Chapter 5.1 Residues: Veterinary Medicines Directorate (VMD) National Surveillance Scheme
Sections 1. Overview 2. Sampling 3. Suspect Substances, Animals and Carcases updated [4. Follow up samples] 5. Annexes
Sections
3. Suspect Substances, Animals and Carcases
1. Overview
In this section
1.1 Introduction
1.1.1 Statutory requirements
The UK has in place a statutory veterinary residue surveillance scheme in fulfilment of its obligations retained under the Official Controls Regulation 2017/625 and, Council Directive 96/22/EC.
This programme helps to ensure that consumers are protected against potentially harmful residues of veterinary medicines.
1.1.2 Co-ordination and collection
The Veterinary Medicines Directorate (VMD) is responsible for the co-ordination and management of the UK programme and for the management and operation of the National Surveillance Scheme (NSS) in GB.
The total number of samples required to fulfil GB’s obligation is determined annually by the VMD, who will then request samples from individual slaughterhouses.
The FSA undertakes the collection of samples from licensed slaughterhouses under contract to the VMD.
1.2 Legislation
1.2.1 Applicable legislation
The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 and The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) Regulations 2019 SI No. 569 (W 125) implement the requirements of Council Directives 96/22/EC.
The legislation requires targeted sampling for veterinary residues by trading partners. They lay down the frequency of sampling required for substances.
1.2.2 Sampling of suspect animals
The Directives also require sampling to be undertaken where the Official Veterinarian (OV) suspects or has evidence that animals have been treated with unauthorised substances or may contain residues of authorised substances above the Maximum Residue Limits (MRL). Casualty animals without any reference of treatments (and compliance with its withdrawals periods) in the food chain information (FCI) should be considered for testing if their condition is likely to have required treatment.
1.2.3 Authorisation
The authorisation certificate brings FSA staff within the definition of “authorised officer”. FSA staff must not undertake work for which they have not been authorised. If in doubt, please consult your ITL for advice.
1.2.4 Powers of the Authorised Officer (AO)
The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (England and Scotland) Regulations 2015 and Regulation 2019 (Wales) give FSA authorised officers:
- the power to detain and inspect animals prior to slaughter
- the power to detain animals for a further examination to be carried out and if necessary samples of tissues / fluids to be taken for analysis
- the power to detain the animal / carcase or group of animals / carcases until the results of the analysis is available
An AO has the power to take a sample from any animal, whether or not intended for human consumption.
1.3 FBO responsibility
1.3.1 Information on origin
Only animals for which full information of the farm submitting for slaughter or source (for example, market or collection centre) is available can be sampled. This information will be essential in tracing the owner, should further action requiring definite identification be necessary.
Slaughterhouse operators are required to keep such records on all animals and it is an offence not to do so.
Reference: The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 Regulation 31 (1) & The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) SI No. 569 (W 125) Regulations 2019 32 (1).
1.4 FSA role
1.4.1 OV responsibility
The OV must:
- ensure that only authorised FSA staff carry out sampling under their supervision
- ensure continuity of evidence when samples are collected, prepared, labelled, stored and despatched
- always obtain indisputable evidence for the origin of the animals sampled
- where the farm submitting for slaughter is unknown, determine the most recent origin by giving the name and status of the person supplying the animal to the slaughterhouse
- file a hardcopy or an electronic copy of the FCI accompanying the animal/batch, signed and dated by the OV, along the local copy of the RIM 1 form
- record in the daybook a confirmation that the above has been completed for the RIM samples (including RIM ref. numbers) taken on that day
- agree with the FBO a method for informing their representative of the collection of the samples after selecting the animals to be sampled and the residue it will be tested for. Some FBO may need to know this information for complying with customer’s requirements (for example, certain export markets)
- encourage the FBO to request from VMD and check the results for the RIM testing and to incorporate them as part of verification records of the HACCP for chemical hazards.
- agree witnessing of sampling by FBO representative
1.4.2 FSA duties
FSA staff must check that the FBO keeps source records according to the requirements of the regulations.
Reference: The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 Regulation 31 (1) & The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) SI No. 569 (W 125) Regulations 2019 32 (1).
1.4.3 Action if no or inadequate records
The AO must bring to the attention of plant management and the OV if no records of the farm submitting for slaughter are kept, or if the records are deficient.
The OV is to follow the hierarchy of enforcement, and:
- record any discussion with the FBO in the daybook
- confirm the deficiency in writing
- Note: a specimen letter (see annex 1) suitable for this purpose is included in this chapter
- keep a copy in the plant file
- enter details onto the ENF 11/5 (Enforcement Programme)
- make a further check of records within 28 days of delivery of the above letter
- if records are still inadequate, make a referral for investigation
Reference: See chapter 7 on ‘Enforcement’ for additional information.
Reference: See chapter 9 on ‘Forms.
1.4.4 Examples of inadequate records
Here are two examples of inadequate FBO records:
- name and address of producer / last owner not recorded
- FSA records indicate 20 animals were presented for ante-mortem inspection – FBO records only show 18 animals have been delivered
1.5 Cross contamination of samples
1.5.1 Purpose of National Surveillance Scheme (NSS)
The aim of the NSS is to detect whether authorised, unauthorised Veterinary Medicinal Products (VMPs), contaminants and prohibited substances are being used in food producing animals and that the conditions attached to authorised VMPs are being observed.
1.5.2 Follow-up action
The Directives and the Animals and Animal Products (Examination of Residues and Maximum Residue Limits) (England and Scotland) Regulations 2015 / (Wales) 2019 require follow-up action to be taken where:
- samples are found to contain residues of VMPs above the permitted maximum residue limit, or
- where residues of unauthorised substances have been detected
This could involve legal proceedings and consequently it is important that the instructions given in this chapter are followed.
1.5.3 On-farm investigation where a positive test result is recorded
When a sample tests non-compliant for a VMP, a Veterinary Officer (VO) from Animal and Plant Health Agency (APHA) visits the farm of origin of the sample to carry out an investigation as to how the residue in the sample may have occurred.
1.5.4 When FSA staff should not act as sampling officers
Laboratory analytical methods are extremely sensitive in identifying and measuring banned substances, down to less than 1 part per billion (the equivalent of a grain of sand in an area the size of an Olympic swimming pool). It is because of this sensitivity that sampling officers, who may have been exposed to certain medicinal products taken by them, by members of their family or by pets, should not take samples during the course of the treatment.
A list of those compounds that are, potentially, the most likely to cause problems is shown in the following table; some of these substances can also be prescribed to companion animals.
Sampling officers should not carry out any sampling during the treatment period.
| Type of Medication | Active Ingredients | May be used in the treatment of: |
|---|---|---|
| Inhaler (containing beta-agonists) |
|
Asthma |
| Skin creams (containing steroids) |
|
Skin conditions, such as dermatitis |
| Non-steroidal anti-inflammatory gels |
|
Pain relief, headaches, arthritis, fever |
| Other topical preparations |
|
Bacterial eye infections |
| Tablets |
|
Joint disease, auto-immune disease |
2. Sampling
In this section
2.3 Red meat: Collecting samples
2.4 Red meat: Collecting blood
2.5 Red meat: Collecting blood for serum analysis
2.6 Red meat: Packing blood samples for despatch
2.8 Poultry meat: Collecting blood for serum analysis
2.9 Poultry meat: Packing blood samples for despatch
2.1 Sampling programme
2.1.1 Sampling requests
Establishments will receive requests each quarter from VMD to collect samples from cattle, sheep, goats, pigs, horses, poultry and game for residue analysis. The residues in meat (RIM) 1 form is the Primary Sample Request form and contains pre-printed information on animals to be selected for sampling.
VMD will send RIM 1 forms to individual plants, unless a base plant has been designated by prior agreement.
Note: Samples must be collected exactly as described in the month specified on the RIM 1.
The animal(s) selected for sampling must fit the information on the RIM 1.
Reference: See annex 2 for a sample RIM 1 form.
2.1.2 When to collect
Samples required for a specified month must be collected during the month stated, spread as evenly as possible throughout the month and not collected on the same day.
Only one sample for a specified residue from animals from the same farm submitted for slaughter must be collected on the same day. More than one sample from the same producer can only be submitted in cases when the species required are not available from another producer.
2.1.3 RIM 1 reference number
Each RIM 1 form has a unique Sample Reference Number (RIM No), which must not be altered. The number must be quoted in any correspondence about the sample and recorded in the daybook on the day of sampling and, for cross-reference, in the copy of the associated FCI to be filed.
2.1.4 RIM labels
Each RIM 1 form is accompanied by an adhesive label printed with:
- the Sample Description
- Sample Reference Number (RIM No) and bar-code.
2.1.5 Samples from animals intended for human consumption
Samples must only be taken from animals, poultry or game intended for human consumption, and not from cull schemes.
2.1.6 Selection criteria
Animals must be selected taking into account the criteria that appear on the RIM 1 form and the criteria below:
- species, sex, age and farming system
- information about the producer
- indication of the use of pharmacologically active substances
- normal use of pharmacologically active substances in the particular production system
- other factors which may make it appropriate to ‘target’ certain animals for sampling; for example:
- animals selected for hormonal growth promoters should be well muscled and a good size for their age
- animals that are small for their age may be appropriate for sampling for antimicrobials since illness could affect their growth, and therefore they are more likely to have been treated
Instructions on the sampling of ‘suspect’ animals can be found in section 3 on ‘Suspect substances, animals and carcases’ in part 1.
2.1.7 Identification of animals
Animals suitable for sampling are to be individually identified and/or clearly marked before slaughter. FBO electronic ID systems are also suitable for the tracing of the movement of an animal through the process In the absence of an electronic system, the identification of the animal must be preserved at flaying or robustly correlated with the kill number by using one of the following methods:
- attach a talisman tag
- apply a cut mark
- attach a detained tag
- note the slap mark / tattoo
- using a record correlating kill number and animal identification number (for example, ear tag). Using a gap in the line may help in keeping the correlation through the processing line.
- Both kill number and animal ID should be recorded on the RIM 1 form
Note: In the case of poultry, it is sufficient to identify the batch from which the sample(s) are to be taken.
2.1.8 Exception to identification of animals pre-slaughter
Where an animal selected in the lairage from the specific group of sheep fails to produce the required quantity of urine, the sampling officer may select another animal which has a full bladder from the same group of animals.
The sampling officer must ensure that the animal can be traced back to the farm or market of origin.
2.1.9 Sample security and continuity of evidence
The results of analyses for all substances could lead to legal proceedings. It is important that there is continuity of evidence; therefore, samples must be accurately identified and secured in a FSA freezer.
Where there is no FSA freezer available in the establishment, alternative arrangements for the storage of samples will need to be made in agreement with the FSA local management team. FSA SLA and Contracts team should be notified of this agreement.
The names of all AOs involved in collecting or handling samples must be recorded in the daybook. The name and signature of the sampling officer must be the same as that on the RIM 1 form and the tamperproof bag.
2.1.10 Completing the summary worksheet
Record the following information on the Summary Worksheet:
- date of collection
- date of despatch
- consignment note number
2.1.11 Sampling not possible
Where a sample collection fails due to insufficient material or where sampling is not possible (for example, due to plant closure, killing pattern or availability of species requested), the OV is to:
- If a sample is temporarily unavailable, this can be taken in the next month provided it is in the same sample quarter (for example a July sample can be taken in September)
- complete the RIM 1 form remarks box, giving the reasons why the sample cannot be taken and
- return RIM 1 form to:
Veterinary Medicines Directorate
Residue Section
Woodham Lane
New Haw
Surrey
KT15 3LS
Send an email to the SLA and Contracts Team (sla.contracts@food.gov.uk), explaining why the sample was not collected.
Note: Due to health and safety considerations, poultry serum samples are not to be taken from unstunned birds in Halal establishments. The remarks box of the RIM 1 should be completed accordingly in the event that any such sampling requests are received by a plant.
2.2 Sampling equipment
2.2.1 Use of containers
It is important that only the specified sampling containers are used, as failure to do so may result in the sample being rejected by the laboratory as unassailable.
2.2.2 Supplies
VMD will supply:
- RIM 1 forms
- adhesive labels
- summary worksheets
- sampling equipment
- tamperproof bags
Maintain sufficient supplies of polystyrene boxes, outer cartons and Freezella packs at the slaughterhouse.
Note: The laboratory will return RIM boxes after use to Topspeed for one to one exchange on the next collection.
If a replacement box is not left at the point of collection, please email rim@topspeedcouriers.co.uk immediately.
2.2.3 Sampling equipment orders
Sampling equipment can be re-ordered by emailing:
2.3 Red meat: Collecting samples
2.3.1 Samples to collect
Kidney, kidney fat, liver, muscle, blood and urine must be collected from the identified, marked carcases that have been inspected and passed as fit for human consumption. The quantity of material collected from each species must be that specified as in the table below.
Samples to collect
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Kidney | Sheep / goats | At the post-mortem inspection point | A pair of kidneys | Sealable plastic bag |
| Kidney | Pigs | At the post-mortem inspection point | One whole kidney | Sealable plastic bag |
| Kidney | Cattle / horses | At the post-mortem inspection point | A portion of kidney; at least 100g taken from one pole so as to exclude pelvic tissue | Sealable plastic bag |
| Kidney | Calves | At the post-mortem inspection point | A pair of kidneys | Sealable plastic bag |
| Kidney fat | Cattle / sheep / goats / pigs | At the post-mortem inspection point | At least 50g of kidney fat | Sealable plastic bag |
| Liver | Cattle / sheep / goats / pigs / horses | At the post-mortem inspection point | At least 100g of liver | Sealable plastic bag |
| Muscle | Cattle / sheep / goats / pigs / horses | At the carcase inspection point | At least 200g of muscle from the diaphragm region of the animal | Sealable plastic bag |
| Urine | Cattle / sheep / pigs | After removal from carcase by incision into the bladder | At least 50ml | 80ml pot then sealable plastic bag |
2.3.2 Special surveys
For specific surveys, sample requests may require muscle and kidney samples to be taken from the same animal. Details will be printed on the RIM 1 form which will be marked ‘Special Survey’.
2.3.3 After sampling
Immediately after collection, the container or bag must be correctly sealed to avoid leakage, and placed into a tamperproof bag with the absorbent pad.
Reference: See the topic ‘Tamperproof bags’ in this section for additional information.
2.4 Red meat: Collecting blood
2.4.1 When and where to collect
Collect blood for serum samples and plasma analysis from the identified, marked carcase. This should be done directly at the sticking point, into the plastic vending cup provided and after the initial flow of blood has slowed.
2.4.2 Alternative collection site
Where collection at the sticking point poses a potential risk to the AO, for example, from carcase kicking, blood should be taken from the heart on the pluck line into the plastic vending cup provided.
A small incision can be made into one of the four chambers of the heart and blood carefully poured into the cup.
2.4.3 Sample handling
These samples must be:
- packaged according to the instructions in this topic
- despatched separately from other samples
- despatched on the same day of collection for bovine animals not requiring a bovine spongiform encephalopathy (BSE) test
- despatched on the day following collection for bovine animals requiring a BSE test, after receipt of a negative test result
Reference: See topic 2.14 on ‘Packaging and despatch of samples’ in part 1 for additional information.
Note: This will require that the courier is booked prior to taking the sample.
Caution:
- Samples can be refrigerated or kept in a cool dark place until collected by Topspeed.
- Samples must not be frozen.
- Please ensure that you place 2 unfrozen Freezella packs in the box. The polystyrene casing may be chilled before use.
- Keep box out of direct sunlight.
- Despatch Monday to Thursday only.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Blood (serum) | Cattle / horses |
At the sticking point or pluck point (heart) Reference: See topic 2.5 on ‘Collecting blood for serum analysis’ in part 1 for additional information |
At least 30ml | 3 x Sarstedt blood tubes then into absorbent wallet, keeping tubes upright |
| Blood (plasma) | Cattle / horses |
at the sticking point or pluck point (heart) Reference: See topic 2.5 on ‘Collecting blood for plasma analysis’ in part 1 for additional information |
At least 75ml | 2 x Li-heparin LH / 25ml monovette then absorbent wallet, keeping tubes upright |
2.5 Red meat: Collecting blood for serum and plasma analysis
2.5.1 Serum analysis
You must follow the correct procedure for collection of blood for serum analysis as described in the table below:
| Step | Action |
|---|---|
| 1 | Collect at least 50ml of blood into the plastic vending cup provided for immediate transfer into 3 x 10ml serum tubes. |
| 2 | Remove the screw cap on the top of the serum tube ensuring that the beads are in the bottom of the tube. |
| 3 |
Pour the blood into the tubes, filling to the line below the threaded top. Caution: Do not overfill or some beads may float to the top and be lost. The beads are coated in a substance that acts as a clotting activator to ensure that the blood clots and the serum becomes separated. |
| 4 | Replace the screw cap on each tube. |
| 5 |
Invert each tube gently 4-5 times to ensure the blood is mixed with the beads. Note: THE TUBE SHOULD NOT BE VIOLENTLY SHAKEN; doing so may cause haemolysis and the sample would therefore be deemed unassailable by the laboratory. |
| 6 | Write the RIM numbers on each tube in the space marked ‘Ref No’. Keep the test tubes stored upright in the four bay absorbent wallet and in a cool place (preferably in a refrigerator) prior to despatch. Each wallet can accommodate one sample of three tubes. |
| 7 | When ready for despatch, place the wallet inside the tamperproof bag. |
| 8 |
Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. Place the tamperproof bag securely inside the polystyrene box. Note: Do not tape the tamperproof bag to the inside of the polystyrene box. |
2.5.2 Plasma analysis
You must follow the correct procedure for collection of blood for plasma analysis as described in the table below:
| Step | Action |
|---|---|
| 1 | The blood should be immediately transferred from the plastic vending cup into 2 x 25ml heparin syringes. |
| 2 | Remove the small orange cap on the top of the syringe and pull the plunger fully back ensuring that the heparin coated beads fall to the bottom. |
| 3 |
Unscrew the lid and pour the blood into the syringe, filling to the line below the threaded top. Caution: Do not overfill or some beads may float to the top and be lost. The beads are coated with the anti-coagulant heparin and are essential to ensure the blood does not clot. |
| 4 | Replace the screw cap and the small orange cap. Break off the plunger at the base of the tube. |
| 5 |
Invert the syringe gently 4-5 times to ensure the blood is mixed with the anti-coagulant. Note: THE TUBE SHOULD NOT BE VIOLENTLY SHAKEN; doing so may cause haemolysis and the sample would therefore be deemed unassailable by the laboratory. |
| 6 | Carefully place the syringes into the tamperproof bag. |
| 7 | Label the tamperproof bag and follow instructions on the bag to seal. |
| 8 |
Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. Place the tamperproof bag securely inside the polystyrene box. Note: Do not tape the tamperproof bag to the inside of the polystyrene box. |
2.6 Red meat: Packing blood samples for despatch
2.6.1 Packing serum and plasma samples for despatch
Samples are to be packed for despatch as follows:
| Step | Action |
|---|---|
| 1 | Place the tamperproof bag containing the samples securely into the polystyrene box. |
| 2 | Seal the polystyrene box. |
| 3 | Place the top two copies of RIM 1 form on top of the polystyrene lid. |
| 4 | Place polystyrene box in cardboard outer carton. |
| 5 | Apply the adhesive address label provided by the carrier to the outer carton across the box flaps. Ensure all other labels on the carton are removed. |
| 6 | Mark the box with ‘This Way Up’ to ensure careful handling. |
Caution: RIM 1 forms must not be sent separately from the samples to which they relate.
2.7 Poultry meat samples
2.7.1 Samples to collect
Samples of liver and muscle must be taken from identified birds that have been inspected and passed as fit for human consumption. The following table gives details of the types of samples and the quantity required.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Liver | Chickens and ducks | Evisceration inspection point | 50g pooled from at least 6 birds | Sealable plastic bag |
| Liver | Turkeys | Evisceration inspection point | 50g pooled from at least 2 birds | Sealable plastic bag |
| Breast muscle | Chickens, ducks and geese | Taken off line to enable muscle to be cut off | 200g from 1 bird | Sealable plastic bag |
| Breast muscle | Turkeys | Taken off line to enable muscle to be cut off | 200g from 1 bird. Sample can be taken from more than one bird in a co-located cutting plant where there is sufficient traceability | Sealable plastic bag |
2.7.2 Special surveys
For specific surveys, sample requests may require muscle and liver samples to be taken from the same group of birds. Details will be printed on the RIM 1 form which will be marked ‘Special Survey’. Tissues should be pooled from the required number of birds as indicated in the table below:
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Liver | Chickens (Broilers) | Evisceration inspection point | 50g liver pooled from at least 6 birds | Sealable plastic bag |
| Muscle | Chickens (Broilers) | Taken off line to enable muscle to be cut off | 100g muscle pooled from at least 6 birds | Sealable plastic bag |
2.7.3 After sampling
Tissue samples must be placed immediately into the sealable plastic bag provided, then into a tamperproof bag.
2.8 Poultry meat: Collecting blood for serum analysis
2.8.1 When and where to collect
Collect blood for serum analysis from at least six birds from the same flock. Only sample birds from single sheds, do not sample birds from mixed sheds. This should be done shortly after neck cutting, into the plastic vending cup provided.
2.8.2 Sample handling
These samples must be:
- packaged according to the instructions in this topic
- despatched separately from other samples
- despatched on the same day of collection
Reference: See topic 2.14 on ‘Packaging and despatch of samples’ in part 1 for additional information.
Note: This will require that the courier is booked prior to taking the sample.
Caution:
- Samples should be refrigerated but must not be frozen.
- Please ensure that you place 2 unfrozen Freezella packs in the box.
- The polystyrene box may be chilled before use.
- Keep box out of direct sunlight.
- Despatch Monday to Thursday only.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Blood (serum) | Chickens, ducks and turkeys |
Shortly after cutting point Reference: see topic 2.8 on ‘Collecting blood for serum analysis’ in part 1 for additional information |
30ml from at least 6 birds | 3 x Sarstedt blood tubes |
2.8.3 Serum analysis
Follow the procedure for the collection of blood for serum analysis as described in the table below.
Blood can be safely collected once birds have ceased swinging after cutting.
| Step | Action |
|---|---|
| 1 |
Using the plastic vending cup provided, collect at least 30ml of blood from at least six birds in the same group. Note: Blood can coagulate quickly so collect enough for one tube at a time. |
| 2 |
Remove the screw caps from the tops of the three Sarstedt serum tubes ensuring that the beads are in the bottom of the tube. Note: These beads are coated in a substance that acts as a clotting activator to ensure the blood clots and the serum becomes separated. |
| 3 |
Pour the blood into each tube, filling to the line below the threaded top. Caution: Do not overfill or some beads may float to the top and be lost. |
| 4 | Replace the screw cap on each tube. |
| 5 |
Invert each tube gently 4-5 times to ensure the blood is mixed with the beads. Note: THE TUBE SHOULD NOT BE VIOLENTLY SHAKEN; doing so may cause haemolysis making the sample unassailable. |
| 6 | Write the RIM number on each tube in the space marked ‘Ref No’. Keep the tubes stored upright in the four-bay absorbent wallet and in a cool place (a dark place or refrigerator) prior to despatch. Each wallet should contain one sample only. One sample = 3 tubes from 6 birds from the same batch. |
| 7 | When ready for despatch, place the wallet inside the tamperproof bag. |
| 8 | Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. Place the tamperproof bag securely inside the polystyrene box. |
2.9 Poultry meat: Packing blood samples for despatch
2.9.1 Packing serum samples for despatch
Samples are to be packed for despatch following the steps detailed below:
| Step | Action |
|---|---|
| 1 |
Place the tamperproof bag securely inside of the polystyrene box ensuring the tubes are not free to move around. Note: Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. |
| 2 | Seal the polystyrene box. |
| 3 |
Place the two copies of the RIM 1 form on top of the polystyrene lid. Caution: RIM 1 forms must not be sent separately from the samples to which they relate. |
| 4 | Place polystyrene box in cardboard outer carton. |
| 5 | Apply the adhesive address label provided by the carrier to the outer carton across the box flaps. Ensure all other labels on the carton are removed. |
| 6 | Mark the box with ‘This Way Up’ to ensure careful handling. |
2.10 Game samples
2.10.1 Definition of farmed game
Farmed game is animals which are not domestic but have been reared within a restricted area.
2.10.2 Farmed game samples
Samples will be requested from deer, partridge, pheasant, red grouse, quail and breeding boar.
2.10.3 Definition of wild game
Wild game is animals that are hunted and shot in the wild for human consumption.
2.10.4 Wild game samples
Samples will be requested from deer, pheasant and partridge.
2.10.5 Large game samples to collect
Samples of kidney, kidney fat, liver and muscle must be taken from deer carcases which have been passed fit for human consumption and for which the origin or source can be identified.
2.10.6 Small game samples to collect
Samples of muscle consist of an entire oven ready carcase of a bird and must be taken from a batch of birds which have been passed as fit for human consumption and for which the origin or source can be identified.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Kidney | Deer | After post-mortem inspection | A whole kidney | Sealable plastic bag |
| Kidney Fat | Deer | After post-mortem inspection | At least 50g of kidney fat | Sealable plastic bag |
| Liver | Deer, partridge, pheasant, red grouse | After post-mortem inspection | At least 100g liver | Sealable plastic bag |
| Muscle | Deer | After post-mortem inspection | At least 50g of diaphragm muscle | Sealable plastic bag |
| Muscle | Partridge, pheasant, quail | Inspection point at the end of the line | An oven ready carcase | Sealable plastic bag |
2.10.7 After sampling
Tissue samples must be placed immediately into the sealable plastic bag provided, and then into a completed tamperproof bag.
2.11 Completing the RIM form
2.11.1 Details to record
The following details must be fully recorded on the RIM 1 form:
- sex and age of animal sampled
- identification of the animal sampled; this enables the AO to cross check with the slaughter records to establish the source of the animal – types of identification:
- ear tag number for cattle, sheep and goats
- slap mark, ear tag or tattoo for pigs
- farm address for poultry
- for cattle the breed of animal sampled (including cross breeds)
- whether the animal is from organic production
- obtain from the slaughterhouse or game handling
- establishment records:
- the farm submitting for slaughter, or if unavailable, the source of animals sampled such as market and lot number, and
- the name and status of the person supplying the animal to the slaughterhouse
- any extra information, for example, kill numbers, which may help in any subsequent tracing
- the date of collection of the sample
- the date of despatch of the sample
- name and designation of collecting officer; this must be the same as on the tamperproof bag
- carrier consignment reference number
Note: If you make an error when recording any of the above data on the RIM 1 form, or anything is unclear that might need going over again, cross through the entry and enter the correct details, then initial the change. Any necessary amendments must be made before the copies of the RIM 1 form are separated. Do not use correction fluid. The original ‘incorrect’ entry must be legible.
2.12 Tamperproof bags
2.12.1 Use of tamperproof bags
Tamperproof bags are an important stage in maintaining continuity of evidence, since the detection of residues in a sample may result in an investigation and potential legal proceedings.
2.12.2 Sealing
Tamperproof bags should be sealed:
- remove the blue strip
- press the orange strip down over the glue firmly
- check the bag is sealed properly before labelling
- check the bag has been signed by the sampling officer and witnessed by the FBO representative
Note: The sampling officer must be the same person that signed the RIM 1 form.
Wherever possible this should be done in the presence of the FBO or person responsible for the source of the sample.
2.12.3 How to label tamperproof bags
Labelling must be carried out immediately after each sample is taken. As far as reasonably possible, completion of labelling should be done in the continued presence of the FBO or person responsible for the source of the sample.
| Step | Action |
|---|---|
| 1 |
Attach the white bar-coded sample label to the front of the bag in the marked space before putting the sample in the tamperproof bag. Caution: Ensure that the bar code label is not creased or otherwise damaged whilst sticking it to the bag. |
| 2 |
Sign and date in the space provided (must be the same person that signed the RIM 1 form). Note: Use only ballpoint to write on the bag. |
| 3 |
The owner or person responsible should also sign and date the tamperproof bag, confirming that the information recorded on it is correct. Note: Refusal to sign should be noted on the front of the bag. |
| 4 | Place the sample in the tamperproof bag and seal by removing the blue strip. |
| 5 | Once sealed, the bag must not be opened until the sample has reached the laboratory. |
| 6 | Record the names of all authorised staff involved in collecting samples in the daybook. |
2.13 Storage of samples
2.13.1 Chilling and freezing
Once the sample has been sealed in the tamperproof bag and the bag has been labelled, samples must be kept chilled from the time of collection and during preparation. With the exception of blood collected for serum and plasma analysis, samples should then be hard frozen on the day of collection.
Note: If necessary, samples must be kept cool by means of insulated containers containing frozen Freezella packs / Biotherm dry ice shippers.
Samples must be frozen for a minimum of 48 hours. Maintain the samples hard frozen until despatch.
Note: The freezer compartment of a domestic refrigerator is not adequate for hard freezing samples.
2.13.2 Freezing of samples prior to despatch
When freezing samples:
- in large chest freezers:
- place samples in the polystyrene box
- leave the lid off the polystyrene box and freeze the whole box containing samples
OR
- in small freezers:
- leave samples in freezer until ready for despatch, then place in polystyrene box
To avoid samples defrosting prior to testing do not over fill the box, and send two boxes if necessary.
2.13.3 Storage
With exception of serum and plasma, all prepared samples must be stored prior to despatch:
- in secure, dedicated FSA freezers
- at a temperature between -15°C and -20°C
Samples must not be allowed to thaw once frozen.
2.14 Packing despatch of samples
2.14.1 Packing samples for despatch
Note: These instructions apply to all surveillance samples except serum and plasma.
Reference: See topics 2.5 and 2.8 on ‘Collecting blood for serum analysis’ and 2.6 and 2.9 on ‘Collecting blood for plasma analysis’ in part 1 for additional information.
Samples are to be packed for despatch as follows:
| Step | Action |
|---|---|
| 1 | Place a frozen Freezella pack / Biotherm dry ice shipper at the base of the polystyrene box. |
| 2 | Place the frozen samples in the box. |
| 3 | Place the second Freezella pack / Biotherm dry ice shippers on top of samples. |
| 4 | Seal the polystyrene box. |
| 5 | Place the top two copies of RIM 1 form on top of the polystyrene lid. |
| 6 | Place polystyrene box in cardboard outer carton. |
Note: To prevent movement, small samples should be wrapped with the Freezella pack / Biotherm dry ice shipper in insulating material before being placed into a polystyrene box.
In periods of hot weather, add an extra Freezella pack / Biotherm dry ice shipper to avoid thawing.
2.14.2 Labelling cardboard outer cartons
Boxes are to be labelled as follows:
| Step | Action |
|---|---|
| 1 | Apply the adhesive address label provided by the carrier to the outer carton across the box flaps. |
| 2 | Mark the box with ‘this way up’ to ensure careful handling. |
2.14.3 Despatching samples
Samples are to be despatched to the laboratory after a minimum of 48 hours hard freezing. Despatch must be no more than five working days after collection, including the day of collection, as this can lead to sample deterioration, and delay on-farm investigation of positives that may result. Where an FBO only operates one day a week, this can be extended to six working days.
The despatch process is detailed in Annex 17.
Note: Serum and plasma samples from bovine animals not requiring a BSE test must be despatched on the same day as collection.
Note: Serum and plasma samples from bovine animals requiring a BSE test must be despatched on the day following receipt of a negative test result.
Note: Samples must not be sent on Fridays or on days preceding public holidays.
2.14.4 Despatch of all residue samples
FSA officers at slaughterhouses must send all red meat, poultry meat, game meat and suspect samples to:
Residues Statutory Programme
Fera Science Ltd
Room 50G30
Sand Hutton
York
YO41 1LZ
2.14.5 Despatch failure
Should despatch fail, you must make an attempt to rearrange despatch:
- ensure the samples have not thawed
- follow points 1 to 3 in sub-topic 2.14.3 on ‘Despatching samples’ in part 1, explaining the reasons behind the failure
Then telephone VMD
(01932 338329) to explain the failure and what follow up action has been taken.
VMD will contact the laboratory to advise them to expect samples arriving with incorrectly dated paperwork.
2.14.6 Retention of documents
After completion of each month’s sampling, the completed Summary Worksheet and RIM 1 form should be retained in plant for 1 year.
2.14.7 Complaints procedure
Should Topspeed fail to collect samples within the agreed timeframe, contact the SLA and Contracts Team by phone (access contact details in chapter 1 on ‘Introduction’), who will escalate the failure to Topspeed headquarters.
2.15 Actions on positive samples
In case of positive results for routine RIM samples, the action to be taken by the OV will depend on the Risk Management Advice provided by the Incidents team and shared subsequently with Field Operations. Before a decision is taken regarding the affected carcase/product, the OV should liaise with the relevant Field Veterinary Coordinator (FVC)/Field Veterinary Leader (FVL).
3. Suspect Substances, Animals and Carcases
3.4 Sampling and despatch procedures for suspect live animals and suspect carcases
3.1 Suspicion of unauthorised substances: Suspected use of authorised veterinary medicines above the MRL and contaminants
3.1.1 Sampling of suspect animals
The legislation requires sampling to be undertaken where the OV suspects or has evidence that animals have been treated with unauthorised substances or may contain residues of authorised substances above the MRL.
3.1.2 Procedures
This topic covers the action to be taken when there are grounds to suspect that a carcase or live animal contains:
- prohibited substances
- unauthorised substances
- residues of an authorised substance at concentrations above the MRL
- a contaminant above the threshold level (see annexes for signs that would give rise to suspicion)
- contaminants in compounds of concern
| Term used | Meaning |
|---|---|
| Prohibited substance | Means any beta-agonist, hormonal or thyrostatic substance, and those specified in Table 2 GB MRL www.gov.uk/guidance/maximum-residues-limits-mrl. |
| Unauthorised substance | Means any substance not included in Table 1 GB MRL. |
| Authorised substance | Means a substance specified in Table 1 GB MRL. |
The following table contains a list of those substances contained in Table 2 of Commission Regulation (EU) 37/2010.
| Annex IV Substances |
|---|
| Aristolochia ssp and preparations thereof |
| Chloramphenicol |
| Chloroform |
| Chlorpromazine |
| Colchicine |
| Dapsone |
| Dimetridazole |
| Metronidazole |
| Nitrofurans (including Furazolidone) |
| Ronidazole |
3.2 Suspect live animals
3.2.1 Inspection of animals under Regulation 20
Under the Residues Regulations, AOs have the power to detain an animal or group of animals for inspection to ascertain whether they have been treated with an unauthorised substance.
Reference: The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 Regulation 20 and The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) Regulations 2019 SI No. 569.
3.2.2 Suspicion of illegal substances
If the AO suspects that an animal has been illegally treated with an unauthorised substance you must notify the OV immediately of your suspicions.
The OV should serve a Form E notice if the FBO or slaughterhouse staff do not co-operate in allowing the inspection to take place.
Reference: See annex 9 for a sample Form E notice.
3.2.3 Signs of hormone growth promoters: live animal
The following signs in a live animal may indicate the illegal use of hormone growth promoters:
- secondary sexual characteristics:
- crest development
- teat development
- restlessness; animals do not settle in the lairage, mill around
- behavioural changes:
- mounting
- aggression
- an even level of finish in a group of cattle of different breed / types
3.2.4 Signs of beta-agonist: live animal
The following signs in a live animal may indicate the illegal use of beta–agonist growth promoters:
- good conformation with little fat
- hyperaesthesia and tachycardia may be present
3.2.5 Result of inspection
If after carrying out the inspection, the OV is satisfied that the animal has not been treated with an unauthorised substance, you should lift the Form E notice by serving a Form F notice on the owner.
Reference: See annex 10 for a sample Form F notice.
3.2.6 Examination of animals under Regulation 21
If as a result of the inspection referred to above, the OV still suspects that the animal or group of animals may contain an unauthorised substance, a Form G notice should be served on the owner of the animal(s) to detain them for further examination. This notice will remain in place until the results of the examination, including analysis of samples, are known.
Reference: See annex 11 for a sample Form G notice.
The OV should make a detailed examination of the animals. This must include checking for evidence of implants and other signs which could indicate the use of unauthorised substances.
3.2.7 Samples to take
Where an implant is not found but the OV is suspicious of the illegal use of other prohibited substances, you should take the following samples:
- hormones - take blood and either urine or faeces
- beta-agonists - take urine
If other unauthorised substances are suspected then advice should be sought from the VMD or veterinary auditor (VA) (Residues) on the appropriate samples to be collected.
3.2.8 Slaughter of detained animals
Animals must not be held in the lairage for more than 48 hours. As it is unlikely that the results of analysis on the sample will be available, the animal should be slaughtered and the carcase and offals detained under Regulation 34(2).
Reference: See topic 3.3 on ‘Suspect carcases’ in part 1 for additional information.
3.2.9 Signs of a suspect substance in live animals
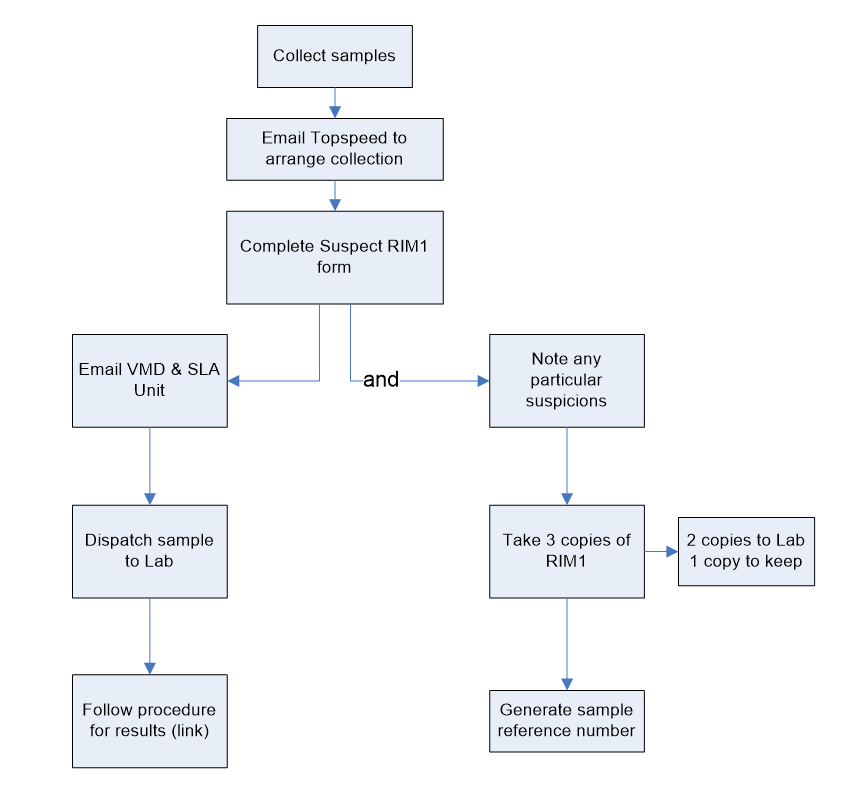
After collection of samples send an e-mail to Topspeed to arrange Collection and complete Suspect RIM1 Form. Upon completion:
Action 1: Notify by email to VMD and SLA Unit, then dispatch sample to the lab, and follow procedure for results.
Action 2: Note any particular suspicions observed in the RIM forms. Send 2 Copies of the completed RIM forms to the lab, keeping the third one on site. Finally, generate a sample reference number.
3.3 Suspect carcases
3.3.1 Detention under Regulation 34(2)
The OV has the power under Regulation 20(2)a and 21(2)b of the Residues Regulations to detain and sample any carcase if they suspect the illegal use of unauthorised substances, or if they suspect that an authorised substance in excess of the MRL may be present in the animal concerned.
The OV must serve Form C on the owner or person in charge of carcase(s). This will remain in force until investigations are completed.
Reference: See annex 7 for a sample Form C notice.
3.3.2 Signs of authorised substances above the MRL
The following signs may raise concerns that a carcase contains authorised substances, such as veterinary medicines, above the MRL:
- signs of recent illness, particularly:
- mastitis (signs may be seen prior to removal of udder)
- lameness / arthritis
- pleurisy / pneumonia
- poor condition
- metritis (signs may be seen prior to evisceration or during inspection of the offal)
- emergency slaughter animals
- injection sites, particularly:
- bruising / discoloration
- smell (especially with tetracyclines)
- swellings
Note: For sites with an oily adjuvant, consider illegal hormone treatment.
The conditions listed above should prompt evaluation regarding whether animals have received recent treatment and whether the withdrawal period has been properly observed by the farmer before sending them for slaughter. See summary table in section 3.4.5.
OVs working at establishments that handle Emergency Slaughter (ES) on a routine basis should prioritize sampling those animals using the table ”Summary of conditions to be considered for suspect sampling” in section 3.4.5. OVs should aim to test two carcasses from ES per year for the presence of non-steroidal anti-inflammatory drugs (NSAIDs) as a priority and/or antimicrobials where relevant. Additional residue samples can be taken for non-ES if deemed appropriate.
3.3.3 Signs of hormone abuse: carcases
The following signs may indicate the illegal use of hormones in a carcase:
- presence of implants or pellets
- injection site
- if detected and an oily adjuvant is present, or when the site is in an unusual place, the possibility of the presence of injectable hormones should be considered
3.3.4 Signs of beta-agonists: carcases
The following signs may indicate the illegal use of beta-agonists in a carcase:
- good conformation with little fat
- flaccidity of the trachea
3.3.5 Evidence of implants
If there is evidence of an implant in the ear, you must detain the carcase and submit the whole ear containing the implant for analysis.
If the implant is discovered in any other part of the carcase, then the surrounding tissue should be excised with the implant and submitted for analysis. Do not attempt to dissect the implant out before despatch.
3.3.6 Types of implant
The table below lists the types of hormonal growth promoter implants which may be found:
| Name of Product | Type of Implant | Active Ingredients | Withdrawal period | Sex of animal used in |
|---|---|---|---|---|
| Compudose 200 | Cylinder |
17 beta-oestradiol 24mg |
0 | Steers |
| Compudose 365 | Cylinder |
17 beta-oestradiol 45mg |
0 | Steers |
| Finaplix | 15 yellow pellets | Trenbolone 140mg | 60 days | All |
| Forplix* | No description available |
Trenbolone 140mg Zeranol 36mg |
Never licensed in the UK | * |
| Implixa BF | 10 white pellets |
Testosterone 200mg oestradiol 20mg |
90 days | Females |
| Implixa BM | 10 white pellets |
Progesterone 200mg oestradiol 20mg |
90 days | Males |
| Ralgro | 3 white pellets | Zeranol 36mg | 70 days | All |
| Revlor | 8 yellow pellets |
Trenbolone 140mg oestradiol 20mg |
60 days | Steers, male and female veal calves |
| Synovex C | 4 yellow pellets |
Progesterone 100mg oestradiol benzoate 10mg |
0 | Males |
| Synovex H | 8 white pellets |
Testosterone 200mg oestradiol 20mg |
0 | Females |
| Synovex S | 8 yellow pellets |
Progesterone 200mg oestradiol 20mg |
0 | Males |
3.4 Sampling and despatch procedures for suspect live animals and suspect carcases
3.4.1 Sampling equipment
If an animal needs to be tested as a suspect, use the Suspect sampling kit provided by VMD.In the case that more than on sample is required, use the sampling kit provided for routine requests and replenish by emailing the SLA and Contracts Team (sla.contracts@food.gov.uk).
A consolidated order will be sent to VMD each Friday and the kit will be despatched to the specified plant.
3.4.2 Suspect RIM1 form
Complete the RIM 1 form marked ‘SUSPECT’ (see example at annex 15). A copy of the ‘Suspect’ RIM 1 form can be obtained by contacting the SLA and Contracts Team or a sample form can be found at annex 15.
If a particular hormone or unauthorised substance is suspected note it on the form. Take three copies of the completed form:
- two for despatch to the laboratory
- one to be retained in the plant file for 12 months from the date of sampling
3.4.3 Sample reference number to use
The OV should generate their own sample reference number using the following:
- slaughterhouse approval number
- the last two digits of the year
- a sequential number (approval / year / number)
One sample number per sample sent must be generated.
Note: Record the numbers used in the daybook.
3.4.4 Reporting suspicious cases
When animals or carcases are detained and sampled under the Residues Regulations, an on-farm investigation may be required. As a result, the OV must inform:
- the VMD via email using the following address: residues@vmd.gov.uk
- the SLA and Contracts Team via email (access contact details in chapter 1 on ‘Introduction’)
| Step | Action |
|---|---|
| 1 |
Detain animal / carcase for examination. Note: All samples MUST be collected, prepared and despatched in accordance with the procedures covered previously in this chapter. |
| 2 | Collect samples as detailed in section 2 on ‘Sampling’ of part 1 with the exception of hard freezing. |
| 3 | Arrange collection by Topspeed using the process at Annex 18. |
| 4 | Complete SUSPECT RIM 1 documentation. |
| 5 | Email VMD and the SLA and Contracts Team. They will alert the laboratory and ensure that the sample is analysed as soon as possible after arrival. |
| 6 | Despatch sample to the laboratory – Residues Statutory Programme, Fera Science Ltd. |
Process:
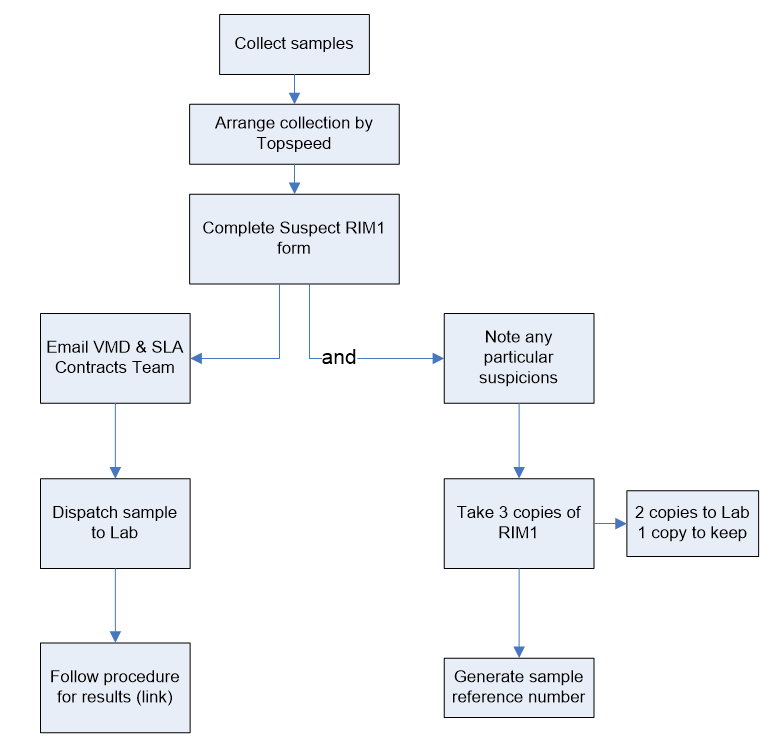
After collection of samples send an e-mail to Topspeed to arrange Collection and complete Suspect RIM1 Form. Upon completion:
Action 1: Notify by email to VMD and SLA Unit , then dispatch sample to the lab and follow procedure for results.
Action 2: Note any particular suspicions observed in the RIM forms. Send 2 Copies of the completed RIM forms to the lab, keeping the third one on site. Finally, generate a sample reference number.
3.4.5 Samples required
A list of the types of analyses and the samples required is given in the following table. For advice on the type of sample to collect for authorised substances not listed, you should contact the VA (Residues).
Example: For antimicrobial or sulphonamide analysis, a kidney sample should be collected.
Samples from carcases
| Analyses | Species | Sample Type |
|---|---|---|
| Antimicrobials | Cattle, sheep, pigs, horses, deer | Kidney |
| Antimicrobials | Poultry | Muscle |
| Table 2** | Cattle, sheep, pigs, deer, horses | Kidney |
| Table 2** | Pheasant, partridge, poultry, deer | Muscle |
| Sulphonamides | Cattle, sheep, pigs, horses | Kidney |
| Sulphonamides | Poultry | Muscle |
| Quinolones / fluoroquinolones | Poultry | Muscle |
| Tetracyclines | Poultry | Muscle |
| Thiamphenicol | Poultry | Muscle |
| Altrenogest | Pigs | Kidney fat |
| Metals | Cattle, sheep, pigs, horses | Kidney |
| Metals | Poultry | Liver |
| Metals | Pheasant, partridge, deer | Muscle |
| Anti-endoparasitic substances | Cattle, sheep, pigs, poultry, deer | Liver |
| Nicarbazin, lasalocid and ionophores | Poultry, deer, Cattle, sheep | Liver |
| Sedatives / beta-blockers | Cattle, sheep, pigs, horses | Liver |
| NSAIDS | Cattle, sheep, pigs, horses | Kidney |
| NSAIDS | Poultry | Liver |
| Paracetamol | Poultry | Liver |
| Pyrethroids | Cattle, sheep, pigs, poultry, deer | Liver |
| Carbamates | Poultry, deer | Liver |
| Beta-agonists | Cattle, sheep, pigs, poultry, deer | Liver |
| Synthetic hormones | Cattle, sheep, pigs | Urine |
| Synthetic hormones | Poultry | Liver |
| Thyrostats | Cattle, sheep, pigs | Urine |
| Thyrostats | Poultry | Liver |
| OCs, PCBs and OPs | Cattle, sheep, pigs | Kidney fat |
| OCs, PCBs and OPs | Poultry, deer | Liver |
| Dexamethazone / methazone | Pigs | Liver |
| Carbadox | Pigs | Kidney |
| Gestagens | Cattle, sheep, pigs | Kidney fat |
| Natural hormones | Cattle | Serum |
| Natural hormones | Poultry | Liver |
| Methyl-testosterone | Pigs, sheep | Urine |
| Nortestosterone | Cattle, sheep, pigs | Urine |
| Synthetic hormones | Cattle, sheep, pigs | Urine |
| Zeranol | Cattle, sheep, pigs | Urine |
| Nortestosterone | Cattle, sheep, pigs | Urine |
| Natural hormones | Cattle, sheep, pigs | Serum |
| Thyrostats | Cattle, sheep, pigs | Urine |
| Beta-agonists | Cattle, sheep, pigs | Urine |
| Gestagenic substances | Cattle, sheep, pigs | Urine |
Note for antimicrobial samples: if a suspect sample is to be tested for antibiotics, the OV is required to select a “panel” for testing at the time of submission. These can be broadly categorised into four ”AMS panels”: AMS1 (penicillins, sulphonamides and tetracyclines), AMS2 (quinolones), AMS4 (aminoglycosides) and AMS5 (macrolides).
Summary of conditions to be considered for suspect sampling.
| - | Condition | Substance to test |
|---|---|---|
| AMI |
Secondary sexual characteristics: crest development teat development, restlessness; animals do not settle in the lairage, mill around Behavioural changes like mounting, aggression An even level of finish in a group of cattle of different breed/types |
Hormones |
| AMI | Good conformation with little fat, hyperaesthesia and tachycardia. | Beta-agonist |
| PMI | Signs of recent illness: mastitis, lameness/arthritis, pleurisy/pneumonia poor condition, metritis | For OV to decide |
| PMI | Emergency slaughter animals | For OV to decide |
| PMI | Injection sites, particularly: bruising / discolouration smell (especially with tetracyclines), swelling | Tetracyclines if bad odour, Hormone if oily adjuvant Others: For OV to decide |
| PMI | Good conformation with little fat, flaccidity of the trachea | Beta-agonists |
| PMI | Evidence of implants | Hormone Growth promoter |
3.5 Results: live animals
3.5.1 Notification of results
The VMD will inform the OV of the results by written confirmation as soon as results are available.
3.5.2 Compliant results
In the event of compliant results, the OV must serve a Form H notice cancelling Form G. Send a copy of the completed Form H to the VMD.
Reference: See annex 12 for a sample Form H notice.
3.5.3 Non-compliant results
In the event of non-compliant results, further action depends on the type of substance found; the VMD will issue specific instructions for each case.
3.5.4 Prohibited substances found
If prohibited substances are found the VMD will request that the OV serve a Form I notice on the owner or person in charge of the animal(s). This notice gives conditions and the time within which the animal(s) must be disposed of as a Category 1 Animal By-Product.
Reference: See annex 13 for a sample Form I notice.
3.5.5 Failure to comply
If the owner or person in charge of the animal(s) fails to comply with Form I you should serve a Form J notice and make arrangements for the disposal of the animal(s). The costs of such action will be recovered from the owner or person in charge of the animals.
Reference: See annex 14 for a sample Form J notice.
3.5.6 Investigation
The detection of residues of unauthorised substances will be immediately investigated by APHA. The OV responsibility is to support the investigation
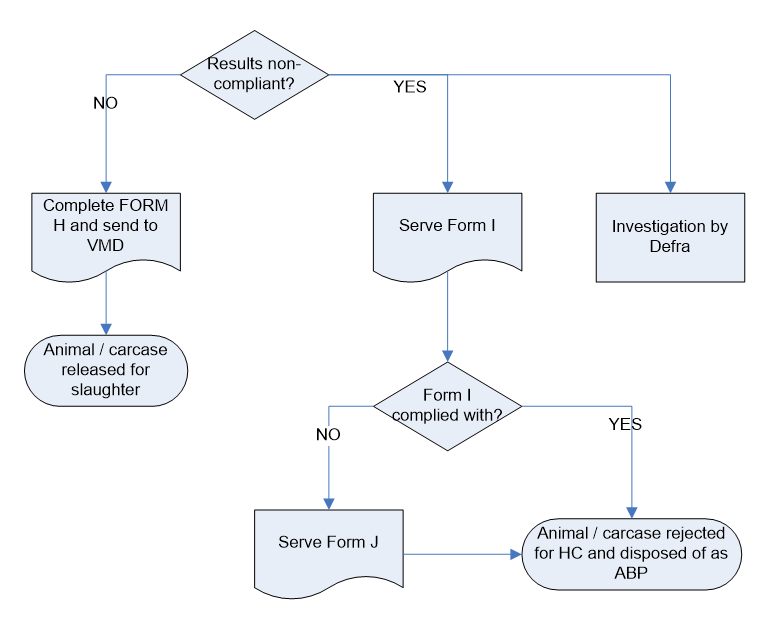
Sampling results
Compliant:
Complete Form H, Send to VMD and release Animal/carcase for slaughter.
Non-Compliant:
Branch 1: Serve Form I
- If not complied: serve Form J, reject Animal/carcase for HC and dispose of as ABP.
- If complied: reject animal/carcase for HC and dispose of as ABP.
Branch 2: on farm Investigation by APHA/Defra.
3.6 Results: Suspect carcases
3.6.1 Results
The OV will be the result is available by written confirmation.
3.6.2 OV action on receipt of positive results
If the results are positive, the OV who was responsible for sending the sample(s) will be sent Form A and Form B and a copy of the original RIM 1 form by the laboratory.
Reference: See annex 5 on ‘Form A’ and annex 6 on ‘Form B’ for samples.
The OV is to:
- give the forms to the owner or person in charge of the carcase
- declare the meat unfit for human consumption
- request voluntary surrender of the carcase
If the FBO refuses to surrender the carcase, you must put in writing the reason why the meat is being formally declared as unfit for human consumption in accordance with retained Regulation (EU) 2019/627, Article 45(f).
Note: Where the FBO continues to refuse to dispose of meat that has been declared unfit, follow the ABP provisions relating to the treatment of meat declared unfit for human consumption in chapter 2.8 on ‘Animal by-products’.
Reference: (EU) 2019/627, Article 43(i)
See chapter 7 on ‘Enforcement’ for additional information.
Caution: If the result is non-compliant for an unauthorised substance, the OV will be contacted by VMD and given further specific instructions.
3.6.3 Compliant results
If the result is compliant, complete Form D and release the carcase for slaughter.
Reference: See annex 8 for a sample Form D.
3.6.4 Follow-up investigation
A follow-up investigation will be carried out and may also be considered for further action.

Results Non-Compliant
If No:
Complete Form D and release carcase for slaughter.
If Yes:
Branch 1: Lab sends Form A and B and RIM1 to the OV who gives those to the owner or person in charge. OV to declare the meat for unfit for human consumption rejecting the animal/carcase for HC and to be disposed of as ABP.
Branch 2: On farm Investigation by APHA/DEFRA
updated [4. Follow up samples]
There may be occasions where Veterinary Medicines Directorate (VMD) will ask FSA staff to attempt to take, target or ‘follow-up’ a sample for a particular producer or County Parish Holding (CPH) number, based on the positive result of a sample previously processed.
To increase chances of obtaining a sample, given that a producer can choose to send animals to a number of Food Business Operators (FBOs) for slaughter (or not send any further animals to slaughter at all), VMD will email the SLA and Contracts team a central spreadsheet with the details of the original sample. The spreadsheet will show the original sample in this kind of format:
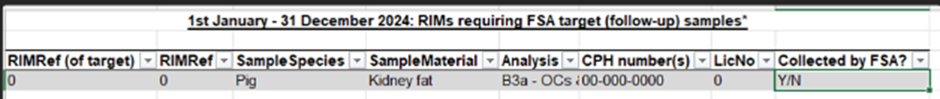
The SLA and Contracts team will send information about the required sample to:
- the FSA ITL of the plant for awareness that additional samples to be taken
- the FSA Field Veterinary Coordinator for the area where the plant is located for awareness that additional samples need to be taken
- the Service Delivery Partner mailbox for appropriate cascade through their local management channels. They should send the information to the OV at local plants and/or the relevant FSA OAs/MHIs responsible for in-plant sampling, so they can be on the lookout for an opportunity to collect such a sample. Samples should be taken the next time that animals from that farmer are received, although there is not a cut out date for the submission of each sample
- RIM form for the relevant follow up sample will be sent with the forms for routine testing on the same month that the follow up sample has been triggered
The next time that animals from the relevant farms are received in the slaughterhouse, the follow up sample must be taken. Regular RIM sample processes shall be followed.
In the case that animals requiring follow up sampling are received in a different plant and identified by the OV/FSA team, the follow up sample must be taken at this abattoir. Suspect sample kit and annex 2. (RIM sample) should be used.]
5. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 3: How to complete a RIM 1 form
Annex 4: How to complete a tamperproof bag
Annex 5: Form A: Primary Analysis Certificate
Annex 6: Form B: Reference Analysis Certificate
Revision log
Published: 20 January 2022
Last updated: 16 December 2024