Review of allergen analytical testing methodologies: measurement parameters and sensitivity of methods
Food allergies affects between 1-2% of the UK population, with some allergens responsible for hospital admissions with anaphylaxis. Food businesses have a legal responsibility to provide food that is safe, which means declaring allergens present as ingredients and warning consumers about their potential unintended presence due to cross-contact. A system needs to be implemented for testing allergens in foods, responding to incidents, and manage risks to protect consumers. This report explores the current state of the art of allergen testing methodologies and the remaining challenges.
Executive Summary
The Food Information Regulation (FIR) states that accurate and understandable allergen information needs to be supplied to consumers for the 14 priority allergens. Food allergies affects between 1-2% of the UK population, with some allergens responsible for hospital admissions with anaphylaxis.
Food businesses have a legal responsibility to provide food that is safe, which means declaring allergens present as ingredients and warning consumers about their potential unintended presence due to cross-contact. A system needs to be implemented for testing allergens in foods, responding to incidents, and manage risks to protect consumers.
This review was prepared to inform FSA on the current state of the art of allergen testing methodologies and the remaining challenges. This project combined a critical literature review of testing methods with assessments of allergen proficiency testing data, consultation with stakeholders from the food industry, and consultation with industry experts regarding multiplex methodologies and the harmonisation of methods in an unbiased review of the current status of testing capabilities for the 14 EU-retained regulated food allergens.
Gaps in testing capabilities were highlighted in order to inform future direction, including a lack of transparent public data for the performance and applicability of commercial test kits. Cross-reactivities of kits were also highlighted along with the need for development of fast and accurate point-of-use tests to support food production. A review of allergen proficiency testing data revealed gaps in testing capabilities and variations between the outputs of different test kits when testing for the same allergen.
This review critically compares current testing methods to progress towards a suitable harmonised testing protocol that facilitates allergen risk management, and to mitigate limitations and evidence gaps. Suitable workflows outlining recommended testing protocols are presented for priority allergens to provide a resource for compliant testing and incident management. Estimations of the cost of setting up new testing laboratories to support allergen workflows are also included in addition to detailing the cost of testing by established laboratories.
Introduction
The Food Information Regulation (FIR) states that accurate and understandable allergen information needs to be supplied to consumers for the 14 priority allergens. Food allergies affects between 1-2% of the UK population, with some allergens responsible for hospital admissions with anaphylaxis.
Food businesses have a legal responsibility to provide food that is safe, which means declaring allergens present as ingredients and warning consumers about their potential unintended presence due to cross-contact. A system needs to be implemented for testing allergens in foods, responding to incidents, and manage risks to protect consumers.
This project brings together literature review information, allergen proficiency testing data from testing laboratories around the world, along with details of consultation with experts involved in method development and standardisation and harmonisation of methods and stakeholders from the food industry, in an unbiased review of the current status of testing capabilities for the 14 EU-retained regulated food allergens. The project identifies strengths and limitations of current allergen testing capabilities and it complements ongoing Codex work and the current EFSA ThRAll project, that advises on threshold levels and harmonised methods of detection.
A wide range of routine and emerging methods are applied to food allergen testing. While certain methods are long-established and at various stages of validation and accreditation on a range of food matrices by their manufacturers, little or no cross-evaluation data are available to compare limitations, consider the potential to inter- convert reporting units and identify evidence gaps. There is much variation in the level of information provided with kits. Some kit manufacturers simply stating the limit of detection (LOD) and limit of quantitation (LOQ) of the kit. Other’s kits manuals provide much more kit validation data, such as that relating to applicability and also to recovery, precision, cross-reactivity when testing certain food matrices. Whether the food matrices were tested raw or incurred (the latter usually providing more suitable information due to the number of allergen-containing foods which are processed in some way prior to consumption. Another evidence gap involved both kits and data published in peer-reviewed manuscripts for which there is often little or no information as to whether the LOD and LOQ were determined by measuring the lowest levels at which an allergen can be detected when spiked in a buffer (the simplest scenario), whether it was spiked into a processed food, or whether it was an incurred product (the most challenging scenario, with allergen materials incorporated into the food formulation prior to relevant processing) to provide a ‘real life’ food matrix for testing.
This literature review investigates this, in addition to critically comparing current testing methods to progress towards a suitable harmonised testing protocol that facilitates allergen risk management, and to mitigate limitations including cross- reactivity and evidence gaps. Validation and standardisation data from UK, Europe or international initiatives are highlighted. The project also benefits from unique access to Fapas® proficiency testing data to review variability of inter- and intra- method data.
Alongside a literature review and expert consultations to align the project to the latest activities in allergen management, outputs include a comprehensive table (Table 1, Appendix 1) comparing and contrasting the commercial methods, highlighting limitations in testing methods, measurement parameters and evidence gaps.
Estimations of the cost of setting up testing laboratories to support allergen workflows are also included. Consultations with a range of industry stakeholders regarding allergen management in the supply chain have been conducted and the interview questionnaire and responses are included in this report.
Suitable workflows outlining the recommended testing protocols are presented for priority allergens to provide a resource for compliant testing and incident management.
The outcomes of this project can also be aligned to data issued by the Food and Agriculture Organisation of the United Nations (FAO) and the World Health Organisation (WHO) regarding their review and establishment of threshold levels in foods for the priority allergens. The incidence and types of food allergy vary across the globe, with individuals in certain global populations showing sensitivity to food types for which other populations show little or no sensitivity. Different food commodities are also consumed at varying intake levels across different cultures and populations. The FAO/WHO Reference Doses (RFD), the maximum single day oral exposure which is anticipated to be without appreciable risk for the general population, are expressed in milligrams of total protein from the allergenic food. The Action Level (AL) relates to the reference dose divided by the mass of the food in kilograms, expressed as milligrames of total protein from the allergenic food per kilogram of food. The action level for a given allergen in foods will therefore differ depending on likely intake mass of that food type. Action levels for priority allergens, based on recommended reference doses and calculated for pre-defined intake categories, ranging from 10g to 1 kg, are detailed in the FAO/WHO Risk Assessment of Food Allergens Part Two (WHO, 2022).
This review compares and contrasts current testing methods to determine strengths and limitations of methods to inform FSA on the current state of the art of allergen testing methodologies.
A table detailing the various objectives for this project is shown below (Table of Objectives) along with an index of sections, each of which aligns with a project deliverable.
Table of Objectives
| Objective number/Task number at project inception | Title of Deliverable or milestone | Status |
|---|---|---|
| 01/01 | Kick-off meeting with FSA to agree deliverables and search terms for the literature review. | Complete |
| 02/01 | Commencement of unbiased literature review and initial filtering. | Complete |
| 02/02 | Completion of filtering of literature. | Complete |
| 02/03 | Completion of unbiased review of literature. | Complete |
| 03/01 | Aligning project with Codex alimentarius activities and EFSA project ThRAll outcomes. Stakeholder engagement. | Complete |
| 04/01 | Assessment of variability of analysis from Fapas® proficiency testing data. | Complete |
| 05/01 | Review of Fapas® proficiency testing data. | Complete |
| 06/01 | Preparation of cost estimates to implement the required methods in a testing laboratory. | Complete |
| 07/01 | Delivery team project meetings and communications | Complete |
| 08/01 | Final report in draft | Complete |
| 09/01 | Final project meeting with FSA | Complete |
| 10/01 | Submission of final report | Complete |
| 10/02 | Submission of draft manuscript | Complete |
2.1 Introduction
The aim of this literature review was to review current methods to detect food allergens and to understand the new methodologies being developed for which information is available in the public domain. The fourteen groups of food allergens for which UK food suppliers must declare presence are: celery, cereals containing gluten (such as wheat, barley and oats), crustaceans (such as prawns, crabs and lobsters), eggs, fish, lupin, milk, molluscs (such as mussels and oysters), mustard, peanuts, sesame, soybeans, sulphur dioxide and sulphites (if the sulphur dioxide and sulphites are at a concentration of more than ten parts per million) and tree nuts (such as almonds, hazelnuts, walnuts, Brazil nuts, cashews, pecans, pistachios and macadamia nuts).The most common methods identified include immunochemistry methods, PCR methods and mass spectrometry methods. With the exception of sulphur dioxide and sulphites, each food allergen relates to a protein molecule.
Although this review is not specific to certain methodologies only, of the commercial methods, particular focus was placed on the commercial methods selected for implementation by international food testing laboratories, represented by the participants in each of the Fapas® allergen testing rounds over the last five years.
This is represented by data submissions by 1009 UK lab submissions, 3470 from Europe and 2124 from the rest of the world.
Current methods for allergen detection were identified to understand the state of the art and to identify the limitations and gaps in current capabilities. It is important to note that commercial allergen testing kits are regularly developed as manufacturers seek improvements in method performance and applicability. Changes in parameters such as the limit of detection (LOD) and limit of quantitation (LOQ) are not always immediately apparent when comparing kits in the literature, especially since kit manufacturers tend to retain the name of each kit, even when improvements or other alterations are made. It is therefore not easily possible to compare the performance of one kit against another at the present day when based on analysis of literature
captured over a series of years. For this reason, where available, this literature review provides both the name and the LOD/LOQ of the kit at the time of publishing, along with other performance data provided in the manuscript. Various different testing kits, such as for the ELISA (Enzyme-Linked ImmunoSorbent Assay) kits which is based on detection by antibodies of the allergenic protein(s), or PCR (Polymerase Chain Reaction) which is based on detection of a DNA sequence found in a particular food species, have been developed and are commercially available from a range of manufacturers. The composition of the kit, and the target protein or DNA sequence varies. However, when performing laboratory studies to compare the performance of these testing kits, many authors have opted to anonymise kit details when reporting performance data. Relating to ELISA kits, there are also necessarily variations in the performance of the kits from batch to batch, since performance will alter depending on the reactivity of new batches of antibodies. For this reason, the data in Table 1 (Appendix 1) have been prepared as far as possible from the current manufacturer kit manuals at the time of writing and may conflict with information details by authors included in the literature review.
The units in which data is reported vary from author to author. For the sake of transparency, the units are retained from the manuscripts. As a term of reference, the term ppm (parts per million) is equivalent to mg/kg (milligrammes per kilogramme of food). The term ppb refers to parts per billion (microgrammes per kilogramme of food).
2.2 Literature review search terms
This literature review commenced with the following searches which required at least one of the following terms in the title or abstract of articles published between 1993 and September 2022: “hypersensiti*” , “hyper-sensiti*”, “allerg*“ and at least one of the following: “celer*“ , “egg“ , “fish“ , “gluten“ , “gliadin“ , “wheat” , “lupin“ , “milk“ , “casein“ , “lactoglobulin“ , “mustard“ , “peanut“ , “sesame“ , “shellfish“ , “shell-fish” , “crustacea*“ , “mollus*“ , “soy*“ , “*nut“ , “almond“ , “brazil nut“ , “cashew“ , “coconut“ , “hazelnut“ , “macadamia*“ , “pecan“ , “pistachio“ , “pine*“ , “shea*“ , “walnut“ , “sulphur dioxide” , “sulfur dioxide” , “sulphite” , “sulfite” and at least one of the following “detect*“ , “quant*“ , “immunoassay“ , “immunosorbent assay” , “ELISA“ , “PCR“ , “polymerase“ , “mass spectro*“ , “LC-MS*” , “LCMS*”. This search was run through the following search engines (number of hits from a title and abstract search in brackets): Web of Science (4326), Pub Med (5551), BASE (6284), Lanl Library (173), BLDSC (10), Google Scholar (7500). Once these references were collected in EndNote 20 the duplicates were removed, leaving 10,320 references. These papers were then categorised based on their relevance to the topic on a scale of 1 to 5 according to the technology and allergen, with books and reviews excluded at this stage as our search was expected to capture any relevant work which would be cited by these. Student theses were also discounted along with methods which were still under development, under the assumption that the most pertinent research would be published in a peer-reviewed journal and captured during the review. The least relevant papers were classified with a 1, these were papers which were not about food or not about allergies. Papers classified as 2 related to the clinical side of allergy study, the biological background to allergic responses and papers regarding food labelling regulations. Papers with a rating of 3 or greater reflected methods used to detect allergens, including commercial, non-commercial and emerging methods and were considered for this review when some form of method verification or validation was included. Additionally, as mentioned above, publicly available information regarding the performance of the commercial ELISA and PCR kits implemented among users of the Fapas® testing programme was used to form part of this review and some of the content of Table 1 (Appendix 1).
2.3 Tabulated summary of testing methods
Table 1 (Appendix 1) was prepared during the literature review. This table summarises the scope and performance of testing methods with a particular focus on the commercial testing kits used by participants in Fapas® allergen testing proficiency trials during the past five years. Fapas® proficiency testing is undertaken by laboratories across the globe, using the testing methods they apply in their routine allergen testing services. These laboratories, experienced in allergen testing, will have naturally adopted the kits and other methods over time which provide the most reliable results for their requirements and matrices. Information provided in the user manual is summarised in the table along with data identified from reviewing the literature. Since testing kits are updated on a regular basis, often maintaining the same kit name which does not reflect that the kit has been developed, it is difficult to relate the literature to the current iteration of the kit. Few kit manuals reference or publish the data relating directly to the development of that kit, either online or in the contents of the kit. If required, kit users can approach kit manufacturers and request whether further details and validation data are available to receive. The detail of the validation data shared can vary between kit manufacturers. It can therefore be challenging to confidently align the literature with test kit data. Unless the specific commercial test kit to which a publication refers is stipulated in the manuscript, no attempt has been made to align data with test kits due to concerns over misaligning the data. As shown in Table 1, although the target protein is stipulated for some test kits, for other test kits the target is either unknown (often the case for kits for which polyclonal antibodies underpin the method which have been raised against the allergenic food as a whole so the precise protein/epitope is not known) or is withheld for proprietary reasons. This lack of transparency makes the comparison of kits, and the determination of the most suitable kits to use during an incident, very challenging and therefore is a knowledge gap. Conversion factors, when available in the manual, have also been included in Table 1 and another knowledge gap is the easy conversion between the data of different kits and the conversion of data into meaningful terms.
2.4 Literature review of methodologies for determining food allergens
The testing methodologies identified for each of the groups of food allergens are discussed below.
2.4.1 Celery
2.4.1.1 Introduction
The prevalence of celery human allergenic responses are raised in some European countries such as Switzerland, Germany and France. As a result it is mandatory to label food products containing celery in European regulations, however it is not mandatory in the United States and other countries where rates of celery allergenicity are lower. The major celery allergen is Api g 1, however in total six allergens have been characterised in celery (Api g 1-6). Api g 1 is homologous to the pollen allergen Bet v 1 and cross reactivity has been reported between celery and birch pollen sensitivities. (EFSA, 2014)
2.4.1.2 ELISA and immunoassay
As is common for allergen testing in food when foods are processed compared to native/raw, Jankiewicz et al. 1997 reported that the specificity and reactivity of IgE antibodies to celery reduced with thermal processing, using celery root as the target food (Jankiewicz, Baltes et al. 1997). The study compared heating by microwave, cooking, drying, gamma radiation, high voltage impulse treatment and ultra-pressure treatment. In contrast, the reactivity of the antibodies was only mildly reduced during non-thermal processing techniques. However, current methodologies to detect celery tend not to use immunochemistry technology as a consequence of the cross- reactivity between the Api g 1 celery allergen and the homologous birch allergen Bet v 1. Instead, PCR is the favoured approach for its specificity. Many publications, and indeed the only commercial testing method used in celery determination in Fapas® proficiency testing rounds, are based on PCR methods.
2.4.1.3 PCR
The EvaGreen® Real-Time PCR method was used for detection of celery, Apium graveolens. (Škultéty and Jurčáková 2011) A primer designed to target the mannitol dehydrogenase gene region was used for specific celery identification in sample. The results showed the possibility to create a calibration curve using artificially adulterated samples. The increasing variability between parallel calibration of celery samples was observed from 0.1 % to 100% and the detection limit was 0.1% celery (equating to 1000 mg celery/kg food).
Luber et al. 2015 reported the development of a tetraplex real-time PCR method (Luber, Demmel et al. 2015). The approach was validated with DNA extracted from lysate mixtures of boiled sausage. Recovery, repeatability and robustness were successfully evaluated and the LOQ was determined as 3.7 mg/kg. However, quantification was achieved using standard addition of the allergen to the prepared food rather than by the more usual route of analysing incurred samples.
A 2017 ring trial of real-time multiplex PCR methods with a spike level of 40 and 100 mg/kg celery was conducted by Waiblinger et al. (Waiblinger, Boernsen et al. 2017) using the published method of a multiplex real-time PCR method to combine the detection and quantification of brown/black mustard, white mustard, celery and soybean was validated (Luber, Demmel et al. 2015) showing that the method was capable of reliably detecting and quantifying incurred boiled sausages containing 40 mg/kg celery. PCR had been shown to cross-react with coriander and lovage previously at the 0.01% level (Waiblinger et al. 2017). The LOD of this method was determined as <10 copies for celery but did not detail how to equate this to the level of celery allergen protein. Details of any commercial kits used in the ring trial were omitted. Current commercial methods detect down to 0.4 mg/kg celery (LOD 1 mg/kg) and it would be interesting to learn the performance of the method used in the ring trial but involving lower levels of allergen detection.
In a study by Wu et al. 2010, a celery mannitol transporter (Mat3) gene-based detection method for celery was established by means of SYBR Green real-time PCR technique (Wu, Chen et al. 2010). The method was found to be applicable to Chinese celery, Western celery and fragrant celery. No cross-reactivity was found between celery and the other food materials (parsley, shallot, carrot, potato, fennel, soybean, rice, peach, apple, orange, walnut, cauliflower, maize, chili, peanut, sesame, pumpkin, and sunflower seed pork, beef, chicken, and mutton along with eight processed products which declared celery as an ingredient). The LOD was determined through experiments on pure celery DNA, DNA mix, and spiked food samples. The method was able to detect 0.001% raw food sample and 0.01% heated food sample. The utility of the method was confirmed by the investigation of 13 commercial foods. The LOD was determined as 5 picograms (pg) celery DNA, indicating that theoretically 0.001% celery could be detected from 100 ng/mL (nanograms per millilitre) DNA template.
Daems et al. 2017 developed a rapid, one-step quantification method of celery DNA by Fiber Optic Surface Plasmon Resonance PCR which allowed for the cycle-to- cycle quantification of the target sequence by melting analysis (Daems, Peeters et al. 2017). The developed bioassay was benchmarked against qPCR followed by high resolution melting analysis, showing excellent agreement (R2 = 0.96).
A commercial PCR method (SureFood® Celery) exists with an LOD of 0.4 mg/kg (of celery powder spiked into corn flour) and an LOQ of 1.0 mg/kg in the same matrix. The performance of the method on other food matrices is not detailed in the manual so users must determine the suitability of their matrices independently. The precise basis of this method is not detailed in the manual, perhaps for proprietary reasons. Methods detailed in the literature do not match always match this LOD or LOQ, however are detailed below as these methods do detail detection in additional matrices.
2.4.1.4 Mass Spectrometry
Mass spectrometry combining two mass analysers (MS/MS), particularly liquid chromatography mass spectrometry (LC-MS/MS), is a technology which has been emerging for allergen detection over approximately the last 10-20 years. Compared to ELISA and PCR methodologies, this is a much more recent application being implemented for allergen detection.
Using nanoLC-ion-trap MS/MS, initial method development was conducted to detect proteins belonging to celery, potato and carrot (Faeste et al. 2010). Among others, a novel patatin (Sola t 1)-like protein was detected in celery and a flavin adenine dinucleotide binding domain-containing protein (Api g 5)-like glycoprotein was identified in carrot. The data also suggested the presence of a Sola t 4- like protease inhibitor in celery. Several unique precursor ion-to-product ion transitions were determined for each species, suggesting the feasibility of developing an MS-based screening method to specifically detect celery allergens in foods. This group initially developed an ELISA assay targeting celery but the antibody showed cross-reactivity with carrot, parsnip and potato.
2.4.1.5 Conclusions – Celery testing methods
From Fapas® data, we see that the method used by food testing laboratories to determine celery is PCR, with one vendor monopolising the market (Table 1, Appendix 1). This commercial method provides details in the manual of LOD and LOQ based on corn flour, presumably spiked with celery powder. Little data is provided regarding cross-reactivity. Data is available in the public domain to show that certain PCR methodology does benefit from low LOD/LOQ and also does not cross-react with a range of food types (Wu, Chen et al. 2010). However, it is impossible to know whether this is the PCR method upon which the commercial method is based. Increased transparency by commercial kit manufacturers regarding the validation data of their kits, including but not restricted to listing the matrices tested, cross-reactivities identified and the manner in which validation samples were prepared and whether they are cooked or raw, incurred or spiked, would greatly benefit testing laboratories in determining the suitability of kits prior to purchase.
Since only one method dominates the market (a PCR kit) it would benefit consumers if a confirmatory method was also available, based on a different technology.
2.4.2 Cereals containing gluten
2.4.2.1 Introduction
Gluten is a class of proteins present in wheat, rye (as secalins) and barley (hordeins) within the grass genus Triticum, including semolina, triticale, spelt, emmer, einkorn, Kamut™(Khorasan wheat), and club wheat. The use of gluten in foodstuffs is common due to benefits concerning texture, moisture retention and flavour. The term ‘gluten’ is a collective term for a structural protein found in certain cereal grains which can trigger celiac disease. The prevalence of sensitivity for the allergens in wheat, barely, rye and oats is <2%. (EFSA, 2014) Wheat gluten is composed of mainly two types of proteins: the glutenins and the gliadins. In barley, gluten proteins are referred to as hordeins, in rye, secalins, and in oats, avenins.
Since these proteins have sequences which differ slightly in different species are not present in the same ratios in the different species, the ability to accurately quantify the overall amount of gluten in various food matrices is challenging.
Current gluten analysis is mainly conducted using ELISA. The main concern with this allergen is detection in partially hydrolysed or fermented products. There is also concern that gliadin is the only target for wheat so there is little diversity between methods.
Lacorn et al. (Lacorn, Lindeke et al. 2018) warn that, ‘For production, starch is cleaned up by the very thorough cold-water washing-out of gluten, or gluten is additionally fragmented by enzymes into peptides. In the latter case, remaining gluten fragments are potentially too small to be detected by sandwich ELISA systems in a quantitative way due to the fact that only one epitope remains in the peptide. In this case, the use of a competitive ELISA assay format is strongly advisable that is also able to detect very small fragments of proteins. However, competitive assays usually have to use less stringent extraction buffers, which may lead to incomplete extraction in heat-treated materials.’
2.4.2.2 ELISA Methods
Holzhauser et al. 2020 reported that a few major limitations of the methodology have been extensively investigated with numerous studies reporting that results of different kits very often show considerable variation (Geng, Westphal et al. 2008, Bugyi, Torok et al. 2013, Scharf, Kasel et al. 2013, Alvarez and Boye 2014, Scherf 2017, Holzhauser, Johnson et al. 2020). Major causes of variability, reviewed by Holzhauser et al., include differences in antibody affinity (Lexhaller, Tompos et al.
2016, Lexhaller, Tompos et al. 2017, Panda, Boyer et al. 2017, Allred and Ritter 2019), the effects of processing and the matrix (Bugyi, Torok et al. 2013, Gomaa and Boye 2013, Gomaa and Boye 2015, Panda, Zoerb et al. 2015) and the genetic and environmental variability of proteins (Pahlavan, Sharma et al. 2016, Hajas, Scherf et al. 2018). These issues demonstrate an urgent need of harmonisation in this field, and indeed this has been the case for over a decade. These issues demonstrate the need for harmonisation in this field, as discussed further in Section 3.
The detection of wheat products is typically achieved through the detection of gluten, with the Voluntary Incidental Trace Allergen Labelling (VITAL) expert panel advising individuals with IgE-mediated wheat allergies that they would be “largely protected when selecting gluten-free products manufactured in conformity to Codex guidance” (Taylor, Baumert et al. 2014). The target protein of commercially available kits is typically gliadin, based on R5 monoclonal antibodies which are specific for proteins from wheat, rye, and barley. The two ELISAs Wheat Protein ELISA Kit (Gliadin kit) and a FASTKIT Wheat ELISA Kit (Wheat ELISA kit) which are supplied by Cosmo Bio Ltd, Japan, were found to have detection limits of 1 ng/ml for matrices of sausage, sauce, pasta sauce, fish paste and cereal (although only the abstract could be accessed of this paper and the method of determining the LOD (whether in buffer, spiked or incurred into the matrix is not clear) (Akiyama, Nakamura et al. 2004). In a ring trial across ten laboratories the ELISAKits FASTKIT ELISA Ver. II Series and the FASPEK® Allergenic Substances Detection Kit (Morinaga) were evaluated on a variety of matrices and gave recoveries of gluten in sausage of around 100% for sausage, boiled beef, tomato sauce, and orange juice but <30% for jam (which can be a vector for gluten contamination) (Akiyama, Nakamura et al. 2004).
The extraction protocol is a crucial step in ELISA analysis and forms part of the manufacturer instructions. Extraction protocols are kit-specific and, for example, should they include reducing agents, these need to be diluted out prior to analysis to avoid disruption to the activity of the kit components. In their 2009 study, van den Broeck et al. (van den Broeck, America et al. 2009) compared different extraction buffers, assessing the proteins which were extracted by each method by gel separation analysis by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). A two-step extraction method was optimised which extracted a wider range of gluten proteins than single-step methods, including extraction of low molecular weight proteins. Different antibodies were also used compared to the R5 antibody, which, when used to probe an immunoblot of the SDS-PAGE-prepared profile using the two-step method, detected different low molecular weight peptides compared to R5. The authors separated gliadin and glutenin extraction; extraction using reducing agent; extraction in 60% ethanol and; a two-step gluten extraction (van den Broeck, America et al. 2009). Of these, the typical commercial kits use a 60% ethanol solution, including the kit used in this work, and the RIDASCREEN® Gliadin competitive ELISA, this work found no significant difference between the 60% ethanol extraction method and the two-step extraction when the extracts were analysed using the RIDASCREEN® kit.
A comparison study between five ELISA kits for gluten included the following kits (LOQ in brackets): RIDASCREEN® Gliadin by R-Biopharm AG, Darmstadt, Germany (5 ppm), wheat protein ELISA kit by Morinaga Institute of Biological Science, Inc., Yokohama, Japan (0.3 ppm), BioKits gluten assay kitby Neogen Corp., Lansing, MI (3 ppm), ALLER-TEK gluten ELISA assay by ELISA Technologies, Inc., Gainesville, FL (5 ppm), AgraQuant® gluten assay by Romer Labs UK Ltd, North Wales, UK (4 ppm), and Gliadin kit by ELISA Systems, Queensland, Australia (5.0 ppm) (Sharma 2012). All LOQs quoted in this manuscript match those declared in current documentation provided with kits, except the kit from ELISA Systems which currently quotes a LOQ of 2.0 ppm (ELISA, 2020) This work tested cornflour spiked with gluten and wheat flour at a range of concentrations and observed different interactions with allergens. The kits provided by R-Biopharm, Morinaga, and Romer Labs reacted strongly with the gliadin fraction, whereas those from BioKits, ALLER- TEK, and ELISA Systems reacted strongly with the glutenin fraction. All kits gave a positive response to gluten spiked at 5 ppm, as would be expected given their stated LOQs. The recovery responses were varied with the R-Biopharm reporting a wheat flour recovery of 74%. The Morinaga and Biokits products exhibited average recoveries between 100-200%, while the Aller-tek, Romer Labs and ELISA Systems had significantly higher recoveries for wheat flour.
The development of incurred gluten contamination standards, where gluten is added to the sample prior to processing to a final product, better-represent real-life challenges to the food industry. With a view to this, Sharma et al. developed cornbread with either gluten or wheat flour incursion assessing the performance of each kit against both (Sharma, Khuda et al. 2013). The variation of gluten source affected the accuracy between the different ELISA kits tested: RIDASCREEN Gliadin (R7001; R-Biopharm AG, Darmstadt, Germany), wheat protein ELISA kit (181GD; Morinaga Institute of Biological Science, Inc., Yokohama, Japan), BioKits gluten assay kit (802002Y; Neogen Corp., Lansing, MI), and AgraQuant Gluten G12 (COKAL02002; Romer Labs UK, Ltd., Cheshire, U.K.). The kits (which may or may not have changed since the study) used different antibody types: BioKits used a Skerritt (401/21) monoclonal antibody; Morinaga used an anti-gluten polyclonal; R- biopharm used the R5 monoclonal; and Romer Labs used the G12 monoclonal antibody. Positive detection of gluten was possible with each kit tested at each level of spiking gluten (0−500 ppm) and wheat flour (20−1000 ppm), and different baking conditions (204.4 °C for 20, 27, and 34 min). The stability and immunoreactivity of gluten proteins, as measured by western blot using three different antibodies, were not adversely affected by the baking conditions. Dependant on the kit and source of gluten, the gluten recovery variation was high, affecting the accuracy of gluten quantification: BioKits 9−77%; Morinaga 91−137%; R-Biopharm 61−108%; and
Romer Labs 113−190%. Gluten recovery was reduced with increased baking time for most ELISA kits analysed. The Morinaga and R-Biopharm kits gave lower recoveries using the wheat flour compared to gluten incurred cornbread, whereas the Biokit gave the opposite observation. The predicted analytical coefficient of variation associated with all ELISA kits was below 12% for all incurred levels, indicative of good analytical precision. This study reveals a wide range of recoveries, both within- kit and between kits, with accuracy affected by kit type and baking conditions, with most kits reporting lower gluten levels as baking time increased. A reduction to zero following a longer baking time may lead to false negative results, putting gluten- sensitive consumers at risk. The variation in the recoveries will impact on the level of gluten estimated by each kit and highlights a gap in the (consistent) capabilities of kits to measure gluten in food.
In their 2013 development of a RM for gluten, a study by Bugyi et al. compared seven different commercially available ELISA kits (Bugyi, Torok et al. 2013). The data were however anonymised in reporting relevant results. This lack of transparency increases the challenge of understanding gaps in the effectiveness of specific kits, however it provides an opportunity to examine the harmonisation across the market. Between the kits, significant differences in average recovery were observed. The differences between kits result from different antibodies, extraction solutions and calibrations, with authors highlighting the fact that R5 and Skerritt antibodies are both developed against prolamines, however their affinities for glutenins and gliadins differs, and this makes the conversion of gliadin units to gluten units inconsistent. Additionally, each kit may be calibrated using a different standard. As highlighted elsewhere in this report, this would benefit from being standardised to ensure that the protein sources across different manufacturers can be accurately compared and contrasted.
Studying baked cookies in work which interrogated ELISA kits and flow cytometry for casein, egg, gluten and soy sensitivity, the following ELISA kits were studied: R- Biopharm RIDASCREEN (exact gluten kit not specified) (R-Biopharm AG, Darmstadt, Germany) and the Neogen Veratox (exact gluten kit not specified) (Neogen Corp., Lansing, MI) (Gomaa and Boye 2013). This work was published in 2013 and while it uses commercial ELISA kits which are still on the market, these kits can be developed and altered constantly, and results presented in this work may not represent current sensitivity. Both ELISA kits and flow cytometry were able to detect gluten allergens under all processing conditions with recoveries of: 93–31% for the RIDASCREEN kit, 72–27% for the Veratox kit, and 75–21% for flow cytometry. The detection of allergenic proteins with both increasing cookie size and temperature is a positive indicator for the robustness of these kits with the internal temperature of the small cookies reaching 155 °C. At temperatures greater than
100 °C, the Maillard reaction occurs which alters protein-carbohydrate interactions and this can mask allergenic epitopes. Robustness must of course be formally assessed during a full validation exercise.
Work from Lacorn et al. which presents case studies highlighting the gaps in the application of ELISA kits for detecting allergens (Lacorn, Lindeke et al. 2018) also highlights the potential for allergenic wheat proteins which are too small for detection by sandwich ELISA to remain in gluten-free wheat starch, when the ‘gluten-free’ flour is produced from wheat by cold water washing-out of gluten. Conversely when using a competitive ELISA, the extraction buffer may be insufficient to extract heat-treated materials, which may arise when the starch is heated to dryness after the cold-water washing. Therefore, to minimise the risk of either method being insufficient, the authors recommend the use of both competitive and sandwich ELISA kits to indicate how the gluten-free wheat starch was produced.
The validation of an ELISA kit, the Morinaga M2103 for Wheat/Gluten, was published in 2019 (Saito, Doi et al. 2019). For the test materials, a blank sample of gluten-free bread was spiked with either gliadin or gluten and additionally an incurred reference bread was made using a gluten-free bread mix and wheat protein spiking solution.
The Association of Official Analytical Chemists (AOAC) Research Institute Performance Tested MethodSM (PTM) program was used to validate the linearity study, selectivity, both incurred and spike matrix studies, LOD, LOQ, robustness and the lot-to-lot consistency/stability studies. An independent laboratory was additionally included in the testing protocol. The analysis of 38 different substances revealed no cross-reactivity above the LOQ except for oats. The method was shown to be robust in terms of altering the extraction times. This manuscript is a rare example of kit validation data being published and thus accessible for stakeholders.
In the past year a comparison of the following sandwich ELISAs was performed by Amnuaycheewa et al. RIDASCREEN® Gliadin kit (R-Biopharm AG, Darmstadt, Germany; Art. No. R7001; the AOAC-RI license #120601, the AOAC-OMA license #2012.01, and the AACC International Approved Method 38–50.01), the Veratox® for Gliadin R5 kit (Neogen Corporation, Lansing, MI, USA; Product No. 8510; the AOAC-RI license# 061201), the Wheat Protein ELISA kit (Gliadin) (Morinaga Institute of Biological Science, Inc., Yokohama, Japan; Cat. No. 181GD), and the AgraQuant® Gluten G12 assay (Romer Labs UK Ltd., Cheshire, UK; Product No. COKAL02002; the AOAC-OMA license #2014.03 and the AACC International Approved Method 38–52.01) (Amnuaycheewa, Niemann et al. 2022). Much of the study focussed on determining gluten levels in 32 foods containing gluten around the 20 ppm target level for gluten-free status, as determined previously by the RIDASCREEN® Gliadin kit or the RIDASCREEN® FAST Gliadin kit. Each of these kits used gliadin as a calibration standard although the RIDASCREEN and Veratox detect the R5 antibody and the Morinaga and AgraQuant detect the G12 antibody.
Tested against 32 foods and ingredients and also against sixteen spiked powders of wheat, barley, rye, triticale, oat and sorghum, representing a wide range of food types and processing conditions, the results were evaluated. As reported by the authors, as expected, similar results were yielded from the two R5 kits. The G12 kit and the Morinaga kit, though reporting result as wheat protein, not gluten, also yielded similar results to the two R5 kits for most samples but yielded substantially different results for a few samples including samples of yeast extract, hemp protein powder and cookie. Those differences could be caused by any one of the several reasons: (a) differences in the grain source of glutens and related proteins, (b) differences in the efficiency of extraction and detection, (c) subsampling differences with particulates, or (d) some combination.
The Romer AgraQuant Gluten G12 Assay (COKAL02002) and the R-Biopharm RIDASCREEN Gliadin Assay (R7001) assays compared similarly in a study to determine gluten in wheat cultivars. The kits apply different antibodies (monoclonal G12 and monoclonal R5, respectively) and are calibrated differently (vital wheat gluten extract and PWG-gliadin, respectively). Both kits showed similar recoveries, around 100% for some cultivars and both kits reacted significantly differently to certain cultivars (Hajas et al 2018).
2.4.2.3 Mass Spectrometry
Authors considering the quantification of low-level (trace level) gluten peptides by mass spectrometry have focused on a variety of different target peptides. Six targets in enzymatically digested food samples were identified by Sealey-Voyksner et al. and they were characterised to LODs ranging from 1 to 30 pg mg-1and the method was capable of detecting and quantifying select target peptides in food over a range of 10 pg/mg (0.01–100 ppm) with good reproducibility (Sealey-Voyksner, Khosla et al. 2010). Reproducibility of the assay was demonstrated for the calibration data and for data collected from the analysis of QC standards over a period of four days. The average coefficient of determination (R2) for each peptide was greater than 0.995.
The detection of a range of peptides to identify five different proteins was published by Manfredi et al. and for spiked rice flour gave good sensitivity, however with incurred test materials the recovery varied from 3-30% (Manfredi, Mattarozzi et al. 2015). This highlights a common issue in allergen detection whereby processed foods are often far more of a challenging matrix for both mass spectrometry and ELISA methods and allergen in processed food may be underestimated by methods.
It was the 33-mer peptide, from the alpha2-glandin protein, which was the focus of quantification work by Schalk et al. (Schalk, Lang et al. 2017). Using rye flour, which does not contain the target peptide, as a matrix, an LOD of 13.1 μg g-1 LOQ of 47.0 μg g-1 was established, significantly lower than the content of the peptide in wheat cultivars. In subsequent work from the same group an attempt to quantify wheat glutens involved the identification of 16 reference proteins which could be summed into an estimate of gluten concentrations. This was compared to established methods, an R5 ELISA and gel permeation high-performance liquid chromatography (HPLC) with fluorescence detection and a strong correlation was found.
2.4.2.4 Conclusions – Cereals containing gluten
The crucial challenge in ELISA detection is the variability across different kits, the calibration and RMs, the antibodies which are used (typically either G12 or R5), and whether the data is reported as gliadin or wheat proteins (Bugyi, Torok et al. 2013). It is essential that the future direction of allergen detection harmonises these concepts so that food manufacturers can test with certainty. The recent work from Amnuaycheewa et al. which used kits testing for two different antibodies found comparable results between them all, suggesting that modern iterations of each kit may be approaching this goal (Amnuaycheewa, Niemann et al. 2022).
It must also be considered that the recovery of gluten can vary considerably between kits so gluten levels could be seriously under-estimated (or over-estimated) depending on the kit used and therefore kit users must have validation data for their typical sample type, with validation samples comprising incurred products. With validation data for a kit, one option is that a correction factor can be applied to calculate the level of gluten in a product. However, labs need to prepare their own validation data in order to apply this. Since gluten can be deliberately fragmented by enzymes during processing, it will be interesting to determine if peptide detection methods develop further in the future (Schalk, Koehler et al. 2018).
2.4.3 Crustacea
2.4.3.1 Introduction
Crustaceans form a large part of many diets across the world, however the prevalence of self-reported allergies varies, from 0.3% in children in the UK to 5.5% in France, with decapods, such as shrimp, lobster, prawn and crab the main allergy causing foods. (Pereira et al., 2005; Touraine et al., 2002) Tropomyosin has been characterised as the major crustacean allergen found in decapods with at least 80% of shrimp allergic individuals reacting to it, however other compounds such as arginine kinase, sarcoplasmic calcium-binding protein and myosin light chains have also been identified as allergy causing. (EFSA, 2014)
2.4.3.2 ELISA, immunochemistry and PCR
A comparison of seven commercial methods for the detection of shrimp allergens in kimchi (salted, fermented vegetables) tested three PCR kits (SureFood Allergen ID Crustaceans from R-Biopharm, Darmstadt, Germany; PowerChek shrimp & crab real-time PCR kit from KogeneBiotech, Seoul, South Korea; and Cruskit real-time PCR from 4LAB Diagnostics, Vicomoscano, Italy) and four ELISA kits (Ridascreen Fast Crustacean from R-Biopharm; Veratox for crustacea allergen supplied by Neogen, MI, USA; AgraQuant ELISA crustacea from Romer Labs, Newark, DE, USA; and Crustacean Residue from ELISA Systems, Queensland, Australia) (Jeong and Kim 2020). Only the ELISA kits were capable of quantification as the PCR kits do not contain standards of known concentrations and the sensitivity of the three PCR kits differed quite significantly at 0.4, 100, and 25 ppm for SureFood, PowerChek, and Cruskit kit, respectively. Both the SureFood and PowerChek kits were capable of amplifying the shrimp DNA with the Ct values of the SureFood kit closely matching the relative allergen concentrations in the traditional Korean dish of kimchi and its ingredients saeu-jeot (salted shrimp) and saeu-aekjeot (fish sauce).
For all tested samples, no positive result was obtained for the Cruskit kit, but tiny shrimp (A. japonicas) was absent from its target species list. For two allergenic proteins in shrimp, tropomyosin (TM) and sarcoplasmic calcium-binding protein (SCA), the four ELISAs were compared, with three kits being sensitive to tropomyosin with an LOQ of 0.003 to 0.01 μg/mL while the Crustacean residue kit was sensitive to TM and SCA only at higher concentrations (0.1 μg/mL). This is still sufficiently sensitive to detect levels at the threshold known to elicit anaphylaxis, although the method of LOQ determination is not available and so it is unclear if it was established in buffer or matrix. This is a curious outcome as while the Ridascreen and Veratox kits offer a broad range of target proteins, the AgraQuant kit and ELISA Systems Crustacean Residue kit target tropomyosin specifically and would be expected to detect this protein at low levels.
Otto et al. 2016 reported an immunoassay for the simultaneous detection of milk, egg, peanut, mustard and crustaceans in cookie samples at sub-100 ppm levels (Otto, Lamote et al. 2016). The method was based on a combination of flow cytometry with competitive ELISA where microbeads coated in antibodies were used as sorbent surface. The lowest concentration of crustacea inducing a significant difference of signal between non-contaminated controls and test samples was 5 mg/kg. The authors reported that the test was sufficiently sensitive to detect crustaceans at the reference doses established by the VITAL expert panel. Assay sensitivity was influenced by the concentration of primary antibodies added to the sample extract for the competition and by the concentration of allergenic proteins bound to the surface of the microbeads. No cross-reactivity was observed with the anti-crustacea antibodies. The authors stated that flow-cytometry-based immunodetection may, in the near future, improve upon the performances of classic ELISAs by adding a new feature: simultaneous detection/quantification of multiple allergens.
Relating to cross-reactivity of ELISA methods, there is much potential for cross- reactivity between crustacean allergens and insect food allergens, due to the commonality of certain proteins between the animal groups. De Marchi et al. 2021 investigated the allergenic potency of the cricket (Acheta domesticus) and shrimp (Litopenaeus vannamei) (De Marchi, Mainente et al. 2021) assessing the effect of thermal processing and gastrointestinal digestion on allergenic properties. A. domesticus is considered a potential nutrient source due to its attractive nutritional profile and lower feed conversion ratio compared to other animals. Cricket proteins relating to sarcoplasmic calcium-binding protein and tropomyosin were detected by the sera of 20 shrimp-sensitive patients, with tropomyosin being the more relevant in terms of reactivity. The assessment of the stability upon food processing and gastrointestinal digestion of cricket proteins, when used as ingredients to enrich food products, is crucial to infer essential data about the risk associated with their ingestion. Of concern, while shrimp tropomyosin was unstable to simulated gastric digestion, cricket tropomyosin showed different properties and was resistant to digestion and would potentially represent a risk of primary sensitization to crustacean allergy from consumption of crickets and cross-reactivity. Indeed, it is possible that the co-sensitization to other allergens, such as house-dust mites, cockroach, mealworm etc. might contribute to the variability of the IgE-binding profiles (van Broekhoven, Bastiaan-Net et al. 2016). Tested on shrimp powder- or cricket flour- incurred biscuits, thermal treatment (baking) enhanced the stability of the allergenic proteins to gastric digestion. Rather than becoming more susceptible to digestion as a consequence of the thermal treatment, TM was recognized by patients’ sera IgE after the gastric digestion and also up to 1 h of intestinal digestion. The high IgE- cross-reactivity between shrimp and cricket tropomyosin indicates that current testing methods may be incapable of discriminating between crustacea and insect protein in food.
2.4.3.3 Mass Spectrometry and other methods
A biomarker approach was adopted for a mass spectrometric method for the quantification of crustacean proteins in salmon lasagne spiked with lobster or shrimp. Proteotypic peptides were identified in combination with enhanced MS sensitivity using MRM3. (Korte, Monneuse et al. 2016) MRM3 is a modern development in mass spectrometry which offers increased sensitivity compared to traditional MRM triple quadrupole instruments through the inclusion of a second fragmentation step. This study demonstrated LODs of 100-1000 mg kg-1 using MRM and 10-100 mg kg-1 in MRM3. A typical LOD for ELISA methods, which benefit from years of development, is currently approximately 0.1-2.6 mg/kg. Another LC-MS/MS method, built around stable isotope-labelled standards for the quantification of tropomyosin and arginine kinase (AK), was able to detect both proteins with recoveries of 94.11-102.16% (Li, Zhou et al. 2022). The LOD ranged between 0.03-0.52 ng mL-1 across the signature peptides for both proteins. This method was tested on commercially available products and detected both TM and AK in all products for which the allergen was included in the ingredients list and also for those for which the allergen was listed in the precautionary (‘may contain’) allergen labelling. No allergen residues were detected in products that claimed to be allergen-free. The LC-MS methodology would benefit from further development with an aim to bring sensitivity in line with that of ELISA methods.
The authors are also aware of research and development work to prepare Surface Plasmon Resonance biosensor detection of shellfish tropomyosin (Zhou et al. 2020). Aptamer methods are also in development for shrimp tropomyosin (Chinnappan et al. 2020) It will be interesting to determine in the future whether such methods become commercialised or whether LC-MS/MS methods are preferred (Li, Zhou et al. 2022).
2.4.3.4 Conclusions - Crustacea testing methods
The literature review has shown that a limited amount of data is available for the comparison of performance of testing methods for crustacea. PCR methods offer only qualitative analysis while ELISA offers semi-quantitation at highly sensitive levels. MS methodology appears to be in the early stages of development with requirements to increase the sensitivity.
2.4.4 Egg
2.4.4.1 Introduction
The chicken egg is widely eaten and used in the food industry, either as a main ingredient or used in a variety of products for its binding, emulsification, coagulation and adhesion properties. Comprised of both a yolk, containing nutrients, and the white, which contains proteins and water most egg-allergic subjects were allergic to proteins found in egg whites, however both egg white and egg yolks can be allergy causing. Multiple allergens have been characterised both in the yolk (serum albumin and YGP42) and the white (ovomucoid, ovalbumin, ovotransferrin and lysosome C).(EFSA, 2014) The prevalence of egg allergy in a challenge proven study found sensitivity levels of 0.1% of adults in both Denmark and Turkey. (Oseterballe et al., 2005; Gelincik et al., 2008)
2.4.4.2 ELISA and immunochemistry-based methods
Working with the Veratox for Egg Allergen Test from Neogen, Williams et al. studied the detection of egg white proteins (ovalbumin, ovotransferrin, ovomucoid, and lysozyme ) in snack foods and noodles (Williams, Westphal et al. 2004). This study used dried egg powder (SRM 8415) from the National Institute of Standards and Technology (NIST) as a reference. The ELISA kits were able to detect egg in dry noodles at a significantly higher level than in boiled noodles.
Comparative results from sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), however, suggested that the protein profiles between the cooked and uncooked egg noodles differed with ovalbumin being undetectable, while ovomucoid proteins were not affected by heating. This indicated that ovomucoid would be a better target for analysis where the sample is subject to thermal processing. This work also tested a variety of matrices, cookies, crackers, salad dressing, noodles and ice cream to demonstrate that recovery was not affected by matrix, with recoveries ranging from 23% to 32%. This egg RM reacted differently by a factor of 10 in a 2010 study by Lacorn and Immer compared to a non- irradiated RM (Lacorn and Immer 2010), highlighting the importance of having a range of RMs for each allergen type, each prepared with different levels of processing. The use of irradiated RMs arises from a need to reduce microbial contaminants and destroy pathogens, such as Salmonella. However, it can also affect the proteins, causing degradation through glycosylation, impacting the concentration of intact proteins and their binding to antibodies. Therefore, validation studies need to carefully evaluate the impact of irradiation on the properties of RMs and ensure that any alterations do not compromise the accuracy and reliablilty of allergen detection using these methods.
The impact of heating egg proteins on detection by three different ELISAs: Neogen’s Veratox Egg Allergen Test, Tepnel Biosystems’ Biokits Egg Assay, and Morinaga’s Egg Protein ELISA Kit, was investigated by Fu et al., the first of which was found to greatly underestimate the levels of protein, which agreed with the finding of Williams et al. (Williams, Westphal et al. 2004, Fu, Maks et al. 2010). For the Biokits, which uses antibodies to ovomucoid marker proteins, higher levels of egg proteins in boiled samples were detected. When the samples were dry heated to temperatures > 176 °C both the Veratox and Biokits gave significant under estimations of egg protein of
< 25%, decreasing further with additional heating. The Morinaga kit has an extraction buffer developed to detect proteins in thermally processed foods and for samples boiled and dry heated to 176 and 204 °C the recovery was greater than that of either of the other two tests.
Thermal processing was investigated through the medium of cookies in work by Gomaa et al. which looked at Morinaga’s Egg Protein ELISA Kit, Neogen’s Veratox Egg Allergen Test and flow cytometry coupled with competitive ELISA where microbeads were used as the sorbent surface (Gomaa and Boye 2013). The objectives of this research were to investigate the effects of baking time (unbaked, 10- 15- and 25-minutes cooking), temperature profile and cookie dimensions and weight on the detection of four allergens (casein, egg, gluten and soy) simultaneously incurred in a non-wheat flour cookie using enzyme linked immunosorbent assay (ELISA) and flow cytometry. As shown in Figure 1, there was a wide disparity when comparing the performance of the three methods. In general, allergen recovery decreased as baking time increased and cookie size was decreased. Temperatures at the centre of the cookies also increased with decreasing cookie size and increased baking time. The recoveries of egg allergens in the baked cookies were less than 50% for the ELISA kits (Morinaga and Veratox kits) and flow cytometry. Nevertheless, the Morinaga kit had significantly higher recoveries of egg allergens in the baked cookies than either the Veratox kit or flow cytometry. Whereas the Morinaga kit had the maximum allergen recovery at 48% for the large cookies baked for 10 min, recoveries with the Veratox kit and flow cytometry did not exceed 5%. While the Moringa kit detected egg in all samples, the Veratox and flow cytometry methods did not detect egg, or reported very low levels, for cookies baked for 15 and 25 minutes, although higher levels of egg were detected by the Veratox kit in raw cookies.
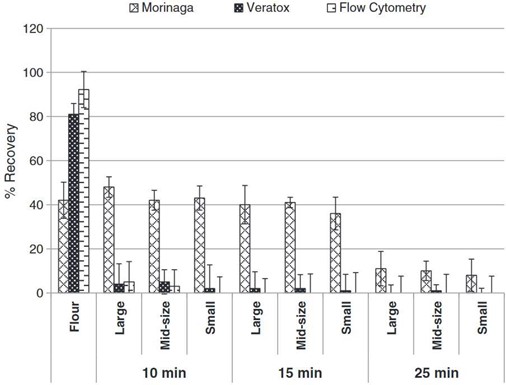
Figure 1. Taken from Gomaa et al. 2013 (Gomaa and Boye 2013). Percentage recoveries of egg in incurred cookie as detected by ELISA and flow cytometry. (Large, mid-size, and small refers to cookie sizes of 10 mm thickness and 38, 58 and 76 mm diameters, respectively; 10, 15, 25 min refers to cookie baked for these different times in an oven at 177 °C).
Two new ELISA kits, the FASTKIT ELISA Ver. II Series (Cosmo Biokit Ltd) and the FASPEK® Allergenic Substances Detection Kit (Morinaga Institute of Biological Sciences) for egg detection were tested in a ten laboratory-wide ring study in work published by Matsuda et al. (Matsuda, Yoshioka et al. 2006). Multiple different ELISA kits using an extraction buffer developed in this work were tested in this study against a variety of matrices, the egg kits were tested in the following with the relative standard deviation (RSD) given as a percentage for each: sausage (4.6%), boiled beef (5.4%), cookie (2.7%), orange juice (2.9%) and strawberry jam (4.7%).
This suggested that the kits were able to detect egg in a range of matrices and with good reproducibility across different labs.
As briefly described above, Otto et al. 2016 reported an immunoassay for the simultaneous detection of milk, egg, peanut, mustard and crustaceans in cookie samples at sub-100 ppm levels (Otto, Lamote et al. 2016). The method was based on a combination of flow cytometry with competitive ELISA where microbeads were used as sorbent surface. Polyclonal antibodies raised to purified casein, the NIST reference standard egg (National Institute of Standards and Technologies, USA), and extracts of crustacean (Panaeus vannamei), peanut (Arachis hypogaea) and mustard (Sinapis alba) were associated with the microbeads. The method was able to detect the presence of the five allergens with median inhibitory concentrations (IC50) ranging from 2.5 to 15 mg/kg according to the allergen to be detected. The lowest concentrations of contaminants inducing a significant difference of signal between non-contaminated controls and test samples were 2 mg/kg of peanut, 5 mg/kg of crustaceans, 5 mg/kg of milk, 5 mg/kg of mustard and 10 mg/kg of egg. The authors reported that the test was sufficiently sensitive to detect peanut and crustaceans at the reference doses established by the VITAL expert panel. Further improvement is needed for mustard, egg, and milk for which the calculated thresholds for a serving of 50 g of cookies are respectively 0.1, 0.6 and 2 ppm. Since the egg used in method development had been irradiated, it may be that the egg epitope had altered due to thermal processing, possibly explaining the reduced sensitivity to egg. Assay sensitivity was influenced by the concentration of primary antibodies added to the sample extract for the competition and by the concentration of allergenic proteins bound to the surface of the microbeads. The anti-casein antibodies cross-reacted with apple (0.7%), the anti-peanut antibodies cross-reacted with turmeric (1%) and the anti-egg antibodies cross-reacted with salmon (0.2%). No cross-reactivity was observed with the anti-crustacea antibodies. The authors stated that flow-cytometry-based immunodetection may, in the near future, improve upon the performances of classic ELISAs by adding a new feature: simultaneous detection/quantification of multiple allergens.
2.4.4.3 Mass Spectrometry
Comparative work was carried out by Heick et al. (including Popping) in 2011 to compare the semi-quantitative capability of 4 ELISA kits with the qualitative capability of an LC-MS/MS method. The ELISA kits again considered the Tepnel Biosystems Biokits Egg Assay and Morinaga Egg Protein ELISA Kit in addition to the R-Biopharm RIDASCREEN FAST Egg Protein, ELISA Systems Egg Residue kit, and a newly developed MS method (Heick, Fischer et al. 2011). The detection capabilities of this novel method were demonstrated by analysing raw and baked bread incurred with seven allergens including egg, with the egg data reported as egg white. Of the four ELISA methods tested, only one could detect egg residues in the processed bread product, although all could detect it in the flour. The levels of egg were significantly underestimated by all kits. The mass spectrometry method, which targeted 4 ovalbumin peptides, did detect egg in the bread, however this was with a signal intensity decreased by 80% when analysing baked bread compared to raw bread. The LC-MS MRM multianalyte method was capable of detecting egg in the processed matrix along with milk, soy, hazelnut, peanut, walnut and almond. The chosen marker peptides were implemented into one method that is capable of the simultaneous detection of milk (casein alpha S1 peptides), egg (ovalbumin peptides), soy (glycinin), hazelnut (11S globulin peptides), peanut (Ara h1 and Ara h3/4 peptides), walnut (Jur r1 peptides) and almond (prunin peptides), incurred in bread material prepared from a standard recipe provided by industry with baking for 60 minutes at 200 °C. The LOD was 42 mg/kg for ‘egg’ incurred’ (no detail was provided as to whether this was whole egg or egg protein) in bread and 0.45mg/L for egg extract spiked into bread, showing the importance of using incurred test materials when determining the suitability of a method to quantitatively determine allergen in real-world samples. The correlation co-efficient for egg detection in incurred bread was 0.9998. Only one ELISA kit (identity of kit anonymised by authors) could detect the allergen in the processed bread. However, all the kits detected egg in the unprocessed matrix, indicating that heat destroys, at least partially, the structures recognized by the kits’ antibodies or that the extractability of the allergens is reduced by processing. This work demonstrates the power of LC-MS compared to the majority of ELISA technologies, although the signal intensity and therefore the LOD is currently lower than for ELISA for LC-MS technologies which is a relative new- comer in the allergen detection field.
As a follow-up to their previous methods, Pilolli et al. (2017) developed an selective reaction monitoring (SRM) LC-MS method using both incurred cookie samples and spiked cookie samples (Pilolli, Chaudhari et al. 2017). The LOD was 9 µg egg allergen (ovalbumin) per gramme of food. By comparing the levels of allergens detected in the incurred samples compared to those for the spike samples, the authors were able to determine the effect of processing on the level of detection of the allergens. Figure 1 clearly shows how the detection is dramatically reduced for each of the 5 allergens under investigation when the samples are processed.
Work by Parker et al. 2015 also developed an LC-MS/MS method for the detection of egg proteins having demonstrated that ELISA Systems’ Egg Residue kit and Neogen’s Biokit are incapable of detecting egg proteins in a baked cereal bar and muffins, the Neogen’s Veratox Egg Allergen Test and R-Biopharm’s RIDASCREEN FAST Egg Protein give recoveries of < 10%. The Morinaga’s Egg Protein ELISA Kit again performed well with the processed foods with recoveries of 76.7% for the cereal bar and 99.6% for the muffin. While not as sensitive as the Morinaga kit (with recoveries of 60.8% and 45.2% respectively) the LC-MS/MS method outperformed the other four commercially available ELISA kits.
Thermally processed egg proteins incurred and spiked in cookies were the subject of an LC-MS/MS method, using a cookie containing whole egg, skimmed milk, soy flour, ground hazelnut and ground peanut to create incurred samples for investigation (Pilolli, De Angelis et al. 2017). The LOD of this newly developed method for egg in incurred cookies was found to be 9 μg g-1 (9 mg/kg) which is higher than for the well-established methods involving ELISA (for which the LOD is typically 0.1-0.3 mg/kg). The authors highlight that the effect of thermal processing was greatest for the egg compared to the other allergens (milk, soy, hazelnut and peanut) with a 97% decrease in sensitivity calculated for incurred samples compared to spiked samples. A matrix- matched calibration curve, prepared using serial dilutions prepared from incurred cookies, yielded a linear correlation coefficient of 0.995. LOD tends to be much lower for ELISA for example between 0.1 and 0.3 mg/kg.
Egg allergens were successfully identified in LC-MS work by Fan et al. on different food types spiked with ovalbumin (Fan, Ma et al. 2023). Ovalbumin was detected by targeting 13 different peptides, with five selected for the purposes of quantitative analysis. Using stable isotope-labelling and LOD for ovalbumin was in the range 17.71–35.43 mg per 100 g with an LOQ of 53.14–70.86 mg per 100 g. The effect of matrices such as a bread and cookies was minor with a range of 82% to 123% while the test was able to detect egg proteins in supermarket products including chocolate pie, vermicelli and Snickers bars. It would be interesting in the future to understand the performance of this method on incurred matrices rather than on matrices spiked with ovalbumin, since heat processing is known to affect the detection of ovalbumin.
Although using the less challenging spiked rather than incurred samples to develop a method to detect egg, Monaci et al. 2014 developed a method using SRM LC-MS for multiallergen measurement for milk, egg and soy, selecting the top 2 performing peptides from a list of 11 (Monaci, Pilolli et al. 2014). LODs were achieved at 0.3 µg ovalbumin per gramme of food.
Gavage et al. 2020 proposed the future development of the application of concatenated peptides for quantitation by multiple reaction monitoring (MRM) mass spectrometry. Concatemers are artificial proteins composed of concatenated, proteotypic peptides originating from different proteins of interest and labelled with stable isotopes. In contrast to the use of labelled synthetic peptides for the same purpose, concatemers need to be proteolytically digested to release their peptides, and thus, this peptide release is also affected by the interference caused by the matrix during the digestion step, in a manner similar to the analyte of interest.
Another advantage of concatemers is their potential for multiplexing. Gavage et al. compared a method applying concatemers to one isotopically labelling proteins on cookies, chocolate, and unbaked lyophilized cookie dough which were screened for egg (ovalbumin, ovotransferrin and vitellogenin-1), milk (αs1-casein and β-LG), peanut (Ara h 1 and Ara h 3), and hazelnut (Cor a 9) allergens. Although the former method gave the superior matrix-matched calibration curves (2.5-50 mg total allergen protein per kg of matrix) with a constant peak area ratio among matrices, the authors highlighted that future development of concatemer methods may be of benefit to food testing capabilities due to the more accurate alignment of the method with the sample extraction protocol.
2.4.4.4 PCR
Eggs are not a suitable material for PCR detection since eggs do not contain significant amounts of DNA for analysis. An entire egg contains only a single copy of DNA in the egg yolk and none in the egg white. Additionally, it is not possible to distinguish the results for chicken’s egg and chicken meat owing to their genetic similarity. Furthermore, it would be completely impossible to identify egg whites using PCR as, although rich in allergenic proteins, they do not contain any genetic material and therefore a false negative result would be obtained if the manufacturer was separating eggs for the recipe, which is a common practice.
2.4.4.5 Conclusions: Egg testing methods
These studies highlight the challenge of creating an ELISA which is sensitive to products containing cooked egg proteins. Firstly, it is essential that the method targets ovomucoid proteins as compared to ovalbumin proteins which can denature with heating (Williams, Westphal et al. 2004). The Morinaga ELISA kit performed well even in comparison to mass spectrometry and Gomaa et al. have speculated that this may be a result of better protein extraction through the use of denaturant and surfactant in the kit buffer (Gomaa and Boye 2013). Perhaps due to issues relating to reliably determining egg levels in processed matrices, LC-MS methodology is relatively advanced for egg allergens compared to for other allergens, with some LC-MS methods challenging the LOD of some of the egg ELISA kits. The availability of two different technologies capable for testing for egg allergen could offer a useful screen (ELISA) and confirmatory test (LC-MS) for this allergen and the differences between the technologies may offer consumers the benefits of allergen detection in a wider range of food types than other allergens for which only one testing technology exists.
2.4.5 Fish
2.4.5.1 Introduction
The major allergens in fish are parvalbumins which are calcium-binding proteins found in fish muscle, of which twelve different allergens have been identified. The prevalence of sensitivity to fish was found to be the lowest among 24 foods tested with just 0.2% of subjects showing signs of IgE mediated sensitivity. (Burney et al. 2010) Methods for the detection of fish allergens are often limited due to the wide variation of fish species. It is important to note that this review will not address allergic reactions to the fish parasite Anisakis simplex or scombroid poisoning (which can result from the improper storage of fish), but instead focus on IgE-mediate food allergies exclusively. (EFSA, 2014)
2.4.5.2
2.4.5.3 ELISA
The cross-reactivity of different parvalbumins was studied by Sharp et al. and immunochemistry methods were developed when antibodies were raised against parvalbumins from four different species of fish (barramundi, basa, pilchard and Atlantic salmon) (Sharp, Stephen et al. 2015). The greatest cross-reactivity was seen for barramundi parvalbumin antibodies with 87.5% of the 40 fish species analysed giving positive responses, however mahi mahi, swordfish and yellow tuna tested negative for each set of parvalbumin antibodies. This illustrates the challenge of creating an ELISA sensitive to all fish parvalbumins. The study additionally highlighted the reduced binding of allergens following thermal processing, a common challenge in allergen ELISA technology.
Three commercial fish ELISA kits were the subject of a study by Ruethers et al.: AgraQuant Fish ELISA kit from Romer Labs (UK, Austria) and Common Bone Fish Antigen EIA ELISA kit, versions 2 and 3 from XEMA (Russia) (Ruethers, Taki et al. 2020). For each ELISA, cod is the reference fish species, although the AgraQuant kit uses antibodies against parvalbumin, while the Common Bone Fish Antigen EIA kits use monoclonal antibodies against a protein of the muscular tropomyosin complex.
The results for each kit were not individually disclosed by the authors, but rather anonymised. Of 57 bony fish the detection rates of raw and heated ranged from 26% to 61%; for canned bony fish products the detection rate was 65% to 86%; and no cartilaginous fish detected. These results demonstrate the challenge still remaining within the food industry with the safety of fish products.
2.4.5.4 PCR
While research methods under development are mentioned in the literature (Kuehn, Hilger et al. 2017, Daga, Cau et al. 2018, Cau, Daga et al. 2022) and PCR methods are available for the species identification of fish in food, commercial manuscripts detailing commercial PCR kits for the purpose of fish allergen detection were not found in this review.
However, research behind the development of fish testing methods was reviewed by Dong and Raghavan, 2022. Processing methods such as application of heat and pressure to fish generally increases allergenicity, although there are examples where allergenicity increases with processing (for example, Sletten et al. 2010, Nugraha et al. 2021). While ELISA methods for fish detection tend to suffer reduced sensitivity with processing due to changes in protein biochemistry and stability (as reviewed by Dong and Raghavan, 2022), PCR methods tend show a more stable performance, especially in thermal treatments, since the DNA target is more robust in uncooked and cooked fish compared to the target proteins. PCR inhibitors can also be removed prior to analysis to improve the performance of PCR assays. For example, a real-time PCR method was used to detect cod and pollock with detection limits of 1-10 mg/kg. However, this work was not linked to commercial test kits, but rather research and development.
2.4.5.5 Mass spectrometry
Numerous fish allergens including parvalbumin, enolase, aldolase and vitellogenin have been reported (Kuehn, Swoboda et al. 2014) and parvalbumins beta (β-PVs) are identified as the major allergens. β-PVs are calcium-binding globular muscle protein consisting of two alpha helixes, having a molecular mass of 10–12 kDa and an acidic isoelectric point (pI) (3–5). Due to this structure, especially the Ca2+ binding site, β-PVs are resistant to tryptic digestion and heat treatment (Swoboda, Bugajska- Schretter et al. 2002).
Sun et al. 2019 developed an LC-MS MRM method to quantify β-PV in flounder (Paralichthys olivaceus), based on the detection of three peptides (Sun, Xu et al. 2019). Quantitative determination was based on one of these three peptides, that which provided the greatest sensitivity. The method was validated within the guidelines of European Medicines Agency (ICH Q2(R2)). Incurred matrices were prepared from the following species, each containing 10% flounder muscle: turbot (Scophthalmus maximus), brown-marbled grouper (Epinephelus fuscoguttatus), small yellow croaker (Pseudosciaena polyactis) and silver carp (Hypophthalmichthys molitrix), pork, shrimp, chicken muscle and beef. To validate the method, incurred matrices were prepared by the addition of 1.0 g of the minced muscle of flounder to
9.0 g non-contaminated minced matrices with homogenization. Validation studies showed having a linear range from 0.10 to 1179.36 nM with r > 0.999. The parvalbumin beta in flounder (Paralichthys olivaceus) has been quantified at a low level down to 0.10 µg/g with satisfactory precision (RSD < 18.35%) and accuracy (<13.3%). The new approach was successfully applied for the determination of parvalbumin beta in the other fish matrices. This method shows great promise to detect fish allergen in flounder and it would be interesting to understand how this method would perform on a much wider variety of fish consumed in the UK.
In a study aimed at determining fish species, work by Mazzeo et al. 2008 involved development of a method using matrix-assisted laser desorption/ionization-time of flight (MALDI-ToF) mass spectrometry, targeting species-specific peptides from 25 fish species (Mazzeo, De Giulio et al. 2008). Many of these peptides originated from parvalbumin, permitting the method to determine not only species, but also fish allergen proteins from cod (Gadus morhua), Merliccius genus, Trisopterus minutus, Sparus auratus and Evynnis japonica. Being an inherently multiplex method, MALDI- ToF lends itself well to identification of an allergen for which there are many different species and therefore different sequences for the allergenic protein. Another benefit of this method is, unlike for methods targeting one allergen sequence such as some ELISA methods, no prior knowledge of the species is required to confirm presence. It would be interesting to learn how this method would perform on cooked samples rather than the raw samples used, and whether more fish allergens can be determined by MALDI-ToF.
Similar to the above study, and using LC-MS with SRM and targeting parvalbumin beta, 12 β-PV biomarker peptides relating to fish species, along with five peptides which are shared with other organisms such as frogs, monotremes, lizards and birds, plus two peptides used for QC purposes (relating to poultry and frog species) were selected which represent 163 β-parvalbumin sequences (Carrera, Canas et al. 2012). The method was also applied to six thermally processed commercial fish products to challenge the heat-resistant properties of the allergen sequences. Using a heat-treated extraction method at 70 °C and ultrafast protein digestion using ultrasound, the entire method was reported to require only two hours which is a great benefit for an LC-MS method to test for allergens belonging to several fish species.
2.4.5.6 Conclusions – Fish testing methods
While it is evident, both from this review and the evidence in Table 1 (Appendix 1), that there is challenge with the more traditional methods of allergen detection in fish including ELISA, much progress is being made in mass spectrometry. Multiplex methods, using thermal-stable proteins, are under development and validation and it will be interesting to understand the scope of these methods in relation to the wide range of fish species commonly consumed in the UK.
2.4.6 Lupin (also known as lupine)
2.4.6.1 Introduction
Lupin (genus Lupinus) belongs to the Leguminosae family, and it can cause allergic reaction in susceptible individuals. Allergy to lupin is often reported in patients with allergy to other legumes, mainly peanut. Lupins contain seed storage proteins, including β-Conglutin which is considered the major lupin allergen, the structure of this is homologous to other major allergens Ara h 1 in peanut and soy β-conglycinin. (EFSA, 2014) The use of lupin, in particular lupin flour, in food products has vastly increased over the last decade and numerous analytical tests have been developed, some of which are commercially available.
2.4.6.2 ELISA
Koeberl et al. (Koeberl, Sharp et al. 2018) studied the ability of three commercial kits to detect three common species of lupin as well as their cross-reactivity to other species. Lupin residue detection kit (ELISA Systems®, Australia); RIDASCREEN® fast lupine (R-Biopharm®, Germany) and AgraQuant® lupine (Romer Labs®, Austria) were used but performance results were anonymised. The calculated lupin concentration for all samples tested varied for the different test kits, and the authors suggest that more comparable analytical methods and CRMs are needed. The study revealed that the levels of cross-reactivity to related legumes also differed across kits and did not necessarily match the clinical cross-reactivity, which the authors highlighted as an area where further research is required.
The ELISA tests NutriLinia Lupine-E (NC-6003/96, Nutricor s.r.o., Slovakia), RIDASCREEN®- FAST Lupine (R- Biopharm, Darmstadt, Germany) and Veratox Lupine (Neogen, Ayr, UK), were used by Röder et al. (Röder, Kleiner et al. 2013) in a study aiming to investigate the detectability of lupin from different cultivars. The authors reported extraordinarily large relative differences in quantitative response between cultivars of 390% - 5050%. The recovery of ‘lupin protein’ (information provided was limited other than this included γ-conglutin) varied extensively depending on the cultivar and across ELISA test kits, one showing particularly low recoveries with 11 out of 14 cultivars tested. This is highlighted in the paper as a limitation that may preclude accurate quantification, in particular when the inter- cultivar response is high and if the detected cultivar is unknown or RM is unavailable. The authors suggest that there is a need to generate data about the quantitative responses of methods to different cultivars, not only of lupin, but also other allergenic foods.
2.4.6.3 Mass Spectrometry
An LC-MS/MS method was reported by Mattarozzi et al. for the reliable identification and quantification of lupin major allergens (conglutinins) in pasta and biscuits (Mattarozzi, Bignardi et al. 2012). They established an LOD of around 2 ppm. The method was validated, obtaining good precision (both inter- and intra-day variability), relative standard deviation lower than 23% and recoveries of 95 ± 10 to 118 ± 12% and 103 ± 1 to 110 ± 12% for biscuits and pasta, respectively. The applicability of the method was tested successfully on market samples with lupine declared in the ingredients or on a precautionary label.
Huschek et al. developed a targeted LC-MS/MS method for the simultaneous analysis of soy, sesame and lupin using isotope-labelled peptides (Huschek, Bonick et al. 2016). The method included three peptides from the β-conglutin protein in white lupin. The performance of the method was evaluated by analysing six replicates of each sample, namely wheat flour, cookies and bread incurred with the allergenic foods. The LOQ achieved for lupin in food was 20 ppm (wheat flour), 10 ppm (cookie) and 50 ppm (bread) and the recovery rates were 113%, 91% and 72%, compared to 0.32 ppm lupin protein for current ELISA methods. The method is described as accredited with regards to DIN EN ISO/IEC 17025.
Another HPLC-MS/MS method was developed by Hoffmann et al. for the simultaneous screening of lupin, pea and soy proteins (Hoffmann, Munch et al. 2017). Four marker peptides were used for determination of lupin, with an LOD of 2 ppm when prepared in meat products. The authors reported that this sensitivity is comparable to that shown by commonly PCR and ELISA techniques and that it is significantly below the reference dose for lupin established by the VITAL Expert panel (50 mg if 100 g portion is consumed) (Taylor, Baumert et al. 2014).
2.4.6.4 Conclusions – lupin testing methods
A crucial challenge in lupin detection is that of cross reactivity with homologous legume proteins and as such the specificity of ELISA testing kits needs to be evaluated to ensure consistent reporting. Additionally the effect of changing cultivars is demonstrated to have a considerable impact quantitative response and this is certainly an area for future research. Mass spectrometry offers a promising alternative with comparable sensitivity to ELISA methods and good reproducibility.
2.4.7 Milk
2.4.7.1 Introduction
Although it is a common component of diets across the world, the protein content of milk can trigger an Ig-E mediated immunological reaction in affected individuals. The prevalence of milk allergies varies across ages, population and studies, but the self- reported prevalence in the UK was 2.7% in a 1994 study. (Young et al., 1994) Cow’s milk allergies can arise from multiple different proteins, caseins, alpha-lactalbumin (ALA), beta-lactoglobulin (β-LG), bovine serum albumin (BSA), immunoglobulin and lactoferrin.
2.4.7.2 ELISA and immunochemistry methods
The first available literature detailing the development of three ELISA kits for detecting casein, beta-lactoglobulin and ‘milk’ (“FASTKIT”) (Cosmo Bio Ltd, Japan) was published in 2004 by Akiyama et al. (Akiyama, Isuzugawa et al. 2004). Although this work is only published in Japanese, the abstract details that the LOD for each kit is 1 ng mL-1-, although and that each kit was used on a variety of matrices including sausage, sauce, cookie, cereal and pasta sauce, with a mean recovery over 40%. Although it must be noted that the method used to establish the LOD is not declared so it cannot be determined if this in matrix or a buffer solution.
A second generation of these kits was presented as the FASTKIT ELISA Ver II and the FASPEK® Allergenic Substances Detection Kit in work by Matsuda in 2006 (Matsuda, Yoshioka et al. 2006). The series of ELISA kits target egg, milk, wheat, buckwheat and peanut and the milk kit was tested by spiking into the following test materials: sausage, boiled beef, cookies, orange juice and strawberry jam. The mean recoveries, from a ring trail of ten laboratories, for all test materials were >50% which is low (ideally this would be 70-120% to meet AOAC guidelines), however the recoveries of milk proteins in cookies for the FASTKIT were only slightly under this level. The authors report cross-reactivity for both milk kits with both goat and sheep milk.
The effect of thermal processing on the detection of milk proteins using ELISA kits was investigated by Downs and Taylor in 2010. (Downs and Taylor 2010) This work was published in 2010 and while it uses three commercial ELISA kits which are still on the market, these kits are developed constantly, and results presented in this work may not represent current sensitivity. The kits used were Neogen Veratox Total Milk (Lansing, MI), ELISA Systems β-LG, and ELISA Systems casein (Windsor, Queensland, Australia). In this work, spiked dough was cooked by boiling (100 °C), baking (190 °C) frying (190 °C) and retorting (121 °C). Using the β-LG kit, poor recoveries of 2-10% were yielded for all processed samples. For the casein and total milk (casein and whey protein) kits, only boiled samples gave recoveries greater than 50%. These decreases in recovery with thermal processing are dramatic and the authors highlight the dangers to the food industry of these under-estimations of milk content.
ELISA kits for casein detection, namely R-Biopharm RIDASCREEN (R-Biopharm AG, Darmstadt, Germany) and the Neogen Veratox (Neogen Corp., Lansing, MI), were studied alongside flow cytometry for casein, egg, gluten, and soy sensitivity in baked cookies (Gomaa and Boye 2013). This work was published in 2013 and while it uses commercial ELISA kits which are still on the market, these kits are developed constantly, and results presented in this work may not represent current sensitivity. For unbaked cookies, recovery for all three methods was ≥90%. Recovery decreased with decreasing cookie size and increased baking time with the recovery range for Ridascreen 89-35%, for Veratox 77-21% and the flow cytometry 75-19%. These results are in line with those observed by Downs and Taylor, indicating that while a positive response was always obtained for the samples, testing methods can struggle to detect casein following thermal processing (Downs and Taylor 2010).
In a major cross-laboratory study of ELISA detection of egg and milk allergens reported in 2014, five casein analysis kits, four kits which targeted beta-lactoglobulin (β-LG) and one kit which determined total milk were compared (Johnson, Rigby et al. 2014). These kits were ELISA Systems casein ESCASPRD-48 (Windsor, Queensland, Australia); Neogen 8460 Veratox casein (Lansing, MI); Morinaga Casein 10152 (Yokohama, Japan); R-Biopharm RIDASCREEN FAST casein kit R4612 (Darmstadt, Germany); Romer AgraQuant casein COKAL1200 (Cheshire, UK), ELISA systems β-LG ESMRDBLG-48 (Windsor, Queensland, Australia); Neogen 8470- Veratox for total milk (Lansing, MI); Morinaga beta-lactoglobulin 10172 (Yokohama, Japan); R-Biopharm RIDASCREEN FAST beta-lactoglobulin kit R4902 (Darmstadt, Germany); Romer AgraQuant Beta-lactoglobulin assay COKAL1048 (Cheshire, UK); Zeu ZE/PR/LS: proteon beta-lactoglobulin (Zaragoza, Spain). The matrix was dessert mix spiked with allergen in the form of skimmed milk powder, spiked at 3, 6, 15 and 30 mg/kg. For the trial, the data for all kits were anonymised. All kits were used to detect the lowest level of milk protein, 3 mg/kg, however all kits under-reported the level of skimmed milk powder (except one which consistently over-estimated milk levels). The aim of this work was to generate a
naturally incurred QC material for food allergen analysis and authors recognise that a more robust material may be required that can challenge detection following processing procedures such as heat, pressure and pH changes which can alter protein binding.
Studying both ELISA and LC-MS/MS methods, Parker et al. produced industry- processed model foods incurred with egg, milk and peanut allergens (Parker, Khuda et al. 2015). The ELISAs used in this work were Morinaga Milk (Yokohama, Japan); Neogen Veratox Milk (Lansing, MI, USA); and R-Biopharm RIDASCREEN FAST Milk (Darmstadt, Germany). These kits were compared in 2015 and their composition may be different to those commercially available currently. When tested against an unprocessed cereal bar sample, both Morinaga and Neogen kits slightly underestimated the milk content, while the R-Biopharm overestimated. Recovery levels for all kits were all higher for a muffin matrix. Baking the samples resulted in reduced recoveries, this was more pronounced for the Neogen kit with a recovery of 17.3% for the muffin final product, compared to 93.8% for the Morinaga product. A crucial difference between the kits is the detection of casein (the Morinaga kit) or casein and β-LG (R-biopharm) compared to total milk protein (Neogen). In milk, the proteins with the greatest heat stability are casein and serum albumin and consequently kits which target these proteins rather than whey proteins demonstrate better detection of thermally processed milk (Nowak-Wegrzyn and Fiocchi 2009). The mass spectrometry method targeted an alphaS1-casein and a β-LG peptide marker for quantitation with secondary peptides used for confirmation. Stable isotope-labelled reference peptides were used for confirmation and concentration calculations and the recoveries of milk from both unprocessed and thermally processed products were >50%. The comparison of methods suggest that the target protein is crucial to method development with casein being less impacted by thermal processing in comparison to the total milk protein content. Additionally, the mass spectrometry method shows less variance between the cereal bar and muffin matrices and consequently may be more tolerant to a range of matrices, although there is not enough evidence in this paper to conclude this.
The use of ELISA kits for the detection of milk in cheese was investigated by Ivens et al. (Ivens, Baumert et al. 2017(1), Ivens, Baumert et al. 2017(2)). At the time of publication (2017) commercial ELISA kits were not validated for the detection of milk residues in hydrolysed or fermented food products. The ELISA kits used in this kit were Neogen Veratox Total Milk and Casein ELISA (Neogen Corp., Lansing, Mich., U.S.A.) ELISA Systems™ Casein kits from ELISA Systems (Windsor, Queensland, Australia). R-Biopharm RIDASCREEN Fast Casein kits (Darmstadt, Germany), the kits tested were commercially available at the time of publication, but their composition and sensitivity may have changed in the interim. During cheese production the whey, which contains the proteins α-lactalbumin and β-lactoglobulin, is separated so the key allergens of milk are the caseins, of which alpha, beta and kappa are considered major allergens. Therefore, to provide meaningful insight into the allergenicity of cheese, the ELISA kit must detect casein. In this work, the R- Biopharm and Neogen Veratox Total Milk kits primarily detected kappa-casein, while the ELISA Systems Casein kit detected only alpha-casein and the Neogen Veratox Casein Kit detected alpha and beta casein. Of the cheeses studied (Mozzarella, Swiss, Blue, Limburger and Brie) the blue cheese was the hardest to detect with all alpha- and beta-casein hydrolysed and milk was only detected in this matrix using the Neogen Casein and Total Milk kits. The authors suggest that while milk residues are detectable in cheese by ELISA kits, it would be important to select the right kit for the level of proteolysis the milk has undergone and they stress that it is possible that there are fragments of proteolysed casein in processed milk which may not be detectable by modern ELISA kits.
Otto et al. 2016 reported an immunoassay using flow cytometry for the detection of milk based on polyclonal antibodies raised to purified casein (Otto, Lamote et al. 2016). The lowest concentrations of contaminants inducing a significant difference of signal between non-contaminated controls and milk test samples was 5 mg/kg of milk and therefore an improvement in the method is required to meet the VITAL threshold of 2 ppm (Taylor, Baumert et al. 2014). The alpha-casein antibodies cross- reacted with apple (0.7). It would be interesting to learn the results of a full validation of this method.
The development of a CRM for milk detection was part of work from the JRC in Geel. It was their aim to improve the harmonisation of reported milk levels, following observations that in a ring trial detecting total cow’s milk protein in baked cookies reported levels were significantly varied. (Cordeiro et al., 2021) A reference method was created to characterise a reference material in work from Martinez-Esteso et al. and given an indicative value from this method. (Martinez-Esteso et al., 2020) In this work the quantity is expressed as “mass of total allergen protein per mass of food” and SI traceability is used to allow comparison between results, this was established by Breidbach et al. (Breidbach et al., 2022) In a ring trail of 23 EU laboratories using principally ELISA methods of detection, a large scatter of results was reported, even where the same ELISA kit was used. (Cordeiro et al., 2021) This suggests that the instructions provided with ELISA kits need to provide more unambiguous instructions. An additional point was raised by the authors that several labs reported using the standard reference material NIST 1549a, a whole milk powder which is supplied with only a “informative (non-certified) protein value”. As it was not intended for allergen detection, it is not suitable for use to calibrate or validate a method for total cow’s milk protein, highlighting the importance of appropriate CRMs.
2.4.7.3 PCR
Currently there are no available commercial kits for the detection of milk allergens. This may be in part due to the fact that there is no difference between the DNA found in milk products and bovine tissues and therefore no test could provide confidence that milk allergens are present.
2.4.7.4 Mass Spectrometry
The aim of Christina et al. in their work on LC-MS/MS detection of milk in baked food products was to create a robust method as a confirmatory technique alongside the use of ELISA kits (Cristina, Elena et al. 2016). The target peptides were selected from the alphaS1 casein protein and the method was validated with baked incurred cookies with powdered milk (SRM-1549). An LOD of 1.3 mg kg-1 and LOQ of 4 mg kg-1 was established, which is not as sensitive as ELISA technology and recoveries varied from 20-26%.
As a follow-up to their previous methods, Pilolli et al. (2017) developed an SRM multiplex LC-MS method using both incurred cookie samples and spiked cookie samples (Pilolli, De Angelis et al. 2017). The LOD was 7 µg milk allergen(alpha-1 casein) per gramme of food. By comparing the levels of allergens detected in the incurred samples compared to those for the spike samples, the authors were able to determine the effect of processing on the level of detection of the allergens. Figure 1 clearly shows how the detection is dramatically reduced for each of the five allergens under investigation when the samples are processed.
In work comparing the effectiveness of ELISA kits against an MS method for milk detection, Heick et al. identified a milk peptide which could be detected in both flour and baked bread products with the recovery of milk 45% of that in bread compared to the unprocessed flour (Heick, Fischer et al. 2011). For the two ELISA kits investigated R-Biopharm RIDASCREEN FAST Casein (Darmstadt, Germany) and Neogen Tepnel Biokits Casein Assay (Lansing, MI) (which were anonymised in the study), the responses were significantly lower with one kit unable to detect any milk in the processed product and the other with a recovery of 17%. It is important to note that while these are commercially available kits their contents may have changed since these tests were performed. In this work, development of a LC-MS MRM multianalyte method was also reported, for which the LOD for milk, based on detection of casein alpha S1 peptides, was 5 µg/g in incurred bread with a correlation coefficient of 0.8985.
In a method targeting milk (alongside egg, soy, hazelnut and peanut), Pilolli et al. determined a LOD of 7 mg kg-1 using spiked cookie dough as a matrix, however this sensitivity dropped by 80% when the cookie dough was baked, again highlighting the challenges of different matrices and incurred allergen detection (Pilolli, De Angelis et al. 2017).
Peptide markers for both alphaS1-casein and β-LG were identified for mass spectrometer analysis by Bianco et al. to monitor both casein and whey fractions of milk (Bianco, Calvano et al. 2022). With good recovery and precision reported, the method was used to detect levels of milk protein, casein, in meat products which were labelled as milk-free at levels 10-fold higher than the action level of these allergens. Although using spiked rather than incurred samples, Monaci et al. 2014 applied an SRM method to determine milk, capable of detecting 0.1 µg alpha-S1 casein (Monaci, Pilolli et al. 2014).
As described above, Gavage et al. highlighted that future development of concatemer MRM mass spectrometry methods may be of benefit to food testing capabilities due to the more accurate alignment of the method with the sample extraction protocol and their initial work involved egg, milk, peanut and hazelnut allergens.
2.4.7.5 Conclusions – Milk testing methods
Modern research is highlighting the challenge in detecting milk proteins in food products with the selection of target allergenic proteins crucial to method development. The effect of thermal processing is greater on proteins such as β-LG, compared to casein, and therefore the method which is most appropriate will vary based on the matrix of the samples. This is particularly appropriate for the detection of cheese as, during the cheesemaking process, the whey is removed and so a kit which targets β-LG would not be appropriate for this application. Improvements regarding recovery levels are required if ELISA kits continue to be applied to determine milk levels in foods, particularly in processed foods. There are clear examples where the use of multiple ELISA kits, or the use of ELISA with LC-MS as a confirmatory test when ELISA data is negative, reaps rewards in the detection of milk allergens. It seems that future development is required to improve the sensitivity of LC-MS methods to detect allergens near the action levels and more data regarding the variance of LC-MS data for a wider range of matrices would be beneficial in determining the suitability of this methodology.
2.4.8 Molluscs
2.4.8.1 Introduction
The animal group ‘shellfish’ comprises two invertebrate phyla; arthropods and molluscs. Although all shellfish are invertebrate animals, these two groups are very distinct in evolutionary terms and subsequently contain different molecular repertoires of allergenic proteins. Co-sensitisation of patients with crustacean and mollusc allergy is often described, however. Sensitising to molluscs and crustaceans can occur due to inhalation of house-dust mite, with paramyosin being the cross- reactive allergen (Yu, Ding et al. 2022). Consequently, mollusc allergy is clinically under-reported and allergens are ill-defined.
2.4.8.2 ELISA and immunochemistry methods
In a 2018 clinical study by Rolland et al., allergens of four frequently ingested Asia- Pacific molluscs were characterized: Sydney rock oyster (Saccostrea glomerata), blue mussel (Mytilus edulis), saucer scallop (Amusium balloti), and southern calamari (Sepioteuthis australis) (Rolland, Varese et al. 2018). Examining cross- reactivity between species and with blue swimmer crab tropomyosin, Por p 1. Unlike for detection of many other allergens, in clinical studies, patient serum IgE antibody studies showed that cooking increased IgE reactivity of mollusc extracts, suggesting that any testing methods developed with these antibodies would apply well to cooked molluscs. Immunoblotting and mass spectrometry analysis identified the allergenic proteins, including one corresponding to heat-stable tropomyosin in all species (37- 40 kDa). IgE-reactive Sydney rock oyster proteins were identified by mass spectrometry, and the novel major oyster tropomyosin allergen was cloned, sequenced, and designated Sac g 1. Oyster extracts showed highest IgE cross- reactivity with other molluscs, while mussel cross-reactivity was weakest. Inhibition immunoblotting demonstrated high cross-reactivity between tropomyosins of mollusc and crustacean species. While this work did not implement testing methods used in food analysis, the method demonstrates the potential to raise antibodies to support development of ELISA tests to screen for heat-stable mollusc allergens.
Kamath et al. 2013 also reported increased recognition of multiple tropomyosin monoclonal antibodies upon heating of shellfish (Kamath, Rahman et al. 2013). These authors also reported cross-reactivity of tropomyosin in all 11 crustacean species, with partial detection in molluscs (cross-reacting with mussels, scallops and snails but not in oyster, octopus and squid). The authors conclude that specific monoclonal antibodies, targeting the N-terminal region of tropomyosin, must be developed to differentiate tropomyosins in crustaceans and molluscs.
2.4.8.3 PCR
Suh et al. 2020 reported a multiplex PCR method for the detection of amplicons of tropomyosin genes for each of oyster (Crassostrea gigas), mussel (Mytilus edulis), abalone (Haliotis discus hannai) and clam (Ruditapes philippinarum ) against a reference set of eight seafood types in total plus eukaryote tropomyosin, with a detection limit of 16 pg of target DNA and was successfully applied to the detection of tropomyosin in 19 processed seafood products in the Republic of Korea including raw, frozen and dried seafood as well as seasoned, porridge and canned seafood products (Suh, Kim et al. 2020). No cross-reactivity was found.
2.4.8.4 Conclusion – Molluscs testing methods
As shown in Table 1 (Appendix 1), methods to detect molluscs by ELISA and PCR techniques are commercially available. No further available methods, or suitable mass spectrometry methods under development, were identified. This reveals a lack of research in this area and a lack of method comparisons to determine method suitability.
2.4.9 Mustard
2.4.9.1 Introduction
Mustard is an edible plant which belongs to the Brassicaceae family, with its seeds commonly used both in cuisine and processed foods, with different seeds combined to make different regional varieties of mustard powder. The most common seeds are white/yellow (Sinapis alba L.), black (Brassica nigra L.) and brown/oriental mustard (Brassica juncea L.). The prevalence of sensitisation can be varied, across reports levels were as low as1% to as high as 28% in children in France. (Moneret-Vautrin D et al., 1983; Rancé F et al., 1999) The characterised allergens are seed storage proteins, in white/yellow mustard (Sinapis alba L.) these are Sin a 1-4 and in brown/oriental mustard (Brassica juncea L.) the allergen Bra j 1 is found. (EFSA, 2014)
2.4.9.2 ELISA, immunochemistry and PCR methods
No peer-reviewed data regarding testing methods used by participants in Fapas® proficiency testing rounds were identified. The performance of two ELISA tests, Mustard ELISA Kit-specific and Mustard ELISA Kit-total (SEDIUM R&D, Pardubice- Nemosice, Czech Republic) was investigated by Palle-Reisch et al. (Palle-Reisch, Hochegger et al. 2015) and compared with real-time PCR methods for the analysis of mustard in two sets of model sausages, both raw and cooked at 75–78 °C for 15 min in a water bath. The sausages in set 1 contained 1 ppm of each white and black mustard and set 2 contained 1 ppm of each white and brown mustard. Applicability to commercial samples was studied by analysing 15 food products.
The Mustard ELISA Kit-total, intended for the quantitative determination of white, black and brown mustard, allowed the detection of mustard in raw and brewed model sausages down to 2 ppm. The LOQ was determined to be 10 ppm mustard in set 1, corresponding to a mustard protein concentration of 2.4 ppm. In raw and brewed model sausages of set 2, the LOQ was found to be 2 ppm mustard, corresponding to 0.5 ppm mustard protein. In raw sausages, the recovery ranged from 93.2% to
113.1% (set 1) and from 104.3% to 129.0% (set 2). In brewed sausages, recovery was in the range from 71.7% to 83.3% (set 1) and 113.0% to 170.8% (set 2).
The Mustard ELISA Kit-specific, targeted for the quantitative determination of white mustard, detected and quantify white mustard down 1 ppm, which corresponds to a white mustard protein concentration of 0.27 ppm (in the presence of 1 ppm black or brown mustard) in both raw and brewed sausages. In general, the Mustard ELISA Kit-specific resulted in recoveries between 46.1% and 70.4% in raw and between 43.1% and 84.3% in brewed sausages.
The analysis of commercial samples carried out by Palle-Reisch et al. showed discrepancy between the levels that the two kits could detect or quantify in a few of the samples, although there was agreement in most cases (Palle-Reisch, Hochegger et al. 2015). However, the sample number was small and the mustard content was very low, with only two products presenting quantifiable levels for only one of the kits (Kit-total). Overall, there was good correlation between ELISA and PCR results, although the ELISA kits demonstrated higher sensitivity than real-time PCR.
Luber at al 2015 reported the development of a tetraplex real-time PCR method for soybean, celery and brown and white mustard. The approach was validated with DNA extracted from lysate mixtures of boiled sausage. The parameters recovery, repeatability and robustness were evaluated and the limits of quantification of brown and white mustard were 2.6 mg/kg and 36.8 mg/kg respectively.
As detailed previously, Otto et al., 2016 reported an immunoassay for the five analytes, including mustard, based on a combination of flow cytometry with competitive ELISA where microbeads were used as sorbent surface (Otto, Lamote et al. 2016). The polyclonal antibodies used to detect mustard were raised against Sinapis alba. The lowest concentrations of mustard detectable was 5 mg/kg which is a great deal higher than the 0.1 ppm VITAL threshold (Taylor, Baumert et al. 2014). The mustard antibodies cross-reacted with rapeseed, Brassica napus (100%) but unexpectedly did not cross-react with turnip oil (Brassica rapa). Cross-reactivities between members of the Brassica genus (B. napus, B. rapa, Brassica oleracea, B.nigra, B. juncea and S. alba) have been reported in the past due to genetic homology. The authors stated that flow-cytometry-based immunodetection may, in the near future, improve upon the performances of classic ELISAs by adding a new feature: simultaneous detection/quantification of multiple allergens.
2.4.9.3 Mass spectrometry
An LC-MS/MS method for analysis of mustard in food was published by Posada- Ayala et al. in 2015 (Posada-Ayala, Alvarez-Llamas et al. 2015). The method targets the storage protein Sin a 1, one of the major allergenic proteins in yellow mustard seed, and it is based on selected reaction monitoring (SRM) of five marker peptides. Three of these peptides and their transitions showed good reproducibility and suitability for allergen quantification. The authors determined an LOD of 0.25 ppm and an LOQ of 0.75 ppm. Method applicability was tested in seven different commercial food products, where Sin a 1 was quantified at 19±3 mg/kg of food.
2.4.9.4 Conclusions – Mustard testing methods
Information relating to methods used in Fapas® proficiency testing rounds was limited (mustard is a relatively recent addition to the proficiency testing programme). The scope and size of studies described by the authors above was also limited.
Further information is required to determine the suitability of tests to determine the various mustard species. It is crucial to ensure that methods do not give false positive results, as was recently the case for the seeming detection of mustard in wheat, which was subsequently determined to be Sinapis arvensis or rapeseed (Remington et al., 2022).
2.4.10 Peanut
2.4.10.1 Introduction
Despite its name, peanut (Arachis hypogea) is a legume rather than a tree nut and is a common cause of allergy in the UK. The legume family, which also includes pea, bean, soybean, lupin, lentil and fenugreek. The prevalence of peanut allergy has been reported at as high as 15% of self reported individuals or as low as 0% among 18-month old children in Iceland (Touraine, 2002; Kristjansson, 1999). Peanut proteins, even in very small quantities, in the range of mg/kg, can cause extremely severe allergic reactions in individuals and therefore the detection of residues has been a major focus of the scientific community. Peanut is typically considered to be one of the most common Ig-E mediated allergies. Peanuts are high in protein content (23-27% by weight) and contain 50 different proteins, of these 13 have been characterised as causing Ig-E mediated responses in allergic individuals called Ara h 1-13. Of these Ara h 1, Ara h 2 and Ara h 3 are considered the major peanut allergens, however regional variations in populations mean that whilst these are the most common sensitisers in the USA, in Spain Ara h 9 and in Sweden Ara h 8 are more common triggers for sensitised patients. (EFSA, 2014) Methodologies described in the literature for the detection of peanut in food are described herein.
2.4.10.2 ELISA
Processing of foods can have a detrimental effect on the detection of peanut and extraction protocols may benefit from optimisation (Khuda, Jackson et al. 2015). The RIDASCREEN Fast Peanut ELISA (R-Biopharm AG, Darmstadt, Germany) was evaluated under the AOAC Research Institute to gain the Performance Tested Method Status in 2003 (Immer, Reck et al. 2004). Across a range of matrices, including chocolate and ice cream, recovery averaged 97%, with an LOD of 1.5 ppm and an LOQ of 2.5 ppm. Additionally, no cross reactivity was found against 34 substances. By using the AOAC evaluation programme different ELISAs can be more easily compared against each other and any gaps in their capabilities can be more easily identified.
Almost twenty years later the RIDASCREEN Peanut ELISA (R-Biopharm AG, Darmstadt, Germany) was evaluated against the AOAC Standard Method Performance Requirement 2017.020, a more comprehensive test build upon the VITAL methodology which applies real-world eliciting dose values to testing. (Lacorn, Dubois et al. 2022a) tested against 91 substances, no cross-reactivity of the ELISA kit was found, the LOD was estimated to be 0.45 mg kg-1 and an LOQ of 0.75 mg kg- 1 of ‘total peanut’ was determined with mean recoveries for incurred cookies and milk chocolate in an independent laboratory between 99 and 104%. Comparing these two reports demonstrates both the increased sensitivity of these kits but also the increased stringency of the accreditation with more substances evaluated for cross contamination, the use of incurred samples as matrices and increased focus on repeatability.
The National Institute of Standards and Technology (NIST) have a large portfolio of Standard Reference Material (SRM) which is used across all scientific measurement services. In terms of SRMs suitable to support allergen testing services, the NIST SRM 2387, peanut butter, was used to evaluate the performance of Veratox for Peanut Allergen Test (Neogen Corp., Lansing, MI), Ridascreen Peanut (R-Biopharm AG, Darmstadt, Germany), and Bio-Kit Peanut Protein Assay Kit (Tepnel, Neogen Corp., Lansing, MI) (this work was published in 2004 so if these kits are still available on the market they may contain different components) (Trucksess, Brewer et al.
2004). The RM was used in a suspension and recoveries varied from 94-107% for the Veratox kit, from 55-60% for the Ridascreen kit and from 86-94% for the BioKit. When the Veratox and Ridascreen kits were used with spiked foods (ice cream, cookies, and breakfast cereals) they gave recoveries of 85-111% and 60-81% respectively. Whilst these were not incurred samples this work demonstrated the capability of commercially available ELISA kits. These three kits, except for the Ridascreen Fast Peanut being used instead of the Ridascreen Peanut variation, were used in a study by Park et al. in 2005 (Park, Coates et al. 2005). Using the AOAC Performance Tested Method, these three kits were evaluated at three laboratories and successfully identified 60 samples spiked with peanut and 60 blank samples. This work recommended that two kits be used in conjunction to give greater confidence in results. Cross- reactivity to 32 substances was also investigated, with the Ridascreen kit being cross-reactive to chickpeas, green peas, and lima beans.
Four ELISA kits were compared in work from Whitaker et al., namely the ProLisa Peanut Pak Kit (ProLab Diagnostics, Ontario Canada), the Veratox Peanut Allergen Test (Neogen Corp., Lansing, MI), the RIDASCREEN Peanut (R-Biopharm AG Darmstadt, Germany), and the Bio-Kit Peanut Protein Assay Kit (ELISA Technologies, distributor, Gainesville, FL) (this work was published in 2005 so if these kits are still available on the market they may contain different components) (Whitaker, Williams et al. 2005). Highlighting the lack of commercially available peanut protein reference standards as a reason for variations in measured protein levels between kits the study reported that across four matrices (breakfast cereal, milk chocolate, ice cream and cookies) and spiking levels (0-10 μg/g) no kit was the most precise or the most accurate, however for incurred samples Neogen and R- Biopharm kits were the most accurate and precise test kits, respectively.
For their study comparing commercial ELISA kits to a newly developed MS method for detecting incurred allergens in bread, Heick et al. used the Ridascreen Fast Peanut (R-Biopharm AG, Darmstadt, Germany), and Bio-Kit Peanut Protein Assay Kit (Tepnel, Neogen Corp., Lansing, MI), although they anonymised the results so as to not provide a direct comparison between kits (this work was published in 2011 so if these kits are still available on the market they may contain different components) (Heick, Fischer et al. 2011). Both ELISA kits detected the peanut in the raw and incurred samples with no loss of sensitivity in thermal processing, however for the MS method the peak signal decreased with baking, suggesting that either the peptide was less extractable or experienced chemical modification during thermal processing. Thermal processing was the focus of work by Fu et al. (Fu and Maks 2013) comparing the Veratox Peanut Allergen Test (Neogen Corp., Lansing, MI) which assesses total peanut proteins and Bio-Kit Peanut Protein Assay Kit (Neogen Corp., Lansing, MI) which employs antibodies specific to the marker protein Ara h 1. A BCA total protein assay was used to determine the effect of heating on protein content which revealed that when heating by boiling or autoclaving the protein extracted decreased by 50%, however dry heating to 100 to 120 °C did not have a significant effect on the extractability, although this decreased at higher temperatures. Both ELISA kits underestimated the level of peanut protein at temperatures above mild conditions, the Bio-Kit gave lower estimations of peanut protein content which can be explained by the fact that it targets Ara h 1 which has been found to be relatively heat labile (Koppelman, Bruijnzeel-Koomen et al. 1999). The authors of this study suggest that ELISA kits should be designed to use target markers which are structurally and immunochemically stable and users of ELISA kits need to be aware of any limitations to minimise the risk of allergens not being detected. This is not always made clear to users in the instruction manual, for example that raw foods are often more suitable for allergen detection by certain kits compared to more processed foods.
Working to identify appropriate peanut proteins for targeting by ELISA kits, Jayasena et al. used the SRM 2387 (peanut butter) to determine the specificity of each kit to Ara h 1 Ara h 2, Ara h 3 and Ara h 6 (Jayasena, Smits et al. 2015). The six ELISA kits used in this study were Veratox for peanut allergen (Neogen, Lansing, MI, USA); BioKits peanut assay kit (Neogen, Lansing, MI, USA); AgraQuant peanut assay (Romer Laboratories UK Ltd.); RIDASCREEN fast peanut (R-Biopharm, Germany); Peanut protein ELISA kit (Morinaga Institute of Biological Science, Inc. Japan); and Peanut residue (ELISA Systems Pty Ltd. Australia). This work was published in 2015 so if these kits are still available on the market they may contain different components. All kits, except the Morinaga, had the greatest sensitivity for Ara h 3 while the Morinaga was most sensitive to Ara h 2 and Ara h 6. The authors note that while the Ara h 3 is the most abundant protein in peanuts and therefore a good indicator of the presence of peanut, the proteins Ara h 2 and Ara h 6 are the most potent allergens and less susceptible to denaturation and aggregation. This would suggest that the allergens Ara h 2 and Ara h 6 would be better target proteins for ELISA kits for processed food products, although this work demonstrated that all kits recovered peanut from the standard RM.
Studying both ELISA and LC-MS/MS methods, Parker et al. produced industry- processed model foods incurred with egg, milk and peanut allergens (Parker, Khuda et al. 2015). The ELISAs used in this work were ELISA Systems Peanut (Queensland, Australia); Morinaga Peanut (Yokohama, Japan); Neogen Veratox Peanut (Lansing, MI, USA); and R-Biopharm RIDASCREEN FAST Peanut (Darmstadt, Germany), (this work was published in 2015 so if these kits are still available on the market they may contain different components). The samples, cereal bars and muffins, were incurred with dark roast peanut flour with all ELISA kits giving recoveries less than 40% for the cereal bar and less than 30% for the muffins. An LC-MS/MS method developed in this work gave far better recoveries at 60 and 70% for the two sample types respectively.
Six different ELISA kits: Veratox® for peanut allergen and BioKits peanut assay kit (Neogen, Lansing, MI, USA); AgraQuant® peanut assay (Romer Laboratories UK Ltd.); RIDASCREEN® FAST peanut (R-Biopharm, Germany); Peanut residue (ELISA Systems Pty Ltd. Australia); and Peanut ELISA kit (Morinaga Institute of Biological Science, Inc. Japan) were assessed against peanut flours with increasing levels of roasting (this work was published in 2019 so if these kits are still available on the market they may contain different components) (Jayasena, Koppelman et al. 2019). This work found that for the minimally processed samples the ELISA kits from Romer, R-Biopharm and Veratox were the most sensitive, with increased thermal processing the Morinaga kit showed highest sensitivity, particularly at low concentrations. A decrease in sensitivity with thermal processing was seen across all ELISA kits and this is likely to result from the reduced solubility and reduced reactivity of the target proteins. This work again demonstrates the advantages of targeting the Ara h 2 protein where a product is likely to contain dark roasted peanut flours and communicating this to the food industry should help ensure that an ELISA kit with sufficient sensitivity for a sample is used.
The effect of changing the extraction buffer in commercial ELISA kits was investigated by Jayasena et al., the kits used in this work were Veratox® for peanut allergen (Neogen, Lansing, MI, USA); and Peanut ELISA kit (Morinaga Institute of Biological Science, Inc. Japan) (this work was published in 2019 so if these kits are still available on the market, they may contain different components) (Jayasena, Wijeratne et al. 2019). For unprocessed samples the Veratox kit gave higher recoveries than the Morinaga kit and these were increased further with the buffers developed in this work Na2CO3, pH 9.6 and PBS containing 1 M Guanidine Hydrochloride, however the Morinaga consistently gave the highest recoveries with the buffer included in the kit. When the samples were more intensively processed the Morinaga kit gave better recoveries than the Veratox kit and across most thermal processing methods its included extraction buffer gave the best recoveries. This suggests that the extraction buffer supplied with the Morinaga kit is well suited to peanut detection and additionally that the crucial factor in good sensitivity to thermally processed peanut allergens is the detection of heat stable allergens, in this case namely Ara 2 h.
As reported by Holzhauser (Holzhauser, Johnson et al. 2020), information provided by ELISA kit manufacturers and peer reviewed assessments of commercially available ELISA kits suggest that most of the commercially available ELISA kits that were reviewed are able to detect peanut protein to the levels required to measure the VITAL 2.0 reference dose at the smallest portion size of 5 g, which equates to a 40 mg kg−1 of peanut protein per food (Koch, Schappi et al. 2003, Park, Coates et al. 2005, Poms, Agazzi et al. 2005, Whitaker, Williams et al. 2005, Jayasena, Smits et al. 2015, Parker, Khuda et al. 2015). In support of this, a number of peer reviewed publications using allergen incurred matrices have demonstrated the detection of peanut at 5 mg kg−1, (Whitaker, Williams et al. 2005, Khuda, Slate et al. 2012) and at 0.625 mg kg−1 (Poms, Agazzi et al. 2005).
In 2005, Park et al. compared the Veratox for Peanut Allergen Assay manufactured by Neogen Corporation (Lansing, MI), the RIDASCREEN FAST Peanut Assay manufactured by R-Biopharm, and the BioKits Peanut Assay Kit manufactured by Tepnel BioSystems in an inter-laboratory trial. All kits were capable of detecting 5 ppm peanut in all samples, which were food matrices spiked with peanut butter. In samples containing high levels of peanut, the RIDASCREEN FAST Peanut kit showed cross-reactivity with green peas, chickpeas, lima bean, however no such cross-reactivity was observed when the extracts were diluted to 100 ppm. It is important to note that this peanut testing kit may have been further developed since 2005. This inter-lab validation would have benefited from the use of incurred samples to determine the effect of thermal processing.
The results of an inter-laboratory study by Poms et al. 2005 with five commercially available peanut ELISA test kits to detect and quantify peanut residues in an incurred biscuit sample and a spiked biscuit and dark chocolate at four different concentrations (0–10 mg peanut per kg matrix corresponding to about 0–2.5 mg peanut protein per kg matrix) are reported (Poms, Agazzi et al. 2005). The test kits were Biokits Peanut Assay Kit from (Tepnel Biosystems,UK), targeting Ara h 1, Peanut Residue ELISA Kit from Elisa Systems (Australia), targeting Ara h 2, Prolisa Peanut PAK ELISA Kit from Pro-Lab Diagnostics (Canada), targeting total soluble peanut protein, Ridascreen Peanut from R-Biopharm (Germany), targeting total soluble peanut protein and Veratox from Neogen (USA), targeting total soluble peanut protein. All kits showed false negatives in biscuit, at a rate varying from 1.9 to 18%. Three of the kits showed false negatives to dark chocolate, at a rate of 5.9- 25.5% but two kits detected zero false negatives. The authors reported that generally all five commercially available ELISA test kits investigated performed well in the concentration range 5–10 mg/kg rather than in the low concentration range (2 or 2.5 mg/kg). The variation in the recoveries of peanut between the different test kits varied between 44–191% across all concentrations. It is important to note that all five kits may have evolved since this study in 2005 and their performance may be very different using the current versions of the kits.
2.4.10.3 PCR
A multiplex PCR method was developed by Engler-Blum et al. which has a practical detection limit of 10 mg kg-1 for both peanuts and hazelnut with no other cross- reactivities found (Engler-Blum, Raiss et al. 2007). Commercially the R-Biopharm SureFood Congen advertises an LOQ of 1 mg kg-1 and reports no cross reactivities.
2.4.10.4 Lateral Flow Tests (LFT)
The Reveal 3-D for Peanut test (Neogen, Lansing, MI) was validated against the AOAC Performance Tested Method 111901, this is an extensive test which assesses robustness, selectivity, cross-reactivity against 29 common food commodities, and matrix tolerance (Le, Vance et al. 2020). The total peanut detection level in CIP rinses ranged from 2-4 ppm and environmental surface swabs at a range of 3-4 µg/100 cm2. These kits are designed for use in detecting allergens in clean-in-place rinses and environmental swabs to provide confidence in allergen risk management in the food manufacturing industry.
2.4.10.5 Mass Spectrometry
A challenge of cross-reactivity in ELISA kits was demonstrated in work by Daly et al. whereby an ELISA kit from an undisclosed company detected both almonds and peanuts in an allergen-free product (Daly, Ansari et al. 2018). The ELISA kit AgraQuant peanut assay (Romer Laboratories UK Ltd.) also found a significantly lower concentration of peanut in the sample, however an LC/MS-MS method found no peanut protein in the sample. The authors concluded that it was possible that both ELISAs showed degrees of cross-reactivity to another similar protein in the sample, although they could not rule out that the LOD of the MS method was too high to detect the peanut levels in this matrix.
As a follow-up to their previous methods, Pilolli et al. (2017) developed an SRM LC- MS method using both incurred cookie samples and spiked cookie samples (Pilolli, De Angelis et al. 2017). The LOD was 13 µg peanut allergen (Conarachin) per gram of food. By comparing the levels of allergens detected in the incurred samples compared to those for the spike samples, the authors were able to determine the effect of processing on the level of detection of the allergens. Figure 1 clearly shows how the detection is dramatically reduced for each of the 5 allergens under investigation when the samples are processed.
A novel approach achieved an LOD of 3 mg kg-1 with LC-MS/MS spectrometry using magnetic beads coated with antibodies to extract Ara h 3 and Ara h 4 from complex matrices (Careri, Elviri et al. 2008a). This work was designed to be used in combination and as a confirmatory tool with ELISA screening procedures.
The impact of matrix effects on MS detection of peanut proteins was mitigated using microfluidic separation in work by Sayers et al. (Sayers, Gethings et al. 2018). With a target detection limit of 3 mg kg-1 and quantification limit of 10 mg kg-1, following VITAL guidance (Allen, Remington et al. 2014) two sample matrices of a chocolate dessert and chocolate bar were incurred with peanut protein. The peptide targets of this work were Ara h 1, Ara h 2, Ara h 3, Ara h 6 and Ara h 7 but only Ara h 2 could be quantified at 10 mg kg-1. As previously discussed, Ara h 2 is considered a good candidate for peanut allergen detection due to its potency and stability with thermal processing. The recoveries of this work were compared to a commercial ELISA kit, the kit AgraQuant peanut assay (Romer Laboratories UK Ltd.) (which while still commercially available may not contain the same components as when this work was published in 2018) and were found to be consistent, reporting around 30-50% of the spiking level.
Planque et al. (Planque, Arnould et al. 2017) described an LC-MS/MS protocol for the detection and quantification of ten allergens in processed foods. They observed an LOQ of 2.5 mg/kg for peanut, cashew, pistachio, and hazelnut proteins. The method can be completed in one day and the authors suggest that it is suitable for routine laboratories. They emphasised the importance of introducing suitably labelled standards in order to correct for matrix effects.
A multi-allergen targeted method was also described by Gu et al. (Gu, Chen et al. 2018) for the determination of allergens in chocolate (milk, soybean, peanut, hazelnut, walnut, almond, cashew and pistachio). Enhanced sensitivity was obtained by introduction of a rapid solid-phase extraction step using MonoSpin PBA spin column. Quantification was based on matrix-matched calibration curves. LOQ values of 2.5–4 μg/g were obtained for peanut.
A multi-allergen LC-MS/MS method was developed by Sealey-Voyksner et al. (Sealey-Voyksner, Zweigenbaum et al. 2016) for the detection of trace levels of peanut and tree nuts in food. Marker peptides were selected only if present in both processed and unprocessed foods and based on abundance for sensitivity, sequence size, and specificity. For peanut-specific peptides along with two peptides specific for each of peanut, almond, pecan, cashew, walnut, hazelnut, pine nut, Brazil, macadamia, pistachio, chestnut and coconut were used as targets for the method. All allergens were detected at 1 ppm spike levels in food samples, and some were also detected at 0.1 ppm, depending on matrix interferences. The method was applied to a wide range of processed commercial samples, being able to confirm declared allergens, identify allergens indicated by PAL and discover undeclared allergens with minimal cross-reactivity due to the specificity of the peptide sequences selected. The method was used to identify undeclared nuts in commercial foods.
Highly specific identification of peanut, almond, cashew, hazelnut, pistachio, and walnut by a technique using MS(3) reconstructed chromatograms on a signature of secondary ions issued from a trapped primary product ion, termed multiple reaction monitoring cubed (MRM3) reported by Korte and Brockmeyer (Korte and Brockmeyer 2016). The analytical performance of the method was assessed for three relevant food matrices with different chemical compositions. Limits of detection were around 1 mg/g or below in fortified matrix samples, not accounting for the effects of food processing. Compared to an MRM-based approach, the MRM3-based method showed an increase in sensitivity of up to 30-fold. Regression analysis demonstrated high linearity of the MRM3 signal in spiked matrix samples together with robust inter- sample reproducibility, confirming that the method is highly applicable for quantitative purposes.
Sagu et al. (Sagu, Huschek et al. 2021) developed a targeted LC-MS/MS method for the detection and quantification of almond, cashew, hazelnut, peanut, pistachio and walnut. The method was validated according to the International Conference on Harmonisation (ICH), determining linearity, selectivity, sensitivity, recovery, repeatability and reproducibility (based on triplicate experiments). In addition, the limits of detection (LOD) and the limits of quantification (LOQ) were calculated for the different nuts. For peanut, an LOD of 9.1 ng flour and an LOQ of 30.6 ng flour (equivalent defatted nut flour injected) were obtained. The study focused on the comparison of various methods for extraction and digestion from the different nuts based on the results obtained with the LC-MS/MS method developed. Of note, this study was conducted using raw unsalted nuts only, further analysis would be needed to investigate the performance of the approach on processed foods as well as the effect of food matrices.
Quantitative methods for peanut have been developed and demonstrated to work in various matrices (chocolate, baked goods, cereals). Four studies identified here describe LODs which are below the 0.4 mg/kg. Peanut has been determined in a food matrix, namely chocolate (Bignardi, Mattarozzi et al. 2013) cookie, (Careri, Elviri et al. 2008b) and biscuit, ice cream or milk chocolate (Korte, Lepski et al. 2016). Interestingly, none of these studies utilized nano-liquid chromatography, thought to be required for the most sensitive methodology.
As described above, Gavage et al. highlighted that future development of concatemer MRM mass spectrometry methods may be of benefit to food testing capabilities due to the more accurate alignment of the method with the sample extraction protocol and their initial multiplex method involved peanut alongside three other allergens.
2.4.10.6 Conclusions – Peanut testing methods
The development of analytical methods for peanut detection over the past twenty years has revealed the importance of target selection. For ELISA kits the use of Ara h 3 as a target protein gives high recoveries in unprocessed substances as it is a highly abundant seed storage protein (Jayasena, Smits et al. 2015, Jayasena, Koppelman et al. 2019). Conversely the less abundant but more potently allergenic protein Ara h 2 has greater thermal processing tolerance. It is important that the differences in the capabilities of methods which detect different proteins or peptides is communicated to responsible individuals within the food industry to ensure that the best techniques to confirm the absence or presence of peanut protein in foods are selected. Additionally, for the peanut allergen, it is encouraging to see the use of VITAL dose levels forming a standard in the literature for methods to target in combination with validating methods against AOAC Standard Methods (Immer, Reck et al. 2004, Le, Vance et al. 2020, Lacorn, Dubois et al. 2022a). This approach should be more widely taken in assessing and advertising commercial methods to increase standardisation across the food industry. A combination of testing technologies, combining detection of different allergen proteins, would provide the most robust workflow to determine the presence of peanut in food matrices.
2.4.11 Sesame
2.4.11.1 Introduction
Sesame (Sesamum indicum L.) is a plant cultivated for its seeds which are used in food products, in particular the bakery goods, fast-foods, processed meats, vegetarian and ethnic dishes. In diets where sesame based foods, such as halva and tahini, are common sesame can be a major cause of food allergy. In the UK the prevalence of sesame sensitivity was found to be 0.1% in six year olds and 0.6% in three year olds. (Venter et al., 2006a; Venter et al., 2006b) The characterised allergens in sesame are known as Ses I 1-7 and they are seed proteins, with the 2S albumin Ses I 1 was the first identified allergen and is a major allergen, alongside its homologue Ses I 2 and the 7S vicilin-like globulin Ses I 3. Studies into the cross reactivity between sesame and other allergens has found increased prevalence of sensitivity to peanuts (84.8%), hazelnut (82.9%), egg (81.5%), walnut (80.6%) and almond (76.3%) amongst children sensitised to seasame. (Stutius et al., 2010)
2.4.11.2 ELISA
The development of a sandwich ELISA for the detection of sesame in foods was detailed by Koppelman et al. and compared to kits from Tepnel Biokits (Deeside, United Kingdom) and ELISA Systems (Windsor, Australia) (Koppelman, Soylemez et al. 2015). The stated LOQs given by the manufacturers for these kits at the time of publication were 6 ppm and 0.5 ppm respectively. A comparison of the recovery of white and black sesame seed in bread incurred with sesame seed flour gave mean recoveries for the developed method and the ELISA Systems kit were 6.5% and 13%, while the Tepnel-Neogen kit was used to give a higher recovery with a mean of 39%. A second sample, peanut butter spiked with tahini was also used as a test for all three kits, in this case the developed method had a mean recovery of 83%, the ELISA Systems kit had a mean recovery of 6% but could not detect the lowest spike at 1 ppm. The Tepnel-Neogen assay resulted in an overestimation of sesame contamination with a mean recovery of 239%. The specificity of the developed kit was investigated against 92 food ingredients with around a quarter of ingredients responsive undiluted. The conclusion from the authors suggests that different assays may be required for measuring residues of sesame seeds in food products. The authors argue that polyclonal antibodies are more appropriate for processed samples, stating, “A monoclonal antibody may become less reactive if its epitope is affected by food processing. For polyclonal antibodies, more epitopes play a role and overall reactivity will only partly be affected when a certain epitope is affected by food processing. Furthermore, because we choose to work with whole extracts rather than purified or isolated proteins, the number of epitopes is even larger, diminishing the chance of losing reactivity in the ELISA when the reactivity of one of the epitopes is affected by food processing”.
The effect of food processing on the allergenicity of sesame seeds was investigated by Achouri et al. using an ELISA kit produced by Tepnel BioSystems Ltd., (Deeside, Flintshire, United Kingdom) (Achouri and Boye 2013). This determined that an increased ELISA response was seen following boiling and dry roasting samples, whereas microwaving decreased the response. With Fourier transform infrared spectroscopy (FTIR) significant changes were observed following thermal processing with significant changes to protein confirmation, for example unfolding and denaturation, impacting the antibody binding to allergenic epitopes. This demonstrates the importance of testing ELISA kits against incurred samples to observe the impact of processed sesame as the binding can be significantly altered while allergenic proteins remain in the sample.
2.4.11.3 Mass Spectrometry
The detection of sesame proteins in a qualitative and quantitative method by LC- MS/MS by Ma et al. was achieved using stable isotope labelled internal standard peptides (Ma, Li et al. 2020). The seven allergenic peptides gave LODs in the range 0.1 to 140.0 fmol μL-1 and LOQs in the range 0.4-400 fmol μL-1. The recovery of 20 ppm incurred protein is reported as 90% for non-processed material and 92% in processed material. The LOQ is 10 times higher than that for commercially available ELISA kits, illustrating the current gap in performance between the two methods for sesame detection, accordingly the VITAL study by Holzhauser in 2020 which reported that no MS methods met the inclusion criteria (Holzhauser, Johnson et al. 2020).
Huschek et al. (Huschek, Bonick et al. 2016) developed a targeted LC-MS/MS method for the simultaneous analysis of soy, sesame and lupine using isotope- labelled peptides. The method included three peptides from the cupin protein Ses i6 in sesame. The performance of the method was evaluated by analysing six replicates of each sample, namely wheat flour, cookies and bread incurred with the allergenic foods. The LOQ achieved for sesame in food was 20 ppm (wheat flour), 10 ppm (cookie) and 50 ppm (bread) and the recovery rates were 113%, 91% and 72%, respectively. The method is described as accredited with regard to ISO 17025.
2.4.11.4 PCR
A commercial PCR test for sesame is available from R-Biopharm and has an LOQ of
≤ 0.4 ppm but publications regarding this kit were not identified. Other PCR methods are quoted in the Koppelman paper: “The sensitivity of the DNA-amplification method was in one report 50 ppm and 5 ppm in another report. At least one of these methods by Schöringhumer et al. may not detect clinically relevant levels of sesame seed residues based upon the VITAL Reference Dose. Furthermore, the DNA- amplification methods detect DNA rather than the allergenic proteinaceous part of sesame seeds. Results obtained with DNA-amplification methods should therefore be interpreted with more care especially for processed food products containing sesame seed-based ingredients where the allergenic protein part is separated from the DNA/R” (Ehlert, Demmel et al. 2009, Schoringhumer, Redl et al. 2009, Taylor, Baumert et al. 2014, Koppelman, Soylemez et al. 2015).
2.4.11.5 Conclusions – Sesame testing methods
The sesame allergen testing market is dominated by ELISA tests, with only one PCR kit available (Table 1, Appendix 1). Currently, alternative techniques such as LC-MS, do not show the limits of detection typical of the ELISA kits.
2.4.12 Soybean
2.4.12.1 Introduction
A legume, the soybean (Glycine max) is a protein rich seed (~38% protein), as vegetarian diets have increased across Europe in recent years, soy consumption has increased as a cheap protein source. In two studies the prevalence of soy sensitivity in the UK was found to be 0.3% and 0.2% in children aged four and eight respectively. (Arshad et al., 2001; Roberts et al. 2005) Although 16 protein fractions capable of causing an IgE mediated reaction, only eight allergens are included in the IUIS database, named Gly m 1-8. The most common storage proteins in soybeans are β-conglycinin (7S, Gly m 1 and 5) and glycinin (11S; Gly m 6). The major allergens are Gly m 1 and Gly m 4 with >90% and 86% of patients reacting to each respectively. (Djurtoft et al., 1991; Baur et al., 1996) Cross reactivities for other legumes including peanuts, green peas, lima beans and string beans have been reported. (EFSA, 2014)
Lacorn et al. illustrated in 2016 that closely-related plants show cross-reactivity to soybean ELISA (R-Biopharm Ridascreen Fast) (Lacorn, Dubois et al. 2016). However, although from a regulatory point of view, these cross-reactivities could be considered undesirable, they may still be relevant due to potential co-sensitivity amongst soy-sensitive consumers. Eighteen phylogenetically closely related species were tested. No cross-reactivities were observed for Lupinus angustifolius, L. albus, and L. luteus. In contrast, cross-reactivities were observed against Pisum sativum (dried and fresh seeds), Vicia pannonica, Lens culinaris, Arachis hypogea (roasted and raw), Cicer arietinum, Trigonella foenum-graecum, Trifolium pratense, Phaseolus vulgaris, P. lunatus, V. faba, P. coccineus, Vigna radiate, and V. angularis.
2.4.12.2 ELISA
Work using R-Biopharm, Veratox-Neogen and Romer ELISA test kits with a matrix of biscuits spiked with soybean concentrate as an allergen found that the R-Biopharm kit was capable of quantifying soybeans from 25 ppm (Binaghi, Greco et al. 2017). Conversely the Veratox kit was not capable of detection of the soybeans at the highest tested concentration (5000 ppm), while the Romer kit did not allow detection at 50 ppm soybean which was the highest soybean concentration tested with this kit. However, it is claimed on the latest version of the Veratox kit that the LOD and LOQ are 2.5 ppm. When the kits were tested against extruded product with soybean, quantitation was achieved using both R-Biopharm and Romer kits when the soybean level was 5 ppm of concentrated soyabean added while no soybean was detected when using the Veratox kit, even at 5000 ppm. This difference is believed by the authors of the study to result from the changes to the soybean protein during cooking. This is illustrated by the fact that in cookie samples the R-Biopharm kit were under-quantified, while extruded cookie samples were over-quantified.
Soybean proteins which had been partially hydrolysed by pepsin (to simulate partial hydrolysis which is used to remove bitterness or improve amino acid or protein functionality) were the subject of a study on the robustness of ELISA techniques by Cucu et al. (Cucu, Platteau et al. 2013). Looking at three different kits (Veratox Soy Allergen, Biokit Soy Allergen and Soy Residue kit), when the sample contained untreated soybean the levels of soybean were overestimated by both the Veratox and Biokit kits (at 260% and 240% respectively), however the Soy Residue kit was more accurate. Increasing hydrolysis caused the detection to decrease, in the case of the Veratox kit, only 20% of the soybean protein could be detected after 180 minutes of hydrolysis. The same kits were used in work on the effect of glycation from the Maillard reaction (Platteau, Cucu et al. 2011). This study found that heating the RM for both the Veratox and Soy Residue kits revealed a complete lack of robustness, likely a result of the denaturation of the 7S conglycinin and trypsin inhibitors these kits use. Conversely the Biokit demonstrated a far less dramatic decrease in detection. In a similar study looking at the effect of oxidation both in the presence and absence of lipids, again for the Veratox and Soy Residue kits the heat treatment almost completely prevented the detection of soybean protein, both with and without the lipids present (Platteau, Cucu et al. 2013). However, for the Biokit ELISA the detection of soybean allergens increased both with and without sunflower oil being present, suggesting that lipid induced oxidation of proteins is compatible with ELISA receptor-based detection. Conversely, hypochlorous acid-induced oxidation severely depressed detection and whilst this is not a food ingredient it is used as a cleaning agent and may impact the detection of trace residues on the production line.
The impact of thermal processing on soybean detection was investigated by Gomaa et al. comparing ELISA kits, Veratox and the ELISA systems kit, with flow cytometry all methods performed reasonably well for non-baked cookie samples with recoveries of 86%, 74% and 89% respectively (Gomaa and Boye 2013). When the cookies were baked, these recoveries fell to 33 to 1% for the Veratox, 1 to 0% for the ELISA systems kit and 21- to 0% for flow cytometry, with larger cookies giving greater recoveries than smaller cookies. An interlaboratory investigation conducted in 2010 by Sakai et al. focused on the FASTKIT Ver. II (supplied by CosmoBio Ltd, Japan) for soybean detection which uses polyclonal antibodies to Gly m Bd 30 K (p34) (Sakai, Adachi et al. 2010). This looked at five different incurred matrices: rice gruel, sausage, sweet adzuki bean soup, sweet potato cake and tomato sauce each containing 10 μg soybean protein per gram of food. Eleven different laboratories were involved in the ring trial which demonstrated high levels of recovery (97-114%) and the reproducibility was also acceptable with an RSDR ranging from 9.3 to 13.4% across the five matrices.
2.4.12.3 PCR
Commercial PCR kits are available (R-Biopharm AG, Darmstadt, Germany; Biotecon Diagnostics GmbH, Potsdam, Germany), however peer-reviewed literature citing the performance of these kits was not identified.
2.4.12.4 Mass Spectrometry
The detection of soybean proteins using mass spectrometry has typically focussed on the proteins from soybean conglycinin (Gly m 5), soy glycinin (Gly m 6), and additionally some studies have used peptides from prolamine and lipid transfer proteins (Holzhauser, Johnson et al. 2020). In their 2017 work Huschek et al. developed a sensitive method for the detection of soybean glycinin employing isotopically labelled proteins for quantitation, an approach also taken by Croote and colleagues (Huschek, Bonick et al. 2016, Croote, Braslavsky et al. 2019). The former work achieved an LOQ of 10 ppm of the target protein converting this to 25 ppm allergenic food per 100 g consumption.
Multi-allergen approaches have also been taken. The development of a mass spectrometry method to detect soybean and other allergens was proposed by Heick et al. in 2011 and compared this to two ELISA kits, the Soy Residue Enhanced Assay and the Tepnel Biokits Soya Assay (Heick, Fischer et al. 2011). Using the related matrices of flour and bread, this study again investigated the impact of baking on limits of detection with only one of the two (anonymised) ELISA kits reported to detect the soybean protein in the processed product with detection at 13% of that found in flour. The MS method, directed at glycinin soybean allergen peptide markers had an 80% decrease in signal response in bread compared to that in flour and the benefits of a multiplex method (this one also capable of analysing for milk and egg in a single analysis) was highlighted by the authors. The LOD was 24 µg/g soybean in incurred bread, with a correlation coefficient of 0.9879.
Gu et al. reported a multi-allergen MRM method on chocolate incurred with a range of allergens. The method, targeting conglycinin and glycinin peptides, was capable of detection down to 0.4 µg/g, quantitative range up to 240 µg/g with recovery in the 61- 86% range (depending on which of the three soy peptides was targeted). The authors highlighted the importance of the special type of monolithic silica gel column bonding of benzene boric acid-based group which can specifically adsorb substances containing o-hydroxy groups and efficiently clean-up complex food extracts for the sensitive detection of trace food allergens. The authors believe that the use of internal standards will lead to a robust quantitative multi-allergen method in the future and they acknowledge that foods involving a wider range of processing formats must be tested in the future.
Monaci et al. 2014 developed a method using SRM LC-MS for multiallergen measurement for milk, egg and soybean, selecting the top 2 performing peptides from a list of 11 (Monaci, Pilolli et al. 2014). LODs were achieved at 0.1 µg/g milk, 0.3 µg/g ovalbumin and 2 µg beta-conglycinin-alpha chain soybean allergen per gram of food, although the samples were spiked rather than incurred, so the level of challenge was considerably lower than for methods developed using incurred food matrices.
As a follow-up to their previous methods, Pilolli et al. (2017) developed an SRM LC- MS method using both incurred cookie samples and spiked cookie samples (Pilolli, De Angelis et al. 2017). The LOD was 6 µg allergen /g food for soybean, 7 µg/g for milk and hazelnut, 9 µg/g for egg and 13 µg/g for peanut. By comparing the levels of allergens detected in the incurred samples compared to those for the spike samples, the authors were able to determine the effect of processing on the level of detection of the allergens. Figure 2 clearly shows how the detection is dramatically reduced for each of the 5 allergens under investigation when the samples are processed. It is interesting that, as in detection by ELISA, detection (of peptides) can also be reduced with increased sample processing.
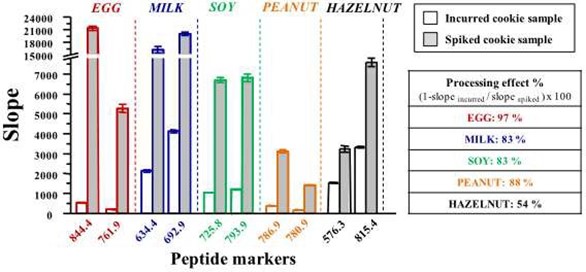
Figure 2. Taken from Pilolli et al. 2017 (Pilolli, De Angelis et al. 2017). Comparison of the calibration curve slopes obtained by interpolation of peak areas in the SRM mode for incurred and spiked samples. The table on the right reports the % processing effect expressed as the percent decrease of the sensitivity calculated in incurred samples compared to spiked samples.
Planque et al. 2017, concentrating on detection by LC-MS of egg, milk, soybean and peanut peptides highlighted the effect of detection of allergens with processing and highlighted the need for labelled allergen peptides to use as internal standards in order to develop quantitative methods (Planque, Arnould et al. 2017). In the meantime, the authors recommend using standard addition methods to estimate allergen levels by LC-MS.
2.4.12.5 Conclusions – Soybean testing methods
These studies highlight the importance of choosing the correct commercial ELISA for the specific food product being analysed. Given the poor performance of many
ELISA kits when samples have undergone processing, there are needs for either improvement of methods or clear and transparent data concerning the levels of processing for which each kit is suitable and lists of the expected recovery for each form of processing. Much LC-MS work has focussed on soybean (not soya protein) detection, compared to the level of LC-MS methods targeting other allergens and methods are capable of detecting down to similar detection limits as ELISA methods (for example LOD 0.24 mg/kg for ELISA and 0.4 mg/kg for mass spectrometry) and therefore mass spectrometry methods for soybean seem more advanced than for other allergens, now requiring full validation and the development of internal standards to more accurately evaluate the quantitative capability of these methods.
2.4.13 Sulphur Dioxide
2.4.13.1 Introduction
Sulphur dioxide (SO2) is a preservative commonly used in a variety of foods and beverages including wine, dried fruits, pickled vegetables, sausages, fruit and vegetable juices, cider and vinegar. It slows oxidation and inhibits growth of fungal and bacterial cultures and thus is applied to extend the shelf life of food products. Sulphur dioxide residues in food are considered to be food allergens. Where SO2 and/or sulphite-based preservatives (even as carryover in an ingredient) have been used and the levels in the finished product are above 10 mg/kg or 10 mg/litre, it must be declared on the label as an allergen. The prevalence of sulphite allergies is higher among asthmatics than the general population, with the FDA estimating that 5% of asthmatic people are sensitive to sulfites, compared to 1% of the general population. (FDA, 1996)
2.4.13.2 Wine and related products
In wine, SO2 occurs naturally (generated by yeast) and is a very common additive due to the importance of its antioxidant and antimicrobial functions. Sulphites and SO2 levels are commonly measured by distillation and titration. Since sulphites can convert to SO2, sulphites are measured and expressed as SO2.
SO2 levels in wine are carefully controlled to ensure effective performance without negative sensory impacts. Industry reference methods are available. The most common method used for the determination of total SO2 is the aeration/oxidation method (AO), intially developed by Monier-Williams and subsequently enhanced by Rankine & Pocock , and is the basis of one of the approved International Code of Oenological Practices methods (OIV-MA-AS323-04A). (Burns, Walker 2018; Rankine, Pockock 1970) As this method liberates the SO2 from the sample matrix, it is relatively robust in terms of interference from other wine components. The equipment and reagents required are, however, relatively specialised and need to be carefully used and maintained, operated by a trained technician so are perhaps less suitable for on-site testing by small producers.
There are a number of alternative methods that allow SO2 analysis to be automated to speed up analysis time and carry out larger batches of samples. Iodic titration (more commonly known as the Ripper method, described in the OIV method OIV- MA-AS323-04B) allows a rapid determination of SO2 with limited equipment and can be automated for use on autotitrators; however, it suffers from significant interferences from other wine components and often gives artificially high results.
Red wines in particular are known to give erroneously high results due to the reaction of some of the colour compounds. The presence of any ascorbic or erythorbic acid also quantitatively reacts with iodine and this method cannot be used in wine that may contain these preservatives, unless they have first been quantified. It is generally acknowledged that iodic titration methods give a less accurate determination of total SO2 than AO methods for these reasons (Wilkes 2018).
Wilkes of the Australian Wine Research Institute states that: “Independent of the method used, the volatile nature of SO2 and its sensitivity to oxidation mean that careful sample handling and rigorous quality assurance procedures are required to achieve accurate and precise results” (Wilkes 2018). When using the available methods, Wilkes states, “the importance of appropriate validation of all laboratory methods (rather than simply following a published method) cannot be overstated. Control samples must be analysed at every first and tenth sample and a known standard should be prepared at a known level and analysed every week with recovery ±5%. Equipment should also be checked weekly to verify that acetic acid is being correctly removed from the samples during analysis since acetic acid is one of the few interferents of aeration/oxidation methods”.
Commercial methods also exist, based on spectrophotometry or sequential analysers. Porter et al. 2017 reported a new high-throughput photometric method capable of measuring SO2 levels in addition to levels of glucose, fructose, malic acid and acetic acid (Porter 2017). This work reported analysis of free and total SO2 in wines and juices using a commercial photometry method (Discrete Analyser by Thermo Scientific) in an adaptation of method OIV-OENO 391–2010. The data published correlated well to those from the industry reference AO method, with high throughput.
An enzymatic method performed by automated spectroscopy to measure SO2 in vinegar was reported by Dini et al., demonstrating validation data, as a rapid alternative to the Ripper method (Dini, Di Lorenzo et al. 2020). The data compared to the industry standard tests with excellent correlation, (LOD 0.946 ppm) and LOQ 2.00 ppm) with good precision and uncertainty. There were a few outlying data points but it will be interesting to see whether this method is adopted by the industry in the future.
2.4.13.3 Finished foods
The level of sulphite in finished food can also be determined by the modified Monier- Williams (MW) method (as published in the AOAC Official Method 962.16). The modified MW method is not applicable to dried onions, leeks, cabbage and certain other vegetables due to the presence of interfering volatile sulphur-containing compounds. The method was further optimised, developing the Optimized Monier- Williams (OMW) method. This method is the most widely used for the quantitative determination of sulphites in food and beverages (see, e.g., AOAC Official Method 990.28) and is sensitive at 10 mg/kg. A gap in this method is that false positive results are obtained from garlic powder, soy protein, onions, leeks, kale, brussels sprouts, horseradish, cabbage, and ginger.
A more recent method developed by AOAC (Method 990.31, 1995) focuses on the use of ion-exclusion chromatography coupled to electrochemical (amperometric) detection. The method is not applicable to dark coloured foods or ingredients where SO2 is strongly bound e.g. caramel colour. The method does not detect naturally occurring sulphite.
2.4.13.4 Conclusions – Sulphur dioxide and sulphites testing methods
AOAC and OIV official methods dominate the testing procedures for sulphites and sulphur dioxide in food and beverages. This conclusion is supported by method information submitted by participants in relevant Fapas® proficiency tests. No further routine method development was identified by this literature review.
2.4.14 Tree nuts
Details of methods which have been discussed in the literature for tree nuts are discussed below. This section discusses a variety of tree nuts which are fruits or seeds of various seeds contained within a hard shell. Tree nuts are common in many diets, either eaten as a whole nut, roasted or raw, or as a component of a dish or baked good. The prevalence of self-reported tree nut allergies across all ages in the UK population in 1994 was 1.7%. (EFSA, 2014; Young et al., 1994)
2.4.14.1 Multi-allergen detection by LC-MS
An LC-MS MRM multianalyte method was reported by Heick et al. 2011 to detect seven allergens: milk, egg, soy, hazelnut, peanut, walnut and almond (Heick, Fischer et al. 2011). The chosen marker peptides were implemented into one method that is capable of the simultaneous detection of milk, egg, soy, hazelnut, peanut, walnut and almond, incurred in bread material prepared from a standard recipe provided by industry with baking for 60 minutes at 200 °C. The four peptides identified for hazelnut detection all originated from 11S globulin. The LOD was 5 mg/kg for hazelnut incurred in bread and 0.42 mg/L for hazelnut in bread spiked with hazelnut, showing the importance of using incurred test materials when determining the suitability of a method to quantitatively determine allergen in real-world samples. The correlation co-efficient was 0.9998 for hazelnut incurred in bread, 0.9977 for peanut, 0.9986 for walnut and 0.9996 for almond. The LOD for milk, hazelnut, peanut and almond was 5,5,11 and 3 mg/kg respectively, 42 and 24 mg/kg respectively for egg and soy and 70 mg/kg for walnut.
Bignardi et al. reported multiplex determination of five nut allergens in biscuit and in dark chocolate complex matrices was obtained by LC-MS SRM analysis with a short LC-MS gradient (12 min) (Bignardi, Mattarozzi et al. 2013). Limits of detection in the 0.1-1.3 mg nut/kg and 5-15 mg nut/kg ranges for biscuit and dark chocolate samples as well as high recoveries (84(±6)-106(±4)% for biscuits and 98(±5)-108(±6)% for dark chocolate) proved the excellent capabilities of the exploited sample treatment method combined with the LC-MS2 analysis. Good precision in terms of intra- and inter-day repeatability was calculated, being always lower than 19 % (n = 75).
Linearity was demonstrated up to four and three orders of magnitude for biscuit and dark chocolate, respectively.
A multi-allergen LC-MS/MS method was developed by Sealey-Voyksner et al. (Sealey-Voyksner, Zweigenbaum et al. 2016) for the detection of trace levels of roasted and raw peanut and tree nuts (almond, pecan, cashew, walnut, hazelnut, pine nut, Brazil nut, macadamia nut, chestnut and coconut) in food. Marker peptides were selected only if present in both processed and unprocessed foods and based on abundance for sensitivity, sequence size, and specificity. At least two species- specific peptides were selected for each tree nut and four for peanut. All allergens were detected at 1 ppm spike levels in food samples, and some of them were also detected at 0.1 ppm. The method was applied to a wide range of processed commercial samples, being able to confirm declared allergens, identify allergens indicated by PAL and discover undeclared allergens. The authors argue that these levels of sensitivity align well with those typical of ELISA kits. The concentration of peptide detected can be equated to the concentration of allergenic protein, in line with FAO/WHO recommendations, which is a benefit of this methodology. Another benefit is the multiplex capability which would allow time savings compared to conducting a separate ELISA test for each individual nut type. The authors also argue that this method should benefit from less cross-reactivity than ELISA methods due to the specificity of the peptide sequences selected, thus reducing the number of false positive responses for the target compound.
Highly specific identification of peanut, almond, cashew, hazelnut, pistachio, and walnut by a MRM3-based LC-MS/MS method was reported by Korte and Brockmeyer (Korte and Brockmeyer 2016). The analytical performance of the method was assessed for three relevant food matrices with different chemical compositions. Limits of detection were around 1 mg/kg or below in fortified matrix samples, not accounting for the effects of food processing. Compared to an MRM- based approach, the MRM3-based method showed an increase in sensitivity of up to 30-fold. Regression analysis demonstrated high linearity of the MRM3 signal in spiked matrix samples together with robust inter-sample reproducibility, confirming that the method is highly applicable for quantitative purposes.
Planque et al. (Planque, Arnould et al. 2017) described an LC-MS/MS protocol for the detection and quantification of ten allergens in processed foods. They observed an LOQ of 2.5 mg/kg for peanut, cashew, pistachio, and hazelnut proteins. They observed an LOQ of 5 mg/kg for soybean, almond, walnut, and pecan nut proteins. The method can be completed in one day and the authors suggest that it is suitable for routine laboratories. They emphasised the importance of introducing suitable labelled standards in order to correct for matrix effects. A multi-allergen targeted method was also described by Gu et al. for the determination of allergens in chocolate (milk, soybean, peanut, hazelnut, walnut, almond, cashew and pistachio).(Gu, Chen et al. 2018) Enhanced sensitivity was obtained by introduction of a rapid solid-phase extraction step using MonoSpin PBA spin column. Quantification was based on matrix-matched calibration curves. LOQ values of 1–3 mg/kg were obtained for tree nuts. Sagu et al. (Sagu, Huschek et al. 2021) developed a targeted LC-MS/MS method for the detection and quantification of almond, cashew, hazelnut, peanut, pistachio and walnut. The method was validated according to the International Conference on Harmonisation (ICH), determining linearity, selectivity, sensitivity, recovery, repeatability and reproducibility (based on triplicate experiments). In addition, the limits of detection (LOD) and the limits of quantification (LOQ) were calculated for the different nuts. For pistachio, the authors quote that an LOD of 20.4 ng flour and an LOQ of 80.1 ng flour (equivalent defatted nut flour injected) were obtained. For walnut, an LOD of 34.4 ng flour and an LOQ of 114.8 ng flour (equivalent defatted nut flour injected) were obtained. For almond, an LOD of 35.6 ng flour and an LOQ of 118.6 ng flour (equivalent defatted nut flour injected) were obtained. The study focused on the comparison of various methods for extraction and digestion from the different nuts based on the results obtained with the LC-MSMS method developed. It concluded that for almond, an SDS extraction buffer and microwave-assisted trypsin digestion provided the best performance. Of note, this study was conducted using raw unsalted nuts only, further analysis would be needed to investigate the performance of the approach on processed foods as well as the effect of food matrices.
The choice of marker peptides is key for the development of reliable MS methods for allergen detection since the sensitivity, robustness and specificity of the method will depend on them. With this in mind, and as part of the development of a multi-analyte reference method, Pilolli et al. undertook a high-resolution MS discovery approach to select the most reliable peptide markers for six allergenic ingredients in two incurred food matrices (Pilolli, Van Poucke et al. 2021). The allergens studied included milk, egg, peanut, soybean, hazelnut, and almond, and they were added to chocolate at 40 ppm and to broth powder at 200 ppm to represent complex matrices incurred with low levels of allergens. Different conditions for protein extraction and purification were assessed, and the results indicated that a two-step extraction (including shaking and sonication at room temperature), followed by sample purification based on size exclusion chromatography at protein level and solid phase extraction of the trypsin digests was the most promising option for both incurred matrices. The authors concluded that this sample preparation workflow and the candidate peptide markers identified show potential to enable the development of a targeted multi- allergen SRM method using a triple quadrupole MS platform which could act as a prototype MS reference method for allergen analysis.
2.4.14.2 Almond
Almond (Prunus dulcis) major protein (AMP or amandin) is the primary storage protein in almond and the major allergen recognised by almond-allergic patients. The protein accounts for approximately 65% of aqueous extractable almond protein and it is substantially heat stable. Almond belongs to the Prunus genus which contains over 400 species including apricot, cherry, sour cherry, peach, plum and mahaleb (a spice produced from the seeds of mahaleb cherry).
2.4.14.3 ELISA and PCR
In the case of almonds, ELISA methods show cross-reactivities against phylogenetically closely related species, such as apricot stones or mahaleb cherry
(Prunus mahaleb) which is used as a spice. Apricot stones are used in the marzipan alternative persipan.
The BioFront MonoTrace almond ELISA kit (BioFront Technologies, Tallahassee, Fla., USA) was studied by Liu et al. (Liu, Chhabra et al. 2017) in parallel with a laboratory-developed monoclonal antibody-based sandwich ELISA (4C10) for the detection and quantification of almond. They reported that both kits were comparable and demonstrated their sensitivity, robustness and specificity for almond detection and quantification. LODs and LOQs of both ELISAs were below 5 ppm full fat almond, and the intra- and inter-assay variabilities were within 15%. Cross-reactivity was not observed with 156 food ingredients at a concentration of 100000 ppm whole sample. The target antigens were stable and detectable in whole almond seeds subjected to autoclaving, blanching, frying, microwaving, and dry roasting. The almond recovery ranges for spiked food matrices were between 81.2% and 127.4% for MonoTrace ELISA whilst for commercial and laboratory prepared foods with declared/known almond amounts recovery rates were between 38.1% to 207.6%. No false-positive or negative results were obtained. The only food ingredient found to interfere with antigen detection was dark chocolate, which resulted in a decreased antigen recovery. However, addition of 5% (w/v) non-fat dried milk in the extraction buffer and extraction at 60 °C, as recommended by the MonoTrace kit, reduced the interference from dark chocolate and increased recovery.
The development and validation of the Neogen Veratox for Almond Allergen ELISA test was published in 2018 (Slotwinski, Almy et al. 2018). The test enables the quantitative analysis and screening of almond protein in food products such as cereals, beverages, crackers, cookies, chocolate bars as well as clean-in-place rinses. The quantification range is 2.5 to 25 ppm and no significant cross-reactivity was detected across 39 commercial food products. Cross-reactivity was detected with other Prunus genus seeds (apricot, nectarine, cherry, plum, peach) but not with the flesh of these fruits.
Röder et al. compared the performance of two commercially available ELISA tests - Neogen Veratox® for almond allergen (Ayr, Scotland, UK) and RIDASCREEN®FAST
Almond (R-Biopharm, Darmstadt, Germany) – with that of a Taqman® real-time PCR method for almond developed in their laboratory (Röder, Vieths et al. 2011). The authors reported high levels of cross-reactivity of both assays to other kernels from Prunoideae fruits: plum, peach, nectarine and cherry, in addition to cross-reactivity to apricot kernel that is denoted in the manuals of both ELISA kits. However, the real- time PCR test exhibited only negligible cross-reactivity with a small number of Prunoideae foods and the authors therefore suggest that the PCR-based method is a superior strategy with regard to specificity compared with ELISA tests. The study also revealed differences in response of almond quantification of approximately 1:2 between Neogen and R-Biopharm ELISAs.
The performance of the RIDASCREEN®FAST Almond test with almonds roasted at various temperatures was investigated by Perner et al. (Perner, Heupel et al. 2019). They reported recovery levels close to 100% in cookies containing almonds roasted at 110 °C and 120 °C, however, almond was not detected when the roasting temperature was greater than 120 °C. SDS-PAGE analysis of nuts roasted at different temperatures showed that the total protein extracted decreased dramatically at roasting temperatures >120 °C, suggesting that the lack of ELISA response from these samples is linked to a reduced extractability/solubility of the proteins in question.
Following an incident in the UK 2015 which revealed the cross-reactivity of ELISA kits targeting almond with the Prunus species, real-time PCR methods were developed. Burns et al. 2016 designed a real-time PCR method shown by the authors to be specific for Prunus mahaleb. Other work has also been completed using real-time PCR to distinguish almond and Prunus mahaleb to provide greater species specification compared to ELISA (Walker et al. 2018). Cumin alleged to have been contaminated with almond was later found to be contaminated with the Prunus species Prunus mahaleb. Paprika was found to be contaminated with P. dulcis (almond). R-Biopharm’s Ridascreen Fast Almond ELISA, Romer’s AgraQuant ELISA and ELISA System’s Mandel/Almond residue kit all showed cross-reactivity to Prunus mahaleb, although the response of the ELISA Systems kit was much diminished compared to the other two kits. PCR methods were developed to distinguish the two species. A confirmatory method was also developed by SRM LC- MS, to develop a staged process to be implemented in any future incidents. Almond ELISAs are also known to cross-react with apricot (Table 1, Appendix 1).
2.4.14.4 Mass Spectrometry
Heick et al. compared the performance of an LC-MS/MS method developed in-house for detection of seven allergens with that of commercial test kits (Heick, Fischer et al. 2011). Almond was one of the allergens covered, and the ELISA kit used was RIDASCREEN® Fast Almond (R-Biopharm AG, Darmstadt, Germany). The study was conducted on wheat flour that had been spiked with the seven allergens as well as on baked bread in order to compare the effectiveness of the methods on raw and processed flour. Samples were tested in triplicate. The LC-MS/MS method targeted four peptides from the protein prunin, with an LOD of 3 µg/g and correlation coefficient of 0.996 in incurred bread. The authors described that the LC-MS/MS method was able to detect higher signals in the processed samples than in the raw flour, suggesting that this may be due to signal suppression in the flour matrix. However, the ELISA test detected less almond in the bread than in the unprocessed flour, which may be due to partial destruction of the epitopes recognised by the antibodies caused by heating. Similar results were observed by Perner et al., (Perner, Heupel et al. 2019) who investigated the effect of roasting on almond and hazelnut allergen detection by various methods, including non-targeted and targeted LC-MS/MS as well as the RIDASCREEN® Fast Almond ELISA test.
2.4.14.5 Conclusions – almond testing methods
Commercial ELISA kits are available for the detection of almond and it is apparent that much development of LC-MS methods has been completed to detect almond at low levels in incurred matrices. The presence of two different technologies to detect almond allergen protein is beneficial when determining workflows to determine almond in foods. However care must be taken to avoid cross-reactivity with Prunus mahaleb and using ELISA and PCR in combination can be an effective method of guarding against this.
2.4.14.6 Hazelnut
Hazelnut (Corylus avellana) has both pollen and non-pollen related allergens. Hazelnut allergens include Cor a 1,2,8,9,11,12,13,14, with the first identified allergen Cor a 1 binding IgE in 63 of 65 patients, both the Cor a 1 and 2 allergens are homonlogous to the major birch pollen allergen Bet v 1.(Ortolani et al., 2000) Pollen unrelated allergy presents as sensitivity to the allergen Cor a 8, which is related to peach allergy. (EFSA, 2014)
2.4.14.7 ELISA and PCR
A 2002 study from Holzhauser et al. involved the SureFood Hazelnut-PCR ELISA, favouring the PCR-ELISA approach over a regular polyclonal protein sandwich-type ELISA (antibody raised to corylin hazelnut protein) owing to the high stability of hazelnut DNA (Holzhauser, Stephan et al. 2002). Both methods were highly sensitive and allowed the detection of <10 ppm of hazelnut in complex food matrixes. The protein-ELISA was highly specific for hazelnut. However, some foods could lead to false-positive results at the 10 ppm level. This method showed no cross- reactivities with non-hazelnut food and when tested against 27 products containing hazelnut only gave one false negative result which contained <1 ppm for the PCR- ELISA and two for the protein-ELISA. This PCR-ELISA was compared to a protein sandwich ELISA with both methods showing an LOD of less than 10 ppm.
During food processing, oxidation processes can take place which can lead to modification of amino acids, formation of protein bound carbonyls and aggregation These modifications can influence the protein-antibody interaction upon which ELISA assays are based. To investigate this, model systems were prepared in which hazelnut proteins were oxidised under different conditions. Platteau et al. then compared the performance of four commercial ELISA kits to determine the effects of oxidation by either lipids from sunflower oil (composite foods containing hazelnut often have a high lipid content) or hypochlorous acid (used to clean factory production lines) (Platteau, Cucu et al. 2013). The ELISA kits compared for hazelnut protein detection were: Veratox for hazelnut from Neogen, Michigan Lansing, USA; Ridascreen FAST Hazelnut from R-Biopharm, Darmstadt, Germany; BioKits Hazelnut Assay from Tepnel, Deeside, Flintshire, UK; and Hazelnut Residue from ELISA Systems, Windsor, Queensland, Australia. The detectability of protein extracted from nine different commercial brands of hazelnuts, being eight virgin and one roasted product, was compared for the four kits. A number of variables were measured in this study, including the type of protein extraction but, overall, the authors found that while the presence of sunflower oil had a minimal impact on detectability, when hypochlorous acid induced oxidation, there was a significant decrease in detection. Also, all four kits underestimated the amount of hazelnut in the native reference samples (with or without oxidation) with the detection reduced to 10-70% of the actual level.
A PCR method for the detection of hazelnut DNA, was developed by Enger-Blum et al., with a practical detection limit of 10 mg/kg (Engler-Blum, Raiss et al. 2007), although there is no evidence regarding whether a commercial method was developed from this. Detecting the hazelnut specific sequence Cor a 1, 60 samples were tested and all those which were declared as containing hazelnuts were found to contain them. Some samples which claimed to contain “hazelnut aroma” did not test positive for hazelnut DNA and the authors reason that it is likely that an artificial flavour has been used in these cases. No samples which did not declare nut content were found to contain any nuts. This manuscript describes development of a method rather than a commercially available method.
Both a real-time PCR and an ELISA method were used to determine whether spiking (roasted) hazelnut paste into peanut paste would create a model of contamination of confectionery, involving peanut pastes containing different levels of hazelnuts, each analysed in three independent experiments and six real-time PCR replicates which showed good reproducibility when a calibration line was prepared for each assay (Piknova, Janska et al. 2018). The PCR used in this work was developed previously by the authors oriented to the hsp1 gene encoding for a low-molecular-weight heat- shock protein, while ELISA used in this work was the commercially available Ridascreen FAST Hazelnut (R-Biopharm, Darmstadt, Germany) (this work was carried out in 2018 and this ELISA may include different components compared to when it was tested in this work) (Piknová, Pangallo et al. 2008). The LOQ for PCR was 2 mg/kg and that for the ELISA was 1 mg/kg, the latter in accordance with previous work cited by Poms et al. (Poms, Klein et al. 2004). The authors argue that the lower cost of PCR, along with a linear calibration curve and larger quantification range are benefits of this method compared to ELISA, however, in our view, the improved sensitivity of the ELISA over the PCR (LOQ 1 ppm for ELISA) will better support the screening of foods to protect consumers who are sensitive to hazelnut.
2.4.14.8 Mass spectrometry
Corylin and oleosin have been reported as potential protein targets for determining hazelnut by LC-MS (Weber et al. 2009). Costa et al. 2014 compared in-house sandwich ELISA, real-time PCR and LC-MS/MS methods to determine hazelnut in chocolate matrices. The real-time PCR primers and probe targeted the hsp1 gene, which encodes a low molecular weight heat-shock protein with the same name, were selected from the available literature. The ELISA comprised monoclonal and polyclonal antibodies raised against hazelnut protein. The LC-MS/MS method was developed based on eight peptides from hazelnut allergens (Cor a 801, Cor a 901, Cor a 902, Cor a 903, Cor a 904, Cor a 1101, Cor a 1102 and Cor a 1103). While this is relatively early LC-MS/MS work for allergen detection, the method showed great promise with an LOD of 1 mg/kg, correlation coefficients R2 above 0.98 and recoveries for most peptides within the acceptance criteria of 70-120%.
As described above, Gavage et al. highlighted that future development of concatemer MRM mass spectrometry methods may be of benefit to food testing capabilities due to the more accurate alignment of the method with the sample extraction protocol and their initial work involved hazelnut (targeting Cor a 9) alongside egg, milk and peanut.
2.4.14.9 Conclusions – hazelnut testing methods
The reduction in ELISA kit sensitivity for hazelnut down to 1 ppm for processed material represent great improvement in this technology and suggest that despite greater cost it may be a more appropriate method for allergen detection than PCR which has a sensitivity of 2 ppm. Using LC-MS in multi-allergen detection including hazelnut detection offers a promising screening method for processed products, although limits of detection remain higher than DNA and immunological methods.
2.4.14.10 Walnut
The Walnut allergens arise either from black or English walnuts, with allergens from the former named Jug n 1,2 and the latter named Jug r 1-4. For the English walnut the major allergen is Jug r 1, the 2S albumin which is a protein similar to those found in Brazil nuts, castor beans, cottonseed and mustard seed. (EFSA, 2014) Both allergens for black walnut Jug n 1,2 are both seed storage proteins and are highly homologous to Jug r 1 and 2 respectively. (EFSA, 2014).
2.4.14.11 ELISA and PCR
The Tepnel Biokits Walnut Assay (Neogen, Lansing, MI, USA) was studied by Heick et al. (Heick, Fischer et al. 2011) in parallel with an LC-MS/MS method in a study conducted on wheat flour spiked with the seven allergens as well as on baked bread in order to compare the effectiveness of the methods on raw and processed flour.
Walnut spiked at 1000 μg/g could be detected with ELISA in flour and bread samples, although the recovery in raw flour was 530% and in baked bread it was 40%, indicating an overestimation of the allergen in raw flour as well as the impact of heating on the antigen recognised by the antibody.
Linacero et al. (Linacero, Ballesteros et al. 2016) used the AgraQuant walnut assay (Romer Labs, UK) as an ELISA test against which to validate the performance of real-time PCR tests. The paper only presents qualitative results obtained with the ELISA test on 12 commercial products, but it does demonstrate good sensitivity, with walnut detected in all the products declaring walnut as an ingredient, some other products labelled as “may contain” and all three foods with walnut not declared. These results overall agreed with those of their best real-time PCR, although the latter detected walnut in one additional product. The authors suggest that the real- time PCR method may be more reliable, but a wider range of processed foods would be required to confirm this suggestion.
Vencia et al. (Vencia, Minale et al. 2019) studied the effect of thermal treatment on the ability of two ELISA test kits to detect walnut. The selected kits were Euroclone (Pero, Italy) and Neogen (Lansing, MI, USA). The response to walnut was different between the kits, with Neogen showing higher recoveries. The work highlighted that boiling for 10 minutes and intense and prolonged roasting (180 °C, 30 minutes) showed a high influence on sensitivity of both kits, concluding that these tests may not be suitable for accurate quantification of walnut in highly processed foods.
A walnut ELISA kit from Morinaga (Walnut Protein [2S-Albumin] Kit; Morinaga Institute of Biological Science, Inc.; “walnut kit”) was the subject of an inter-laboratory study published in 2010 (Sakai, Adachi et al. 2010). The LOD and LOQ values were 0.39 ppm (equivalent to 0.16 mg/g of food sample) and 0.78 ppm (equivalent to 0.31 mg/g of food sample), respectively. The results showed good reproducibility in all processed model foods tested, good repeatability and high recoveries, concluding that the walnut kit could be used as a reliable tool for determination of walnut in foods. However, no walnut ELISA kit is currently available on the Morinaga website.
2.4.14.12 Mass Spectrometry
A study by Downs et al. (Downs, Baumert et al. 2016) used a label-free non-targeted LC-MS/MS approach evaluate changes in the solubility and detectability of allergens from roasted walnuts. The results indicated that the detection and quantification of allergens from roasted walnuts was affected differentially depending on the individual allergenic protein in question, the degree of heat treatment, and the sample preparation method. A conclusion of this work was that a much more comprehensive knowledge of food genomes is required for mass spectrometry methods to work to their full potential in the analysis of food allergens, especially those from plant foods. In addition, the properties of the individual proteins should be considered when developing MS methods for the analysis of food allergens.
A study by Xiong et al. (Xiong, McFarland et al. 2019) explored the importance of high-quality protein databases for the development of fit for purpose LC-MS/MS methods for allergen analysis in food. The utility of supplementing incomplete protein sequence databases with translated genomic sequencing data was evaluated for English walnut in a proteomics approach to identify marker peptides. As anticipated, this provided enhanced selection of candidate peptide markers and differentiation between closely related species. The authors concluded that “Future improvements of protein databases and release of genomics-derived sequences are expected to facilitate the development of robust and harmonised LC–tandem MS-based methods for food allergen detection”.
2.4.14.13 Conclusions – walnut testing methods
Commercially available immunological methods for the detection of walnuts demonstrate tolerance to processing, which is crucial to reliably detecting walnuts in food products. LC-MS methods are not currently as sensitive as these other methods, however, as multiple authors have noted, once the genome of walnut is better characterised the sensitivity of this technique may improve.
2.4.14.14 Pecan
Pecan allergy is triggered by two different proteins, Car I 1, which is an albumin seed storage protein, and Car I 4, which is a hexameric legumin seed storage protein.
2.4.14.15 ELISA
The BioFront Technologies monoclonal antibody-based direct sandwich enzyme- linked immunosorbent assay (ELISA) for pecan detection was evaluated by Liu et al. (Liu, Zaffran et al. 2019). Flours prepared from autoclaved, blanched, fried, microwaved and dry roasted whole pecan nuts were incurred into Cornflakes, sponge cakes, and sugar cookies at the 0.5-5% (w/w) level. The LOD was 0.5 ppm (± 0.2 ppm) and the LOQ was 1.5 ± 0.6 ppm. This was poorer, by a small amount, than the manufacturer reported detection limit of other ELISA kits. The intra- and inter-assay variabilities were less than 14%. The detection antibody did not exhibit cross-reactivity with 155 foods/ingredients tested at 100,000 ppm, although it registered 0.6% and 0.8% cross-reactivity with 10,000 ppm of English walnut and black walnut, respectively. The target antigen was stable against autoclaving, blanching, frying, microwaving, and roasting. The antigen was detected in a variety of food matrices with 80.5–111.6% and 22.2–154.5% recoveries for pecan-spiked and incurred samples, respectively. The assay did not yield any false negative results among tested commercial and in-house prepared samples.
2.4.14.16 Conclusions – Pecan testing methods
Low levels of cross reactivities with non-walnut foods and high sensitivity following processing suggest that ELISA techniques for pecans are robust. In LC-MS both LOD and LOQs are higher for pecan, however these are in multi-allergen testing regimes.
2.4.14.17 Pistachio
Pistachio nut is responsible for triggering IgE-mediated reactions in allergic individuals, caused by several proteins.
2.4.14.18 ELISA
The AgraQuant pistachio ELISA assay kit (Romer Labs, UK) was used by Sanchiz et al. (Sanchiz, Ballesteros et al. 2017) to validate the performance of two real-time PCR methods (based on SYBR®Green and locked nucleic acid (LNA) probe) for the analysis of commercial products. The study only reported qualitative results from the ELISA assay (presence/absence of pistachio), and it showed concordance with in- house real time PCR (Pis v 1 primers designed by the authors) methods in 12 out of 14 food products analysed. The ELISA kit (LOQ 1–40 mg/kg) detected two false positives: pesto sauce, which contains 5% of cashew nut, and chocolate with hazelnut, suggesting cross-reactivity with these two nuts. The LNA probe-real time PCR method was the more sensitive, reliable and specific of the PCR methods with an LOD of 10 mg/kg pistachio and resisted gentle thermal processing.
2.4.14.19 Conclusions – Pistachio testing methods
It is apparent that much work has been underway to develop mass spectrometry methods to detect pistachio. The commercial ELISA method represented in the literature showed cross-reactivity to other matrices, as do other commercially available ELISA kits targeting pistachio (Table 1, Appendix 1). While the MS methods have low sensitivity, this level does not match the sensitivity of ELISA kits (commercial ELISA methods purport an LOD of approximately 0.1 mg/kg), so the suitability of these methods must be assessed against eliciting levels. As determined by the EFSA ThRAll project, it may be that optimisation of the extraction buffer may improve the sensitivity of the method towards tree nut species.
2.5 Conclusions to the literature review
This literature review has considered published studies relating to methods to determine allergens and highlights the strengths and limitations of such methods. Commercial ELISA kits have historically been the preferred methods for food allergen detection and quantification, especially by the food industry and enforcement agencies for the detection of contamination levels of many food allergens, although for certain allergens (for example celery), only PCR methods are available. However, ELISA methods are susceptible to erroneous results, partly due to the modification of the allergens (proteins) during food processing which can lead to reduced recoveries. Limitations include variable sensitivities and the performance specified by the manufacturer cross-reactivity, and a potential for low levels of protein recovery. PCR methods detect DNA and not protein (of which all fourteen allergen foods are comprised with the exception of sulphites and sulphur dioxide) and are not applicable to the testing of all fourteen allergens. PCR methods also suffer from limitations due to thermal processing of foods. As highlighted by Walker et al. 2018, Mass Spectrometry for the detection of allergen proteins is a developing area, promising a number of advantages over ELISA and PCR. Mass spectrometry can be more specific (less cross-reactivity) for detection of the target protein to be quantified due to careful selection of the species-specific sequence to be detected, provides protein identity information, permits a wider linear dynamic range, is less prone to be affected by food processing and, if appropriately applied, can be used as a reference method or for the production of CRMs. However, LC-MS/MS methods currently tend not to show the levels of sensitivity of ELISA methods and can also show low recovery, depending on the extraction method used. All methods (ELISA, PCR and MS) suffer from issues in accurate quantification of allergens due to a lack of harmonised incurred reference methods.
In the absence of perfect methods which are not blighted by cross-reactivities, low sensitivities and false results, incident management would benefit from a combined method approach, as is detailed in the workflow section of this report (Section 9).
Table 1 (Appendix 1) is presented to accompany this literature review to detail the scope and the various performance data of the commercial methods which have been used by testing laboratories over the past five years in their submission of proficiency testing data to Fapas®. Much of the data in this table requires the kit manufacturers to disclose the performance data for the kit and to fully declare how performance was monitored, how test samples were prepared for method validation, which foods were included, the number of replicates of each sample tested. There is a great variation in the amount of data disclosed in the kit manual, depending on the supplier and the target allergen of the kit. Some kit manufacturers/suppliers disclose very little data, choosing to simply declare the LOD, LOQ and the units of measurement. Other manufacturers/suppliers provide additional data, for example listing recovery, precision and cross-reactivity data and providing information regarding the sample types tested during method development and whether they were incurred or raw. Some suppliers choose to disclose a comprehensive list of all of the matrices tested (for example, more than 30), others include a much shorter list (4-6 matrices), and it is therefore unknown whether a comprehensive range of matrices has been tested during the development phase and how the kit performs. Some suppliers provide no information regarding the applicable matrices for the kit.
The method of LOD determination for commercial test kits are stated in a negligible number of kits. The most simple method of determining LOD would be to analyse a buffer spiked with a low level of allergen, the simplicity of this matrix would be expected to give the lowest LOD. Alternatively a finished food may be spiked with the allergen following processing, this would provide a more reflective matrix to a real sample compared to a buffer. Finally using an incurred product requires the allergen being added before all processing in line with industry recipes to produce a final product.
This last form of test material comprises the most challenging matrix. While this form of sample would likely show the lowest recovery of the allergen (and higher LOD and LOQ compared to ‘in buffer’ experiments) due to the effects of processing on the integrity of the proteins under investigation, it represents a ‘real’ food scenario and provides testing laboratories with the most comprehensive information when selecting an appropriate test kit for a test material. If data were more transparent, test kit users could compare the ‘real world’ capability of the test kits available to better inform regarding kit suitability.
While certain suppliers state in their manuals that validation data is available on request, others do not. In the interests of fairness, it was agreed that Table 1 (Appendix 1) would therefore be prepared from information provided in the user manuals. As highlighted in this table, there is often not a great deal of transparency regarding many of the validation parameters. This is a gap identified by this project. It would be beneficial to testing laboratories if full validation data were declared by kit manufacturers so that testing labs have a basis upon which to select methods which they can then perform some basic in-house performance measurements to determine suitability to their food types of interest before investing heavily in testing.
Further limitations to food allergen testing capabilities are discussed throughout the various sections of this project and in the conclusions of this project.
Dr. Popping is Chief Executive Officer at FOCOS strategic food consulting company. FOCOS advises food manufacturers, start-up companies, not-for-profit organisations, investors, laboratories and governments on strategic food safety solutions and emerging technologies. Dr. Popping previously worked as Chief Scientific Officer and Director Scientific Development and Regulatory Affairs for multi-national contract laboratories in global roles. He also serves on numerous standardisation committees and government working groups related to food allergens, including FAO/WHO Food Allergen Expert Consultations, European Committee for Standardisation (CEN) CEN/TC 275/WG 12, BRCGS Gluten working group and Association of Official Analytical Chemists (AOAC) allergen working groups. He previously served as AOAC General Referee for food allergen methods. Dr. Popping has also assessed the Codex Alimentarius priority allergen list through risk assessment. He has authored over 75 peer-reviewed publications on food safety, food allergens, food authenticity, food analysis, validation and regulatory assessments (FOCOS, 2023). Dr Popping serves on the board of directors of AOAC International and is a member of the editorial board of J. Food Additives and Contaminants and J. Food Analytical Methods. He co-edited a 76-chapter reference book “Present Knowledge in Food Safety” (ISBN 978-0-12-819470-6). Dr Popping’s contribution will support the alignment of the project outcomes to BSI, Codex and AOAC standardisation activities.
3.1. Consultation: Standardisation activities
Standardisation in the allergen testing field involves the preparation of Certified Reference Materials (CRMs) which can be used as a standard when testing for each allergen to calibrate the various kits that testing labs use to standardise the data.
Standardisation is proving to be a very long and challenging process and progress has been slow. To facilitate food allergen testing and protect allergen-sensitive consumers, the Food Allergen Working Group at the AOAC is preparing a guidance document highlighting the need for Quality Control materials (QCs) rather than CRMs due to the relative simplicity and speed of preparing QCs compared to CRMs.
3.2. Definitions:
Certified Reference Material (CRM):
Reference material, accompanied by documentation issued by an authoritative body and providing one or more specified property values with associated uncertainties and traceabilities, using valid procedures. Traceability includes metrological traceability, metrological traceability is a property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty (JCGM, 2012)
A CRM must demonstrate compliance to International Organisation for Standardisation Standard ISO 17034:2016 and ISO Guidelines 35:2006 and 31:2015. Therefore, CRMs tend to incur a high cost of preparation. A key point is that a reference method is necessary to establish a CRM. Such a reference method is available (currently) for only one allergen, cows’ milk. This has been established in a series of studies from the JRC Geel (See section 2.4.7.2).
Few CRMs are available. Work is ongoing at the European Commission Joint Research Centre for which certain CRMs are planned for completion within the next two years.
Reference Material (RM):
Material, sufficiently homogeneous and stable with reference to specified properties, which has been established to be fit for its intended use in measurement or in examination of nominal properties (JCGM, 2012)
The producer of an RM does not have to be ISO 17034 compliant.
Projects with aims to produce RMs include:
• FSA-funded project FS101206 for which an output was a chocolate paste RM containing skimmed milk powder, egg white powder, almond, hazelnut and walnut flours at the 10 mg/kg level along with a negative chocolate paste RM.
• the NIST Food Allergen Program (USA). Current food allergen CRMs from NIST include a value for total commodity as determined by non-specific approaches, such as total nitrogen measurement, and contain no direct link between the measured food allergen protein(s) and the reported total commodity value. NIST are now working to provide RMs which improve the connection between the measured protein food allergen and the reported total commodity value.
Quality Control material (QC): No regulations are imposed, matrix containing a specified level of allergen. Lower cost of preparation compared to RMs or CRMs. However, information on traceability and uncertainty may be lacking.
• In terms of similar Codex Alimentarius, ISO and CEN activities, the absence of CRMs and RMs may have adversely impacted the development of standards for allergen detection methods. CRMs for egg and milk had been developed as part of the initiative of the MoniQA Association (the International Association for Monitoring and Quality Assurance in the Total Food Supply Chain) and were commercially available. However, there have been recent challenges to these particular CRMs and they are no longer available
QCs are already available on the market, often from proficiency testing providers and these going some way to mitigate issues relating to the lack of RM until RMs are available.
One of the complications of preparing CRMs or QCs is that not all matrices respond in the same way during allergen testing, for example the allergen level yielded by a test may be impacted by the level of processing of a product, or the matrix types, for example oils matrices compared to baked goods. There is also a significant difference in the level of allergen yielded in a RM or QC which has been prepared by spiking with allergen post-production compared to an incurred RM (i.e. a matrix into which the allergen is spiked prior to processing). Since the production of incurred materials is time consuming and costly, it may only be feasible to prepare incurred materials for some key matrices. Therefore, the production of a range of RMs for each allergen, comprising a variety of matrix types so that data can be compared to matrix-matched RMs, may not be possible.
One of the recently emerging methods of allergens detection is the quantitation of multiple allergens by mass spectrometry. These methods require RMs. One of the objectives of the European Food Safety Authority (EFSA) project, “Detection and Quantification of Allergens in Foods and Minimum Eliciting Doses in Food Allergic Individuals (or ThRAll Project, reference GP/EFSA/AFSCO/2017/03) is to develop a harmonised quantitative MS-based prototype reference method for the detection of multiple food allergens in standardised incurred food matrices. This will be undertaken for cow's milk, hen's egg, peanut, soybean, hazelnut, and almond incurred into two highly processed food matrices, chocolate and broth powder. This project is co-funded by EFSA, United Kingdom Food Standards Agency and Belgian Federal Agency for the Safety of the Food Chain (FASFC) (see Section 5).
Commercial interests often override efforts to achieve standardisation, partly since the testing companies and the standardisation organisations both have commercial interests which may conflict. Nevertheless, efforts have been made by many of the standardisation bodies to determine the performance requirements of each method so that standardisation bodies could state which kits meet the performance characteristics (and therefore should be used by testing laboratories). For example, AOAC have developed the approach of SMPRs (Standard Method Performance Requirements) which facilitates the development of new methods, unhampered by existing, single-method-specific standards. If performance requirements were agreed unilaterally, this may facilitate standardisation activities in addition to supporting method development. Existing, method-specific standards may hamper the development of improved methods. A unilateral agreement of Method Performance criteria would allow such improved method development without having to perform a long and laborious standardisation process for each method.
3.3. Harmonisation activities
In comparison to the complexities impeding the development of activities aimed at standardisation, taking steps towards achieving harmonisation provides the opportunity to expedite the process to support comparative allergen analyses. With harmonisation, all testing facilities would use the same Quality Control standard (or the same CRM) to calibrate a test method to achieve more consistent results.
In Japan, an approach was adopted whereby the government organised the validation of allergen test kits manufactured by three providers for which test kits provided comparable results in validation studies. The three kit manufacturers were Morinaga Institute of Biological Science Inc., R-Biopharm AG and Nippon Chemiphar Co. Ltd. The Japanese government only recognises data generated using these three successfully validated kits, hence the majority of manufacturers producing food for the Japanese market follow suit and use only testing labs which use these kits. It could be that FSA, Defra or British Retail Consortium organise a similar kit validation process to harmonise allergen testing in UK. There is also a possibility that AOAC could offer the certification of certain commercial kits.
Aside from the evidence gaps in methodological testing capability of the various commercial test kits available (detailed in Table 1, Appendix 1), other gaps in allergen management are discussed below.
4.1. Current evidence gaps in testing
4.1.1. Determination of reference doses
While allergen consumption thresholds are available based on clinical studies, more work is required to determine the threshold of foods. Most recently, the ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens recommended reference doses for the global priority allergens, but other important allergens that are listed in UK and European regulations have not yet been assigned reference doses. Here, VITAL® 3.0 levels can serve as a guide.
4.1.2. Implementation of testing within the supply chain
(i) There is a gap here in that the level of control in the supply chain needs to be more tightly controlled to mitigate issues concerning undeclared allergens. While larger manufacturers and larger suppliers tend to invest in allergen risk assessment and confirmatory allergen testing, there are risks associated with many small-to-medium sized businesses which often perform no risk assessment and little or no testing.
(ii) Allergen testing costs approximately £150-250 per allergen by ELISA, which screens for a single allergen in each test. Multi-allergen tests are required to reduce costs and test times. Infrared spectroscopy (IR) methods have been developed for on-site detection or screening to detect multiple allergens. However, the sensitivity of IR methods is not sufficient (being in the percentage range rather than the required parts per million or parts per billion range) to support the required levels, given the threshold levels/reference doses suggested by the Joint Food and Agriculture Organisation and World Health Organisation (FAO/WHO) Food Standards Programme (FAO/WHO) Expert Group (WHO, 2021).
(iii) Methods are under development by loop-mediated isothermal amplification (LAMP) assays which could provide confirmative on-site testing of ingredients. The false positive rate of such a test (much like lateral flow tests) is low.
(iv) Methods of point-of-use testing of ingredients by technologies including LAMP and Near-Infrared Spectroscopy (NIR) are under development for areas such as quality control and authenticity of products, for example protein, sugar and fat composition. It is anticipated that new, validated low-cost methods will be available for point-of-use ingredients testing for allergens within 5-10 years. This may permit manufacturers to gain fast knowledge regarding their raw material safety and quality at the time of use in the factory.
(v) Increased energy costs and shortage of supplies (for example linked to issues in transportation, and/or to the war in Ukraine) are causing changes in supply chains. Manufacturers are increasingly resorting to spot buying of ingredients when regular (and trusted) suppliers cannot meet demand or are using alternative (undeclared) ingredients. The practice of spot buying can inherently result in a reduced level of audit data and can result in increased risks of food fraud and safety and quality concerns.
4.1.3. Analytical gaps and testing service provision
(i) Those in the supply chain must become better-versed regarding the testing types which they request from the laboratories and which are fit-for-purpose for their sample types. For raw materials this is not a severe issue as many testing methods are fit-for-purpose for raw ingredients. However, when foods are processed, the proteins can be altered and, in general, it is more difficult to detect the allergenic protein or peptides, and detection can be reduced or the method may no longer be fit for purpose. For those needing accurate testing results of processed products, it must be understood which types of testing methods are fit for purpose. For example, there are laboratories that offer testing services by PCR for egg to detect egg-specific DNA. Here the challenge is that an entire egg contains only a single copy of DNA in the egg yolk, which is very little for detection by most DNA-based detection methods currently used. In addition, the food industry used fractionated products, e.g. egg white powder, which contains no DNA but large quantities of (allergenic) proteins that can trigger severe allergic reactions in susceptible individuals. Therefore, DNA-based detection methods such as PCR may not be fit-for-purpose for detecting the presence of egg and its derivatives like egg white powder. Similarly, PCR methods for milk tend to lack sensitivity. Alternative methods to PCR, e.g. ELISA, should be offered and non-fit-for-purpose tests should not be offered.
(ii) Full validation of testing methods is required (involving a series of studies to determine accuracy, precision, sensitivity [meaning the slope of the calibration curve], specificity, robustness, applicability, repeatability, reproducibility, LOD, LOQ and range of LOQ studies) for a wide range of matrices. The risk of false negative results would need to be investigated, especially for matrices with a high risk of false negatives. An example of such matrices includes tomato-based matrices due to the low pH of tomato, and tomato ketchup which also includes vinegar. At low pH, the DNA auto-catalyses and DNA may not be detectable by PCR, a false negative result may be yielded by PCR. In such cases, analysis by Mass Spectrometry or ELISA would be preferable. PCR testing is also less useful when a matrix has a high protein load and a low DNA load, for example egg.
(iii) As discussed in more detail under Harmonisation Activities (Section 3), there is a need for RMs or, as a minimum, quality control material against which all testing laboratories should calibrate their methods. Such RM is needed for each allergen, and ideally prepared in a range of applicable food matrices for each allergen to account for matrix interferences of certain ingredients and/or processing conditions. As discussed elsewhere in this report, RMs are currently completely lacking for most allergens. There are difficulties in preparing RMs to cover all possible food matrix types. Producing a RM for matrices such as mayonnaise for example (egg and mustard allergen concern) which contains significant levels of oil and has low pH is challenging due to challenges in extracting protein from such oil-based samples.
(iv) There are also requirements to consider the fraction of a protein which is being tested. For example, in milk, there are commercial kits containing antibodies against the casein protein. However, if the milk is fractionated during processing, and the whey proteins fraction is being used for food production, the casein allergen may no longer be present and thus cannot be detected, which does not mean that no allergens are present. Also, incorrect levels may be determined if a particular protein fraction that the antibody recognises is enriched or depleted during fractionation.
(v) Further method development is required. A reliable method of evaluating the performance of a particular method is for the method to be the subject of an inter-lab trial. Examples of inter-lab trial data are provided in the literature review section of this report but, as an example, twelve laboratories participated in a trial to determine sesame in a mayonnaise containing 47 mg/kg sesame, which is a high level of sesame given that the reference dose is 2 mg. Only two of the twelve labs detected sesame, and both under-estimated the level by approximately 50%. (Besler-Scharf, 2021) Mayonnaise is a challenging matrix due to its acidic pH and oil content, but this demonstrates that further method development is required to protect consumers, to detect even high levels of allergens in some matrices.
(vi) It is important that more method validation data and quality data are included in the manufacturers’ kit instructions so that it is clear exactly which matrices have been used in their validation studies to inform users. However, several of the allergenic commodities are so versatile that they are an ingredient (in one form or another) in many foods. For allergens such as soya, it is estimated that soya and its derivatives can be found in over 30,000 products worldwide.(personal comms) As an example, soya can be used as flour, lecithin, oil, phospholipid fraction, phytoestrogen. It is likely that milk and its derivatives are contained in a similar number of products. While method validation requires investment, the more products for which validation data is available, the more is known about the applicability of each method/test kit. In an ideal situation, such validation data could be held in a central database, allowing other laboratories to access it, thereby significantly reducing the workload and sharing the efforts across laboratories. However, this will only work if validation has been performed to national or international standards.
(vii) Similarly, a gap exists in that manufacturers are currently not obliged to publicise their day-to-day allergen issues so many issues are resolved before recalls are required. Therefore, the information the public and governments can access may not reflect the real situation. Changes are required so that all allergen-related issues are logged centrally so that we are aware of the scale of allergen issues in the UK.
(viii) ELISA is currently the most popular method for allergen detection, due mainly to the simplicity and wide availability of commercial kits. In addition, ELISA tend to be appropriately sensitive and specific for allergen proteins, particularly in raw ingredients. The acceptable cost and required instrumentation make this technique attractive for laboratories, in addition to the comparatively low level of expertise that is required by laboratory staff to conduct the method and evaluate the results. Given that there are gaps in the testing capability, some ELISA kit manufacturers however include a caveat in the user guide to their methods of using PCR as a confirmatory method. PCR is a lower cost method compared to ELISA and is often available and well-established in laboratories which also use ELISA, which may be the reason that kit manufacturers suggest this confirmatory methodology to users. As described above, PCR is not always an appropriate confirmatory method. Furthermore, it is wise to apply a confirmatory method which can detect the allergenic substance i.e. the peptide or protein, rather than aiming to detect the DNA of the host ingredient. However, a more appropriate confirmatory method would be liquid chromatography mass spectrometry (LC-MS) as this method detects the allergenic peptides and proteins themselves. Arguably, fewer laboratories which use ELISA would also have LC-MS facilities which tend to be high cost in terms of purchase, running and maintaining, and require specialist training outside of the molecular biology skills used for PCR and ELISA. It seems that there is a funding gap for developing the use of LC-MS/MS for allergen detection and quantitation compared to the development of PCR and that there should be more focus on future work to prepare LC-MS methods, which also have the benefit that they can be multiplex methods to detect multiple allergens in a single analysis. EFSA has recently funded a project (please refer to section 5 on EU Project ThRAll) with the goal to develop reference (harmonised) methodologies for the detection and quantification of allergens in foods using mass spectrometric approaches.
(ix) Point-of-use testing could be improved for use in factories. The Titan Project (providing digital technologies that increase transparency throughout the food value chain to save money, resources, people, and the planet) includes the development of emerging nanotechnology devices for use by manufacturers in order to reduce the burden of allergen testing on the manufacturer.
Sampling regimes in factories require careful planning. Single-point sampling is a risk. A two-prong method of testing may be preferable:
(1) Perform tests on incoming raw materials to check for cross-contamination or mislabelling
(2) Environmental mapping on site, performing point-of-use testing of areas identified as high-risk areas on site and also on the production line to check for contamination.
The Titan project will test these scenarios. Artificial Intelligence methods may also prove beneficial to access historical data to determine particular areas of a site, or specific suppliers, where the risk has been shown to be elevated, to manage and prioritise testing efforts.
4.1.4. Method Performance Criteria
(i) Lessons can be learned from other countries to improve method performance criteria for allergen management. In Germany, a working group exists to support allergen management in the food chain, comprising approximately 75% members from government and 25% from industry. The aim of the working group is to develop official government control methods (analytical methods) for the detection of food allergens. While, as previously mentioned, ELISA is by far the most commonly deployed detection method for food allergens, very few commercial ELISA methods have been validated by this group, which is in part because the government aims to
avoid commercial imbalance by validating only one or two commercial kits for an allergen while there are more ELISA kit producers offering detection kits for the allergen of interest. Also, since many of the validated methods are submitted for international standardisation, preferring certain methods over others would make it difficult for newer, potentially even better, methods to be accepted. As this is a general problem for methods which are standard methods, an approach at Codex Alimentarius was launched to establish Method Performance Criteria (MPC), also referred to as Method Performance Requirements (MPRs), rather than standardising individual methods. Setting such MPCs/MPRs would allow new methods to be accepted as long as they fulfil the criteria. AOAC International has adopted this route and developed an SMPR® (Standard Method Performance Requirements) program for different types of methods and commodities, including food allergens. Here, AOAC SMPR® 2017.020 and AOAC SMPR® 2018.003 lay down the requirements under which such methods could be accepted by AOAC, either as a Performance Tested Method (PTM) or Official Method of Analysis (OMA). Irrespective of the approach, single method validation or setting method performance criteria, the availability of appropriate RM may facilitate these approaches.
However, according to work by Rzychon et al 2017, RMs will not necessarily improve measurements where this lack of correlation is observed, although their use will highlight the variation which will be of interest to risk assessors. (Rzychon et al., 2017) With correlation between test kits, RMs improved comparability of results.
However, beneficial effects were not observed equally by all kits or even for all matrices on the same kit. While this work was based on gluten ELISA tests, the conclusions may well apply to any protein ELISA.
(ii) Auditing within the food chain in the UK and EU is deemed to be of a high quality. It is estimated that contractual agreements between buyers and sellers on safety and hygiene standards and of food certificate checking further afield would yield improvements in allergen management and safety, with independent auditing of overseas suppliers. It is known for example that, as part of their risk assessment, some companies in non-European countries do not test for all allergens held in their facilities or handled in their factories, due to the financial burden of testing. An example is a company outside of Europe known to handle much dried fruit but also handles tree nuts in the same facilities, although this is not declared on the dried fruit labels(personal comms). Without audit and inspection, we cannot be sure of the quality standards within the supply chain with its ever-growing complexity. There is also a trade-off here as more affluent countries such as the UK can afford to invest in high- end detection equipment such as LC-MS/MS but poorer exporting countries will rely on lower cost technologies, which may or may not be appropriate for testing the particular ingredient or compound. Audits evaluate the status of a commodity at a point in time only and are used to confirm that measures have been implemented.
For this reason, the key here again is education about the risks of food allergens and their adverse health impact, especially at the beginning of the food supply chain, so that the correct checks and tests can be implemented. With this knowledge, then the appropriate analytical testing can be applied and high-quality audits could be implemented to verify conformation to high standards. Without audits, the UK is unaware of practices in the global supply chain, since financial issues, education and perception of food-associated risk differ across the globe.
4.2. Emerging risks
The main emerging risk at present in terms of allergen management concerns alternative proteins. In order to feed a growing global population, much innovation is currently underway worldwide to prepare proteins from alternative sources compared to those used today, or to use the same protein sources but in new ways, such as from livestock meat, dairy foods, fresh vegetables, grains, nuts, beans, pulses and seeds. Insect proteins are being widely considered and developed to appeal to Western palates. Much in the same way as a much higher proportion of Asian consumers are sensitive to milk compared to Western consumers, there are considerations here that when new protein food sources such as insects or more highly processed vegetable proteins are introduced to a new population of consumers, data may emerge of increased levels of inherent allergen incidence due to the biology of this particular population. Also, insects such as cockroach contain tropomyosin which is a protein structurally largely identical to the tropomyosin of crustacean, a known allergen. Therefore, allergen labelling of insect foods or insect matrices used in food production is recommendable to protect crustacean-allergic consumers.
Aside from insect protein, novel ways to use plant-based proteins are being developed. Plants including soya and pea are being processed in novel ways to produce vegetable ingredients which simulate the texture of meat to offer a meat- free alternative to products such as beef steak. During this processing, the proteins are often enriched. It is important that we understand how processing is affecting the protein, and thereby the allergen, even for plant proteins we are familiar with but in other, differently processed, forms. As an example, a protein containing a sequence or structure known to trigger an allergy, may be ‘hidden’ and non-reactive inside the folded protein when in its native form. However, processing may release the allergenic sequence/structure or simply increase the level of allergen within the ingredient which could lead to an increased risk of allergic reactions.
It is already known that some consumers are sensitive to pea proteins, however, insufficient cases have been reported to initiate a response in declaring pea protein as a food allergen of concern. Pea protein is of major interest to current novel protein innovations. There is a requirement to be proactive to develop testing methods for pea before the alternative proteins market grows to the stage where the prevalence increases to a level which would justify declaring pea as a regulated food allergen.
Also, since there are risks that processing methods may change the allergenicity of a commodity, there is a need to educate those who are developing these innovations, often small enterprises, so that risk assessment and testing strategies can be implemented to avoid innovation of products which unintentionally increase the risk of eliciting an allergic reaction. An example of such failed innovation in the GMO area is the transgenic soybean which carried a Brazil-nut allergen (albumin). (Nordlee, Taylor, et al., 1996) This product was never marketed.
4.3. Conclusions
Evidence gaps in allergen testing have been discussed above. Necessary improvements to testing services have been highlighted. Improvements and gaps in the methodologies used for allergen testing have been highlighted here and are also discussed in detail in the literature review section (Section 2). Reliable, independent auditing to the same standard is required for all global suppliers in the UK supply chain. Finally, we must act now develop tests and risk assessment approaches to inform risk management regarding issues that may arise from emerging risks such as novel foods.
As highlighted by many stakeholders and our expert consultants, there is a need for the development and commercialisation of a fast multi-allergen test, which can be used at point-of-use in factories to mitigate cross-contamination risks. Funding to develop such a test is required. In addition, more funding to improve current multiplex mass spectrometry methods would support rapid, single test confirmatory testing of allergens in foods.
As the co-leader of the EFSA ThRAll project (detection and quantification of allergens in food and minimum eliciting doses in food allergic individuals), Clare’s collaboration will align the literature review with the outcomes of the ThRAll project. Clare currently holds a joint appointment between the Universities of Manchester and Surrey. Her laboratory is based at present in the Manchester Institute of Biotechnology at the University of Manchester and is part of the Respiratory and Allergy Research team at the Wythenshawe Hospital and the Immunology Section at the University of Surrey. She led the EU integrated projects iFAAM (integrated approaches to Food Allergen and Allergy Management) and EuroPrevall (the prevalence, cost and basis of food allergy across Europe) and coordinated the European Food Safety Authority project ThRAll and currently leads the UK Food Standards Agency project PAFA (Patterns and Prevalence of Adult Food Allergy).
Clare is also a partner in a recently awarded project from EFSA led by EuroFIR on allergenicity prediction. Professor Mills is a member of the FSA Advisory Committee on Novel Foods and Processes and was involved in the recent FAO/WHO Expert Consultation on Food Allergens. Her personal research interests are focused on structure-function relationships in food proteins particularly with regards what makes some proteins, and not others, become allergens, including the effects of the food matrix and processing on resistance of food proteins to digestion and the role this plays in determining the allergenicity of foods.
5.1. Report on EFSA project GP/EFSA/AFSCO/2017/03. “Detection and Quantification of Allergens in Foods and Minimum Eliciting Doses in Food- Allergic Individuals” (ThRAll).
Project partnership: This contract/grant was awarded by EFSA to Professor Clare Mills, School of Biological Sciences, Division of Infection, Immunity and Respiratory Medicine, Manchester Academic Health Science Centre, Manchester Institute of Biotechnology, The University of Manchester (UNIMAN) UK.
Following the University of Manchester not renewing its article 36 membership the contract was transferred to Partner 1 (Dr Linda Monaci, Institute of Sciences of Food Production, National Research Council of Italy (CNR-ISPA), via Giovanni Amendola 122/O - 70123 Bari, Italy).
Contractor/Beneficiary: The University of Manchester (until 18th December 2019); CNR-ISP (19th December 2019-30th September 2022).
Other Partner Organisations were as follows:
Partner 2: Flanders Research Institute for Agriculture, Fisheries and Food (ILVO), Brusselsesteenweg 370, 9090 Melle, Belgium.
Partner 3: CER Groupe, Rue du point du Jour, 8, 6900 Marloie, Belgium. Partner 4: INRAE UMR 1163 Biodiversité et Biotechnologie Fongiques (BBF), F- 13288 Marseille, France, INRAE UR1238 BIA, Rue de la Géraudière, BP 71327, 44313 Nantes, France and INRAE-CEA, Service de Pharmacologie et d'Immunoanalyse, Laboratoire d'Immuno-Allergie Alimentaire, Bât. 133-CEA de Saclay, 91191 - Gif-sur-Yvette, France.
The project was co-funded by the United Kingdom Food Standards Agency (FS101209) and the Federal Agency for the Safety of the Food Chain (FASFC, Belgium).
5.2. Summary of Results
5.2.1. ThRAll Objective 1: Develop reference (harmonised) methodologies for the detection and quantification of allergens in foods.
The focus of Objective 1 of ThRAll was the development of a prototype multi-analyte mass spectrometry-based reference method for determination of allergenic food ingredients (Mills et al., 2019). A systematic review of the literature on food allergen analysis using mass spectrometry (MS) was performed and peptide markers for the six allergenic food ingredients collated. The peptides were evaluated and filtered based on their length, their type, food matrix and level of processing investigated, and whether they were identified using discovery or targeted MS analysis. Peptides containing amino acid residues prone to modifications, such as methionine or asparagine-glycine motifs, were excluded. Peptide specificity and potential sequence similarity with homologous proteins from related species was also assessed. Based on this analysis a preliminary list of candidate marker peptides was developed (Pilolli et al., 2020).
To support further evaluation of the candidate peptides, test method comparisons and validation two difficult-to-analyse food matrices were prepared for the project based on a chocolate bar and a powdered broth. These were incurred with six different allergenic food ingredients namely cow’s milk, hen’s egg, peanut, soya, hazelnut and almond. These included RMs developed through the UK FSA call FS101206 Development of Quality Control Materials for Food Allergen Analysis) and RMs from NIST and MoniQA. The materials were assessed for homogeneity and the IgE-binding capacity of the allergenic ingredients assessed using in vitro test methods using serum samples from relevant food allergic subjects (Huet et al., 2022).
Analysis of these materials provided data which were then used to further filter peptide markers to give a preliminary list of around fifty candidate marker peptides
(Pilolli et al., 2021). These were then systematically evaluated with optimization using multiple reaction monitoring (MRM) experiments executed on triple quadrupole instruments. Since the broth powder proved to be very highly processed with many allergens poorly detected by either immunoassay or MS-based methods, the method optimisation was undertaken using allergens incurred into the chocolate bar matrix. The method optimisation assessed methodological parameters including extraction and purification of allergenic ingredient proteins and optimisation of digestion protocols (Henrottin et al., 2023). This analysis identified the key methodological parameters and allowed a subset of the peptide markers to be identified which were synthesised in stable isotopically labelled forms for use as external calibrants in further method validation.
The MS results will be compared with analytical results obtained on the same incurred materials using ELISA but an assessment of droplet digital PCR based methods (ddPCR) showed they were not suitable for use with such complex incurred matrices. Thus, a comparison of test method data is only possible with ELISA. Working with the community, an approach to develop harmonised conversion factors has been developed. These were then applied to analysis of an inter-laboratory assessment of the prototype test method. This demonstrated the transferability of the method, despite its complexity, across laboratories experienced in allergen analysis. The method has the sensitivity required to quantify the allergens from egg, milk, peanut, almond and hazelnut at the action levels identified for these foods by the recent FAO/WHO expert consultation (FAO/WHO, 2022). Further refinement to improve the sensitivity by approximately 3-fold will be required to enable the method to be fully deployed in line with the FAO/WHO expert consultation recommendations for test method performance. Further refinement to bring the detection of peanut and whey in line with that of egg, soyabean, hazelnut and almond is also required, perhaps developing an optimised extraction buffer. Furthermore, given that there is indication that the boiling step during the preparation of the broth powder may have impacted on the diminished detection of peanut in this matrix, work to understand the impact of extensive processing on the clinical reactivity of food among sensitive consumers would be beneficial, to determine if, for example, the highly processed peanut in this matrix would still elicit an allergic reaction.
5.2.2. ThRAll Objective 2: Generate good quality data on Minimum Eliciting Doses (MED) and Minimum Observed Eliciting Doses (MOED).
Through systematic mapping of clinical record forms, a harmonised approach for coding of food allergy data was developed which will support collation of data on minimum eliciting doses from low-dose oral food challenges undertaken in food allergic patients. This was used as the basis for developing an electronic record using the REDCap secure web application for managing online databases and surveys to which data were either uploaded directly or entered from the literature. Data gaps identified included the lack of challenge data for foods such as Brazil nut, macadamia nut, molluscan shellfish and lupin. For many other foods, fewer than 60 patient records could be identified for inclusion, as is required for best practice modelling (Klein Entink et al., 2014). Many of the foods for which data were lacking represent less prevalent food allergies which makes it more difficult for clinical studies to identify many patients to include in any threshold study.
Options for modelling dose distributions were explored using fish as a case study. Data from two published studies were harmonised, the dose distributions modelled using interval censoring survival analysis and the MEDs calculated. This analysis demonstrated the benefits of combining studies in providing dose estimates with narrower confidence intervals. The combined data set provides ED 05 values close to those published by EuroPrevall and a little lower than those published in the recent FAO/WHO expert consultation. It was not possible to apply novel model averaging approaches since the code available was designed for use with only one particular database. This approach provides a framework for the future curation of oral food challenge data.
5.3. Conclusions: Aligning the ThRAll outcomes with this review
The new LC-MS method developed in this project benefits from detection of allergens at the action levels identified for these foods by the recent FAO/WHO expert consultation (FAO/WHO, 2022). This method, along with the incurred RMs
developed, would therefore strongly support current methods (mainly ELISA), acting as a confirmatory method during incident management, to interrogate foods for hen’s egg, milk, peanut, almond and hazelnut.
UK food suppliers were contacted and answered questions regarding their allergen testing procedures, either during an interview or in response to receiving the questions in a questionnaire format. The responses are tabulated and enclosed. Identifying information has been removed from the responses so that the answers cannot be traced back to individual companies or employees. For this reason, products produced by each company are simply referred to as ‘own brand’ in the documents.
A questionnaire was issued to each of the stakeholders. The provided table of responses is included on the following pages.
Company A
| Question | Response |
|---|---|
| Overview of business | Global manufacturer. Respondent works in strategy, policy and incident management. |
| Can you describe your allergen management regime | Allergen management regime is primarily based on the HACCP, on which Risk Assessments (RA) are based. This included quality policies and involves every stage of manufacture from the procurement of raw materials, to goods received, to controls in manufacture, packaging and distribution. Effort is made to maximise choice and minimise risk. A significant part of minimising risk involves cleaning of the production lines and validating this cleaning process. Critical Control Points on the lines have been identified. The company only uses PAL if it has been determined by the RA that sporadic cross-contamination may arise. The company has introduced a programme for labelling guidance based on research data from working with stakeholders such as the Anaphylaxis Campaign, that 30% of consumers ignore labelling. The company has a maximum level of allergen carryover that it permits. |
| Which specific allergens from the 14 food allergen groups does your company test for? | Most of the 14 groups are tested for, testing for allergens known to be in the foods prepared. Testing is performed following intelligence such as Horizon Scanning data or information from Trade Associations, but most testing is conducted on the production line during validation to verify cleaning processes so test for the allergens known to be included in those products (see later). So mostly peanuts, tree nuts (almond and hazelnut), milk, eggs and, in some factories, crustacea. |
| Which allergens are not tested for and why? | Most allergen testing occurs to validate and verify cleaning of the production line. For example, if the predominant (in terms of quantity) allergen in Product X is egg, following cleaning, products prepared at 8 times points following cleaning between Time=0 and Time=60 minutes will be tested for presence of egg. This way, if egg is not present, the cleaning process has been successful in terms of removing egg and there is confidence that allergens which may have been present at lower levels in Product X will also have been removed during the cleaning process. |
| What factors do you consider when determining which items will undergo testing for allergens? | A risk-based approach is adopted. For a production line, the product which will be prepared on that production line with the highest allergen loading will be used for the basis of validation of a Critical Control Point (CCP) study. Following production of that product, various swabs will be taken at various points on the production line by a specialist sanitation team, including swabbing of different materials which are present on the line such as rubber and stainless steel. The next product to be prepared on the line is also tested at 8 timepoints from T=0 to T=60 minutes on three different occasions to prove that PAL is not required. This risk assessment stays in place until something changes and verification checks are also performed annually. Regarding products with 'free from' claims, these are prepared in a dedicated facility only handling 'free from' ingredients so no environmental monitoring is needed but raw materials are tested on site by ELISA. |
| For a given product, how do you decide which allergens to test for? | The allergen testing is targeted to the predominant allergen which has been present on the production line (see above). Following a consumer complaint, as much detail is established as possible, including clinical diagnosis of allergic status and testing is performed on the outcomes of this investigation. Suppliers must provide details of allergens handled on their sites, provide certification of audit which is carried out by a third party inspector organised by Company A and suppliers must provide details of allergens handled by their previous supplier, so that stock is traced 'one step back' before the supplier to the respondent's company. |
| What do you test for allergens? | Most testing is of environmental monitoring samples and final products. Less emphasis on testing of intermediate products as allergen monitoring is risk assessed for the final product, as described above. |
| Can you describe your sampling procedure. | In terms of the HACCP standard, the number and location of swabs taken for environmental monitoring are dictated in this document. Data generated from respondent's horizon scanning activities of incidents and vulnerabilities (e.g. due to commodity shortage) in the supply chain and data from Trade Bodies and trade consortia (including nation bodies for allergy research) feed into intelligence and may incite testing. In the cleaning of the lines, a 'worst case scenario' approach is taken to determine that allergens used on the production line has been removed by cleaning processes (as described above). |
| How many replicates of each sample are tested by each testing laboratory? | At least duplicate analysis, further replicates are dependent on the protocols of the testing lab. |
| What factors do you consider when selecting a testing laboratory? | The laboratory must provide suitable data for spike recovery testing on matrices provided by Company A. For new matrices, spike recovery data must be generated. Spike recovery data must be within 80-130% of the spiked level. Company A also sends a Quality Control sample with each sample for testing which contains the allergen to check that allergen is detected in that sample by the testing lab. Laboratory must be accredited to ISO 17025 and the laboratory's performance in FAPAS® proficiency testing rounds is considered. These criteria are checked annually by the respondent's company. |
| Are the data qualitative or quantitative? | Quantitative, but the respondent's company recognise there is uncertainty of measurement with data generated by ELISA. Company A has also organised testing performed by RT-PCR in the past but it is difficult to interpret since there is no direct quantitative link between PCR copy number and the level of allergen present so PCR is only used for information and ELISA is relied on for quantitation. |
| What are the units of measurement |
mg/kg of allergen (mg/kg of allergen protein, where available) |
| Is there a particular LOD or LOQ that you require of the test. | Low ppm required. Company A are concerned if the LOD or LOQ is lower than 1-2.5 ppm since such tests run the risk of detecting artefacts. The respondent's company insist that the laboratory verify their own LOD and LOQ when using the test kit, rather than relying on the LOD and LOQ the kit manufacturers state should be yielded. |
| Which technologies are used in your testing of allergens? | ELISA only, with some information generated by RT-PCR but confirmed by ELISA. The respondent's company does not trust data generated by lateral flow device (LFD), which is qualitative only). Some matrices are difficult to analyse, e.g. chocolate, in which the fat and polyphenols interfere with LFD testing so ELISA is preferred over LFD. |
| What measures do you have in place for Quality Control? | Testing laboratories must take part in FAPAS® proficiency testing rounds and must perform as expected, supplying this data on the test report. Matrix validation studies must be completed by the testing lab and spike recovery testing must yield data within the 80-130% tolerance of the expected level. The testing lab must be accredited to ISO 17025 and use RMs. If a conversion factor has been used to convert the test data to the level of total allergen protein detected, this must be detailed in the report. |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? | For routine CCP verification, colleagues in food safety/sanitation interpret the data. Data resulting from testing resulting from an incident is interpreted by the most senior colleague in Incident Management, who performs a risk assessment to determine if there is a safety risk for consumers. For testing for claims on a 'free from' product, the food safety team interprets the data. |
| Do you use action levels/VITAL® reference eliciting doses to determine safe levels? | Yes, maximum/indicative levels are based on VITAL® 3.0 reference doses. In the future, the respondent is hoping that Codex recommend use of VITAL® 3.0 data and that the UK regulations will reflect this. |
| How do you decide when to recall a product? | A food safety risk assessment is prepared, based on information including the number of affected units outside of the company's control, the prevalence allergy in the affected geographic area, severity of reaction (known from clinical studies, where >ED10 tends to give more severe reactions and ED05 gives non-severe reactions which do not require medical intervention). The respondent's company use ED05 as a cut off value. Market action is taken depending on these considerations. FAO and WHO use ED05 as their recommended level for PAL so this is a conservative/precautionary level. The cost of recall to the company is cognisant that food allergy and acute reactions are high on the company's agenda which is why this company uses guidance from research for labelling allergens as safety is non-negotiable to the company. |
| Do you use any tools or calculations to interpret the data? |
The data is added to the RA or HACCP. Trends are compared to determine annual performance to identify any areas of risk. |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime. When you consider the costs in allergen testing, where do the direct costs to your business lie? | Cost of testing is £50-80 per sample, therefore the cost of each CCP is £1,200-1,920. Testing is undertaken when risk assessment dictates it is required, along with annual verifications. Testing products with claims of being free from allergens is higher so is undertaken in-house. |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | Safety and testing requirements are non-negotiable. |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? | Outside of the respondent's remit. |
| What type of assessments do you generally perform and do these differ by product / allergen? | Probability Risk Assessment and Deterministic Risk Assessment. |
| Are there any other challenges you face when considering the possible allergen content of your products? | Biggest challenge is the requirement of approved testing labs. The differences in commercial testing kits used by these labs is also a big challenge as the performance of the kits is different when analysing cooked and processed goods compared to less processed or raw goods, so the Quality Control samples are very important. The respondent considers themselves fortunate not to deal much with matrices for which there is known cross-reactivity of the test kits such as mustard cross-reactivity with oilseed rape. The respondent states that there is a role for the lab to play in open dialogue with its customers, being transparent about the evidence gaps and challenges to the performance of the test kits they are using and that this open dialogue should be encouraged. Another challenge is the need for the lab to be able to report results in mg/kg allergen protein, this is straight-forward with some ELISA kits, with others a conversion factor is required and this is not possible for allergens tested by PCR. There is also a need for more incurred RMs to be commercially available for labs to use as part of their QC considerations. There is a need for all testing laboratories to be transparent about the kit they used, the conversion factor, QC used and to provide the data from the QC. |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | It would be ideal if it would be possible in the future to have a low cost, instant, quantitative dipstick-type point-of-use test which would allow a faster turnaround time in allergen testing. Usually, testing takes 10 working days, or can pay for priority testing when required. Also, chocolate is a difficult matrix for testing and more quality controls should be available for this matrix. |
| Do you have any points you would like to raise, or further comments? | It would be ideal if the regulations could include a requirement for the use of VITAL® 3.0 data for setting acceptable allergen levels in foods. A regulatory system is required for PAL, based on VITAL® 3.0 data. There should be harmonisation between the regulations by UK, EFSA and FDA to facilitate international supply of raw ingredients and final products. |
Company B
| Question | Response |
|---|---|
| Overview of business | UK retailer, selling fresh, frozen and ambient foods. Products are both branded and unbranded and there are different processes for each regarding allergen management. We also sell imported foods and have cafes/counters. |
| Can you describe your allergen management regime | For own brand goods, RA and HACCP are in place and all testing is conducted by UKAS-accredited laboratories. Cleaning processes are validated. All suppliers for 'own label' products must be working to at least BRC A grade for unannounced audits. Allergen management of branded goods is the responsibility of the supplier. Minimum standards are set for suppliers and suppliers of high-risk matrices (e.g. raw meat, cured/dried meats, pufferfish, or supplied by microbusinesses) must be working to at least BRC A grade for unannounced audits when taken on by the respondent company. Cleaning processes are validated and all testing is performed by UKAS- accredited laboratories. A HACCP must have been put in place by the supplier and the respondent has issued a food safety manual with a section on management of allergens and on preparing products with ‘free from’ claims and vegetarian and vegan products. Suppliers must also risk assess their labelling process and determine if PAL is needed. Suppliers must provide data showing that their cleaning process is validated. A risk assessment is prepared regarding the Finished Product Surveillance System. Suppliers must use UKAS-accredited testing laboratories. Emerging risks are assessed in-business. Incident management is both local (supplier-specific) and can also involve discussions with FSA. Allergen management of branded goods is the responsibility of the supplier. All suppliers must comply with legislation. |
| Which specific allergens from the 14 food allergen groups does your company test for? | All allergen groups are tested for if there is a risk. Lupin is the least tested for since few of the respondent's products carry a lupin risk. |
| Which allergens are not tested for and why? | None. All allergens are tested for if required by the RA. The respondent only tests own brand products. |
| What factors do you consider when determining which items will undergo testing for allergens? | Risks arising from cross-contamination, PAL labelling, products with free from claims, if emerging risks have come to light, previous supplier performance, previous testing results for this matrix. |
| For a given product, how do you decide which allergens to test for? | Test for allergens of risk in that supply chain, considering allergens handled on site and at the raw material site. Also perform random due diligence testing for allergens not handled on the site. |
| What do you test for allergens? | Finished products mainly, although consider risks for raw materials and may implement testing according to RA. |
| Can you describe your sampling procedure. | A routine surveillance programme occurs. A prescribed number of products per category are selected using random selection techniques by a third party and tested each period. The randomisation can be deliberately skewed if required, e.g. due to emerging issues or intelligence. |
| How many replicates of each sample are tested by each testing laboratory? | One sample is taken for testing and an audit sub-sample is retained in case results require confirmation at a later date. The number of replicates of the sample depends on the standard procedure of the laboratory. |
| What factors do you consider when selecting a testing laboratory? | Laboratory must meet spike recovery parameters prescribed by the respondent company. Testing is repeated if data are close to the LOD of the kit. Laboratory and method must be UKAS-accredited. Reliability of lab, history of performance, inclusion of QCs, as required for UKAS. |
| Are the data qualitative or quantitative? | A mix. Quantitative data is preferred, majority of methods are semi-quantitative (ELISA). Celery is determined by PCR due to concerns about cross-reactivity but there is a challenge in interpreting these results. |
| What are the units of measurement | Depends on the units of measurement provided by the particular kit used by testing laboratory. Reported in mg/kg of protein or mg/kg of allergen protein. Units in which data are reported impacts on the RA. |
| Is there a particular LOD or LOQ that you require of the test. | The LOQ of any test used must be achieved in a repeatable manner, so repeatable, reliable and statistically accurate. Seek most accurate LOD, not the lowest. In products with a ‘free from’ claim, a maximum LOQ is set and the LOQ must be stated in the report - this LOQ is dictated by gluten Regulation in the case of gluten. For other allergens, maximum LOQ limits have been set following consultation with numerous labs and supply bases to establish 'the norm'. |
| Which technologies are used in your testing of allergens? | Prefer to use ELISA. Use PCR for celery detection only and also to support investigations if an ELISA kit cross-reacts with certain ingredients. |
| What measures do you have in place for Quality Control? | Those required for UKAS accreditation. Key Performance Indicators - poor quality relating to a testing lab is flagged. Any known cross-reactivity of the testing kit used must be reported in the results. |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? |
Data is interpreted from a central perspective. Data can also be interpreted by managers in relevant product areas. If an allergen is reported as 'not detected' this sample data is determined as 'safe' by the teams. If a positive result is reported, the risk assessment is referred to and factors are considered concerning, for example, if whether the data is quantitative or qualitative, cross-reactivity of the testing method, supplier's historical test record, consideration of how and likelihood that cross-contamination has occurred, consider target consumer (particularly if this is a product with ‘free from’ claims, threshold data from WHO/EFSA. Ultimately the team managing that category of food type make the decision of how to interpret data. |
| Do you use action levels/VITAL reference eliciting doses to determine safe levels? | Yes, used as a tool as part of the RA described above, including VITAL® 3.0, EFSA and WHO limits to set safe levels. |
| How do you decide when to recall a product? | Based on the RA, considering likelihood of risk, target consumer, any complaints relating to the product, test results, VITAL® 3.0 data and if expiry is still within date. |
| Do you use any tools or calculations to interpret the data? |
Use tools to determine the amount of allergen (in mg) which may have been consumed and compare this to VITAL® 3.0 acceptable level. |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime. When you consider the costs in allergen testing, where do the direct costs to your business lie? |
One colleague manages all testing and allergen management is also a third of the role of another colleague. Technical managers and technical leadership team also feed into this. The team operates to a budget but this budget is for all testing so it is difficult to calculate how much is spent on allergen testing. The testing labs source the samples from the retailer's stores and charge them for the samples and for the testing. Between 650 and 700 samples are tested per year, equivalent to 10% of the total product range. Costs are £52 per ELISA sample for quantitative testing, £48 for semi-quantitative ELISA testing, £80 for lactose-free testing, £65 for qualitative PCR testing and £120 for semi-quantitative PCR testing. |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | Consider risk to business so test more products with ‘free from’ claims and have annual audits of suppliers of products with ‘free from’ claims suppliers. Testing of other products is risk-based. The whole testing programme has its own budget, including microbiology testing, so it's difficult to state the exact amount spent on allergen testing, although allergen testing is an important part of this budget. |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? | Difficult to estimate (see response above). Annual testing costs exceed £150K, then there are the costs of staff and auditors. |
| What type of assessments do you generally perform and do these differ by product / allergen? | Test products, taking into account the target consumer and whether there is a PAL or if the product is prepared on a shared line in the factory. 'Free from' products carry more weight in terms of the number of products tested. Sulphites and gluten are tested against the defined limits. |
| Are there any other challenges you face when considering the possible allergen content of your products? | There is no threshold for the amount of soya in the final product, there is a threshold in the risk assessment conducted by the FSA in September 2014 which used a probabilistic approach for the action level of soya flour (236 ppm) but no threshold for the amount of soya, which is unhelpful. Challenges relating to the potential cross-contamination of wheat with mustard. Allergen-free manufacturing carries many challenges around validation of cleaning processes - testing of the line with a swab may give a positive result but this doesn't reflect the amount of an allergen contained in the final product. If this retailer has any detection of allergen in the swabs for products with a ‘free from’ claim, they don't sell the product as ‘free from’. There are challenges regarding how to know if cleaning processes are sufficient. The supply base struggle to know how many steps back in the supply chain to require checking of their raw materials - more guidance on this would be beneficial. The respondent does not specify the cleaning validation process for suppliers, other than the swabs must be analysed by UKAS-accredited labs. Guidance on this would also be beneficial. |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | Cross-reactivity of testing kits is a real challenge. The respondent feels that the kit manufacturers should be looking into this more and publishing this information. Concerns with mustard in wheat flour. The fact that celery can currently only be reliably determine by PCR is a concern, given the disadvantages of PCR data for allergen data interpretation. |
| Do you have any points you would like to raise, or further comments? | There is no industry-wide agreement on threshold data for allergens. The respondent stated that more research is required on threshold data and feels that more weight should be placed on WHO limits compared to VITAL® 3.0 data. More guidance from the FSA would be beneficial on validation of line cleaning processes and how to interpret data reported in mg/cm3 to mg/kg. The respondent states that more research is required on the affect of food processing on allergenicity. Since the retailer prefers not to use PAL unless absolutely necessary, to support their food-sensitive consumers, VITAL® 3.0 data is required for processed products. |
Company C
| Question | Response |
|---|---|
| Overview of business | Retailer selling fresh, grocery, formulated non food and constructed non food across stores within UK and Northern Ireland. Some own brand products produced on our behalf, some by our own manufacturing as well as via production in store of PPDS products. |
| Can you describe your allergen management regime | Labelling policy compliant with 1169 Allergen policy Allergen training Allergen council Incident management process (including out of hours) Surveillance programme Supplier approval and ongoing management Specifications approved and auditing programme in place Complaints management |
| Which specific allergens from the 14 food allergen groups does your company test for? |
Main focus on nuts, egg, cereals, fish, milk, sulphur dioxide, crustaceans, mustard, sesame and soya as these are commonly handled within processing environments |
| Which allergens are not tested for and why? | Lupin as not used on factory sites within our supply chain and customers unfamiliar. Celery not used significantly within our supply chain. Small impact within UK population. |
| What factors do you consider when determining which items will undergo testing for allergens? | Product labelling ; contains vs may contains ‘Free from’ Claim on product What is handled within the factory environment Industry incidents |
| For a given product, how do you decide which allergens to test for? | Product labelling ; contains vs may contains ‘Free from’ Claim on product What is handled within the factory environment Industry incidents |
| What do you test for allergens? E.g. finished products, ingredients, production lines, other. | Finished products and raw material sampling – own brand suppliers are tasked with conducting their own allergen risk assessment and then testing programme |
| Can you describe your sampling procedure. | Risk assessed surveillance testing programme with result trending. Horizon scanning to determine raw material testing programme. |
| How many replicates of each sample are tested by each testing laboratory? | Our testing is surveillance for due diligence so we would test one product from the shelf and then test further samples (same or different date codes) if an issue was detected as well as follow up with the supplier on their test results |
| What factors do you consider when selecting a testing laboratory? | ISO 17025 accreditation (lab and method), methodology used and specialist skill set |
| Are the data qualitative or quantitative? | Both |
| What are the units of measurement of the testing procedures? |
Depends on the method used but normally ppm |
| Is there a particular LOD or LOQ that you require of the test. |
Use lowest LOQ available for allergen testing method |
| Which technologies are used in your testing of allergens? | Mainly ELISA and PCR |
| What measures do you have in place for Quality Control in the allergen testing? | Method must be part of lab ISO 17025 accreditation, internal auditing and EQA (which is part of 17025). |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? | Same process for raw materials and final products. The data interpretation will be conducted by a combination of the laboratory, the supplier, technical manager for the product category and food safety supply manager |
| Do you use action levels/VITAL® reference eliciting doses to determine safe levels? | Yes VITAL 3 as part of risk assessment |
| How do you decide when to recall a product? | Risk assessment completed (using VITAL as one of the tools) by technical manager and multi-disciplinary team. Incident meeting held as required. |
| Do you use any tools or calculations to interpret the data? | Yes VITAL 3 |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime. When you consider the costs in allergen testing, where do the direct costs to your business lie? | Routine testing costs are manageable however in an incident, where you need a quick turnaround, the price can increase significantly. |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | Not cost driven. Risk assessment driven. |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? | Not cost driven. Risk assessment driven. |
| What type of assessments do you generally perform and do these differ by product / allergen? | For own brand product will review supplier allergen management programme and their risk assessments. Supplier scorecard completed for products with a Free From claim. |
| Are there any other challenges you face when considering the possible allergen content of your products? | There are several industry issues currently and I will use wheat and mustard as an example – natural cross contamination in the field, a variety of mustard types and no test method. Are all the mustard strains allergenic? Once product leaves the factory then down to customer and their understanding – how do they handle in home, particularly in a multiple household where only 1 person has an allergy. Loose food management. |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | Not all allergen methods are available eg : wheat - you can test for gluten but that is not the same as a wheat allergy. Not all allergen methods can give quantitative results – problem if Codex thresholds come in. There are several industry issues currently and I will use wheat and mustard as an example – natural cross contamination in the field, a variety of mustard types and no test method. Are all the mustard strains allergenic? |
| Do you have any points you would like to raise, or further comments? | Support codex thresholds and mandatory minimum levels however we must have industry guidance on what, where and how much to test. |
Company D
| Question | Response |
|---|---|
| Please can you provide a brief overview of your business | Retailer operating in Great Britain and the Republic of Ireland. |
| Can you describe your allergen management regime | Not directly applicable as we do not directly manufacture product. The manufacturers would be responsible for implementing an allergen management regime in line with our requirements. |
| Which specific allergens from the 14 food allergen groups does your company test for? | Beta-lactoglobulin (β-LG) Casein Crustacean (Tropomyosin) Whole Egg Protein Gluten Tree nuts: Hazelnut, Walnut, Almond, pistachio, cashew, pine Mustard Peanut Sesame Soya SO2 Lupin Molluscs Fish Celery Hydrolysed gluten |
| Which allergens are not tested for and why? | We can test for all allergens; some allergens are more commonly tested for than others. |
| What factors do you consider when determining which items will undergo testing for allergens? | Products making claims e.g., milk free, nut free, gluten free etc. would be prioritised for testing. Also, products may be tested in response to industry issues e.g., peanut in soya lecithin. |
| For a given product, how do you decide which allergens to test for? |
Claims on pack would be considered e.g., gluten free, milk free or suitable for coeliac. |
| What do you test for allergens? E.g., finished products, ingredients, production lines, other. | Finished products |
| What type of assessments do you generally perform and do these differ by product / allergen? | As a retailer we test finished products, audit production sites, review and approve product specifications and artwork for labels. |
| Can you describe your sampling procedure. | Product samples are purchased from store by an independent third party and transported securely to the nominated laboratory. Once at the laboratory food samples are homogenised by the laboratory using blenders before testing (if needed), unless it is a powder or homogeneous liquid/sauce. |
| What factors do you consider when selecting a testing laboratory? | Reputation, accreditation, capability to handle the volume of testing required for our business, competitive pricing. |
| Which technologies are used in your testing of allergens? | ELISA and PCR |
| How many replicates of each sample are tested by each testing laboratory? |
1 extracted sample, with duplicate ELISA measurement. |
| Are the data you receive from testing in a qualitative or quantitative format? | Quantitative data for food samples. |
| What are the units of measurement of the testing procedures? | Mg/kg (ppm) allergen (commodity) or allergen protein, depending on the test. |
| Is there a particular LOD or LOQ that you require of the test? | Food samples are analysed quantitatively. The laboratory we use applies a lower LOQ as the reporting limit. Each method has an upper LOQ which is the maximum LOQ. The maximum limit can be increased through dilutions testing. |
| What measures do you have in place for Quality Control in the allergen testing? | Analytical quality control sample (internal quality control), Blank of extraction, Calibration curve, Negative control, ELISA duplicate measurements consistency check Participation in external quality assurance (EQA) schemes – proficiency tests, Environmental monitoring, Calibrations, Segregation of areas (Sample preparation separate from ELISA lab) Documented procedures, including risk management. |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? | The laboratory conducting the testing determines whether the result indicates a food safety issue. |
| Do you use action levels/VITAL reference eliciting doses to determine safe levels? | VITAL thresholds may be used if required. |
| How do you decide when to recall a product? | Risk assessment which may consider: • If the product carries a “may contain” statement for the allergen in question • Level of contamination/potential to cause a reaction Where the product is in the supply chain - e.g., in store or in a storage facility |
| Do you use any tools or calculations to interpret the data? | The results which pass QC, are reported with a Red, Amber, or Green grade in line with our requirements. |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime? When you consider the costs in allergen testing, where do the direct costs to your business lie? |
Approximate cost per sample ranges from £40 to £85 for the most common allergen tests - gluten, egg, milk |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | Potential risk to consumers and what is reasonable. |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? Please state if this is not plausible. |
Not possible to answer, would vary for each manufacturing site and their product range |
| Are there any other challenges you face when considering the possible allergen content of your products? | Complex global supply chain for raw materials e.g., the Indian government’s investigation into peanut contamination in soya lecithin demonstrated the challenges UK businesses face when assessing cross contamination risks of small amounts of allergen being present in the final product. Food sensitivity varies in different parts of the world. A globally recognised list of the most common allergens would be helpful. |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | Agreed action levels for allergen presence would be a useful tool to help understand risk and provide clarity for Regulators and industry. |
| Do you have any points you would like to raise, or further comments? | N/A |
Company E
| Question | Response |
|---|---|
| Please can you provide a brief overview of your business | Convenience store retailer with multiple distribution partners who manage the retail stores Central office function manages National Brand product supply Distribution Partners manage local brand source and supply |
| Can you describe your allergen management regime | For the Central Office National Brand function: All suppliers complete an allergen risk assessment (ARA). This covers; allergens handled at raw material site, allergens handled at manufacturing site, validation/verification programmes, process RA. The ARA is designed to determine risk of allergen cross contamination and alibi labelling requirements. All ARAs and evidence (lab reports / verification plans etc) are approved by QA technologist or Quality and Responsible Retailing Controller. All suppliers are required to comply with our internal Allergen management policies which details the testing requirements including methodology and frequency. |
| Which specific allergens from the 14 food allergen groups does your company test for? | Varies for each supplier. Most common include; Nuts, gluten, milk (β-LG/Casein), fish, egg but can be any of the allergens. |
| Which allergens are not tested for and why? | Allergen would not be tested for if it is not handled on same line as finished product in manufacturing site and/or raw material site. |
| What factors do you consider when determining which items will undergo testing for allergens? | Worst case scenario, the form of the allergen (powder is higher risk than solid product), finished product claims eg; free from, known risks eg mustard cross contamination into wheat products from the farm |
| For a given product, how do you decide which allergens to test for? |
Allergens that are handled on the same line but not declared in final product packaging Final product testing to demonstrate positive result and requirement for alibi labelling If sulphites are used on site, test to demonstrate <10ppm in final product |
| What do you test for allergens? E.g. finished products, ingredients, production lines, other. |
Finished products, production lines, rinse water |
| What type of assessments do you generally perform and do these differ by product / allergen? | Both external and internal lab analysis Rapid swabs can be used by sites internally to detect specific allergens. Labs can analyse swabs and final product samples |
| Can you describe your sampling procedure. | As per laboratory procedure. |
| What factors do you consider when selecting a testing laboratory? | All external labs accredited ISO17025 |
| Which technologies are used in your testing of allergens? | For lab analysis, ELISA and DNA testing Environmental monitoring – rapid allergen swabs |
| How many replicates of each sample are tested by each testing laboratory? | As per laboratory procedure. |
| Are the data you receive from testing in a qualitative or quantitative format? | Both |
| What are the units of measurement of the testing procedures? | Ppm or LOD mg/kg |
| Is there a particular LOD or LOQ that you require of the test? | Legally Gluten <20ppm We do not state LOD/LOQ’s required, we require validated test kits appropriate to the allergen of concern and quantitative and qualitative results in support |
| What measures do you have in place for Quality Control in the allergen testing? | All external labs accredited ISO17025, for internal site testing, all sites are BRC/GFSI accredited so working to international standard. |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? | Teams at supplier sites and our QARR team |
| Do you use action levels/VITAL reference eliciting doses to determine safe levels? | No, we do not accept calculations as a determination of presence of allergen |
| How do you decide when to recall a product? | When there is a risk of allergen cross contamination identified for a product on sale that has not been communicated to the consumer. |
| Do you use any tools or calculations to interpret the data? | No – VITAL is not included in our policy |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime? When you consider the costs in allergen testing, where do the direct costs to your business lie? | Do not currently test ourselves |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | N/A |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? Please state if this is not plausible. | N/A |
| Are there any other challenges you face when considering the possible allergen content of your products? | • Reliability of test methods available • Changes in supply chains • Production rota changes • The effect to processing eg – production processes eliminating the allergenic protein in the allergen raw material • Working with international suppliers where allergies and allergen management are considered a lesser problem than in the UK |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | To be determined |
| Do you have any points you would like to raise, or further comments? | No |
Company F
| Question | Response |
|---|---|
| Please can you provide a brief overview of your business | Global food manufacturer of herbs, spices, condiments, and flavours. |
| Can you describe your allergen management regime | Global allergen management procedure deployed to all plants this covers site risk assessment expectations, control of allergens, cleaning validation and verification program requirements. The procedure utilises industry best practice guidelines such as FDE allergen management guideline and FARRP. Raw material data / vendor data management process that includes allergens uploaded into internal systems to drive labelling and scheduling requirements. |
| Which specific allergens from the 14 food allergen groups does your company test for? | Typically, we test for gluten, egg, milk and sesame seed as part of our factory monitoring although from time to time we test for the presence of other allergens as well if we have intelligence or risk assessment information to suggest there is a concern. |
| Which allergens are not tested for and why? | Testing regimes are based upon risk which is why some allergens are not tested. Additionally due to the challenges of testing our product matrices for some allergens we do not routinely test them however have multi allergen test protocols in place if there was a specific concern. |
| What factors do you consider when determining which items will undergo testing for allergens? | Risk profile of the product, form of the raw material if any free from claims are made on the product. |
| For a given product, how do you decide which allergens to test for? | Based upon risk assessment, ‘allergens’ is a blanket term however we recognise that the profile for the allergen of concern will change according to the risk i.e. field level contaminant in a commodity vs factory cross contamination vs making a free from claim to target a vulnerable consumer group. |
| What do you test for allergens? E.g. finished products, ingredients, production lines, other. | Finished products where a claim is made or as part of validation study Raw materials – where a risk has been identified Product lines – As part of annual verification and validation studies, as well as positive release of manufacturing lines where a free from claim is being made |
| What type of assessments do you generally perform and do these differ by product / allergen? | We conduct raw material risk assessments looking at risk of contamination with allergens, factory assessments looking at cleaning and also airborne contamination. These assessments may be quantitative or qualitative depending on the input data. |
| Can you describe your sampling procedure. | Finished product – cleaning validation first off Finished product claim –3 samples taken through the batch and tested (x3) Raw materials – ISO sampling guidelines applied |
| What factors do you consider when selecting a testing laboratory? | Accreditation of lab and method. Validation studies completed on testing methods / kits used. Clear understanding on the challenges of testing spice matrix. |
| Which technologies are used in your testing of allergens? | Lateral flow devices / ELISA / PCR |
| How many replicates of each sample are tested by each testing laboratory? | Depends – typically there is one sample however we may make take multiple samples across the same batch. |
| Are the data you receive from testing in a qualitative or quantitative format? | Depends on the testing method used |
| What are the units of measurement of the testing procedures? | Depends on the testing method used – typically for ELISA it is PPM and for PCR it is report as positive / negative at the LOD of the test |
| Is there a particular LOD or LOQ that you require of the test? | We do not specify the LOD / LOQ for the tests we use, just that the sample matrix has been validated including where appropriate spike recovery testing. |
| What measures do you have in place for Quality Control in the allergen testing? | PCR / ELISA testing is conducted in an external laboratory that has undergone approval as per our process (Review of accreditation, procedures on testing including internal QC, kit validation) labs also need to participate in ring testing scheme. Lateral Flow devices – These are internally validated to ensure that they detect allergens at the LOD determined by the kit. |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? | Trained / competent personnel review data and make decisions on if the product is safe for consumer. Process utilises protein action levels. |
| Do you use action levels/VITAL reference eliciting doses to determine safe levels? | VITAL levels are utilised by trained / competent personnel to determine safe levels. |
| How do you decide when to recall a product? | If a risk to customers have been identified then product would be recalled. |
| Do you use any tools or calculations to interpret the data? | Calculator based on VITAL action levels is used. External allergen expertise is sought when required. |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime? When you consider the costs in allergen testing, where do the direct costs to your business lie? | Generally, the cost of allergen testing is high when compared with micro testing, however it is a cost the business recognises needs to be spent. Our current regime fits within our cost model. |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | Our allergen mitigation programs are built on risk that recognise that testing is only one of the many mitigation strategies that can be utilised. |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? Please state if this is not plausible. | Not plausible. |
| Are there any other challenges you face when considering the possible allergen content of your products? | Lack of understanding of allergens and protein action levels amongst regulators. Lack of harmony from a global perspective on action levels and labelling. |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | Testing in a spice matrix for ELISA can be challenging and the use of PCR does not give a quantitative result to allow a VITAL risk assessment. Further research and development of Mass spec methods would be useful. |
| Do you have any points you would like to raise, or further comments? | None. |
Company G
| Question | Response |
|---|---|
| Overview of business | A retailer selling ambient and chilled products globally |
| Can you describe your allergen management regime | Alongside any legal obligations, we lay out specific standards our suppliers are expected to meet with regards to allergen management and specifically our free from product ranges. This covers both factory standards, allergen risk assessment and mitigations, as well as testing requirements that our suppliers must comply with |
| Which specific allergens from the 14 food allergen groups does your company test for? | As part of our independent product testing (IPT), we test products where a specific free from claim is made for that specific allergen e.g. gluten free rather than an ingredient label declaration |
| Which allergens are not tested for and why? | As part of our surveillance we are only testing for allergens where we are making a front of pack claim that the product is free from a specific allergen e.g. gluten free, dairy free |
| What factors do you consider when determining which items will undergo testing for allergens? | We target all products where a free from claim is made. |
| For a given product, how do you decide which allergens to test for? | Determined by the free from claim. |
| What do you test for allergens? | All product types where a free from claim is made. |
| Can you describe your sampling procedure. | Shopped from store against a planned schedule targeting products with a front of pack claim eg gluten free. Frequency altered by risk but aim to test all products twice per year. |
| How many replicates of each sample are tested by each testing laboratory? | One sample is tested. |
| What factors do you consider when selecting a testing laboratory? | We work directly with one service provider who assess testing laboratories on our behalf. They are assessed for performance, responsiveness, technical competence, sample preparation controls, reporting style, turn around times etc. Our contracted service provider has extensive experience of working with testing laboratories. |
| Are the data qualitative or quantitative? | Quantitative for ELISA, PCR/NGS are semi quantitative. |
| What are the units of measurement | Test and legal requirements dependent. |
| Is there a particular LOD or LOQ that you require of the test. |
We target tests with defined LOQ where possible. |
| Which technologies are used in your testing of allergens? | We have access to a full range of methodologies. |
| What measures do you have in place for Quality Control? | All laboratories used are UKAS accredited to ISO 17025. |
| Who is responsible for interpreting the data and how is it decided whether a sample is determined as safe for consumers? How does this differ for ingredients versus final product testing (if applicable)? | We target finished product testing and results are interpreted by our service provider to determine “in spec” vs “out of spec”. Any out of spec result would be reviewed and interpreted by us and where applicable incident management procedures would be applied. We also have access to an expert toxicologist and expertise with our service provider to support and advise any risk based decisions. |
| Do you use action levels/VITAL® reference eliciting doses to determine safe levels? | Not answered |
| How do you decide when to recall a product? | We have an incident management procedure that covers information gathering, risk assessment and action for food safety incident. In the event of an identified issue information would be gathered from the supplier and an incident management team formed. The team would include business subject matter experts in relation to allergens if the incident was related to allergens. Gathered information would be reviewed and risk assessed to determine next steps. We would consider - • The allergen of concern and if there are known detection limits that would cause a reaction (e.g., gluten, sulphites), is it known to cause anaphylaxis? • Is the product being marketed to a vulnerable customer (e.g., a free from range) • Is the allergen hidden within the product (e.g., wrong ingredient used verses a clear mispack - would the customer see the issue? What is the alibi information on pack?) • Amount of product impacted and where it is in our supply chain (e.g., can this be isolated before it reaches the customer or not Has testing taken place? If so, is it accurate? |
| Do you use any tools or calculations to interpret the data? | Yes, we use an internally developed PPM calculator. |
| We are interested to learn about the costs involved in testing for businesses. How affordable is the testing regime. When you consider the costs in allergen testing, where do the direct costs to your business lie? | Testing costs are best obtained from the labs and service providers. |
| What considerations do you take into account when deciding how much to invest in allergen mitigation? | Testing costs are best obtained from the labs and service providers. Allergen management is one of a number of food safety risks that require effective management. Due to the potential severity of food allergens, we invest a significant amount of time and resources into the effective management of these. We employ a team of food technologist and subject matter experts to ensure that the risks from allergens are carefully managed. |
| Could you estimate approximately what % of net profit is invested in monitoring for food allergens? | Testing is only a part of the management of allergens. We complete ~700 supplier audits a year / employ a team of ~60 Technologists / develop and invest in systems to ensure product and supplier controls are in place. The total food safety programme is run at a cost of multiple millions of (£). |
| What type of assessments do you generally perform and do these differ by product / allergen? | Not answered |
| Are there any other challenges you face when considering the possible allergen content of your products? | When considering the manufacture of free from products we give careful consideration to the supplier, the processing environment and the procurement of raw materials. The supporting risk assessments and audits must support the decision to all production to commence from any new supplier. |
| Considering allergen management, are there any gaps in food testing services which you wish were available or different to support your business? Are there any products for which you find it challenging to find a testing service? | Rapid, accurate and cheap testing for raw materials intake would be advantages for our supplier to essentially positively release high risk raw materials onto site. |
| Do you have any points you would like to raise, or further comments? | Not answered |
An expert consultation with Mr. Mark Sykes and Mr. Dominic Anderson on the variability of analysis between methods for the same allergen. The tables referenced in this section are appended in Appendix 2.
This section of the study concerns the variability of analysis between methods for the same allergen. Although regarding tree nut-allergens, much testing is progressing to LC-MS/MS methods, the majority of food testing laboratories world-wide continue to use ELISA kits which are specific not only to the allergen/matrix combination being tested but also specific to the design by the kit manufacturer. There is a fundamental lack of equivalence and reproducibility between different ELISA kit manufacturers (and sometimes between different types of kit for a single allergen by the same manufacturer). Batch-to-batch variation due to variations in kit-supplied buffers and calibration standards exists. Also, the antibodies used to detect the allergen and the composition of calibration standards differs between manufacturers for kits detecting the same allergen, leading to variations in PT data depending on the method applied. The potential to convert units of measurement in order to compare data between methods for the same allergen is discussed. The inter-conversion of units of measurement such as measurements of genomic sequence, peptide or single protein into a measurement of total food allergen is essential to evaluate and compare methods. Furthermore, there is a variability within the ELISA kit analysis (repeatability precision). This is evidenced by the data for homogeneity testing for proficiency test samples. This part of the project interrogates several years’ worth of homogeneity data and proficiency test data for a range of allergens analysed by ELISA kit with a view to determining an objective measure of precision that is actually present in real-world data.
7.1. Background introduction
A proficiency test (PT) is the performance assessment of a laboratory by interlaboratory comparison. (FAPAS, 2023) The assessment is in the form of a z- score;
z = (X – Xpt)/σp
X is the laboratory’s result, Xpt is the assigned property value for the analysis in question, σp is the standard deviation for proficiency assessment. Xpt is usually derived from the consensus of participants’ results. The successful operation of a PT depends on the test sample being sufficiently homogeneous between samples (such that each participant in the PT receives effectively the same sample). The assessment by z-score must be fit-for-purpose, defined by the calculation of the assigned value Xpt and the application of an appropriate value for σp.
In the majority of PTs (of any kind in the food sector), there is no observable method dependency and no mandated method; participants in the PT are free to use their normal, routine method and all PT results are treated as equivalent. The principal exception is for allergens, where the vast majority of participants use ELISA kits and different ELISA kits provide significantly different results. (Owen et al., 2009; Sykes et al., 2012; Hamide et al., 2019)
In this situation, participants in a PT are still free to use their normal, routine ELISA method but their result must be submitted against the ELISA kit they have used. Results from the PT are then treated as groups corresponding to specific ELISA kits, with their separate assigned values.
Homogeneity data and assigned values are considered fit-for-purpose if they meet statistical criteria for acceptable precision, dependent on the value of σp. In allergens PTs, the value of σp is set as 25% relative to the assigned value. This relative standard deviation was derived as a result of examination of early Fapas® allergens PTs. (Owen et al., 2009)
Homogeneity data are acquired as a set of 20 data points; 10 samples analysed in duplicate. By analysing each sample in duplicate, this provides a measure of the repeatability precision of the analysis, prior to its use in assessing the sample-to- sample variance. An analysis which is imprecise might not provide sufficient evidence of acceptable homogeneity. The acceptable analytical precision is determined by the ratio san/σp where san is the observed analytical standard deviation. The critical value is 0.5, above which the analytical imprecision could compromise the power of the statistical test to detect inhomogeneity. (Fearn, T. et al., 2001)
The PT assigned values are derived from the consensus of results corresponding to each allergen/ELISA kit combination. The fitness-for-purpose of the consensus is determined by the ratio of the uncertainty u of the consensus to the value of σp, where the critical value is normally 0.3 (ISO, 2015) but, for practical purposes of rounding data, a critical value up to 0.35 is acceptable. (Fapas, 2023) A ratio above the critical value risks compromising the usefulness of the z-scores but assessments will sometimes be provided for information only where the critical value is slightly exceeded, in order to provide some guidance to participants.
This study against Section 7 of the project has investigated Fapas® allergens PTs for an up-to-date summary of the current state of allergens testing by ELISA kit.
7.2. PT Data set
Summary data from Fapas® allergens PTs were collated from January 2018 to November 2022. The PT data include the matrix, allergen, ELISA kits used, consensus assigned values Xpt per ELISA kit, uncertainty (u) of the consensus and σp values. Summary data were also collated for these PTs from the homogeneity testing in the form of the mean value, σp, observed analytical standard deviation for repeatability (san) and the ratio san/σp. The PT data in total summarized 14295 individual submitted results (although this includes qualitative results, so the total quantitative results would be approximately half this number, i.e. about 7000 individual submitted results). Earlier data sets do not include results not assigned to a specific ELISA kit, so the overall number of submitted results to the PTs will be in excess of 7000. The homogeneity data numbered 133 sets of data, of which each set comprises 20 individual data points, i.e. a total of 2660 individual data points. In addition, comments relating to the use of PCR methods were collated (25 comments).
NB. The data cover a period of about five years. During this period, some ELISA kits have been either discontinued, revised, replaced or entirely new kits have come on the market. The data reflects as accurately as possible the ELISA kits that were contemporary at the time of the PT.
The majority of allergens PTs comprise two test materials, labelled A and B. Superficially, one will be ‘blank’ (i.e. not spiked with allergen) and the other spiked with allergenic ingredient. This adds challenge to the participants in correctly identifying the spiked sample. Some PTs, however, will have both samples spiked but at different levels. Some PTs contain only one test material (usually the processed sample matrices). Therefore, the data sets include PT references with A, B or no specific material reference.
Part-way through the five-year period of this data collection, a change was made to how participants could report results. The earlier PTs required participants to enter their result and then choose the ELISA kit from a drop-down menu. The later PTs required participants to enter their result directly against a list of ELISA kits. The change in reporting was an efficiency improvement in how Fapas® was able to handle the data. Hence, the earlier PTs list only the ELISA kits against which assigned values could be calculated but later PTs additionally list all ELISA kits, regardless of whether an assigned value could be calculated.
The median number of registrations in the PT data studied was 57.5, of which a median of 54 (94%) submitted results. Participants in PTs fail to submit results by the deadline for various reasons, including equipment failure, unexpected staff resource unavailable, business priorities coming before PT samples. For the PT data set in question, this includes the principal periods of the global coronavirus pandemic and, while most food testing laboratories continued operating as essential services, they would have been compromised in staff availability. The 94% of results submission for allergens PTs therefore represents a very high proportion.
The majority (53%) of participants were from the EU, 15% were UK laboratories and 32% were rest-of-world (comprising North America, South America, Middle East Africa, Asia-Pacific). The UK laboratories comprise official control laboratories and third-party testing laboratories.
Appendix 2 presents the list of PTs included in the study, their registration numbers and results returns, and the broad geographic location of laboratories.
7.3. Section 7b. Short review of applicable analysis of existing Fapas® proficiency testing data
This part of the study benefits from the use of large volumes of Fapas® data which are not publicly available free of charge. The data from the last five years’ worth of Fapas® proficiency test reports will be reviewed. The allergens proficiency tests attract between 30 and 150 participating laboratories per test, of which there are 25- 30 such tests per year. Participants are assessed on the basis of the correct detection of allergen (qualitatively) and on the accuracy of quantitation of the detected allergen. Results are assessed separately according to the ELISA kit (or alternative method) used. There are unequal sub-populations of laboratories using different ELISA kits, so the most popular kits are more likely to be performance assessed than the less popular kits (due to insufficient numbers of data points). For processed foods in particular, the less popular or available kits tend to be those most suited to denatured allergenic proteins. Although the majority of laboratories are routinely using ELISA kits, a few laboratories report results from LC-MS/MS or PCR methods. This section of the study describes the effect of the different populations of data and what that means for the interpretation of allergen analyses.
7.3.1. PT Data analysis and interrogation
7.3.1.1. Homogeneity data
The mean ratio san/σp was calculated for each allergen and an overall mean calculated ( Appendix 2). The mean ratios varied between 0.277 (lupin) and 0.567 (β-LG), with an overall mean of 0.432. The critical value is 0.5 for the normal acceptability of repeatability precision. The mean ratios of two other analytes (gluten and milk) exceeded the critical ratio (at 0.516 for gluten and 0.549 for milk). Of the individual data sets, 48 (36%) exceeded the critical value of 0.5. Of the β-LG data, 5 of 6 data sets exceeded the critical value (but mean concentrations tend to be low for β-LG). Hence, the overall premise of using 25% RSD for σp appears to be appropriate in evaluating homogeneity data with regard to the risk of not detecting true inhomogeneity. However, the data are also clearly showing that the repeatability limit of ELISA kits has been reached. The mean ratio san/σp is only just being maintained (overall mean of 0.432 is just less than the critical value of 0.5), so the repeatability of ELISA kits has not improved. If the repeatability had improved, we would expect to observe a much lower san/σp ratio.
7.3.1.2. Proficiency Testing data
The PT data were separated by allergen determination corresponding to gluten, egg, milk, soya, tree nut, peanut and other (itself comprising celery, mustard, lupin, sesame, fish). The other category corresponds to more recently added PTs into the programme, so there are fewer data sets for these allergens. Some PTs combined more than one allergen.
The value of u/σp was calculated for PT data and summarised by allergen, with the mean and standard deviation calculated for the grouped data. The results of this analysis are shown in Appendix 2. The mean u/σp ranged between 0.228 and 0.328, so within the Fapas® critical value of 0.35. The standard deviations ranged between 0.109 and 0.208, not insignificant given the values of the means. Hence, the overall premise of using 25% RSD for σp appears to be appropriate in evaluating PT consensus assigned values with regard to the risk of compromising the usefulness of z-score performance assessments. However, the data are also clearly showing that the reproducibility limit of ELISA kits has been reached. If the reproducibility of ELISA kit use had improved, we would expect to observe much lower u/σp values.
The PT data were visually inspected for general trends and anomalies (data in Tables 4-10 (Appendix 2). Two trends become immediately obvious: the majority of results from R-Biopharm ELISA kits and the discrepancy of assigned values between different ELISA kits. The latter issue has been reported previously and the data in the current project simply demonstrate that the situation has not changed in more than 13 years. (Owen et al., 2009; Sykes et al., 2012; Hamide et al., 2019) It is recognised that some ELISA kits report against whole ingredient (e.g. peanut) and some against the protein component (e.g. peanut protein). There still remains a discrepancy in assigned values between kits that purport to report like-for-like. The former trend of over-representation by one ELISA kit manufacturer has been suspected for a long time (and known in individual Fapas® PT reports) but the data of the current project reinforces this view. In addition, it is also apparent in the data that, where there are multiple R-Biopharm ELISA kits represented in the PTs, one R- Biopharm kit will be distinctly more popular than others. For example, in gluten analysis, the R-Biopharm RIDASCREEN Gliadin (R7001) kit will have many more results submitted against it than the R-Biopharm RIDASCREEN Fast Gliadin (R7002) or R-Biopharm RIDASCREEN FAST Gliadin sensitive (R7051) kits. This is because the R-Biopharm RIDASCREEN Gliadin (R7001) kit, in combination with the Mendez Cocktail solution, is the Gold Standard method recommended by CODEX for the analysis of gluten (Lacorn, Dubois, et al., 2022b; R-Biopharm, 2022)
In addition to the issue of significantly different results being associated with different ELISA kit manufacturers, a further issue is evident in this data. This issue relates to the lack of comparability between ELISA kits from the same manufacturer. To provide one example, in PT 27316 (milk in infant soya formula) test material A, three R-Biopharm kits are represented: R-Biopharm RIDASCREEN Fast β-Lactoglobulin (R4912), R-Biopharm RIDASCREEN Fast Casein (R4612) and R-Biopharm RIDASCREEN Fast Milk (R4652). The consensus assigned values were, respectively, 1.56 mg/kg, 31.0 mg/kg and 17.8 mg/kg. Clearly, the assigned value for casein should not be nearly 2x that of milk. This issue is particularly prevalent for egg and milk determinations.
7.3.1.3. PCR method comments
The majority of laboratories represented in PTs continue to use ELISA as the primary method for allergens analysis. The relative lack of other methods is evident in that, of the data sets studied in this project, only 25 comments related to the use of other methods (24 using PCR and one using LC-MS/MS), out of thousands of PT results. Non-ELISA methods are used either as a primary method or as confirmation of an ELISA result or to verify the absence of an allergen that is not expected to be detected by ELISA. Most of the PCR kits were the Congen SureFood kit (19 responses), even though some comments refer to R-Biopharm as the kit, R- Biopharm is the distributor of the kit. Two PCR kits were described as ‘in-house’, two PCR kits were unknown and one kit was from ALScreen.
An estimation of costs associated with setting up a testing service for the main technologies identified for current allergen testing techniques (ELISA, PCR and LC- MS with multiple reaction monitoring) in an established laboratory that already offers testing services and with personnel already trained in the relevant technology is set out in Appendix 3.
This estimate assumes that the laboratory already has access to common laboratory equipment including centrifuge, vortex, incubator, fridge freezer, pipettes. Equipment servicing and calibration are not included in this cost estimate. Average prices have been taken for consumables (included testing kits, RMs). Costs of method-specific items (plate reader, real-time PCR instrument, LC-MS instrument) have been averaged from recent quotes received. In terms of analyst time, 1 day equates to 7.5 hours.
Costs to gain UKAS-accreditation have not been included as these will, to some extent, depend on the size of the organisation, the policies and procedures already in place, the number of test services offered and the current UKAS status. There will be costs linked provision of general policies and procedures, record keeping, quality management such as non-conformance investigations, complaints procedure, equipment performance checks, training, laboratory information management system, equipment maintenance, calibration and servicing. There will also be costs linked to ISO 9001 certification and audit.
Regarding the costs of the different techniques, ELISA is a relatively low-cost laboratory technique with the instrumentation involved costing around £16K and each test kit costing around £250 to accommodate the analysis of approximately 14 samples in duplicate along with QCs and standards. PCR is more expensive, with the associated hardware costing around £61.5K and test kit costs are approximately in line with ELISA kits. Both techniques are often offered by a wide range of laboratories and are used for a wide range of food (and other) testing requirements in addition to allergen testing. Mass spectrometry methods tend to be offered by a smaller number of testing laboratories, requiring a hardware investment of upwards of approximately £416K.
Regarding costs to businesses for submitting samples for allergen testing, depending on the allergen required, analysis can be completed by testing labs, charging from approximately £55 to £141 per sample for UKAS-accredited testing with a standard turnaround of five working days.
RMs play a crucial role in allergen analysis to provide a means of:
(a) deriving conversion factors, especially in relation to mass spectrometry methods where there is a need to convert from peptide mass to mg allergenic food protein;
(b) supporting effective test method validation; and
(c) harmonisation of test method results as has been shown for determination of gluten. (Rzychon et al 2017)
They can include the allergenic ingredient itself alone and incurred in a food matrix. There are at least allergen CRMs available i.e. materials which have been certified (for example, ISO 17034) and demonstrate traceability to national or international standards and provide a statement of uncertainty. Although CRMs for allergens have been prepared in the past, we have identified issues with these materials no longer being available. Furthermore, there is a limit to CRMs which are incurred. Where such CRMs are lacking, in the interim, QC materials can be used, and are available for a wide range of allergenic food ingredients and incurred food matrices, e.g. surplus materials from proficiency testing providers. They may also be prepared in- house to provide closer matrix matching of food products. However, these may lack an assigned allergenic protein content, limiting their usefulness.
Since allergen testing is impacted by processing and its effect on the detectability of the protein allergen target, and due to the inherent lack of knowledge regarding the level of processing when presented with a sample suspected of eliciting allergenic reactivity, rather than relying on a single test to determine if an allergen is present, a workflow comprising multiple complimentary tests must be implemented.
Workflows include testing by validated fit-for-purpose methods. Since the allergenic hazard comprises proteins (apart from sulphites and sulphur dioxide), methods used should target the allergen proteins or their constituent peptides and provide test results in mg allergenic ingredient protein/kg food product, as recommended by the
FAO/WHO expert consultation. Only when no such method is available should test methods targeting non-protein measurands, such as DNA-based methods, be considered. As discussed previously, PCR methods are not suitable for egg and are less sensitive for certain allergens such as milk. As demonstrated by incidents in the supply chain linked to cross-reactivities displayed by allergen testing kits, workflows must include more than one test method in order to gain confidence in a negative result and to protect allergen-sensitive consumers (Walker et al 2018, see Section 2). Ideally testing will target different analytes and will also target the same or different analytes using more than one applicable technology. Testing only for the allergen protein/peptide is important for example when testing beef products for cow’s milk allergen or chicken products for egg.
Testing should be conducted by an ISO 17025-accredited laboratory using a validated test method and the sample should be analysed in duplicate. Incurred RMs with an established uncertainty factor must be extracted and analysed in the same batch to verify method performance and to build up QC plots to track variations in kit performance between kit batches and over time.
The laboratory’s performance in the most recent proficiency testing rounds must be transparent on the test report and must be at least ‘satisfactory’ for correct identification in qualitative analysis. For a quantitative method the proficiency test z- score must be ≤±2. The methods used should all be validated, with validation data published, including all performance criteria, the composition and preparation conditions of the samples, the RM used.
For the purposes of this project, the Action Level, where available, corresponds to those recently published in the FAO/WHO ‘Risk Assessment of Food Allergens Part 2: Review and establish threshold levels in foods for the priority allergens’ (WHO, 2022). The consultation also identified associated test method performance criteria for the global priority allergenic foods identified by the expert consultation. These action levels are based on health-based guidance values for global priority allergenic food ingredients that have been identified by the expert consultation and the food consumption data. Published data are available for other allergenic food ingredients on which health based guidance values may be identified using a similar approach (Houben et al 2019) which could allow an interim action level to be derived for other priority allergenic foods such as soybean.
In terms of incident management, in all cases where the food allergen is a protein (so excluding sulphites and sulphur dioxide), all testing should use only methods which target the allergenic proteins or their constituent peptides. PCR methods would ideally only be used as a surrogate testing method where an alternative protein-based method does not exist, as is the case for celery for example. At present, these methodologies are represented by ELISA and peptide LC-MS/MS. A combination of orthogonal methods would improve the robustness of the testing. While LC-MS/MS methods currently lack sensitivity, they benefit from enhanced specificity. As highlighted in this report, ELISA methods are sensitive and are specific but for a range of epitopes, as shown in Section 5. Given the current lower sensitivity of LC-MS compared to ELISA methods, we recommend that the initial analysis should be based on ELISA, specifically a kit which can detect allergens down to the Action Levels prescribed by FAO/WHO for the priority allergens, as detailed in Table Section 9-Table 1. In the absence of action levels for the other recognised UK food allergens, there is a need for indicative Health-Based Guidance Values (HBGVs) to be set. The specific method for use would consider the sensitivity, specificity, alignment of the LOD with the ED10 (while further work on reference dose derivation for ED05 continues as recommended in FAO/WHO ‘Risk Assessment of Food Allergens Part 2: Review and establish threshold levels in foods for the priority allergens) and the performance of the kit on the particular matrix.
Some kits are preferred by users due to the rapidity of the testing, however methods with more involved extractions, for example, often perform better on processed products for example kits manufactured by Morinaga Institute of Biological Science Inc., so may be more suitable to protect consumers. In order to provide meaningful data to inform suppliers, producers and enforcement, the kit would allow reporting of the data in mg of allergenic protein per kg of food. When a suspect sample is under investigation following an allergen incident, it must be determined whether the sample contains the allergen or is negative for the allergen. In most cases, primary analyses by ELISA kits will be most suitable, using a range of kits targeting different allergenic proteins when available, with further investigative analysis by LC-MS if the ELISA tests provide a negative result. In the eventuality of a negative result for LC- MS/MS, this should be confirmed by an alternative ELISA kit, selecting a kit based upon a different antibody to that used for the initial analysis. This testing regime is designed to avoid yielding false negative data which can be the case when only single tests are applied (Walker et al 2017).
Due to the reduced cost of testing, ELISA and PCR testing is more appropriate in the first instance. In the case of egg, PCR is not suitable, as discussed previously and consideration must be given to the sensitivity of the testing method. PCR methods targeting milk for example often lack sensitivity and ELISA should be performed in the first instances.
9.1. Recommended workflow for allergen incident management
When interrogating a suspect sample (for example a product for which testing has given a non-routine unexpected result, or is linked to an allergy incident, product recall or complaint), believed to have elicited an allergic response for an allergen, a representative sample of the product should be taken (100g-1kg) and homogenised into powder (or slurry, if a liquid) prior to analysis, taking validated laboratory precautions to avoid cross-contamination. At least two sub-samples should be taken for analysis, of at least 1g in mass each and extracted alongside suitable positive and negative RMs, ideally a CRM and analysed alongside a blank (ELISA well containing only the kit dilution buffer). A third aliquot of the sample should be spiked with allergen and tested, as described above.
In the first instance, an ELISA test should be conducted for the allergen(s) under suspicion. Where feasible, multiple ELISA tests should be worked through, ideally until an ELISA has been performed for each available target protein for that allergen, although this information is not always disclosed or is not known. The information is not always known when the polyclonal antibodies underpinning the method have been raised against the allergenic food as a whole, so the precise protein/epitope is not known. Testing must also encompass kits which support the appropriate level of processing (typically 1-3 different tests). Known cross-reactivities of the kits must be considered for the matrix in question. Table 15 of the FAO/WHO Risk Assessment of Food Allergens Part 2: Review and establish threshold levels in foods for the priority allergens (WHO, 2022) has been adapted (Section 9-Table 1) to inform regarding suitable available methodologies to pursue in the workflow for the allergens for which an Action Level is available. Should the suspect sample still test negative after ELISA analysis, an LC-MS method to target the allergen should be sought. Where there are gaps in capabilities to meet action levels, temporary action levels need to be set.
For the rare instances for which only PCR tests are available (e.g. celery), a PCR should be performed. Should the data be negative, then an alternative PCR, if available, targeting an alternative DNA sequence, should be instigated. For some of the tree nuts, the ELISA kits available are limited. For example, for Brazil nut, only one ELISA test is in common use, along with lateral flow tests and PCR, so these alternatives should be applied as secondary methods following a negative result from an ELISA. Ideally LC-MS methodology would then be sought and the sample analysed alongside RMs.
9.2. An example workflow for egg
Samples, as detailed above, should be tested alongside RMs. RMs available are NIST SRM 8445 (whole egg), ThRAll RMs (hen’s egg in broth and in chocolate), FSA RMs prepared under FSA-funded projects (FS101206,egg white in chocolate). QC materials comprising egg in a range of matrices such as cake mix are available from proficiency testing providers. A third sub-sample of the matrix should be over- spiked with allergen and tested to determine matrix effects and the recovery of the allergen in that matrix.
As discussed previously, ELISA testing must be used for determination of egg and not PCR. The first test kit should target an egg white protein. Should a food sample be found negative for that allergen, a second ELISA test should target a different egg white protein. Consideration should be given to performing ELISA using kits which are more suited to hydrolysed products, for example the Morinaga test kits. Finally, a confirmatory test by LC-MS is required to provide a robust framework targeting the detection of allergen proteins and peptides. The LC-MS method developed during the EFSA ThRAll project (Detection and Quantification of Allergens in Foods and Minimum Eliciting Doses in Food-Allergic Individuals) has the sensitivity required to quantify egg at the action levels identified for these foods by the recent FAO/WHO expert consultation (FAO/WHO, 2022).
9.3. An example workflow for milk
For the determination of milk, ELISA methods must be used in preference to PCR methods. The first test kits should target casein and β-lactoglobulin. Should a food sample be found negative for that allergen by these methods, an alternative test provider’s kit should be used to determine whole milk. Finally, a confirmatory test by LC-MS is required to provide a robust framework targeting the detection of allergen proteins and peptides. The LC-MS method developed during the EFSA ThRAll project (Detection and Quantification of Allergens in Foods and Minimum Eliciting Doses in Food-Allergic Individuals) has the sensitivity required to quantify milk at the action levels identified for these foods by the recent FAO/WHO expert consultation (FAO/WHO, 2022). Applicable RMs available include ThRAll RMs (skimmed milk in broth and in chocolate), RMs prepared during FSA-funded projects (FS101206, chocolate paste containing skimmed milk). QC materials comprising egg in a range of matrices such as cake mix are available from proficiency testing providers.
9.4. An example workflow for peanut
Reference materials are available for peanut and should be used during testing, namely NIST SRM 2387 (qualitative) incurred peanut butter and ThRAll RM (quantitative for incurred chocolate). For the determination of peanut, ELISA tests must be used, first of all targeting the highest number of known target analytes such as a kit which is sensitive to Ara h1, Ara h2 and Ara h3. Should allergen not be detected, an alternative kit should be applied to screen for alternative target proteins such as kits which detect the Ara h2 and Ara h6 combination. Should a suspect sample continue to be found as negative, a PCR test could be applied before confirmatory testing by LC-MS. The LC-MS method developed during the EFSA ThRAll project has the sensitivity required to quantify the allergens from egg, milk, peanut, almond and hazelnut at the action levels identified for these foods by the recent FAO/WHO expert consultation (FAO/WHO, 2022), while further refinement to improve the sensitivity by approximately 3-fold would be required to enable the method to be fully deployed in line with the FAO/WHO expert consultation recommendations for test method performance.
9.5. An example workflow for mustard
In the case of mustard, less information is available regarding the target proteins of the ELISA kits, and a widely accepted confirmatory LC-MS method has not been developed. CRMs are not available but reference materials from proficiency testing companies are available, and should be used in the absence of a CRM. Also, little information is available regarding the identity of the target proteins of the available ELISA kits. It is therefore recommended in the scope of this review that one of the two ELISA tests available will be applied and, if mustard is not detected in a suspect sample, one of the two PCR kits is applied. Failing detection, the remaining ELISA and PCR kits could be applied. This will be the scenario until a confirmatory test is available, although no suitable LC-MS methods have been identified by this review that have undergone an inter-lab validation.
These workflows, combining available methods and preferring ELISA over PCR unless ELISA is not available, can be applied to all food allergens other than sulphur dioxide and sulphites and available methods are detailed in Table 1 (Appendix 1).
For detection of sulphur dioxide and sulphites, users should refer to official methods (for example AOAC Official Method 990.28, OIV-MA-AS323-04A). Suitable RMs are detailed in Section 9-Table 2.
Section 9-Table 1. Action levels and desired kit LOQ for allergens depending on matrix. Taken from Table 15 of FAO/WHO Risk Assessment of Food Allergens Part 2: Review and establish threshold levels in foods for the priority
| Allergenic food ingredient | Matrix | P75 intake (Portion size for the 75th percentile of consumers)(g) | Proposed action level (mg protein/kg food) | Desired method LOQ (mg protein/kg food) | Are at least two ELISA methods available at this LOQ? (target) | LC-MS method available/under development? (LOQ) |
|---|---|---|---|---|---|---|
| Milk | Cookies/ biscuits | 50 | 40 | 13.3 | Yes (casein, β- LG) | R&D methods exist for baked goods at this LOQ e.g. Christina et al. 2016 |
| Milk | Chocolate | 40 | 50 | 16 | Yes (casein, β- LG) | Validated (inter-lab) R&D methods exist for chocolate at this LOQ e.g. Gavage et al. 2022. |
| Egg | Cookies/biscuits | 50 | 40 | 13.3 | Yes (ovalbumin, ovomucoid, lysozyme) | EFSA ThRAll method applies to broth and chocolate. Further evaluation on cookies required. R&D methods available at this LOQ e.g. Monaci et al 2014. |
| Peanut | Chocolate | 40 | 50 | 16 | Yes (Ara h 1, Ara h 2, Ara h 3, Ara h 6) | R&D methods exist for chocolate at this LOQ, e.g. Sayers, Gethings et al. 2018 |
| Almond | Chocolate, ice cream, pasta sauce | 40 | 25 | 8.3 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al. 2017 |
| Almond | Cookies | 50 | 20 | 6.6 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al. 2017 |
| Almond | Pasta sauce | 80 | 10 | 3.3 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al. 2017 |
| Almond | Ice cream | 100 | 10 | 3.3 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al. 2017 |
| Hazelnut | Bread roll | 120 | 25 | 8.3 | Yes (target undisclosed) | R&D methods exist for hazelnut in bread but not at this LOQ (LOD was 24mg/kg) |
| Hazelnut | Chocolate | 40 | 75 | 25 | Yes (target undisclosed) | EFSA ThRAll method available at this LOQ |
| Hazelnut | Cookies | 50 | 60 | 20 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al 2017 |
| Hazelnut | Tomato sauce | 80 | 35 | 11 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al 2017 |
| Hazelnut | Ice cream | 100 | 30 | 10 | Yes (target undisclosed) | R&D methods available at this LOQ e.g. Planque et al 2017 |
| Walnut | Chocolate | 40 | 25 | 8.3 | Yes (target undisclosed) | R&D method exists at this LOQ e.g. Gu et al 2018, Planque et al 2017 |
| Walnut | Cookies | 50 | 20 | 6.6 | Yes (target undisclosed) | R&D method exists but not for this LOQ / matrix. Further development required. |
| Walnut | Sauce | 80 | 10 | 3.3 | Yes (target undisclosed) | R&D method exists but not for this LOQ / matrix. Further development required. |
| Walnut | Ice cream | 100 | 10 | 3.3 | Yes (target undisclosed) | R&D method exists but not for this LOQ / matrix. Further development required. |
| Walnut | Bread roll | 120 | 8 | 2.6 | Yes (target undisclosed) | R&D method exists but not for this LOQ / matrix. Further development required. |
| Pecan | Chocolate | 40 | 25 | 8.3 | Yes (target undisclosed) | R&D method exists at this LOQ e.g. Planque et al. 2017 |
| Pecan | Cookies | 50 | 20 | 6.6 | Yes (target undisclosed) | R&D method exists at this LOQ e.g. Planque et al. 2017 |
| Pecan | Sauce | 80 | 10 | 3.3 | Yes (target undisclosed) | R&D method exists but LOQ is 5 mg/kg e.g. Planque et al. 2017 |
| Pecan | Ice cream | 100 | 10 | 3.3 | Yes (target undisclosed) | R&D method exists but LOQ is 5 mg/kg e.g. Planque et al. 2017 |
| Cashew | Chocolate | 40 | 25 | 8.3 | May depend on level of processing (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Cashew | Cookies | 50 | 20 | 6.6 | May depend on level of processing (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Cashew | Sauce | 80 | 10 | 3.3 | May depend on level of processing (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Cashew | Ice cream | 100 | 10 | 3.3 | May depend on level of processing (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Cashew | Bread roll | 120 | 8 | 2.6 | May depend on level of processing (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Pistachio | Chocolate | 40 | 25 | 8.3 | Yes (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Pistachio | Cookies | 50 | 20 | 6.6 | Yes (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Pistachio | Sauce | 80 | 10 | 3.3 | Yes (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Pistachio | Ice cream | 100 | 10 | 3.3 | Yes (target undisclosed) | Yes, R&D method exists at this LOQ e.g. Planque et al 2017 |
| Pistachio | Bread roll | 120 | 8 | 2.6 | Yes (target undisclosed) | Methods exist for pistachio but data required for bread |
| Wheat | Cookies | 50 | 100 | 33 | Yes, (gliadin) for hydrolysed, fermented and unhydrolysed foods. Apply kits which differ in the antibody used (R5 and G12 antibodies). | Development of methods required |
| Wheat determined as gluten | Infant semolina | 200 | 25 | 8.3 | Yes, (gliadin) for hydrolysed, fermented and unhydrolyzed foods | Development of methods required |
| Fish | Wine | 283 | 15 | 5 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Soy sauce | 30 | 150 | 50 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Chicken meatball | 126 | 35 | 11 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Pork meatball dumpling |
126 | 35 | 11 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Vegetable and chicken soup |
400 | 10 | 3.3 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Tofu soup Mushroom soup |
400 | 10 | 3.3 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Soy sauce | 30 | 150 | 50 | Yes (parvalbumin) | Development of methods required |
| Fish | Almond coconut muesli |
60 | 80 | 26 | Only when the species is cod (parvalbumin) | Development of methods required |
| Fish | Chicken corn soup | 400 | 10 | 3.3 | Only when the species is cod (parvalbumin) | Development of methods required |
| Crustacean shellfish | Fish ball sausages | 150 | 1000 | 333 | Yes (tropomyosin) | R&D methods under development for this allergen, require development for this matrix |
| Crustacean shellfish | Chicken meatball | 130 | 1500 | 500 | Yes (tropomyosin) | R&D methods under development for this allergen, require development for this matrix |
| Crustacean shellfish | Freeze- dried egg soup | 400 | 500 | 166 | Yes (tropomyosin) | R&D methods under development for this allergen, require development for this matrix |
Section 9-Table 2. Suitable RMs and QC materials.
| Food allergen type | Reference/Descriptor | Matrix | Reference material status | Incurred status | Availability |
|---|---|---|---|---|---|
| Milk | MoniQA MQA092014 | Negative and positive skimmed milk powders |
CRM | Incurred | No longer available |
| Milk | NIST SRM whole milk 1549 | - | CRM | Incurred | Not currently available |
| Milk | ThRAll RM | Chocolate | EFSA ThRAll RM | Incurred | Project ongoing |
| Milk | ThRAll RM | Broth | EFSA ThRAll RM | Incurred | Project ongoing |
| Milk | Product code LGC7421 | Skimmed milk powder | RM, FSA-funded | Powder, not applicable | Available in kit reference LGC746- KT |
| Milk | Product code LGC7462 | Chocolate paste containing milk egg white hazelnut powder walnut powder | RM, FSA-funded | Spiked | Available in kit reference LGC746- KT |
| Egg | NIST SRM 8445 | Whole egg | CRM | Incurred | Available |
| Egg | NIST SRM 8415 | Whole egg powder | CRM | Incurred | Not currently available |
| Egg | ThRAll RM | Chocolate | EFSA ThRAll RM | Incurred | Project ongoing |
| Egg | ThRAll RM | Broth | EFSA ThRAll RM | Incurred | Project ongoing |
| Egg | Product code LGC7422 | Egg white powder | RM, FSA-funded | Powder, not applicable | Available in kit reference LGC746- KT |
| Egg | Product code LGC7462 | Chocolate paste containing milk egg white hazelnut powder walnut powder |
RM, FSA-funded | Spiked | Available in kit reference LGC746- KT |
| Peanut | NIST SRM 2387 (qualitative) | Peanut butter | CRM | Incurred | Available |
| Peanut | ThRAll RM | Chocolate | EFSA ThRAll RM | Incurred | Project ongoing |
| Peanut | ThRAll RM | Broth | EFSA ThRAll RM | Incurred | Project ongoing |
| Soya | ThRAll RM | Chocolate | EFSA ThRAll RM | Incurred | Project ongoing |
| Soya | ThRAll RM | Broth | EFSA ThRAll RM | Incurred | Project ongoing |
| Hazelnut | ThRAll RM | Chocolate | EFSA ThRAll RM | Incurred | Project ongoing |
| Hazelnut | ThRAll RM | Broth | EFSA ThRAll RM | Incurred | Project ongoing |
| Hazelnut | Product code LGC7425 | Hazelnut powder, partially defatted | RM, FSA-funded | Powder, not applicable | Available in kit reference LGC746-KT |
| Hazelnut | Product code LGC7462 | Chocolate paste containing milk powder, egg white powder, hazelnut powder (partially defatted), walnut powder (partially defatted) | RM, FSA-funded | Spiked | Available in kit reference LGC746-KT |
| Almond | ThRAll RM | Chocolate | EFSA ThRAll RM | Project ongoing | |
| Almond | ThRAll RM | Broth | EFSA ThRAll RM | Project ongoing | |
| Almond | Product code LGC7424 | Almond powder | RM, FSA-funded | Available in kit reference LGC746-KT | |
| Almond | Product code LGC7462 | Chocolate paste containing milk powder, egg white powder, hazelnut powder (partially defatted), walnut powder (partially defatted) |
RM | Available in kit reference LGC746-KT | |
| Walnut | Product code LGC7426 | Walnut powder, partially defatted | RM, FSA-funded | Powder, not applicable | Available in kit reference LGC746-KT |
| Walnut | Product code LGC7426 | Chocolate paste containing milk powder, egg white powder, hazelnut powder (partially defatted), walnut powder (partially defatted) | RM | Spiked | Available in kit reference LGC746-KT |
| No allergenic ingredients |
LGC7461 | Chocolate paste | RM, FSA-funded | Not applicable | Available in kit reference LGC746-KT |
| Celery | QC materials available from proficiency testing providers | e.g. soup powder | QC Material | Various, spiked or incurred | Available |
| Fish | QC materials available from proficiency testing providers | e.g. cod muscle, fish in sauce | QC Material | Various, spiked or incurred | Available |
| Cereals containing Gluten | QC materials available from proficiency testing providers | e.g. soya formula, cake mix, oat- based food, cumin powder | QC Material | Various, spiked or incurred | Available |
| Lupin | QC materials available from proficiency testing providers | e.g. wheat flour | QC Material | Various, spiked or incurred | Available |
| Molluscs | QC materials available from proficiency testing providers | e.g. soup powder, sauce | QC Material | Various, spiked or incurred | Available |
| Mustard | QC materials available from proficiency testing providers | e.g. soup powder | QC Material | Various, spiked or incurred | Available |
| Sesame | QC materials available from proficiency testing providers | e.g. cumin powder | QC Material | Various, spiked or incurred | Available |
| Crustaceans | QC materials available from proficiency testing providers | e.g. sauce | QC Material | Various, spiked or incurred | Available |
| Other tree nuts | QC materials available from proficiency testing providers | e.g. chocolate | QC Material | Various, spiked or incurred | Available |
| Sulphites | QC1541 | Water | CRM | Incurred | Available |
| Sulphites | Calibrants from titration instrument manufacturers |
For use with wine | Unknown | Not known | Available |
10.1 Evidence Gaps
A number of gaps in knowledge and challenges to the allergen testing of foods have been highlighted in this report. They are summarised below:
As highlighted in the literature review, when tested by different kits, for some matrices cross-kit results are highly comparable. However, as reported by (Amnuaycheewa, Niemann et al. 2022 (in this case comparing gluten/gliadin- sensitive ELISA kits) while reporting similar results for some matrix types, yielded substantially different results for a few samples including samples of yeast extract, hemp protein powder and cookie. Those differences could be caused by any one of the several reasons: (a) differences in the grain source of glutens and related proteins, (b) differences in the efficiency of extraction and detection, (c) subsampling differences with particulates, or (d) some combination.
It is highlighted throughout this report that there is a lack of data and transparency regarding the applicability of test kits and public knowledge is often lacking regarding matrices for which kits have been found to be applicable and not applicable. This is demonstrated in the literature review for example by Röder, Kleiner et al. 2013 who state that there is a need to generate and publish data about the quantitative responses of methods to multiple cultivars of a given crop, and also to other allergenic foods.
Gaps in capability were also raised throughout the literature review relating to the inconsistency of data when a sample is processed compared to the testing of the raw product. This is perhaps best described by the detection of milk in processed products but applied to a wide range of allergens throughout the review. A crucial difference between kits designed to detect milk allergens is the detection of casein or casein and β-LG compared to detection of total milk protein. In milk, the proteins with the greatest heat stability are casein and serum albumin and consequently kits which target these proteins rather than whey proteins demonstrate better detection of thermally processed milk (Nowak-Wegrzyn and Fiocchi 2009). In this work, the comparison of methods suggest that the target protein is crucial to method development with casein being less impacted by thermal processing in comparison to the total milk protein content. Additionally, the mass spectrometry method showed less variance between the cereal bar and muffin matrices and consequently may be more tolerant to a range of matrices, although there was not enough evidence in this paper to conclude this. As highlighted in our studies of PT data, for processed foods in particular, the less popular or available kits tend to be those most suited to denatured allergenic proteins. There is a fundamental lack of clarity in the public domain regarding the suitability of many test kits for more processed samples. This needs to be addressed to support accurate allergen testing.
The use of ELISA kits for the detection of milk in cheese was investigated by Ivens et al. (Ivens, Baumert et al. 2017(1), Ivens, Baumert et al. 2017(2)). The authors suggest that while milk residues are detectable in cheese by ELISA kits, it would be important to select the appropriate kit for the level of proteolysis the milk has undergone and they stress that it is possible that there are fragments of proteolysed casein in processed milk which may not be detectable by modern ELISA kits.
Similarly, outcomes were reported in Section 7 which focussed on the study of proficiency testing data submitted to Fapas® between January 2018 and November 2022 relating to the variability of analysis between methods for the same allergen. Although regarding tree nut-allergens, much testing is progressing to LC-MS/MS methods, the majority of food testing laboratories world-wide continue to use ELISA kits which are specific not only to the allergen/matrix combination being tested but also specific to the design by the kit manufacturer. There was a fundamental lack of equivalence and reproducibility between different ELISA kit manufacturers (and sometimes between different types of kit for a single allergen by the same manufacturer). Batch-to-batch variation due to variations in kit-supplied buffers and calibration standards exists.
In addition to the issue of significantly different results being associated with different ELISA kit manufacturers, a further issue is evident in the PT data. This relates to the lack of comparability between ELISA kits from the same manufacturer. To provide one example, in PT 27316 (milk in infant soya formula) test material A, three kits from a single manufacturer were represented: at kit sensitive to β-Lactoglobulin (R4912), a kit sensitive to Casein (R4612) and a kit for ’milk’. The consensus assigned values were, respectively, 1.56 mg/kg, 31.0 mg/kg and 17.8 mg/kg. Clearly, the assigned value for casein should not be nearly 2x that of milk. This issue is particularly prevalent in the PT data for egg and milk determinations across various kit manufacturers.
During Section 7, analysis of PT data clearly showed that the repeatability limit of ELISA kits has been reached. The mean ratio san/σp was only just being maintained, so the repeatability of ELISA kits had not improved during the course of the data analysis. The data also clearly showed that the reproducibility limit of ELISA kits had been reached. Separately, it was noted in Section 7 that the antibodies used to detect the allergen and the composition of calibration standards differs between manufacturers for kits detecting the same allergen, leading to variations in PT data depending on the method applied.
The potential to convert units of measurement in order to compare data between methods for the same allergen was also discussed in Section 7. The inter-conversion of units of measurement such as measurements of genomic sequence, peptide or single protein into a measurement of total food allergen is essential to evaluate and compare methods but conversion factors are often not supplied by kit manufacturers.
Throughout the literature review and the comparison of available test kits for each allergen (Appendix 1), there was limited data regarding how the LOD and LOQ were determined for each kit. Were they determined simply by analysing a buffer spiked with an allergen which would provide the lowest (i.e. most sensitive) LOD and LOQ data. Or were raw or processed samples used, spiked with allergen (which provided moderately challenging samples to interrogate). Or had the LOD and LOQ been determined by the most challenging and also most informative method, namely preparing a range of samples incurred with allergen and processed to a level representative of consumer foods. Due to the lack of transparency as to how LOD and LOQ was determined for each kit, the true comparison of kit capabilities is impossible for users taking the values published in the kit instructions. Instead, users would be required to prepare samples and compare kits experimentally, an exercise which is costly in time and finances. More details of this nature are required of manufacturers in order that users can make calculated decisions as to which kit or kits to pursue for their own intra-laboratory validations before they can offer testing services.
Given the data above regarding the suitability of different protein types depending on the level of processing of the samples, there is also a requirement that better transparency of the target protein of each kit would support allergen testing. There are issues here relating to Intellectual Property but the lack of this data for many test kits does not support food test. Instead, testing laboratories must either rely on those kits for which the target protein is stated by the manufacturer, or perform costly studies themselves, comparing different kits and their suitability for different sample types.
All methods (ELISA, PCR and MS) suffer from issues in accurate quantification of allergens due to a lack of harmonised incurred reference methods, which is also a gap in testing capability.
A gap identified for allergen testing by LC-MS/MS is that a much more comprehensive knowledge of food genomes is required for mass spectrometry methods to work to their full potential in the analysis of food allergens, especially those from plant foods.
As highlighted by many stakeholders and our expert consultants, there is a need for the development and commercialisation of a fast, accurate, low-cost, multi-allergen tests, which can be used at point-of-use in factories to identify allergens, such as Near Infrared Spectroscopy or other innovative approaches. Relevant to the minimum sensitivity required for test kits, more work is also required to determine allergen thresholds of different foods for all allergens which must be declared on foods in the UK.
Finally, another evidence gap relating to allergen testing is this issue of the cross- reactivities which lead to false positive results when testing for some allergens. This is discussed below in more detail.
10.2 Cross-reactivity of testing methods
Cross-reactivities of individual test kits are detailed in Appendix 1. It is challenging to assess the full scope of cross-reactivities and whether there are kits on the market which compensate for the cross-reactivities in other kits for the same allergen due to the lack of cross-reactivity data provided by some kit manufacturers. A statement as to whether cross-reactivity testing has been conducted would be helpful, and a full list of those matrices tested would be ideal. However, cross-reactivities of note when considering the range of kits available for each allergen are:
As reported in the literature review, there are concerns regarding cross-reactivities including mustard antibodies cross-reacting with rapeseed (Brassica napus) at a level of 100%. This is a concern since rapeseed oil is used in a range of foods. .
Various milk testing kits report cross-reactivity goat and sheep milk. (Park, Coates et al. 2005) reported cross-reactivity between a peanut kit and chickpea, green pea, and lima bean.
There is much potential for cross-reactivity between crustacean allergens and insect food allergens, due to the commonality of certain proteins between the animal groups. In GB, interest in insects as a source of protein is growing. Insects that were legally marketed before 1 January 2018, submitted a novel foods application to the EU by 1 January 2019 and submit an application to the GB authorities by 31 December 2023, can remain in the market. Crustacean allergens such as tropomyosin are known to be highly homologous to their insect counterparts (Romero et al., 2016), and IgE cross-reactivity has been shown (De Marchi et al.,2021). It is therefore possible that antibody-based tests for crustaceans might detect insect proteins via species cross-reactivity.
Kamath et al. 2013 also reported increased recognition of multiple tropomyosin monoclonal antibodies upon heating of shellfish (Kamath, Rahman et al. 2013). These authors also reported cross-reactivity of tropomyosin in all 11 crustacean species, with partial detection in molluscs (cross-reacting with mussels, scallops and snails but not in oyster, octopus and squid). The authors conclude that specific monoclonal antibodies, targeting the N-terminal region of tropomyosin, must be developed to differentiate tropomyosins in crustaceans and molluscs.
Lacorn et al. illustrated in 2016 that closely-related plants show cross-reactivity to soybean ELISA (Lacorn, Dubois et al. 2016). However, although from a regulatory point of view, these cross-reactivities could be considered undesirable, they may still be relevant due to potential co-sensitivity amongst soy-sensitive consumers. Eighteen phylogenetically closely related species were tested. No cross-reactivities were observed for Lupinus angustifolius, L. albus, and L. luteus. In contrast, cross- reactivities were observed against Pisum sativum (dried and fresh seeds), Vicia pannonica, Lens culinaris, Arachis hypogea (roasted and raw), Cicer arietinum, Trigonella foenum-graecum, Trifolium pratense, Phaseolus vulgaris, P. lunatus, V. faba, P. coccineus, Vigna radiate, and V. angularis.
In the case of almonds, ELISA methods show cross-reactivities against phylogenetically closely related species including Prunus genus seeds (apricot, nectarine, cherry, plum, peach) but not with the flesh of these fruits (Slotwinski, Almy et al. 2018). Mahaleb cherry (Prunus mahaleb) is used as a spice. Apricot stones are used in the marzipan alternative persipan. Real-time PCR methods were developed. Burns et al. 2016 designed a real-time PCR method shown by the authors to be specific for Prunus mahaleb. Other work has also been completed using real-time PCR to distinguish almond and Prunus mahaleb to provide greater species specification compared to ELISA (Walker et al. 2018).
However, care must be taken to avoid cross-reactivity with Prunus mahaleb and using ELISA and PCR in combination can be an effective method of guarding against this.
As detailed further in Appendix 1, various of the nut kits show low levels of cross- reactivity with other nuts, and greater kit specificity is required. For example Sanchiz et al. (Sanchiz, Ballesteros et al. 2017) determined cross-reactivity of a pecan test kit with a food containing 5% of cashew nut, and chocolate with hazelnut, suggesting cross-reactivity with these two nuts.
Mass spectrometry can be more specific (less cross-reactivity) for detection of the target allergen protein to be quantified due to careful selection of the species-specific sequence to be detected, providing protein identity information, permitting a wider linear dynamic range, being less prone to be affected by food processing and, if appropriately applied, can be used as a reference method or for the production of CRMs. However, LC-MS/MS methods currently tend not to show the levels of sensitivity of ELISA methods and can also show low recovery, depending on the extraction method used.
The fact that celery can currently only be reliably determine by PCR is a concern, given the disadvantages of PCR data for allergen data interpretation.
As shown in Appendix 1, lupin ELISA kits cross-react with chickpea (0.0003%), lentil (0.0004%), soy flour (unroasted, 0.0700%), soy flour (roasted, 0.0009%) and soy lecithin (0.002%) although there are cross-sensitivities of these within allergen sufferers.
This cross-reactivity information, which is not always included in the manuals of testing kits, highlights the necessity for test kit manufacturers to perform a comprehensive range of testing to determine cross-reactivity, and to make this list public, to support allergen testing and management. Overall, a lack of transparency regarding the validation studies conducted (or not conducted) on each test kit is a fundamental limitation when it comes to determining the capabilities and limitations of test kits.
10.3 Overall Conclusions
This review of methods used for food allergen testing involved a literature review of data (laboratory evaluations and data from kit manufacturers) published in the public domain in this field over the last twenty years (included in Sections 2 and 3). One issue which arose time and again was the concern that little or no data about which version of the various commercial testing kits are detailed in the literature. Commercial food testing kits often undergo development and alteration which will change the performance of the kits. For example, ELISA kits necessarily undergo alterations, when new batches of antibody are prepared when stocks are depleted. Also, kit manufacturers often update each of their kits, for example introducing altered or improved extraction buffers or other reagents, and performing more validation studies and, on occasion, the name and reference number of the kit is not altered. Much caution is therefore required when relating current kit performance to data published in previous years. The protein target of the kits is not always known (this is often the case when kits are underpinned by polyclonal antibodies which were raised against the allergenic food as a whole, so the precise protein/epitope is not known) or the protein identity is not disclosed due to proprietary issues. This lack of knowledge regarding the target protein poses an issue during incident management when application of range of tests which are sensitive to different protein targets would be beneficial to gain confidence when trying to detect an allergen in a sample or to rule out a suspect sample for allergen presence. The crucial challenge in ELISA detection is the variability of data generated across different kits targeting the same allergen. Another gap in the information included in many test kits is a lack of interconversion factors to convert from one allergenic protein to another or to ‘total allergen protein per kg food.’
The performance of allergen detection methods is affected, often but not exclusively, in a negative manner, when foods undergo thermal processing, a very common treatment of most food products unless commonly eaten raw. Thermal processing can, for example, alter protein folding and oxidation processes can take place which can lead to modification of amino acids, formation of protein bound carbonyls and aggregation. Each of these changes can affect the detectability of the parts of the protein which are detected in methods such as ELISA and can also alter the level of protein extractability/solubility which affects the recovery of testing procedures. Limitations involving detection of milk in cheese due to proteolysis during processing were also discussed, as were cross-reactivities which can lead to false positive data. Kit manufacturers should release their entire validation reports as a matter of course, and manuals should clearly state the food matrices for which their kit has been validated, whether these products were raw or processed and the form (e.g. time, temperature, pressure) of processing.
To help to overcome complications linked to determining any changes in recovery (and therefore LOD) linked to food processing, it would also be beneficial if it was stipulated that methods must be tested against standardised RMs (where available), ideally indicating which RMs should be used for each allergen and the disclosure of the RM used should be included in the validation data. This way, test users could compare the performance of the kit in their hands on the same RM compared to the performance declared by manufacturers. Ideally, harmonisation of methods would be achieved, and CRMs would be available for all allergens and for a much wider range of levels of processing, in order that users can reliably calibrate their methods against these RMs. Preferably, more resource would be allocated to preparing a much wider and comprehensive range of CRMs for each allergen. Currently, CRMs are lacking (incurred RMs in particular) with certain of those previously prepared now removed from the market. RMs are expensive to prepare and verify as homogenous and commercial incentive for their preparation is often low, hence their poor representation on the market. Realistically, without RMs against which to calibrate methods, the capability of current methods is only semi-quantitative. It should also be encouraged that kit manufacturers provide detailed information regarding the manner by which the amount of allergenic protein present in a sample can be calculated from the data per mass of food product, in line with the conclusions of the FAO/WHO expert consultation. As is currently the focus of FAO/WHO, more data are also required to determine allergen thresholds of different foods. These data will be valuable in terms of allergen management in the UK.
The review has determined that allergen test kit suppliers are often not transparent in publishing the entire validation studies completed before marketing the kit. A likely benefit of full publication of validation results in the public domain would be improved protection of consumers. This would include how the test materials used for measuring kit performance were prepared. For example, were they simply a buffer spiked with the allergen - this comprises the simplest form of test material and is not representative of ‘real’ food samples. Was a raw food used and spiked or was a finished food product spiked with the allergen? Were incurred products prepared, accurately mimicking food processing techniques? Spiking approaches for test method validation are not representative of true food samples as the allergenic food ingredient used for spiking had not undergone the same level of processing as the food matrix. The use of incurred materials is the ideal scenario to mimic ‘real’ foods. As the choice of analytical method is crucial to ensure consumer safety, we need to have confidence in the testing methods used and standardisation to compare data. This relates to all allergens but soyabean is one key area where detection of the allergen is kit- or method-dependent, often relating to the level and type of processing. So many foods contain soyabean and each involves a different level of processing. This is discussed in this report (Section 3 and also Section 10).
To further complicate our understanding of the range of commercial testing kits available, it has become apparent during the preparation of Table 1 that not all suppliers detail much information regarding the performance of the kit. There is evidence from comparing some of the kit parameters that some suppliers re-brand other kits and sell under a different name, which further complicates our understanding of current capabilities and the range/number of different kits available on the market.
In order to protect consumers against accidental consumption of allergenic foods and associated allergic reactions, there is a need to improve education across the supply chain relating to the need for testing at each stage in the chain. It is also important that suppliers of foods and their ingredients understand the limitations of each particular test (Sections 3 and 4). The need for improved harmonisation of methods and auditing throughout the supply chain was also covered in these sections and legislative gaps highlighted. Alignment of food testing methods developed in the EFSA ThRAll project was also discussed.
This project also comprised statistical analysis of the Fapas® allergen proficiency testing data generated over the last five years. This analysis (Section 7) demonstrated that the repeatability and reproducibility limits of ELISA kits have been reached. New methods are clearly required to fill this gap. Furthermore, analysis of the Fapas® data reinforced issues raised by other authors that ELISA kits do not report like-for-like data and that data cannot be inter-converted. Finally, this statistical analysis exercise of Fapas® data raised the issue of significantly different results being associated with different ELISA kit manufacturers. A further issue is evident in this data, and it relates to the lack of comparability between ELISA kits from the same manufacturer, demonstrating that the use of different kits from one provider for a single allergen yields a different final level of allergen in a given sample.
It has been clearly demonstrated throughout this project that there are limitations in allergenic food ingredient detection and quantification. Each of the main testing methodologies show advantages and disadvantages and hybrid testing will yield higher efficiency in successful testing in most cases. Reliance on a single method per allergen ingredient leaves testing workflows vulnerable to not detecting the presence of an allergenic ingredient due to the differing limitations of all methods. Workflows have therefore been developed to support incident management, combining different technology formats in order to maximise the scope of the testing for each allergen and applicability depending on the level of processing (Section 9). To increase our understanding of allergen risk management in the supply chain, stakeholder interviews are included in this report detailing the various strategies used across UK suppliers to manage food allergen risk.
10.4 Future direction
In Japan, much progress has been made to align food regulation with allergen testing methods. While this approach may not be perfect, especially since the range of permitted test methods is mandated, thus perhaps stifling innovation while also removing some commercial testing methods from the market, this may be a useful model aiming to standardise testing and offer improved protection to consumers.
Since a different model is used compared to that in the UK and EU, there may be areas of learning that can be made from discussing allergen management with countries which have adopted a contrasting approach. For example, it is possible that establishing an approval system where manufacturers submit their validation data to be assessed by experts, or that a requirement is made for companies to publish up-to-date validation and performance data with their kits, would bring benefits to UK procedures for food testing.
The Japanese are recognised as being advanced compared to other nations regarding management of allergens and also have different views regarding how RMs should be prepared (personal comms) or how calibrants should be applied and it may be interesting to learn more on their methods in an interview with relevant academics in Japan.
As highlighted by many stakeholders and our expert consultants, there is a need for the development and commercialisation of a fast, accurate, low-cost, multi-allergen tests, which can be used at point-of-use in factories to identify allergens. One candidate rapid technology may be Near Infrared Spectroscopy (NIR) although development of such methods is required.
While ELISA, lateral flow test strips and PCR testing methods have dominated the allergen testing scene for a number of decades and therefore benefit from years of method development and validation, new methods are becoming increasingly apparent, with increased specificity and robustness to thermal processing. Mass spectrometry methods fall under this category. While LC-MS methods show benefits in specificity compared to ELISA, sensitivity is often lacking in these relatively new mass spectrometry methods at present. However, some mass spectrometry methods deliver to the expected guidelines of FAO/WHO and, as highlighted in the EFSA ThRAll project, method optimisation for certain allergens has the potential to further improve sensitivity. In order to progress more recent methods of allergen detection, increased funding for R&D is required.
Concerning emerging technologies, methods using alternative technologies are under development but are not being disclosed in the public domain at present for proprietary reasons(personal comms). It would be beneficial to discuss these technologies in confidence, to better inform FSA of emerging technologies. No matter the platform (e.g. ELISA, PCR, lateral flow, LC-MS/MS, emerging technologies), the development of reliable, rapid and point-of-use allergen detection methods showing high accuracy and sensitivity is paramount to protect allergen-sensitive consumers.
Achouri, A. and J. I. Boye (2013). "Thermal processing, salt and high pressure treatment effects on molecular structure and antigenicity of sesame protein isolate." Food Research International 53(1): 240-251.
Akiyama, H., K. Nakamura, N. Harikai, H. Watanabe, K. Ijima, H. Yamakawa, Y. Mizuguchi, R. Yoshikawa, M. Yamamoto, H. Sato, M. Watai, F. Arakawa, T. Ogasawara, R. Nishihara, H. Kato, A. Yamauchi, Y. Takahata, F. Morimatsu, S. Mamegoshi, S. Muraoka, T. Honjoh, T. Watanabe, K. Sakata, T. Imamura, M. Toyoda, R. Matsuda and T. Maitani (2004). "Inter-laboratory evaluation studies for establishment of notified ELISA methods for allergic substances (Peanuts)." Journal of the Food Hygienic Society of Japan 45(6): 325-331.
Allen, K. J., B. C. Remington, J. L. Baumert, R. W. R. Crevel, G. F. Houben, S. Brooke-Taylor, A. G. Kruizinga and S. L. Taylor (2014). "Allergen reference doses for precautionary labeling (VITAL 2.0): Clinical implications." Journal of Allergy and Clinical Immunology 133(1): 156-164.
Allred, L. K. and B. W. Ritter (2019). "Recognition of Gliadin and Glutenin Fractions in Four Commercial Gluten Assays." Journal of AOAC INTERNATIONAL 93(1): 190- 196.
Alvarez, P. A. and J. I. Boye (2014). "Comparison of gluten recovery in gluten- incurred buckwheat flour using different commercial test kits." Food and Agricultural Immunology 25(2): 200-208.
Amnuaycheewa, P., L. Niemann, R. E. Goodman, J. L. Baumert and S. L. Taylor (2022). "Challenges in Gluten Analysis: A Comparison of Four Commercial Sandwich ELISA Kits." Foods 11(5):706.
Baseggio Conrado A, Ierodiakonou D, Gowland MH, Boyle RJ, Turner PJ. (2021). “Food anaphylaxis in the United Kingdom: analysis of national data, 1998–2018.” British Medical Journal. 17:372.
Baur X, Pau M, Czuppon A and Fruhmann G, (1996). “Characterization of soybean allergens causing sensitization of occupationally exposed bakers.” Allergy, 51: 326-330.
Besler-Scharf, M. (2021). Allergens IV: celery, mustard and sesame in mayonnaise (Report No. DLA ptAL04 (2021)), DLA Proficiency Tests. PDF available here. (Accessed: 25 January 2023)
Bianco, M., C. D. Calvano, G. Ventura, I. Losito and T. R. I. Cataldi (2022). "Determination of hidden milk allergens in meat-based foodstuffs by liquid chromatography coupled to electrospray ionization and high-resolution tandem mass spectrometry." Food Control 131:108443.
Bignardi, C., M. Mattarozzi, A. Penna, S. Sidoli, L. Elviri, M. Careri and A. Mangia (2013). "A Rapid Size-Exclusion Solid-Phase Extraction Step for Enhanced Sensitivity in Multi-Allergen Determination in Dark Chocolate and Biscuits by Liquid Chromatography-Tandem Mass Spectrometry." Food Analytical Methods 6(4): 1144- 1152.
Binaghi, M. J., C. B. Greco, M. E. Martin, S. R. Drago, P. A. R. de Ferrer and L. B. Lopez (2017). "Soya traces quantification in cookies and extruded products with ELISA kits." Acta Bioquimica Clinica Latinoamericana 51(4): 653-660.
Breidbach, A. et al. (2022) ‘Assignment of a Reference Value of Total Cow’s Milk Protein Content in Baked Cookies Used in an Interlaboratory Comparison’, Foods, 11(6): 869.
Bugyi, Z., K. Torok, L. Hajas, Z. Adonyi, B. Popping and S. Tomoskozi (2013). "Comparative study of commercially available gluten ELISA kits using an incurred reference material." Quality Assurance and Safety of Crops & Foods 5(1): 79-87.
Burney P, Summers C, Chinn S, Hooper R, van Ree R and Lidholm J, (2010). “Prevalence and distribution of sensitization to foods in the European Community Respiratory Health Survey: a EuroPrevall analysis”. Allergy, 65: 1182- 1188.
Burns, M., Walker, M. Wilkes, T, Hall, L., Gray, K and Nixon, G. (2016). Development of a Real-Time PCR Approach for the Specific Detection of Prunus mahaleb. Food and Nutrition Sciences Vol.7 (8) 2016. DOI: 10.4236/fns.2016.78071.
Careri, M., L. Elviri, J. B. Lagos, A. Mangia, F. Speroni and M. Terenghi (2008a). "Selective and rapid immunomagnetic bead-based sample treatment for the liquid chromatography-electrospray ion-trap mass spectrometry detection of Ara h3/4 peanut protein in foods." Journal of Chromatography A 1206(2): 89-94.
Careri, M., L. Elviri, M. Maffini, A. Mangia, C. Mucchino and M. Terenghi (2008b). "Determination of peanut allergens in cereal-chocolate-based snacks: metal-tag inductively coupled plasma mass spectrometry immunoassay versus liquid chromatography/electrospray ionization tandem mass spectrometry." Rapid Communications in Mass Spectrometry 22(6): 807-811.
Carrera, M., B. Canas and J. M. Gallardo (2012). "Rapid direct detection of the major fish allergen, parvalbumin, by selected MS/MS ion monitoring mass spectrometry." Journal of Proteomics 75(11): 3211-3220.
Cau, S., C. Daga, C. Spanu, B. Soro, T. Tedde, S. Salza, R. Melillo, G. Piras, S. Virgilio, B. Vodret and A. G. Mudadu (2022). "Detection of Fish Allergens in Foods Using an In-House Real-Time PCR Targeting the Ribosomal 18S rRNA Gene." Foods 11(22): 3686.
Chinnappan, R., Rahamn, A.a., AlZabn, R., Kamath, S., Lopata, A.L., Abu-Salah, K and Zourob, M. (2020) Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem. 314:126133. doi: 10.1016/j.foodchem.2019.126133.
Cordeiro, F., Cubero-Leon, E., Nørgaard, J. et al. (2021) "Total cow’s milk protein in cookies: the first interlaboratory comparison with a well-defined measurand fit for food allergen risk assessment." Accred Qual Assur 26, 177–181. https://doi.org/10.1007
Costa, J., Ansari, P., Mafra, I., Oliveira, M.B.P. and Baumgartner, S., (2014). “Assessing hazelnut allergens by protein-and DNA-based approaches: LC-MS/MS, ELISA and real-time PCR.” Analytical and bioanalytical chemistry, 406: 2581-2590
Cristina, L., A. Elena, C. Davide, M. Giribaldi, D. Lucia, G. Cristiano, A. Marco, R. Carlo, L. Cavallarin and G. M. Gabriella (2016). "Validation of a mass spectrometry- based method for milk traces detection in baked food." Food Chemistry 199: 119- 127.
Croote, D., I. Braslavsky and S. R. Quake (2019). "Addressing Complex Matrix Interference Improves Multiplex Food Allergen Detection by Targeted LC-MS/MS." Analytical Chemistry 91(15): 9760-9769.
Cucu, T., C. Platteau, I. Taverniers, B. Devreese, M. De Loose and B. De Meulenaer (2013). "Effect of partial hydrolysis on the hazelnut and soybean protein detectability by ELISA." Food Control 30(2): 497-503.
Daems, D., B. Peeters, F. Delport, T. Remans, J. Lammertyn and D. Spasic (2017). "Identification and Quantification of Celery Allergens Using Fiber Optic Surface Plasmon Resonance PCR." Sensors 17(8): 1754.
Daga, C., S. Cau, M. G. Tilocca, B. Soro, A. Marongiu and B. Vodret (2018). "Detection of fish allergen by droplet digital PCR." Ital J Food Saf 7(4): 7264.
Daly, M., P. Ansari, G. Haubl, A. Rogers and K. Brunner (2018). "Assessing Almond and Peanut Allergens Using Commercially Available Immunoanalytical Kits and LC- MS/MS: A Case Study." Journal of AOAC International 101(1): 96-101.
De Marchi, L., F. Mainente, M. Leonardi, S. Scheurer, A. Wangorsch, V. Mahler, R. Pilolli, D. Sorio and G. Zoccatelli (2021). "Allergenicity assessment of the edible cricket Acheta domesticus in terms of thermal and gastrointestinal processing and IgE cross-reactivity with shrimp." Food Chemistry 359: 129878.
Dini, I., R. Di Lorenzo, A. Senatore, D. Coppola and S. Laneri (2020). "Validation of Rapid Enzymatic Quantification of Acetic Acid in Vinegar on Automated Spectrophotometric System." Foods 9(6): 761.
Djurtoft R, Pedersen HS, Aabin B and Barkholt V, (1991). “Studies of food allergens: soybean and egg proteins.” Advances in Experimental Medicine and Biology, 289: 281-293.
Dong, X. and Raghavan, V. (2022). A comprehensive overview of emerging processing techniques and detection methods for seafood allergens. Comprehensive reviews in food science and food safety. 21(7). https://doi.org/10.1111/1541-4337.12987.
Downs, M. L., J. L. Baumert, S. L. Taylor and E. N. C. Mills (2016). "Mass spectrometric analysis of allergens in roasted walnuts." Journal of Proteomics 142: 62-69.
Downs, M. L. and S. L. Taylor (2010). "Effects of Thermal Processing on the Enzyme-Linked Immunosorbent Assay (ELISA) Detection of Milk Residues in a Model Food Matrix." Journal of Agricultural and Food Chemistry 58(18): 10085- 10091.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), (2014). “Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes.” EFSA Journal 2014; 12( 11):3894
ELISA (2020), Gluten ELISA kit, Available at: https://www.elisakits.co.uk/gliadingluten-elisa-kit/ (Accessed: 25 January 2023)
Ehlert, A., A. Demmel, C. Hupfer, U. Busch and K. H. Engel (2009). "Simultaneous detection of DNA from 10 food allergens by ligation-dependent probe amplification." Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 26(4): 409-418.
Engler-Blum, G., C. Raiss and E. Burgmaier-Thielert (2007). "Detection of allergens in food using real-time polymerase chain reaction and immunoassays: Sensitive methods for detection of peanut and hazelnut - A comparative approach." Deutsche Lebensmittel-Rundschau 103(3): 101-108.
Fan, S., J. Ma, Z. Liu, Y. Ning, M. Cao, Q. Li and Y. Zhang (2023). "Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard." Food Science and Human Wellness 12(3): 728-736.
FAO/WHO. (2022). "Risk Assessment of Food Allergens – Part 2: Review and establish threshold levels in foods for the priority allergens. Meeting Report." Food Safety and Quality Series 5: 152.
Fapas, 2023, Protocol for Proficiency Testing Schemes Part 1- Common Principles, Version 8, Fapas
FDA (Food and Drug Administration), (1996). “Sulphites safe for most, dangerous for some.” FDA Consumer, the magazine of the Food and Drug Administration, 30 December 1996.
Fearn, T. and Thompson, M., 2001, “A new test for sufficient homogeneity”, Analyst,
126: 1414-1417.
FOCOS (2023), "Publications" Available at: https://www.focos-food.com/publications/ (Accessed: 29 June 2023)
Fu, T. J. and N. Maks (2013). "Impact of Thermal Processing on ELISA Detection of Peanut Allergens." Journal of Agricultural and Food Chemistry 61(24): 5649-5658.
Fu, T. J., N. Maks and K. Banaszewski (2010). "Effect of Heat Treatment on the Quantitative Detection of Egg Protein Residues by Commercial Enzyme-Linked Immunosorbent Assay Test Kits." Journal of Agricultural and Food Chemistry 58(8): 4831-4838.
Gavage, M., Van Vlierberghe, K., Van Poucke, C. De Loose, M., Gavaert, K., Dieu, M., Renard, P. Arnould, T., Filee, P and Gillard, N. (2020) Comparative study of concatemer efficiency as an isotope-labelled internal standard for allergen quantification. Food Chemistry 332 127413
Gelincik A, Buyukozturk S, Gul H, Isik E, Issever H, Ozseker F, Colakoglu B, Dal M, Ayvaz O, Gungor G and Akkor A, (2008). “Confirmed prevalence of food allergy and non-allergic food hypersensitivity in a Mediterranean population.” Clinical and Experimental Allergy, 38: 1333-1341.
Geng, T., C. D. Westphal and J. M. Yeung (2008). Detection of Gluten by Commercial Test Kits: Effects of Food Matrices and Extraction Procedures. Food Contaminants, American Chemical Society. 1001: 462-475.
Gomaa, A. and J. Boye (2015). "Simultaneous detection of multi-allergens in an incurred food matrix using ELISA, multiplex flow cytometry and liquid chromatography mass spectrometry (LC-MS)." Food Chemistry 175: 585-592.
Gomaa, A. and J. I. Boye (2013). "Impact of thermal processing time and cookie size on the detection of casein, egg, gluten and soy allergens in food." Food Research International 52(1): 483-489.
Gu, S. Q., N. N. Chen, Y. Zhou, C. M. Zhao, L. N. Zhan, L. Qu, C. Cao, L. Han, X. J. Deng, T. Ding, C. Song and Y. P. Ding (2018). "A rapid solid-phase extraction combined with liquid chromatography-tandem mass spectrometry for simultaneous screening of multiple allergens in chocolates." Food Control 84: 89-96.
Hajas, L., K. A. Scherf, K. Török, Z. Bugyi, E. Schall, R. E. Poms, P. Koehler and S. Tömösközi (2018). "Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis." Food Chem 267: 387-394.
Hamide Z. Senyuva, Ivona Baricevic Jones, Mark Sykes & Sabine Baumgartner (2019), “A critical review of the specifications and performance of antibody and DNA- based methods for detection and quantification of allergens in foods”, Food Additives and Contaminants: Part A, 36(4):507-547
Heick, J., M. Fischer, S. Kerbach, U. Tamm and B. Popping (2011). "Application of a Liquid Chromatography Tandem Mass Spectrometry Method for the Simultaneous Detection of Seven Allergenic Foods in Flour and Bread and Comparison of the Method with Commercially Available ELISA Test Kits." Journal of Aoac International 94(4): 1060-1068.
Heick, J., M. Fischer and B. Popping (2011). "First screening method for the simultaneous detection of seven allergens by liquid chromatography mass spectrometry." Journal of Chromatography A 1218(7): 938-943.
Henrottin, J., Pilolli, R., Huet, A.-C., van Poucke, C., Nitride, C., De Loose, M., . . . Monaci, L. (2023). "Optimization of a sample preparation workflow based on UHPLC-MS/MS method for multi-allergen detection in chocolate: An outcome of the ThRAll project." Food Control, 143: 109256.
Hoffmann, B., S. Munch, F. Schwagele, C. Neususs and W. Jira (2017). "A sensitive HPLC-MS/MS screening method for the simultaneous detection of lupine, pea, and soy proteins in meat products." Food Control 71: 200-209.
Holzhauser, T., P. Johnson, J. P. Hindley, G. O'Connor, C. H. Chan, J. Costa, C. K. Faeste, B. J. Hirst, F. Lambertini, M. Miani, M. C. Robert, M. Roder, S. Ronsmans, Z. Bugyi, S. Tomoskozi and S. D. Flanagan (2020). "Are current analytical methods suitable to verify VITAL (R) 2.0/3.0 allergen reference doses for EU allergens in foods?" Food and Chemical Toxicology 145: 111709.
Holzhauser, T., O. Stephan and S. Vieths (2002). "Detection of potentially allergenic hazelnut (Corylus avellana) residues in food: A comparative study with DNA PCR- ELISA and protein sandwich-ELISA." Journal of Agricultural and Food Chemistry 50(21): 5808-5815.
Houben GF, Baumert JL, Blom WM, Kruizinga AG, Meima MY, Remington BC, Wheeler MW, Westerhout J, Taylor SL. (2020) “Full range of population Eliciting Dose values for 14 priority allergenic foods and recommendations for use in risk characterization.” Food Chem Toxicol. 146:111831. doi: 10.1016/j.fct.2020.111831.
Huet, A. C., Paulus, M., Henrottin, J., Brossard, C., Tranquet, O., Bernard, H., . . . Van Poucke, C. (2022). "Development of incurred chocolate bars and broth powder with six fully characterised food allergens as test materials for food allergen analysis." Anal Bioanal Chem, 414(8): 2553-2570.
Huschek, G., J. Bonick, Y. Lowenstein, S. Sievers and H. Rawel (2016). "Quantification of allergenic plant traces in baked products by targeted proteomics using isotope marked peptides." Lwt-Food Science and Technology 74: 286-293.
Imamura T., Toyoda M., Matsuda R., and Maitani T. (2004). "Inter-laboratory evaluation studies for development of notified ELISA methods for allergic substances (milk)." Journal of the Food Hygienic Society of Japan 45(3): 120-127.
Immer, U., B. Reck, S. Lindeke and S. Koppelman (2004). "RIDASCREEN (R) FAST PEANUT, a rapid and safe tool to determine peanut contamination in food." International Journal of Food Science and Technology 39(8): 869-871.
ISO, 2015, “Statistical Methods for use in Proficiency Testing by Interlaboratory Comparison.”, ISO 13528:2015, International Organization for Standardization
ISPA, "Detection and quantification of allergens in food and minimum eliciting doses in food allergic individuals" Available at https://www.ispacnr.it/thrall-project/ (Accessed: 25 January 2023)
Ivens, K. O., J. L. Baumert, R. L. Hutkins and S. L. Taylor (2017 (1)). "The Effect of Different Methods of Fermentation on the Detection of Milk Protein Residues in Retail Cheese by Enzyme-Linked Immunosorbent Assay (ELISA)." Journal of Food Science 82(11): 2752-2758.
Ivens, K. O., J. L. Baumert, R. L. Hutkins and S. L. Taylor (2017 (2)). "Effect of proteolysis during Cheddar cheese aging on the detection of milk protein residues by ELISA." Journal of Dairy Science 100(3): 1629-1639.
Jankiewicz, A., W. Baltes, K. W. Bogl, L. I. Dehne, A. Jamin, A. Hoffmann, D. Haustein and S. Vieths (1997). "Influence of food processing on the immunochemical stability of celery allergens." Journal of the Science of Food and Agriculture 75(3): 359-370.
Jayasena, S., S. J. Koppelman, B. Nayak, S. L. Taylor and J. L. Baumert (2019). "Comparison of recovery and immunochemical detection of peanut proteins from differentially roasted peanut flour using ELISA." Food Chemistry 292: 32-38.
Jayasena, S., M. Smits, D. Fiechter, A. de Jong, J. Nordlee, J. Baumert, S. L. Taylor, R. H. Pieters and S. J. Koppelman (2015). "Comparison of Six Commercial ELISA Kits for Their Specificity and Sensitivity in Detecting Different Major Peanut Allergens." Journal of Agricultural and Food Chemistry 63(6): 1849-1855.
Jayasena, S., S. S. K. Wijeratne, S. L. Taylor and J. L. Baumert (2019). "Improved extraction of peanut residues from a wheat flour matrix for immunochemical detection." Food Chemistry 278: 832-840.
JCGM, (2012), " International vocabulary ofmetrology – Basic and general concepts and associated terms (VIM)", Available at: https://www.bipm.org/documents/20126/2071204/JCGM_200_2012.pdf/f0e1ad45- d337-bbeb-53a6-15fe649d0ff1?version=1.16&t=1659082802818&download=true (Accessed: 29 June 2023)
Jeong, S. G. and S. H. Kim (2020). "Application of commercial kits using DNA-based and immunochemical methods for determination of shrimp allergens in kimchi and its ingredients." Journal of Food Science 85(10): 3638-3643.
Johnson, P. E., N. M. Rigby, J. R. Dainty, A. R. Mackie, U. U. Immer, A. Rogers, P. Titchener, M. Shoji, A. Ryan, L. Mata, H. Brown, T. Holzhauser, V. Dumont, J. A. Wykes, M. Walker, J. Griffin, J. White, G. Taylor, B. Popping, R. Crevel, S. Miguel, P. Lutter, F. Gaskin, T. B. Koerner, D. Clarke, R. Sherlock, A. Flanagan, C. H. Chan and E. N. C. Mills (2014). "A multi-laboratory evaluation of a clinically-validated incurred quality control material for analysis of allergens in food." Food Chemistry 148: 30-36.
Kamath, S. D., A. M. A. Rahman, T. Komoda and A. L. Lopata (2013). "Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies." Food Chemistry 141(4): 4031-4039.
Khuda, S., A. Slate, M. Pereira, F. Al-Taher, L. Jackson, C. Diaz-Amigo, E. C. Bigley, T. Whitaker and K. M. Williams (2012). "Effect of Processing on Recovery and Variability Associated with Immunochemical Analytical Methods for Multiple Allergens in a Single Matrix: Sugar Cookies." Journal of Agricultural and Food Chemistry 60(17): 4195-4203.
Khuda, S. E., L. S. Jackson, T. J. Fu and K. M. Williams (2015). "Effects of processing on the recovery of food allergens from a model dark chocolate matrix." Food Chemistry 168: 580-587.
Klein Entink, R. H., Remington, B. C., Blom, W. M., Rubingh, C. M., Kruizinga, A. G., Baumert, J. L., Houben, G. F. (2014). "Food allergy population thresholds: an evaluation of the number of oral food challenges and dosing schemes on the accuracy of threshold dose distribution modeling." Food Chem Toxicol, 70: 134-143.
Koch, P., G. F. Schappi, R. E. Poms, B. Wuthrich, E. Anklam and R. Battaglia (2003). "Comparison of commercially available ELISA kits with human sera-based detection methods for peanut allergens in foods." Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 20(9): 797-803.
Koeberl, M., M. F. Sharp, R. K. Tian, S. Buddhadasa, D. Clarke and J. Roberts (2018). "Lupine allergen detecting capability and cross-reactivity of related legumes by ELISA." Food Chemistry 256: 105-112.
Koppelman, S. J., C. A. F. M. Bruijnzeel-Koomen, M. Hessing and H. H. J. de Jongh (1999). "Heat-induced Conformational Changes of Ara h 1, a Major Peanut Allergen, Do Not Affect Its Allergenic Properties*." Journal of Biological Chemistry 274(8): 4770-4777.
Koppelman, S. J., G. Soylemez, L. Niemann, F. E. Gaskin, J. L. Baumert and S. L. Taylor (2015). "Sandwich Enzyme-Linked Immunosorbent Assay for Detecting Sesame Seed in Foods." Biomed Research International 2015: 853836.
Korte, R. and J. Brockmeyer (2016). "MRM3-based LC-MS multi-method for the detection and quantification of nut allergens." Analytical and Bioanalytical Chemistry 408(27): 7845-7855.
Korte, R., S. Lepski and J. Brockmeyer (2016). "Comprehensive peptide marker identification for the detection of multiple nut allergens using a non-targeted LC- HRMS multi-method." Analytical and Bioanalytical Chemistry 408(12): 3059-3069.
Korte, R., J. M. Monneuse, E. Gemrot, I. Metton, H. U. Humpf and J. Brockmeyer (2016). "New High-Performance Liquid Chromatography Coupled Mass Spectrometry Method for the Detection of Lobster and Shrimp Allergens in Food Samples via Multiple Reaction Monitoring and Multiple Reaction Monitoring Cubed." Journal of Agricultural and Food Chemistry 64(31): 6219-6227.
Kristjansson I, Ardal B, Jonsson JS, Sigurdsson JA, Foldevi M and Bjorksten B, (1999). “Adverse reactions to food and food allergy in young children in Iceland and Sweden”. Scandinavian Journal of Primary Health Care, 17: 30-34
Kuehn, A., C. Hilger, T. Graf and F. Hentges (2017). "Protein and DNA-based assays as complementary tools for fish allergen detection." Allergol Select 1(2): 120- 126.
Kuehn, A., I. Swoboda, K. Arumugam, C. Hilger and F. Hentges (2014). "Fish Allergens at a Glance: Variable Allergenicity of Parvalbumins, the Major Fish Allergens." Frontiers in Immunology 5: 1-8.
Lacorn, M., T. Dubois, C. Gosswein, R. Kredel, B. Ferkinghoff, S. Brunelle, J. Theolier, S. Dominguez and T. Weiss (2022a). "Validation of the RIDASCREENV (R) Peanut for Determination of Peanut Protein in Cookies, Milk Chocolate, Ice Cream, Trail Mix, Puffed Rice Cereals, and Granola Bar: AOAC Performance Tested Method(SM) 112102." Journal of Aoac International 105(3): 784-801.
Lacorn, M., T. Dubios, T. Weiss, L. Zimmerman, T-M. Schinabeck, S. Loos-Theisen, K. Scherf, (2022b) "Determination of Gliadin as a Measure of Gluten in Food by R5 Sandwich ELISA RIDASCREEN® Gliadin Matrix Extension: Collaborative Study 2012.01”, Journal of Aoac International, 105(2): 442–455
Lacorn, M., T. Dubois, S. Siebeneicher and T. Weiss (2016). “Accurate and Sensitive Quantification of Soy Proteins in Raw and Processed Food by Sandwich ELISA” Food Science and Technology, 4(4):69-77
Lacorn, M. and U. Immer (2010). "Standardization in allergen determination." Accreditation and Quality Assurance 15(4): 207-216.
Lacorn, M., S. Lindeke, S. Siebeneicher and T. Weiss (2018). "Commercial ELISA Measurement of Allergens and Gluten: What We Can Learn from Case Studies." Journal of Aoac International 101(1): 102-107.
Le, Q. N., A. Vance, N. Bakir, D. Almy, E. Slenk, B. Roman, N. Klass, B. Bastin and R. Donofrio (2020). "Validation of the Reveal (R) 3-D for Peanut Lateral Flow Test: AOAC Performance Tested Method(SM) 111901." Journal of Aoac International 103(4): 1112-1118.
Lexhaller, B., C. Tompos and K. A. Scherf (2016). "Comparative analysis of prolamin and glutelin fractions from wheat, rye, and barley with five sandwich ELISA test kits." Analytical and Bioanalytical Chemistry 408(22): 6093-6104.
Lexhaller, B., C. Tompos and K. A. Scherf (2017). "Fundamental study on reactivities of gluten protein types from wheat, rye and barley with five sandwich ELISA test kits." Food Chemistry 237: 320-330.
Li, L. F., J. R. Zhou, K. W. Wang, X. J. Chen, L. L. Fu and Y. B. Wang (2022).
"Screening and Identification of Specific Aptamers for Shellfish Allergen
Tropomyosin with Capillary Electrophoresis-SELEX." Food Analytical Methods 15(6): 1535-1544.
Linacero, R., I. Ballesteros, A. Sanchiz, N. Prieto, E. Iniesto, Y. Martinez, M. M. Pedrosa, M. Muzquiz, B. Cabanillas, M. Rovira, C. Burbano and C. Cuadrado (2016). "Detection by real time PCR of walnut allergen coding sequences in processed foods." Food Chemistry 202: 334-340.
Liu, C., G. S. Chhabra, J. Zhao, V. D. Zaffran, S. Gupta, K. H. Roux, T. M. Gradziel and S. K. Sathe (2017). "Comparison of Laboratory-Developed and Commercial Monoclonal Antibody-Based Sandwich Enzyme-Linked Immunosorbent Assays for Almond (Prunus dulcis) Detection and Quantification." J Food Sci 82(10): 2504-2515.
Liu, C., V. D. Zaffran, S. Gupta, K. H. Roux and S. K. Sathe (2019). "Pecan (Carya illinoinensis) detection using a monoclonal antibody-based direct sandwich enzyme- linked immunosorbent assay." LWT 116: 108516.
Luber, F., A. Demmel, K. Pankofer, U. Busch and K. H. Engel (2015). "Simultaneous quantification of the food allergens soy bean, celery, white mustard and brown mustard via combination of tetraplex real-time PCR and standard addition." Food Control 47: 246-253.
Ma, X. L., H. Li, J. K. Zhang, W. S. Huang, J. X. Han, Y. Q. Ge, J. Y. Sun and Y. Chen (2020). "Comprehensive quantification of sesame allergens in processed food using liquid chromatography-tandem mass spectrometry." Food Control 107:106744.
Manfredi, A., M. Mattarozzi, M. Giannetto and M. Careri (2015). "Multiplex liquid chromatography-tandem mass spectrometry for the detection of wheat, oat, barley and rye prolamins towards the assessment of gluten-free product safety." Analytica Chimica Acta 895: 62-70.
Martinez-Esteso, M.J., O’Connor, G., Nørgaard, J. et al. (2020) "A reference method for determining the total allergenic protein content in a processed food: the case of milk in cookies as proof of concept." Anal Bioanal Chem 412, 8249–8267.
Matsuda, R., Y. Yoshioka, H. Akiyama, K. Aburatani, Y. Watanabe, T. Matsumoto, N. Morishita, H. Sato, T. Mishima, R. Gamo, Y. Kihira and T. Maitani (2006). "Interlaboratory evaluation of two enzyme-linked immunosorbent assay kits for the detection of egg, milk, wheat, buckwheat, and peanut in foods." Journal of Aoac International 89(6): 1600-1608.
Mattarozzi, M., C. Bignardi, L. Elviri and M. Careri (2012). "Rapid shotgun proteomic liquid chromatography-electrospray ionization-tandem mass spectrometry-based method for the lupin ( Lupinus albus L.) multi-allergen determination in foods." J Agric Food Chem 60(23): 5841-5846.
Mazzeo, M. F., B. De Giulio, G. Guerriero, G. Ciarcia, A. Malorni, G. L. Russo and R. A. Siciliano (2008). "Fish Authentication by MALDI-TOF Mass Spectrometry." Journal of Agricultural and Food Chemistry 56(23): 11071-11076.
Mills, E. N. C., Adel-Patient, K., Bernard, H., De Loose, M., Gillard, N., Huet, A. C., . .. Monaci, L. (2019). "Detection and Quantification of Allergens in Foods and Minimum Eliciting Doses in Food-Allergic Individuals (ThRAll)." J AOAC Int, 102(5): 1346-1353
Monaci, L., R. Pilolli, E. De Angelis, M. Godula and A. Visconti (2014). "Multi-allergen detection in food by micro high-performance liquid chromatography coupled to a dual cell linear ion trap mass spectrometry." Journal of Chromatography A 1358: 136-144.
Moneret-Vautrin D and André C, (1983). “Immunopathologie d’Allergie Alimentaire et Fausses Allergies Alimentaires”. Masson Paris, 63.
GW Monier-Williams, (1927), "The Determination of Sulphur Dioxide in Food" Reports on Public Health and Medical Subjects, HMSO, London 43
Nordlee, J. A., Taylor, S. L., Townsend, J. A., Thomas, L. A., Bush, R. K. (1996), “Identification of a Brazil-nut allergen in transgenic soybeans”, New England Journal of Medicine, 334(11): 688-92.
Nowak-Wegrzyn, A. and A. Fiocchi (2009). "Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity." Current Opinion in Allergy and Clinical Immunology 9(3): 234-237.
Nugraha, R., Ruethers, T., Johnston, E.B., Rolland, J.M., O’Hehir, R.E., Kamath, S.D. and Lopata, A.L. (2021). Effects of Extraction Buffer on the Solubility and Immunoreactivity of the Pacific Oyster Allergens. Foods Feb; 10(2): 409. doi: 10.3390/foods10020409.
Ortolani C, Ballmer-Weber BK, Hansen KS, Ispano M, Wuthrich B, Bindslev-Jensen C, Ansaloni R, Vannucci L, Pravettoni V, Scibilia J, Poulsen LK and Pastorello EA, (2000) "Hazelnut allergy: a double-blind, placebo-controlled food challenge multicenter study." Journal of Allergy and Clinical Immunology,105, 577-581.
Otto, G., A. Lamote, E. Deckers, V. Dumont, P. Delahaut, M. L. Scippo, J. Pleck, C. Hillairet and N. Gillard (2016). "A flow-cytometry-based method for detecting simultaneously five allergens in a complex food matrix." Journal of Food Science and Technology-Mysore 53(12): 4179-4186.
Osterballe M., Hansen T.K., Mortz C.G., Host A. and Bindslev-Jensen C., (2005). “The prevalence of food hypersensitivity in an unselected population of children and adults.” Pediatric Allergy and Immunology, 16: 567-573
Owen, L. and Gilbert, J., 2009, “Proficiency testing for quality assurance of allergens methods, Analytical and Bioanalytical Chemistry”, 395(1): 147-153.
Pahlavan, A., G. M. Sharma, M. Pereira and K. M. Williams (2016). "Effects of grain species and cultivar, thermal processing, and enzymatic hydrolysis on gluten quantitation." Food Chemistry 208: 264-271.
Palle-Reisch, M., R. Hochegger, S. Štumr, K. Korycanova and M. Cichna-Markl (2015). "Validation and comparison of two commercial ELISA kits and three in-house developed real-time PCR assays for the detection of potentially allergenic mustard in food." Food Chem 174: 75-81.
Panda, R., M. Boyer and E. A. E. Garber (2017). "A multiplex competitive ELISA for the detection and characterization of gluten in fermented-hydrolyzed foods." Analytical and Bioanalytical Chemistry 409(30): 6959-6973.
Panda, R., H. F. Zoerb, C. Y. Cho, L. S. Jackson and E. A. E. Garber (2015). "Detection and Quantification of Gluten during the Brewing and Fermentation of Beer Using Antibody-Based Technologies." Journal of Food Protection 78(6): 1167-1177.
Park, D. L., S. Coates, V. A. Brewer, E. A. Garber, M. Abouzied, K. Johnson, B. Ritter and D. McKenzie (2005). "Performance Tested Method multiple laboratory validation study of ELISA-based assays for the detection of peanuts in food." J AOAC Int 88(1): 156-160.
Parker, C. H., S. E. Khuda, M. Pereira, M. M. Ross, T. J. Fu, X. B. Fan, Y. Wu, K. M. Williams, J. DeVries, B. Pulvermacher, B. Bedford, X. Zhang and L. S. Jackson (2015). "Multi-allergen Quantitation and the Impact of Thermal Treatment in Industry- Processed Baked Goods by ELISA and Liquid Chromatography-Tandem Mass Spectrometry." Journal of Agricultural and Food Chemistry 63(49): 10669-10680.
Pereira B., Venter C., Grundy J., Clayton C.B., Arshad S.H. and Dean T., (2005). “Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers”. Journal of Allergy and Clinical Immunology,116: 884-892.
Perner, S. P., L. Heupel, L. Zimmermann, Y. Peters, K. U. Vongehr, H. El-Bedewy, S. Siebeneicher, T. Weiss, T. Hektor, B. Lindemann, S. Loos-Theisen and K. Schneider (2019). "Investigation of Reduced ELISA Recovery of Almond and Hazelnut Traces from Roasted Nut Samples by SDS-PAGE and Mass Spectrometry." Journal of Aoac International 102(5): 1271-1279.
Piknova, L., V. Janska, T. Kuchta and P. Siekel (2018). "Comparison of Real-Time Polymerase Chain Reaction and Enzyme-Linked Immunosorbent Assay for Sensitive and Quantitative Detection of Hazelnuts in Nut Pastes." Journal of Aoac International
101(6): 1864-1867.
Piknová, L., D. Pangallo and T. Kuchta (2008). "A novel real-time polymerase chain reaction (PCR) method for the detection of hazelnuts in food." European Food Research and Technology 226(5): 1155-1158.
Pilolli, R., R. Chaudhari, F. Palmisano and L. Monaci (2017). "Development of a mass spectrometry immunoassay for unambiguous detection of egg allergen traces in wines." Analytical and Bioanalytical Chemistry 409(6): 1581-1589.
Pilolli, R., E. De Angelis and L. Monaci (2017). "Streamlining the analytical workflow for multiplex MS/MS allergen detection in processed foods." Food Chemistry 221: 1747-1753.
Pilolli, R., Nitride, C., Gillard, N., Huet, A. C., van Poucke, C., de Loose, M., . . . Monaci, L. (2020). "Critical review on proteotypic peptide marker tracing for six allergenic ingredients in incurred foods by mass spectrometry." Food Res Int, 128: 108747
Pilolli, R., C. Van Poucke, E. De Angelis, C. Nitride, M. de Loose, N. Gillard, A. C. Huet, O. Tranquet, C. Larre, K. Adel-Patient, H. Bernard, E. N. C. Mills and L. Monaci (2021). "Discovery based high resolution MS/MS analysis for selection of allergen markers in chocolate and broth powder matrices." Food Chemistry 343: 128533.
Planque, M., T. Arnould, M. Dieu, P. Delahaut, P. Renard and N. Gillard (2017). "Liquid chromatography coupled to tandem mass spectrometry for detecting ten allergens in complex and incurred foodstuffs." Journal of Chromatography A 1530: 138-151.
Planque, M., T. Arnould, P. Renard, P. Delahaut, M. Dieu and N. Gillard (2017). "Highlight on Bottlenecks in Food Allergen Analysis: Detection and Quantification by Mass Spectrometry." Journal of Aoac International 100(4): 1126-1130.
Platteau, C., T. Cucu, B. De Meulenaer, B. Devreese, M. De Loose and I. Taverniers (2011). "Effect of protein glycation in the presence or absence of wheat proteins on detection of soybean proteins by commercial ELISA." Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 28(2): 127-135.
Platteau, C., T. Cucu, I. Taverniers, B. Devreese, M. De Loose and B. De Meulenaer (2013). "Effect of oxidation in the presence or absence of lipids on hazelnut and soybean protein detectability by commercial ELISA." Food and Agricultural Immunology 24(2): 179-192.
Poms, R. E., M. E. Agazzi, A. Bau, M. Brohee, C. Capelletti, J. V. Nørgaard and E. Anklam (2005). "Inter-laboratory validation study of five commercial ELISA test kits for the determination of peanut proteins in biscuits and dark chocolate." Food Addit Contam 22(2): 104-112.
Poms, R. E., C. L. Klein and E. Anklam (2004). "Methods for allergen analysis in food: a review." Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 21(1): 1-31.
Porter, B. B., M.; Newell, B.; Hoxey, L. and Wilkes, E. (2017). "A new validated method for the determination of free and total sulphur dioxide using a discrete analyser." AWRI Tech. Rev 231: 6-11.
Posada-Ayala, M., G. Alvarez-Llamas, A. S. Maroto, X. Maes, E. Munoz-Garcia, M. Villalba, R. Rodriguez, M. Perez-Gordo, F. Vivanco, C. Pastor-Vargas, and J. Cuesta-Herranz. (2015) “Novel liquid chromatography-mass spectrometry method for sensitive determination of the mustard allergen Sin a 1 in food.” Food Chem. 183:58–63.
R-Biopharm (2022) RIDASCREEN® Gliadin Art. No.: R7001, R-Biopharm website, Accessed 3 July 2023, Available at: https://food.r-biopharm.com/products/ridascreen- gliadin/
Rancé F, Kanny G, Dutau G and Moneret-Vautrin DA, (1999). “Food hypersensitivity in children: clinical aspects and distribution of allergens.” Pediatric Allergy and Immunology,10:33-38
Rankine, B.C., Pocock, K.F. (1970) "Alkalimetric determination of sulphur dioxide in wine." Aust. Wine Brew. Spirit Rev. 88(8):40,42,44.
Remington et al. (2022), ILSI Europe Practical Guidance on the Application of Food Allergen Quantitative Risk Assessment (QRA), Report Series, 13/06/2022, https://doi.org/10.5281/zenodo.6651934
Roberts G, Peckitt C, Northstone K, Strachan D, Lack G, Henderson J, Golding J and Team AS, (2005). “Relationship between aeroallergen and food allergen sensitization in childhood.” Clinical and Experimental Allergy, 35: 933-940.
Rodriguez, M. Perez-Gordo, F. Vivanco, C. Pastor-Vargas and J. Cuesta-Herranz (2015). "Novel liquid chromatography-mass spectrometry method for sensitive determination of the mustard allergen Sin a 1 in food." Food Chemistry 183: 58-63.
Röder, M., K. Kleiner, A. Sachs, N. Keil and T. Holzhauser (2013). "Detectability of Lupine Seeds by ELISA and PCR May Be Strongly Influenced by Potential Differences between Cultivars." Journal of Agricultural and Food Chemistry 61(25): 5936-5945.
Röder, M., S. Vieths and T. Holzhauser (2011). "Sensitive and specific detection of potentially allergenic almond (Prunus dulcis) in complex food matrices by Taqman (R) real-time polymerase chain reaction in comparison to commercially available protein-based enzyme-linked immunosorbent assay." Analytica Chimica Acta 685(1): 74-83.
Rolland, J. M., N. P. Varese, J. B. Abramovitch, J. Anania, R. Nugraha, S. Kamath, A. Hazard, A. L. Lopata and R. E. O'Hehir (2018). "Effect of Heat Processing on IgE Reactivity and Cross-Reactivity of Tropomyosin and Other Allergens of Asia-Pacific Mollusc Species: Identification of Novel Sydney Rock Oyster Tropomyosin Sac g 1." Molecular Nutrition & Food Research 62(14):e1800148.
Romero, M.R., Claydon, A.J., Fitches, E.C., Wakefield, M.E. and Charlton, A.J. (2016) "Sequence homology of the fly proteins tropomyosin, arginine kinase and myosin light chain with known allergens in invertebrates." Journal of Insects as Food and Feed, 2(2): 69-81
Ruethers, T., A. C. Taki, J. Khangurha, J. Roberts, S. Buddhadasa, D. Clarke, C. E. Hedges, D. E. Campbell, S. D. Kamath, A. L. Lopata and M. Koeberl (2020). "Commercial fish ELISA kits have a limited capacity to detect different fish species and their products." Journal of the Science of Food and Agriculture 100(12): 4353- 4363.
Rzychon, M., Brohée, M., Cordeiro, F., Haraszi, R., Ulberth, F. and O'Connor, G., (2017). The feasibility of harmonizing gluten ELISA measurements. Food chemistry, 234: 144-154.
Sagu, S. T., G. Huschek, T. Homann and H. M. Rawel (2021). "Effect of Sample Preparation on the Detection and Quantification of Selected Nuts Allergenic Proteins by LC-MS/MS." Molecules 26(15):4698.
Saito, E., H. Doi, K. Kurihara, K. Kato, K. Aburatani, M. Shoji and Y. Naka (2019). "The Validation of the Wheat Gluten ELISA Kit." Journal of Aoac International 102(4): 1162-1173.
Sakai, S., R. Adachi, H. Akiyama, R. Teshima, H. Doi, H. Shibata and A. Urisu (2010). "Determination of Walnut Protein in Processed Foods by Enzyme-Linked Immunosorbent Assay: Inter laboratory Study." Journal of Aoac International 93(4): 1255-1261.
Sakai, S., R. Adachi, H. Akiyama, R. Teshima, N. Morishita, T. Matsumoto and A. Urisu (2010). "Enzyme-Linked Immunosorbent Assay Kit for the Determination of Soybean Protein in Processed Foods: Interlaboratory Evaluation." Journal of Aoac International 93(1): 243-248.
Sanchiz, A., I. Ballesteros, A. Martin, J. Rueda, M. M. Pedrosa, M. D. Dieguez, M. Rovira, C. Cuadrado and R. Linacero (2017). "Detection of pistachio allergen coding sequences in food products: A comparison of two real time PCR approaches." Food Control 75: 262-270.
Sayers, R. L., L. A. Gethings, V. Lee, A. Balasundaram, P. E. Johnson, J. A. Marsh, A. Wallace, H. Brown, A. Rogers, J. I. Langridge and E. N. C. Mills (2018). "Microfluidic Separation Coupled to Mass Spectrometry for Quantification of Peanut Allergens in a Complex Food Matrix." Journal of Proteome Research 17(1): 647-655.
Schalk, K., P. Koehler and K. A. Scherf (2018). "Targeted liquid chromatography tandem mass spectrometry to quantitate wheat gluten using well-defined reference proteins." PLOS ONE 13(2): e0192804.
Schalk, K., C. Lang, H. Wieser, P. Koehler and K. A. Scherf (2017). "Quantitation of the immunodominant 33-mer peptide from α-gliadin in wheat flours by liquid chromatography tandem mass spectrometry." Scientific Reports 7(1): 45092.
Schall, E., Scherfb,K.A., Bugyia, Z., Hajasa, L., Töröka, K., Koehlerd, P., Pomse, R.E., D'Amicof, S., Schoenlechnerg, R., Tömösközi, S. (2020). Characterisation and comparison of selected wheat (Triticum aestivum L.) cultivars and their blends to develop a gluten reference material. Food Chemistry 313, 126049.
Scharf, A., U. Kasel, G. Wichmann and M. Besler (2013). "Performance of ELISA and PCR Methods for the Determination of Allergens in Food: An Evaluation of Six Years of Proficiency Testing for Soy (Glycine max L.) and Wheat Gluten (Triticum aestivum L.)." Journal of Agricultural and Food Chemistry 61(43): 10261-10272.
Scherf, K. A. (2017). "Gluten Analysis of Wheat Starches with Seven Commercial ELISA Test Kits—Up to Six Different Values." Food Analytical Methods 10(1): 234- 246.
Schoringhumer, K., G. Redl and M. Cichna-Markl (2009). "Development and Validation of a Duplex Real-Time PCR Method To Simultaneously Detect Potentially Allergenic Sesame and Hazelnut in Food." Journal of Agricultural and Food Chemistry 57(6): 2126-2134.
Sealey-Voyksner, J., J. Zweigenbaum and R. Voyksner (2016). "Discovery of highly conserved unique peanut and tree nut peptides by LC-MS/MS for multi-allergen detection." Food Chem 194: 201-211.
Sealey-Voyksner, J. A., C. Khosla, R. D. Voyksner and J. W. Jorgenson (2010). "Novel aspects of quantitation of immunogenic wheat gluten peptides by liquid chromatography-mass spectrometry/mass spectrometry." Journal of Chromatography A 1217(25): 4167-4183.
Sharma, G. M. (2012). "Immunoreactivity and Detection of Wheat Proteins by Commercial ELISA Kits." Journal of Aoac International 95(2): 364-371.
Sharma, G. M., S. E. Khuda, M. Pereira, A. Slate, L. S. Jackson, C. Pardo, K. M. Williams and T. B. Whitaker (2013). "Development of an Incurred Cornbread Model for Gluten Detection by Immunoassays." Journal of Agricultural and Food Chemistry 61(49): 12146-12154.
Sharp, M. F., J. N. Stephen, L. Kraft, T. Weiss, S. D. Kamath and A. L. Lopata (2015). "Immunological cross-reactivity between four distant parvalbumins-Impact on allergen detection and diagnostics." Molecular Immunology 63(2): 437-448.
Škultéty, O. and A. Jurčáková (2011). "Eva green real-time PCR used to detect celery as an allergen in food.", Potravinarstvo Slovak Journal of Food Sciences, 5(2): 70-72
Sletten, G., Van Do, T, Lindvik, H., Egaas, E. and Florvaag, E. (2010). Effects of industrial processing on the immunogenicity of commonly ingested fish species. Int. Arch. Allergy Immunol. 151(3):223-36.
Slotwinski, E., D. Almy, R. Viator, M. Abouzied, F. Klein and J. Rice (2018). "Development and Validation of a Quantitative ELISA for the Detection of Almond Residues in Foods." Journal of Aoac International 101(1): 118-123.
Stutius LM, Sheehan WJ, Rangsithienchai P, Bharmanee A, Scott JE, Young MC, Dioun AF, Schneider LC and Phipatanakul W, (2010). “Characterizing the relationship between sesame, coconut, and nut allergy in children.” Pediatric Allergy and Immunology, 21: 1114-1118
Suh, S. M., M. J. Kim, H. I. Kim, H. J. Kim and H. Y. Kim (2020). "A multiplex PCR assay combined with capillary electrophoresis for the simultaneous detection of tropomyosin allergens from oyster, mussel, abalone, and clam mollusk species." Food Chemistry 317: 126451.
Sun, L. R., L. L. Xu, Y. H. Huang, H. Lin, I. Ahmed and Z. X. Li (2019). "Identification and comparison of allergenicity of native and recombinant fish major allergen parvalbumins from Japanese flounder (Paralichthys olivaceus)." Food & Function 10(10): 6615-6623.
Swoboda, I., A. Bugajska-Schretter, P. Verdino, W. Keller, W. R. Sperr, P. Valent, R. Valenta and S. Spitzauer (2002). "Recombinant carp parvalbumin, the major cross- reactive fish allergen: A tool for diagnosis and therapy of fish allergy." Journal of Immunology 168(9): 4576-4584.
Sykes, M, Anderson, D, Parmar, B, (2012) Normalisation of data from allergens proficiency tests, Anal Bioanal Chem, 403(10): 3069
Taylor, S. L., J. L. Baumert, A. G. Kruizinga, B. C. Remington, R. W. R. Crevel, S. Brooke-Taylor, K. J. Allen and G. Houben (2014). "Establishment of Reference Doses for residues of allergenic foods: Report of the VITAL Expert Panel." Food and Chemical Toxicology, 63: 9-17.
Touraine F, Ouzeau J, Boullaud C, Dalmay F and Bonnaud F, (2002). “Survey on the prevalence of food allergy in school children.” Revue Française d'Allergologie et d'Immunologie Clinique, 42: 763-768.
Trucksess, M. W., V. A. Brewer, K. M. Williams, C. D. Westphal and J. T. Heeres (2004). "Preparation of peanut butter suspension for determination of peanuts using enzyme-linked immunoassay kits." Journal of Aoac International 87(2): 424-428.
Turner, P.J., Baumert, J.L., Beyer, K., Brooke-Taylor, S., Comberiati, P., Crevel, R.W.R., Gerdts, J.D., Hazel Gowland, M., Houben, G.F., Hourihane, J.O., Konstantinou, G.N., La Vieille, S., Moya, B., Muraro, A., Mills, E.N.C., Patel, N., Podestà, M., Popping, B., Reese, I., Roberts, G., Said, M., Santos, A.F., Schnadt, S., Taylor, S.L., Vlieg-Boerstra, B., Remington, B.C. and the EAACI Taskforce on Food allergen thresholds (2022), “‘Too high, too low’: The complexities of using thresholds in isolation to inform precautionary allergen (‘may contain’) labels.” Allergy, 77: 1661- 1666. https://doi.org/10.1111/all.15202
van Broekhoven, S., S. Bastiaan-Net, N. W. de Jong and H. J. Wichers (2016). "Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species." Food Chem 196: 1075-1083.
van den Broeck, H. C., A. H. P. America, M. J. M. Smulders, D. Bosch, R. J. Hamer, L. J. W. J. Gilissen and I. M. van der Meer (2009). "A modified extraction protocol enables detection and quantification of celiac disease-related gluten proteins from wheat." Journal of Chromatography B 877(10): 975-982.
Vencia, W., P. Minale, L. Migone, F. Lazzara, G. Vito, A. Ferrari and E. Razzuoli (2019). "Effects of thermal treatment on walnut detection and allergenicity." Journal of the Science of Food and Agriculture 99(5): 2636-2640.
Venter C, Pereira B, Grundy J, Clayton CB, Arshad SH and Dean T, (2006a). “Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study”. Pediatric Allergy and Immunology, 17: 356-363.
Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B and Dean T, (2006b). “Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life.” Journal of Allergy and Clinical Immunology, 117: 1118-1124.
Waiblinger, H. U., B. Boernsen, C. Geppert, A. Demmel, V. Peterseil and R. Koeppel (2017). "Ring trial validation of single and multiplex real-time PCR methods for the detection and quantification of the allergenic food ingredients mustard, celery, soy, wheat and rye." Journal of Consumer Protection and Food Safety 12(1): 55-72.
Walker, M.J., Burns, M., Quaglia, M., Nixon, G., Hopley, C.J., Gray, K.M et al. (2018) “Almond or Mahaleb? Orthogonal Allergen Analysis During a Live Incident Investigation by ELISA, Molecular Biology, and Protein Mass Spectrometry” Journal of AOAC International 101 (1): 162-169
Whitaker, T. B., K. M. Williams, M. W. Trucksess and A. B. Slate (2005). "Immunochemical analytical methods for the determination of peanut proteins in foods." Journal of Aoac International 88(1): 161-174.
Wilkes, E. (2018). "Sulfur dioxide measurement: Practical measurement of total SO2 in wine." Wine & Viticulture Journal 33(4): 32-34.
Williams, K. M., C. D. Westphal and L. C. Shriver-Lake (2004). "Determination of egg proteins in snack food and noodles." Journal of Aoac International 87(6): 1485-1491.
World Health Organization, World Heath Organisation International, “Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens - Part 2: Review and establish threshold levels in foods of the priority allergens”, Available online here (Accessed: 25 January 2023)
World Health Organization, World Heath Organisation International, "Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens Part 3: Review and establish precautionary labelling in foods of the priority allergens. Summary Report.", 13 December 2021, Available at: https://cdn.who.int/media/docs/default-source/food-safety/jemra/3rd-all… (Accessed: 25 January 2023)
Wu, Y. J., Y. Chen, B. Wang, Y. H. Gao, L. Q. Bai and H. Y. Wang (2010). "SYBR Green Real-Time PCR Used to Detect Celery in Food." Journal of Aoac International 93(5): 1530-1536.
Xiong, W. L., M. A. McFarland, C. Pirone and C. H. Parker (2019). "Selection of Tree Nut Allergen Peptide Markers: A Need for Improved Protein Sequence Databases." Journal of Aoac International 102(5): 1263-1270.
Young E, Stoneham MD, Petruckevitch A, Barton J and Rona R (1994). "A population study of food intolerance." Lancet 343: 1127-1130.
Yu, C., X. Ding, X. Gao, H. Lin, M. Ullah Khan, H. Lin, X. Dang and Z. Li (2022). "Immunological Cross-Reactivity Involving Mollusc Species and Mite-Mollusc and Cross-Reactive Allergen PM Are Risk Factors of Mollusc Allergy." J Agric Food Chem 70(1): 360-372.
Zhou, J., Wang, Y., Qian, Y., Zhang, T. Zheng, L. and Hu, L. (2020). Quantification of shellfish major allergen tropomyosin by SPR biosensor with gold patterned Biochips. Food Control 107, 106547. https://doi.org/10.1016/J.FOODCONT.2019.02.041