Chapter 2.4 Post-Mortem, Health and Identification Marking
Chapter 2.4 Post-Mortem, Health and Identification Marking
Sections
6. Poultry Post-Mortem Inspection
7. Judgements at Poultry Post-Mortem Inspection
8. Wild Game Post-Mortem Inspection
9. Health and Identification Marking
11. Slaughter Hygiene Verification System in Red Meat
1. Introduction
In this section
1.1 Overview
1.1.1 Purpose
The principal purpose of post-mortem inspection is to supplement ante-mortem inspection and to detect:
- diseases of public health significance
- diseases of animal health significance
- residues or contaminants in excess of the levels allowed by legislation
- the risk of non-visible contamination
- other factors which might require the meat to be declared unfit for human consumption or restrictions to be placed on its use
- visible lesions that are relevant to animal welfare such as beating or long-standing untreated injuries
1.2 Legislation
1.2.1 Regulations
Retained Regulations (EU) 2017/625, 2019/624 and 2019/627 details:
- who can undertake the post mortem-inspection
- the purpose of post-mortem inspection
- the post-mortem inspection procedures
- the decisions to be taken concerning meat
Retained Regulation (EU) 853/2004 details the standards that the Food Business Operator (FBO) should provide and achieve for post-mortem inspection.
1.2.2 Post-Mortem inspection requirements
Specific requirements for each species are listed in Retained Regulation (EU) 2019/627
Reference: See Annex 1 for a summary of post-mortem inspection requirements.
2. FSA role
In this section
2.1 Introduction to post-mortem inspection
2.1 Introduction to post-mortem inspection
2.1.1 Key principles
Post-mortem inspection should:
- take into account ante-mortem inspection results
- view all external surfaces
- pay particular attention to the detection of zoonotic and notifiable diseases
- take into account food chain information (FCI) or trained hunter’s declaration
- take place without delay after slaughter
- include carcases and accompanying offal
2.1.2 Contamination during inspection
During inspection, precautions must be taken to ensure that contamination of the meat by actions such as palpation, cutting or incision is kept to a minimum. Minimal handling of the carcase and offal should take place.
Bovine animals under 8 months old can undergo visual only inspection in accordance with Retained Regulation (EU) 2019/627, Article 18.
In relation to pig meat, the European Food Safety Authority (EFSA) adopted a Scientific Opinion which concluded that palpation or incisions in carcase and offal at post-mortem inspection should be omitted for pigs subjected to routine slaughter, because of the risk of microbial cross-contamination being higher than the risk associated with potentially reduced detection of conditions targeted by those techniques.
The use of palpation and / or incision should be limited to suspect pigs (see sub-topics 2.4.1 to 2.4.3 for further information).
2.1.3 Accuracy
The speed of the slaughter line and the number of inspection staff present must ensure proper inspection is completed and records maintained. Food business operators should be instructed to take immediate corrective action, including a reduction in the speed of slaughter, where:
- contamination is detected on external surfaces of a carcase or its cavities and the food business operator does not take appropriate action to rectify the situation; or
- if good hygiene practices are jeopardised.
Reference: Retained Regulation (EU) 2019/627 Article 12, 4 and Article 46, 1
MHI post-mortem inspection is for defect detection. OV post-mortem inspection is for disease diagnosis.
2.1.4 Additional examination requirements for post-mortem inspection
Where it is thought necessary, additional examinations are to take place such as palpation and incision of the carcase and offal and laboratory tests to:
- reach a definitive diagnosis
- detect the presence of:
- an animal disease
- residues or contaminants in excess of the levels allowed by community legislation
- non-compliance (NC) with microbiological criteria
- other factors that might require the meat to be declared unfit for human consumption or restrictions to be placed on its use
Note: Special attention should be taken in the case of animals having undergone emergency slaughter
- assess whether animal welfare is being compromised
2.1.5 OV presence (on the line)
The OV need not be present at all times on the line during post-mortem inspection if:
- an MHI carries out post-mortem inspection and puts aside abnormal meat with uncommonly occurring conditions and all other meat from the same animal
- the MHI documents their procedures and findings in a manner that allows the OV to be satisfied that standards are being met
- the OV subsequently inspects all such meat
The MHI may discard meat from poultry and rabbits with abnormalities and the OV need not systematically inspect all such meat.
2.1.6 MHI post-mortem decision tree

2.1.7 Abnormal meat
To consider an abnormal carcase meat/offal as ‘uncommon’, we could take into consideration different aspects such as:
- prevalence of the condition in the area
- prevalence of the condition in the flock / herd (degree of infection or infestation)
- the possible human health implications of the condition (such as zoonoses)
- the possible animal health implications of the condition (such as lesions which may indicate a possible notifiable disease such as classical swine fever, foot and mouth disease)
- possible animal welfare problems on farm, during transport or in the lairage
- the need to refer it to the veterinarian to do a differential diagnosis
- economic importance of the condition for the farming industry (degree of infestation)
Based on all the above, the MHI will need to make a judgement and notify the OV of the findings.
2.1.8 Examples of abnormal conditions that can be classified as common or uncommon
The table below outlines abnormal conditions and their classification.
| Abnormal condition | Comments | Occurrence |
|---|---|---|
| Broilers septicaemia / toxaemia | Very prevalent condition. It represented 14.75% of total conditions rejected in 2004. | Common |
| Mastitis in older cattle | Common condition in all species, especially cows. No need to inform the OV as the farmer is already aware and will receive notification when he is informed about the post-mortem inspection records. | Common |
| Sheep caseous lymphadenitis |
Is becoming more common but the OV needs to be made aware because of the economic importance of the disease (responsible for 1% of condemnations at meat inspection).
|
Uncommon |
| Cattle (30 month or younger) fascioliasis | Common in ungulates. The OV does not need to be informed. The disease is of great economic importance because of liver condemnations. The farmer will be informed when he receives notification of the post-mortem inspection findings. | Common |
| Pigs pleurisy / pneumonia | Inflammation of the pleurae is a common meat inspection lesion in pigs. It requires the stripping of the pleura or removal of the rib cage, but carcase condemnation is not normally necessary. There is positive correlation between the number of carcases requiring lung condemnation and the number of those requiring pleura stripping. The OV does not need to be informed. | Common |
| Sheep anthrax | Normally identified at ante-mortem inspection if a suspect animal is found dead in the lairage. It is a notifiable disease, and it is a zoonoses. The OV must be informed and should immediately inform the APHA Duty Veterinarian. | Uncommon |
| Broilers mechanical damage | This is normally the result of poor functioning of the poultry plant machinery. The FBO has to be informed by the MHI if he has not already identified the problem. | Common |
| Cattle sarcocystis | The incidence is higher in older cattle but is an uncommon condition. Depending on the degree of infestation, the carcase and viscera have to be rejected. The OV should be informed. | Uncommon |
| Pigs ascariasis (milk spot) | The second most recorded condition at post-mortem in pigs (17% of total rejections in 2004). It is mainly identified in livers (‘milk spot’) which are unfit for human consumption. The farmer will be informed when he receives the post-mortem inspection report. The OV does not need to be informed. | Common |
2.2 FSA Duties
2.2.1 Outline
The following table outlines the duties of the FSA Operations Group with regard to post-mortem inspection.
| Role | By | Frequency |
|---|---|---|
| Carry out post-mortem inspection | An OV or MHI appropriately authorised under (EU) 2019/624, (or appropriately authorised slaughterhouse staff in poultry or rabbit slaughterhouses) working under the supervision of an OV | All carcases and accompanying offal without delay after slaughter |
| Carry out post-mortem inspection for animals subject to emergency slaughter outside the slaughterhouse | An OV only; this cannot be delegated to a MHI |
All carcases and offal as soon as possible.
|
| Carry out PM for animals accompanied by a farmer’s declaration | OV or MHI | All carcases and offal as soon as possible |
| Record post-mortem inspection results | OV or MHI (or plant inspection assistant (PIA) | At the time of post-mortem inspection |
| Apply Health Mark |
The Health Mark must be applied under the supervision of the OV
|
Immediately after post-mortem inspection (this may be prior to results of any examination for trichinella being available, if OV satisfied meat will only be placed on market if results are satisfactory) See chapter 2.6 on ‘TSE testing’ for health marking Bovine Spongiform Encephalopathy (BSE) tested cattle |
| Disease sampling / testing | OV or MHI | When disease is suspected |
| Monitoring sampling / testing | OV or MHI or specifically trained plant staff | When monitoring of disease is required, for example, TSE, trichinella |
2.3 Post-mortem inspection guidelines
2.3.1 Options in post-mortem inspection
Specific requirements for all species are listed in retained regulation (EU) 2019/627 Articles 14 to 28.
2.3.2 Splitting carcases
Carcases of domestic solipeds, bovine animals over eight months old and domestic swine more than five weeks old must be submitted for post-mortem inspection split lengthways into half carcases down the spinal column.
Reference: Retained Regulation (EU) 2019/627 Article 15, 2.
However, to take account of particular eating habits, technological developments or specific sanitary situations, the official veterinarian may authorise the submission for post-mortem inspection of carcases of domestic solipeds, bovine animals more than eight months old and domestic swine more than five weeks old that are not split in half.
In low-capacity slaughterhouses or low-capacity game-handling establishments handling fewer than 1 000 livestock units per year, the official veterinarian may, for sanitary reasons, authorise the cutting into quarter carcases of adult domestic solipeds, adult bovine animals and adult large wild game before post-mortem inspection.
Reference: Retained Regulation (EU) 2019/627 Article 15, 3 and 4.
The OV may also require any head or any carcase to be split lengthways if the inspection so necessitates.
Caution: Splitting the head of cattle carries a health and safety risk, and if the animal is required to be sampled for BSE it may only take place after the sample has been taken.
2.3.3 Minimal handling by inspectors
During inspection, precautions must be taken to ensure that contamination of the meat by actions such as palpation, cutting or incision is kept to a minimum.
Note: Whilst still allowing for adequate post-mortem inspection care must be taken not to de-value the carcase or offal when making post-mortem incisions.
2.3.4 Visual inspection only
Carcases and offal of pigs of all ages are to undergo visual inspection procedures. Further inspection procedures (FIP) (palpation and / or incision) can be carried out when one of the following indicates a risk to public health, animal health or animal welfare:
- checks on the FCI
- checks on any other data from the holding of provenance
- ante-mortem or post-mortem findings
Note: Further inspection can also be carried out if gathering of evidence is required for enforcement purposes (for example, welfare investigation).
2.3.5 Examples of conditions found in pigs at ante-mortem that might justify further inspection procedures at post-mortem
For the majority of the conditions listed on the current ante mortem inspection sheet there would be no need for pigs to be marked to undergo FIP at post-mortem.
However, the following may justify FIP:
- mastitis (if associated with general signs)
- moribund / recumbent
- orchitis (marked to consider Brucella, occupational zoonoses)
- suspect emaciation, poor condition
- suspect fever
- slaughtered in lairage
Note: the OV is not limited to these conditions and should use their professional judgement.
2.3.6 Examples of conditions found in pigs at post-mortem that might justify FIPs
For localised conditions on pig carcases, FIPs are not normally justified unless a generalised and-or septic condition is also observed / suspected.
The following localised conditions may justify detaining the carcase for FIP at post-mortem:
- multiple abscesses
- TB like lesions (in cases of enlarged lymph nodes)
When the OV / MHI suspects a generalised condition, in some cases the appropriate decision about the fitness of the meat for human consumption cannot be made without further examinations.
If any of the following conditions is observed / suspected, this may justify detaining the carcase or offal for FIP at post-mortem inspection:
- anaemia (may be part of other generalised condition)
- badly bled (may mask some other post-mortem signs)
- contamination gut content (may mask other conditions)
- emaciation / generalised oedema
- erysipelas
- generalised TB, tumours, melanosis
- jaundice
- machine damage (if may mask other conditions)
- poly-arthritis
- septic peritonitis
- septic pleurisy
- suspect pyaemia / multiple abscesses-tail bite-other
- suspect uraemia / abnormal odour
- suspect fever / septicaemia
- suspect residues
Note: The OV / MHI is not limited to these conditions and should use their professional judgement.
2.4 Decisions concerning meat
2.4.1 Animal carcases for which a ‘suspect animal card’ was completed
The OV must have a suitable system in place to inform the person(s) performing the post-mortem inspection of any condition that may help in the post-mortem judgement for that carcase. This includes any animals for which a ‘Suspect Animal Card’ has been completed and also pigs identified at ante mortem inspection as requiring further post-mortem inspection procedures other than visual inspection.
2.4.2 Possible outcomes
After the inspection, the OV/MHI can:
- pass the meat as fit for human consumption
- declare the meat unfit for human consumption
- detain the meat for further examination following rectification
2.4.3 Reasons for declaring meat unfit
Meat may be declared unfit for human consumption if it:
- derives from animals that have not undergone ante-mortem inspection, except for hunted wild game
- derives from animals the offal of which has not undergone post-mortem inspection, unless otherwise permitted under Regulation 853/2004 or Regulation 2019/627 Article 45(b).
- derives from animals which are dead before slaughter, stillborn, unborn, or slaughtered under the age of seven days
- results from the trimming of sticking points
- derives from animals affected by animal diseases for which animal health rules are laid down in Annex I to Council Directive 2002/99/EC except if it is obtained in conformity with the specific requirements provided for in that legislation, unless otherwise provided for in Section IV (Reference: Retained Regulation (EU) 2019/627 Article 45(e)
- derives from animals affected by a generalised disease, such as septicaemia, pyaemia, toxaemia or viraemia
- is not in conformity with microbiological criteria laid down under community legislation to determine whether food may be placed on the market
- exhibits parasitic infestation, unless otherwise provided for in Section IV
- contains chemical residues or contaminants in excess of the levels laid down in community legislation; any overshooting of the relevant level should lead to additional analyses whenever appropriate
- without prejudice to more specific community legislation, derives from animals or carcases containing residues of forbidden substances or from animals that have been treated with forbidden substances
- consists of the liver and kidneys of animals more than two years old from regions where plans approved in accordance with Article 5 of Directive 96/23/EC has revealed the generalised presence of heavy metals in the environment
- has been treated illegally with decontaminating substances
- has been treated illegally with ionising or UV-rays
- contains foreign bodies (except, in the case of wild game, material used to hunt the animal)
- exceeds the maximum permitted radioactivity levels laid down under community legislation
- indicates patho-physiological changes, anomalies in consistency, insufficient bleeding (except for wild game) or organoleptic anomalies, in particular a pronounced sexual odour
- derives from emaciated animals
- contains specified risk material, except as provided for under community legislation
- shows soiling, faecal, or other contamination
- consists of blood that may constitute a risk to public or animal health owing to the health status of any animal from which it derives or contamination arising during the slaughter process
- in the opinion of the OV, after examination of all the relevant information, it may constitute a risk to public or animal health or is for any other reason not suitable for human consumption
Where there is total rejection the whole carcase, offal and blood and the rest of body parts must be disposed of as an ABP.
Reference: Retained Regulation (EU) 2019/627, Article 45.
2.4.4 Reference link to pathological conditions
For poultry, consult the poultry condition cards found on Digital Workplace and linked from section 7 on ‘Judgements at poultry post-mortem inspection’ of this chapter.
2.4.5 Meat declared unfit
Where the OV is not satisfied that the meat is fit for human consumption, the health mark / identification mark must not be applied in accordance with retained Regulation (EU) 2019/627, Article 48, 2(a). The FBO should be asked to voluntarily surrender meat rejected as unfit for human consumption. Where surrender is not forthcoming, the OV should put in writing the reasons why they are formally declaring the meat unfit for human consumption in accordance with Retained Regulation 2017/625 Article 138,3.
Note: Where the FBO continues to refuse to dispose of meat that has been declared unfit, follow the ABP provisions relating to the treatment of meat declared unfit for human consumption. See chapter 2.8 on ‘Animal by-products’.
2.4.6 Further inspection required
If the OV / MHI considers that the carcase and offal require further inspection, the carcase and the associated offal must be detained and kept under control of the OV pending the inspection.
2.4.7 When partial rejection may be appropriate
Partial rejection of the meat or offal may be appropriate where only part of the carcase or a single organ is affected. Reject only the affected carcase part or offal and the tissue immediately surrounding it as an ABP.
2.4.8 Detention procedure
When detaining a carcase for further inspection it is important to maintain correlation of the detained carcase and all relevant parts until post-mortem inspection has been completed and any additional examinations have taken place.
The detention method and any other examinations that are carried out must be done in a manner that prevents the risk of cross-contamination with meat intended for human consumption, for example, prevention of contact between carcases.
Note: It is inappropriate to detain meat that has been declared unfit for human consumption with a formal food detention notice, as the product becomes an ABP, and no provision exists to detain an ABP.
2.4.9 Rectification FBO responsibility
It is the responsibility of the FBO to present carcases and offal to the FSA for final inspection free from contamination by faeces, gut content, hair, wool, bile, and any other pollutants in accordance with the FBO’s procedures based on HACCP principles.
2.4.10 FSA Operations group responsibilities
FSA Operations Group staff should have regard to the following:
- Meat showing signs of pathology or contamination must not be health marked/passed as fit and should be detained for rectification by the FBO.
- Where contamination on a series of carcases/offal is persistent and represents a failure in the FBOs hygienic procedures, the OV should immediately be informed, to establish the cause and rectify the problem; this may involve the OV stopping the line to resolve the issue.
Note: All line stoppages should be recorded in the day book and in the enforcement programme in Chronos.
- The OV must discuss the dressing procedures and HACCP based plan with the FBO where persistent deficiencies are identified.
Note: Deficiencies in dressing should be recorded using the Slaughter Hygiene Verification (SHV) K2 form in red meat and in poultry.
FSA staff must not carry out any type of meat rectification work, including for quality reasons, as this is the responsibility of the FBO.
2.4.11 Use of scabbards by FSA staff
Scabbards should only be used to transport knives to and from the post-mortem inspection stations. Once at the post-mortem inspection station, sterilizers should be used to store knives when not in use.
3. FBO responsibility
In this section
3.1 Responsibility
3.1.1 Responsibility
It is the responsibility of the FBO to produce safe meat. FSA Operations Group inspectors confirm FBO actions and identify any specific risks.
3.1.2 Timelines
Stunning, bleeding, skinning, evisceration, and further dressing are carried out without undue delay and in a manner that avoids contaminating the meat.
3.1.3 FSA facilities
The FBO follows the instructions of the OV to ensure that post-mortem inspection of all slaughtered animals is carried out under suitable conditions.
3.1.4 FBO facilities
Until post-mortem inspection is completed all parts of a slaughtered animal:
- must remain identifiable as belonging to a given carcase
- must not come into contact with any other carcase, offal, or viscera
- must not be washed
The FBO must ensure that:
- slaughtered animals are dressed and treated in such a manner as not to prevent or hinder inspection
- no carcases are cut up unless retained Regulation (EU) 2017/627 Article 15 applies see paragraph 2.3.2
- no action is taken to destroy or alter evidence of disease
- no part, except the hide or skin, is removed from the establishment until post-mortem inspection is completed and any required samples are taken
Exceptions
- for all species: the penis, if not intended for human consumption
- for sheep and goats: the head if no part of it is intended for human consumption
Reference: 2019/627 Articles 19, 20 and 21.
Any visible contamination must be removed without delay.
Reference: (EC) 853/2004 Annex III, Section I, Chapter IV.
3.1.5 Skinning
All carcases and other parts of the body intended for human consumption must undergo complete skinning, except for:
- porcine animals
- the heads of ovine and caprine animals and calves
- the muzzle and lips of bovine animals
- feet of bovine, ovine and caprine animals
Unskinned feet must be handled so as to avoid contamination of other meat.
Note: When destined for further handling, and before leaving the slaughtering establishment, feet of all species must be skinned or scalded and depilated.
Reference: Retained Regulation (EU) 853/2004 Annex III, Section I, Chapter IV, 18.
3.1.6 Spleens
Spleens must be removed completely and, wherever possible, whole. The operator must present spleens correlated to carcases for inspection.
3.1.7 Delayed uteri removal
For the grading and classification of female bovines as heifers or cows the uteri may be left attached to the carcase until the grading is completed.
Meat and Livestock Commercial Services Ltd (MLCSL) officers are being advised to speak to the FBO where they have a need for the uteri to be retained for grading purposes. The OV must be satisfied that a suitable system can be adopted before the procedure can start.
3.1.8 Uteri removal: FBO responsibility
In order to facilitate the process, the FBO must have a suitable system in place. The procedure must:
- be agreed between the FBO and the OV
- ensure that post-mortem inspection is completed, and that no carcase is released for human consumption until the uteri has been completely removed and the carcase found fit for human consumption
- in addition, the uteri should be hygienically removed as soon as is practical following classification / grading
3.1.9 Uteri removal: OV responsibility
The OV must be satisfied that:
- suitable procedure can be adopted to ensure that hygienic production is maintained, for example, keeping correlation between the uteri and the carcase without a risk of cross contamination
- health marks are not applied until the carcases have had the uteri removed and have passed post-mortem inspection
3.1.10 Storage facilities
There are lockable facilities for the refrigerated storage of detained meat and separate lockable facilities for the storage of meat declared unfit for human consumption.
3.1.11 After post-mortem inspection
Retained Regulation (EU) 853/2004, Annex III, Section I, Chapter IV, 16 states:
- the tonsils of bovine animals, porcine animals and solipeds must be removed hygienically
- meat declared unfit for human consumption must be removed as soon as possible from the clean sector of the establishment
- meat detained or declared unfit for human consumption and inedible by-products must not come into contact with meat and offal declared fit for human consumption
4. Guidance on conditions
In this section
4.1 Judgements at red meat post-mortem inspection
4.1 Judgements at red meat post-mortem inspection
4.1.1 Introduction
It is the duty of the OV, or the MHI acting under their authority, during post-mortem inspection to make a judgement based on the specific case presented and the requirements of Regulation 2019/627 Articles 29 to 35.
4.1.2 Legislation
Retained Regulation (EU) 2019/627 lays down eight specific hazards:
- TSE
- Cysticercosis
- Glanders
- Tuberculosis
- Brucellosis
- Trichinosis
- Salmonella
- Campylobacter
4.1.3 Guidance
There follows guidance on the following specific topics:
- TSE
- Glanders
- Brucellosis
- Cysticercus bovis
- Arthritis
- Tumours in bovines
- Trichinella
- Aujeszky’s Disease
4.2 Transmissible spongiform encephalopathy
4.2.1 Guidance on TSE
Official controls carried out in relation to TSE are to take account of the requirements of Retained Regulation (EU) No 999/2001 and other relevant community legislation.
Reference: See chapter 2.6 on ‘TSE testing’ for additional information.
4.3 Glanders
4.3.1 Guidance on Glanders
Where appropriate, solipeds are to be examined for glanders. Examination for glanders in solipeds is to include a careful examination of mucous membranes from the trachea, larynx, nasal cavities and sinuses and their ramifications, after splitting the head in the median plane and excising the nasal septum.
Meat from horses in which glanders has been diagnosed are to be declared unfit for human consumption.
Reference: Retained Regulation (EU) 2019/627 Article 32.
4.4 Brucellosis
4.4.1 Guidance on Brucellosis
When animals have reacted positively or inconclusively to a brucellosis test, or there are other grounds for suspecting infection, they are to be slaughtered separately from other animals, taking precautions to avoid the risk of contamination of other carcases, the slaughter line and staff present in the slaughterhouse.
Meat from animals in which post-mortem inspection has revealed lesions suggestive of acute infection with brucellosis is to be declared unfit for human consumption. In the case of animals reacting positively or inconclusively to a brucellosis test, the udder, genital tract, and blood must be declared unfit for human consumption even if no such lesion is found.
Reference: Retained Regulation (EU) 2019/627 Article 34
Note: All FSA staff should be aware that, when dealing with brucellosis suspects, they must always wear eye protection, disposable masks, and gloves.
4.5 Cysticercus bovis
4.5.1 Introduction
Updated: [Meat infected with Cysticercus bovis is to be declared unfit for human consumption. However, in some circumstances , the parts not infected may be declared fit for human consumption after having undergone a cold treatment. See guidance chart in 4.5.2.]
At this time the derogations from post-mortem inspection in Article 30(1) do not apply.
Reference: Retained Regulation (EU) 2019/627 Article 30.
4.5.2 Guidance on C. bovis
Use the table below as a guide to judgement when cases of C.bovis are detected.
Guidance of Cysticercus bovis
| Number (Post-mortem findings) | Location (Post-mortem findings) | Status (Post-mortem findings) | Judgement |
|---|---|---|---|
| One cyst | Localised* | Viable | Reject the affected organ or carcase part |
| One cyst | Localised* | Non-viable (caseous / calcified) | Require cold updated: [treatment] for remainder |
| More than one cyst | Localised* | Viable | Reject the affected organ or carcase part |
| More than one cyst | Localised* | Non-viable (caseous / calcified) | Require cold updated: [treatment] for remainder |
| More than one cyst | Generalised** | Viable | Reject the affected organ(s) or carcase(s) part |
| More than one cyst | Generalised** | Non-viable (caseous / calcified) | Require cold updated: [treatment] for remainder |
* only one area or part affected (such as heart or diaphragm)
** more than one area or part affected (such as heart and diaphragm)
Updated [*** live cyst that can develop into the next stage of the parasite]
4.5.3 Cold storage of carcases and offal with a localised or non-viable generalised C. bovis infestation
After rejection of the relevant carcase part or offal, the remainder of the carcase and offal must updated [remain under the competent authority control and] undergo a ‘cold treatment’ as follows:
Cold storage of carcases and offal with a localised Cysticercus bovis infestation
| Temperature | Minimum time (weeks) |
|---|---|
| not exceeding -7°C | not less than 3 weeks |
| not exceeding -10°C | not less than 2 weeks |
Updated: [No health mark is to be applied at PMI where cold treatment is needed before the product is fit for human consumption. A health mark/ ID mark (where cut and boned) must be applied once the required cold treatment has been verified as completed. See 4.5.6.
FBOs that intend to arrange for cold treatment of carcases and offal with a localised or non-viable generalised C. bovis infestation, must have in place a written procedure, previously agreed with the OV. The procedure must cover how they intend to comply with the freezing requirements and how the product will be handled. It must be signed by the FBO and be version controlled.
Whenever C. bovis is identified at post-mortem inspection, a note in the plant Daybook must be made, including kill number, ear tag of the animal and decision taken regarding the carcase and offal. When the carcase/offal is eligible for cold treatment and the FBO arranges for such storage, no formal detention of the meat is required.
If the FBO does not follow their own C. bovis handling SOP or takes possession of the meat prior to the appropriate treatment being verified, the meat will be declared unfit for human consumption.
Reference: Article 45(h) of Assimilated Regulation (EU) 2019/627
It is acceptable for the carcase to be boned-out prior to the commencement of the cold treatment, provided boning takes place under supervision of the AO and that the meat remains under the competent authority control. The identity of the meat must be maintained throughout boning, packaging and storage.]
4.5.4 Updated: [Permitted destinations for boning under supervision and cold treatment
If the slaughterhouse has a co-located cutting plant (CP) and a freezer, the meat can be boned under supervision and cold treatment applied on site.
Where the slaughterhouse has no co-located CP, the carcass can be despatched for boning under supervision and cold treatment at an FSA approved stand-alone CP that has a freezer able to perform such cold treatment. The transport arrangements should be done by the FBO with agreement from the OV. In advance of the transport taking place, the OV must liaise with the relevant FVC and ITL covering the cluster in which the destination stand-alone CP is located. The ITL covering the destination stand-alone CP will be responsible for arranging the AO resources required for supervised boning and subsequent verification if cold treatment conditions were met and for subsequently releasing the meat.
Where the slaughterhouse has a co-located CP but no freezer, the meat can be boned under supervision at the co-located CP and transported for cold treatment to an FSA approved stand-alone CP with freezing facilities or LA approved cold store (CS). The transport arrangements should be done by the FBO with agreement from the OV.
If the destination plant is a stand-alone CP, the OV must liaise with the relevant FVC and ITL in advance of the transport taking place. The ITL covering the destination stand-alone CP will be responsible for arranging AO resources required for the verification of the cold treatment conditions.
If the destination plant is a stand-alone CS, the OV is to liaise with the relevant LA in advance of the transport taking place. The LA covering the stand-alone CS will be responsible for arranging LA resources required for verification of the cold treatment conditions and sharing that information with the OV.
Contact details for the relevant LA Food Safety Team can be found at https://www.food.gov.uk/contact/consumers/find-details/contact-a-local-food-safety-team
4.5.5 FSA controls during boning under supervision / Transport to an approved establishment
An AO must attend the co-located cutting plant (CP) or the stand-alone CP to supervise the boning and conduct further examination of the meat. A charge will be made for this, coded against the plant in which the supervised boning activity takes place.
When boning and freezing is to take place at the co-located CP the Cysticercus Bovis Detention Label PMI 4/15 C must be applied to the packaging before boned out meat is transferred to the on-site freezer.
When boning and freezing is to take place at a stand-alone CP the numbered security seals must be applied to the quarters, before they are transported from the slaughterhouse. Once the carcase is boned out under supervision at the stand-alone CP the Cysticercus Bovis Detention Label PMI 4/15 A must be applied to the packaging before boned-out meat is transferred to the freezer for cold treatment.
When boning is to take place at the co-located CP and freezing is to take place at a stand-alone cold store/CP the packaged meat should be labelled with the Cysticercus bovis Detention Label PMI 4/15 B.
Where the meat is to be consigned to another approved establishment with boning/ cold treatment facilities, a Transfer Permit PMI 4/16 must accompany the consignment:
- part 1 of the Transfer Permit must be completed at the slaughterhouse of origin, the original to go with the consignment and a copy to be retained at the slaughterhouse
- part 2 of the Transfer Permit should be completed at the destination establishment by the FBO
Reference: See chapter 9 on ‘Forms’, for sample copies of the PMI 4/15 A, PMI 4/15 B and PMI 4/15 C, Cysticercus bovis Detention Labels and the Transfer Permit PMI 4/16.
Note: The AO can be an OV or MHI.
4.5.6 Releasing the meat
The OV / AO must verify compliance with the cold treatment provisions before the FBO can move the product. An AO should visit the cold treatment facility to verify that cold storage times/temperatures were compliant.
When the cold treatment takes place in a stand-alone cutting plant / cold store the AO must complete part 3 of the Transfer Permit PMI 4/16. The original is to be retained at the cold treatment facility. A copy is to be sent back to the FSA office at the originating slaughterhouse and should be kept on file for a minimum of 12 months.
4.5.7 Application of the ID mark:
When the cold treatment takes place in a co-located CP and the AO verifies compliance with the treatment conditions, the meat can be ID marked and released.
When the boning and cold treatment are undertaken at a stand-alone cutting plant the ID mark of that cutting plant must be applied.
When the boning is undertaken at a cutting plant co-located with a slaughterhouse, and the cold treatment is undertaken at a standalone cutting plant/ cold store the meat must be returned to the cutting plant where the meat was worked on for the application of the ID mark.
Note: The AO can be an OV or MHI (FSA approved plants) or local authority (LA) Inspector (LA approved cold stores).
A charge will be made for work associated with releasing the meat.]
4.6 Tuberculosis (TB)
4.6.1 Guidance on TB
Full instructions on TB are now contained within chapter 6 on ‘Notifiable diseases’, section 7.
4.7 Arthritis
4.7.1 Guidance on arthritis
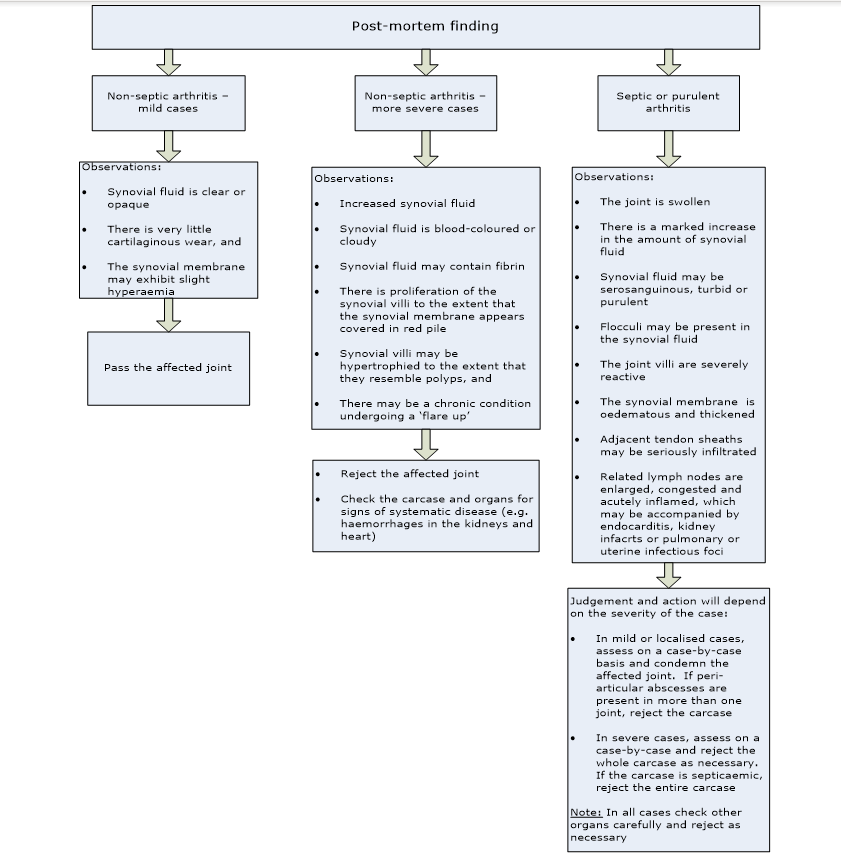
Arthritis is an inflammatory condition of the joint, synovial membrane and articular surfaces. It is a routine and common cause of partial and total rejection of carcases. The flowchart below lists the post-mortem findings and guidance on the judgement of arthritic conditions:
4.8 Tumours in bovines
4.8.1 Guidance on tumours in bovines
Where tumours are encountered in the carcases or offal of bovines, Enzootic Bovine Leukosis must be a consideration.
- The OV must inform APHA.
- Samples from the carcase might be required.
- Before contacting APHA, the OV should gather all possible information about the animal, including date of birth and number of permanent incisors erupted.
Reference: See chapter 6 on ‘Notifiable diseases’ for additional information.
4.9 Aujeszky’s disease: National Serum Survey
4.9.1 Purpose
To demonstrate continuing freedom from Aujeszky’s disease a serum sample must be submitted for serological examination from every slaughtered breeding boar.
4.9.2 Who collects samples
The OV is responsible for collecting samples or delegating the task to a suitably trained MHI.
4.9.3 Restocking of sampling equipment
Sampling equipment can be obtained from SLA and Contracts Team. The equipment for this survey includes ELISA discs, plastic bags, address labels and photographic slide magazines used to dry the discs.
A training note has been produced by the SLA and Contracts Team detailing the new sampling procedure with photographs.
4.9.4 Method for collecting serum samples on ELISA discs
Samples must be obtained from carcases at a sufficient distance from the point of kill when there is no risk from post slaughter carcase movement and from FBO activities. Where possible this should be done at the post-mortem inspection site.
Caution: Avoid contaminating the disc with water or dirt.
The disc should be grasped by the body of the disc and not by the peripheral discs. Dry the saturated discs in the photographic slide magazines provided, ensuring effective separation between discs to prevent cross contamination.
Wash, rinse and dry the photographic slide magazines between uses.
Note: The ‘clotted blood’ method of sampling is no longer to be used.
Method for collecting serum samples on ELISA discs:
Step 1: Use one ELISA disc for each boar. Pre-number the discs.
Step 2: Each peripheral disc must be saturated with blood. Partially saturated peripheral discs are of no use.
Step 3: Place saturated discs in a clearly identified photographic slide magazine. Place discs in every second compartment of the slide magazine to allow effective separation while they dry.
Step 4: Note sufficient information on the sample submission form to identify the owner of each boar.
Step 5: Drying: Discs should be allowed to dry at room temperature, out of direct sunlight, for at least 12 hours. Discs must be completely dry before despatch to the laboratory.
Step 6: Punch out a central hole in each disc once dry. Thread the discs onto file tags in a sequence that corresponds with the submission sheet and place into plastic bags for despatch to the laboratory with the completed submission form.
4.9.5 Storage prior to despatch
Prepared ELISA disc samples should be stored at 4°C until posted.
4.9.6 Posting and packaging details
The following points are to be observed:
- Samples may be batched and posted weekly (no more than 14 days from sampling to posting).
- 1st class post-must be used.
- Each batch of samples must be accompanied by a completed submission form.
- The package must be marked AD SURVEY SAMPLES.
- Avoid posting samples on a Friday as they may be delayed in transit over a weekend.
4.9.7 Submission address
Serum samples from all slaughterhouses in England and Wales must be sent to:
APHA Weybridge
Woodham Lane
New Haw
Addlestone
Surrey
KT15 3NB
4.9.8 Sample submission form
Each sample submission form must provide sufficient information to identify the person who was the owner of each boar at the time that it was consigned to or purchased by the slaughterhouse.
The sample submission form must be completed and printed to go with the samples to APHA.
Retain a copy of each submission form for at least 1 year.
Reference: See Annex 2 for a sample copy of the sample submission form.
4.9.9 Notification
Notification by email to APHA is no longer required. The form should be printed to accompany the samples to APHA Weybridge.
4.9.10 Results
Results are reported to Defra and SLA and Contracts Team. The SLA and Contracts Team will correlate the results and send them to the FVC to cascade.
5. Trichinella Testing
In this section
5.4 Packaging and despatch of samples
5.1 Introduction
5.1.1 Background
Trichinellosis is an infestation of the muscles of animals and man with the larvae of Trichinella spiralis. Infection occurs through the eating of raw or undercooked meat.
Meat from animals infected with Trichinae is declared unfit for human consumption.
5.1.2 Legislation
Retained Regulation (EU) 2019/627 Article 31 requires the carcases of swine (domestic, farmed game and wild game), solipeds and other susceptible species to be examined for trichinosis.
Commission Regulation 2015/1375 lays down the technical details of trichinella testing.
Reference: Retained Regulation (EU) 2015/1375 – amends Regulation 2075/2005 and 216/2014, and sets out requirements for trichinella testing, derogations, and conditions for controlled housing.
5.1.3 FSA role
Trichinella testing is an official control. The OV is to ensure that sampling takes place and samples are appropriately identified, handled, and sent for testing to an accredited laboratory.
Reference: Retained Regulation (EU) 2019/627 Article 37, 2
Sampling and preparation of samples can be carried out by the OV or a MHI.
However, slaughter staff that have received training can, under the supervision of the OV, carry out sampling and testing tasks.
Reference: Retained Regulation (EU) 2019/624 Article 14
5.1.4 Sampling of carcases (including exemptions)
Under retained regulation (EU) 2015/1375, samples must be collected from carcases of the following animals:
- breeding domestic swine (sows and boars)
- wild boar (any age, whether wild or farmed)
- solipeds (any age)
- all pigs that have not been reared in controlled housing conditions (this information will be captured on the FCI accompanying the pigs to the slaughterhouse)
Meat from domestic swine that has been subject to a freezing treatment under official control is exempt from testing.
5.1.5 Retention of parts for human consumption
Carcases, and parts from carcases sampled for trichinella testing must not leave the establishment before the examination has been found negative.
Similarly, other parts of the animal intended for human consumption containing striated muscle must be retained until a negative result is received.
Parts of the animal not containing striated muscle are not subject to any restrictions and can leave the slaughterhouse. In that case, care must be taken to prevent pieces of striated muscle, such as diaphragm or sphincters being left attached.
5.1.6 Controlled housing conditions
‘Controlled housing conditions’ are defined in Retained Regulation (EU) 2015/1375, Annex IV, Chapter 1 and include a range of measures that reduce the risk of the pigs being infected with trichinella. Importantly, the definition does not exclude pigs that have outdoor access, provided that the outdoor access does not present a risk of introducing trichinella into the holding.
Republic of Ireland (RoI) has, to date, not put in place a mechanism whereby housing can be deemed to meet the conditions specified in Article 1 and Annex IV of Retained Regulation (EU) No 2075/2005. Therefore, all pigs born and reared in RoI, which are slaughtered in slaughterhouses in England or Wales, shall be tested for trichinella, regardless of the housing system recorded on the FCI.
5.1.7 Retention of animal by-products
ABP containing striated muscle and intended for animal consumption (Category 3 by-products) must not leave the establishment before the examination has been found negative.
There is no need to retain:
- ABP that do not contain striated muscle
- ABP that contain striated muscle but that are not intended for animal consumption (Category 2 by-products)
5.1.8 Health marking carcases
Where a procedure is in place in the slaughterhouse to ensure that no part of carcases examined leaves the establishment until the result of the trichinella examination is found to be negative and the procedure is formally approved by the OV, the health mark may be applied before the results of the trichinella examination are available.
The FBO must have a written procedure agreed with the OV in place.
Where such system is not in place, the health mark must not be applied until a negative test result has been received.
5.1.9 Cutting or carcases
Pending the results of the trichinella examination, such carcases may be cut up into a maximum of six parts in a slaughterhouse or in a co-located cutting plant.
If the test result is positive and correlation between carcase parts lost, the whole batch of cuts must be disposed of as a by-product.
5.2 Cold treatment methods
5.2.1 Cold treatment for pig meat
Cold treatment may be used as an alternative to trichinella testing for domestic pig meat. The storage temperatures specified for cold treatment are significantly lower than those for the normal storage of frozen meat.
The following conditions must be followed when the cold treatment method is used:
- meat brought in already frozen must be kept in this condition
- the technical equipment and energy supply of the refrigerating room must be such as to ensure that the required temperature is reached very rapidly and maintained in all parts of the room and of the meat
- insulated packaging should be removed before freezing, except for meat which has already reached throughout the required temperature when it is brought into the refrigeration room
- consignments in the refrigeration room must be kept separately and under lockable conditions
- the date and time when each consignment is brought into the refrigeration room must be recorded
5.2.2 Time and temperature for cold treatment
The time / temperature combination for cold treatment is dependent upon the thickness of the pieces of meat. These combinations are summarized in the table below:
| Method | Maximum thickness of the pieces of meat | Maximum temperature of the storage room | Minimum consecutive time for cold treatment |
|---|---|---|---|
| 1 | Up to 15 cm (6 ") | -15°C | 20 days |
| 1 | Up to 15 cm (6 ") | -23°C | 10 days |
| 1 | Up to 15 cm (6 ") | -29°C | 6 days |
| 2 | 15 - 50 cm (6" - 20") | -15°C | 30 days |
| 2 | 15 - 50 cm (6" - 20") | -25°C | 20 days |
| 2 | 15 - 50 cm (6" - 20") | -29°C | 12 days |
| 3 | Up to 25 cm (10 ") | -25°C | 10 days |
| 3 | 25 - 50 cm (10" - 20") | -25°C | 20 days |
5.2.3 Specified times when core temperature is monitored
The following time / temperature combinations are permissible providing the core temperature of the meat is monitored:
| Maximum core temperature of the meat | Minimum consecutive time period for the cold treatment |
|---|---|
| -18°C | 106 hours |
| -21°C | 82 hours |
| -23½°C | 63 hours |
| -26°C | 48 hours |
| -29°C | 35 hours |
| -32°C | 22 hours |
| -35°C | 8 hours |
5.2.4 Cold treatment in other species
Cold treatment is not an alternative for the testing of wild boar or solipeds.
5.3 Collecting samples
5.3.1 Sampling responsibility
The OV must ensure that sampling takes place and samples are correctly identified and handled, and sent for testing to:
Biobest Laboratories Ltd
6 Charles Darwin House
The Edinburgh Technopole
Milton Bridge
Nr. Penicuik
Midlothian
EH26 0PY
Telephone: 0131 440 2628
Fax: 0131 440 9587
Email: enquiry@biobest.co.uk
Website: biobest.co.uk
Collection and handling of samples and testing tasks may be carried out by an MHI or delegated to plant staff if they have received specific training and the OV is satisfied that the sampling procedure is carried out correctly. For self-testing abattoirs see topic 5.7 on ‘Use of on-site labs’.
Samples must be collected using a clean knife and disposable forceps.
5.3.2 Sample description
A sample of the size specified below must be collected from the described sampling site.
Note: Take samples as a single piece of meat.
If this preferred sample site is not available, then the alternative sample must be collected.
The weight of meat specimens refers to a meat sample free of all fat and fascia. Particular attention should be made collecting muscle samples from the tongue to avoid sample contamination with the superficial layer of the tongue, which is indigestible and can prevent reading of the sediment.
| Animal Categories | Sample size | Sampling site | Alternative sample |
|---|---|---|---|
| Boars and Sows | Between 2 and 4g | Pillar of the diaphragm at the transition to the sinewy part | 4g, to be taken from the rib part or the breastbone part of the diaphragm, from the jaw muscle, tongue, or the abdominal muscles |
| Solipeds | Between 10 and 11.5g | Lingual or jaw muscle | Larger size specimen from the diaphragm pillar at the transition to the sinewy part |
| Wild Boar | Between 10 and 11.5g |
Foreleg, tongue, or diaphragm | None |
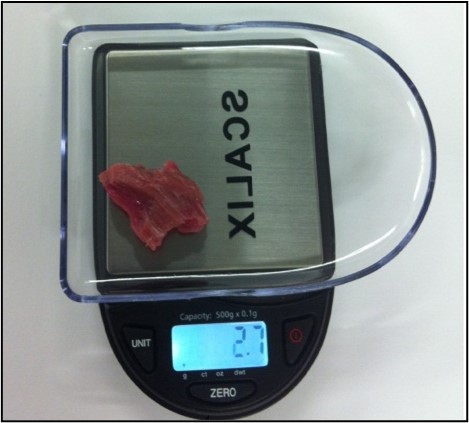
5.3.3 Sample size guide
- Use the scales provided to ensure the correct weight.
- Each specimen must consist of a single piece of meat free of fat or fascia and be of the correct weight.
- Large samples reduce the pooling ability in the lab and result in increased cost to the FSA.
- Underweight samples will be rejected by the lab and not tested.
Note: New plants must request scales from the corporate support unit transactions team York (CSU) csu@food.gov.uk.
2-4g boars and sows

10-11.5 grams wild boars and solipeds
5.3.4 Sampling point
Samples may be collected at any point during dressing or chilling providing the identity of the carcase can be ascertained.
5.3.5 Pooling of samples
Up to 100g of samples from different animals can be pooled as a single batch for testing. The number of samples in a batch will depend on the animal category, as the sample size is different, for example, 50 sows and boars, 10 solipeds.
You can pool samples from different producers.
Reference: See sub-topic 5.3.2 on ‘Sample description’ for additional information.
However, samples from different animal categories, such as domestic pigs and wild boars, must not be pooled in the same batch as digestion times may be different.
5.3.6 Sampling procedure
The following procedure must be followed when collecting samples for testing:
Step 1: Open the small sealable Liquitite Pathoseal bag
Step 2: Collect the samples of meat as appropriate for the species and category of animal sample
Step 3: Pool the samples up to 100g in the small Liquitite Pathoseal bag
Step 4: Close the small Liquitite Pathoseal bag. Stick barcode label to the bag and insert into the larger Pathoseal bag with the absorbent pad
Step 5: Place two squares of Techni Ice into the large Pathoseal bag
Step 6: Stick the corresponding barcode to the PMI 4/18 form
Step 7: Complete the PMI 4/17 form
5.3.7 Completion of PMI 4/17 form
Carcases must be identifiable to their farm of origin until a test result has been received so a farm investigation can be carried out if the result is positive.
PMI 4/17 (Trichinella Sampling form) must be completed when the samples are collected. The identity of each sampled carcase must be recorded in a way that allows the farm of origin to be identified, for example, by recording the slap number or the County Parish Holding number (CPH) obtained from the Animal Movement Licence.
Individual carcase identification when a farm supplies several animals is not required, as in the event of a positive all carcases in the batch will be re-tested.
To keep correlation with the sample and PMI 4/18, (Trichinella Testing Submission Form), the serial number of the barcode label used to identify those must be inserted in the Reference Number box.
5.3.8 Completion of PMI 4/18 form
PMI 4/18 (Trichinella Testing Submission Form) must be completed by FSA staff and accompany the sample to the lab.
One form with one barcode must be completed for every batch of up to 100g of samples. Make sure the number of samples correlates with the number of animals entered on the form so Biobest Laboratories do not report incorrect number of samples supplied.
Note: An email address must be supplied to the lab for notification of the test result and a mobile phone number for text notification that results are available.
Affix the barcode label correlated to the sample bag to the PMI 4/18.
Send the original to the lab in a clean sealed A4 bag and keep a photocopy on file.
5.4 Packaging and despatch of samples
5.4.1 Transport containers
Samples are transported in Pathoshield packaging. The courier Topspeed collects for next day delivery to Biobest Laboratories.
5.4.2 Chilling
Samples are kept chilled by two squares of Techni Ice. The Techni ice squares must be held frozen until use.
5.4.3 Pathoshield packaging procedure
The table below lists the steps that must be followed using a Pathoshield box to despatch samples:
Pathoshield packaging procedure
| Step | Action |
|---|---|
| 1 | Attach the Biobest Laboratories barcode to the small pathoseal bag and attach the corresponding barcode onto a trichinella testing submission form (PMI 4/18). |
| 2 | Place the small bag into the larger pathoseal bag, placing 2 Techni Ice squares between the bags |
| 3 | Complete form PMI 4/17 to record the samples and which barcodes they were submitted with |
| 4 | Place sample into the Pathoshield outer box. Affix the peel-off barcode sticker onto the duplicate copy of the page. |
| 5 | Put completed forms PMI 4/17 and PMI 4/18 in a plastic bag before placing them in the box ready for despatch to the laboratory. |
| 6 |
If sending a single box: affix pre-printed Biobest Laboratories address label to box and seal the box using the blue security seal provided. If sending multiple boxes: Re-package into a larger box and attach address label and consignment note to outer box. |
| 7 | Place the Pathoshield box in a plastic refuse bag to protect the surface of the box from contamination while carrying it through the slaughterhouse and during storage. |
| 8 | Close the plastic refuse bag with a cable tie or other secure means. |
5.4.4 Storage pending despatch
On completion of sampling, place the Pathoshield box in the detained chiller until transferring them to the collection point. Topspeed will collect at the agreed collection time for delivery to Biobest Laboratories.
5.4.5 Notify lab of Saturday testing
If testing is required on a Saturday, FSA staff need to telephone Biobest Laboratories on the Thursday beforehand to advise them that trichinella samples are being sent for Saturday morning delivery:
Biobest Laboratories – 0131 440 2628
Topspeed need to be informed that the sample needs to arrive before 9am on Saturday in order to be tested.
No notification is required for samples dispatched for Monday to Friday testing.
5.4.6 Despatch from base plants
When, for practical reasons, samples cannot be despatched from the plant where the animals are slaughtered, they can be taken to a different plant to be despatched from there.
However, when completing the PMI forms, the sampling plant details must be entered.
In that case all the original documentation must be filed in the plant where the sample was taken as soon as practical.
5.5 Courier collection services and procedures
5.5.1 Next day before noon service
Trichinella samples should be despatched using the Topspeed ‘Next Day Service’.
Note: Topspeed will only collect samples between 09:00 – 17:00 unless out of hours arrangements have been agreed.
5.5.2 Saturday service
In addition to the standard service, Topspeed provide a ‘Saturday Service’. This service may only be requested if prior permission is obtained from the SLA and Contracts Team as it incurs increased costs and Biobest must be informed on the preceding Thursday that samples will be arriving at the lab for testing.
This service is only to be used for samples that need to be tested on a Saturday.
Test results for Saturday testing will be received on the same day.
5.5.3 Booking sample collection
The following steps should be taken when booking sample collection:
Step 1: Go to Topspeed and complete the online booking form. See Annex 7 for information on completing the online booking form.
Step 2: Provide Topspeed with the following information:
- number of items (boxes) in consignment
- kill date and time
- the name of person making the booking
Step 3: Write the barcode numbers as reference for the collection; Topspeed to collect as arranged
5.5.4 Sample collection point
Immediately prior to the agreed collection time the Pathoshield box containing the sample(s) should be removed from the plastic refuse bag and placed at the agreed collection point.
5.5.5 Despatch failure
Should Topspeed fail to collect samples within the agreed timeframe, contact Topspeed to arrange collection immediately and inform the SLA and Contracts Team by email at sla.contracts@food.gov.uk.
5.6 Consumables
5.6.1 Ordering consumables
To request stocks of consumables, updated: [complete the Trichinella – Plant Order Form that can be accessed through the following link: Ops forms page].
The minimum order is 1 box of the following options:
- Pathoshield P7 kit x 12 for trichinella testing - recommended for plants processing small number of animals for testing
- bespoke Pathoshield 7 comprising
- A5 Pathoseal
- 200ml Absorbent
- A6 Liquitite
- Techni Ice x 24 squares
- Forceps
- Security Seal
- Outer compliant box
- bespoke Pathoshield 7 comprising
- Pathoshield P3 kit x 10 for trichinella testing - recommended for plants processing larger number of animals for testing
- bespoke Pathoshield 3 comprising
- A4 Pathoseal
- 200ml Absorbent
- A5 Liquitite
- Techni Ice x 20 squares
- Forceps
- Security Seal
- Outer compliant box
- bespoke Pathoshield 3 comprising
Note: Allow 5 days lead time for delivery of the consumables.
5.6.2 Barcodes
The barcodes can be obtained from the CSU by email csu@food.gov.uk.
5.7 Use of on-site facilities, private laboratories, and other arrangements
5.7.1 Background
Slaughterhouses that have facilities and trained staff available for the collection and testing of trichinella samples may use their own arrangements instead of having the samples dispatched to Biobest Laboratories. Where these arrangements are in place, the lab will operate as a supplier providing a service to the FSA Operations Group.
In order to carry out trichinella testing, on-site self-testing facilities must be accredited by United Kingdom Accreditation Service (UKAS) and participate in the FSA Quality Assurance Scheme conducted by the UK National Reference Laboratory (UKNRL). Other FBOs may also send samples to such “self-tester” sites as an alternative to Biobest Laboratories.
Private testing laboratories may also be used in place of Biobest Laboratories. These laboratories must also participate in the FSA UKNRL Quality Assurance Scheme, as above.
5.7.2 Requirements for on-site labs
Any plant that wishes to start trichinella testing in an ‘on site’ laboratory must be assessed by the UK National Reference Laboratory (UKNRL) and be permitted by FSA to undertake testing.
The NRL will arrange for an on-site inspection and produce a report which will either recommend approval for self-testing or highlight areas that need to be addressed prior to recommendation for approval being issued.
The NRL offer training to staff under the VetQAS scheme to ensure Sampling Officers have the relevant skills and knowledge to undertake testing.
FSA Operations Group will issue a designated lab status letter once the above criteria have been satisfied to ensure compliance with Retained Regulation (EU) No. 2015/1375.
5.7.3 Responsibilities of the lab operator
Once contracted by the FSA Operations Group to carry out trichinella testing, the lab operator is responsible for:
- the collection and identification of the samples
- the identification and correlation of sampled carcases
- the supply of equipment and disposables
- the operation of the lab
- the examination of the digested samples
- the maintenance of all records
- the training of staff
5.7.4 Quality assurance
All laboratories undertaking testing must take part in the quarterly QA scheme organised by the UKNRL. All laboratories must take action to rectify any deficiencies noted either in the assessment or following a QA test. Failure to do so will result in the removal of designated lab status.
The OV will receive a copy of the QA report and will be responsible for ensuring the results are returned within the specified timescale and that any deficiencies identified are addressed.
5.7.5 Non-compliance with SOP
Where the OV / FVC is not satisfied that the lab operator is complying with the standard operating procedure (SOP) agreed with the FSA Operations Group, advice must be given to rectify the breach.
Failure to comply with the SOP is a breach of the terms of the contract and if the deficiency is not rectified, the OV must inform the SLA and Contracts Team. The FSA Operations Group can then suspend the SOP.
When the SOP is suspended, the FSA Operations Group will collect the samples and dispatch them to Biobest Laboratories.
The health mark must not be applied to any carcase when there are no guarantees that the result of the testing is reliable.
5.8 Test results
5.8.1 Receipt of test results
Trichinella testing is an official control, and the FSA is responsible for obtaining the test result.
By default, a laboratory report containing results will be sent by e-mail to the address specified on the submission form.
Biobest Laboratories currently offer SMS reporting of results for other tests and aims to add this option for trichinella. To register interest in this service, contact Biobest Laboratories on 0131 440 2628.
5.8.2 Negative results
On receipt of a negative result, the health mark and identification mark can be applied.
ABP containing striated muscle that were being retained can be released.
5.8.3 Positive or doubtful results
If the initial result received from the laboratory is positive or doubtful, Biobest Laboratories will contact the SLA and Contracts Team, who will immediately contact the OV to advise on the procedure for despatching samples to NRL - APHA York for re-test. The OV must also advise the local APHA office.
Commission Regulation (EC) No 2015/1375 requires positive or doubtful results to be confirmed, collecting samples from the suspect carcases, and digesting them in smaller pools.
5.8.4 Re-sampling carcases with positive or doubtful results
The SLA and Contracts Team will contact the OV / FVC to request samples for re-testing.
These samples must be of the correct weight and from the correct sample site for the species concerned. A PMI 4-18 must be completed per pool and be sent to NRL - APHA York.
The SLA and Contracts Team will confirm which courier service should be used.
Samples for re-test should be sent to:
Trichinella National Reference Laboratory
APHA York
Biotech Campus
York
YO41 1LZ
The carcases and all body parts must remain detained, pending the outcome of the re-testing.
5.8.5 Traceability report
Pending the result of the re-test, the OV / FVC should obtain the FCI to create a traceability report for the detained carcases, to identify the farm of origin should a positive result be confirmed.
5.8.6 Notification of positive results
The SLA and Contracts Team will notify the OV / FVC and APHA if a positive result is confirmed.
On receiving confirmation of a positive result, the OV / FVC should email their traceability report to the SLA and Contracts team in York (access contact details in chapter 1 on ‘Introduction’).
If the positive result has been confirmed by NRL - APHA York, the positive carcase and all body parts must be disposed of as a Category 2 animal by-product and confirmation of action emailed to the SLA and Contracts Team sla@food.gov.uk.
For pigs from RoI, positive results shall be reported by the FSA to the Department of Agriculture, Food and the Marine (DAFM), the RoI competent authority. This will activate the RoI contingency plan with regard to the investigation of the source of infestation and any associated spread among other pigs or other susceptible species.
6. Poultry Post-Mortem Inspection
In this section
6.1. Correlation and Inspection
6.1 Correlation and inspection
6.1.1 Inspection requirements
The inspector is required to inspect the external surface of all carcases and accompanying offal.
6.1.2 Whole bird inspection point
Inspection of the whole bodies of birds is recommended so that diseased birds can be removed early in the process and this should be included in the HACCP plan.
6.1.3 Evisceration line inspection
Correlated carcases and offal either attached or detached are inspected.
6.1.4 Carcase presented for post-mortem inspection without offal
If poultry carcases are presented without offal at the post-mortem inspection point as a result of the accidental removal of all or part of the offal they do not need to be rejected. They should be inspected and if the carcases pass post-mortem inspection, they can be considered fit for human consumption. However, such cases should be judged according to the merits of each case.
This scenario is not intended to cover inadequate presentation / correlation of offal due to malfunctioning evisceration equipment or inadequate manual evisceration practices.
Offal and viscera that have not undergone PM inspection should be disposed of as Category 2 ABP.
Note: In the event of a significant increase in presentation of carcases without offal, follow the usual hierarchy of enforcement to address the root of the problem.
6.1.5 Delayed evisceration
(EC) 853/2004 Annex III, Section II, Chapter IV, 7 (c) states ‘viscera or parts of viscera remaining in the carcase, except for the kidneys, must be removed entirely, if possible, and as soon as possible, unless otherwise authorised by the competent authority.’
FBOs intending to carry out delayed evisceration should develop a procedure based on the HACCP principles detailing how the process is going to take place, assess the risks, and implement measures to ensure these risks are minimised.
When discussing with the OV the following conditions need to be considered prior to the process commencing:
- The FBO has to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles for this process. This can be in the form of a Standard Operating Procedure (SOP).
- Viscera can be left in the carcase after slaughter for not longer than 15 days at a temperature of not more than 4ºC (this mirrors the requirements in Annex III, Section II, Chapter VI, paragraph 9 of Regulation 853/2004, for the delayed evisceration of poultry slaughtered on-farm). If FBOs wish to apply other time/temperature combinations, they will need to produce a risk assessment to support any deviation from these parameters.
- Un-eviscerated carcases should either be kept in a separate chiller, or if this is not possible, sufficiently separated from any other carcases or food stuffs to prevent the risk of cross-contamination.
- When the delayed evisceration takes place, the viscera in the body cavity will need to be completely removed in a hygienic manner. In cases where the intestinal tract is ruptured and subsequently contaminates the carcase or offal the contaminated parts must be either trimmed or thoroughly washed with potable water or, where required, disposed of as animal by-products.
- FBOs will need to adjust the processing lines for this operation to ensure that post-mortem inspection can be carried out effectively by the OV, MHI or a PIA under the FSA supervision.
Although establishments undertaking delayed evisceration do not require specific approval or authorisation, the OV shall inform their FVL/FVC of the FBO’s intention to implement delayed evisceration. Once the FVC and the OV are satisfied with the process, the OV shall notify the approvals team at approvals@food.gov.uk once the FBO has commenced this type of production in order to have the information updated in E&P.
In cases where the hygienic conditions are not complied with by the FBO, the established hierarchy of enforcement as per any other deficiency shall be followed. If FBOs are unable to achieve compliance the delayed evisceration process can be stopped using the standard enforcement procedures.
6.1.6: Partial evisceration: effilé or roped poultry
Partial evisceration or effilé is defined in Regulation (EU) 543/2008 (the Poultry Meat Marketing Regulations), as the process of leaving the heart, liver, lungs, kidneys, crop, proventriculus and gizzard inside the body cavity of the bird.
Annex III, Section II, Chapter IV, Paragraph 7 (c) of Regulation (EC) 853/2004 states that viscera or parts of viscera remaining in the carcase, except for the kidneys, must be removed entirely, if possible, and as soon as possible, unless otherwise authorised by the competent authority.
The FSA, as the competent authority, can authorise a derogation from the “removed entirely” criterion described above. Unlike for delayed evisceration, authorisation for effilé or partial evisceration has to be granted on a case-by-case basis, following the procedure described in 6.1.7 below.
For the production of partially eviscerated poultry or effilé, the following requirements will need to be fulfilled:
HACCP based procedures
- The FBO has to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles for this process. This can be in the form of a Standard Operating Procedure (SOP).
Ante-Mortem Inspection/FCI
- Only healthy flocks are eligible for partial evisceration. If the FCI suggests that there have been health problems at farm level, the OV can reject the batch for effilé production, and all carcasses must be fully eviscerated.
- It is recommended that un-tested (for example, exempt from testing under the National Control Plan) or Salmonella positive batches are not used for the production of partially eviscerated poultry. Should the FBO decide to use these batches they will have to be treated with special precautions. In any case, the OV can request a complete evisceration if preliminary post-mortem findings are of concern (see PMI paragraph below).
Operational requirements
- Intestinal tract to be removed in a hygienic manner and in such a way that spillage of digestive content is prevented.
- In case of rupture of the intestinal tract and subsequent contamination of the carcass/offal, the carcass will need to be fully eviscerated and washed as per normal production.
- In partially eviscerated poultry, inside wash is not recommended.
- Only the heart, liver, lungs, kidneys, crop, proventriculus and gizzard can remain inside the bird.
Post-Mortem inspection
- All external carcase surfaces and body cavities will need to be visually inspected.
- Green offal must be presented to the Official (OV, OA or PIAs) and subject to post-mortem inspection.
- In addition, the remaining offal in the body cavity from a minimum of 20 birds or 10% of the batch, whichever is bigger, will have to be inspected in full. There are two possibilities that the FBO can choose from:
- Viscera inspected inside the bird (the FBO will have to adapt the speed of the production to allow for this). From a practical point of view, this might be challenging in certain circumstances.
- FBO fully eviscerates at least 20 birds or 10% of the batch and the viscera are inspected outside the bird ensuring correlation between the carcases and the viscera is adequately kept (likely to be quicker).
- If the preliminary post-mortem inspections show an unusual level of rejections, the inspection level of the viscera should be increased by the OV to his/her satisfaction and, if necessary, up to 100% of the batch.
- The speed of the line will be limited to the speed at which the official carrying out post-mortem inspection is able to cope with.
- If the evisceration is completed on a table, adequate hygienic practices will need to be adhered to (for example, washing of hands, regular cleaning of the table, etc).
Commercialisation
- For this product to be marketed, it should be presented for sale labelled or identified as partially eviscerated (“effilé”, “roped”).
6.1.7: Authorisation process for partially eviscerated poultry (“effilé” or “roped”)
Establishments wishing to produce partially eviscerated poultry will require specific authorisation and will need to complete the application form provided in Annex 15.
Parts 1 and 2 of the application form will need to be completed by the FBO that wishes to undertake the process in consultation with the Official Veterinarian (OV). The completed application shall be submitted to the Approvals and Registrations Team approvals@food.gov.uk.
Parts 3 and 4 refer to the authorisation by the FVL/FVC following an on-site assessment. An onsite trial can be arranged between the FBO, the OV and the FVL/FVC to ascertain if the procedures put in place by the FBO are satisfactory.
The completed form with the final recommendation has to be emailed to the Approvals and Registrations Team.
Once the completed form is received by the approvals team, they will inform the FBO in writing of the possible outcomes, as follows:
- Authorisation: If the FVL/FVC is satisfied with the proposal, facilities and the hygiene practices observed during operations on site, authorisation can be immediately granted.
- Refusal: If the FVL/FVC is not satisfied with the proposed arrangements and/or with the hygiene of the operations, the authorisation should not be granted. The FVL/FVC should provide evidence of the reasons for the refusal in the boxes provided in Part 4 of Annex 15.
If after being authorised, the agreed procedures are not complied with and subsequently hygiene and food safety are compromised, the authorisation for partial evisceration or effilé can be withdrawn. A notification letter will be sent by the approvals team to the FBO confirming the decision and the reasons for the withdrawal.
If the FBO disagrees with the outcome of the process, they can appeal in writing to the Operations Head Veterinarian using the approvals address approvals@food.gov.uk.
6.2 Poultry feet for human consumption
6.2.1 Inspection requirements
Feet harvested for human consumption must be inspected.
Feet that are not separately identifiable, such as feet belonging to carcases rejected at evisceration, must not be released for human consumption.
Feet can be exported under an agreed health certificate signed by a Local Veterinary Inspector.
6.3 General Contamination
6.3.1 Meat that is unfit for human consumption
Meat, carcases and / or offal affected with generalised contamination by faecal material, bile, grease, or disinfectants should be considered unfit for human consumption.
6.3.2 Contamination from the alimentary tract and faecal material
A hygienic trimming system must be in place if the FBO decides to trim contaminated carcases.
Any part of the carcase or offal affected with bile staining should be trimmed. Where plucking machines break the skin of poultry the underlying musculature should be considered to be contaminated and trimmed from the carcase.
6.3.3 Meat falling from the line / conveyor
The FBO should have a system in place to deal with carcases or offal that fall on the floor. The OV / MHI should verify that the FBO has a system in place to ensure meat contaminated after post-mortem inspection is not released for human consumption.
6.4 Guidelines on trimming poultry
6.4.1 Trimming supervision
Rectification resulting from post-mortem findings must be carried out under the responsibility of the FSA Operations Group team (supervision of trimming may be carried out by a plant inspection assistant (PIA). Plant operatives should carry out removal of unfit meat identified at post-mortem inspection. Identification of unfit meat for trimming must not be delegated to untrained individuals.
6.4.2 Location of trimming point
Trimming of minor blemishes such as bruising is at the discretion of the FBO
Removal of significant quantities of meat is usually impracticable with high line speeds, and in these cases an adjacent trimming area should be provided.
6.4.3 Trimming after chilling
Trimming of carcases may be delayed until after chilling, providing that:
- there is no risk of contamination to other carcases
- for example, faecal contamination has to be trimmed before chilling
- arrangements are in place for the trimming to be done under the supervision of the OV / MHI at regular times
Note: The OV and the FBO should agree recognised methods (marking and identification of parts to be trimmed) to ensure that trimming is effectively completed by plant staff.
7. Judgements at Poultry Post-Mortem Inspection
In this section
7.1 Poultry condition cards
Click a condition to follow the link:
Abnormal colour (septicaemia – toxaemia)
AM rejects (cull / runts)
Ascites – oedema
Bruising – fractures
Cellulitis
Contamination
DOA / DIL
Dead other than slaughter (uncut–badly bled)
Dermatitis
Emaciation
Hepatitis
Joint lesions
Machine damage
Overscald
Pericarditis
Perihepatitis / peritonitis
Respiratory disease (airsacculitis)
Salpingitis
Tumours
Other factory (processing)
Other farm (for example, jaundice, oregon, white muscle)
Wooden breast
7.2 Introduction
7.2.1 Post-mortem judgements in poultry
Twenty-one poultry condition cards have been developed to achieve standardisation of post-mortem findings in poultry slaughterhouses in the United Kingdom.
These condition cards are to be used as a guidance which inspection teams must follow.
Notwithstanding, the professional expertise of the OV, based on local knowledge and the FCI received for each flock, may result in judgements differing from the advice provided in the condition cards for specific flocks of birds.
7.2.2 Trimming
Where the OV considers the entire carcase is not unfit, the affected parts of the carcase may be removed, and the rest of the carcase may be allowed to enter the food chain. This is to be carried out by plant operatives.
The OV must be content that the FBO has developed a system and trimming is carried out in such a manner that all affected parts are removed to the OV’s entire satisfaction.
7.3 Breast blisters
7.3.1 Breast blisters
Judgement:
Infected, haemorrhagic, or enlarged breast blisters should be trimmed. The affected tissue may be adherent to the keel bone and when this happens part of the bone will have to be removed with the affected tissues. Trimming of small, uninfected, non-haemorrhagic blisters may be deferred until after chilling, when a proportion of them will have disappeared.
Note: The OV needs to consider that breast blisters might be the result of poor husbandry on the farm. If appropriate, the local ROD / DVM should be informed.
7.4 Avian Tuberculosis and Erysipelas
7.4.1 Avian tuberculosis
Avian tuberculosis usually affects older birds with lesions seen most commonly in:
- the liver
- kidneys
- intestinal tract
- bone marrow.
The lesions are irregular shaped greyish-white nodules varying in size from that of a pin's head to large masses. The tubercles can be shelled out from the surrounding tissue. When cut through, the nodules are firm with a dry, cheesy, appearance. If the long bones are split lengthwise, small spherical nodules may be found in the bone marrow.
Confirmation can be made by microscopic examination for the causal organism.
Judgement: Carcases and offal should be considered unfit.
7.4.2 Erysipelas
Erysipelas is primarily a disease of turkeys and the affected birds are listless with, rarely, a swelling of the snood. Mature domestic fowl may also be affected.
Where possible, affected birds should be rejected by the pre-slaughter health inspection but if they inadvertently reach the post-mortem inspection station, they will show signs typical of septicaemia.
- the liver is often enlarged, congested, friable and sometimes light brown in colour
- the intestines are commonly congested and there may be catarrhal enteritis
- a valvular endocarditis may be present in more chronic cases
Judgement: Carcases and offal should be considered unfit.
8. Wild Game Post-Mortem Inspection
In this section
8.1 Introduction
8.1.1 Purpose
This section provides guidance on how to carry out official controls at approved game handling establishments (GHE).
Reference: (EC) 853/2004 overview, (22).
8.1.2 Attendance
An Assessment for OV Flexible Attendance policy (see Chapter 2.10 on ‘Inspection and Attendance’, Annex 1) has been developed to provide a means for assessing the required OV attendance in these types of establishments.
In summary:
- either an MHI or OV, but not both, is required for post-mortem inspection, except that OV presence throughout such inspection is required in specified cases
- additional OV visits are required where the MHI has put aside meat with abnormalities for inspection by the OV, meaning visits for the purpose of inspection of such meat
- operating hours agreements will need to be obtained with each approved GHE; however, due to the nature of the business this may prove difficult – approved GHEs are obliged to inform the FSA when they are operating in order that FSA attendance can be arranged, if required
Note: PIAs are no longer permitted in approved GHEs and should not be performing post-mortem inspections.
8.1.3 Chilling
Carcases have to be collected and transferred to the approved GHE, which may be remote from the hunting area; therefore, some delay in chilling may occur.
However, the chilling must begin within a reasonable period of time after killing and achieve a temperature throughout the meat of not more than 7°C in the case of large wild game and 4°C in the case of small wild game. This does not preclude completion of dressing in the approved GHE before these temperatures have been achieved.
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, 5 and (EC) 853/2004 EC, Annex III, Section IV, Chapter III, Point 4.
8.1.4 Separation of different types of game
In establishments that are approved for the handling of wild game, precautions are to be taken to prevent cross-contamination between species by separation either in time or in space of operations carried out on the different species.
In premises that are approved for the processing of both wild and farmed game, separate facilities for the reception and storage of carcases of farmed game slaughtered at the farm, and for wild game, must be available.
In-fur and in-feather wild game may be stored in separate parts of the same larder / chiller, although separate larder / chillers are preferable.
8.2 Trained hunters
8.2.1 Trained hunter’s examination
A trained person must carry out an examination of the body and, in the case of large wild game, of any viscera removed, to identify any characteristics which may indicate that the meat presents a health risk. The examination must take place as soon as possible after killing.
Reference: (EC) No 853/2004 Annex III, Section IV, Chapter II (Large Wild Game) and Chapter III (Small Wild Game).
8.2.2 Trained hunter’s declaration: large wild game
Following the examination referred to above, large wild game carcases eviscerated in the field require a declaration from a trained person. This must bear the date, time, and place of killing and carry a declaration that, based on an examination of the carcase and viscera:
- there is no suspicion of environmental contamination
- no abnormal behaviour was observed before killing
- no abnormal characteristics were found during the examination
The declaration must be numbered and should be attached to the carcase unless it covers more than one animal body. The declaration may cover more than one animal body, provided that a clear link between the animal bodies and the declaration is established and guaranteed. In these circumstances, the declaration would make reference to a group of numbered carcases and each carcase would be clearly identified with numbered tags or firmly attached labels.
Note: If abnormal characteristics are found during the examination, abnormal behaviour was observed before killing, or environmental contamination is suspected, the trained person must inform the competent authority.
8.2.3 Head and viscera
Where the trained hunter’s declaration is provided stating that no abnormalities were found, the head and the viscera need not accompany the body, except in the case of species susceptible to trichinosis, whose head (except for tusks) and diaphragm must accompany the body. The exception to this is that if the head is required for further use as a trophy, it may be sent to an ABP processing plant that has been approved for the production of trophies. In these circumstances, the head may be dispatched pending a satisfactory trichinella test, provided that the identification of the head is maintained throughout the process.
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, 4 (a).
8.2.4 Acceptance in GHE
Carcases not accompanied by the head and viscera must be the subject of a declaration signed by the trained hunter.
If there is no signed declaration, such carcases must not be accepted in approved GHEs, and are not eligible for human consumption.
If any of the information required to be included in the hunter’s declaration in sub-topic 8.4.2 is missing, the carcases must not be accepted in the approved GHE and the carcase is not eligible for human consumption unless the missing information is provided by the FBO.
The declaration must be signed by a trained hunter. The FBO should keep a copy of the hunter’s training certificate for verification purposes or other suitable method that can verify that the hunter is trained.
Unskinned large wild game may be received by a GHE from another Member State only if it is accompanied by a certificate issued and signed by an OV. A template of this certificate can be found in Annex 7
Reference: (EC) No 853/2004, Annex III, Section IV, Chapter II, 4 (c).
8.2.5 Trained person (hunter) unexpectedly unavailable
In the event that the trained person (hunter) is unexpectedly unavailable, carcases accompanied by the head and all the viscera (with the exception of the stomach and intestines) may be accepted into an approved GHE without the declaration from a trained person.
8.2.6 Offal
In the case of carcase and offal presented without the trained hunter’s declaration, (as in the circumstances detailed above), they cannot be accepted unless clear identification and correlation marks between carcase and offal are present.
Where the carcase has a hunter’s declaration stating no abnormalities were identified, in most cases the offal will not be present. In the event that the offal is present, it must be clearly correlated to the carcase; if it is not, then the offal cannot be used for human consumption.
Where the carcase has a hunter’s declaration stating that abnormalities were found, then the offal must accompany the carcase and must be correlated to it.
(As an example of correlation, the hunter’s declaration is often made on a tie-on label attached to the hock of the carcase; a duplicate label can be tied to the offal where present.)
Reference: (EC) No 853/2004, Annex III, Section IV, Chapter II, 3.
8.2.7 Specimen trained hunter’s declarations
Specimen declarations for wild game animals may be found in the ‘Wild Game Guide’.
8.2.8 Small wild game
In the case of small wild game, a trained hunter’s declaration is not a legal requirement. However, if abnormal characteristics are found during the examination, abnormal behaviour was observed before killing, or environmental contamination is suspected, the trained person must inform the competent authority. The declaration may be attached to trays or cartons to inform the competent authority of any abnormal characteristics, behaviour, or environmental contamination.
In general, if small game exhibits abnormal behaviour, they should not be considered to be fit for human consumption.
Reference: (EC) No 853/2004 Annex III, Section IV, Chapter III, 2.
8.3 Carcase handling
8.3.1 Transport of carcases with hunter’s declarations
There are no provisions under 625/2017 permitting anybody to convey this information on behalf of the trained person instead of a declaration being provided.
Declarations attached to carcases (of large wild game) must not be removed before delivery to the approved GHE where it will be processed, as otherwise the carcase may be disposed as ABP. Similarly, if identification marks which link to a declaration covering several animals are removed or destroyed, those unidentified carcases will be disposed of as ABP.
8.3.2 Skinning
Unskinned large wild game:
- may be skinned and placed on the market only if:
- before skinning, it is stored and handled separately from other food and not frozen, and
- after skinning, it undergoes a final inspection in accordance with Regulation 2019/627 Article 28.
- may be sent to a game handling establishment in another Member State only if, during transport to that game handling establishment, it is accompanied by a certificate issued and signed by an official veterinarian; a template of this certificate can be found in Annex 7
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, 8.
8.4 FSA role
8.4.1 Receipt of carcases and timing of inspection
The inspector (MHI or OV) shall perform the post-mortem inspection activities. It is not essential that there is inspection of carcases prior to the beginning of processing (that is, before skinning), but it is good practice.
Where applicable or practical the FBO may segregate unprocessed carcases that they intend to reject and present them to the inspector prior to disposing of them, for example:
- carcases show signs consistent with death other than by hunting (for example, by road accident)
- carcases are so contaminated that entry would jeopardise operational hygiene or that show evidence of advanced or generalised decomposition
If the FBO decides to proceed with skinning and / or dressing the inspection needs to take place soon after skinning / dressing and / or evisceration.
FBO rejection of carcases before presentation for inspection is often part of the plant HACCP. Inspectors should be aware of this control and audit it in the same way as other plant controls, particularly the evidence, and extent, of corrective action. Discrepancies in intake records and controls should be noted in the plant daybook for future reference.
8.4.2 Read declaration
The OV or inspector is to take account of the declaration or information the trained person involved in hunting the animal has provided in accordance with (EC) 853/2004.
The FBO should provide a copy to the OV or inspector of the hunter’s training certificate or any alternative method so they can verify that the hunter signing the declaration is trained to do so. If there is no evidence of the training of the hunter, and the carcase is not accompanied by the head and the viscera, then the carcase must be detained pending the information of the hunter’s training. The FBO should be given the opportunity to provide such evidence. If the FBO can’t prove that the hunter is trained to sign the declaration, then the carcase cannot be health marked and must be disposed of as an ABP.
The OV or the inspector will need to verify that the hunter’s declaration includes all the information required by (EC) 853/2004 and that it is signed by a trained hunter. Refer to sub-topic 8.2.2 on ‘Trained hunter’s declaration: large wild game’.
According to (EC) 853/2004 the hunter’s declaration must include:
- the date, time, and place of killing and
- carry a declaration that, based on an examination of the carcase and viscera:
- there is no suspicion of environmental contamination
- no abnormal behaviour was observed before killing
- no abnormal characteristics were found during the examination
The declaration must be numbered and should be attached to the carcase unless it covers more than one animal body.
If the required details are missing, the carcase must be detained and the FBO should be given the chance to provide the missing information.
If the FBO cannot provide the missing information, then the carcase must not be health marked and should be disposed of as an ABP.
Where the declaration makes reference to TB, the carcase and offal lymph nodes should be examined in detail and appropriate records made. The carcase and offal should be detained for the OV to provide professional judgement and inform APHA using TB50 form as a template. The incidence and significance of TB varies in different parts of the UK. The advice of APHA should therefore be sought on what further action to take in relation to wild deer where TB infection is suspected (such as collection of samples). The OV will need to make a decision on the fitness of the carcase and offal. In plants where flexible attendance is implemented, the above course of actions must be detailed in a protocol included in the agreed flexible attendance procedures.
This biological hazard must also be considered and analysed in the HACCP plan accordingly.
8.4.3 Inspections
During post-mortem inspection, the inspector is to carry out a visual examination of the carcase, its cavities and, where appropriate, organs with a view to:
- detecting any abnormalities not resulting from the hunting process; for this purpose, the diagnosis must take account of any information that the trained person has provided concerning the behaviour of the animal before killing
- checking that death was not caused by reasons other than hunting, for example, road traffic accident, disease, injury
The inspection of large game should pay particular attention to contamination associated with gralloching (green offal removal), around the pelvis sternum and cut flanks. In carcases that have not been head shot, contamination may be extensive and may result in rejection of the whole carcase – although pre-inspection checks by the FBO should normally identify such carcases.
The carcases must be presented free of contamination to the inspector at post-mortem inspection point. Carcases presented with contamination will not be health marked until the carcase is rectified. The OV or inspector may need to spend extra time in the approved GHE until the carcases are rectified. This time is chargeable to the FBO.
If high number of carcases with contamination are presented at post-mortem inspection, then the FBOs procedures based on HACCP principles should be checked and enforced if appropriate and OV flexible attendance reviewed.
If an assessment cannot be made on the basis of visual examination alone, further palpation and cuts of relevant parts of body may be undertaken and, if necessary, a more extensive inspection must be carried out in a laboratory.
Reference: EU) 2019/627 Article 28.
If the approved GHE is subject to OV flexible attendance, then the OV should verify at least once per month the post-mortem inspection performance of the inspector (MHI or OV) via post-mortem inspection verification checks. Local arrangements should be in place and detailed in the flexible attendance agreement, to allow post-mortem inspection verification checks.
8.4.4 Small wild game contamination
The carcases of small wild game may be contaminated during plucking and evisceration. Where exposed meat, breasts or carcases are contaminated with feathers, down or gut contents they must be rejected.
The use of cloths or paper towels to wipe contamination from carcases is not acceptable. Clean paper towels may be used once to remove feather debris and blood from the vent after evisceration.
Breast meat can only be removed from plucked carcases or in circumstances when the plucked breast has been protected from contamination from other feathers. The removal of breast meat without associated plucking is not acceptable.
8.4.5 Sample inspection of small wild game
Setting the size of the sample is a decision for the inspector taking into account:
- information supplied by the trained hunter (if available)
- species of animal / bird presented for inspection
- general impression gained of the wild game presented for inspection (including uniformity of the sample and signs of decomposition)
- previous history of the source, such as the pattern of disease and proportion of decomposed and contaminated carcases in previous batches
- prevailing climatic conditions
- FBO’s procedures based on HACCP principles and acceptance of birds from hunters
Provided the batch of carcases is relatively uniform, is made up of the same species and came from the same source on the same day, a minimum of 5% of the carcases and viscera must be examined. Batches of less than 20 carcases should be subject to 100% inspection.
8.4.6 No FSA daily attendance
Where there is no daily FSA attendance, the OV may arrange with the FBO a day for the inspection of 5% of each batch present and due to be processed. If they pass inspection, the FBO may proceed to the processing of those batches without the need for several FSA visits. Similarly, if 5% of a batch is retained for inspection, the remainder could be processed and held pending a satisfactory inspection of the 5%, with rejection of the whole batch if the inspection is unsatisfactory.
8.4.7 Other batch factors
In agreeing to inspect a proportion of carcases from a batch, the inspector is making an assessment of the FBO’s competence to recognise unfit or contaminated meat and to take appropriate corrective action. The proportion of a batch to be inspected should reflect the competence of the FBO and evidence of effective processing and hygiene management during uninspected and unattended processing periods.
As with conventional red meat and poultry inspection, decisions must be based on overall hygiene during the dressing process and particularly evidence of cross contamination or contamination associated with dressing procedures.
Poor practice during FSA inspection would provide little confidence that the remainder of the batch was dressed hygienically or that appropriate corrective action and rejections were made during dressing.
The proportion of a batch to be inspected may therefore be larger than 5%, but it must not be less than this.
8.4.8 FBO records
The inspector’s checks should address the following aspects of the FBO records:
- Are there accurate intake records showing numbers of rejections and reasons for rejections?
- Are there records of rejections during processing and are they categorised?
- Can these records be reconciled with ABP records?
- Are there appropriate records of corrective actions?
8.4.9 Other inspection checks
Other checks which the inspector should consider include:
- Are dressing procedures, particularly contamination controls, satisfactory?
- During processing, are hand washing, knife practices and other sanitising procedures satisfactory?
- Are the levels of rejection comparable with those for the previously processed birds or animals from that batch?
- Are birds / animals for FSA inspection presented after the other part of the batch has been processed, or before?
8.4.10 Wild boar
Wild boar are susceptible to the same diseases as domestic pigs and thus it can be expected that a range of lesions similar to that found in farmed pigs will be encountered.
Note: Trichinella testing is required in wild boar. If the head is required for further use as a trophy, it may be sent to an ABP processing plant that has been approved for the production of trophies. The head may be dispatched pending a satisfactory trichinella test, provided that the identification of the head is maintained throughout the process.
8.4.11 FVC verification visits
The FVC must visit all the approved GHE in their area at least once per season to verify that official controls are carried out as per MOC instructions. To ensure all activities are verified during this visit you can use the aide memoires found at annex 12 (large wild game) and annex 13 (small wild game). These aide memoires may be used by the OV or inspector as a check list to ensure that all the official tasks are carried out.
When serious FBO NCs are identified, The FVC should discuss with the FVL the possibility of increasing the OV attendance in the plant and the OV flexible attendance should be reviewed.
Any findings identified during the FVC verification visit should be discussed with the service delivery partners (SDP) and if necessary, dealt with via contract management.
8.4.12 Communication between OV and inspector
In approved GHE where there is OV flexibility implemented, there must be a written communication procedure in place between the inspector and the OV. The OV must be aware of any NCs identified by the inspectors when the OV is not present so enforcement action is taken if necessary.
This written communication procedure must be available for the FSA officers (FVL, FVC, veterinary auditor (VA) during the day of their visit for their assessment.
8.5 Inspection of deer
8.5.1 When to inspect
The carcases of deer should be inspected after skinning in conjunction with the available correlated red offal, where available.
Note: Red offal will only be presented for inspection where the trained person has noted an abnormality or where they are unexpectedly unavailable.
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, Paragraph 4 (a)-(c).
8.5.2 Minimum post-mortem requirements
Post-mortem inspection must consist of a visual examination of the carcase, its cavities and accompanying offal. In most cases, offal will not be available and in these circumstances, if a declaration from a trained person is not attached to the carcase or it is not identified to a declaration, it must be disposed of as ABP.
8.5.3 Bullet wounds
Carcases with damage caused by the entry of the bullet will require trimming of any bruised or contaminated meat.
Carcases where the bullet entered through the shoulder or the anterior thorax may have shattered bones and muscle damage requiring extensive trimming and rejection of the shoulder or quarter.
Where the bullet has entered through the abdomen, bruising, bone damage and contamination can be extensive and may warrant rejection of the entire carcase.
8.5.4 Contamination
Some damage to the heart, liver and lungs may occur as a result of shooting. Decomposition and contamination are common findings. As a consequence of rupture of the abdominal organs following shooting, or as a consequence of poor gralloching, leakage of gut contents into the abdominal cavity may occur.
The carcase may also become contaminated as a result of poor handling in the field or during transportation to the processing establishment. Any part of the carcase with visible contamination must be trimmed and rejected.
The retention of heavily contaminated meat in close proximity to potentially fit carcases should be avoided. In those circumstances, where trimming precedes inspection, and to minimise potential contamination, trimmed meat should be hygienically retained so that a decision can be made based on the condition of the whole of the carcase. It may not be possible to make a decision if all parts of the carcase have not been retained and identified.
8.5.5 Total rejection
When carcases have been stored under unacceptable conditions (such as high ambient temperatures or exposed to pests) conditions such as generalised decomposition or blowfly infestation will be encountered, and total rejection is necessary.
8.6 Processing in fur / in feather (IFIF) carcases
8.6.1 IFIF trade
Approved premises, such as red or white meat cutting plants, cannot be regarded as a local retailer and therefore cannot receive exempt game or game meat directly from local producers or hunters.
If game is not supplied under any of the exemptions listed in the wild game guide, it must ultimately be processed and inspected in an approved GHE.
Approved GHEs can sell on unprocessed game that has not been subject to an inspection but only to another approved GHE either here or elsewhere in the EU. An identification mark should be applied to small wild game if it has been handled in some way in an approved GHE before it is sent on to another approved GHE.
Temperature requirements apply (4°C small wild game and 7°C large wild game)
Reference: (EC) 853/2004, Article 1, 3 (c) and (e).
8.6.2 Trade of unplucked / unskinned and uneviscerated small wild game
FSA staff shall be aware that where small wild game are to be traded unskinned / unplucked and uneviscerated they:
- may be frozen or deep frozen
- should be stored separately from fresh meat, poultry meat, and other wild game already skinned and plucked
- can be traded only to another approved GHE; sealed boxes and uneviscerated wild game cannot be factored by approved cutting plants even though the packaging is not opened
Note: Smithfield Market is not an approved GHE.
Reference: (EC) 853/2004, Annex III, Section II, Chapter V 1 (c).
8.6.3 FBO duties
Where the FBO intends to trade small game unskinned / unplucked and uneviscerated they must inform FSA staff for monitoring and verification of this activity during the plant audit.
They should have procedures in place to ensure that there is no undue extra food risk in transporting the uneviscerated animals, for example, FBO presented procedures in place to ensure that chill chain is maintained when the viscera are still within the body cavity.
8.6.4 Inspection of small wild game to be traded
Where the FBO intends to trade small wild game, which is unskinned / unplucked and uneviscerated, the FSA staff must monitor and verify this activity as part of the establishment audit. Post-mortem inspection will take place at the receiving approved GHE.
8.6.5 ID marking of small wild game to be traded
An identification mark should be applied to unskinned / unplucked and uneviscerated small wild game, if it has been handled or graded in some way in an approved GHE before it is sent on to another approved GHE.
8.6.6 Intra-community trade
In-skin, in-feather and processed wild game can be consigned to and received from other Member States, subject to any animal health restrictions, and subject to the appropriate export / import certification being in place. If you are unclear as to whether exports or imports may take place during outbreaks of notifiable disease, contact APHA.
All game intended for export or import must have been examined by a trained person (where applicable) immediately after shooting and the game must be handled and transported hygienically in refrigerated transport. The Regulations place a responsibility on the supplier of such game to ensure that it is only consigned to approved premises and transported in hygienic conditions. Unskinned large wild game may be sent to a game handling establishment in another Member State only if it is accompanied by a certificate issued and signed by an OV. A copy of the certificate is at Annex 7.
8.7 Recording of inspection results
8.7.1 Duty of FSA Operations Group
If inspections reveal the presence of any disease or condition that might affect public or animal health or indicate that animal welfare has been compromised the OV is to inform the FBO.
Where lesions suggestive of TB are recorded on the trained person’s declaration, the OV or MHI should confirm that this information has been passed to APHA. APHA should also be contacted if potential TB lesions are found during the inspection of large wild game carcases.
Where the OV is not present the MHI shall contact the OV as soon as possible and discuss necessary action, as per procedures detailed in the agreed flexible attendance document. In certain cases, this may require attendance of the OV at the approved GHE.
Where the problem arose during primary production, the OV shall gather all the information and cascade it to APHA where appropriate, as detailed in section 8.4.2.
8.7.2 FBO’s trained hunter’s declaration and inspection record
The FBO must have a system in place to file the trained person’s declarations (including trained person’ inspection records) in such a way that the declarations can be identified clearly to the individual carcases or batch of carcases.
For large game, the declaration or a number repeated on and relating to the declaration must be attached to the carcase when it is presented for inspection. Carcases without an attached hunter’s declaration label or link to a declaration must be disposed of as ABP (unless presented with the head and all the viscera except for the stomach and intestines).
8.7.3 Post-mortem inspection results and recording of data
Results of post-mortem inspection should be recorded on IRIS. Where there is no IT system available in the plant, forms PMI 4/2 (Deer – Daily Record of Rejection Conditions), PMI 4/5 (Daily Record of Rejection Conditions Large Wild Game), PMI 4/10 (Daily Record of Rejection Conditions Small Wild Game in Feather) and PMI 4/13 (Daily Record of Rejection Conditions Small Wild Game in Fur) can be used to record condition data to be entered onto IRIS at a later date / time. This should be completed at the earliest opportunity, subject to IT availability.
The FSA and FBO must have a system in place to ensure that the results of ante and post-mortem inspections are recorded accurately and can be identified clearly to the batch of animals, or in some cases to the individual animal. The OV must be satisfied with the system for collecting the data at all points.
Reference: See chapter 9 on ‘Forms.
8.7.4 Database
Information is logged on an FSA national database and will be used by:
- Defra to analyse disease trends
- FSA to monitor disease status, for example, trichinella
- FVC when establishing OV attendance
Note: Additional information on Assessment for OV Flexible Attendance is available in the ‘Policy and Procedure for Flexible Attendance at Slaughterhouses and Game Handling Establishments’.
9. Health and Identification Marking
In this section
9.1 Health marking
9.1.1 Overview
The health mark indicates that the animals and the resulting carcase have undergone ante and post-mortem inspection in accordance with (EU) 2019/624 and (EU) 2019/627 and there are no grounds for declaring the meat unfit for human consumption.
Reference: See the topic 9.2 on ‘Identification marking’ in this section for additional information.
9.1.2 Responsibility and health marking
The OV is responsible for ensuring the correct application of the health mark. The actual application of the health mark may be delegated to an MHI or to an FBO member of staff, but only under the effective supervision of the OV.
The health mark shall be applied when official controls have not identified any deficiencies that would make the meat unfit for human consumption and, where appropriate, TSE testing has been carried out with negative results.
9.1.3 Delegation of application of the health mark to plant staff
Article 18 (4) of Regulation (EU) 2017/625 allows for the OV to delegate the application of the health mark to plant staff as long as they comply with the conditions laid down in paragraph 3 of the same article which state that staff:
a) act independently from the production staff of the slaughterhouse;
b) have undergone appropriate training to carry out this task; and
c) carry out this task in the presence and following the instructions of the OV or the OA.
The requirement in c) does not mean that the OV/OA needs to observe FBO staff applying the health mark on every occasion but the OV will need to implement supervision systems and guarantee that the health mark is kept and used appropriately.
SOP
The OV will discuss the delegation requirements with the FBO and will develop a SOP in agreement with the FBO. The SOP will detail the procedures for releasing and recovering the health mark each time it is delegated to plant staff (including during breaks, plant breakdowns), how the task is going to be carried out (species, positions on the line) and the training requirements for plant staff.
The training must be provided by the OV and/or an appropriately briefed OA ensuring that the instructions provided in Section 9 of Chapter 2.4 of the MOC are correctly understood by appropriate plant staff identified by the FBO.
Training will be recorded and the OV must keep an up-to-date list of all the FBO staff authorised to apply the health mark on their behalf and make this list available to the FBO and to FSA officials at any time.
Risk Assessment
For allowing the delegation, it is essential that the OV has confidence in the implementation of the Food Safety Management Systems at the plant.
Before proceeding with the delegation of the health mark to plant staff, the OV will carry out a risk assessment (form provided in Annex 14) and complete a one-week trial. The OV must be satisfied that there are adequate procedures in place to ensure that the health mark is only applied on carcases deemed fit for human consumption and for dealing with carcases that are declared unfit and/or detained.
As part of the risk assessment, the OV will also discuss with the ITL for the area any potential impact on OAs’ resource requirements for the plant. Once the OV is satisfied with the procedures, they will permit the delegation by signing the risk assessment form provided in Annex 14 and will inform the ITL for updating the SOP as necessary.
Supervision and performance monitoring
The delegation and return of the health mark must be recorded on each occasion. Plant staff must return the health mark to the OV or the OA every time it is not in use; this includes during breaks, breakdowns, or any other circumstances.
Records must include at least the following:
- the date and time of delegation of the health mark
- the name of the person receiving the health mark
- the initials of the OV/OA delegating the health mark
- the date and time of return
- the initials of the OV/OA receiving the health mark
The performance of FBO staff carrying out this task on behalf of the OV will be monitored daily by the OV/OA.
Records of both, supervision of the health mark and performance monitoring of authorised FBO staff must be recorded in the relevant form provided in Chapter 9 (HM DEL).
Withdrawal of the delegation
Where supervision or monitoring indicates that the delegation and/or application of the health mark is not in accordance with the agreed SOP, the OV can either reinstate the application of the health mark from a particular FBO member to OAs and/or the OV or withdraw the delegation for the whole establishment if there is evidence or suspicion that continuing with the delegation might lead to a risk to food safety.
Records of reinstatement of the application of the health mark for individual members of FBO staff will be recorded in form HM DEL (Chapter 9).
If the OV decides to withdraw the delegation for the whole establishment they will record this in Part 3 of the risk assessment form (Annex 14).
9.1.4 Meat that should be health marked
The health mark is only applied to carcases and wholesale cuts of:
- cattle, including buffalo and bison
- sheep, goats, and pigs
- horses
- camelids
- ratites
- farmed deer and wild boar
- large wild game, deer, and wild boar
Reference: (EU) 2019/627 Article 48, 2.
9.1.5 Application
The health mark should be applied in the slaughterhouse or game-handling establishment so that if carcases are cut into half or quarters or half carcases are cut into 3 pieces, each bear such a health mark. The FBO should inform the AO how many pieces the carcase will be cut into if they wish the minimum number of marks to be applied.
9.1.6 Wild game
Meat from wild game can only bear a health mark if it is skinned in a game handling establishment, has undergone post-mortem inspection, and been found fit for human consumption.
Reference: (EU) 2019/627 Article 48.
9.1.7 Application at inspection
A system should be in place so that the line speed and inspection facilities allow the health mark to be applied to the carcase at the time of post-mortem inspection.
9.1.8 Blurring
Blurred health marks are unacceptable and, if this is a problem, a system should be arranged so that:
- one health mark is applied if the carcase is fit at the time of inspection
- health marking is completed once the carcase has dried (in the chiller)
9.1.9 Health mark and trichinosis
Where a procedure is in place in the slaughterhouse to ensure that no part of carcases examined leaves the premises until the result of the trichinella examination is found to be negative and the procedure is formally approved by the OV, the health mark may be applied before the results of the trichinella examination are available.
The operator must have a written procedure agreed with the OV in place.
Where such system is not in place, the health mark must not be applied until a negative test result has been received.
9.1.10 Withheld health mark
The health mark can only be applied to the carcase of animals which have undergone ante and post-mortem inspections in accordance with (EU) 2019/627 and there are no grounds for declaring the meat unfit for human consumption. Examples of where the health mark should be withheld are:
- failure of ante-mortem and / or post-mortem inspection
- presence of SRM (except Vertebral Column of over 30-month bovines)
- carcases presented for inspection with evidence of visible contamination or gross pathology
- where residues or contaminants are suspected
- carcases produced in a slaughterhouse where the water supply is found to have been contaminated and a risk to public health exists
- where adequate facilities for inspection are not available and there is a risk that carcases with visible contamination or gross pathology could be inadvertently health marked (that is it has not been possible to perform adequate inspection)
- carcases from animals suffering from a notifiable disease
- meat declared by the OV to be unfit for human consumption
9.1.11 Recording marks used
To prevent fraudulent use of health marks and other stamps all members of the FSA staff must record in the daybook:
- the time of issue
- the number of the health mark stamp
- the time stamps are returned to secure storage
9.1.12 Security of the health mark
The security of the health mark stamp is the responsibility of the officer to whom it was issued.
- The health mark stamp must be kept in secure lockable facilities when not in use.
- The OV must be able to demonstrate the security of health marking equipment.
- The OV must have an auditable system in place to check that all health mark stamps have been returned at the end of each operational day.
- Anyone possessing or using health marking equipment, without the authority of the OV is committing an offence.
9.1.13 Reporting missing stamps
If a health mark stamp is stolen or lost, there is potential that it can be used for fraudulent activities and used for illegally killed animals. Missing stamps whether lost or stolen must be reported immediately to CSU transactions team.
9.1.14 Meat not health marked
Unmarked meat that is required to be health marked cannot be sold for human consumption. The FBO is responsible for disposing of the meat in compliance with the ABP regulations.
Reference: (EC) 853/2004, Article 5
9.1.15 Health mark labels
For the health marking of lamb, kid, and piglet carcases the hygiene regulations no longer permit the use of health marks in the form of a label or tag instead of ink / hot branding as was permitted under the previous legislation.
Reference: (EC) No 2076/2005, Article 5.
9.2 Identification marking
9.2.1 Requirements
Carcases and wholesale cuts of red meat species, farmed game mammals (other than lagomorphs) and large wild game that have passed official controls at a game handling establishment should all be health marked. Other products of animal origin only require an identification mark.
9.2.2 Application
Identification marks are applied by the FBO. The FSA is required to verify compliance with the application of identification marks.
10. Edible co-products
In this section
10.1 Edible co-products
10.1.1 Definition
Edible co-products are parts of slaughtered animals unsuitable for human consumption at the time of production in the slaughter house, but which can later be processed for use in human food.
Examples of edible co-products include:
- rendered animal fat and greaves
- treated stomachs bladders and intestines
- gelatine
- collagen
Reference: (EC) 853/2004, Annex III, Sections XII, XIII, XIV and XV.
Detailed guidance is contained in the FSA guide: Industry Guide on Edible Co-products and Animal By-products. This can be found in Annex 1, Chapter 18 ‘Waste Management (including Animal By-Products)’ of the Meat Industry Guide.
10.1.2 Feet for human consumption
Feet intended for human consumption are treated as edible offal. All feet intended for human consumption must be inspected.
Feet processed on site:
Post-mortem inspection can be done before or after further treatment (such as dehairing) on an individual basis or in batches. If post-mortem inspection takes place before treatment, a further spot check will be needed to ensure that these feet are free from any pathology.
Feet processed at a different approved site:
Post-mortem inspection can be done before or after cleaning (washing) on an individual basis or in batches. If post-mortem inspection takes place before cleaning, a further spot check will be needed to ensure that these feet are visibly clean before shipping for further processing.
In both cases a full correlation system must be implemented by the FBO to ensure that if a carcase is condemned, the correlated feet of the entire batch are disposed of as unfit for human consumption. FBOs may assist the inspection process and set aside feet with identified abnormalities.
Feet which have not been inspected, are not visibly clean or have not been processed cannot be despatched from the establishment as intended for human consumption.
10.1.3 FBO responsibility
The FBO should identify handle, process, store, and despatch edible co-products in accordance with the guidance contained in the meat industry guide.
Co-products should be stored and despatched to appropriate destinations separate from ABP, in accordance with the guidance.
Co-products should be despatched with the correct documentation, containing the information outlined in the specimen documents in the co-products guidance.
10.1.4 FSA responsibility
The OV is to check that:
- the FBO handles the co-products in accordance with the FSA guidance having due regard to hygienic processing, separation, storage, and temperature requirements
- that the edible co-products are consigned to appropriate premises
- that adequate separation from ABP’s is maintained, such as cattle hides intended for the production of gelatine for human consumption are stored and despatched with adequate separation from all other hides
- that a control system is in place for hides from bovines that require BSE testing, pending a negative test result
11. Slaughter hygiene verification system in red meat
In this section
11.2 Slaughter hygiene verification
11.3 Process – hygiene verification
11.4 Product – carcase verification
11.5 Plant – establishment verification
11.1 Introduction
11.1.1 Purpose
The SHV system focuses on gathering qualitative measures to assess FBO processing standards.
The SHV system monitors contamination at final inspection as a key point to satisfy FSA regulatory requirements, but also creates a more holistic approach to provide a more complete picture of the processing standards of the FBO, with the ultimate objective of providing clear evidence of improvements to carcase hygiene when required. The SHV system focuses on the need for FBOs to take the necessary corrective actions, quarantine and rectify contaminated carcase, take effective actions, and prevent re-occurrence.
This guidance outlines a consistent approach on how and when OVs / AOs shall verify that FBOs have implemented effective slaughter hygiene practices and procedures which prevent contamination of carcases with enteric pathogens and faecal contamination throughout the entire slaughter and dressing operation and that their food safety management systems demonstrate this control.
The results of verification checks can be used to:
- provide advice to assist the FBO with root cause analysis
- provide data for further trend analysis by FSA
- provide evidence for enforcement action
- justify health and identification marking
- inform the FBO audit process
- inform veterinary certification for third country export
11.1.2 Background
The FSA has developed SHV procedures by looking at the regulatory official control verification requirements at abattoirs.
With particular reference to slaughter hygiene, official controls must verify:
- FBO compliance with Regulations (EC) 852/2004 and (EC) 853/2004
- that FBOs apply procedures to ensure good hygiene practices continuously and properly
- that FBOs apply HACCP-based procedures continuously and properly
Verification is the responsibility of the OV, but information regarding good hygiene practices and HACCP based procedures can be gathered by the Official Auxiliary (OA) to assist the OV.
11.2 Slaughter hygiene verification
11.2.1 Key elements of the verification system
The verification system applies predetermined minimum frequencies of verification tasks, which provide information on the delivery of official controls, enforcement activity and objective evidence to support FBO audits.
Key summary points of the verification system are as follows:
- SHV checks should be carried out by OVs and AOs
- SHV must be completed in each establishment
- the number of verification checks can decrease or increase depending on findings
- the SHV system can be utilised by OVs / AOs and technical contract managers to assess performance and official control delivery to focus attention and discussions
11.2.2 Slaughter hygiene verification method
The verification system includes a number of tasks that must be carried out for each of the processed species and should cover the whole of the production process. Verification tasks are divided into the four following categories and have different frequencies based on the associated risks and possible impact on public health:
- process – hygiene verification
- product – carcase / offal verification
- plant – establishment verification
- HACCP and microbiological verification
A summary of all verification tasks and their frequencies can be found at Annex 8, a SHV Task Schedule at Annex 10 and a SHV flow chart at Annex 11.
The initial selection of carcases for process hygiene and product verification should be random. However, based on the findings, the OV / AO may wish to target a specific type of process or animals to better assess FBO’s controls.
If the outcome of the verification checks indicates poorly implemented FBO procedures, then the documented SOPs and records should also be considered as part of SHV verification checks.
11.2.3 Minimum requirements – assessment of samples
The OV / AO should select a point on the production line where suitable facilities are available to allow a thorough examination of all surfaces of the sampled carcases.
Sufficient time must be allocated by the OV / AO to ensure a thorough examination of the carcase / side is performed and accurate data is collected, and consistency is maintained.
11.2.4 Outcomes
Each verification area must be assessed by the OV / AO and scored-based on the outcome (compliant / NC) and the level of the enforcement action taken.
| Outcome | Description |
|---|---|
| Compliant: green |
Food business is operating in accordance with its food safety management systems, food safety standards and has met the requirements of the regulations No enforcement action taken |
| Non-compliant: yellow | A non-compliance that resulted in a verbal advice |
| Non-compliant: amber | A non-compliance that resulted in a written advice |
| Non-compliant: red | A non-compliance that resulted in a formal enforcement action (service of legal notices, referral for investigation) |
Using objective evidence, the type of deficiencies identified during the daily / weekly / monthly SHV checks and FBO’s corrective action reflect the extent and effectiveness of performance and compliance.
11.2.5 Reporting arrangements
The K2 system will produce data reports with results of verification activity. The information must be utilised by OVs / AOs to monitor individual plant performance during the interim FBO audit period.
OV / AO must use the FSA Slaughter Hygiene Checklist (Annex 4) to record the outcome of verification checks and store it at the plant.
11.2.6 Use of verification data
The recorded daily outcomes of verification tasks will provide information about the level of current performance / compliance.
The gathered data will assist the OV / AO in defining reasonable expectations of operating standards.
Establishment trend analysis and professional judgement from the OV / AO is required for appropriate action. This will assist in compliance decisions and achieve consistency of approach.
The OV / AO should review the results on a daily, weekly, monthly basis and take the appropriate action as detailed in topics 12.3 to 12.6.
11.3 Process – hygiene verification
11.3.1 OV / AO responsibility
The OV / AO is expected to verify hygienic standard of the process to assess if the FBO has adequate controls in place to minimise contamination and if corrective actions are taken when contamination incidents occur.
11.3.2 Process scope
| Verification Steps | Scope (all species) |
|---|---|
| 1 Cleanliness of animals |
Animals clean on arrival or measures taken by FBO to ensure that animals are clean before dressing commences or other measures taken to prevent cross contamination from dirty animals. OV / AO to record in K2 the number of carcases checked, and the number of carcases found not clean. |
| 2 Bleeding |
Bleeding does not result in carcase contamination. OV / AO to record in K2 the number of carcases checked, and the number of carcases found contaminated with faeces / ingesta after bleeding. |
| 3 Skinning / hair removal |
Meat contamination avoided (for example, contact between outside skin and carcases prevented, operator / equipment in contact with the outside of hide / fleece not touching the meat) Skinning completed (no pieces of skin left) and bristles removed. OV / AO to record in K2 the number of carcases checked, and the number of carcases found not completely skinned (pieces of skin left / bristles not removed) and/or contaminated with faeces / ingesta / milk after skinning / hair removal. |
| 4 Evisceration / udder removal |
Spillage of digestive tract content prevented, and removal of udder does not result in contamination of the carcase with milk or colostrum. OV / AO to record in K2 the number of carcases checked, and the number of carcases found contaminated with faeces / ingesta / milk after evisceration/udder removal. |
| 5 Presentation for inspection |
Carcases and offal presented for inspection free from any visible contamination. OV / AO to record in K2 the number of carcases / offal checked, and the number of carcases / offal found contaminated with faeces / ingesta / milk at the step of presentation for inspection. |
11.3.3 Process frequency and sample size
The verification checks in process hygiene areas have to be carried out every day for every species slaughtered. However, the frequency of verification checks at the steps: ‘Cleanliness of animals’, ‘Bleeding’, ‘Skinning / hair removal’ and ‘Evisceration / udder removal’ can be reduced to once a week, if the OV is satisfied with the hygienic standard of the establishment and certain conditions are met.
In order for the OV to consider the reduced frequency of verification checks, the establishment should meet the following criteria:
- ‘Good’ or ‘Generally Satisfactory’ outcome of the last FBO audit
- no formal enforcement related to the hygiene of production (no hygiene improvement notices (HINs), remedial action notices (RANs, referrals for investigation) in the last 4 weeks
- less than 5% of carcases presented contaminated for inspection in the last 4 weeks (daily percentage).
| Verification Step | Basic frequency | Reduced frequency |
|---|---|---|
| 1 Cleanliness of animals | Daily | Once a week |
| 2 Bleeding | Daily | Once a week |
| 3 Skinning / hair removal | Daily | Once a week |
| 4 Evisceration / udder removal | Daily | Once a week |
| 5 Presentation for inspection | Daily | Daily |
Note: The frequency of the verification checks at the step ‘Presentation for Inspection’ cannot be reduced and they should be always carried out daily.
The table below demonstrated how many carcases – based on the establishment’s daily throughput – have to be verified daily at steps: ‘Cleanliness of animals’, ‘Bleeding’, ‘Skinning / hair removal’ and Evisceration / udder removal’.
The numbers provided in the previous table are a minimum and can be increased by the OV dependant on findings during checks.
| Daily throughput |
Minimum number of carcases to be checked (at steps: ‘Cleanliness of animals’, ‘Bleeding’, ‘Skinning / hair removal’, Evisceration / udder removal’) |
|---|---|
| 0-100 | 2 |
| 101-250 | 4 |
| 251-500 | 7 |
| More than 500 | 11 |
The daily number of carcases and offal that must be verified at the ‘Presentation for inspection’ step, with outcome recorded, depends on the daily throughput of each slaughtered species.
The following table demonstrates how many carcases / offal should be selected for verification. All slaughtered species should be verified daily.
| Daily throughput |
Minimum number of carcases and offal to check daily (at ‘Presentation for inspection’ step) |
|---|---|
| 0-24 | 4 |
| 25-100 | 10 |
| 101-250 | 30 |
| More than 250 | 60 |
Note: Any decision to increase the number of checks, above the minimum recommended in the table above, should be recorded in the plant daybook.
11.3.4 Process – unit size
For all species, a unit is defined as a whole carcase (with offal), regardless if split or not e.g. if a carcase is split into two sides, then two sides have to be inspected to count it as a unit.
Due to different line set ups and arrangements it is possible to assess part carcases / sides at random to achieve the required sample size, (for example: assess a run of beef hindquarters on the high stand and complete the monitoring from the low stand with a later run of forequarters).
Where carcases or sides are divided into sections for assessment, all defects from the sections that make up one complete carcase must be added together to determine how the defect is scored for that carcase.
Carcase verification can be carried out ‘on-line’ at normal processing speeds or at a designated area.
11.3.5 Process – contamination
Any visible trace of faecal, ingesta and milk contamination must be counted and recorded. Each contaminated carcase or offal counts as one incident, regardless of the amount of contamination present.
In cases where contamination identified during verification checks is different to digestive tract content (faecal / ingesta) or milk, the OV / AO should bring it to the attention of the FBO and ask for it to be removed / trimmed. Such cases, however, do not have to be recorded. Examples of contamination other than digestive tract content or milk include rail dust, hide / wool, bile, and oil / grease. Excessive and frequent contamination of this type should trigger enforcement action.
Additional points for consideration when scoring:
- retained udder fragments are evidence of milk contamination
- gut segments, including oesophagus, are classified with faeces, ingesta, milk
- contamination issues already identified by the FBO (such as clearly marked carcases for further rectification) are not to be added to the SHV form as those were already identified as part of the FBO’s HACCP system
- however, excessive carcases being removed from the processing line is a significant issue and appropriate OV action should be taken regardless of whether the FBO has identified these; detention logs and rejected meat records (IRIS) will provide appropriate evidence to utilise
11.3.6 Process – enforcement
The FSA supports a ‘zero tolerance’ approach to visible contamination on carcases, which requires that all identified visible contamination on meat is removed by the FBO without delay by trimming or alternative method having an equivalent effect.
In cases where frequent and regular contamination problems are identified by OV / AO, an enforcement action must be taken in accordance with Chapter 7 ‘Enforcement’.
11.3.7 Process – digestive tract content
OVs / AOs are to identify foreign material as faeces or ingesta based on the characteristics of colour and texture and only when they are able to identify either colour or texture. Size is unimportant in identifying faecal or ingesta contamination however, as size decreases, colour and texture become more difficult to identify.
- The colour of faecal or ingesta contamination is:
- cattle – yellow, green, or brown
- pigs – tan to dark brown
- sheep and goats – brown to black
- Faecal or ingesta contamination has a fibrous or plant-like texture; for example, sheep and goat faeces and ingesta may be tarry, whilst pig faeces and ingesta may include identifiable grain particles
11.3.8 Process – milk
OVs / AOs are to identify foreign material as milk based on two factors: colour and consistency.
- The colour of milk ranges from clear to white to light yellow.
- The consistency of milk ranges from watery to ropy or curdy.
Milk, if present, tends to be found on the midline, during or after removal of mammary glands (udder).
11.4 Product – carcase / offal verification
11.4.1 Product – carcase / offal verification
On an ongoing basis, the OV will verify a sample of carcases and offal (including fifth quarter product) that have been health marked. The verification checks should reflect the full range of species and age / type of animal being processed. Only the final product (carcases or offal) should be verified, and the following production stages could be selected for carrying out the checks:
- immediately after inspection points (after final rectification by the FBO) – to ensure real time checks
- in the chiller
Verification of offal includes parts that are fit for human consumption at the inspection point (such as liver, heart, and skirt). Others intended as edible co-products which require further processing prior to being eaten (for example, tripe and casings) should also be included in the verification checks.
11.4.2 Product – carcase / offal verification scope
Product verification replaces the previous PMI verification checks and focuses on the FSA’s performance. Therefore, the outcome should not be used as direct indication of the FBO’s performance. However, frequent findings in this area could trigger additional checks as part of the process verification.
The following table details the scope of verification during product checks:
| Area of verification | Scope |
|---|---|
| 1 Pathology | Meat is free from all pathological conditions |
| 2 Statutory requirements | Post-mortem inspection has been carried out in accordance with legal requirement |
| 3 Faecal / ingesta / milk | Meat is free from faecal / ingesta / milk contamination |
| 4 Health marking | Meat is correctly and legibly health marked |
| 5 Other | Record any identified deficiency (for example, contamination with bile / hair / wool, tonsils, stick wounds, SRM, rail flake) |
11.4.3 Product – carcase / offal verification – frequency
Verification must be carried out on three operational days a week (if possible) or spread over the whole week in establishments with a very low throughput (less than 100 a week).
The number of carcases to be checked depends on the weekly throughput (as in the following table):
| Weekly throughput | Weekly total of carcases and offal to check |
|---|---|
| More than 1000 | 60 carcases and 60 sets of offal (20 carcases and 20 sets of offal per species per day, 3 days per week) |
| 101-1000 | 30 carcases and 30 sets of offal (10 carcases and 10 sets of offal per species per day, 3 days per week) |
| 0-100 | 5 carcases and 5 sets of offal (spread over the whole week, if possible) |
Note: In OV-only establishments and plants with recognised OV flexibility (such as cold inspection) the product verification checks should be carried out during routine FVC or contractor management visits and documented on the K2 system by the FSA / service delivery partner (SDP) at least every three months. The FVC is accountable for ensuring these checks have been carried out and documented and is responsible for establishing the number of carcases and offal that should be verified during those visits. The verification system should not impact on agreed resource and business agreements as outlined in the Statement of Resources for the individual establishment.
Note: Any decision to increase the number of checks, above the minimum recommended in the table above, should be recorded in the plant daybook.
11.4.4 Product – carcase / offal verification – assessing results
Although product verification aims to measure the FSA’s effectiveness as the inspection service, it is also an indication of the effectiveness of FBO controls.
The product verification is not subject to scoring. The OV is only required to record and input in the system the number of deficiencies identified and the total number of carcases / offal checked.
Verification results should be assessed by the OV / FVC to monitor team performance. Variables in each establishment should be considered if concerns are raised following verification checks (for example, lighting, available inspection time and space, FBO performance, plant layout).
Note: The OV / FVC should maintain realistic expectations during the checks when assessing team performance from the product verification results, as minor incidents of contamination become more evident post-chilling, particularly with pig hair and wool.
11.5 Plant – establishment verification
11.5.1 Plant – establishment verification
Establishment verification tasks focus mainly on different parts of the establishment, equipment, cleanliness, hygiene arrangements and procedures.
The minimum frequency of establishment verification tasks depends on the FBO audit outcome. However, the OV can increase the frequency if considered necessary and should always score a relevant section when an intervention takes place that resulted in verbal, written or formal enforcement.
Some establishment verification tasks are considered essential and should be carried out and scored every day, regardless of the audit score awarded.
The following table lists the establishment verification areas and the minimum frequency of checks based on FBO audit outcome.
Plant - establishment verification (FBO audit outcome)
| Establishment verification tasks and their frequency | Improvement necessary / Urgent improvement necessary | Good / Generally satisfactory |
|---|---|---|
| 1 Intake / FCI | Daily | Daily |
| 2 Ante-mortem arrangements | Daily | Daily |
| 3 Correlation of carcases and offal | Daily | Daily |
| 4 Operational break / cleaning | Daily | Daily |
| 5 General hygiene (1) | Daily | Daily |
| 6 Handling of carcases / offal during storage and despatch (2) | Daily | Daily |
| 7 Co-products and animal by-products (3) | Daily | Daily |
| 8 FBO pre-operational cleaning | Weekly | Monthly |
| 9 Carcase and offal chilling | Weekly | Monthly |
| 10 Premises (4) | Weekly | Monthly |
(1) ’General Hygiene’ includes verification of hygienic practices (including staff movement, PPE provisions and practices, hand washing), hygienic facilities provided (hot water, soap, sterilisers), door policy, cross contamination controls.
(2) ’Handling of carcases / offal during storage and dispatch’ includes 5th quarter products (such as bones, tendons, feet).
(3) ’Co-products and animal by-products’ includes verification of separation of edible and non-edible materials and captures outcome of daily/weekly SRM checks (for details see MOC Chapter ‘2.7 Specified risk material controls’ and Chapter ‘2.8 Animal By-Products’).
(4)’Premises’ includes verification of lairage / intake area, processing / dressing area, chillers, packing / packaging storage area, dispatch area, plant surrounds, fly screening / vermin entry prevention, control of waste water, drainage and effluent, and water testing.
Note: SHV K2 form should be also used to record monthly summary of FBO compliance with SRM controls. Details should be input in the relevant part of the SHV K2 form.
11.6 HACCP verification
11.6.1 HACCP verification
The verification of the FBO’s HACCP-based procedures is focused primarily on two areas: monitoring of CPs/CCPs and corrective actions.
The OV / AO is not expected to check all records but must verify a sample to be satisfied that the FBO is following their own procedures for monitoring control points and that the FBO is taking and recording pre-established corrective actions when the control is lost.
| Area of verification | Scope |
|---|---|
| 1 Monitoring of CPs/CCPs | Monitoring procedures implemented; accurate records that reflect reality maintained (up to date) |
| 2 Corrective actions | Correct actions taken when monitoring indicate loss of control, such as CPs/CCPs outside of limits (as per HACCP plan) |
11.6.2 HACCP verification – frequency
The minimum frequency of verification of the FBO’s HACCP-based procedures is pre-set and linked with the outcome of the last FBO audit. However, the OV can modify the frequency of those checks depending on the outcome or other findings indicating that the HACCP based procedures are not adequately implemented and / or risks are not sufficiently controlled (for example, high numbers of contaminated carcases found during the process or product verification checks).
The following table specifies the minimum frequency of HACCP verification checks based on the audit score.
FBO audit outcome
| Area of verification | Improvement necessary / Urgent improvement necessary’ | Good / Generally satisfactory |
|---|---|---|
| 1 Monitoring of CPs/CCPs | Weekly | Monthly |
| 2 Corrective actions | Weekly | Monthly |
11.7 Microbiological verification
11.7.1 Microbiological verification
All FBOs are required to comply with current EU law and ensure that meat and carcases in the slaughterhouse are tested in accordance with (EC) 2073/2005. The OV / AO should verify on a monthly basis that the microbiological sampling is taking place as per the legislative requirement. This includes observing the FBO sampling procedures as well as verification of sampling frequency, sample size and parameters tested.
| Area of verification | Scope |
|---|---|
| 1 FBO sampling procedures | Microbiological sampling carried out as per legislative requirement (in accordance with (EC) 2073/2005, correct frequency of testing followed, correct sample size) |
| 2 FBO analysis of results | Results / trends analysed, and action taken when results indicate a problem |
Note: In premises where microbiological testing is done less frequently than monthly, the verification frequency should be adjusted and aligned with that of the FBO’s testing regime.
12. Slaughter hygiene verification system in poultry
In this section
12.2 Slaughter hygiene verification system
12.3 Process – hygiene verification
12.4 Product – carcase verification
12.5 Plant – establishment verification
12.1 Introduction
12.1.1 Purpose
This section describes the official control procedures for SHV in poultry abattoirs. The SHV system provides an ongoing assessment of FBO compliance with food hygiene requirements from acceptance of the animals for slaughter, through processing, offal harvesting and chilling to carcase and offal / co-product packing for despatch.
The verification objective is to provide assurance that only meat that is produced in accordance with legislative requirements is placed on the market.
This guidance outlines how and when OVs / AOs shall verify that FBOs have developed effective slaughter hygiene practices and that they are implementing effective procedures which:
- prevent contamination of carcases with enteric pathogens and faecal contamination throughout the entire slaughter and processing operation, and that their food safety management systems demonstrate this control
- ensure that carcases with visible faecal contamination are identified and rectified
- verify the monitoring procedures following findings of visible contamination and the corrective actions undertaken to bring the process back under control
The results of verification checks can be used to:
- provide advice to assist the FBO with root cause analysis
- provide evidence for enforcement action
- justify identification marking
- inform the FBO audit process
- inform veterinary certification for third country export
12.1.2 Background
FSA has developed SHV procedures by looking at the regulatory official control verification requirements at abattoirs.
With particular reference to slaughter hygiene, official controls must verify:
- FBO compliance with Regulations (EC) 852/2004 and 853/2004
- that FBOs apply procedures to ensure good hygiene practices continuously and properly
- that FBOs apply HACCP based procedures continuously and properly regarding:
- acceptance for slaughter
- compliance with microbiological criteria
- freedom from foreign bodies
- that FBO procedures guarantee to the best possible extent that meat:
- does not contain patho-physiological abnormalities or changes
- does not bear faecal or other contamination
Verification is the responsibility of the OV, but information regarding good hygiene practices and HACCP based procedures can be gathered by Official Auxiliaries (OAs) to assist the OV.
The verification system focuses on gathering qualitative measures to assess FBO processing standards.
The SHV system creates a more holistic approach to provide a more complete picture of the FBOs processing standards with the ultimate objective of providing clear evidence of improvements to carcase hygiene when required.
12.2 Slaughter hygiene verification
12.2.1 Key elements of the verification system
The verification system applies predetermined minimum frequencies of verification tasks, which provide information on the delivery of official controls, enforcement activity and objective evidence to support FBO audits.
Key summary points of the verification system are:
- SHV checks should be carried out by OVs and AOs
- the number of checks can increase or decrease depending on findings
- the SHV system can be utilised by the OV / AO and technical contract managers to assess performance and official control delivery to focus attention and discussions
12.2.2 SHV method
The verification system includes a number of tasks that must be carried out and should cover the whole production process. Verification tasks are divided into the four following categories and have different frequencies based on the associated risks and possible impact on public health:
- process – hygiene verification
- product – carcase / offal verification
- plant – establishment verification
- HACCP and microbiological verification
A summary of all verification tasks and their frequencies can be found in Annex 9.
The initial selection of carcases for process hygiene and product verification should be random. However, based on the findings, the OV / AO may wish to target a specific type of process or animal to better assess FBO controls.
12.2.3 Minimum requirements – assessment of samples
The OV / AO should select a point on the production line where suitable facilities are available to allow an examination of surfaces of the sampled carcases.
Adequate time must be allocated by the OV / AO to ensure an examination of the carcase is performed and accurate data is collected, and consistency is maintained.
12.2.4 Outcomes
Each verification area must be assessed by the OV / AO and scored based on the outcome (compliant / NC) and the level of the enforcement action taken. The score is recorded in the K2 system.
| Outcome | Description |
|---|---|
| Compliant: Green | Food business is operating in accordance with its food safety management system, food safety standards and has met the requirements of the regulations; no enforcement action taken |
| Non-compliant: Yellow | A non-compliance that resulted in verbal advice |
| Non-compliant: Amber | A non-compliance that resulted in written advice |
| Non-compliant: Red | A non-compliance that resulted in formal enforcement action, such as service of legal notice, referral for investigation |
12.2.5 Reporting arrangements
The K2 system will produce daily, weekly, and monthly data reports of verification activity results. The OV/ AO must utilise the information monitor individual plant performance during the interim FBO audit period with the following objectives:
- drive consistency of enforcement
- encourage continuous improvement in FBO slaughter hygiene activities
- determine the level of current compliance within a production method
OV / AO must use the Poultry Slaughter Hygiene Checklist (Annex 9) to record the outcome of verification checks when K2 system access is not available on-site and store it at the plant until the information is entered into the system –local FSA team should have procedures in place to ensure the information is entered in the K2 system as soon as possible–.
12.2.6 Use of verification data
The recorded daily outcomes of verification tasks will provide information about the level of current performance / compliance within a production method.
The data collection at plant level will assist the OV / AO in defining reasonable expectations of operating standards.
Establishment trend analysis and professional judgement from the OV / AO is required for appropriate action. This will assist in compliance decisions and achieve consistency of approach.
The OV / AO should review the results on a daily, weekly, and monthly basis and take the appropriate action as detailed in sub-sections 12.3 to 12.6.
12.3 Process – hygiene verification
12.3.1 OV / AO responsibility
The OV / AO is expected to verify the efficacy of the evisceration and the hygiene standard of the process to assess:
- if the FBO has adequate controls in place to minimise contamination
- if corrective actions are taken when contamination incidents occur
- if corrective actions are taken when carcases are not correctly eviscerated
12.3.2 Process scope
| Inspection verification steps: | Scope (all species) |
|---|---|
| 1. Contamination | Processing does not result in carcase contamination Measures taken to prevent the spillage of the digestive tract content during evisceration |
| 2. Evisceration | Carcases are eviscerated; offal is not missing and is presented for post-mortem inspection |
12.3.3 Process frequency and sample size
The verification checks in the process hygiene area have to be carried out every day and at least 150 carcases should be verified.
For small size slaughterhouses, sample size can be reduced and at least 40 carcases should be verified per day.
For the purpose of SHV, small size slaughterhouses are defined as those processing 1500 or less birds a day.
The number can be increased by the OV based on findings. Carcases should be selected randomly, and the checks should be spread throughout the day and cover the full range of species and age / type of animals being processed.
During process hygiene checks it is not necessary to lift carcases from the line. It is sufficient to inspect carcases in a manner that is similar to regular post-mortem inspection at this point.
Note: It is not necessary to check 150 or 40 (for small size abattoirs) carcases of each species slaughtered every day; sample size is the combined number of all species processed on site.
| Inspection verification steps: | Frequency |
|---|---|
| 1. Contamination | Daily |
| 2. Evisceration | Daily |
12.3.4 Process – location
Process hygiene verification checks should be carried out at, or prior to, the Evisceration post-mortem inspection point, where the OV / AO can visually assess the carcases.
12.3.5 Process – contamination
The OV / AO must record in relevant sections of the K2 system all instances of carcases with faecal or ingesta contamination identified during the process hygiene verification checks, as well as the number of carcases that were not eviscerated or presented for inspection without offal (offal was missing).
Any visible trace of faecal or ingesta contamination must be counted and recorded. Each contaminated carcase counts as one incident, regardless of the amount of contamination present.
In cases where contamination identified during verification checks is different to digestive tract content (faecal / ingesta), the OV / AO should bring it to the attention of the FBO. Such cases, however, do not have to be recorded in the SHV system. Examples of contamination other than digestive tract content include bile and oil / grease. Excessive and frequent contamination of this type should trigger enforcement action.
Note: When the nature of the process and product require that parts of viscera remain inside the bird, for example, delayed evisceration or partial evisceration – effile, those parts should not be counted as evisceration failure.
12.3.6 Process – enforcement
In cases where frequent and regular contamination problems are identified by the OV / AO, enforcement action must be taken in accordance with Chapter 7 Enforcement.
12.3.7 Process – digestive tract content
The OV / AO is to identify foreign material as faeces or ingesta based on the characteristics of colour, texture, and composition. Size is unimportant in identifying faecal or ingesta contamination; however, as size decreases, colour and texture become more difficult to identify. The characteristics below are only listed as guidance and the OV / AO should use their professional judgement when making the decision.
Identification of contamination
| Faecal | Ingesta | |
|---|---|---|
| Colour | Varying shades of yellow to green, brown, and white | Varies with diet |
| Consistency | Frequently semi-solid to paste | Characteristically solid or granular, occasionally digestive fluids are present |
| Composition | May or may not include plant material | Contains identifiable plant material |
12.4 Product – carcase / offal verification
12.4.1 Product – carcase / offal verification
On an ongoing basis, the OV / AO will verify a sample of carcases and offal destined for human consumption (including fifth quarter product) that have passed post-mortem inspection and are considered a final product (such as the FBO having finished any required rectification work). The verification checks should reflect the full range of species and age / type of animals being processed. Additionally, the OV / AO is required to verify the ID marking arrangements.
Carcase verification checks should be carried out after the final carcase washing process at a point that allows the OV / AO to lift carcases and perform a detailed inspection (for example, grading, packing and despatch).
Verification of offal refers to parts that are to be sold as fit for human consumption (such as liver and heart). Other parts intended as edible co-products that require further processing prior to being placed on the market (such as feet and tongues) should also be included in the verification checks. Offal verification checks should be carried out after the post-mortem inspection is completed and the product has been initially processed (separated, trimmed, washed).
12.4.2 Product – sample size / frequency
Each day, at least 60 carcases and sets of offal (if fit for human consumption) should be checked.
For small size abattoirs at least 30 carcases and sets of offal (if fit for human consumption) should be checked per day.
The checks should be spread across each day of production and carcases / offal should be selected randomly. The detailed inspection of carcases should include the inspection of external surfaces, the body cavity, and the neck area.
The following guidance details how the product (carcase) inspection could be carried out:
| Outside back | While holding the carcase, with the back of the carcase towards the observer, and starting at the hock area, observe the hock, back part of the legs, tail area, back of the carcase and top side of the wings. |
|---|---|
| Outside front | Turn the carcase and observe the bottom side of the wings, breast, and front part of the legs. |
| Inside | Observe the inside surfaces of the carcase and the abdominal flaps and fat. |
| Neck flap area | Observe the neck flap and the thoracic inlet area. |
12.4.3 Product – verification scope
Product verification focuses on the FSA’s post-mortem inspection performance and the FBO’s hygienic standard of operation and the effectiveness of rectification procedures; therefore, the product verification checks are separated into two areas:
- verification of post-mortem arrangement
- verification of FBO controls
Both areas can be verified at the same time when assessing the same sample of carcase / offal; however, verification of post-mortem arrangement cannot be carried out by OAs.
12.4.4 Product – verification of post-mortem arrangement
Verification of post-mortem arrangement must be carried out by the OV, and since it focuses on FSA’s performance, it is not subject to scoring.
In the K2 system, the OV is only required to record the number of carcases / offal checked and the number found affected by pathology missed by the inspection team.
The following table details the scope of product checks focused on verification of post-mortem arrangement.
| Area of verification | Scope |
|---|---|
| Pathology | Meat is free from all pathological conditions |
Verification results should be assessed by the OV / FVC to monitor team performance. Variables in each establishment should be considered if concerns are raised following verification checks (for example, lighting, available inspection time and plant layout). The OV / FVC should maintain realistic expectations during the checks when assessing team performance based on the product verification results.
Note: In:
- OV only establishments
- poultry establishments with a hybrid post-mortem inspection system (where the OV also undertakes post-mortem inspection along with OAs or PIAs)
- plants with recognised OV flexibility
the effectiveness of FSA’s post-mortem performance should be verified during routine FVC or SDP management visits and documented on the K2 system by FSA / SDP at least every three months. The FVC is accountable for ensuring these checks have been carried out and documented and is responsible for establishing the number of carcases and offal that should be verified during those visits. The verification of FSA’s post-mortem performance should not impact on agreed resource and business agreements as outlined in the Statement of Resources for the individual establishment.
12.4.5 Product – verification of FBO controls
Product verification checks that assess FBO controls focus on evisceration, contamination, ID marking and other deficiencies and are therefore subject to scoring.
The OV / AO is required to record and input into the K2 system:
- the number of carcases and offal found to be non-compliant
- the total number of carcases / offal checked
- the score indicating enforcement action taken (if any)
In addition, the OV / AO is required to verify the ID marking arrangements.
The following table details the scope of product checks focused on verification of FBO controls:
| Area of verification | Scope |
|---|---|
| Evisceration | Carcases fully eviscerated* |
| Faecal / ingesta | Meat is free from faecal / ingesta contamination |
| ID marking | Meat is correctly and legibly ID marked |
| Other | Record any identified deficiency (such as contamination with bile / grease, poor defeathering) |
* Instances where a piece of digestive tract is found inside a carcase should be counted as evisceration failure. However, in those cases, it also should be verified if contamination with faeces / ingesta is visible and if it is found it should also be recorded under ‘Faecal / Ingesta’ contamination.
In cases where frequent and regular problems (such as contamination) are identified by the OV / AO during the product verification checks focused on FBO controls, enforcement action must be taken in accordance with Chapter 7 Enforcement.
12.4.6 Rejected carcase checks
The OV is required to carry out a detailed inspection of a random sample, from each batch of birds having the same origin, of parts of birds or entire birds declared unfit for human consumption following post-mortem inspection on a daily basis. The number of birds checked, and the outcomes, should be recorded in the day book, not in the K2 system.
12.5 Plant – establishment verification
12.5.1 Plant – establishment verification
Establishment verification tasks focus mainly on different parts of the establishment, equipment, cleanliness, hygiene arrangements and procedures.
The minimum frequency of establishment verification tasks depends on the FBO audit outcome. However, the OV can increase the frequency if considered necessary and should always score a relevant section when an intervention takes place that resulted in verbal, written or formal enforcement.
Some establishment verification tasks are considered essential and should be carried out and scored every day, regardless of the audit score awarded.
The following table lists the establishment verification areas and the minimum frequency of checks based on FBO audit outcome.
FBO Audit Outcome
| Establishment verification tasks and their frequency | Improvement necessary / Urgent improvement necessary | Good / Generally satisfactory |
|---|---|---|
| 1. Intake / food chain information | Daily | Daily |
| 2. Ante-mortem arrangements / presentation | Daily | Daily |
| 3. Correlation of carcases and offal | Daily | Daily |
| 4. Operational / break cleaning | Daily | Daily |
|
5. General hygiene Includes verification of hygienic practices (inc staff movement, PPE provisions and practices, hand washing), hygienic facilities provided (inc hot water, soap, sterilisers), door policy and cross contamination controls |
Daily | Daily |
|
6. Handling of carcases / offal during storage and despatch Includes fifth quarter products (inc tongues and feet) |
Daily | Daily |
|
7. Co-products and animal by-products Includes verification of separation of edible and non-edible materials |
Daily | Daily |
| 8. FBO’s pre-operational cleaning | Weekly | Monthly |
|
9. Carcase and offal chilling Includes verification that equipment was emptied, cleaned, and disinfected at least once a day |
Weekly | Monthly |
|
10. Premises Includes verification of lairage / intake area, cleaning and disinfection of crates and modules, processing / dressing area, chillers, packing / packaging storage area, despatch area, plant surrounds, fly screening / vermin entry prevention, control of waste water, drainage and effluent, water testing |
Weekly | Monthly |
12.6 HACCP verification
12.6.1 HACCP verification
The verification of the FBO’s HACCP based procedures is focused primarily on two areas: monitoring of control points (CPs), critical control points (CCPs) and corrective actions.
The OV / AO is not expected to check all records, but must verify a sample to be satisfied that the FBO is:
- following their own procedures for monitoring CPs/CCPs
- taking and recording pre-established corrective actions when the control is lost
| Area of Verification | Scope |
|---|---|
| Monitoring of CPs/CCPs | Monitoring procedures implemented, accurate records that reflect reality maintained (up to date) |
| Corrective Actions | Correct actions taken when monitoring indicates loss of control, such as CPs/CCPs outside limits, as per HACCP plan |
12.6.2 HACCP verification – frequency
The minimum frequency verification of the FBO’s HACCP based procedures is pre-set and linked with the outcome of the last FBO audit. However, the OV can modify the frequency of those checks depending on the outcome or other findings, indicating that the HACCP based procedures are not adequately implemented and / or risks are not sufficiently controlled (for example, high numbers of contaminated carcases found during the process or product verification checks).
The following table specifies the minimum frequency of HACCP verification checks based on the audit score.
FBO Audit Outcome
| Area of verification | Improvement necessary / Urgent improvement necessary | Good / Generally satisfactory |
|---|---|---|
| Monitoring of CPs/CCPs | Weekly | Monthly |
| Corrective actions | Weekly | Monthly |
12.7 Microbiological verification
12.7.1 Microbiological verification
All FBOs are required to comply with current EU law and ensure that meat and carcases in the slaughterhouse are tested in accordance with Regulation (EC) 2073/2005. The OV / AO will verify on a monthly basis if the microbiological sampling is taking place as per the legislative requirements. This includes observing the FBO’s sampling procedures as well as verification of other areas, such as sampling frequency, sample size, parameters tested.
| Area of verification | Scope |
|---|---|
| FBO’s sampling procedures | Microbiological sampling carried out as per legislative requirements (in accordance with (EC) 2073/2005, correct frequency of testing followed, correct sample size |
| FBO’s analysis of results | Results / trends analysed, and action taken when results indicate a problem |
Note: In premises where microbiological testing is done less frequently than monthly, the verification frequency should be adjusted and aligned with that of the FBO’s testing regime.
13. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms.
Annex 1: Post-mortem inspection requirements summary
Annex 2: Sample: Aujeszky’s disease – National Serum Survey submission form
Annex 3: Sample: APHA1 data collection form
Annex 4: Slaughter hygiene checklist
Annex 5: Model document: Health certificate for the trade of unskinned large wild game
Annex 6: Updated: [REMOVED Trichinella sampling kit order request form]
Annex 7: Sample despatch process
Annex 8: Summary of verification checks and their frequencies
Annex 9: Poultry slaughter hygiene checklist
Annex 12: Aide memoire: Large wild game
Revision log
Published: 30 December 2021
Last updated: 14 April 2025