Stakeholder responses: Consultation on mechanically separated meat (MSM) guidance
The Food Standards Agency (FSA) sought feedback relating to new mechanically separated meat (MSM) guidance that is intended to provide support for Food Business Operators (FBO) following court judgments that clarify how the definition of MSM should be interpreted and applied.
Introduction
About the consultation
This 12-week consultation was issued on 28 February 2024 and closed on 22 May 2024.
The Food Standards Agency (FSA) sought views on a proposed Guidance document regarding mechanically separated meat (MSM). The Guidance is intended primarily to support Food Business Operators (FBOs) to achieve compliance with regulatory requirements following the Supreme Court Judgment that clarified how the definition of MSM is to be interpreted and applied.
The consultation was published on the FSA website and communicated to key stakeholders. Responses were received via an online survey and by email.
Views were sought specifically on:
- The effectiveness of the MSM Guidance document in providing support to achieve compliance with regulatory requirements in light of the Supreme Court Judgment.
- The impacts of FBOs adapting their activities and operations in line with the Supreme Court Judgment.
- Whether there are wider issues around MSM that the FSA, or wider government, should be seeking to address and why.
This report provides a summary of the comments received. It sets out an overview of the respondents and summarises the recurring themes from the responses. The Annexes provide a more detailed breakdown of responses and the individual responses to the consultation’s open-ended questions. The FSA’s comments on the responses are presented as part of the thematic summary and also alongside each of the individual comments in Annex B. The FSA is grateful to all stakeholders who responded.
Characteristics of respondents
Responses were received from a total of 60 respondents. There were 46 submissions via the online survey. There were 14 responses submitted via email, 2 of which included answers specifically to the questions asked in the online survey and those answers have been combined with the submissions to the online survey giving a total of 48 responses to the online survey questions.
Responses were submitted by businesses (22 FBOs and 1 producer of machinery used by FBOs), individual consumers (15), trade associations (12), local authorities (4), and those that opted not to identify themselves (6).
The trade associations that responded represent extensive UK memberships, many of which have direct interest in this matter.
Questions and responses reflected in this document
In the online survey, all respondents were asked a series of closed-ended (multiple choice) and open-ended (open text) questions relating to the Guidance.
In addition, those who replied as FBOs were specifically presented with a series of questions regarding the impacts on their businesses of adapting activities and processes in line with the Supreme Court Judgment. Responses to questions on business impacts (questions 15-28) have been omitted from this summary given they are of a commercially sensitive nature. They will, however, be considered as part of an assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment which will be published.
Closed-ended questions and responses are presented in Annex A, open-ended questions and responses in Annex B, and email responses in Annex C.
Summary of consultation responses
Useability and relevance of the Guidance
All respondents were invited to give views on the effectiveness of the MSM Guidance document in providing support to achieve compliance with regulatory requirements in light of the Supreme Court Judgment. Respondents were asked a set of closed-ended questions and invited to elaborate with further comments. The intention was to gather views on the readability and relevance of the Guidance and how useful the Guidance is to assist businesses to comply with the law as clarified by the Supreme Court Judgment. Below are the key statistics in the summary of closed-ended survey questions. The statistics indicate that, while the Guidance is relevant, there are improvements to be considered on clarity and scope.
- 37% of the responses to whether the Guidance was easy to understand were positive, 23% were neutral and 40% were negative.
- 43% of respondents provided a positive response when asked if the Guidance aids understanding of the legislation, 14% were neutral and 43% were negative.
- On whether the Guidance was relevant to businesses/organisations, 71% of responses were positive, 15% were neutral and 6% were negative. 8% felt this question was not relevant to them.
- On whether the Guidance helps businesses understand how to comply with the MSM regulatory requirements, 37% of responses were positive, 17% were neutral and 34% were negative. 12% of respondents advised this was not relevant to them.
- 46% of respondents had a positive opinion on the usefulness of the Q&A annex to the Guidance, 28% had a neutral opinion and 26% had a negative opinion.
- 26% of respondents provided a positive response on whether the Guidance covered all relevant matters in relation to MSM, 20% provided a neutral response and 54% provided a negative response.
Additional views submitted by respondents
For respondents that selected 'disagree' or 'strongly disagree' regarding statements relating to the Guidance, the opportunity to explain their reasoning was provided in the form of an open-ended follow-up question. Several respondents, when invited to elaborate, highlighted fundamental disagreements with the legislation and/or FSA’s view of the Supreme Court Judgment rather than specific comments on the Guidance document.
We acknowledge that some industry stakeholders fundamentally disagree with the legislation, the Supreme Court Judgment and/or the FSA’s understanding of the Supreme Court Judgment. However, the FSA as Regulator must uphold the law and the FSA stands by its understanding of the Supreme Court Judgment. The Guidance is designed to support businesses to comply with regulatory requirements.
There were helpful responses regarding the suitability of the Guidance that the FSA will consider when finalising the Guidance to ensure that it is understandable and best supports FBOs in determining whether a product is MSM to ensure their compliance in line with regulatory requirements as clarified by the Courts. These responses typically highlighted a need for greater detail, including in the following areas:
- Clarifying how the criteria that determine whether MSM is produced will be assessed, to aid understanding and to ensure consistency;
- Differentiating between meat classifications;
- Specific hygiene controls associated with MSM production;
- Impacts on existing products e.g., transitional arrangements; and
- Any appeals procedures for businesses in the event of enforcement-related disputes.
Thematic analysis
Respondents were asked a series of open-ended questions to allow them the opportunity to elaborate and provide qualitative evidence to support their views. These responses have been combined with email submissions. Initial analysis has been provided here and will be considered further as we finalise the Guidance.
Common themes recurred across the survey and email responses. Broadly, these can be categorised as (1) points raised regarding the Guidance itself, (2) consumer concerns around MSM, (3) points around enforcement, and (4) broader points regarding impacts beyond those identified in the Guidance. These themes are set out below, accompanied by our initial responses to the points raised.
Points raised regarding the Guidance document
Requests for more detail: some respondents felt that important details were missing; some respondents felt that the wording in the Guidance should be more precise, as the current wording was confusing and could therefore be open to interpretation Some respondents felt that definitions of different meat classifications were not demonstrably based on science.
- These comments have been noted. The points raised will be considered when finalising the Guidance.
Technological advances in meat processing: some industry respondents stated that technological advances in the mechanisation of meat processing have occurred since the legislation pertaining to MSM was created. Respondents reported that these technological advances maximise yields and provide economic benefit for consumers. Their concerns are that the legislation is not reflective of modern-day production of MSM, and that the Guidance should take technological advances into greater consideration.
- We support safe innovation that allows businesses to improve and modernise their operations. However, FBOs must continue to meet legal requirements when upgrading technology and processing methods. The definition of MSM in legislation is designed to take into account technological advances. As stated in assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in NI) “the definition of mechanically separated meat (MSM) should be a generic one covering all methods of mechanical separation. Rapid technological developments in this area mean that a flexible definition is appropriate.”
- We have noted these comments and will consider as we develop the Guidance further, including the need for the Guidance to reflect developments in technology. Crucially, FBOs must continue to meet legal requirements when upgrading technology and processing methods.
“Poultry wishbone meat”: some industry respondents expressed the opinion that the Guidance is fundamentally flawed because they consider that the FSA has misinterpreted that the Supreme Court Judgment applies to poultry wishbone meat. Furthermore, some industry respondents felt that the issuing of the Guidance would therefore result in meat harvested from poultry wishbones being incorrectly, in their view, classified as MSM.
- We acknowledge this view; however, the FSA as Regulator must uphold the law and stands by its understanding of the Supreme Court Judgment, notwithstanding that some respondents may disagree. The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
- The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat, in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
- No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court, are met, including the process chosen to separate the meat from the bone.
Consumer comments and concerns about MSM
There were three main issues raised by consumers. One was in relation to labelling where respondents highlighted the need for transparent and clear information on food labels so as not to mislead consumers. The second was the need for more straightforward information on MSM for the wider public and the third was an objection to the use of MSM as an ingredient in food.
- We agree that transparent and clear food information is important. The FSA's main objectives in law are to protect public health from risks arising from the consumption of food and generally to protect the interests of consumers in relation to food. As laid down in assimilated Regulation (EC) 178/2002 (commonly referred to as 'General Food Law'), it is a general principle of food law to provide a basis for consumers to make informed choices in relation to food they consume and to prevent any practices that may mislead the consumer.
- This applies to MSM just as it does to all food. MSM must be labelled as such on products to allow consumers to make an informed choice. The Court Judgment clarifies the definition of MSM and therefore informs what must be labelled as MSM. Labelling requirements for MSM are set out in law. They are primarily laid out in assimilated Regulation (EU) No 1169/2011 in GB / Regulation (EU) No 1169/2011 in NI (commonly referred to as 'Food Information for Consumers' or 'FIC'). Defra leads on labelling legislation in England; the FSA in Wales and NI; Food Standards Scotland (FSS) in Scotland.
- Legislation permits the production and use of MSM, and there are no plans to change this. MSM produced in compliance with hygiene regulations is a safe product that can be used as an ingredient in a wide variety of foods.
- There are stricter legislative requirements for MSM than for other classifications of meat to ensure food safety (e.g., on permitted raw materials, permitted uses, temperature controls and microbiological criteria). Where MSM is produced in line with legislative requirements, microbiological risks are similar to those for meat preparations and minced meat. MSM producers must meet the specific hygiene rules laid down in assimilated Regulation (EC) No 853/2004 / Regulation (EC) No 853/2004 in Northern Ireland, and microbiological criteria for foodstuffs in assimilated Regulation (EC) No 2073/2005 in GB / Regulation (EC) No 2073/2005 in NI, in so far as they concern the production of MSM.
Points raised around enforcement
Practicalities of enforcement: respondents expressed concerns about enforcement and the practicalities associated with this. For example, issues were raised in relation to potential increased workloads and training requirements for enforcement officers. Respondents also raised concerns around subjectivity and the potential for inconsistencies in enforcement across the UK.
- We have noted these concerns. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement and identification of training requirements.
Appeals process: respondents queried whether there would be an appeals process, specifically in relation to meat classification decisions or other enforcement decisions that a business may wish to contest.
- Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
Broader points regarding impacts beyond those identified in the Guidance
Underestimation of impacts: several respondents believed there are wider impacts which have not been considered. Concerns were raised particularly in relation to cost implications for FBOs due to loss of value associated with any products being reclassified as MSM; increased raw material costs e.g., for businesses having to replace anything reclassified as MSM with alternative meat preparations; potential job losses related to increased costs; and costs of production process changes. It was highlighted that cost implications for FBOs would in turn lead to increased costs for consumers. Some respondents wanted clarity on implications for products already on the market, and environmental and animal welfare impacts to be considered e.g., due to the potential need for increased slaughter of animals to replace any material reclassified as MSM which could not be used in many final food products. Some respondents wanted to know whether there would be an impact assessment conducted.
- The consultation invited respondents to provide data as evidence of any potential impacts of businesses adapting in line with the Court Judgment. We are keen to understand the wider impacts and welcome the evidence submitted which is being considered and analysed. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Imported food: some respondents raised issues concerning imports. For example, checks at GB borders to ensure MSM products comply with UK legislation.
- We note these concerns. The Government has robust border controls for food entering the UK in order to protect consumers and maintain high levels of public health. Imported products must meet UK requirements and there are comprehensive control measures in place to ensure these are met. For further guidance on the importation of MSM please note the 'Checks at the border' section found here - Import food and drink from the EU to Great Britain - GOV.UK (www.gov.uk)
Conclusion
All responses have been acknowledged and will be considered during the process of finalising the Guidance, which will be published in due course.
As referred to above, an assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Responses to closed-ended questions about the respondents
Q1. Which of the following options best describes you as a respondent?
46 out of 48 respondents answered this question:
- Food business that uses mechanical separation equipment - 8
- Food business that uses MSM as an ingredient - 8
- Local Authority - 4
- Trade Association - 6
- Consumer - 14
- Other - 6
Q2. Have you read through the ‘Guidance on Mechanically Separated Meat (MSM)’ and ‘Consultation on Mechanically Separated Meat (MSM) Guidance’ documents prior to completing this survey?
44 out of 48 respondents answered this question:
- No - 2
- Yes - 42
Q3. Select where you are based
45 out of 48 respondents answered this question (please note multiple answers were possible):
- England - 39
- Wales - 7
- Northern Ireland - 8
- None of the above - 2
Q4. How many staff does your organisation employ?
16 out of 17 respondents answered this question:
- 9 or fewer (micro) - 1
- 10 - 49 employees (small) - 1
- 50 - 249 employees (medium) - 3
- 250 or greater employees (large) - 11
Q5. Is your food business approved in accordance with assimilated Regulation (EC) No. 853/2004 in GB / Regulation (EC) No. 853/2004 in Northern Ireland?
7 out of 17 respondents answered this question:
- None of the above - 1
- Yes, approved by the Food Standards Agency - 6
- Yes, approved by the Local Authority - 0
- Don't know - 0
Q6. Does your business produce MSM or meat preparations?
8 out of 17 respondents answered this question:
- None of the above - 1
- Yes, my organisation currently produces MSM in compliance with the Regulations and new Guidance - 4
- Yes, my organisation currently produces MSM but is adapting its activities/processes in line with the Court judgments regarding the definition of MSM - 1
Yes, my organisation produces meat preparations - 2 - Don't know - 0
Q7. Does your business manufacture products containing MSM meat or meat preparations?
9 out of 17 respondents answered this question:
- None of the above - 1
- Yes, my organisation manufactures products containing MSM in compliance with the Regulations and new Guidance - 0
- Yes, my organisation manufactures products containing MSM but is adapting its activities/processes in line with the Court judgments - 5
- Yes, my organisation manufactures products containing meat preparations - 3
- Don't know - 0
Responses to closed-ended questions on the MSM Guidance
The following information was obtained from responses to the online survey.
Q8. To what extent do you agree or disagree with the following statements?
Q8a: The Guidance on MSM is easy to understand.
35 out of 48 respondents answered this question:
- Strongly Agree - 3
- Agree - 10
- Neither Agree nor Disagree - 8
- Disagree - 6
- Strongly Disagree - 8
- Don’t Know - 0
- Not Relevant - 0
Q8b. To what extent do you agree or disagree that the Guidance on MSM aids understanding of the Regulations.
35 out of 48 respondents answered this question:
- Strongly Agree - 3
- Agree - 12
- Neither Agree nor Disagree - 5
- Disagree - 6
- Strongly Disagree - 9
- Don’t Know - 0
- Not Relevant - 0
Q8c. The Guidance on MSM is relevant to me and/or my business/organisation.
34 out of 37 respondents answered this question:
- Strongly Agree - 11
- Agree - 13
- Neither Agree nor Disagree - 5
- Disagree - 0
- Strongly Disagree - 2
- Don’t Know - 0
- Not Relevant - 3
Q8d. To what extent do you agree or disagree that the Guidance helps businesses understand how to comply with regulatory requirements regarding MSM.
35 out of 48 respondents answered this question:
- Strongly Agree - 3
- Agree - 10
- Neither Agree nor Disagree - 6
- Disagree - 3
- Strongly Disagree - 9
- Don’t Know - 0
- Not Relevant - 4
Q8e. The Q&A annex to the Guidance provides additional information that is useful.
35 out of 48 respondents answered this question:
- Strongly Agree - 5
- Agree - 11
- Neither Agree nor Disagree - 10
- Disagree - 2
- Strongly Disagree - 7
- Don’t Know - 0
- Not Relevant - 0
Q8f. The Guidance covers all relevant matters in relation to MSM.
35 out of 48 respondents answered this question:
- Strongly Agree - 5
- Agree - 4
- Neither Agree nor Disagree - 7
- Disagree - 6
- Strongly Disagree - 13
- Don’t Know - 0
- Not Relevant - 0
The FSA’s considered comments in answer to consultation responses are given under each response.
To aid readability, two categories of respondent shown as food business that uses mechanical separation equipment and food business that uses MSM as an ingredient in the online survey have been merged and shown as ‘FBO’ (Food Business Operator) in this document.
The categories of respondent listed in the tables are shown as follows:
- consumer – Consumer
- Food Business Operator – FBO
- Trade Association – TA
- organisation not listed – Other
- did not identify themselves – DNID
Where “three cumulative criteria” are mentioned throughout the FSA comments section, this is referring to the criteria laid out in the Supreme Court Judgment, which must be read in conjunction with one another when determining whether a product is MSM. A product that satisfies all three criteria is classified as MSM:
- the use of bones from which the intact muscles have already been detached, or of poultry carcases, to which meat remains attached
- the use of methods of mechanical separation to recover that meat
- the loss or modification of the muscle fibre structure of the meat thus recovered by reason of the use of those processes
Q9: Explain why you disagree that ‘The Guidance on MSM is easy to understand’. If possible, suggest how it could be made easier to understand.
Response 1: FBO (Q9)
The draft guidance is not easy to understand and it does not fulfil its aims as set out in paragraph 11 of the guidance. For example micro criteria guidance with regard to MSM is not covered. The guidance also fails to provide clarity on:
- where in the judgements it expressly mentions portions/cuts of meat (para 26)
- it doesn’t provide specific scenario based examples e.g. pork ribs / poultry wishbone cuts to aid the reader
- it is not clear on visual assessment of meat and what that actually is. This would therefore be open to interpretation by the site Official Veterinarian (OV) or enforcement officials. There needs to be clearly defined criteria for what the visual assessment is looking for when it comes to looking at loss or modification of muscle fibre structure.
- the guidance needs to provide more details on the characteristics of MSM e.g. temperature controls / micro criteria requirements.
FSA comments on response 1 (Q9)
Comments noted. These points will be considered when finalising the Guidance.
Response 2: FBO (Q9)
It is our company's belief that the FSA is in error in extrapolating its interpretation of the Supreme Court's judgement (on a different product and involving a single company) to include poultry wishbone meat. It is our belief that the Supreme Court had no intention of including products specifically outside of the 2011 moratorium. In view of this we believe the FSA guidance document to be majorly defective and inappropriate in relation to poultry wishbone meat – for this reason all responses in this section are given as 'strongly disagree'.
FSA comments on response 2 (Q9)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 3: TA (Q9)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 3 (Q9)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 4: FBO (Q9)
The guidance in response to the supreme court ruling has been stretched to include wishbone meat. it is believed poultry meat was never intended to be captured by the ruling as such is flawed and has significant impact which need to be taken into account.
FSA comments on response 4 (Q9)
The FSA stands by its understanding of the Supreme Court Judgment.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 5: DNID (Q9)
The guidance is illogical - it states that a simple macroscopic visual will determine if the structure of a muscle fibre has been damaged which is patently impossible.
FSA comments on response 5 (Q9)
Comments noted and will be considered when finalising the Guidance.
Response 6: FBO (Q9)
The guidance is contradictory and does not explain the requirements for the production and differentiation between various meat products. It also does not provide scientific reasons for the differing definitions provided.
FSA comments on response 6 (Q9)
Comments noted and will be considered when finalising the Guidance.
Response 7: Other (Q9)
Technology has moved on to reduce human interactions and maintain costs, the mechanisation of carcase processing in effect creates MSM for all primal cuts and deboned products.
FSA comments on response 7 (Q9)
In assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
The use of methods of mechanical separation to recover meat does not on its own determine that MSM is produced. It is one of three cumulative criteria. MSM is only produced when all three criteria are met.
Response 8: FBO (Q9)
The Guidance has made specific reference to Baader, Sepamatic machines used in mechanical separation, the industry will seek to request clarification if there is other equipment implicated and why were these mentioned specifically. In addition the 1st criteria of “meat on bone” needs to be confirmed to determine if there is a qty of bone allowed Other concerns include that the modification of meat would become a point of view issue and there will be inconsistency so what type of measures will be taken to ensure consistency in approach and application of the guidance when it comes to enforcement. There is also no mention of an appeals process, potentially there is one or certainly there needs to be one. The issue ref that the MSM meat cannot be used as part of the Protein calculation for nutritional decs, which is contrary to some EU jurisdictions where the MSM is labelled and the meat can be used as part of the Protein for Nutritional calculation. An impact assessment by the FSA should be undertaken to consider all associated costs of making this change for FBO’s and the industry, including changes to labels, new artwork origination costs and potential restriction of choice and availability issue, cost of living concerns and the impact of imported products into UK -which could be incorrectly labelled. There is reference to “Relevant Exemptions” the industry will request clarification as to what these are. The Guidance covers England, Wales, and NI – It does not cover Scotland (so what is happening in Scotland?) Where material is reclassified to MSM – there will be material in the market already and thus there will need to be a period of Run out and Run in. As any enforcement has the potential to cause uncertainty and recalls situations need to be avoided. E.g. Frozen stock in the supply chain and in the trade is all potentially implicated and this stock should be allowed to be used during this implementation period. FSA to provide clarification on FBO’s who are currently unaware that they are producing MSM, hence will require re classification and to re-apply for new licenses. This changeover will require a defined period of time. Following this public consultation period the FSA to issue the outcome from it and explain to the public fully what the main areas of concerns and issues were.
FSA comments on response 8 (Q9)
Comments noted. These points will be considered when finalising the Guidance.
Regarding the point on Scotland, FSS wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 9: FBO (Q9)
The definitions list that MSM is as a result of passing bones or carcasses of poultry through a mechanical means to extract flesh still attached but that this also requires the element of "loss or modification of the fibre" - what is this exactly as this will help businesses to determine whether a material which may have been produced this way meets the definition. Without it we are still left wondering if the fibres are modified to the extent whereby they now meet the definition of MSM.
FSA comments on response 9 (Q9)
The concept of loss or modification of the muscle fibre structure is part of the legal definition of MSM, given in assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in Northern Ireland). The legislation is unchanged.
Consideration will be given to how the criterion relating to loss or modification of the muscle fibre structure may be best explained in the finalised Guidance.
It may also be useful to refer to the relevant court cases, referenced with links in the draft Guidance, to further understand the considerations and conclusions of the Judgment regarding this criterion and how it should be applied: https://www.food.gov.uk/our-work/draft-guidance-on-mechanically-separated-meat-msm-summary.
Response 10: TA (Q9)
The guidance has not taken into account technical changes in the meat industry - eg the mechanical deboning of chicken thighs - technically means boneless thigh meat is MSM!
FSA comments on response 10 (Q9)
In assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
The use of methods of mechanical separation to recover meat does not on its own determine that MSM is produced. It is one of three cumulative criteria. MSM is only produced when all three criteria are met.
Response 11: Other (Q9)
The FSA has changed it's views on MSM and DSM, These rules no longer apply in the EU, MSM/DSM from ruminant bones is produced without restrictions in most EU countries
FSA comments on response 11 (Q9)
The Guidance pertains only to UK legislative requirements. DSM is not a category of meat recognised in UK or EU legislation. The previous FSA Guidance that regarded MSM/DSM ('Guidance to the moratorium on Desinewed Meat (DSM)') was withdrawn on 14 November 2022 (as was the moratorium it provided guidance on) upon the conclusion of a series of legal cases that had begun in 2012.
In accordance with UK legislation (assimilated Regulation (EC) No 999/2001 in GB; Regulation (EC) No 999/2001 in Northern Ireland), the use of bovine, ovine and caprine (cattle, sheep and goat) (ruminant) bones, or bone-in cuts, as raw material for MSM is prohibited and has been since 2001 due to public health concerns. Specifically, the potential risk of Transmissible Spongiform Encephalopathies (TSE) including Bovine Spongiform Encephalopathy (BSE).
Response 12: TA (Q9)
More detail is required - see email info (BMPA email response)
FSA comments on response 12 (Q9)
Comment noted.
Response 13: TA (Q9)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 13 (Q9)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Q10: Explain why you disagree that ‘The Guidance on MSM aids understanding of the Regulations’.
Response 1: FBO (Q10)
The guidance on MSM does not aid understanding of the regulations because, we disagree with the FSA’s interpretation of the Supreme Court Judgement in including wishbone meat as falling within the definition of MSM. We consider that, in all likelihood, the Supreme Court did not intend for products outside of the 2012 moratorium to be under its judgement. The judgement related to one company, a different product, with different production/manufacturing practices.
FSA comments on response 1 (Q10)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 2: FBO (Q10)
It is our company's belief that the FSA is in error in extrapolating its interpretation of the Supreme Court's judgement (on a different product and involving a single company) to include poultry wishbone meat. It is our belief that the Supreme Court had no intention of including products specifically outside of the 2011 moratorium. In view of this we believe the FSA guidance document to be majorly defective and inappropriate in relation to poultry wishbone meat – for this reason all responses in this section are given as 'strongly disagree'.
FSA comments on response 2 (Q10)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 3: TA (Q10)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 3 (Q10)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 4: FBO (Q10)
The guidance in response to the supreme court ruling has been stretched to include wishbone meat. it is believed poultry meat was never intended to be captured by the ruling as such is flawed and has significant impact which need to be taken into account.
FSA comments on response 4 (Q10)
The FSA stands by its understanding of the Supreme Court Judgment.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 5: DNID (Q10)
It is lacking important details.
FSA comments on response 5 (Q10)
Comment noted.
Response 6: DNID (Q10)
The guidance doesn't assist understanding the Regulations as it appears to try and interpret the regulations in an unscientific manner. The broad interpretation of 'boning' and meat being 'removed' along with the view that damage to microfibrils can be determined by a visual inspection is an over-interpretation and should be determined by the Courts.
FSA comments on response 6 (Q10)
The Supreme Court determined how the definition of MSM is to be read and applied. The FSA position follows the Supreme Court Judgment that gave clarity on interpreting the legislation and the Guidance is written on that basis, to aid understanding of the MSM definition in light of the Judgment.
Response 7: FBO (Q10)
Although the guidance references the regulations it provides no further clarity on the types of products the guidance is supposed to be defining. It appears that it is simply up to a FSA representative, Trading standards officer or EHO to determine by sight what a product is. This is a most unscientific method for a science based organisation. This type of determination is so subjective to the point of being un-defendable, as it leaves no scope to challenge. Enforcement of regulations cannot be determined by a single subjective opinion with no recourse. As this guidance is designed to cover all aspects of automated meat production in the UK meat industry, we fail to see how this can be comprehensively trained out to all FSA/TSO/EHO officers to ensure a competent and consistent standard across all areas.
FSA comments on response 7 (Q10)
The Guidance is not designed to cover all aspects of automatic meat production in the UK meat industry. It is intended to aid understanding of the definition of MSM in light of Supreme Court Judgment, to support FBOs in determining whether a product is MSM to ensure their compliance in line with regulatory requirements.
Determining whether a product is MSM is not achievable purely by looking at the product. As the Guidance outlines, it is necessary to consider three cumulative criteria in determining whether MSM is produced.
Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
Other comments noted. These points will be considered when finalising the Guidance.
Response 8: Other (Q10)
The document is generalised and does not take into account the economic changes to food production. Technologies have been introduced to improve the quality and safety of process trimmings - removal of cartilage and damaged bones in poultry.
FSA comments on response 8 (Q10)
Comments noted. In assimilated Regulation (EC) No. 853/2004 (Regulation (EC) No. 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
Response 9: FBO (Q10)
The Guidance has made specific reference to Baader, Sepamatic machines used in mechanical separation, the industry will seek to request clarification if there is other equipment implicated and why were these mentioned specifically. In addition the 1st criteria of “meat on bone” needs to be confirmed to determine if there is a qty of bone allowed Other concerns include that the modification of meat would become a point of view issue and there will be inconsistency so what type of measures will be taken to ensure consistency in approach and application of the guidance when it comes to enforcement. There is also no mention of an appeals process, potentially there is one or certainly there needs to be one. The issue ref that the MSM meat cannot be used as part of the Protein calculation for nutritional decs, which is contrary to some EU jurisdictions where the MSM is labelled and the meat can be used as part of the Protein for Nutritional calculation. An impact assessment by the FSA should be undertaken to consider all associated costs of making this change for FBO’s and the industry, including changes to labels, new artwork origination costs and potential restriction of choice and availability issue, cost of living concerns and the impact of imported products into UK -which could be incorrectly labelled. There is reference to “Relevant Exemptions” the industry will request clarification as to what these are. The Guidance covers England, Wales, and NI – It does not cover Scotland (so what is happening in Scotland?) Where material is reclassified to MSM – there will be material in the market already and thus there will need to be a period of Run out and Run in. As any enforcement has the potential to cause uncertainty and recalls situations need to be avoided. E.g. Frozen stock in the supply chain and in the trade is all potentially implicated and this stock should be allowed to be used during this implementation period. FSA to provide clarification on FBO’s who are currently unaware that they are producing MSM, hence will require re classification and to re-apply for new licenses. This changeover will require a defined period of time. Following this public consultation period the FSA to issue the outcome from it and explain to the public fully what the main areas of concerns and issues were.
FSA comments on response 9 (Q10)
Comments noted. These points will be considered when finalising the Guidance.
Regarding the point on Scotland, FSS wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 10: FBO (Q10)
As above - it is the definition of fibres being modified - what is this exactly?
FSA comments on response 10 (Q10)
The concept of loss or modification of the muscle fibre structure is part of the legal definition of MSM, given in assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in Northern Ireland). The legislation is unchanged.
Consideration will be given to how the criterion relating to loss or modification of the muscle fibre structure may be best explained in the finalised Guidance.
It may also be useful to refer to the relevant court cases, referenced with links in the Guidance, to further understand the considerations and conclusions of the Judgment regarding this criterion and how it should be applied.
Access via this link.
Response 11: TA (Q10)
The regulations when initially drafted were pertinent to traditional methods of manual labour. Technology has moved on and the regulations has not kept up to date.
FSA comments on response 11 (Q10)
In assimilated Regulation (EC) No. 853/2004 (Regulation (EC) No. 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
The use of methods of mechanical separation to recover meat does not on its own determine that MSM is produced. It is one of three cumulative criteria. MSM is only produced when all three criteria are met.
Response 12: Other (Q10)
The FSA has changed it's views on MSM and DSM, These rules no longer apply in the EU, MSM/DSM from ruminant bones is produced without restrictions in most EU countries.
FSA comments on response 12 (Q10)
The Guidance pertains only to UK legislative requirements. DSM is not a category of meat recognised in UK or EU legislation. The previous FSA Guidance that regarded MSM/DSM ('Guidance to the moratorium on Desinewed Meat (DSM)') was withdrawn on 14 November 2022 (as was the moratorium it provided guidance on) upon the conclusion of a series of legal cases that had begun in 2012.
In accordance with UK legislation (assimilated Regulation (EC) No 999/2001 in GB; Regulation (EC) No 999/2001 in Northern Ireland), the use of bovine, ovine and caprine (cattle, sheep and goat) (ruminant) bones, or bone-in cuts, as raw material for MSM is prohibited and has been since 2001 due to public health concerns. Specifically, the potential risk of Transmissible Spongiform Encephalopathies (TSE) including Bovine Spongiform Encephalopathy (BSE).
Response 13: TA (Q10)
It is lacking detail. [link to email provided here by FSA]: See email response BMPA
FSA comments on response 13 (Q10)
Comment noted.
Response 14: TA (Q10)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 14 (Q10)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Q11: Explain why you disagree that ‘The Guidance on MSM is relevant to me and/or my business/organisation.
Response 1: TA (Q11)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 1 (Q11)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 2: FBO (Q11)
The guidance in response to the supreme court ruling has been stretched to include wishbone meat. It is believed poultry meat was never intended to be captured by the ruling as such is flawed and has significant impact which need to be taken into account.
FSA comments on response 2 (Q11)
The FSA stands by its understanding of the Supreme Court Judgment.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 3: Other (Q11)
The guidance is generalised and fails to define high pressure and low pressure processing of poultry meats, pork meats such as the pork buttons and cartilage trimmings produced as part of the bacon production industry.
FSA comments on response 3 (Q11)
Comments noted. These points will be considered when finalising the Guidance.
Response 4: TA (Q11)
As above. [link to email provided here by FSA]: See email response BMPA.
FSA comments on response 4 (Q11)
Comment noted.
Response 5: TA (Q11)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 5 (Q11)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Q12 Explain why you disagree that ‘The Guidance helps businesses understand how to comply with regulatory requirements regarding MSM.’ If possible, suggest what could further help businesses to understand.
Response 1: FBO (Q12)
Again this links back to A9 and 10. • There is no clarity on transitional arrangements for products already on the market e.g. frozen stock and finished products– what will happen to this material/product? • What does visibly bone-free actually mean? (Q&A Q9)
FSA comments on response 1 (Q12)
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Comments noted. The ‘visibly bone-free’ point will be considered when finalising the Guidance.
Response 2: FBO (Q12)
It is our company's belief that the FSA is in error in extrapolating its interpretation of the Supreme Court's judgement (on a different product and involving a single company) to include poultry wishbone meat. It is our belief that the Supreme Court had no intention of including products specifically outside of the 2011 moratorium. In view of this we believe the FSA guidance document to be majorly defective and inappropriate in relation to poultry wishbone meat – for this reason all responses in this section are given as 'strongly disagree'.
FSA comments on response 2 (Q12)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 3: TA (Q12)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 3 (Q12)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 4: FBO (Q12)
A full impact assessment is required before any such guidance is fully implemented and/ or amended.
FSA comments on response 4 (Q12)
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 5: FBO (Q12)
It has always been the standpoint of the FSA that is it FBO's who are responsible for ensuring they are compliant with the regulations. As a business we cannot invest in automation of processes (due to lack of available skilled employees, which is a national problem) which could be reclassified as something else at a future point. We have found from past experience that it is very difficult to get clarity from the Competent Authority on any matter which are subjective.
FSA comments on response 5 (Q12)
The use of methods of mechanical separation to recover meat does not on its own determine that MSM is produced. It is one of three cumulative criteria. MSM is only produced when all three criteria are met.
Response 6: FBO (Q12)
The Guidance has made specific reference to Baader, Sepamatic machines used in mechanical separation, the industry will seek to request clarification if there is other equipment implicated and why were these mentioned specifically. In addition the 1st criteria of “meat on bone” needs to be confirmed to determine if there is a qty of bone allowed Other concerns include that the modification of meat would become a point of view issue and there will be inconsistency so what type of measures will be taken to ensure consistency in approach and application of the guidance when it comes to enforcement. There is also no mention of an appeals process, potentially there is one or certainly there needs to be one. The issue ref that the MSM meat cannot be used as part of the Protein calculation for nutritional decs, which is contrary to some EU jurisdictions where the MSM is labelled and the meat can be used as part of the Protein for Nutritional calculation. An impact assessment by the FSA should be undertaken to consider all associated costs of making this change for FBO’s and the industry, including changes to labels, new artwork origination costs and potential restriction of choice and availability issue, cost of living concerns and the impact of imported products into UK -which could be incorrectly labelled. There is reference to “Relevant Exemptions” the industry will request clarification as to what these are. The Guidance covers England, Wales, and NI – It does not cover Scotland (so what is happening in Scotland?) Where material is reclassified to MSM – there will be material in the market already and thus there will need to be a period of Run out and Run in. As any enforcement has the potential to cause uncertainty and recalls situations need to be avoided. E.g. Frozen stock in the supply chain and in the trade is all potentially implicated and this stock should be allowed to be used during this implementation period. FSA to provide clarification on FBO’s who are currently unaware that they are producing MSM, hence will require re classification and to re-apply for new licenses. This changeover will require a defined period of time. Following this public consultation period the FSA to issue the outcome from it and explain to the public fully what the main areas of concerns and issues were.
FSA comments on response 6 (Q12)
Comments noted. These points will be considered when finalising the Guidance.
Regarding the point on Scotland, FSS wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 7: FBO (Q12)
Because of the same point. If the definition was simply bones or carcasses passed through a mechanical means once meat has been removed from the bones or carcass, then it would be straightforward but there is the added rider of "IF" there is modification of the fibres. It is the word "IF" that leaves businesses unsure whether their material complies with the definition or not.
FSA comments on response 7 (Q12)
The concept of loss or modification of the muscle fibre structure is part of the legal definition of MSM, given in assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in Northern Ireland). The definition and the regulation are unchanged.
Consideration will be given to how the criterion relating to loss or modification of the muscle fibre structure may be best explained in the finalised Guidance.
It may also be useful to refer to the relevant court cases, referenced with links in the Guidance, to further understand the considerations and conclusions of the Judgment regarding this criterion and how it should be applied.
Access via this link.
Response 8: TA (Q12)
Business operations and changes in technology have significantly moved on. Legislation needs to be reviewed to capture and understand the changes that have taken place.
FSA comments on response 8 (Q12)
In assimilated Regulation (EC) No. 853/2004 (Regulation (EC) No. 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
Response 9: TA (Q12)
There has been far too little engagement on this matter, the FSA have had plenty of opportunity and not taken this up.
FSA comments on response 9 (Q12)
The FSA has offered and provided various opportunities for stakeholder engagement on this matter over the past few years. This has included engagement directly with the MSM Technical Group, which was established in 2022, and further ad hoc engagement opportunities with other interested parties’ representatives. Stakeholder engagement has also been conducted by Senior FSA officials, including the FSA Chief Executive and Chair. There will be further stakeholder engagement prior to the Guidance being finalised.
Response 10: TA (Q12)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 10 (Q12)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Q13: Explain why you disagree that ‘The Q&A annex to the Guidance provides additional information that is useful'.
Response 1: FBO (Q13)
It is our company's belief that the FSA is in error in extrapolating its interpretation of the Supreme Court's judgement (on a different product and involving a single company) to include poultry wishbone meat. It is our belief that the Supreme Court had no intention of including products specifically outside of the 2011 moratorium. In view of this we believe the FSA guidance document to be majorly defective and inappropriate in relation to poultry wishbone meat – for this reason all responses in this section are given as 'strongly disagree'.
FSA comments on response 1 (Q13)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 2: TA (Q13)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 2 (Q13)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 3: FBO (Q13)
Does not take into account or properly differentiate between high pressure machine separated MSM and low pressure wishbone meat.
FSA comments on response 3 (Q13)
Comment noted. This point will be considered when finalising the Guidance.
Response 4: FBO (Q13)
The Q&A at the end of the document does not define in any detail what the requirements of the guidance are. The only area which is covered in any detail is wishbone meat however even this area is ambiguous at very best. The scientific methodology determined by Leatherhead and used initially by the FSA has been discarded despite costing them over £100,000. Point 15 on the document regarding food waste is simply wrong. There will be an inevitable increase in food waste as the product may be available for businesses to use, however as MSM is not QUID able this makes the product worthless to manufacturers.
FSA comments on response 4 (Q13)
Comments noted. These points will be considered when finalising the Guidance.
MSM produced in compliance with hygiene regulations is a safe product that can be used as an ingredient in a wide variety of foods. MSM is also required to be labelled as such on products to allow consumers to make an informed choice.
Response 5: FBO (Q13)
The Guidance has made specific reference to Baader, Sepamatic machines used in mechanical separation, the industry will seek to request clarification if there is other equipment implicated and why were these mentioned specifically. In addition the 1st criteria of “meat on bone” needs to be confirmed to determine if there is a qty of bone allowed Other concerns include that the modification of meat would become a point of view issue and there will be inconsistency so what type of measures will be taken to ensure consistency in approach and application of the guidance when it comes to enforcement. There is also no mention of an appeals process, potentially there is one or certainly there needs to be one. The issue ref that the MSM meat cannot be used as part of the Protein calculation for nutritional decs, which is contrary to some EU jurisdictions where the MSM is labelled and the meat can be used as part of the Protein for Nutritional calculation. An impact assessment by the FSA should be undertaken to consider all associated costs of making this change for FBO’s and the industry, including changes to labels, new artwork origination costs and potential restriction of choice and availability issue, cost of living concerns and the impact of imported products into UK -which could be incorrectly labelled. There is reference to “Relevant Exemptions” the industry will request clarification as to what these are. The Guidance covers England, Wales, and NI – It does not cover Scotland (so what is happening in Scotland?) Where material is reclassified to MSM – there will be material in the market already and thus there will need to be a period of Run out and Run in. As any enforcement has the potential to cause uncertainty and recalls situations need to be avoided. E.g. Frozen stock in the supply chain and in the trade is all potentially implicated and this stock should be allowed to be used during this implementation period. FSA to provide clarification on FBO’s who are currently unaware that they are producing MSM, hence will require re classification and to re-apply for new licenses. This changeover will require a defined period of time. Following this public consultation period the FSA to issue the outcome from it and explain to the public fully what the main areas of concerns and issues were.
FSA comments on response 5 (Q13)
Comments noted. These points will be considered when finalising the Guidance.
Regarding the point on Scotland, FSS wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 6: TA (Q13)
The Q&A is based on old systems of production at the disadvantage of industry and the consumer.
FSA comments on response 6 (Q13)
Comment noted and will be considered in the finalising of the Guidance.
Response 7: TA (Q13)
See email info.
FSA comments on response 7 (Q13)
Comment noted. Please see email responses for further information.
Response 8: TA (Q13)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 8 (Q13)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Q14: Explain why you disagree that ‘The Guidance covers all relevant matters in relation to MSM’
Response 1: FBO (Q14)
Links back to A9 and A10 – does not detail production of MSM requirements.
FSA comments on response 1 (Q14)
Comment noted and will be considered in finalising the Guidance.
Response 2: FBO (Q14)
It is our company's belief that the FSA is in error in extrapolating its interpretation of the Supreme Court's judgement (on a different product and involving a single company) to include poultry wishbone meat. It is our belief that the Supreme Court had no intention of including products specifically outside of the 2011 moratorium. In view of this we believe the FSA guidance document to be majorly defective and inappropriate in relation to poultry wishbone meat – for this reason all responses in this section are given as 'strongly disagree'.
FSA comments on response 2 (Q14)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 3: TA (Q14)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 3 (Q14)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Response 4: Other (Q14)
There is not enough detail, I have emailed a separate note already.
FSA comments on response 4 (Q14)
Comment noted. Please see email responses for further information.
Response 5: FBO (Q14)
It moves a good quality breast meat from the wishbone into MSM which has significant impact on cost, food waste and loss of value. the impact need proper consideration.
FSA comments on response 5 (Q14)
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of non-compliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 6: DNID (Q14)
There is no appeals process - what happens if you disagree on the reclassification of the material you are producing? It would be a vary costly mistake to make!
FSA comments on response 6 (Q14)
Neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency.
Response 7: FBO (Q14)
This is due to the fact that the guidance is not detailed enough and is flawed in its analysis of the usage of this type of product. There are several areas of impact that have not been correctly analysed and have more relevance to the potential outcome than anything contained within the guidance. An example of this, is the potential increase in food costs to the most vulnerable consumers in our society. This is despite the fact there is no food safety risk from these products.
FSA comments on response 7 (Q14)
Comment noted. These points will be considered when finalising the Guidance.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 8: FBO (Q14)
The Guidance has made specific reference to Baader, Sepamatic machines used in mechanical separation, the industry will seek to request clarification if there is other equipment implicated and why were these mentioned specifically. In addition the 1st criteria of “meat on bone” needs to be confirmed to determine if there is a qty of bone allowed Other concerns include that the modification of meat would become a point of view issue and there will be inconsistency so what type of measures will be taken to ensure consistency in approach and application of the guidance when it comes to enforcement. There is also no mention of an appeals process, potentially there is one or certainly there needs to be one. The issue ref that the MSM meat cannot be used as part of the Protein calculation for nutritional decs, which is contrary to some EU jurisdictions where the MSM is labelled and the meat can be used as part of the Protein for Nutritional calculation. An impact assessment by the FSA should be undertaken to consider all associated costs of making this change for FBO’s and the industry, including changes to labels, new artwork origination costs and potential restriction of choice and availability issue, cost of living concerns and the impact of imported products into UK -which could be incorrectly labelled. There is reference to “Relevant Exemptions” the industry will request clarification as to what these are. The Guidance covers England, Wales, and NI – It does not cover Scotland (so what is happening in Scotland?) Where material is reclassified to MSM – there will be material in the market already and thus there will need to be a period of Run out and Run in. As any enforcement has the potential to cause uncertainty and recalls situations need to be avoided. E.g. Frozen stock in the supply chain and in the trade is all potentially implicated and this stock should be allowed to be used during this implementation period. FSA to provide clarification on FBO’s who are currently unaware that they are producing MSM, hence will require re classification and to re-apply for new licenses. This changeover will require a defined period of time. Following this public consultation period the FSA to issue the outcome from it and explain to the public fully what the main areas of concerns and issues were.
FSA comments on response 8 (Q14)
Comments noted. These points will be considered when finalising the Guidance.
Regarding the point on Scotland, FSS wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
Regarding enforcement decisions at approved meat premises, neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency. Any appeals against enforcement action undertaken by a Local Authority should be raised and discussed with them directly.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 9: FBO (Q14)
The guidance has not fully reviewed the impacts of changing the definition of 3mm Wishbone which is from a defined process and material used as a meat preparation since 2004. The implications would lead additional birds to be slaughtered, additional farming to support, higher CO2 emissions, higher costs leading to job losses and consumer food inflation.
FSA comments on response 9 (Q14)
No definitions have been changed or are being changed, including those of MSM and meat preparations (both defined in assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in Northern Ireland)). The Supreme Court Judgment has clarified how the already existing legal definition of MSM must be interpreted and applied. In light of that clarification, the previous FSA Guidance pertaining to MSM was withdrawn (in November 2022) and new Guidance has been drafted to aid understanding of when and how MSM is produced.
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of non-compliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 10: TA (Q14)
It does not contain a specific definition of "loss or modification of fibres".
FSA comments on response 10 (Q14)
The concept of loss or modification of the muscle fibre structure is part of the legal definition of MSM, given in assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in Northern Ireland). The definition and the regulation are unchanged.
Consideration will be given to how the criterion relating to loss or modification of the muscle fibre structure may be best explained in the finalised Guidance.
It may also be useful to refer to the relevant court cases, referenced with links in the Guidance, to further understand the considerations and conclusions of the Judgment regarding this criterion and how it should be applied.
Access via this link.
Response 11: Consumer (Q14)
I couldn’t see a simple explanation of what the consumer labelling should be for MSM on food packaging. Presumably that is laid out in other regulations or guidance?
FSA comments on response 11 (Q14)
Labelling requirements for MSM are primarily laid out in assimilated Regulation (EU) No 1169/2011 in GB / Regulation (EU) No 1169/2011 in NI (commonly referred to as 'Food Information for Consumers' or 'FIC'). Specific hygiene rules for food of animal origin are laid out in assimilated Regulation (EC) No 853/2004 / Regulation (EC) No 853/2004 in Northern Ireland.
Response 12: TA (Q14)
The guidance does not take into account processing methodology affecting operators Health & Safety and the upgrade in technology to maximise company yield and to offer an economic benefit to the consumer.
FSA comments on response 12 (Q14)
Comment noted.
In assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
Response 13: TA (Q14)
See email info.
FSA comments on response 13 (Q14)
Comment noted. Please see email responses for further information.
Response 14: TA (Q14)
The FSA has made a significant error in stretching its interpretation of the Supreme Court judgement (on a different product and single company) to include wishbone meat. We believe that the Court had no intention of including products specifically outside of the moratorium created in 2011. As such the guidance document is fundamentally flawed and therefore no part of it can be relied on in relation to wishbone meat – hence ‘strongly disagree’ on all of the above question.
FSA comments on response 14 (Q14)
The FSA stands by its understanding of the Supreme Court Judgment.
The Supreme Court Judgment set out the criteria to be met, to be applied across all cuts of meat. It will be a question of fact whether the process for removing meat from the bone, or bone from the meat, meets the test laid out in the Judgment and this is the purpose behind the Guidance.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat. The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
Questions 15 to 28
In the consultation document, it was stated that the FSA is seeking evidence from industry to inform assessment of the total costs and benefits, and that information is sought regarding the impacts of FBOs adapting activities and processes in line with the Supreme Court Judgment.
Online survey questions 15-28 were presented to businesses responding. The questions are shown below. The responses are omitted from this summary document. The responses are of a commercially sensitive nature, and they will be considered as part of an assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment which will be published later in the process.
| Question | Number of responses |
|---|---|
| Q15. Indicate the roles of staff that will be responsible for reading and understanding the Guidance on Mechanically Separated Meat. | 12 |
| Q16. How many staff in each role do you estimate will spend time reading and understanding the Guidance. For example, there will be 1 supervisor and 1 manager responsible for reading and understanding the Guidance. | 11 |
| Q17. How much time, on average, will each member of staff responsible for reading and understanding the Guidance take to complete this task? Provide your answer in minutes. For example, it will take each supervisor 30 minutes to read and understand the new Guidance. | 11 |
| Q18. Indicate the roles of staff that will be responsible for disseminating the information from the Guidance to colleagues. | 12 |
| Q19. How many staff in each role do you estimate will spend time disseminating information about the Guidance to colleagues. For example, there will be 2 team leaders responsible for explaining the Guidance to colleagues. | 9 |
| Q20. How much time, on average, will each employee responsible for disseminating information about the Guidance to colleagues spend doing so? Provide your answer in minutes. For example, it will take each team leader 45 minutes to deliver training on the Guidance to colleagues. | 9 |
| Q21. For this question, assume a hypothetical scenario of the Guidance being implemented immediately post consultation. Some products may need to be relabelled as food businesses adapt their activities/processes in line with the Court Judgments regarding the definition of MSM. Estimate the number of stock keeping units that your business will relabel as a result of adapting activities/processes in line with the Court Judgments. Stock keeping units are defined as the unique identification number that a business gives each of their products. | 10 |
| Q22. For this question, assume a hypothetical scenario of the Guidance being implemented immediately post consultation. Some products may need to be relabelled as food businesses adapt their activities/processes in line with the Court Judgments regarding the definition of MSM. Already existing labels/packaging may need to be disposed of. Provide an estimated quantity of the stock held of labels/packaging that you expect your business would have to dispose of, and an estimate of the amount of labelling/packaging stock your business currently uses each month. If possible, include a unit of measurement in your answer. Examples: 5 stock keeping units of packaging will need to be disposed of and my business currently uses 3 stock keeping units of labelling each month; or 2 tonnes of labelling will be disposed of, and we currently use 0.5 tonnes of labelling each month. Stock keeping units are defined as the unique identification number that a business gives each of their products. | 10 |
| Q23. Provide information about how you will dispose of incorrect labels and, if possible, the associated cost of this. | 10 |
| Q24. Food businesses adapting their activities/processes in line with the Court Judgments regarding the definition of MSM may mean manufacturers deciding to reformulate some products, potentially using higher priced ingredients. How likely or unlikely is it that your business will reformulate products to maintain its activity type as meat preparation e.g., by replacing material that is reclassified as MSM with material that remains classified as meat preparations? | 7 |
| Q25. Provide additional information about how your business will reformulate products to maintain its activity type as meat preparation. For example, additional information might include the processes involved, the time taken for each process, and the associated cost. | 6 |
| Q26. The FSA understands that food businesses adapting activities/processes in line with the Court Judgments may mean taking decisions that affect the structure of the business. For example, by changing the business model, adjusting the scale of operations, or changing production processes to maintain the current meat establishment activity type. How likely or unlikely is it that your business will take a decision that affects the structure of the business? | 12 |
| Q27. Provide additional information about the decisions that you may consider and how they will impact the business. For example, additional information might include details of changes, time required, and associated costs. | 9 |
| Q28. If you expect that your business will not have adapted activities/processes in line with the Court Judgments by the close of this consultation on Wednesday 22nd May, estimate how much longer the business requires to adapt. Provide your answer in weeks | 6 |
Q29: The FSA assumes that the impact on enforcement officers from local authorities will be minimal as the implementation of the Guidance will not result in any new tasks outside of the existing duties of enforcement officers. To what extent do you agree or disagree with this assumption?
- Strongly agree - 4
- Agree - 6
- Neither agree nor disagree - 7
- Disagree - 5
- Strongly disagree - 8
- Don't know - 3
- Not relevant - 0
Q30. Explain why you disagree.
Response 1: Other (Q30)
The consultation quite rightly highlights some food standards (labelling and composition) issues. These include the need for the correct labelling/relabelling of MSM and the absence of MSM from meat content percentages. What appears lacking, however, is a consideration of the impact of the proposed changes on the practicalities of enforcing these food standards requirements. Specifically, are they enforceable or will they create opportunities for food crime. The re-classification of product as MSM is likely to lead to a shortfall in supply at the necessary price within the UK and an increased reliance on imported product. In addition, the analysis of samples provides no definitive indication as to whether product is or is not MSM. It would therefore appear prudent to consider these, and other factors impacting on the feasibility of food standards enforcement, prior to implementation of the MSM guidance.
FSA comments on response 1 (Q30)
Comments noted. The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 2: TA (Q30)
Looking at MSM as a whole this policy will require national resource and enforcement as the FSA currently has little concept of who and where the material previously covered by the moratorium is being produced. Relying on self-declaration will result in a two-tier system for both approvals and enforcement, which may compromise commercial viability and healthy competition.
FSA comments on response 2 (Q30)
Comments noted.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 3: FBO (Q30)
There are many factors to consider including raw material in storage , product in storage , existing production method, mgt of waste and making sure this is equally applied across all business sectors.
FSA comments on response 3 (Q30)
Comments noted.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 4: DNID (Q30)
At local authority level there will be a complete lack of knowledge of detail in meat processing. What training are they and the FSA going to undertake? We have not seen anything from the FSA on this matter.
FSA comments on response 4 (Q30)
Comments noted.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement and identification of training requirements.
Response 5: FBO (Q30)
As this whole guidance is based upon a single subjective, non-scientific opinion of a product, which will determine its market value. This will inevitably cause conflict between FBO's and regulatory officials. Which will in turn draw challenges from industry which will significantly increase the workload of regulatory personnel.
FSA comments on response 5 (Q30)
The Supreme Court determined how the definition of MSM is to be read and applied. The FSA position follows the Supreme Court Judgment that gave clarity on interpreting the legislation and the Guidance is written on that basis, to aid understanding of the MSM definition in light of the Judgment.
Neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency.
Response 6: Other (Q30)
The role of officers will be increased and open the risk of reduced food safety where companies will disregard the changes to maintain their commercial benefit and opportunities.
FSA comments on response 6 (Q30)
Comment noted. The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement and identification of training requirements.
Response 7: Consumer (Q30)
Mechanically made meat is not really for humans in my belief and lots can go wrong. [ ] You are the people who monitor and protect the publics food supple, so it falls on your shoulders and your conscience.
FSA note: Further comments omitted about the importance of transparent labelling to ensure trust in the food system, not specifically relevant to this consultation.
FSA comments on response 7 (Q30)
Comments noted. The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
Response 8: TA (Q30)
There is a significant lack of technical processing understanding, the enforcement officers will revert to legislation interpretation. The legislation needs to be updated to reflect the technological changes.
FSA comments on response 8 (Q30)
In assimilated Regulation (EC) No. 853/2004 (Regulation (EC) No. 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
Response 9: Consumer (Q30)
All MSM should be banned.
FSA comments on response 9 (Q30)
Comments noted. Legislation permits the production and use of MSM, and there are no plans to change this. MSM produced in compliance with hygiene regulations is a safe product that can be used as an ingredient in a wide variety of foods. MSM is also required to be labelled as such on products to allow consumers to make an informed choice.
Q31. Do you agree or disagree that all relevant direct impacts have been identified within the MSM Consultation?
33 out of 48 respondents answered this question:
- Agree - 11
- Disagree - 19
- Don't know - 3
Q32. If you believe there are additional direct impacts beyond those already identified, identify them here. Where possible, provide evidence that is monetised or in numerical form measuring the impact on a one-off or annual basis. This will help the FSA to monetise the cost robustly.
Response 1: FBO (Q32)
The FSA has only identified impacts associated will the time taken to read the document and to replace some labels.
- Product already on market what happens to this product?
- Financial impact to businesses – increased production costs and loss of profit for investment into the business
- Can we be sure that imported products comply with this guidance e.g. minced meat is actually minced meat and not MSM?
FSA comments on response 1 (Q32)
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of non-compliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment. Nevertheless, businesses must meet legal requirements.
Imported products must meet UK requirements and there are import control measures in place.
Response 2: FBO (Q32)
If ultimately poultry wishbone meat is to be classified as MSM, then the outcome will impact negatively on the already low product value MSM market.
FSA comments on response 2 (Q32)
Comment noted.
Response 3: TA (Q32)
See separate submission made via email.
FSA comments on response 3 (Q32)
Comment noted. Please see email responses for further information.
Response 4: Other (Q32)
The impact of this I believe has been seriously underestimated, we believe that the cost would be something like £10 to £12 million. It will lead to food inflation and huge food waste for the pork sector. Reformulation will need to take place and may result in lower meat content due to material being reclassified. What are you going to do about imported material that is wrongly labelled and currently being processed as meat?
FSA comments on response 4 (Q32)
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Court Judgment will be undertaken and published later in the process.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment. Nevertheless, businesses must meet legal requirements.
Imported products must meet UK requirements and there are import control measures in place. This has not changed.
Response 5: FBO (Q32)
Answered in previous questions but, raw material on cost 34.9m waste / loss of value 9,5m waste generated 5600t consumer on cost? environmental impact?
FSA comments on response 5 (Q32)
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 6: DNID (Q32)
Are the FSA going to conduct an impact assessment? There will also be FBO's that are wrongly registered e.g. meat preps when they should have approval for the production of MSM - how are you going to find out who they are? Our estimate is that it will cost between £10 to £12 million of the businesses that we know. The question is how many are there producing this material that you don't know?
FSA comments on response 6 (Q32)
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 7: FBO (Q32)
As the guidance is not clear enough for FBO's to determine the classification each product, should the regulatory officer determine that the products we produce are all MSM then the impact would be devastating and cost our business over £20 million per annum, or possible total factory closure with the loss of over 80 jobs. From detailed analysis of the industries current usage of this type of product, we believe this total loss will be more than £172 million per annum to the meat industry. This does not include any additional factors such as waste removal, increased carbon footprint costs, job losses and the inevitable increase of food costs to the most vulnerable consumers.
FSA comments on response 7 (Q32)
Comments noted. Consideration will be given to making the Guidance as clear as possible.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 8: Other (Q32)
Totally disagree with the nature of the question. A lack of communication and a failure to fully engage with industry on costs and understanding the supply chain and processes to maximise yields to the benefit of the consumer and the business has not been understood.
FSA comments on response 8 (Q32)
Comment noted. The FSA has offered and provided various opportunities for stakeholder engagement on this matter over the past several few years. This has included engagement directly with the MSM Technical Group, which was established in 2022, and further ad hoc engagement opportunities with other industry interested parties’ representatives. Stakeholder engagement has also been conducted with by Senior FSA officials, including the FSA Chief Executive and Chair. There will be further stakeholder engagement prior to the Guidance being finalised.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of non-compliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
In assimilated Regulation (EC) No 853/2004 (Regulation (EC) No 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 9: FBO (Q32)
The cost of alternative materials will result in an additional £3.5m per annum, the equivalent of 5.2m of chickens per annum, resulting in an additional 35m kgs of C02 per annum. The environmental impact must be considered as many products will not be able to MSM and alternatives will need materials will need to be sourced.
FSA comments on response 9 (Q32)
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 10: FBO (Q32)
Supply Chain impacts on volume availability of alternate materials which may result in use on increased Non UK sourced materials. Costs impacts from the use of alternate materials which mean that labelling is not impacted. Consumer impact on the eat of products as this material is often used in products for a softer texture which we will now need to find an alternate solution for.
FSA comments on response 10 (Q32)
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 11: Consumer (Q32)
Not relevant to fake food/crops other than I have said money shouldn't overrule people's health or it be on your conscience who pass such laws.
FSA comments on response 11 (Q32)
Comments noted. The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
Response 12: FBO (Q32)
Reviewing the specification cost Training cost.
FSA comments on response 12 (Q32)
Comment noted.
Response 13: TA (Q32)
The removal of MSM, driven by retailers, will increase final products such as ready meals, sausages, burgers and other value-added products by at least 25% on sales value.
FSA comments on response 13 (Q32)
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 14: TA (Q32)
There are FBO's that have approvals for meat preps and producing MSM material. You will need to check every premises in England and Wales with LA's. Scotland and NI will need to do the same. In short the FSA and LA's will need to physically check every FBO's premises. Why has there been NO impact assessment?
FSA comments on response 14 (Q32)
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 15: TA (Q32)
The FSA seems to believe that the only impact will be the time taken to read the document and to replace some labels. See accompanying briefing paper along with impact figures.
FSA comments on response 15 (Q32)
Comment noted and briefing paper will be considered.
Q33. Provide information about the impacts that the clarified definition of MSM will have on you as a consumer.
Response 1: Consumer (Q33)
lots as if it not prevented or labelled not to mislead how can anyone know???????????????????????????????????????
Response 2: Consumer (Q33)
I feel reassured that the clarification, if followed correctly, will result in a more accurate description of the proportion/amount of MSM present in any pork- or poultry-based meat products that I might wish to buy in the future.
Response 3: Consumer (Q33)
As a consumer I don’t like ultra processed meats as these mechanical methods make everything look and feel surreal.
Response 4: Consumer (Q33)
I don't want to eat any MSM meat products , no matter how cheap or cost effective to a business it is.
Response 5: Consumer (Q33)
I think the wider public needs more straight forward information on msm.
FSA comments on responses 1 - 5 (Q33)
The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
Q34. Do you believe there are wider challenges associated with MSM that the FSA or wider government might seek to address?
33 out of 48 respondents answered this question:
- Yes - 25
- No - 1
- Don't know - 7
Q35. Please provide details of any wider challenges, with supporting evidence where possible and explain how any potential changes or measures could benefit stakeholders.
Response 1: FBO (Q35)
The definition of MSM in the EU assimilated law 853/2004 is not clear. The UK government has an opportunity to affect legislative change and to focus more on the ‘outputs’ of a process (i.e. the physical and histological properties of the product itself) rather than focusing on input material. Any change, however, must consider the importance of a UK level playing field i.e. the impact of the Windsor Framework.
Para 30 in the consultation letter the FSA assumes no food waste – retailers don’t want their products to include material categorised as MSM in their products resulting in this material going to petfood.
- There are no benefits identified in the consultation for changing the guidance.
- Consistency of application of this guidance across the UK – varying interpretations by officials especially in stand-alone plants.
- Enforcement of this guidance is not clear – what is the process? – will there be an appeals process? How is enforcement to be applied consistency across all of the UK?
- Impact on consumers o We conducted a number of surveys in May to understand consumer views on MSM o We had a minimum of 520 respondents answering each of the surveys
- Attached is a 2 page summary of the key findings
- In summary, consumer views on the inclusion of MSM are complex; although the majority accept its use in principle, our research suggests that when confronted with ingredient decs which contain MSM, this has a detrimental effect on propensity to purchase.
- The research shows that 21% of consumers could be lost by just the presence of MSM in an ingredients list, and that over a third, when asked directly, say they would be less likely to buy if MSM were included in the product.
FSA comments on response 1 (Q35)
The definition of MSM is not solely input-based. The input is one of three cumulative criteria that need to be considered together to determine whether MSM is produced – the other two being the process applied to the input, and characteristics of the output as a result of the process. This is also the case for other definitions e.g., for meat preparations – also defined by considering the input, process applied, and characteristics following the process. Comment regarding level playing field noted. MSM produced in compliance with hygiene regulations is a safe product that can be used as an ingredient in a wide variety of foods. MSM is also required to be labelled as such on products to allow consumers to make an informed choice.
Neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency.
Other comments noted. These points, including the research submitted, will be considered when finalising the Guidance.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process.
Response 2: Other (Q35)
Determining whether imported products are MSM if otherwise labelled.
FSA comments on response 2 (Q35)
Comment noted.
Response 3: FBO (Q35)
Poultry wishbone meat is a high quality meat. Downgrading its status to that of MSM will destroy its value as its nutritional value could not then be used as part of a final product's declared meat content. UK food retailers/supermarkets will not use ingredients designated as MSM within their meat products. As a consequence, there will be an estimated £75Million increased cost for consumers buying coated poultry and poultry meat preparations. Poultry wishbone meat being downgraded to MSM will result in increased food production waste - despite being high quality meat. There will be an environmental impact as a consequence of additional food waste and it will also be necessary to rear more birds to make up for the loss of wishbone meat in meat supply chains - a further environmental impact in agriculture and primary/secondary poultry processing. All parts of the meat supply chain (growers, retailers, traders, meat preparation manufacturers and food consumers) will be affected significantly affected by the FSA's position regarding poultry wishbone meat - should it ultimately be re-classified as MSM. There will be an additional enforcement task for the FSA to check imports for compliance without more resources being made available.
FSA comments on response 3 (Q35)
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court, are met, including the process chosen to separate the meat from the bone.
Other comments noted.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 4: TA (Q35)
See separate submission made via email.
FSA comments on response 4 (Q35)
Comment noted. Please see email responses for further information.
Response 5: Other (Q35)
There are no benefits for the food industry at all only extra costs when you start to reclassify material, it will only lead to more food inflation and food waste.
FSA comments on response 5 (Q35)
Comment noted.
Response 6: FBO (Q35)
The legislation and interpretation if same is out of date. it penalise advancement in automation and fails to differentiate wishbone meat from MSM as produced under high pressure separation ( pink slime). the cost impacts have been previously covered.
FSA comments on response 6 (Q35)
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court, are met, including the process chosen to separate the meat from the bone.
Response 7: DNID (Q35)
Here are 100's of tonnes of material entering the UK weekly wrongly labelled going into meat products that is undeclared. As you are basically going to stop UK manufactures using material produced here by reclassifying just about all of it, what are you going to do about imported material?
FSA comments on response 7 (Q35)
The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
Imported products must meet UK requirements and there are robust import control measures in place.
Response 8: TA (Q35)
Sustainability and environmental impact - high quality protein will be removed from the food chain for no apparent benefit. Food waste - a vital source of cheap high quality protein will be diverted to waste following adoption of this policy. Food safety - no evidence has been presented at any stage of any food safety challenge as a result of this product being produced. To the contrary there is many years of collected evidence which confirms that this product is fit and safe for human consumption. Cost of living crisis - the removal of a core ingredient of many wholesome food items important to the more resource challenged in society will impact negatively on the cost of living and the viability of businesses dependent on providing good food at an affordable cost.
FSA comments on response 8 (Q35)
There is no suggestion that MSM is unsafe or unfit for human consumption nor that it should be diverted to waste. MSM produced in compliance with hygiene regulations is a safe product that can be used as an ingredient in a wide variety of foods. MSM is also required to be labelled as such on products to allow consumers to make an informed choice.
Other comments noted.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 9: FBO (Q35)
The main challenges of the implementation will be to replace the current products with suitable alternatives at similar pricing (which we do not believe exist). Increased carbon footprint for the whole of the meat industry as the removal of the source of protein needs to be replaced in a growing society. There are no benefits to anyone in the implementation of these changes. We believe these changes will lead to jobs losses and financial difficulties for many FBO's particular smaller concerns. The inevitable increase in food prices for the most vulnerable in our society.
FSA comments on response 9 (Q35)
Comments noted.
The law is not being changed or consulted on; rather, it is being interpreted in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 10: Other (Q35)
The methodology and changes in technology as well as production processes does not reflect and respond to the legislation created over 20 years ago.
FSA comments on response 10 (Q35)
In assimilated Regulation (EC) No. 853/2004 (Regulation (EC) No. 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
Response 11: FBO (Q35)
Ask the industry bodies BMPA, BPC, BRCGS for what their views are directly in relation to this MSM guidance.
FSA comments on response 11 (Q35)
The FSA has offered and provided various opportunities for stakeholder engagement on this matter over the past several few years. This has included engagement directly with the MSM Technical Group, which was established in 2022, and further ad hoc engagement opportunities with other industry interested parties’ representatives. Stakeholder engagement has also been conducted with by Senior FSA officials, including the FSA Chief Executive and Chair. There will be further stakeholder engagement prior to the Guidance being finalised.
Response 12: FBO (Q35)
Environmental, Welfare and Cost The 3mm Wishbone is an industry standard cut from the chicken. The cut is known to the public and there are industry controls to ensure a consistent reputable finished consumer product. A government and industry framework should be explored; otherwise, a great number of chickens will need to be slaughtered.
FSA comments on response 12 (Q35)
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 13: FBO (Q35)
Impact on material availability. Impact on costs for consumers. Impact on consumer acceptance of finished products
FSA comments on response 13 (Q35)
Comment noted.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Response 14: Consumer (Q35)
Fake food should not be sold here and it will eventually affect stakeholders and /or their conscience.
FSA comments on response 14 (Q35)
Comment noted. MSM is a genuine food ingredient. The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
Response 15: TA (Q35)
The technological changes and the automation of processing in the poultry industry have created a massive grey area in interpretations of the legislative requirement. Low pressure mechanical mincing and High pressure produce two very different products. The current range of value-added minced poultry and pork product on offer to the consumer, provides an economic support to many low-income families as a source of protein.
FSA comments on response 15 (Q35)
In assimilated Regulation (EC) No. 853/2004 (Regulation (EC) No. 853/2004 in NI), the MSM definition is designed to be a generic one covering all methods of mechanical separation, specifically in recognition of rapid technological developments in this area which mean that a flexible definition is appropriate.
FBOs must continue to meet legal requirements when upgrading technology and processing methods.
MSM, assuming it is produced in compliance with legislative requirements, is a safe product that can be used as an ingredient in a wide variety of foods.
Response 16: Consumer (Q35)
The continuation of businesses using MSM and palming these products off as being halal hasn’t been clarified.
FSA comments on response 16 (Q35)
Comment noted.
Response 17: Other (Q35)
Allowing ruminant bones to be used in pet food would have a benefit to the abattoirs due to being able to command a higher price for the bones. Also, why is it a ruminant bone can be passed through a mincer and used and yet can't be put through a seperater to make a superior product?
FSA comments on response 17 (Q35)
Comments noted. The consultation relates to the production of MSM in food produced for human consumption.
Ruminant bones are already permitted to be used in pet food, with the exception of those considered Specified Risk Material (SRM).
In accordance with UK legislation (assimilated Regulation (EC) No 999/2001 in GB; Regulation (EC) No 999/2001 in Northern Ireland), the use of bovine, ovine and caprine (cattle, sheep and goat) (ruminant) bones, or bone-in cuts, as raw material for MSM is prohibited and has been since 2001 due to public health concerns. Specifically, the potential risk of Transmissible Spongiform Encephalopathies (TSE) including Bovine Spongiform Encephalopathy (BSE).
Response 18: Consumer (Q35)
Some businesses may find ways to avoid complying with this new bill.
FSA comments on response 18 (Q35)
This does not relate to a bill but to a guidance document only. The legislation is already in force.
The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
Response 19: Consumer (Q35)
Simpler information for wider public.
FSA comments on response 19 (Q35)
Comment noted.
Response 20: DNID (Q35)
Huge waste of a good food resource.
FSA comments on response 20 (Q35)
Comment noted.
Response 21: TA (Q35)
Lack of engagement is your Achilles heel. I have spoken about engagement for years - your culture needs to change. Take a look at the way the governments of Australia & New Zealand work with industry. You have an industry very willing to engage!
My view of this whole situation we have ended up in is that the FSA have seriously underestimated the impact this will have. The worst is yet to come, the only advice I can offer is to be very careful how you manage this process going forward.
FSA comments to response 21 (Q35)
Comment noted.
The FSA has offered and provided various opportunities for stakeholder engagement on this matter over the past several few years. This has included engagement directly with the MSM Technical Group, which was established in 2022, and further ad hoc engagement opportunities with other industry interested parties’ representatives. Stakeholder engagement has also been conducted with by Senior FSA officials, including the FSA Chief Executive and Chair. There will be further stakeholder engagement prior to the Guidance being finalised.
Response 22: TA (Q35)
The definition of MSM in the EU retained legislation 853/2004 is widely acknowledged as a terribly drafted piece of regulation. Now is the UK Government’s opportunity to move away from this awful ‘input-based’ definition (i.e. what goes into a machine), and towards an ‘outputbased’ definition (i.e. the physical and histological properties of the product itself). All other food hygiene legislation is risk-based focusing on the properties and safety of the final product. Additionally, any legislative clarification or change must consider the importance of a UK level playing field i.e. the impact of the Windsor Framework.
See accompanying briefing paper along with impact figures.
FSA comments on response 22 (Q35)
Comment noted.
The definition of MSM is not input-based. The input is one of three cumulative criteria that need to be considered together to determine whether MSM is produced – the other two being the process applied to the input, and characteristics of the output as a result of the process. This is also the case for other definitions e.g., for meat preparations – also defined by considering the input, process applied, and characteristics following the process.
The legislative clarification is in respect of legislation applicable in GB and also in NI so there remains a level playing field across the UK and there will be no impact on the Windsor Framework.
1. FBO
We do not do Mechanically Separated meat and therefore cant really do your survey as we don’t know anything about it.
FSA response to enquiry 1
Comment noted.
2. FBO
Just reading the Mechanically Separated Meat Guidance, and hope that you can advise me on the impacts on Feed?
Establishments manufacturing and/or handling products subject to requirements under Annex III to the Regulations must be approved for the manufacture and/or handling of products that they wish to place on the market unless a relevant exemption applies.
It doesn’t state that Feed / ABP are exempt , but as a food of animal origin can I assume that Pet food sites handling MSM do not need a separate approval?
When MSM is used as an ingredient MSM as defined in Annex I of the Regulations must be labelled in the ingredients list as ‘Mechanically Separated Meat’ with the name(s) of animal species from which it derives.
And therefor Labelling of MSM does not impact Pet food.
FSA response to enquiry 2
MSM is a term to refer to a category of food for human consumption; it is not applicable to pet food. Pet food production is governed by different legislation so the contents of the MSM Guidance are not relevant to pet food businesses.
Pet food businesses that require approval or registration for the manufacture of pet food would not need need separate approval relating to MSM.
On ingredients labelling, the requirement to declare MSM in a product list of ingredients as 'Mechanically Separated Meat' is laid out in the Food Information to Consumers (FIC) assimilated Regulation (EU) 1169/2011 and only applies to food intended for human consumption, not pet food products.
3. Producer of Food Processing Machines
I feel some injustice brewing … in so much that the SEPAmatic and the Baader are being categorised as “MSM” machines..
t’s not the machine but the product produced which should questioned.
Eg people die in cars but we don’t blame cars!
The SEPAmatic or the Baader are machines which can be used to safeguard the integrity of the meat by reducing (if not eliminating) unwanted gristle / sinew and without altering the functionality of the meat.
May I have a clarification?
FSA response to enquiry 3
The Guidance states the intended audience to whom the document might be of interest. This includes FBOs currently using, or intending to use, mechanical meat separation equipment in their production processes. Some examples of manufacturers of meat separation equipment commonly used in industry are provided to aid understanding of the Guidance: “for example, Baader, SEPAmatic, Marel and other food processing machines”. Reference is also made, in this context, to the need for the three cumulative criteria to be met in order for a product to be classified as MSM.
It is noted that it is not the machine, but the product produced which is MSM but the way in which some machines operate means that the product produced will be classified as MSM and FBOs will need to consider how the machine operates in determining if it produces MSM.
This response regarding the naming of machine manufacturers will be considered in the finalising of the Guidance.
4. East of England Trading Standards Association
This response represents a consensus view of the East of England Trading Standards Association (EETSA) members to the consultation paper on The Mechanically separated Meat (MSM) Guidance Consultation. It does not necessarily reflect the opinions of the employing authorities.
We offer the following comments:
We note that feedback is sought specifically on:
- The effectiveness of the MSM Guidance document in providing support in light of the court judgments.
- The impacts of FBOs adapting their activities and operations in line with the court judgments.
- Whether there are wider issues around MSM that the FSA, or indeed wider government, should be seeking to address and why.
- We believe that the proposed revised guidance is effective. It incorporates the court decision. It is easy to understand and is quite clear.
- We do not have experience of relevant FBOs so cannot comment on how this will impact on their activities and operations.
- The wider issue is about clear labelling and consumer education. The most important thing is that consumers are not misled about the quality of the meat that they are buying. Certainly, no-one expects a frankfurter to be anything less than highly processed.
We think it is useful for consumers to be aware what is highly processed food, so they can make an informed judgement.
(Response submitted by EETSA Specialist)
If you have any queries or would like any further information regarding the EETSA response to this consultation, please contact
FSA response to enquiry 4
Comments noted. These points, including those on labelling, will be considered when finalising the Guidance.
Please note that consumer awareness of processed food is a distinct issue from those that the Guidance is intended to address on MSM. Please see the following link for the FSA position on ultra-processed foods: https://www.food.gov.uk/safety-hygiene/ultra-processed-foods#what-is-an-ultra-processed-food.
5. Zwanenberg Food Group UK
Ref Draft Guidance notes on Mechanically Separated Meat
We are writing as a Food Business Operator (FBO) in reference to the [draft] Guidance notes on Mechanically Separated Meat (MSM). Our Taste Original business produces a range of quality, ready-to-eat meat snack products that are proven to be highly popular with consumers nationwide. We have worked hard to develop products that offer great tasting snacks whilst representing value for money through both Taste Original and private label channels. Our established recipes have been developed from accredited and respected supply-chains to ensure consistently high standards with complete traceability. All of this has led to a growing business that, with more than 600 colleagues, is one of the largest employers in the Corby area. At Taste Original we use a significant quantity of 3mm wishbone chicken in our product enabling us to create that all important value proposition for our customers whilst having the additional benefits of facilitating carcass utilisation in partnership with our suppliers.
It is our considered opinion that the potential negative impact of the draft Guidance notes on Mechanically Separated Meat on the wider food industry in general and our business specifically will be considerable.
[The respondent provided details of business impacts here. The areas covered included carbon footprint, manufacturing costs, sales, business operations and structure, and economic impacts. The details have been omitted from this publication by the FSA. See FSA comments column for details.]
FSA response to enquiry 5
Comments noted.
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
On the omission of impacts details: as outlined in the consultation document, information was sought regarding the impacts of FBOs adapting activities and processes in line with the Supreme Court Judgment. In line with the approach to responses to survey questions 15-28, that information is omitted from this publication as it is of a commercially sensitive nature.
6. Princes Group
In reference to the Consultation on mechanically separated meat (MSM) guidance. The Food Standards Agency (FSA) is seeking feedback relating to new MSM guidance that is intended to provide support for Food Business Operators (FBO) following court judgments that clarify how the definition of mechanically separated meat (MSM) should be interpreted and applied. I would like to highlight issues, concerns, and feedback on this consultation, please find my points below.
- The Guidance has made specific reference to Baader, Sepamatic machines used in mechanical separation, the industry / Princes will seek to request clarification if there is other equipment implicated and why were these mentioned specifically.
- In addition, the 1st criteria of “meat on bone” can the FSA clarify as it needs to be confirmed to determine if there is a minimum quantity of bone allowed.
- Other concerns include that the modification of meat would become a point of view issue and there will be inconsistency in interpretation, so what type of measures will be taken to ensure consistency in approach and application of the guidance when it comes to enforcement.
- There is also no mention of an appeals process, potentially there is one or certainly there needs to be one.
- The issue ref that the MSM meat cannot be used as part of the Protein calculation for nutritional decs, which is contrary to some EU jurisdictions where the MSM is labelled, and the meat can be used as part of the Protein for Nutritional calculation.
- The issue in point 5 requires that all current recipes would have to be reformulated so that current nutrition claims for Protein remain unchanged or totally new formulations are created which directly impacts on label changes resulting in increased costs for new artwork and label generation.
- An impact assessment by the FSA should be undertaken to consider all associated costs of making this change for FBO’s and the industry, including changes to labels, new artwork origination costs and potential restriction of choice and availability issue, cost of living concerns and the impact of imported products into UK - which could be incorrectly labelled.
- There is reference to “Relevant Exemptions” the industry / Princes will request clarification as to what these are.
- The Guidance covers England, Wales, and NI – It does not cover Scotland (so what is happening in Scotland). There is a potential for further inconsistencies as the rules in Scotland will be different to the rest of UK meaning any enforcement as a result is difficult to justify. Technically, if product is produced in Scotland and not declared as MSM can I trade this product in England, Wales, and NI.
- Where material is reclassified to MSM – there will be material in the market already and thus there will need to be a period of Run out and Run in. As any enforcement has the potential to cause uncertainty and recalls situations need to be avoided. E.g. Frozen stock in the supply chain and in the trade is all potentially affected and this stock should be allowed to be used during this implementation period.
- FSA to provide clarification on FBO’s who are currently unaware that they are producing MSM, hence will require re-classification and to re-apply for new licenses. This changeover will require a defined period of time.
- Following this public consultation period the FSA to issue the outcome from it and explain to the public fully what the main areas of concerns and issues were.
I look forward to receiving a response from the FSA and would welcome the opportunity to discuss these points in detail. However, in the meantime if any further information is required then please do not hesitate to contact me.
FSA response to enquiry 6
- The intention is not to implicate equipment. The Guidance states the intended audience to whom the document might be of interest. This includes FBOs currently using, or intending to use, mechanical meat separation equipment in their production processes. Some examples of manufacturers of meat separation equipment commonly used in industry are provided to aid understanding of the Guidance: “for example, Baader, SEPAmatic, Marel and other food processing machines”. However, this response regarding the naming of some machine manufacturers will be considered in the finalising of the Guidance
- This point on providing clarity will be considered in the finalising of the Guidance.
- Ensuring consistency of enforcement is an important matter across competent authorities when implementing any new Guidance.
- Neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency.
- The Guidance pertains only to UK legislative requirements.
- There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
- See comment 6.
- "Relevant exemptions" is in reference to the approvals process. Businesses that intend to undertake certain activities (e.g., produce MSM) need to be approved to do so unless relevant exemptions apply. The approvals process is unchanged. Links are provided in the Guidance to details of the approvals process.
- As the Guidance covers England, Wales and NI... the Guidance is not therefore the place to state what is happening in Scotland. Food Standards Scotland (FSS) wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS is not intending to consult on this as there is no change in the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
- See comment 6.
- The principal point of the Guidance is to help businesses to understand when/how MSM is produced following the Supreme Court Judgment. This point will be considered in finalising the Guidance.
- Noted.
7. BMPA
As I can’t progress any further with your online survey, I will submit the other points I would like to make formally to you:
- There is NO appeals process if the FBO believes the person from the FSA or Local Authorities reclassification is wrong.
- What training are government officials going to receive?
- After the consultation closes what are the time frames for publishing the results and what happens next?
- Are you going to carry out an impact assessment of the costs and food waste?
- Will there be any issues with products already on the market?
- Is there going to be a cutoff date for production?
- Long life products need to be considered as they may have MSM in them if you reclassify the material a manufacturer is using
- What is the period FBO’s will be given for packaging changes and reformulation?
- Reclassification will lead to food inflation as this material will need to be replaced or the meat content will need to be lowered.
- What are you going to do about wrongly labelled material from the EU?
- Do you have an estimate of how many SKU’s might be affected?
- We are assuming that an FBO producing material that meets all three criteria will be deemed to be producing MSM – is that correct?
- We are assuming that there will be no separate deal made on Poultry Wishbone Meat as the guidance covers poultry in its entirety?
- If material is reclassified on the spot what happens to material already in the supply chain and products on retail shelves?
- What is your estimate for the total cost to industry?
- How many FBO’s do you think are producing material that will be reclassified as MSM?
- How are you going to manage this whole process going forward?
- Industry bodies request a round table discuss on the subject matter
Having spoken to (name omitted) we would encourage you to at least engage with us both as there is mush to discuss going forward.
Kind regards,
FSA response to enquiry 7
Points addressed where appropriate:
- Neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency.
- Ensuring consistency of enforcement is an important matter across competent authorities when implementing any new guidance. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement and identification of training requirements.
- All consultation responses will be considered in the finalising of the Guidance. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
- Imported products must meet UK requirements and there are import control measures in place.
- If the three cumulative criteria are met, MSM is produced.
- As outlined in the consultation document, there will be further stakeholder engagement prior to the Guidance being finalised and published.
- There is no separate arrangement for poultry wishbone meat. Whether it constitutes MSM will depend on whether it meets the three criteria.
Other comments are noted and will be considered in the finalising of the Guidance.
8. 2 Sisters Food Group
We are writing to set out our response to the consultation document launched on 28th February 2024, Consultation on Mechanically Separated Meat (MSM).
Specifically, we will address the value of wishbone meat from poultry which is a valuable source of breast meat and will move from a long-standing classification, since 2004, of a meat prep to MSM under the consultation.
The wishbone meat is used to produce a 3mm chicken mince which is used extensively in the manufacture of breaded chicken products.
The meat is generated at the cutting plant by deboning the carcass either by hand or automation leaving a fully intact wishbone. At the processing stage the separation of bones, sinew and cartilage from meat is done using a low pressure separator with a filter of a diameter of 3mm with no significant loss of structure or muscle fibre.
The resultant 3mm mince is used extensively in the manufacture of breaded chicken products which are sold through retail and food service markets. They provide an affordable healthy protein from wishbone breast meat used in the manufacture of reformed added value (breaded) poultry products e.g. chicken goujons. These products may be sold as chilled or frozen formats.
The breaded chicken market offers products that are convenient, affordable and a good source of protein. The value of this market at retail is £1,14bn,
Fresh breaded £516m,
Frozen breaded £630m.
Growth is also being seen in the food service market; it is predicted that the UK chicken restaurant market will overtake its 2022 value of £2.3 billion to reach £2.7 billion in 2027.
The basis of our objection to this guidance is as follows
- High value, good quality breast meat attached to the wishbone and used as wishbone meat will no longer be able to be classified as meat and contribute to the meat content of the product. This will completely devalue a perfectly good source of protein. Loss of value £9.5m / annum.
- If an alternative market cannot be found for the product, which is highly probable, as there is no market for wishbone meat, significant food waste will be generated. Volume lost/waste generated, 5600 tonnes/year.
- The replacement raw material to retain breast meat in the product will result in significant costs to the consumer at a time when we are in a cost of living crisis and the breaded chicken market is a market in growth. This has additional raw material oncost to our supply chain of £34.9m per annum
- There may be insufficient material available to make up the deficit in meat if wishbone meat is removed from the supply chain. This together with the significant price increase will most certainly lead to an increase in foreign imports e.g. Thai and Brazilian chicken, putting British farmers and British protein at a disadvantage. This goes against all the Government narrative to support British farming and protect food security.
- There is also an impact on operational and production efficiencies where any replacement of the 3mm mince used in reformed products has to be switched to other whole muscle products. At a production level this translates to,
- A reduction in throughout of 10% less per hour
- An increase in labour of 30% more per production line
- An increase in operational costs of 37% per tonne
- A reduction in packing efficiency of 15-20%
In summary if this change occurs then there will be
- Significant increase in cost to consumer during a cost of living crisis.
- Limiting consumer choice of a nutritious affordable and low impact protein.
- Increasing the environmental impact through moving a high quality food to generating food waste.
- Allows cheaper imports to come in putting British farming at a competitive disadvantage.
- Increased cost of production.
- Impact on UK food security.
We trust these material impacts will be taken into consideration as part of the consultation and that Government will work with Industry to find a satisfactory solution, protect food security and support the consumer.
FSA response to enquiry 8
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
The FSA is grateful for your detailed comments, and they will be considered when finalising the Guidance.
MSM produced in compliance with hygiene regulations is a safe product that can be used as an ingredient in a wide variety of foods. MSM is also required to be labelled as such on products to allow consumers to make an informed choice.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
9. International Meat Trade Association
Good Afternoon,
I am responding to the FSA consultation on MSM on behalf of IMTA.
The International Meat Trade Association (IMTA) is a UK trade association, representing predominantly UK companies importing and exporting meat. Our goal is the facilitation of the trade in meat ensuring UK consumer choice, food security and carcass balance through import and export. IMTA represents 65 trading members and 20 associate members including freight forwarders, shipping lines, and levy boards. Two-way trade is essential in the meat sector, and we need to ensure that the UK regulatory landscape facilitates trade in both directions for the cuts we import and those that we export. For background, please see IMTA two-way trade infographics.
Response to MSM consultation:
Owing to the many consultations and changes (including the border target operating model) that our industry is faced with, IMTA has not been able to provide a bespoke response to this consultation. However, we understand that The British Poultry Council has detailed at length the reasons why FSA should not change the definition for wishbone meat to MSM. There is no evidential basis for doing so and to do so would be to the detriment of the UK consumer as well as the industry, at a time when consumers are being squeezed and extremely price conscious (as monitored by FSA in its own consumer monitoring surveys). We would call for FSA to assess the clear case that BPC has made against a change to the definition for wishbone meat.
FSA response to enquiry 9
No definitions have been changed or are being changed. The Supreme Court determined how the definition of MSM is to be read and applied. The FSA position follows the Supreme Court Judgment that gave clarity on interpreting the legislation and the Guidance is written on that basis, to aid understanding of the MSM definition in light of the Judgment.
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
Comments noted. These points will be considered when finalising the Guidance. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
10. BPC
The British Poultry Council (BPC) is the trade association for the poultry meat supply chain in the United Kingdom. It represents companies that produce meat from chickens, turkeys, ducks, geese, and laying hens, as well as those involved in poultry genetics, breeding, hatching, farming, and processing.
This response is in three parts:
- Part One: General Stance and Impact Summary
- Part Two: Responses to online survey questions
- Part Three: Call for a Statutory Instrument
Part One: General Stance and Impact Summary
The BPC utterly rejects the grounds for the introduction of the Guidance document presented by the FSA. BPC members are only concerned with wishbone meat in this context and believe that the FSA has over-reached in suggesting wishbone meat (an accepted meat preparation since the introduction of EU 853/2004 and subsequent UK retained EU law) is now Mechanically Separated Meat (MSM). Wishbone meat was specifically named as outside of the moratorium upon which the subsequent Court judgements were made, and therefore should not be considered as part of this Guidance.
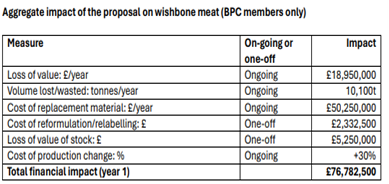
Other impacts are not quantifiable at this time but should be taken into account as part of a Government impact assessment. These include:
- Sustainability impact: disposal of wishbone meat as waste and additional production of breast meat to replace wishbone meat
- Enforcement: the FSA does not have the resources there is no appeals process
- Imports: the same material entering the UK must be assessed to the same standard, and the FSA should commit resources to inspect/check the flow of goods
Absence of an impact assessment
In the absence of an impact assessment, it is essential that the FSA understands the damage that it will be responsible for should this policy be pursued. If this consultation does not meet a threshold of majority of supportive responses, then the Guidance it is based on should be immediately withdrawn and a route forward created in partnership with industry and other stakeholders.
Part Two: Responses to online survey questions [the responses have been incorporated]
The online survey is unfairly biased towards the outcome that the FSA has pre-determined for this issue. It limits and guides questions shown depending on previous responses, it does not touch on the major impacts of the proposal, and it does not include a Government impact assessment that is legally required for public consultations.
Part Three: Call for a Statutory Instrument
Food Standards Agency’s stance will increase the cost of production of breaded products by 30%British Poultry Council’s response to the Agency’s intention to rebrand wishbone meat as MSM
22nd May 2024
We believe there is an urgent need to introduce a Statutory Instrument (SI) to prevent over £75 million (1) in added food costs, increased environmental impact, and the waste of good quality UK-produced food.The Food Standards Agency (FSA) consultation 2 on the definition of Mechanically Separated Meat (MSM) intends to change the long-standing definition of wishbone meat from a valuable meat preparation into valueless MSM (3). Wishbone meat – that part we all know from a roast chicken - is good quality meat and, crucially, counts towards the meat content of food products whereas MSM does not. Many stakeholders across various supply chains will be critically impacted by this decision: meat sectors, farmers, retailers, traders, chilled food producers, and consumers. The burden and cost of this change will threaten UK food security and business viability.
The FSA’s action will:
- Cost consumers over £75 million.
- Limit consumer choice of a nutritious, affordable, and low-impact protein.
- Add to food waste with disposal of up to 10,000 tonnes of good quality meat.
- Increase the environmental impact of food production through food waste and the need to source additional alternatives.
- Increase the regulatory burden (and cost thereof) on businesses, with no appeals process.
- Place an additional enforcement burden on the FSA and trade colleagues to inspect and assess imports without giving them any means of carrying out that enforcement.
Breaded products have become a fixture on the nation’s dinner tables. These convenient products are affordable and accessible in part because they use minced breast meat taken from around the wishbone. Since 2004 under EU law this country has considered wishbone meat as a meat preparation (4), and under retained EU food hygiene law the UK has continued to define it as such.
If this proposed change of definition occurs, the FSA’s interpretation will be responsible for costing consumers over £75 million in a cost-of-living crisis, with an estimated 10,000 tonnes (5) of good quality food going to waste every year. Off the back of record food inflation and drastic increases to the cost of production facing domestic producers, this change will only make it more difficult for people to access nutritious, low-impact and affordable food. The UK poultry meat industry is seriously concerned about the detrimental effect this will have on UK food security.
The Rt Hon Mark Spencer MP, Minister for Food, Farming and Fisheries, agrees. He has publicly stated that wishbone meat ‘is meat’ and has given verbal assurance that Defra will help find a solution. This is encouraging as poultry is half the meat the UK eats and plays a key role in keeping people fed, so we need a plan in place as an output from this consultation.
Wishbone meat is an important and valuable part of people’s diets; it is commonly used in breaded products, which are some of the most popular in the country. There is no market for MSM in the UK, and since retailers will never specify MSM in a product, the overall cost to the industry is estimated at around £75 million (1)
The FSA’s actions based on a 2019 Supreme Court ruling (6) are about a different product and process. The Agency is taking an excessively broad interpretation of this judgement, particularly for a product that was specifically named as being outside of the 2011 moratorium. As a result it is overreaching its mission to ‘protect the interests of consumers in relation to food’. It is difficult to see how the FSA is achieving this when in one decision, during a cost-of-living crisis, it would be knowingly imposing such a burden on businesses and consumers, despite claims that food security and food affordability are factors that “cut across their entire mission” (7).
We need Government to work with industry to ensure producers and consumers are not unfairly burdened.
Notes
1. The ‘over £75 million’ figure is an estimate based on:
- £19 million of direct loss of value as wishbone meat will no longer be specified
- £50 million of cost of replacement material, which will be whole muscle sourced from UK and EU
- £7.5 million in year one costs of reformulation, relabelling, and loss of existing stock
2. https://www.food.gov.uk/news-alerts/consultations/consultation-on-mecha…
3. https://www.legislation.gov.uk/eur/2004/853/annex/I/division/1/division…
4. https://www.legislation.gov.uk/eur/2004/853/annex/I/division/1/division…
5. The 8,000 tonnes figure is an aggregate of production across BPC members
6. R (on the application of Newby Foods Ltd) (Appellant) v Food Standards Agency (Respondent) (supremecourt.uk)
7. FSA Quote “cut across their entire mission” The FSA strategy for 2022 to 2027 | Food Standards Agency
Annex: What a Statutory Instrument will achieve
We are asking Defra to create a Statutory Instrument (SI) to establish an exception for wishbone meat from the FSA’s proposed MSM definition, allowing it to exist as a meat preparation as part of the UK regulatory framework. There is precedent for using an SI in this manner. In doing so itwill achieve specific objectives without impinging on, or needing to change, other legislation.
To retain the value of wishbone meat with no interruption to the value chain
Reclassifying this material as MSM would eliminate its value, require substitutes, increase cost to consumers, increase food waste, and increase environmental impact (evidence for which will be submitted as part of the FSA's consultation). Retaining its definition of a meat preparation will avoid all this impact.
To allow FSA to satisfy the ‘clarification’ on the definition of MSM given by the Supreme Court
By creating a discrete exception using an SI, it allows the FSA to fully comply with both UK legislation and the Court's interpretation, i.e. Parliament has precedence.
To allow Defra to satisfy the requirement to inform and not mislead consumers
Wishbone meat has been considered a meat preparation since 2004 and, in that time, noconsumers have been misled or misinformed. In keeping the definition through an SI, we wouldbe maintaining that consistency and not creating any opportunities to mislead or misinform.
To avoid disruption to trade with the EU
The use of an SI would mean that when this material is traded (inwards or outwards) it wouldhave to comply with the legislation of the receiving country. Material coming into the UK wouldbe identified as a meat preparation and would need safeguards/checks. Material going into theEU would be identified according to EU legislation - as it currently has to be.
To satisfy the requirements of the Northern Ireland framework
An SI would need to be applicable to all parts of the UK. If so, material produced and movedaround the UK internal market would fall under the SI. Material moving GB to NI to EU, or NI toEU would have to comply with EU legislation - as it currently does.
To reduce the burden of regulation and enforcement
An SI can be used to place limits on the material of aspects that are measurable, and the requirement for producers (and users of imported material) to monitor and report. This would give a framework, traceability, and clear boundaries for enforcement (which does not exist for MSM) that would alleviate the burden on both inspection of UK production, and on checks of imports.
To sustain business viability
The British poultry meat industry contributes £6.8bn GVA to the UK economy and generates £1.2bn in tax revenue to the exchequer, without any Government subsidies. In addition to the complete loss of value of wishbone meat, disposal costs and sourcing a replacement, this change would come at a time when the industry is already facing soaring cost of production expenses. The breaded meat market is one of the only product areas that has seen significant growth over the last few years. With no means to recoup these losses, the industry will be forced to further scale back production.
In addition, if the UK were to stop producing wishbone meat it would leave the domestic market at risk from imports, particularly in the absence of border checks. This would only serve to undermine domestic food producers further. The FSA has no means of analysing, and therefore no way to enforce, what is and is not MSM. If our authorities are unable to police imports, then the FSA will be knowingly putting our domestic producers at a significant disadvantage to their EU competitors.
To aid food accessibility
UK retailers and consumers do not want MSM. Wishbone meat has been defined as a meat preparation since 2004, and losing this valuable source of protein and stripping a product of its meat content will only exacerbate the cost-of-living crisis we find ourselves in. We know people are already struggling to put food on the table as demonstrated by the 37% increase in the use of food banks for emergency food parcels from April 2022 and March 2023 as reported by the Trussell Trust. This change to wishbone meat will make it even more difficult for families to feed themselves, and for British businesses to keep food moving.
To avoid food waste
Given the absence of a domestic market for MSM in the UK and the refusal by important trading markets to accept this product, around 8,000 tonnes of high-quality food suitable for human consumption will go to waste every year.
To keep food affordable
If followed through, the FSA’s decision will ultimately mean an increase in food prices. The estimate from the poultry meat supply chain is £80 million added to consumers’ food bills.
Breaded products are a staple for consumers and have seen a 25% increase over the last year alone, so this change by the FSA would have an unwanted and unwarranted impact on the price we all pay for food.
FSA response to enquiry 10
The FSA is grateful for your detailed comments, and they will be considered when finalising the Guidance.
On Part One
The Supreme Court determined how the definition of MSM is to be read and applied. The FSA position follows the Supreme Court Judgment that gave clarity on interpreting the legislation and the Guidance is written on that basis, to aid understanding of the MSM definition in light of the Judgment.
The FSA does not agree that the Supreme Court Judgment was limited to certain types of meat in the same way that the definition of MSM in the legislation is not limited to certain types of meat.
The Judgment did not draw a distinction between MSM produced from poultry and MSM produced from other animals.
No cut or type of meat (e.g., poultry wishbone meat) inherently produces MSM. MSM is produced only where the three cumulative criteria, as defined by the Supreme Court Judgment, are met, including the process chosen to separate the meat from the bone.
Comments on impacts are welcome and noted.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Neither the Supreme Court Judgment nor the Guidance, once finalised and published, changes the existing means of challenging Competent Authority decisions about meat premises. Details regarding FSA-approved establishments are available on the FSA website via the following link: Appeal a decision about a meat premises | Food Standards Agency.
On Part Two – responses to the survey questions have been added to those submitted via the online survey.
On Part Three
The FSA continues to work closely with FSA NI and FSA Wales, DEFRA and other areas of Government to ensure that UK legislation relating to this matter and other food matters is appropriate.
The FSA’s fundamental mission is food you can trust. By this, we mean that people can trust that the food they buy and eat is safe and what it says it is, and food is healthier and more sustainable.
As stated in the consultation document, there will be further stakeholder engagement prior to the Guidance being finalised.
11. Provision Trade Federation
Introduction The Provision Trade Federation is a long-established UK-wide trade association representing companies of all sizes involved in supplying dairy products (including milk powders, cheese, butter, yogurt and other dairy desserts), bacon, pigmeat and fish. Collectively these categories account for about £24 billion a year, or roughly 20% of total UK household expenditure on food. Our members include importers and exporters, as well as processors and manufacturers. The members of our Bacon and Pigmeat Section are here and the members of our Bacon and Pigmeat Section Committee are here. Within our Bacon and Pigmeat Section we have members who produce MSM and those who source MSM from established suppliers. 2 PTF Overall Position Although the consultation is on the definition of MSM, an equally important question is how MSM is treated for labelling purposes and this needs further discussion. Given the negative environmental and economic impacts of the strict definition of MSM, combined with the current labelling requirements, a full review of the latter is essential and long overdue. There is a strong sense that the current approach to the labelling of MSM is based on a historical perception which is no longer valid. In the 1990’s there was a backlash against the product which was mechanically removed and ‘flowed like a puree’ from the bone as a ‘pink slime’. This product was considered sufficiently different from meat that it should be treated differently. However, over the last 40 years there have been many changes in the way the meat industry carries out butchery operations, and modern machinery using low pressure can remove a quality material which is not dissimilar to mince. This may fall within the definition of MSM under the court judgements and as such would be downgraded, probably going to waste and certainly reducing the return to the business. However, it makes no sense, economically or environmentally, to insist that this product is not ‘meat’ when the average consumer would almost certainly consider it to be ‘meat’. A full review of the justification for the current MSM labelling requirements is long overdue and should include research to properly understand what the consumer understands to be MSM, particularly with respect to the low pressure product, and how it should be treated for labelling purposes. PTF would be happy to support the FSA and DEFRA in taking such a project forward. PTF, 17 Clerkenwell Green, London EC1R 0DP 3 Additional Points on the Guidance 3.1 We are aware of concerns that the guidance goes further than the court judgements and adopts a strict approach to the definition of MSM which will lead to increased costs to industry associated with the production of MSM and the potential for reduced sales as a consequence of products being labelled as containing MSM and the resulting reduced meat content. Where there is any difference of opinion, it would be helpful if there were clear links from the guidance to the court judgements, with references and extracts to add clarity. 3.2 Given that the definition has been blurred for so long, there is confusion over whether products are MSM. Even with the guidance, businesses may genuinely believe that they are not producing MSM, particularly given the strict interpretation that has been adopted. It will take time for the knowledge and understanding to filter through and any adjustments made. This needs to be reflected in the enforcement approach. 3.3 If not already planned, the FSA will need to invest in a campaign to communicate the interpretation in the guidance both to industry in the UK and those countries exporting product to the UK which would fall within the definition of MSM. More work is needed to identify product that is imported that falls within the definition of MSM.
FSA response to enquiry 11
The FSA is grateful for your detailed comments, including those on the related matter of MSM labelling as laid out in the Food Information to Consumers (FIC) assimilated Regulation (EU) 1169/2011/ Regulation (EU) 1169/2011. They will be considered when finalising the Guidance.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
On 3.1, this will be considered in the development of a finalised Guidance document.
On 3.2, this will indeed be considered in the development of the approach to enforcement
On 3.3, this will also be considered in the development of the approach to enforcement. There will also be further stakeholder engagement prior to the Guidance being finalised.
12. BRC
Dear Sir or Madam, Thank you for giving us the opportunity to respond to the consultation on the draft guidance on mechanically separated meat (MSM). The BRC is the lead trade association for UK retail. Our purpose is to make a positive difference to the retail industry and the customers it serves, today and in the future. Retail is the ‘everywhere economy’, a vital part of the socio-economic fabric of the UK. The industry makes up 5% of the UK GDP and is the largest private sector employer, providing 3 million direct jobs and 2.7 million more in the supply chain. Retail has a presence in every village, town and city across the country. Over 200 major retailers are members of the BRC, with thousands of smaller, independents represented by BRC’s trade association members. Together, these businesses operate across all retail channels and categories and deliver over £350 billion of retail sales per year. We build the reputation of the retail industry, work with our members to drive change, develop exceptional retail leaders, and use our expertise to influence government policy so retail businesses thrive and consumers benefit. Our work helps retailers trade legally, safely, ethically, profitably and sustainably. On food, our membership comprises over 5,000 businesses, accounts for £180 bn of grocery sales and employs over 1.5 million people in food outlets and distribution. Although we appreciate that this consultation is not seeking views on the definition of MSM, the suppliers to our members are very concerned about the implication of the definition and the Courts’ judgements reflected in the guidance document. For this reason we have actively discussed it with our members. MSM currently used in chicken nuggets and hot dogs is not too different from minced chicken meat, and when cooked it is unlikely one could tell the difference. However, this is also true for many products, and they are still described according to their true nature. We came to the conclusion that meat mechanically separated should be described as MSM and not meat, since if a description of meat was allowed, this could set a precedence for other less quality ingredients to be described in a potentially misleading way, and be more widely used. The Form Rooms, 22 Tower Street, London, WC2H 9NS +44 (0)20 7854 8900 info@brc.org.uk brc.org.uk British Retail Consortium - a company limited by guarantee Registered in England and Wales No. 405720 On the three points on which the FSA is seeking comments: The effectiveness of the MSM Guidance document in providing support in light of the court judgments – We believe the guidance appropriately covers the court judgements. The impacts of FBOs adapting their activities and operations in line with the court judgments – The majority of our members have policies against the use of mechanically separated meat. The change in the status of some ingredients from meat to MSM based on the court judgement will mean that retailers will be looking into the composition of products, and this may lead to some members to have to reformulate or change their labels, if their policies allow the use of MSM. Both these routes will take some time and will result in additional costs which may need to be passed onto consumers. A realistic timescale to allow these changes must be agreed. Whether there are wider issues around MSM that the FSA, or indeed wider government, should be seeking to address and why – we are not aware of any other issues. However, we have noticed that the scope of the guidance document is England, Wales and Northern Ireland. It is important for our members to understand that Scotland has the same understanding as the other countries. Ideally we would like to see this guidance to be published jointly with Food Standards Scotland (FSS). We hope our response and comments are clear. Please do not hesitate to contact us if you want to follow up on any of our points.
FSA response to enquiry 12
The FSA is grateful for your detailed comments, including those on the effectiveness of the Guidance in reflecting the Supreme Court Judgment and on business impacts They will be considered when finalising the Guidance.
There may be increased costs depending on the commercial decisions that businesses take in moving from a position of noncompliance with legislative requirements to one of compliance, in line with the Supreme Court Judgment.
An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
Regarding the point on Scotland, FSS wrote to Scottish businesses in November 2022 to advise that they must comply with the law. FSS is not intending to consult on this as there is no change to the law. FSS advises that anyone with queries regarding Scottish businesses should contact FSS for confirmation of the position.
13. Campaigner on E.coli O157
Dear Sirs,
The final Court Rulings on what the definition of Mechanically Separated Meat (MSM) in the High Court Judgment of 5 July 2022 was a victory in terms of protecting the consumer’s interest.
As stated in your consultation guidance document under point 14 “it must be “labelled in the ingredients list as ‘Mechanically Separated Meat’ with the name(s) of animal species from which it derives”.
This means that Mechanically Separated Meat is an ingredient and not Meat. Therefore, the consumer benefits as prior to the court ruling it may have been possible to add Mechanically type Separated Meat to the contents of meat in order to increase its weight and monetary value.
The Labelling Regulations No 1169/2011 Annex VII makes it clear that products classified as MSM may not be labelled as ‘meat’. In declaring the meat content of food, MSM must not be considered meat content.
Article 7.1 of the Regulation “requires that information may not be provided which is misleading as to the characteristics of food, particularly its nature, composition, method of manufacture or production.”
“Regulations state that packages intended for the final consumer containing meat preparations that contain MSM must bear a notice indicating that such products should be cooked before consumption”.
“In terms of MSM labelling during production and before manufacturing into the final products, Article 18 of assimilated Regulation (EC) No 178/2002 in GB / Regulation (EC) No 178/2002 in NI states that traceability of products must be established at all stages of production, processing and distribution. Article 3 of assimilated Regulation (EC) No 931/2011 in GB / Regulation (EC) No 931/2011 in NI also provides traceability requirements about information to be made available to suppliers and competent authorities”.
When the final guidance is produced, we would wish to see all the above points stated within such documentation and robust enforcement of these legal requirements thereafter.
It is a pity that in 2012 the Food Standards Agency (FSA) was allowing a category of meat to be marketed in the UK as desinewed meat (DSM). As DSM is not a category recognised in law, it appears that the Agency may have taken the side of the Meat Industry, rather than put the interests of the consumer as the overriding priority.
FSA response to enquiry 13
The FSA is grateful for your detailed comments, including those on legislation that is outside of the hygiene regulations yet relevant to MSM e.g., labelling and traceability requirements. They will be considered when finalising the Guidance.
14. Moy Park/Pilgrim's
Further to completing the online survey please find attached response and supplementary documents.
[Email and attachments comprised tables to show impacts (including economic impacts – omitted from this publication in line with the approach to commercially sensitive information submitted in response to the request for evidence of business impacts), responses to the consultation questions (these have been incorporated into the survey responses), and a presentation paper regarding consumer views on MSM].
FSA response on enquiry 14
Email and supplementary information noted.
These points will be considered when finalising the Guidance. An assessment of the impacts of businesses adapting activities and processes in line with the Supreme Court Judgment will be undertaken and published later in the process. The assessment will support the approach to enforcement.
List of respondents
This list does not include responses from individuals
- 2 Sisters Food Group
- BMPA (British Meat Processors Association)
- BPC (British Poultry Council)
- BRC (British Retail Consortium)
- BRENSTECH Ltd
- Campaigner on E. coli O157
- Consultant
- Daphne's Welsh Lamb
- East of England Trading Standards Association Limited
- IMTA (International Meat Trade Association)
- Jeremy Townsend Meats Ltd
- Moy Park/ Pilgrim’s
- Princes Group
- PTF (Provision Trade Federation)
- Responding on behalf of myself as a Good Standards Officer
- SEPAmatic
- UK Pet Food
- Zwanenberg Food Group UK
- Responses from members of the public