisation.
9.3.8 Meat controls from a VZ
If vaccination is used for the control of the disease, Defra and the Welsh Government will issue guidance for the meat controls from a VZ.
9.3.9 Meat treatments
These procedures are required for meat and offal from susceptible animals from a PZ and for offal and certain meat from a SZ.
Meat requiring treatment to ensure the destruction of FMD virus must have undergone any of the following treatments:
- heat treatment in a hermetically sealed container at a level of at least FO3;
- heat treatment at a minimum temperature of 70°C, reached throughout the meat;
- heat treatment in a hermetically sealed container to at least 60°C for a minimum of 4 hours, during which the core temperature must be at least 70°C for 30 minutes;
- natural fermentation and maturation of not less than nine months, resulting in the following characteristics
- Aw value of not more than 0.93, or
- pH value of not more than 6.0;
- heat treatment ensuring a core temperature of at least 65°C is reached for the time necessary to achieve a pasteurisation value equal to or more than 40.
9.3.10 Trimming-offal standards
The following procedures are required for meat from susceptible animals from a SZ:
- heart from which lymphatic glands, connective tissue and adhering fat has been completely removed,
- liver from which lymphatic glands, adhering connective tissue and fat has been completely removed,
- whole masseter muscles,
- tongues with epithelium and without bone, cartilage and tonsils,
- lungs from which the trachea and main bronchi and the mediastinal and bronchial lymphatic gland have been removed,
- other offal without bone or cartilage from which lymphatic glands, connective tissue, adhering fat and mucous membrane have been removed.
9.3.11 De-boning standards
Meat (together with diaphragms but excluding offal) is deboned if the bone and main accessible lymphatic glands have been removed.
9.3.12 Maturation
Carcases are matured if they:
- have been matured at a temperature of more than 2°C for at least 24 hours; and
- have a pH value in the middle of the Longissimus dorsi recorded at less than 6.0.
9.4 FBO responsibilities
9.4.1 Application for FMD designation
The FBO should apply to be pre-designated or designated to the FSA Approvals Team (Approvals@food.gov.uk), who will provide the latest version of the application form containing all the requirements and instructions necessary for the designation.
Updated: [Refer to section 12 of this chapter for further details.]
9.4.2 Biosecurity
Good biosecurity standards in slaughterhouses must be implemented at all times but, during an FMD outbreak, they must be heightened.
Guidance on biosecurity is available on the Defra pages of Gov.uk.
9.4.3 Marking of meat
Updated: [Controlled meat (meat from Protection Zones (PZ) or Surveillance Zones (SZ)) must have a special health mark or ID mark clearly applied to it. This can be either:
- ‘A diagonal cross, superimposed on the Health Mark or Identification Mark applied under article 5 of assimilated Regulation No 853/2004 laying down specific hygiene rules for food of animal origin, or
- A single oval Health Mark stamp, 6.5 cm wide and 4.5 cm high with the letters UK on the upper part, the establishment approval number in the centre, two straight lines crossing at the centre of the stamp in such a way that the information is not obscured, and information which identifies the veterinarian who inspected the meat.
All meat with an ‘over-stamped’ health mark or identification mark is ‘controlled meat’ and therefore must be treated in a designated establishment using approved treatments to destroy the FMD virus.
Every consignment of controlled meat dispatched from the slaughterhouse must be accompanied by an accurate description of the products, including clear wording stating which disease has caused the meat to be controlled and the phrase ‘controlled meat’.
Controlled meat can be transported to a designated treatment centre for an approved treatment to ensure any undetected FMD virus is destroyed.
Meat of animals from the Restricted zone but outside the Protection and Surveillance zone is not considered controlled meat and cannot be marked as such. However, once a Restricted zone is declared, if there are additional requirements for the marking of that meat, they must be followed.]
Regulated meat can be transported to a designated treatment centre for an approved treatment to ensure any undetected FMD virus is destroyed. After treatment, the restricted markings can be removed and the normal oval (or round mark if the EU Decision requires this) health mark be applied.
9.4.4 Traceability & record keeping
Traceability of the meat is a legal requirement in all circumstances. The FBO should be aware that robust internal traceability systems will help to minimize the costs of the required tracing of the meat produced from the PZ before the declaration of the PZ.
The occupier of every premises in a PZ or SZ where susceptible animals are kept shall create and maintain the records regarding the number of each species of animal kept and the stock of meat, meat products, carcases, hides and skins, manure, fodder and used litter. The occupier shall maintain these records updating them within 24 hours of any change.
9.5 FSA responsibilities
9.5.1 FSA presence
During an FMD outbreak, OV flexibilities at slaughterhouses and Game Handling Establishments are suspended. At least one OV must be present at all times when slaughtering until all animals have passed post-mortem inspection.
Additional FSA attendance may be required to provide the controls and verification required for the control of the outbreak.
Strict biosecurity practices must be implemented by FSA staff at all times. Particular attention must be paid to the use, handling and disposal of protective clothing and the C and D of footwear and equipment.
9.5.2 Confirmation of FMD designation of the slaughterhouse
OVs must obtain confirmation from the FSA that the slaughterhouse is designated by the FSA in England or by Welsh Government in Wales for handling regulated meat and / or operating within a PZ / SZ before releasing animals from PZ or SZ for slaughter.
OVs must obtain confirmation from the FSA that the cutting plants or treatment establishments to where ‘regulated meat’ is dispatched are designated for handling and treating ‘regulated meat’.
FSA staff should encourage FBOs to apply for pre-authorisation to the FSA even if they do not need it, so they can operate without disruption should they require it at a later stage.
9.5.3 Movement of animals
On arrival to the slaughterhouse the OV or MHI must inspect the movement licences and accompanying documents for every animal or batch of animals to verify:
- the origin of the animals
- that the consignment is intended for that slaughterhouse
- that where the animals originate from a PZ / SZ, they are kept separated from other animals.
9.5.4 C and D
Additional FSA checks are required to verify compliance with the C and D conditions attached to the licences.
After unloading at the premises of the destination, the parts of the vehicle used to transport anything which might be contaminated with mud, slurry, animal faeces, excretions or any other similar matter including the wheels and wheel arches must be C and D on site.
The provisions for signing the driving declaration and leaving the establishment without the vehicle being C and D are not applicable in control zones during an FMD outbreak.
FSA staff must carry out 100% checks of C and D of vehicles used to transport animals from a PZ / SZ and of all vehicles if the slaughterhouse is located in a PZ / SZ. Additional FSA staff may be required to perform those checks.
The hard standing area used for the C and D of the livestock transport must be maintained clean and free of animals / vermin / pets. This area must be C and D after finishing operations. Other vehicles should not have access to this loading area for the duration of operation as a designated establishment.
C and D must include the wheels and wheel arches.
All transport vehicles must be thoroughly C and D after unloading the animals and before leaving the slaughterhouse. Special care must be taken to avoid any recontamination of vehicles after C and D, particularly through soil and dirt adhering to the wheel arches and surrounding parts (as this is not controlled by the wheel mats at the exit). This may necessitate spraying the exterior of the vehicle at the boundary of the site.
The abattoir must be clean prior to commencing killing. Naturally vermin and poultry should not have access to the abattoir to avoid transmission of undetected disease.
No additional FSA C and D checks are required in a slaughterhouse located outside of the PZ / SZ where the transport of animals originated from outside the PZ / SZ. However, the FBO must maintain high standards of C and D of all vehicles and no transport vehicle must leave any designated slaughterhouse without being C and D.
9.5.5 Confirmation of slaughter
The OV or an MHI under the OV supervision, must confirm that the animals arriving under a Specific Licence have been slaughtered by endorsing the licence presented by the FBO.
Where any problems arise relating to animal movements the OV must contact the local Trading Standards Office and APHA.
The OV should verify that the FBO is returning the completed Specific Licence to the issuing APHA office.
9.5.6 Enhanced post-mortem inspection
The designation of slaughterhouses includes the requirement of the presentation of all the heads and feet from all the susceptible animals to the official post-mortem inspection.
The FSA is committed to provide additional resources when necessary for allowing the post-mortem inspection of heads and feet but in certain circumstances the speed of the line may need to be reduced for allowing that inspection.
9.5.7 FSA verification of FBO controls in slaughterhouses
The OV must verify that the FBO complies with the objectives of the FMD legislation, movement licences and, where applicable the conditions of the FMD authorisation. In particular:
- FMD designation status of the slaughterhouse.
- Movement licences of animals admitted for slaughter.
- C and D of ALL the livestock transport vehicles before leaving the establishment.
- Slaughtering of animals no later than 24 hours after unloading.
- ‘Regulated meat’ is meat from animals within the designated protection or SZ. Such meat must:
- be marked as ‘restricted meat’ by ‘over-stamping’ of the Health Mark or Inspection Mark.
- be kept separately from other meat at all times
- be transported separately and only to designated premises
- not be traded or sold in the UK
- not be traded with other EU states
- not be exported from the EU
- Full traceability of ‘regulated meat’
- Verification of the destination of all the dispatched ‘regulated meat’.
- Application of Special Mark to ‘unregulated’ meat.
- Adequate handling, storage and disposal of ABP in compliance with designation conditions and FMD Order.
- Adequate handling, storage and disposal of manure in compliance with designation conditions and FMD Order.
9.5.8 FSA verification of FBO controls in cutting plants
UAIs must be organised for verifying that FBO complies with the objective of the FMD legislation, movement licences and, where applicable, the conditions of the FMD designation. In particular:
- FMD designation status of the cutting plant.
- Traceability documentation of meat received at the establishment.
- ‘Regulated meat’ is meat from animals within the designated protection or SZs. Such meat must:
- be marked as ‘regulated meat’ by ‘over-stamping’ of the Health Mark or Inspection Mark.
- be kept separately from other meat at all times
- be transported separately and only to designated premises
- not be traded or sold in the UK
- not be traded with other EU states
- not be exported from the EU
- Full traceability of ‘regulated meat’.
- Verification of the destination of all the dispatched ‘regulated meat’.
- Application of Special Mark to “unregulated” meat.
- Adequate handling, storage and disposal of ABP in compliance with designation conditions and FMD Order.
9.5.9 FSA verification of FBO controls in treating establishments
UAIs must be organised for verifying that the FBO complies with the objective of the FMD legislation, movement licences and, where applicable, the conditions of the FMD designation. In particular:
- FMD designation status of the treating establishment.
- Traceability documentation of meat received at the establishment.
- ‘regulated meat’ is meat from animals within the designated protection or SZs before applying a specific treatment as per FMD legislation. Such meat must:
- be kept marked as ‘regulated meat’ by and ‘over-stamped’ Health Mark or Inspection Mark.
- be kept separately from other meat at all times
- be transported separately and only to designated premises
- not be traded or sold in the UK
- not be traded with other EU states
- not be exported from the EU
- Full traceability of ‘regulated meat’.
- HACCP-based procedures demonstrating that the treatment comply with the specific legal requirement and, therefore, would ensure that the FMD is destroyed. (See 9.6.9)
- After treatment, the meat is considered unrestricted and the ‘over-stamped’ markings can be removed.
- Application of Special Mark to ‘unregulated’ meat when required.
- Adequate handling, storage and disposal of ABPs.
9.6 Animal by-products and co-products
9.6.1 ABPs and co-products produced in a PZ or a SZ or from animals originating in such a zone.
ABPs and co-products other than hides, skins, wool, ruminant hair or pig bristles must not been sold or consigned for sale unless they satisfy one of the following requirements:
- They were produced more than 21 days before the earliest infection date in the PZ, or in the case of a SZ, the associated PZ and at all times stored and transported separately from animal products not so produced.
- They have undergone one of the following treatments:
- heat treatment in a hermetically sealed container at a level of at least FO3;
- heat treatment in which the centre temperature is raised to at least 70°C for at least 60 minutes.
- Blood and blood products used for technical purposes have undergone any of the treatments referred to in point B(3)(e)(ii) of Chapter IV of Annex VIII to Regulation (EC) No. 1774/2002.
- Lard and rendered fats have undergone the heat treatment referred to in point B(2)(d)(iv) of Chapter IV of Annex VII to Regulation (EC) No. 1774/2002.
- Petfood and dog chews complying with the requirements of points B(2), (3) or (4) of Chapter II of Annex VIII to Regulation (EC) No. 1774/2002.
- Game trophies of ungulates complying with the requirements of points A(1), (3) or (4) of Chapter VII of Annex VIII to Regulation (EC) No. 1774/2002.
- Animal casings have been cleaned, scraped and either salted with sodium chloride for 30 days or bleached or dried after scraping and were protected from recontamination after treatment.
- It forms part of a composite product (that is, a manufactured or processed product containing more than one ingredient at least one of which is an animal product) and each ingredient which is an animal product has been treated as above or was not produced from susceptible animals originating on IP, suspect premises or contact premises or in a temporary control zone, protection zone, surveillance zone or vaccination zone.
9.6.2 Hides, skins, wool, ruminant hair and pig bristles produced in a PZ or a SZ or from animals originating in such a zone
Hides, skins, wool, ruminant hair and pig bristles of susceptible animals originating in a PZ or SZ must not been sold or consigned for sale unless either:
- they were produced more than 21 days before the earliest infection date in the PZ, or in the case of a SZ, the associated PZ, and at all times stored separately from hides and skins which were not so produced; or
- it has been treated for complying with the requirements in article 20 of Regulation (EC) No. 1774/2002 and points:
- In the case of hides and skins: A(2)(c) or (d) of Chapter VI of Annex VIII to Regulation (EC) No.1774/2002
- In the case of wool, ruminant hair and pig bristles: A(1) of Chapter VIII to Regulation (EC) No. 1774/2002
9.6.3 Manure produced in a PZ
Particular controls apply to manure from premises in a PZ where susceptible animals are kept; or collected from vehicles carrying susceptible animals from or within a PZ.
It must only be dispatched under a licence granted by an APHA inspector.
9.7 Enforcement
9.7.1 Enforcement of licence requirements
Updated: [Refer to section 13 of this chapter for further details.]
9.7.2 Enforcement of C and D requirements
Updated: [Refer to section 13 of this chapter for further details.]
Additionally, the breach of the terms of their licence under the FMD Order should be enforced.
9.7.3 Designated establishments
Updated: [Refer to section 13 of this chapter for further details.]
9.8 Timesheet code
Any time spent by FSA officials on additional controls related to FMD must be coded as GFMD in the timesheet.
10. Outbreak of Bluetongue Virus Disease (BTV)
In this section
10.2 Ante-Mortem Requirements for BTV
10.1 Introduction
10.1.1 Background
Bluetongue Virus Disease (BTV) is a notifiable disease of ruminants, including sheep, cattle, deer, goats and camelids. It is generally accepted that BTV does not cause disease in other animals or humans.
The severity of the infection depends upon the strain of the virus and may be affected by serotype. There are currently 29 different BTV serotypes.
The serotype involved in the outbreak of BTV in Northern Europe was identified as serotype 8 (BTV 8). Other serotypes common in mainland Europe include BTV 1 and BTV 4.
Spread between animals is almost exclusively via vector transmission - infected midges moving between and biting animals susceptible to the disease. It is not spread through carcases or fomites, such as contaminated vehicles, boots or clothing. However, infected midges trapped inside animal transport vehicles could potentially spread disease over large distances.
BTV is mainly spread by adult infected midges biting an animal susceptible to the disease. Although susceptible animals are vulnerable throughout the warmer months of the year, the peak populations of the vector midge (various Culicoides species) occur in the late summer and autumn, particularly at dawn and dusk. The midge however can also overwinter in buildings.
The virus can also be transmitted via infected germinal products (semen, ova, and embryos) as well as passed on maternally from mother to unborn offspring.
The latest information about BTV can be found online.
These instructions are applicable in case of outbreak of BTV. However, part of these instructions may be applicable to certain scenarios (situations) where there is a risk of introduction of the disease through the import of animals.
10.1.2 Zones for BTV
Once circulation of disease is officially confirmed, appropriate areas are declared as a Control Zone (CZ) or a Temporary Control Zone (TCZ) (which must include the infected premises) and a Restricted Zone (RZ) (made up of a Protection Zone (PZ) and Surveillance Zone (SZ)).
- CZ or TCZ - at least 20km around infected premises.
- PZ - at least 100km around infected premises (with flexibility to adjust according to epidemiological circumstances).
- SZ - at least 150km around the infected premises (with flexibility to adjust according to epidemiological circumstances).
Illustration of the relationship of disease control zones from GB Bluetongue Virus Disease Control Strategy
The rest of England, Scotland and Wales that are not under movement restrictions are known as the ‘free area’ (FA).
A map with the current zones will be available on the relevant devolved government policy websites. The size of the zones, which are centred around the infected premises, will take account of geographical barriers, whether or not individual or multiple serotypes are circulating, and the circumstances of the outbreak. Where they overlap and could be combined a practical and proportionate approach will be taken.
An outbreak is only confirmed once disease is found to be circulating in the midge vector populations. A single infected animal alone would NOT therefore constitute an outbreak, unless it can be proven to have become infected by resident midge vectors via another animal in the vicinity.
During a BTV outbreak, restrictions may apply to red meat slaughterhouses approved for processing ruminants and game handling establishments (GHE) operating within RZ.
Only specifically designated slaughterhouses outside the PZ are permitted to receive ruminants from the BTV zones under a General Licence.
There are no restrictions on the movement of non BTV susceptible species such as pigs and poultry within the BTV RZ. Slaughterhouses processing such species alone (for example, pigs) will operate as normal.
FSA duties (for example, audits) in operating cutting establishments will continue as normal.
Each movement is also subject to special conditions as stipulated on the license which has implications for both the transporter and the slaughterhouse operator on arrival.
The conditions of the movement licence for animals from any BTV zone to a slaughterhouse in a free area will require that the slaughterhouse processing them is designated.
On some occasions, movements within and into the zones will also require a movement licence, e.g., movements of animals to a slaughterhouse in the TCZ.
The operator of the slaughterhouse in an SZ also needs to have a licence to operate if they receive animals from a PZ.
10.1.3 Legislation
England
Bluetongue Regulations 2008 as amended. These regulations implement Council Directive 2000/75 and enforce Commission Regulation 1266/2007.
Wales
The Bluetongue (Wales) Regulations 2008 as amended. These Regulations implement Council Directive 2000/75/EC and enforce Commission Regulation (EC) No1266/2007
Scotland
The Bluetongue (Scotland) Order 2012 No. 199. This Order implement Council Directive 2000/75 and enforces Commission Regulation 1266/2007
You can view these at www.legislation.gov.uk.
10.2 Ante-Mortem Requirements for BTV
10.2.1 Importance of official ante-mortem inspection
Although BTV has no public health implications, it has major significance for animal health and substantial economic consequences.
It is important for the OV to be vigilant for BTV clinical signs at ante-mortem and post-mortem inspections on all ruminants.
Photographic images of clinical signs could be found online.
If you suspect the presence of the disease, contact APHA immediately following the procedure of reporting suspect cases of Notifiable Diseases established in the Section 2 of this MOC Chapter.
The Food Chain Information (FCI) should reflect any treatment applied to the animal such as insecticides or repellents and the OV must verify compliance with the withdrawal periods. Action in line with MOC instructions for suspected residues (Chapter 5) should be considered.
Licensed insecticides or repellents which must be applied to lorries prior to loading or buildings, in accordance with the manufacturer instruction do not need to be declared in the FCI.
Flexibilities on OV attendance are not applicable in slaughterhouses receiving animals covered by BTV movement licences.
10.2.2 ‘Dusk’ rule
Animals moved under a General Licence from a RZ to a slaughterhouse within the PZ have to be moved and slaughtered at least two hours before sunset at the latest (the ‘dusk’ rule).
The OV is to report any non-compliance with the requirements of the ‘dusk rule’ to their Local Authority Trading Standards Office and to the Field Veterinary Leader who may consider the recommendation of the suspension of the designation of the slaughterhouse.
10.2.3 Identification of a suspect case
On identification of a suspect case of the disease (or if in doubt) the OV must notify the APHA immediately, inform the FBO of the slaughterhouse and report it to the Field Veterinary Leader and the Incidents team.
After notification, the OV must follow the instructions issued by the APHA Vet.
FBO’s should be aware that if a BTV positive case is identified in a slaughterhouse, APHA will serve a Notice declaring the slaughterhouse as an infected premises and certain conditions will then be imposed.
Note: See Section 2 in particular 2.2.9 ‘Procedure for suspect notifiable disease’ in this chapter.
10.2.4 Residues
BTV movement controls may involve the use of insecticides and repellents at farm level and during transport. This should be taken into consideration when carrying out the ante-mortem inspection and, particularly, during the assessment of the FCI.
Withdrawal periods prior to slaughter must be adhered to as stipulated by the manufacturers’ instructions for any pour on insecticides or residual sprays that are applied to animals.
The insecticides required for premises and vehicles are not usually licensed for being used directly on the animals. Those environmental insecticides or repellents must be licensed by the HSE and used as per manufacturer’s instructions. As they are not applied directly on the animals, they are not required to be recorded in the FCI.
Insecticides or repellents used on the animals must be licensed by the VMD, used as per manufacturer’s instructions, the withdrawal periods observed and declared in the FCI.
10.3 Movements Rules for BTV
10.3.1 FBO duties
FBO should be aware that for animals moved under a General Licence the movements must be timely reported to the British Cattle Movement Service (BCMS) for cattle and Livestock Information Service (LIS) for sheep and goat in England and EIDCymru in Wales.
The FBO should endorse the movement licence by signing it to confirm that the animals have arrived and been slaughtered immediately.
The FBO must send the completed movement licence (including the FBO and OV / MHI parts as per point 10.3.4) to the issuing APHA licensing office.
10.3.2 Movement of live animals
The movement of susceptible live animals (for example, ruminants) will be subject to restriction.
The direct movement to slaughter of ruminants is likely to be allowed soon after the start of an outbreak providing the movement takes place under a general licence or a specific licence issued by APHA.
A sample of the specific movement licence is shown below:




For movement licences and most up to date licensing requirements FSA staff / FBOs may consult the DEFRA website/Welsh Government website or contact their local APHA office in the first instance.
10.3.3 Movement of Fallen Stock & Deer Carcases
There are no movement restrictions on fallen stock from slaughterhouses and deer carcases to GHE.
10.3.4 FSA duties
The OV is to report any non-compliance with the requirements of the movement licences to their Local Authority Trading Standards Office.
The OV / MHI should endorse the movement licence by completing the part dedicated to the OV / MHI for confirming that all ruminant animals covered by the licence have been slaughtered in compliance with the movement licence conditions. Once this part has been completed, the licence must be returned to the FBO.
On arrival at the slaughterhouse the animals must be slaughtered no later than two hours prior to sunset on the same day as arrival and the journey must be planned accordingly in conjunction with the slaughterhouse operator.
The OV should verify that the FBO is complying with the licence requirement of returning the completed movement licence to the issuing APHA licensing office.
10.4 Designation of Slaughterhouses for BTV
10.4.1 Designation requirement for slaughterhouses in relation to BTV
The following establishments will require designation by Defra / by the Welsh Ministers based on FSA recommendation before receiving and processing animals:
- Slaughterhouses located in a Bluetongue free zone receiving animals from Protection, Surveillance, or Restricted zones.
- Slaughterhouses in surveillance zones receiving animals from protection zones.
- Slaughterhouses processing imported animals representing a higher risk of introduction of the disease.
- Slaughterhouses receiving animals that require designation as a condition of the movement license, usually including movements of animals originating in Temporary Control Zones, Control Zones and from premises under Bluetongue restriction notices.
- Any slaughterhouse as required as a condition from an animal movement licence
For a designation to be issued the following conditions shall also be met:
- animals must not be resident within the confines of the slaughterhouse, including its lairage
- Slaughterhouses with nearby farming activity shall have facilities and procedures in place to fully manage biosecurity risks presented by the farming operations
- Field lairages not to be used for receiving animals moving under a licence to a designated slaughterhouse
- Specific midge control measures, controls on the times of arrival and killing of animals, biosecurity controls, controls for the manure and measures for cleaning of the lairages and associated areas
The conditions to obtain designation are intended to minimise potential disease risk presented by biting midges, the vector for this disease. They are specified in Part 1 of the designation application form. Annex 29.
10.4.2 FBO responsibility
The slaughterhouse operators can apply for a pre-designation anytime. On request of the FBO, the pre-designation may be subsequently activated during an outbreak or any of the circumstances named in 10.4.1.
FBO requiring pre-designation / designation should contact the FSA Approvals team for requesting the required application. A sample of the pre-designation/designation form which includes the requirements can be found in Annex 29.
FBO of pre-designated slaughterhouse will require the activation of the designation after verifying that all the information included in the pre-designation form remains accurate and the requirements will be complied.
If the slaughterhouse is located within a SZ, the FBO would require to contact APHA for obtaining a general licence to slaughter animals in the BTV SZ which have moved direct from premises in the BTV PZ.
10.4.3 FSA duties
The OVs are to encourage the FBOs to apply for pre-designation to the FSA as part of their contingency plans and to facilitate preparedness. This would help them to operate without disruption during the outbreak.
The OV will have to verify compliance with the designation rules and, once satisfied with that, recommend the pre-designation/designation of the slaughterhouse if all the rules are complied with.
The specific pre-designation and designation conditions are described in the pre-designation/designation application form. They include:
- Controls related to the compliance of the “dusk rule”. The rules are only applicable for animals moved under a BTV licence. The procedures in place should be documented for facilitating staff training and implementation when the designation is activated.
- Controls of slaughtering other animals without undue delay. Movement rules require that animals should be slaughtered no later than 48 hours after arrival (for animals moved under BTV movement licences, the “dusk rule” apply). This applies at both pre-designation and designation stages. If the FBO is not complying with this condition, the OV should not recommend the pre-designation.
- Requirements in relation to biosecurity. In particular the slaughterhouse has to keep yards and surrounds clean and minimise areas which could harbour vectors. This applies at both pre-designation and designation stages. If the FBO is not complying with this condition, the OV should not recommend the pre-designation.
- Requirements in relation to the management of manure. When the designation is activated, Manure and other material likely to attract midges must be removed from in and around the animal areas of the abattoir. It shall be removed completely from the site at least once in the 7 days preceding and at least once in the 7 days after the slaughter of any animals that are required to be slaughtered at a designated slaughterhouse, to minimise sites likely to harbour the vector.
- Pre-designated slaughterhouses must have documented procedures reflecting the capability of applying that requirement when the designation is activated.
- Requirement in relation to the cleaning and disinfection of lairage and associated areas. Although the lairage should be cleansed and disinfected as part of the abattoir’s normal operating protocol, there are no additional requirements for disinfection under BTV designation. For BTV controls, the cleaning procedures for the lairage, unloading areas and walkways must be clearly documented including frequency. Those procedures must be implemented as per established procedures in both pre-designation and designation stages and these can mirror regular abattoir cleaning SOPs. The procedures may establish different frequencies during outbreaks, to meet the requirements of the designation, in particular:
o Manure and other material likely to attract midges must be removed from in and around the animal areas of the abattoir at least once in the 7 days preceding and at least once in the 7 days after the slaughter of any animals that are required to be slaughtered at a designated slaughterhouse.
o Unloading facilities for vehicles and any walkways likely to become soiled with dung must be fully cleansed and disinfected on at least a daily basis. - Requirement in relation to control of vector The slaughterhouse must have controls in place to minimise risks of vector attack. These may include:
- Adequate cleaning of yards and other areas within slaughterhouse curtilage to minimise areas which could harbour vectors.
- Appropriate use of insecticides, ensuring they are dry before animals enter the treated area.
- As an alternative or in addition to the use of insecticides, the use of electronic or other means to control flying insects.
- Adequate storage away from animal areas for and disposal of the manure and other material likely to attract midges.
On granting the pre-designation / designation, the OV should keep a copy in the plant file and verify compliance.
FSA auditors should verify compliance with the conditions established in the pre-designation / designation document and that the information on it remains accurate.
Failure to comply with the conditions should trigger the consideration of the suspension or revocation of the pre-designation / designation. The OV should contact the FVL in those cases.
10.4.4 Designation and activation process
Updated: [The FBO must contact Approvals@food.gov.uk to request an application form (Annex 29).
Refer to section 12 of this chapter for further details.]
10.5 Meat controls in BTV
There are no specific meat restrictions (for example, special mark or treatment) related to BTV.
10.6 Enforcement
10.6.1 Enforcement of licence requirements including “dusk rule”
The LA are the enforcing authorities for movement controls.
FSA staff are authorised to verify compliance with the conditions of the licence. Any suspected breach of the movement controls or licences must be reported to the LA Trading Standards Department and the local APHA office. The FVL must be informed.
10.6.2 Designated and licensed establishments
Updated: [Refer to section 13.3 of this chapter for further details.]
10.7 Timesheet code
Work undertaken by the OV to verify compliance with the designation rules should be coded to GDIS.
11. Outbreak of African Swine Fever (ASF)
In this section
11.3 ASF case in a slaughterhouse/GHE (Slaughterhouse case)
11.4 ASF case in a farm (Infected Premises)
11.5 ASF controls related to Protection Zone and Surveillance Zone
11.6 Pre-designation & Designations of meat establishments
11.1 Introduction
11.1.1 Background
This section describes how an outbreak of African Swine Fever (ASF) would be managed at FSA Field Operations level, particularly the controls applicable in FSA approved slaughterhouses, wild game establishments, cutting plants and treatment centres when an ASF outbreak is confirmed and control zones are declared.
ASF is a notifiable disease of animals. The domestic legislation requires that any person who suspects that a domestic or feral pig or carcase is infected with the disease must immediately notify the appropriate authority through Animal and Plant Health Agency (APHA). Animals of the Suidae family including pigs and wild boar are susceptible to ASF.
The ASF virus can remain active for months or years in pig meat products and can be a significant source of spread and dispersal of ASF in pigs. Updated: [ASF can be spread from pig to pig through direct contact with infected pigs, faeces or body fluids; indirect contact via fomites such as equipment, vehicles or people who work with pigs between pig farms/slaughterhouses with ineffective biosecurity; pigs eating infected pig meat or meat products.]
For suspected cases of ASF found in slaughterhouses or in wild game establishments, section 2 (Action on suspicion of notifiable diseases) of this chapter must be followed but further detailed guidance specific for ASF is included in this section (point 11.2).
Before the confirmation of any outbreak of ASF, particularly when the international epidemiological situation indicates higher risk of introduction of the disease in GB, the FBO and the OV should establish contingency plans which may include the application for pre-designation of the slaughterhouse for processing pigs from controlled zones. Further guidance is provided in this section (point 11.2).
Updated: [The latest information about ASF in UK can be found online. Pictures of clinical signs and lesions of ASF are available online.
Upon confirmation that ASF has been found in domestic pigs or wild boar in GB, animal health protection measures are imposed to prevent the spread of disease.
In relation to ASF controls, the following definitions apply:
- “Controlled animal” (“restricted animal” under the definition of the Products of Animal Origin (Disease Control) Regulations 2008),] is an animal of a species susceptible to ASF which is at, in or from:
- suspect premises
- an establishment where a disease is suspected
- infected premises
- an establishment where a disease is confirmed
- an infected area
- a protection zone, or
- a surveillance zone.
- “Controlled meat” is meat, including meat that has come into contact with meat produced on or after the date that a protection zone or a surveillance zone is declared, or an earlier date where the Secretary of State specifies such a date for the purpose of disease control from a restricted animal. Where restricted meat has been treated in accordance with the relevant legislation at a treatment centre it ceases to be regarded as restricted meat.
- “Designation of premises, slaughterhouses and game handling establishments”: The Secretary of State may designate any establishment or premises for the purposes of slaughtering animals, or cutting, preparing, processing, packing, wrapping, storage or treatment of meat.
- During an outbreak, the animals and other potentially infectious material (for example, manure, restricted meat, animal by-products) would be moved under specific or general licences.
- A person moving any pig under the authority of a specific licence must: carry the licence or a copy of it at all times during the movement; and on demand by an inspector or other officer of the appropriate authority, produce the licence or a copy and allow a copy or extract to be taken.
- A person moving any pig under the authority of a general licence must at all times during the movement, carry a document containing details of what is being transported, including the quantity; the date of the movement; the names of the persons responsible for the pig or thing being moved at the place of departure and the place of destination; the addresses of the place of departure and the place of destination; on demand by an inspector or other officer of the appropriate authority, produce the document and allow a copy or extract to be taken; and keep the document for at least six months.
Where disinfection of vehicles transporting pigs and areas where live animals are handled is carried out during the outbreak, the disinfectants must be approved by the appropriate authority and shown on the list of approved disinfectants published under the “General Orders” and used at the authorised dilution rate and in accordance with the manufacturer’s instructions.
The disinfectant that is used needs to be approved, a list of approved disinfectants can be found online.
11.1.2 Legislation and disease control strategy
The main legislation applicable during an ASF outbreak is:
- The Diseases of Swine Regulations 2014
- Animal Health Act 1981 as amended by the Animal Health Act 2002
- The Products of Animal Origin (Disease Control) (England) Regulations 2008 (SI 2008/465) (as amended)
- The Products of Animal Origin (Disease Control) (Wales) Regulations 2008 (SI 2008/1275) (as amended)
- The Diseases of Animals (Approved Disinfectants) (England) Order 2007
- The Diseases of Animals (Approved Disinfectants) (Wales) Order 2007
The Disease Control Strategy for African and Classical Swine Fever in Great Britain describes how government and others would manage an outbreak of either ASF or Classical swine fever (CSF) in Great Britain (GB) and is available online.
11.2 Preparedness for ASF
11.2.1 FBO preparedness for a potential outbreak of ASF
FBO handling or processing pigs, wild boars or their meat should be prepared for the possibility of an ASF outbreak affecting GB. This preparation should include the implementation of good biosecurity standards in the premises, the establishing of procedures for minimizing the impact of a potential withdraw/recall and the establishing of procedures for the adequate handling of restricted meat.
FBO must provide the required facilities and equipment for the cleansing and disinfecting of vehicles transporting pigs on site. FBO should promote and supervise the adequate implementation by drivers of the sequence of stages for dry-cleaning, washing and disinfection using disinfectant authorised under the general order as required by the legislation and the good practices promoted by the industry which can be found online.
FBO should establish a cleaning schedule and a documented procedure for the frequent and effective cleansing and disinfection of lairages, animal by-product areas and associated areas and implements.
FBO should clearly define the batches, establish robust internal traceability of meat at slaughterhouse and cutting plant and implement an adequate cleansing and disinfection of the food contact surfaces between batches (for example, by using cleaning with detergent followed by use of effective food-grade disinfectant effective against the virus following their manufacturer instructions and using adequate concentration and contact time).
Further guidance for traceability and preparedness for recalls/withdrawals is available at the FSA’s Guidance on Food Traceability, withdrawals and recalls within the UK Food Industry.
FBO should consider applying for a pre-designation for ASF which would improve their preparedness and speed up the designation process in case of ASF outbreak (see point 11.5 for further details).
FBO should schedule the arrival of animals for allowing their slaughter, in peacetime, within 48 hours of arrival and, when possible, the day of arrival. The schedule for arrival of animals and slaughtering should allow the adequate cleaning and disinfecting of lairages ensuring the adequate contact time for the disinfectants to act and their rising and/or drying as per manufactures instructions for preventing the risk of residues from disinfectants in the meat.
Slaughterhouses are animal holdings and therefore, the FBO’s slaughterhouses have a responsibility to prevent animal diseases. General guidance for animal diseases prevention including biosecurity measures, staff and visitors’ controls, buildings, equipment and vehicles controls are available online.
The industry has produced further guidance and materials.
11.2.2 FSA team preparedness
The OV at pig slaughterhouses and Game Handling Establishments processing wild boars should discuss regularly with the FBO their preparedness for ASF outbreaks using the guidance provided in this section and particularly the points raised under the heading 11.2.1.
When the establishment has been pre-designated for ASF, the OV should review the pre-designation at least once a year, using the opportunity for providing advice for improving the FBO preparedness and reviewing FSA team preparedness including awareness and procedures in place.
The FSA team can improve the preparedness for an ASF outbreak by:
- Being able to identify the signs of ASF infection at the ante-mortem and port-mortem inspection and the reporting procedures to APHA (Points 11.1.1 and section 2 of this MOC chapter) on suspicion of notifiable diseases.
- Applying strict biosecurity practices such as:
- Comply with FBO’s biosecurity procedures.
- Ensure a good separation of personal clothing and protective clothing using designated changing areas.
- Keep the FSA room tidy and clean.
- Eat only in designated areas separated from protective clothing and inspection equipment.
- Use dedicated protective clothing in the lairage and yards.
- Comply with the FSA procedures in relation to PPE
- HSW3 – Personal Protective Equipment (PPE) found on Share Point, particularly:
- Staff must not take protective clothing home for laundering. If overalls need to be transported to another site for laundering, they should be placed in special bags for transport and then placed directly into laundry bin. Those special bags are available from procurement.
- When carrying out ante-mortem inspection, non-white coats may be worn with waterproof over trouser or leggings. Coats must not be taken outside the establishment and must be sent to laundry as per other protective clothing. Waterproof over trouser/leggings must be disinfected with an approved disinfectant.
- Arrange systems for the frequent change or/and disinfection of the protective clothing used in the lairage, yards and animal by-product areas.
- The use of disposable PPE may be considered particularly during an outbreak when dealing with restricted animals or meat. When used, the disposal must be established observing good biosecurity practices.
- Use of designated parking.
- Ensure robust cleaning and disinfection practices for the PPE and tools.
- Ensure a safe handling, storage and dispatching of dirty protective clothing.
- Plan a suitable system for overcrossing the Health Mark in case of being necessary during the outbreak.
11.3 ASF case in a slaughterhouse/GHE (Slaughterhouse case)
11.3.1 Definition
Live animals, carcases and offal with suspicious ASF clinical signs or lesions found during routine ante-mortem or post-mortem inspection, are called ‘slaughterhouse cases’. The animals may or may not have come from premises included in ASF Surveillance Zone/Protection Zone.
11.3.2 FBO duties
The FBO should assist to OV and APHA Vet during the investigation of the suspected case and, as per legislation, must comply with
- not to move, or permit to be moved
- the pig or carcase which is the subject of the notification from the premises where it is located;
- any other pig or carcase to or from those premises;
- any other animal from those premises if the veterinary inspector is of the opinion that it is likely to spread disease;
- anything off those premises unless the veterinary inspector is of the opinion that it is not likely to spread disease;
- to ensure that any person who has been in contact with any pig or carcase, or who has been on any part of the premises that may be contaminated with disease, takes all necessary biosecurity precautions to reduce the risk of spreading disease before leaving the premises; and
- not to permit any pig to be slaughtered unless authorised by a veterinary inspector; and
- to identify and isolate any carcase in which ASF has been suspected, any carcase originating from the same premises (and any carcase that has been in contact with any such carcase) so that such carcases do not come into contact with any other pig or carcase at the slaughterhouse.
11.3.3 FSA duties
On suspicion of ASF during the ante-mortem or post-mortem inspection, the OV must follow the instruction laid out in Section 2 in particular point 2.2.9 ‘Procedure for suspect notifiable disease’ in Chapter 6 (Notifiable Diseases) of MOC Volume 1.
If ASF is identified/suspected at the slaughterhouse, the OV must report to APHA who will provide further instructions and inform the FBO of the suspicion requesting to stop the production as established by the legislation.
When APHA is notified of suspicion of disease in pigs at an establishment, the establishment will be placed under restrictions and further movements of animals onto the premises prohibited whilst investigations take place. The killing of pigs will be halted by the FBO until the APHA Vet arrives and assesses the situation. Suspect animal(s) must be kept isolated and no product of animal origin (for example, meat, animal by-products) or potentially contaminated items/vehicles/person must leave the establishment.
If instructed by the APHA Vet, any remaining pigs will be killed without delay and the meat detained and kept separate from other meat.
Meat and any product (for example, animal by-products, edible co-products, manure, digestive tract contain) that has come from the suspect pig/s or may have come into contact with such meat/products, will be detained pending the outcome of the investigation. If swine fever is confirmed these meat/products will be handled and disposed of as category 2 material (category 1 material if originated from wild animals) in leak-proof and closed containers. All meat at the premises will temporarily be detained until the APHA Vet has assessed the risk of the meat/products being infected or contaminated with swine fever virus. Where there is no risk of ASF infection or contamination, meat/products may be released otherwise it will be detained pending test results.
If ASF can be negated by the APHA Vet on clinical grounds, restrictions can be lifted and normal business resumed. All meat that had been detained will be released for sale subject to it continuing to comply with food hygiene requirements.
The establishment is not treated as an Infected Premises (IP) as it is likely pigs will have arrived already infected with ASF, although this may have been transmitted to others in the lairage or carcasses contaminated with ASF virus.
If samples need to be taken to confirm or negate the presence of ASF then restrictions will remain in force for 24-48 hours; this will prevent further animals being brought into the abattoir for slaughter. The APHA Vet will assess which pigs in the lairage may be infected and take the necessary samples.
Cross-contamination is a risk at both production areas (between meat/products) and lairages (between animals). The APHA Vet, working with the OV and FBO, will determine which animals and products may be at risk and thus detained and potentially disposed of. Withdrawal/recall of meat/products exposed to the risk of contamination may be necessary.
All animal waste (including manure, digestive tract content, hair, bones) and animal-by products have to be handled and transported preventing the risk of spread of the virus and disposed in a way assuring inactivation of virus.
In case of requiring a recall/withdrawal, the quantities/batches affected will depend on the batch size, segregation during processing & traceability. It will also depend on the quality and frequency of staff hand washing and sterilisation of tools and the cleansing and disinfection procedures and their robust implementation particularly for food contact surfaces, scalding tank/steam chambers and depilation machinery.
The potential need of recall/withdrawal would likely be for animal health purposes only as there would not be public health concerns. Depending of the risk assessment, the product may be subjected to a withdrawal rather than a recall. In that case, the withdrawal may be applied up to retail or retail distribution but not consumers and probably not from shop shelves. In order to minimize the size of the recall/withdraw, small batches, cleaning and disinfection between batches and robust traceability systems are recommended.
Similar procedures would be applied in Game Handling Establishments where ASF is suspected.
The OV must verify the compliance with the FBO’s duties established in the legislation, reminding the FBO of their obligations and reporting any breach to the APHA Vet.
11.4 ASF case in a farm (Infected Premises)
11.4.1 Background
Animals at a farm where ASF has been confirmed will be disposed of at the farm and will never be sent to a slaughterhouse. However, animals from that farm may have been sent to slaughter before disease was confirmed, whilst the disease was incubating.
Therefore, the meat from these pigs may be affected by ASF and will need to be traced, withdrawn, and disposed of. Animal products potentially infected with ASF will be disposed of as category 2 animal by-products.
11.4.2 FBO duties
The FBO and OV will be notified by APHA that the slaughterhouse has received pigs from an IP and the products from these pigs must be withdrawn and disposed of.
The FBO is responsible for disposing of the carcase/meat and derived products including animal by-products, coproducts. If the product has already left the establishment the FBO is responsible for notifying the recipient that they have similar responsibilities to dispose of the meat or notify other premises if the meat has been moved. Records must be retained for inspection.
Meat must be withdrawn by processors, manufacturers, distributors and retailers as far as retail shelves but not from end consumers.
Any person who is in possession of meat from a restricted animal originating from the “relevant date” from suspect premises, or meat that has come into contact with such meat, must detain that meat until those premises are no longer suspect premises. “Relevant date” means the date the suspect premises or infected premises became subject to disease restrictions, or any earlier date where the Secretary of State specifies such a date for disease control purposes.
Any person in possession of meat produced from a restricted animal originating from infected premises from the relevant date, or meat that has come into contact with such meat, must destroy that meat without delay.
Any person who has owned or been in possession of meat referred above must endeavour to trace that meat and inform the recipient of that meat, other than a consumer, that it is from infected premises.
11.4.3 FSA duties
FSA must follow APHA instructions. APHA will determine the earliest estimated date of introduction of the ASF virus on the IP farm following the epidemiological investigation and will provide instructions about the requirements for tracing, withdrawal and disposal of any meat and animal-by-products.
Pigs moved from the IP to slaughter in the period after ASF may have been introduced at the farm, but before disease restrictions were imposed may have been infected with ASF. Therefore, will be subjected to APHA epidemiological investigation and risk analysis. The meat from these pigs may be infected with ASF and will need to be traced, withdrawn and disposed of.
The FBO and OV will be notified by APHA that the slaughterhouse has received pigs from an IP and the products from these pigs must be withdrawn and disposed of. The FBO is responsible for disposing of the carcasses/meat and derived products including animal by-products and co-products). If the product has already left the establishment, the FBO is responsible for notifying the recipient and they have similar responsibilities to dispose of the meat or notify other premises if the meat has been moved. Records must be retained for inspection. Meat must be withdrawn by processors, manufacturers, distributors and retailers as far as retail shelves but not from end consumers.
The OV should verify:
- the FBOs batching procedures in place for assessing the risk of other meat being contaminated. This would require the consideration of the FBO’s internal traceability procedures, cleaning schedules, frequencies and procedures applied in the lairage, scalding tank/depilation machine and food contact surface (for example, evisceration/cutting equipment, cutting board).
- the FBO’s traceability records for the affected meat liaising with the FSA Incidents team for the wider supervision of the any withdraw needed.
- The FBOs traceability records for any category 3 material and manure/digestive tract contain generated or potentially contaminated with the virus. This would require the assessment of the batching of animal by-products and the frequency of collection and liaison with APHA.
11.5 ASF controls related to Protection Zone and Surveillance Zone
11.5.1 Background
After the confirmation of ASF, control zones will be established around the Infected Premises (IP):
- The Protection Zone (PZ) will centre around the IP with a radius of at least 3 km
- The Surveillance Zone (SZ) will centre around the IP with a radius of at least 10 km. The PZ will be found within the SZ.
11.5.2 Movement restrictions
An automatic ban on movements of pigs across GB is not proposed for ASF. Therefore, the movement restrictions are likely to affect mainly premises and animals in the PZ/SZ.
Pigs cannot be moved off or onto premises in the PZ or SZ. Pigs may be moved within a premise as long as they do not cross a public or private road. Derogations are unlikely to be available in the period following declaration of the zones. However, after a few weeks have passed since the last confirmed case in the area, government may start to consider the case to allow limited movement of pigs off premises in the PZ or SZ:
- for immediate slaughter in a designated slaughterhouse
- to other premises within the same zone due to welfare problems
- for culling and movement of the carcase to a rendering plant for processing
- pigs may be licensed from outside the control zones onto premises within zones.
APHA may license for the movement of a pig from outside the surveillance zone (SZ) to a designated slaughterhouse within the zone (SZ) for immediate slaughter provided that the vehicle transporting the pig is thoroughly cleansed and disinfected at the slaughterhouse after the pig has been unloaded. The licence may be granted by a veterinary inspector to allow movement of a pig after the expiry of the relevant period specified in the legislation, if the pig is transported directly to a designated slaughterhouse. The APHA Vet has individually inspected the pigs on the premises of origin, samples have been taken and the pigs are transported in a vehicle sealed by an APHA Vet.
APHA may license the movement of a pig from outside the protection zone (PZ) to a designated slaughterhouse inside the zone for immediate slaughter provided that the vehicle transporting the pig is thoroughly cleansed and disinfected at the slaughterhouse after the pig has been unloaded. The licence may be granted by APHA Vet only after the expiry of the relevant period specified in the legislation.
Trucks and vehicles that have carried live pigs or other livestock or material which may be contaminated with ASF virus are prohibited from leaving premises in the PZ/SZ unless they have undergone cleansing and disinfection (C&D). In the PZ, C&D of such vehicles must be inspected and authorised by a Veterinary Inspector.
11.5.3 Operation of meat establishments within the PZ/SZ
Pig slaughterhouses and GHE located in the PZ/SZ would need to be designated for being able to operate during the outbreak. Pre-designation of the slaughterhouses before the outbreak would facilitate the designation when required.
The movement of pigs from outside the PZ/SZ to a slaughterhouse located within the zones may be licensed from early in the outbreak as the movement is from a low disease risk area to a slaughterhouse for immediate slaughter. Slaughterhouses operating within a control zone must be designated. There are no specific controls on the meat produced from pigs originating from outside the zones. The practice of allowing C&D of vehicles away from the slaughterhouse will be suspended in these circumstances and they must fully C&D prior to leaving the slaughterhouse.
Cutting plants located in within the PZ/SZ do not need to be designated for ASF unless that they are accepting restricted meat.
11.5.4 Operation of establishments outside the PZ/SZ
Pigs originating outside the PZ/SZ and slaughtered at a slaughterhouse outside the PZ/SZ will not be subject to any additional controls, save any imposed in wider movement restriction or other control zones. There is no requirement for the slaughterhouse to be designated or for the meat to be controlled or (heat) treated.
The practice of allowing C&D of vehicles away from the slaughterhouse may be suspended if the disease situation requires.
Slaughterhouse located outside the PZ/SZ may be designated for processing pigs from the PS/SZ. Once pigs originating from within the PZ/SZ can be licensed to slaughter they must go to a slaughterhouse designated to slaughter animals from the PZ/SZ. Ideally the slaughterhouse will be located within the PZ/SZ, but regardless of location it must be designated.
Cutting plants and treatment establishments accepting restricted meat must be designated for ASF.
11.5.5 Operation of GHE
On confirmation of disease in feral pigs, the appropriate authority may declare a feral pig control zone.
Meat from a feral pig hunted in any feral pig control zone must not be placed on the market by any person unless the carcase has tested negative for ASF and an APHA Vet considers there is no risk of the spread of disease.
GHE accepting feral pigs located in the feral pig control zone or receiving bodies from that area have to be designated.
11.5.6 FBO duties
The legislation requires that meat establishments may only receive restricted meat/animals if it is a designated establishment. Therefore, an FBO at a pig slaughterhouse must prevent the acceptance of restricted animals unless the slaughterhouse has been granted with a designation for ASF.
Upon confirmation of an ASF outbreak in Great Britain, the FBOs should enhance their Food Chain Information (FCI) controls and establish procedures for ensuring that movement rules are observed by the pig suppliers and lorries transporting pigs to the slaughterhouse. FBO should be mindful that the potential slaughtering of restricted pigs from control zones in non-designated slaughterhouse is a breach of the legislation and will involve withdrawals/recalls and production disruptions.
Specific movement licences accompanying the pigs may require countersigning by the FBO slaughtering the pigs and/or the OV of the establishment. FBO should read and act upon the instructions provided in the movement licences.
When the vehicle transporting the pigs has been sealed by APHA, the FBO must request the presence of the OV for verifying the unsealing of the vehicle.
If a pig slaughterhouse is located in a PZ/SZ, the FBO would not be able to operate during the outbreak unless it is designated for ASF.
Manure or slurry, including digestive tract contain, from pig origin must not be transported or spread unless that it is done under a licence granted by an APHA Vet.
11.5.7 FSA duties
The OV must ensure that all the pigs accepted into the slaughterhouse:
- In the case of pigs from outside the PZ/SZ, the conditions of the general licence have been complied with.
- In the case of pigs from PZ/SZ, the conditions of the specific licences have been complied and they have been accepted into a slaughterhouse designated for ASF.
- In case of slaughterhouses located within or receiving pigs from PZ/SZ, the slaughterhouse is designated for that outbreak of ASF. A pre-designation does not qualify the slaughterhouse for receiving animals from PS/SZ.
When the vehicle transporting the pigs has been sealed by APHA, the OV must verify the unsealing of the vehicle.
In the case of the movement licence requiring an endorsement from FSA staff at the establishment, the OV should complete it as appropriate and ensure the instructions of the movement licence are followed.
When any breach in the movement licence conditions or standards is identified, the OV must report it to the relevant Local Authority (LA) recording the details in the daybook and inform to the Field Veterinary Leader (FVL)/Field Veterinary Coordinator (FVC) providing details of the breach and the response from the LA.
When any breach in the movement licence conditions has been committed by the FBO of a designated slaughterhouse, the request of a suspension of the designation should be considered by the OV in liaison with the FVL.
Pig slaughterhouse located within a PZ/SZ would require a designation for ASF for operating during the outbreak. When a pig slaughterhouse falls within a PZ/SZ while it has not been pre-designated for ASF yet, the OV should discuss with the FBO the need of pre-designation for being able to operate and provide advice for applying for a designation if needed (the FBO may consider to stop operations as well).
On confirmation of an ASF outbreak in GB, when a pig slaughterhouse is pre-designated, the OV should review and verify the compliance with the designation conditions for being able to recommend or not its designation and advice the FBO of any identified shortcoming which would need to be corrected before the FBO can apply for the designation.
When inspecting cutting plants during an outbreak, the inspector must ensure that pig carcases and pig meat bare a valid health mark or identification mark. Meat bearing crossed health marks or crossed identification marks must not be in cutting plants which have not been designated for ASF.
11.6 Pre-designation & Designations of meat establishments
11.6.1 Background
During an outbreak of ASF, the following designation requirements apply:
- Updated: [Slaughterhouses/GHEs accepting susceptible animals from PZ/SZ (or Feral Pig Investigation or Control Zones) would need to be level 2 designated.
- Slaughterhouses located in PZ/SZ would need to be at least level 1 designated.]
- Cutting plants accepting restricted meat must be designated for that.
- Treatment establishments receiving restricted meat for ASF treatment must be designated.
The pre-designation process allows the FBO to be prepared for applying for a designation in the case of an outbreak and will make the designation process quicker.
Animal By-Products from restricted animals must be categorised as Category 2 material (category 1 material if originated from wild animals). Any other animal by-product contacting directly or indirectly those products or meat from restricted animals must be categorised as Category 2 material as well.
11.6.2 Pre-designation process in peace-time
The aim of the pre-designation process is to allow the FBO and the resident FSA officials to be prepared in advance for applying for a designation if needed during an outbreak and for speeding up the designation process during an outbreak.
FBOs of slaughterhouses, GHEs, cutting plants and treatment establishments may apply for a pre-designation for ASF anytime. If the pre-designation is granted, on request of the FBO, the pre-designation may be subsequently activated during an outbreak.
A site approved for several co-located activities (slaughter, cutting, processing) under the same approval number would require a separate pre-designation /designation for each activity.
FBO requiring pre-designation should contact the FSA Approvals team and request an application. A sample of the pre-designation/designation form for slaughterhouses and GHEs which includes the requirements can be found in Annex 30.
11.6.3 Designation process during an ASF outbreak
FBO of pre-designated establishments will require the activation of the designation after verifying that all the information included in the pre-designation form remains accurate and the requirements will be complied with.
Designations cannot be granted unless there is an official confirmed an outbreak and ASF restrictions are in place. The pre-designation does not guarantee the designation.
Establishments which are not pre-designated at the time of the outbreak can apply for a designation. However, the designation process may take longer than in the case of pre-designated establishments.
During an ASF outbreak, FBO of pre-designated establishment requiring designation should contact the FSA Approvals team for requesting the activation of the designation. FBO of other establishment requiring designation should contact the FSA Approvals team for requesting the designation. A sample of the pre-designation/designation form for slaughterhouses and GHEs which includes the requirements can be found in Annex 30 A.
Updated: [Refer to section 12 of this chapter for further details.]
11.7 Meat and animal by-product controls in ASF designated establishments
11.7.1 Background about meat controls
Meat produced from pigs originating from the PZ/SZ (regardless of where they were slaughtered) is termed “restricted meat”. Such meat receives a special mark (a crossed through oval health mark) and cannot be sold fresh. It must be treated at a designated treatment centre and prior to treatment only handled at designated premises.
In some circumstances Third Countries may require the implementation of additional safeguard measures that apply to pigs, pork and pork products produced within the UK or a region of the UK. Should this happen, additional measures, such as special marking and trade restrictions may be imposed. Where a special stamp is proposed to indicate meat is restricted to the domestic market, it is likely a round stamp will be adopted.
It is paramount that “controlled meat” is continuously and robustly identified, separated and clearly marked with crossed identification mark or crossed health mark and that its traceability is thorough and consistent at all the stages.
Any risk of direct or indirect cross-contamination from “controlled meat” to other meat must be prevented.
The legislation requires that records related to “controlled meat” must be kept for 3 years from the date of slaughter, movement or treatment.
The FBO must not place on the market or export any “controlled meat”.
Updated: [Once treated, “controlled meat” ceases to be regarded as “controlled meat”.]
11.7.2 Background about animal by-products controls
Updated: [Animal by-products from controlled animals are to be handled, stored, and dispatched separately from all other animal by-products. They are not to be used in raw pet food and are to be dealt with in accordance with assimilated Regulation (EC) 1069/2009 (as amended).
Any animal by-products which come into contact with animal by-products from controlled animals, must be treated as animal by-products from controlled animals.
Animal by-products must be stored and dispatched in closed leak-proof containers, observing adequate biosecurity practices. Yards are to be kept clean and cleansed and disinfected immediately and thoroughly if spillage occurs.
Animal by-products containers must be cleansed and disinfected immediately after their use. When the establishment is in a controlled zone or has been handling controlled meat, the disinfection of the containers should be carried out using an approved disinfectant at the appropriate concentration.
If animal by-products from controlled animals are moved within or from a PZ/SZ, they must have the appropriate movement licence in place, and this must be checked before dispatch. Manure, litter and slurry including digestive tract content from designated slaughterhouses, must be removed from in and around the live animal areas of the slaughterhouse daily, treated with disinfectant and either stored separately on the premises or removed from site completely. This material can only be dispatched under a special licence issued by APHA.].
The yards and the animal by-products handling and storage areas must be kept clean and, when necessary, disinfected.
11.7.3 Marking of restricted meat
Restricted meat must be marked with a specific Health Mark/Identification Mark which is Health Mark or Identification Mark over-stamped with a diagonal cross. This can be:
- a diagonal cross, superimposed on the health mark or identification mark applied under article 5 of Regulation (EC) No 853/2004 laying down specific hygiene rules for food of animal origin, or;
- a health mark oval stamp, 6.5 cm wide and 4.5 cm high with two straight lines crossing at the centre of the stamp in such a way that the information of the Health Mark is not obscured.
11.7.4 Movement of restricted meat
Restricted meat can only be moved or dispatched:
- From designated slaughterhouses to designated cutting plants. When the cutting plant is collocated, it requires a separate designation and the movement records between the slaughterhouse and the cutting plant must be kept.
- From designated slaughterhouses or designated cutting plants to designated treatment establishments.
Movements of restricted meat must be reduced to the minimum and re-dispatching of restricted meat is not allowed.
Freezing of restricted meat should not be carried out. The restricted meat must be treated without delay. Once it has been treated in a designated treatment centre, the meat product may be frozen as it will not be restricted any more.
The movement of restricted meat must be carried out preventing any risk of contamination from the restricted meat to other products or the environment during the handling, loading/unloading and transport.
The commercial document accompanying the meat must clearly state that the meat is “restricted meat”.
Any person who is in possession of restricted meat must make records of the following:
- the quantity of meat handled;
- the disease which caused the meat to be subject to restrictions under the disease legislation;
- the quantity of meat placed into and removed from cold storage;
- the date of such movement into or out of cold storage;
- the quantity of such meat that is no longer intended for human consumption.
Records must be kept for 3 years from the date of slaughter, movement or treatment.
11.7.5 Treatment of restricted meat
One the following treatments must be applied to “restricted meat” in designated treatment establishments:
- Heat treatment in a hermetically sealed container with an F value of 3 or more (where F is the killing effect on bacterial spores: an F value of 3 means that the coldest point in the product has been heated sufficiently to achieve the same killing effect as 121°C in three minutes with instantaneous heating and chilling).
- Heat treatment at a minimum temperature of 80°C which must be reached throughout the meat.
- Heat treatment in a hermetically sealed container to at least 60°C for a minimum of 4 hours during which time the core temperature must be at least 70°C for 30 minutes.
- Natural fermentation and maturation of not less than nine months for boneless meat resulting in the following characteristics: a Water Activity (Aw) value of not more than 0.93 or a pH value of not more than 6.
- Treatment of hams and loins involving natural fermentation and maturation for at least 190 days for hams and 140 days for loins.
In addition to the HACCP records, the occupier of a treatment centre where restricted meat is treated must keep records of the following:
- the date of the treatment;
- the species of animal from which the meat came;
- the quantity of meat treated
- the treatment applied.
Records must be kept for 3 years from the date of slaughter, movement or treatment.
11.7.6 FBO duties
The occupier of an establishment must ensure that restricted meat is marked with the special mark (over crossed health mark or identification mark) required by the legislation.
A person must not be in possession or control of restricted meat unless it is marked with the required special mark and the establishment is designated for ASF.
A person must not remove the special mark except to enable cutting, preparing, processing, packing or treatment of the restricted meat. Any person who removes the special mark, other than a person treating meat at a designated treatment centre, must reapply the special mark, with the appropriate plant approval number, after cutting, preparing, processing or packing the meat. The reapplication of the special mark should be immediate to avoid keeping the meat unattended at any time without being properly identified with the special mark.
Any person who is in possession of restricted meat must make records of the following:
- the quantity of meat handled;
- the disease which caused the meat to be subject to restrictions under the disease legislation;
- the quantity of meat placed into and removed from cold storage;
- the date of such movement into or out of cold storage;
- the quantity of such meat that is no longer intended for human consumption.
The occupier of a treatment centre where restricted meat is treated must keep records of the following:
- the date of the treatment;
- the species of animal from which the meat came;
- the quantity of meat treated;
- the treatment applied.
Records must be kept for 3 years from the date of slaughter, movement or treatment.
11.7.7 FSA duties
FSA staff delivering official controls in approved establishments during an ASF must verify compliance with the rules for “restricted meat” in particular:
- Restricted animals and restricted meat can only be present in establishment specifically designated for ASF. The OV/MHI must request a copy of the designation to the FBO for verification purposes.
- Requirements established in the designation for ASF.
- Complete separation from other meat / animals / animal by-products.
- Continuous and adequate application of the required marking at all the times.
- Dispatching of restricted meat to exclusively ASF designated premises
- Records regarding traceability of restricted meat and animal by-products
- Treatment parameters and practices.
FSA staff must detain any meat when in doubt and seek clarification.
FSA staff inspecting designated establishments must record in the daybook details of the verification of “restricted meat”.
FSA must report any incident related to “restricted meat” to FSA Incidents, inform immediately the FVL and refer it to the relevant Local authority.
11.8 Regionalisation
In addition to the controls related to Protection and Surveillance Zones, regionalisation may be implemented as part of the ASF controls.
Regionalisation is allowed at international level for the handling of outbreaks of ASF while reducing the impact of ASF outbreaks in international trade. The legal basis for regionalisation or zoning is in the World Trade Organisation (WTO) – World Organisation for Animal Health (OIE) is in the Article 6 of the Sanitary and Phytosanitary (SPS) Agreement and the chapter 4.3 of the OIE Terrestrial Animal Health Code.
The OIE defines zone/region as a clearly defined part of a territory containing an animal subpopulation which a distinct health status with respect to specific disease for which required surveillance, control and biosecurity measures have been applied for the purpose of international trade.
Regionalisation is applied in the European Union for the management of the ASF outbreaks in its member states. The Implementing Decision 2014/709/EU establishes the criteria for demarcating Parts I, II, III and IV subjected to specific restrictions in relation to animals, meat and by-products:
- Part IV: occurrence of ASF in both domestic pigs and wild boar. The disease control presents specific challenges due to the systemic and high-level non-compliance by stakeholders with the relevant EU requirements, in particular in relation to identification, registration and traceability of pigs and there are certain difficulties for the veterinary authorities to ensure the conformity with those requirements.
- Part III: occurrence of ASF in both domestic pigs and wild boar.
- Part II: occurrence of ASF only in wild boar.
- Part I: higher risk area with no cases, nor outbreaks, of ASF and where higher surveillance (in particular passive) is applied adjacent to a Part II, III or IV.
The restrictions relevant for meat controls applied to the parts can be summarised as:
- Live pigs cannot be dispatched from Parts II, III or IV.
- Pig meat, pig meat preparations and pig meat products cannot be dispatched from Parts III or IV.
- Animal by-products from porcine animals cannot be dispatched from Parts III or IV.
A map summarising the current regionalisation applied in the EU member states is available online.
11.9 Enforcement related to ASF controls
The Diseases of Swine Regulations 2014 are enforced by the relevant local authority.
A person must not intentionally obstruct or impede anyone acting in the execution or enforcement of The Diseases of Swine Regulations; without reasonable cause, proof of which lies on the person charged, fail to give to any person acting in the execution or enforcement of these Regulations any assistance or information that is reasonably required; provide to anyone acting in the execution or enforcement of these Regulations any information knowing it to be false or misleading or not believing it to be true; or fail to produce a record when required to do so by any person acting in the execution or enforcement of these Regulations.
The Product of Animal Origin (Disease Control) Regulations are enforced by the Secretary of State or by local authority in England, by the Welsh Ministers or by local authority in Wales.
FSA staff are authorised to act on matters arising under the following legislation:
- The Diseases of Swine Regulations 2014 (to be confirmed)
- The Product of Animal Origin (Disease Control) Regulations 2008 (to be confirmed)
Therefore, FSA staff can verify compliance with those Regulations in an FSA approved establishment while breaches would need to be reported to the relevant Local Authority for its enforcement.
The FSA can hold the granting of pre-designations/designations and recommend the suspension or withdrawal of them to Defra and Welsh Government at any time when their conditions are not complied with.
11.9.1 Enforcement of licence requirements
Updated: [Refer to section 13.1 of this chapter for further details.]
11.9.2 Designated establishments
Updated: [Refer to section 13.3 of this chapter for further details.]
11.9.3 Restricted meat
Whenever any need for clarification or suspected breach affecting the meat in an FSA approved establishment is identified in relation to ASF controls, the OV/AO should initially detain the meat using a Detention Notice (ENF 11/26) and consider the derived animal by-products detaining them if applicable. This will allow the gathering of all the relevant information and its sharing with FSA Incidents and the relevant enforcement authority.
11.10 Timesheet code
Work undertaken by the OV to verify compliance with the designation rules and the additional tasks carried out during the ASF outbreaks should be coded in the timesheets as GDIS.
12.Updated: [Pre-designation & Designations of meat establishments.
12.1 Introduction
12.2 Pre-designation of establishments
12.3 Designation process during a notifiable disease outbreak
12.1 Introduction
During a Notifiable disease outbreak some meat establishments might need to be designated to be able to operate or to receive animals/meat originating from controlled areas.
The FBO can apply for pre-designation at any time, and once an outbreak has been confirmed they will need to apply for designation. Designations cannot be granted unless there is an official outbreak has been declared, and notifiable disease restrictions are in place.
12.2 Pre-designation of establishments
The pre-designation process allows the FBO to be prepared for applying for a designation in the case of an outbreak, making the designation process quicker.
Local teams (OVs, TMs, FVCs and FVLs) play a key role in the designation process, but the overall responsibility for the recommendation to Defra or Welsh Government rests with the FVL. Support can be sought from notifiable-disease.portfolio@food.gov.uk at any time during the process.
The FBO must contact Approvals@food.gov.uk to request an application form for the relevant disease:
- Annex 25: Avian Influenza and Newcastle Disease
- Annex 29: Bluetongue
- Annexes 30: African Swine fever, Classical Swine fever, Swine Vesicular Disease, Rinderpest (Cattle plague), Pest Des Petis Ruminant (Sheep and Goat plague) and Food and Mouth Disease.
Upon completion by the FBO of part One of the “Application for Designation of a Slaughterhouse” and once the OV is satisfied that the abattoir has the necessary SOPs in place and meets the criteria for the level required, the OV should complete and sign Part Two of the aforementioned document. The FBO can then submit the application form to the approvals team Approvals@food.gov.uk
The approvals team will subsequently assign a FVL to each application. An on-site visit to the establishment by the relevant SDP representative, FVC or FVL to assess the procedures and SOPs needs to be considered on a case-by-case basis. For designations with different levels of compliance:
- Level 1: Compliant FBOs with experienced OVs might not need a specific visit, and the recommendation for the establishment to be designated can be made as a desktop exercise.
- Level 2 or new applications, might require a more in-depth assessment, and an on-site visit can be more appropriate. It is for the FVL to decide what the best approach is in each situation.
Once the FVL is satisfied that the SOPs are satisfactory and the establishment meets the requirements, Part Three should be signed and sent back to the Approvals team.
The establishment is then considered as pre-designated.
12.3 Designation process during a notifiable disease outbreak
Establishments which are not pre-designated at the time of the outbreak can apply for a designation. These shall go through the complete designation process as detailed in 12.1. Pre-designated establishments, although are not guaranteed an immediate designation, are likely to obtain their designation much quicker.
The FBO of pre-designated establishments will require the activation of the designation when an outbreak is declared. The FBO should ask the resident OV to verify that all the information included in the pre-designation form remains accurate, the SOPs are up to date, and the requirements will be complied with during the outbreak.
Once this verification is completed, the OV will recommend the re-activation of the designation to the Approvals Team in the FSA via email.
The approval team will liaise with a Defra representative for the signature of Part Four if the establishment is located in England, or to a Welsh Government representative if it is located in Wales.
Once the designation is activated by Defra/Welsh government, the Approvals team will send the FBO, copying in the ND portfolio, FVL, FVC, SDP manages and OV, the designation documents via email and will update the information in the Establishments and People database.
13. updated: [Enforcement during a Notifiable disease outbreak
13.1 Enforcement of licence requirements
13.2 Enforcement of Cleansing and Disinfection requirements
13.3 Designated establishments – breach of designation conditions
The LA is the enforcing authority for movement controls.
FSA staff are authorised to verify compliance with the conditions of the licence. Any suspected NC must be reported to the LA Trading Standards Department. The following parties should be also copied in for awareness:
- FVL / FVC
- APHA licensing (outbreak.licensing@apha.gov.uk)
- Defra (outbreakndccvetoperations@defra.gov.uk)
13.2 Enforcement of Cleansing and Disinfection requirements
Additional FSA checks are required to verify compliance with the Cleansing and Disinfection conditions attached to the licences.
In accordance with the Transport of Animals (Cleansing and Disinfection) (No 3) (England) (Wales) (2003) Order, Defra/WG can ask FSA staff to carry out 100% checks of C&D of crates, modules and vehicles used to:
- transport animals from a PZ or SZ
- transport animals to a slaughterhouse situated within a PZ or SZ
Where Cleansing and Disinfection is unsatisfactory, FSA officers must serve a notice on the person in charge of the vehicle and report the incident to the LA.
Reference: See Chapter 2.2 Section 5 on ‘Cleansing and Disinfection’.
13.3 Designated establishments – breach of designation conditions
If the OV or AO finds that the FBO is not meeting the conditions of the designation at pre-designation stage or when the designation is active, they should ask the FBO to address the issue promptly, if the issue is not resolved within the agreed deadline another request to take action can be sent in writing, noting this advice in the daybook.
If this approach does not resolve the issue, the OV should then write to the Approvals team and FVL/FVC suggesting in writing that the designation should be suspended or revoked, along with evidence of the breaches and actions taken].
14. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms.
Annex 1: Update: [REMOVED]
Annex 2: Update: [REMOVED]
Annex 3: Update: [REMOVED]
Annex 4: Updated BS112 – Licence
Annex 5: Updated BS15B – Notice
Annex 6: Updated EBL9 – Licence
Annex 7: Updated EBL7 – Submission form
Annex 8a: CS117 – TB/EBL FSA consumables for other red meat abattoirs form
Annex 8b: CS118 – TB/EBL FSA consumables for APHA contracted abattoirs
Annex 9: Updated Sample: TB24
Annex10: Updated Sample: TB24c
Annex 11: Updated Sample: TB16b
Annex 12: Update: [REMOVED]
Annex 14: Update: [REMOVED]
Annex 15a: Updated Sample: TB110 Reactor sampling and submission form
Annex 15b: Guidance for preparing protocols in contracted TB reactor slaughterhouses
Annex 17: Description of lesion template
Annex 18: Updated Sample: TB24h
Annex 20: FSA consumables requisition form - REMOVED
Annex 21: CS115 – DNA equipment form
Annex 22: Material for DNA analysis
Annex 23: Sample Despatch Process
Annex 24: Aujeszky’s Disease Training Note
Annex 25: Application for designation of slaughterhouses for Avian influenza or Newcastle Disease
Annex 26: Avian Influenza restricted meat: Communication lines for FBOs
Annex 27: Avian Influenza: Traceability of restricted meat
Annex 28: Specific licence – movement of poultry to slaughter from premises in a PZ or SZ
Annex 29: Application for designation of a slaughterhouse for Bluetongue
Annex 30: Updated: [REMOVED]
Annex 30A Updated: [Application for designation of a slaughterhouse for Exotic Diseases]
Annex 30B Updated: [Application for designation/authorisation of a cutting plant]
Annex 31a: FSA TB Ready check list
Annex 31b: TR558 - Private TB Reactor Slaughter/Direct Contacts (DCs) Arrangements Agreement Form (also to be used for private slaughter of Inconclusive Reactors)
Annex 32: Updated Sample: TB24c licence form
Revision log
Published: 25 January 2022
Last updated: 14 April 2025