Chapter 4.3 Verifying Operator’s Own Checks
Sections 1. Introduction 2. Lactic acid to reduce microbiological surface contamination in bovine carcases 3. Verification of Microbiological Criteria 4. Traceability 5. Procedures for the verification of the manufacture of beef patties and burgers intended to be consumed less than thoroughly cooked at retail level 6. Annexes
Sections
2. Lactic acid to reduce microbiological surface contamination in bovine carcases
3. Verification of Microbiological Criteria
6. Verification of Water Testing Procedures
1. Introduction
In this section
1.1 Background
1.1.1 General obligations regarding the organisation of official controls
Regulation (EU) 2017/625 requires official controls to be undertaken regularly, on a risk basis and with appropriate frequency to achieve the objectives of the regulations, taking account of:
- identified risks associated with animals and goods; the activities under the control of operators; the location of the activities, and the use of products, processes, materials or substances that may influence food safety, integrity and wholesomeness, or feed safety, animal health or animal welfare;
- any information indicating the likelihood that consumers might be misled, in particular as to the nature, identity, property, composition, quantity, durability, country of origin or place of provenance, method of manufacture or production of food;
- feed or food business operators' (FBOs) past record as regards compliance with feed or food law or with animal health and animal welfare rules
- the reliability and results of own checks controls performed by the operators or by a third party at their request;
- any information that might indicate non-compliance with the food and feed safety, animal health or animal welfare requirements set out in these regulations.
Reference: (EU) 2017/625, Article 9(1).
2. Lactic acid to reduce microbiological surface contamination in bovine carcases
2.3 Concentration and applications of solution
2.4 Exceptions to the use of lactic acid
2.1 Background
2.1.1 Substances to remove surface contamination
EU hygiene legislation provides for the use of potable water to remove surface contamination from products of animal origin. However, it does also provide for other substances to be used for this purpose, provided that they have been approved under a procedure laid down in Regulation 853/2004.
The first substance approved for this purpose is lactic acid used to reduce microbiological surface contamination on bovine carcases. It was adopted by the European Commission as Commission Regulation 101/2013 on 4 February 2013 and entered into force on 25 February 2013.
The measure was preceded by a thorough risk assessment by the European Food Safety Authority (EFSA), which resulted in a favourable opinion published on 26 July 2011 on the safety and efficacy of lactic acid.
2.2 Legislative references
2.2.1 Relevant legislation
- Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs
- Regulation (EC) No 1333/2008 on food additives
- Commission Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008
- Commission Regulation (EU) No 380/2012 amending Annex II to Regulation (EC) No 1333/2008 as regards the conditions of use and the use levels for aluminium-containing food additives
- Commission Regulation (EU) No 101/2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcases
2.2.2 Commission Regulation (EU) No 101/2013
Commission Regulation (EU) No 101/2013 allows FBOs to choose to use lactic acid to reduce microbiological surface contamination on bovine carcases, half carcases or quarters at the slaughterhouse, in compliance with the conditions set out in the Annex to the Regulation.
2.3 Concentration and application of solutions
2.3.1 Requirements for lactic acid solutions
Solutions which may be used must be prepared from lactic acid that meets the specifications for use as a food additive, set out in Regulation (EU) No 231/2012.
Note: The specifications set out in Regulation (EU) No 231/2012 are reproduced in Annex 1 at the end of this chapter.
2.3.2 Concentration of prepared lactic acid solution
The prepared solution must be between 2% to 5% lactic acid solution in potable water.
2.3.3 Application of prepared lactic acid solution
The prepared solution must be:
- applied and used at temperatures of up to a maximum of 55°C
- applied either by spraying or misting
- applied under controlled and verifiable conditions integrated into a HACCP-based management system including, at least, the criteria set out below under HACCP
2.3.4 To what may the prepared lactic acid solution be applied?
The prepared solution must only be applied to entire carcases, half-carcases or quarters of meat from domestic bovine animals (including buffalo, water buffalo and bison), at the slaughterhouse.
2.4 Exceptions to the use of lactic acid
2.4.1 Visible faecal contamination
Lactic acid solutions must not be applied to carcases with visible faecal contamination.
2.4.2 Irreversible physical changes
The application of lactic acid solutions must not result in any irreversible physical changes to the meat.
2.5 Minimum HACCP requirements
2.5.1 HACCP
The FBO’s HACCP plan should, as a minimum, incorporate the following elements:
- Sampling of carcases for the purposes of assessing compliance with microbiological criteria within the meaning of Regulation (EC) No 2073/2005 must be carried out before the application of lactic acid solutions to the carcases, half-carcases or quarters.
- Lactic acid concentration during treatment must be monitored as part of the HACCP plan, verified by periodic monitoring, documented and recorded.
- The temperature of the solution during treatment must, as part of the HACCP plan, be documented and recorded and continuously monitored using measuring instruments.
2.6 FBO duties
2.6.1 Use of lactic acid
The FBO must ensure that lactic acid is only used at the dilution specified in the legislation.
The FBO should, where possible, notify the FSA OV of their intention to use lactic acid as a decontamination agent and ensure that the OV is familiar with the relevant sections of the HACCP plan.
2.6.2 Update to HACCP plans
The FBO must ensure that their HACCP plan includes a section detailing the conditions for the use of, controls and verification of the procedures for the use of lactic acid.
2.6.3 Communication of information
Slaughterhouse FBOs using lactic acid solutions to reduce microbial surface contamination of entire carcases, half-carcases or quarters, must inform the FBO receiving the treated carcases or half-carcases or quarters of such use.
This information should be documented- for example, included in the commercial documents which accompany treated meat.
2.7 FSA role
2.7.1 Check suitable HACCP plan in place
The OV and FSA team must ensure that where the FBO intends to use lactic acid as a decontamination agent, there is a suitable HACCP plan in place as detailed in the legislation.
The FBO HACCP plan and associated records should be verified during audit with particular reference to the records required by the legislation.
2.7.2 Monitor use
The use of lactic acid should be monitored to ensure that it is not applied to carcases that have faecal contamination and is used at the correct dilution and within the specified temperature range.
2.7.3 Frequency of verification at audit
Until further instructions are provided, should the FBO choose to use lactic acid as a decontaminant, the FVC should contact the Field Operations helpline on 01904 232083 to discuss the frequency at which the verification at audit as detailed in the following paragraph should take place.
2.7.4 Verification at audit
When carrying out an audit of FBO controls where lactic acid is being used, OVs should verify the controls the FBO has in place to ensure that the requirements of EU 231/2012 have been met, namely:
- The lactic acid meets the requirements of Regulation (EU) No 231/2012.
- The lactic acid is made up in a solution of between 2% and 5% in potable water.
- The lactic acid solution is applied at a temperature below 55°C.
- The lactic acid solution is only applied to carcases free from visual faecal contamination.
- Microbiological testing is carried out before the use of lactic acid solution.
- The FBO is notifying customers receiving treated carcases of the treatment applied with lactic acid.
These checks should be recorded on the audit report form in Part 2 on ‘HACCP based procedures’.
2.7.5 Health mark legibility
If the application of the lactic acid solution interferes with the legibility of the health mark, this should be resolved between the FBO and the OV, in full consultation with the FVC and Area Veterinary Manager (AVM).
3. Verification of Microbiological Criteria
In this section
3.2 Legislation and guidance documents
3.3 Testing requirements: slaughter operations
3.4 Testing requirements: other operations
3.5 Testing requirements: ready to eat products
3.8 OV role: all establishments
3.1 Background
3.1.1 Purpose of Microbiological Testing
Assimilated Regulation (EC) 2073/2005 (as amended) lays down the microbiological criteria for certain microorganisms and the implementing rules to be complied with by Food Business Operators (FBOs) when implementing the general and specific hygiene measures referred to in Article 4 of assimilated Regulation (EC) 852/2004. The competent authority shall verify compliance with the rules and criteria laid down in this regulation under Article 18 of assimilated Regulation (EU) 2017/625.
The purpose of microbiological testing is to ensure that:
- results support validation or verification of the correct functioning of FBO’s procedures based on HACCP principles and good hygiene practice
- the supply, handling and processing of meat under the FBO control are carried out in such a way that the process hygiene criteria are met
- the food safety criteria are met throughout the shelf life of the product under reasonable conditions of distribution, storage and intended use as described in the food safety management system; and
- process controls are reviewed when microbiological results indicate that these processes are out of control
- corrective actions are taken to protect the health of consumers when test results are unsatisfactory under the process hygiene criteria (for example, improve food safety systems) or the food safety criteria (for example, by withdrawing or recalling non-compliant products). More details on corrective actions are provided in point 3.7.2 below.
3.1.2 Microbiological criteria
Two different types of criteria are established in assimilated Regulation (EC) 2073/2005, Food Safety Criteria and Process Hygiene Criteria. Food safety criteria assess the safety of a product; process hygiene criteria assess the hygienic functioning of production processes. The main difference between them is the additional action required when the results are not within limits.
When food safety criteria are not met, the batch of food affected should be removed from (recalled) or not placed on (withdrawn) the market.
Failure to meet either type of criteria should always result in an investigation to find the root cause of the contamination and regain control to prevent contamination of future production. The FBO must take action under assimilated Regulation (EC) 2073/2005, Article 7, paragraphs 1 to 4, as well as the appropriate corrective action defined in their HACCP plans and any additional action to protect public health.
Depending on which microbiological limits have been exceeded, to fully comply with the criteria, the FBO is required to take different actions in accordance with point 3.7 below.
3.1.2.1 Food safety criteria
Food safety criteria have been set for fresh poultry meat, minced meat, meat preparations, meat products, mechanically separated meat, gelatine and collagen and ready-to-eat foods. Other than meat, these criteria are also set for milk and dairy products, eggs products and fishery products. For the full list of food safety criteria, microbiological requirements refer to Annex I, Chapter 1 of Regulation (EC) 2073/2005 EUR-Lex – 02005R2073-20200308 – EN – EUR-Lex (europa.eu).
Demonstration of compliance with food safety criteria for meat and processed meat is required as follows:
- Absence of Salmonella spp in:
- minced meat and meat preparations intended to be eaten raw
- minced meat and meat preparations intended to be eaten cooked
mechanically separated meat (MSM)
meat products intended to be eaten raw
meat products made from poultry meat intended to be eaten cooked
fresh poultry meat (this is applicable only if Salmonella Typhimurium or Salmonella Enteritidis are identified)
gelatine and collagen
- Listeria monocytogenes less than 100 cfu/g in ready-to-eat meats that either do not support the growth of Listeria or have evidence that Listeria will not reach levels greater than 100 cfu/g during their shelf-life.
- Absence of Listeria monocytogenes before the food is placed on the market for foods that support growth and do not have shelf-life assessment data.
3.1.2.2 Process Hygiene Criteria
The purpose of testing against the process hygiene criteria set for carcases and certain processed meats (and other products of animal origin) is not to assess the fitness of individual carcases or processed meats for human consumption. The results provide an indication of performance and hygienic control during slaughter, dressing and/or production process at the time of sampling and therefore will allow to confirm if the food safety management systems at all stages of production are effective.
For the full list of process hygiene criteria, microbiological requirements refer to Annex I, Chapter 2 of Regulation (EC) 2073/2005 EUR-Lex – 02005R2073-20200308 – EN – EUR-Lex (europa.eu).
Demonstration of compliance with process hygiene criteria for meat and processed meat is required as follows:
- Aerobic Colony Count (ACC) and Enterobacteriaceae – on cattle, sheep, goats, horses and pig carcases (below specified limits).
- Salmonella spp – on cattle, sheep, goats, horses, pig, broiler and turkey carcases (absence from a specified number of samples per 50 samples examined).
- Campylobacter spp – on broiler carcasses (below specific limits from a specified number of samples per 50 samples examined).
- Aerobic Colony Count (ACC) and E. coli – in minced meat and mechanically separated meat (below specified limits).
- E. coli – in meat preparations (below specified limits).
3.2 Legislation and guidance documents
3.2.1 Assimilated Regulation (EC) 2073/2005
Assimilated Regulation (EC) 2073/2005 (as amended) sets out the microbiological criteria for certain micro-organisms and the implementing rules to be complied with by FBOs, when implementing the general and specific hygiene measures referred to in Article 4 of Assimilated Regulation (EC) No 852/2004.
3.2.2 Assimilated Regulation (EC) 2160/2003
Assimilated Regulation (EC) 2160/2003 (as amended) on the control of Salmonella and other specified food-borne zoonotic agents applies in relation to Salmonella testing.
3.2.3 Assimilated Regulation (EC) 178/2002
Assimilated Regulation (EC) 178/2002 lays down general food safety requirements, according to which food must not be placed on the market if it is unsafe. Article 19, point 4 of this regulation states that FBOs shall collaborate with the competent authorities on action taken to avoid or reduce risks posed by a food which they supply or have supplied. As a result of this provision, the FSA expects FBOs to inform the FSA Food Incidents branch at foodincidents@food.gov.uk if they consider that a food placed on the market may be injurious to health. FBOs have an obligation to withdraw or recall any unsafe food from the market.
3.2.4 Assimilated Regulation (EC) 852/2004
FBOs are required to comply with microbiological criteria under Article 4 of assimilated Regulation (EC) 852/2004.
3.2.5 Food Hygiene Regulations
The Food Hygiene (S/W) Regulations 2006 (as amended) / The Food Safety and Hygiene (England) Regulations 2013 make it an offence for any person to contravene or fail to comply with the specified community provisions.
Schedule 2 of these Regulations lays out the requirement in respect of assimilated Regulation (EC) 2073/2005, in that the FBO will have to take the appropriate measures laid down in Article 7, Points 1 to 4 when test results prove unsatisfactory.
3.2.6 Assimilated Regulation (EU) 2017/625
Article 15 establishes that FBOs shall give staff of the competent authorities access to their computerised information management systems, their documents and any other relevant information when required for the completion of official controls.
3.2.7 Guidance for Veterinary Auditors: FBO Audit Aide Memoire Appendix 1
OVs will find it useful to refer to the Audit Aide Memoire in Annex 1 of Chapter 4.1, in particular:
- Section 3.9 (micro criteria in slaughterhouses)
- Section 3.13 (micro criteria in cutting plants)
- Section 5.13 (using results to verify HACCP based procedures)
and to the tables provided in Appendix 1 of the Audit Aide-Memoire where full details of the microbiological criteria that are laid down by Assimilated Regulation (EC) 2073/2005 under both Food Safety Criteria and Process Hygiene Criteria, and sampling frequencies are reproduced.
Reference: Chapter 4.1 Audit, Annex 1 ‘FBO Audit Aide Memoire’, Appendix 1.
3.3 Laboratory requirements
3.3.1 Analytical reference method
The laboratory undertaking testing for the food business operator should use the organism-specific method:
- for Salmonella EN/ISO 6759
- for Listeria monocytogenes EN/ISO 11290 -1 and 2
- for Enterobacteriaceae ISO 21528-2
- for E. coli ISO 16649-1
- for Aerobic Colony Count (ACC) ISO 4833
- for Campylobacter ISO10272-2
It is best practice (not a legal requirement) that the laboratory undertaking testing for the food business operator (FBO) is accredited by UKAS for the examinations required in meat samples https://www.ukas.com/find-an-organisation/.
It is advisable (not a legal requirement) that the laboratory takes part in a recognised proficiency testing scheme for the examinations required, for example, FAPAS proficiency testing: https://fapas.com.
The use of alternative analytical methods is acceptable when the methods are validated against the reference method in Annex I of Assimilated Regulation (EC) 2073/2005 and if a proprietary method, certified by a third party in accordance with the protocol set out in EN/ISO standard 16140 or other internationally accepted similar protocols, is used. Links to internationally accredited bodies that provide a list of their alternative methods can be found below:
The testing laboratory will be able to supply the equipment and consumables necessary for sampling.
3.3.2 Laboratory test portions - processed meat
The test portion size for minced meat, mechanically separated meat, meat preparations and meat products is specified in the Regulation for Salmonella as either 25g or 10g and for Listeria Monocytogenes in ready-to-eat meat as 25g. SOPs should state the sample size. If FBOs decide to use a larger portion than required by the regulations, this should be to the satisfaction of the competent authority and, if results are unsatisfactory in a larger portion, the same corrective action is to be taken as for a smaller portion.
The laboratory test portion weight for minced meat, mechanically separated meat or meat preparations for ACC and E. coli examination is not specified in the Regulation so the ISO standard (6887-2) that establishes specific rules for the preparation of meat and meat products samples should be followed which specifies a 25g sample.
The laboratory must be able to obtain both test portions (for ACC and for E. coli testing) from each sample it receives. Test portions should be taken from throughout the sample including the surface and the interior.
3.3.3 Pooling of samples for Salmonella testing
The pooling of samples for Salmonella testing is permitted only if it takes place at the testing laboratory and where evidence is available to show that the sensitivity of the method is not reduced. A note explaining how to undertake pooling is included in the reference method for Salmonella EN ISO 6579: 2002.
3.3.4 Sample information
Information about the batch sent to the lab must be recorded on a sample form. This should include:
- name and species of the product (e.g., beef burger, turkey mince)
- pack description (for example, retail 500g pack)
- physical state (for example, fresh or frozen)
- type of packaging (for example, MAP)
- date of production
- traceability code and source (slaughterhouse, cutting plant)
3.4 Testing requirements: Slaughter operations
3.4.1 Testing requirements - Red meat slaughterhouses
Testing in red meat slaughterhouses is to verify process hygiene criteria only; there are currently no requirements for food safety microbiological criteria. Process hygiene criteria set indicative microbiological values above which corrective actions are required to maintain the hygiene of the process. For details on corrective actions see point 3.7.2 below.
Note: There are some exceptions on microbiological testing for small slaughterhouses according to their annual throughput. Details on testing frequency and exemptions are in the table in point 3.4.3.1. Seasonal throughput increases are to be taken into consideration when calculating the annual throughput. Updated [Throughput for specific slaughterhouses can be gathered from the IRIS application, E&P – View or requested to the FSA CBI team at CBI@food.gov.uk.]
Testing at red meat slaughterhouses shall include carcases of cattle, sheep, goats, horses and pigs. See point 3.10 below for details on sampling methods.
Carcases of cattle, sheep, goats and horses:
Five carcases of each species are required to be sampled per sampling session. One sample is from one carcase.
- for Aerobic Colony Count (ACC) and Enterobacteriaceae (ENT), use the specified mean log level below for the five samples. The limits given in the regulation are for an excision method; the limits for the swab or sponge method are lower and are given in (brackets) in the table below under the figures for excision.
- for Salmonella (Sal), the criterion is equal to or below 2 positives in 10 consecutive sampling sessions (that is 50 samples) using a sponge method.
| Result | ACC | Ent | Sal |
|---|---|---|---|
| Unacceptable - mean log /number of positives is above |
5.0 (4.3) |
2.5 (1.8) |
>2/50 |
| Acceptable - mean log below |
5.0 (4.3) |
2.5 (1.8) |
- |
| Satisfactory - mean log /number of positives is equal to or below |
3.5 (2.8) |
1.5 (0.8) |
≤2/50 |
Carcases of pigs:
Five carcases are required to be sampled per sampling session. One sample is from one carcase.
- For Aerobic Colony Count (ACC) and Enterobacteriaceae (ENT), use the specified mean log level below for the five samples. The figures given are for the excision method, the figures for the swab or sponge method are lower and are given in the table below in (brackets) under the figures for excision.
- For Salmonella (Sal), the criterion is equal to or below 3 positives in 10 consecutive sampling sessions (that is 50 samples) using a sponge method.
| Result | ACC | ENT | Sal |
|---|---|---|---|
| Unacceptable - mean log /number of positives is above |
5.0 (4.3) |
3.0 (2.3) |
>3/50 |
| Acceptable - mean log below |
5.0 (4.3) |
3.0 (2.3) |
- |
| Satisfactory - mean log /number of positives is equal to or below |
4.0 (3.3) |
2.0 (1.3) |
≤3/50 |
Note: The mean log value for ACC and ENT of the five carcases tested per sampling session can be calculated by adding the 5 individual log results together and dividing by 5. Results shall be reported as cfu/cm2. The lab can give the results as cfu/swab; in this case, the FBO needs to know how many cm2 are covered in the swab and divide the result to provide the results as cfu/cm2. Results for Salmonella will be presence/absence in the tested area.
3.4.2 Testing requirements - Poultry slaughterhouses
Broilers and turkeys are tested for Salmonella to check food process hygiene criteria.
The samples taken to check process hygiene criteria can also be used to verify compliance with food safety criteria requirements. To this effect, FBOs must carry out further tests where Salmonella spp results have been positive to identify whether Salmonella enteritidis or Salmonella typhimurium are present.
Broilers are also tested for Campylobacter to check the process hygiene criteria in the slaughterhouse.
FBO Campylobacter results should be entered into CaPTa (Campylobacter Process Hygiene Criteria Testing Application) at the required frequency. An internal guide on how to enter the data in the system has been produced by the FSA and can be found in the following link CaPTa User Guidance v1.
Note: Slaughterhouses with an annual throughput below 1,000,000 broilers or turkeys (less than 20,000 broilers/turkeys per week) are exempt from Salmonella testing requirements. No exception is currently considered for Campylobacter testing.
Note: For details on testing frequencies and exemptions see table in point 3.4.3.2. Seasonal throughput increases are to be taken into consideration when calculating the annual throughput. Updated [Throughput for specific slaughterhouses can be gathered from the IRIS application, E&P – View or requested to the FSA CBI team at CBI@food.gov.uk.]
Testing at poultry slaughterhouses should include carcases of broilers (for Salmonella and Campylobacter) and turkeys (for Salmonella only):
Neck skins from at least 15 carcases are required to be sampled per sampling session. One sample is composed of three pooled neck skins collected after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase; the neck skins from 3 carcases from the same flock or origin shall be pooled to form 5 x 25g final samples. The same samples can be used for testing Salmonella and Campylobacter in broilers if tested in the same laboratory (see note below if not processed at the same laboratory).
- For Salmonella (Sal), in broilers and turkeys, the criterion is equal to or below 5 positives (presence) in 10 consecutive sampling sessions (that is 50 samples). If a sample tests positive for Salmonella spp. in any sampling session it must be serotyped for S. enteritidis and S. typhimurium to check compliance with the food safety criteria.
- For Campylobacter (Campy), only in broilers, the criterion is equal or below a specified number of positives (more than 1000 cfu/g) in 10 consecutive sampling sessions (that is 50 samples). The current criterion accepts up to 15/50 positive samples to comply. The number of positives accepted will decrease to 10/50 from 1 January 2025.
| Result | Sal | Campy |
|---|---|---|
| Unacceptable - presence /number of positives is above | >5/50 | >15/50 |
| Satisfactory - absence /number of positives is equal to or below | ≤5/50 | ≤15/50 |
Note: when testing for Salmonella and Campylobacter in broilers is carried out in two different laboratories, neck skins from a minimum of 20 carcases shall be sampled at random after chilling during each sampling session. One sample is composed of four pooled neck skins. Each sample will be then split in two to be tested for Salmonella and Campylobacter separately. See point 3.10 below for sampling methods and Annex 2 for Campylobacter testing requirements.
3.4.3 Sampling frequency at slaughterhouses (red meat and poultry)
Carcases of red and poultry meat are to be tested weekly initially. The day of sampling shall be changed each week to ensure that each day of the week is covered. Reduced sampling frequency can be applied if results are satisfactory after a number of weeks; this will be different according to the annual throughput of the slaughterhouse. For details on sampling frequency and reduced sampling frequency refer to tables 3.4.3.1 for red meat and 3.4.3.2 for poultry.
3.4.3.1 Sampling frequency in red meat slaughterhouses
| Category | Annual throughput per species | Initial sampling frequency | Reduced sampling frequency if results are satisfactory |
|---|---|---|---|
| Standard 1 |
Over:
(>400 or 2,000/week) |
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 2 |
|
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 3 |
(>30 or 150/week) |
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 4 |
|
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 5 |
|
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
3.4.3.2 Sampling frequencies in poultry meat carcases
| Category | Annual throughput of turkeys or broilers | Initial sampling frequency (one sample is three neck skins) | Reduced frequency if results are satisfactory |
|---|---|---|---|
| Standard 1 | Over 7,500,000 (>150,000/week) |
Salmonella: Campylobacter: |
Salmonella: Campylobacter: |
| Small 2 | Below 7,500,000 but over 1,000,000 (>20,000week) |
Salmonella: Campylobacter: |
Salmonella: Campylobacter: |
| Small 3 | Below 1,000,000 (<20,000/week) |
Salmonella: Campylobacter: |
Salmonella: Campylobacter: |
3.4.4 OV to monitor FBO sampling results at the slaughterhouse
The OV should regularly monitor the sampling technique, the procedures for transporting the samples to the laboratory, the laboratory reference method used and the provision of results at slaughterhouses where sampling and testing are required.
The requirement for the OV to verify the sampling arrangements is part of the Slaughter Hygiene Verification (SHV) system for both red and poultry slaughterhouses. The verification must be completed at least monthly or aligned with the FBO testing frequency if this is less than monthly. (see Chapter 2.4, Sections 12.7 for red meat and 13.7 for poultry).
The microbiological testing arrangements and actions taken following receipt of the test results will also be verified by Field Veterinary Leaders (FVLs) and Field Veterinary Coordinators (FVCs) during the approval process and by Veterinary Auditors during full audits.
Verification aims to ensure the FBO has complied with the sampling requirements and to ensure non-compliant results are acted upon. Assimilated Regulation (EU) 2019/627 also requires the competent authority to supervise the FBO action plan where there are repeated failures, and that may require the OV to apply the hierarchy of enforcement (see Enforcement in point 3.9 below).
Note: Species for which criteria are not specified, for example, game, rabbits, ducks and geese carcases, are not required to be sampled. Turkey carcases are not required to be sampled for Campylobacter.
3.5 Testing requirements: Other activities
3.5.1 Criteria requirements: other activities
Testing is required for minced meat, meat preparations, meat products, gelatine, collagen and mechanically separated meat (MSM). Also required for milk and dairy products, egg products and fishery products. For details see Annex I, Chapters 1 and 2 of Regulation 2073/2005.
Sampling is on a batch basis.
Note: A batch is defined as a group or set of identifiable products obtained from a given process under practically identical circumstances, produced in a given place and within one defined production period. FBO’s documentation should explain how a batch, for the purposes of microbiological sampling, has been established.
3.5.2 Testing for Food Safety Criteria - Other activities
Minced meat and meat preparations intended to be eaten raw: Salmonella should be absent on 5 x 25g samples from a batch of minced meat or meat preparations intended to be eaten raw made from any species of meat, for example, steak tartare or burgers intended to be consumed less than thoroughly cooked (LTTC). Guidance for LTTC is available in Section 5 of this chapter.
Minced meat and meat preparations from poultry meat intended to be eaten cooked: Salmonella should be absent on 5 x 25g samples from a batch of minced meat or meat preparations made from poultry meat intended to be eaten cooked. This applies to poultry meat of all species including ducks, geese, turkeys spent hens and broilers. For example, minced chicken, turkey burgers, chicken sausages, chicken and turkey escalopes.
Minced meat and meat preparations from red meat intended to be eaten cooked: Salmonella should be absent on 5 x 10g samples from a batch of minced meat or meat preparations made from other species than poultry intended to be eaten cooked. This applies to all species of red meat including game, for example, minced meat for bolognese sauce or shepherd’s pie, sausages, and burgers.
Mechanically separated meat (MSM): Salmonella should be absent on 5 x 10g samples from a batch of MSM when produced with the techniques referred to in paragraph 3 of Chapter III of Section V of Annex III to Regulation (EC) 853/2004.
Meat products intended to be eaten raw: Salmonella should be absent on 5 x 25g samples from a batch of meat products intended to be eaten raw, for example, air-dried smoked duck, partially fermented sausages or biltong. This does not apply to products where the manufacturing process or the composition of the product will eliminate the Salmonella risk such as certain types of salami, nor does it apply to fully cooked ready-to-eat meat products such as cooked ham.
Meat products from poultry meat intended to be eaten cooked: Salmonella should be absent on 5 x 25g samples from a batch of meat products made from poultry meat intended to be eaten cooked, for example, turkey bacon and chicken nuggets.
Gelatine and collagen: Salmonella should be absent on 5 x 25g samples from a batch of products placed on the market during their shelf-life.
Fresh poultry meat (other than poultry carcases): Absence of Salmonella enteritidis and Salmonella typhimurium from 5 x 25g fresh poultry meat samples from a batch including meat from hens, broilers and turkeys (including breeders).
3.5.3 Testing for Process Hygiene Criteria – Other activities
Minced meat and mechanically separated meat (MSM)– five samples must be taken from one batch per sampling session and tested:
- for Aerobic Colony Count (ACC) – all five samples must be less than 5 x 106 cfu/g of which at least three samples must be less than 5 x 105 cfu/g.
- for E. coli (EC) – all five samples must be less than 500 cfu/g of which at least three samples must be less than 50 cfu/g.
Meat preparations – five samples must be taken from one batch per sampling session and tested:
- for E. coli (EC) – all five samples must be less than 5000 cfu/g of which at least three samples must be less than 500 cfu/g.
If a process hygiene criterion is not met, the meat can be placed or remain on the market, but the FBO must review the production processes and improve process hygiene to ensure future production will meet the criteria. The actions should be included in the food safety management procedures, which should also include relevant actions specified in Annex I (Chapter 2) of the Regulation. Enforcement authorities will require sufficient evidence that the food business operator has taken the appropriate corrective action. See point 3.7.2 below for corrective actions.
3.5.4 Sampling frequency for other activities
Weekly samples are required from one batch of minced meat, meat preparations or MSM. The day of sampling shall be changed each week (if possible) to ensure that each day of the week is covered. The weekly frequency can be reduced to once every two weeks after 6 consecutive weeks of satisfactory results for E. Coli or ACC and after 30 consecutive weeks of satisfactory results for Salmonella.
| Product | Test | Initial frequency | Reduced frequency if results are satisfactory |
|---|---|---|---|
| Minced meat and meat preparations | E. Coli and Aerobic Colony Count | Weekly | After 6 consecutive weeks of satisfactory results Once every 2 weeks |
| Minced meat and meat preparations and fresh poultry meat | Salmonella | Weekly | After 30 consecutive weeks of satisfactory results Once every 2 weeks |
3.5.5 Exception to testing - Other activities
Establishments producing an average of less than 2 metric tonnes per week of combined minced meat and meat preparations intended to be eaten cooked are currently not required to take any samples. This exception is based on of a risk analysis carried out by FSA as the competent authority.
Note: This exception does not apply to Mechanically Separated Meat (MSM) or minced meat / meat preparations intended to be eaten raw or undercooked (for example, burgers intended to be eaten less than thoroughly cooked) or if production increases during short periods (for example, summer or Christmas seasonal production peaks), where the FBO shall test during the period exceeding the 2 tonnes per week.
3.5.6 Labelling requirements
Article 6 of assimilated Regulation (EC) 2073/2005 requires that minced meat and meat preparations (made from species other than poultry) which are intended to be eaten cooked, must be clearly labelled when placed on the market to inform the consumer of the need for thorough cooking before consumption.
Note: This labelling requirement does not apply to minced meat or meat preparations made from poultry meat.
3.6 Testing requirements: Ready-to-Eat (RTE) products
3.6.1 Listeria monocytogenes (foodstuff)
Ready-to-eat (RTE) products are defined as food intended by the producer or the manufacturer to be consumed directly without the need for cooking or other processing. RTE products are to be tested for food safety criteria in accordance with the following:
a) For RTE foods that can support the growth of L. monocytogenes (for example cooked meats, soft cheeses or smoked fish), 5 x 25 g samples from a batch should be taken and there are two criteria:
- not exceeding 100 cfu/g for the duration of the shelf-life of the product, or
- absence in 25g before the food has left the immediate control of the FBO if the FBO cannot demonstrate that the product will not exceed 100 cfu/g during its shelf -life.
b) For RTE foods unable to support the growth of Listeria monocytogenes: less than 100 cfu/g throughout their shelf-life.
The following are considered to fall into this category
- meat products which have received heat treatment or other processing effective to eliminate L. monocytogenes, when recontamination is not possible after this treatment (for example, products heat treated in their final package)
- products with pH ≤ 4,4
- products with aw ≤ 0.92
- products with pH ≤ 5 and aw ≤ 0,94
- products with a shelf-life of less than 5 days
Note: Regular testing under normal circumstances is not required for products that have received heat treatment or other processing effective to eliminate L. monocytogenes when recontamination is not possible after this treatment (for example, products heat treated in their final package such as hams or other cooked meats).
3.6.2 Processing areas and equipment (environmental testing for RTE processing plants)
Article 5 of Regulation (EC) 2073/2005 requires that FBOs manufacturing RTE products sample the processing areas and equipment for Listeria spp. as part of their sampling scheme. If Listeria spp. are detected, serotyping to identify Listeria monocytogenes will be required.
Environmental testing programmes should include food contact and non-food contact surfaces.
Food contact surfaces may include conveyor belts, working tables, knives and utensils, gloves and aprons, slicers, dicers, filling/packaging machines, transport racks, trays, scales, hoppers, etc.
Non-food contact surfaces may include the exterior of food contact equipment, control panels, sides of weigh scales, floors, walls, refrigeration units, drains, etc.
All samples should show negative results as the presence of Listeria monocytogenes in production areas, particularly in high-risk areas, can re-contaminate RTE products. If found, it is important to investigate the possible sources and establish measures to prevent the introduction of Listeria monocytogenes in the products.
3.6.3 Frequency of testing for RTE products
The legislation does not set a testing frequency for Listeria monocytogenes in RTE foods or the environment.
FBOs shall decide the appropriate sampling frequencies based on their HACCP principles and take into account the intended use of the foodstuff. It is for the FBO to demonstrate that the testing shows satisfactory results and based on this, determine the sampling interval.
Initially, it may be best to test weekly, or at whatever frequency the FBO produces RTE foods if less than weekly. Information on testing for Listeria is available online as well as FSA advice.
Once the FBO has results over a period of time and there are no failures, then the FBO may increase the testing interval based on the evidence of testing and their food safety programme. Changes in the factors affecting the process and product such as change in suppliers or batches or changes in the processes will determine the need for further testing.
If the OV has any concerns surrounding the frequency of testing, they should escalate the matter to the FVC in their area.
3.7 Unsatisfactory results
3.7.1 Unsatisfactory results
In the event of unsatisfactory results as regards both food safety and process hygiene criteria, the actions to be taken by the FBO laid down in Article 7 of assimilated Regulation (EC) 2073/2005 shall be taken together with other corrective actions defined in their HACCP-based procedures.
3.7.2 Corrective actions
Trend analysis of the results should be undertaken, and corrective actions might include:
- improvements in slaughter hygiene
- review of process controls
- review of the origin of animals
- review of biosecurity measures in the farms of origin
- improvements in production hygiene and cleaning procedures
- improvements in the selection and/or origin of raw materials
In addition, FBOs shall take measures to find the cause of the unsatisfactory results to prevent the recurrence of the unacceptable microbiological contamination. Those measures may include modifications to the HACCP plan(s) or other food hygiene control measures in place.
The FBO should ensure test results are retained for inspection by the OV / FVL / FVC / Veterinary Auditor. As a minimum, results should be retained for at least 1 audit period or 52 samples, whichever is greater.
If food safety criteria are exceeded, it indicates that the batch tested is unsatisfactory and should be removed from (recalled) or not placed on (withdrawn) the market. However, products which are not yet at the retail level may be submitted for further processing by a treatment that eliminates the hazard in question. This treatment may only be carried out by FBOs other than those at the retail level. The product may be treated at the same establishment or at another approved establishment under Regulation (EC) 853/2004. The treatment has to be effective in destroying the microorganism and evidence of corrective action taken and traceability must be kept. The FBO shall notify the competent authority (FSA Incidents team). Guidance on how to undertake the notification and a link to the incident report form can be found here.
A batch of mechanically separated meat (MSM) with unsatisfactory results with respect to the Salmonella criterion may be used in the food chain only to manufacture heat-treated meat products in establishments approved under Regulation (EC) 853/2004.
In some circumstances, withdrawal or recall of the affected product will not be possible due to the product having been consumed by the final consumer because of the length of time that it takes for some testing to be completed (for example, Salmonella serotyping). In these circumstances, the FBO should review their procedures to ensure the root cause is identified and process controls streamlined to prevent any re-occurrence.
The OV / FVL / FVC / Veterinary Auditor shall verify that the FBO has taken action and has reported the unsatisfactory results for food safety criteria to the FSA Incidents Team.
Reference: Regulation (EC) 2073/2005, Annex I, Chapters 1 and 2 and Article 7 Regulation (EC) 853/2004, Annex III, Section V, Chapter III, Point 3(e)
3.8 OV and other Authorised Officers' role: all establishments
3.8.1 OV / FVL / FVC /Veterinary Auditor responsibility
FVLs will verify FBO procedures and initial results during the approval process of both, slaughterhouses and cutting plants.
FVCs undertaking unannounced inspections (UAI) in standalone cutting plants and OVs undertaking UAIs in RTE cutting plants shall undertake verification checks on sampling and testing in every visit.
Veterinary Auditors will verify procedures, sampling frequency, sampling reference method and results at every full audit in all establishments.
The role of the OV in slaughterhouses and co-located cutting plants is to:
- monitor the FBO’s compliance with microbiological criteria testing as required by the established SHV Procedures (see Chapter 2.4, Sections 12.7 and 13.7 for details) including regular verification of the sampling methods;
- verify that testing has been carried out following the requirements of the appropriate legislation
- verify the method of despatch to the testing laboratory
- verify that the laboratory methods used are the reference method or an alternative in accordance with Article 5 of Regulation 2073/2005
- verify that the results fall within the required limits and are produced at the required frequency in accordance with the establishment's throughput
- verify that where any further action by the FBO is required, this action is taken promptly and is documented within their HACCP-based procedures and;
- take appropriate enforcement action if this is necessary.
Unsatisfactory results shall be followed up in further visits / partial audits as appropriate.
3.8.2 Verify testing requirements and sampling frequencies
The testing requirements and sampling frequencies which the FBO must follow are detailed in Annex I to Regulation (EC) No 2073/2005. Refer to the tables provided in points 3.4.3.1 and 3.4.3.2 above for a summary of requirements for slaughterhouses and to points 3.5 and 3.6 for requirements in cutting plants.
The Authorised Officer should refer to these resources as required, and ensure that they are familiar with the requirements and testing frequencies for the establishments at which they are based or approving /inspecting /auditing.
3.8.3 Verify that the results fall within the required limits
Assimilated Regulation (EC) 2073/2005 Article 9, requires the food business operator to analyse the trend of results and if the trend is towards unsatisfactory results, take action to prevent microbiological risks.
The OV must periodically review the test results at least monthly, or as required by the established SHV criteria, at slaughterhouses and co-located establishments and during audits in non-co-located establishments, or UAIs in RTE standalone cutting plants. The OV / Veterinary Auditor / FVC must follow up on unsatisfactory results closely and verify that the FBO takes adequate corrective actions until controls are re-gained.
Microbiological sampling results
| Hazard | Process hygiene criteria |
|---|---|
| Salmonella spp. (carcases) |
Results are reported as ‘detected’ or ‘absent’. Results from a number of samples throughout the specified sampling period must be returned as ‘absent’. |
| Campylobacter spp. (broiler carcases) | The limit is 1,000 cfu/g. Satisfactory results if a maximum of 15 samples out of 50 (10 consecutive sampling sessions) are below this limit and unsatisfactory if more than 15 samples out of 50 are above this limit. The established maximum number of samples above the limit will decrease to 10 in 2025. |
| Enterobacteria- ceae (Enteros) (red meat carcases) | For the process hygiene criteria in slaughterhouses processing cattle, sheep, goats, horses or pig carcases. Use the specified mean log level established for the five samples. See point 3.4.1 |
| Aerobic colony count (ACC) |
For minced meat and MSM, all five samples must return results of less than 5 x 106 cfu/g and of those five samples, at least three must return results of less than 5 x 105 cfu/g. |
|
E coli (minced meat /meat preparations) |
For minced meat and MSM, all five samples must return results of less than 500 cfu/g and of those five samples, at least three must return results of less than 50 cfu/g. For meat preparations, all five samples must return results of less than 5,000 cfu/g and of those five samples, at least three must return results of less than 500 cfu/g. |
| Salmonella (minced meat /meat preparations) |
If any of the test results from samples of minced meat, MSM or meat products is positive for Salmonella spp, then the batch must be removed from the market. Please refer to instructions later in this chapter in point 3.9 on ‘Enforcement: microbiological criteria’. If the product is at retail and is intended to be cooked before eating, it must be withdrawn as a minimum. The FBO may decide to instigate a recall. If the product is RTE, then a recall is required. |
|---|---|
| Listeria monocytogenes (RTE foods) |
In foods that support the growth of Listeria monocytogenes: Absence in 25g before the food is placed on the market if the FBO is not able to demonstrate that the product will not exceed the limit of 100 cfu/g throughout the shelf-life or less than 100 cfu/g where the FBO can satisfactorily demonstrate that the product will not exceed the limit of 100 cfu/g at the end of the shelf-life. The operator may fix intermediate limits during the process that must be low enough to guarantee that the limit of 100 cfu/g is not exceeded at the end of shelf-life. In foods that do not support the growth of Listeria monocytogenes: less than 100 cfu/g throughout shelf life. The following are considered to fall into this category:
|
3.9 Enforcement: microbiological criteria
3.9.1 OV / Veterinary Auditor advisory role
Where the FBO is not following the sampling, testing and corrective action requirements contained in Assimilated Regulation (EC) 2073/2005, the OV / Veterinary Auditor, as a first step on the hierarchy of enforcement, should consider informal action to achieve compliance. Where this includes failure to comply with the process hygiene criteria for either Salmonella or Campylobacter on several occasions this will include requiring the FBO to submit to them an action plan to achieve compliance which shall be supervised by the OV. In other cases, this can include educating the FBO and encouraging rectification and providing advice.
Reference: Regulation (EU) 2019/627, Articles 35 and 36.
3.9.2 OV / FVC / Veterinary Auditor Actions
The following table contains examples of FBO non-compliance and the possible enforcement actions that the OV / FVC or the Auditor may take.
In addition, where the FBO has exceeded a reduced testing interval, the OV / FVC or the Auditor should inform the FBO that they must commence testing at the shortest interval and demonstrate that they meet the requirements of testing before moving to a reduced testing frequency again.
Before taking formal action the OV or the Auditor must ensure that enforcement actions are in line with the MOC chapter 7 on ‘Enforcement’.
| FBO fails to | OV / FVC /Auditor informal action | OV /FVC / Auditor formal action |
|---|---|---|
| Comply with the size, number of samples and frequency of testing for the required microorganisms, use the reference method or an alternative that complies with Article 5 of Regulation 2073/2005 | Verbal advice / Written advice | Remedial Action Notice (RAN) |
| Perform removal from the market or not place on the market (for unsatisfactory food safety criteria) | Verbal advice / Written advice (for inadequate procedures) |
|
| Undertake trend analysis of results and take adequate corrective actions | Verbal advice / Written advice | HIN – if FBO is asked to provide trend analysis for results already received. RAN – If FBO is asked for a trend analysis for microbiological results from the point of serving the notice. |
| Take adequate corrective actions (for unsatisfactory process hygiene criteria) | Verbal advice / Written advice |
RAN - if there is evidence of the process resulting in unacceptable levels of contamination. |
| Heat treat MSM produced in accordance with 853/2004, Annex III, Section V, Chapter III, Point 3 with unsatisfactory Salmonella results if it is to go into the food chain | Verbal advice / Written advice | Detain, seek voluntary surrender or seizure in accordance with procedures in MOC chapter 7 on 'Enforcement', Section 3. |
3.10 Sampling Methods
3.10.1 Sampling Methods for red meat carcases
The destructive and non-destructive sampling methods, the selection of the sample sites and the rules for storage and transport of samples are described in standard BS EN ISO 17604.
Five carcases shall be sampled at random during each sampling session. Sample sites must be selected taking into account the slaughter technology used in each plant. The purpose is to sample the sites with the highest levels of contamination.
When sampling for analysis of Enterobacteriaceae and ACC, four sites of each carcase shall be sampled. Four tissue samples representing a total of 20 cm2 shall be obtained by the destructive method (excision). When the samples are taken from different sample sites on the carcase, they shall be pooled before examination. When using the non-destructive method (sponge swab), the sampling area shall cover a minimum of 100 cm2 (50 cm2 for small ruminant carcases) per sampling site.
When sampling for Salmonella analysis, an abrasive sponge sampling method shall be used selecting the areas most likely to be contaminated. The total sampling area shall cover a minimum of 400 cm2 (200 cm2 for small ruminant carcases). The surface is calculated by multiplying the width of the sponge by the length of the swabbing on the carcase. Excision cannot be used for Salmonella testing.
A single sponge sampling method can be used for the three tests (Enterobacteriaceae, ACC and Salmonella).
Use sterile dry abrasive sponge swabs (10x10 cm or 5x10 cm, folded in half) in sterile plastic sample bags (waffle-style cellulose sponge dishcloths and stomacher bags).
Diluent sterile 0.9% unbuffered sodium chloride solution. The sponge should be rehydrated in the sample bag with approximately 10ml diluent. The sponges should be damp without excess diluent in the bag. Alternatively, sponges can be rehydrated, stored frozen and defrosted before use.


Trained operatives should grasp the sponge through the bag folding the bag back over the hand avoiding that the sponge, the diluent, or the internal surface of the bag come into contact with other surfaces.
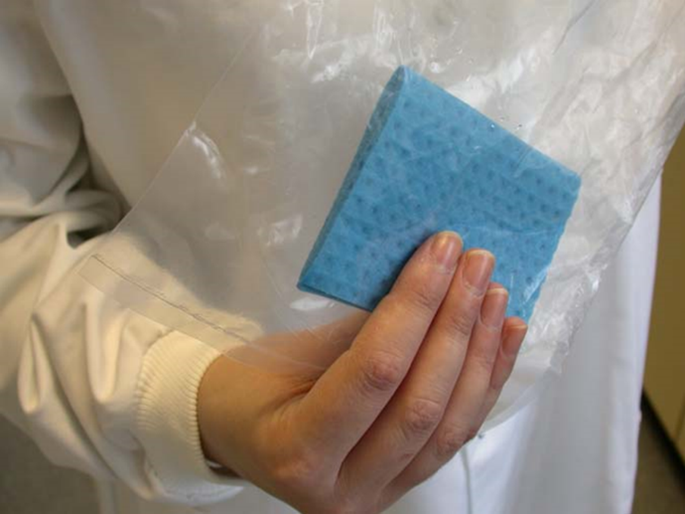


A randomly chosen side of a randomly chosen carcase after inspection and before chilling is to be tested. The wipe with the sponge is to be applied with firm pressure and a slight side-to-side movement down one side of the carcase starting at the back leg and moving across the carcase. A firm consistent pressure is to be used. The length of the wipe should be approximately 100 cm for adult sheep, goats and pigs and 150 cm for adult cattle and horses.


Once finished, the operative should refold the bag over the sponge and secure the bag with a closure.
The bag should be labelled and the following information recorded:
- date of sampling
- species
- origin of animal (farm postcode, slaughtering reference)
- length of wipe for red meat (an estimate is sufficient)
Sponge samples should be kept cool and delivered to the laboratory within two hours. If longer than two hours the samples should be placed into an insulated cool box containing frozen freezer blocks or crushed ice. The samples should be kept cold but not allowed to freeze.
Sample testing in the lab should commence within 24 hours of sampling.
Reference: Regulation (EC) 2073/2005, Annex I, Chapter 3
3.10.2 Sampling Methods for poultry carcases
For the Salmonella analyses, a minimum of 15 carcases shall be sampled at random during each sampling session and after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase. On each occasion, the neck skin samples from three carcases shall be pooled before examination to form 5 x 25g final samples.
For the Salmonella and Campylobacter analyses, if carried out in the same laboratory, a minimum of 15 carcases shall be sampled at random during each sampling session after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase. On each occasion, the neck skin samples from the three carcases shall be pooled before examination to form 5 x 26g final samples.
If Salmonella and Campylobacter analyses are carried out in different laboratories, a minimum of 20 carcases shall be sampled at random during each sampling session and after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase. On each occasion the neck skin samples from four carcases shall be pooled before examination in order to form 5 x 35g final samples which will then be split to form 5 x 25g final samples to be tested for Salmonella and 5 x 10g final samples to be tested for Campylobacter.
If the slaughterhouse is exempt from carrying out Salmonella testing and only
Campylobacter testing is required, a minimum of 15 carcases shall be sampled at random during each sampling session and after chilling. On each occasion the neck skin samples from three carcases shall be pooled before examination to form 5 x 10g final samples.
A poster summarising the different options can be found in Annex 2.
Operatives taking the sample should use gloves and scissors wiped with an alcohol wipe. Grip the plastic bag at the bottom and fold it back over the gloved hand. Avoid the internal surface of the bag or the scissors contacting other surfaces.

Grasp the neck skin through the bag and cut off approximately 10g with the clean scissors, repeat with two further neck skins to make a total of three (four in the case of broilers when Salmonella and Campylobacter are going to be tested in separate laboratories) in one bag. Fold the bag back over the sample and tie it to secure the neck skin samples inside. Clean gloves and scissors with alcohol wipes and repeat.

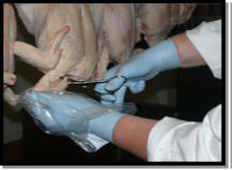

For pieces of poultry meat (other than carcases): aseptically remove 25g taking any skin as a priority and supplementing with surface muscle slices. Whole pieces can be taken and sent to the laboratory for skin and muscle slice removal.
Bags should be labelled and the following information recorded:
- date of sampling
- species
- origin of birds (farm postcode, slaughtering reference)
Salmonella and Campylobacter samples shall be transported to the laboratory at a temperature not lower than 1°C and not higher than 8°C. The time between the sampling and the testing for Campylobacter shall be less than 48 hours to ensure the integrity of the sample is maintained. Samples that have reached a temperature of 0°C shall not be used to verify compliance with the Campylobacter criterion. Testing should commence within 24 hours of sampling.
Reference: Regulation (EC) 2073/2005, Annex I, Chapter 3
3.10.3 Environmental testing
FBOs manufacturing RTE foods, which may pose a Listeria monocytogenes risk for public health, are required to sample the processing areas and equipment for Listeria monocytogenes as part of their sampling scheme. Regulation (EC) 2073/2005 does not establish a sampling frequency, but this should be based on risk and trends analysis should be followed to decide if the frequency can be reduced.
For other establishments, FBOs may choose to sample the process environment to validate and verify the cleaning procedures as part of their HACCP plan(s). Rapid methods can be useful.
When the criteria for carcases or processed meat are not met, sampling of the processing environment should be considered as part of the investigatory action.
FBOs should determine what sites to sample throughout their establishment (from raw material intake to final dispatch) taking into account previous microbiological results from environmental monitoring. Sampling should be focused at sites where contamination is most likely to occur and include both, food contact and non-food contact surfaces.
The BS EN ISO standard 18593 provides useful information and can be used as the reference method.
4. Traceability
In this section
4.5 FBO responsibilities: provision of information on frozen food of animal origin
4.1 Introduction
4.1.1 Definition and scope
Traceability, as defined by article 3, paragraph 15 of Regulation (EC) No. 178/2002, means ‘the ability to trace and follow a food, feed, food-producing animal or substance intended to be, or expected to be incorporated into a food or feed, through all stages of production, processing and distribution.’
The following pages provide further background, a summary of the FBO’s responsibilities and guidance on the role of the OV in monitoring and verifying FBO compliance with the traceability requirements.
4.2 Legislative references
4.2.1 Traceability legislation
(EC) No. 178/2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority (EFSA) and laying down procedures in matters of food safety, as read with …
Commission Implementing Regulation (EU) No. 931/2011 on the traceability requirements set by Regulation (EC) No 178/2002 of the European Parliament and of the Council for food of animal origin.
Commission Regulation (EC) No. 853/2004, Annex II, Section I, Part A, Paragraph 4, laying down specific hygiene rules for food of animal origin.
Commission Regulation (EU) No. 16/2012 amending Annex II to Regulation (EC) No. 853/2004 of the European Parliament and of the Council as regards the requirements concerning frozen food of animal origin intended for human consumption.
4.3 Background
4.3.1 Comprehensive system of traceability to be established
The aim is to ensure that unsafe food is not placed on the market and that the systems in place to identify and respond to food safety problems allow for the proper functioning of the internal market and the protection of public and / or animal health.
This level of protection can be jeopardised where it is impossible to trace food and feed. It is therefore necessary for FBOs to ‘establish a comprehensive system of traceability within their businesses so that targeted and accurate withdrawals can be undertaken or information can be easily provided to consumers or control officials when required, thereby avoiding the potential for unnecessary wider disruption in the event of food safety problems.’
Reference: (EC) No. 178/2002, Recital 28
To achieve the traceability of food as set out in Article 18 of Regulation (EC) No 178/2002, the names and addresses of both the FBO supplying the food and the FBO to whom the food was supplied are needed (except when they are final consumers).
In the sector of food of animal origin additional information is required such as the volume or quantity of the food, a reference identifying the lot, batch or consignment, as appropriate, a detailed description of the food and the date of dispatch.
There is however no legal requirement for the origin of food to remain identifiable during production at an establishment.
4.3.2 Insufficient documentary records
Food or feed business operators must ensure that traceability of food, feed, animals or substances which may be incorporated into a further product can be assured at all stages.
Food crises in the past have revealed that documentary records were not always sufficient to allow full traceability of suspect foods. Furthermore, recent experience has shown that FBOs do not generally possess the information needed to ensure that their systems identifying the handling or storage of foods is adequate, in particular in the sector of food of animal origin.
Reference: Commission Implementing Regulation (EU) No. 931/2011
4.3.3 One step back, one step forward
To achieve the traceability of food as set out in Article 18 of Regulation (EC) No. 178/2002, the names and addresses of both the FBO supplying the food and the FBO to whom the food was supplied are needed. The requirement relies on the ‘one step back – one step forward’ approach which requires that FBOs have in place a system enabling them to identify their immediate supplier(s) and customer(s), except when they are the final consumer.
With regards to food, the implementation of a traceability system is an essential element in ensuring food safety and the reliability of information provided to consumers.
Traceability does not itself make food safe, but it is an essential way of providing assurance and assisting in containing food safety problems.
4.4 FBO responsibilities
4.4.1 FBO to identify suppliers and direct recipients
Traceability is a requirement to be complied with in addition to the food bearing a health mark or an identification mark.
FBOs are required to identify the suppliers and direct recipients of their food / feed.
The responsibility to devise such traceability systems rests with FBOs that place such food or feed on the market as they are best placed to identify and manage their suppliers and customers.
4.4.2 Format of relevant information
Without prejudice to specific requirements, industry is allowed some flexibility concerning the format in which relevant information is made available. However, it requires both food businesses and the control authorities to take an active role in ensuring effective implementation.
It is the need to maintain and provide traceability information that is of primary importance, rather than the format in which it is kept.
However, the information needs to be sufficiently organised to enable availability ‘on demand’, without undue delay.
4.4.3 Traceability to be established at all stages
The traceability of food, feed, food-producing animals, and any other substance intended to be, or expected to be, incorporated into a food or feed must be established at all stages of production, processing and distribution along the food / feed chain.
4.4.4 Identify suppliers
FBOs must be able to identify any person from whom they have been supplied with a food, a feed, a food-producing animal, or any substance intended to be, or expected to be, incorporated into a food or feed.
To this end, such operators must have in place systems and procedures which allow for this information to be made available to the competent authorities on demand.
4.4.5 Identify businesses supplied
Food and feed business operators must have in place systems and procedures to identify the other businesses to which their products have been supplied. This information must be made available to the competent authorities on demand.
4.4.6 Food adequately labelled or identified
Food or feed which is placed on the market or is likely to be placed on the market in the Community must be adequately labelled or identified to facilitate its traceability, through relevant documentation or information in accordance with the relevant requirements.
Reference: (EC) 178/2002, Article 18.
Labelling legislation is generally enforced by Local Authorities (LAs) or by the Rural Payments Agency (Beef Labelling Scheme).
4.4.7 Information to be made available by the FBO
Commission Implementing Regulation (EU) No. 931/2011, Article 3, states that:
- FBOs shall ensure that the following information concerning consignments of food of animal origin is made available to the FBO to whom the food is supplied and, upon request, to the CA:
- an accurate description of the food
- the volume or quantity of the food
- the name and address of the FBO from which the food has been dispatched
- the name and address of the consignor (owner) if different from the FBO from which the food has been dispatched
- the name and address of the FBO to whom the food is dispatched
- the name and address of the consignee (owner), if different from the FBO to whom the food is dispatched
- a reference identifying the lot, batch or consignment, as appropriate
- the date of despatch
- The information referred to in paragraph 1 is to be made available in addition to any information required under relevant provisions of EU legislation concerning the traceability of food of animal origin.
4.4.8 Updated on a daily basis
The information referred to in paragraph 1 (as quoted above) is to be updated on a daily basis and kept at least available until it can be reasonably assumed that the food has been consumed. The period during which this information must be available depends on the shelf life of product and guidance is available (see later in this chapter).
Reference: Commission Implementing Regulation (EU) No. 931/2011, Article 3, 3.
4.4.9 Provision of information without undue delay
When requested by the CA, such information is to be provided without undue delay. The appropriate form in which the information must be made available is up to the choice of the supplier of the food, as long as the information requested in paragraph 1 is clearly and unequivocally available to and retrievable by the business operator to whom the food is supplied.
4.4.10 Internal traceability
The regulations do not require a link between incoming and outgoing products, (so called ‘internal traceability’), nor is there any requirement for records to be kept identifying how batches are split and combined within a business to create particular products or new batches.
The decision on whether to implement an internal traceability system, and when implemented the level of detail of such an internal system, is a commercial decision left to the FBO and may be commensurate with the size and nature of the food business.
Nevertheless, an internal traceability system would contribute to more targeted and accurate withdrawals. FBOs are likely to save costs in terms of time of a withdrawal and in avoiding unnecessary wider disruption which in turn would help maintain consumer confidence. Traceability systems can also provide information within food businesses to assist in process control and stock management.
4.4.11 Always applicable
The traceability requirements of Article 18 of Regulation 178/2002 are general requirements and are always applicable to all food / feed.
FBOs should determine whether specific sectorial traceability provisions applicable to their sector or specific regulations laying down marketing and quality standards for certain products (for example, Beef Labelling Scheme, Poultry Meat Marketing Standards) already meet the requirements of the regulations.
4.4.12 Retention period for traceability records
The minimum period of time for keeping traceability records is not specified in the Regulations and it is for the business to decide. However, failure to produce adequate records would constitute a breach of the requirements.
Current European Commission guidance suggests that a general rule of a 5 year period from the date of manufacturing or delivery to destination would meet the objective of the regulations.
Reference: Guidance on the Implementation of Articles 11, 12, 14, 17, 18, 19 and 20 of Regulation (EC) No. 178/2002 on General Food Law.
4.4.13 Specific examples of suggested record retention periods
The common rule above can be adapted for products with a short shelf life:
- for highly perishable products with a ‘use by’ date less than 3 months or without a specified date, destined directly to final consumer, records could be kept for 6 months after date of manufacturing or delivery
- for products with a ‘best before’ date, records could be kept for the period of the shelf life plus 6 months
- for products without a specified durability date, the 5 years period could apply
4.5 FBO responsibilities: provision of information on frozen food of animal origin
4.5.1 Information requirements for frozen food of animal origin
For frozen food of animal origin, Regulation (EC) No. 853/2004 (as amended by Regulation (EU) No. 16/2012) requires FBOs to make available to the FBOs they supply information concerning the date of production and, if different, also the date of freezing.
4.5.2 Date of production
In this context, ‘date of production’ means:
- the date of slaughter in the case of carcases, half carcases or quarter carcases
- the date of killing in the case of bodies of wild game
- the date of harvesting or catching, in the case of fishery products
- the date of processing, cutting, mincing or preparation, as appropriate, for any other food of animal origin
Reference: (EC) No. 853/2004 (as amended by (EU) No. 16/2012), Annex II, Section IV.
4.5.3 Information to be made available
Until the stage at which frozen food of animal origin is labelled for the consumer in accordance with Directive 2000/13/EC (the EU Food Labelling Directive) or used for further processing, FBOs must ensure that they make the following information available to the FBOs they supply and, upon request, to the CA:
- the date of production; and
- the date of freezing, if different from the date of production
Where a frozen food of animal origin is made from a batch of raw materials with different dates of production and of freezing, the oldest dates of production and / or of freezing, as appropriate, must be made available.
Reference: (EC) No. 853/2004 (as amended by (EU) No. 16/2012), Annex II, Section IV.
4.5.4 Format of the information
The appropriate format in which the information must be made available is for the FBO supplying the frozen food of animal origin to decide, but they must ensure that the required information is clearly and unequivocally available to, and retrievable by, the FBO to whom the food is supplied.
Reference: (EC) No. 853/2004 (as amended by (EU) No. 16/2012), Annex II, Section IV.
4.6 FSA role
4.6.1 AO responsibility
As part of the official controls carried out by the CA for food, the AO has responsibility for ensuring that the traceability requirements are complied with.
4.6.2 OV to monitor traceability system
The AO should monitor the FBO’s traceability system in place.
This will be achieved by learning about how the FBO created it, uses it and how the system works in practice. Each FBO will have their own traceability system(s) and the OV should familiarise themselves with it in order to understand and monitor it.
The AO should ensure that any other relevant legislation with an impact on traceability data is also implemented by relevant FBOs in addition to the general traceability requirements. For example, the requirement for labelling bovine carcases with red stripe label when the removal of the vertebral column is required or the need for commercial documents to contain this information as required by Regulation (EC) No. 999/2001 (as amended), Annex V, 11.3, b; or the requirement for internal traceability to be maintained for beef under the Beef Labelling Scheme (the latter is not enforced by the FSA).
Reference: See chapter 2.7 on ‘SRM’, section 2 for details relating to the example quoted above.
4.6.3 AO to verify traceability system
The AO should verify that the traceability system in place is being carried out in accordance with the requirements of the relevant legislation. This should include a traceability check ‘in situ’ in addition to a check on the historical traceability records.
The traceability check ‘in situ’ should take the form of selecting a product from the intake or dispatch bays where finished products or ingredients are found, identifying the information available on the products and seeking the relevant traceability records: both intake and despatch documents should have all the required information.
This check ‘in situ’ may be performed in the event of finding raw materials, ingredients and / or products with poor or unclear traceability data, when there is suspicion that product may have been mislabelled (for example, meat substitutions) and / or as often as the OV considers necessary to ensure that the FBO satisfies the requirements of the regulations with regards to traceability.
4.6.4 AO to verify FBO takes further action
The AO should verify that where further action by the FBO is required, this action is taken promptly and efficiently.
Where traceability details on the product and / or records are not available and / or are proven to be wrong, the FBO will need to demonstrate what action is taken to correct it.
4.6.5 AO to take enforcement action where appropriate
The AO should take appropriate and proportionate enforcement action when necessary, as described in MOC chapter 7 on ‘Enforcement’. Some specific examples are given on the following pages.
4.7 Enforcement action: examples
4.7.1 Health marked product
Where health marked products fail to comply with the traceability requirements of Article 18, Regulation (EC) No. 178/2002 as read with Commission Implementing Regulation (EU) No. 931/2011, this will constitute an offence under Regulation 4(c) of the General Food Regulations 2004.
4.7.2 Health marked product: enforcement action in cases of non-compliance
Health marked carcases and primal cuts which have not left the slaughterhouse or been further cut or processed in a cutting plant, may not be certified under Regulation 27 of the Food Hygiene (Wales) Regulations 2006 / Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013 as failing to comply with the ‘Hygiene Regulations’. This is because Regulation (EC) No. 178/2002, Article 18, as read with Commission Implementing Regulation (EU) No. 931/2011 are excluded from the definition of ‘Hygiene Regulations’ and the requirements for traceability of ID marked products contained in Regulation (EC) No. 853/2004, Annex II, Section I, will not apply in these circumstances.
Where health marked products which have not left the approved slaughterhouse or been cut or further processed in a cutting plant have associated commercial documentation that fails to comply with the traceability requirements, they should be formally detained until the commercial documentation has been altered to accurately detail the products being consigned from the establishment in accordance with the legal requirements.
Where products bearing a health mark have been despatched without adequate traceability information, the FBO must be advised in the first instance in order that they take corrective action. Enforcement should be escalated to ensure that commercial documents reflect the information required under Article 18, Regulation (EC) 178/2002 as read with Commission Implementing Regulation (EU) No. 931/2011. If serious / repetitive breaches have been identified, the FBO should be referred for investigation.
4.7.3 Health mark legibility
Where the traceability deficiency identifies a failure to comply with the food safety requirements, the FBO shall initiate procedures to withdraw or recall the food in accordance with Article 19 of Regulation (EC) 178/2002. Where health marked products have been consigned to another establishment, the FBO of the recipient plant(s) should be informed, as their ability to comply with the traceability requirements may be hampered as a result of the inaccurate information they receive, which may cause them to inadvertently mislabel products they subsequently supply.
The AO / enforcement authority responsible for enforcement action at subsequent establishments must be informed so that all appropriate action is taken. This may include formally detaining product until commercial documentation has been provided that accurately details the products consigned by the supplier.
4.7.4 ID marked product
Where ID marked products fail to comply with the traceability requirements of Article 18, Regulation (EC) No. 178/2002 as read with Commission Implementing Regulation (EU) No. 931/2011, they will also breach the provisions of Regulation (EC) No. 853/2004, Annex II, Section I, Part A, paragraph 4.
This will constitute an offence under Regulation 17 of the Food Hygiene Wales) Regulations 2006 / Regulation 19 of the Food Safety and Hygiene (England) Regulations 2013, as well as Regulation 4 (c) of the General Food Regulations 2004 (Wales).
Failure to comply with Regulation (EC) No. 853/2004, Annex II traceability requirements will apply only to products that have been further cut or processed and have received an Identification Mark.
4.7.5 ID marked product: enforcement action in cases of non-compliance
Where ID marked products fail to comply with the traceability requirements of Regulation (EC) No. 853/2004, enforcement should be escalated in accordance with MOC chapter 7 on ‘Enforcement’.
An assessment should also be made with respect to any potential fraud, the FBO’s ability to trace all meat to comply with any product recall or withdrawal requirements and to determine whether the raw materials for the product were processed lawfully in approved establishments.
Where appropriate, non-compliant ID marked products may be formally certified under Regulation 27 of the Food Hygiene (Wales) Regulations 2006 / Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013, as not having been produced, processed or distributed in accordance with the ‘Hygiene Regulations’ due to its failure to comply with Regulation (EC) No. 853/2004. Where voluntary surrender is not forthcoming, non-compliant products may be formally seized and a Condemnation Order applied for at the Magistrates / Sheriffs Court. Non-compliant product that has been so certified will be deemed to fail to comply with the food safety requirements and the FBO must initiate withdrawal or recall of the product in line with Article 19 of Regulation (EC) 178/2002. A referral for investigation may also be appropriate for serious or repetitive breaches or where public health protection is being compromised.
4.8 Summary
4.8.1 Summary
Checks on compliance with traceability requirements will be achieved initially over the duration of the approval process, followed by audits at the appropriate frequency and during UAI to approved establishments.
Reference: The FBO Audit Aide Memoire located at Chapter 4.1 Audit, Annex 1, contains pointers for the auditing AO to consider in relation to traceability. A traceability system can be considered acceptable when it delivers accurate information in a timely manner. Assurance of this may be attained by checking product and records data against the system in place.
It is essential that the FBO’s traceability system is designed to follow the physical flow of the product and helps to identify its location at a given moment in time.
This means that the FBO must provide evidence of the traceability for animals, raw materials and/or ingredients received at the premises, allowing for the identification of their location. The same applies to any products manufactured on site that are to be despatched.
5. Procedures for the verification of the manufacture of beef patties and burgers intended to be consumed less than thoroughly cooked at retail level
In this section
5.1 Background
5.1.1 History and key issues
The sale and consumption of beef patties and burgers served less than fully cooked is a trend that has been steadily increasing in the UK. A number of catering chains and outlets now offer this option to customers. The FSA has produced guidance for FBOs and LAs with advice on safe systems for serving less than thoroughly cooked patties and burgers and on the enforcement approach.
This section is intended to complement the LA document, by giving FBOs and FSA officials information about how to approach, audit and assess approved manufacturers that supply catering establishments (for example, restaurants and pubs) that intend to serve less than thoroughly cooked beef patties and burgers to the final consumer.
5.1.2 Identification of retail establishments
New and existing FBOs who intend to serve less than thoroughly cooked patties and burgers are required to notify their LA before they commence this activity in line with requirements in food hygiene legislation. This will allow LAs to assess the FBO’s proposed HACCP based procedures and discuss as appropriate.
It will also allow the identification of establishments involved in the supply chain, particularly those that provide minced meat or meat preparations to the caterer.
5.2 Legislative references
5.2.1 Regulation (EC) No 178/2002
(EC) No 178/2002 lays down general food safety requirements, according to which food must not be placed on the market if it is unsafe.
5.2.2 Regulation (EC) No 852/2004
Regulation (EC) No 852/2004 lays down the principles of food hygiene. In particular article 4 requires FBOs to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles.
5.2.3 Regulation (EC) No 853/2004
Regulation (EC) No 853/2004 lays down specific requirements for the manufacture of minced meat and meat preparations. In particular Section V, Chapter III of Annex III concerns the age and / or temperature of meat used for minced meat and meat preparations.
5.2.4 Regulation (EC) No 2073/2005
Regulation (EC) No 2073/2005 (as amended) sets out the microbiological criteria for certain micro-organisms and the implementing rules to be complied with by FBOs when implementing the general and specific hygiene measures referred to in Regulation (EC) No 852/2004.
5.2.5 Food hygiene regulations
The Food Hygiene (S/W) Regulations 2006 (as amended) / The Food Safety and Hygiene (England) Regulations 2013 make it an offence for any person to contravene or fail to comply with the specified community provisions.
5.3 Understanding the hazards
5.3.1 E. coli O157 and other STEC (Shiga-toxin producing E. coli) and Salmonella
The key microbiological hazards that can be associated with raw beef are E. coli O157 and other STEC (Shiga-toxin producing E. coli) and Salmonella.
E. coli O157 and other STEC are of particular concern because although uncommon they can have a low infectious dose and can cause serious illness and lead to death in some cases.
The main source of E. coli is the intestines of cattle and sheep. When cattle and sheep are slaughtered there is the potential for E.coli O157 from the animal’s gut and hide to contaminate the carcases during the slaughter and preparation of the meat.
Salmonella can also be present in the gut of animals, particularly poultry. While the number of cases of disease associated with
Salmonella is decreasing it can also cause death.
Contamination of whole cuts of beef and lamb therefore tends to be only on the outside of meat.
5.4 FBO role – minimum HACCP requirements
5.4.1 HACCP
The law requires FBOs to have food safety management procedures in place, based on HACCP principles, which effectively control the risks at all stages of the food chain. Consequently, the FBO’s documentation has to consider the key SOPs / CP / CCPs in the process at all stages.
Operators in the supply chain of meat that is intended for the production of burgers and patties intended to be consumed less that thoroughly cooked, must be able to demonstrate that their own procedures are effective in reducing the risk to an acceptable level and adapt their food safety management systems accordingly.
5.4.2 At slaughterhouse level
The FBO has to consider at least the following steps to assess the hazards and subsequently implement controls to ensure food safety:
- intake of livestock (for example clean livestock policy)
- removal of the hide
- evisceration process
- chilling of meat
- intervention techniques (for example, use of lactic acid or steam vacuum)
Further guidance on food safety management for businesses can be found on the FSA website.
Further guidance on acceptable standards for clean cattle and sheep can be found online.
5.4.3 At cutting plant level
Likewise, the FBO must assess the hazards associated to the cutting plant processes, including the mincing, mixing and the forming of patties and burgers. To that effect, the FSA has produced a model HACCP plan that can be used to guide FBOs when developing controls in their production.
5.4.4 Application of microbiological criteria
The Microbiological Criteria Regulation contains two food safety criteria applicable to the manufacture of minced meat and meat preparations:
- minced meat and meat preparations intended to be eaten raw (absence of Salmonella in 25g)
- minced meat and meat preparations made from species other than poultry intended to be eaten cooked (absence of Salmonella in 10g)
Burgers that are not thoroughly cooked will contain some meat that is not cooked all the way through. The FSA therefore considers that the more stringent of the two criteria (absence in 25g, for mince or meat preparations intended to be eaten raw) should be applied.
Note: Current UK exemption under the microbiological criteria for small establishments producing less than 2 tonnes of minced meat / meat preparations per week is not applicable to those establishments producing patties and burgers intended to be eaten less than thoroughly cooked. Therefore, even if only a small amount of such products is produced, microbiological testing is required.
5.4.5 Ageing of meat
Establishments supplying or manufacturing raw materials for retail establishments serving beef patties or burgers less than thoroughly cooked shall comply either with the requirements set in regulation 853/2004 in relation to the age of the meat used for mincing:
- no more than 6 days from slaughter unless boned and vacuum packed where the age limit is 15 days
- for frozen meat there is not a specific requirement other than it must be boned before freezing and stored only for a limited period
or comply with the FSA guidance when exceeding the established age for the manufacture of minced meat (patties).
Note: This requirement is only applicable to the manufacture of patties / minced meat only products. Meat preparations (for example, burgers) are not obliged to comply with age limit requirements.
5.4.6 Raw materials
For the manufacture of burger patties (minced meat shaped into burger patties but meeting the definition of minced meat), the raw material used must:
- comply with the requirements for fresh meat and derive from skeletal muscle
- not derive from:
- scrap cuts or scrap trimmings
- MSM
- the region of the carpus or tarsus
- meat containing bones or skins
- meat of the head (except masseters)
- bone scrapings
- the diaphragm (unless the serosa has been removed)
- the non-muscular part of the linea alba
For the manufacture of burgers (also raw patties, but due to the addition of seasonings or other ingredients they meet the definition of meat preparations), the raw materials used must meet the requirements of fresh meat or the above requirements. However, if the final product is intended to be heat treated (as in the case of burgers, even those that will not be thoroughly cooked), scrap cuts or scrap trimmings and MSM complying with the microbiological requirements for minced meat can be used (see sub-topic 5.4.3 At cutting plant level).
5.5 FSA role – verification and audit
5.5.1 OV role
The OV in slaughterhouses or cutting plants sourcing establishments involved in the serving of beef patties and burgers less than thoroughly cooked must ensure that the FBO has developed a suitable HACCP plan and carry out spot checks to ensure this is implemented accordingly.
5.5.2 Verification at audit
The FBO’s food safety management procedures based on HACCP principles, associated records and processes will be verified during audit both at slaughterhouses and at cutting plants involved in the supply chain.
Attention should be paid to the validation procedures, particularly where the minced meat or meat preparations are supplied ready for use by the caterer with only minimal cooking required before it is served to the final consumer.
In addition, some of the treatments described in the guidance for caterers and LAs may be applied at the cutting plant; officials must therefore familiarise themselves with its content.
5.5.3 Other guidance
In addition to the guidance for caterers and LAs, and the FSA guides above, the FSA guidance on the control of cross-contamination may be of assistance.
6. Verification of Water Testing Procedures
6.1 Introduction
6.2 ‘Potable’ water
6.3 Legal requirements for water usage
6.4 Monitoring of Water Supply (private and/or public)– FBO Obligations
6.5 Private Water Supplies
6.6 Public Mains Supply
6.7 Water Testing Programme
6.8 Links to the Water Supply (Water Quality) Regulations
6.1 Introduction
Water can be a potential source of microbiological and chemical hazards. Micro-organisms that cause food poisoning can survive for days or even months in water.
Procedures are needed to minimise the risk of such hazards causing contamination and therefore illness to consumers.
Examples demonstrating the importance of monitoring the water supply:
| Problem | Effect | Outcome |
|---|---|---|
| Water supplies can become polluted with human sewage or agricultural waste containing faecal contamination from animals | Contaminated water supply (biological contaminants) | A source of microbiological contamination |
| Water supplies can be a source of chemical contaminants, such as heavy metals, pesticides, nitrates, and industrial pollutants, which can be transferred from water used in processing or cleaning onto food | Contaminated water supply (chemical contaminants) | A source of chemical contamination |
| Water distribution system not kept clean, adequately maintained, or infrequently used water storage tanks and/or pipes | Bacteria can, in specific circumstances, multiply or create biofilms in water distribution systems and chemical residues can be present and spread to other parts of the food production system and transferred to food | A source of microbiological and/or chemical contamination |
6.2 'Potable' water
There are multiple provisions within the retained EU Food Hygiene Regulations that require an FBO to use ‘potable’ water in food production.
6.2.1 The definition of 'potable' water
‘Potable water’ is defined in Article 2 of Retained Regulation (EC) 852/2004 (as amended following EU exit):
(i) as regards England, water meeting the requirements laid down in the Private Water Supplies (England) Regulations 2016 (as amended);
(ii) as regards Wales, water meeting the requirements laid down in the Private Water Supplies (Wales) Regulations 2017.
Whilst Article 2 refers to the ‘Private’ Water Supplies Regulations to define ‘potable’ water, the water requirements referred to in these regulations (see point 2.2 below) are applicable whether the FBO is getting their water from a private or a public (mains) water supply.
6.2.2 The Private Water Supplies Regulations
The requirements / standards for water that FBOs must achieve where potable water is required, are as follows:
PART 1 of the Private Water Supplies regulation defines ‘water intended for human consumption’ as all water:
(a) either in its original state or after treatment, intended for drinking, cooking, food preparation or other domestic purposes, regardless of its origin and whether it is supplied from any distribution network, from a tanker, or in bottles or containers;
(b) used in any food production undertaking for the manufacture, processing, preservation or marketing of products or substances intended for human consumption unless, in accordance with retained Regulation (EC) 852/2004, the competent authority, is satisfied that the quality of the water cannot affect the wholesomeness of the foodstuff in its finished form.
PART 2 states that for water to be regarded as wholesome the following conditions are to be met:
(a) it does not contain any micro-organism, parasite, or substance, alone or in conjunction with any other substance, at a concentration or value that would constitute a potential danger to human health,
(b) it complies with the concentrations or values prescribed in Part 1 of Schedule 1 for each parameter, and
(c) the water satisfies the formula “[nitrate]/50 + [nitrite]/3 ≤ 1”, where the square brackets signify the concentrations in mg/l for nitrate (NO3) and nitrite (NO2).
6.3 Legal requirements for water usage
6.3.1 Retained Regulation (EC) 852/2004
Annex II, Chapter II (2) “Requirements in rooms where foodstuffs are prepared, treated or processed” require adequate facilities to be provided, where necessary, for the cleaning, disinfecting and storage of working utensils and equipment. These facilities are to be constructed of corrosion-resistant materials, be easy to clean and have an adequate supply of hot and cold water.
Annex II, Chapter II (3) requires that adequate provision is to be made, where necessary, for washing foods. Every sink or other such facility provided for the washing of food is to have an adequate supply of hot and/or cold potable water consistent with the requirements of Chapter VII and be kept clean and, where necessary, disinfected.
Annex II, Chapter VII, refers to hygiene requirements for FBOs with regards to Water Supply:
- There is to be an adequate supply of potable water, which is to be used whenever necessary to ensure that foodstuffs are not contaminated.
- Where non-potable water is used, for example for fire control, steam production, refrigeration, and other similar purposes, it is to circulate in a separate duly identified system. Non-potable water is not to connect with or allow reflux into, potable water systems.
- Recycled water used in processing or as an ingredient is not to present a risk of contamination. It is to be of the same standard as potable water, unless the competent authority is satisfied that the quality of the water cannot affect the wholesomeness of the foodstuff in its finished form.
- Ice which comes into contact with food, or which may contaminate food is to be made from potable water and handled and stored under conditions that protect it from contamination.
- Steam used directly in contact with food is not to contain any substance that presents a hazard to health or is likely to contaminate the food.
- Where heat treatment is applied to foodstuffs in hermetically sealed containers it is to be ensured that water used to cool the containers after heat treatment is not a source of contamination for the foodstuff.
6.3.2 Retained Regulation (EC) 853/2004
Article 3(2) of Chapter II refers to Food Business Operators obligations: FBOs shall not use any substance other than potable water to remove surface contamination from products of animal origin, unless use of the substance has been prescribed by the appropriate authority.
Annex III, Section I, Chapter IV (9): when not skinned, porcine animals must have their bristles removed immediately. The risk of contamination of the meat with scalding water must be minimised. Only approved additives may be used for this operation. Porcine animals must be thoroughly rinsed afterwards with potable water.
6.3.3 Water Fittings Regulations
In order to protect the water supply from contamination, it is important that fixtures and fittings are correctly installed and in a good state of repair. The Water Supply (Water Fittings) Regulations 1999 set out requirements for the design, materials, installation and maintenance of plumbing systems, water fittings and water-using appliances. Businesses have the legal duty to ensure that their systems satisfy these requirements. The regulations apply in England and Wales to all plumbing systems, water fittings and equipment supplied, or to be supplied, with water from the public water supply. This applies to systems in all types of premises. The FSA is not the enforcement authority for these regulations but should be aware of the possibility of contamination from this source.
6.4 Monitoring of Water Supply (private and/or public)– FBO Obligations
Supply – FBOs will have to consider the need for adequate water supplies for food processing, cleaning and other requirements in the design and construction of premises or when buildings are rebuilt, altered, or refurbished.
Capacity – FBOs are to make sure that the water distribution system has sufficient capacity to meet demand at peak times (for example, during cleaning).
Water storage tanks – when used, these should be made of inert material to avoid chemical contamination of water and corrosion. They must be kept covered and secured to prevent contamination. Tanks should be cleaned regularly to prevent any build-up of organic or mineral material that could act as a source of microbial growth and contamination. Disinfection systems, if used, should follow ‘specialists' advice. Examples of disinfection systems can include chlorination, ozonation or UV light.
Pipes – the composition and condition of the pipes can influence what chemicals may be released into the system and the types of bacteria that colonise water distribution systems and pipe surfaces. Some pipe materials may break down and release bio source compounds, such as iron, hydrogen, and phosphate, that can encourage biofilm growth and bacteria to multiply. Biofilms coating the interior surfaces of water distribution pipes usually develop slowly and can take years to reach maturity. Older pipes are therefore more prone to developing large amounts of scale and rust. Water distribution systems should be kept in good condition through regular inspections for signs of damage, corrosion and leaks and records of these checks should be kept by the FBO as part of their pre-requisites controls.
Plans – water distribution systems can be complex, especially in larger premises. Detailed water distribution plans will help to identify any redundant pipe work that could act as a reservoir of microbiological contamination and to define an area to be isolated if contamination occurs. FBOs should keep an accurate and dated plan of the potable water system (and any non-potable water system if applicable), including pipe work, point of entry of water into the premises and numbered outlets. The plan should be submitted with applications of approvals of new premises and should be kept updated if alterations are made.
Non-potable water – may be used in food premises for certain purposes such as fire control, non-food contact steam generation or refrigeration. Potable and non-potable water systems are to be clearly identified, particularly water outlets to avoid misuse of non-potable water.
Ice used in contact with food – FBOs need to make sure ice is made from potable water and is handled and stored under conditions that protect it from contamination (for example containers are kept covered and are cleaned and disinfected periodically).
Water testing – regular testing of water samples from cold or mixed hot/cold water outlets where the water could come into direct contact with food, food processing equipment, or food handlers, will indicate whether contamination is occurring and if water is potable. Water testing programmes, as described in point 7 below, should be established by FBOs at their premises as part of their pre-requisite programmes and at a frequency based on a risk assessment.
6.5 Private Water Supplies
Water that does not originate from public mains is described as private water supply. It can be from ground waters (for example, boreholes, wells, and springs) or from surface waters (for example, streams, rivers, lakes, and lochs). A Local Authority (LA) will carry out a risk assessment for every private water supply in their area and review and update that risk assessment at least every 5 years (or earlier if considered that the existing risk assessment is inadequate) in accordance with the requirements of the Private Water Supplies Regulations. FBOs can and should request a copy of the risk assessment to their LA where a private water supply is feeding their business.
Private water supplies are not treated and therefore are more likely to be contaminated with micro-organisms and/or chemicals. Private water supplies may become contaminated with harmful pathogens if the borehole headworks are not sealed properly, there is ingress of surface water or access to spring sources allowing water sources to be contaminated by faecal matter.
Where water is drawn from a private supply it may require disinfection treatment (for example, filtration, ultra-violet light, chlorination). FBOs using private water supplies must consult a water treatment specialist to help identify the most effective method and requirements for regular maintenance.
6.6 Public Mains Supply
Water may be drawn from the public ‘mains supply’ network operated by a water company (a water undertaker or licensed water supplier). Water suppliers are required to monitor the microbiological and physical-chemical quality of mains water entering the premises to demonstrate that it meets the standards required in the Water Supply Regulations. A copy of their test results can be obtained but usually with a delay.
FBOs must demonstrate that the water used at their premises is potable so will need to carry out their own independent verification tests as described in point 7 below.
Water tanks are common habitats for water microbes; systems with water tanks that do not turn over frequently can encourage microbiological activity. If mains water is stored in tanks before use and/or if the water distribution system is complex and/or old, the water can become contaminated after entering the premises. This is to be taken into consideration as part of the FBO’s risk assessment.
Regular testing of water samples from cold or mixed hot/cold water outlets where the water could come into direct contact with food, food processing equipment or food handlers will indicate whether contamination is occurring on site. FBOs will need to carry out a risk assessment at their premises to decide their own water testing programme as part of their pre-requisite programme.
6.7 Water Testing Programme
6.7.1 Microbiological Parameters
The testing programme, for both public and private water supplies, shall include testing for the following microbiological parameters to demonstrate that the water is potable:
- E. coli and Enterococci as per Part 1 of Schedule 1 of the Private Water Supplies Regulations Presence of these organisms in the water is an indication of faecal contamination and is considered high risk.
- Indicator Parameters listed in Part 2 of Schedule 1 of the Private Water Supplies Regulations, including Coliforms, TVC at 22°C, and, in the case of surface water or groundwater that has been influenced by surface water, Clostridium Perfringens. Routine sampling of TVC at 22°C will help to understand baseline levels and therefore identify any significant deviation from that baseline (when there are abnormal changes).
- The frequency of testing shall be decided by the FBO based on a risk assessment taking into consideration the particularities at their premises and the type of production. As an example; when the risk is considered low (e.g. small cutting plant where water is from mains supply, pipe systems are well maintained, there is no storage tank, water is not used as an ingredient and where previous test results have been satisfactory), yearly testing for all the microbiological parameters detailed in Parts 1 and 2 of Schedule 1 (as above) might be sufficient. In any case, a risk assessment would be necessary to ascertain the sampling frequency.
- FBOs with a private water supply should liaise with their local authority to establish any additional parameters they should be testing for and the frequency of testing, taking into account any risk factors specific to their area and to their establishment (including type of production). FSA officials should ensure they are aware of any additional information provided by the local authority when assessing whether a water testing programme is adequate.
- FBOs who use a public mains supply, although they will likely need to test less frequently, their water testing programme will have to take into consideration any particular risks in the area (for example through information provided by their water supplier) and any particular risks linked to their establishment and type of production (for example if they use water storage tanks or if the water is used as an ingredient).
6.7.2 Physical-chemical Parameters
- Private water supplies should be tested by local authorities for residues of pesticides that may come from crop spray runoff after heavy rain and heavy metals that may be present in the rock strata where the water originates. FBOs should request a copy of the test of the private water supply undertaken by the local authority in their area and this information should be considered when determining how often to test and what to test for.
- Public mains water suppliers should provide an annual summary of the physical-chemical analysis of the water in the area. A copy of these results can be obtained from the supplier for the previous year.
6.7.3 Water sampling procedures
Water samples need to be taken carefully so that no contamination is introduced when the sample is taken. Staff taking the samples should receive specific training. All samples are to be identified with the specific sample point location (all sample points should be identified in the water distribution plan).
FBOs should use laboratories that are accredited by a recognised body for the relevant test methods used in water sampling such as UKAS or at least participate in proficiency testing schemes.
6.7.4 FBO follow-up actions
Presence of E. coli or Enterococci: These faecal indicators are high risk and must not be present in potable water (the limit is 0/100ml).
If the water supply has become contaminated before entering the premises, it is the water supplier’s or local authority’s responsibility to communicate the issue to the FBO and restore potable water quality.
Regardless of whether the contamination occurred before or after entering the FBO premises, if the water supply is contaminated at the establishment, the FBO must take urgent corrective actions to ensure food safety. Corrective actions may include (this list is not exhaustive):
- isolating affected water outlets / tanks until satisfactory microbiological test results are obtained
- retesting at the point of entry, at the outlet from which the contaminated sample was obtained and at any other associated outlets
- stopping production where no potable supply can be provided
- dealing with any product that has potentially been contaminated
- investigating the potential source of the contamination and, if necessary, contact the water supplier or the local authority
- establishing and, where necessary, eliminating the underlying cause to prevent similar contamination incidents in the future, such as, for example the installation of either a water filtration and chlorination system or a water filter and ultra-violet sterilisation system
- reviewing sampling and testing procedures and improve staff instructions and training as required
Presence of Indicator Parameters such as:
- Coliforms: 0/100ml
- Abnormal changes (deviations from the trend identified during routine testing or considerable deviations from results provided by the water supplier or the local authority) to TVC at 22°C: number/ml
- Clostridium perfringens: 0/100ml (only in the case of surface water or groundwater that has been influenced by surface water)
Coliform bacteria are microbes found in the digestive systems of warm-blooded animals, in soil, on plants, and in surface water. These microbes typically do not make people sick; however, because microbes that do cause disease are hard to test for in the water, “total coliforms” are tested instead. If the total coliform count is high, then it is very possible that harmful germs like viruses, bacteria, and parasites might also be found in the water.
Incidents where the maximum concentration of indicator parameters has been exceeded or where abnormal changes in TVC numbers are observed, require a similar approach to investigating failures of E. coli and/or Enterococci, although the risk to human health is likely to be lower, which may allow the company to take different investigation paths.
When deviations occur, this may trigger further investigation and follow-up actions should include, at the very least, re-sampling of the outlet from which the non-compliant sample was taken as well as from any other sampling point considered relevant. Re-sampling should include testing for E. coli and Enterococci. If the test is again positive for the indicators but there is no evidence of faecal or other contamination, FBOs should investigate the source of the problem and keep re-testing until a compliant result is received.
| Microbiological Parameter | Satisfactory level (number/100 ml) | Satisfactory level (number/ml) |
|---|---|---|
| E.coli | 0 | N/A |
| Enterococci | 0 | N/A |
| Clostridium Perfringens (In the case of surface water or groundwater that has been influenced by surface water) | 0 | N/A |
| Coliforms | 0 | N/A |
| TVCs at 22°C | N/A | No abnormal change |
6.7.5 FSA oversight and follow-up actions
FSA officials should be satisfied that the risk assessment undertaken by the FBO to establish the water testing programme is appropriate for their establishment and has taken into consideration relevant factors, for example (this list is not exhaustive):
- the size of the establishment
- the origin of the water supply (private or mains)
- the use/non-use of water storage tanks
- the type of production (water/ice used as an ingredient)
and that the programme is documented, includes follow up actions in the event of unsatisfactory results and is effectively implemented. Field Veterinary Leaders will undertake an initial assessment of the documented procedures during the Approval Visits. Copies of all relevant documents should be retained. Not presenting evidence of water testing results, might lead to the approval being refused.
If an FBO does not implement an adequate water testing programme, enforcement action may be appropriate based on failure to ensure that the water they use at their premises is potable as required by Article 2 of retained (EC) 852/2004 Regulation, and failure to adopt appropriate hygiene measures.
When there is evidence of E. coli and/or Enterococci being present in the water and the FBO does not take immediate action, enforcement action should be considered by FSA Officials. Enforcement action may include the use of FSA powers to make FBOs take the steps outlined in section 7.4 above.
If enforcement is proposed based on the presence of indicator parameters or suspected contamination other than E. coli or Enterococci, expert advice will be needed to determine if the water in question falls outside the definition of potable water set out at 2.2 above. FSA Officials should contact the Meat Hygiene Policy Team at meathygiene@food.gov.uk to facilitate this.
At all stages when considering enforcement actions, FSA officials should ensure that the strength and reliability of any evidence of contamination is considered. If there is high risk and strong evidence, then enforcement action is more likely to be appropriate than if there is low risk with weak evidence. If the evidence is poor then further investigation may be needed before taking enforcement, this will always depend on the facts and risk of the individual case.
6.8 Links to the Water Supply (Water Quality) Regulations
It should be noted that the FSA is not the enforcement authority for these regulations. They are included here for reference.
6.8.1 Public water supply
England
The Water Supply (Water Quality) Regulations 2016 (legislation.gov.uk)
The Water Supply (Water Quality) (Amendment) Regulations 2018 (legislation.gov.uk)
Wales
The Water Supply (Water Quality) Regulations 2018 (legislation.gov.uk)
Northern Ireland
The Water Supply (Water Quality) Regulations (Northern Ireland) 2017
Scotland
The Public Water Supplies (Scotland) Regulations 2014
6.8.2 Private water supplies
England
The Private Water Supplies (England) Regulations 2016 (legislation.gov.uk)
The Private Water Supplies (England) (Amendment) Regulations 2018 (legislation.gov.uk)
Wales
The Private Water Supplies (Wales) Regulations 2017 (legislation.gov.uk)
Northern Ireland
The Private Water Supplies (Northern Ireland) Regulations 2017
Scotland
The Water Intended for Human Consumption (Private Supplies) (Scotland) Regulations 2017
7. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: The specification for lactic acid
Annex 2: Poster for Campylobacter sampling in broiler carcases under the Process Hygiene Criteria
Revision log
Published: 19 January 2022
Last updated: 6 June 2024