Annex A: Risk Analysis and Regulated Products Service Report
FSA 23-09-08 Annex A Risk Analysis and Regulated Products Service Report gives updates on Risk Analysis and applications.
Risk Analysis updates
Overview
| Issues in risks analysis internal system* | Totals |
|---|---|
| Total active issues | 38 |
| Issues flagged for additional board scrutiny | 7 |
*Active issues are those progressing and logged in FSA internal system. These are published to the online register when they reach the risk assessment and evidence gathering stage. Data correct as of 30 June 2023.
Issues that are at risk assessment and beyond are published to an online register on a quarterly basis.
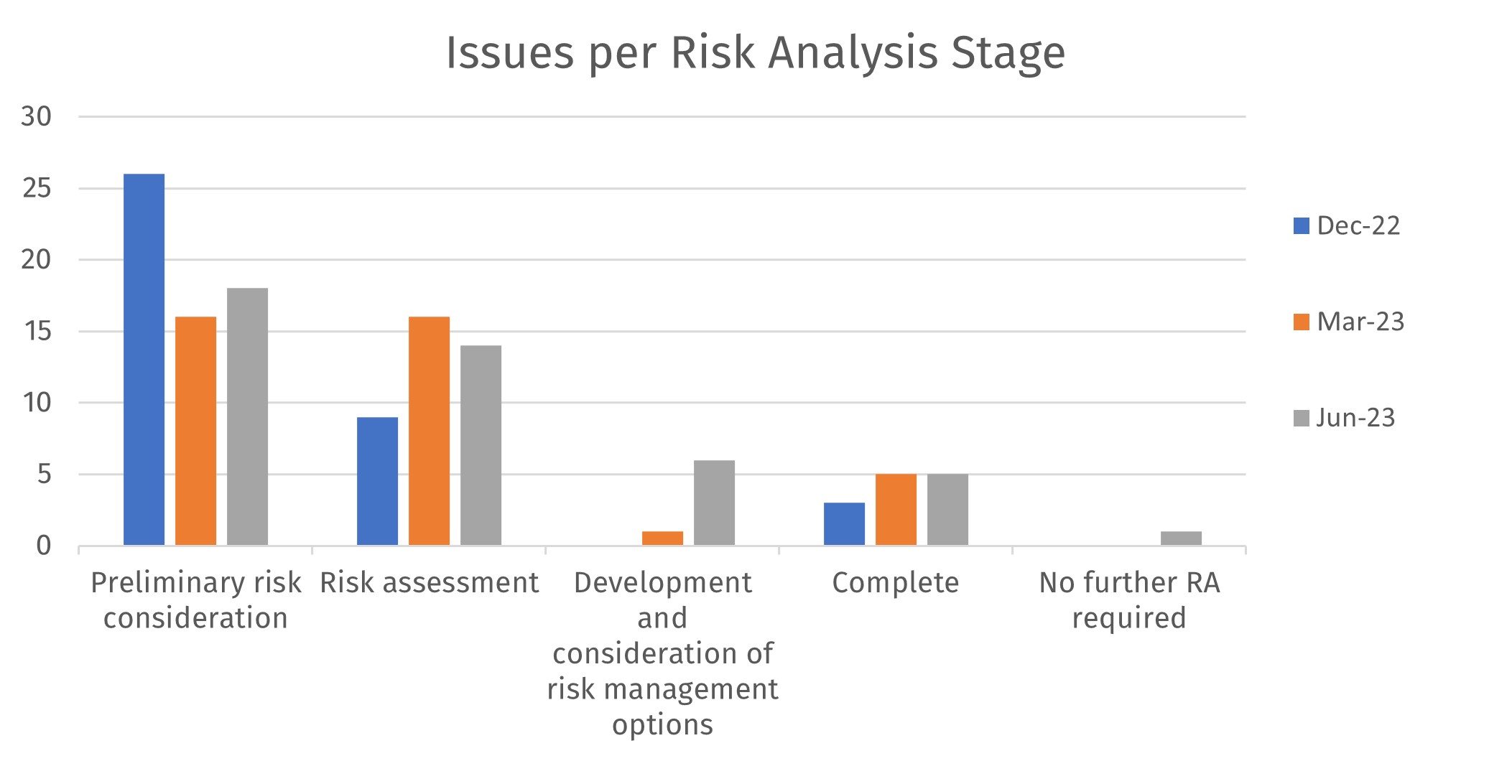
Three issues have progressed to risk management and four have been added to the register since the last report. (listed in later slide).
Issues per Risk Analysis Stage
Active Issues by policy area

Performance measures
Compliance indicators (risk analysis principles)* (please note figures for financial year 2023/24 are year to date.
| Compliance indicators | 2021/22 | 2022/23 | 2023/24 |
|---|---|---|---|
| Evidence based** Issues with sufficient evidence package and sign off | 100% | 100% | 100% |
| Open and transparent: Completed issues recorded in public register with full information pack published*** | 100% | 100% | 100% |
| Four country working: Active issues where dispute mechanism triggered | 0 | 0 | 0 |
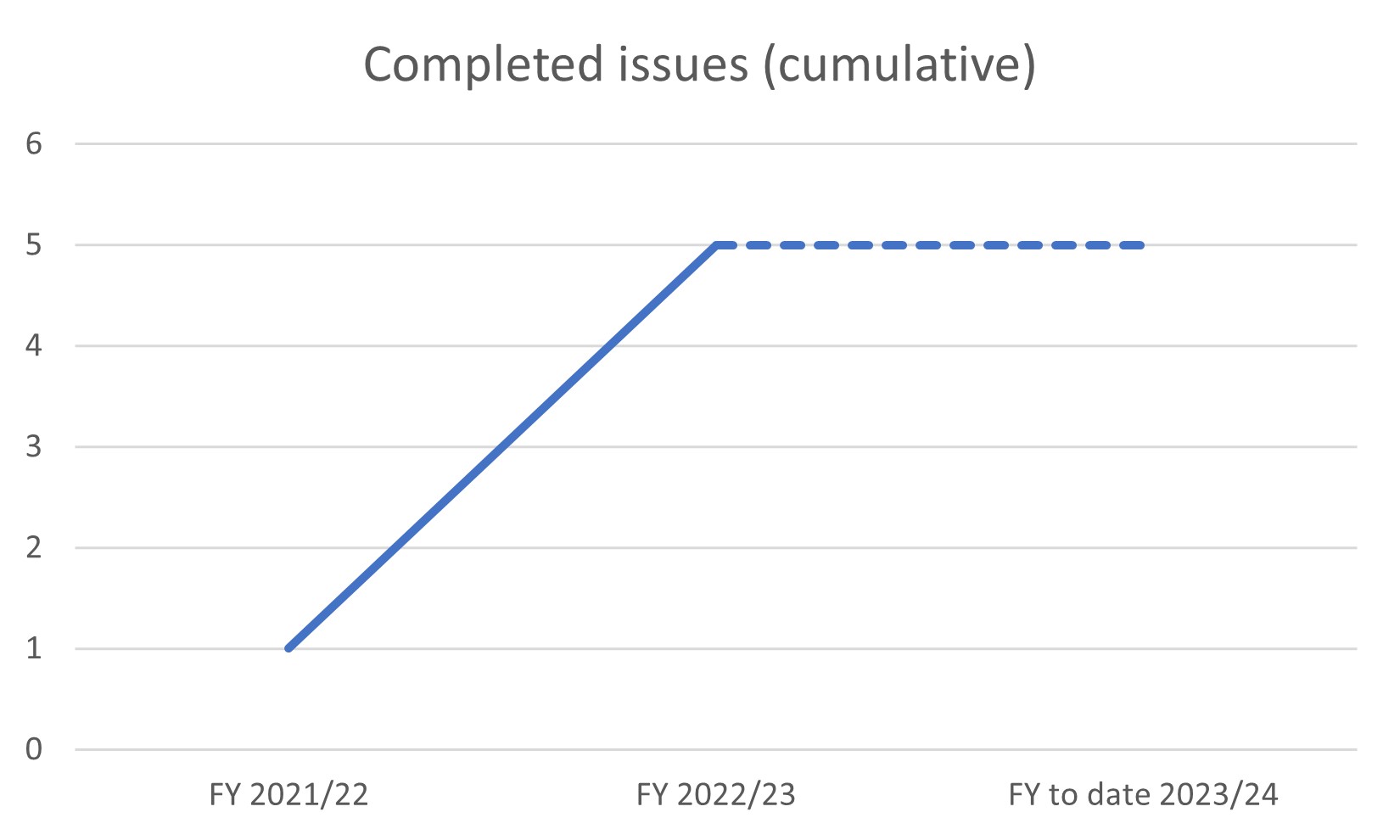
Completed issues (cumulative)
| Monitoring indicators | Total |
|---|---|
| Issues completed (financial year to date) | 0 |
| issues completed (total) | 5 |
* Compliance indicators relate to the following figures
Issues with sufficient evidence package and sign-off, and Completed issues recorded in public register with full information pack published:
- 2021/22 – one complete (end of March 22)
- 2022/23 – five complete (end of March 23)
- 2023/24 – five complete (2023/24 FY to 26th June 23)
Active issues where dispute mechanism triggered:
- 2021/22 – seven active (end of March 22)
- 2022/23 – 22 active (end of March 23)
- 2023/24 – 38 active (2023/24 FY to 26th June 23)
**Our evidence-based indicator ensures that the correct level of
assurance took place during evidence generation.
***Publication of information in line with FSA Code of Practice on Openness
Risk analysis register updates since last Board meeting in June
Details of issues undergoing risk analysis are published to an online register following initial consideration, once it is confirmed that risk assessment or other evidence is required, and the risk assessment phase of the process commences.
| Issue | Description | Status |
|---|---|---|
| Country Profiles - Imported Food of Non-Animal Origin (FNAO) Phase 1 | The FSA Market Access Assurance Team will support the FSA Trade Risk Assessment Team in the production of Country Profiles for trading partners exporting food of non-animal origin (FNAO) to the UK. These profiles will assist the Market Access Assurance Team in monitoring the risk associated with each country and to inform on the need for follow-up action. |
Previously in register – Country profile not required. No evidence produced, and further risk analysis deemed unnecessary. |
| Risk assessment on Avian Influenza infection via food chain | Production of an updated risk assessment for Avian Influenza in food, triggered by changes to consumer advice regarding egg consumption and the geographically widespread nature of Avian Influenza generating over 100 confirmed cases in the 2021/22 Avian Influenza season. This work has been initiated to ensure risk management advice for the consumption of poultry, wild game and raw eggs remains appropriate and is supported by the latest evidence on risks associated with Avian Influenza, especially with respect to vulnerable groups, taking into account developments in the spread of Avian Influenza. | Progressed to development and consideration of risk management options. |
| Assessment of the risk to vulnerable consumers from Listeria monocytogenes in blue cheese | Assessment of the risk to vulnerable consumers from listeria monocytogenes in blue cheeses. There are inconsistencies in the advice provided by government partners to pregnant women on the consumption of blue cheese. A RA will assess the risk to vulnerable consumers. FSA and FSS are working together to present consistent RM advice underpinned by science and evidence. | Progressed to development and consideration of risk management options. |
| Assessment of the risk to vulnerable consumers from Listeria monocytogenes in smoked fish | Assessment of the risk to vulnerable consumers, including pregnant women and people with weakened immune systems, from Listeria monocytogenes in smoked fish. This follows confirmation there has been an increase over the period 2020-22 in the number of cases of listeriosis linked to the consumption of smoked salmon by vulnerable groups. FSA and FSS are working with other Government departments to present consistent RM advice underpinned by science and evidence. |
Progressed to development and consideration of risk management options* *Risk management advice has been published and this issue moved to complete. However, this occurred outside the performance window for this paper. This update will be incorporated in the next risk analysis register update. |
The register of risk analysis issues was last updated on 26 June 2023, incorporating updates to the end of May. The next update will incorporate updates to the end of August and will be published by the end of September at the earliest.
New Risk analysis register additions since last Board meeting in June
Details of issues undergoing risk analysis are published to an online register following initial consideration, once it is confirmed that risk assessment or other evidence is required, and the risk assessment phase of the process commences.
| Issue | Description | Status | Earliest estimate of completion date* |
|---|---|---|---|
| Microbiological Risk Assessment to support development of advice and guidance to manage outbreaks of norovirus associated with consumption of raw oysters with respect to the amount of Norovirus contamination detected by real time reverse transcription polymerase chain reaction ISO 15216 | The FSA is seeking to develop risk management advice in relation to Norovirus testing of oysters during outbreak situations, taking into account the amount of norovirus contamination detected and how this may lead to resumption of harvesting from oyster harvesting areas implicated in an outbreak. | Added to register – development and consideration of risk management options. | Risk management advice is expected to be completed by the end of October 2023. |
| 2023 review of imported food and feed controls under Retained EU Commission Implementing Regulation 2019/1793 | To ensure that the proposed changes to the list of certain controlled food and feed products not of animal origin (FNAO) in the annexes of Retained EU Commission Implementing Regulation 2019/1793 outlined are appropriate. | Added to register – development and consideration of risk management options. | Risk management advice is expected to be completed by Summer 2023 at the earliest. |
| Flexibility to increase the threshold for designation of low-capacity slaughterhouses | Flexibility under Article 7 (1) (b) of REUL 2019/624 to increase the threshold for the designation of low-capacity slaughterhouses. This will allow the post-mortem inspection in those eligible slaughterhouses to be performed by the official auxiliary without the official veterinarian (OV) being at the slaughterhouse when this is carried out. | Added to register – risk assessment and evidence gathering. | Risk assessment was completed in April 2023. No further information on risk management deadlines are available. |
| Risk Profile - Live Bivalve Molluscs (LBMs): Oysters | FSA work with the UK Office for SPS Trade Assurance (in Defra) to identify and characterise the hazards associated with oysters from different global regions. | Added to register – risk assessment and evidence gathering. | The Risk Profile is expected to be completed by the end of August 2023 at the earliest . |
*Earliest estimated completion dates are provisional at this stage and dependent on the progress of the RA phase and subsequent RM approach. Issues may be reprioritised if other issues emerge that take priority on public health grounds.
The register of risk analysis issues was last updated on 26 June 2023, incorporating updates to the end of May. The next update will incorporate updates to the end of August and will be published by the end of September at the earliest.
Regulatory Products Service Update
Key update
On 20 June 2023, CMS was launched as the new method to receive, track and progress applications to the FSA. Since implementation, there were no new applications between 20 and 30 June 2023. We believe this is due to additional information requested from applicants before a submission can be made.
The following reporting is based on applications received through the Application Service platform. Future reporting will include CMS data.
Cohorts by quarter
The below table shows the breakdown and progress of applications in the Regulated Products Service by quarter.
| Date | Total Contacts | Incomplete Applications | Applications progressing | Pre-Validation | Risk Assessment | Risk Management | Completed |
|---|---|---|---|---|---|---|---|
| Jan to March 2021 | 993 | 790 | 177 | 85 | 82 | 10 | 26 |
| April to June 2021 | 183 | 135 | 36 | 5 | 29 | 2 | 12 |
| July to September 2021 | 105 | 82 | 19 | 3 | 11 | 5 | 4 |
| October to December 2021 | 111 | 73 | 34 | 4 | 27 | 3 | 4 |
| Jan to March 2022 | 108 | 82 | 23 | 5 | 18 | - | 3 |
| April to June 2022 | 123 | 97 | 26 | 7 | 19 | - | 0 |
| July to September 2022 | 113 | 90 | 23 | 16 | 6 | 1 | 0 |
| October to December 2022 | 129 | 86 | 42 | 40 | 2 | - | 1 |
| Jan to March 2023 | 128 | 105 | 23 | 23 | - | - | 0 |
| April to June 2023 | 70 | 47 | 23 | 23 | - | - | 0 |
| Total | 2063 | 1587 | 425 | 211 | 194 | 21 | 50 |
| Stop the clock | - | - | - | 44 | 38 | - | - |
Note: ‘Completed’ refers to applications which have reached the final stage in the process and have been either approved or rejected.
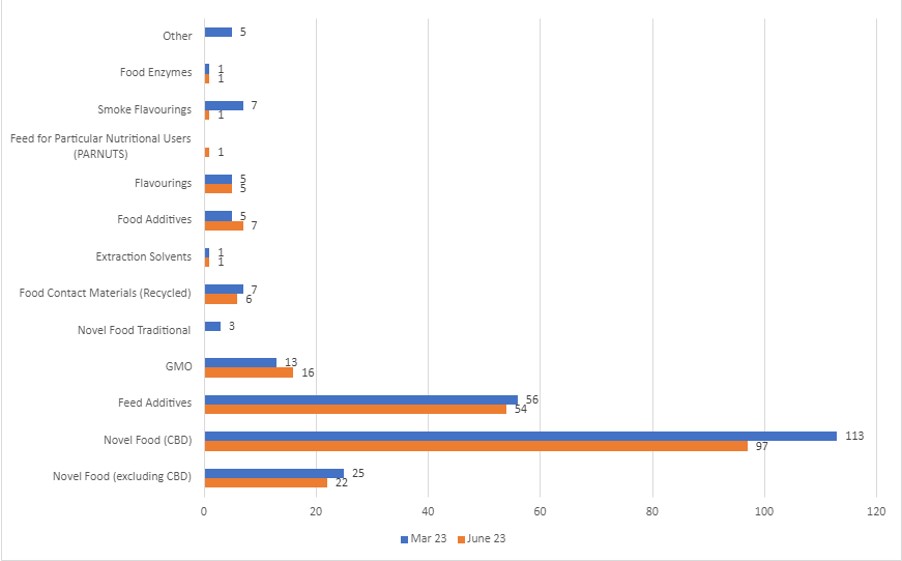
This table breaks down the applications by regime at different stages in the Regulated Products Service: March 2023 v June 2023.
| Regime | Applications progressing | Applications at pre-validation | Applications at Risk assessment | Applications at Risk Management | Applications at authorisation | Applications completed |
|---|---|---|---|---|---|---|
| Novel food (excluding CBD) |
March: 51 June: 52 |
March:25 June: 22 |
March: 24 June: 30 |
- |
March: 2 June: 0 |
March: 6 June: 8 |
| Novel Food (CBD) |
March: 130 June: 122 |
March: 113 June: 97 |
March: 17 June: 25 |
- |
- |
- |
| Feed Additives |
March: 158 June: 166 |
March: 56 June: 54 |
March: 83 June: 92 |
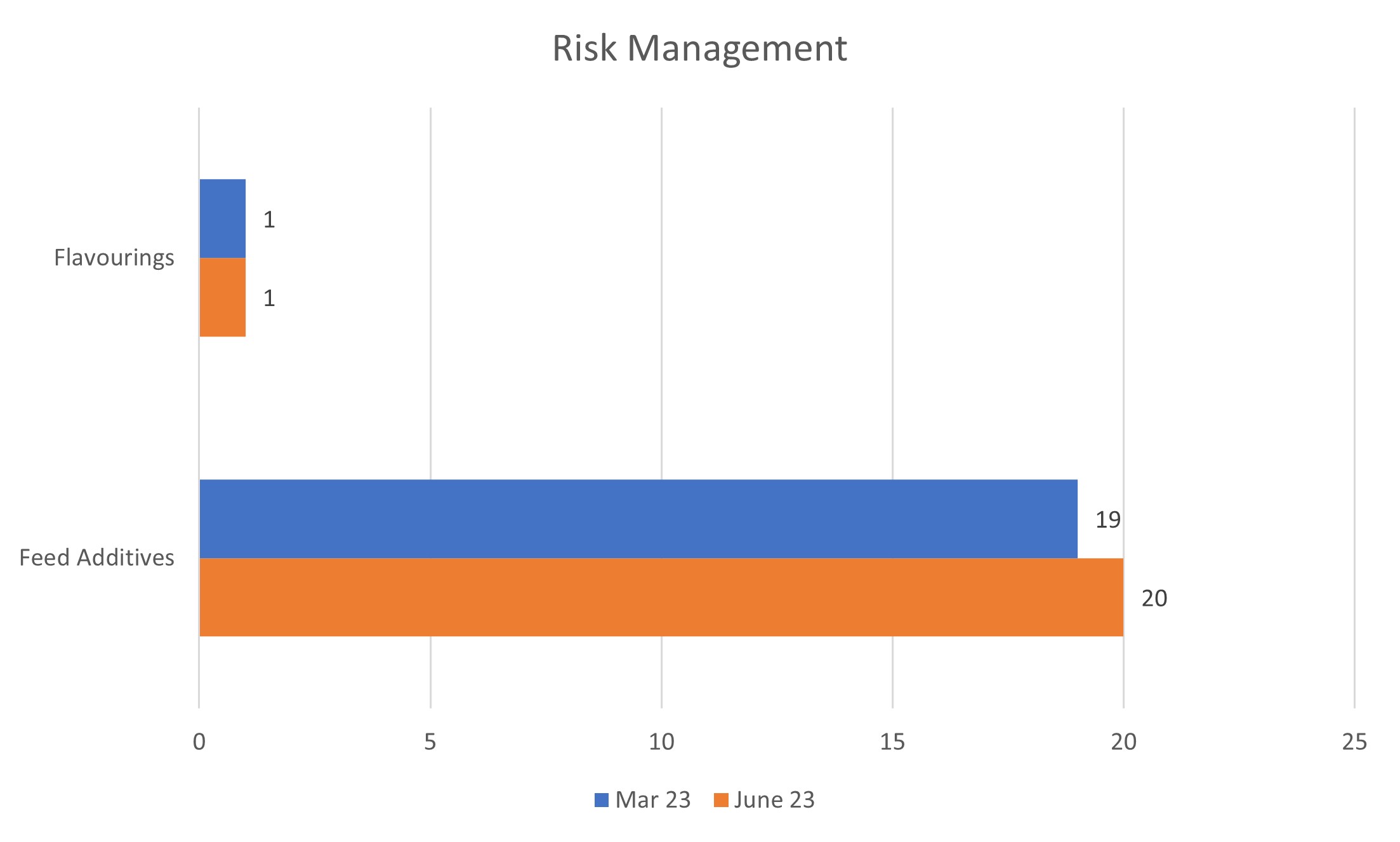
March: 19 June: 20 |
March: - June: - |
March: 11 June: 11 |
| GMO |
March: 35 June: 29 |
March: 13 June: 16 |
March: 11 June: 13 |
- |
March: 11 June: 0 |
March: 10 June: 21 |
| Novel Food Traditional |
March: 3 June: 0 |
March: 3 June: 0 |
- |
- |
- |
March: 2 June: 3 |
| Food Contact Materials (Recycled) |
March: 12 June: 12 |
March: 7 June: 6 |
March: 5 June: 6 |
- |
- |
- |
| Food Contact Materials (Plastics) |
March: 3 June: 3 |
- |
March: 3 June: 3 |
- |
- | - |
| Extraction Solvents |
March: 1 June: 1 |
March: 1 June: 1 |
- |
- |
- |
- |
| Food Additives |
March: 18 June: 19 |
March: 5 June: 7 |
March: 12 June: 12 |
- |
March: 1 June: 0 |
March: - June: 1 |
| Flavourings |
March: 10 June: 9 |
March: 5 June: 5 |
March: 3 June: 3 |
March: 1 June: 1 |
March: 1 June: 0 |
March: - June: 1 |
| Feed for Particular Nutritional Users (PARNUTS) |
March: 2 June: 3 |
March: - June: 1 |
March: 2 June: 2 |
- | - | - |
| Novel Food Status | - | - | - | - | - |
March: 2 June: 2 |
| Smoke Flavourings |
March: 9 June: 9 |
March: 7 June: 1 |
March: 2 June: 8 |
- | - |
March: 3 June: 3 |
| Food Enzymes |
March: 1 June: 1 |
March: 1 June: 1 |
- | - | - | - |
| Feed Detoxification Processes | - | - | - | - | - | - |
| Other |
March: 5 June: - |
March: 5 June: - |
- | - | - | - |
| Total |
March: 438 June: 426 |
March: 241 June: 211 |
March: 162 June: 194 |
March: 20 June: 21 |
March: 15 June: 0 |
March: 34 June: 50 |
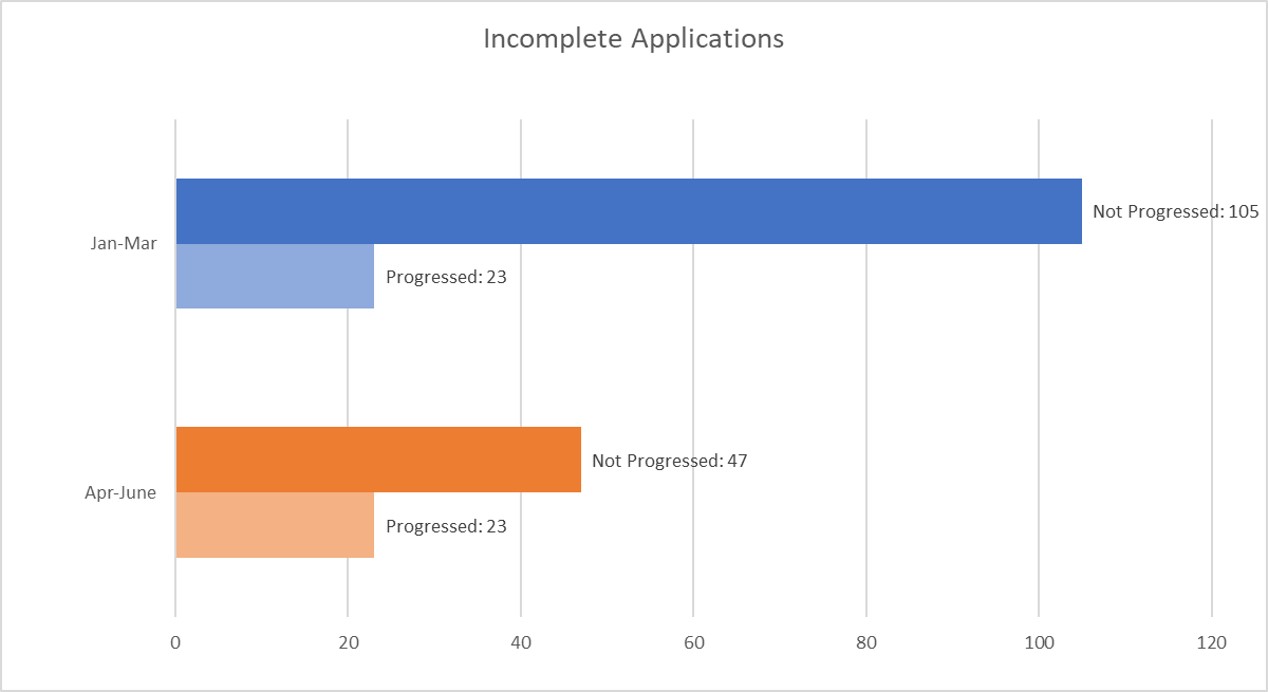
Incomplete Applications
Incomplete applications describe any communication submitted through the online portal that is not taken forward. This can include questions and comments as well as applications that do not contain sufficient information to proceed. This quarter, we have continued to receive a high number of incorrect, abandoned or incomplete applications at 69%. As the new Case Management System has been introduced, we expect a significant reduction in incomplete applications.
Total number of contacts: January to March 2023 = 128
Total number of contacts: April to June 2023 = 70
Incomplete applications
Pre-Validation
49% of all applications are at the Pre-Validation stage. CBD applications make up 46% and are being actively managed. As of 30 June 2023, there were 211 applications at this stage, compared to 241 at March 2023. The regime distribution is shown in the below graph.
Risk Assessment
46% of all applications are currently at the Risk Assessment stage. As of 30 June 2023, there were 194 applications, compared to 162 in March 2023. The regime distribution is shown in the below graph:

Risk Management
5% of all applications are at the Risk Management stage. As of 30 June 2023, there were 21 applications at this stage, compared to 20 in March 2023. The regime distribution is shown in the below graph:
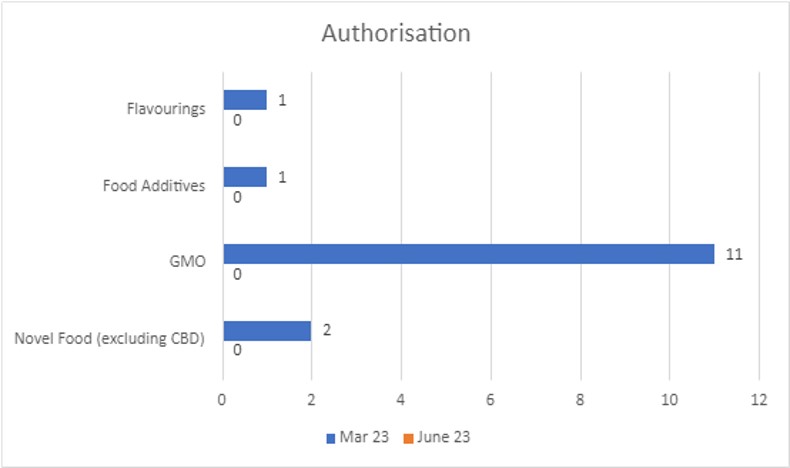
Authorisation
As of 30 June 2023, there were 0 applications in the Authorisation stage, compared to 15 at March 2023 (which have now been completed). The regime distribution is shown in the below graph:
Completed Applications
As of 30 June 2023, the Regulated Products Service has completed 50 applications, an increase of 16 from 31 March 2023. The regime distribution is shown in the below graph:
