Manual for Official Controls
Manual for Official Controls - multi-page guide.
Sections
1. Background and functions
In this section
1.1 Food Standards Agency
1.1.1 Food Standards Agency remit
The Food Standards Agency (FSA) is an independent Government body, established in 2000 to protect public health and consumer interests in relation to food.
The FSA is the national authority responsible for food safety and food hygiene across England, Wales and Northern Ireland.
FSA’s remit includes:
- negotiations on behalf of England, Wales and Northern Ireland by legal experts and policy officials to ensure that assimilated law reflects the interests of consumers
- providing Food Business Operators (FBOs) responsible for placing unsafe food on the market with appropriate advice to withdraw or recall it and working with other enforcement authorities to take appropriate action
- ensuring good food hygiene, through various initiatives delivered in partnership with Local Authorities (LAs)
- ensuring minimum standards of meat hygiene are maintained within approved slaughterhouses and meat establishments throughout Great Britain. In Northern Ireland, this role is carried out by the Department of Agriculture Environment and Rural Affairs (DAERA)
This guidance manual, the Manual for Official Controls (MOC), contains details of the tasks, responsibilities and duties FSA staff and veterinary contractors undertake in approved meat establishments.
This version of the Manual for Official Controls has been updated to take account of new EU Official Control requirements which came into effect on the 14 December 2019. It is important that FSA Authorised Officers, FSA and LA staff implement the legislation as set out in this manual.
Guidance on some aspects of the new Official Control package has not yet been introduced as these require more consideration. The FSA is working on this and as guidance is finalised it will be communicated to staff and included within the MOC. In the meantime, staff must continue to undertake official controls as outlined within the MOC.
1.2 Food Standards Agency operations (meat hygiene)
1.2.1 Principal functions
The principal functions of FSA staff working in approved meat establishments are listed in the table below together with the Government department that holds the policy lead.
Principle function table
| Function | Policy lead |
|---|---|
| Provision of meat inspection and health marking | FSA |
| Enforcement of food safety and hygiene legislation in approved slaughterhouses, cutting plants and game handling establishments, together with co-located plants, processing minced meat, meat preparations, mechanically separated meat and meat products. | FSA |
| Enforcement of controls over Specified Risk Material (SRM) and other animal by-products (ABP) | FSA, (TSE) Defra and Welsh Government |
| Enforcement of welfareatslaughter regulations | Defra and Welsh Government |
| Collection and despatch of samples for statutory veterinary medicines residue testing and testing of suspect cases | Defra, Veterinary Medicines Directorate (VMD) |
| Collection and despatch of sheep and goat brain stem samples for the testing of Transmissible Spongiform Encephalopathy (TSE) | Defra and Welsh Government |
| Supervision of Bovine Spongiform Encephalopathy (BSE) testing | Defra and Welsh Government |
| Collection and despatch of samples for examination and testing for some notifiable diseases | Defra, Animal and Plant Health Agency (APHA) |
|
Provision of export certification when required by the importing authority or by assimilated law |
Defra / APHA |
| Inspection of imported meat in approved premises | FSA |
| Provision of services to British Cattle Movement Service (BCMS) for the Cattle Tracing System | Defra (Rural Payments Agency) |
| Provision of other services on a repayment basis to other Service Level Agreement customers | Other customers |
1.3 Contacts
FSA teams:
Corporate Support Unit York Transactions Team (Tel: 01904 232177)
Approvals and Registrations Team (email: approvals@food.gov.uk Tel: 01904 232060)
SLA and Contracts Team (email: sla.contracts@food.gov.uk Tel: 01904 232093)
Updated [FSA Helpline (0330 332 7149)]
Data and Performance Team (email:operations.data@food.gov.uk)
MOC Guidance Team (email:MOC@food.gov.uk)
National Food Crime Unit (email:foodcrime@food.gov.uk Tel: 800 028 1180. For non-UK mobiles or calls from overseas use 0207 276 8787.)
Throughput Team (email:Throughput@food.gov.uk Tel: 01904 232209)
Food Incidents Team (email:foodincidents@food.gov.uk Tel: 020 7276 8448)
Imported Food Team (email:Imported.food@food.gov.uk)
Other:
Department for Environment, Food and Rural Affairs
Animal and Plant Health Agency (APHA)
Find a relevant APHA regional office (Tel: 03000 200 301)
Local authorities
Companies House (email:enquiries@companies-house.gov.uk Tel: 0870 33 33 636)
Food Standards Scotland – Operations (email:operations@fss.scot)
2. Relationships with other bodies
In this section
2.2 Department for Environment, Food and Rural Affairs
2.1 Introduction
2.1.1 Stakeholders and customers
The FSA has many stakeholders and customers, primarily consumers and the public, but also including:
- other government departments and agencies
- health ministers in England, Scotland and Wales
- meat industry
- halal, kosher and religious slaughter groups
- staff and their professional / representative organisations
- Local authorities (LAs)
- farming industry
- animal welfare organisations
- competent authorities of other countries
- European Commission (EC)
- DAERA / Veterinary Public Health Unit (VPHU)
2.1.2 Service standards
Our approach to customers and stakeholders, including Food Business Operators (FBOs) and their staff, must be:
- courteous
- professional
- considerate
- patient
FSA officials must always make a clear distinction between statutory requirements and recommendations of best practice. All advice and enforcement action should be proportionate and comply with the Enforcement Policy in Meat Plants.
Reference: For additional information see chapter 7 on ‘Enforcement’.
2.2 Department for Environment, Food and Rural Affairs
2.2.1 Responsibility
Defra are the central competent authority for animal health and animal welfare legislation in England.
2.2.2 Defra executive agencies
FSA Operations carries out work on behalf of the following Defra Executive Agencies:
- Rural Payments Agency (RPA), which incorporates BCMS for cattle identification matters
- VMD for medicinal residues
- APHA for animal welfare, identification and disease control and monitoring
2.2.3 Work on behalf of Defra
The work that FSA undertake on behalf of Defra and its agencies is outlined in this chapter at section 1.2.
2.2.4 Contact information
Defra can be contacted via their website.
2.3 Animal and Plant Health Agency
2.3.1 Background
FSA Operations works closely with APHA on matters relating to animal welfare and disease control.
2.3.2 Contact information
More information on the operational responsibilities and structure of APHA can be found via their website.
2.4 Local Authorities
2.4.1 LA purpose
LAs provide services directly to local communities. These services include trading standards and environmental health.
2.4.2 LA Animal Health Enforcement Officers
County Councils, Unitary Authorities, Metropolitan Borough Councils and London Boroughs are responsible for the enforcement of legislation relating to the health and welfare of farmed animals. The Trading Standards departments within these authorities usually provide this service, but this is dependent on the individual structure of each authority.
LA Animal Health Officers are responsible for the enforcement of legislation related to the health and welfare of animals on farms and during transport. The legislation covers animal identification, animal movements, disease control, animal welfare, animal by-products, bio-security and contingency planning.
LA Food Officers may visit food premises and have right of entry.
2.4.3 LA Food Enforcement Officers
LA Environmental Health Officers are responsible for enforcement of all food safety and hygiene legislation in all registered and approved food establishments, except for slaughterhouses, cutting plants and game handling establishments requiring approval by the FSAand those approved meat processing plants co-located to slaughter, cutting and game handling premises.
LA’s Trading Standards Officers are also responsible for all food standards enforcement, animal health and animal welfare in transport provisions.
Food establishments falling within the remit of the LA range from major national manufacturers to stand-alone cold-stores where the FSA is not present.
LA Food Enforcement Officers are also responsible for the prevention of illegal slaughter outside approved establishments, and for investigating food complaints from consumers.
2.4.4 FSA and LAs
Our officials work closely with LAs. Particularly Animal Health Officers, Trading Standards Officers (TSOs) and Environmental Health Officers (EHOs). LA officers deal specifically with:
- welfare of animals in transit
- confirmation of validity of cattle passports
- processing of animal by-products
- animal identification
- movementand documentation relating toanimals
- bio-security
- beef labelling requirements
- durability marking of food
- complaints from the general public relating to physical, chemical and microbiological contamination of meat
- issues relating to meat hygiene outside approved establishments
2.4.5 Relationships
Effective communication between FSA staff and LA enforcement officers is essential. Communication can:
- identify local risks, consumer complaints and concerns
- promote understanding of individual pressures and priorities
- facilitate successful enforcement activities that make the most effective use of local resource
2.4.6 Means of communication
Local communication channels between FSA officials and the LA officers must be maintained, for example:
- proactive and informal communication when LA enforcement officers are attending slaughterhouses
- maintaining ongoing discussions in relation to referrals or areas of common interest
- inviting FSA staff to LA regional meetings where appropriate, to discuss LA priorities and issues; FSA staff attending LA regional meetings where resource allows, and contribute to discussions
- considering possible joint local training opportunities, and sharing of information
2.4.7 Partnership working
Effective partnership working by the FSA and LAs will help achieve enforcement objectives and will ensure they are resourced effectively.
The FSA should always provide LAs with referral information at the earliest opportunity.
LAs should respond to the referrals at the earliest opportunity or advise when and how they will be able to respond. Where LAs are unable to respond straight away, the LA should offer advice to FSA staff on any immediate action (for example, appropriate evidence gathering) required to ensure future enforcement action is successful.
LAs should proactively advise FSA staff on the outcomes of any non-compliance detected at a slaughterhouse and explain why such an enforcement approach has been taken.
2.4.8 LA food complaints
Periodically LAs receive complaints from consumers and retailers about meat, meat products / meat preparations / MSM produced in approved establishments.
In order that the matter is investigated, the LA should refer the complaint to Corporate Support Unit (CSU) at York.
CSU will assign an appropriate officer at the establishment concerned to investigate. The assigned officer will be provided with a Food Complaint Investigation Report for completion.
Once the investigation is concluded and the form completed by the assigned officer, it must be returned to CSU and any physical evidence handed back to the LA officer who referred the matter (observing all security and continuity of evidence issues).
2.5 Meat industry
2.5.1 Liaison with industry
As well as day-to-day dealings with FBOs, the FSA also liaises with industry representatives through their respective organisations.
3. Communication and guidance
In this section
3.1 Lines of communication
3.1.1 Communication procedure
All staff follow a standard set of procedures when dealing with communications and queries. These procedures allow FSA Operations staff to work efficiently and effectively. There are situations where the lines of communication are different to those detailed here and are outlined in the relevant instructions.
3.1.2 Summary
The table below summarises the point of contact for technical advice, and also provides points of contact where non-technical advice is required.
Note: In Urgent Improvement Necessary establishments, technical matters should be discussed by the Field Veterinary Co-ordinator (FVC) and the contactor’s Technical Manager (TM). The FVL should be involved in these discussions when necessary.
Point of contact summary
| Advice required by | Technical Advice given by | Non-Technical Advice given by |
|---|---|---|
| Meat Hygiene Inspector (MHI) | OV | Inspection Team Leader (ITL) |
| cOV | FVC (following their contractors’ procedure via their TM) | Refer to their contractor’s TM |
| eOV | FVC | ITL |
| FVC | FVL | Operations Manager (OM) / Head of Operational Delivery (HOD) |
| ITL | FVC | Area Manager (AM) / OM |
| AM | FVC | OM / HOD |
| OM | FVL | HOD |
3.1.3 FBOs seeking advice
FBOs should be made aware that they should ask for advice in the first instance from their OV.
3.1.4 Technical advice for OMs / HODs
AMs / OMs / HODs should always seek technical veterinary advice from the FVC / FVL and / or FSA legal when making decisions relating to application or enforcement of official controls.
3.1.5 Internal communication of non-compliance reporting
When reporting an incident regarding any consignment arriving at the premises, which does not comply with the Regulations, the Authorised Officer (AO) must complete an Internal Communication of Non-Compliance Report (ENF 11/22).
Records of AO reports are monitored in York. This analysis allows the premises regularly dispatching non-conforming product to be identified and enables follow-up action to be initiated.
Reference:See chapter 9 on ‘Forms’.
3.1.6 Liaison with other authorities
There will be occasions where it will be necessary for the OV to contact other authorities, such as APHA, LAs, The Environment Agency etc. For ease of reference, the OV should be aware of their local points of contact.
The ‘Farm to Fork’ wall poster, was issued to all FSA facilities in slaughterhouses may be used to record contact details for each Authority.
Reference: See Annex 1 for an example of the poster.
3.2 Manuals and guides
3.2.1 Manual for Official Controls
The MOC provides details of the tasks, responsibilities and duties FSA staff and veterinary contractors undertake in approved meat establishments.
Volume 1 contains detail of the official controls and forms.
Volume 2 contains relevant legislation.
The manual includes guidance for staff on:
- inspection
- verification and audit
- health marking
- decision making and actions to be taken following official controls
- enforcement
- sampling procedures
- monitoring and surveillance programmes
Note:
- The OV is responsible for ensuring that all members of the team read and understand the instructions and is also responsible for making the FBO aware of any changes to the manual
- All staff must be aware of and follow the instructions in the manual unless there is good reason to depart from it. Where the guidance in the MOC is departed from the rationale for this should be clearly recorded
3.2.2 User identifies requirement for MOC amendment
Users of the MOC may identify areas of the manual where they feel that an amendment to existing instructions is warranted. In this case, they should email the Guidance Team, providing full details of their suggestion for improvement or amendment.
The Guidance Team will evaluate the suggestion and commission to the relevant Portfolio Group.
3.3 Daybook
3.3.1 Daybook maintenance
An official daybook must be maintained by FSA staff at each approved establishment. The day and date of operation must be entered by the AO on arrival at the premises. All operational staff should contribute to the daybook when necessary. The daybook is the property of the FSA and must remain under official control at all times. The daybook should not be used in place of other operational records or to needlessly duplicate information recorded elsewhere.
After completion of the day’s entries, the AO or Inspection Team Leader (ITL) should enter their signature then rule a line across the page, immediately below the signature. This is to prevent further, non-contemporaneous, entries being made.
3.3.2 Daybook access
All FSA operational staff must have ready access to the daybook. The OV should inform the FBO that they are entitled to read and make entries in the daybook and reasonable access should be provided.
3.3.3 Daybook security
The Daybook is FSA property and needs to remain under the control of the FSA or its SDP at all times. It is a requirement that the AO ensures that the Daybook is appropriately secured in a lockable storage unit where provided and available. In establishments where it has not been possible to provide a lockable storage unit, it may be appropriate to make local arrangements to keep the Daybook as secure as possible in an alternative location with the agreement of the local management team including the FVC. Those establishments should inform CBI to record them on a central record.
Evidence recorded contemporaneously in a Daybook, such as records of conversations with FBOs and accounts given by different AOs, is often essential evidence in a case and scanned copies of such entries are often used as exhibits. The defence is entitled to see the best evidence (the original version) of the Daybook to ensure the version being exhibited correlates to the original and has not been changed or added to. Therefore, it is vital that the security of the Daybook is maintained so any evidence within, is retained in its original form to comply with the Criminal Procedures and Investigations Act 1996 (CPIA). [See MOC Chapter 7, Section 2.5 for further guidance on gathering and preserving evidence in accordance with CPIA].
3.3.4 Other daybook functions
The daybook should be used to:
- record the health mark number(s) issued to or used by operational staff
- record the serial numbers of seals applied
- record the start and end times of regulatory duties
- record the time of the first kill and last carcase inspected each day, togetherwith any relevant comments; inspection teams may choose to record specific times for each species slaughtered at their establishment
- create a daily record of significant incidents, events or actions which occur at the establishment
- record specific actions taken by the FSA
- provide a means of communication between members of the operational team
- record details of non-compliances or offences that may become a source of evidence for legal proceedings
- record details of enforcement action taken by operational staff
- record verbal technical advice given by Veterinary colleagues or management to all AOs or other operational staff
Note: Contemporaneous notes should be recorded in your personal official notebook where access to the daybook is not readily available. These notes need not be transcribed into the daybook although a reference to their existence may be made.
Reference: See topic 3.4 on ‘Official notebooks’ in this section for additional information.
3.3.5 Arrival and departure
For health and safety purposes all members of the FSA team and their visitors must print their name, designation and time of arrival and departure at the establishment. If necessary, extend the vertical lines by ruling down. After all expected staff have arrived, the ITL should rule across the daybook page leaving four blank lines to accommodate other FSA officials who may visit the establishment.
Note: These entries must be signed by the team member or visitor at the time of departure from the premises.
3.3.6 Use and recording of stamps
It is very important that Health Marks (HMs) are controlled to prevent fraudulent use. All stamps used by FSA staff working in approved establishments must be kept in secure storage when not in use and be recorded in the daybook when issued and returned.
3.3.7 Health mark stamp
All members of staff using a HM stamp must record:
- the number of the HM and the time of issue
- the time stamps are returned to storage
3.3.8 Guidance on daybook entries
All entries in the daybook may be disclosed, for example, to the FBO, and must be professional and courteous. The daybook is an open document and it may be used as evidence in court.
Entries in the daybook:
- must not be written in offensive language
- must not be derogatory about any individuals
- must adhere to the facts
- must state professional opinions that the author is prepared to defend in court if necessary
- must not be used to record disagreements within the team
- must not be used to record criticism of any FSA staff or policy
Daybook entries must be:
- indelible (in ink or ballpoint pen, not pencil)
- relevant
- factual
- legible
- concise
- unambiguous
- written in clear English
- contemporaneous
- signed with the person's name (not just initialled)
- dated
3.3.9 Record of incidents
The format to be used to record incidents should include:
- time of the incident
- description of the incident
- action taken, including details of evidence collected and held under official control and advice given
- names of FSA and FBO staff involved
3.3.10 Retention
In accordance with FSA retention policy, all daybooks should be securely retained for a period of 6 years prior to disposal.
Older daybooks should not be sent for disposal without the approval of the OM / HOD.
3.4 Official notebooks
3.4.1 Official notebook use
These are to be used for recording contemporaneous notes where the daybook is not readily available; for example, where an incident occurs in the lairage that requires facts to be recorded immediately or where the OV is making notes at a meeting with the FBO.
The use of the notebook is not to replace the plant daybook for recording of day-to-day activities and is only to be used for recording factual information, which may need to be presented in court at a subsequent prosecution.
3.4.2 Reference to notebook entries
Where information is recorded in an official notebook, this need not be transcribed into the daybook; however, an entry should be made in the daybook referring to the fact that notes have been taken.
3.4.3 Important points
The notebook may be inspected in court and the following guidance must be adhered to maintain validity:
- record name on front cover, designation and date started
- make all entries with ink or ballpoint pen
- include only original entries and do not copy notes from elsewhere
- record the date and time at commencement of an entry, and upon completion
- enter the notes at the time ‘the offence’ is witnessed or as soon as possible afterwards whilst the facts are fresh in the memory
Note: Include names of other FSA staff present at the time
- if makingalterations, strikeapen through the words, and make the correction,initialling in left hand column; notes must not be erased
- do not remove pages from the notebook
- sign and date each entry at the base of each page
- do not use the notebook for any purpose not connected with your official duties
The notebook may have to be produced in court and read by all parties so entries must be relevant, factual, legible, concise and written in plain English.
3.4.4 Security
You are responsible for ensuring the security of your notebook and producing it in court. Further notebooks are available from CSU on return of your completed notebook.
3.4.5 Return of all notebooks
Notebooks remain the property of the FSA and must be returned prior to leaving the FSA or when requesting a further notebook.
3.4.6 Storage of completed notebooks
Completed notebooks which have been returned as above will be stored and may be required for evidence in the future.
3.5 Operations staff personal conduct
3.5.1 Staff conduct
All staff should adopt, maintain and demonstrate best practice in the course of their duties and conduct themselves in a professional way at all times.
The FSA takes incidents of bullying and harassment very seriously. Information, including policies and other resources can be found on the FSA Intranet Bullying and Harassmentpages.Please note:these pages can only be accessed by FSA staff on FSA devices.
3.5.2 Health and safety
All employees will remain aware of their legal obligations and take seriously the responsibility for their own health and safety and that of other persons who may be affected by their acts or omissions. Information, including policies and other resources can be found on the FSA Intranet Health, safety and wellbeing pages.Please note:these pages can only be accessed by FSA staff on FSA devices.
3.5.3 Personal standards
Every person working in a food handling area is to maintain a high degree of personal cleanliness and is to wear suitable, clean and, where necessary, protective clothing.
Reference: Regulation(EC) 852/2004 Annex II Chapter VIII.
3.5.4 Personal hygiene
FSA staff are to:
- wear white, clean protective clothing when handling exposed meat
- wear hairnets (and beard snoods if appropriate) to cover the hair of the head and where necessary the neck
- wear clean waterproof footwear
- wear designated waterproof footwear and lairage coats when working in dirty areas or with livestock
- not wear watches, jewellery (except plain wedding rings), aftershaves and perfumes in production areas
3.5.5 Operational hygiene
When working in an approved establishment, FSA staff must:
- keep personal equipment clean and change protective clothing as necessary
- use the proper hygiene facilities at all times and in such a way that there is no risk of contamination of meat
- wash contaminated aprons in the apron wash facilities
- use a dedicated hygiene facility
- wash hands, or gloves, whenever they become soiled, and always after handling detained or rejected product
- use a rubber glove over a chain mail glove to reduce the risk of cross contamination
- use blue, food safe, waterproof dressings to protect cuts
Note: some FBOs may require that dressings are also metal detectable.
3.5.6 Health status
FSA staff handling food or entering any food handling area in any capacity where there is any likelihood of direct or indirect contamination must not be:
- suffering from a disease likely to be transmitted through food
- a carrier of a disease likely to be transmitted through food
- afflicted, for example, with infected wounds, skin infections, sores or diarrhoea
3.6 Authorisation documents
3.6.1 OA / MHI title
The (EU) 2017/625 official control package uses the title of Official Auxiliaries (OAs) for Meat Hygiene Inspection (MHI) staff.
MHIs can continue to use the title of MHI except when participating in enforcement action, when the title Official Auxiliary must be used. Authorisation certificates will also use the title Official Auxiliary.
3.6.2 Authorisation documents
FSA staff are issued with authorisation documents depending on their designations, together with photo ID cards for some staff.
There is a legal requirement that AOs must produce a " duly authenticated document showing authorisation"when requested to utilise their powers of entry etc.
Authorisation documents are now issued electronically as a pdf document. AOs have options of how they produce such a document if requested and this may be:
- by downloading the pdf document onto their phone and saving it, to be accessed if or when requested,
- by downloading the pdf document and printing it off as a hard copy, to be produced if or when requested
AOs must have access to their authorisations document in hard copy or electronic formwhilst engaged in official duties, and be prepared to produce them on request. AOs must also ensure they possess all relevant authorisations for the type of establishment where they work and activities which they are performing.
When ceasing to work on behalf of the FSA authorisation documents and letters of confirmation must be returned to their line manager.
Any lost or found authorisation documents must be reported to CSU.
3.6.3 Devolved administrations and other government departments
The FSA does not directly authorise officers for functions that are the policy area of Defra or the Welsh Government (except animal welfare). The FSA receives a delegated authority letter that authorises its staff as inspectors / "persons" to act on their behalf. The general authorisation document provided reflects the officer's authority to act on behalf of both the FSA and those other government departments.
3.6.4 Powers of entry
Authorisation documents provide evidence of the legislation under which they may act. This includes the officer’s power to enter approved establishments at all reasonable hours to identify contraventions of the legislation under which they are authorised and for the performance of all statutory duties.
3.6.5 Action without authorisation
All officers acting on behalf of the FSA must never take enforcement action where they have not been appropriately authorised, as such action would not be valid.
If officers are in any doubt as to whether they are appropriately authorised they should seek technical advice as detailed in the topic ‘Lines of Communication’ previously in this section.
3.7 Modern Slavery and Human Trafficking (MSHT)
Issues of MSHT as defined in the Modern Slavery Act 2015 may be encountered during the operation of food businesses. The indications of such offending taking place may be subtle, but present non the less.
Incidents of suspected MSHT may impact on the FBO, by way of criminal investigation; the consumer, by way of untrained / unqualified staff being involved in the production process and importantly the Potential Victim of Trafficking (PVoT).
Concerns and suspicions of MSHT incidents that do not cause food hazards should be reported to NFCU Food Crime inbox immediately, or alternatively:
- your local Police Force,
- Modern Slavery Helpline (tel. 08000 121 700),
- the Gangmasters and Labour Abuse Authority (tel. 0800 432 0804)
MSHT incidents which have a direct cause of food hazards should be reported immediately to the Incidents Team.
In identifying signs of MSHT, consideration should be given to the following factors:
- FBO staff who live on site. This accommodation may be located within the FBO’s premises or externally in temporary accommodation such as caravans, out houses and vehicles, or even just mattresses located in a room
- FBO staff who appear to have been subject to physical violence or show fear when in the presence of management, allowing the management to answer questions directed at staff
- FBO staff who are not in possession of their identity documents as they are held by a third party or are not receiving any wage for their work
- FBO staff who are not allowed to leave the workplace and have little to no contact with the outside world including friends or family
- FBO staff who do not appear to have access to health care, clean clothing or food and water
- FBO staff who appear to be juveniles under the age of 18, working full time and living with persons who are not members of their family
- FBO staff who are dropped off and collected for work always in the same way, especially at unusual times
- FBO staff who are in a situation of dependence, maybe unfamiliar with the local language or show signs of control, which may include psychological control, (for example, through religion, witchcraft, juju)
- FBO staff who have no contract of employment and are unable to negotiate their working conditions, working excessively long hours, with little to no days off, do not have the correct protective clothing, training or professional knowledge to conduct their job
- FBO staff who appear to be distrustful of authorities and act as if instructed by a third party
4.Annexes
Please note: these pages can only be accessed by FSA staff onFSA devices.
Annex 1a: Sample Farm to Fork poster (England)
Sections
3. Collection and Communication of Inspection Results
1. Introduction
In this section
1.1 Purpose of FCI and CCIR
1.1.1 Purpose of food chain information (FCI)
FCI should be used by slaughterhouse Food Business Operators (FBOs) to assess any potential hazards presented by the animals intended for slaughter as part of their Hazard Analysis and Critical Control Point (HACCP)-based food safety management systems. FBOs should act upon the information provided in the FCI by making decisions about accepting animals and any special processing arrangements, for example, slaughter at the end of a run, additional dressing requirements, reduced line speed. This helps to ensure that certain veterinary medicines or animals affected by disease do not enter the food chain.
Information that must be confirmed on the FCI declaration includes:
- health status of the farm. That the holding is not under any movement restrictions for animal disease or public health reasons
- withdrawal periods have been observed. That there are no known veterinary medicine residues in the meat
- the animal's health status. That the animal to be slaughtered has not been exposed and does not show any signs of disease that may affect the safety of the meat
FCI is required for every animal intended for human consumption. The producer must provide FCI to the FBO for all animals presented for slaughter.
It is the FBOs responsibility to evaluate the FCI and then make it available to the OV without delay.
The OV must review the FCI before ante-mortem inspection to determine the inspection procedures required. It is also the OV’s responsibility to verify the FBOs HACCP plan includes and assesses all potential hazards contained in the FCI in line with the HACCP principles and that the HACCP established procedures are correctly implemented.
1.1.2 Veterinary Attestation Number (VAN)
From 13 December 2023, all livestock farmers who produce livestock or livestock products destined for the food chain, and which may be exported to the European Union, will require proof of an annual veterinary visit. This requirement can be fulfilled in several ways:
- Proof of participation in a Defra qualifying assurance scheme, such as Red Tractor, Quality Meat Scotland (QMS), Farm Assured Welsh Livestock (FAWL), RSPCA, Lion Quality or Poultry Health Scheme. The Food Business Operator (FBO) will verify membership details (no VAN required). The assurance schemes operate their own audit and compliance processes which provide the level of confidence that assurance scheme members are meeting the requirement and therefore the VAN is not required
- In England, if a farm has had an annual visit as part of the Defra Animal Health and Welfare Review Pathway Scheme, then this visit will fulfil the requirement. The visiting vet will fill in the Pathway form and provide the VAN of the veterinary visit
- If the farm is neither part of a recognised farm assurance scheme nor receives a Pathway vet visit (for England only), then a visit must be organised with a private veterinarian and an attestation is required from the veterinarian stating the visit has taken place. A VAN number will be issued by the attending veterinarian
The vet visit will review the farm and all its livestock species for signs of notifiable diseases and biosecurity risks. The vet will generate a 20-digit Veterinary Attestation Number (VAN), which must be included on movement licences and the FCI. It will comprise of:
- the visiting vet’s RCVS number,
- the County Parish Holding (CPH) number of the establishment visited, and
- the date of validity of the declaration
For example, 1234567 [MRCVS number] 12/345/6789 [CPH number] 0624 [Valid to the end of June 2024].
This requirement applies to farm-to-slaughter and farm-to-processor movements only (for example, animal markets); farm-to-farm movements are not affected.
If the farm is not a member of the qualifying assurance scheme or a VAN is not provided, the OV at the slaughterhouse will not be able to sign a Support Health Attestation (SHA) facilitating products derived from the animals on that consignment to be exported to the EU.
Even when meat from animals is not intended for export to the EU, there is a high likelihood that some of the animal products or by-products derived from them may be included in exports to the EU. Therefore, Defra is strongly recommending that all farm businesses ensure a veterinary visit has taken place at their farm.
Note: A 4-month implementation period starting from 13 Dec 2023 has been agreed with RCVS, to monitor compliance with the requirement for the Veterinary Health Attestation visits and to enable verification and addressing of any potential issues. During the implementation period farmer self-declarations can still be accepted at the abattoir, but OVs should encourage FBOs to communicate back to their suppliers and highlight that the implementation period will end on 12 April 2024 and from 13 April 2024, if there is no VAN or other evidence that a farm visit has been carried out, the OVs will not issue an SHA.
Reference: Regulation (EU) 2016/429, Article 8
Regulation (EU) 2020/692
1.1.3 Purpose of collection and communication of inspection results (CCIR)
CCIR is information provided to the producer to initiate any actions required on the farm to improve animal health, animal welfare and subsequently food safety.
Where inspection procedures reveal animal health or welfare problems that have arisen at primary production, the FSA must report direct to the producer.
Where inspection procedures reveal the presence of any disease or condition that might affect public or animal health or indicate compromised animal welfare, the OV should inform the slaughterhouse FBO.
1.2 Information cycle (FCI and CCIR)

1.3 Legislation
1.3.1 Regulations
The information cycle (FCI and CCIR) is required by Regulations (EC) 852/2004, (EC) 853/2004 and (EU) 2017/625.
Information Cycle Table
| Regulation | Requirement | Responsibility |
|---|---|---|
| (EC) 852/2004 | Lays down the records which FBOs rearing animals are required to keep. | FBOs for the holding of provenance (farmer or producer) |
| (EC) 853/2004 | Describes the FCI that FBOs must request, receive and act upon. | Slaughterhouse FBOs |
| (EU) 2019/624 and (EU) 2019/627 |
Requires the OV to check and analyse the FCI and to take account of this when carrying out ante and post-mortem inspections.
|
OV |
1.3.2 FCI implementing measures
Regulation (EC) 853/2004 establishes that slaughterhouse operators must not accept animals onto the slaughterhouse premises unless they have requested, and been provided with, relevant FCI.
Commission Implementing Regulation (EU) 2019/627, requires the Competent Authority (CA) to inform the FBO at the holding of provenance of the minimum elements of FCI to be supplied to the slaughterhouse.
Reference: (EC) 853/2004, Annex II, Section III. (EU) 2019/627, Article 9, Paragraph 1.
1.3.3 Additional FCI requirements: broilers
Council Directive 2007/43/EC lays down the minimum rules for the protection of chickens kept for meat production.
The Welfare of Farmed Animals (Amendment) Regulations 2010 (England / Wales) implement Council Directive 2007/43/EC and specify additional Food Chain Information requirements in respect of conventionally reared meat chickens. This regulation requires that the daily mortality rate and cumulative daily mortality rate and the hybrid or breed of chickens from a flock with a stocking density above 33 kilograms per m2 of usable area are treated as relevant food safety information and included in the FCI. See points 2.1.3 and 2.1.4 below.
References: Council Directive 2007/43 (EC)
SI No 3033/2010 The Welfare of Farmed Animals (England) (Amendment) Regulations 2010
SI No 2713/2010 (W229) The Welfare of Farmed Animals (Wales) (Amendment) Regulations 2010
1.4 FSA Operational staff role
Food Standards Agency Operational staff role
| Inspection and verification | By | Frequency | Time code |
|---|---|---|---|
| Review FCI and use information for ante-mortem inspection | OV / MHI if AM on farm of any species | One per batch from a producer or for individual animals | INSP |
| Carrying out ante-mortem inspection and recording data | OV | Individual animals Batches of poultry Recording by animal or batch | INSP |
| Carrying out post-mortem inspection and recording data | OV or MHI OV for meat with abnormalities. | Individual carcases and offal. Recording by carcase or batch | INSP |
1.4.1 Implementation of CCIR
The OV shall record and evaluate the results of official controls. IT tools have been developed allowing the collection and communication of the inspection results to abattoir FBOs and producers. The IRIS system is now available for all species.
The link to access IRIS support pages, along with Inspection Results Templates and Condition Reference cards is: IRIS2 guidance - Home (sharepoint.com).
The link to access IRIS to input information is: IRIS_Mobile (onk2.com).
Reference: (EU) 2019/627, Article 39
2. Food Chain Information
In this section
2.1 FCI: Poultry
2.1.1 Background
Since 01 January 2006, it has been a requirement that FCI is supplied in respect of poultry intended for human consumption.
The minimum information to be provided by the FBO rearing animals (farmer or producer), not less than 24 hours before the arrival of the poultry at the slaughterhouse, is contained in the form ‘Poultry FCI’ in Annex 11. This form has been provided by the FSA to all slaughterhouse FBOs.
Reference: (EC) 853/2004, Annex II, Section III, 3 (a) - (h).
2.1.2 Categories of chickens
For the purposes of entry of the FCI details into IRIS, one of three categories should be used for chickens:
Categories of chickens
| Category | Description |
|---|---|
| Broilers | All chickens reared specifically for food production (as meat) This includes poussin, slow-growing organic birds and cockerels specifically reared for meat |
| Hens | Reared for the production of eggs for food consumption |
| Poultry | Cockerels and hens used for breeding and not the prime purpose of food production, or rare cases of other poultry that do not classify as ‘broilers’ or ‘hens’ |
2.1.3 Council Directive 2007/43/EC
EU Council Directive (EC) 2007/43 (The Broiler Directive) lays down minimum rules for the protection of conventionally reared meat chickens (broilers) on holdings with 500 or more birds.
Under this Directive, the maximum on-farm stocking density (SD) for conventionally reared meat chickens is 33 kg/m².
SD above 33 kg/m² and up to 39 kg/m² is allowed, providing that the keeper complies with the extra requirements as detailed in the legislation listed below.
Reference:
SI No 3033/2010 The Welfare of Farmed Animals (England) (Amendment) Regulations 2010
SI No 2713/2010 (W229) The Welfare of Farmed Animals (Wales) (Amendment) Regulations 2010
2.1.4 Additional poultry FCI requirements under Council Directive 2007/43/EC
In relation to FCI, several pieces of data are considered relevant food safety information for flocks above 33 kg/m².
These are:
- the cumulative daily mortality rate (CDMR) for each house
- information on the hybrid or breed of chicken for each house
Note: See Annex 1 for an example of a completed CDMR table.
2.1.5 Poultry slaughterhouse- FBO responsibility
The FBOs of establishments processing poultry must request, receive, check and act on FCI. They must not accept poultry for slaughter unless they have requested, received and acted upon the information.
Receipt should normally be no less than 24 hours before delivery of the birds.
The FBO must make the FCI, including details of the numbers of dead on arrival, available to the OV. The FBO must notify the OV of health concerns before the OV carries out an ante-mortem inspection.
Reference: (EC) 853/2004, Annex II, Section III Points 1, 2 and 5.
2.1.6 OV responsibility
The OV must check the FCI provided for completeness and contents as a part of the ante-mortem inspection.
The OV is entitled to request any additional data from the producer. For example, when presented with a very high CDMR and no explanation is on the FCI for this, it is reasonable to request the complete set of daily mortality rates (for that particular flock’s production cycle) to fully understand at what stage of the production cycle significant mortality occurred. This should help the OV evaluate the health and welfare status of the birds on arrival at the slaughterhouse and determine whether there are immediate concerns regarding the health and welfare of any remaining birds at the site.
FCI should also be taken into consideration when post-mortem inspection is carried out.
The hierarchy of enforcement should be followed if any of the required FCI elements are missing, or the information is misleading (see point 4.2 below).
Legislation establishes that the OV must impose conditions under which animals must be dealt with under a specific scheme for the eradication or control of a specific disease, such as brucellosis or tuberculosis, or zoonotic agents such as Salmonella, under direct supervision. The Competent Authority (CA) must also determine the conditions under which such animals may be slaughtered. These conditions are designed to minimise the contamination of other animals and the meat of other animals.
Reference: Regulation (EU) 2019/627, Chapter III, Article 43 Point 6.
2.1.7 FCI: Salmonella on-farm testing (National Control Programme)
There is a statutory requirement for Salmonella on-farm testing of most chicken and turkey flocks under the requirements of the UK Salmonella National Control Programmes (NCPs). Producers are required to take boot-swab samples (or other sample types permitted under the NCP) from the poultry bedding or the environment at the farm. The sectors covered, and the producers to which the statutory NCP requirements are applicable, are detailed in the tables below.
All birds (unless exemptions explained in the tables apply) must arrive at the slaughterhouse with the Salmonella NCP test(s) result(s) and the date(s) of the sampling of their specific flock recorded in the Food Chain Information (FCI). The FCI for the batch must include:
- the NCP Salmonella test result(s), negative or positive
- the date(s) the sample(s) was/were taken from the flock
There may be flocks that have had more than one Salmonella test done; all relevant tests must be included on the FCI.
Salmonella Enteritidis and Salmonella Typhimurium have accounted for the majority of cases of human salmonellosis and have consistently been the most commonly implicated pathogens in general outbreaks of food-borne disease. These are referred to as ‘salmonella-regulated serovars’.
If an operator or an official sample test positive for Salmonella Enteritidis or Salmonella Typhimurium (including monophasic Salmonella Typhimurium) the flock will be considered high-risk positive, and the operator must declare it on the FCI and agree to its acceptance in advance with the FBO at the slaughterhouse.
APHA will also take random NCP official samples each calendar year from a single flock on 10% of holdings with more than 5,000 broilers or more than 500 fattening turkeys or in cases where there is no evidence of the required level of NCP testing or NCP rules have not been followed in a specific flock or flocks.
Note: If the date of on-farm sampling is more than the number of weeks permitted in the tables below, the birds can still be slaughtered. This should be according to the specific measures set in point 2.1.12 ‘OV action where the Salmonella result has not been recorded on the FCI or sampling is outside the sampling window’.
| Type | Sampling requirements | Applicable to | Exclusions |
|---|---|---|---|
| Broilers (flock of chickens reared for their meat) | Within a period of 3 weeks before the birds are slaughtered | All broiler flocks |
Farms with fewer than 2000 chickens. OR
OR
|
| Birds over 81 days old such as Certified Organic Birds produced according to REUL No. 889/2008 | Up to 6 weeks before they are slaughtered. | All broiler flocks |
Farms with fewer than 2000 chickens. OR
OR
|
Footnote 1: Operators of these farms do not have to take NCP samples, but their flock may be subject to official NCP sampling, in which case the test result should be included in their FCI.
| Type | Sampling Requirements | Applicable to | Exclusions |
|---|---|---|---|
| Adult breeding chickens (Gallus gallus) |
At least every 3 weeks during the laying period. The exception is when the flock has tested positive for a Salmonella strain covered by the NCP (regulated serotype or serovar). In this case, sampling must be at least every 2 weeks. |
All breeding chicken flocks with 250 or more birds. |
OR
|
| Type | Sampling Requirements | Applicable to | Exclusions |
|---|---|---|---|
| Laying chickens producing eggs for human consumption |
Pullets (also known as young/rearing hens) must be sampled within two weeks before moving to the laying unit. Adult egg-laying flocks must start the sampling between 22-26 weeks old and at least every 15 weeks during the laying production. |
All commercial laying chicken flocks that produce table eggs (Class A eggs) for human consumption |
|
Footnote 2: If such a flock is to be slaughtered for human consumption, a Salmonella NCP sample must be taken before slaughter at the timings described under Birds over 81 days old.
| Type | Sampling Requirements | Applicable to | Exclusions |
|---|---|---|---|
| Fattening turkeys (birds reared to produce meat for human consumption) |
Birds slaughtered at less than 101 days of age: Sampling within 3 weeks before slaughter. Birds slaughtered at more than 100 days of age (or younger but are organic turkeys produced according to REUL No. 889/2008): Sampling within 6 weeks before the slaughter. |
Farms of more than 500 fattening turkeys over a calendar year. |
In GB only, in farms rearing between 500 and 10,000 turkeys in a 12-month period AND only selling locally, producers can apply for an exemption to APHA. (footnote 4)3 and (footnote 5)4 |
| Turkey breeders |
Adult flocks must be sampled every 3 weeks during production either:
Birds slaughtered at more than 100 days of age (or younger but are organic turkeys): Sampling within 6 weeks before the slaughter. |
Farms of breeding turkeys if they have 250 or more birds at any time in a 12-month period. | Farm with fewer than 250 breeding turkeys in a 12-month period. |
Footnote 3: Operators of these farms do not have to take NCP samples, but their flock may be subject to official NCP sampling, in which case the test result should be included in their FCI.
Footnote 4: The farmer must apply to APHA for an exemption and must be declared on the FCI documentation.
‘Local/Locally’ in the exemption columns of the above tables refers to producers selling directly to consumers at farmers’ markets or retailers in any of the following:
- The county where the holding is
- The counties next to the holding’s county
- Anywhere up to 30 miles (or 50 Km) from the borders of the holding’s county
Nevertheless, the rules on only selling locally are lifted in the fortnights leading up to Easter and Christmas for fattening turkeys.
The requirement for statutory Salmonella sampling at the farm does not apply to other poultry species (for example ducks, quails). However, whilst there is no testing requirement, Salmonella status may be required to be included in the FCI under voluntary assurance or good practice schemes. The FCI must state:
- the date(s) on which the Salmonella NCP sample was taken
- whether the result(s) was/were positive or negative
- if positive, detail of the serotype or at least the serogroup (A,B,C,D,E) result
In such cases, it is expected that the FBO has procedures in place to deal with this hazard by establishing procedures based on HACCP principles to minimise the risk of potential cross-contamination at all stages when handling positive batches.
2.1.8 On farm restrictions: OV actions
In some circumstances, the NCP test results can lead to a flock being placed under restriction when positive for Salmonella enteritidis, Salmonella typhimurium or monophasic strains of Salmonella typhimurium (antigenic formula Salmonella 1,4,[5],12:i-).
In these cases, the birds can only move under an APHA movement licence. The OV can expect to receive the APHA movement licence either at the time the FCI documents are received or on arrival of the birds at the slaughterhouse. The number of birds in the batch should be cross checked with the details on the movement licence (which may cover more than one consignment of birds) and any further batches expected at the slaughterhouse. If any anomalies are detected, the APHA office that issued the movement licence should be contacted. Such licences may have been issued by either the local APHA office or by Business Support (SSC), Worcester.
However, a restriction notice is not always served on a Salmonella positive flock. If no restriction notice has been served, no movement licence will have been issued by APHA, even if the FCI states that the birds have tested positive for Salmonella. Whether or not a restriction notice is issued to a particular farmer will depend on the situation and the specific sector NCP. If no movement licence is received with a high-risk positive flock, the OV should contact the APHA office to confirm if the farm is under restriction.
Reference:
REUL No 2160/2003
REUL No 200/2010 (implementing legislation for breeding chickens)
REUL No 517/2011 (implementing legislation for laying chickens)
REUL No 200/2012 (implementing legislation for broilers)
REUL No 1190/2012
SI No 2007/3574 The Control of Salmonella in Poultry (England) Order 2007
SI No 2008/524(W50) The Control of Salmonella in Poultry Scheme (Wales) Order 2008
SI No 2008/263 the Control of Salmonella in Poultry Scheme Order (Northern Ireland) 2008
SI No 2009/229 The Control of Salmonella in Poultry (Breeding, Laying and Broiler Flocks) (Scotland) Order 2009
SI No 2009/260 The Control of Salmonella in Broiler Flocks (England) Order 2009
SI No 2009/441(W46) The Control of Salmonella in Broiler Flocks (Wales) Order 2009
SI No 2009/205 The Control of Salmonella in Broiler Flocks Scheme Order (Northern Ireland) 2009
SI No 2009/3271 The Control of Salmonella in Turkey Flocks (England) Order 2009
SI No 2010/65(W15) The Control of Salmonella in Turkey Flocks (Wales) Order 2010
SI No 2010/248 The Control of Salmonella in Turkey Flocks Scheme Order (Northern Ireland) 2010
SI No 2009/417 the Control of Salmonella in Turkey Flocks (Scotland) Order 2009
The primary framework legislation, REUL 2003/99/ and 2160/2003, implementing legislation for NCPs specifically deals with Salmonella control at all relevant stages of the food chain, but principally at the farm.
2.1.9 FBO action where a positive test result for a regulated Salmonella serovar (high risk) is recorded in the FCI
Where a positive test result indicates the presence, or the suspicion of the presence, of a regulated Salmonella serovar in the FCI, these flocks must be treated as high risk to public health. This applies to the following:
- Salmonella enteritidis
- Salmonella typhimurium
- monophasic Salmonella typhimurium (1,4,[5],12:i-)
- group D Salmonella (suspect enteritidis)
- group B (suspect typhimurium / monophasic typhimurium)
Important Note: where a group B or group D result has been partially serotyped and the initial / partial antigenic result is available indicating that the Salmonella detected is not enteritidis or typhimurium, this flock can be treated as positive for a lower risk serotype (refer to points 2.1.10 and 2.1.13).
FBO actions when accepting a high-risk Salmonella flock:
- Alert the OV to the FCI content regarding Salmonella and inform the OV of the procedures in place to process the flock
- Organise the slaughter plan for the day so that the affected batch(es) are slaughtered at the end of the production day to minimise the risk of cross-contamination
- After slaughter of the affected batch(es), undertake a full cleansing and disinfection of all equipment and machinery, including changing the water in the scalding tank(s), and renewing the water in the spin chiller(s)
- Where a high-risk Salmonella positive batch has been slaughtered during the production day (either in error or on welfare grounds), then the production should be stopped as soon as the affected batch has been slaughtered, and full cleansing and disinfection as above must take place before any further slaughtering commences
- Measures should be taken to minimise the risk of potential cross-contamination at all stages when handling high risk Salmonella positive batches
- The FBO must follow their own documented procedures, based on the HACCP principles, as regards placing the meat on the market
FBO actions regarding decisions concerning meat from a high-risk Salmonella flock:
The carcases from high risk Salmonella positive batches cannot be released for human consumption unless they meet the requirements of the table below.
The following 3 options are available at the slaughterhouse if the FBO accepts processing the high-risk Salmonella flock(s):
- Undertaking a Sentinel test. The FBO has the option of accepting high-risk Salmonella positive flocks and carrying out a sentinel test of the affected flock, by sampling 15 neck flaps from a batch of 150 birds at the abattoir under Regulation (EC) 2073/2005, point 1.28 of Annex I, Chapter 1 –absence in 5 samples of 25 gr each (neck flap)–. If the result of this test is negative, the flock should still be processed as a high-risk Salmonella positive as a preventive measure to ensure the protection of public health and to minimise any potential cross-contamination of the slaughterhouse facilities, but the meat can be released for human consumption as fresh meat. The remaining birds from this flock have to be processed in the same abattoir, and immediately after the results are obtained. Thinning would not be an option in these circumstances. The 150-batch sample is considered to be representative of assessing the risk status of the fresh meat to be placed on the market
- Undertaking poultry carcases (neck flap) or poultry portions sampling. Accept the flock and test the carcases for Salmonella spp. (neck flap) to ensure they comply with the Process Hygiene Criteria under point 2.1.5 of Annex I, Chapter 2 of Regulation (EC) 2073/2005. If Salmonella is isolated, serotype the samples for S. enteritidis and S. typhimurium to ensure compliance with the Food Safety Criteria under point 1.28 of Annex I, Chapter 1 (absence in 5 samples of 25 gr each). For the Salmonella analysis for fresh poultry meat other than poultry carcases (for example, from portions or when the neck flap has been removed) 5 samples of at least 25 g of the same batch shall be collected ensuring that the sample contains skin and a thin surface muscle slice to form each sample unit
- Processing the meat by heat treatment or other treatment capable of eliminating the hazard at an establishment other than retail. If the meat is not tested or if positive for a Salmonella regulated serovar after testing as described in points 1 to 3 above, the meat can be processed by a treatment eliminating the hazard (for example industrial heat treatment). This treatment may only be carried out by FBOs other than those at the retail level. Untreated meat that has tested positive will have to be discarded as category 2 animal by-product
Note: Until test results are received, the meat will have to be retained in the slaughterhouse (if necessary frozen) adequately identified and stored separately from other meats.
Note: For the purpose of these instructions, a flock is defined as a group of birds reared in the same house within the same farm. For birds not confined solely to a house, a flock is equivalent to a group of birds that physically share a designated area.
Note: for further details on how to take Salmonella samples at the slaughterhouse refer to Chapter 4.3 (Verification of microbiological criteria).
FBO Actions at the slaughterhouse for NCP high-risk Salmonella positive flocks
| Salmonella enteritidis or typhimurium fresh meat test result (sampled at the slaughterhouse) | FBO Action | Meat and offal | Animal by Products (ABP) |
|---|---|---|---|
| Negative (-) | None | Fit for human consumption as fresh meat in accordance with the food hygiene regime | Category 3 in accordance with the normal ABP regime |
| Positive (+) | Processing by a treatment eliminating the hazard in question (for example, industrial heat treatment or another treatment that eliminates salmonella). This treatment may only be carried out by food business operators other than those at retail level. | Fit for human consumption as meat product in accordance with the food hygiene regime | Category 3 in accordance with the normal ABP regime |
| Positive (+) | Not treated (because of a commercial decision) | Unfit for human consumption | Category 2 |
| Not tested | Processing by a treatment eliminating the hazard in question (for example, industrial heat treatment or another treatment that eliminates salmonella). This treatment may only be carried out by food business operators other than those at retail level. | Fit for human consumption as meat product in accordance with the food hygiene regime | Category 3 in accordance with the normal ABP regime |
| Not tested | Not treated (because of a commercial decision) | Unfit for human consumption | Category 2 |
| Not tested | Already placed in the market or ready to be placed in the market (for example, incorrectly completed FCI at the time of slaughter) | Withdrawal of products that are not at retail level for either further treatment or disposal |
If ABPs still traceable: Category 2 if meat not treated Category 3 if meat treated |
| Not tested –culled at the abattoir – not intended for human consumption | Culling in a slaughterhouse should be permitted only in exceptional circumstances and after being permitted by the CA. Further information provided in subtopic 2.1.15 | Unfit for human or animal consumption | Category 2 |
2.1.10 FBO action where a positive result for lower risk Salmonella serovar is recorded in the FCI
Where a positive test result for a lower risk Salmonella serotype (other than Salmonella enteritidis or Salmonella typhimurium as detailed in point 2.1.9 above) is indicated on the FCI, the FBO should take the following action:
- Alert the OV to the FCI content regarding Salmonella and inform the OV of the procedures in place to process the flock
- Organise the slaughter plan for the day so that the affected batch(es) are slaughtered at the end of the production day, or if this is not possible on welfare grounds, at the end of a production run or just before an operational break
- Where a positive batch has been processed in the middle of a production run, as soon as the affected batch has been processed, a thorough wash down (full cleansing and disinfection as detailed above for high risk is not necessary) of the plucking and evisceration room (including equipment) must be undertaken before any further processing re-commences. This is to minimise the risk of cross contamination for the following batches
- In any case, after the finish of production for the day, a full cleansing and disinfection of all equipment and machinery, including changing the water in the scalding tanks, and renewing the water in the spin chillers must be undertaken
- Following production, in the absence of any relevant AM or PM findings, the carcases can enter the food chain as normal
Note: Poultry meat preparations, poultry minced meat and meat products tested under Regulation (EC) 2073/2005 must be negative for all Salmonella serotypes, not just S. typhimurium or S. enteritidis. For more information please refer to MOC Chapter 4.3 Verification of microbiological criteria.
Note: Legislation requires that FBOs check FCI and act upon the information received. In the case of Salmonella positives, the FBO should have the procedure to follow included in their HACCP-based food safety management system.
2.1.11 OV action where a positive Salmonella test result is recorded in the FCI
The OV is to:
- check which Salmonella serotype is detailed on the FCI (or if serotyping is still pending, assume serogroups B and D are high-risk flocks unless salmonella enteritidis or salmonella typhimurium have already been excluded)
- check the date of the sampling and confirm compliance with the period required as per the table above in point 2.1.7
- check that the high / low-risk procedure has been followed following the FBO’s HACCP-based food safety management system
- notify the inspection team that the flock is positive, and ensure that the appropriate judgement on pericarditis is followed in accordance with the information contained on the Pericarditis Poultry Condition card (see chapter 2.4 on ‘Post-mortem, health and identification marking’, section 7)
- in case of specific incidents or “force majeure” such as lengthy breakdowns or road accidents (as an example) that might have a considerable impact on the welfare of the animals, the situation might need to be dealt with on a case by case basis. If this occurs, the OV must contact the poultry portfolio representative for further assessment of the situation and guidance on how to proceed with the Salmonella positive flock(s)
- ensure that the relevant cleansing and disinfection procedure is followed (as detailed in the previous sub-topics) after processing Salmonella positive flocks
Where non-compliance is found, action should be taken in accordance with the hierarchy of enforcement as outlined in Chapter 7 on ‘Enforcement’.
2.1.12 OV action where the Salmonella result has not been recorded on FCI, or the NCP Salmonella sampling is outside the sampling window.
In the first instance, the OV should request that the FBO contact the primary producer of the batch to determine whether an oversight has occurred and request the appropriate information is made available.
Where the flock
- was not eligible to be tested under the requirements of the NCP, the batch can be slaughtered as per normal procedures
- was eligible for testing, and the primary producer confirms that the test result is available, the OV must ensure that a copy of the test result is sent to the slaughterhouse. Once received by the FBO, action should be taken with the consignment in accordance with the test result received
Where this fails to resolve the issue, and no test results are available, the batch must be considered to be of unknown Salmonella status.
- If the flock is still at the farm, then the OV is to contact APHA within 2 working days to discuss the case
- If the birds are already in the abattoir, these should be processed as if a high-risk Salmonella result had been received. The OV is to contact APHA within 2 working days for information purposes
APHA contact details: APHA SSC CSCOneHealthSalmonella@apha.gov.uk and telephone 0345 601 4858.
Details for APHA should include contact details of the affected farm and specific flock(s) as per the FCI.
When the examination of the sample does not start within 48 hours following the time of receipt of the samples by the laboratory and within four days from the date of sampling, as per Regulation 200/2012 requirements, the sample is rendered as not valid for preslaughter NCP purposes, and the flock must be processed as a high-risk Salmonella flock as described in section 2.1.9 of this chapter. These cases must be reported to APHA.
Alternatively, the flock can be sampled/tested again if the birds are still on the farm or have been returned to the farm following the identification of this issue.
Finally, where the most recent test was taken earlier than permitted under NCP rules and outside the sampling window, the case is to be discussed individually with the FSA poultry portfolio team (poultry.portfolio@food.gov.uk).
A decision will be made based on flock status, past history, epidemiological assessment and length of time outside the window.

2.1.13 Salmonella group rather than serotype provided
In instances where the Salmonella group is provided instead of the serotype, the batches can still be processed as follows:
Result
- Salmonella groups D or B*
- Salmonella groups C, G or E
Action:
- As high-risk Salmonella positive
- As low-risk Salmonella positive
* The current serotyping process, for Salmonella typhimurium and monophasic strains especially, can be lengthy. The test process can, at an earlier stage, rule out the serotype being Salmonella typhimurium. It has therefore been agreed that an official or a NCP approved laboratory report, confirming that the flock is Salmonella positive, serogroup B, but that the isolate is not Salmonella typhimurium (based on initial antigen determination) is acceptable for the flock to be processed as low-risk salmonella positive.
2.1.14 Additional information to consider regarding Salmonella positive results
- Once a Salmonella positive result is obtained in a flock, the Salmonella status does not usually change, even if subsequently collected NCP sample test results for that flock are negative.
The exception is if a subsequent officially collected confirmatory sample negates this result (official confirmatory samples are only collected by APHA (GB) or DAERA (NI) and are not collected in every positive breeding or laying flock).
Flocks are to be processed as Salmonella positive high/low risk if there has ever been a positive Salmonella result unless a subsequent officially collected confirmatory sample was negative (in which case the original NCP sample result is officially deemed a false positive).
- Long term rearing birds (for example, fattening turkeys, slow reared broilers or breeding flocks) can recover to negative after an initial Salmonella positive result.
In these cases, the statutory Salmonella testing required before slaughter should confirm the latest negative test of the flock.
The FCI must however show all Salmonella testing results and the birds will still be considered positive for a regulated serovar under the NCP. The FSA internal procedure for these cases is that, if there are no other concerns, each case must be discussed individually with the Poultry Portfolio team (poultry.portfolio@food.gov.uk) that will confirm if the flock should be treated as positive or as negative. - If the portfolio confirms that the latest negative result can be considered, in the case of a previous positive for high-risk Salmonella serovars, the flock can be slaughtered as if it was a low-risk Salmonella serovar. In the case of a previous positive for low-risk salmonella serovars, the flock can be slaughtered as any normal flock
- If the Salmonella positive result is linked to a serovar used for vaccinating the flock (which should be stated in the laboratory result), this flock is not considered as Salmonella positive for the purposes of the birds being slaughtered for human consumption, and the flock can be processed as any other normal flock
2.1.15 Culling of a flock positive to Salmonella regulated serovar in the slaughterhouse
Culling flocks positive to a Salmonella regulated serovar in a slaughterhouse is permitted only in exceptional circumstances, if authorised by the competent authority (FSA), and when all alternative options have been exhausted. Permission will be granted on a case-by-case basis.
An example of exemptional circumstances could be welfare grounds in cases where culling companies are not available to cull the birds on the farm in time to prevent welfare issues due to increased stocking density.
When the results of an NCP test are positive for a regulated Salmonella serovar in a flock of birds, APHA will inform the Poultry Portfolio. If the bird producer cannot cull the birds at the farm, they will contact the slaughterhouse directly, asking the FBO if they would accept the birds either for processing, following one of the options described in point 2.1.9, or for being culled at their site and disposed of as Category 2 animal by-products.
If the FBO is willing to accept the birds, the decision to grant permission for the culling will be undertaken by the local FSA team (FVL and FVC) in consultation with the Poultry Portfolio poultry.portfolio@food.gov.uk.
The decision will be based on the establishment demonstrating that they have specific procedures to process the birds hygienically and minimise the risk of spreading the disease. These procedures must cover at least the following:
- Total segregation of the positive flock from other live birds
- The disposal of the culled birds, blood and feathers as Category 2 animal-by-products
- After culling the affected batch(es), the FBO undertakes a full cleansing and disinfection of all equipment and machinery, including the scalding tank(s), and the spin chiller(s) once emptied
- A thorough cleansing and disinfection of crates and modules used to transport live birds, vehicles and all areas used to process the birds, for example bleeding area, plucking equipment, etc
- Evisceration is not recommended, but if the particularities of the line do not allow the eviscerations to stop, these areas must be subsequently cleansed and disinfected before processing birds intended for human consumption
- Verification that the cleansing and disinfection have been satisfactory
The OV shall supervise the culling process and verify that the cleaning and disinfecting procedures have been effective.
Legal reference - Annex III, Section II, Chapter IV, point 10 of Regulation (EC) 853/2004 allows the use of white meat slaughterhouses, when permitted by the competent authority, for slaughtering sick or suspect animals and when applying disease eradication or control programmes. Please note that this provision is not available for red meat slaughterhouses.
2.2 FCI: Pigs
2.2.1 Background
FCI for pigs was fully implemented from 1 January 2008.
2.2.2 Pigs slaughterhouse- FBO responsibility
FBOs must not accept pigs for slaughter unless they have requested, received and acted upon the FCI.
After deciding to accept the pigs for slaughter, the FBO must make the FCI available to the OV without delay. The FBO must notify the OV of health concerns before the OV carries out an ante-mortem inspection.
Reference: (EC) 853/2004, Annex II, Section III, Points 1, 2 and 5
It is the responsibility of slaughterhouse FBOs to decide on the FCI that they require and to request this FCI from the FBO rearing the animals (farmer or producer). Guidance on the minimum requirements for FCI can be found on the FSA website.
Reference: (EC) 853/2004, Annex II, Section III, Point 3 (a) - (h).
2.2.3 Methods of receiving pig FCI
Since 1 April 2012, pig keepers in England and Wales are required to report movements (including pigs from Scotland) using the electronic AML2 online system (eAML2) operated by the British Pig Executive (BPEX). Refer to Chapter 2.5, Section 2.11 for further details on pigs’ identification requirements.
To be legally compliant, pig movements must be reported through the eAML2 online system or by contacting the eAML2 free-to-use bureau service by phone (to get a printed copy for the movement).
eAML2
Email: eaml2@ahdb.org.uk
Telephone: 0844 335 8400
Monday to Friday, 9 am to 5 pm
The FBO should receive the FCI by at least one of the following routes:
- via eAML2 online system
- included in the haulier summary (HS), a document required by Trading Standards to accompany every load in transit, which contains the movement and FCI details
- on the ‘old style’ FCI paper form
Note: In Scotland, with effect since 1 December 2011, details of pigs moving to slaughter should be notified to the ScotEID movement reporting database electronically, by telephone or in writing. This includes movements of pigs from Scotland to England and Wales for slaughter.
Reference: Regulation (EC) 853/2004, Annex II, Section III, Point 3 (a) - (h).
2.2.4 Pigs arriving without FCI
FCI must be provided for all animals slaughtered for human consumption, within 24 hours of their arrival.
The OV may permit animals without FCI to be slaughtered, but the health mark must be withheld until the FCI has been provided and examined.
Pending final judgement, the carcases and offal must be stored separately from other meat (subject to the provision below).
When animals arrive without FCI but are slaughtered and the meat held pending the arrival of the FCI, the meat shall be declared unfit for human consumption and disposed of as an animal by-product if no FCI is provided within the 24-hour period, as required by the Regulations.
Note: See section 4 below on ‘Verification and Enforcement’ for further information.
2.2.5 Housing system/Controlled housing conditions
Pig producers must declare the housing system used for their pigs on the FCI.
‘Controlled housing conditions’ include a range of measures that reduce the risk of the pigs being infected with Trichinella and therefore these pigs are exempt from trichinella testing at the slaughterhouse. Importantly, the definition does not exclude pigs that have outdoor access, provided that the outdoor access does not present a risk of introducing Trichinella into the holding.
Republic of Ireland (RoI) has, to date, not put in place a mechanism whereby housing can be deemed to meet the conditions specified in Article 1 and Annex IV of retained Regulation (EU) No 2075/2005. Therefore, all pigs born and reared in RoI, which are slaughtered in slaughterhouses in England or Wales, shall be tested for trichinella, regardless of the housing system recorded on the FCI.
Refer to Chapter 2.4, Section 5 for detailed information on Trichinella testing.
Reference: Regulation (EU) 2015/1375, Annex IV, Chapter I
2.3 FCI: Horses
2.3.1 Background
FCI for horses was fully implemented from 1 January 2009.
It is the responsibility of slaughterhouse FBOs to request this FCI from the FBO rearing the animals (producer, owner or keeper).
With effect from 23 February 2015, FCI, in addition to the passport for individual equines, must accompany all equines consigned for slaughter for human consumption. Note that FCI is also required for horses originated from wild and semi-wild horses living in designated areas to which certain identification derogations apply.
Requesting and receiving FCI is the responsibility of the slaughterhouse operator.
Note: the rules for horses apply to all equidae (donkeys, asinine, mules). Instructions in this chapter refer to horses for purposes of simplification.
Reference: (EC) 853/2004, Annex II, Section III, 3 (a) - (h).
2.3.2 Horse slaughterhouse FBO responsibility
FBOs of establishments processing horses must request, receive, check and act on FCI. They must not accept horses for slaughter unless they have requested, received and acted upon the information.
A revised model FCI document is attached at Annex 3.
After deciding to accept the horses for slaughter, and after conducting identity checks, the FBO must make the passport and FCI available to the OV without delay. The FBO must notify the OV of health concerns before the OV carries out AMI.
Reference: (EC) 853/2004, Annex II, Section III, 1, 2, 5.8.
Note: See chapter 2.5 on ‘Animal identification’, section 4.10 on ‘Verifying eligibility of horses’ for detailed guidance.
2.4 FCI: Other species
2.4.1 FCI implementation for other species (other than poultry, pigs, horses and veal calves)
For species other than poultry, pigs, horses and veal calves, FCI was implemented from 1 January 2010.
As with other species, the FSA has provided guidance on the ‘minimum elements’ of FCI required and model documents have been developed. This has been made available on the FSA website. FBOs may however choose to request additional information.
For farmed game, information should be provided in the FBO’s declaration made at the time of slaughter which, if correctly completed, contains all the elements required for FCI.
There is no requirement for the provision of FCI for wild game animals; this is replaced by the hunter’s declaration.
2.4.2 FCI in cases of on farm emergency slaughter
The declaration that accompanies the bodies of animals subjected to on farm emergency slaughter, if correctly completed, contains all the elements required for FCI, and therefore additional FCI documentation will not be required.
2.4.3 Additional FCI requirement for cattle
There is a requirement within the ‘minimum elements’ of FCI for cattle that a declaration is made by the keeper, specifying the bovine tuberculosis (TB) status of the holding.
This will assist in identifying cattle that have tested negative but come from restricted herds, or young animals aged less than 8 weeks, which arrive under a general licence and are indistinguishable from animals arriving from non-restricted herds.
In such cases, the FCI will determine the origin of the animals, and there is a requirement for the OV to be in attendance during slaughter.
2.4.4 Additional FCI requirement for other species susceptible to bovine TB
Food chain information, incorporating a declaration regarding the TB status of the holding, must be provided for farmed animals such as camelids, bison, water buffalo and deer that are slaughtered on farm. The FCI must accompany the carcases to the slaughterhouse.
2.5 FCI receipt and check
2.5.1 FCI receipt by the OV
The OV should receive the FCI report from the slaughterhouse FBO at least 24 hours in advance of arrival of the animals. However, FCI can be received at the same time as the animals providing that:
- it does not jeopardise the objectives of (EC) 853/2004
- it does not cause serious disruption in the slaughterhouse activity
Where animals have undergone ante-mortem inspection by an OV at the holding of provenance and have the relevant certificate, the FCI may accompany the animals rather than arriving 24 hours in advance.
Reference: (EU) 2019/624, Article 5, Paragraph 2(f).
Poultry note: FSA considers that, because of the organisation of the poultry industry and the need to use FCI to plan the slaughter of flocks, it is necessary that FCI is received in advance of the arrival of the poultry at the slaughterhouse.
FSA is encouraging slaughterhouse FBOs to treat the 24 hours period as a minimum period and to request FCI further in advance if this is necessary to make appropriate arrangements for specific flocks (for example, to plan the slaughter of a flock which has tested positive for Salmonella at the end of a shift / day).
Other species: The FSA has elected to allow FCI to be sent to the slaughterhouse operator with the animals (FCI is not required to be sent 24 hours in advance). However, if there is any information on the FCI which might result in serious disruption to the slaughterhouse activity, the FCI must be received in good time before the animals arrive. In addition, FBOs are recommended to obtain FCI long enough in advance of delivery to the slaughterhouse to enable them and the OV to take any necessary action.
2.5.2 OV role
The OV is to check and analyse relevant information from the FCI report and may take any of the following decisions:
- animals with a disease or condition that may be transmitted to animals or humans through the handling or eating of meat must be rejected for slaughter and killed separately under conditions such that other animals cannot be contaminated and declared unfit for human consumption
- change slaughterhouse process (for example, reduce line speed or increase number of inspectors)
- slaughter animals / batch of animals last (for example, if known to carry a pathogenic organism)
- detain animal(s) or carcase(s) for further testing
2.5.3 Animals with no FCI
If animals arrive at the slaughterhouse without FCI, the FBO must notify the OV. The OV should agree the procedure with the FBO in advance.
Reference: (EC) 853/2004, Annex II, Section III, 6.
The OV may permit the slaughter of animals if the FCI is not available. In such cases the OV must detain carcases of animals slaughtered in the absence of FCI, and their related offal, pending receipt of FCI.
Reference: (EU) 2019/627 Article 40
Before permitting the slaughter of animals without FCI, the OV must ensure that:
- there are adequate facilities for the separate storage of carcases and their offal
- arrangements are in place to identify these in the slaughter line so that they are not inadvertently health marked
If the OV decides to permit slaughter they will need to confirm this in writing to the FBO.
The Regulations provide that, if FCI is not received within 24 hours of the animal’s arrival at the slaughterhouse, all meat from the animal is to be declared unfit for human consumption.
FCI must be provided, within 24 hours of their arrival, for all animals slaughtered for human consumption.
When the OV does not permit the slaughter of animals (for example, where there are no facilities to store carcases separately), the animals may, subject to animal health and welfare considerations, be kept in the lairage until the food chain information is provided. If this information cannot be supplied or the FBO does not wish to keep animals in the lairage then the animal(s) must be killed separately from other animals and the meat declared unfit.
Reference: (EC) 2019/627 Article 40. Further information is available in section 4 on ‘Verification and enforcement’ in this chapter.
2.5.4 The Cascade principle in the use of medicines
The cascade principle allows veterinary surgeons to legally prescribe medicines that are not authorised, at a different concentration or for another specie for a relevant clinical case. The Cascade is a risk-based decision tree to help veterinary surgeons decide which product to use when and at which concentration when no authorised veterinary medicine is available or authorised.
A veterinary surgeon prescribing or administering a medicine to food-producing animals under the Cascade principle must specify an appropriate withdrawal period. When setting this withdrawal period, the veterinary surgeon must consider known information about the use of the product on the authorised species and concentrations when prescribing under the Cascade principle.
Where the product is not used as authorised, for example, when a higher dose or longer duration of treatment is used, or a species for which the product is not indicated is treated, care needs to be taken to ensure a reasonable withdrawal period is set. This ensures that no residues of veterinary medicines above the Maximum Residue Limit remain at the time of slaughter or when produce is taken.
Unless the medicine indicates a withdrawal period for the species concerned and at the required concentration, this should not be less than:
- 7 days for eggs
- 7 days for milk
- 28 days for meat from poultry and mammals, including fat and offal
- 500-degree days for fish meat
Reference: The Veterinary Medicines Regulations 2013, Schedule 4, Point 2.
Guidance for prescribing vets on the use of the cascade.
2.6. Livestock Information Service
The Livestock Information Service (LIS), launched in 2022, is used to record movement data for sheep, goats and deer by users in the supply chain, including farmers, markets and slaughterhouses in England.
The LIS enables suppliers to record sheep, goat and deer movements online (LIS-1 and LIS-2 forms), replacing the paper version of the movement document.
The information included in a LIS movement document is the same as in the paper version, including the Food Chain Information (FCI) and the Veterinary Attestation Number (VAN).
The LIS movement document and FCI report should be available for the OV to check and analyse all the relevant information regarding the animals accepted for slaughter.
When mistakes or missing information are identified, the FBO must ensure that the issue is addressed with the supplier as it occurs with the paper versions.
3. Collection and communication of inspection results
In this section
3.1 Introduction
3.1.1 Duty of the FSA
If inspections reveal the presence of any disease or condition that might affect public or animal health or compromise animal welfare the OV is to inform the FBO.
Where the problem arose during primary production, the OV is to inform:
- the farmer
- the farmer’s veterinary surgeon
- where appropriate APHA
Reference: (EC) 2019/627 Article 39(5).
3.2 Recording of inspection data
3.2.1 Recording of data
The Operations Group team in each establishment should have a system in place to ensure that the results of ante and post-mortem inspections are recorded accurately and where possible be identified clearly back to the batch of animals (and specifically to the flock / shed for poultry or by slap mark for pigs, as appropriate for the information supplied on the FCI).
The farmer may use this information to improve the health status of his stock. Defra will use the data for disease surveillance therefore the accuracy of the information is vital.
3.2.2 Recording ante and post-mortem inspection results
All inspection results must be recorded on IRIS2. The data input should be completed in a timely manner. Where possible, this should be on the same day, but it must be completed within 48 hours of slaughter (not including non-operating days). Procedures for data input should be agreed and communicated to each FSA inspection team by the establishment management team.
Note: The CIR 12/1, CIR 12/2 and the PMI 4/8 will still be available for staff to use if required due to local circumstances.
3.2.3 Plants with no IT connection
Where an establishment has no FSA IT connectivity, the FCI, AMI and PMI data is to be collected at that plant and then entered at a later point in time by the MHI or OV when a suitable connection is available.
3.2.4 CCIR for poultry
CCIR plays an important role in meeting the requirements of the Broiler Directive, by reporting where welfare triggers are exceeded, based on conditions observed during ante and post-mortem inspection (welfare indicators).
Where welfare triggers are exceeded, IRIS2 will automatically generate a report. These reports are sent to the SLA and Contracts team and are then checked by a FVL. The SLA and Contracts team then send the individual reports to APHA, with a copy to FSA staff at the relevant establishment.
APHA follow up these reports (for instance, by visiting the relevant farm or requiring an action plan from the producer).
APHA then provide feedback to FSA regarding the outcome of their actions.
Reference: EU Directive 2007/43
3.3 IRIS2 for all species
3.3.1 IRIS2 application
All guides for IRIS2 can be found here: IRIS2 guidance - Home (sharepoint.com).
IRIS2 can be accessed through: IRIS_Mobile (onk2.com)
3.4 CCIR: electronic feedback
3.4.1 Electronic feedback
Although it is possible for completed inspection reports to be printed from IRIS2, this is discouraged. Electronic feedback should be sent directly from the system to the FBO.
3.4.2 Automated reports
Once the inspection records are marked as complete, the report will be automatically generated and sent via email at 2:30 am (subject to operational need) the following day.
3.4.3 FBO reports
Any FBO wishing to receive the automated reports can do so by supplying a name and email address. FVLs / FVCs, OVs or ITLs should discuss this with the FBO and send the email address(es) to: iris@food.gov.uk
Note: FBOs may have the reports sent to a maximum of 3 email addresses if they wish.
4. Verification and enforcement
In this section
4.1 Verification guidelines for species: FCI full Implementation
4.1 Verification guidelines for species: FCI full Implementation
Verification guidelines for species: Food chain information full Implementation
| Process | Responsibility |
|---|---|
| FCI is provided for all animals sent to slaughter. | FBO rearing animals |
| FCI may arrive with the animals (with the exception of poultry, for which it must arrive at least 24 hours in advance) but any item of FCI which might result in serious disruption to the slaughterhouse activity must be received in good time before the animals arrive. | Slaughterhouse FBO |
| The FBO checks the FCI as per HACCP procedures and acts upon it by accepting / rejecting the animals for slaughter. | Slaughterhouse FBO |
| FBO makes FCI available to the OV (OV / MHI if ante-mortem inspection on farm) and notifies them of any anomalies in FCI and of animals that have arrived without this information. | Slaughterhouse FBO |
| Animals are not slaughtered unless FCI is provided, or the OV permits slaughter and carcases and offal are detained until FCI is provided. Note: see section 4.2 on ‘Enforcement’ in this chapter. | OV |
| AM Records: Enter the data for the applicable species on IRIS2 | OV / MHI |
|
PM Records: Enter data on IRIS2 Note: In general (and there may be exceptions to this), a ‘batch’ relates to a group of animals, all of which:
|
OV / MHI |
4.1.1 Rejected meat receipts
The purpose of PMI 4/8 (Rejected Meat Receipt) is to provide a receipt for, and a record of, rejected meat to verify that the FBO has agreed to the voluntary surrender of the meat.
Form PMI 4/8 is no longer required when the inspection data has been entered on IRIS2. If needed a copy of the reports can be printed from IRIS2 and handed to the FBO. The only occasion a PMI 4/8 form should be used is when there is no FSA IT system at the establishment.
Note: In general (and there may be exceptions to this), a ‘batch’ relates to a group of animals, all of which:
- are from the same producer
- and arrive on the same means of transport
- and arrive on the same day
Ensure that a responsible member of plant staff signs the PMI 4/8 and that a copy of the receipt is filed by Operations Group staff. Once signed by the FSA representative and the slaughterhouse FBO, pass the relevant copies of PMI 4/8 to the slaughterhouse FBO.
4.1.2 Recording information on ante / post-mortem form (CCIR)
Information must be accurate. The OV must be satisfied with the system for accurately collecting data in the lairage and at all points on the slaughter line.
4.2 Enforcement
4.2.1 When FCI is not received
FCI must be available for all animals sent for slaughter or, in the case of emergency slaughtered animals or animals slaughtered on the farm, sent for dressing to the abattoir.
FCI must be provided for all animals slaughtered for human consumption, within 24 hours of their arrival.
The OV may permit the animals without FCI to be slaughtered but the health mark must be withheld until the FCI has been provided and examined. Pending final judgement, the carcases and offal must be stored separately from other meat (subject to the provision below).
When animals arrive without FCI but are slaughtered and the meat held pending the arrival of FCI, the meat shall be declared unfit for human consumption and disposed of as an animal by-product if no FCI is provided within this 24-hour period, as required by the Regulations. Refer to Chapter 7 for information on Enforcement.
Note: The declaration that accompanies the bodies of animals subjected to on farm emergency slaughter, if correctly completed, contains all of the required elements for FCI, therefore additional FCI documentation for such animals will not be required.
4.2.2 Measures in cases of wrong or misleading FCI
If FCI, or any required element(s) of the FCI, is/are wrong or misleading, which has implications for public health and/or animal health or welfare, the OV must inform the FBO and request clarification about this information.
If it is believed that the accompanying records, documentation or other information do not correspond with the true situation on the holding of provenance, or the true condition of the animals, and could mislead the OV, the following actions must be taken:
- The OV must report the issue to the relevant Local Authority (Trading Standards), copying the communication to National Food Crime Unit (NFCU) on foodcrime@food.gov.uk and APHA –when applicable–. The OV must also inform the FSA local veterinary team for awareness
- Additionally, if there is evidence that the FBO at the slaughterhouse knew that the information provided in the FCI was inaccurate/false and they were not acting on the information received in good faith, enforcement for obstruction action against the slaughterhouse FBO shall also be taken accordingly
Reference: Regulation (EU) 2019/627, Article 42.
4.2.3 FCI incomplete
If FCI, or any required element(s) of the FCI set out in Retained (EU) 853/2004, is/are missing, and this has implications for public health and/or animal health or welfare, the OV must inform the FBO and request the missing information.
Where relevant food chain information is not available within 24 hours of an animal's arrival at the slaughterhouse, the official veterinarian shall declare all meat from the animal unfit for human consumption. If the animal has not yet been slaughtered, it shall be killed separately from other animals taking all necessary precautions to safeguard animal and human health.
If any of the legally required FCI details are absent, the normal hierarchy of enforcement should be followed, for example, FBOs that are regularly failing to identify missing parts of the FCI, relying on the OV to identify them to then act.
4.2.4 Circumstances when records indicate that meat must be declared as unfit for human consumption
Where animals are already present at the slaughterhouse, and accompanying records, documentation or other information demonstrates that:
- the animal(s) come from a holding or an area subject to movement prohibition for reasons of animal or public health or
- the rules on the use of veterinary medicinal products have not been complied with or
- other conditions adversely affecting human or animal health are present
the animals must be slaughtered separately and declared unfit for human consumption.
Note: The list of authorised veterinary medicinal products, including withdrawal periods, can be found online via VMD and NOAH.
These links can also be found on K2 applications pages in the ‘Link Applications’ listings. Withdrawal Periods (noahcompendium.co.uk).
4.2.5 Disposal of meat declared as unfit for human consumption
When the meat cannot be health marked due to absence of FCI or due to the information provided, the meat must be declared unfit for human consumption and the OV should seek voluntary surrender of the meat.
Where surrender is not forthcoming, the OV should put in writing the reasons why they are formally declaring the meat unfit for human consumption, in accordance with (EU) 2019/627, Article 40 and 41.
Note: Where the FBO continues to refuse to dispose of meat declared unfit, follow the ABP provisions relating to the treatment of meat that has been declared unfit for human consumption in chapter 2.8 on ABPs.
5. Annexes
Update: [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 1: Cumulative Daily Mortality Rate (CDMR)
Annex 2: Model document: Letter to FBO permitting slaughter
Annex 3: Model document: FCI for equines
Annex 4: IRIS User Guide REMOVED
Annex 5: IRIS Q&A REMOVED
Annex 6: IRIS Ante-Mortem Conditions REMOVED
Annex 7: IRIS Post-Mortem Conditions REMOVED
Annex 13: FCI Farmed game and fractious bovine slaughtered on farm
Annex 14: FCI Poultry slaughtered on farm
Annex 15: FCI Emergency slaughter
Annex 16: FCI Live farmed game
Annex 17: FCI Farmed game animals susceptible to bovine TB
-
Footnote 1: Operators of these farms do not have to undertake NCP samples, but their flock may be subject to official NCP sampling, in which case the result should be included in their FCI
-
Footnote 1: Operators of these farms do not have to undertake NCP samples, but their flock may be subject to official NCP sampling, in which case the result should be included in their FCI
-
Footnote 2: If such a flock is to be slaughtered for human consumption, a Salmonella NCP test must be undertaken before slaughter at the timings described under Birds over 81 days old.
-
Footnote 3: Operators of these farms do not have to undertake NCP samples, but their flock may be subject to official NCP sampling, in which case the result should be included in their FCI.
-
Footnote 4: The farmer must apply to AHPA for an annual exemption and must be declared on the FCI documentation.
Sections
1. Introduction
In this section
1.1 FSA roles
1.1.1 Purpose
The purpose of ante-mortem inspection is:
- to determine whether there is any sign of any condition which might adversely affect human or animal health
- to enable the Official Veterinarian (OV) to make the decisions as to whether the animal can be slaughtered for human consumption
- to inform possible adjustments during post-mortem inspection
- to determine whether any test should be carried out in relation to disease diagnosis or for residues of veterinary medical products
- to determine whether welfare has been compromised
- examination of food chain information
Particular attention should be given when zoonotic or notifiable diseases are a possible diagnosis.
1.1.2 Regulations
Ante-mortem inspection is covered by Regulation (EC) 853/2004 (Food Business Operator (FBO) duties) and Regulation (EU) 2017/625, Regulation, 2019/624 and Regulation 2019/627 (FSA requirements).
1.1.3 Inspection tasks
The OV must carry out the ante-mortem inspection.
The Meat Hygiene Inspector (MHI) may assist the OV with some pre-slaughter tasks. In relation to ante-mortem inspection and welfare checks they can make an initial check and help with purely practical tasks.
The MHI must alert the OV to abnormal animals identified pre-slaughter.
1.1.4 Circumstances where ante-mortem inspection has been undertaken by an OV at the holding of provenance
Where the ante-mortem inspection has been carried out by an OV at the holding of provenance a MHI can undertake the ante-mortem inspection required in the slaughterhouse on all species when certain criteria are met, under the responsibility of the OV, for example, without the OV being present.
The MHI must alert the OV to abnormal animals identified pre-slaughter and the OV must then carryout the ante-mortem inspection.
MHIs cannot be involved in ante-mortem inspections in the following circumstances:
- animals have undergone emergency slaughter;
- animals suspected of having a disease or condition that may adversely affect human health;
- bovine animals from herds that have not been declared officially free of Tuberculosis (TB) or the officially free status of which has been suspended;
- bovine animals from herds and to ovine and caprine animals from holdings that have not been declared officially free of brucellosis or the officially free status of which has been suspended;
- in the case of an outbreak of animal diseases to animals coming from a region as defined in Article 2 of Council Directive 64/432/EEC in which animal health restrictions are applied;
- where animals are subject to stricter controls due to the spread of emerging diseases or particular diseases listed by the World Organisation for Animal Health.
Abnormal animals identified pre-slaughter
| Inspection and verification | By |
|---|---|
| Observing animals at unloading (random) | OV / MHI |
| Initial checks | OV assisted by MHI |
| Ante-mortem inspection | OV assisted by MHI |
| Completion of ante-mortem record | OV / MHI |
| Completion of pen cards | OV / MHI |
| Enforcement | OV |
| Checks following ante-mortem at the holding of provenance | OV or MHI acting under the responsibility of the OV |
Reference: Regulation (EU) 2019/624, Article 3, 2 and Article 5, 3 and Regulation (EU) 2019/627, Section 2, Article 11.
1.2 FSA role: verification of FBO responsibilities
1.2.1 Introduction
The FBO has a number of responsibilities that the FSA are required to verify are fulfilled. Informal or formal enforcement action may be necessary where these responsibilities are not met.
1.2.2 Ante-mortem inspection
The FBO must follow the instructions of the OV to ensure that ante-mortem inspection of every animal to be slaughtered is carried out under suitable conditions.
Regulation (EC) 853/2004, Annex III, Section I, Chapter IV, Paragraph 5
Regulation (EC) 853/2004, Annex III, Section II, Chapter II, Paragraph 1
Where animals are slaughtered without ante-mortem inspection, the OV must declare the meat from such animals unfit for human consumption in accordance with the requirements of Article 45(a) Regulation (EU) 2019/627.
Where the FBO refuses to dispose of the meat, see chapter 2.8 on ‘Animal by Products (ABP)’ section 5 on ‘Enforcement’.
1.2.3 Ante-mortem inspection at the holding of provenance
The competent authority (FSA) may allow ante-mortem inspection of all species of animals intended for slaughter to be performed at the holding of provenance. This must be first agreed by the food business operator responsible for the holding of provenance and the FSA.
The following checks must be carried out at the slaughterhouse:
- on records or documentation at the holding of provenance, including verification of the food chain information;
- to ascertain if there is evidence or reason to suspect that the animals may contain chemical residues in excess of the levels laid down in legislation, or residues of forbidden substances;
- for signs indicating problems related to animal welfare, including excessive dirtiness;
- that they were fit for transport.
The animals fit for slaughter shall be properly identified and separated from other animals and sent directly to the slaughterhouse together with health certificate completed by the OV which can be found at annex 7.
Where animals are not slaughtered within three days from the date of issue of the health certificate then:
- where the animals have not been dispatched from the holding of provenance to the slaughterhouse, an additional ante-mortem inspection shall be carried out and a new health certificate shall be issued; or
- where the animals are already on their way to or are at the slaughterhouse, the slaughter may be authorised as soon as the reason for the delay has been assessed, provided that the animals undergo an additional ante-mortem inspection in accordance at the slaughterhouse.
- In the case of farmed game where slaughter is carried out at a slaughterhouse, by way of derogation from the above, EU Countries may allow slaughter until 28 days from the date of the issue of the health certificate if:
- only small quantities of the farmed game meat are directly supplied by the producer to the final consumer or to local retail establishments directly supplying to the final consumer; and
- not more than 50 animals are slaughtered per year at the holding of provenance
Where farmed game is not presented for slaughter within 28 days the animals must be re-presented for ante-mortem inspection as described in the case of other animals above.
Reference: Regulation (EU) 2019/624 Article 5.
Reference: Regulation (EU) 2019/624 Article 5 Paragraph 2(f) Health Certificate for animals that undergo ante-mortem inspection at the holding of provenance.
Reference: Regulation (EU) 2019/624 Article 6 Paragraph 5.
1.2.4 Cleanliness of the livestock
The OV must verify that the FBO is presenting animals that have a clean hide, skin or fleece, so as to avoid any unacceptable risk of contamination of fresh meat during slaughter.
Reference: Regulation (EU) 2019/627 Article 11, Paragraph 4.
1.2.5 Health status and identity
The FBO must have procedures in place ensuring that each animal or batch of animals accepted into the slaughterhouse:
- are properly identified
- are accompanied by the relevant information from the holding of provenance
- are not from areas under disease control with movement restrictions unless the Competent Authority (CA) so permits
- are clean
- are healthy, as far as the FBO can judge
- are in a satisfactory state as regards to welfare
If the FBO is aware of any animals that do not comply with any of the above then the OV must be notified.
Isolation facilities must be used for suspect animals.
Note: The AO must not complete the animal movement licences. This is an FBO responsibility.
1.2.6 Welfare
The FBO complies with the requirements of: The Welfare of Animals at the Time of Killing (England) 2015, The Welfare of Animals at the Time of Killing (England) 2015 and Welfare of Animals (Transport) Order 2006 (as amended) (WATO) and immediately takes necessary corrective measure to prevent recurrence of any non-compliance.
1.2.7 Animals accepted at the slaughterhouse
The FBO only accepts animals that are alive into the slaughter establishments, with the exception of:
- animals that have undergone emergency slaughter outside the slaughterhouse (red meat)
- delayed eviscerated poultry, geese and ducks raised for the production of ‘foie gras’
- animals (including farmed game, ratites and bison) slaughtered at the place of production
- wild game
Note: (EC) 853/2004 permits bison to be slaughtered on farm in ‘exceptional circumstances’ such as those that would put human health or animal welfare at risk if the animal(s) were transported live.
1.2.8 Acceptance of animals slaughtered on farm
Any animals (including farmed game and bison) slaughtered on farm, must be inspected at ante-mortem by an OV as soon as practicable before the slaughter date.
Please note that the OV is a veterinarian designated by the CA to carry out specific official controls on holdings on its behalf.
The bodies must be accompanied by the relevant documents:
- Veterinary health certificate (see annex 7 of this chapter).
- FCI (see annex 2 of this chapter).
- Certificate of Competence (CoC) for emergency slaughter. Should the animal be intended for human consumption then the person that carries out the emergency slaughter must hold a CoC for these operations. Alternatively, a Veterinary Surgeon can slaughter an animal for human consumption as part of their professional duties in exceptional circumstances (as long as there is not conflict of interest).
Bodies of animals slaughtered on farm must be transported hygienically and without undue delay. If transport takes more than two hours, it should be under refrigeration.
If necessary, the animals may be eviscerated at the site of slaughter under the supervision of the OV.
Reference: (EC) 853/2004, Annex III, Section III point 3
Reference: Annex 2 on ‘Additional food chain information for Cattle and Calves’ and annex 7 on ‘Health certificate for farmed game slaughtered at the holding’ of this chapter.
1.2.9 Dead on arrival (DOA) and dead in lairage (DIL)
Bovines:
The FBO reports DOA and DIL bovines O48M of age to a collector (their normal collector or the National Fallen Stock Company (NFSCo) on 0845 054 8888). They will arrange for the body to be collected and tested for Transmissible Spongiform Encephalopathies (TSE).
1.2.10 Lairage period
In England and Wales, animals must be slaughtered without undue delay.
Reference: (EC) 853/2004, Annex III, Chapter IV, 6.
1.2.11 Movement from slaughterhouse
Please see 4.1 for further details.
2. Procedures
In this section
2.1 Ante-mortem inspection procedures
2.2 Specific ante-mortem issues for poultry
2.1 Ante-mortem inspection procedures
2.1.1 Observations
The OV must observe each animal (except poultry) moving and at rest. The inspection must be sufficient to identify animals showing neurological symptoms, respiratory symptoms, alimentary tract abnormalities, change in gait, or external abnormalities.
Where there is a field lairage or buildings within the same County Parish Holding (CPH) number of the slaughterhouse then animals can move freely between the slaughterhouse lairage and the field lairage.
Where animals are held in fields or buildings associated with the slaughterhouse but which have a different CPH number the OV should under no circumstances carry out ante mortem inspection or any other Official duties in these areas. Animals cannot move from the slaughterhouse back to the field or buildings with a different CPH number.
2.1.2 Initial check
Routine ante-mortem inspection may begin with an initial check done by the MHI.
Where a suitably trained MHI assists the OV in carrying out the initial check, the OV should subsequently observe all the animals interacting with each other in their pens during ante-mortem inspection.
The initial check, if undertaken by the OV, may suffice as to constitute an adequate ante-mortem inspection.
2.1.3 Clinical inspections
In addition to routine ante-mortem inspection, the OV is required to carry out a clinical inspection of all animals which do not appear to be ‘normal’ and those that the FBO or an MHI may have put aside.
Reference: See Topic 2.3 on ‘Suspect live animals’ for the procedure to be followed for suspect animals.
Note: Clinical inspection does not necessarily require the OV to undertake a clinical examination, although this should be undertaken if warranted.
2.1.4 Considerations
Two important variables must be taken into consideration by the OV when performing ante-mortem inspection:
- every slaughterhouse and lairage layout are different
- the OV may require different conditions for inspecting the different species
Consequently, the OV must explain their ante-mortem requirements to the FBO to ensure that appropriate facilities and access are available.
2.1.5 Facilities and equipment requirements
To be able to carry out satisfactory ante-mortem inspections, clinical inspections and detailed examinations, the OV must have available the following facilities and equipment:
- adequate lighting
- adequate space
- adequate access
- adequate separate facilities for detailed examination (a crush or equivalent is desirable but not legally required – the OV should arrange suitable facilities with the FBO)
- isolation pen(s) for suspect animals with separate drainage and situated as to avoid contamination of other animals (not needed in all establishments)
- staffing assistance (for handling or restraint)
- sufficient time
- proper equipment, for example, thermometer and stethoscope
2.1.6 Time of ante-mortem inspection
The inspection must take place within 24 hours of arrival at the slaughterhouse and less than 24 hours before slaughter. Where ante-mortem is carried out at the holding of provenance then it must be undertaken within 3 days of the animals being slaughtered or in the case of farmed game, where this has been authorised, within 28 days of slaughter.
In some cases, ante-mortem inspection may need to be repeated. The OV may inspect the animal(s) at any other time.
Reference: Regulation (EU) 2019/627, Section 2, Article 11, Paragraph 2.
2.1.7 Exception to OV ante-mortem inspection at the slaughterhouse
OVs must carry out ante-mortem inspection at the slaughterhouse except in the following situation:
- An appointed OV has carried out ante-mortem inspection at the holding of provenance and the Food Chain Information (FCI) has been received by the FBO where additional checks will be carried out and may be conducted by a meat hygiene inspector as described in paragraph 1.1.3 above.
- The MHI is satisfied that the:
- FCI does not point to any possible problem for food safety
- checks indicate the animal’s welfare has not been compromised
- the MHI has checked the health certificate and found it to be satisfactory
- the OV must ensure, through regular checks that the MHI is carrying out such actions properly.
Note: Animals that have undergone ante-mortem at the farm by an appointed OV must come with a health certificate.
Reference: Specimen health certificate for live animals as set out in Part I of Annex IV to Implementing Regulation (EU) 2019/628.
Note: The MHI must have undergone required training in these duties before undertaking this work.
2.1.8 Emergency slaughter at the slaughterhouse
If an animal has an accident in a slaughterhouse, it must have veterinary ante-mortem inspection either before or just after the accident in order for it to be slaughtered for human consumption. To protect animal welfare where such an accident occurs out of hours, and the OV is not available, the ante-mortem inspection may be carried out by an appointed OV.
The OV must complete and sign the certificate for emergency slaughter.
The FBO is responsible for any costs incurred in such a circumstance; the carcase and all body parts must be retained for cold inspection by the OV.
Meat from animals that undergo slaughter following an accident in a slaughterhouse may be used for human consumption if:
- the animal had a veterinary ante-mortem inspection either before or after the accident
- the post-mortem inspection is performed personally by the OV
- at post-mortem inspection, no serious lesions other than those due to the accident are found
- a bovine is over O48M, TSE testing will be required (including bison)
Meat is to be declared unfit for human consumption if it derives from animals that have not undergone ante-mortem inspection, except for hunted wild game.
See sub-section 2.4 on ‘Emergency slaughter on farm’ for more information.
Reference: Regulation (EU) 2019/627 Articles 16, 45(a) and 47.
2.1.9 FBO responsibility
The FBO shall ensure that any animal that has experienced pain or suffering during transport or following arrival at the slaughterhouse is slaughtered or killed immediately.
Reference: WATOK Part 3 paragraph 12 (a) Schedule 1.
2.1.10 FCI
When the FBO passes FCI to the OV, the OV should use their professional judgement and take into account the information provided when performing ante and post-mortem inspection.
Reference: See chapter 2.1 ‘FCI and CCIR’ for additional information.
For Pigs
When FCI and / or other data (from farm of origin or ante-mortem inspection) indicates possible risk to public health, animal health or animal welfare, the OV can instruct the FBO to mark / identify those animals for further inspection procedures (FIP) at post-mortem inspection to decide if the meat is fit for human consumption.
Reference: Regulation (EU) 2019/627 Article 10, Paragraph 1.
2.1.11 Examples: pig conditions in ante-mortem justifying FIP at post-mortem
For the majority of the conditions listed on the current ante-mortem inspection sheet there would be no need for pigs to be marked to undergo FIP at post-mortem.
However, the following may justify FIP:
- mastitis (if associated with general signs)
- moribund / recumbent
- orchitis (marked to consider brucella, occupational zoonoses)
- suspect emaciation, poor condition
- suspect fever
- slaughtered in lairage
- gathering of evidence for enforcement purposes (welfare breach suspect)
Note: the OV is not limited to these conditions and should use their professional judgement.
2.1.12 Cleanliness of animals for slaughter
The OV must verify that the FBO complies with his duty to slaughter only animals that are sufficiently clean and record the details, if in their opinion the animals are too dirty to be processed hygienically.
The procedures in place to deal with animals that are soiled and the ability of the FBO to process those animals must be taken into consideration.
Reference: Regulation (EU) 2019/627, Articles 11(4) and 43(2).
2.1.13 Ante-mortem inspection records
Legislation requires the FSA to keep records of ante-mortem inspection.
Reference: Regulation (EU) 2019/627 Article 39(1).
Reference: See topic 3.4 on ‘Use of ante-mortem inspection records’ for details of where to record ante-mortem inspection results.
2.1.14 When animals can be moved from lairage to slaughter
The OV must be satisfied that an effective positive release system is in operation to ensure that every animal that requires ante-mortem inspection:
- receives it when it is required
- is only slaughtered for human consumption when ante-mortem inspection has been carried out and has been passed fit for slaughter
Animals must not be moved from the lairage to be slaughtered for human consumption until:
- ante-mortem inspection has been completed, recorded and signed by the OV, and
- any conditions for suspect animals have been met
2.1.15 Disease eradication programmes
The OV is to impose the conditions, already determined by the CA, for slaughtering animals under schemes for the control of:
- a specific disease, such as brucellosis or TB
- zoonotic agents such as salmonella
to permit taking more precautionary hygiene measures and allow a more thorough post-mortem inspection.
2.1.16 Ante-mortem inspection summary
The flowchart below summarises the procedure for ante-mortem inspection.
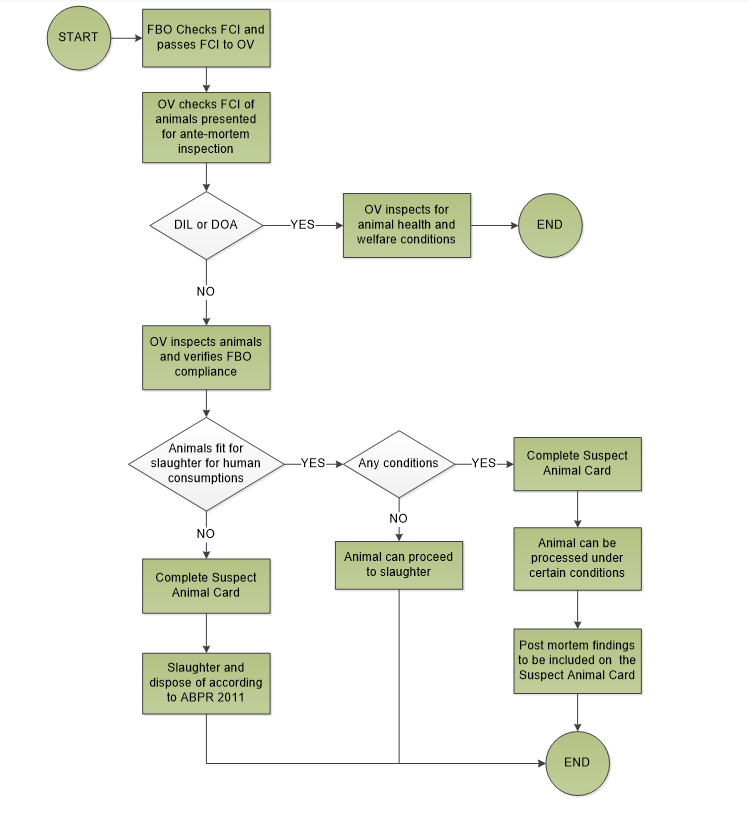
2.2 Specific ante-mortem issues for poultry
2.2.1 Ante-mortem inspection of poultry
A) When ante-mortem inspection is carried out at the holding of provenance:
When poultry arrives accompanied by a Health Certificate for live animals transported from the holding to the slaughterhouse, full OV ante-mortem inspection at the slaughterhouse is not required. In that case, ante-mortem inspection at the slaughterhouse can be performed by a suitably trained MHI under the responsibility of the OV. Inspection is to verify:
- animal identification
- animal welfare
- any condition that may adversely affect human or animal health
When the birds are not slaughtered within three days of the issue of the Health Certificate, the following should take place:
- birds that have not left the holding must be re-examined and a new Health Certificate issued
- birds that have arrived at or are en route to the slaughterhouse may be slaughtered for human consumption after they have passed an ante-mortem inspection performed by the OV
Reference: Regulation (EU) 2019/624 Article 5 and MOC 2.1.7 above.
Reference: Regulation (EU) 2017/625 Article 18(2)(a).
B) When ante-mortem inspection is not carried out at the holding of provenance:
Birds shall be subjected to ante-mortem inspection at the slaughterhouse. When doing ante-mortem inspection the OV must:
- assess the overall health and welfare of the birds
- listen to the birds and observe a random sample checking posture, wattle colour and cleanliness, and
- consider the information available from the FCI
Ante-mortem inspection at the slaughterhouse can be limited to a representative sample of birds from each flock.
Under Regulation (EC) No 2160/2003 ‘flock’ means all poultry of the same health status kept on the same premises or in the same enclosure and constituting a single epidemiological unit; in the case of housed poultry, this includes all birds sharing the same airspace. In practical terms this equates to “a group of birds reared in the same house within the same farm. For birds not confined solely to a house a flock is equivalent to a group of birds that share physically a designated area.”
When ante-mortem is limited to a representative sample from the flock, the OV, as a minimum, must perform ante-mortem inspection of the first load from each flock arriving to the slaughterhouse. That means that if more than one load of poultry from the same house arrives to the slaughterhouse on the same day, the OV must inspect at least the first lorry for that flock or, if the first lorry contains less than 500 birds (footnote 1) , a number of lorries until ante-mortem has been carried out on at least 500 birds from the flock.
If another load of birds from the same house is sent for slaughter the following day, the OV is required to carry out ante-mortem inspection of at least the first load from that house that day (or several lorries until ante-mortem has been carried out on at least 500 birds from the flock). This is because ante-mortem inspection in the slaughterhouse must take place less than 24 hours before slaughter and the conditions of the flock might change within that period of time.
The OV can carry out ante-mortem inspection of more than one load per house per day as and when needed or considered necessary by them, particularly in cases where the FCI or ante-mortem checks on the first load raise possible public health, animal health or animal welfare concerns for a given flock. Each establishment will be required to develop a positive release system agreed between the FBO and the OV to ensure the required sample of birds has received ante-mortem inspection prior to slaughter.
Routine welfare checks carried out by the OV or the OA should be spread out to ensure that loads that have not been subjected to ante-mortem inspection are also covered by those welfare checks.
Details of the systems in place to carry out ante-mortem inspection of a representative sample of birds from each flock should be agreed between the OV and the FBO and should be documented for the benefit of other officials attending the plant. It is recommended that a Standard Operating Procedure (SOP) is developed by the FBO in agreement with the OV.
The SOP (or any chosen documented form) must detail the positive release system agreed with the FBO, the systems put in place by the OV to ensure that welfare checks undertaken by the OV/OA are spread out to include loads that have not received ante-mortem inspection and actions to be taken if ante-mortem of the first load, or subsequent checks indicate welfare issues or disease concerns (for example, inform the FBO that the number of checks for a particular flock are going to increase to ensure the positive release system is consequently adapted). The SOP should be reviewed regularly and as often as necessary.
If there is an outbreak of a notifiable disease in poultry (such as Avian Influenza or Newcastle Disease), to carry out AMI on a representative sample of the flock is not permitted when poultry is moved to the slaughterhouse under a movement licence (e.g. from a restricted zone). In those circumstances, the OV must undertake immediate full veterinary Ante-Mortem Inspection (AMI) upon unloading from the vehicle.
Reference: Regulation (EU) 2019/627 Articles 2(21) and 11(1)
Reference: Regulation (EC) 2160/2003 Article 2(b)
2.2.2 Poultry testing positive for salmonella
Where the FCI received shows that the batch has tested positive for Salmonella, the OV must ensure that the appropriate arrangements are in place to:
- slaughter the batch at the end of the production day where possible, or at the end of a production run where necessary on welfare grounds and
- undertake cleaning, and where required disinfection, after slaughter
Note: See chapter 2.1 on ‘FCI and CCIR’, topic 2.1 on ‘FCI-Poultry’ for full details of the required actions in the case of Salmonella positive poultry batches.
2.2.3 Poultry showing signs of disease
If the birds show clinical symptoms of a disease, they may not be slaughtered for human consumption. However, killing of these birds on the slaughter line may take place at the end of the normal slaughter process, if precautions are taken to avoid the risk of spreading pathogenic organisms and to clean and disinfect the facilities after killing.
Reference: Regulation (EU) 2019/627 Article 43 Paragraph 3.
2.2.4 Poultry slaughtered at the holding
In the case of poultry slaughtered at the farm and sent for delayed evisceration, the ante-mortem inspection will be done at the holding by an OV or an AV.
Delayed eviscerated poultry obtained at the farm of production may be kept for up to 15 days at a temperature of not more than 4°C. It must be then eviscerated in a slaughterhouse or in a cutting plant located in the same member state as the farm of production and must undergo post-mortem inspection. Non-eviscerated carcases must be accompanied to the slaughterhouse or cutting plant with a ‘Health Certificate for poultry intended for the production of foie gras and delayed eviscerated poultry slaughtered at the holding of provenance’.
Reference: Regulation (EU) 2019/624, Article 6(2) – Health Certification.
2.2.5 Health certificate
The post-mortem inspection at the slaughterhouse or cutting plant must include a check on the certificate accompanying the carcases.
Reference: Regulation 2019/627 Article 10(2)
2.3 Suspect live animals
2.3.1 Slaughter of suspect animals
Suspect animals are to undergo detailed ante-mortem examination in order for the OV to make a decision whether the animal is fit for slaughter for human consumption.
The OV must defer the slaughter of animals suspected of having a disease or condition that may adversely affect human or animal health. The FBO should hold the animal(s) in isolation pending the OVs final decision.
Reference: Regulation 2019/627 Article 43(4)
2.3.2 OV judgement
Each OV should make a professional judgement based on the FCI, ante-mortem inspection or any other information presented, as to which animal/s should be further examined. Such an examination may include taking of appropriate samples.
2.3.3 Examples of suspect animals
Examples where the OV might identify suspect animals are where:
- animals show clinical signs of illness, disease or disorder
- animals show clinical signs of a disease transmissible to man or animals, especially a notifiable disease
- Example: animals are found or suspected to have any form of clinical TB
- animals show clinical signs of a disease or disorder likely to make fresh meat unfit for human consumption
- animals show signs of fatigue or stress
Note: they must be rested for not less than 24 hours unless the OV has determined otherwise
OR
- there is evidence or suspicion that illegal or unauthorised substances have been administered, or there may be veterinary medicines in excess of maximum residue limits present in the animal
Reference: Regulation 2019/627 Article 43(3) and (4).
2.3.4 Veterinary medicines
Animals that might contain residues of veterinary medicinal products in excess of the levels allowed or residues of forbidden substances are to be sampled with in accordance with The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) Regulations 1997.
Reference: Regulation (EU) 2019/627 Article 43(5).
2.3.5 OV action
Where a suspect animal has been identified the animal should be removed to an isolation pen and a suspect animal card (AMI 2/2) completed.
The MHI conducting the post-mortem inspection of a suspect animal must be informed of the circumstances by the OV to ensure that ante-mortem and post-mortem findings are correlated and recorded on the AMI 2/2.
2.4 Emergency slaughter on farm
2.4.1 Local Authorities (LA) purpose
LAs are the CA for enforcement during the transit of animals from the farm to the slaughterhouse. The LA and APHA One Health must be contacted if any breaches are suspected during the transport of the animals. See Chapter 2.3 on Animal Welfare sub-section 3.3 ‘Referral to LA / APHA’ for the referral process.
2.4.2 Definition
Updated [Meat from a red meat animal that has undergone emergency slaughter outside the slaughterhouse, following a veterinary ante-mortem, may be used for human consumption only if it is from an otherwise healthy animal that has suffered an accident that prevented its transport to the slaughterhouse for welfare reasons.]
2.4.3 OV attendance
Updated [The OV needs to be present during the post-mortem inspection of any animal that has undergone emergency slaughter.
Delayed or cold inspection is not permitted for animals slaughtered on farm under the provisions in (EC) 853/2004 Annex III, Section I, Chapter VI.
The OV should note any injuries that would indicate an on-farm welfare problem and if these are present, APHA must be informed (reference number provided must be kept and recorded in the FSA Day Book at the slaughterhouse) as detailed in chapter 2.3 on ‘Animal welfare’.]
2.4.4 Declarations
Where an ungulate is required to undergo emergency slaughter a health certificate is required to be completed indicating a favourable outcome of the ante-mortem and accompany the slaughtered animal to the slaughterhouse for examination by the OV.
Any observations relevant for subsequent meat inspection shall be recorded in the health certificate including details of any treatment administered by the PVS to the animal.
Reference: (EC) 853/2004, Annex III, Section I, Chapter VI, 5 & 6 and (EU) 2019/624, Article 4.
Reference: See Annex 1 for the combined ‘Model Declaration for Emergency Slaughter for Human Consumption of Bovine Animals Outside the Slaughterhouse’ and Regulation (EU) 2019/628, Part I of Annex IV ‘Veterinary Surgeon’s Declaration’.
2.4.5 Eligibility
Please follow the steps in the table below with regards to eligibility:
| Step | Action |
|---|---|
| 1 | Confirm animal eligible by checking:
|
| 2 | Check if any testing is required, for example, TSE. |
| 3 |
Post-mortem inspection must be carried out by an OV at the slaughterhouse.
|
| 4 | The oval health mark should be applied to fit carcases. |
2.4.6 Exceptional circumstances: bison
In exceptional circumstances, bison may be slaughtered on farm in accordance with the provisions relating to farmed game.
Reference: (EC) 853/2004 Annex III, Section III, Para 4.
2.4.7 OV action in the case of discrepancy
The following table details the actions that the OV should take where discrepancies in eligibility occur.
| Step | Action |
|---|---|
| 1 | In all cases, first contact the PVS who attended the animal.: |
| 2 | Where the declaration is not completed correctly and in particular does not have the date and time of emergency slaughter completed, please refer to the section on Declarations (above) for appropriate action to be taken. |
| 3 | Keep the PVS declaration received in a secure file and record the circumstances in the Day Book. |
| 4 | Record the results of post-mortem Inspection and correlate with the PVS declaration. |
| 5 | Any welfare concerns should be reported to APHA and the LA. |
| 6 | Where discrepancies in the declaration are identified and the PVS is unable to supply a correct declaration the carcase should not be health marked and must be rejected as unfit for human consumption because it is not in compliance with the requirements of (EC) 853/2004, Annex III, Section I, Chapter VI. |
2.4.8 Examples of possible discrepancies
The following list is provided for illustrative purposes and is not considered to be exhaustive:
- The OV has verifiable evidence to support the opinion that the animal has not suffered a genuine accident.
- The declaration has been altered after completion without initials to confirm authenticity.
- The PVS was not present at the time of slaughter of the animal and / or the declaration does not conform to the requirements of the specimen declaration at Annex 1 of this chapter, including failure to record the date and time of emergency slaughter.
- Transport / time / chilling requirements have not been adhered to.
- The number of animals received from a single source is excessive.
- The OV has obtained verifiable evidence to demonstrate animal welfare breaches.
2.4.9 Declaration
An animal may be accompanied by a farmer’s declaration that the animal is known or suspected to be injured or showing signs of abnormality.
Reference: See Annex 2 of this chapter for an example of a model document to accompany animals showing signs of a disease or condition that may affect the safety of meat derived from them
2.4.10 FBO responsibility
The farmer/owner who reared the animal must ensure that:
- A declaration stating the identity of the animal and indicating any veterinary products or other treatments administered to the animal, dates of administration and withdrawal periods, has accompanied the dead animal to the slaughterhouse.
- A veterinarian has carried out an ante-mortem inspection of the animal and completed a health declaration (date and time, reason for the emergency slaughter and the nature of any treatment).
- The slaughtered and bled animal has been transported to the slaughterhouse hygienically and without undue delay. Removal of the stomach and intestines, but no other dressing, may have taken place on the spot, under the supervision of the veterinarian. Any viscera removed has accompanied the dead animal to the slaughterhouse and has been identified as belonging to that animal.
If more than two hours elapse between slaughter and arrival at the slaughterhouse, the animal must be refrigerated. Where climatic conditions so permit, active chilling is not necessary.
The FBO of the slaughterhouse must verify that all the above requirements have been followed. The FBO must provide all the above documents and details to the OV at the slaughterhouse and follow any instructions during the post-mortem inspection (including any additional tests required) concerning the use of the meat.
Reference: (EC) 853/2004 Annex III Section I Chapter VI ‘Emergency slaughter outside the slaughterhouse’
2.5 Slaughter of fractious animals (bovine)
Domestic bovines that are considered fractious, dangerous or not handleable and which the FBO intends to enter into the human food chain can be slaughtered on-farm in exceptional circumstances.
This is a national policy which is permitted on a case-by-case basis, where such animals cannot be transported because transportation would present either a health or safety risk to their handler or a welfare risk to the animal.
Authorisation by the CA is required and must be informed in advance of the date and time of slaughter of the animal(s).
Other conditions that must be met are:
Slaughter of fractious bovines conditions and actions
| Step | Action |
|---|---|
| 1 | The owner of the bovine must submit a request to the FBO (at the slaughterhouse). |
| 2 | The herd undergoes regular veterinary inspection and details are available. |
| 3 | The circumstances are discussed between the AV (or OV carrying out the veterinary inspection on-farm) and the FBO and OV (at the receiving approved slaughterhouse) before any action is taken. |
| 4 | Prior agreement has to be reached between the OV at the slaughterhouse and FBO that the body of the animal can be consigned to the slaughterhouse for processing. |
| 5 | Animal welfare requirements are complied with. |
| 6 |
The AV/OV on-farm must issue a health certificate attesting to a favourable result of the ante-mortem inspection, correct slaughter and bleeding and the date and time of slaughter.
See Annex 7 of this chapter. |
| 7 |
The appropriate declaration (FCI) must be duly completed by the farmer/owner who reared the animals, confirming their identity and indicating any veterinary products or other treatments administered, dates administered and any appropriate withdrawal periods. This must accompany the slaughtered animal to the slaughterhouse.
|
| 8 | Correct animal identification must be provided (passport up to date with readable ear tags in bovines) |
| 9 | Slaughtered and bled animals are transported to the slaughterhouse hygienically and without undue delay. If transport takes more than two hours, the animals are, if necessary, refrigerated. Where climatic conditions so permit, active chilling is not necessary |
| 10 | The OV must be present when the body arrives, and carry out the post-mortem inspection |
Some of the reasons for declaring the meat unfit for human consumption may include;
- Where the document/declaration accompanying the animal fails to comply with the information requirements for FCI in (EC) No 853/2004, this failure must result in the animal or meat from the animal being declared unfit for human consumption by the OV.
- If the decisions concerning live animals have not been complied with, the OV must declare the animal unfit for human consumption; this could include where the animal’s identification is not reasonably ascertainable or where animals are subjected to treatment with veterinary medicinal products in excess of permitted levels or where there is suspected presence of veterinary residues because the required withdrawal period has not been complied with.
- Where any of the many decisions concerning meat have not been complied with, the meat must be declared unfit for human consumption: this may include failure to have undertaken an ante-mortem inspection (by the AV/OV on the farm); failure to have conducted a post mortem inspection (for example. when the offal has been discarded prior to post-mortem inspection) may also be a reason for declaring the animal or meat derived from it as being unfit for human consumption. The carcase and all by products would be required to be disposed of as ABP.
3. Record keeping
In this section
3.1 Record keeping purpose
3.1.1 Purpose
- To record disease conditions for disease surveillance purposes.
- To indicate to the FBO that the OV has passed or rejected the animal for slaughter for human consumption.
- To meet statutory obligations to maintain records and supply FBOs, producers, veterinarians and the CA with relevant information.
3.2 Positive release pen card
3.2.1 Purpose
The pen card may be used to identify, to the FBO that the animals have been inspected and found as suitable to be slaughtered for human consumption without additional conditions. It should be clearly displayed on the pen after satisfactory ante-mortem inspection.
3.2.2 Completion
Plant operatives enter stock details.
The OV authorises slaughter by signing the positive release pen card.
3.2.3 Retention period
Completed pen cards are retained for 6 months. They may be used to check that only animals which have undergone ante-mortem inspection are slaughtered.
3.2.4 Alternative arrangements
The pen card system may be unsuitable in some establishments. An equivalent system that achieves the same outcome may be used.
3.3 Suspect animal card
3.3.1 Purpose
This card must be used to identify animals considered suspect but which may still be suitable for slaughter. It must be completed by the OV and accompany the animal on transfer to an isolation pen.
3.3.2 Multiple suspect animals
Suspect animal cards may be used for groups of animals where the OV considers it impractical to use one card for one animal, for example, a flock of sheep.
3.3.3 Where to display
The suspect animal card must be attached to a suitable position on the isolation pen.
3.3.4 Retention of suspect animal cards
The OV must notify the MHI(s) of any suspect animal and ensure the subsequent post-mortem findings are recorded on the AMI 2/2 (Ante-Mortem Health Inspection Suspect Animal card).
Reference: See chapter 9 on ‘Forms’.
The suspect animal card must be used to record the post-mortem findings and retained for 12 months.
3.4 Use of ante-mortem inspection records
3.4.1 ante-mortem inspection records: red meat
The AMI 2/3 (Ante-Mortem Inspection Record: Red Meat) must be used for all red meat species excluding pigs, unless the FBO provides an alternative containing at least the same information.
Note: The AMI 2/3 may still be used for pigs, as an aide-memoire, if required. See sub-topic below ‘Ante-Mortem data recording for pigs’
Reference: See chapter 9 on ‘Forms’ AMI 2/3
3.4.2 Who completes the AMI 2/3
Competent lairage staff may complete details on the left-hand side of the form. FSA staff must complete details in the remaining columns on the right-hand side of the form.
3.4.3 Information to include
The OV / MHI must complete details of the date and time of inspection and the OV signs the appropriate column. The ‘Comments / Action’ column must include:
- details of any abnormalities noted and any subsequent actions required
- arrival of emergency slaughter animals
Note: ante-mortem details must be completed and the positive release box initialled by the OV / MHI to indicate that the animal and the documentation have been checked and found satisfactory; the MHI may initial this box in the absence of the OV
- arrival of farmed game slaughtered on the farm
Note: ante-mortem details to be completed on the joint declaration certificate. See Annex 7 in this chapter
- any welfare concerns and action taken
- the arrival of over 48 months old cattle or imported cattle requiring BSE testing as described in Chapter 2.6 (TSE), point 2.1.3 (Animals that require testing).
3.4.4 AMI 2/5
The AMI 2/5 (Daily Ante-Mortem Inspection Record, Red Meat, excluding pigs) must be completed and signed by the OV on each day the establishment operates.
Reference: See chapter 9 on ‘Forms’.
3.4.5 Information to include on the AMI 2/5
The OV must record on the AMI 2/5:
- each occurrence of a condition identified at ante-mortem inspection
- each occurrence of a condition resulting in the animal not being processed for human consumption
- each occurrence that required bovine brain stem testing
- the total number of each species presented for ante-mortem inspection
Note: The OV is to retain the completed daily form to record details on the Weekly Ante-Mortem Inspection Report, Red Meat (AMI 2/6).
3.4.6 AMI 2/6
The AMI 2/6 (Weekly Ante-Mortem Inspection Report, Red Meat) must be completed by the OV on the first day of operation the following week (Monday if worked).
Reference: See chapter 9 on ‘Forms’
3.4.7 Information to include on the AMI 2/6
The OV must use the figures recorded for the previous seven days on the AMI 2/5 to complete this report. The OV must sign and return the report to CSU and retain a copy at the plant.
Note: Nil returns are required
3.4.8 Ante-mortem data recording in pigs
Ante-Mortem inspection records for pigs must be recorded by FSA staff on IRIS.
The AMI 2/3 (Ante-Mortem Inspection Record – Red Meat) may be used as an aide-memoire prior to input of the data on to IRIS.
Reference: See chapter 9 on ‘Forms’ for AMI 2/3 and chapter 2.1 FCI CCIR for the IRIS user guide.
3.4.9 Ante-mortem data recording for poultry
Ante-Mortem inspection records for poultry must be recorded by FSA staff on IRIS.
The AMI 2/4 (Ante-Mortem Inspection Record – Avian) may be used as an aide-memoire prior to input of the data on to IRIS.
Reference: See chapter 9 on ‘Forms’ for AMI 2/4 and chapter 2.1 FCI CCIR for the IRIS user guide.
3.4.10 Who completes the AMI 2/4 (where used as an aide memoire)
Competent lairage staff may complete details on the left-hand side of the form. FSA staff must complete details in the remaining columns on the right-hand side of the form.
4. Movements
In this section
4.1 Movement of live animals from slaughterhouse
4.2 Fields and buildings used in connection with the slaughterhouse operations
4.1 Movement of live animals from slaughterhouse
4.1.1 OV action
Animals that are presented to a slaughterhouse for slaughter must be slaughtered there, other than in exceptional circumstances.
Reference Regulation (EU) 2019/627 Article 43.
The Disease Control (England)/(Wales) Order 2003 Article 10.
Disease Control Order, (DCO): 10 Restrictions on movements to and from slaughterhouses.
No person shall— (a) move any animal to a slaughterhouse save for the purpose of slaughter within 48 hours of its arrival there; or (b) receive any animal from a slaughterhouse unless, in the case of any animal other than a pig, under the authority of a licence issued by a veterinary inspector.
“animals” means cattle (excluding bison and yak), deer, goats, pigs and sheep.
4.1.2 Unidentified animals
Animals whose identity cannot be reasonably ascertainable must be declared unfit for human consumption. They cannot be returned to their farms of origin.
4.1.3 Movement of cattle in England and Wales
Where Cattle have been sent to the wrong type of abattoir it may be possible in exceptional circumstances to move them to another abattoir. The FBO should apply to APHA for a licence.
Reference: See DCO Annex 6 for a sample Exceptional Licence for the Movement of Cattle from One Slaughterhouse to Another.
4.1.4 Movement of sheep, goats and deer in England and Wales
These animals may only be sent to other abattoirs in exceptional circumstances, such as a line breakdown.
The FBO must apply to APHA for a licence.
4.1.5 Movement of pigs in England and Wales
Under no circumstances may pigs be moved from a slaughterhouse under the Disease Control Order. In exceptional circumstances, such as a major line breakdown, that might compromise the animal welfare, pigs may be sent to another abattoir under the Miscellaneous Provision Order. The FBO must apply to APHA for a licence.
4.1.6 Movement of horses in England and Wales
Horses delivered to a slaughterhouse that are incorrectly identified must not be allowed to leave the premises. A horse incorrectly identified is not eligible for slaughter for human consumption.
However, a horse may be correctly identified and still be ineligible for slaughter for human consumption (for example, the Section IX is signed, withdrawals periods have not been observed). Horses correctly identified but not eligible for slaughter for human consumption can leave the abattoir provided no other restrictions apply (for example, disease controls).
4.1.7 Movement of poultry in England and Wales
Since poultry species are not covered by the Disease Control Order, these can be returned in exceptional circumstances to the farm of origin, or another abattoir, on welfare grounds, without a licence from APHA. For example, in case of a major line breakdown, when birds originated from a premises in a disease free area and no other restrictions apply.
4.2 Fields and buildings used in connection with the slaughterhouse operations
4.2.1 Fields which are part of curtilage
Field lairages within the curtilage of the approved slaughterhouse are part of the slaughterhouse and therefore the responsibility of the FBO. These field lairages are part of the approved slaughterhouse and must share the same CPH number.
Animals can therefore move into such a lairage.
The slaughter of the animals must not be unduly delayed, and the OV should monitor this, informing FBOs of their responsibilities.
Reference: (EC) 853/2004, Annex III, Section I, Chapter IV, 1.
4.2.2 Fields which are not part of curtilage
If the field (and this can also be extended to barns or other buildings used to keep animals) used in connection with the slaughterhouse business has a different CPH number, then even if adjacent it is effectively a different holding and subject to different rules.
These adjacent fields (or buildings) are not part of the approved slaughterhouse and must not be included in the curtilage.
These adjacent fields (or buildings) must comply with rules under The Disease Control (England)/(Wales) Order, the livestock identification and movements legislation and TB legislation and the movement of the animals on to the field and from the field on to the slaughterhouse must be reported, including the use of movement licences. They cannot serve as destination for animals moving direct to slaughter or via a slaughter market or collection while under standstill (DCO Schedule 1). Animals from TB restricted farms, or untested animals cannot be moved to an adjacent field with different CPH.
The OV must report to the LA Trading Standards for the abattoir any instance where the movement from animals from these adjacent fields (or buildings) to the slaughterhouse occurs unreported.
Under APHA’s implementation of the ID Regulations, the FBO would be considered responsible for two holdings and subject to the biosecurity rules pertaining to such situations, defining effective operational separation of the two.
Animals cannot move from the slaughterhouse back to these fields (or buildings).
5. Cleansing and disinfection for biosecurity
In this section
5.2 Cleansing and disinfection (Cand D) of lairages
5.1 Overview
5.1.1 Introduction
Adequate C and D facilities and standards are essential for disease control and biosecurity. This topic details the requirements in England and Wales in both red meat and white meat slaughterhouses.
This chapter includes the official verification of the C and D standards of:
- lairages (including any area within the curtilage of the slaughterhouse in which live animals are handled).
- vehicles transporting livestock or poultry.
- crates and modules used for transporting poultry.
5.1.2 Legislation
The relevant legislation referred to in this topic is listed below:
- (EC) 853/2004
- (EC) 852/2004
- The Transport of Animals (Cleansing and Disinfection) (England)/(Wales) (No. 3) Order 2003 (as amended)
- The Disease of Animals (Approved Disinfectant) (England) Order 2007 (as amended).
- The Disease of Animals (Approved Disinfectant) (Wales) Order 2006 (as amended).
5.1.3 Regulatory requirements
Red meat slaughterhouses must have adequate and hygienic lairage facilities or, climate permitting, waiting pens that are easy to cleanse and disinfect. The drainage of the wastewater must not compromise food safety.
There must be a separate place with appropriate facilities for the cleaning, washing and disinfection of livestock vehicles.
However, slaughterhouses need not have these places and facilities if the CA so permits and official authorised places and facilities exist nearby.
Reference: (EC) 853/2004, Annex III, Section 1, Chapter II, 1(a) and 6.
Poultry and lagomorph slaughterhouses must have a room or covered space for the reception of the animals and for their inspection before slaughter.
Crates for delivering animals to the slaughterhouse and modules, where used, must be made of non-corrodible material and may be easy to cleanse and disinfect. Immediately after emptying and, if necessary, before re-use, all equipment used for collecting and delivering animals must be cleansed, washed and disinfected.
There must be a separate place with the appropriate facilities for the cleaning, washing and disinfection of:
a. transport equipment, such as crates; and
b. means of transport
However, these places and facilities are not compulsory for b if officially authorised places and facilities exist nearby.
Reference: (EC) 853/2004, Annex II, Section II, Chapter I, 1(3) and Chapter II, 1 and 6.
5.1.4 Location of facilities
C and D facilities for livestock vehicles, crates and modules (for poultry and lagomorphs), must be provided in approved slaughterhouses.
Legislation allows the approval of slaughterhouses without having these places and facilities if official authorised places and facilities exist nearby and the competent authority has given permission. Where this is the case, the FBO must have a high degree of control over the facilities which must be:
- capable of being reached easily
- situated within a reasonable distance from the slaughterhouse
- regarded by the FBO as part of the slaughterhouse
- possible for the OV to supervise.
However, poultry crates and modules must be always be cleansed and disinfected on site and before leaving the slaughterhouse.
5.1.5 Advice to FBO
The OV may provide the FBO with advice on C and D of their lairage and on the C and D facilities they must provide for livestock / poultry vehicles
5.1.6 OV action where the facilities are inadequate
When the facilities provided by the FBO are insufficient or fail to operate correctly, the OV must follow the guidance contained in chapter 7 on ‘Enforcement’.
5.2 C and D of lairages
5.2.1 Introduction
The legislative requirements for the C and D of lairages in slaughterhouses are limited to those under the Hygiene Regulations and are therefore focussed on public health.
5.2.2 Lairage C and D requirements
The FBO must keep the lairage easy to cleanse and disinfect. Lairages and yards are part of the food premises and the FBO must keep them clean and maintained in good repair and condition. Consideration must also be taken of the need to prevent animals becoming soiled, and of the welfare of the animals while they await slaughter.
As part of the FBO’s legal responsibility for establishing procedures for the C and D of the establishment, the FBO should establish procedures for the C and D of the lairages and related areas (for example, unloading areas, races). The frequency and procedures including the disinfectant in use should be documented as part of the HACCP-based procedures (for example as pre-requisite programmes or SOP’s).
The frequency and standards of the C and D of the lairage should be established by the FBO based on risk. For example, slaughterhouses receiving animals from TB restricted farms would require a more frequent C and D of the lairage and using disinfectants effective for TB.
In the case of specifically designated slaughterhouses for operating during outbreaks of exotic notifiable diseases, the designation conditions establish the minimum frequency for the C and D of lairage (for example, daily).
5.3 C and D of livestock vehicles
5.3.1 Introduction
C and D of livestock and poultry vehicles is essential for disease control and biosecurity, and not just with regard to zoonotic diseases. This topic details the requirements in England and Wales for ensuring compliance with C and D of livestock vehicles in both red and white meat slaughterhouses.
The responsibilities of vehicle drivers and FBOs are outlined as well as the FSA responsibilities in monitoring and enforcement of the C and D process, and LA enforcement responsibilities.
5.3.2 Vehicle C and D requirements (excluding horses and rabbits)
A vehicle which has delivered livestock or poultry to a slaughterhouse in England and Wales must be C and D as soon as reasonably practicable after unloading, and in any case within 24 hours or before the vehicle is next used for carrying livestock or poultry, whichever is sooner.
Note: the transport of animals between the same two points over the course of a single day and in a means of transport used exclusively for that purpose does not need to be C and D each time.
The C and D may take place at the slaughterhouse or elsewhere, in line with current regulatory requirements.
Reference: Transport (Cleansing and Disinfection) (England) / (Wales) (No 3) Order 2003 (as amended). Article 3
5.3.3 Vehicle C and D requirements (horses and rabbits)
Before horses or non-hoofed mammals are loaded for transport, the means of transport must have been previously cleaned and, where necessary, disinfected. Disinfection is required in all cases where the means of transport was last used to transport livestock or poultry.
Soiled bedding and excreta must nevertheless be removed from the means of transport as soon as possible.
5.3.4 Vehicle C and D requirements (poultry)
Crates for delivering birds to the slaughterhouse and modules, where used, must be made of non-corrodible material and be easy to cleanse and disinfect. Immediately after emptying and, if necessary, before re-use, all equipment used for collecting and delivering live animals must be cleaned, washed and disinfected.
Disinfectant should be applied after the crates / modules have been cleaned. The disinfectant must be listed in approved disinfectants list on the Defra website for approved disinfectants and must be used at the established concentration.
In absence of an outbreak of Notifiable Avian Disease, the disinfectant must comply with the General Order. In case of an outbreak of Avian Influenza or Newcastle Disease, the designation of the slaughterhouse will require the use of disinfectants and concentration approved for the Poultry Orders.
Crates and modules must be cleaned on site so the use of Drivers’ declaration is not applicable for them. In order to prevent the risk of recontamination, cleansed and disinfected crates / modules must only be loaded in cleansed and disinfected lorries.
The requirement for C and D crates and modules is a hygiene requirement and verification of compliance should be carried out during the daily attendance as any other hygiene verification checks. In case of identifying any breach, the hierarchy of the enforcement must be followed.
5.3.5 Method of C and D
The interior and exterior of the vehicle and any containers and equipment used to transport birds or hoofed animals (other than horses) must be C and D.
In the case of animals not transported in a container; all the inside surfaces of those parts of the means of transport that the animals may have had access to during the journey must be cleansed whether or not they are soiled.
Any detachable fittings not used during the journey, any other part of the means of transport and any equipment must be cleansed if they are soiled.
In the case of animals transported in a container, the interior of the container shall be cleansed whether or not it is soiled, and the exterior of the container and any parts of the means of transport carrying the container must be cleansed if they are soiled.
The wheels, mudguards and wheel arches of the means of transport shall be cleansed whether or not they are soiled and whether or not the animals were transported in a container.
Overall, the only part of a vehicle specifically exempted is the driver’s cab.
Disinfectant must be applied at the adequate concentration (as per approved disinfectant list published by Defra) after the full cleaning of those parts. The completion of C and D in a single operation is not satisfactory as disinfectant would not be effective in presence of organic material and the use of water during the disinfection would dilute the required concentration and / or contact time for the disinfectant.
Re-contamination of the lorry after disinfection should be prevented by ensuring an adequate separation, layout of establishment and procedures.
Containers may be destroyed rather than C and D (for example, single use containers).
Nothing in the legislation requires the use of disinfectant inside the driver's cab of any means of transport.
Reference: The Transport of Animals (Cleansing and Disinfection) (England) (Wales) (No.3) Orders 2003 (as amended) Schedule 2.
5.3.6 Approved disinfectants
Vehicles or means of transport for animals that need to be disinfected after cleansing must be done so using a disinfectant approved under the Diseases of Animals (Approved Disinfectants) Orders, at the concentration required in the Orders.
A list of Defra approved disinfectants for animal diseases and their approved dilution rates is available.
For general disinfection, any approved disinfectant listed under the General Orders can be used. For specific disinfection for listed diseases (for example, bovine TB) only those disinfectants listed against the specific order may be used.
The following orders apply:
- General Order: to be used in all the lorries except when a specific order is required.
- TB Order: to be used in all the lorries transporting animals covered by specific or general TB licences (for example, bTB negative cattle from a farm under bTB restriction, bTB reactors, bTB direct contacts, bTB inconclusive reactors).
- Diseases of Poultry Order: to be used during outbreaks of Avian Influenza or Newcastle disease in lorries moved under licence (e.g. birds from PZ or SZ, mammals exposed to AI) and in designated slaughterhouses.
- Foot and Mouth Disease Order: to be used during outbreaks of FMD in lorries transporting animals requiring licence and in designated slaughterhouses.
- Swine Vesicular Disease Order: to be used during outbreaks of SVD in lorries transporting animals requiring licence and in designated slaughterhouses.
Reference: Diseases of Animals (Approved Disinfectants) (England) Order 2007
Diseases of Animals (Approved Disinfectants) (Wales) Order 2006
5.3.7 Livestock vehicle driver responsibility
Where a vehicle arrives at a slaughterhouse in England and Wales loaded with birds or hoofed animals (other than horses), and is to leave the establishment unloaded without first being C and D on site, the driver must complete and sign a declaration form ‘Undertaking to Cleanse and Disinfect Vehicle’ (FM/AW 27) stating where C and D will take place.
Poultry crates, and where applicable modules, must be cleansed and disinfected before they leave the slaughterhouse (see 5.3).
The form FM/AW 27 is available online only and will no longer be available in hard copy from APHA. There is only a requirement for a single copy of the form.
The FBO should provide the driver with the FM/AW 27 for completion and signature. The driver should hand the completed form to the FBO for retention.
Reference: See Annex 3 for an example of form FM/AW 27. The form is available online.
Reference: The Transport of Animals (Cleansing and Disinfection) (England) (Wales) (No.3) Orders 2003 (as amended), Article 8.
5.3.8 FBO responsibility, England and Wales
It is the responsibility of the FBO to:
- provide the driver with the declaration form (FM/AW 27) for completion
- ensure the driver signs the declaration form
- keep the single copy for three months and produce it to an inspector on request
- send a copy of the completed declarations to his LA on the same day that he receives them.
5.3.9 Alternative forms
The driver’s declaration has to be in a form approved by the Secretary of State.
There may be specific circumstances where, if agreed with Defra or the Welsh Government and the relevant Local Authorities, an alternative form may be used (see Annex 3b of this chapter).
Example: Integrated poultry businesses where vehicles always cleanse and disinfect at the same site.
5.4 Enforcement responsibilities
5.4.1 FSA responsibility
FSA authorised staff in England and Wales are responsible for monitoring and enforcement of the C and D process in approved red and white meat slaughterhouses by:
- verifying the availability of C and D facilities, equipment and approved disinfectant (for example, adequate water supply, suitable space, tools)
- verifying the C and D of poultry crates and modules.
These could be verified during the routine checks (during pre-operational checks or during ante-mortem inspection). The hierarchy of enforcement should be followed in case of failure to comply with this legal requirement (Regulation 853/2004, Annex III, Section I or II, Chapter II, paragraph 6 and Annex III, Section II, Chapter I, paragraph 3).
And by:
- undertaking random or targeted spot checks on the actual procedures followed for the C and D of vehicles transporting livestock or poultry.
- Monitoring the operation of the driver declaration system (if used in compliance with the article 8 of the Transport of Animals (Cleansing and Disinfection) (England) (Wales) (No.3) Orders 2003 (as amended) by undertaking checks to ensure that:
- declarations are being given to and signed by the driver of the vehicle
- declarations are being sent daily to the LA
- FBO is retaining copies of the declarations.
- Issuing verbal warnings to the FBO and / or to the haulier where non-compliance of C and D rules has been observed.
- Serving a notice (Note: there are separate notices for England and Wales) to cleanse and disinfect a vehicle or container upon the person in charge of the vehicle or container where a means of transport, a container or any equipment either:
- has not been cleansed and disinfected in accordance with the Orders; or
- needs to be cleansed and disinfected because it may give rise to a risk of transmission of disease.
- Immediately reporting to the LA the details of any cases of non-compliance with C and D rules, including where a notice has been served and has been disregarded, or needs to be followed up.
- Immediately reporting to APHA any breach of the licence movement conditions including the general movement licence for transporting cattle from TB restricted farms (see sample of TB24c in Annex 10 of the MOC Chapter 6).
- Informing the FBO.
The actual number or proportion of vehicles / declarations to be checked will be dependent on the level of checks authorised to be carried out at the plant, FSA authorised staff will have to undertake one of the following:
- Weekly checks – FSA authorised staff are to undertake 1.5 hours of checks each week, this can be split over multiple days or can be carried out on one day. Information from these checks must be recorded on K2. Plants undertaking weekly checks will be notified of the frequency of checks by the SLA and Contracts Team in advance and will be required to continue to check at that frequency for at least 3 months.
- Monthly checks – FSA authorised staff are to undertake 45 minutes of checks once a month within a set week (A schedule is available from the SLA and Contracts team) FSA staff can select any day within that set week but they must not select the same day as was selected in the previous month; for example, if in July checks were carried out on a Monday, in August checks can be carried out on any day but Monday. Information is to be recorded on K2. All approved red and white meat slaughterhouses will undertake the monthly check unless otherwise informed by the SLA and Contracts Team.
Note: There is no requirement to alternate days when checks are carried out if a plant only operates one day a week or vehicles only enter a premises on the same day each week.
Checks carried out weekly / monthly must include either a check of vehicles being correctly cleansed or disinfected or that declarations of ‘off site’ cleaning are being handled correctly. A combination of both must be undertaken whenever possible if vehicles at plants undertake either.
Checks carried out must be recorded on K2 and must include the number of vehicles checked for adequate C and D or the completion of the FM/AW 27 form, any issues that arise and what action was taken. Copies of any notices served should be retained at the premises for one year.
Reference: The K2 form is available online.
Guidance for completing the K2 records are available at Annex 9.
Details reported to the LA should include the following:
- the vehicle registration
- the name of the driver
- where possible the suspected destination
- details of the breach
- copies of any notices served
The OV should make a note of any referrals to the LA in the plant enforcement records.
Reference: The Transport of Animals (Cleansing and Disinfection) (England) (Wales) (No.3) Orders 2003 Article 9.
FSA staff must also report any non-compliances in relation to completing the declaration forms to LAs detailing what has been incorrectly completed. The OV should note any referrals to the LA in the plant enforcement records.
Reference: The Transport of Animals (Cleansing and Disinfection) (England) and (Wales) (No.3) Order 2003 Article 8.
In relation to rabbits and horses, FSA staff should be aware of the animal welfare requirements to cleanse and, where necessary, disinfect animal transport after each use. Suspected breaches should be reported to the LA.
Reference: The Transport of Animals (Cleansing and Disinfection) (England) / (Wales) (No.3) Order 2003 Articles 4 and 5.
5.4.2 OV responsibility in relation to drivers’ declarations
The OV is to discuss with the FBO any non-compliance where declaration forms are not being issued to drivers or not being sent to the LA as required. The FBO is to be reminded of their responsibilities and the importance of the declaration system in enforcing C and D rules.
Adequate C and D facilities and equipment are required in all the slaughterhouses regardless the implementation of drivers’ declaration.
Note: Breaches must be reported to the LA.
5.4.3 Local authority responsibility England and Wales
LAs and the FSA have shared enforcement responsibilities in relation to the C and D of vehicles at slaughterhouses. Appropriately authorised LA inspectors will discharge their responsibilities by:
- carrying out spot checks on drivers who have completed a declaration stating where they propose to carry out C and D of their vehicle as part of their risk based enforcement activity
- making announced or unannounced inspections to licensed establishments as part of a routine inspection programme; the OV present will be advised of their attendance and reason for the visit on their arrival
- responding to information received from FSA staff on NC with C and D of vehicles at the plant or the declarations procedure
- taking appropriate enforcement action where a notice has been served by the FSA under Article 9; LAs have sole responsibility for carrying out prosecutions
Reference: The Transport of Animals (Cleansing and Disinfection) (England)/(Wales) (No 3) Order 2003.
6. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Model document: Emergency slaughter / Veterinary surgeon’s declaration
Annex 2: Model document: Additional food chain information for cattle, sheep, goats and calves
Annex 3a: Sample document: FM/AW 27 Undertaking to cleanse and disinfect vehicle form (Word)
Annex 3b: Annex 3a alternative format (Excel)
Annex 4: Notice to cleanse and disinfect a vehicle (England)
Annex 5: Notice to cleanse and disinfect a vehicle (Wales)
Annex 8: Sample document: Emergency slaughter certification letter
Annex 9: Guidance for completing the K2 application for recording checks on C and D of vehicles
-
The selection of minimum 500 birds follows EFSA’s advice in Opinion on poultry meat inspection:
Indicating high level batch sensitivity to detect disease and conditions at sample size of 500 and informing that Ante-mortem inspection (if used correctly) has a relatively high probability of detecting most diseases and conditions in infected batches.
Sections
1. Introduction
In this section
1.1 Purpose
1.1.1 Key principle
Assimilated Regulation (EC) No 1099/2009, Article 3(1) states ‘Animals shall be spared any avoidable pain, distress or suffering during their killing and related operations’.
This requirement is the key principle underpinning the guidance which follows in this chapter.
1.1.2 FSA role
FSA Operations staff should verify compliance with relevant domestic legislation on animal welfare prior to and during slaughter and killing, taking proportionate enforcement action.
Every time animal welfare is compromised, and an animal is suffering, enforcement action should be taken. A risk based and proportionate approach to enforcement should not be perceived as supporting bad practices or compromising animal welfare.
Retained Council Regulation (EC) No 1099/2009 only refers to Business Operator (BO) not Food Business Operator (FBO). Throughout this chapter this reference is used where appropriate to ensure the correct use of terminology as used in the regulation.
The hygiene and welfare regulations require the BO to have procedures in place to guarantee that the welfare of each animal is not compromised on farm, during transport and on arrival at the slaughterhouse and the Official Veterinarian (OV) is required to verify compliance with this requirement.
1.1.3 Data collection
The FSA collects data to inform Defra and the Welsh Government of trends in animal welfare in England and Wales.
1.1.4 Welfare surveillance
The OV and FSA team must identify welfare issues in live / dead animals and carcases at post-mortem, which appear to have originated on the farm of provenance or during transport, gather evidence and report as appropriate.
- Serious breach: Failure to comply with legislation relating to the transport of animals has led to suffering being caused to animal(s) and that suffering is serious, unnecessary and directly attributable to the actions, or the failure to act, of the transporter and/or the keeper who caused the journey to be undertaken
- All other breaches: All other instances where the condition of animals and/or transport give cause for concern and represent potential non-compliance with legislative requirements. This would include less severe welfare issues, accidents and incidents that could not reasonably have been avoided. Annex 4 (report to APHA for Triage) should reflect the OV’s assessment of why the incident has been determined as non-urgent
For serious breaches, the local authority must always be contacted immediately and given the opportunity to attend.
Reporting to APHA should be carried out as below.
Delays in reporting incidents may affect the ability to gather further evidence. In cases of serious breaches, the LA Inspector must be given the opportunity to visit the abattoir as soon as possible and collect evidence gathered by the OV or gather evidence themselves. Alternatively, the LA may wish to direct the OV as to evidence gathering requirements in their absence if they are unable to attend the abattoir immediately. Annex 4 should reflect any contact made with the LA for serious cases and the outcome of the request to attend.
The OV should inform the BO about any Annex 4 referrals they make to APHA, for information and to allow the BO to take rectification action with their suppliers. A copy of the Annex 4 referral must be provided to the BO upon request.
In every case the owner of the animal must be given the opportunity to examine the evidence. They will usually be informed by the BO or their representative. It is not the role of the OV to inform the owner.
The BO should be informed by the OV and asked to contact the owner of the animal (where they are not the owner) giving the owner the opportunity to examine the evidence. A record must be made in the day book of this request.
Do not delay action to safeguard the welfare of animals. If there is immediate pain or suffering that cannot be resolved, (for example a broken leg) then the animal must be killed or slaughtered as soon as possible. Photographs and / or video evidence should always be taken. When an animal has an injury that is only apparent on moving, video evidence must be obtained. Detain the carcase for further examination and retain any relevant body parts as evidence for any enforcement action. Ensure that you maintain correlation of body parts with the animal’s ID should this be required as evidence.
Fitness for human consumption should not be a consideration in the decision to kill an animal on welfare grounds.
Other FSA staff, particularly Meat Hygiene Inspectors (MHI), must take an active role in welfare monitoring and when made aware of welfare issues, record them in the day book or personal note book and report to the OV for action to be taken. If there is suffering, the MHI, and/or OV, must take immediate action to prevent further suffering. In the absence of the OV, the MHI must ensure that the BO is instructed on action to be taken.
1.2 Legislation
1.2.1 Welfare legislation - EC legislation is now referred to as “Assimilated Regulation”
At the point of the UK’s exit from the EU, most operative EU Regulations and Decisions were incorporated into domestic law as retained direct EU legislation under the European Union (Withdrawal) Act 2018. From 1 January 2024, under the Retained EU Law (Revocation and Reform) Act 2023, retained EU law became ‘assimilated law’. Legislation that was, prior to 1 January 2024, referred to as “retained direct EU legislation” (which may often have ‘EU’ or ‘EC’ included in its title) should now be identified as ‘assimilated law’ where applicable to GB.
Key pieces of legislation relating to welfare include:
- Assimilated Regulation (EC) No 1099/2009 on the protection of animals at the time of killing
- The Welfare of Animals at the Time of Killing (Wales) Reference 2014 SSI 951 (WATOK)
- The Welfare of Animals at the Time of Killing (England) Reference 2015 SI 1782 (WATOK)
- Assimilated Regulation No (EC) 1/2005 on the protection of animals during transport and related operations
- Animal Welfare Act 2006
- Welfare of Animals (Transport) (England) Order 2006 SI 3260
- Welfare of Animals (Transport) (Wales) Order 2007 SI1047
- Council Directive 2007/43/EC (the ‘Broiler Directive’)
- The Mandatory Use of Closed Circuit Television in Slaughterhouses (England) Regulations 2018 (‘CCTV regulation’) SI556
- The Mandatory Use of Closed Circuit Television in Slaughterhouses (Wales) Regulations 2024 (Senedd Wales) SL(6)471
1.2.2 Assimilated Council Regulation No (EC) 1099/2009
Provides key animal welfare standards which must be achieved. It places a responsibility on the BO to ensure animals are provided with physical comfort and protection; protected from injury; handled and housed appropriately; do not show signs of avoidable pain or fear or exhibit abnormal behaviour; do not suffer prolonged withdrawal of food or water (Annex III); and are prevented from avoidable interaction with other animals which may harm their welfare.
Key requirements of the Regulation are:
- Standard Operating Procedures (SOP)
- Guides to Good Practice (GGP)
- Animal Welfare Officer (AWO)
- Certificate of Competence (CoC)
- keeping of records for 1 year
The regulation is also directly applicable across all Member States and is implemented through domestic legislation in the UK.
1.2.3 Domestic legislation
Domestic implementing legislation provides powers to appoint inspectors, details enforcement powers, specifies penalties, time limits for taking formal action, and maintains national rules.
England: The Welfare of Animals at the Time of Killing (England) Regulations 2015 SI 1782
Wales: The Welfare of Animals at the Time of Killing (Wales) Regulations 2014 SSI 951
1.2.4 Guides to good practice
Assimilated Regulation (EC) No 1099/2009 Chapter II, Article 13 requires Member States to encourage the development of guides to good practice. The following guides have been developed by industry and are validated by the Competent Authority (Defra).
British Meat Processors Association Guidance
British Poultry Council Guidance
Animal Transport Guides Guidance
Updated [Transporting animals in Great Britain]
European Animal Welfare Network Guidance
1.2.5 Assimilated Regulation No (EC) 1/2005 and Welfare of Animals (Transport) (England) Order 2006 SI 3260 and (Wales) Order 2007 WSI 1047 (WATO)
Assimilated Regulation No (EC) 1/2005 on the protection of animals during transport sets out the statutory provisions that apply to the welfare of animals transported for an economic activity. National implementing legislation is required to provide for enforcement, powers, penalties, time limits, derogations, and additional national measures. This is done through The Welfare of Animals (Transport) Orders / Regulations in England and Wales (WATO).
Assimilated Regulation No (EC) 1/2005 provides key welfare standards that must be achieved during transport.
The FSA does not enforce WATO or Assimilated Regulation No (EC) 1/2005. If the OV or MHI identifies a suspected non-compliance with welfare during transport, they must
- ensure appropriate action is taken immediately to safeguard the welfare of the animal, this may be slaughter where the animal is injured
- advise the haulier and the BO of the non-compliance
- where the incident is serious, telephone the relevant LA as soon as possible, see 3.2.2 for guidance on serious breaches
- collect evidence and report to the APHA dedicated email: CSCOneHealthWelfare@apha.gov.uk
- record details of the haulier, including driver name, vehicle registration number and trailer number; this should be recorded on Annex 4
- complete Annex 5 evidence form to accompany and be submitted with Annex 4 report form
- upload Annex 4 & 5 and evidence documents to Digital Asset Management System (DAMS), see Annex 21 of Chapter 2.3 for DAMS User Guide
1.2.6 Legislative responsibilities
Assimilated Regulations No (EC) 853/2004, No (EC) 2017/625, No (EC) 2019/624 and No (EC) 2019/627 all refer to duties in relation to animal welfare.
In addition, the FSA collects information required under assimilated Regulation No (EC) 2007/43/.
1.2.7 Assimilated Regulation No (EC) 853/2004
BOs transporting live animals to slaughterhouses must ensure that during collection and transport, animals are handled carefully without causing unnecessary distress.
Reference: Assimilated Regulation (EC) No 853/2004 Annex III, Section 1, Chapter I, 1.
1.2.8 Assimilated Regulation No (EC) 2017/625
Article 21, 1 of assimilated Regulation No (EC) 2017/625 (Specific rules on official controls and for action to be taken by the competent authorities in relation to the welfare requirements for animals) require official controls to verify compliance to be performed at all relevant stages of production, processing and distribution along the agri-food chain.
Article 17(c) defines ante-mortem inspection as:
‘… the verification, prior to slaughtering activities, of human and animal health and animal welfare requirements, including, where appropriate, the clinical examination of each individual animal, and the verification of the food chain information as referred to in Section III of Annex II to Regulation (EC) No 853/2004’.
1.2.9 Assimilated Regulation No (EC) 2019/624
Assimilated Regulation No (EC) 2019/624 establishes certain rules around the use of MHIs in the process of verification that animals presented for slaughter comply with animal health and welfare requirements. These are described in more detail in Chapter 2.2 of the MOC.
1.2.10 Assimilated Regulation No (EC) 2019/627
Title III, Chapter II, Section 2, Article 11, 3 of Assimilated Regulation No(EC) 2019/627 requires that ante-mortem inspection shall determine whether, as regards the particular animal inspected, there is any sign that the health and welfare of the animal has been compromised. Section 5 of this Regulation establishes controls on animal welfare, in particular Article 38 (verification of rules concerning protection of animals during transport and at the time of slaughter) and Article 44, setting measures in all cases on non-compliance with requirements for animal welfare.
1.2.11 Assimilated Regulation No (EC) 2007/43 (the Broiler Directive)
Lays down minimum rules for the protection of conventionally reared meat chickens (broilers) on holdings with 500 or more birds (known as the ‘Broiler Directive’).
Under this Directive, the maximum on-farm stocking density (SD) for conventionally reared meat chickens is 33 kg/m².
SD in excess of 33 kg/m² and up to 39 kg/m² is allowed, providing that the keeper complies with and records on the Food Chain Information (FCI) the extra requirements as detailed in the implementing legislation as listed below:
- cumulative daily mortality rate
- breed / line information
If FCI is received with SD over 39 kg/m², please refer this to APHA immediately and notify the Animal Welfare team.
Whilst foot pad dermatitis (FPD) is also one of the on-farm welfare indicators monitored under the Broiler Directive, this is only to be scored on an exception basis where there is a welfare concern that the FPD is severe. Please see section 3.3.5 and link below for further details on reporting FPD: The Code of Practice for Meat Chickens and Meat Breeding Chickens
Note: refer to chapter 2.4 on ‘Post-Mortem, Health and Identification Marking’, section 7.
Defra is the Competent Authority (CA) under the terms of this Directive. FSA collects the information required under Annex III of the Directive (required elements of poultry FCI) and reports back to the CA where there are indications of poor animal welfare conditions.
2. Business Operator Role
In this section
2.1 Standard operating procedure
2.4 Delay in slaughter in white meat slaughterhouses and the provision of food and water
Updated [2.5 Movement of oversize animals to another slaughterhouse]
2.1 Standard operating procedure
2.1.1 Introduction
Assimilated Regulation No (EC) 1099/2009 on the protection of animals at the time of killing requires all slaughterhouses to have an SOP. This means written instructions aimed at achieving uniformity in the performance of specific functions or standards. There should be SOPs for all the different operations, for example, lairage, restraint, stunning, bleeding and assessment.
The SOP should detail the process such that it explains fully the role of each individual in the activity it covers and all of their duties and responsibilities. It should also detail who is responsible for ensuring that the process is carried out correctly, carrying out assessments on the key parameters as in Annex 1, including monitoring for signs of unconsciousness, and taking any action necessary to ensure that the process is fully compliant with welfare legislation.
Reference: Assimilated Regulation No (EC) 1099/2009, Chapter II, Article 6
Recital 27 states: Standard operating procedures should be developed at all stages of the production cycle and should be risk-based. They should include clear objectives, responsible persons, modus operandi, measurable criteria, as well as monitoring and recording procedures. Key parameters set out for each stunning method should be specified in a way ensuring proper stunning of all animals submitted to the process.
Annex 16 of this chapter links to further guidance on SOPs.
2.1.2 SOP requirements
Assimilated Regulation (EC) No 1099/2009, Chapter II, Article 6 states:
- BOs shall plan in advance the killing of animals and related operations and shall carry them out in accordance with standard operating procedures. (EC) 1099/2009 Article 2, (i) states: "standard operating procedures" means a set of written instructions aimed at achieving uniformity of the performance of a specific function or standard;
- BOs shall draw up and implement such standard operating procedures to ensure that killing and related operations are carried out in accordance with Article 3(1)
Note: Article 3(1) states ‘Animals shall be spared any avoidable pain, distress or suffering during their killing and related operations’.
A business operator may use standard operating procedures as described in the guides to good practice.
As regards stunning, the SOP shall:
a) take into account the manufacturers' recommendations
b) define for each stunning method used, on the basis of available scientific evidence, the key parameters set out in Chapter I of Annex I, ensuring their effectiveness to stun the animals
c) specify the measures to be taken when the checks indicate that an animal is not properly stunned or, in the case of animals slaughtered in accordance with Article 4(4) (religious slaughter), that the animal still presents signs of life
- The BO must show SOPs to the OV on request. Article 6, (4) states: BOs shall make available to the competent authority their standard operating procedures upon request
- When non-compliances are found during normal operations for which there is a SOP, the SOP should be checked by the OV. Where the circumstances leading to the non-compliance are not covered, the BO must be required to make amendments to the SOP in order to prevent further similar non-compliances occurring
- The SOP in pigs and poultry establishments using gas stun systems, should detail action to be taken in the event of a breakdown. WATOK Schedule 1, requires that there is a means of flushing the gas stunner with atmospheric air with the minimum of delay and that there is a means of access to any pigs or poultry with the minimum of delay. (Pigs: WATOK, Schedule 1, paraph 29 (2)(e) and (f) and Poultry WATOK, Schedule 1, paraph 30 (3)(c) and (d))
- Poultry gas stun systems only. Defra have agreed that where the business can demonstrate flushing of the system with a high concentration of CO2 will kill all the birds there is no requirement to remove birds with the minimum of delay in the event of a breakdown
2.2 Animal Welfare Officer
2.2.1 Legislative requirements
Assimilated Regulation (EC) No 1099/2009 requires the BO to designate an AWO if the establishment slaughters more than 1,000 livestock units (definition of livestock unit below) or 150,000 poultry or rabbits per annum. The BO can appoint more than one AWO. For example, the BO could appoint an AWO for the lairage operations and another for the restraint and stunning / bleeding operations. Where slaughter without prior stunning takes place the AWO for that operation must be the holder of a CoC for activity 61. The responsibilities of the AWO are detailed in the legislation and should be specified in the SOP as they apply to the slaughterhouse operation.
Reference: Assimilated Regulation (EC) No 1099/2009, Chapter III, Article 17.
2.2.2 Advisory booklet for BOs
The EU Commission has produced an advisory booklet.
This provides an outline of the envisaged role and examples of the checks that an AWO could carry out with a suggested record keeping format.
2.2.3 Role of the AWO
The AWO must:
- report directly to the BO on matters relating to the welfare of the animals; they shall be in a position to require that slaughterhouse personnel carry out any remedial action necessary to ensure compliance with the rules laid down in Assimilated Regulation (EC) No 1099/2009
- comply with the responsibilities set out in the standard operating procedures of the slaughterhouse; these responsibilities shall be effectively brought to the attention of the personnel concerned
- hold a CoC issued for all the operations taking place in the slaughterhouses for which they are responsible; where activities include slaughter by religious rites, it may be necessary to designate the person holding a CoC for code 61 activities as the AWO for those activities
Note: Refer to section 5 on ‘CoC’ further details.
Keep a record of the action taken to improve animal welfare in the slaughterhouse in which they carry out their tasks.
Keep the above record for at least one year and make it available to the competent authority upon request.
2.2.4 Definition of a livestock unit
‘Livestock unit’ (LU) means a standard measurement unit that allows the aggregation of the various categories of livestock (red meat species) in order to enable them to be compared.
The conversion rates for 1 LU are summarised below:
- 1 adult bovine animal or horse
- 2 bovine animals under 8 months
- 5 pigs with a live weight of over 100 kg
- 6.66 other pigs
- 10 sheep and goats
- 20 lambs, kids and piglets less than 15 kg live weight
2.2.5 Restraining and stunning
Assimilated Regulation No (EC) 1099/2009 on the protection of animals at the time of killing Article 9 requires that the AWO (BO) ensures all equipment used for restraining or stunning animals is maintained and checked in accordance with the manufacturers' instructions by persons specifically trained for that purpose.
The AWO (BO) must keep a record of maintenance on restraining and stunning equipment and retain these records for at least one year.
The OV should regularly ensure that these are kept up to date. In the event of any equipment failure, the OV should examine the maintenance records. If they are missing or not up to date, the OV should take appropriate enforcement action.
Appropriate back up stunning equipment must be immediately available, on the spot, at all times the slaughterhouse is operating. This may differ from the first method used.
Updated [For slaughter by religious rites without prior stunning, in poultry only, this is not a requirement and a repeat incision with a knife is permitted. When stunning is used for religious slaughter in poultry a suitable back up method must be available at all times and used in the case of a failure to stun.]
Animals must not be placed in restraining equipment, including head restraints, until the person in charge of stunning or bleeding is ready to stun or bleed them as quickly as possible.
2.3 Restraining and stunning
2.3.1 Checks on stunning
Assimilated Regulation (EC) No 1099/2009 on the protection of animals at the time of killing Article 5 requires the BO to ensure that persons responsible for stunning, or other nominated staff e.g. AWO, carry out regular checks to ensure that the animals do not present any signs of consciousness or sensibility in the period between the end of the stunning process and death.
These checks must be carried out on a representative sample of animals and the frequency established by taking into account the outcome of previous checks and any factors which may affect the efficiency of the stunning process. A record must be kept of the checks carried out. It is for the BO to determine how these checks are carried out and detail in the SOP
The SOP should determine the frequency of checks and any factors used to reduce the checks. When the checks indicate that an animal is not properly stunned, the person in charge of stunning must immediately take appropriate measures as specified in the standard operating procedures.
European Food Safety Authority (EFSA) has produced guidance on the signs of consciousness in bovine, sheep and goats, pigs and poultry:
2.4 Delay in slaughter in white meat slaughterhouses and the provision of food and water
2.4.1 BO responsibility: contingency plan for delayed poultry slaughter
BOs should have contingency plans in place for delays in slaughter as part of the SOP, and as normal practice, arrange the catching, transport and the delivery of poultry in such a way that waiting times are kept to a minimum.
Assimilated Regulation No (EC) 1/2005 and Welfare of Animals (Transport) (England) Order 2006 SI 3260 and (Wales) Order 2007 WSI 1047 (WATO) require that poultry are provided with food and water after 12 hours from the start of their journey.
Poultry that are still aboard the transport vehicle are considered to still be in transit and the journey not completed. The journey starts when the animals start to be loaded onto the vehicle and ends at the point they start to be unloaded. When they are unloaded, this should be done without delay.
If it will not be possible to unload the birds and put them in the lairage within 12 hours of their journey starting, arrangements must be made by the FBO or haulier to provide them with feed and water or make alternative arrangements in accordance with their contingency plan procedures.
Liaison will be required with the LA if the 12-hour transport time is likely to be exceeded when birds remain on the transport as this comes under the responsibility of the LA.
In the case of a major breakdown in plant operations, the welfare of birds is paramount, and the BO should:
- Immediately notify farms and hauliers to ensure that no further birds are despatched to the slaughterhouse until the problem has been resolved
- Put in place measures to ensure bird welfare is protected whilst the breakdown is rectified. This may include:
- Keeping vehicles moving to ensure ventilation or placing transport containers in the shade and / or providing forced air ventilation. It is important to monitor both ambient temperatures as well as those within the transport vehicle
- Contact other slaughterhouses with compatible crate handling systems which may be able to accept live birds for killing / slaughtering
- Consider whether to return birds to the farm of origin for water and feeding. Sending birds to farms other than that of origin is not good biosecurity practice and may increase disease risk. Birds must be returned to the farm and unloaded within 12 hours of the start of the journey or feed and water will need to be provided
- Take all necessary action to rectify the breakdown
When the modules / containers are unloaded from the vehicle, (EC)1099/2009 will then apply and the provisions in Annex III for feed and water will need to be considered if slaughter is delayed.
The provision of feed and water may be by a method other than using water or feed in a recognisable form. FSA are aware that there are products available that provide a fluid and nutrient source to poultry. If clarification is required on the suitability of a product used, please consult with your FVL, FVC or E&J Technical Manager.
The BO should be able to demonstrate that the method used to provide feed and water is capable of achieving nutrient and fluid intake.
BOs must ensure that they plan the delivery and processing of birds taking into account their operational hours or make alternative arrangements where slaughter of all the birds delivered is not possible within the working day.
In exceptional cases, when birds remain between operating shifts, they must be supplied with water (and feed after 12 hours) and adequate ventilation provided, or alternative arrangements be put in place, such as transport to an alternative slaughterhouse.
Updated [2.5 Movement of oversize animals to another slaughterhouse]
2.5.1 Moving oversize cattle when not unloaded at the slaughterhouse
When an animal is identified as too large or has horns that prevent it being moved to the abattoir’s stun box the following procedure agreed with APHA shall be followed.
Cattle that have not been unloaded at the slaughterhouse, are deemed not to have been delivered and no licence is required for their onward movement. The DCO (Disease Control Order) general licence allows drop-off at more than one slaughterhouse as long as these are the last points of call for the vehicle involved.
It is vital that slaughterhouses have routine procedures in their SOP to assess the size of animals before unloading so that oversize cattle can be moved to an abattoir with suitable facilities.
2.5.2 Protocol for movement of oversize cattle to another abattoir after being unloaded.
Cattle that have been unloaded at the slaughterhouse and are then found to be too large to enter the stunning box can potentially be moved in exceptional circumstances to another abattoir with suitable facilities under a licence issued by an APHA veterinary inspector.
This process will not allow an animal to be returned to the farm of origin, only to another slaughterhouse that has suitable facilities for larger cattle.
The DCO Article 10b states: No person shall receive any animal from a slaughterhouse unless, in the case of any animal other than a pig, under the authority of a licence issued by a veterinary inspector.
The policy intention behind this is to allow such moves only in exceptional circumstances, not for commercial expediency. Any movement out of a slaughterhouse constitutes a potential extra risk and needs to be considered carefully before any such movement is licensed, though the risk will be less for moves to other slaughterhouses as opposed to moves elsewhere.
‘Exceptional circumstances’ mean that an animal’s welfare is compromised with no other resolution than to move it elsewhere. Legitimate reasons include:
- production-line breakdowns
- fire / flood
- a situation compromising welfare in a lairage with no other obvious solution (e.g. an oversized animal that cannot safely be killed in the lairage)
Commercial expediency cannot not be considered a legitimate reason.
When the slaughterhouse operator determines that they require a movement licence they should request the OV to assess the case and verify that the circumstances will meet the requirements for a licence. For example, that the animal is too large to handle safely within the raceway and stun box, that it cannot safely be killed in the lairage, and all other solutions have been considered and exhausted.
The OV assessment should consider where and when the animal is to be moved, as well as the fitness of the animal to be moved.
The FBO must maintain an up-to-date list of slaughterhouses that have suitable facilities for handling oversize cattle that can be referred to in the event of an animal needing to be moved for slaughter elsewhere.
Once satisfied that the animal should and can be moved, the OV shall contact APHA providing full details of the case and their personal contact details.
The OV at the receiving abattoir shall also be contacted to familiarise them with the case details.
Contact with APHA will be through the advice line:
- in England (0300 0200 301)
- in Wales (0300 303 8268)
- or alternatively information about the incident and OV contact details can be emailed to customeradvice@apha.gov.uk between the hours of 08:30 am and 17:00 pm (Monday to Friday) where this will be communicated to the duty vet who can contact the OV directly.
The OV must inform the FVC and animal welfare team animalwelfare@food.gov.uk of all movements approved with details of the animal, including ID and the premises from which the animal will be moved and those it will move to.]
3. FSA role
In this section
3.2 Verification of animal welfare
3.4 Entry of compliance level in animal welfare database
3.1 Inspection duties
3.1.1 OV checks
The OV should carry out checks:
- to monitor welfare of live animals
- to monitor slaughter operations
- to monitor slaughter by a religious method*
- on restraint facilities for religious slaughter*
* Note: See section 4 on ‘Religious slaughter’ in this chapter.
The OV must record welfare scores of 2, 3 or 4 on the FSA animal welfare and enforcement system (Chronos).
3.1.2 FSA duties
The following inspection and verification duties are to be undertaken at the given frequency.
| Duty | By | How often |
|---|---|---|
| Inspect and verify BO compliance with welfare legislation – see following paragraphs for details of daily welfare reports to be completed. |
OV and MHI MHIs must report any welfare incident to the OV. |
During each killing period and at least several times daily. The FSA team should establish clear guidelines on welfare checks and frequency. This must be reflected in the agreed welfare checks protocol. A copy of this must be retained in the site FSA office. |
| Confirmation of compliance with welfare legislation, or where welfare incidents occur, appropriate enforcement action taken and entry of details in Chronos, the animal welfare and enforcement system. | OV and MHI | Daily |
| MHI’s should record in the day book any welfare non compliances noted during post-mortem examination and in particular during head inspection if there are multiple captive bolt shots, ensure that the OV is made aware. Tec Files 117 captive bolt stuns in cattle. TEC Files - Tec Files 117.pdf - All Documents (sharepoint.com) | MHI | Daily when identified |
| The communication of inspection results to farmers and private veterinary surgeons. |
OV Management of the database may be by an MHI. |
Same day |
| Reporting of animal welfare incidents to APHA / LA. | OV | Same day where possible; non-urgent cases by 17:00 the next day. |
| CoC checks. The FSA team should maintain a welfare folder containing copies of SOPs, where not readily available, and a record of TCoC’s and CoC’s (name, ID number, species, activities, and expiry date of the TCoC) for all staff handling live animals, verified with the WATOK team, Corporate Support Unit (CSU) York. The CoC record should be updated each time a new member of staff begins work in the premises together with any updates to the CoCs held. This record may be either electronic or hard copy. | OV | For new staff and as required for all BO staff handling live animals. |
| Log welfare incidents on Chronos. Ref. chapter 7 on ‘Enforcement’. | OV | As required |
| Welfare surveillance | OV | As required – see below |
3.1.3 Welfare checks: OV responsibility
The OV has overall responsibility to ensure daily welfare inspections are conducted in plant. The delegation of specific monitoring duties to MHIs should be established at plant level by the OV. This will include CCTV monitoring in England or where permitted in Wales. The OV and MHIs should work together to ensure the highest standards of welfare are maintained. The Animal Welfare Verification Protocol pro-forma should be completed and maintained as a plant specific record of the duties carried out and an up to date copy retained in the site FSA office. This document can be found at Annex 8.
The OV will need to establish that each MHI is aware of the required welfare standards and that their welfare training is up to date.
On each day of operation, the OV and MHIs should ensure that the BO and staff follow the procedures laid down in the SOP.
The SOP should be reviewed regularly – at least monthly or following any change to the slaughter process introduced by the BO.
The database for CoCs can be accessed via K2.
You will need the date of birth or CoC ID number to check the details.
EU CoCs are no longer valid.
Citizens from the Republic of Ireland (ROI) can apply for a UK CoC when they have a current ROI CoC.
3.1.4 Daily welfare assessment reports
The WEL 3/1 (Red Meat) / WEL 3/2 (White Meat) Welfare Assessment Reports must be completed as appropriate on a daily basis when slaughter is carried out on the premises. The forms are located in Chapter 9 ‘Forms’.
3.1.5 Completion of daily welfare assessment reports
The workload in plants and time restraints will often result in these assessments being populated as a collective effort from the FSA team over the course of the working day. The time of each check should be entered and initialled in the relevant boxes by the MHI / OV who conducted the check for the specific area.
Where Health and Safety considerations mean that it is not considered safe to enter a stunning area or other areas to verify welfare, then checks should be carried out regularly using CCTV, (both live and recorded) where that is available. The person carrying out the checks should record that they were done remotely for H&S reasons. The ITL should be informed so that a review of H&S can be carried out.
Welfare competent MHIs should be designated by the OV for specific assessments. In OV only plants, the OV should initial the box after completion of the checks.
In the event that the MHI is the only FSA team member remaining (due to OV flexibility), then the OV should check the assessment form when next attending the plant. The OV should verify that the daily welfare sheet has been completed, where MHIs complete the checks and make a note of this in the daybook.
In plants where ‘cold inspection’ is carried out, the frequency of welfare checks should be established in consultation with the local field veterinary team. Additional visits by a Welfare Assurance Team member should be facilitated to ensure local managers are assured that welfare standards are maintained when FSA staff are not present.
3.1.6 Team responsibility
Where non-compliance with legislative requirements is identified by an MHI they must inform the OV immediately, having first taken action to ensure the welfare of the animal(s) is protected.
In doing so, the evidence gathering for potential enforcement should not be jeopardised and prompt action must be taken to seek witnesses to corroborate events. Carcase parts or the entire carcase, as appropriate, must be detained when they are evidence for a potential welfare case.
Where appropriate, a WATOK Enforcement Notice (WEN), suspension or in serious cases a revocation of the CoC should be used by the OV in liaison with the VEDM. Updated [Details on the procedure for extension of a WEN when requested by the BO can be found at 7.1.5.]
The use of the contemporaneous notebook, camera and video equipment by the OV is essential during these checks. OVs and MHIs should record details of any welfare incident observed in their contemporaneous notebook or in the day book as soon as possible after the incident. A camera should be used to obtain further evidence of a welfare incident if the OV is not present or unable to attend.
In England FSA staff must monitor the CCTV of the stunning and killing process at random during each working day. In Wales the FSA has agreed a CCTV viewing protocol with the main industry bodies and this should be followed by the OV in agreement with the BO.
The OV must close off the assessment report at the end of the day by making a note in the daybook.
A proactive approach to welfare monitoring should be exercised at all times and reality checks undertaken throughout the day by all FSA staff where the opportunity arises. These reality checks may sit outside recorded checks already undertaken and be reported and acted upon as with any other welfare issue and recorded in the daybook.
3.2 Verification of animal welfare
3.2.1 Introduction to the animal welfare assessment database
Chronos is the verification programme for animal welfare in slaughterhouses. Data is shared with Defra and the Welsh Government on a monthly basis.
It is used where welfare incidents have occurred, to record the level and nature of such welfare incidents and action taken.
3.2.2 Daily input
When a welfare incident is recorded, appropriate enforcement action should always be taken and recorded. A WEN should always be considered for scores of 4, other than in cases where the incident is an unavoidable accident, or the evidence is lacking. In every case, a score of 4 should be actioned by giving at least written advice to the BO except, where an incident or accident is considered to be unavoidable and no fault of the operator when a 4 score should still be applied and a verbal warning given. This should be clearly explained in Chronos and recorded in the daybook.
Should the welfare incident relate to an incident in transport or on farm, referral to APHA is required. In serious cases, the LA must be informed as soon as possible.
Where welfare issues are identified in live/dead animals, the OV should give consideration to the nature of the condition and whether the animal has suffered unnecessarily either on farm, in transport or both, and also whether that suffering is attributable to an act, or a failure to act, by a person responsible for that animal at any time. There are a number of outcomes depending on the circumstances and the OV’s considered judgement.
- Fitness for transport - where the condition of the animal would have been present on the farm and the welfare of the animal would have been seriously compromised during transport (and where the transporter could reasonably be expected to be fully aware of the condition when loading the animal), for example, broken legs, severely lame animals, very sick/dehydrated animals; the OV should treat these as serious breaches and make immediate contact with the LA, gather all available evidence (either on behalf of or in conjunction with the LA) and refer to the APHA email contact. Any potential offences committed by the keeper and/or transporter will be investigated further by the LA and any appropriate action taken. APHA may also consider regulatory action in order to prevent further non-compliance by the transporter
- Where the condition of the animal would have been present on farm but the welfare of the animal would not have been compromised during transport, for example, sheep scab, ringworm; the OV should refer these to the dedicated APHA email contact as a non-urgent breach referral and collect any available evidence (photos). These may trigger an on-farm inspection
- Where the condition of the animal would have been present on the farm and the welfare of the animal may have been compromised to some degree during transport but there is the possibility that the transporter may not have been aware of the condition when loading the animal for example, bruising, pig with prolapsed rectum, last 10% of pregnancy, slight lameness; the OV should refer these to the dedicated APHA email contact as a non-urgent breach referral and collect any available evidence (photos). These may trigger an investigation referral to the LA in relation to the transporter and/or a farm inspection
- Where the condition of the animal is the result of an accident or an unexpected event (and includes death) and the cause cannot reasonably be attributed to the actions, or failures, of the transporter. For example, injuries from unavoidable emergency stopping, animals unexpectedly experiencing a heart attack and knocked horns. The OV should refer these to the dedicated APHA email contact as non-urgent breach referrals which APHA will keep a record of and, if necessary, discuss further with transporters if levels become unacceptable and could be representative of a wider issue
- Where the OV observes actions of the transporter that are not in line with legislative requirements and cause unnecessary suffering to animals. For example, beating/kicking animals, excessive goad use and dragging animals, these should be treated as serious breaches and reported to the LA and to APHA for the attention of the Welfare In Transport team
Where animal welfare complies with all aspects of the legislation and a 1 score applies the OV will not need to take any action.
3.2.3 Frequency of observation
Some aspects require observing several times each day – this will depend on the throughput and risk:
Example: Effectiveness of stunning, bleeding operations.
Other aspects can be checked on a less frequent basis – at least monthly.
Example: The provision of a SOP as required by assimilated Council Regulation (EC) No 1099/2009 (see page 2-1).
These will also be verified at audit but the OV should ensure that checks are carried out monthly to verify that the BO has procedures in place to comply with all legislative requirements. It is a good idea to initial and date documents when a check is made.
3.2.4 Welfare incident recording
Select a welfare score when entering daily data in Chronos and provide explanatory details in the action boxes. See below for guidance on scoring.
Also, include any reference number that you may have been given by the LA or APHA and / or use the unique reference number allocated in the format: plant number / date / time offence was observed.
3.3 Referral to LA / APHA
3.3.1 Referral process
Where a suspect animal has arrived at the slaughterhouse, the OV should determine if, in their opinion, avoidable pain, suffering or distress has been caused to the animal(s), if this can be attributed to the person transporting the animal or causing the transport of the animal, or both, and ensure evidence is gathered (assimilated Regulation (EC) No 1099/2009 uses the word ‘avoidable’ but in other legislation the word ‘unnecessary’ is used).
- The OV shall ensure that the welfare of the animal is protected (for example, immediate slaughter, isolation, etc)
- Welfare breaches concerning transport considered to be serious and urgent, per section 3.2.2, should be reported as soon as possible by phone to the Local Authority (Trading Standards) for the slaughterhouse in addition to following the Annex 4 and 5 reporting process. Welfare breaches concerning the farm should be reported to LAs and APHA by phone as soon as possible. The OV shall ask for instructions from LAs/APHA with regards to evidence collection
Completed Annex 4 and Annex 5 reports must be returned to the APHA One Health mailbox: CSCOneHealthWelfare@apha.gov.uk.
OVs must identify each Annex 4 and Annex 5 with a unique serial number made up of Establishment no / date (ddmmyy) / time (hhmm) (for example, UK 1234 / 110517 / 1330).
Any incident that requires the completion of an Annex 4 must be reported to the FBO (and the driver/haulier when possible or deemed appropriate) with the aim of ensuring that the haulier and/or originating farm is made aware and can take any appropriate action as soon as possible to ensure that a similar incident does not occur in the future.
Serious breaches (covered by part (a) and (e) in section 3.2.2 Daily input)
Where a serious welfare breach requires urgent attendance of the LA, either to gather/ collect evidence in relation to animals or the means of transport/ transporter, the OV must contact the LA as soon as possible to explain the situation. It is expected that the LA will engage to either attend in person or explain what they require the OV to do in terms of evidence gathering/ retention. Where the LA is able to attend, please provide details of the action taken. Reasons for non-attendance or lack of assistance by the LA must be recorded on the Annex 4 and in the daybook, together with the details of the individual contacted, where known.
It is likely the animal will need to be immediately slaughtered so the OV must first ensure that a member of the FBO staff is contacted in order to slaughter the animal without undue delay. If the animal is suffering this must be done even if the LA plan to attend.
A member of the BO staff at a slaughterhouse who has had suitable training but no CoC may kill an animal in the lairage where it is injured, suffering or in pain, provided that the individual is suitably competent and the animal does not enter the food chain.
Retain the carcase or body parts affected as evidence (for the LA) and secure with appropriate identification, as per MOC instructions.
For all potential welfare in transport breaches, the required information is the transporter name and address and/or transporter authorisation number, full driver name and certificate of competence number (if available), vehicle registration / trailer number and vehicle approval number if available.
Where there are concerns that welfare of animals on the premises of origin could also be at immediate risk, an urgent visit by APHA and or the LA must be triggered within the next 24-48 hours. Therefore, the OV must contact the APHA customer advice team providing full details including which LA they have contacted:
- in England (0300 0200 301 options 3-2-1)
- or alternatively information about the incident and OV contact details can be emailed to customeradvice@apha.gov.uk between the hours of 8.30am and 5pm (Monday to Friday) where this will be communicated to the duty vet who can contact the OV directly to discuss and assign a visit as appropriate
- in Wales (0300 303 8268 working hours or 0700 0780 144 out of hours) as soon as possible to inform them of the incident and provide the welfare reference number
The associated Annex 4 and Annex 5 should be emailed by 5pm that day to CSCOneHealthWelfare@apha.gov.uk (and for England also to customeradvice@apha.gov.uk).
APHA will log the incident but the expectation will be that the LA has already been notified by the OV. Any failure to contact/liaise with the LA immediately by the OV at the time of incident may lead to further investigation not being possible.
APHA may also consider taking regulatory action alongside any investigation to prevent further non-compliance or bring a transporter back into compliance.
The relevant LA will be the primary authority for the area in which the slaughterhouse is based.
A summary of any communications with the LA/APHA should be entered in the FSA daybook or personal notebook if away from the establishment.
Non-urgent breaches (covered by parts (b), (c) and (d) in section 3.2.2 Daily input)
Reports must be submitted to CSCOneHealthWelfare@apha.gov.uk by 17:00 the next working day. The LA will be notified in a later email from APHA.
In all cases, gather the evidence as you would for a serious breach. The OV should contact a member of the FBO staff in order to ensure that any animals with identifiable welfare conditions that are causing pain, distress or suffering are slaughtered without undue delay. Body parts or carcasses required for evidence in a potential investigation should be retained as per MOC instructions.
A diagram for the referral process is provided at Annex 7.
3.3.2 Dead on Arrival (DOA) animals
Red Meat Animals DOAs
All red meat DOA animals should be reported to APHA. The OV should not carry out a post-mortem because this requires suitable facilities and expertise should the case result in a prosecution. The OV should consider whether there are any indications of why the animal may have died and whether there are any identifiable reasons as to why the animal should not have been transported. Animals that are dead on arrival may indicate serious breaches or may be unexplainable and unavoidable incidents. The OV should determine which, in their opinion, is the case when inspecting the animal.
Where it is suspected that the Stocking Density in transport is too high, the details of the vehicle should be recorded together with the number of animals, approximate weights, and type (for example, sheared sheep would require less space than those in full wool).
Poultry DOAs
In white meat premises if, after carrying out a sample of post-mortem examinations, the OV believes that welfare in transport or on farm has been compromised this should be reported to APHA as a suspected non-compliance of Welfare on farm or in Transport.
Broiler chickens over 1.5% DOA should be reported for investigation and other large poultry should be referred where there are over 2.5% DOA. Where there are regular occurrences at levels below these, they should also be reported after discussion with the E&J Technical Manager, FVL or FVC.
Although the legislation sets out minimum stocking densities, it does permit some flexibility on this for animal welfare reasons. The aim is to ensure the physical and thermal comfort of the birds. To determine whether an adjustment to a stocking density level is appropriate, transporters should consider the weight, size, and physical condition of the birds, as well as the weather conditions and overall journey time including loading and unloading. Where stocking density levels are thought to have caused welfare issues the incident must be reported and include the details of the vehicle, the number of animals, approximate weights, and type.
Poultry SD can be calculated from the table below, these figures may vary depending not only on the weight and size of the birds but also on their physical condition, the meteorological conditions and the likely journey time.
| Kgs | Min cm²/kg |
|---|---|
| < 1.6 kg | 180 - 200 |
| 1.6 – 3 kg | 160 |
| 3 – 5 kg | 115 |
| > 5 kg | 105 |
Reporting Procedure
If a welfare issue is suspected to originate on-farm then it should be reported to APHA following the process previously described.
In urgent cases, initial contact with the LA and APHA should ideally be by telephone (leave an answerphone message if necessary), with details of the case and confirm referral using the Annex 4 notification form and a completed Annex 5 evidence form. All calls and emails should be logged in the day book or personal note book to support the evidential chain.
The LA will inform the owner of any referral for investigation and the owner will be allowed to invite their own veterinarian to attend any post-mortem (diagnostic) examination undertaken in the course of an investigation. Failure to do so could jeopardise any court action. The BO should contact the owner of the animals where they have not been purchased by the BO.
Annexes 4 and 5 must be retained in the premises Animal Welfare folder.
All reports must be sent by email to APHA and a unique identifying number allocated to each case. This should be in the format:
WRN Plant number / date / time offence was observed. For example: WRN 7312/23.02.2015/10.36
This number should be used as the title for emails and other correspondence allowing traceability of all messages.
3.3.3 Assessing lame animals
When examining a lame animal, it is useful to record the degree of lameness using a scoring system. The descriptions below should be used to score the lameness and can then be referred to in statements and notes.
| Score | Level of Lameness |
| 1 | Visibly lame but can keep up with the group |
| 2 | Unable to keep up with the group |
| 3 | Requires assistance to rise; non-weight bearing on one or more legs |
| 4 | Requires assistance to rise; non-weight bearing on one or more legs; reluctant to walk; halted movement; unable to climb steep ramps |
| 5 | Unable to rise or remain standing; extreme discomfort or vocalisation with assisted movement |
Animals with lameness scores 3, 4 and 5 should be killed immediately where they lie. (assimilated Regulation (EC) No 1099/2009, Annex III, 1.11.) Slaughter does not have to be for human consumption and this should not determine the approach and timescale for dealing with the animal. Lame animals must not be loaded for transport to a slaughterhouse and transport of a lame animal must be reported to LA/APHA.
In some cases adult cattle (usually dairy cows) may be transported with shackles on the hind legs where they have had difficulty standing. It is permissible for these animals to be transported to a slaughterhouse only. They should be separated from other animals in transport. Should the animal have become recumbent in transport then this should be reported to LA/APHA.
Examples of a serious lameness breach:
- The condition of the animal would have been present before loading, resulting in the welfare of the animal being seriously compromised during transport. The transporter could reasonably be expected to be fully aware of the condition when loading
- The animal was likely injured during transport and this injury can be attributed to the actions of the transporter or the construction of the vehicle
Examples of a non-urgent lameness breach:
- The condition of the animal may have been present before loading, resulting in the welfare of the animal being compromised to some degree during transport. There is the possibility that the transporter may not have been aware of the condition
- The animal was likely injured during transport through no fault of the transport or transporter
When determining whether a case is to be reported as a serious breach or a non-urgent breach it should be judged on its own merits regardless of the lameness scoring.
For animals that may have been injured during transport, the OV should assess whether this can be attributed to the actions of the transporter or the construction of the vehicle. If this is the case, then they should indicate this on the Annex 4 referral (and consider immediate LA engagement for serious breaches). If the injury and circumstances surrounding it cannot be explained, then this should be referred via the Annex 4 process and the OV should clearly state that an accident/unavoidable incident may have occurred.
The OV must complete the declaration that avoidable/unnecessary suffering has occurred where they have suitable evidence that the condition of the animal is such that it should not have been transported or should not have been transported in the manner that it has been.
When collecting evidence, video footage of the lame animals is essential to convey the degree of lameness and suffering. Still photographs are of very little use in these cases.
3.3.4 Assessing heavily pregnant animals
It is illegal to transport an animal that is unfit for travel. What is deemed as ‘unfit for travel’ includes heavily pregnant females where more than 90 per cent of the expected gestation period has passed, unless they are being transported for veterinary treatment.
FSA responsibility When it is suspected that a heavily pregnant animal has been transported, the OV must record this on the daily welfare exception report. In determining if the animal is in late pregnancy, the OV should take into account the following
3.3.4.1 Ante-mortem examination - potential signs of heavily pregnant animal:
- obvious udder development, this occurs earlier in younger animals
- obvious enlargement of the abdomen and in cattle this will be particularly noted on the right hand side
- enlargement and swelling of the vaginal area and vulval lips
- mucous discharge from the vagina in advanced pregnancy
- tail slightly raised and appear in discomfort if very close to birthing
Where these or other findings are noted at ante-mortem, the OV should notify the MHI and ensure they monitor the carcase during dressing and that their observations are recorded.
3.3.4.2 Slaughter
It is best practice that foetuses should not be removed from the uterus until at least 15-20 minutes after the maternal neck or chest cut. Foetal death or irreversible brain damage will usually have occurred by 15-20 minutes after slaughter of the dam. In some cases the foetus may show obvious signs of recovery and even commence breathing. In such cases the foetus should immediately be humanely killed.
When uterine, placental or foetal tissues are to be collected arrangements must be made to ensure that the foetus is humanely killed as soon as possible. If the OV is not present at the time of dressing the foetus must be examined by the OV and a professional opinion reached. Photographs are essential in any cases where an element of doubt may exist should the decision be challenged or result in prosecution.
3.3.4.3 Post-mortem examination - potential signs of heavily pregnant animal:
- enlarged uterus
- foetus will be obviously close-to-term:- showing hair formation over the entire body, eyelashes, open eyes and teeth in cattle, sheep and goats. In cattle, there will usually be ‘golden slippers’ present on the feet
The foetus, after removal from the uterus, must be examined by the OV before a decision is made on the stage of pregnancy.
Any of these findings will need to result in consideration by the OV of whether the animal was in the last 10% of pregnancy. The stage of gestation can be calculated using the formula below.
3.3.4.4 Estimating gestation
The OV should take measurements of the Crown to Rump Length (CRL) in cms (top of head to buttocks) to be able to estimate the gestational period. Calculate this based on the formula:
The stage of gestation can be calculated using the following SRUC published formula:
Days of gestation = 2.5 x (*CRL+21) *CRL = top of head to buttocks length (in cms)
This formula is applicable to all animal species.
Typical duration of pregnancy in common species
| Species | Duration |
|---|---|
| Solipeds (Horses) | 330-342 days |
| Cattle | 279-292 days |
| Goats | 145-155 days |
| Sheep | 144-151 days |
| Pigs (Sows) | 112-115 days |
3.3.4.5 Evidence gathering
Ensure that all evidence is recorded, signed and dated and secured appropriately The OV should ensure that the calf (evidence) can be cross referenced to the dam’s carcase and identity.
Photographs or video capture of the dam and the parts of the foetus identified as being indicative of late pregnancy must be obtained. Use a ruler or similar to show scale / size.
Freeze a fore and hind limb as evidence for further investigation. Freezing the mammary gland and blood samples from the dam and foetus will provide additional evidence and allow DNA checks. In all cases the owner of the animal must be contacted and allowed to have an independent examination of the foetus and dam’s carcase.
3.3.4.6 Biosecurity Implications
Additional PPE should be worn during the collection of samples and evidence gathering. Take into consideration potential hazards, (Brucellosis Leptospira and Q fever).
Any person likely to have contact with a woman that is or might be pregnant must consider the risks posed by handling foetal material and if necessary arrange for a colleague to gather evidence under supervision.
3.3.4.7 Legislation and MOC references
MOC Chapter 2.3 Annex 2 section 1.4. Condition of animals at unloading applies.
Assimilated Regulation No (EC) 1/2005 on the protection of animals during transport and related operations Annex I, Chapter 1 states: “Ill, injured, infirm or fatigued animal are not transported e.g. pregnant females for whom 90% or more of the expected gestation period has already passed.”
3.3.5 Assessing Foot Pad Dermatitis (FPD) in Poultry
FPD is one of the measures to assess whether welfare standards are being met during rearing, alongside post-mortem rejections and on-farm mortality.
A number of factors have been shown to influence the occurrence of FPD such as litter type and quality, litter depth, water drinker type, bird age, ventilation and drinker management, feed source and its quality, rearing system, and breed (genotype) used among others. Enteric disease may be a predisposing factor.
The severity of FPD will depend on the area affected and the type of lesion. The OV should score FPD from 0 to 2 as described in the condition card in Annex 3. Scoring is broadly based on the size of the lesion but any lesions with deep ulceration will be scored 2 regardless of the size.
When severe FPD is suspected in a flock, the OV should score feet from a minimum of 100 birds and the number of feet in each category 0, 1 and 2 should be recorded. The score shall be calculated using the Swedish Score System:
a) (number of birds with score 1x1) + (number of birds with score 2x2) = A b) Threshold A≥ 167
When exceeded threshold is detected within a flock, the OV should inform the FBO, and also should inform APHA through an Annex 4 that will be automatically generated when the info is introduced in the relevant animal welfare section in Chronos. APHA will decide if they need to investigate the rearing conditions further.
All FPD verification checks carried out by the OV, regardless of these triggering reporting or not, are to be entered in the IRIS system alongside the rest of PMI data for the flock.
For further information please check The Code of Practice for Meat Chickens and Meat Breeding Chickens
3.3.6 Guidance for OVs to aid enforcement authorities
If the welfare of an animal is compromised, always ensure it is killed as soon as possible.
When a welfare incident is suspected, it is important that appropriate evidence is obtained at the earliest stage of the investigation. The following notes should be used as a guide together with the OV checklist at Annex 5 when gathering evidence.
- In every case the OV must ensure that suitable evidence is gathered for the investigation. The aim should be to take pictures / video showing any lameness and or wounds while the animal is still alive. Clear notes at this stage will help in the preparation of a statement, should this be required
- Where the relevant enforcement officer cannot attend the slaughterhouse or the animal needs to be dispatched immediately to protect welfare, the OV must gather the following evidence:
- a) Video and photographic evidence of the ‘live’ animal must clearly show the cause of any welfare or health issue and any identification markers on the animal. It is useful to record yourself on the video evidence stating date and time of location with details of the case
- b) Photographs must be annotated with the date, time and by whom the pictures are taken
- c) Any relevant physical evidence including whole carcasses or parts of carcasses. Without physical evidence, further investigation is often not possible. The OV should also provide any veterinary evidence in relation to the samples for example the aging of lesions, etc
- d) Details of the livestock vehicle (name of the driver, transporter including their authorisation number, registration number and trailer number) should be recorded. The APHA welfare in transport team will monitor and log these details
- e) Details of other livestock arriving from the same holding (numbers and conditions)
- f) Copies of original paperwork including passports and relevant documentation that indicates the identification of the animal, details of the owner and transporter and time of arrival
- The OV must provide a clear, professional opinion of the case, verbally in the first instance then in writing (see paragraph 5). The OV in this event is a witness of fact (not an ‘expert’) asked by the court to analyse evidence and give an opinion on their observations in relation to their professional role. However, if it becomes necessary to give evidence in court in a prosecution case, the OV would have to comply with the Expert Witness Rules in order to give specific parts of their evidence. If the OV’s opinion is that the animal has been caused ‘unnecessary or avoidable pain, distress or suffering’ this should be stated in the initial written report. (Assimilated Council Regulation (EC) No 1099/2009 uses the word ‘avoidable’ but in other legislation the word ‘unnecessary’ is used.)
- The evidence will be collected at the earliest opportunity. The date until which evidence will be retained should be completed on the Annex 4 referral form. This should allow reasonable time for collection of the evidence where suitable storage facilities are available. If large items need to be retained as evidence, contact your Inspection Team Leader (ITL) or Area Manager (AM) who should be able to locate premises with more suitable storage facilities
- There may be a request for a witness statement. This should be produced without delay and must be clear, detailed and unambiguous. The statement needs to tell the factual points of the events in a chronological order:
- a) Identify the person making the statement and their qualifications, experience and job profile
- b) Clearly state what the issue / concern is and when applicable state that the animal ‘has been caused unnecessary or avoidable pain, distress or suffering’, plus reasons why. Without this statement there is no offence. The wording used should reflect the legislative requirement
- c) If veterinary terminology is used, this needs to be explained in layman’s terms
- d) Include any video footage / photographs taken and refer to this in the statement
- Include any ante / post-mortem report and exhibit this as an item in the statement
3.4 Entry of information in Chronos
3.4.1 Purpose
Compliance data is collected daily at all slaughterhouses to provide reports on welfare issues in plants on a monthly basis that subsequently allows trends in animal welfare to be assessed by Defra and the Welsh Government.
3.4.2 OV responsibility
The OV should verify compliance with relevant national rules on animal welfare prior to and during slaughter and killing, using a systematic approach, taking proportionate enforcement action where necessary.
Suspected breaches occurring outside the slaughterhouse must be reported immediately to LA and APHA when serious. Non-urgent reports must be made before 5pm the next working day.
The OV must log all calls and contact in the daybook or their personal note book with details of date time and reason for contact, even where no reply or response was received from the officer being contacted.
Where a lack of positive response is evident from the BO, after taking proportionate enforcement action, then the OV must report the circumstances to the local FSA Field Veterinary team.
Where agreed by the FSA, compliance with animal welfare can be verified as part of ante-mortem checks at the holding of provenance on those animals being sent to a slaughterhouse by an OV/AV. Further checks will then be carried out at the slaughterhouse on the health and welfare of the animals and to verify information supplied with the animals concerning the ante-mortem checks undertaken at the holding of provenance. More information on ante-mortem checks at the holding of provenance and subsequent checks at the slaughterhouse can be found in chapter 2.2 of the MOC.
3.4.3 Score definition
Reporting requires a score to be given for each score 2, 3 or 4 incident in accordance with the information below.
| Score | Description |
|---|---|
| 1 Welfare compliant | Compliant with welfare regulations; the BO is operating fully in compliance with the regulations and their own welfare controls and SOPs. |
| 2 No immediate risk to welfare | Low risk of compromising animal welfare or an isolated low risk situation that poses no immediate risk to the welfare of animals. |
| 3 Potential risk to welfare | Potential to risk of significantly compromising animal welfare but where there is no immediate risk to animals. This may lead to a situation that poses a risk to animals, causing pain, distress or suffering. |
| 4 Welfare critical | Poses a serious and imminent risk to animal welfare or one where avoidable pain, distress or suffering has been caused. |
Examples:
2 score – minor issue with SOP
3 score – slippery floor with potential risk of animals falling
4 score – animal not effectively stunned and no corrective action taken
| Score | Definition | The OV should apply the score if |
|---|---|---|
| 2 |
An isolated low risk situation observed with the requirements of legislation but with no immediate risk of injury, avoidable pain distress or suffering. There was a technical infringement that does not impact on the welfare of animals. |
Lapses in compliance are observed which are rectified immediately on request and no harm occurred. BO compliant and good records. These may be subject to the use of enforcement notices. |
| 3 |
Welfare practices were observed as failing to comply with the requirements of legislation and there was no potential risk to animals. There were no animals suffering any avoidable pain, distress or suffering during their killing and related operations. This may lead to a situation that poses a risk to animals, causing pain, distress or suffering, which would result in a 4 score. Welfare of animals during transportation was suspected to be compromised. |
Any technical NCs have been noted during the period. These may be subject to the use of enforcement notices. |
| 4 |
Welfare practices were observed as failing to comply with legislative requirements, and there was evidence of animals suffering avoidable pain, distress or suffering during their killing and related operations or a contravention poses a serious and imminent risk to animal welfare. Welfare of animals during transportation was seriously compromised with evidence of animals suffering unnecessary or avoidable pain, distress or suffering. DOAs, female red meat animals transported in the last 10% of pregnancy and a failure to provide water at all times all require a 4 score. DOA and females transported in the last 10% of pregnancy must be referred to the LA. |
There have been NCs causing or that can cause actual harm to animals (whether prosecutable or non- prosecutable) during the period. In every case appropriate enforcement will have been carried out. Transport / on farm incidents will have been referred to the LA / APHA immediately. |
Where an incident or accident is considered to be unavoidable and no fault of the operator a 4 score should be applied and only a verbal warning given. This must be clearly explained in the Chronos entry and recorded in the daybook
3.4.4 ‘1’ scores
No entry is required on Chronos for a score of ‘1’ as this indicates compliance.
3.4.5 ‘2’ ‘3’ or ‘4’ scores
If a '2' '3' or '4' score is awarded, the deficiency and action taken boxes must be completed with a brief description of the issue (for example, ‘no water available for X time’). The OV must record the number of animals on Chronos.
If a '4' (and '3' where applicable) is scored, the OV must select from the ‘action drop down list’ and if relevant provide the following:
- the date it has been referred to the LA and / or APHA
- if an enforcement notice was served at the time of the incident as per section 5 guidance; enter in the enforcement section of Chronos
- if a CoC is suspended or revoked
- the date referred for investigation by the FSA and the investigation reference number, if held
- justification details if the incident has not been referred for investigation, for example unavoidable accidental incident, not witnessed
3.5 Suspected breach of animal welfare
3.5.1 LA / APHA contact
All referrals must be made to the dedicated APHA email contact point in the first instance: CSCOneHealthWelfare@apha.gov.uk
The LA for the area where the slaughterhouse is located will be notified of any suspected breach of welfare, either in transport or on farm by APHA. They will then liaise with the LA responsible for the premises where the breach occurred when necessary.
In serious and / or urgent cases both the LA and APHA in the area where the slaughterhouses are located must also be contacted and informed of the suspected breach.
APHA can be contacted by using their central contact number:
England: 0300 020 0301
Wales: 0300 303 8268
A summary of any communications with LA / APHA should be entered in the FSA daybook or notebook if away from the establishment.
3.5.2 Inadequate neck cut (red neck) birds using a ‘simple stunning’ method
Assimilated Regulation No (EC) 1099/2009 states: “The methods referred to in Annex I which do not result in instantaneous death (hereinafter referred to as ‘simple stunning’) shall be followed as quickly as possible by a procedure ensuring death, such as bleeding, pithing, electrocution or prolonged exposure to anoxia.”
Identifying an inadequately cut bird indicates that the bird did not receive a neck cut or adequate neck cut resulting in a rapid loss of blood and death. Such incidents indicate non-compliance with a number of requirements in (EC) 1099/2009 and WATOK legislation.
Where the BO uses a stun (kill) method, the bird will be dead and an inadequately cut bird will not be a non-compliance unless there has been a failure of the stun system.
3.5.3 FSA action
Where an OV, MHI or Plant Inspection Assistant (PIA) identifies an uncut / inadequately cut bird (red neck) on the shackle line, they must gather evidence and record the details.
The FSA team must identify NCs that have been observed using the legislative references shown below.
| Scenario | Legislative reference |
|---|---|
| Bird could have been subject to avoidable pain when using ‘simple stunning’ | Assimilated Regulation No (EC) 1099/2009 Article 3 (1) states that “Animals shall be spared any avoidable pain, distress or suffering during their killing and related operations.” |
| Procedure (neck cut) did not ensure death in a ‘simple stunned’ bird | Assimilated Regulation No (EC) 1099/2009 Article 4 (1) states that “Animals shall only be killed after stunning in accordance with the methods and specific requirements related to the application of those methods set out in Annex I. The loss of consciousness and sensibility shall be maintained until the death of the animal.” |
| Procedure failed to ensure bird unconscious until death |
Assimilated Regulation No (EC) 1099/2009 Article 5 (1) states that “Business operators shall ensure that persons responsible for stunning, or other nominated staff, carry out regular checks to ensure that the animals do not present any signs of consciousness or sensibility in the period between the end of the stunning process and death. Those checks shall be carried out on a sufficiently representative sample of animals and their frequency shall be established taking into account the outcome of previous checks and any factors which may affect the efficiency of the stunning process. When the outcome of the checks indicates that an animal is not properly stunned, the person in charge of the stunning shall immediately take the appropriate measures as specified in the standards operating procedures drawn up in accordance with Article 6 (2). |
| BO has not ensured line speed allowed sufficient time for adequate neck cut | WATOK Schedule 1, Paragraph 21 (1) (b) states that “No person may operate a shackle line unless the speed at which the shackle line is operated is such that any act or operation intended to be performed in relation to, or on, poultry suspended from it can be performed without undue haste and with proper regard for the welfare of the poultry.” |
| BO or slaughterer has not ensured neck cut is such that bleeding is rapid and profuse |
WATOK Schedule 1, Paragraph 31 (1) states that “A person engaged in the bleeding or pithing of an animal that has been simple stunned must ensure that the animal has been bled or pithed without delay after it has been simple stunned.” Paragraph 31 (2) states that “A person engaged in the bleeding of an animal which has been simple stunned must ensure that the bleeding is: (a) rapid, profuse and complete; and (b) completed before the animal regains consciousness. Paragraph 31 (3) states that “Without prejudice to the generality of paragraph 3.1 of Annex III of WATOK, if an animal is bled after simple stunning, no person may cause or permit any further dressing procedure or any electrical stimulation to be performed on the animal before the bleeding has ended and in any event not before the expiry of: (a) in the case of a turkey or goose, a period of not less than two minutes (b) in the case of any other bird, a period of not less than 90 seconds.” |
After each incident, the OV must give written advice to the BO detailing the non- compliance and actions required to achieve the objectives of the legislation. Where the line speed, shortage of slaughterers or lack of rotation is a contributory factor, a WEN should be served to slow down the line.
3.5.4 Evidence gathering
The OV and FSA team must ensure that every incident where a bird is identified as not bled or poorly bled is recorded and evidence gathered at the time of the incident. This will help to demonstrate that the BO is failing to take action to spare animals’ avoidable pain, distress or suffering. It will also help avoid systemic failures of SOPs and prevent problems recurring.
Notes to include as evidence:
- the line speed on the day and time of the incident, and whether it is different to normal for that premises
- the daily throughput and hours worked on the day of the incident
- the names of slaughterers at the time of the incident; this will help to identify if there is a pattern with some slaughterers more often involved
- where there is clearly one slaughterer failing to carry out adequate neck cuts, the OV should consider suspension or revocation of the CoC
- the amperage and voltage of the equipment to demonstrate that it was a simple stun / stun to establish that the bird will have suffered
- any discussions with BO staff, and allow the BO to add comments to the day book
The OV / MHIs should record the outcomes of the discussions carried out with the BO, animal welfare officer and operatives including their comments and intentions about how to solve the problem.
Evidence should be obtained on whether the SOPs have been reviewed by the BO in order to prevent recurrence.
Photographs of the affected bird(s) must be taken and secured as advised in MOC Chapter 7 Enforcement.
Where possible, a representative sample of affected birds should be retained if there are suitable freezer facilities available. If not, facilities may need to be secured off site.
If, after discussion with the FVL or FVC, it is considered necessary to send birds for post-mortem, arrangements will be made by the FSA.
3.5.5 Verification procedures
SOPs should describe the procedures and checks necessary to ensure there is adequate stunning and bleeding of the animals. The AWO should keep records on the checks they carry out and of the actions taken when shortcomings are identified. The FSA team should check the SOPs and records regularly, record any deficiencies identified and discuss these with the BO representative.
Verification by the FSA team should include checks on the stunned animals at the point before they enter the plucking machine in order to verify that they are unconscious and properly bled at that moment. Evidence should be recorded of any animal that is inadequately cut, poorly cut or conscious along with actions taken and the reason why this happened.
3.6 Welfare folder
The OV must have and keep an up to date welfare folder. This must contain, as a minimum, the following information:
- The approval document showing the species that can be processed and the layout map. Also, cattle box approval if applicable (hard copy and / or electronic copy)
- A list showing methods of killing that have been used, including back up and religious slaughter (hard copy and / or electronic copy)
- The CCTV cameras location map (hard copy and / or electronic copy)
- If partnership / sole trader: FBO name, address and contact details or incorporation details (as registered with Companies House or equivalent) including full company name, registered office address (including postcode) and company registration number (always hard copy)
- AWO names and contact details (for example, e-mails, phone numbers) as applicable (always hard copy)
- Either a version-controlled copy of the SOPs if they are not readily available (hard copy and / or electronic copy) or if they are readily available, a note with the relevant contact point (hard copy). Note: The OV must always verify with the BO that the SOP is the most recent version before taking enforcement action
- A record of the Temporary Certificate of Competence (TCoC) and CoCs. This record can be either electronic or hard copy. Note: A check must be made with the WATOK team in York that CoCs are valid when new staff commence employment
- Local LA and APHA contact numbers and e-mails (always hard copy)
- The FSA welfare checks protocol, showing how the work is organized and shared within the FSA team (always hard copy)
- FSA aide memoire for the daily welfare checks (always hard copy)
- Blank copies and records of the daily welfare checks (always hard copy)
- Blank copies of the relevant enforcement notices that might need to be served immediately / urgently (always hard copy)
- Letters and notices served (always hard copy)
- Updated local protocols and agreements (hard copy and / or electronic copy)
- Copies of relevant meetings and communications (hard copy and / or electronic copy)
Note: The folder must be organized in such a way that the information is easily identifiable and should document what information is stored electronically and how it can be accessed.
4. Religious Slaughter
In this section
4.1 Compliance with religious slaughter requirements: BO responsibility
4.2 Compliance with religious slaughter requirements: FSA responsibility
4.1 Compliance with religious slaughter requirements: BO responsibility
4.1.1 Legislation: slaughter
WATOK (England), (Wales) Schedule 3, 1, (c) states:
‘killing in accordance with religious rites’ means killing without the infliction of unnecessary suffering:
a) by the Jewish method (Shechita) for the food of Jews by a Jew who is licensed by the Rabbinical Commission and holds a certificate for that purpose
b) by the Muslim method (Halal) for the food of Muslims by a Muslim who holds a certificate for that purpose.
4.1.2 Legislation: species
WATOK (England) and (Wales) Schedule 3, Part 1 2, (1) sets out the species that are permitted to be slaughtered by a religious method. These are sheep, goats, bovine animals or birds killed in a slaughterhouse.
WATOK (England) and (Wales) Schedule 3, Part 1, 1 (a) defines ‘bovine animal’ as an ox, bullock, cow, heifer, steer or calf; and 1 (b) defines ‘bird’ as a turkey, domestic fowl, guinea-fowl, duck, goose or quail.
These are the only species permitted to be slaughtered according to religious rites without prior stunning.
4.1.3 Legislation: method of slaughter
WATOK (England) and (Wales) Schedule 3, Part 2, 5 for sheep goats and bovines and Part 3, 7 for birds, requires that:
Any person that kills an animal in accordance with religious rites must inspect the knife immediately before killing to ensure that it is:
a) undamaged
b) of sufficient size and sharpness to kill the animal
For ruminants, the incision must ensure it is killed by the severance of both its carotid arteries and jugular veins by rapid, uninterrupted movements of a hand- held knife.
For birds, the incision must ensure the bird is killed by the severance of both of its carotid arteries by rapid, uninterrupted movements of a hand-held knife.
4.1.4 Compliance with religious slaughter requirements
In establishments where killing by a non-stun religious method takes place, there must be checks by the slaughterer, verified by the BO (this could be through the AWO), that animals are unconscious before being released from restraint and checks that the animal does not present any sign of life before undergoing dressing or scalding.
European Food Safety Authority (EFSA) has produced guidance on the signs of consciousness in bovine, sheep and goats, pigs and poultry – see section 2.3.1 above. A guide to the signs of effective stun and unconsciousness can also be found in the welfare indicator cards at Annex 11 Signs of properly stunned or dead poultry and Annex 12 Signs of a properly stunned or dead animal by stunning method.
Note: The requirements in WATOK Schedule 3 for killing by a religious method also apply.
4.1.5 Legislative requirements
The EC Regulations permit slaughter by a religious method without prior stunning. Reference: assimilated Regulation No (EC) 1099/2009 Article 4 (4).
Where animals are killed without stunning by a religious method the persons responsible for slaughtering must carry out systematic checks to ensure that animals do not present any signs of consciousness or sensibility before being released from restraint and do not present any sign of life before undergoing dressing or scalding. The person carrying out these checks must have a CoC for the activities being checked and report to the AWO. A record should be kept of the checks carried out. Reference: assimilated Regulation No (EC) 1099/2009 Article 5 (2).
BOs engaged in religious slaughter without stunning must specify in the SOP measures to be taken when the checks indicate the animal still presents signs of life. Reference: assimilated Regulation No (EC) 1099/2009 Article 6 (2), (c).
BOs must also ensure that all animals that are killed by religious rites without prior stunning are individually restrained; in particular, ruminants should be individually mechanically restrained. Reference: Assimilated Council Regulation (EC) 1099/2009 Article 15 (2).
Bovines can only be killed in an approved restraining pen. Reference: WATOK (England) (Wales), Schedule 3, Part 2, 3.
The animals must not be placed in restraining equipment until the CoC holder is ready to make the incision immediately. Reference: assimilated Regulation No (EC) 1099/2009 Article 9 (3); WATOK (England) (Wales), Schedule 3, 6 (1) a.
If the restraining equipment was not in operation before 1 January 2013, then it must also comply with the requirements in paragraph 3, Annex II of assimilated Regulation No (EC) 1099/2009; that is, it must:
- optimise the application of the killing method
- prevent injury or contusions to the animal
- minimise struggle and vocalisation when an animal is restrained and
- minimise the time of restraint
Note: The use of V-shaped restrainers for sheep / goats is permitted since these are a type of mechanical restraint. Only one live animal can be restrained at a time. The belt must be stationary for the duration of the bleeding, until the animal is unconscious and for 20 seconds as a minimum when the animal is killed without prior stunning.
Reference: assimilated Regulation No (EC) 1099/2009 Art 15, 2 and Article 9(3).
Restraining equipment must be checked and maintained in accordance with the manufacturer’s instructions.
4.1.6 Adult bovine restraint
Note that adult bovines can only be restrained upright in a stunning box approved under:
- Schedule 3 Part 2 of WATOK (England) and (Wales)
Young bovines must be individually mechanically restrained for non-stun religious slaughter. The process / restraint used must not result in any avoidable pain distress or suffering and in particular consideration should be given to the contact of any body parts with parts of the equipment which would result in avoidable pain.
WATOK (England) and (Wales) Schedule 3, 6 (1) requires that animals are not placed in restraining equipment until the person is ready to make the incision.
FBOs shall contact approvals for an application form which must be submitted along with copies of the plans of the box. An on-site assessment will then be conducted by a Field Veterinary Leader. If they are content the way the restraint is operated, they may recommend approval. The decision maker for approval will be the Head of Operational Assurance and Excellence. The restraint facilities must be approved by the FSA prior to their use.
If during routine checks on approved adult bovine restraint facilities the OV is of the opinion that the facility is not being used as originally approved or may cause adult bovine’s pain distress or suffering they should discuss this with their Field Veterinary team who may make a recommendation for an approval review. If a review is required the restraint box cannot be used until the review has been completed. A WEN may be required to prevent the use of the box for non stun slaughter.
The SOP for the bovine restraint box slaughter method should be reviewed at least monthly and include the process that the BO uses to assess signs of unconsciousness and after what period of time a post cut stun is applied.
If a review is required or the restraint facility is causing pain distress or suffering during use then the OV should serve an enforcement notice preventing its use with immediate effect. See section 6 for enforcement details.
4.1.7 Handling of sheep, goats and bovine animals
Where sheep, goats or bovines are killed by religious rites without stunning, the slaughterer and BO must ensure:
- that animals are not shackled, hoisted or moved in any way until the animal is unconscious and in any event not released from restraint before the expiry of:
- sheep and goats: a period of not less than 20 seconds
- bovines: a period of not less than 30 seconds
- there is appropriate back up stunning equipment close to the restraining pen / equipment for use on ruminants in case of emergency; this is to be used immediately where the animal is subject to avoidable pain, suffering or agitation or has injuries or contusions
Note: The animal must be unconscious before it is moved; some animals may require longer than the above times before they become so. The BO must ensure that checks for consciousness / unconsciousness are made by the responsible CoC holder before animals are released from restraint. A record must be kept of the checks carried out.
The BO, and any person engaged in the killing of a bird in accordance with religious rites without prior stunning, must ensure that where the bird has not been stunned without bleeding, no further dressing procedure or any electrical stimulation is performed on the bird if it presents any sign of life and in any event not before the expiry of:
- turkey or goose: a period of not less than 2 minutes
- any other bird: a period not less than 90 seconds
No dressing or scalding can take place until the absence of signs of life has been verified.
England only:
A premises stunning and then killing by a religious method with Jewish or Muslim slaughterers does not have to comply with the parameters set out in (REUL) No 1099/2009 Annex I. The animal must be immediately rendered unconscious and remain unconscious until dead before any further procedures take place.
4.2 Compliance with religious slaughter requirements: FSA responsibility
4.2.1 OV checks
The OV should carry out checks:
- to monitor slaughter by a non-stun religious method
- on restraint facilities for non-stun religious slaughter
- to monitor the period of time that the animal remains restrained after neck cut has taken place and ensure this complies with both domestic and EU regulations requiring unconsciousness and minimum standstill times
- ensure that animals are checked by persons responsible for slaughtering and they carry out systematic checks to ensure that the animals do not present any signs of consciousness or sensibility before being released from restraint and do not present any sign of life before undergoing dressing or scalding
- monitor records of checks carried out by the BO on signs of unconsciousness
- during non-stun religious slaughter it is usually necessary for the slaughterman to hold the head in sheep, goats and poultry to make the neck cut. This is not considered as restraint, therefore no additional restraint code is required and an individual with only code 61 can carry this out
4.2.2 Welfare incident recording
All welfare incidents observed must be recorded in Chronos.
Where animals are released from restraint whilst still conscious or before the required time has elapsed, appropriate enforcement action must be immediately taken.
This could include using an enforcement notice to slow down the slaughter process, which would allow sufficient time for the required monitoring of signs of unconsciousness / consciousness; restraint times to be observed and / or in cases where the CoC holder is failing to comply with these requirements, suspension or revocation of the CoC.
Details of animals released before unconsciousness should be recorded.
4.2.3 Enforcement
For guidance on enforcement of animal welfare regulations refer to:
- topic 6 on ‘Enforcement’ of this chapter
- chapter 7 on ‘Enforcement’
Note: In the event that users require technical guidance on enforcement issues, they should follow the escalation through lines of communication as detailed in chapter 1 on ‘Introduction’, section 3.
5. Mandatory use of CCTV
In this section
The Mandatory Use of Closed Circuit Television in Slaughterhouses (England) Regulations 2018 (‘CCTV regulation’) and SL(6)471 - The Mandatory Use of Closed Circuit Television in Slaughterhouses (Wales) Regulations 2024 (Senedd. Wales) (‘CCTV legislation’) lay down rules on the installation, operation and retention of CCTV systems, images and information.
Coming into force, Welsh Regulation.
Subject to Senedd approval, the Regulations will come into force in two parts:
- Part one will come into force on 1 June; these are the requirements to install and operate a CCTV system and keep CCTV footage and information
- Part two will come into force on 1 December; these are the offences and powers to inspect, seize and enforce the Regulations
Please note that all entries below in relation to Wales have to be read in conjunction with the updates provided as the implementation is rolled out.
5.1 Requirements
Regulation 3(1) of the CCTV legislation requires that the BO of a slaughterhouse must install a CCTV system that provides a complete and clear image of killing and related operations in all areas of the slaughterhouse (includes any land, building, shed, pen, receptacle or vehicle of any description) where live animals are present.
CCTV cameras should cover unloading, lairage, handling, restraining, stunning, bleeding and killing areas. This should include animals that are alive but unconscious post stunning or during bleeding.
Slaughter by a method that results in instantaneous death would not require CCTV after that point in the process. For example, bullet (free projectile). Regulation 3(3) requires that the system be operational at all times when live animals are present.
This includes overnight and on non-working days if animals are delivered or held at the slaughterhouse outside of normal operating hours. Where there are fields or buildings associated with the slaughterhouse that have the same CPH number as the slaughterhouse, these are classed as field lairages so will require CCTV coverage and the following rules apply:
- CCTV in the field lairage is mandatory
- Animals can move to and from the slaughterhouse to the field lairage without further requirements
- Animals from a holding under standstill, can be moved to slaughter, directly or indirectly via an approved slaughter-market or collection centre (DCO Schedule 1). In doing so they may go into the slaughterhouse field lairage
- Animals from TB restricted farms, or untested animals, can be moved either directly or through exempt markets and approved collection centres to a slaughterhouse. In doing so they may go into the slaughterhouse field lairage
If the field used in connection with the slaughterhouse business has a different CPH number then, even if adjacent, it is effectively a separate holding and subject to different rules, including:
- they are not field lairages, and therefore CCTV is not mandatory
- the animals cannot move from the slaughterhouse back to the field
Regulation 4(1) requires that the BO retains and stores any images and information obtained by the CCTV system for 90 calendar days from the date the images or information are obtained. The OV and FSA Inspection staff (Inspector) can view live and historical CCTV images and the OV should verify monthly that images are retained for at least 90 calendar days.
Regulation 5(1) (England) gives an inspector power to view, copy or seize CCTV equipment as well as images or information obtained by a CCTV system when executing and enforcing The Welfare of Animals at the Time of Killing (England) Regulations 2015 or the EU Regulation.
Regulation 5(1) (Wales) gives an inspector power to view, copy or seize CCTV equipment as well as images or information obtained by a CCTV system when executing and enforcing The Welfare of Animals at the Time of Killing (Wales) Regulations 2014 or the EU Regulation or the Welfare of Animals (Transport) (Wales) Order 2007.
Access to CCTV equipment, images and information must be provided by the BO to any inspector appointed by the CA.
An inspector is any authorised officer for the purposes of executing and enforcing the Welfare of Animals at the Time of Killing (England) Regulations 2015 or the EU Regulations. Inspectors include OVs, E&J Technical Managers, MHIs, FSA Field / Audit veterinarians and criminal investigators. In Wales an inspector can also be a person authorised under section 51 of the Animal Welfare Act 2006.
England only. Should an LA officer in England wish to obtain footage in relation to a transport non compliance the OV cannot request footage under the CCTV regulations on their behalf. Should the FBO agree to share the footage then the LA should liaise directly with the FBO. CCTV footage can only be formally requested where the suspected offence has taken place under WATOK (England) or Regulation 1099/2009 in England.
Wales only. An inspector means a person appointed under section 51 of the Animal Welfare Act 2006. The LA officer will normally be authorised under this section. CCTV footage can be formally requested where the suspected offence has taken place under WATOK (Wales), Regulation 1099/2009 or the Welfare of Animals (Transport) (Wales) Order.
5.2 Viewing and monitoring CCTV
FSA staff must ensure that CCTV footage is monitored on each operational day. CCTV footage viewed should be a combination of live footage and, where possible, footage randomly selected from historical operational and non- operational periods. If it is not possible to review historical footage on each operational day this should be noted in the daybook and a process should be put in place to review it at regular intervals. The OV should ensure that all cameras are fully operational. As a minimum the OV must view CCTV at least daily for a total of 15 minutes.
If Meat Hygiene Inspectors (MHI) form part of the team, they can also assist the OV by completing additional CCTV checks where time permits. A protocol should be in place to outline which team members will view the CCTV. Viewing of CCTV must be recorded in the daybook with the time and length viewing period observed. The WEL 3-1 or 3-2 must also be completed under the CCTV section.
CCTV is a very useful source of evidence where a welfare breach is suspected. FSA OVs have powers to seize CCTV footage if a breach of the welfare regulations is suspected.
A request should be made in writing to the BO for a copy of CCTV footage relating to any suspected breach using the CCTV Regulations provisions, Regulation 5.
The CCTV footage should be handed to the FSA by the BO or their representative to maintain the evidential chain The OV must not allow the CCTV copy to pass out of the direct control of the OV or BO until such time as an FSA investigator collects the footage where appropriate.
Updated [An Inspector may record CCTV footage on their mobile phone as evidence at any point in time up to the receipt of the CCTV footage from the BO that has formally been requested.]
5.3 Enforcement
The CCTV Regulations give enforcement powers to the FSA. Failure to have CCTV that complies with the CCTV Regulations, failure to retain images for 90 days and failure to provide access or obstruction of an inspector will constitute offences under the CCTV Regulations.
You should encourage the BO to inform you of any issues or problems with any CCTV equipment and the likely timescale that problems will persist. All enforcement action should be proportionate and in line with the hierarchy of enforcement at Chapter 7 of the MOC. An inspector may issue a written CCTV Enforcement Notice (WEL 11/38) requiring the BO and/or their staff to take specific actions, which may include timescales to rectify contraventions of the CCTV Regulations.
Once an inspector is satisfied that the contravention has been remedied, they must serve a CCTV Completion Notice (WEL 11/39).
If an inspector is not satisfied that the contravention has been remedied in the timescale required and so the CCTV Enforcement Notice has not been complied with, they may choose not to issue a CCTV Completion Notice but, in this case, must provide a CCTV Refusal to Issue a Completion Notice (WEL 11/40), this will detail:
- the reasons for the decision not to serve a Completion Notice
- provide details on how to appeal the appointed inspector’s decision
An inspector may vary or withdraw a CCTV Enforcement Notice in writing at any time, for example where rectification of an issue becomes more urgent or it is evident a solution will take more time, or where an alternative solution is presented. A breach of a CCTV Enforcement Notice will be referred for formal investigation.
5.4 Appeals
A person may appeal against a decision
- to serve a CCTV Enforcement Notice
- not to issue a CCTV Completion Notice
England updated [and Wales].
The appeal will be heard by the First-tier tribunal in the General Regulatory Chamber.
A CCTV Enforcement Notice will not be suspended pending the appeal, unless the First Tier Tribunal orders otherwise.
Updated [Appeals should be made to: grc@justice.gov.uk
Alternatively, the Ministry of Justice form T98 may be lodged with the General Regulatory Chamber, HM Courts and Tribunals Service, PO Box 9300, Leicester, LE1 8DJ within 28 days of the decision.
Where an OV is aware that an individual intends to appeal a decision, they should notify the SLA and Contracts team in York and FSA legal.]
6. CoC
In this section
6.1 Training and registration of BO staff
6.1 Training and registration of BO staff
6.1.1 BO responsibility
It is the BO’s responsibility to ensure that staff are correctly trained, with the relevant CoC, to carry out tasks assigned to them.
6.1.2 Requirements for BO staff: Certificates of Competence
Assimilated Regulation (EC) No 1099/2009 requires that any person involved in the handling or slaughter of animals has a CoC for all operations they will carry out.
Reference: Assimilated Regulation (EC) No 1099/2009, Chapter II, Article 7.
Animal handlers, lairage workers and poultry shacklers (plus those who kill an animal by means of a free bullet in the field for human consumption) require a CoC.
Employees or others that are carrying out ancillary duties not related to the slaughter process in the lairage, for example clipping / shearing cattle and sheep prior to slaughter or fork truck drivers in poultry premises that do not carry out any other handing of live animals will not need a CoC.
6.1.3 Who needs a CoC?
CoCs will be required by persons undertaking the following operations, for human consumption in an approved slaughterhouse:
- the handling and care of animals before they are restrained
- the restraint of animals for the purpose of stunning or killing
- the stunning (including methods resulting in instantaneous death) of animals
- the pithing and assessment of effective pithing of animals
- the assessment of effective stunning
- the shackling or hoisting of live animals
- the bleeding of live animals
- the slaughtering in accordance with Article 4(4) (slaughter in accordance with religious rites)
Reference: Assimilated Regulation (EC) 1099/2009 Chapter II, Article 7.
When an animal is unable to enter the food chain due to ante-mortem issues, for example cattle with no ear tags, Defra have confirmed that as the animal is not being slaughtered for human consumption a WATOK licence holder can kill the animal and remove the carcase from the slaughterhouse. The requirements for restraint, particularly for cattle, will still apply.
A member of the BO staff at a slaughterhouse site at night who has had suitable training but no CoC may kill an animal in the lairage where it is injured, suffering or in pain, provided that the individual is suitably competent and the animal does not enter the food chain.
Where animals are delivered outside normal operating hours, the driver of the livestock lorry, owner of the animals or their employee may unload animals without holding a CoC as they are considered competent as animal handlers. Where the FBO allows this to take place there must be clear signage indicating pen capacity, the need to ensure bedding and water in pens and a contact number should any animals be injured so that a slaughter person can be called in to kill the animal. The provision for BO staff on site with suitable training would also apply. In either case as the animal will not have had a Veterinary ante mortem it cannot enter the food chain.
Animals killed on farm for Emergency slaughter or fractious animals must be slaughtered by a CoC holder. When an animal escapes from a slaughterhouse, and subject to satisfactory ante-mortem, is killed outside the slaughterhouse this must be done by a CoC holder. There are exemptions from having a CoC which include a Veterinary Surgeon in the pursuance of their Professional duties.
Where calves are slaughtered a new V code was introduced in May 2017. Anyone with a CoC prior to that date and code A for cattle can also slaughter calves. After that date an operative must have the V code for calves. There has been no retrospective update of CoC cards.
Additional guidance for restraint and religious slaughter.
When the animal is released after the required period of restraint it is likely to be unconscious but still alive. The operative carrying out shackling is required to have a CoC with activity 43 (shackling or hoisting of live animals) if the animal is unconscious but not dead.
Where there is a delay in shackling an assessment should be made on the absence of signs of life at this point. If the OV is satisfied that there are no signs of life at the point of shackling, then a CoC with activity 43 (shackling or hoisting of live animals) will not be required.
Holding the head of an animal in non-stun religious slaughter (sheep/goat/poultry) to make a neck cut, is not considered restraint. Holding the head or the leg to make a neck cut of a stunned animal is not considered restraint. CoC holders do not require Activity 42 (restraint). The activity 61 code (bleeding using religious rites) is intended for slaughter without prior stunning.
Halal slaughter of previously stunned animals and birds does not require code 61, the CoC holder needs activity 53 only for bleeding in these circumstances.
6.1.4 Slaughterers transferring to or commencing duties at an alternative slaughterhouse
Where a person transfers to or commences work at another slaughterhouse to carry out duties involving the slaughter or handling of animals, the OV must verify that the individual holds a valid CoC for the operations that they will be undertaking.
The OV should check new individual’s CoCs on the FSA’s slaughterers registration database by contacting the WATOK team in CSU.
If you have access to K2, the CoC details can be accessed online.
You will need the name and date of birth or CoC ID number to search.
These checks should be completed as soon as possible, and before the individual undertakes any duties involving the handling, restraint or stunning of animals. Obtain a copy and retain in the welfare file.
Note: EU CoCs other than those issued in the UK are no longer valid.
Citizens from the Republic of Ireland can apply for a UK CoC when they have a current CoC. More details can be obtained from the WATOK team at WATOK@food.gov.uk
6.1.5 Training for CoCs
Training for a CoC and the award of qualifications is carried out by external bodies. The body awarding qualification certificates in England and Wales is currently only Food and Drink Qualifications (FDQ).
Reference: Retained Council Regulation (EC) 1099/2009, Chapter V, Article 21.
To access a list of the training modules and link to TCoC / CoC species and activities see the qualification specification sheet on the FDQ website.
6.1.6 Types of CoC
(EC) No 1099/2009 states which tasks require a CoC. The following table details the various types of CoC / certification which will be issued.
| Type | Purpose |
|---|---|
| CoC (TCoC) |
This allows person, while training, to carry out those tasks requiring a CoC. A TCoC holder must work under the permanent supervision of a full CoC holder at all times for the tasks and activities being undertaken. The applicant must register for training with FDQ prior to applying for a TCoC. Further guidance on the TCoC application process is available at: Temporary Certificate of competence to slaughter or kill animals application (GOV.UK) The digital application form is available at: Apply for a temporary certificate of competence to slaughter or kill animals For activity codes: Temporary Certificate of competence to slaughter or kill animals application, get an activity code (GOV.UK) For on farm game slaughter establishments where an individual requires a TCoC an application can be made online or alternatively a paper copy can be downloaded and sent to the WATOK team. A copy of the paper application form can be found online at: Temporary certificate of competence to slaughter or kill animals - GOV.UK (publishing.service.gov.uk) Please send your completed application form to: WATOK CoC Transactions Team Food Standards Agency Foss House, Room 112 Peasholme Green York YO1 7PR A temporary CoC can only be issued for 3 months and will not normally be renewable for the same species / operations. (In exceptional circumstances, a TCoC can be extended if evidence is supplied to show that the failure to complete training was beyond the control of the individual. The applicant should submit an appeal to the FSA.) Further guidance on application procedures is available on the FSA website. Conversion of a TCoC to a full CoC will involve the applicant obtaining a qualification certificate. On receipt of a copy of the qualification certificate and payment of a fee, a full CoC will be issued by FSA. |
| Qualification certificate | This is issued by the training award body when the candidate has been assessed as competent. A qualification certificate is required to obtain a full CoC. |
| Certificate of Competence | This will allow a person to carry out those tasks specified on the CoC without supervision. |
| WATOK Licence | Issued only in England and Wales. Not valid for slaughterhouse activities. This allows a person to carry out those tasks specified on the licence outside of a slaughterhouse only. It is assessed by APHA. |
6.1.7 Obtaining a CoC
There are two methods of obtaining a CoC:
- holding a qualification certificate issued by an awarding body or a licence granted by the Rabbinical Commission
- holding a veterinary qualification, recognised by Royal College of Veterinary Surgeons (RCVS), along with suitable RCVS recorded Continual Professional Development
Qualification certificates are granted by awarding bodies regulated by Ofqual in England and Wales (currently FDQ).
BOs asking for guidance on training and assessment of their staff should be directed to the awarding bodies in their country for a list of providers. In England and Wales, FDQ is currently the only awarding body. As a government department, the FSA cannot recommend individual suppliers.
A list of the FDQ modules and link to species and activities can be found on the FDQ website.
Applicants using a qualification certificate to apply for a CoC should complete the relevant form in the guidance.
Further guidance on application procedures is available on the FSA website.
6.1.8 Working following an assessment
A TCoC holder who has passed their assessment can be allowed to work unsupervised for the species and operations for which they have been successfully assessed for a period of 30 days.
The assessor must communicate the decision to the OV either verbally or in writing and a note should be made by the OV in the day book that the individual has been assessed and which species and activities they were successful in.
The OV must verify that any person who has been successfully assessed has obtained a full CoC within 30 days of the assessment and ensure that a copy of the CoC is retained in the premises welfare folder.
Where the individual is unable to provide a full CoC within 30 days, they must provide evidence of their application and the OV must verify with the WATOK team that the application has been received.
If the CoC or application cannot be verified after 30 days, the OV must instruct the individual to stop working with the species and activities they were assessed for until such time that the person can demonstrate that they have a full CoC.
6.2 Certificate of Competence: species and operations
6.2.1 CoC species and operations
The chart on the following pages shows species and operations from which the applicant will select the operations required for their CoC. A check will be made when the application is processed that the applicant has a qualification certificate for these operations.
6.2.2 White meat
Operation 15 cervical dislocation: it should be noted that manual dislocation is only permitted up to 3 kg live weight and between 3 and 5 kg mechanical cervical dislocation must be used. A person cannot do more than 70 birds per day using manual cervical dislocation.
There is currently only one device for mechanical cervical dislocation which Defra consider is mechanical and effective. HSA give details of the device considered by Defra to be mechanical cervical dislocation in their practical slaughter of poultry publication at Manual - Humane Slaughter Association (hsa.org.uk)
This cannot be used as a routine method, but only for back up stunning.
6.2.3 Summary of species and activities
Summary of species and activities
| Red Meat |
Cattle Bison Water Buffalo |
Calves After 1 May 2017 |
Horses | Sheep & Goats | Pigs | Large Game |
|---|---|---|---|---|---|---|
| Reference | A | V | B | C | D | E |
| 11.Penetrative captive bolt device | - | - | - | - | - | - |
|
12.Non-penetrative captive Bolt device Simple Stun Only |
N/A | up to 10 Kg | N/A | up to 10 Kg | N/A | up to 10 Kg |
|
12 (a) Non-penetrative captive bolt device England only 2022 amendment STUN (KILL) |
- | - | - |
Lamb less than 6kg Kids less than 4kg |
Piglets less than 10kg | |
| 13.Firearm with free projectile | - | - | - | - | - | - |
| 14.Pithing and the assessment of effective pithing | Not for human or animal consumption | Not for human or animal consumption | - | Not for human or animal consumption | - | - |
| 21.Head-only electrical stunning | - | - | - | - | - | - |
| 22.Head-to-Body electrical stunning | - | - | - | - | - | - |
| 31.Carbon dioxide at high concentration | N/A | N/A | N/A | N/A | - | N/A |
| 32.Carbon dioxide in two phases | N/A | N/A | N/A | N/A | N/A | N/A |
| 33.Carbon dioxide associated with inert gases | N/A | N/A | N/A | N/A | - | N/A |
| 34.Inert gases | N/A | N/A | N/A | N/A | - | N/A |
| 41.the handling and care of animals before they are restrained; | - | - | - | - | - | - |
| 42.the restraint of animals for the purpose of stunning or killing; | - | - | - | - | - | - |
| 43.the shackling or hoisting of animals; | - | - | - | - | - | - |
| 51.the stunning of animals; | - | - | - | - | - | - |
| 52.the assessment of effective stunning; | - | - | - | - | - | - |
| 53.the bleeding of live animals; monitoring the absence of signs of life | - | - | - | - | - | - |
| 54. WATOK ONLY. monitoring the absence of signs of life | - | - | - | - | - | - |
| 61.Slaughtering in accordance with Article 4 (4) of Regulation EC 1099/2009 | - | - | N/A | - | N/A | N/A |
| Poultry, Ratites and Lagomorphs. | Chicken & Guinea Fowl | Quail | Turkey | Ratites | Ducks | Geese | Lagomorphs (Rabbits & Hares) |
|---|---|---|---|---|---|---|---|
| Reference | K | L | M | N | Q | P | R |
| 11.Penetrative captive bolt device | - | - | - | - | - | - | - |
| 12.Non-penetrative captive bolt device | - | - | - | - | - | - | - |
| 13.Firearm with free projectile | - | - | - | - | - | - | - |
| 14.Cervical dislocation | Manual to 3Kg mechanical to 5 Kg | Manual to 3Kg mechanical to 5 Kg | Manual to 3Kg mechanical to 5 Kg | N/A | Manual to 3Kg mechanical to 5 Kg | Manual to 3Kg mechanical to 5 Kg | N/A |
|
15. Percussive blow to the head (WATOK Schedule 1 - 26. (1) No person may stun an animal using a non-mechanical percussive blow to the head. (2) But the prohibition in sub-paragraph (1) does not apply to rabbits, provided that the operation is carried out in such a way that the rabbit is immediately rendered unconscious and remains so until it is dead. N |
N/A | N/A | N/A | N/A | N/A | N/A | Rabbits only up to 5 Kg |
| 21.Head-only electrical stunning | - | - | - | - | - | - | - |
| 22.Head-to-Body electrical stunning | - | - | - | - | - | - | - |
| 23.Electrical waterbath | - | - | - | N/A | - | - | N/A |
| 31.Carbon dioxide at high concentration | Not in abattoir | Not in abattoir | Not in abattoir | N/A | N/A | N/A | N/A |
| 32.Carbon dioxide in two phases | - | - | - | N/A | - | - | N/A |
| 33.Carbon dioxide associated with inert gases | - | - | - | N/A | - | - | N/A |
| 34.Inert gases | - | - | - | N/A | - | - | N/A |
| 35. low Atmospheric pressure stunning | Chicken less than 4kg | N/A | N/A | N/A | N/A | N/A | N/A |
| 41.the handling and care of animals before they are restrained; | - | - | - | - | - | - | - |
| 42.the restraint of animals for the purpose of stunning or killing; | - | - | - | - | - | - | - |
| 43.the shackling or hoisting of animals; | - | - | - | - | - | -- | - |
| 51.the stunning of animals; | - | - | - | - | - | - | - |
| 52.the assessment of effective stunning; | - | - | - | - | - | - | - |
| 53.the bleeding of live animals; monitoring the absence of signs of life | - | - | - | - | - | - | - |
| 54. WATOK ONLY. monitoring the absence of signs of life | - | - | - | - | - | - | - |
| 61.Slaughtering in accordance with Article 4 (4) of Regulation EC 1099/2009 | - | - | - | N/A | - | - | N/A |
Assessment for Activity 53 (the bleeding of live animals and monitoring the absence of signs of life) includes monitoring the effectiveness of stunning and the absence of signs of life. CoC holders with Activity 53, do not require Activity 52 (assessment of effective stunning) in their CoC to carry out bleeding operations. Note: See Annex IV of 1099/2009.
6.3 Suspension or revocation
6.3.1 Terms
The term CoC includes a Temporary CoC (TCoC).
6.3.2 Return of a suspended or revoked CoC by holder
The OV should consider either CoC suspension or revocation if, during welfare assessments, they are of the opinion that the holder:
- is no longer a fit and proper person
- is no longer competent to carry out the operations which the CoC authorises
- has failed to comply with any provision of the assimilated Regulation or WATOK
- has been convicted of an offence under any animal welfare legislation, this includes incidents outside approved premises
6.3.3 Contraventions
If an individual is responsible for an incident that poses a serious and imminent risk to animal welfare or one where avoidable pain, distress or suffering has been caused, suspension or revocation of the CoC must always be considered.
In cases where there is clear evidence that it is not the fault of the individual, or as a result of the actions of the individual, suspension or revocation of the CoC would not be appropriate.
Contraventions may occasionally occur where the OV deems that suspension or revocation of the CoC is not required. However, the OV must verbally advise the person and BO of the contravention, following up with written confirmation, and record the details in the daybook and / or pocketbook and on Chronos. The OV may still consider issuing a WEN in such circumstances.
When retraining is considered the appropriate course of action, suspension of the CoC should be used.
Any incident that results in a referral for investigation would usually result in a revocation of the CoC of any individuals that are considered responsible for the incident.
6.3.4 Immediate action
Where an individual has caused pain, distress or suffering to an animal, the OV should consider immediate suspension or revocation of their CoC. Evidence of breaches should always be gathered to justify the decision and this should include CCTV footage where available. Only when this was an accident would this action not be appropriate.
When suspension or revocation is considered the correct course of action, the OV must issue the CoC holder with a signed and dated letter (available at Annex 2b or 2c) as soon after the incident as possible, setting out the reasons for the suspension / revocation, the date from which it is effective and details of any rights of appeal.
Following an incident, the OV should inform the BO and CoC holder as soon as possible, that the incident will be investigated, and suspension or revocation will be considered, setting out the reasons and possible consequences. If it is not possible to speak to the CoC holder the OV should ensure that the BO is informed and has made the CoC holder aware.
The CoC holder should be advised of the procedure for a non statutory review as per 6.3.5 below.
The OV must ask the BO to remove the CoC holder from any duties that require a CoC. Where the BO does not comply, the OV must request the VEDM to issue the BO with a WEN prohibiting the CoC holder from carrying out any activity that requires a CoC.
Suspensions should be used for the purposes of retraining only. The suspension letter must set out the retraining activities to be undertaken before the suspension is lifted. Suspensions are intended to be temporary in nature and we would expect training to be completed within 3 months. If the FSA has not been provided with evidence of the retraining activities within this period, the FSA will contact the CoC holder to check if it is the intention for the retraining activities to be completed. Failure to complete the retraining activities may result in revocation.
It is possible for the suspended CoC holder to request a reasonable extension to the three month suspension to permit retraining to take place. Extensions of any agreed time limits should be put in writing and the CoC holder notified that failure to complete retraining will result in revocation.
The current incident, together with any previous suspensions of the individual, should always be considered when deciding whether to revoke or suspend the CoC. Revocation should always be considered where the individual has previously been suspended and retrained for the same offence.
In some cases, it may be appropriate to provide written advice for the BO and / or AWO to implement additional controls such as increased supervision of the suspended individual. The FSA team led by the OV should check that the AWO is taking any additional action to ensure that the individual is working correctly in line with legislative requirements.
Any formal letters sent to notify CoC holders of the suspension or revocation of CoCs must be copied to the WATOK Team, the Animal Welfare Team, the BO, FVL, the Veterinary Enforcement Delivery Manager (VEDM), Head of Delivery, Veterinary Delivery Leader and to the Operations Head Veterinarian.
6.3.5 Certificate of competence suspension or revocation triage
Process for OV decision making following a 4 score welfare incident
| Step in decision making process | Details |
|---|---|
| Step 1: Evaluating the incident |
The initial welfare incident will be reviewed by the OV (including viewing CCTV where available) and placed into one of three categories using the OVs professional judgement:
In each case, the enforcement hierarchy should be used dependent on the severity of the incident. For 4 score welfare incidents, written (not verbal) advice must always be used and revocation considered. Where a case is to be referred for investigation, revocation of the CoC should normally be used. Where a specific individual cannot be identified as responsible, in the case of slaughter teams for example, there will be no revocation or suspension of a CoC but as an alternative response to the incident the OV may consider using a WEN requiring retraining of the team. A WEN may also be used in these cases to slow down the line speed allowing each slaughterer more time to complete their duties. 1. Error of judgement by the individual This may involve a usually competent person involved in a one-off welfare incident. This must not be as a result of:
Where the OV considers that the actions of the CoC holder are not those usually seen and are as a result of an isolated failure, consideration can be given to using written advice. This written advice must be shared with the WATOK team who will log the written advice on the individual’s CoC record. When considering written advice, the CoC database must be checked and if there is already written advice recorded on two occasions within the previous 12 months, further written advice is not an appropriate course of action. When retraining is considered the appropriate course of action, suspension of the CoC should be used. Any retraining must follow the process laid down in Step 4 and should address the cause of the failure, the correct procedures, the plant SOP and applicable legislation. Retraining must be carried out by an FDQ trainer / assessor who is responsible for the content and timing of any retraining. The OV has no role in determining the time taken for retraining or in the practical assessment. If the incident is a serious error of judgement, or occurs on more than one occasion, the OV must consider revocation of the CoC. A person whose CoC has been revoked may apply for a new TCoC however this will not normally be considered where criminal investigations or proceedings are underway. Where an individual has been convicted of welfare matters, they may apply for a new TCoC but must declare the previous conviction within the previous 3 years and the application will then be considered on its merits. REUL 1099/2009 Article 21, 6. There is a right of appeal to the First Tier Tribunal (FTT) up to 28 days after the date of revocation or suspension. 2. Unjustifiable or deliberate act by the individual Where an individual is observed to be involved in systematic welfare abuse, or an unjustifiable or deliberate act that causes avoidable pain, distress or suffering, the OV must take immediate action to prevent that person working with live animals. These cases should always result in immediate revocation of the CoC where there has been a breach of welfare legislation. There is a right of appeal to the FTT up to 28 days after the date of revocation. 3. Accidental incident When the OV identifies an incident involving a live animal and a 4 score applies due to no fault of the individual, suspension or revocation of a CoC will not usually be appropriate. No action will be required to be taken against individuals. The SOP must be reviewed to ensure that any factors leading to the incident are covered. If not, a WEN must be used to require the BO to modify and / or to properly implement the SOP. A WEN must be served if the incident was caused by equipment failure. BO actions will need to be monitored by the OV. If the BO fails to take action, the case must be referred for investigation. In all circumstances where formal action is not considered appropriate, the OV must send a warning letter recording details of the incident and providing information as to good practice. The letter must inform the individual that a copy will be held on file and the incident may be taken into consideration should any further incident occur. A copy of the letter should be retained by the WATOK team. Verbal advice is not suitable for such incidents and must not be used. The OV must ensure that the decision, as above, is made and communicated to the BO as soon as possible. A justification of the decision should be recorded in Chronos. |
| Step 2: Review of the evidence within 14 days |
The FSA will carry out an internal review of a suspension or revocation decision, when requested and evidence is supplied for a review within 14 calendar days of notification to the CoC holder of the incident. All suspended or revoked CoC holders should be asked to provide the OV with any evidence they think will assist in the review and which could result in a change to the decision. The evidence must be provided within 7 calendar days from notification of the suspension or revocation, after which time the available information (for example, plant day book, pocket book entries, CCTV) supporting the decision will be reviewed by the OV and E&J Technical Manager. In each case, the available information will be copied by the E&J Technical Manager to the FVL (FVC), VEDM who will then review the decision. On review of the case, the FVL (FVC), VEDM with assistance of the E&J Technical Manager if required, will consider the circumstances and information available, including any available CCTV footage. The FVL is the ultimate decision maker and must ensure that the CoC holder is informed of the outcome when a review is carried out.
The WATOK team will issue a reminder to all those involved in assessing the decision at 14 days post suspension to ensure that where a review takes place this is properly recorded and where appropriate notified to the CoC holder, BO and WATOK team. |
| Step 3: Appeals to FTT |
If the CoC is suspended or revoked, the individual concerned has 28 days to appeal the decision to the FTT. This right of appeal also applies to the use of a WEN and also to substituted decisions. If the decision is appealed, the case will pass to FSA Legal. Early responses to FSA legal in these cases is essential to ensure that the FSA can meet the timescales for defending an appeal. |
| Step 4:Retraining after a CoC or TCoC suspension |
This must be carried out by an FDQ trainer / assessor. Retraining will be based on the following:
The trainer for the retraining must receive the above information before developing the bespoke session for the individual. The duration of the training is a matter for the trainer to ascertain. The practical appraisal of the individual can only take place AFTER the training. THE TRAINING The individual’s understanding of what “suspension” means. The individual’s understanding of why they were suspended (including any CCTV). Discussion between the individual and the trainer about lessons that could be learned from the alleged incident. Review of the SOP(s) of that activity & consideration to the circumstances surrounding the suspension – this should be pitched at a level appropriate to the individual. Overview of the general requirements (not activity specific) of the requirements including:
The FDQ unit/s directly related to the species and operations that the suspension is for. It is essential that the OV clearly states what the suspension relates to in the suspension letter so that training can be correctly targeted. THE APPRAISAL In line with assessment criteria for practical activities for recognised WATOK qualifications, the trainer will need to observe the individual undertaking the task or activity that was the cause of the suspension. The means and method of observation is down to the trainer, and may be in person, or remotely, depending on accessibility. The observation must, however, be “live”, i.e., in real time and not pre-recorded. As the individual’s CoC/TCoC is suspended, the OV will return the CoC or TCoC at the time of retraining to allow the individual to carry out the relevant tasks for a practical assessment. THE FEEDBACK The trainer should supply the individual, the OV, and the BO with confirmation that the training has been undertaken, and the outcome of the appraisal. Where the trainer appraises the individual to be competent the suspension is considered immediately lifted, allowing the individual to return to duties permitted by the CoC/TCoC. The VEDM should then supply a letter (annex 2d) to the individual that confirms the lifting of the suspension, the letter must be copied to the WATOK team to update their records. Where the trainer appraises the individual not to be competent, the CoC or TCoC must be returned to the OV pending further action. The trainer may feed back to the BO on the content of the relevant SOP(s). |
6.3.6 Return of a suspended or revoked CoC by holder
Where a CoC has been suspended or revoked, the holder is required by WATOK (England and Wales) to return the CoC to the FSA within 14 days of the letter of suspension or revocation. In the case of immediate suspension, the CoC should be returned to the OV who suspended the CoC. The OV will normally retain the CoC pending successful retraining. When the OV retains the CoC it must be held in a secure place in the FSA office. Where the retraining does not take place within a period of 3 months from the date of the letter of suspension then the OV should return the COC to the WATOK team. In the case of CoC revocation the CoC should be returned within 14 days to the WATOK team either by the OV or the operative.
Where revocation / suspension of a CoC takes place and the case is referred for further investigation, the WATOK team / CSU should liaise with FSA Legal Services to ensure that the Investigating Officer (IO) responsible for the investigation conducts the interview as quickly as possible.
Reference: WATOK (England) (Wales) Chapter 3, Article 19 (3).
6.3.7 Issuing a CoC refusal notice
When the person making an application for a temporary CoC has failed to answer all the relevant questions on the application form, or has declared a previous welfare offence, the FSA WATOK team will refuse to issue a temporary CoC.
If a period of more than three years has elapsed since a previous revocation or conviction of a welfare offence a person can still apply for a TCoC provided that they declare the previous revocation or conviction. Such an application will be considered on an individual basis.
The evidence will be reviewed by an FVL who will request further evidence if they feel it necessary. You should notify the person that they have a right to appeal a refusal to grant a temporary CoC. The FVL will liaise with the WATOK team / CSU and notify the OV and the applicant of their decision.
6.4 Appeal process
6.4.1 Applicant / holder rights
An applicant or CoC holder may appeal against the decision to:
- refuse to grant a temporary or full CoC
- suspend a CoC
- revoke a CoC
The appeal will be heard by the FTT.
Appeals should be made to: updated [grc@justice.gov.uk ]
Alternatively, the Ministry of Justice form T98 may be lodged with the General Regulatory Chamber, HM Courts and Tribunals Service, PO Box 9300, Leicester, LE1 8DJ within 28 days of the decision.
Where an OV is aware that an individual intends to appeal a decision, they should notify the SLA and Contracts team in York and FSA legal.
6.4.2 Return of CoC after FTT decision
Where a CoC is to be returned or replaced after a decision has been made by FTT to overturn a suspension or revocation, the WATOK team / CSU should use the template letter at Annex 2g.
7. Enforcement
In this section
7.1 Introduction
7.1.1 Purpose
These enforcement arrangements apply to all slaughterhouses and farmed game establishments approved in England and Wales and under veterinary control.
Enforcement action is taken in accordance with the FSA enforcement policy.
This section must be read in conjunction with chapter 7 on ‘Enforcement’ of the MOC.
7.1.2 Terminology
Note that for the purposes of enforcing welfare legislation the operator responsible is referred to as BO and this term should be used in formal notices.
7.1.3 Provisions of Assimilated Regulation No (EU) 1099/2009
Article 22 of 1099/2009 is repealed by Article 159 of assimilated Regulation No (EU) 2017/625 and replaced with Article 138 of 2017/625. This sets out that where the non-compliance is established, the competent authorities shall take:
a) any action necessary to determine the origin and extent of the non-compliance and to establish the operator’s responsibilities; and
(b) appropriate measures to ensure that the operator concerned remedies the non-compliance and prevents further occurrences of such non-compliance.
Examples of these measures might include (but not be limited to):
Article 138(2)(e) - order the operator to increase the frequency of own controls;
Article 138(2)(i) - order the cessation for an appropriate period of time of all or part of the activities of the operator concerned;
Article 138(2)(j)- order the suspension or withdrawal of the registration or approval of the establishment
Article 138(2)(k) - order the slaughter or killing of animals provided that this is the most appropriate measure to safeguard human health as well as animal health and welfare.
The competent authorities shall provide the operator concerned, or its representative, with:
(a) written notification of their decision concerning the action or measure to be taken under Article 138 together with the reasons for that decision; and
(b) information on any right of appeal against such decisions and on the applicable procedure and time limits with respect to such right of appeal.
7.1.4 Use of welfare notices
Authorised Officer (AO) has powers under Regulation 38 of WATOK to serve a formal WEN (WEL 11/34). The same notice can be used in three ways:
- To require steps to be taken to remedy contraventions of the regulations (for example updating the SOP or where equipment did not have a regular maintenance record)
- To require the rate of operations to be reduced until steps have been taken to remedy contraventions of the regulations (for example, slowing down the line where animals are not adequately stunned as a result of high line speed or to slow down the slaughter operation where a premises undertaking religious slaughter without pre stunning does not comply with the requirements in WATOK Schedule 3 for animals not to be moved until unconscious)
- To stop an activity, process or operation or the use of facilities or equipment until steps have been taken to remedy contraventions of the regulations (for example, stopping the use of equipment that is causing pain distress or suffering, for example, if equipment is not giving an effective stun)
Regulation 38,9 of WATOK permits an AO at any time to withdraw or vary an enforcement notice in writing. A template letter for the withdrawal of a WEN is provided at annex 19.
Once the BO or the person on whom the notice is served has complied with the WEN, the AO must serve a Welfare Completion Notice (WEL 11/35) to confirm that they are satisfied the BO / person has taken the steps specified to correct the contravention.
You may refuse to serve a Welfare Completion Notice if you are not satisfied that the terms of the WEN have been met. In such cases, you must complete and serve a Refusal to Serve a Welfare Completion Notice (WEL 11/36), setting out the reasons for the refusal.
It is an offence to fail to comply with a WEN under Regulation 30 (2) of WATOK. Where a notice has been breached, the OV must gather evidence of the contravention that led to the service of the formal notice, together with evidence to demonstrate the breach of the notice and refer both matters for investigation.
The BO can appeal an enforcement notice through the FTT details can be found earlier in this chapter.
The BO or person upon whom a notice has been served can appeal against the inspector’s decision to serve the WEN or issue a Welfare Completion Notice through the FTT.
A WEN may remain in force if not complied with until FTT considers the case. If the WEN has been complied with a completion notice must be issued in accordance with Regulation 38 of WATOK.
Details can be found earlier in this chapter.
Note: Where enforcement action is being taken against an individual responsible for any pain, distress or suffering to an animal, the OV should consider whether it is also appropriate to suspend or revoke the persons CoC in line with the provisions of Article 19 of the domestic regulations.
Note: Where a CoC is suspended, notify the WATOK team / CSU York Transactions Team at: WATOK@food.gov.uk.
7.1.5 Extension of a WEN when requested by the BO
Whilst there is no legal basis for the inspector to extend a notice, Regulation 38(9) of WATOK allows inspectors to withdraw or vary any enforcement notice.
If a BO wishes to request an extension to a WEN, they must do so in writing prior to the expiry of the notice and must:
- confirm that welfare will not be compromised by the extension
- explain how they are going to manage the welfare risk in the interim period
- identify genuine reasons why they are seeking an extension
- provided details of the length of the extension they are seeking and copies of agreements for the work if it is to be carried out by external contractors
If the inspector is happy with the BO’s past record of compliance, and agrees with the proposed extension period and reasons, they should agree with the BO the length of time required to comply and confirm this agreement in writing.
The inspector must review the works carried out by the BO after the agreed extension date has expired and:
- withdraw the WEN if compliance has been achieved through the service of a Welfare Completion Notice, or
- serve a Refusal to Issue a Welfare Completion Notice, with reasons for the refusal if they are not content with the actions; the inspector must refer the breach of a WEN for formal investigation
7.1.6 Prosecutable non-compliance
In terms of welfare enforcement, a prosecutable non-compliance is one where there is evidence of a clear contravention of the regulations and / or the incident involves any avoidable pain, distress or suffering.
The OV should gather evidence to assist an investigation if there is a suspicion that an offence has taken place (WATOK Part 5 lists offences).
7.2 Non-compliances
7.2.1 Non-compliance assessment
The OV must use their professional judgment to assess if what has been observed is a ‘prosecutable’ non-compliance, before referring the matter for investigation. The assessment should include the following:
- details of the regulation(s) that have been contravened
- the severity of the incident (for example, were any animals subject to any avoidable pain, distress or suffering during their killing and related operations?)
- evidence of avoidable pain, distress or suffering caused to the animal(s) (for example, whole bodies of dead animals, post-mortem examination results, detained carcase, heads or other body parts, animal behaviour such as panting, evidence of thirst / hunger, video evidence)
- whether the incident was accidental or caused by negligence
- details of all relevant suspects, names, positions and any training they have received
- the species and operations listed on the CoC
- details of specific directors (for limited companies) where contraventions have occurred, and it can be additionally demonstrated that they have occurred through the direct consent, connivance or neglect or those directors
- whether there have been previous incidents of a similar nature
- whether the abuse was deliberate (for example, kicking or inappropriate use of goads)
- Whether any previous verbal or written warnings have been issued
- whether a Welfare Notice has been used and not complied with
- the nature of the response from the AWO or BO when the OV advised them of the incident
- whether the operative / BO took effective corrective action
- details of all available witnesses
- whether there is evidence to confirm the witness statements (for example, clinical signs, contemporaneous notes, drawings, maps, photographs, temperature readings, calculations and measurements showing SD)
7.2.2 Body parts for evidence that are SRM
Bovine heads / whole bodies and body parts of various species may be SRM by definition; however, it should be remembered that such bodies or body part must not be disposed of as ABP where they are required as evidence.
This material must be identified, seized, secured and stored safely until after all appropriate enforcement action has been taken by the FSA or where it has been passed to another enforcement authority as essential evidence for their case.
Once the case is concluded, the material will then be disposed of as the appropriate class of ABP.
7.2.3 Enforcement notice and / or suspension / revocation of CoC
If a non-compliance is observed, then appropriate enforcement action must be taken.
Where an individual is responsible for a non-compliance, then Suspension or Revocation of the CoC should always be considered and taken forward along with any routine enforcement action. Notify the WATOK team / CSU York Team at: WATOK@food.gov.uk.
Providing a verbal warning only, without further escalation, is not proportionate action to take in cases of potential or suspected cruelty to animals.
7.2.4 Recording evidence
All action taken on non-compliances must be transferred to the Enforcement Programme.
Where a Welfare Enforcement Notice (WEN) is issued requiring immediate action the Remedial Action Notice (RAN) column should be populated with the date of service. Where a WEN is issued requiring an improvement to be made, the Hygiene Improvement Notice (HIN) column should be used.
All welfare incidents where there is non-compliance with legislation must be recorded in Chronos. Where the breach causes no risk of pain, distress or suffering and is corrected immediately, a 2 score should be used. If the contravention is not a 2 score, but there is still no evidence of pain, distress or suffering, a 3 score should be used and if there is evidence of pain, distress or suffering a 4 score should always be used.
Incidents not leading to a referral for investigation:
If the incident was assessed as ‘non-prosecutable’ and no further action is required on this occasion, the appropriate ‘Action required’ box of Chronos must be selected. An advisory letter must be applied as a minimum intervention for 4 score incidents and a brief summary of the reason for non-referral should be included when entering details of the non-compliance, for example, ‘unavoidable accidental incident’ or ‘no witness’.
7.2.5 Reporting Non-compliance for WATO
Non-compliances observed for welfare during transport should be documented and referred to the LA immediately, and subsequently confirmed in writing, using the notification form at Annex 4 (see referrals process in paragraph 3.3.1) and should be recorded on the Chronos. All cases must have a unique identification number allocated to them to aid traceability of cases. Keep a copy in the plant welfare folder.
7.2.6 Reporting of on farm welfare NCs
Identified welfare issues which appear to have originated on the holding of provenance, should be documented and referred in writing to APHA as well as being reported to the LA. Keep a copy of the report in the plant welfare folder.
7.2.7 Use of CCTV footage
The use of cameras and video equipment is essential to evidence an animal’s ability to move, support their weight on all limbs or their general condition.
If the BO has CCTV installed and a welfare issue has been identified, request access to the footage and ask for a copy to be provided as soon as possible, both verbally and in writing.
The CCTV footage should be requested from BOs if a breach of the welfare regulations is suspected. Failure to provide footage of a contravention should be referred for investigation under the obstruction provisions.
8. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 1: Examples of Certificate of Competence; photo ID card
Annex 2a: Aide memoire CoC suspension and revocation letters
Annex 2b: CoC suspension letter
Annex 2c: CoC revocation letter
Annex 2d: Return of CoC after suspension letter
Annex 2e: Failure to retrain revocation letter
Annex 2f: Return of CoC after review letter
Annex 2g: Return of CoC after FTT decision letter
Annex 3: Foot pad dermatitis condition card
Annex 4: LA notification form: welfare breaches
Annex 5: OV checklist for animal welfare incident on farm or during transport
Annex 6: Procedures for heavily pregnant animals
Annex 8: Animal Welfare Verification Protocol pro-forma
Annex 9: Aide Memoire – welfare checks in red meat
Annex 10: Aide Memoire – welfare checks in poultry
Annex 11: Signs of properly stunned or dead poultry
Annex 12: Signs of a properly stunned animal by stunning method
Annex 14: Welfare Referral Aide Memoire
Annex 16: Questions and Answers on Annex II of Council Regulation (EC) No 1099/2009
Annex 17: CCTV Viewing Protocol
Annex 18 REMOVED
Annex 19: Withdrawal of a Welfare of Animals at the Time of Killing Enforcement Notice
Annex 20: REMOVED
Sections
6. Poultry Post-Mortem Inspection
7. Judgements at Poultry Post-Mortem Inspection
8. Wild Game Post-Mortem Inspection
9. Health and Identification Marking
11. Slaughter Hygiene Verification System in Red Meat
1. Introduction
In this section
1.1 Overview
1.1.1 Purpose
The principal purpose of post-mortem inspection is to supplement ante-mortem inspection and to detect:
- diseases of public health significance
- diseases of animal health significance
- residues or contaminants in excess of the levels allowed by legislation
- the risk of non-visible contamination
- other factors which might require the meat to be declared unfit for human consumption or restrictions to be placed on its use
- visible lesions that are relevant to animal welfare such as beating or long-standing untreated injuries
1.2 Legislation
1.2.1 Regulations
Retained Regulations (EU) 2017/625, 2019/624 and 2019/627 details:
- who can undertake the post mortem-inspection
- the purpose of post-mortem inspection
- the post-mortem inspection procedures
- the decisions to be taken concerning meat
Retained Regulation (EU) 853/2004 details the standards that the Food Business Operator (FBO) should provide and achieve for post-mortem inspection.
1.2.2 Post-Mortem inspection requirements
Specific requirements for each species are listed in Retained Regulation (EU) 2019/627
Reference: See Annex 1 for a summary of post-mortem inspection requirements.
2. FSA role
In this section
2.1 Introduction to post-mortem inspection
2.1 Introduction to post-mortem inspection
2.1.1 Key principles
Post-mortem inspection should:
- take into account ante-mortem inspection results
- view all external surfaces
- pay particular attention to the detection of zoonotic and notifiable diseases
- take into account food chain information (FCI) or trained hunter’s declaration
- take place without delay after slaughter
- include carcases and accompanying offal
2.1.2 Contamination during inspection
During inspection, precautions must be taken to ensure that contamination of the meat by actions such as palpation, cutting or incision is kept to a minimum. Minimal handling of the carcase and offal should take place.
Bovine animals under 8 months old can undergo visual only inspection in accordance with Retained Regulation (EU) 2019/627, Article 18.
In relation to pig meat, the European Food Safety Authority (EFSA) adopted a Scientific Opinion which concluded that palpation or incisions in carcase and offal at post-mortem inspection should be omitted for pigs subjected to routine slaughter, because of the risk of microbial cross-contamination being higher than the risk associated with potentially reduced detection of conditions targeted by those techniques.
The use of palpation and / or incision should be limited to suspect pigs (see sub-topics 2.4.1 to 2.4.3 for further information).
2.1.3 Accuracy
The speed of the slaughter line and the number of inspection staff present must ensure proper inspection is completed and records maintained. Food business operators should be instructed to take immediate corrective action, including a reduction in the speed of slaughter, where:
- contamination is detected on external surfaces of a carcase or its cavities and the food business operator does not take appropriate action to rectify the situation; or
- if good hygiene practices are jeopardised.
Reference: Retained Regulation (EU) 2019/627 Article 12, 4 and Article 46, 1
MHI post-mortem inspection is for defect detection. OV post-mortem inspection is for disease diagnosis.
2.1.4 Additional examination requirements for post-mortem inspection
Where it is thought necessary, additional examinations are to take place such as palpation and incision of the carcase and offal and laboratory tests to:
- reach a definitive diagnosis
- detect the presence of:
- an animal disease
- residues or contaminants in excess of the levels allowed by community legislation
- non-compliance (NC) with microbiological criteria
- other factors that might require the meat to be declared unfit for human consumption or restrictions to be placed on its use
Note: Special attention should be taken in the case of animals having undergone emergency slaughter
- assess whether animal welfare is being compromised
2.1.5 OV presence (on the line)
The OV need not be present at all times on the line during post-mortem inspection if:
- an MHI carries out post-mortem inspection and puts aside abnormal meat with uncommonly occurring conditions and all other meat from the same animal
- the MHI documents their procedures and findings in a manner that allows the OV to be satisfied that standards are being met
- the OV subsequently inspects all such meat
The MHI may discard meat from poultry and rabbits with abnormalities and the OV need not systematically inspect all such meat.
2.1.6 MHI post-mortem decision tree

2.1.7 Abnormal meat
To consider an abnormal carcase meat/offal as ‘uncommon’, we could take into consideration different aspects such as:
- prevalence of the condition in the area
- prevalence of the condition in the flock / herd (degree of infection or infestation)
- the possible human health implications of the condition (such as zoonoses)
- the possible animal health implications of the condition (such as lesions which may indicate a possible notifiable disease such as classical swine fever, foot and mouth disease)
- possible animal welfare problems on farm, during transport or in the lairage
- the need to refer it to the veterinarian to do a differential diagnosis
- economic importance of the condition for the farming industry (degree of infestation)
Based on all the above, the MHI will need to make a judgement and notify the OV of the findings.
2.1.8 Examples of abnormal conditions that can be classified as common or uncommon
The table below outlines abnormal conditions and their classification.
| Abnormal condition | Comments | Occurrence |
|---|---|---|
| Broilers septicaemia / toxaemia | Very prevalent condition. It represented 14.75% of total conditions rejected in 2004. | Common |
| Mastitis in older cattle | Common condition in all species, especially cows. No need to inform the OV as the farmer is already aware and will receive notification when he is informed about the post-mortem inspection records. | Common |
| Sheep caseous lymphadenitis |
Is becoming more common but the OV needs to be made aware because of the economic importance of the disease (responsible for 1% of condemnations at meat inspection).
|
Uncommon |
| Cattle (30 month or younger) fascioliasis | Common in ungulates. The OV does not need to be informed. The disease is of great economic importance because of liver condemnations. The farmer will be informed when he receives notification of the post-mortem inspection findings. | Common |
| Pigs pleurisy / pneumonia | Inflammation of the pleurae is a common meat inspection lesion in pigs. It requires the stripping of the pleura or removal of the rib cage, but carcase condemnation is not normally necessary. There is positive correlation between the number of carcases requiring lung condemnation and the number of those requiring pleura stripping. The OV does not need to be informed. | Common |
| Sheep anthrax | Normally identified at ante-mortem inspection if a suspect animal is found dead in the lairage. It is a notifiable disease, and it is a zoonoses. The OV must be informed and should immediately inform the APHA Duty Veterinarian. | Uncommon |
| Broilers mechanical damage | This is normally the result of poor functioning of the poultry plant machinery. The FBO has to be informed by the MHI if he has not already identified the problem. | Common |
| Cattle sarcocystis | The incidence is higher in older cattle but is an uncommon condition. Depending on the degree of infestation, the carcase and viscera have to be rejected. The OV should be informed. | Uncommon |
| Pigs ascariasis (milk spot) | The second most recorded condition at post-mortem in pigs (17% of total rejections in 2004). It is mainly identified in livers (‘milk spot’) which are unfit for human consumption. The farmer will be informed when he receives the post-mortem inspection report. The OV does not need to be informed. | Common |
2.2 FSA Duties
2.2.1 Outline
The following table outlines the duties of the FSA Operations Group with regard to post-mortem inspection.
| Role | By | Frequency |
|---|---|---|
| Carry out post-mortem inspection | An OV or MHI appropriately authorised under (EU) 2019/624, (or appropriately authorised slaughterhouse staff in poultry or rabbit slaughterhouses) working under the supervision of an OV | All carcases and accompanying offal without delay after slaughter |
| Carry out post-mortem inspection for animals subject to emergency slaughter outside the slaughterhouse | An OV only; this cannot be delegated to a MHI |
All carcases and offal as soon as possible.
|
| Carry out PM for animals accompanied by a farmer’s declaration | OV or MHI | All carcases and offal as soon as possible |
| Record post-mortem inspection results | OV or MHI (or plant inspection assistant (PIA) | At the time of post-mortem inspection |
| Apply Health Mark |
The Health Mark must be applied under the supervision of the OV
|
Immediately after post-mortem inspection (this may be prior to results of any examination for trichinella being available, if OV satisfied meat will only be placed on market if results are satisfactory) See chapter 2.6 on ‘TSE testing’ for health marking Bovine Spongiform Encephalopathy (BSE) tested cattle |
| Disease sampling / testing | OV or MHI | When disease is suspected |
| Monitoring sampling / testing | OV or MHI or specifically trained plant staff | When monitoring of disease is required, for example, TSE, trichinella |
2.3 Post-mortem inspection guidelines
2.3.1 Options in post-mortem inspection
Specific requirements for all species are listed in retained regulation (EU) 2019/627 Articles 14 to 28.
2.3.2 Splitting carcases
Carcases of domestic solipeds, bovine animals over eight months old and domestic swine more than five weeks old must be submitted for post-mortem inspection split lengthways into half carcases down the spinal column.
Reference: Retained Regulation (EU) 2019/627 Article 15, 2.
However, to take account of particular eating habits, technological developments or specific sanitary situations, the official veterinarian may authorise the submission for post-mortem inspection of carcases of domestic solipeds, bovine animals more than eight months old and domestic swine more than five weeks old that are not split in half.
In low-capacity slaughterhouses or low-capacity game-handling establishments handling fewer than 1 000 livestock units per year, the official veterinarian may, for sanitary reasons, authorise the cutting into quarter carcases of adult domestic solipeds, adult bovine animals and adult large wild game before post-mortem inspection.
Reference: Retained Regulation (EU) 2019/627 Article 15, 3 and 4.
The OV may also require any head or any carcase to be split lengthways if the inspection so necessitates.
Caution: Splitting the head of cattle carries a health and safety risk, and if the animal is required to be sampled for BSE it may only take place after the sample has been taken.
2.3.3 Minimal handling by inspectors
During inspection, precautions must be taken to ensure that contamination of the meat by actions such as palpation, cutting or incision is kept to a minimum.
Note: Whilst still allowing for adequate post-mortem inspection care must be taken not to de-value the carcase or offal when making post-mortem incisions.
2.3.4 Visual inspection only
Carcases and offal of pigs of all ages are to undergo visual inspection procedures. Further inspection procedures (FIP) (palpation and / or incision) can be carried out when one of the following indicates a risk to public health, animal health or animal welfare:
- checks on the FCI
- checks on any other data from the holding of provenance
- ante-mortem or post-mortem findings
Note: Further inspection can also be carried out if gathering of evidence is required for enforcement purposes (for example, welfare investigation).
2.3.5 Examples of conditions found in pigs at ante-mortem that might justify further inspection procedures at post-mortem
For the majority of the conditions listed on the current ante mortem inspection sheet there would be no need for pigs to be marked to undergo FIP at post-mortem.
However, the following may justify FIP:
- mastitis (if associated with general signs)
- moribund / recumbent
- orchitis (marked to consider Brucella, occupational zoonoses)
- suspect emaciation, poor condition
- suspect fever
- slaughtered in lairage
Note: the OV is not limited to these conditions and should use their professional judgement.
2.3.6 Examples of conditions found in pigs at post-mortem that might justify FIPs
For localised conditions on pig carcases, FIPs are not normally justified unless a generalised and-or septic condition is also observed / suspected.
The following localised conditions may justify detaining the carcase for FIP at post-mortem:
- multiple abscesses
- TB like lesions (in cases of enlarged lymph nodes)
When the OV / MHI suspects a generalised condition, in some cases the appropriate decision about the fitness of the meat for human consumption cannot be made without further examinations.
If any of the following conditions is observed / suspected, this may justify detaining the carcase or offal for FIP at post-mortem inspection:
- anaemia (may be part of other generalised condition)
- badly bled (may mask some other post-mortem signs)
- contamination gut content (may mask other conditions)
- emaciation / generalised oedema
- erysipelas
- generalised TB, tumours, melanosis
- jaundice
- machine damage (if may mask other conditions)
- poly-arthritis
- septic peritonitis
- septic pleurisy
- suspect pyaemia / multiple abscesses-tail bite-other
- suspect uraemia / abnormal odour
- suspect fever / septicaemia
- suspect residues
Note: The OV / MHI is not limited to these conditions and should use their professional judgement.
2.4 Decisions concerning meat
2.4.1 Animal carcases for which a ‘suspect animal card’ was completed
The OV must have a suitable system in place to inform the person(s) performing the post-mortem inspection of any condition that may help in the post-mortem judgement for that carcase. This includes any animals for which a ‘Suspect Animal Card’ has been completed and also pigs identified at ante mortem inspection as requiring further post-mortem inspection procedures other than visual inspection.
2.4.2 Possible outcomes
After the inspection, the OV/MHI can:
- pass the meat as fit for human consumption
- declare the meat unfit for human consumption
- detain the meat for further examination following rectification
2.4.3 Reasons for declaring meat unfit
Meat may be declared unfit for human consumption if it:
- derives from animals that have not undergone ante-mortem inspection, except for hunted wild game
- derives from animals the offal of which has not undergone post-mortem inspection, unless otherwise permitted under Regulation 853/2004 or Regulation 2019/627 Article 45(b).
- derives from animals which are dead before slaughter, stillborn, unborn, or slaughtered under the age of seven days
- results from the trimming of sticking points
- derives from animals affected by animal diseases for which animal health rules are laid down in Annex I to Council Directive 2002/99/EC except if it is obtained in conformity with the specific requirements provided for in that legislation, unless otherwise provided for in Section IV (Reference: Retained Regulation (EU) 2019/627 Article 45(e)
- derives from animals affected by a generalised disease, such as septicaemia, pyaemia, toxaemia or viraemia
- is not in conformity with microbiological criteria laid down under community legislation to determine whether food may be placed on the market
- exhibits parasitic infestation, unless otherwise provided for in Section IV
- contains chemical residues or contaminants in excess of the levels laid down in community legislation; any overshooting of the relevant level should lead to additional analyses whenever appropriate
- without prejudice to more specific community legislation, derives from animals or carcases containing residues of forbidden substances or from animals that have been treated with forbidden substances
- consists of the liver and kidneys of animals more than two years old from regions where plans approved in accordance with Article 5 of Directive 96/23/EC has revealed the generalised presence of heavy metals in the environment
- has been treated illegally with decontaminating substances
- has been treated illegally with ionising or UV-rays
- contains foreign bodies (except, in the case of wild game, material used to hunt the animal)
- exceeds the maximum permitted radioactivity levels laid down under community legislation
- indicates patho-physiological changes, anomalies in consistency, insufficient bleeding (except for wild game) or organoleptic anomalies, in particular a pronounced sexual odour
- derives from emaciated animals
- contains specified risk material, except as provided for under community legislation
- shows soiling, faecal, or other contamination
- consists of blood that may constitute a risk to public or animal health owing to the health status of any animal from which it derives or contamination arising during the slaughter process
- in the opinion of the OV, after examination of all the relevant information, it may constitute a risk to public or animal health or is for any other reason not suitable for human consumption
Where there is total rejection the whole carcase, offal and blood and the rest of body parts must be disposed of as an ABP.
Reference: Retained Regulation (EU) 2019/627, Article 45.
2.4.4 Reference link to pathological conditions
For poultry, consult the poultry condition cards found on Digital Workplace and linked from section 7 on ‘Judgements at poultry post-mortem inspection’ of this chapter.
2.4.5 Meat declared unfit
Where the OV is not satisfied that the meat is fit for human consumption, the health mark / identification mark must not be applied in accordance with retained Regulation (EU) 2019/627, Article 48, 2(a). The FBO should be asked to voluntarily surrender meat rejected as unfit for human consumption. Where surrender is not forthcoming, the OV should put in writing the reasons why they are formally declaring the meat unfit for human consumption in accordance with Retained Regulation 2017/625 Article 138,3.
Note: Where the FBO continues to refuse to dispose of meat that has been declared unfit, follow the ABP provisions relating to the treatment of meat declared unfit for human consumption. See chapter 2.8 on ‘Animal by-products’.
2.4.6 Further inspection required
If the OV / MHI considers that the carcase and offal require further inspection, the carcase and the associated offal must be detained and kept under control of the OV pending the inspection.
2.4.7 When partial rejection may be appropriate
Partial rejection of the meat or offal may be appropriate where only part of the carcase or a single organ is affected. Reject only the affected carcase part or offal and the tissue immediately surrounding it as an ABP.
2.4.8 Detention procedure
When detaining a carcase for further inspection it is important to maintain correlation of the detained carcase and all relevant parts until post-mortem inspection has been completed and any additional examinations have taken place.
The detention method and any other examinations that are carried out must be done in a manner that prevents the risk of cross-contamination with meat intended for human consumption, for example, prevention of contact between carcases.
Note: It is inappropriate to detain meat that has been declared unfit for human consumption with a formal food detention notice, as the product becomes an ABP, and no provision exists to detain an ABP.
2.4.9 Rectification FBO responsibility
It is the responsibility of the FBO to present carcases and offal to the FSA for final inspection free from contamination by faeces, gut content, hair, wool, bile, and any other pollutants in accordance with the FBO’s procedures based on HACCP principles.
2.4.10 FSA Operations group responsibilities
FSA Operations Group staff should have regard to the following:
- Meat showing signs of pathology or contamination must not be health marked/passed as fit and should be detained for rectification by the FBO.
- Where contamination on a series of carcases/offal is persistent and represents a failure in the FBOs hygienic procedures, the OV should immediately be informed, to establish the cause and rectify the problem; this may involve the OV stopping the line to resolve the issue.
Note: All line stoppages should be recorded in the day book and in the enforcement programme in Chronos.
- The OV must discuss the dressing procedures and HACCP based plan with the FBO where persistent deficiencies are identified.
Note: Deficiencies in dressing should be recorded using the Slaughter Hygiene Verification (SHV) K2 form in red meat and in poultry.
FSA staff must not carry out any type of meat rectification work, including for quality reasons, as this is the responsibility of the FBO.
2.4.11 Use of scabbards by FSA staff
Scabbards should only be used to transport knives to and from the post-mortem inspection stations. Once at the post-mortem inspection station, sterilizers should be used to store knives when not in use.
3. FBO responsibility
In this section
3.1 Responsibility
3.1.1 Responsibility
It is the responsibility of the FBO to produce safe meat. FSA Operations Group inspectors confirm FBO actions and identify any specific risks.
3.1.2 Timelines
Stunning, bleeding, skinning, evisceration, and further dressing are carried out without undue delay and in a manner that avoids contaminating the meat.
3.1.3 FSA facilities
The FBO follows the instructions of the OV to ensure that post-mortem inspection of all slaughtered animals is carried out under suitable conditions.
3.1.4 FBO facilities
Until post-mortem inspection is completed all parts of a slaughtered animal:
- must remain identifiable as belonging to a given carcase
- must not come into contact with any other carcase, offal, or viscera
- must not be washed
The FBO must ensure that:
- slaughtered animals are dressed and treated in such a manner as not to prevent or hinder inspection
- no carcases are cut up unless retained Regulation (EU) 2017/627 Article 15 applies see paragraph 2.3.2
- no action is taken to destroy or alter evidence of disease
- no part, except the hide or skin, is removed from the establishment until post-mortem inspection is completed and any required samples are taken
Exceptions
- for all species: the penis, if not intended for human consumption
- for sheep and goats: the head if no part of it is intended for human consumption
Reference: 2019/627 Articles 19, 20 and 21.
Any visible contamination must be removed without delay.
Reference: (EC) 853/2004 Annex III, Section I, Chapter IV.
3.1.5 Skinning
All carcases and other parts of the body intended for human consumption must undergo complete skinning, except for:
- porcine animals
- updated [the heads of ovine and caprine animals and calves
- the muzzle and lips of bovine animals
- feet of bovine, ovine and caprine animals]
Unskinned feet must be handled so as to avoid contamination of other meat.
Note: When destined for further handling, and before leaving the slaughtering establishment, feet of all species must be skinned or scalded and depilated.
Reference: Retained Regulation (EU) 853/2004 Annex III, Section I, Chapter IV, 18.
3.1.6 Spleens
Spleens must be removed completely and, wherever possible, whole. The operator must present spleens correlated to carcases for inspection.
3.1.7 Delayed uteri removal
For the grading and classification of female bovines as heifers or cows the uteri may be left attached to the carcase until the grading is completed.
Meat and Livestock Commercial Services Ltd (MLCSL) officers are being advised to speak to the FBO where they have a need for the uteri to be retained for grading purposes. The OV must be satisfied that a suitable system can be adopted before the procedure can start.
3.1.8 Uteri removal: FBO responsibility
In order to facilitate the process, the FBO must have a suitable system in place. The procedure must:
- be agreed between the FBO and the OV
- ensure that post-mortem inspection is completed, and that no carcase is released for human consumption until the uteri has been completely removed and the carcase found fit for human consumption
- in addition, the uteri should be hygienically removed as soon as is practical following classification / grading
3.1.9 Uteri removal: OV responsibility
The OV must be satisfied that:
- suitable procedure can be adopted to ensure that hygienic production is maintained, for example, keeping correlation between the uteri and the carcase without a risk of cross contamination
- health marks are not applied until the carcases have had the uteri removed and have passed post-mortem inspection
3.1.10 Storage facilities
There are lockable facilities for the refrigerated storage of detained meat and separate lockable facilities for the storage of meat declared unfit for human consumption.
3.1.11 After post-mortem inspection
Retained Regulation (EU) 853/2004, Annex III, Section I, Chapter IV, 16 states:
- the tonsils of bovine animals, porcine animals and solipeds must be removed hygienically
- meat declared unfit for human consumption must be removed as soon as possible from the clean sector of the establishment
- meat detained or declared unfit for human consumption and inedible by-products must not come into contact with meat and offal declared fit for human consumption
4. Guidance on conditions
In this section
4.1 Judgements at red meat post-mortem inspection
4.1 Judgements at red meat post-mortem inspection
4.1.1 Introduction
It is the duty of the OV, or the MHI acting under their authority, during post-mortem inspection to make a judgement based on the specific case presented and the requirements of Regulation 2019/627 Articles 29 to 35.
4.1.2 Legislation
Retained Regulation (EU) 2019/627 lays down eight specific hazards:
- TSE
- Cysticercosis
- Glanders
- Tuberculosis
- Brucellosis
- Trichinosis
- Salmonella
- Campylobacter
4.1.3 Guidance
There follows guidance on the following specific topics:
- TSE
- Glanders
- Brucellosis
- Cysticercus bovis
- Arthritis
- Tumours in bovines
- Trichinella
- Aujeszky’s Disease
4.2 Transmissible spongiform encephalopathy
4.2.1 Guidance on TSE
Official controls carried out in relation to TSE are to take account of the requirements of Retained Regulation (EU) No 999/2001 and other relevant community legislation.
Reference: See chapter 2.6 on ‘TSE testing’ for additional information.
4.3 Glanders
4.3.1 Guidance on Glanders
Where appropriate, solipeds are to be examined for glanders. Examination for glanders in solipeds is to include a careful examination of mucous membranes from the trachea, larynx, nasal cavities and sinuses and their ramifications, after splitting the head in the median plane and excising the nasal septum.
Meat from horses in which glanders has been diagnosed are to be declared unfit for human consumption.
Reference: Retained Regulation (EU) 2019/627 Article 32.
4.4 Brucellosis
4.4.1 Guidance on Brucellosis
When animals have reacted positively or inconclusively to a brucellosis test, or there are other grounds for suspecting infection, they are to be slaughtered separately from other animals, taking precautions to avoid the risk of contamination of other carcases, the slaughter line and staff present in the slaughterhouse.
Meat from animals in which post-mortem inspection has revealed lesions suggestive of acute infection with brucellosis is to be declared unfit for human consumption. In the case of animals reacting positively or inconclusively to a brucellosis test, the udder, genital tract, and blood must be declared unfit for human consumption even if no such lesion is found.
Reference: Retained Regulation (EU) 2019/627 Article 34
Note: All FSA staff should be aware that, when dealing with brucellosis suspects, they must always wear eye protection, disposable masks, and gloves.
4.5 Cysticercus bovis
4.5.1 Introduction
Meat infected with cysticercus is to be declared unfit for human consumption. However, when the animal is not generally infected with cysticercus, the parts not infected may be declared fit for human consumption after having undergone a cold treatment.
At this time the derogations from post-mortem inspection in Article 30(1) do not apply.
Reference: Retained Regulation (EU) 2019/627 Article 30.
4.5.2 Guidance on C. bovis
Use the table below as a guide to judgement when cases of C.bovis are detected.
Guidance of Cysticercus bovis
| Number (Post-mortem findings) | Location (Post-mortem findings) | Status (Post-mortem findings) | Judgement |
|---|---|---|---|
| One cyst | Localised* | Viable | Reject the affected organ or carcase part |
| One cyst | Localised* | Non-viable (caseous / calcified) | Require cold storage for remainder |
| More than one cyst | Localised* | Viable | Reject the affected organ or carcase part |
| More than one cyst | Localised* | Non-viable (caseous / calcified) | Require cold storage for remainder |
| More than one cyst | Generalised** | Viable | Reject the affected organ(s) or carcase(s) part |
| More than one cyst | Generalised** | Non-viable (caseous / calcified) | Require cold storage for remainder |
* only one area or part affected (such as heart or diaphragm)
** more than one area or part affected (such as heart and diaphragm)
4.5.3 Cold storage of carcases and offal with a localised or non-viable generalised C. bovis infestation
After rejection of the relevant carcase part or offal, the remainder of the carcase and offal must undergo a ‘cold treatment’ as follows:
Cold storage of carcases and offal with a localised Cysticercus bovis infestation
| Temperature | Minimum time (weeks) |
|---|---|
| not exceeding -7°C | not less than 3 weeks |
| not exceeding -10°C | not less than 2 weeks |
It is acceptable for the carcase to be boned-out prior to the commencement of the cold treatment, provided boning takes place under supervision of the AO and that the identity of the meat can be maintained throughout boning, packaging and storage.
4.5.4 Permitted destinations for cold storage
If cold storage facilities are not available at the slaughterhouse, the meat can be transported to a suitably equipped approved establishment for cold treatment. This arrangement should be done by the FBO with agreement from the OV.
4.5.5 Transport to an approved establishment
Where the meat is to be consigned to another approved establishment with cold storage facilities:
- the packaged meat should be labelled with Cysticercus bovis detention labels, or if part carcases use talisman seals
- part 1 of the transfer permit must be completed at the slaughterhouse, the original to go with the consignment and a copy to be retained at the slaughterhouse
- part 2 of the transfer permit should be completed at the receiving establishment by the FBO
Reference: See chapter 9 on ‘Forms’, for sample copies of the PMI 4/15 Cysticercus bovis detention label and the Transfer Permit PMI 4/16.
4.5.6 Releasing the meat
An AO should visit the destination cold store to check and release the meat. A charge will normally be made for this.
- If the AO is satisfied the treatment of the meat has been done satisfactorily and has no cause for concern, then the meat can be ID marked at the cold store and released.
- The AO should complete part 3 of the transfer permit and send it back to the FSA office at the originating slaughterhouse.
- Once the transfer permit is returned to the originating slaughterhouse it should be kept on file for a minimum of 12 months.
Note: The AO can be an OV, MHI or local authority (LA) Inspector.
4.6 Tuberculosis (TB)
4.6.1 Guidance on TB
Full instructions on TB are now contained within chapter 6 on ‘Notifiable diseases’, section 7.
4.7 Arthritis
4.7.1 Guidance on arthritis
Arthritis is an inflammatory condition of the joint, synovial membrane and articular surfaces. It is a routine and common cause of partial and total rejection of carcases. The flowchart below lists the post-mortem findings and guidance on the judgement of arthritic conditions:
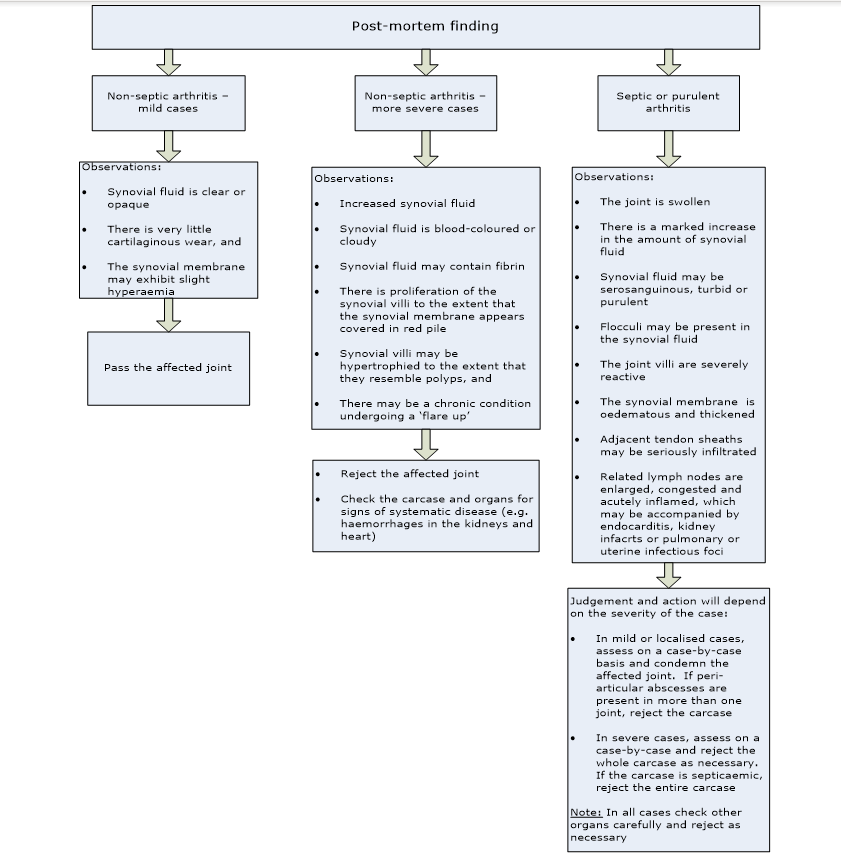
4.8 Tumours in bovines
4.8.1 Guidance on tumours in bovines
Where tumours are encountered in the carcases or offal of bovines, Enzootic Bovine Leukosis must be a consideration.
- The OV must inform APHA.
- Samples from the carcase might be required.
- Before contacting APHA, the OV should gather all possible information about the animal, including date of birth and number of permanent incisors erupted.
Reference: See chapter 6 on ‘Notifiable diseases’ for additional information.
4.9 Aujeszky’s disease: National Serum Survey
4.9.1 Purpose
To demonstrate continuing freedom from Aujeszky’s disease a serum sample must be submitted for serological examination from every slaughtered breeding boar.
4.9.2 Who collects samples
The OV is responsible for collecting samples or delegating the task to a suitably trained MHI.
4.9.3 Restocking of sampling equipment
Sampling equipment can be obtained from SLA and Contracts Team. The equipment for this survey includes ELISA discs, plastic bags, address labels and photographic slide magazines used to dry the discs.
A training note has been produced by the SLA and Contracts Team detailing the new sampling procedure with photographs.
4.9.4 Method for collecting serum samples on ELISA discs
Samples must be obtained from carcases at a sufficient distance from the point of kill when there is no risk from post slaughter carcase movement and from FBO activities. Where possible this should be done at the post-mortem inspection site.
Caution: Avoid contaminating the disc with water or dirt.
The disc should be grasped by the body of the disc and not by the peripheral discs. Dry the saturated discs in the photographic slide magazines provided, ensuring effective separation between discs to prevent cross contamination.
Wash, rinse and dry the photographic slide magazines between uses.
Note: The ‘clotted blood’ method of sampling is no longer to be used.
Method for collecting serum samples on ELISA discs:
Step 1: Use one ELISA disc for each boar. Pre-number the discs.
Step 2: Each peripheral disc must be saturated with blood. Partially saturated peripheral discs are of no use.
Step 3: Place saturated discs in a clearly identified photographic slide magazine. Place discs in every second compartment of the slide magazine to allow effective separation while they dry.
Step 4: Note sufficient information on the sample submission form to identify the owner of each boar.
Step 5: Drying: Discs should be allowed to dry at room temperature, out of direct sunlight, for at least 12 hours. Discs must be completely dry before despatch to the laboratory.
Step 6: Punch out a central hole in each disc once dry. Thread the discs onto file tags in a sequence that corresponds with the submission sheet and place into plastic bags for despatch to the laboratory with the completed submission form.
4.9.5 Storage prior to despatch
Prepared ELISA disc samples should be stored at 4°C until posted.
4.9.6 Posting and packaging details
The following points are to be observed:
- Samples may be batched and posted weekly (no more than 14 days from sampling to posting).
- 1st class post-must be used.
- Each batch of samples must be accompanied by a completed submission form.
- The package must be marked AD SURVEY SAMPLES.
- Avoid posting samples on a Friday as they may be delayed in transit over a weekend.
4.9.7 Submission address
Serum samples from all slaughterhouses in England and Wales must be sent to:
APHA Weybridge
Woodham Lane
New Haw
Addlestone
Surrey
KT15 3NB
4.9.8 Sample submission form
Each sample submission form must provide sufficient information to identify the person who was the owner of each boar at the time that it was consigned to or purchased by the slaughterhouse.
The sample submission form must be completed and printed to go with the samples to APHA.
Retain a copy of each submission form for at least 1 year.
Reference: See Annex 2 for a sample copy of the sample submission form.
4.9.9 Notification
Notification by email to APHA is no longer required. The form should be printed to accompany the samples to APHA Weybridge.
4.9.10 Results
Results are reported to Defra and SLA and Contracts Team. The SLA and Contracts Team will correlate the results and send them to the FVC to cascade.
5. Trichinella Testing
In this section
5.4 Packaging and despatch of samples
5.1 Introduction
5.1.1 Background
Trichinellosis is an infestation of the muscles of animals and man with the larvae of Trichinella spiralis. Infection occurs through the eating of raw or undercooked meat.
Meat from animals infected with Trichinae is declared unfit for human consumption.
5.1.2 Legislation
Retained Regulation (EU) 2019/627 Article 31 requires the carcases of swine (domestic, farmed game and wild game), solipeds and other susceptible species to be examined for trichinosis.
Commission Regulation 2015/1375 lays down the technical details of trichinella testing.
Reference: Retained Regulation (EU) 2015/1375 – amends Regulation 2075/2005 and 216/2014, and sets out requirements for trichinella testing, derogations, and conditions for controlled housing.
5.1.3 FSA role
Trichinella testing is an official control. The OV is to ensure that sampling takes place and samples are appropriately identified, handled, and sent for testing to an accredited laboratory.
Reference: Retained Regulation (EU) 2019/627 Article 37, 2
Sampling and preparation of samples can be carried out by the OV or a MHI.
However, slaughter staff that have received training can, under the supervision of the OV, carry out sampling and testing tasks.
Reference: Retained Regulation (EU) 2019/624 Article 14
5.1.4 Sampling of carcases (including exemptions)
Under retained regulation (EU) 2015/1375, samples must be collected from carcases of the following animals:
- breeding domestic swine (sows and boars)
- wild boar (any age, whether wild or farmed)
- solipeds (any age)
- all pigs that have not been reared in controlled housing conditions (this information will be captured on the FCI accompanying the pigs to the slaughterhouse)
Meat from domestic swine that has been subject to a freezing treatment under official control is exempt from testing.
5.1.5 Retention of parts for human consumption
Carcases, and parts from carcases sampled for trichinella testing must not leave the establishment before the examination has been found negative.
Similarly, other parts of the animal intended for human consumption containing striated muscle must be retained until a negative result is received.
Parts of the animal not containing striated muscle are not subject to any restrictions and can leave the slaughterhouse. In that case, care must be taken to prevent pieces of striated muscle, such as diaphragm or sphincters being left attached.
5.1.6 Controlled housing conditions
‘Controlled housing conditions’ are defined in Retained Regulation (EU) 2015/1375, Annex IV, Chapter 1 and include a range of measures that reduce the risk of the pigs being infected with trichinella. Importantly, the definition does not exclude pigs that have outdoor access, provided that the outdoor access does not present a risk of introducing trichinella into the holding.
Republic of Ireland (RoI) has, to date, not put in place a mechanism whereby housing can be deemed to meet the conditions specified in Article 1 and Annex IV of Retained Regulation (EU) No 2075/2005. Therefore, all pigs born and reared in RoI, which are slaughtered in slaughterhouses in England or Wales, shall be tested for trichinella, regardless of the housing system recorded on the FCI.
5.1.7 Retention of animal by-products
ABP containing striated muscle and intended for animal consumption (Category 3 by-products) must not leave the establishment before the examination has been found negative.
There is no need to retain:
- ABP that do not contain striated muscle
- ABP that contain striated muscle but that are not intended for animal consumption (Category 2 by-products)
5.1.8 Health marking carcases
Where a procedure is in place in the slaughterhouse to ensure that no part of carcases examined leaves the establishment until the result of the trichinella examination is found to be negative and the procedure is formally approved by the OV, the health mark may be applied before the results of the trichinella examination are available.
The FBO must have a written procedure agreed with the OV in place.
Where such system is not in place, the health mark must not be applied until a negative test result has been received.
5.1.9 Cutting or carcases
Pending the results of the trichinella examination, such carcases may be cut up into a maximum of six parts in a slaughterhouse or in a co-located cutting plant.
If the test result is positive and correlation between carcase parts lost, the whole batch of cuts must be disposed of as a by-product.
5.2 Cold treatment methods
5.2.1 Cold treatment for pig meat
Cold treatment may be used as an alternative to trichinella testing for domestic pig meat. The storage temperatures specified for cold treatment are significantly lower than those for the normal storage of frozen meat.
The following conditions must be followed when the cold treatment method is used:
- meat brought in already frozen must be kept in this condition
- the technical equipment and energy supply of the refrigerating room must be such as to ensure that the required temperature is reached very rapidly and maintained in all parts of the room and of the meat
- insulated packaging should be removed before freezing, except for meat which has already reached throughout the required temperature when it is brought into the refrigeration room
- consignments in the refrigeration room must be kept separately and under lockable conditions
- the date and time when each consignment is brought into the refrigeration room must be recorded
5.2.2 Time and temperature for cold treatment
The time / temperature combination for cold treatment is dependent upon the thickness of the pieces of meat. These combinations are summarized in the table below:
| Method | Maximum thickness of the pieces of meat | Maximum temperature of the storage room | Minimum consecutive time for cold treatment |
|---|---|---|---|
| 1 | Up to 15 cm (6 ") | -15°C | 20 days |
| 1 | Up to 15 cm (6 ") | -23°C | 10 days |
| 1 | Up to 15 cm (6 ") | -29°C | 6 days |
| 2 | 15 - 50 cm (6" - 20") | -15°C | 30 days |
| 2 | 15 - 50 cm (6" - 20") | -25°C | 20 days |
| 2 | 15 - 50 cm (6" - 20") | -29°C | 12 days |
| 3 | Up to 25 cm (10 ") | -25°C | 10 days |
| 3 | 25 - 50 cm (10" - 20") | -25°C | 20 days |
5.2.3 Specified times when core temperature is monitored
The following time / temperature combinations are permissible providing the core temperature of the meat is monitored:
| Maximum core temperature of the meat | Minimum consecutive time period for the cold treatment |
|---|---|
| -18°C | 106 hours |
| -21°C | 82 hours |
| -23½°C | 63 hours |
| -26°C | 48 hours |
| -29°C | 35 hours |
| -32°C | 22 hours |
| -35°C | 8 hours |
5.2.4 Cold treatment in other species
Cold treatment is not an alternative for the testing of wild boar or solipeds.
5.3 Collecting samples
5.3.1 Sampling responsibility
The OV must ensure that sampling takes place and samples are correctly identified and handled, and sent for testing to:
Biobest Laboratories Ltd
6 Charles Darwin House
The Edinburgh Technopole
Milton Bridge
Nr. Penicuik
Midlothian
EH26 0PY
Telephone: 0131 440 2628
Fax: 0131 440 9587
Email: enquiry@biobest.co.uk
Website: biobest.co.uk
Collection and handling of samples and testing tasks may be carried out by an MHI or delegated to plant staff if they have received specific training and the OV is satisfied that the sampling procedure is carried out correctly. For self-testing abattoirs see topic 5.7 on ‘Use of on-site labs’.
Samples must be collected using a clean knife and disposable forceps.
5.3.2 Sample description
A sample of the size specified below must be collected from the described sampling site.
Note: Take samples as a single piece of meat.
If this preferred sample site is not available, then the alternative sample must be collected.
The weight of meat specimens refers to a meat sample free of all fat and fascia. Particular attention should be made collecting muscle samples from the tongue to avoid sample contamination with the superficial layer of the tongue, which is indigestible and can prevent reading of the sediment.
| Animal Categories | Sample size | Sampling site | Alternative sample |
|---|---|---|---|
| Boars and Sows | Between 2 and 4g | Pillar of the diaphragm at the transition to the sinewy part | 4g, to be taken from the rib part or the breastbone part of the diaphragm, from the jaw muscle, tongue, or the abdominal muscles |
| Solipeds | Between 10 and 11.5g | Lingual or jaw muscle | Larger size specimen from the diaphragm pillar at the transition to the sinewy part |
| Wild Boar | Between 10 and 11.5g |
Foreleg, tongue, or diaphragm | None |
5.3.3 Sample size guide
- Use the scales provided to ensure the correct weight.
- Each specimen must consist of a single piece of meat free of fat or fascia and be of the correct weight.
- Large samples reduce the pooling ability in the lab and result in increased cost to the FSA.
- Underweight samples will be rejected by the lab and not tested.
Note: New plants must request scales from the corporate support unit transactions team York (CSU) csu@food.gov.uk.
2-4g boars and sows

10-11.5 grams wild boars and solipeds
5.3.4 Sampling point
Samples may be collected at any point during dressing or chilling providing the identity of the carcase can be ascertained.
5.3.5 Pooling of samples
Up to 100g of samples from different animals can be pooled as a single batch for testing. The number of samples in a batch will depend on the animal category, as the sample size is different, for example, 50 sows and boars, 10 solipeds.
You can pool samples from different producers.
Reference: See sub-topic 5.3.2 on ‘Sample description’ for additional information.
However, samples from different animal categories, such as domestic pigs and wild boars, must not be pooled in the same batch as digestion times may be different.
5.3.6 Sampling procedure
The following procedure must be followed when collecting samples for testing:
Step 1: Open the small sealable Liquitite Pathoseal bag
Step 2: Collect the samples of meat as appropriate for the species and category of animal sample
Step 3: Pool the samples up to 100g in the small Liquitite Pathoseal bag
Step 4: Close the small Liquitite Pathoseal bag. Stick barcode label to the bag and insert into the larger Pathoseal bag with the absorbent pad
Step 5: Place two squares of Techni Ice into the large Pathoseal bag
Step 6: Stick the corresponding barcode to the PMI 4/18 form
Step 7: Complete the PMI 4/17 form
5.3.7 Completion of PMI 4/17 form
Carcases must be identifiable to their farm of origin until a test result has been received so a farm investigation can be carried out if the result is positive.
PMI 4/17 (Trichinella Sampling form) must be completed when the samples are collected. The identity of each sampled carcase must be recorded in a way that allows the farm of origin to be identified, for example, by recording the slap number or the County Parish Holding number (CPH) obtained from the Animal Movement Licence.
Individual carcase identification when a farm supplies several animals is not required, as in the event of a positive all carcases in the batch will be re-tested.
To keep correlation with the sample and PMI 4/18, (Trichinella Testing Submission Form), the serial number of the barcode label used to identify those must be inserted in the Reference Number box.
5.3.8 Completion of PMI 4/18 form
PMI 4/18 (Trichinella Testing Submission Form) must be completed by FSA staff and accompany the sample to the lab.
One form with one barcode must be completed for every batch of up to 100g of samples. Make sure the number of samples correlates with the number of animals entered on the form so Biobest Laboratories do not report incorrect number of samples supplied.
Note: An email address must be supplied to the lab for notification of the test result and a mobile phone number for text notification that results are available.
Affix the barcode label correlated to the sample bag to the PMI 4/18.
Send the original to the lab in a clean sealed A4 bag and keep a photocopy on file.
5.4 Packaging and despatch of samples
5.4.1 Transport containers
Samples are transported in Pathoshield packaging. The courier Topspeed collects for next day delivery to Biobest Laboratories.
5.4.2 Chilling
Samples are kept chilled by two squares of Techni Ice. The Techni ice squares must be held frozen until use.
5.4.3 Pathoshield packaging procedure
The table below lists the steps that must be followed using a Pathoshield box to despatch samples:
Pathoshield packaging procedure
| Step | Action |
|---|---|
| 1 | Attach the Biobest Laboratories barcode to the small pathoseal bag and attach the corresponding barcode onto a trichinella testing submission form (PMI 4/18). |
| 2 | Place the small bag into the larger pathoseal bag, placing 2 Techni Ice squares between the bags |
| 3 | Complete form PMI 4/17 to record the samples and which barcodes they were submitted with |
| 4 | Place sample into the Pathoshield outer box. Affix the peel-off barcode sticker onto the duplicate copy of the page. |
| 5 | Put completed forms PMI 4/17 and PMI 4/18 in a plastic bag before placing them in the box ready for despatch to the laboratory. |
| 6 |
If sending a single box: affix pre-printed Biobest Laboratories address label to box and seal the box using the blue security seal provided. If sending multiple boxes: Re-package into a larger box and attach address label and consignment note to outer box. |
| 7 | Place the Pathoshield box in a plastic refuse bag to protect the surface of the box from contamination while carrying it through the slaughterhouse and during storage. |
| 8 | Close the plastic refuse bag with a cable tie or other secure means. |
5.4.4 Storage pending despatch
On completion of sampling, place the Pathoshield box in the detained chiller until transferring them to the collection point. Topspeed will collect at the agreed collection time for delivery to Biobest Laboratories.
5.4.5 Notify lab of Saturday testing
If testing is required on a Saturday, FSA staff need to telephone Biobest Laboratories on the Thursday beforehand to advise them that trichinella samples are being sent for Saturday morning delivery:
Biobest Laboratories – 0131 440 2628
Topspeed need to be informed that the sample needs to arrive before 9am on Saturday in order to be tested.
No notification is required for samples dispatched for Monday to Friday testing.
5.4.6 Despatch from base plants
When, for practical reasons, samples cannot be despatched from the plant where the animals are slaughtered, they can be taken to a different plant to be despatched from there.
However, when completing the PMI forms, the sampling plant details must be entered.
In that case all the original documentation must be filed in the plant where the sample was taken as soon as practical.
5.5 Courier collection services and procedures
5.5.1 Next day before noon service
Trichinella samples should be despatched using the Topspeed ‘Next Day Service’.
Note: Topspeed will only collect samples between 09:00 – 17:00 unless out of hours arrangements have been agreed.
5.5.2 Saturday service
In addition to the standard service, Topspeed provide a ‘Saturday Service’. This service may only be requested if prior permission is obtained from the SLA and Contracts Team as it incurs increased costs and Biobest must be informed on the preceding Thursday that samples will be arriving at the lab for testing.
This service is only to be used for samples that need to be tested on a Saturday.
Test results for Saturday testing will be received on the same day.
5.5.3 Booking sample collection
The following steps should be taken when booking sample collection:
Step 1: Go to Topspeed and complete the online booking form. See Annex 7 for information on completing the online booking form.
Step 2: Provide Topspeed with the following information:
- number of items (boxes) in consignment
- kill date and time
- the name of person making the booking
Step 3: Write the barcode numbers as reference for the collection; Topspeed to collect as arranged
5.5.4 Sample collection point
Immediately prior to the agreed collection time the Pathoshield box containing the sample(s) should be removed from the plastic refuse bag and placed at the agreed collection point.
5.5.5 Despatch failure
Should Topspeed fail to collect samples within the agreed timeframe, contact Topspeed to arrange collection immediately and inform the SLA and Contracts Team by email at sla.contracts@food.gov.uk.
5.6 Consumables
5.6.1 Ordering consumables
To request stocks of consumables, contact CSU by email at csu@food.gov.uk using the order form at Annex 6 in this chapter.
The minimum order is 1 box of the following options:
- Pathoshield P7 kit x 12 for trichinella testing - recommended for plants processing small number of animals for testing
- bespoke Pathoshield 7 comprising
- A5 Pathoseal
- 200ml Absorbent
- A6 Liquitite
- Techni Ice x 24 squares
- Forceps
- Security Seal
- Outer compliant box
- bespoke Pathoshield 7 comprising
- Pathoshield P3 kit x 10 for trichinella testing - recommended for plants processing larger number of animals for testing
- bespoke Pathoshield 3 comprising
- A4 Pathoseal
- 200ml Absorbent
- A5 Liquitite
- Techni Ice x 20 squares
- Forceps
- Security Seal
- Outer compliant box
- bespoke Pathoshield 3 comprising
Note: Allow 5 days lead time for delivery of the consumables.
5.6.2 Barcodes
The barcodes can be obtained from the CSU by email csu@food.gov.uk.
5.7 Use of on-site facilities, private laboratories, and other arrangements
5.7.1 Background
Slaughterhouses that have facilities and trained staff available for the collection and testing of trichinella samples may use their own arrangements instead of having the samples dispatched to Biobest Laboratories. Where these arrangements are in place, the lab will operate as a supplier providing a service to the FSA Operations Group.
In order to carry out trichinella testing, on-site self-testing facilities must be accredited by United Kingdom Accreditation Service (UKAS) and participate in the FSA Quality Assurance Scheme conducted by the UK National Reference Laboratory (UKNRL). Other FBOs may also send samples to such “self-tester” sites as an alternative to Biobest Laboratories.
Private testing laboratories may also be used in place of Biobest Laboratories. These laboratories must also participate in the FSA UKNRL Quality Assurance Scheme, as above.
5.7.2 Requirements for on-site labs
Any plant that wishes to start trichinella testing in an ‘on site’ laboratory must be assessed by the UK National Reference Laboratory (UKNRL) and be permitted by FSA to undertake testing.
The NRL will arrange for an on-site inspection and produce a report which will either recommend approval for self-testing or highlight areas that need to be addressed prior to recommendation for approval being issued.
The NRL offer training to staff under the VetQAS scheme to ensure Sampling Officers have the relevant skills and knowledge to undertake testing.
FSA Operations Group will issue a designated lab status letter once the above criteria have been satisfied to ensure compliance with Retained Regulation (EU) No. 2015/1375.
5.7.3 Responsibilities of the lab operator
Once contracted by the FSA Operations Group to carry out trichinella testing, the lab operator is responsible for:
- the collection and identification of the samples
- the identification and correlation of sampled carcases
- the supply of equipment and disposables
- the operation of the lab
- the examination of the digested samples
- the maintenance of all records
- the training of staff
5.7.4 Quality assurance
All laboratories undertaking testing must take part in the quarterly QA scheme organised by the UKNRL. All laboratories must take action to rectify any deficiencies noted either in the assessment or following a QA test. Failure to do so will result in the removal of designated lab status.
The OV will receive a copy of the QA report and will be responsible for ensuring the results are returned within the specified timescale and that any deficiencies identified are addressed.
5.7.5 Non-compliance with SOP
Where the OV / FVC is not satisfied that the lab operator is complying with the standard operating procedure (SOP) agreed with the FSA Operations Group, advice must be given to rectify the breach.
Failure to comply with the SOP is a breach of the terms of the contract and if the deficiency is not rectified, the OV must inform the SLA and Contracts Team. The FSA Operations Group can then suspend the SOP.
When the SOP is suspended, the FSA Operations Group will collect the samples and dispatch them to Biobest Laboratories.
The health mark must not be applied to any carcase when there are no guarantees that the result of the testing is reliable.
5.8 Test results
5.8.1 Receipt of test results
Trichinella testing is an official control, and the FSA is responsible for obtaining the test result.
By default, a laboratory report containing results will be sent by e-mail to the address specified on the submission form.
Biobest Laboratories currently offer SMS reporting of results for other tests and aims to add this option for trichinella. To register interest in this service, contact Biobest Laboratories on 0131 440 2628.
5.8.2 Negative results
On receipt of a negative result, the health mark and identification mark can be applied.
ABP containing striated muscle that were being retained can be released.
5.8.3 Positive or doubtful results
If the initial result received from the laboratory is positive or doubtful, Biobest Laboratories will contact the SLA and Contracts Team, who will immediately contact the OV to advise on the procedure for despatching samples to NRL - APHA York for re-test. The OV must also advise the local APHA office.
Commission Regulation (EC) No 2015/1375 requires positive or doubtful results to be confirmed, collecting samples from the suspect carcases, and digesting them in smaller pools.
5.8.4 Re-sampling carcases with positive or doubtful results
The SLA and Contracts Team will contact the OV / FVC to request samples for re-testing.
These samples must be of the correct weight and from the correct sample site for the species concerned. A PMI 4-18 must be completed per pool and be sent to NRL - APHA York.
The SLA and Contracts Team will confirm which courier service should be used.
Samples for re-test should be sent to:
Trichinella National Reference Laboratory
APHA York
Biotech Campus
York
YO41 1LZ
The carcases and all body parts must remain detained, pending the outcome of the re-testing.
5.8.5 Traceability report
Pending the result of the re-test, the OV / FVC should obtain the FCI to create a traceability report for the detained carcases, to identify the farm of origin should a positive result be confirmed.
5.8.6 Notification of positive results
The SLA and Contracts Team will notify the OV / FVC and APHA if a positive result is confirmed.
On receiving confirmation of a positive result, the OV / FVC should email their traceability report to the SLA and Contracts team in York (access contact details in chapter 1 on ‘Introduction’).
If the positive result has been confirmed by NRL - APHA York, the positive carcase and all body parts must be disposed of as a Category 2 animal by-product and confirmation of action emailed to the SLA and Contracts Team sla@food.gov.uk.
For pigs from RoI, positive results shall be reported by the FSA to the Department of Agriculture, Food and the Marine (DAFM), the RoI competent authority. This will activate the RoI contingency plan with regard to the investigation of the source of infestation and any associated spread among other pigs or other susceptible species.
6. Poultry Post-Mortem Inspection
In this section
6.1. Correlation and Inspection
6.1 Correlation and inspection
6.1.1 Inspection requirements
The inspector is required to inspect the external surface of all carcases and accompanying offal.
6.1.2 Whole bird inspection point
Inspection of the whole bodies of birds is recommended so that diseased birds can be removed early in the process and this should be included in the HACCP plan.
6.1.3 Evisceration line inspection
Correlated carcases and offal either attached or detached are inspected.
6.1.4 Carcase presented for post-mortem inspection without offal
If poultry carcases are presented without offal at the post-mortem inspection point as a result of the accidental removal of all or part of the offal they do not need to be rejected. They should be inspected and if the carcases pass post-mortem inspection, they can be considered fit for human consumption. However, such cases should be judged according to the merits of each case.
This scenario is not intended to cover inadequate presentation / correlation of offal due to malfunctioning evisceration equipment or inadequate manual evisceration practices.
Offal and viscera that have not undergone PM inspection should be disposed of as Category 2 ABP.
Note: In the event of a significant increase in presentation of carcases without offal, follow the usual hierarchy of enforcement to address the root of the problem.
6.1.5 Delayed evisceration
(EC) 853/2004 Annex III, Section II, Chapter IV, 7 (c) states ‘viscera or parts of viscera remaining in the carcase, except for the kidneys, must be removed entirely, if possible, and as soon as possible, unless otherwise authorised by the competent authority.’
FBOs intending to carry out delayed evisceration should develop a procedure based on the HACCP principles detailing how the process is going to take place, assess the risks, and implement measures to ensure these risks are minimised.
When discussing with the OV the following conditions need to be considered prior to the process commencing:
- The FBO has to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles for this process. This can be in the form of a Standard Operating Procedure (SOP).
- Viscera can be left in the carcase after slaughter for not longer than 15 days at a temperature of not more than 4ºC (this mirrors the requirements in Annex III, Section II, Chapter VI, paragraph 9 of Regulation 853/2004, for the delayed evisceration of poultry slaughtered on-farm). If FBOs wish to apply other time/temperature combinations, they will need to produce a risk assessment to support any deviation from these parameters.
- Un-eviscerated carcases should either be kept in a separate chiller, or if this is not possible, sufficiently separated from any other carcases or food stuffs to prevent the risk of cross-contamination.
- When the delayed evisceration takes place, the viscera in the body cavity will need to be completely removed in a hygienic manner. In cases where the intestinal tract is ruptured and subsequently contaminates the carcase or offal the contaminated parts must be either trimmed or thoroughly washed with potable water or, where required, disposed of as animal by-products.
- FBOs will need to adjust the processing lines for this operation to ensure that post-mortem inspection can be carried out effectively by the OV, MHI or a PIA under the FSA supervision.
Although establishments undertaking delayed evisceration do not require specific approval or authorisation, the OV shall inform their FVL/FVC of the FBO’s intention to implement delayed evisceration. Once the FVC and the OV are satisfied with the process, the OV shall notify the approvals team at approvals@food.gov.uk once the FBO has commenced this type of production in order to have the information updated in E&P.
In cases where the hygienic conditions are not complied with by the FBO, the established hierarchy of enforcement as per any other deficiency shall be followed. If FBOs are unable to achieve compliance the delayed evisceration process can be stopped using the standard enforcement procedures.
6.1.6: Partial evisceration: effilé or roped poultry
Partial evisceration or effilé is defined in Regulation (EU) 543/2008 (the Poultry Meat Marketing Regulations), as the process of leaving the heart, liver, lungs, kidneys, crop, proventriculus and gizzard inside the body cavity of the bird.
Annex III, Section II, Chapter IV, Paragraph 7 (c) of Regulation (EC) 853/2004 states that viscera or parts of viscera remaining in the carcase, except for the kidneys, must be removed entirely, if possible, and as soon as possible, unless otherwise authorised by the competent authority.
The FSA, as the competent authority, can authorise a derogation from the “removed entirely” criterion described above. Unlike for delayed evisceration, authorisation for effilé or partial evisceration has to be granted on a case-by-case basis, following the procedure described in 6.1.7 below.
For the production of partially eviscerated poultry or effilé, the following requirements will need to be fulfilled:
HACCP based procedures
- The FBO has to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles for this process. This can be in the form of a Standard Operating Procedure (SOP).
Ante-Mortem Inspection/FCI
- Only healthy flocks are eligible for partial evisceration. If the FCI suggests that there have been health problems at farm level, the OV can reject the batch for effilé production, and all carcasses must be fully eviscerated.
- It is recommended that un-tested (for example, exempt from testing under the National Control Plan) or Salmonella positive batches are not used for the production of partially eviscerated poultry. Should the FBO decide to use these batches they will have to be treated with special precautions. In any case, the OV can request a complete evisceration if preliminary post-mortem findings are of concern (see PMI paragraph below).
Operational requirements
- Intestinal tract to be removed in a hygienic manner and in such a way that spillage of digestive content is prevented.
- In case of rupture of the intestinal tract and subsequent contamination of the carcass/offal, the carcass will need to be fully eviscerated and washed as per normal production.
- In partially eviscerated poultry, inside wash is not recommended.
- Only the heart, liver, lungs, kidneys, crop, proventriculus and gizzard can remain inside the bird.
Post-Mortem inspection
- All external carcase surfaces and body cavities will need to be visually inspected.
- Updated [Green offal must be presented to the Official (OV, OA or PIAs) and subject to post-mortem inspection.]
- In addition, the remaining offal in the body cavity from a minimum of 20 birds or 10% of the batch, whichever is bigger, will have to be inspected in full. There are two possibilities that the FBO can choose from:
- Viscera inspected inside the bird (the FBO will have to adapt the speed of the production to allow for this). From a practical point of view, this might be challenging in certain circumstances.
- FBO fully eviscerates at least 20 birds or 10% of the batch and the viscera are inspected outside the bird ensuring correlation between the carcases and the viscera is adequately kept (likely to be quicker).
- If the preliminary post-mortem inspections show an unusual level of rejections, the inspection level of the viscera should be increased by the OV to his/her satisfaction and, if necessary, up to 100% of the batch.
- The speed of the line will be limited to the speed at which the official carrying out post-mortem inspection is able to cope with.
- If the evisceration is completed on a table, adequate hygienic practices will need to be adhered to (for example, washing of hands, regular cleaning of the table, etc).
Commercialisation
- For this product to be marketed, it should be presented for sale labelled or identified as partially eviscerated (“effilé”, “roped”).
6.1.7: Authorisation process for partially eviscerated poultry (“effilé” or “roped”)
Establishments wishing to produce partially eviscerated poultry will require specific authorisation and will need to complete the application form provided in Annex 15.
Parts 1 and 2 of the application form will need to be completed by the FBO that wishes to undertake the process in consultation with the Official Veterinarian (OV). The completed application shall be submitted to the Approvals and Registrations Team approvals@food.gov.uk.
Parts 3 and 4 refer to the authorisation by the FVL/FVC following an on-site assessment. An onsite trial can be arranged between the FBO, the OV and the FVL/FVC to ascertain if the procedures put in place by the FBO are satisfactory.
The completed form with the final recommendation has to be emailed to the Approvals and Registrations Team.
Once the completed form is received by the approvals team, they will inform the FBO in writing of the possible outcomes, as follows:
- Authorisation: If the FVL/FVC is satisfied with the proposal, facilities and the hygiene practices observed during operations on site, authorisation can be immediately granted.
- Refusal: If the FVL/FVC is not satisfied with the proposed arrangements and/or with the hygiene of the operations, the authorisation should not be granted. The FVL/FVC should provide evidence of the reasons for the refusal in the boxes provided in Part 4 of Annex 15.
If after being authorised, the agreed procedures are not complied with and subsequently hygiene and food safety are compromised, the authorisation for partial evisceration or effilé can be withdrawn. A notification letter will be sent by the approvals team to the FBO confirming the decision and the reasons for the withdrawal.
If the FBO disagrees with the outcome of the process, they can appeal in writing to the Operations Head Veterinarian using the approvals address approvals@food.gov.uk.
6.2 Poultry feet for human consumption
6.2.1 Inspection requirements
Feet harvested for human consumption must be inspected.
Feet that are not separately identifiable, such as feet belonging to carcases rejected at evisceration, must not be released for human consumption.
Feet can be exported under an agreed health certificate signed by a Local Veterinary Inspector.
6.3 General Contamination
6.3.1 Meat that is unfit for human consumption
Meat, carcases and / or offal affected with generalised contamination by faecal material, bile, grease, or disinfectants should be considered unfit for human consumption.
6.3.2 Contamination from the alimentary tract and faecal material
A hygienic trimming system must be in place if the FBO decides to trim contaminated carcases.
Any part of the carcase or offal affected with bile staining should be trimmed. Where plucking machines break the skin of poultry the underlying musculature should be considered to be contaminated and trimmed from the carcase.
6.3.3 Meat falling from the line / conveyor
The FBO should have a system in place to deal with carcases or offal that fall on the floor. The OV / MHI should verify that the FBO has a system in place to ensure meat contaminated after post-mortem inspection is not released for human consumption.
6.4 Guidelines on trimming poultry
6.4.1 Trimming supervision
Rectification resulting from post-mortem findings must be carried out under the responsibility of the FSA Operations Group team (supervision of trimming may be carried out by a plant inspection assistant (PIA). Plant operatives should carry out removal of unfit meat identified at post-mortem inspection. Identification of unfit meat for trimming must not be delegated to untrained individuals.
6.4.2 Location of trimming point
Trimming of minor blemishes such as bruising is at the discretion of the FBO
Removal of significant quantities of meat is usually impracticable with high line speeds, and in these cases an adjacent trimming area should be provided.
6.4.3 Trimming after chilling
Trimming of carcases may be delayed until after chilling, providing that:
- there is no risk of contamination to other carcases
- for example, faecal contamination has to be trimmed before chilling
- arrangements are in place for the trimming to be done under the supervision of the OV / MHI at regular times
Note: The OV and the FBO should agree recognised methods (marking and identification of parts to be trimmed) to ensure that trimming is effectively completed by plant staff.
7. Judgements at Poultry Post-Mortem Inspection
In this section
7.1 Poultry condition cards
Click a condition to follow the link:
Abnormal colour (septicaemia – toxaemia)
AM rejects (cull / runts)
Ascites – oedema
Bruising – fractures
Cellulitis
Contamination
DOA / DIL
Dead other than slaughter (uncut–badly bled)
Dermatitis
Emaciation
Hepatitis
Joint lesions
Machine damage
Overscald
Pericarditis
Perihepatitis / peritonitis
Respiratory disease (airsacculitis)
Salpingitis
Tumours
Other factory (processing)
Other farm (for example, jaundice, oregon, white muscle)
Wooden breast
7.2 Introduction
7.2.1 Post-mortem judgements in poultry
Twenty-one poultry condition cards have been developed to achieve standardisation of post-mortem findings in poultry slaughterhouses in the United Kingdom.
These condition cards are to be used as a guidance which inspection teams must follow.
Notwithstanding, the professional expertise of the OV, based on local knowledge and the FCI received for each flock, may result in judgements differing from the advice provided in the condition cards for specific flocks of birds.
7.2.2 Trimming
Where the OV considers the entire carcase is not unfit, the affected parts of the carcase may be removed, and the rest of the carcase may be allowed to enter the food chain. This is to be carried out by plant operatives.
The OV must be content that the FBO has developed a system and trimming is carried out in such a manner that all affected parts are removed to the OV’s entire satisfaction.
7.3 Breast blisters
7.3.1 Breast blisters
Judgement:
Infected, haemorrhagic, or enlarged breast blisters should be trimmed. The affected tissue may be adherent to the keel bone and when this happens part of the bone will have to be removed with the affected tissues. Trimming of small, uninfected, non-haemorrhagic blisters may be deferred until after chilling, when a proportion of them will have disappeared.
Note: The OV needs to consider that breast blisters might be the result of poor husbandry on the farm. If appropriate, the local ROD / DVM should be informed.
7.4 Avian Tuberculosis and Erysipelas
7.4.1 Avian tuberculosis
Avian tuberculosis usually affects older birds with lesions seen most commonly in:
- the liver
- kidneys
- intestinal tract
- bone marrow.
The lesions are irregular shaped greyish-white nodules varying in size from that of a pin's head to large masses. The tubercles can be shelled out from the surrounding tissue. When cut through, the nodules are firm with a dry, cheesy, appearance. If the long bones are split lengthwise, small spherical nodules may be found in the bone marrow.
Confirmation can be made by microscopic examination for the causal organism.
Judgement: Carcases and offal should be considered unfit.
7.4.2 Erysipelas
Erysipelas is primarily a disease of turkeys and the affected birds are listless with, rarely, a swelling of the snood. Mature domestic fowl may also be affected.
Where possible, affected birds should be rejected by the pre-slaughter health inspection but if they inadvertently reach the post-mortem inspection station, they will show signs typical of septicaemia.
- the liver is often enlarged, congested, friable and sometimes light brown in colour
- the intestines are commonly congested and there may be catarrhal enteritis
- a valvular endocarditis may be present in more chronic cases
Judgement: Carcases and offal should be considered unfit.
8. Wild Game Post-Mortem Inspection
In this section
8.1 Introduction
8.1.1 Purpose
This section provides guidance on how to carry out official controls at approved game handling establishments (GHE).
Reference: (EC) 853/2004 overview, (22).
8.1.2 Attendance
An Assessment for OV Flexible Attendance policy (see Chapter 2.10 on ‘Inspection and Attendance’, Annex 1) has been developed to provide a means for assessing the required OV attendance in these types of establishments.
In summary:
- either an MHI or OV, but not both, is required for post-mortem inspection, except that OV presence throughout such inspection is required in specified cases
- additional OV visits are required where the MHI has put aside meat with abnormalities for inspection by the OV, meaning visits for the purpose of inspection of such meat
- operating hours agreements will need to be obtained with each approved GHE; however, due to the nature of the business this may prove difficult – approved GHEs are obliged to inform the FSA when they are operating in order that FSA attendance can be arranged, if required
Note: PIAs are no longer permitted in approved GHEs and should not be performing post-mortem inspections.
8.1.3 Chilling
Carcases have to be collected and transferred to the approved GHE, which may be remote from the hunting area; therefore, some delay in chilling may occur.
However, the chilling must begin within a reasonable period of time after killing and achieve a temperature throughout the meat of not more than 7°C in the case of large wild game and 4°C in the case of small wild game. This does not preclude completion of dressing in the approved GHE before these temperatures have been achieved.
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, 5 and (EC) 853/2004 EC, Annex III, Section IV, Chapter III, Point 4.
8.1.4 Separation of different types of game
In establishments that are approved for the handling of wild game, precautions are to be taken to prevent cross-contamination between species by separation either in time or in space of operations carried out on the different species.
In premises that are approved for the processing of both wild and farmed game, separate facilities for the reception and storage of carcases of farmed game slaughtered at the farm, and for wild game, must be available.
In-fur and in-feather wild game may be stored in separate parts of the same larder / chiller, although separate larder / chillers are preferable.
8.2 Trained hunters
8.2.1 Trained hunter’s examination
A trained person must carry out an examination of the body and, in the case of large wild game, of any viscera removed, to identify any characteristics which may indicate that the meat presents a health risk. The examination must take place as soon as possible after killing.
Reference: (EC) No 853/2004 Annex III, Section IV, Chapter II (Large Wild Game) and Chapter III (Small Wild Game).
8.2.2 Trained hunter’s declaration: large wild game
Following the examination referred to above, large wild game carcases eviscerated in the field require a declaration from a trained person. This must bear the date, time, and place of killing and carry a declaration that, based on an examination of the carcase and viscera:
- there is no suspicion of environmental contamination
- no abnormal behaviour was observed before killing
- no abnormal characteristics were found during the examination
The declaration must be numbered and should be attached to the carcase unless it covers more than one animal body. The declaration may cover more than one animal body, provided that a clear link between the animal bodies and the declaration is established and guaranteed. In these circumstances, the declaration would make reference to a group of numbered carcases and each carcase would be clearly identified with numbered tags or firmly attached labels.
Note: If abnormal characteristics are found during the examination, abnormal behaviour was observed before killing, or environmental contamination is suspected, the trained person must inform the competent authority.
8.2.3 Head and viscera
Where the trained hunter’s declaration is provided stating that no abnormalities were found, the head and the viscera need not accompany the body, except in the case of species susceptible to trichinosis, whose head (except for tusks) and diaphragm must accompany the body. The exception to this is that if the head is required for further use as a trophy, it may be sent to an ABP processing plant that has been approved for the production of trophies. In these circumstances, the head may be dispatched pending a satisfactory trichinella test, provided that the identification of the head is maintained throughout the process.
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, 4 (a).
8.2.4 Acceptance in GHE
Carcases not accompanied by the head and viscera must be the subject of a declaration signed by the trained hunter.
If there is no signed declaration, such carcases must not be accepted in approved GHEs, and are not eligible for human consumption.
If any of the information required to be included in the hunter’s declaration in sub-topic 8.4.2 is missing, the carcases must not be accepted in the approved GHE and the carcase is not eligible for human consumption unless the missing information is provided by the FBO.
The declaration must be signed by a trained hunter. The FBO should keep a copy of the hunter’s training certificate for verification purposes or other suitable method that can verify that the hunter is trained.
Unskinned large wild game may be received by a GHE from another Member State only if it is accompanied by a certificate issued and signed by an OV. A template of this certificate can be found in Annex 7
Reference: (EC) No 853/2004, Annex III, Section IV, Chapter II, 4 (c).
8.2.5 Trained person (hunter) unexpectedly unavailable
In the event that the trained person (hunter) is unexpectedly unavailable, carcases accompanied by the head and all the viscera (with the exception of the stomach and intestines) may be accepted into an approved GHE without the declaration from a trained person.
8.2.6 Offal
In the case of carcase and offal presented without the trained hunter’s declaration, (as in the circumstances detailed above), they cannot be accepted unless clear identification and correlation marks between carcase and offal are present.
Where the carcase has a hunter’s declaration stating no abnormalities were identified, in most cases the offal will not be present. In the event that the offal is present, it must be clearly correlated to the carcase; if it is not, then the offal cannot be used for human consumption.
Where the carcase has a hunter’s declaration stating that abnormalities were found, then the offal must accompany the carcase and must be correlated to it.
(As an example of correlation, the hunter’s declaration is often made on a tie-on label attached to the hock of the carcase; a duplicate label can be tied to the offal where present.)
Reference: (EC) No 853/2004, Annex III, Section IV, Chapter II, 3.
8.2.7 Specimen trained hunter’s declarations
Specimen declarations for wild game animals may be found in the ‘Wild Game Guide’.
8.2.8 Small wild game
In the case of small wild game, a trained hunter’s declaration is not a legal requirement. However, if abnormal characteristics are found during the examination, abnormal behaviour was observed before killing, or environmental contamination is suspected, the trained person must inform the competent authority. The declaration may be attached to trays or cartons to inform the competent authority of any abnormal characteristics, behaviour, or environmental contamination.
In general, if small game exhibits abnormal behaviour, they should not be considered to be fit for human consumption.
Reference: (EC) No 853/2004 Annex III, Section IV, Chapter III, 2.
8.3 Carcase handling
8.3.1 Transport of carcases with hunter’s declarations
There are no provisions under 625/2017 permitting anybody to convey this information on behalf of the trained person instead of a declaration being provided.
Declarations attached to carcases (of large wild game) must not be removed before delivery to the approved GHE where it will be processed, as otherwise the carcase may be disposed as ABP. Similarly, if identification marks which link to a declaration covering several animals are removed or destroyed, those unidentified carcases will be disposed of as ABP.
8.3.2 Skinning
Unskinned large wild game:
- may be skinned and placed on the market only if:
- before skinning, it is stored and handled separately from other food and not frozen, and
- after skinning, it undergoes a final inspection in accordance with Regulation 2019/627 Article 28.
- may be sent to a game handling establishment in another Member State only if, during transport to that game handling establishment, it is accompanied by a certificate issued and signed by an official veterinarian; a template of this certificate can be found in Annex 7
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, 8.
8.4 FSA role
8.4.1 Receipt of carcases and timing of inspection
The inspector (MHI or OV) shall perform the post-mortem inspection activities. It is not essential that there is inspection of carcases prior to the beginning of processing (that is, before skinning), but it is good practice.
Where applicable or practical the FBO may segregate unprocessed carcases that they intend to reject and present them to the inspector prior to disposing of them, for example:
- carcases show signs consistent with death other than by hunting (for example, by road accident)
- carcases are so contaminated that entry would jeopardise operational hygiene or that show evidence of advanced or generalised decomposition
If the FBO decides to proceed with skinning and / or dressing the inspection needs to take place soon after skinning / dressing and / or evisceration.
FBO rejection of carcases before presentation for inspection is often part of the plant HACCP. Inspectors should be aware of this control and audit it in the same way as other plant controls, particularly the evidence, and extent, of corrective action. Discrepancies in intake records and controls should be noted in the plant daybook for future reference.
8.4.2 Read declaration
The OV or inspector is to take account of the declaration or information the trained person involved in hunting the animal has provided in accordance with (EC) 853/2004.
The FBO should provide a copy to the OV or inspector of the hunter’s training certificate or any alternative method so they can verify that the hunter signing the declaration is trained to do so. If there is no evidence of the training of the hunter, and the carcase is not accompanied by the head and the viscera, then the carcase must be detained pending the information of the hunter’s training. The FBO should be given the opportunity to provide such evidence. If the FBO can’t prove that the hunter is trained to sign the declaration, then the carcase cannot be health marked and must be disposed of as an ABP.
The OV or the inspector will need to verify that the hunter’s declaration includes all the information required by (EC) 853/2004 and that it is signed by a trained hunter. Refer to sub-topic 8.2.2 on ‘Trained hunter’s declaration: large wild game’.
According to (EC) 853/2004 the hunter’s declaration must include:
- the date, time, and place of killing and
- carry a declaration that, based on an examination of the carcase and viscera:
- there is no suspicion of environmental contamination
- no abnormal behaviour was observed before killing
- no abnormal characteristics were found during the examination
The declaration must be numbered and should be attached to the carcase unless it covers more than one animal body.
If the required details are missing, the carcase must be detained and the FBO should be given the chance to provide the missing information.
If the FBO cannot provide the missing information, then the carcase must not be health marked and should be disposed of as an ABP.
Where the declaration makes reference to TB, the carcase and offal lymph nodes should be examined in detail and appropriate records made. The carcase and offal should be detained for the OV to provide professional judgement and inform APHA using TB50 form as a template. The incidence and significance of TB varies in different parts of the UK. The advice of APHA should therefore be sought on what further action to take in relation to wild deer where TB infection is suspected (such as collection of samples). The OV will need to make a decision on the fitness of the carcase and offal. In plants where flexible attendance is implemented, the above course of actions must be detailed in a protocol included in the agreed flexible attendance procedures.
This biological hazard must also be considered and analysed in the HACCP plan accordingly.
8.4.3 Inspections
During post-mortem inspection, the inspector is to carry out a visual examination of the carcase, its cavities and, where appropriate, organs with a view to:
- detecting any abnormalities not resulting from the hunting process; for this purpose, the diagnosis must take account of any information that the trained person has provided concerning the behaviour of the animal before killing
- checking that death was not caused by reasons other than hunting, for example, road traffic accident, disease, injury
The inspection of large game should pay particular attention to contamination associated with gralloching (green offal removal), around the pelvis sternum and cut flanks. In carcases that have not been head shot, contamination may be extensive and may result in rejection of the whole carcase – although pre-inspection checks by the FBO should normally identify such carcases.
The carcases must be presented free of contamination to the inspector at post-mortem inspection point. Carcases presented with contamination will not be health marked until the carcase is rectified. The OV or inspector may need to spend extra time in the approved GHE until the carcases are rectified. This time is chargeable to the FBO.
If high number of carcases with contamination are presented at post-mortem inspection, then the FBOs procedures based on HACCP principles should be checked and enforced if appropriate and OV flexible attendance reviewed.
If an assessment cannot be made on the basis of visual examination alone, further palpation and cuts of relevant parts of body may be undertaken and, if necessary, a more extensive inspection must be carried out in a laboratory.
Reference: EU) 2019/627 Article 28.
If the approved GHE is subject to OV flexible attendance, then the OV should verify at least once per month the post-mortem inspection performance of the inspector (MHI or OV) via post-mortem inspection verification checks. Local arrangements should be in place and detailed in the flexible attendance agreement, to allow post-mortem inspection verification checks.
8.4.4 Small wild game contamination
The carcases of small wild game may be contaminated during plucking and evisceration. Where exposed meat, breasts or carcases are contaminated with feathers, down or gut contents they must be rejected.
The use of cloths or paper towels to wipe contamination from carcases is not acceptable. Clean paper towels may be used once to remove feather debris and blood from the vent after evisceration.
Breast meat can only be removed from plucked carcases or in circumstances when the plucked breast has been protected from contamination from other feathers. The removal of breast meat without associated plucking is not acceptable.
8.4.5 Sample inspection of small wild game
Setting the size of the sample is a decision for the inspector taking into account:
- information supplied by the trained hunter (if available)
- species of animal / bird presented for inspection
- general impression gained of the wild game presented for inspection (including uniformity of the sample and signs of decomposition)
- previous history of the source, such as the pattern of disease and proportion of decomposed and contaminated carcases in previous batches
- prevailing climatic conditions
- FBO’s procedures based on HACCP principles and acceptance of birds from hunters
Provided the batch of carcases is relatively uniform, is made up of the same species and came from the same source on the same day, a minimum of 5% of the carcases and viscera must be examined. Batches of less than 20 carcases should be subject to 100% inspection.
8.4.6 No FSA daily attendance
Where there is no daily FSA attendance, the OV may arrange with the FBO a day for the inspection of 5% of each batch present and due to be processed. If they pass inspection, the FBO may proceed to the processing of those batches without the need for several FSA visits. Similarly, if 5% of a batch is retained for inspection, the remainder could be processed and held pending a satisfactory inspection of the 5%, with rejection of the whole batch if the inspection is unsatisfactory.
8.4.7 Other batch factors
In agreeing to inspect a proportion of carcases from a batch, the inspector is making an assessment of the FBO’s competence to recognise unfit or contaminated meat and to take appropriate corrective action. The proportion of a batch to be inspected should reflect the competence of the FBO and evidence of effective processing and hygiene management during uninspected and unattended processing periods.
As with conventional red meat and poultry inspection, decisions must be based on overall hygiene during the dressing process and particularly evidence of cross contamination or contamination associated with dressing procedures.
Poor practice during FSA inspection would provide little confidence that the remainder of the batch was dressed hygienically or that appropriate corrective action and rejections were made during dressing.
The proportion of a batch to be inspected may therefore be larger than 5%, but it must not be less than this.
8.4.8 FBO records
The inspector’s checks should address the following aspects of the FBO records:
- Are there accurate intake records showing numbers of rejections and reasons for rejections?
- Are there records of rejections during processing and are they categorised?
- Can these records be reconciled with ABP records?
- Are there appropriate records of corrective actions?
8.4.9 Other inspection checks
Other checks which the inspector should consider include:
- Are dressing procedures, particularly contamination controls, satisfactory?
- During processing, are hand washing, knife practices and other sanitising procedures satisfactory?
- Are the levels of rejection comparable with those for the previously processed birds or animals from that batch?
- Are birds / animals for FSA inspection presented after the other part of the batch has been processed, or before?
8.4.10 Wild boar
Wild boar are susceptible to the same diseases as domestic pigs and thus it can be expected that a range of lesions similar to that found in farmed pigs will be encountered.
Note: Trichinella testing is required in wild boar. If the head is required for further use as a trophy, it may be sent to an ABP processing plant that has been approved for the production of trophies. The head may be dispatched pending a satisfactory trichinella test, provided that the identification of the head is maintained throughout the process.
8.4.11 FVC verification visits
The FVC must visit all the approved GHE in their area at least once per season to verify that official controls are carried out as per MOC instructions. To ensure all activities are verified during this visit you can use the aide memoires found at annex 12 (large wild game) and annex 13 (small wild game). These aide memoires may be used by the OV or inspector as a check list to ensure that all the official tasks are carried out.
When serious FBO NCs are identified, The FVC should discuss with the FVL the possibility of increasing the OV attendance in the plant and the OV flexible attendance should be reviewed.
Any findings identified during the FVC verification visit should be discussed with the service delivery partners (SDP) and if necessary, dealt with via contract management.
8.4.12 Communication between OV and inspector
In approved GHE where there is OV flexibility implemented, there must be a written communication procedure in place between the inspector and the OV. The OV must be aware of any NCs identified by the inspectors when the OV is not present so enforcement action is taken if necessary.
This written communication procedure must be available for the FSA officers (FVL, FVC, veterinary auditor (VA) during the day of their visit for their assessment.
8.5 Inspection of deer
8.5.1 When to inspect
The carcases of deer should be inspected after skinning in conjunction with the available correlated red offal, where available.
Note: Red offal will only be presented for inspection where the trained person has noted an abnormality or where they are unexpectedly unavailable.
Reference: (EC) 853/2004, Annex III, Section IV, Chapter II, Paragraph 4 (a)-(c).
8.5.2 Minimum post-mortem requirements
Post-mortem inspection must consist of a visual examination of the carcase, its cavities and accompanying offal. In most cases, offal will not be available and in these circumstances, if a declaration from a trained person is not attached to the carcase or it is not identified to a declaration, it must be disposed of as ABP.
8.5.3 Bullet wounds
Carcases with damage caused by the entry of the bullet will require trimming of any bruised or contaminated meat.
Carcases where the bullet entered through the shoulder or the anterior thorax may have shattered bones and muscle damage requiring extensive trimming and rejection of the shoulder or quarter.
Where the bullet has entered through the abdomen, bruising, bone damage and contamination can be extensive and may warrant rejection of the entire carcase.
8.5.4 Contamination
Some damage to the heart, liver and lungs may occur as a result of shooting. Decomposition and contamination are common findings. As a consequence of rupture of the abdominal organs following shooting, or as a consequence of poor gralloching, leakage of gut contents into the abdominal cavity may occur.
The carcase may also become contaminated as a result of poor handling in the field or during transportation to the processing establishment. Any part of the carcase with visible contamination must be trimmed and rejected.
The retention of heavily contaminated meat in close proximity to potentially fit carcases should be avoided. In those circumstances, where trimming precedes inspection, and to minimise potential contamination, trimmed meat should be hygienically retained so that a decision can be made based on the condition of the whole of the carcase. It may not be possible to make a decision if all parts of the carcase have not been retained and identified.
8.5.5 Total rejection
When carcases have been stored under unacceptable conditions (such as high ambient temperatures or exposed to pests) conditions such as generalised decomposition or blowfly infestation will be encountered, and total rejection is necessary.
8.6 Processing in fur / in feather (IFIF) carcases
8.6.1 IFIF trade
Approved premises, such as red or white meat cutting plants, cannot be regarded as a local retailer and therefore cannot receive exempt game or game meat directly from local producers or hunters.
If game is not supplied under any of the exemptions listed in the wild game guide, it must ultimately be processed and inspected in an approved GHE.
Approved GHEs can sell on unprocessed game that has not been subject to an inspection but only to another approved GHE either here or elsewhere in the EU. An identification mark should be applied to small wild game if it has been handled in some way in an approved GHE before it is sent on to another approved GHE.
Temperature requirements apply (4°C small wild game and 7°C large wild game)
Reference: (EC) 853/2004, Article 1, 3 (c) and (e).
8.6.2 Trade of unplucked / unskinned and uneviscerated small wild game
FSA staff shall be aware that where small wild game are to be traded unskinned / unplucked and uneviscerated they:
- may be frozen or deep frozen
- should be stored separately from fresh meat, poultry meat, and other wild game already skinned and plucked
- can be traded only to another approved GHE; sealed boxes and uneviscerated wild game cannot be factored by approved cutting plants even though the packaging is not opened
Note: Smithfield Market is not an approved GHE.
Reference: (EC) 853/2004, Annex III, Section II, Chapter V 1 (c).
8.6.3 FBO duties
Where the FBO intends to trade small game unskinned / unplucked and uneviscerated they must inform FSA staff for monitoring and verification of this activity during the plant audit.
They should have procedures in place to ensure that there is no undue extra food risk in transporting the uneviscerated animals, for example, FBO presented procedures in place to ensure that chill chain is maintained when the viscera are still within the body cavity.
8.6.4 Inspection of small wild game to be traded
Where the FBO intends to trade small wild game, which is unskinned / unplucked and uneviscerated, the FSA staff must monitor and verify this activity as part of the establishment audit. Post-mortem inspection will take place at the receiving approved GHE.
8.6.5 ID marking of small wild game to be traded
An identification mark should be applied to unskinned / unplucked and uneviscerated small wild game, if it has been handled or graded in some way in an approved GHE before it is sent on to another approved GHE.
8.6.6 Intra-community trade
In-skin, in-feather and processed wild game can be consigned to and received from other Member States, subject to any animal health restrictions, and subject to the appropriate export / import certification being in place. If you are unclear as to whether exports or imports may take place during outbreaks of notifiable disease, contact APHA.
All game intended for export or import must have been examined by a trained person (where applicable) immediately after shooting and the game must be handled and transported hygienically in refrigerated transport. The Regulations place a responsibility on the supplier of such game to ensure that it is only consigned to approved premises and transported in hygienic conditions. Unskinned large wild game may be sent to a game handling establishment in another Member State only if it is accompanied by a certificate issued and signed by an OV. A copy of the certificate is at Annex 7.
8.7 Recording of inspection results
8.7.1 Duty of FSA Operations Group
If inspections reveal the presence of any disease or condition that might affect public or animal health or indicate that animal welfare has been compromised the OV is to inform the FBO.
Where lesions suggestive of TB are recorded on the trained person’s declaration, the OV or MHI should confirm that this information has been passed to APHA. APHA should also be contacted if potential TB lesions are found during the inspection of large wild game carcases.
Where the OV is not present the MHI shall contact the OV as soon as possible and discuss necessary action, as per procedures detailed in the agreed flexible attendance document. In certain cases, this may require attendance of the OV at the approved GHE.
Where the problem arose during primary production, the OV shall gather all the information and cascade it to APHA where appropriate, as detailed in section 8.4.2.
8.7.2 FBO’s trained hunter’s declaration and inspection record
The FBO must have a system in place to file the trained person’s declarations (including trained person’ inspection records) in such a way that the declarations can be identified clearly to the individual carcases or batch of carcases.
For large game, the declaration or a number repeated on and relating to the declaration must be attached to the carcase when it is presented for inspection. Carcases without an attached hunter’s declaration label or link to a declaration must be disposed of as ABP (unless presented with the head and all the viscera except for the stomach and intestines).
8.7.3 Post-mortem inspection results and recording of data
Results of post-mortem inspection should be recorded on IRIS. Where there is no IT system available in the plant, forms PMI 4/2 (Deer – Daily Record of Rejection Conditions), PMI 4/5 (Daily Record of Rejection Conditions Large Wild Game), PMI 4/10 (Daily Record of Rejection Conditions Small Wild Game in Feather) and PMI 4/13 (Daily Record of Rejection Conditions Small Wild Game in Fur) can be used to record condition data to be entered onto IRIS at a later date / time. This should be completed at the earliest opportunity, subject to IT availability.
The FSA and FBO must have a system in place to ensure that the results of ante and post-mortem inspections are recorded accurately and can be identified clearly to the batch of animals, or in some cases to the individual animal. The OV must be satisfied with the system for collecting the data at all points.
Reference: See chapter 9 on ‘Forms.
8.7.4 Database
Information is logged on an FSA national database and will be used by:
- Defra to analyse disease trends
- FSA to monitor disease status, for example, trichinella
- FVC when establishing OV attendance
Note: Additional information on Assessment for OV Flexible Attendance is available in the ‘Policy and Procedure for Flexible Attendance at Slaughterhouses and Game Handling Establishments’.
9. Health and Identification Marking
In this section
9.1 Health marking
9.1.1 Overview
The health mark indicates that the animals and the resulting carcase have undergone ante and post-mortem inspection in accordance with (EU) 2019/624 and (EU) 2019/627 and there are no grounds for declaring the meat unfit for human consumption.
Reference: See the topic 9.2 on ‘Identification marking’ in this section for additional information.
9.1.2 Responsibility and health marking
The OV is responsible for ensuring the correct application of the health mark. The actual application of the health mark may be delegated to an MHI or to an FBO member of staff, but only under the effective supervision of the OV.
The health mark shall be applied when official controls have not identified any deficiencies that would make the meat unfit for human consumption and, where appropriate, TSE testing has been carried out with negative results.
9.1.3 Delegation of application of the health mark to plant staff
Article 18 (4) of Regulation (EU) 2017/625 allows for the OV to delegate the application of the health mark to plant staff as long as they comply with the conditions laid down in paragraph 3 of the same article which state that staff:
a) act independently from the production staff of the slaughterhouse;
b) have undergone appropriate training to carry out this task; and
c) carry out this task in the presence and following the instructions of the OV or the OA.
The requirement in c) does not mean that the OV/OA needs to observe FBO staff applying the health mark on every occasion but the OV will need to implement supervision systems and guarantee that the health mark is kept and used appropriately.
SOP
The OV will discuss the delegation requirements with the FBO and will develop a SOP in agreement with the FBO. The SOP will detail the procedures for releasing and recovering the health mark each time it is delegated to plant staff (including during breaks, plant breakdowns), how the task is going to be carried out (species, positions on the line) and the training requirements for plant staff.
The training must be provided by the OV and/or an appropriately briefed OA ensuring that the instructions provided in Section 9 of Chapter 2.4 of the MOC are correctly understood by appropriate plant staff identified by the FBO.
Training will be recorded and the OV must keep an up-to-date list of all the FBO staff authorised to apply the health mark on their behalf and make this list available to the FBO and to FSA officials at any time.
Risk Assessment
For allowing the delegation, it is essential that the OV has confidence in the implementation of the Food Safety Management Systems at the plant.
Before proceeding with the delegation of the health mark to plant staff, the OV will carry out a risk assessment (form provided in Annex 14) and complete a one-week trial. The OV must be satisfied that there are adequate procedures in place to ensure that the health mark is only applied on carcases deemed fit for human consumption and for dealing with carcases that are declared unfit and/or detained.
As part of the risk assessment, the OV will also discuss with the ITL for the area any potential impact on OAs’ resource requirements for the plant. Once the OV is satisfied with the procedures, they will permit the delegation by signing the risk assessment form provided in Annex 14 and will inform the ITL for updating the SOP as necessary.
Supervision and performance monitoring
The delegation and return of the health mark must be recorded on each occasion. Plant staff must return the health mark to the OV or the OA every time it is not in use; this includes during breaks, breakdowns, or any other circumstances.
Records must include at least the following:
- the date and time of delegation of the health mark
- the name of the person receiving the health mark
- the initials of the OV/OA delegating the health mark
- the date and time of return
- the initials of the OV/OA receiving the health mark
The performance of FBO staff carrying out this task on behalf of the OV will be monitored daily by the OV/OA.
Records of both, supervision of the health mark and performance monitoring of authorised FBO staff must be recorded in the relevant form provided in Chapter 9 (HM DEL).
Withdrawal of the delegation
Where supervision or monitoring indicates that the delegation and/or application of the health mark is not in accordance with the agreed SOP, the OV can either reinstate the application of the health mark from a particular FBO member to OAs and/or the OV or withdraw the delegation for the whole establishment if there is evidence or suspicion that continuing with the delegation might lead to a risk to food safety.
Records of reinstatement of the application of the health mark for individual members of FBO staff will be recorded in form HM DEL (Chapter 9).
If the OV decides to withdraw the delegation for the whole establishment they will record this in Part 3 of the risk assessment form (Annex 14).
9.1.4 Meat that should be health marked
The health mark is only applied to carcases and wholesale cuts of:
- cattle, including buffalo and bison
- sheep, goats, and pigs
- horses
- camelids
- ratites
- farmed deer and wild boar
- large wild game, deer, and wild boar
Reference: (EU) 2019/627 Article 48, 2.
9.1.5 Application
The health mark should be applied in the slaughterhouse or game-handling establishment so that if carcases are cut into half or quarters or half carcases are cut into 3 pieces, each bear such a health mark. The FBO should inform the AO how many pieces the carcase will be cut into if they wish the minimum number of marks to be applied.
9.1.6 Wild game
Meat from wild game can only bear a health mark if it is skinned in a game handling establishment, has undergone post-mortem inspection, and been found fit for human consumption.
Reference: (EU) 2019/627 Article 48.
9.1.7 Application at inspection
A system should be in place so that the line speed and inspection facilities allow the health mark to be applied to the carcase at the time of post-mortem inspection.
9.1.8 Blurring
Blurred health marks are unacceptable and, if this is a problem, a system should be arranged so that:
- one health mark is applied if the carcase is fit at the time of inspection
- health marking is completed once the carcase has dried (in the chiller)
9.1.9 Health mark and trichinosis
Where a procedure is in place in the slaughterhouse to ensure that no part of carcases examined leaves the premises until the result of the trichinella examination is found to be negative and the procedure is formally approved by the OV, the health mark may be applied before the results of the trichinella examination are available.
The operator must have a written procedure agreed with the OV in place.
Where such system is not in place, the health mark must not be applied until a negative test result has been received.
9.1.10 Withheld health mark
The health mark can only be applied to the carcase of animals which have undergone ante and post-mortem inspections in accordance with (EU) 2019/627 and there are no grounds for declaring the meat unfit for human consumption. Examples of where the health mark should be withheld are:
- failure of ante-mortem and / or post-mortem inspection
- presence of SRM (except Vertebral Column of over 30-month bovines)
- carcases presented for inspection with evidence of visible contamination or gross pathology
- where residues or contaminants are suspected
- carcases produced in a slaughterhouse where the water supply is found to have been contaminated and a risk to public health exists
- where adequate facilities for inspection are not available and there is a risk that carcases with visible contamination or gross pathology could be inadvertently health marked (that is it has not been possible to perform adequate inspection)
- carcases from animals suffering from a notifiable disease
- meat declared by the OV to be unfit for human consumption
9.1.11 Recording marks used
To prevent fraudulent use of health marks and other stamps all members of the FSA staff must record in the daybook:
- the time of issue
- the number of the health mark stamp
- the time stamps are returned to secure storage
9.1.12 Security of the health mark
The security of the health mark stamp is the responsibility of the officer to whom it was issued.
- The health mark stamp must be kept in secure lockable facilities when not in use.
- The OV must be able to demonstrate the security of health marking equipment.
- The OV must have an auditable system in place to check that all health mark stamps have been returned at the end of each operational day.
- Anyone possessing or using health marking equipment, without the authority of the OV is committing an offence.
9.1.13 Reporting missing stamps
If a health mark stamp is stolen or lost, there is potential that it can be used for fraudulent activities and used for illegally killed animals. Missing stamps whether lost or stolen must be reported immediately to CSU transactions team.
9.1.14 Meat not health marked
Unmarked meat that is required to be health marked cannot be sold for human consumption. The FBO is responsible for disposing of the meat in compliance with the ABP regulations.
Reference: (EC) 853/2004, Article 5
9.1.15 Health mark labels
For the health marking of lamb, kid, and piglet carcases the hygiene regulations no longer permit the use of health marks in the form of a label or tag instead of ink / hot branding as was permitted under the previous legislation.
Reference: (EC) No 2076/2005, Article 5.
9.2 Identification marking
9.2.1 Requirements
Carcases and wholesale cuts of red meat species, farmed game mammals (other than lagomorphs) and large wild game that have passed official controls at a game handling establishment should all be health marked. Other products of animal origin only require an identification mark.
9.2.2 Application
Identification marks are applied by the FBO. The FSA is required to verify compliance with the application of identification marks.
10. Edible co-products
In this section
10.1 Edible co-products
10.1.1 Definition
Edible co-products are parts of slaughtered animals unsuitable for human consumption at the time of production in the slaughter house, but which can later be processed for use in human food.
Examples of edible co-products include:
- rendered animal fat and greaves
- treated stomachs bladders and intestines
- gelatine
- collagen
Reference: (EC) 853/2004, Annex III, Sections XII, XIII, XIV and XV.
Detailed guidance is contained in the FSA guide: Industry Guide on Edible Co-products and Animal By-products. This can be found in Annex 1, Chapter 18 ‘Waste Management (including Animal By-Products)’ of the Meat Industry Guide.
10.1.2 Feet for human consumption
Feet intended for human consumption are treated as edible offal. All feet intended for human consumption must be inspected.
Feet processed on site:
Post-mortem inspection can be done before or after further treatment (such as dehairing) on an individual basis or in batches. If post-mortem inspection takes place before treatment, a further spot check will be needed to ensure that these feet are free from any pathology.
Feet processed at a different approved site:
Post-mortem inspection can be done before or after cleaning (washing) on an individual basis or in batches. If post-mortem inspection takes place before cleaning, a further spot check will be needed to ensure that these feet are visibly clean before shipping for further processing.
In both cases a full correlation system must be implemented by the FBO to ensure that if a carcase is condemned, the correlated feet of the entire batch are disposed of as unfit for human consumption. FBOs may assist the inspection process and set aside feet with identified abnormalities.
Feet which have not been inspected, are not visibly clean or have not been processed cannot be despatched from the establishment as intended for human consumption.
10.1.3 FBO responsibility
The FBO should identify handle, process, store, and despatch edible co-products in accordance with the guidance contained in the meat industry guide.
Co-products should be stored and despatched to appropriate destinations separate from ABP, in accordance with the guidance.
Co-products should be despatched with the correct documentation, containing the information outlined in the specimen documents in the co-products guidance.
10.1.4 FSA responsibility
The OV is to check that:
- the FBO handles the co-products in accordance with the FSA guidance having due regard to hygienic processing, separation, storage, and temperature requirements
- that the edible co-products are consigned to appropriate premises
- that adequate separation from ABP’s is maintained, such as cattle hides intended for the production of gelatine for human consumption are stored and despatched with adequate separation from all other hides
- that a control system is in place for hides from bovines that require BSE testing, pending a negative test result
11. Slaughter hygiene verification system in red meat
In this section
11.2 Slaughter hygiene verification
11.3 Process – hygiene verification
11.4 Product – carcase verification
11.5 Plant – establishment verification
11.1 Introduction
11.1.1 Purpose
The SHV system focuses on gathering qualitative measures to assess FBO processing standards.
The SHV system monitors contamination at final inspection as a key point to satisfy FSA regulatory requirements, but also creates a more holistic approach to provide a more complete picture of the processing standards of the FBO, with the ultimate objective of providing clear evidence of improvements to carcase hygiene when required. The SHV system focuses on the need for FBOs to take the necessary corrective actions, quarantine and rectify contaminated carcase, take effective actions, and prevent re-occurrence.
This guidance outlines a consistent approach on how and when OVs / AOs shall verify that FBOs have implemented effective slaughter hygiene practices and procedures which prevent contamination of carcases with enteric pathogens and faecal contamination throughout the entire slaughter and dressing operation and that their food safety management systems demonstrate this control.
The results of verification checks can be used to:
- provide advice to assist the FBO with root cause analysis
- provide data for further trend analysis by FSA
- provide evidence for enforcement action
- justify health and identification marking
- inform the FBO audit process
- inform veterinary certification for third country export
11.1.2 Background
The FSA has developed SHV procedures by looking at the regulatory official control verification requirements at abattoirs.
With particular reference to slaughter hygiene, official controls must verify:
- FBO compliance with Regulations (EC) 852/2004 and (EC) 853/2004
- that FBOs apply procedures to ensure good hygiene practices continuously and properly
- that FBOs apply HACCP-based procedures continuously and properly
Verification is the responsibility of the OV, but information regarding good hygiene practices and HACCP based procedures can be gathered by the Official Auxiliary (OA) to assist the OV.
11.2 Slaughter hygiene verification
11.2.1 Key elements of the verification system
The verification system applies predetermined minimum frequencies of verification tasks, which provide information on the delivery of official controls, enforcement activity and objective evidence to support FBO audits.
Key summary points of the verification system are as follows:
- SHV checks should be carried out by OVs and AOs
- SHV must be completed in each establishment
- the number of verification checks can decrease or increase depending on findings
- the SHV system can be utilised by OVs / AOs and technical contract managers to assess performance and official control delivery to focus attention and discussions
11.2.2 Slaughter hygiene verification method
The verification system includes a number of tasks that must be carried out for each of the processed species and should cover the whole of the production process. Verification tasks are divided into the four following categories and have different frequencies based on the associated risks and possible impact on public health:
- process – hygiene verification
- product – carcase / offal verification
- plant – establishment verification
- HACCP and microbiological verification
A summary of all verification tasks and their frequencies can be found at Annex 8, a SHV Task Schedule at Annex 10 and a SHV flow chart at Annex 11.
The initial selection of carcases for process hygiene and product verification should be random. However, based on the findings, the OV / AO may wish to target a specific type of process or animals to better assess FBO’s controls.
If the outcome of the verification checks indicates poorly implemented FBO procedures, then the documented SOPs and records should also be considered as part of SHV verification checks.
11.2.3 Minimum requirements – assessment of samples
The OV / AO should select a point on the production line where suitable facilities are available to allow a thorough examination of all surfaces of the sampled carcases.
Sufficient time must be allocated by the OV / AO to ensure a thorough examination of the carcase / side is performed and accurate data is collected, and consistency is maintained.
11.2.4 Outcomes
Each verification area must be assessed by the OV / AO and scored-based on the outcome (compliant / NC) and the level of the enforcement action taken.
| Outcome | Description |
|---|---|
| Compliant: green |
Food business is operating in accordance with its food safety management systems, food safety standards and has met the requirements of the regulations No enforcement action taken |
| Non-compliant: yellow | A non-compliance that resulted in a verbal advice |
| Non-compliant: amber | A non-compliance that resulted in a written advice |
| Non-compliant: red | A non-compliance that resulted in a formal enforcement action (service of legal notices, referral for investigation) |
Using objective evidence, the type of deficiencies identified during the daily / weekly / monthly SHV checks and FBO’s corrective action reflect the extent and effectiveness of performance and compliance.
11.2.5 Reporting arrangements
The K2 system will produce data reports with results of verification activity. The information must be utilised by OVs / AOs to monitor individual plant performance during the interim FBO audit period.
OV / AO must use the FSA Slaughter Hygiene Checklist (Annex 4) to record the outcome of verification checks and store it at the plant.
11.2.6 Use of verification data
The recorded daily outcomes of verification tasks will provide information about the level of current performance / compliance.
The gathered data will assist the OV / AO in defining reasonable expectations of operating standards.
Establishment trend analysis and professional judgement from the OV / AO is required for appropriate action. This will assist in compliance decisions and achieve consistency of approach.
The OV / AO should review the results on a daily, weekly, monthly basis and take the appropriate action as detailed in topics 12.3 to 12.6.
11.3 Process – hygiene verification
11.3.1 OV / AO responsibility
The OV / AO is expected to verify hygienic standard of the process to assess if the FBO has adequate controls in place to minimise contamination and if corrective actions are taken when contamination incidents occur.
11.3.2 Process scope
| Verification Steps | Scope (all species) |
|---|---|
| 1 Cleanliness of animals |
Animals clean on arrival or measures taken by FBO to ensure that animals are clean before dressing commences or other measures taken to prevent cross contamination from dirty animals. OV / AO to record in K2 the number of carcases checked, and the number of carcases found not clean. |
| 2 Bleeding |
Bleeding does not result in carcase contamination. OV / AO to record in K2 the number of carcases checked, and the number of carcases found contaminated with faeces / ingesta after bleeding. |
| 3 Skinning / hair removal |
Meat contamination avoided (for example, contact between outside skin and carcases prevented, operator / equipment in contact with the outside of hide / fleece not touching the meat) Skinning completed (no pieces of skin left) and bristles removed. OV / AO to record in K2 the number of carcases checked, and the number of carcases found not completely skinned (pieces of skin left / bristles not removed) and/or contaminated with faeces / ingesta / milk after skinning / hair removal. |
| 4 Evisceration / udder removal |
Spillage of digestive tract content prevented, and removal of udder does not result in contamination of the carcase with milk or colostrum. OV / AO to record in K2 the number of carcases checked, and the number of carcases found contaminated with faeces / ingesta / milk after evisceration/udder removal. |
| 5 Presentation for inspection |
Carcases and offal presented for inspection free from any visible contamination. OV / AO to record in K2 the number of carcases / offal checked, and the number of carcases / offal found contaminated with faeces / ingesta / milk at the step of presentation for inspection. |
11.3.3 Process frequency and sample size
The verification checks in process hygiene areas have to be carried out every day for every species slaughtered. However, the frequency of verification checks at the steps: ‘Cleanliness of animals’, ‘Bleeding’, ‘Skinning / hair removal’ and ‘Evisceration / udder removal’ can be reduced to once a week, if the OV is satisfied with the hygienic standard of the establishment and certain conditions are met.
In order for the OV to consider the reduced frequency of verification checks, the establishment should meet the following criteria:
- ‘Good’ or ‘Generally Satisfactory’ outcome of the last FBO audit
- no formal enforcement related to the hygiene of production (no hygiene improvement notices (HINs), remedial action notices (RANs, referrals for investigation) in the last 4 weeks
- less than 5% of carcases presented contaminated for inspection in the last 4 weeks (daily percentage).
| Verification Step | Basic frequency | Reduced frequency |
|---|---|---|
| 1 Cleanliness of animals | Daily | Once a week |
| 2 Bleeding | Daily | Once a week |
| 3 Skinning / hair removal | Daily | Once a week |
| 4 Evisceration / udder removal | Daily | Once a week |
| 5 Presentation for inspection | Daily | Daily |
Note: The frequency of the verification checks at the step ‘Presentation for Inspection’ cannot be reduced and they should be always carried out daily.
The table below demonstrated how many carcases – based on the establishment’s daily throughput – have to be verified daily at steps: ‘Cleanliness of animals’, ‘Bleeding’, ‘Skinning / hair removal’ and Evisceration / udder removal’.
The numbers provided in the previous table are a minimum and can be increased by the OV dependant on findings during checks.
| Daily throughput |
Minimum number of carcases to be checked (at steps: ‘Cleanliness of animals’, ‘Bleeding’, ‘Skinning / hair removal’, Evisceration / udder removal’) |
|---|---|
| 0-100 | 2 |
| 101-250 | 4 |
| 251-500 | 7 |
| More than 500 | 11 |
The daily number of carcases and offal that must be verified at the ‘Presentation for inspection’ step, with outcome recorded, depends on the daily throughput of each slaughtered species.
The following table demonstrates how many carcases / offal should be selected for verification. All slaughtered species should be verified daily.
| Daily throughput |
Minimum number of carcases and offal to check daily (at ‘Presentation for inspection’ step) |
|---|---|
| 0-24 | 4 |
| 25-100 | 10 |
| 101-250 | 30 |
| More than 250 | 60 |
Note: Any decision to increase the number of checks, above the minimum recommended in the table above, should be recorded in the plant daybook.
11.3.4 Process – unit size
For all species, a unit is defined as a whole carcase (with offal), regardless if split or not e.g. if a carcase is split into two sides, then two sides have to be inspected to count it as a unit.
Due to different line set ups and arrangements it is possible to assess part carcases / sides at random to achieve the required sample size, (for example: assess a run of beef hindquarters on the high stand and complete the monitoring from the low stand with a later run of forequarters).
Where carcases or sides are divided into sections for assessment, all defects from the sections that make up one complete carcase must be added together to determine how the defect is scored for that carcase.
Carcase verification can be carried out ‘on-line’ at normal processing speeds or at a designated area.
11.3.5 Process – contamination
Any visible trace of faecal, ingesta and milk contamination must be counted and recorded. Each contaminated carcase or offal counts as one incident, regardless of the amount of contamination present.
In cases where contamination identified during verification checks is different to digestive tract content (faecal / ingesta) or milk, the OV / AO should bring it to the attention of the FBO and ask for it to be removed / trimmed. Such cases, however, do not have to be recorded. Examples of contamination other than digestive tract content or milk include rail dust, hide / wool, bile, and oil / grease. Excessive and frequent contamination of this type should trigger enforcement action.
Additional points for consideration when scoring:
- retained udder fragments are evidence of milk contamination
- gut segments, including oesophagus, are classified with faeces, ingesta, milk
- contamination issues already identified by the FBO (such as clearly marked carcases for further rectification) are not to be added to the SHV form as those were already identified as part of the FBO’s HACCP system
- however, excessive carcases being removed from the processing line is a significant issue and appropriate OV action should be taken regardless of whether the FBO has identified these; detention logs and rejected meat records (IRIS) will provide appropriate evidence to utilise
11.3.6 Process – enforcement
The FSA supports a ‘zero tolerance’ approach to visible contamination on carcases, which requires that all identified visible contamination on meat is removed by the FBO without delay by trimming or alternative method having an equivalent effect.
In cases where frequent and regular contamination problems are identified by OV / AO, an enforcement action must be taken in accordance with Chapter 7 ‘Enforcement’.
11.3.7 Process – digestive tract content
OVs / AOs are to identify foreign material as faeces or ingesta based on the characteristics of colour and texture and only when they are able to identify either colour or texture. Size is unimportant in identifying faecal or ingesta contamination however, as size decreases, colour and texture become more difficult to identify.
- The colour of faecal or ingesta contamination is:
- cattle – yellow, green, or brown
- pigs – tan to dark brown
- sheep and goats – brown to black
- Faecal or ingesta contamination has a fibrous or plant-like texture; for example, sheep and goat faeces and ingesta may be tarry, whilst pig faeces and ingesta may include identifiable grain particles
11.3.8 Process – milk
OVs / AOs are to identify foreign material as milk based on two factors: colour and consistency.
- The colour of milk ranges from clear to white to light yellow.
- The consistency of milk ranges from watery to ropy or curdy.
Milk, if present, tends to be found on the midline, during or after removal of mammary glands (udder).
11.4 Product – carcase / offal verification
11.4.1 Product – carcase / offal verification
On an ongoing basis, the OV will verify a sample of carcases and offal (including fifth quarter product) that have been health marked. The verification checks should reflect the full range of species and age / type of animal being processed. Only the final product (carcases or offal) should be verified, and the following production stages could be selected for carrying out the checks:
- immediately after inspection points (after final rectification by the FBO) – to ensure real time checks
- in the chiller
Verification of offal includes parts that are fit for human consumption at the inspection point (such as liver, heart, and skirt). Others intended as edible co-products which require further processing prior to being eaten (for example, tripe and casings) should also be included in the verification checks.
11.4.2 Product – carcase / offal verification scope
Product verification replaces the previous PMI verification checks and focuses on the FSA’s performance. Therefore, the outcome should not be used as direct indication of the FBO’s performance. However, frequent findings in this area could trigger additional checks as part of the process verification.
The following table details the scope of verification during product checks:
| Area of verification | Scope |
|---|---|
| 1 Pathology | Meat is free from all pathological conditions |
| 2 Statutory requirements | Post-mortem inspection has been carried out in accordance with legal requirement |
| 3 Faecal / ingesta / milk | Meat is free from faecal / ingesta / milk contamination |
| 4 Health marking | Meat is correctly and legibly health marked |
| 5 Other | Record any identified deficiency (for example, contamination with bile / hair / wool, tonsils, stick wounds, SRM, rail flake) |
11.4.3 Product – carcase / offal verification – frequency
Verification must be carried out on three operational days a week (if possible) or spread over the whole week in establishments with a very low throughput (less than 100 a week).
The number of carcases to be checked depends on the weekly throughput (as in the following table):
| Weekly throughput | Weekly total of carcases and offal to check |
|---|---|
| More than 1000 | 60 carcases and 60 sets of offal (20 carcases and 20 sets of offal per species per day, 3 days per week) |
| 101-1000 | 30 carcases and 30 sets of offal (10 carcases and 10 sets of offal per species per day, 3 days per week) |
| 0-100 | 5 carcases and 5 sets of offal (spread over the whole week, if possible) |
Note: In OV-only establishments and plants with recognised OV flexibility (such as cold inspection) the product verification checks should be carried out during routine FVC or contractor management visits and documented on the K2 system by the FSA / service delivery partner (SDP) at least every three months. The FVC is accountable for ensuring these checks have been carried out and documented and is responsible for establishing the number of carcases and offal that should be verified during those visits. The verification system should not impact on agreed resource and business agreements as outlined in the Statement of Resources for the individual establishment.
Note: Any decision to increase the number of checks, above the minimum recommended in the table above, should be recorded in the plant daybook.
11.4.4 Product – carcase / offal verification – assessing results
Although product verification aims to measure the FSA’s effectiveness as the inspection service, it is also an indication of the effectiveness of FBO controls.
The product verification is not subject to scoring. The OV is only required to record and input in the system the number of deficiencies identified and the total number of carcases / offal checked.
Verification results should be assessed by the OV / FVC to monitor team performance. Variables in each establishment should be considered if concerns are raised following verification checks (for example, lighting, available inspection time and space, FBO performance, plant layout).
Note: The OV / FVC should maintain realistic expectations during the checks when assessing team performance from the product verification results, as minor incidents of contamination become more evident post-chilling, particularly with pig hair and wool.
11.5 Plant – establishment verification
11.5.1 Plant – establishment verification
Establishment verification tasks focus mainly on different parts of the establishment, equipment, cleanliness, hygiene arrangements and procedures.
The minimum frequency of establishment verification tasks depends on the FBO audit outcome. However, the OV can increase the frequency if considered necessary and should always score a relevant section when an intervention takes place that resulted in verbal, written or formal enforcement.
Some establishment verification tasks are considered essential and should be carried out and scored every day, regardless of the audit score awarded.
The following table lists the establishment verification areas and the minimum frequency of checks based on FBO audit outcome.
Plant - establishment verification (FBO audit outcome)
| Establishment verification tasks and their frequency | Improvement necessary / Urgent improvement necessary | Good / Generally satisfactory |
|---|---|---|
| 1 Intake / FCI | Daily | Daily |
| 2 Ante-mortem arrangements | Daily | Daily |
| 3 Correlation of carcases and offal | Daily | Daily |
| 4 Operational break / cleaning | Daily | Daily |
| 5 General hygiene (1) | Daily | Daily |
| 6 Handling of carcases / offal during storage and despatch (2) | Daily | Daily |
| 7 Co-products and animal by-products (3) | Daily | Daily |
| 8 FBO pre-operational cleaning | Weekly | Monthly |
| 9 Carcase and offal chilling | Weekly | Monthly |
| 10 Premises (4) | Weekly | Monthly |
(1) ’General Hygiene’ includes verification of hygienic practices (including staff movement, PPE provisions and practices, hand washing), hygienic facilities provided (hot water, soap, sterilisers), door policy, cross contamination controls.
(2) ’Handling of carcases / offal during storage and dispatch’ includes 5th quarter products (such as bones, tendons, feet).
(3) ’Co-products and animal by-products’ includes verification of separation of edible and non-edible materials and captures outcome of daily/weekly SRM checks (for details see MOC Chapter ‘2.7 Specified risk material controls’ and Chapter ‘2.8 Animal By-Products’).
(4)’Premises’ includes verification of lairage / intake area, processing / dressing area, chillers, packing / packaging storage area, dispatch area, plant surrounds, fly screening / vermin entry prevention, control of waste water, drainage and effluent, and water testing.
Note: SHV K2 form should be also used to record monthly summary of FBO compliance with SRM controls. Details should be input in the relevant part of the SHV K2 form.
11.6 HACCP verification
11.6.1 HACCP verification
The verification of the FBO’s HACCP-based procedures is focused primarily on two areas: monitoring of CPs/CCPs and corrective actions.
The OV / AO is not expected to check all records but must verify a sample to be satisfied that the FBO is following their own procedures for monitoring control points and that the FBO is taking and recording pre-established corrective actions when the control is lost.
| Area of verification | Scope |
|---|---|
| 1 Monitoring of CPs/CCPs | Monitoring procedures implemented; accurate records that reflect reality maintained (up to date) |
| 2 Corrective actions | Correct actions taken when monitoring indicate loss of control, such as CPs/CCPs outside of limits (as per HACCP plan) |
11.6.2 HACCP verification – frequency
The minimum frequency of verification of the FBO’s HACCP-based procedures is pre-set and linked with the outcome of the last FBO audit. However, the OV can modify the frequency of those checks depending on the outcome or other findings indicating that the HACCP based procedures are not adequately implemented and / or risks are not sufficiently controlled (for example, high numbers of contaminated carcases found during the process or product verification checks).
The following table specifies the minimum frequency of HACCP verification checks based on the audit score.
FBO audit outcome
| Area of verification | Improvement necessary / Urgent improvement necessary’ | Good / Generally satisfactory |
|---|---|---|
| 1 Monitoring of CPs/CCPs | Weekly | Monthly |
| 2 Corrective actions | Weekly | Monthly |
11.7 Microbiological verification
11.7.1 Microbiological verification
All FBOs are required to comply with current EU law and ensure that meat and carcases in the slaughterhouse are tested in accordance with (EC) 2073/2005. The OV / AO should verify on a monthly basis that the microbiological sampling is taking place as per the legislative requirement. This includes observing the FBO sampling procedures as well as verification of sampling frequency, sample size and parameters tested.
| Area of verification | Scope |
|---|---|
| 1 FBO sampling procedures | Microbiological sampling carried out as per legislative requirement (in accordance with (EC) 2073/2005, correct frequency of testing followed, correct sample size) |
| 2 FBO analysis of results | Results / trends analysed, and action taken when results indicate a problem |
Note: In premises where microbiological testing is done less frequently than monthly, the verification frequency should be adjusted and aligned with that of the FBO’s testing regime.
12. Slaughter hygiene verification system in poultry
In this section
12.2 Slaughter hygiene verification system
12.3 Process – hygiene verification
12.4 Product – carcase verification
12.5 Plant – establishment verification
12.1 Introduction
12.1.1 Purpose
This section describes the official control procedures for SHV in poultry abattoirs. The SHV system provides an ongoing assessment of FBO compliance with food hygiene requirements from acceptance of the animals for slaughter, through processing, offal harvesting and chilling to carcase and offal / co-product packing for despatch.
The verification objective is to provide assurance that only meat that is produced in accordance with legislative requirements is placed on the market.
This guidance outlines how and when OVs / AOs shall verify that FBOs have developed effective slaughter hygiene practices and that they are implementing effective procedures which:
- prevent contamination of carcases with enteric pathogens and faecal contamination throughout the entire slaughter and processing operation, and that their food safety management systems demonstrate this control
- ensure that carcases with visible faecal contamination are identified and rectified
- verify the monitoring procedures following findings of visible contamination and the corrective actions undertaken to bring the process back under control
The results of verification checks can be used to:
- provide advice to assist the FBO with root cause analysis
- provide evidence for enforcement action
- justify identification marking
- inform the FBO audit process
- inform veterinary certification for third country export
12.1.2 Background
FSA has developed SHV procedures by looking at the regulatory official control verification requirements at abattoirs.
With particular reference to slaughter hygiene, official controls must verify:
- FBO compliance with Regulations (EC) 852/2004 and 853/2004
- that FBOs apply procedures to ensure good hygiene practices continuously and properly
- that FBOs apply HACCP based procedures continuously and properly regarding:
- acceptance for slaughter
- compliance with microbiological criteria
- freedom from foreign bodies
- that FBO procedures guarantee to the best possible extent that meat:
- does not contain patho-physiological abnormalities or changes
- does not bear faecal or other contamination
Verification is the responsibility of the OV, but information regarding good hygiene practices and HACCP based procedures can be gathered by Official Auxiliaries (OAs) to assist the OV.
The verification system focuses on gathering qualitative measures to assess FBO processing standards.
The SHV system creates a more holistic approach to provide a more complete picture of the FBOs processing standards with the ultimate objective of providing clear evidence of improvements to carcase hygiene when required.
12.2 Slaughter hygiene verification
12.2.1 Key elements of the verification system
The verification system applies predetermined minimum frequencies of verification tasks, which provide information on the delivery of official controls, enforcement activity and objective evidence to support FBO audits.
Key summary points of the verification system are:
- SHV checks should be carried out by OVs and AOs
- the number of checks can increase or decrease depending on findings
- the SHV system can be utilised by the OV / AO and technical contract managers to assess performance and official control delivery to focus attention and discussions
12.2.2 SHV method
The verification system includes a number of tasks that must be carried out and should cover the whole production process. Verification tasks are divided into the four following categories and have different frequencies based on the associated risks and possible impact on public health:
- process – hygiene verification
- product – carcase / offal verification
- plant – establishment verification
- HACCP and microbiological verification
A summary of all verification tasks and their frequencies can be found in Annex 9.
The initial selection of carcases for process hygiene and product verification should be random. However, based on the findings, the OV / AO may wish to target a specific type of process or animal to better assess FBO controls.
12.2.3 Minimum requirements – assessment of samples
The OV / AO should select a point on the production line where suitable facilities are available to allow an examination of surfaces of the sampled carcases.
Adequate time must be allocated by the OV / AO to ensure an examination of the carcase is performed and accurate data is collected, and consistency is maintained.
12.2.4 Outcomes
Each verification area must be assessed by the OV / AO and scored based on the outcome (compliant / NC) and the level of the enforcement action taken. The score is recorded in the K2 system.
| Outcome | Description |
|---|---|
| Compliant: Green | Food business is operating in accordance with its food safety management system, food safety standards and has met the requirements of the regulations; no enforcement action taken |
| Non-compliant: Yellow | A non-compliance that resulted in verbal advice |
| Non-compliant: Amber | A non-compliance that resulted in written advice |
| Non-compliant: Red | A non-compliance that resulted in formal enforcement action, such as service of legal notice, referral for investigation |
12.2.5 Reporting arrangements
The K2 system will produce daily, weekly, and monthly data reports of verification activity results. The OV/ AO must utilise the information monitor individual plant performance during the interim FBO audit period with the following objectives:
- drive consistency of enforcement
- encourage continuous improvement in FBO slaughter hygiene activities
- determine the level of current compliance within a production method
OV / AO must use the Poultry Slaughter Hygiene Checklist (Annex 9) to record the outcome of verification checks when K2 system access is not available on-site and store it at the plant until the information is entered into the system –local FSA team should have procedures in place to ensure the information is entered in the K2 system as soon as possible–.
12.2.6 Use of verification data
The recorded daily outcomes of verification tasks will provide information about the level of current performance / compliance within a production method.
The data collection at plant level will assist the OV / AO in defining reasonable expectations of operating standards.
Establishment trend analysis and professional judgement from the OV / AO is required for appropriate action. This will assist in compliance decisions and achieve consistency of approach.
The OV / AO should review the results on a daily, weekly, and monthly basis and take the appropriate action as detailed in sub-sections 12.3 to 12.6.
12.3 Process – hygiene verification
12.3.1 OV / AO responsibility
The OV / AO is expected to verify the efficacy of the evisceration and the hygiene standard of the process to assess:
- if the FBO has adequate controls in place to minimise contamination
- if corrective actions are taken when contamination incidents occur
- if corrective actions are taken when carcases are not correctly eviscerated
12.3.2 Process scope
| Inspection verification steps: | Scope (all species) |
|---|---|
| 1. Contamination | Processing does not result in carcase contamination Measures taken to prevent the spillage of the digestive tract content during evisceration |
| 2. Evisceration | Carcases are eviscerated; offal is not missing and is presented for post-mortem inspection |
12.3.3 Process frequency and sample size
The verification checks in the process hygiene area have to be carried out every day and at least 150 carcases should be verified.
For small size slaughterhouses, sample size can be reduced and at least 40 carcases should be verified per day.
For the purpose of SHV, small size slaughterhouses are defined as those processing 1500 or less birds a day.
The number can be increased by the OV based on findings. Carcases should be selected randomly, and the checks should be spread throughout the day and cover the full range of species and age / type of animals being processed.
During process hygiene checks it is not necessary to lift carcases from the line. It is sufficient to inspect carcases in a manner that is similar to regular post-mortem inspection at this point.
Note: It is not necessary to check 150 or 40 (for small size abattoirs) carcases of each species slaughtered every day; sample size is the combined number of all species processed on site.
| Inspection verification steps: | Frequency |
|---|---|
| 1. Contamination | Daily |
| 2. Evisceration | Daily |
12.3.4 Process – location
Process hygiene verification checks should be carried out at, or prior to, the Evisceration post-mortem inspection point, where the OV / AO can visually assess the carcases.
12.3.5 Process – contamination
The OV / AO must record in relevant sections of the K2 system all instances of carcases with faecal or ingesta contamination identified during the process hygiene verification checks, as well as the number of carcases that were not eviscerated or presented for inspection without offal (offal was missing).
Any visible trace of faecal or ingesta contamination must be counted and recorded. Each contaminated carcase counts as one incident, regardless of the amount of contamination present.
In cases where contamination identified during verification checks is different to digestive tract content (faecal / ingesta), the OV / AO should bring it to the attention of the FBO. Such cases, however, do not have to be recorded in the SHV system. Examples of contamination other than digestive tract content include bile and oil / grease. Excessive and frequent contamination of this type should trigger enforcement action.
Note: When the nature of the process and product require that parts of viscera remain inside the bird, for example, delayed evisceration or partial evisceration – effile, those parts should not be counted as evisceration failure.
12.3.6 Process – enforcement
In cases where frequent and regular contamination problems are identified by the OV / AO, enforcement action must be taken in accordance with Chapter 7 Enforcement.
12.3.7 Process – digestive tract content
The OV / AO is to identify foreign material as faeces or ingesta based on the characteristics of colour, texture, and composition. Size is unimportant in identifying faecal or ingesta contamination; however, as size decreases, colour and texture become more difficult to identify. The characteristics below are only listed as guidance and the OV / AO should use their professional judgement when making the decision.
Identification of contamination
| Faecal | Ingesta | |
|---|---|---|
| Colour | Varying shades of yellow to green, brown, and white | Varies with diet |
| Consistency | Frequently semi-solid to paste | Characteristically solid or granular, occasionally digestive fluids are present |
| Composition | May or may not include plant material | Contains identifiable plant material |
12.4 Product – carcase / offal verification
12.4.1 Product – carcase / offal verification
On an ongoing basis, the OV / AO will verify a sample of carcases and offal destined for human consumption (including fifth quarter product) that have passed post-mortem inspection and are considered a final product (such as the FBO having finished any required rectification work). The verification checks should reflect the full range of species and age / type of animals being processed. Additionally, the OV / AO is required to verify the ID marking arrangements.
Carcase verification checks should be carried out after the final carcase washing process at a point that allows the OV / AO to lift carcases and perform a detailed inspection (for example, grading, packing and despatch).
Verification of offal refers to parts that are to be sold as fit for human consumption (such as liver and heart). Other parts intended as edible co-products that require further processing prior to being placed on the market (such as feet and tongues) should also be included in the verification checks. Offal verification checks should be carried out after the post-mortem inspection is completed and the product has been initially processed (separated, trimmed, washed).
12.4.2 Product – sample size / frequency
Each day, at least 60 carcases and sets of offal (if fit for human consumption) should be checked.
For small size abattoirs at least 30 carcases and sets of offal (if fit for human consumption) should be checked per day.
The checks should be spread across each day of production and carcases / offal should be selected randomly. The detailed inspection of carcases should include the inspection of external surfaces, the body cavity, and the neck area.
The following guidance details how the product (carcase) inspection could be carried out:
| Outside back | While holding the carcase, with the back of the carcase towards the observer, and starting at the hock area, observe the hock, back part of the legs, tail area, back of the carcase and top side of the wings. |
|---|---|
| Outside front | Turn the carcase and observe the bottom side of the wings, breast, and front part of the legs. |
| Inside | Observe the inside surfaces of the carcase and the abdominal flaps and fat. |
| Neck flap area | Observe the neck flap and the thoracic inlet area. |
12.4.3 Product – verification scope
Product verification focuses on the FSA’s post-mortem inspection performance and the FBO’s hygienic standard of operation and the effectiveness of rectification procedures; therefore, the product verification checks are separated into two areas:
- verification of post-mortem arrangement
- verification of FBO controls
Both areas can be verified at the same time when assessing the same sample of carcase / offal; however, verification of post-mortem arrangement cannot be carried out by OAs.
12.4.4 Product – verification of post-mortem arrangement
Verification of post-mortem arrangement must be carried out by the OV, and since it focuses on FSA’s performance, it is not subject to scoring.
In the K2 system, the OV is only required to record the number of carcases / offal checked and the number found affected by pathology missed by the inspection team.
The following table details the scope of product checks focused on verification of post-mortem arrangement.
| Area of verification | Scope |
|---|---|
| Pathology | Meat is free from all pathological conditions |
Verification results should be assessed by the OV / FVC to monitor team performance. Variables in each establishment should be considered if concerns are raised following verification checks (for example, lighting, available inspection time and plant layout). The OV / FVC should maintain realistic expectations during the checks when assessing team performance based on the product verification results.
Note: In:
- OV only establishments
- poultry establishments with a hybrid post-mortem inspection system (where the OV also undertakes post-mortem inspection along with OAs or PIAs)
- plants with recognised OV flexibility
the effectiveness of FSA’s post-mortem performance should be verified during routine FVC or SDP management visits and documented on the K2 system by FSA / SDP at least every three months. The FVC is accountable for ensuring these checks have been carried out and documented and is responsible for establishing the number of carcases and offal that should be verified during those visits. The verification of FSA’s post-mortem performance should not impact on agreed resource and business agreements as outlined in the Statement of Resources for the individual establishment.
12.4.5 Product – verification of FBO controls
Product verification checks that assess FBO controls focus on evisceration, contamination, ID marking and other deficiencies and are therefore subject to scoring.
The OV / AO is required to record and input into the K2 system:
- the number of carcases and offal found to be non-compliant
- the total number of carcases / offal checked
- the score indicating enforcement action taken (if any)
In addition, the OV / AO is required to verify the ID marking arrangements.
The following table details the scope of product checks focused on verification of FBO controls:
| Area of verification | Scope |
|---|---|
| Evisceration | Carcases fully eviscerated* |
| Faecal / ingesta | Meat is free from faecal / ingesta contamination |
| ID marking | Meat is correctly and legibly ID marked |
| Other | Record any identified deficiency (such as contamination with bile / grease, poor defeathering) |
* Instances where a piece of digestive tract is found inside a carcase should be counted as evisceration failure. However, in those cases, it also should be verified if contamination with faeces / ingesta is visible and if it is found it should also be recorded under ‘Faecal / Ingesta’ contamination.
In cases where frequent and regular problems (such as contamination) are identified by the OV / AO during the product verification checks focused on FBO controls, enforcement action must be taken in accordance with Chapter 7 Enforcement.
12.4.6 Rejected carcase checks
The OV is required to carry out a detailed inspection of a random sample, from each batch of birds having the same origin, of parts of birds or entire birds declared unfit for human consumption following post-mortem inspection on a daily basis. The number of birds checked, and the outcomes, should be recorded in the day book, not in the K2 system.
12.5 Plant – establishment verification
12.5.1 Plant – establishment verification
Establishment verification tasks focus mainly on different parts of the establishment, equipment, cleanliness, hygiene arrangements and procedures.
The minimum frequency of establishment verification tasks depends on the FBO audit outcome. However, the OV can increase the frequency if considered necessary and should always score a relevant section when an intervention takes place that resulted in verbal, written or formal enforcement.
Some establishment verification tasks are considered essential and should be carried out and scored every day, regardless of the audit score awarded.
The following table lists the establishment verification areas and the minimum frequency of checks based on FBO audit outcome.
FBO Audit Outcome
| Establishment verification tasks and their frequency | Improvement necessary / Urgent improvement necessary | Good / Generally satisfactory |
|---|---|---|
| 1. Intake / food chain information | Daily | Daily |
| 2. Ante-mortem arrangements / presentation | Daily | Daily |
| 3. Correlation of carcases and offal | Daily | Daily |
| 4. Operational / break cleaning | Daily | Daily |
|
5. General hygiene Includes verification of hygienic practices (inc staff movement, PPE provisions and practices, hand washing), hygienic facilities provided (inc hot water, soap, sterilisers), door policy and cross contamination controls |
Daily | Daily |
|
6. Handling of carcases / offal during storage and despatch Includes fifth quarter products (inc tongues and feet) |
Daily | Daily |
|
7. Co-products and animal by-products Includes verification of separation of edible and non-edible materials |
Daily | Daily |
| 8. FBO’s pre-operational cleaning | Weekly | Monthly |
|
9. Carcase and offal chilling Includes verification that equipment was emptied, cleaned, and disinfected at least once a day |
Weekly | Monthly |
|
10. Premises Includes verification of lairage / intake area, cleaning and disinfection of crates and modules, processing / dressing area, chillers, packing / packaging storage area, despatch area, plant surrounds, fly screening / vermin entry prevention, control of waste water, drainage and effluent, water testing |
Weekly | Monthly |
12.6 HACCP verification
12.6.1 HACCP verification
The verification of the FBO’s HACCP based procedures is focused primarily on two areas: monitoring of control points (CPs), critical control points (CCPs) and corrective actions.
The OV / AO is not expected to check all records, but must verify a sample to be satisfied that the FBO is:
- following their own procedures for monitoring CPs/CCPs
- taking and recording pre-established corrective actions when the control is lost
| Area of Verification | Scope |
|---|---|
| Monitoring of CPs/CCPs | Monitoring procedures implemented, accurate records that reflect reality maintained (up to date) |
| Corrective Actions | Correct actions taken when monitoring indicates loss of control, such as CPs/CCPs outside limits, as per HACCP plan |
12.6.2 HACCP verification – frequency
The minimum frequency verification of the FBO’s HACCP based procedures is pre-set and linked with the outcome of the last FBO audit. However, the OV can modify the frequency of those checks depending on the outcome or other findings, indicating that the HACCP based procedures are not adequately implemented and / or risks are not sufficiently controlled (for example, high numbers of contaminated carcases found during the process or product verification checks).
The following table specifies the minimum frequency of HACCP verification checks based on the audit score.
FBO Audit Outcome
| Area of verification | Improvement necessary / Urgent improvement necessary | Good / Generally satisfactory |
|---|---|---|
| Monitoring of CPs/CCPs | Weekly | Monthly |
| Corrective actions | Weekly | Monthly |
12.7 Microbiological verification
12.7.1 Microbiological verification
All FBOs are required to comply with current EU law and ensure that meat and carcases in the slaughterhouse are tested in accordance with Regulation (EC) 2073/2005. The OV / AO will verify on a monthly basis if the microbiological sampling is taking place as per the legislative requirements. This includes observing the FBO’s sampling procedures as well as verification of other areas, such as sampling frequency, sample size, parameters tested.
| Area of verification | Scope |
|---|---|
| FBO’s sampling procedures | Microbiological sampling carried out as per legislative requirements (in accordance with (EC) 2073/2005, correct frequency of testing followed, correct sample size |
| FBO’s analysis of results | Results / trends analysed, and action taken when results indicate a problem |
Note: In premises where microbiological testing is done less frequently than monthly, the verification frequency should be adjusted and aligned with that of the FBO’s testing regime.
13. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 1: Post-mortem inspection requirements summary
Annex 2: Sample: Aujeszky’s disease – National Serum Survey submission form
Annex 3: Sample: APHA1 data collection form
Annex 4: Slaughter hygiene checklist
Annex 5: Model document: Health certificate for the trade of unskinned large wild game
Annex 6: Trichinella sampling kit order request form
Annex 7: Sample despatch process
Annex 8: Summary of verification checks and their frequencies
Annex 9: Poultry slaughter hygiene checklist
Annex 12: Aide memoire: Large wild game
Sections
1. Legislation
In this section
1.1 European regulations
1.1.1 Regulation (EC) 178/2002
Article 18 of (EC) 178/2002 requires that FBOs must have systems and procedures in place to ensure that the traceability of food and food-producing animals can be established at all stages of production, processing and distribution.
The FBO must be able to:
- identify any person from whom they have been supplied with a food-producing animal
- identify the other businesses to which their products have been supplied
- make this information available to the Competent Authorities on demand
1.1.2 Regulation (EC) 853/2004
The EU Regulations require the FBO to ensure that all animals or, where appropriate, each batch of animals sent for slaughter is identified so that their origin can be traced.
Reference: (EC) 853/2004, Annex III, Section I, Chapter IV, Article 3
1.1.3 Regulation (EU) 2019/627
During the exercise of official controls, the OV is to verify compliance with the FBOs duty pursuant to (EC) 853/2004 to ensure that animals accepted for slaughter for human consumption are properly identified.
The OV is to ensure that animals whose identity is not ascertainable are killed separately and declared unfit for human consumption.
Whenever the OV considers it necessary, official controls are to be carried out on the holding of provenance.
Reference: (EU) 2019/627 Article 43(1).
1.2 Domestic regulations
1.2.1 Domestic legislation
Staff should note that not all of the relevant legislation has been included in Volume 2 of the MOC.
Additional domestic legislation, detailing requirements for animal identification and movement, is available on the Defra website or via the National Archives website as detailed below. This may be of use for reference or to determine if an offence has been committed requiring referral to a Local Authority for enforcement.
Note: There are variations in the legislation applicable in England and Wales and OVs must ensure that they are aware of the legislative requirements applicable in the establishment concerned.
Reference: DEFRA or Legislation.gov
2. Animal identification
In this section
2.2 Cattle ear tag requirements
2.3 Ear tags in imported cattle
2.5 Cattle passport requirements
2.6 Examples of cattle identification documents
2.9 Identification requirements
2.1 Introduction
2.1.1 Cattle identification regulations 2015 (CIR)
The Cattle Identification Regulations 2015 (CIR) (as amended) and the Cattle Identification (Wales) Regulations 2007 (as amended) enforce the requirements of European legislation for identification and registration of bovine animals. Predominantly, this is Regulation (EC) 1760/2000.
CIR provide powers to the competent authorities and detail requirements on keepers with respect to:
- notification of holdings
- ear tags
- registration of cattle
- cattle passports
- notification of movements or death
- record keeping
2.1.2 Definition: Keeper
The keeper is the person responsible for the animals whether on a permanent or temporary basis. It includes slaughterhouse operators, market operators and transporters in some contexts.
2.1.3 Enforcement
The LA Trading Standards staff are the primary enforcement officers for CIR, with a role to advise, educate and enforce the requirements of the Regulations.
2.2 Cattle ear tag requirements
2.2.1 Ear tags: GB cattle
All cattle born and imported into GB must be tagged in at least one ear. Cattle born from 1 January 1998 should have a tag in each ear. There are requirements in CIR 2007 that detail the time periods within which keepers must apply ear tags to cattle and replace lost tags.
Cattle must be tagged properly to be moved.
Reference: CIR 2007, Schedule 1.
2.2.2 Single tagging
Cattle born between 1 April 1995 and 31 December 1997 must be identified with at least one ear tag.
2.2.3 Double tagging
Cattle born from 1 January 1998 must be identified with an approved ear tag in each ear, which show the same official identity.
One of these ear tags is considered the primary ear tag and the other, the secondary ear tag.
If the ear tag is made from two pieces, both sides must be printed and bear the Crown logo.
2.2.4 Primary ear tag
The main ear tag, known in GB as the primary ear tag, is a distance readable yellow plastic two-piece ear tag which requires specific information.
2.2.5 Information required on primary tag
Crown logo, followed by the letters ‘UK’ and the animal’s unique number, which will consist of a six digit all numeric herd mark followed by a six-digit unique animal code. The first digit of the animal code is a check digit to allow officials to check the code is correct, for example, UK 230011 200123.
Note: This information will always be printed not hand written.
Note: Crown logo on ear tags became a requirement on 1 January 1998.
2.2.6 Primary tag: option 1
There are two options for the primary ear tag.
Option 1

2.2.7 Primary tag: option 2
Option 2 is recommended for small-eared breeds (for example, Channel Island breeds, Dexter breeds) and meets the minimum size requirements for the primary ear tag.
Option 2


2.2.8 Secondary ear tag
This ear tag can be the same design as the main ear tag or an approved alternative in a different colour. It should be placed in the other ear to the primary tag, unless an ear is damaged, when they can be fitted to the same ear.
Management information concerning the animal may be added to the lower part of the ear tag.
2.2.9 Information required on secondary tag
Crown logo, followed by the letters ‘UK’ and the animal’s unique number, which will consist of a six digit all numeric herd mark followed by a six-digit unique animal code. The first digit of the animal code is a check digit to allow officials to check the code is correct UK 230011 200123.
Note: This information will always be printed not hand written.
Note: The secondary tag can also contain management information which can be printed or hand written.
2.2.10 Secondary tag: option 1
Examples of second ear tags are as follows:

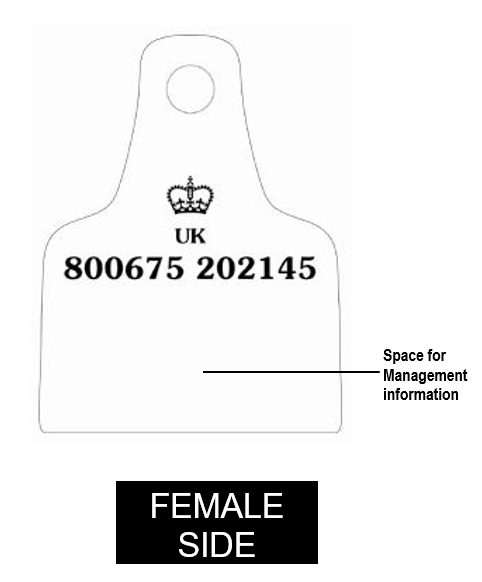
2.2.11 Secondary tag: option 2
This is a small plastic two-piece ear tag, which may be any colour.
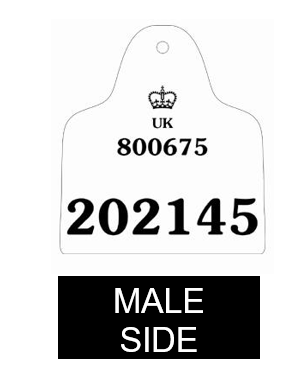

2.2.12 Secondary tag: option 3
The button ear tag is a round two-piece plastic button design, which may be any colour.

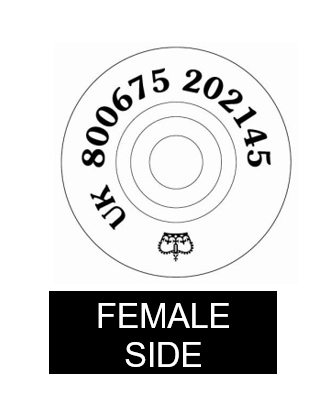
2.2.13 Secondary tag: option 4
The metal ear tag is a one-piece design.

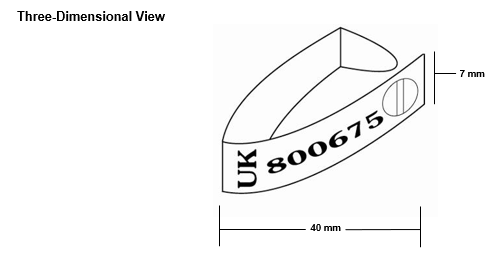
2.2.14 Ear tag requirements
The following table shows the cattle identification requirements in force at various dates of birth.
| Date of birth | Tagging requirements | Example of format |
|---|---|---|
| 15 October 1990 to 1 April 1995 | Single ear tag or tattoo |
A1234 123 B654 3210 D123 123C |
| 1 April 1995 to 31 December 1997 | One ear tag in right ear with unique alpha-numeric identity including UK prefix |
UKAB 1234 56789 UK A 1234 56789 |
| Presentation for slaughter / slaughter of cattle born in, or imported into, the UK before 1 August 1996 for human consumption is prohibited) | Presentation for slaughter / slaughter of cattle born in, or imported into, the UK before 1 August 1996 for human consumption is prohibited) | Presentation for slaughter / slaughter of cattle born in, or imported into, the UK before 1 August 1996 for human consumption is prohibited) |
|---|---|---|
| 1 January 1998 to 31 December 1999 |
Double tagging with same alpha-numeric identity including UK prefix in each ear, for lifetime of the animal |
UKAB 1234 56789 UK A 1234 56789 |
| 1 January 2000 to 30 June 2000 |
Double tagging with same alpha-numeric or numeric identity including UK prefix in each ear, for lifetime of the animal |
UKAB 1234 56789 UK A 1234 56789 UK 666666500046 |
| 1 July 2000 to present | Double tagging, with same numeric identity including UK prefix in each ear, for lifetime of the animal | UK 666666500046 |
2.2.15 Unacceptable official identification
The following other forms of identification cannot be accepted as official identification:
- hand written tags
- tattoos
- printed tags without a country code
- tags where the code appears to have been amended or tampered with (except the addition of management information to secondary ear tags)
- tags with missing information, for example, one missing one number
- unreadable ear tags
2.3 Ear tags in imported cattle
2.3.1 Ear tags: EU cattle
Cattle imported from EU member states and presented for slaughter for human consumption must be identified with a printed ear tag in each ear which shows the same official identity (double tagged) bearing:
- the country logo
- the country code
- an official identity of not more than 12 digits (which identifies the holding of origin and the animal)
This will also apply if cattle have been imported to an EU member state from a third country for onward trade with the UK. They should have been tagged with the importing member state’s tags.
Note: If there is any doubt regarding the validity of tags, further advice should be sought from your FVC.
Reference: See topic 2.4 on ‘List of country codes’ in this section for additional information.
2.3.2 Ear tags: third country cattle
Cattle imported from third countries which go direct to slaughter (within 15 days of arriving in the UK) are identified in accordance with third country rules. They must be accompanied by a veterinary certificate. They will have ear tags and national administrative documents of the country of origin.
Otherwise cattle must be re-tagged within 20 days of passing the veterinary checks and the farmer must apply for a passport within 15 days of arriving.
Re: CIR 2007, Schedule 1, Paragraph 9
Note: Cattle imported from third countries will have UK or other EU Member State’s tags, and the date of import will be shown on the passport. This is the only place where the origin of the animal can be identified, so it is important to examine every passport that is checked, as the origin of the animal may have important consequences for BSE controls.
2.3.3 Ear tags: Northern Ireland cattle
Cattle imported from Northern Ireland and presented for slaughter for human consumption must be identified with a printed ear tag in each ear showing the same official identity (double tagging) bearing:
- unique NI logo (pictured)
- UK prefix
- official identity of 12 digits

Note: All cattle imported into GB from NI must be registered with British cattle movement service (BCMS) (unless slaughtered within 15 days of arrival into GB). They must only be moved to an approved slaughterhouse is accompanied by a cattle passport issued by BCMS. Single tagged cattle must be retagged within 15 days of arrival into GB.
Reference: CIR 2007 Schedule 1 paragraphs 8 and 11
EC 1760/2000 article 4 (1)
2.4 Country codes
2.4.1 Europe
The table below lists the codes for each EU member state:
| Country | Code | Country | Code |
|---|---|---|---|
| Austria | AT | Latvia | LV |
| Belgium | BE | Lithuania | LT |
| Bulgaria | BG | Luxembourg | LU |
| Cyprus | CY | Malta | MT |
| Czechia | CZ | Netherlands | NL |
| Denmark | DK | Poland | PL |
| Estonia | EE | Portugal | PT |
| Finland | FI | Romania | RO |
| France | FR | Spain | ES |
| Germany | DE | Sweden | SE |
| Greece | EL | Slovakia | SK |
| Hungary | HU | Slovenia | SI |
| Ireland | IE | Italy | IT |
Note: Cattle imported from the Channel Islands and Isle of Man have UK ear tags with different logos.
2.4.2 Third countries
Codes for countries outside the EU are contained in the ISO 3166 online browsing platform.
2.5 Cattle passport requirements
2.5.1 Overview
All cattle born or imported into GB from an EU or third country since 1 July 1996 must be registered with BCMS (unless slaughtered within 15 days of arrival into the UK.) They must only be moved to an approved slaughterhouse if accompanied by a cattle passport issued by BCMS.
2.5.2 Passport types
There are five types of official cattle identification documents for cattle in GB.
The table below shows the type of passport or other identification document issued, dependant on the date of birth or import.
Note: Imported cattle must be accompanied by official documentation.
Reference: See sub-topics 2.5.7 and 2.5.8 in this section for additional information.
| Date of birth / import | Document |
|---|---|
| Before 1 July 1996 |
|
| 1 July 1996 to 28 September 1998 |
|
| 28 September 1998 to 31 July 2011 |
|
| From 1 August 2011 |
Note: Movement cards will not be supplied with this type of passport. Keepers must notify cattle movements using CTS Online or via a self-service telephone line. Note: Unless imported less than 15 days previously, cattle (including calves) can only be accepted for slaughter if they have a full passport. |
| For cattle refused a cattle passport |
|
Note: Presentation for slaughter / slaughter of cattle born, or imported into, the UK before 1 August 1996 for human consumption is prohibited
2.5.3 Passport details
From 1 August 2011, the GB passport is the CPP52 single A4 sheet, which shows the following information:
- animal details: date of birth, sex, breed or colour of coat
- official identification number as printed on the ear tag
- identity of genetic or surrogate dam
- holding of birth
- date the passport was issued and reissued
- movement summary: identity of locations and dates of change since the passport was issued
Reference: (EC) 1760/2000 Article 6 (1)
2.5.4 Valid passport
A valid passport has:
- all registration details complete
- an ear tag number matching the ear tags on the animal
- address details of the most recent holdings the animal has moved through, up to a maximum of 6 holdings (the full history will be available via CTS)
- entries which have been signed and dated by each keeper of the animal
- no sign of having been tampered with or amended in any way
- a heat-sensitive diamond shape which will fade when held between finger and thumb (security feature in the bottom right-hand corner)
In all cases, the original documents must be presented with the animal. Photocopies of documents are not acceptable.
Exception: It is acceptable to slaughter an animal on welfare grounds without valid documentation. However, the carcase must not be health marked until receipt of the correct original valid passport.
2.5.5 Valid NI passport
There are no passports in NI. Every animal is recorded on a central database, the Animal and Public Health Information System (APHIS). Normally a print-out of the database containing the animal’s information, movement history and statuses of this particular animal as held on the APHIS database is produced instead of a passport.
Animals are moved within NI on what are called: ‘owners’ declarations’. These can be either hand-written or electronic based on the level of IT knowledge and application at the holding. However, when animals are moved ‘outside’ NI (and the APHIS system) the print-out will accompany the animal. This information has been produced directly from APHIS for the purpose of moving the animal into GB.
All the information is entered on to the APHIS system at the different stages of the animals’ life time and this is centrally stored for access at any stage (only through secure access profiles). APHIS also holds all testing information, ante mortem inspection and post mortem inspection results (when the animal is slaughtered in NI).
Cattle born in NI before September 2008 may have ear tags mismatching against the owners declaration / APHIS print out. This is due to zeros being added to the passport number to ensure that it has 14 characters.
This means that ear tags and passport numbers may not match as the ear tags will still show the ‘old number’ of less than 14 characters while the document shows the 14 characters with zeros included on the herd number and individual number.
2.5.6 Passport: stamped ‘not for human consumption’
There may be circumstances, for example, when cattle have been fed mammalian protein, when a decision is made to prevent certain cattle from entering the food chain.
The passports of any such animals of all ages will be stamped clearly in blue ink ‘Not for human consumption’ and the information retained by BCMS in their central records.
If any such animal is presented in a slaughterhouse the OV must notify:
- Regional Veterinary Manager, APHA, Worcester (01905 763355)
- the local APHA office
- LA Trading Standards Department
- BCMS
Under no circumstances may these animals be slaughtered for human consumption and their carcases must not enter the food chain.
2.5.7 Cattle from EU member states
All cattle imported from another EU Member State or Northern Ireland and sent direct for slaughter must be accompanied by:
- a passport issued by the Member State (an EU passport)
- an export health certificate
- a Permit Authorising Movement of Cattle (MC2L) issued by DAERA (animals from NI only).
Passports issued by EU Member States vary in style.
Example: They can be a computer printout. They may be titled ‘Movement Licence’ or an equivalent description.
Reference: See sub-topic 2.6.7 on ‘Example of a Dutch cattle passport’ subsequently presented in this topic for additional information.
Important: Keepers of imported cattle not slaughtered within 15 days of arrival into GB must obtain a passport from BCMS. The country of origin, date of import and import health certificate number is shown on the front of the CPP52 single sheet passport, or on the inside back cover of the CPP13 cheque book style passport.
2.5.8 Cattle from third countries
A GB passport will accompany animals imported since 1 July 1996 from third countries, unless they are presented for slaughter within 15 days of import.
Animals imported direct for slaughter within 15 days of arrival must be accompanied by an export certificate and must be clearly identified.
2.6 Examples of cattle identification documents
2.6.1 CPP-1
This is an old style passport or CPP-1. It was issued from 1 July 1996 until BCMS started issuing cheque book style passports in September 1998.

2.6.2 Certificate of CTS registration (COR)
This is a Certificate of CTS Registration or COR. They were issued to cattle that were born before 1 July 1996 (when passports were introduced) and animals which also have an old-style passport (CPP-1). These animals are not eligible to be slaughtered for human consumption.

2.6.3 Notice of registration (CPP35)
This is a Notice of Registration (CPP35) and is issued for animals that have been refused a passport. These animals are not eligible to be slaughtered for human consumption.

2.6.4 CPP13
This is a chequebook-style passport (CPP13). BCMS issued these for animals born or imported between 28 September 1998 and 31 July 2011. These were also issued when keepers sent their old-style passports (CPP-1) and certificates of registration (COR) for amendment. This means that some cattle born in or imported into the UK before 1 August 1996 may have a cheque-book style passport because the original identification document has been replaced. These cattle with replacement identification documents are not eligible to be slaughtered for human consumption.

2.6.5 CPP52
This is a single page passport, A4 size, which has been issued by BCMS since 1 August 2011. All passports issued or re-issued since then will be this new style document. BCMS do not intend to recall any previously issued passports, but if a passport is returned for any reason, for example for correction or for extra pages to be added, then a single page passport will be issued as a replacement.
Cattle movement cards are no longer provided with these passports. Keepers of cattle with this single-sheet type of passport will be required to notify cattle movements using the CTS Online website or via a self-service telephone helpline.
Non-bovine imported: front page

Imported bovine: front page
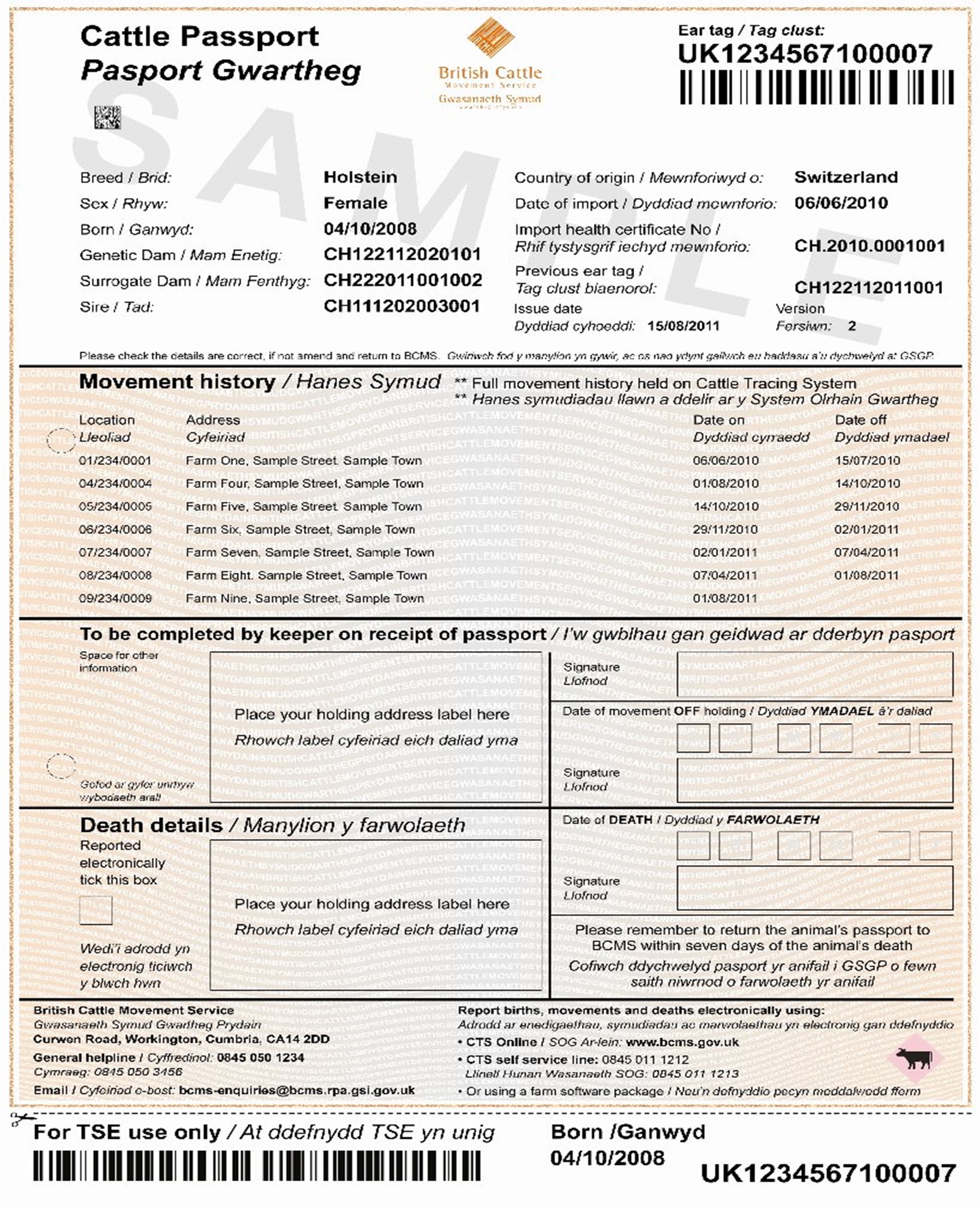
Reverse of page
2.6.6 Example ‘not for human consumption’ stamped passport


2.6.7 Example of a Dutch cattle passport

2.6.8 Example of an Irish cattle passport
Front of document

Reverse of document
2.7 Cattle age requirements
2.7.1 Prohibition on older cattle
The sale of meat derived from cattle born in, or imported into, the UK before 1 August 1996 is strictly prohibited. It is also an offence to consign such an animal to a slaughterhouse. Any meat from such animals is automatically deemed to be an ABP.
Legislation:
- Commission Decision 2007/411(EC), prohibiting the placing on the market of products derived from bovine animals born or reared within the UK before 1 August 1996.
- the TSE (England) Regulations 2010, Schedule 2, paragraph 7
- the TSE (Wales) Regulations 2008, Schedule 2, paragraph 2
2.7.2 Bovines requiring Bovine Spongiform Encephalopathy (BSE) testing
Requirements regarding BSE testing differ according to the age and origin of the bovine.
Reference: Full details of the requirements are located within chapter 2.6 on ‘Transmissible Spongiform Encephalopathy testing’, section 2.
2.7.3 FBO identification responsibilities
FBOs must ensure that they can identify:
- cattle born in, or imported into, the UK before 1 August 1996 and other cattle ineligible for the food supply
- cattle that require BSE testing
- cattle aged over 30 months of age (OTM) carcases must be despatched to a cutting plant authorised to remove OTM bovine vertebral column (VC) SRM
Reference: Guidance on SRM is contained in chapter 2.7 on ‘Specified risk material’.
2.8 Sheep and goats
2.8.1 Background
The identification of sheep and goats arriving at the slaughterhouse involves two separate areas of legislation, namely the EC Hygiene Regulations and the sheep and goats (records, identification and movement) orders (SAGRIMO) Order enforcing the Council Regulation (EC) 21/2004.
The Hygiene Regulations are directly enforceable by the FSA, whereas breaches of SAGRIMO involve Defra and are reported to the LA who will then take appropriate action and report findings to Defra.
New electronic tagging requirements for sheep and goats came into force on 1 January 2010 and the new requirements, as well as historic ones, are all available in Defra guidance. The guidance is available on Defra’s website keeping sheep and goats.
Under the Hygiene Regulations, sheep and goats accepted for slaughter must be properly identified in such a way that their origin can be traced.
2.8.2 Legislation
The following table contains the key pieces of legislation relating to sheep and goat identification:
| Regulation | Responsibilities |
|---|---|
| (EC) 853/2004 | FBO |
| (EU)2019/627 6272017 | Authorised Officers to verify FBO compliance |
| (EC) 21/2004 | The Regulation is directly applicable. However, there is also domestic enforcing legislation; the Sheep and Goats (Records, Identification and Movement) Order 2009 (SAGRIMO) and equivalent legislation in Wales- see page 2-44 in this section for ID requirements under SAGRIMO. |
2.9 Identification requirements
2.9.1 What is ‘properly identified’?
Under Regulation (EC) 853/2004, the FBO may accept for slaughter only animals that are properly identified. For the purposes of the Hygiene Regulations, ‘properly identified’ means identified in such a way that the farm or holding from which a sheep or goat was sent for slaughter can be traced.
This information should be shown on the movement document which accompanies the animals and it should identify the animals in such a way that it enables them to be related to the food chain information (FCI). FCI should either be provided on the movement document or be provided separately.
Additionally, sheep can be deemed to be properly identified within the requirements of SAGRIMO if the animal bears an ear tag showing the farm where the animal was born in accordance with those requirements.
A judgement as to whether an improperly identified animal’s identity is still ‘ascertainable’ has to be made by the OV (in accordance with (EC) 2019/627, Article 43 (1).
2.9.2 What is ‘ascertainable’?
The identity of a sheep may be considered ‘ascertainable’ if it can be traced back to its last holding.
Difficulties may arise from loss of a tag or from lack of correlation with the information on the movement document or in the FCI.
2.9.3 FBO controls for sheep and goat identification
The table below describes the FBO responsibilities and controls to ensure that sheep and goats are ‘properly identified’ before slaughter, depending on whether the animals come directly from a farm or a livestock market.
| Animals transported | FBO responsibilities and controls |
|---|---|
| Directly from farm |
The FBO, under the requirements for HACCP based procedures, should have a system to check that all sheep accepted for slaughter are properly identified. The FBO should also check that the movement document is completed and shows the correct number of sheep in the batch and where required, records animals’ individual identities and that FCI details have been completed appropriately. Under the requirements of SAGRIMO, the FBO should check that all sheep are tagged and correspond to the movement document. This system should be agreed with the OV and include a system of notification of arrival of animals in the slaughterhouses to the OV, taking account of the operating practices of the plant. |
| From livestock market |
Sheep may be consigned to the slaughterhouse in a composite group comprising animals from many different farms. Slaughterhouse FBOs should have a system to check that all sheep accepted for slaughter are properly identified (as above). The FBO should check that the movement document is completed and shows the correct number of sheep, either individually recorded or batch recorded. Under the requirements of SAGRIMO, where a batch of ‘slaughter’ animals (lambs intended for slaughter before the age of 12 months) is received, which originate from more than one holding, the slaughterhouse must record the mix of identities in the batch in their holding register, by recording each of the batch numbers together with the corresponding number of animals originating from each holding. This information is not needed on the movement document, but some slaughterhouses may ask for this information to be included so they do not have to compile it themselves. This system should be agreed with the OV and include a system of notification of arrival of animals in the slaughterhouses to the OV, taking account of the operating practices of the plant. Any discrepancies should be reported to the OV. Factors to be considered by the FBO in determining the size of the proportion of a consignment to be checked include:
Checks on tagging and proper identification may be carried out:
Note: Division of responsibility between the operators of markets and slaughterhouses is a commercial matter between the parties. Slaughterhouse operators should have a written description of the system employed, and should have a procedure for checking the system. for details of Central Point Recording Centres and Critical Control Point (CCP) Systems. |
2.9.4 OV actions
It is the responsibility of the OV (Regulation 2019/627) to verify that the FBO is compliant with the requirement that animals accepted for slaughter are properly identified. They must also ensure that animals whose identity is not ascertainable are killed separately, declared unfit and disposed of in accordance with ABP legislation. FCI must also be checked by the OV (see Chapter 2.1 of the MOC for further guidance on FCI).
All sheep should be identifiable by means of an official identifier. Loss of tags is a recognised problem in sheep, and tags may be lost between the farm or market and the slaughterhouse. When sheep without tags are delivered to the slaughterhouse, and the lack of identification cannot be considered to be the result of loss of tags since leaving the farm (for example, significant numbers of sheep, or no physical evidence of having been tagged), they should not be accepted for slaughter for human consumption.
Details of animals presented for slaughter with a single slaughter tag after 30 June in the year following the lambing season, which you believe are older than 12 months of age and should therefore be identified with double tags, should be recorded and made available to the LA on request.
Details of the consignor’s non-compliance (NC) with SAGRIMO must be reported to the LA.
The following table describes OV actions to be taken, depending where the animals come from:
| Animals from | OV action |
|---|---|
| Multiple pick-up transport directly from farms |
The batch identity of sheep from more than one farm, transported directly to the slaughterhouse on one vehicle, will usually be maintained by separate penning and unloading. Alternatively, temporary marks (for example, paint marks) may be used for batch identification to overcome problems arising from loss of tags. However, where a significant number of sheep are not tagged, and there is no physical evidence that they had been tagged, the identity of such sheep is not ascertainable. They should not be slaughtered for human consumption, but should be killed separately, disposed of as unfit and the details reported to the LA. Note: Temporary marks are an adjunct to proper identification, and do not remove the requirement for compliance with the Hygiene Regulations. Provided the batch identity has been maintained during transport, any minor discrepancies between ear tags and the information on the movement document(s) may not prevent slaughter for human consumption, but must be reported to the LA. |
| Transported from livestock market |
In the case of a sheep in a composite consignment from a market which has a system for checking tags at the market, the incident of a missing tag can be reasonably interpreted as a genuine loss of a tag since leaving the market. In such circumstances, its identity can be considered to be ascertainable, and it can be accepted for slaughter for human consumption. If significant numbers of sheep were found to be unidentified at the slaughterhouse and there was no physical evidence that they had been tagged, this would be a clear indication of failure to carry out checks at the market and to comply with a formal, agreed procedure. Where identity of such sheep could not be ‘ascertainable’, they are not permitted to be slaughtered for human consumption, must be reported to the LA and disposed of as unfit. |
2.9.5 Movement of sheep: Animal Movement Licence
Sheep moving to slaughter require a completed Animal Movement Licence – AML1 form (England and Wales) or a Scottish movement document – which specifies:
- the address, including the postcode, and county parish holding (CPH) number of the holdings from where and to where the sheep is being moved (Scottish forms will have the address of the keeper only on the forms which will not necessarily relate to the CPH)
- the date the movement in taking place
- the number of sheep the document covers
- from 1 January 2011, the numbers of individually identified sheep born after 31 December 2009
Note: These animals will need to have their individual identification numbers recorded on the movement document (attached lists are acceptable). Information about mixed batches is not required but may be supplied. Individual ‘off movement’ (farm to abattoir) information will not be included where the slaughterhouse is acting as a Central Point Recording Centre on behalf of the keeper – this will be indicated by means of a tick box on the movement document.
- FCI declaration (England and Wales only)
The form must be retained for at least three years by the FBO, who must also send a copy to the LA within three days of the arrival of a sheep.
The Livestock Information Service:
The Livestock Information Service (LIS), launched in 2022, is used to record movement data for sheep, goats and deer by users in the supply chain, including farmers, markets and slaughterhouses in England.
The LIS enables suppliers to record sheep, goat and deer movements online (LIS-1 and LIS-2 forms), replacing the paper version of the movement document.
The information included in a LIS movement document is the same as in the paper version, including the Food Chain Information (FCI) and the Veterinary Attestation Number (VAN).
The LIS movement document and FCI report should be available for the OV to check and analyse all the relevant information regarding the animals accepted for slaughter.
When mistakes or missing information are identified, the FBO must ensure that the issue is addressed with the supplier as it occurs with the paper versions.
2.9.6 Recording movements: movement document
Moves can be recorded and reported in the movement document in two ways – individual recording or batch recording as detailed below.
For animals individually identified after 31 December 2009, the movement document must include individual ID numbers.
Exceptions where movements can continue to be batch recorded are:
- animals intended for slaughter within 12 months of age (identified with electronic or non-electronic slaughter tags)
- electronic (ID) animals (double tagged including one electronic ID identifier) moving to an approved central point recording centre.
2.10 Identification requirements under SAGRIMO
2.10.1 ID requirements
Sheep and goat identification and movement rules are based on the principle that each sheep should bear a tag or tags which correlates with the requirements of the Sheep and Goats (Records, Identification and Movement) (England) Order 2009 (and its Welsh and Scottish equivalent legislation) (SAGRIMO).
A sheep officially identified after 31 December 2009 will comply with SAGRIMO if it has one single tag, which can be an electronic slaughter tag or a non-electronic slaughter tag (slaughter animals) and is also accompanied by appropriate documentation. This should be in the form of a movement document made under the 2009 order, from the last holding from which it has moved (or the market from which it was consigned).
Goats will comply with SAGRIMO if they have one single tag (slaughter) or two conventional identifiers. Additionally, they may also be electronically identified, in which case one of the identifiers must be electronic. As for sheep, they need to be accompanied by the same appropriate documentation.
Sheep or goats bearing single tags must be under 12 months of age. It can be difficult to identify whether an animal is less than 12 months of age and therefore correctly identified. For example, a lamb presented for slaughter in May 2011 may have been born any time between December 2009 and May 2010. Given the difficulties of ageing slaughter lambs, Defra recommends a pragmatic approach to enforcement and a single tagged lamb slaughtered before 30 June of the year following their birth cohort can be accepted as compliant with SAGRIMO. In the case of single tagged sheep presented for slaughter after 30 June, which appears to have been born in the previous year’s cohort, the FBO should query their age and whether their identification complies with SAGRIMO (should they be double tagged) and report this to the OV.
The FBO should keep details of consignments presented for slaughter which they suspect includes animals older than 12 months. This information should be made available to the LA on request.
Sheep bearing tags applied under previous requirements (prior to 31 December 2009) will continue to be presented for slaughter for some considerable time in the future. Judgements as to whether they comply with SAGRIMO should be made having regard to the requirements of the legislation at the time. See Defra guidance on sheep tagging requirements.
2.10.2 Breaches of SAGRIMO
In cases where the OV considers that a breach of SAGRIMO has occurred, they should refer the matter to the LA.
2.11 Pigs
2.11.1 Regulations
- England – The Pigs (Records, Identification and Movement) Order 2011.
- Wales - The Pigs (Records, Identification and Movement) (Wales) Order 2011.
All pigs arriving at the slaughterhouse should be identifiable by means of an animal identification herdmark.
2.11.2 Requirements of the pigs' identification
The occupier of a holding who begins to keep pigs on that holding, must notify the competent authority (APHA) who will issue a herdmark.
All pigs arriving at the slaughterhouse should be properly identified with their herdmark by means of an identification mark.
2.11.3 Types of animal identification for Pigs' identification. In England, Scotland and Wales identification may be through any of the means listed in the table below:
| Type | Notes |
|---|---|
| Slapmark: England and Wales | Applied to both shoulders, showing the keeper’s herdmark. Size is not specified; however, the slapmark must be legible before and after slaughter, throughout processing. |
| Slapmark: Scotland | Applied to one shoulder (1), showing either the keeper’s herdmark or an alpha numeric slapmark allocated to the keeper by one of the Scottish marketing or processing groups (2). |
| Ear tag: England, Wales and Scotland |
Ear tags (in one ear) must be:
|
| Tattoo | Tattoo of the herdmark on one ear. If desired, the other ear may have an individual number and / or management information. Size is not specified, but the tattoo must be legible before and after slaughter and throughout processing. |
- Some processors require a slapmark applied to each shoulder of the pig. The keeper should check with the processor in advance of sending pigs, to conform their requirements.
- There are currently 3 groups; Vion (processing group), Scottish Pig Producers and Scotlean (marketing groups).
2.11.4 Imported pigs
Pigs imported from outside the EU must be identified at the destination holding with an ear tag or tattoo containing the letters ‘UK’ followed by the herdmark of the herd into which the pig is introduced and the letter ‘F’ unless the pigs are delivered directly to slaughter.
2.11.5 What is 'properly identified'?
Under Regulation (EC) 853/2004, the FBO may accept for slaughter only animals that are properly identified. For the purposes of the Hygiene Regulations, each batch of pigs sent for slaughter must be identified so that their origin can be traced.
Information regarding identification of the pigs should be shown on the movement document which accompanies the animals and it should identify the animals in such a way that it enables them to be related to the food chain information (FCI). FCI should be provided on the movement document (eAML2) as detailed in point 2.11.7 or as a separate document.
A judgement as to whether an improperly identified animal’s identity is still ‘ascertainable’ has to be made by the OV (in accordance with (EC) 2019/627, Article 43 (1).
2.11.6 What is 'ascertainable'?
The identity of a pig may be considered ‘ascertainable’ if it can be traced back to its last holding.
Difficulties may arise from loss of a tag, unclear slapmarks/tattoos or lack of correlation with the information on the movement document or in the FCI. If a pig (or a batch of pigs) cannot be traced back to the last holding, or if the OV suspects any fraudulent activity, the pig (or the batch of pigs) has to be killed separately, declared unfit for human consumption and disposed of in accordance with the animal by-products legislation.
Inadequate identification issues found in a batch of pigs at the slaughterhouse, must be reported by the OV to APHA.
2.11.7 Movement of pigs: England and Wales
Since 1 April 2012, pig keepers in England and Wales are required to report movements (including pigs from Scotland) using the electronic AML2 online system (eAML2) operated by the British Pig Executive (BPEX).
To be legally compliant, pig movements must be reported through the eAML2 online system or by contacting the eAML2 free-to-use bureau service.
In the case of pigs going to slaughter, the pigs may be processed as normal, provided satisfactory FCI is presented to the OV. No further involvement from FSA is required.
https://www.gov.uk/guidance/moving-pigs-what-keepers-need-to-know
2.11.8 eAML2 system
This system captures the following information:
- the address, including postcode, and CPH number of the holdings from where, and to where, the pig is being moved
- the date the movement is taking place
- the number of pigs being moved
- the identification herdmark of the pigs
- pig movements from a market, the lot numbers of the pigs being moved
2.11.9 FBO responsibility: confirmation of move
The FBO should ensure that all details of the movement are notified within 3 days of the movement taking place.
For IT enabled abattoirs, the FBO should confirm receipt of the animals electronically via the eAML2 system (or via FBO in-house software if integrated with eAML2).
FBO establishments without IT should confirm the move via phone.
eAML2
Email: eaml2@ahdb.org.uk
Telephone: 0844 335 8400
Monday to Friday, 9am to 5pm
If the movement is notified by phone, the FBO should get 2 copies of the movement document from the haulier and use these to report the move to eAML2 by phone. The FBO must keep a copy of the document for 6 months.
The local authority is the enforcing authority for this requirement; any non-compliance will be reported to them.
2.11.10 Movement of pigs to or from Scotland
In Scotland, with effect since 1 December 2011, details of pigs moving to slaughter should be notified to the ScotEID movement reporting database electronically, by telephone or in writing. The notification must specify the following information:
- the address, including postcode, and CPH number of the holdings from where and to where the pigs are being moved
- the date the movement is taking place
- the number of pigs moved
- the identification herd mark of each pig moved
- in the case of pigs moved from a market, the lot number of the pigs being moved
The FBO must check and confirm receipt of the pigs within 3 days of arrival to ScotEID* by one of the movement notification methods mentioned above.
*Note: This also applies to pigs being moved from England and Wales for slaughter in Scotland.
2.12 Deer
2.12.1 The TB (Deer) Order 1989 as amended
Farmed deer must be uniquely identified with an official ear tag, if they have been tested for TB or before they leave the farm of origin.
The tag must show either the Defra herd number, or British Deer Farmers Association (BDFA) herd registration number and the animal’s own unique number.
The letters UK must go before the Defra herdmark, for example, UK AB1234 000001.
2.12.2 Regulation (EC) 853/2004
(EC) 853/2004, Annex II, Section III applies the same identification rules to farmed game as to other red meat animals.
It is required that a declaration by the FBO who reared the animals, stating their identity and indicating any veterinary products or other treatments administered, dates of administration and withdrawal periods, accompanies the slaughtered animals to the slaughterhouse.
2.13 Horse identification requirements
2.13.1 Definition
The term ‘horse’ used throughout the MOC means any wild or domesticated soliped mammals of all species within the genus Equus of the family Equidae, and their crosses.
2.13.2 Regulations
Commission Implementing Regulation (EU) 2015/262
The Equine Identification (England) Regulations 2018
The Equine Identification (Wales) Regulations 2019
2.13.3 Horse identification requirements
There are two elements to horse identification: a single lifetime identification document commonly known as “passport” and an element of identity verification that ensures a unique link between the passport and the horse which is a transponder or “microchip”.
There are derogations concerning the identification of certain horse populations living under wild or semi-wild conditions which will be specifically covered later in this chapter.
There are numerous organisations authorised to issue horse passports. In the UK they are called Passport Issuing Organisations (PIOs) and these may be:
- organisations that do not manage a studbook but authorised to issue horse passports
- organisations that manage studbooks and are authorised to issue horse passports.
PIOs are authorised and regulated by Defra. When a PIO is no longer authorised to issue or update horse passports, passports issued by this organisation whilst they were authorised remain valid and another PIO takes over administration of these passports; the details of both PIOs are recorded in the list below. As part of their requirements, these organisations have a duty to facilitate information to the enforcement authorities and that includes OVs. Defra maintain the list of PIOs, it can be accessed online.
The ‘Equine Identification Booklet’ produced by Weatherby’s, RCVS and BEVA can be found in Annex 4 to this chapter. This booklet was produced to create a system of description of horses for the purpose of identifying individual animals. It is valuable reference material for understanding horse descriptions and horse silhouettes.
Microchips became a compulsory identification element on 1 July 2009 for horses identified for the first time after this date. From this date on it became a legal requirement that, at the time the horse is identified for the first time, the animal is actively marked by the implantation of a microchip.
Some microchips inserted prior to 2009 may not be recorded in the passport. However, if inserted and recorded in the passport it becomes part of the horse’s official identification and must be treated as such.
In any case, horses that are eligible for slaughter for human consumption must have any microchip safely removed before the carcase may be passed as fit for human consumption regardless of when it was implanted and recorded or not in the passport.
Domestic Equine Identification Regulations (England-2018 and Wales-2019) introduced compulsory retrospective implantation of microchips on horses born 30 June 2009 and before. The requirement would apply to equines with a passport from one PIO based in the relevant country. The deadlines for retrospective microchipping in each country are:
England: all equines to be microchipped by 1 October 2020.
Wales: all equines to be microchipped by 12 February 2021.
Similar legislation will come into effect for Scotland on 28 March 2021, but not for Northern Ireland.
The following sections below highlight the changes introduced by the different legislations grouped under the date in which they became compulsory:
Before 1 July 2009
- Horses born after January 1998 and which were voluntarily registered in breed studbooks or for competition or racing were required to have a passport.
- European legislation extended the requirement to all horses and amended the format of the passport to include Section IX ‘Administration of Veterinary Medicinal Products’. A deadline was introduced to update older style passports by adding Section IX pages. The deadline was 30 June 2004.
- From 28 February 2005, to be eligible for slaughter for human consumption, passports must contain Section IX and this section must verify that the animal is eligible i.e. there is no indication that the animal is ‘not intended for human consumption’.
1 July 2009
- Compulsory deadlines for keeper’s submission of passport application forms to the PIOs are introduced; passport applications had to be submitted by the keeper before 31 December of the year of birth of the horse or within 6 months following the date of birth, whatever date occurs latter. Late submissions resulted in the passport being stamped by the PIO that the horse is not intended for human consumption on the Section IX of the passport.
- Microchips became compulsory for horses identified from 1 July 2009 and these microchips had to be inserted by veterinarians. Horse silhouette become not compulsory, however most passport issuing organizations kept requiring one.
- All duplicate and replacement passports had to be stamped by the PIO that the horse is not intended for human consumption; this was not always the case before 1 July 2009.
1 January 2016
- There is a new passport format but existing passports issued before 1 January remain valid.
- The layout of the passport is slightly different, and these passports have the ‘Administration of Veterinary Medicinal Products’ under Section II (instead of Section IX).
- Horses born on or after 1 January 2016 must be identified no later than 12 months following the date of birth. Passports issued after 12 months deadline will be stamped by the PIO that the horse is not intended for human consumption on the Section II of the passport.
- Horse silhouettes are back into the passports as a compulsory element.
1 October 2018
- Domestic legislation is introduced in the UK (W, NI and Scot issued later than Oct-18) implementing EU legislation in force since 1 January 2016.
- Domestic legislation introduces shorter passport application deadlines than the EU legislation and owners of horses must ensure that an application for a passport is received by the PIO within 6 months from the date of birth of the horse or by the 30th of November in the year in which the horse is born, whichever is the later.
- Passports issued from this date and created from pre-printed stock must contain as a minimum a serial number printed on each of the pages which form Sections I to III of the passport as an additional security measure.
2.13.4 Derogation to horse identification requirements: “the designated areas”
There are certain designated areas in England and Wales (none in Scotland or Northern Ireland) containing defined populations of horses living under wild or semi-wild conditions that do not need to be identified with passports and microchips whilst they remain within the defined areas and provided they are not treated with any veterinary medicinal product. Once these horses are removed from those populations or brought into domestic use, they must be identified.
The derogation in England apply to horses that are:
- identified in the lists kept by the Dartmoor Commoners’ Council
- entered in the stud book kept by the Exmoor Pony Society
- identified in the lists kept by the Verderers of the New Forest or entered in the stud book kept by the New Forest Pony Breeding and Cattle Society
- identified in the lists kept by the National Trust as Konik equines located at Wicken Fen
In Wales the derogation applies to horses that are:
- identified in the lists kept by the Hill Pony Improvement Societies of Wales
- identified in the lists kept by the Cymdeithas Merlod y Carneddau
When one of these horses (with no identification) is treated with any veterinary medicinal product, the responsible person must ensure that the horse has identification and is implanted with a microchip within 30 days of the treatment.
The identification requirements for these horses when moved for slaughter vary depending on the age of the horses (please note that FCI is always required regardless of the identification requirements):
Wild or semi-wild horses under 12 months of age:
A wild or semi-wild horse is eligible for slaughter for human consumption and can be presented at the abattoir without a passport and without a microchip if:
the horse is under 12 months of age,
- it is being moved directly from the designated area in which it was born to a place for slaughter,
- it has not previously been treated with any veterinary medicinal product,
- it has a sticker issued by PIO attached to it before it leaves the designated area, and the sticker is marked with a unique identification number and the date on which it was attached to the horse, and
- it is slaughtered within 7 days of the date shown on the sticker.
Wild or semi-wild horses over 12 months of age:
A wild or semi-wild horse aged 12 months or over is eligible for slaughter for human consumption and can be presented at the abattoir if it:
- is being moved directly from the designated area in which it was born to a place for slaughter,
- has a sticker issued by PIO attached to it before it leaves the designated area, and the sticker is marked with a unique identification number and the date on which it was attached to the horse,
- has a passport and the Section II does not show that the animal is not intended for slaughter for human consumption, and
- is slaughtered within 7 days of the date shown on the sticker.
(Note that a microchip is not required in this case)
Wild or semi-wild horses moved to another holding destination other than a slaughterhouse:
A wild or semi-wild horse of any age can be moved from a designated area to another place other than a slaughterhouse if:
- has a sticker issued by PIO attached to it before it leaves the designated area, and the sticker must be marked with a unique identification number and the date on which it was attached to the horse,
- has a passport,
- reaches holding destination within 7 days of the date shown on the sticker, and
- a microchip is inserted into the horse within 30 days of arrival at the holding destination.
(Note that, if in time this horse is presented at the abattoir, the horse should be identified by a passport and a microchip same as any other horse)
See Annex 1 to this chapter for an Example of a rump sticker provided by Pet-ID Equine.
2.13.5 Veterinary treatment and horse identification
A private veterinary surgeon (PVS) or other person administering any veterinary medicinal product to a horse must first check the passport to ascertain whether the horse is intended for human consumption. Section IX (or Section II, where the passport was issued from 1 January 2016) is the part of the passport containing the food status of the horse.
Only certain veterinary medicinal products must be recorded in the horse passport:
- All vaccines must be recorded in the passport regardless of whether the horse is intended for human consumption.
- Food producing horses – passport declares ‘intended for human consumption’ or no declaration made: All vaccines must be recorded and also any of the ‘Essential Substances’ used (substances listed in European Council Regulation 122/2013, the ‘Essential Substances’ List). The withdrawal period for each of the ‘Essential Substances’ is 6 months.
A written record of use for any other veterinary medicine must be kept, although this is done in a separate record (not in the passport, there is no specific section in the passport to do so). Records of administered veterinary medicines must be kept for 5 years even if the animal has been sold or slaughtered during that time.
Only products containing pharmacologically active substances listed in Table 1 of Regulation 37/2010 may be administered to food-producing animals. In the event that a medicine containing any of the substances listed in Table 2 of Regulation 37/2010 is administered to a horse, the animal can never be slaughtered for human consumption and the owner or PVS must sign Section IX of the passport to declare the horse as ‘not intended for human consumption’ (Section II where the passport was issued from 1 January 2016).
2.13.6 Phenylbutazone (Bute) and horse identification
Phenylbutazone is neither listed in Table 1 nor has been included in Table 2 of Regulation (EU) 37/2010. This means that, whilst not a banned active ingredient, it cannot be used in food producing animals.
Horses that have been treated with phenylbutazone must not enter the food chain and their passports must be signed by the owner or PVS on Section IX of the passport to declare the horse as ‘not intended for human consumption’ (Section II where the passport was issued from 1 January 2016).
3. FBO responsibility
In this section
3.1 Introduction
3.1.1 Duty to ensure traceability
The FBO has a duty to ensure that all livestock submitted for slaughter are correctly identified.
These checks should form part of the procedures that they have put in place in accordance with of Article 5 of (EC) 852/2004 to meet the requirements of HACCP.
Reference: (EC) 853/2004 Annex II Section II (1 and 2).
3.1.2 Record keeping
The FBO must ensure that records of all livestock delivered to the establishment are kept in accordance with the species requirements described previously in the legislation section of this chapter.
3.2 Cattle
3.2.1 FBO responsibility
It is the responsibility of the FBO to ensure that cattle presented for slaughter for human consumption:
- comply with the age criteria
- are properly identified
- are accompanied by valid documentation.
Reference: (EC) 853/2004 Annex II Section II (1and 2),
The TSE (England) Regulations 2010
The TSE (Wales) Regulations 2008
When the FBO presents animals that do not comply with age criteria, the FSA may reject the carcase from those animals as unfit for human consumption and take enforcement action as appropriate.
When the FBO presents animals that are not properly identified, the FBO should present whatever further information that is available which allows the OV to make a judgement as to whether the animal’s identity is ascertainable. The keeper, however, has 48 hours to arrange for the correct identification of the animal.
3.2.2 FBO to report movements and death to BCMS
The FBO is required to notify BCMS of the movement of cattle on to the slaughterhouse.
The FBO is also required to notify BCMS of the death of cattle at the slaughterhouse.
It is possible, although not mandatory, for slaughterhouses to report cattle movements and deaths electronically, using CTS online, an approved software package or the self-service telephone line.
3.2.3 FBO to return cattle passports to BCMS
It is the responsibility of the FBO to return cattle passports to BCMS, to reach BCMS within 7 days of slaughter. Updated [(unless the death of the cattle is reported to BCMS electronically- please see 3.2.4)].
Note: The only exception to this is where FSA retain the passport (which will occur only in the event of a discrepancy arising).
Updated [BCMS will provide the FBO with the necessary pre-paid polylope envelopes for the purposes of returning the cattle passports. Any requests for additional envelopes should be made to BCMS by telephoning 0845 050 1234].
3.2.4 Completion of death details on the passport
If the FBO has reported the death to BCMS electronically, then that is the notification of death and the FBO should return the passport to BCMS, after any necessary FSA identity verification checks have been carried out.
Note: Updated [Only those slaughterhouses where the death of the cattle is reported to BCMS electronically and have capacity to store the passports securely, could use the contracted shredding external company () who will collect and safely shred/dispose the cattle passports].
If the FBO is not using an electronic method to notify deaths to BCMS, then the slaughter details must be entered onto the death details section of the cattle passport by a responsible member of the slaughterhouse staff. Updated [Again, the FBO is responsible for returning the passport to BCMS once any necessary FSA verification checks have been satisfactorily completed, through the pre-paid polylope envelopes provided by BCMS].
Completion of the kill number in non-BSE testing cattle is not a legal requirement, but a best practice that should be encouraged. In BSE testing the recording of a kill number is recommended. It is only a requirement if it is in the required methods of operation (RMOP).
Note: Entry of slaughter details or kill number must not be performed by FSA staff.
3.2.5 Completion of death details: on farm slaughter
In the case of cattle slaughtered on farm and sent to a slaughterhouse for dressing (emergency slaughter), the keeper must complete the death details in the passport and send it with the animal and appropriate FCI declaration to the slaughterhouse.
The FBO must then return the passport to BCMS within 7 days of slaughter (unless the passport is retained by FSA for further investigation).
Note: Different BSE test age rules apply to emergency slaughter cattle; see chapter 2.6 on ‘TSE Testing’.
3.2.6 Return of documents to BCMS: summary of FBO responsibilities
- All passports must be received at BCMS within 7 days of the animal’s death.
- Updated [All passports, with the exception of those retained by FSA for further investigation, should be returned by the FBO to BCMS in the pre-paid polylope envelopes supplied for the purpose, unless the slaughterhouse is reporting the death of the cattle electronically (please see further clarification in 3.2.3)]
- Updated [The FBO must include the kill sheet in the pre-paid envelope provided by BCMS]
- The kill sheet should be clearly marked to identify any animals which were slaughtered on-farm.
- Passports for animals which were slaughtered on farm may also be included in the same pouch but must be clearly marked and placed within a separate envelope within the pouch or pre-paid envelope.
- Updated [The FBO should check that the total number of passports in the pre-paid envelope equals the total number of animals processed as detailed on the kill sheet]
3.3 Cattle register
3.3.1 Requirement to keep a register
FBOs are required to keep records of:
- cattle moving on and off the slaughterhouse, and
- cattle deaths
Other keepers are required to keep similar records.
Reference: CIR 2007, Schedule 5 and (EC) 1760/2000, Articles 7(1) and 7(4).
Note: The LA is the enforcing authority for this and the FSA carry out inspections on their behalf. Any non-compliance will be reported to them.
3.3.2 Contents of register
The register may be kept in computerised or paper form and must contain the following information:
- the unique official identification code for each animal from the ear tag
- the breed and sex of the animal
- where the animal came from
- date of arrival at the lairage or slaughterhouse; if the lairage is at a separate location and has a different CPH number, the date of arrival at both the lairage and the slaughterhouse must be recorded
- date of return to keeper and address animal sent to (where movement restrictions permit such movements)
- date of death
3.3.3 Availability for inspection
The register (or a copy of computer printouts) must be available for inspection to an AO of FSA, Defra or the LA.
3.3.4 Use of the cattle register as a kill sheet
A copy of the cattle register, or an alternative kill sheet containing the same information, must be provided daily by the FBO to FSA operational staff. It will be checked to verify the accuracy of the data registered and to confirm throughput information.
The FBO must then send the original kill sheet, with the relevant cattle passports, to BCMS.
3.4 Restrictions on slaughter of cattle
3.4.1 Compliance with age rules
The FBO must ensure that no cattle born in, or imported into, the UK before 01 August 1996 are slaughtered for human consumption.
When cattle that require BSE testing are identified at premises that do not have an approved RMOP, they may be moved, under licence, to an abattoir with an approved RMOP. The FBO should apply to APHA (England and Wales) for licence.
A list of establishments that can slaughter cattle that require a BSE test is available.
Reference: The TSE (England) Regulations 2018, Schedule 2, paragraph 12
The TSE (Wales) Regulations 208, Schedule 2, paragraph 5, 5 (1)
Reference: See chapter 2.2 on ‘Ante-mortem inspection’, section 4 for additional information.
3.4.2 Stamped passports
The FBO must inform the OV if an animal arrives at the slaughterhouse accompanied by a passport stamped ‘NOT FOR HUMAN CONSUMPTION: Animal exposed to mammalian protein’.
Reference: See sub-topic 2.5.6 on ‘Passport: Stamped not for human consumption’ of this chapter for additional information.
3.4.3 FBO duty when errors are found at pre-slaughter checks
If during the FBOs pre-slaughter checks, a bovine is found where:
- one or more ear tags are missing
- ear tags are mismatched or of an unapproved type (for example, hand written)
- the passport details obviously do not match the bovine, is wrong, invalid or missing
- age rules have been breached
the FBO should:
- immediately notify the OV and present the passport to the OV
- detain the animal
- notify the keeper that the animal has been detained, and
- if appropriate, that the keeper has 48 hours to arrange for the animal to be correctly identified
Note: The FBO must not return the passport presented with the animal to the keeper until the correct passport is presented to the OV. (Un-reconciled passports are returned to BCMS with a completed form AID 5-4 by the OV once investigations have been completed; see sub-topic 4.5.17 on ‘Records’).
3.5 Horses: FBO responsibility
3.5.1 FBO responsibilities
The term ‘horse’ used throughout the MOC means any wild or domesticated soliped mammals of all species within the genus Equus of the family Equidae, and their crosses.
The FBO is responsible for:
- ensuring that every horse accepted for slaughter is accompanied by its matching passport, which has been issued by an approved issuing body and complies with the required format
- ensuring that those horses identified under derogation rules, originate directly from one of the designated areas (holding of birth)
- ensuring that every horse or batch of horses accepted for slaughter is accompanied by food chain information (FCI) and that the information provided in the FCI is acted upon
- ensuring the passport contains a Section IX that does not show that the animal is not intended for slaughter for human consumption (Section II for horse passports issued from 1 January 2016)
- checking that neither the Central Equine Database nor the passport exclude the horse from the human food chain
- ensuring that the passport does not show signs of tampering in any section of the passport
- ensuring that any withdrawal period for veterinary medicinal recorded on the passport and FCI have elapsed
- scanning every horse prior to acceptance for slaughter for the presence of a microchip and if present, ensuring that the number matches that recorded in the passport
Note: Some microchips inserted prior to 1 July 2009 may not be recorded in the passport. However, if a microchip was inserted and was recorded in the passport it becomes part of the horse’s official identification and must therefore be treated as such.
Horses delivered to a slaughterhouse that are incorrectly identified must not be allowed to leave the premises. A horse incorrectly identified is not eligible for slaughter for human consumption.
However, a horse may be correctly identified and still be ineligible for slaughter for human consumption (for example, the Section IX is signed, withdrawals periods have not been observed). Horses correctly identified but not eligible for slaughter for human consumption can leave the abattoir provided no other restrictions apply (for example, disease controls).
In the event of failure to comply with any of the requirements listed above, the FBO must notify the OV and take appropriate measures.
Reference: (EC) 853/2004 Annex II, Section II.
3.5.2 FBO action after deciding to accept the horse for slaughter
Once the FBO has carried out the checks detailed above, and decided to accept the animal for slaughter, they must give the passport and FCI to the OV.
Reference: (EC) 853/2004 Annex II, Section III, Paragraph 8.
4. FSA role
In this section
4.5 Verification of age: Cattle
4.6 Cattle ear tag discrepancies
4.7 Cattle passport discrepancies
4.8 Guidance on returning cattle passports
4.9 Verifying eligibility of horses
4.1 Introduction
4.1.1 Verification of FBO duties
The OV is to verify that the FBO complies with (EC) 853/2004 to ensure that animals accepted for slaughter for human consumption are properly identified.
The OV must ensure that animals whose identity is not ascertainable by the FBO are killed separately and declared unfit for human consumption.
If the OV considers it necessary, they should contact APHA to arrange for official controls to be carried out on the holding of provenance.
Reference: See the section 5 on ‘Enforcement’ in this chapter for additional information.
Reference: 6272019, Article 43.
4.1.2 Role of the OV / AO
The role of the OV / AO will vary depending on the species of livestock presented for slaughter.
For sheep, goats, deer and pigs this will entail verification of animal identity by sampling and periodic checks to ensure that the FBO is checking animals are clearly tagged / marked and maintaining accurate records. These checks should be undertaken at least weekly or whenever operations take place if less than weekly, and as part of the audit of FBO procedures. The OV or AO must make a record of the check and outcome in the daybook or as part of audit notes.
For cattle, verification must be undertaken for a minimum of 10% of animals presented, increasing the percentage if required as detailed in the following topics.
For horses, verification must be undertaken for 100% of all those presented, as detailed in the following topics.
Reference: See the relevant species topics in section 2 on ‘Animal identification’ and the requirements listed in section 1 on ‘Legislation’ in this chapter for additional information.
4.2 Verifying cattle ID
4.2.1 Minimum level of cattle identification checks
FSA AOs must conduct identity checks on 10% of cattle as a minimum, dependant on the levels of compliance with cattle identification requirements. This is to verify that age related SRM controls and BSE testing requirements are complied with and to verify that the FBO is fulfilling their responsibilities as far as animal identification is concerned.
4.2.2 Increased level of cattle identification checks
Verification levels should be increased immediately following NC, until the reason for the NC has been established and rectified.
The OV should set an increased level of verification, sufficient to provide assurance that FBO controls are applied effectively. The OV should raise any concerns with the FVC in the first instance.
Once the OV is satisfied that the FBO has addressed and corrected the root cause of the NC, the verification level should return to the 10% level.
4.2.3 Flexibilities on identification checks
Identity checks must be random and spread throughout the day, every day. There is flexibility on the number of animals to be checked each day, as long as the required percentage is achieved by the end of the week. Checks should be spread throughout the day and should not fall into a pattern, for example, concentrated in any particular day or part of a day.
To allow FSA to provide continued assurance to the consumer and customers that BSE testing is conducted on all relevant bovines, the identification of at least one animal should be verified every day to prevent any potential relaxation that may arise without daily checks.
The Cattle NC online form, (available in the ‘Applications’ section of Digital Workplace) has columns for the number of cattle killed and the number of carcases checked.
It is appreciated that, in smaller establishments, operationally it may be more practical to verify cattle identity of a higher percentage.
4.2.4 Recording non-compliances
Every week, the OV must record and submit all identified NCs using the Cattle NC online form.
Note: The Cattle NC online form can be found in the Applications section of Digital Workplace
A NC should only be recorded when an identification problem has not been detected by the FBO and it is identified by FSA operational staff for the first time.
Any questions regarding the use and completion of the form should be directed to the relevant FVC in the first instance.
4.2.5 Non-compliances identified outside the relevant percentage checks
If a problem is identified outside of the percentage in force, action should be taken by the OV / AO as necessary. Issues identified outside of the random checks should be recorded in the cattle ID NC online form and should be considered an additional check for that day.
4.2.6 Non-compliances which must trigger an increase in supervision checks
- Failure to classify and slaughter the animal in the correct age category (under thirty months (UTM), OTM and cattle that require BSE testing).
- Unless authorised, slaughter of a bovine animal that requires BSE testing.
- Slaughter of cattle born in, or imported into, the UK before 1 August 1996.
- No ear tags.
- Different ear tags.
- No passport / wrong passport.
- The sex and / or breed of the bovine obviously does not match details recorded on the passport.
- Passport appears to have been tampered with or amended or any other obvious reason to suspect that it is not valid.
4.2.7 Establish identity to the satisfaction of the OV
It is the responsibility of the keeper to correctly identify the animal.
It is the FBOs responsibility to ensure that animals that are slaughtered are properly identified.
When the animal has already been slaughtered and no valid official cattle identification document can be obtained, the carcase must not be health marked.
Note: Notwithstanding cases referred to the LA for investigation, it is still the OV who must make the final decision regarding the acceptability of the animal for health marking.
4.2.8 Suspect forgery or fraud
In cases of doubt or suspicion of forgery or fraud, when the animal has already been slaughtered, carcases must not be health marked while enquiries are being made.
If the OV has reasonable grounds for suspecting that a cattle passport may be inaccurate, for example, where there is a clear disparity between the age given on the passport and the dentition, the OV must initiate further checks to establish whether the documentation is correct.
4.2.9 FSA check of cattle register
The OV must inspect the FBO’s cattle register each month to ensure that the records are being completed promptly and accurately (see sub-topic 3.3 on ‘Cattle register’). The OV should:
- check at least 1% of the entries made
- sign and date the register with details of the number of entries checked and found acceptable
- if the register is kept electronically, make an entry in the daybook to prove that checks have been made
If the register is found to be deficient, you must take action as detailed in section 5 on ‘Enforcement’ of this chapter.
4.3 Pre-slaughter check: Cattle
4.3.1 FSA verification duties
The FSA is required to verify that FBOs have complied with their responsibilities as far as animal identification is concerned.
Reference: (EU) 2019/627, Article 43 (1).
4.3.2 AO action when a live animal discrepancy is recorded
The AO must take the following actions when the FBO reports a live animal discrepancy:
- confirm that the FBO has taken the actions as specified on page 3-8 of this chapter
- assess the identification or evidence provided by the FBO
- supervise the slaughter and disposal of the carcase if no identification or acceptable evidence of identification is provided by the FBO (see sub-topic 4.3.8 to 4.3.10 of this chapter)
4.3.3 Opportunity to provide evidence
If a live bovine is presented with any of the following, the OV must allow the keeper opportunity to establish the animal’s identity and provide the reason as to why the animal was presented without correct identification:
- no ear tag
- one ear tag (when double tagging is required)
- mismatched ear tags
- the wrong passport
- no passport
Caution: A bovine animal without any ear tag cannot be reconciled against any documentation provided and it is unlikely that sufficient evidence can be presented to authenticate identity and permit re-tagging.
Animals with ear tag discrepancies such as no ear tags or mismatched ear tags must be notified to LA using the form AID 5/7 Part 1 (FSA Referrals to LA).
When more than one bovine animal from the same holding have been submitted with only one official ear tag and/or passports with missing data, and also if repeated cases from the same origin occur within a week, the OV is also to notify the LA using the form AID 5/7 Part 2 (FSA Referrals to LA).
Isolated instances of this kind in which the OV has been able to obtain a satisfactory explanation of the reasons for the discrepancy and also when there is no clear evidence of illegal activity do not need to be reported.
4.3.4 Genuine mistake suspected
If after initial enquiries, the evidence available to the OV clearly shows that a genuine mistake has been made with a cattle passport (for example, passport misplaced, accidentally destroyed or lost), the OV can obtain clarification on the animal status by contacting the BCMS helpline (8:30 to 5:00 pm) on:
0345 050 1234 – England
0345 050 3456 – Wales
18001 0345 050 1234 – Type talk for the hearing impaired
or online at BCMS
When the BCMS reference number is issued, the OV will follow up with an email describing the situation to the BCMS e-mail address: bcmsenquiries@rpa.gov.uk. This email will include pictures of the animal and its ear tag (front and back) to allow BCMS to carry out their investigation.
BCMS will reply to the OV by email, including shots of the CTS database and confirming the animal status (for example, the animal is alive and in the holding). BCMS will also state that there are no objections on their part for the animal to be slaughtered, as far as its identification is concerned.
Should the investigation reveal a different result this will also be clearly stated in the email to the OV.
If the wrong passport was presented to the OV, this can be returned to the keeper once the BCMS investigation has produced a result.
4.3.5 Suspected fraud
If fraud is suspected, the details must be referred to the LA Trading Standards Department and copied to the Deregistration Department at BCMS by emailing bcmsenforcementreferrals@rpa.gov.uk.
Reference: See the section 5 on ‘Enforcement’ in this chapter for additional information.
4.3.6 Identity established
If the identity of the live animal has been established to the satisfaction of the OV and:
- is re-tagged satisfactorily
- the animal is presented with at least one official tag which matches the passport
- the OV may allow the animal to be slaughtered for human consumption
4.3.7 Correct passport supplied
Should the correct passport be provided within 48 hours the animal may be slaughtered for human consumption. If reliable evidence available shows that the passport exists (for example, a legible photocopy is produced when the passport is not presented on arrival to the slaughterhouse), the animal may be slaughtered but the carcase will be detained pending arrival of the original passport.
Please note that cattle passports are not issued for dead animals.
4.3.8 Identity not established: FBO action
If the identity of the animal is not established to the satisfaction of the OV and the animal:
- is not re-tagged
- is re-tagged with tags that do not reconcile with the passport
- no correct passport is provided (including when BCMS investigation results, as triggered by the OV, confirmed that the animal’s identity cannot be verified)
the FBO must slaughter the animal separately, then stain and dispose of the appropriate category of ABP under supervision by FSA operational staff.
4.3.9 Identity not established: AO action
If the identity of the animal is not established to the satisfaction of the OV, the AO must mark the passport ‘not reconciled’ and return to BCMS with a copy of the AID 5/4 (Cattle Identification NC Report) detailing the non-compliance. All non-reconciled passports must be returned to BCMS by FSA staff.
Reference: See chapter 9 on ‘Forms’ for a copy of AID 5/4.
Note: See the section 5 on ‘Enforcement’ in this chapter for additional information.
4.3.10 Daybook entries
Details of all identification issues and their outcomes will be recorded in the plant’s daybook. Likewise copies of the emails related to BCMS enquiries will be kept by the OV in the plant’s files.
4.4 Post-slaughter: Cattle
4.4.1 Check of kill sheet
The FBO will provide FSA staff with copies of kill sheets. These should be checked to verify the accuracy of the data registered and to confirm throughput information.
Note: FBO kill sheets may no longer be used as an alternative to the AID 5-1, as this is now a compulsory form.
4.4.2 FSA verification of identity
The FSA AO is responsible for verifying that the representative sample of bovine animals which have been slaughtered for human consumption are:
- correctly identified
- accompanied by a valid passport (checks include ear tag number, sex and breed)
- in compliance with age criteria applicable in the establishment
- imported animals have the required official documentation
Reference: See the section 1 on Legislation and section 3 on ‘FBO responsibility’ in this chapter for additional information.
Note: AOs should check that when two ear tags are fitted, these both bear the same identity.
4.4.3 FSA post-slaughter identity checks
The following checks must be carried out post-slaughter by an FSA AO for the appropriate percentage in force, and relevant action taken for any discrepancies.
| Stage | Check |
|---|---|
| 1 | The animal has official, valid ear tags |
| 2 | The ear tag details match the passport |
| 3 | The dentition is consistent with the date of birth on the passport and does not indicate any obvious signs of fraud |
| 4 | The passport is valid and the sex and breed of the animal match the passport |
| 5 | The inside back page for import details (if any) |
4.4.4 Requirement for proof of identity
The OV is to notify the operator of any carcases without the correct identification without delay. The FBO should be given the opportunity to present evidence to allow the OV to be confident that the identity of the carcase was ascertainable.
Caution: Clear and unambiguous proof will be required and more than one piece of evidence may be needed to substantiate the identity of the animal.
Reference: See section 5 on ‘Enforcement’ of this chapter for additional information.
4.4.5 Ongoing disputes
Where there is an ongoing dispute regarding the identity of a carcase, and the FBO or primary producer is awaiting the results of DNA testing, the FBO should be offered the option of freezing the carcase.
Reference: See the sub-topic 5.2.2 on ‘Disposal of carcases’ of this chapter for additional information.
4.5 Verification of age: Cattle
4.5.1 FSA responsibility
The principal guide for age estimation of cattle is the date of birth on the passport. The OV may, however, take into account other factors, such as dentition, and carcase characteristics such as ossification, when establishing if the identity of the bovine is ascertainable.
An AO must carry out a dentition check on the relevant percentage of carcases presented as less than 30 months old, and record the results on AID 5/1 (Cattle Identification Record) if 5 or more permanent incisors are erupted.
Reference: See chapter 9 on ‘Forms’.
Reference: See topic 2.7 on ‘Cattle age requirements’ of this chapter for additional information.
4.5.2 Who completes AID 5-1?
The authorised officer who carried out the inspection must complete and initial each entry.
The OV who checks all the entries on the form must then sign the form.
Note: If the FSA authorised officer who completed the entries is the only FSA authorised officer present, they must also sign the OV confirmation section, having first carried out a secondary check of their original entries.
4.5.3 Frequency of completion
The AID 5-1 must be completed daily; fill in as many AID 5-1s as necessary to cover the number of cattle ID checks being undertaken.
4.5.4 Bovines requiring BSE testing
From 1 March 2013, there is no requirement to test for BSE in healthy slaughter cattle born in one of the EU-25 countries.
However, there remains a requirement to test certain ‘risk cattle’, depending on their age and origin and AOs must remain vigilant when undertaking cattle ID checks that such cattle are identified.
Full details of BSE testing requirements are located within chapter 2.6 on ‘TSE testing’ and all AOs undertaking identity checks must be familiar with the testing requirements.
4.5.5 Earliest date of birth (DOB) for slaughter of cattle requiring BSE testing or SRM VC removal
Determine the earliest date of birth for slaughter for O24M / OTM / O48M processing – as appropriate – that corresponds to the current date, using the relevant Bovine Eligibility Checklist.
Locate today’s date on the chart, and enter the corresponding ‘Earliest date of birth for slaughter’ from the right hand column. Enter this date in the relevant box on the AID 5/1.
Reference: Copies of Bovine Eligibility Checklists for O24M, OTM and O48M are available. If you require a spare copy, please contact Corporate Support Unit (CSU) York.
4.5.6 Completing AID 5-1
FBO kill sheets may no longer be used as an alternative to the AID 5/1, as this is now a compulsory form.
The whole of the AID 5-1 must be completed, but only for the relevant percentage of carcases checked.
The following boxes must be completed on the AID 5-1:
- Approval Number
- Establishment Name
- Date
- Earliest date of birth for slaughter of cattle (BSE testing / SRM VC removal) – O24M, OTM and O48M
- Total number of cattle slaughtered
- Total number of cattle checked
- % of cattle checked
Check if the animal was born or imported into the UK before 01/08/96
If imported, check if this was on/after 01/08/1996 and what the BSE testing age is for the country of origin, then:
- enter the kill number
- enter the ear tag number
- enter the date of birth
- indicate ‘yes’ or ‘no’ for imported, BSE test needed and valid passport
- for animals presented as UTM if there are 5 or more permanent incisors enter the number
- in the case of an invalid passport, complete form AID 5/4
Once completed, sign the AID 5-1.
4.5.7 OV confirmation
The OV must check the information provided on the AID 5-1, ensuring that each entry is initialled by the authorised officer who undertook the inspection, and confirm that:
- any cattle that require BSE testing have been identified and that appropriate action has been taken
- any animal born before 1 August 1996 has been identified, appropriate action has been taken
- any discrepancy relating to the animals age has been identified, the appropriate action has been taken
Reference: See chapter 7 on ‘Enforcement’ for additional information.
4.5.8 Discontinuous establishments
Where, in smaller establishments, the OV is not present at the end of the processing day another FSA authorised officer must check and sign the OV confirmation section. Similarly, where there is only one FSA authorised officer at the end of the processing day, they must complete and initial each entry, and then sign the OV confirmation section, having carried out a secondary check of their original entries.
4.5.9 Retention of form
When completed and signed, the AID 5/1s should be retained in the plant file for 2 years.
4.5.10 Principles of cattle dentition
Expert advice and statistical evidence shows that:
- the majority of animals with 6 permanent incisors erupted will be over thirty months of age
- a bovine animal with 7 or 8 permanent incisors erupted must be considered over thirty months of age
4.5.11 Disparity in stated age and dentition
If the OV has reasonable grounds for suspecting that the official cattle documentation may be incorrect, for example, where there is a clear disparity between the age given on the official cattle documentation and the dentition, the OV should initiate further checks to be satisfied that the documentation is genuine.
4.5.12 Guide to ageing cattle
The following table of dentition is based on research but may be helpful in estimating bovine ages.
Note: 24 months = 730 days
30 months = 912 days
| Permanent incisors | Minimum age days | Maximum age days |
|---|---|---|
| 1 erupted | 541 | 806 |
| 2 erupted | 536 | 825 |
| 2 in wear | 584 | 1019 |
| 3 erupted | 689 | 961 |
| 4 erupted | 715 | 937 |
| 4 in wear | 732 | 1275 |
| 5 erupted | 902 | 1277 |
| 6 erupted | 978 | 1304 |
| 6 in wear | 980 | 1498 |
| 7 erupted | 1038 | 1742 |
| 8 erupted | 1098 | 1715 |
| 8 in wear | 1103 | - |
4.5.13 Water buffalo
The temporary incisors in water buffalo are significantly larger than those in other bovine species, and may give rise to confusion.
In case of doubt the gum may be dissected to examine the teeth roots.
4.5.14 Actions where 5 or 6 incisors are erupted
After the AO has carried out the dentition check, and the passport indicates the animal is under thirty months old, but 5 or 6 permanent incisors are identified:
- there is no need to submit a brainstem for BSE testing
- if there is a significant discrepancy in the age compared to the dentition, the case may be referred to LA Trading Standards for further investigation; they may wish to retain some evidence, such as the head, for their investigations
- the carcase may enter the food chain if it has passed post mortem inspection, and VC is treated as SRM
4.5.15 Actions where 7 or more permanent incisors are erupted
Any animal with 7 or more permanent incisors erupted and a passport indicating that it is less than thirty months old should be detained and investigated in the first instance. These cases should be referred to the LA. Where no further information comes to light, the OV’s final decision should be based on the fact that animals with 7 or more incisors erupted cannot be UTM.
Reference: See sub topic 4.5.16 on ‘OV not satisfied’ for further appropriate action.
4.5.16 OV not satisfied
Where the OV is unable to satisfy the identity of the carcase, the FBO should identify the carcase as an ABP, which should be stained and disposed of under FSA supervision. The passport presented with the carcase should be marked ‘not reconciled’ and returned to the BCMS.
4.5.17 Records
When an animal is slaughtered and during the verification of the relevant percentage of carcases any of the following are identified:
- no tag
- illegible tags
- unofficial or unapproved tags
- evidence that a tag has been tampered with,
- 7 or 8 permanent incisors animal with an under thirty months passport
Do not allow the carcase to go for human consumption, and:
- mark the passport ‘not reconciled’ and return to BCMS with a completed AID 5/4 detailing the non-compliance
Note: Do not send passports and AID 5/4 forms to BCMS until all enquiries are complete.
- refer the matter to the LA and copy the referral to the BCMS Enforcement Referrals section (address: BCMS Enforcement Referrals Section, Curwen Road, Workington, CA14 2DD)
- record referrals to LAs / BCMS in the daybook
- record any enforcement action in the daybook and complete ENF 11/5 (Enforcement Programme)
- secure evidence, especially the heads of suspect animals with the ears and ear tags attached
- notify these cases to the FVC
Note: This ensures management are aware of issues which may be referred to them.
4.6 Cattle ear tag discrepancies
4.6.1 OV duties: action to take
When a carcase is presented without satisfactory ear tags, providing other eligibility checks have been completed satisfactorily, the OV should immediately notify the FBO of any carcases without the correct identification and allow the FBO opportunity to present evidence to establish the identity of the carcase.
Note: These will be exceptional cases and the OV will probably need more than one piece of evidence to be convinced as to the identity of the animal.
The OV must be satisfied regarding the identity of the animal. If fraud is suspected the details must be referred to the Trading Standards Department and copied to BCMS by emailing bcmsenforcementreferrals@rpa.gov.uk.
4.6.2 Damaged ears
If the second ear is missing or badly damaged, both tags may be fitted to the same ear.
4.6.3 Illegible tags
If a tag is illegible you should treat the carcase as if its tag(s) were missing.
4.6.4 Carcase with only one tag
Where a carcase is found with only one tag, the following applies:
- if the animal was born before 15 September 1998, such carcases should be accepted with no further action (providing other eligibility checks have been completed satisfactorily)
- if the animal was born after 15 September 1998, there is evidence that a second tag had been fitted and all other eligibility requirements have been met, the OV may accept the carcase for human consumption
- if the animal was born after 15 September 1998, but there is no evidence that a second tag had been fitted, the OV may still accept the carcase for human consumption, provided any necessary checks are made to allow them to reasonably ascertain the identity of the animal
4.7 Cattle passport discrepancies
4.7.1 Definition: wrong passport
A ‘wrong passport’ is where the passport presented with the animal relates to an entirely different animal.
The passport is considered to be the wrong one if:
- it does not match the animal’s ear tag, or
- the size / dentition of the animal indicates that the date of birth shown on the passport is incorrect
- the passport is not one of the three types of the UK passport
Note: Animals imported from EU: the passport is not the type issued by the relevant EC exporting country, when the animal is imported direct for slaughter and / or does not have an export health certificate, and / or does not have a Permit Authorising Movement of Cattle (MC2L) issued by DAERA (animals from Northern Ireland only).
4.7.2 Definition: invalid passport
An ‘invalid passport’ is where the passport presented with the animal does relate to that animal, but some of the details are incorrect or missing.
The AO should consider that the passport presented with a carcase is invalid if:
- it appears to have been tampered with or amended
- the sex or breed of the bovine obviously do not match the information on the passport
- details of the last holding where the animal has been kept are missing or do not match the FCI
Note: The OV may accept a passport as valid if minor information is missing (for example, it has not been signed by the last keeper) provided traceability of the animal has been maintained. The carcase may be passed fit for human consumption if the keeper or his agent signs the passport within 7 days of slaughter.
4.7.3 OV action
If a carcase has been presented for post-mortem eligibility checks with the wrong passport, an invalid passport or without a passport, the OV must follow the steps in the table below:
| Step | Action |
|---|---|
| 1 | Detain the carcase |
| 2 | Notify the FBO that the passport is wrong, invalid or missing |
| 3 |
Allow the keeper 48 hrs to present the correct passport or correct the deficiency Note: The carcase may be held for a period longer than 48 hrs if the OV has evidence that the correct passport has been located (is in possession of a photocopy) Reference: See sub-topic 5.2.1 on ‘Storage of carcase pending investigation’ of this chapter for additional information |
| 4 | Retain control of the passport |
| 5 | Take enforcement action if appropriate |
4.7.4 Pending further investigation
The carcase must not be passed as fit for human consumption until the original, valid passport is presented.
The OV should detain the carcase pending the outcome of investigations and follow the guidance in the table below.
The result of such investigation will determine health marking or disposal as ABP and possible enforcement action.
Reference: See the sub topic 5.2.2 on ‘Disposal of carcases’ of this chapter for additional information.
| If | Then | Passport to be returned to BCMS by |
|---|---|---|
| The correct passport is submitted |
|
FBO |
| The passport is invalid, but the discrepancy is rectified (for example, by the movement section being completed or the keeper signing the passport) |
|
FBO |
| The passport contains a minor breed discrepancy, but the animal’s identity is not in doubt |
|
FBO |
| The correct passport is not submitted |
|
OV |
| The passport displays incorrect sex details |
|
FBO or OV, depending on the outcome of the investigations |
| The passport appears to have been amended or tampered with |
|
OV |
| The number on the ear tag has a ‘UK’ prefix, but the passport does not OR the tag number on the passport has a ‘UK’ prefix, but the number on the ear tag does not |
|
OV |
4.7.5 Action after investigation
If the passport is considered invalid or any doubt remains as to the identity of the animal, the OV must:
- mark the passport ‘not reconciled’ and return to BCMS with a completed AID 5/4 form detailing the non-compliance
- instruct the FBO to identify the carcase as an animal by-product, stain and dispose of it under FSA supervision
- record details in the daybook
- take enforcement action as appropriate
Reference: See the section 5 on ‘Enforcement’ in this chapter for additional information.
4.7.6 Right to request further documents
Pedigree certification that is pre-printed and can be confirmed with the breed society may be used to help establish the date of birth.
The OV may request additional information from the keeper (for example, herd record books) in order to satisfy that the documentation is genuine.
The OV may also contact BCMS to request details of the animal’s records on CTS. The number to use for such queries is 01900 702130, or visit the BCMS website.
4.7.7 Reporting stamped passports
Where cattle are identified live or presented slaughtered with a passport stamped ‘NOT FOR HUMAN CONSUMPTION: Animal exposed to mammalian protein’ the OV must inform, by telephone:
- Regional Veterinary Manager, APHA, Worcester (01905 763355)
- the local APHA office
- LA (Trading Standards Department)
- BCMS
Under no circumstances may carcases from these animals be health marked for human consumption.
Note: Carcases, offal and all other parts of the carcase (with the exception of the hide) must be disposed of as SRM Category 1 ABP.
4.8 Guidance on returning cattle passports
4.8.1 Reconciled passports given back to FBO
Once the eligibility checks have been satisfactorily completed for the relevant percentage of carcases and the carcase has been accepted (or rejected) as eligible for human consumption the OV should give the passport to the FBO, for them to return it to BCMS.
4.8.2 Security of documents
All passports retained by FSA for further investigation must be kept under secure conditions by the FSA and must not be returned to the FBO or to previous keepers. The FBO may take photocopies of passports before slaughter or after slaughter, under FSA supervision.
4.8.3 Return of documents to BCMS: OV
A copy of the completed AID 5/1 should be filed in plant by the OV; there is no longer a requirement to send to BCMS.
In addition to the AID 5/1, copies of any AID 5/4s issued should also be enclosed in the polybag, attached to the passport(s) to which they relate (see following table).
Note: Further supplies of polybags (for the purposes of returning forms and non-reconciled passports to BCMS) are available by contacting BCMS on 0845 050 1234.
4.8.4 Return of non-reconciled documents to BCMS
Non-reconciled passports must be returned to BCMS, as detailed in the step-action table below.
Note: Do not send passports and AID 5-4 forms to BCMS until all enquiries are complete.
| Step | Action |
|---|---|
| 1 | Stamp the passport with the plant document stamp |
| 2 | Write the words NOT RECONCILED in red across the passport |
| 3 | Complete an AID 5-4, detailing the non-compliance |
| 4 | Keep copies of documents on file at the plant |
| 5 | Attach the AID 5-4 and copies of any notices issued, to the appropriate passport |
| 6 | Place the passport and completed forms in the polybag |
4.8.5 Animals rejected on pathological grounds
It is not necessary to complete an AID 5-4 for animals which were not passed fit for human consumption on pathological grounds. Do not write ‘not-reconciled’ on the passports relating to such animals.
4.8.6 Additional instructions: AID 5-4
For all passports marked ‘not-reconciled’, the death details page of the passport must be completed, even if the number on the passport was not the same as the number on the ear tag.
Complete the AID 5-4 with the number that appeared on the ear tag of the animal that was actually slaughtered. This will enable BCMS to update both the record of the animal slaughtered, and the record relating to the number that appeared on the passport.
4.9 Verifying eligibility of horses
4.9.1 Definition
The term ‘horse’ used throughout the MOC means any wild or domesticated soliped mammals of all species within the genus Equus of the family Equidae, and their crosses.
4.9.2 FSA duties
FSA staff are responsible for verifying that the FBO carries out all necessary checks on every horse, their passports, microchips and FCI prior to acceptance for slaughter, ensuring that the tasks are carried out accurately and that the horses presented to the OV for ante-mortem inspection are eligible to enter the human food chain. This includes undertaking checks to verify that the microchips, markings and descriptions in the passport match the horse presented for slaughter, taking into account natural changes that may occur, for example due to age and scarring, and also checks to verify that the details in the paper passport match those contained in the Central Equine Database (CED).
Carcases of solipeds must be examined for trichinosis. Trichinella testing is an official control. The OV is to ensure that sampling takes place and samples are appropriately identified, handled and sent for testing to an accredited laboratory. Full instructions are available in MOC Chapter 2.4, Section 5. Sampled carcases are retained until reception of negative test results.
4.9.3 Central Equine Database (CED)
The CED is a centrally managed database of all equine identification data in the UK. The purpose is to secure the human food chain, and to help agencies deal with lost, fly-grazed, stolen or abandoned horses and combat criminal activity. The CED contains records of every equine registered with any UK PIO (Scotland, Wales, Northern Ireland and England). The CED can be accessed or via Digital Workplace. FSA officials need a name and password provided by Equine Register to log in.
The FSA and Defra have agreed that OVs and MHIs use the CED as a platform to:
- assist with the equine identification verification checks, and
- report the slaughter of horses and the outcome of the carcase (either in or out of the human food chain).
4.10 Verifying eligibility of horses: Pre-slaughter
4.10.1 OV action: pre-slaughter horse identification and FCI checks
The OV must examine the passport and FCI of every horse presented for slaughter for human consumption. Taking all necessary precautions in terms of Health and Safety, the OV also needs to be satisfied that the microchip recorded in the passport is also present in the horse.
Please note that this is verification of the FBO duties, it is part of the official controls and does not replace the FBO’s responsibilities.
The entire passport must be checked to ensure it fully relates to the horse presented with it and to spot potential signs of fraud.
Every UK passport of horses presented for slaughter must be checked against the CED and this constitutes part of the identification verification checks.
Details around horse passports, microchips and specific identification requirements for wild or semi wild horses originated from certain designated areas can be found in Section 2.13 of this chapter. Food Chain Information requirements can be found in MOC Chapter 2.1, Section 2.3
Useful information can be found in Annex 3 to this chapter “Weatherbys Passport Evolution” where Weatherbys has provided us with details around the changes introduced in the appearance of their passports over the years. Also Annex 5 “Weatherbys Passports: Signs of Tampered Passport” provide further guidance on what to look for to spot potential passport fraud.
In the event that FSA staff have a query regarding the passport, in the first instance they should contact the relevant PIO for clarification. If the PIO is unable to satisfactorily resolve the query, FSA staff should email the SLA and Contract Team, with full details.
4.10.2 Common horse identification issues
The table below provides some guidance on certain common horse identification issues and an indication of the most appropriate course of action:
| Problem identified | Action |
|---|---|
| No passport, even if accompanied by breed society documentation | Ineligible for slaughter for human consumption (however, be aware of derogations applicable to wild and semi-wild horses living in designated areas) |
| FCI absent (wild and semi-wild horses originated from the designated areas must be presented with FCI too) | Ineligible for slaughter for human consumption |
| No Section IX (Section II for passports issued from 1 January 2016) | Ineligible for slaughter for human consumption |
| Section IX stamped/signed to indicate that the horse is not intended for human consumption (Section II for passports issued from 1 January 2016) | Ineligible for slaughter for human consumption |
| Microchip number on passport does not match that in the horse | Ineligible for slaughter for human consumption |
| There is more than one microchip in the horse | This is an irregularity that requires further investigation. Occasionally more than one microchip is present in a horse; if PIO and veterinarian can offer a satisfactory explanation, this must be taken into account before a final decision is made. |
| Microchip recorded on passport cannot be detected in the horse | This is an irregularity that requires further investigation. Consider possible faulty microchip present in the neck or migrated to another part of the body. Consider if the wrong passport has been presented with the horse. |
| Microchip detected in horse, but not recorded on passport | This is an irregularity that requires further investigation. Note that some microchips inserted prior to 2009 may not always be recorded in the passport, use the horse markings to decide if identity can be ascertained. Consider if the wrong passport has been presented with the horse. Also, consider if the microchip appears registered to a different horse. |
| CED shows that the horse is not eligible for the food chain | Ineligible for slaughter for human consumption |
| The record for the horse is not found in the CED | If the passport was issued by a UK PIO, the horse is in principle not eligible for slaughter for human consumption. Further investigation might be required. |
| Passport from The National Pony Society (NPS) appears as non-eligible for the human food chain in CED but the Section IX appears as eligible |
Ineligible for slaughter for human consumption. There are some NPS passports where the pony was declared out of the food chain on the PIO database, but this does not appear in the passport. The reason for this is unknown and there is nothing in the passport to indicate the status of the animal. The CED has been updated with the correct food status as per the original PIO database. (Weatherby’s has taken over) |
| Passport from Pleasure Horse Society (PHS) appears as non-eligible for the human food chain in CED |
Ineligible for slaughter for human consumption. All PHS passport have both parts of the Section IX signed and are ineligible for slaughter for human consumption. (Horse Passport Agency has taken over) |
| Passport from The Spotted Horse and Pony Society issued after 12 May 2008 |
Ineligible for slaughter for human consumption. Any passport issued after this date has been issued illegally. (Pet-ID Equine has taken over) |
| Passport from The Gypsy Cob Society Ltd issued after 25/10/2010 |
Ineligible for slaughter for human consumption. Any passport issued after this date has been issued illegally (The Lipizzaner National Stud Book Association of Great Britain has taken over) |
| Passport from Irish Horse Register containing several Section IX pages |
This is an irregularity that requires further investigation. When the layout of the Section IX changed in 2009, the PIO “updated” some passports by adding additional Section IX pages compliant with the new requirements to old passport already containing Section IX page. |
| Passport from Irish Cob Society |
Ineligible for slaughter for human consumption until the validity of the passport is first verified with DAFM. The Department of Agriculture, Food and the Marine in Ireland (DAFM) have advised that no horse with an Irish Cob Society passport should be slaughtered before the validity of the document and eligibility for slaughter for human consumption is first verified with DAFM. The entire passport must be scanned and emailed to HorseID@agriculture.gov.ie Discussing with the FBO in advance will prevent disappointment. |
| Documents issued by Joint Measurement Board (JMB) |
JMB used to issue identity documents before the current PIO system was put in place. They applied to be a PIO but were rejected, so stopped issuing them. JMB was never authorised by Defra to issue passports and for that reason their database has never been transferred to the CED JMB documents are not valid equine passports and an animal presented with one of these documents is ineligible for slaughter for human consumption. |
Older equine born on or before 30th June 2009 presented for slaughter on or after the following dates and with a passport issued by a PIO based on one of these countries which has never had a microchip inserted (compulsory retrospective microchipping):
|
The absence of a microchip in this older equine should not rule it out of the human food chain if the passport is valid with clear food chain information and the equine remains eligible for slaughter for human consumption. Defra has not requested that the CED be amended and for that reason, an equine missing a microchip will not be automatically ruled out of the food chain. |
4.10.3 OV further checks
Once the owner has presented the passport and after the FBO has decided that the horse will be presented for ante-mortem inspection, the OV should make checks to ensure that there is no visible evidence to indicate that substances with a pharmacological effect have been administered which may make the meat unfit for human consumption.
Where there are any drugs listed in a horse passport or FCI, the OV must check the latest position on withdrawal periods and authorisation status by referring to the VMD website.
Any horse treated with a prohibited substance, as detailed in Table 2 of the Annex to Commission Regulation (EU) No 37/2010, or any horse treated with phenylbutazone, can never be used for human consumption.
Table 2 of the Annex to Commission Regulation (EU) No 37/2010 includes:
- Aristolochia spp. and preparations thereof
- Chloramphenicol
- Chloroform
- Chlorpromazine
- Colchicine
- Dapsone
- Dimetridazole
- Metronidazole
- Nitrofurans (including furazolidone)
- Ronidazole
4.10.4 OV action: horses incorrectly identified
The OV is to verify compliance of the FBO’s duty to ensure that animals accepted for slaughter are properly identified. The OV is to ensure that animals whose identity is not ascertainable are killed separately and declared unfit for human consumption.
Only if the welfare of the horse could be compromised by delaying the slaughter, the animal may be slaughtered even if the legally required information concerning its identity has not been supplied, but this information must be supplied before the meat is passed as fit for human consumption.
Horses delivered to a slaughterhouse that are incorrectly identified must not be allowed to leave the premises. However, a horse may be correctly identified and still be ineligible for slaughter for human consumption (for example, the Section IX is signed, withdrawals periods have not been observed). Horses correctly identified but not eligible for slaughter for human consumption can leave the abattoir provided no other restrictions apply (for example disease controls).
Full details of discrepancies or signs of tampering identified at this stage or reported back to the OV by the PIO on reception of the passport after slaughter, must be recorded in both plant Day Book and Kill Sheet. The passport, FCI and any other relevant documents must be retained as evidence and made available to the Local Authorities at the earliest opportunity. Early contact with the Local Authorities is essential to preserve any other evidence they might require.
Signs of tampering identified and reported back to the OV by the PIO on reception of the passport after slaughter must be communicated to the FBO at the earliest opportunity to facilitate the withdrawal of any affected meat from the market as soon as possible.
Horses that are presented incorrectly identified must be slaughtered separately and disposed of as Category 2 Animal By-Product.
Abattoirs are intended for the slaughter of healthy and correctly identified animals for the production of meat intended for human consumption. The admission of animals known to be non-eligible for human consumption with a view to destine the carcases to feeding zoo animals is not acceptable.
4.11 Verifying eligibility of horses: Post-slaughter
4.11.1 Removal of microchips
Following slaughter, FSA staff must:
- verify that the FBO scans the carcase for the presence of microchips
- verify that the FBO identifies and locates any implanted microchips
- verify that the FBO removes the microchip and hands it to the FSA staff and
- dispose of the microchip in the clinical waste container provided by the SLA and Contract Team.
If the microchip is located but cannot be removed that part of the carcase containing the microchip is not eligible for the food chain and must be removed before the rest of the carcase can be released into the food chain.
4.11.2 Age verification for horses presented without passport
Foals under 12 months of age may be moved directly to slaughter from the designated areas without a passport and without a microchip (for full details on identification requirements, please refer to sub-topic 2.13.4 of this chapter). These foals may be eligible for the human food chain if the dentition check performed after slaughter confirms that the animal is under 12 months of age and has visible cups of the temporary lateral incisors. If the dentition check confirms that the animal is older than 12 months, the carcase and all body parts will be totally condemned on the basis of inappropriate identification.
Annex 2 to this chapter contains practical guidance on performing these dentition checks.
Horses accompanied by their passports are not subjected to routine dentition checks.
4.11.3 Reporting slaughter of the horses in the CED
The UK passport of every horse presented for slaughter must be checked against the CED as part of the identification verification checks and then, the date of slaughter of the horse and the outcome of the carcase is to be recorded in the CED to keep it up to date.
4.11.4 Return of passports
Following slaughter, FSA staff must:
- cancel the passport by application of the plant stamp at, as a minimum, the silhouette page and Section IX (Section II for passports issued from 1 January 2016) of the passport
- the stamp must be signed, dated and the outcome of the horse / carcase must be indicated
- invalidate the passport by cutting the top right corner of the passport (all pages)
- retain the passport and FCI for horses subjected to RIM sampling for a period of at least 12 weeks (a copy, either paper or electronic, of the passport must be sent to the PIO with a note explaining why the original passport cannot be sent back yet).
In the event of a positive RIM sample:
- an SLA and Contract team member will contact the FSA team at the establishment and ask them to scan and email specific pages from the passport in question
- FSA staff must then send the passport in question and FCI to the SLA and Contract Team, by special delivery
In the event of horses not being subjected to any tests, FSA staff should:
- return UK issued passports to the approved Passport Issuing Organisation within 7 days of the date of death
- return non-UK passports to the competent authority of the country where the horse passport originates; EU competent authority addresses can be found online.
These passports must be sent using the International Standard Tariff together with a cover letter produced using FSA template (see Annex 6).
If you are unsure of where to send the passport, please contact SLA and Contracts team.
- return rump stickers to the issuing PIO.
4.11.5 Weekly kill record
Following slaughter, FSA staff should record details of all horses slaughtered on to the establishment’s horse passport Excel spreadsheet, which should be emailed to the SLA and Contract team on a weekly basis. Blank copies of the spreadsheet are available on request from the SLA and Contract team.
5. Enforcement
In this section
5.1 Introduction
5.1.1 Failure of FBO duties
In cases where there has been a clear breach of the domestic or EU requirements for the FBO to establish animal identity, action should be taken to refer the matter to the LA Trading Standards Department and escalate the breaches of Regulation (EC) 853/2004 in accordance with the hierarchy of enforcement.
Serious or persistent breaches should be recommended for prosecution in the normal way.
Reference: See chapter 7 on ‘Enforcement’ for additional information.
5.1.2 Inadequate cattle register
If the inspection shows that the register has not been correctly maintained, the OV should:
- notify the LA immediately in writing
- copy the details to the BCMS Enforcement Referrals Section:
Cattle Enforcements and Referrals Section
BCMS
Curwen Road
Workington
CA14 2DD
Email: bcmsenforcementreferrals@rpa.gov.uk
- record details in the plant daybook
- inform the FBO that a report of discrepancies and / or breach of the law are being reported to the LA for consideration
5.1.3 Reasons for suspicion
Several things may give the OV grounds for suspecting cattle identity fraud. Examples include:
- tampered ear tags
- shiny new ear tags
- different character fonts on the same ear tag
- extra holes in the ear with no tag
- short period of residence on any holding on the passport
- passport alterations / omissions
- wrong breed / sex / colour
- absence of thymus in the carcase if presented as UTM
- very little cartilage in the vertebral spinous processes if presented as UTM
- dentition checks
5.1.4 Official controls on farm of provenance
LAs (Trading Standards Department) are responsible for enforcement of Animal Identification legislation and Trades Description legislation. The OV should inform the LA in which the slaughterhouse is situated of any suspect offence regarding:
- the identification of animals
- movement records, and
- suspect fraudulent documents
Reports should be made promptly and in writing, so that enforcement action is not prejudiced where there are time limits for action set down in the legislation.
You must keep detailed records in the daybook and retain any evidence and copies of documentation that could be used in an investigation.
5.1.5 Notifying BCMS
Details of all referrals to the LA regarding cattle identity should be sent to the BCMS for follow up action:
BCMS Enforcement Referrals Section
Curwen Road
Workington
CA14 2DD
Tel: 01900 702130
Email: bcmsenforcementreferrals@rpa.gov.uk
5.2 Storage and disposal of carcases
5.2.1 Storage of carcases pending investigation
The OV should instruct the FBO to inform the keeper that the carcase will not be health marked pending the outcome of any investigation by the LA. This may include DNA testing of suspect animals.
Note: Investigation may take a period of time during which chilled carcases could deteriorate. Formally detain the carcase under Regulation 10 (1) of the Food Safety and Hygiene (England) Regulations 2013 or Regulation 9 (5) of the Food Hygiene (Wales) Regulations 2006, (form ENF 11/26) for further examination / sampling.
The owners of carcases that have not been health marked pending investigation must be kept fully informed of the position and given the opportunity to request that the carcase is frozen or boned and frozen under supervision at their own expense until the investigation has been completed.
The frozen carcase should be marked in accordance with the instructions in chapter 7 on ‘Enforcement’, sub-topic 3.2.1.
The OV must be satisfied that if the carcase is to be frozen away from the slaughterhouse that satisfactory control and detention remain in place.
Note: Ensure that the LA is informed and that any further enforcement and disposal is done in conjunction with them as the enforcement body with responsibility outside the approved establishment.
5.2.2 Disposal of carcases
When the OV is satisfied that a bovine animal’s identity is not ascertainable, the carcase must not be health marked and must be declared unfit for human consumption by the OV. The FBO should dispose of the carcase as follows:
- carcase containing SRM (including the VC in carcases from animals suspected or confirmed as being OTM) should be slashed, stained with adequate blue staining and disposed of as SRM (Category 1 ABP)
- carcases that have had all SRM removed (including vertebral column in bovine carcases suspected of being OTM) should be slashed and stained with a dark colourant of sufficient strength and disposed of as Category 2 ABP; the SRM should be stained with adequate blue staining and disposed of as (Category 1 ABP)
Reference: See chapter 2.6 on ‘TSE Testing’ for additional information.
Where surrender is not forthcoming, the OV should put in writing the reasons why they are formally declaring the meat unfit for human consumption in accordance with Regulation (EU) 2019/627 Article 45.
Note: Where the FBO continues to refuse to dispose of meat that has been declared unfit, follow the ABP provisions relating to the treatment of meat declared unfit for human consumption in chapter 2.8 Animal By-Products.
6. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Example of rump sticker and derogation disposal form
Annex 2: Guide for performing horse dentition checks (under 12 months of age)
Annex 3: Weatherbys passport evolution
Annex 4: Weatherbys RCVS and Beva identification of horses booklet
2. BSE Testing in Cattle
3. Sheep TSE Sampling and Submission
4. Sheep TSE Testing: Form Completion
5. Annexes
Sections
3. Sheep TSE Sampling and Submission
1. Introduction
In this section
1.1 Purpose
1.1.1 Background
Cattle, sheep and goats can be susceptible to a group of brain diseases known as transmissible spongiform encephalopathies (TSEs). The best known of these diseases is Bovine Spongiform Encephalopathy (BSE) (mad cow disease) in cattle. BSE has been linked to the human TSE disease, variant Creutzfeldt-Jakob disease (vCJD).
Since 1990 the European Community (EC) has adopted a series of measures to protect human and animal health from the risk of TSE.
It is appropriate in the view of the magnitude of the risk posed to human and animal health by certain TSEs to adopt specific rules for their prevention, control and eradication.
EC Member States must carry out an annual programme for monitoring BSE and scrapie (a similar disease affecting sheep and goats).
In order to ensure that the rules concerning the prevention, control and eradication of TSEs are observed, it is necessary for samples to be taken for laboratory testing on the basis of an established protocol which would give a full epidemiological picture of the situation as regards TSE.
The TSE disease surveillance statistics for UK are published online.
1.1.2 TSE suspects
These instructions are not intended to apply to animals suspected of suffering from a TSE which must be dealt with in accordance with chapter 6 on ‘Notifiable diseases’.
1.1.3 Health and safety
When following these instructions all FSA staff must adhere to the FSA health and safety guidelines.
Reference: See guidelines in the Health and Safety Manual located on Digital Workplace.
1.2 Legislation
1.2.1 Relevant legislation
(EC) 999/2001 (as amended) lays down rules for the prevention, control and eradication of certain TSEs.
The Transmissible Spongiform Encephalopathies (England) Regulations 2018 and The Transmissible Spongiform Encephalopathies (Wales) Regulations 2018 implement Regulation (EC) 999/2001 in England and Wales.
(EU) 2019/627, Article 29 enquires official controls to check that the FBOs procedures guarantee compliance with relevant community legislation on TSEs.
The Transmissible Spongiform Encephalopathies (England) Regulations 2018 (S.I. 2018 No. 731)
1.2.2 Monitoring programmes
(EC) 999/2001 requires that Member States monitor and test for TSE in certain animals from different categories, including
- bovine animals slaughtered for human consumption
- bovine animals not slaughtered for human consumption
- ovine and caprine animals slaughtered for human consumption
- ovine and caprine animals not slaughtered for human consumption
- monitoring in infected flocks
1.2.3 Legislation relating to TSE testing of cattle
The official controls on cattle slaughtered for human consumption and BSE tested are based on the following pieces of legislation:
- Regulation (EC) 999/2001 (as amended) which sets out the TSE controls including those for Specified Risk Materials (SRM) and Bovine Spongiform Encephalopathy (BSE) testing
- Regulation (EC) 1069/2009 which sets out specific rules for the handling and disposing of animal by-products
- Regulation (EU) 2019/627 Article 29 which sets out specific rules for Official Veterinarians (OVs) in slaughterhouses
- Commission Decision 2009/719/EC (as amended) which authorised certain Member States to revise their annual BSE monitoring programme
- the Transmissible Spongiform Encephalopathies (England) Regulations 2018
- the Transmissible Spongiform Encephalopathies (Wales) Regulations 2018
1.3 Disposal
When the text refers to material being disposed of as Category 1 Animal By-Product (ABP) SRM must be destroyed by incineration or rendering and then incineration at approved premises (as opposed to other Category 1 ABP, which can be rendered and land filled).
The OV must verify that this material is consigned to approved premises and obtain confirmation that it has been incinerated.
Reference: (EC) 999/2001 and (EC) 1069/2009, Chapter II, Article 12 (a).
Reference: See chapter 2.7 on ‘SRM’ and chapter 2.8 on ‘ABP’ for additional information.
Note: List of approved premises in Great Britain can be accessed on the APHA website.
2. BSE testing in cattle
In this section
2.2 BSE testing of bovines in slaughterhouses
2.3 Standard operating procedures for BSE sampling and testing
2.4 Identification of cattle to be tested
2.7 Alternative sampling techniques
2.8 Traceability of sample to carcase
2.9 Samples packaging and delivery
2.10 Traceability of all parts: tested animal to carcase
2.12 Retention of all body parts
2.17 ‘Insufficient test’ result
2.18 ‘Inconclusive test’ result
2.1 BSE testing requirements
2.1.1 Bovines born in UK or certain other Member States- testing requirements from 1 March 2013
The BSE testing requirements outlined in this paragraph apply from 1 March 2013 and relate only to cattle born in one of the EU Member States listed in the table below:
- All ‘at risk cattle’ aged over 48 months (birth date + four years and 1 day) (O48M).
Note: See the sub topic 2.1.3 on ‘Animals that require testing’ for definition of ‘at risk cattle’.
| Country | Eartag ID prefix |
|---|---|
| Austria | AT |
| Belgium | BE |
| Croatia | HR |
| Cyprus | CY |
| Czech Republic | CZ |
| Denmark | DK |
| Estonia | EE |
| Finland | FI |
| France | FR |
| Germany | DE |
| Greece | EL |
| Hungary | HU |
| Ireland | IE |
| Italy | IT |
| Latvia | LV |
| Lithuania | LT |
| Luxembourg | LU |
| Malta | MT |
| Netherlands | NL |
| Poland | PL |
| Portugal | PT |
| Slovak Republic | SK |
| Slovenia | SI |
| Spain | ES |
| Sweden | SE |
| United Kingdom (including Channel Islands and Isle of Man) | UK |
2.1.2 Bovines born elsewhere- testing requirements
Cattle with ear tags that do not have the prefixes listed above must be BSE tested if: over thirty months of age (OTM) if healthy at slaughter or over 24 months of age (O24M) in cases of emergency slaughter or where identified as sick at ante mortem inspection.
Note: See sub topic 2.1.3 on ‘Animals that require testing’ for definition of ‘at risk cattle’.
Note: Cattle born in a Third Country (not one of the 28 EU Member States) and imported into a Member State will be re-tagged with a tag showing the importing Member State’s prefix (unless slaughtered within 20 days). The Third Country import information should be available in the passport.
2.1.3 Animals that require testing
The following animals require testing:
- all O48M (EU26) / O24M (non EU26) bovine animals that have undergone:
- emergency slaughter in accordance with, point 1 of Chapter VI of Section I of Annex III to Regulation (EC) No 853/2004, or
- an ante-mortem inspection with observations concerning accidents, or serious physiological and functional problems, or signs in accordance with 2019/627, Article 43(4):
- that welfare has been compromised; or
- of any condition which might adversely affect human or animal health, paying particular attention to the detection of zoonotic diseases and animal diseases for which animal health rules are laid down in EU legislation.
- animals OTM (non EU26) healthy at slaughter.
2.1.4 Exceptions
Some casualty animals or animals with abnormalities may be exempted from the testing requirement. These animals are:
- TB reactors and TB inconclusive reactors – unless suffering from a concurrent disease or abnormality as outlined in this instruction
- cattle with localised lesions or conditions with no systemic affects – examples include minor foot lameness, ringworm, superficial tumours, minor hernias, minor abscesses, localised mastitis or mild conjunctivitis; in such cases, the OV must be content that there are no signs of concurrent disease
Note: The above examples are intended as a guide. It is for the OV to make a professional judgement in each situation as it arises, in line with these instructions.
The following table illustrates the BSE testing requirements for all cattle:
Exceptions
| Country of birth | Ear tag country codes |
BSE testing age from 1 March 2013 Healthy slaughter cattle |
BSE testing age from 1 March 2013 Emergency slaughter and sick at ante mortem (fit for human consumption) |
BSE testing age from 1 March 2013 Fallen stock (not fit for human consumption) |
|---|---|---|---|---|
| Austria | AT | No Testing Required | Over 48 Months | Over 48 Months |
| Belgium | BE | No Testing Required | Over 48 Months | Over 48 Months |
| Croatia | HR | No Testing Required | Over 48 Months | Over 48 Months |
| Cyprus | CY | No Testing Required | Over 48 Months | Over 48 Months |
| Czech Republic | CZ | No Testing Required | Over 48 Months | Over 48 Months |
| Denmark | DK | No Testing Required | Over 48 Months | Over 48 Months |
| Estonia | EE | No Testing Required | Over 48 Months | Over 48 Months |
| Finland | FI | No Testing Required | Over 48 Months | Over 48 Months |
| France | FR | No Testing Required | Over 48 Months | Over 48 Months |
| Germany | DE | No Testing Required | Over 48 Months | Over 48 Months |
| Greece | EL | No Testing Required | Over 48 Months | Over 48 Months |
| Hungary | HU | No Testing Required | Over 48 Months | Over 48 Months |
| Ireland (ROI) | IE | No Testing Required | Over 48 Months | Over 48 Months |
| Italy | IT | No Testing Required | Over 48 Months | Over 48 Months |
| Latvia | LV | No Testing Required | Over 48 Months | Over 48 Months |
| Lithuania | LT | No Testing Required | Over 48 Months | Over 48 Months |
| Luxemburg | LU | No Testing Required | Over 48 Months | Over 48 Months |
| Malta | MT | No Testing Required | Over 48 Months | Over 48 Months |
| Netherlands | NL | No Testing Required | Over 48 Months | Over 48 Months |
| Poland | PL | No Testing Required | Over 48 Months | Over 48 Months |
| Portugal | PT | No Testing Required | Over 48 Months | Over 48 Months |
| Slovakia | SK | No Testing Required | Over 48 Months | Over 48 Months |
| Slovenia | SI | No Testing Required | Over 48 Months | Over 48 Months |
| Spain | ES | No Testing Required | Over 48 Months | Over 48 Months |
| Sweden | SE | No Testing Required | Over 48 Months | Over 48 Months |
| UK (including Channel Islands and Isle of Man) | UK | No Testing Required | Over 48 Months | Over 48 Months |
| Bulgaria | BG | Over 30 months | Over 24 months | Over 24 months |
| Romania | RO | Over 30 months | Over 24 months | Over 24 months |
| All other countries | UK (if not slaughtered within 20 days of import; import information is shown on the inside back page of the cheque-book style passport) | Over 30 months | Over 24 months | Over 24 months |
2.2 BSE testing of bovines in slaughterhouses
2.2.1 Introduction
FBOs processing bovines that require BSE testing must comply with the specific legal requirements for the testing and should have standard operating procedure (SOP) for ensuring their correct implementation.
An approved required methods of operation (RMOP) is no longer required but FBOs are expected to have agreed a SOP with the FSA. The previously agreed RMOP could be used as an SOP if it remains valid and up-to-date.
The agent of the BSE is a food safety hazard and should be included in the HACCP based procedures for the establishment. The SOP for BSE testing along with the SRM removal should be part of the control measures for that hazard (i.e. prions).
2.2.2 Sampling area
The sampling area must be suitable, safe and clean with hygienic facilities for the taking of brain stem samples, for example, dedicated table or room or sampling on the line.
2.2.3 Trained plant staff
Sampling cannot be undertaken unless the FBO provides staff that are suitably trained and competent to carry out brain stem sampling. The OV should use the FBO’s training records to ascertain that there are sufficient suitably trained staff.
The FBO may contact CSCOneHealthGeneral@apha.gov.uk for the training requirements of their staff. The FBO should keep the training records as set by APHA and update them for any internal cascading among other members of the FBO’s staff.
2.2.4 Passport handling
The FBO must have a system to ensure (as far as reasonably possible) that slaughtered cattle match the details shown on the passport (ear tag number, breed, sex and age).
2.2.5 Traceability system
A robust traceability system must be established through which the ear tag number and kill number is associated with the sample, carcase and body parts.
This system must also:
- define the criteria by which the FBO identifies the animal/s requiring testing
- reconcile the number of cattle that should have been tested and the number of samples despatched to the laboratory
2.2.6 Ensuring eligibility
It is the FBOs responsibility to ensure that cattle presented for slaughter:
- have the correct ear tags in place
- are accompanied by a valid passport
- are eligible for slaughter
The FBO must keep a record of cattle movements onto the premises.
Reference: The Cattle Identification Regulations 2007 and the Cattle Identification (Wales) Regulations 2007.
Reference: See chapter 2.5 on ‘Animal identification’ for additional information.
2.2.7 Storage facilities
Suitable and secure facilities must be available to retain the carcase, body parts including hide and blood, and by-products, until the test results are received.
Note: Hides can be delivered to a hide premises before the test result is received, provided that the hides are kept under official control until the BSE test results are received.
Note: if the test results are not received when the body parts are disposed of, they must be disposed of as SRM by incineration.
2.2.8 Cold inspection
Delayed post-mortem inspection (‘cold inspection’) may be permitted in small establishments after an assessment has been made by the FVC as to the suitability of the premises.
When permitted, removal of the brain stem sample will need to be done in the presence of FSA staff.
The SOP for the establishment must place special emphasis on the measures to be taken to ensure full traceability of carcases and all body parts.
2.2.9 Office equipment
There should be suitable office facilities with:
- a photocopying machine
- a system for the receipt of the test results from the laboratory, either by fax or by other electronic means
2.2.10 FSA pre-requisites
OVs and MHIs must have received training before carrying out official controls on the processing of cattle requiring BSE testing.
The training contains further guidance to that recorded in this document. Refer to training or your line manager for any further assistance.
2.3 SOP for BSE sampling and testing
2.3.1 SOP agreement
FBOs should agree a SOP with the FSA, which will continue to maintain food safety and BSE controls. The SOP should be part of the HACCP-based procedures and could be based on the previously agreed RMOP.
Reference: See Annex 1 for a sample of the former RMOP which could be used as a reference for drafting or checking the SOP.
OVs must ensure that the SOP contains all the steps of production with detailed procedures for each step.
OVs must only accept a SOP when they are satisfied that the controls are robust enough to provide confidence in the security of the entire system.
2.3.2 Audit and verification
SOP will be subject to verification or audit by:
- FVC
- Veterinary Assurance Team
- Internal Audit Unit
- Independent auditors
2.3.3 Hazard identification and control plan
The FBO must review their HACCP plan when slaughtering cattle that require BSE testing, to ensure hazards are identified and controlled. The control measures for BSE related hazards should include the SOP for BSE sampling and testing.
2.4 Identification of cattle to be tested
2.4.1 Identification procedure
Lairage procedures and pre-slaughter checks must be robust to ensure all cattle that require BSE testing are identified. The table on the following page details both FBO and FSA responsibilities.
| FBO responsibility | FSA responsibility |
|---|---|
|
Must implement in the lairage a positive release system for cattle that require BSE testing. Must ensure bovines born in or imported into the UK before 1 August 1996 are rejected for slaughter for human consumption. Must ensure BSE testing of cattle that were born in one of the countries listed in the Annex to Commission Decision 2009/719 [as amended] aged 48 months and one day or more that were subject to emergency slaughter or where the OV judges testing is necessary at ante mortem inspection. Must ensure cattle that were not born in one of the countries listed in the Annex to Commission Decision 2009/719 (as amended) are tested if aged over 30 months or over 24 months if subject to emergency slaughter or where the OV judges testing is necessary at ante mortem inspection. (The countries listed in the Annex are the 25 EU Member States as listed in the table in sub-topic on ‘Bovines born in UK or certain other Member States’ in this chapter.) Bovines for BSE testing must be marked by a suitable, robust and reliable method prior to slaughter (for example, spray marking, tagging). |
FSA staff must verify
FSA staff must also perform 10% physical checks of ear tag / passport reconciliation in the lairage. Passport and ID checks, including DOB and age on the % of animals required according to the percentage in force at the establishment in question (See chapter 2.5 on ‘Animal identification’, section 4). The OV must ensure that a form TSE 6/4 is completed and issued to the FBO for all animals requiring BSE testing intended for human consumption and not identified by the FBO |
2.4.2 Bovines not eligible for human consumption
Animals born in or imported into the UK before 1 August 1996 are prohibited from entering the food chain.
Animals identified by the FBO as being born prior to 1st August 1996 (or having the default birthdate 11/11/1111 on their identity documents) must be notified to the OV, who must inform the FVC.
These cattle must be destroyed as fallen stock and be BSE tested when they are culled or die.
Guidance on fallen stock can be found online.
If the premises are already approved for BSE testing of bovines, the abattoir may submit a sample for BSE testing using BSE test code FSCA2 and arrange for the body to be disposed of as Category 1 ABP for incineration.
OVs must be vigilant that these cattle are not slaughtered for human consumption unless they were imported into the UK on or after 1 August 1996.
Reference: The TSE (Wales) Regulations 2018
The TSE (England) Regulations 2018
2.4.3 Issue of form to test animal not for human consumption
The OV is to issue to the FBO form TSE 6/5 for animals identified as requiring BSE testing, but that are not eligible for human consumption.
Reference: See chapter 9 on ‘Forms’ for a copy of TSE 6/5.
2.4.4 Identification documents
For the different types of documents that accompany the cattle and the different tagging systems, depending on the age, see chapter 2.5 on ‘Animal Identification’.
2.4.5 Lairage facilities
The establishment should have a lairage with suitable facilities to:
- check information on the passports prior to slaughter and make a physical check to match the passport numbers with the ear tags
- segregate cattle that require BSE testing from those that do not
2.5 Slaughtering schedule
2.5.1 Segregation of animals requiring testing
All eligible animals requiring BSE testing must be identified following the means of identification detailed in the SOP (for example, spray marking, tagging) and slaughtered separately (preferably as the last down the line) clearly identifiable from other animals and their products.
2.5.2 Exception
For welfare reasons, an animal requiring BSE testing may need to be slaughtered immediately.
2.6 Sampling procedure
2.6.1 Sampling technique
The collection of the brain stem sample could be carried out either:
- when the head is still attached to the carcase
OR
- after separating the head from the carcase and placing it on a dedicated table or on a line
The low-pressure water / hose and the low pressure compressed air methods may be used only once the head has been separated from the carcase.
The FBO must ensure that they have sufficient stock of consumable equipment required for sampling (including labels where used) before commencing processing.
2.6.2 Sampling on a dedicated table
A dedicated table and associated facilities for record keeping must be made available.
The distance of the sampling area from the line does not need to be excessive but sufficient to avoid any risk of cross-contamination (splashing) of fresh meat during the sampling process.
2.6.3 Sampling with the head attached to or separated from the carcase
If sampling is carried out when the head is still attached to or separated from the carcase, preventive measures such as spoon washing and sterilisation (if a stainless steel spoon is used for sampling) or disposal of sampling equipment as clinical waste (if plastic spoon and forceps are used) after each sample is taken, must be employed to avoid the risk of cross-contamination of the sample.
2.6.4 Operatives undertaking sampling
Brain stem samples must be taken by trained plant operatives only.
The FBO:
- is responsible for ensuring there are sufficient staff trained and competent in taking brain stem samples
- should keep a record of all trained operatives
- MUST notify FSA staff if any training is to be undertaken using heads of bovines that don’t require BSE testing
2.6.5 FSA supervision
FSA staff must not undertake any sampling but must carry out the following supervision:
| Check | Frequency | Records |
|---|---|---|
|
|
Day Book |
|
|
TSE 6/9 |
|
|
TSE 6/11 |
2.6.6 Loss of correlation
If correlation or sample identity has been lost, carcases and offal after the last correctly correlated brain stem sample must be disposed of as Category 1 SRM by incineration. All samples must still be sent for testing.
Reference: See also sub-topic 2.8.7 on ‘FSA action: loss of correlation’ in this chapter.
2.6.7 Brain stem sample quality
The FBO is responsible for ensuring that the brain stem sample submitted for analysis is of adequate quality to allow the lab to carry out the test and provide a result.
Note: All parts of all tested cattle for which results are not yet available must either be retained under official control or destroyed as Category 1 (SRM) by-product by incineration.
2.6.8 Disposal of sampling equipment
Following sampling, all testing equipment (for example, plastic spoon, plastic forceps and gloves) must be disposed of by the FBO as clinical waste in accordance with legal requirements.
The FBO must be aware that if the sampling material is not disposed of after each use there is a potential risk of cross contamination from a possible positive brain stem sample to other brain stem samples.
2.7 Alternative sampling techniques
2.7.1 Sampling with low pressure water/hose method
The following pre-requisites must be met before the extraction of the brain stem sample with low water pressure / hose method is carried out:
- the tongue has been removed
- any harvesting of head meat has been carried out
- the risk of cross- contamination with brain exudate of other meat is minimised or avoided
To minimise or avoid the risk of cross-contamination the following procedures should be carried out before and during the removal of the brain stem sample:
| Step 1 | Minimise or avoid the risk of cross contamination |
| Before the brain stem is removed | After the head is removed from the carcase:
|
| Step 2 | Minimise or avoid the risk of cross contamination |
| During the removal of the brain stem |
Once the above procedures are complete the use of the low-pressure water / hose method in obtaining the brain stem sample can be applied. The primary measures to be used to ensure that cross-contamination of any other meat nearby with brain exudate is minimised or avoided are: (i) Ensuring adequate space between the head and any other meat intended for human consumption so as to minimise cross-contamination dependent on direct contact, splash or possibly aerosol spray. This can be done in a number of ways such as:
(ii) Minimising cross-contamination from personnel by:
Given that each abattoir will be different, it will be for the FBO to agree with the OV and the FVC the most effective combination of methods required to minimise cross-contamination. |
2.7.2 Sampling using low pressure air compression
The following pre-requisites must be met before the extraction of the brain stem sample by means of the low pressure compressed air method is carried out:
- the tongue has been removed
- harvesting of head meat has been carried out
- the risk of cross-contamination with brain exudate of other meat is minimised or avoided
- the head complies with the following criteria:
- the bolt hole is not too large, obstructed or wrongly orientated and multiple bolt holes are absent; good stunning practices will be required to ensure these criteria
- aside from the bolt hole there are no other entry points into the cranial cavity / sinuses; in effect, if horns are removed care should be taken not to breach the cranial cavity
If the above criteria are not complied with, samplers must be competent in resorting to use of the spoon method in extracting the sample.
To minimise or avoid the risk of cross-contamination the following procedures should be carried out before and during the removal of the brain stem sample:
| Step 1 | Minimise or avoid the risk of cross-contamination |
| Before the brain stem is removed | After the head is removed from the carcase:
|
| Step 2 | Minimise or avoid the risk of cross-contamination |
| During the removal of the brain stem |
Once the above procedures are complete, the use of the low pressure compressed air method in obtaining the brain stem sample can be applied. The primary measures to be used to ensure that cross-contamination of any other meat nearby with brain exudate is minimised or avoided are as follows: (i) A plastic bag – ideally 100-gauge, clear polythene, 250 x 400mm – should be placed over the head and lightly knotted at the top (the muzzle area of the head).Then:
(ii) The optimum air pressure of 6 and 8 Bar is used – lower or higher pressures can result in increased spray. (iii) There should be adequate space between the head and any other meat intended for human consumption so as to minimise cross-contamination dependent on direct contact, splash or possibly aerosol spray which may escape beyond the plastic bag. This can be done in a number of ways such as:
(iv) Minimising cross-contamination from personnel by:
Given that each abattoir will be different, it will be for the FBO to agree with the OV and the FVC the most effective combination of methods required to minimise cross-contamination. In all cases it should be ensured that personnel undertaking sampling using the low-pressure air extraction method are adequately trained in both the method itself and the precautions required when doing so. |
2.8 Traceability of sample to carcase
2.8.1 Maintenance of traceability
The FBO is responsible for and must maintain robust traceability from animal to sample to carcase and all retained parts throughout the entire process.
Sample pots must be properly identified and correlated to the head, the carcase and retained body parts of the animal that has been sampled.
2.8.2 Records
The FBO is responsible for implementing a robust recording system for traceability purposes.
2.8.3 Sample identification
FBOs have two options for identification and submission of samples to the laboratory: either an electronic or a manual system. If using a manual system FBOs are responsible for sourcing their own supplies of barcode labels.
FBOs must ensure that all sample pots are identified with the vertical application of a barcode label. Pot lids can also be identified with the kill number as an additional precaution.
Barcode labels take the following formats:
- 9999DDD000001 for ‘dummy run’ or trial samples
- 9999MMM000001 manually submitted data
- 9999EEE000001 for electronically submitted data
Where 9999 is the establishment approval number and 000001 is the serial number of the sample. Barcode labels must not be reused.
| Manual system | Electronic system |
|---|---|
|
Apply a label to the sample pot when the sample is taken. Apply label to the movement card taken from the animal’s passport. Submit movement card with the sample for animal’s ID in barcode form. On the blue / green passports (CCP 01), or where no movement card is available, the FBO must photocopy the front cover of the passport and apply label. |
If using the electronic system, no hard copy passport will be necessary. The details of the barcode must meet the requirements of the testing laboratory and contain sufficient information to allow subsequent tracing of individual carcases and related body parts. It is recommended that the FBO thoroughly tests their bar code system by sending ‘dummy’ labels to the testing laboratory for scanning and reading, before the system is used on actual samples from bovine animals requiring BSE testing. Any failure to read the bar code labels by the testing laboratory will result in a ‘no test’ result and the subsequent disposal of affected carcases and body parts by incineration. |
2.8.4 Test code categories
A table of test codes to be used to submit samples can be found at Annex 3. It is essential the correct codes are used for disease surveillance purposes. The number of animals tested as emergency slaughter, sick at ante-mortem, and healthy are reported as part of the UK’s BSE surveillance programme.
2.8.5 Contaminated / leaking pots
Contaminated / leaking pots will delay the testing process in the lab and could result in delayed results.
Sampling staff must take care to avoid splashes of blood on the outside of the sampling pots and to ensure that the sample pot lid is firmly screwed and securely tightened and checked prior to being packed.
Note: Tamper-evident pot lids are available from an approved laboratory.
2.8.6 FBO responsibility
FBOs must ensure that sample pots are correctly identified and that correlation of the animal and the carcase and parts is maintained at all times.
2.8.7 FSA action- loss of correlation
If FSA checks show correlation has been lost, the OV must
- notify the FBO
- stop processing
- check all animals to the point that correlation has been lost
- instruct the FBO to dispose of all non-correlated carcases / offal as Category 1 SRM by incineration
Note: If the OV is satisfied that all animals that require BSE testing have been sampled, even if individual correlation is lost, carcases may be passed fit for human consumption if all of the submitted sample tests return a negative test result.
This does not affect any enforcement actions to be taken as a non-compliance with the TSE regulations.
2.9 Samples packaging and delivery
2.9.1 Same day delivery
The FBO is responsible for ensuring that samples are despatched as soon as possible on the same day of the sampling
2.9.2 Storage
If samples cannot be despatched the same day of sampling, they can be chilled in a fridge (samples can be stored for up to 4 days at 4oC).
The FBO must ensure that any storage arrangements for brain stem sample pots do not allow cross contamination with food / meat intended for human consumption.
2.9.3 Packaging and delivery
The FBO is responsible for the labelling and packaging of samples, and arranging their delivery to the approved testing laboratory specified in the SOP. A list of approved laboratories can be accessed online.
FBOs must arrange for the samples to be delivered to an approved testing laboratory under the TSE Regulations (England) 2018 Schedule 2, paragraph 9 or the TSE Regulations (Wales) 2018 Schedule 2, paragraph 5. The laboratory can be approved by the UK or another member state of the EU.
The FBO is responsible for ensuring that there are sufficient slaughterhouse staff trained and competent in the labelling, packaging and despatch of brain stem samples.
2.9.4 Packaging and labelling requirements
The FBO must ensure that brain stem samples are packed and labelled in accordance with the packaging instructions P650 of the European Agreement Concerning the International Carriage of Dangerous Goods by Road (version applicable as from 1 January 2005).
2.9.5 FBO verification of number of samples despatched
The FBO must:
- reconcile the number of samples with the number of bovines slaughtered that require BSE testing before the samples are despatched
- notify the number of samples being delivered to the testing laboratory in advance of their despatch by fax or by e-mail
2.9.6 FSA controls
FSA Staff must:
| Check | Frequency | Records |
|---|---|---|
|
Every day sampling takes place. |
TSE 6/11 |
Note: For sample copy of TSE 6-11 see chapter 9 on ‘Forms’.
2.10 Traceability of all parts: tested animal to carcase
2.10.1 FBO traceability system
The FBO must arrange for a reliable and robust traceability system which satisfies the OV that the carcase and all retained parts can be traced to the brain stem sample, with
- identification of both carcase and brain stem sample
- individual or batch identification of offal, hides, blood, trimmings, feet, udders, fat and by-products
If a batching system is adopted, all material in the batch must be disposed of as SRM by incineration if a positive, insufficient or ‘no test’ result is obtained.
2.10.2 FSA responsibility
FSA staff must confirm that the system for traceability is robust and adequate to ensure that all parts of any positive carcase and the one before and two afterwards on the slaughter line (1b2a) and all parts of any ‘no test’ carcase can be identified and disposed of appropriately. Where the obex is not present and there is ‘insufficient’ material to run three check-tests, the carcase will be treated as a positive and the 1b2a will also need to be destroyed.
2.10.3 FBO by- product records
The FBO must keep records of all SRM material requiring incineration despatched from the premises prior to receipt of negative test results.
2.10.4 FSA supervision of FBO records
The OV must verify the FBO records of disposal to confirm that the destination for disposal is approved for incineration of SRM.
2.10.5 Health marking of carcases and offal awaiting BSE test results
Carcases of animals that require BSE testing must only be health marked after satisfactory Post-Mortem inspection. All health marked carcases must be kept under FSA secure controls until a negative test result has been verified.
Offal may also be health marked or identification marked prior to the receipt of a BSE test result, however the offal must be kept under FSA secure controls until a negative test result has been verified.
2.11 Retention of carcases
2.11.1 FBO retention facilities
The FBO must have sufficient and suitable facilities for holding all the carcase(s) (and the one before and two after as appropriate) and all body parts of animals requiring BSE testing until results are received.
Hides, however, may be sent to an approved hide premises under official control.
All carcases and body parts must be stored as per the SOP.
2.11.2 FBO retention of carcases
Carcases retained pending a test result must be held by the FBO in accordance with one of the following options:
Food business operator retention of carcases
| Retention area | Action |
|---|---|
| In a detained chiller: | Carcases awaiting test results must not come into contact with detained carcases awaiting further examination by an inspector or OV. |
| In a chiller other than a detained chiller: | Carcases awaiting test results (including the one before and two after, if appropriate) must be batched and stored together and must not come in to contact with any other carcases. |
2.11.3 Storage in non-sequential kill order in chillers
Carcases awaiting BSE test results must be stored in chillers in kill order unless the criteria below are applied:
- a draft protocol is drawn up by the FBO outlining the procedures to be followed and is agreed with the OV and the FVC for the premises
- the OV and FVC for premises that rely primarily on IT systems for traceability of animals / carcases within the premises should be satisfied that such IT systems demonstrate traceability and also, that there are manual back-up procedures in place in the event of IT systems failing
- the OV and FVC for premises which do not rely primarily on IT systems for traceability of animals / carcases within the premises should be satisfied that their manual traceability systems are such that they provide the necessary assurances with regard to traceability
- in the event that the traceability systems fail to identify the relevant carcases (the positive, insufficient or the ‘no test’ carcase and the 1B2A from the kill line) the OV must consult the FVC as there may be a need to destroy the whole batch
2.11.4 FBO retention of detained carcases
Carcases detained for other reasons, must also be retained pending a test result. They should be held securely and not come in to contact with other detained carcases.
Under these circumstances, it may be inevitable that carcases are not held in the order of kill.
2.11.5 FSA security of the retention facilities
FSA staff must ensure that at the end of each day the holding area is secure and that all parts of tested animal(s) (and one before and two after, if appropriate) are retained under official controls until the results are provided by the FBO to FSA staff.
Chillers must be secured by FSA either sealing the chillers or the rails that contain carcases pending test results.
Seals may not be broken except by FSA staff.
All procedures relating to chiller controls must be recorded by FSA staff on form TSE 6/10:
Reference: See chapter 9 ‘Forms’ for a sample copy of TSE 6/10.
2.11.6 Test carcases or its parts not fit for human consumption
Test carcases or parts of test carcases (pending a test result) found unfit for human consumption must not be stored with other carcases or part carcases that have been passed fit for human consumption.
These carcases or part carcases may be dealt with differently depending on the following situations:
Test carcases or its parts not fit for human consumption
| If... | Action |
|---|---|
| the FBO does not wait for the test result | Dispose of as SRM by incineration |
| a negative test result is received | Retain hygienically and once the negative test result is received dispose of as required depending on the by-product category of the material |
| a positive, insufficient or ‘no test’ result is received | Retain hygienically and once the positive, insufficient or ‘no test’ result is received dispose of the positive and the 1B2A carcases by incineration as appropriate |
2.12 Retention of all body parts
2.12.1 Identification and retention of body parts
After slaughter, all parts of the animal under test which have been retained must be traceable to the carcase held during retention, and must be treated as below.
Identification and retention of body parts
| Part of the carcase | Action |
|---|---|
| Offal intended for human consumption | Must be retained under official control:
|
| Blood | All blood must be retained separately or batched pending a test result, unless disposed of as SRM by incineration before test results are received. FSA controls must be as follows:
|
| Green offal | Parts of green offal not classified as SRM (such as stomachs) must be:
|
| Offal not intended for human consumption | Offal not intended for human consumption must be retained before the test results are received or disposed of as SRM by incineration before a test result is obtained |
| Hides | Reference: See topic 2.13 on ‘Retention of hides’ in this section for additional information |
2.12.2 SRM
SRM removed from carcases must be stained and disposed of by incineration if not held until a negative result is available.
2.12.3 Rumen and gut contents
Rumen contents and gut contents must be disposed of in accordance with existing procedures.
2.13 Retention of hides
2.13.1 Retention at the premises
Hides must be held in the hide room either batched or individually identified.
If batched without individual identification, they must be clearly labelled ‘pending test result’, including the number of hides in the batch and the date of slaughter.
2.13.2 Despatch of hides to a hide premises
Hides may be despatched to a hide market or tannery before a test result is received.
If any of the carcases subsequently test positive or there is no negative test result, the hide must be identified by APHA staff at the hide market or tannery and destroyed by incineration. If batched, the entire batch will need to be destroyed by incineration.
FBOs must agree a hide protocol with APHA to allow hides to be despatched prior to receipt of test results. The OV must be aware of the hide protocol.
2.13.3 Official controls
Hides must be detained under official control until the test results are received.
Official controls
| Hide | FSA control |
|---|---|
| retained at slaughterhouse pending test result | FSA AOs are to do:
|
| removed to an approved hide market before test result obtained | Note: At hide premises APHA checks disposal if a positive result is received. |
2.13.4 Hides from positive, insufficient or ‘no test’ result
If hides are individually identified then any positives must be disposed of as SRM by incineration.
If they are batched, the entire batch must be incinerated in the event of a positive or ‘insufficient’ test result.
2.14 Test results
2.14.1 FBO responsibility
The FBO is responsible for:
- having facilities in place to receive tests results, and
- giving FSA staff in plant copies of the test results
Copies of the test results must be kept by the FBO for 24 months.
2.14.2 FSA responsibility
FSA staff are to read the test results and take all necessary actions. Form TSE 6-7 must be completed and, together with copies of the test results, kept on file in the FSA office for 12 months.
Poor quality samples reported by the lab will need to be investigated. The SLA and contracts team will notify the local team from the plant affected to request an investigation. Results of the investigation and where necessary further preventative action need to forwarded to sla.contracts@food.gov.uk , FVC and FVL.
2.14.3 FSA access to test results
Upon completion of a successful approval trial, the approved laboratory will work with SLA and Contract team to provide access to the results for FSA staff.
2.15 Positive test result
2.15.1 Notification of positive test result
When a positive BSE test is identified, the approved laboratory will inform:
- the FBO
- the FSA staff at plant
- APHA
APHA will be responsible for DNA testing of samples collected by FSA.
2.15.2 DNA Sampling kits
APHA will deliver the sampling kits to the plant.
The sampling kit will include:
- an outer box
- sample pots
- labels
- tamper-evident bag
- freezer pack
2.15.3 FSA responsibility for DNA sampling
FSA staff are to take samples for DNA testing from the positive carcase and the one before and one after.
Samples, approximately the size of a 50p coin, are to be taken from the diaphragm of each carcase ensuring there is no cross contamination between carcases. These must be sealed into separate sample pots and placed into the pot tray and sealed into the tamperproof bag and then frozen for 24 hours under FSA control.
Once the samples are frozen, FSA staff should contact APHA by email at: lab.services@apha.gsi.gov.uk to inform them of the plant number and the samples to expect for the Cattle Microsatellite Identification test (DNA test).
FSA staff should then contact PDP couriers on 01784 420466 using the ‘VETLAB’ account, to organise collection of the samples, which are to be sent to:
Central Sequencing Unit
APHA Weybridge
Woodham Lane
New Haw
Addlestone
KT15 3NB
Once the collection time has been arranged place the samples in the box with the freezer packs for collection.
2.15.4 Carcase and body parts
If a positive test result is received, the carcase and all parts of the positive animal, or the whole batch if a batching system is in operation, together with all parts of the animal slaughtered before and the two animals slaughtered afterwards (‘1B2A’) must be destroyed by incineration unless there have been effective arrangements put in place for preventing cross contamination between carcases during processing and storage.
Note: The 1B2A rule applies.
Note: The FBO is responsible for identification and disposal by incineration of the relevant carcases and parts.
Note: The OV must confirm the identity of the positive carcase (and the one before and two after (‘1B2A’) during the process), offal (which may have been batched), hide and blood and will verify that this material is consigned to a site approved to incinerate ABP Cat 1 SRM.
Note: AOs must check 100% of carcases and body parts for full traceability and disposal and should verify the slashing and staining of positive carcases.
2.15.5 Hides
If the hides are stored in the slaughterhouse hide room until the test result is received and there is a positive result the individual hide or the entire batch (if not individually identified) must be identified under FSA supervision and destroyed by incineration.
Note: The 1B2A rule applies.
Note: For the action to be taken for hides delivered to a hide premises refer to the ‘Hide protocol’.
2.15.6 SRM records
The FBO is responsible for maintaining accurate records of the weight disposed of as SRM by incineration.
2.16 ‘No test’ result
2.16.1 Carcase and body parts
If a ‘no test’ report is received, for example, due to the target area of the obex being unavailable, the carcase and all parts of the ‘no test’ animal must be destroyed by incineration.
Note: The 1B2A rule does not apply. However, the whole batch must be destroyed if a batching system is in operation.
Note: If the target area of the obex is not available, the testing laboratory will carry out three further tests. Only when these further tests return negative, will the sample be reported as a ‘no test’. If the laboratory cannot assign negative results to the three additional tests or there is insufficient material to test, then the 1B2A rule applies
The FBO is responsible for identification and disposal by incineration of the relevant carcases and parts.
2.16.2 Hides
If the hides are stored in the slaughterhouse hide room until the test result is received and there is a ‘no test’, the individual hide or the entire batch (if not individually identified) must be identified and despatched for destruction by incineration.
Note: The 1B2A rule does not apply to individually identified hides.
2.16.3 FSA responsibility
The OV must confirm the identity of the ‘no test’ carcase, offal (which may have been batched), hide and blood and will verify that this material is delivered to be destructed by incineration.
Note: AOs must check 100% of carcases and body parts for full traceability and disposal.
2.16.4 SRM records
The FBO is responsible for maintaining accurate records of the weight disposed of as SRM by incineration.
2.17 ‘Insufficient’ test result
2.17.1 Carcase and body parts
If an ‘insufficient’ test result is received indicating that the approved testing laboratory has not been able to carry out the three further tests on the submitted sample because of a shortage (or complete absence) of suitable brain stem material, the FBO must immediately dispose of as SRM:
- the carcase and all parts of the body (including the blood) of that animal
- the carcase and all parts of the body (including the blood) of the animal immediately preceding that animal on the slaughter line and the two animals immediately following it
- the whole batch if a batching system is in operation
2.17.2 Hides
If the hides are stored in the slaughterhouse hide room until the test result is received and there is an ‘insufficient test’ result, the individual hide or the entire batch (if not individually identified) must be identified and despatched for destruction by incineration.
Note: The 1B2A rule does not apply to individually identified hides.
2.17.3 FSA responsibility
The OV must confirm the identity of the ‘insufficient test’ carcase, offal (which may have been batched), hide and blood and verify that this material is delivered to be destroyed by incineration.
Note: AO’s must check 100% of carcases and body parts for full traceability and disposal.
2.17.4 SRM records
The FBO is responsible for maintaining accurate records of the weight disposed of as SRM by incineration.
2.18 ‘Inconclusive’ test result
2.18.1 Carcase and body parts
An ‘inconclusive test’ should be treated as a ‘positive’.
Note: The EU TSE Regulations set out what action should be taken in the event of an ‘inconclusive test’ result. However, this category is not recognised in the TSE statutory instrument (SI) because in practice, the BSE tests used by approved laboratories provide either positive or negative results.
2.19 ‘Outstanding’ test result
2.19.1 Carcase and body parts
If an ‘outstanding’ test result is received, indicating that some of the paperwork is missing or incomplete, the approved testing laboratory has not been able to correlate the sample to the animal details and further information is required from the FBO. In this case, the FBO must immediately submit any information required.
Meanwhile:
- the carcase and all parts of the body (including the blood) of the ‘outstanding’ test result animal, must remain under official control until the laboratory receives the information from the FBO and is able to release the test result
- the carcase and all parts of the body (including the blood) of the animal slaughtered immediately before the ‘outstanding’ test result animal and the two animals immediately following it (1B2A), must remain under official control until the laboratory receives the information from the FBO and is able to release the test result
- if a batching system is in operation, the whole batch must remain under official control until the laboratory receives the information from the FBO and is able to release the test result
2.19.2 Hides
If the hides are stored in the slaughterhouse hide room until the test result is received and there is an ‘outstanding’ test result, the individual hide or the entire batch (if not individually identified) must be identified and kept under official control or despatched for destruction by incineration.
Note: The 1B2A rule applies.
2.19.3 FSA responsibility
The OV must confirm the identity of the ‘outstanding’ test carcase (and the one before and two after (1B2A) during the process), offal (which may have been batched), hide and blood and will verify that these remain under official control until the test result is obtained from the laboratory.
Note: AO’s must check 100% of carcases and body parts for full traceability and disposal.
2.20 Negative test result
2.20.1 Carcase release
When a negative result is received, the carcase and offal may be released as fit for human consumption unless they need to be destroyed under the 1B2A requirements.
2.20.2 Vertebral column removal
The vertebral column of OTM cattle slaughtered for human consumption must be removed in an approved cutting plant additionally authorised for vertebral column removal, then disposed of as SRM.
Reference: See instructions on the removal of the vertebral column in authorised cutting plants in chapter 2.7 on ‘Specified risk material’.
Note: A list of these cutting plants is available on the FSA website.
2.21 Enforcement
2.21.1 Enforcement responsibility
The FSA is responsible for enforcement within authorised premises, acting on behalf of Defra, Scottish Government and Welsh Government.
The local authority (LA) is responsible for enforcement outside authorised premises, including the consignment of cattle born or reared in the UK before 1st August 1996 to slaughterhouses.
Reference: See chapter 2.5 on ‘Animal Identification’ for additional information on eligibility of cattle for slaughter.
3. Sheep TSE Sampling and Submission
In this section
3.11 Packaging and storage of samples: Bioshield box and Bioshield bottle
3.14 Collection and disposal of sharps and clinical waste
3.1 Survey requirements
3.1.1 Regulations
(EC) 999/2001 requires Member States to have a survey in place to monitor the presence of Transmissible Spongiform Encephalopathies (TSEs) in sheep.
The above regulation is enacted in GB by The Transmissible Spongiform Encephalopathies (England) Regulations 2018 and the Transmissible Spongiform Encephalopathies (Wales) Regulations 2018, as amended.
3.1.2 Relevant establishments
These instructions are relevant to FSA staff at slaughterhouses participating in the Sheep TSE Survey.
3.1.3 Authorisation
Under the TSE Regulations, FBOs and their employees are required to comply with such reasonable requirements as the inspector considers necessary. An inspector in this case is any FSA officer who has been issued with an authorisation, after the satisfactory completion of the necessary training, in accordance with the TSE Regulations.
3.2 Sampling plan
3.2.1 Sheep sampling plan
APHA, in consultation with Defra and the FSA, determine the required percentage to be sampled. The SLA and Contracts team will notify this percentage to FSA staff in participating plants.
The sampling plan enables the epidemiological requirement of the survey to be met and completed by the end of each calendar year.
APHA assesses the sampling survey throughout the year, which means that sampling percentages may be adjusted during the year to achieve the required target.
Brain stem samples must be collected in order to test selected animals in compliance with the sheep survey.
Samples are sent to the TSE Testing Laboratory for testing.
To meet the increased EU requirement (Regulation (EC) 36/2005) for TSE testing and increase the analytical sensitivity of the testing program, it is required that the cerebellum is included in the sample along with the brain stem. This allows classical scrapie to be differentiated from atypical scrapie should the sample test positive.
3.2.2 Suspect scrapie cases
Suspect scrapie cases must be reported immediately to the local APHA Regional Operational Director (ROD) / Divisional Veterinary Manager (DVM) and the animal(s) must not be allowed to enter the slaughterhall.
Reference: See chapter 6 on ‘Notifiable diseases’ for the procedures that must be followed.
3.2.3 Dead on arrival and dead in lairage
(EC) 999/2001 requires a number of sheep over 18 months of age that have died other than by being slaughtered for human consumption to be tested for TSE.
Brain stem and cerebellum samples are collected for testing instead of whole heads being sent to the TSE Testing Laboratory.
3.2.4 Compulsory scrapie flock scheme
On confirmation of TSE in sheep flocks, current EC Regulations ((EC) 999/2001, (as amended), Annex III, Chapter A) require further testing of these flocks over a three year ‘restriction period’ under the Compulsory Scrapie Flock Scheme (CSFS).
A random selection of sheep aged over 18 months slaughtered for human consumption is to be tested. Such animals must not be included in the percentage of sheep required to be tested under the normal sampling plan.
CSFS animals can only go to slaughterhouses currently participating in the survey or that have previously participated and trained staff are available, and where the FBO is happy to take them.
Reference: See sub-topic 3.4.10 on ‘Selection CSFS’ for further information.
3.2.5 Goat sampling plan
At present, FSA staff are not required to sample adult goats.
3.3 When to sample
3.3.1 When to collect samples: sheep/goat surveys
Samples must only be collected Monday to Thursday, and ideally spread out over the four days. Samples must be taken on any of these days when eligible animals are selected.
Note: Sampling of goats, including those dead on arrival (DoA) or dead in lairage (DiL), is currently not required.
Samples must not be collected on Fridays or when the following day is a public holiday.
3.3.2 When to collect samples: CSFS
CSFS animals may be presented on any day of operation but the Specialist Service Centre (SSC), Worcester (formerly the National Scrapie Plan Administration Centre – NSPAC) will endeavour to ensure they are slaughtered Monday to Thursday.
Should any CSFS animals be slaughtered on a Friday, weekend or Public Holiday, samples must be taken and stored in a refrigerator until they can be despatched.
3.3.3 When to collect samples: All
Caution: Failure to comply with these instructions will result in a delay in testing and return of the results to the slaughterhouse, as the laboratory will not be able to cope with incorrect or excessive supply.
Note: During religious festivals, sheep that have been selected by a family may be excluded from the sampling process; however, any shortfall must be made up during the same week.
3.4 Selecting eligible animals to sample
3.4.1 Selection: sheep survey
Sheep are selected by either the OV or MHI (the ultimate responsibility rests with the OV).
Only sheep with a flockmark or individual ear tag are to be selected.
Selection within the pen should be at random, avoiding over-representation with regard to:
- origin
- age (confirmed by a post-slaughter dentition check)
- production system
- any other characteristic
Reference: See chapter 2.5 on ‘Animal identification’ for additional information.
Note: Animals should not be selected and then held overnight for slaughter the following day. However, in exceptional circumstances such as when animals are unacceptably dirty, animals may be held overnight. In this case, the lab must be informed, so they can expect the samples the following day.
Note: Sheep born and raised in Great Britain (GB) are preferred for sampling. If there are no GB sheep available, sheep born and raised overseas may be selected.
Establishments participating in the survey must sample a given percentage of their weekly throughput of ewes and rams aged over 18 months (eligible animals).
Whenever eligible sheep are killed, the minimum number of samples to collect is one, never zero.
Calculate the number of samples required by:
a. divide the number of eligible sheep by 100 (for example, 256 / 100 = 2.56)
b. multiply the result by the given percentage (for example, 2.56 x 1.0 = 2.56 or 2.56 x 0.5 = 1.28)
c. round the result to the nearest whole number (for example, 2.56 = 3 or 1.28 = 1)
The daily percentage number should only be used as a guide.
The total number of samples collected during the week should reflect the given percentage of the weekly throughput of eligible sheep. Any shortfall should be made up the following week.
3.4.2 Worked example: sheep survey
Sheep survey sampling days
| Day | Mon (Sampling day) | Tue (Sampling day) | Wed (Sampling day) | Thu (Sampling day) | Fri | Sat | Sun | Total |
|---|---|---|---|---|---|---|---|---|
| Eligible throughput | 256 | 301 | 124 | 0 | 13 | 104 | 0 | 798 |
| Samples required (1%) | 3 | 3 | 1 | 0 | 1 | 1 | 0 | 9 |
| Samples collected | 4 | 0 | 3 | 0 | 0 | 0 | 0 | 7 |
| +/- | +1 | -3 | +2 | 0 | -1 | -1 | 0 | -2 |
| Eligible throughput | 256 | 301 | 124 | 0 | 13 | 104 | 0 | 798 |
| Samples required (0.5%) | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 6 |
| Samples collected | 4 | 0 | 3 | 0 | 0 | 0 | 0 | 7 |
| +/- | +3 | -2 | +2 | 0 | -1 | -1 | 0 | +1 |
3.4.3 Identifying selected sheep
| Options | Method |
|---|---|
| 1 |
The fleece of all eligible animals must be marked in the lairage using the green colour identifier spray. Mark each animal with a number starting at one (1) each day. This will allow sheep to be presented for slaughter in number order and facilitate correlation. Sprays are ordered by FSA staff from the TSE Testing Laboratory using the TSE Testing Laboratory consumable order form (Annex 5). |
| 2 |
The lairage staff should handle and tag each selected animal with the red ear tag under FSA supervision, post-slaughter but before head removal. This is an FBO requirement and the FSA officer can direct the FBO to do so. If local conditions do not permit this then the lairage staff should restrain the sheep for the OV to insert the tag, although this should be avoided. |
3.4.4 Form completion
At the point of selection you should begin completion of the Daily Record Sheet (TSE 6/6) and the Rapid Testing form (TSE 6/2).
For more information, see topics 4.1 and 4.2 in this chapter.
3.4.5 Selection: goat survey
If the sampling of goats is required, 100% of adult goats presented for slaughter must be sampled.
3.4.6 Dentition check: sheep / goat surveys
A dentition check must be made by FSA staff post slaughter. Only sheep and goats that have more than two permanent incisors erupted may be sampled. Enter the number of incisors for the animal’s TSE 6/2 at this point.
Otherwise, do not take the sample and discard the TSE 6/2 that was completed in the lairage. The carcase can be processed as normal and does not need to be retained.
Older animals that have lost some or all of their permanent teeth (‘broken mouthed’) are still eligible for testing.
3.4.7 Selection: dead on arrival (DoA) and dead in lairage (DiL)
In participating establishments, adult sheep found DoA or DiL must be tested for TSE as part of the fallen stock survey.
The animal(s) may have to be moved by the FBO to a suitable place, for example, the isolation pen in the lairage.
Adult sheep or goats are those with more than two permanent incisors erupted through the gum.
Brain stem samples are collected from DoA and DiL animals and are to be identified with ‘DoA / DiL’ on the pot lids.
Note: If the slaughterhouse is not part of the survey the animal should be disposed of as Category 1 (Cat 1) Animal By-product (ABP) (Specified Risk Material (SRM)), by incineration.
3.4.8 Health and safety
FSA staff must wear suitable protective clothing including disposable gloves.
Reference: See FSA ‘Health and safety manual’.
3.4.9 Tagging
If any sheep do not have an ear tag they must still be sampled. Place a red tag in the ear, and record this number in the ‘other ear tags’ field on the TSE 6/3 form.
3.4.10 Body disposal
After removal of the head for sampling, the body (and any contaminated material) must be disposed of as Cat 1 ABP (SRM) by incineration. An additional allowance of 30-40kg must be allowed per animal in terms of the overall calculation of daily SRM weights.
3.4.11 Selection: CSFS
SLA and Contracts Team will be notified in advance by the SSC (Worcester) of the number of CSFS animals to be slaughtered and sampled to ensure that enough sampling equipment and resources are available.
SLA and Contracts Team will contact the OV and / or Inspection Team Leader (ITL) upon receipt of the details.
Animals will be accompanied by forms NSP 61 and NSP 61A, which will already have been partially completed.
The NSP 61A will show the details of every animal selected to be culled. Some will be identified with an orange ear tag and these are the animals to be sampled (‘‘selected animals’’). This tag can be used to uniquely identify the animal during processing.
Animals not identified with an orange ear tag must not be sampled.
CSFS animals selected for testing (with an orange ear tag) that are found DoA or DiL must be sampled. The relevant sections in form NSP 61 and NSP 61A must be completed. There is no need to complete a TSE 6/3 form.
Sheep that are DoA or DiL which are not selected (do not have an orange tag) must also be tested. See sub-topics 3.4.6 to 3.4.9 in this chapter.
Such animals must not be included in the percentage of sheep required to be tested under the normal sampling plan.
3.5 Slaughter arrangements
3.5.1 Slaughter protocol
The OV should ask the FBO to produce and agree a written protocol for slaughter, sample collection, identification and retention of split carcases, offal and waste and cleaning of equipment and premises. The FBO, OV and FVC must sign these and retain copies.
To ensure traceability, the OV should liaise with the FBO to agree a reliable method for identifying and correlating the retained carcases and body parts (including organs, tissues, blood and fleece / skin), as part of the protocol.
3.5.2 Hygiene
Slaughter procedures for sample animals must not jeopardise hygiene at the premises. Captive bolts can be used in the slaughter process.
3.5.3 Batch slaughter
The most efficient way to collect samples is to slaughter the selected animals in one batch. This may also minimise the time between slaughter and despatch of samples.
3.5.4 Time of slaughter
FSA staff are to record in the plant daybook the time the first sample animal is slaughtered.
3.5.5 On-line splitting
Splitting of carcases may be undertaken on the slaughter line during dressing where the OV is satisfied that there is full compliance with the legislative requirements relating to cross-contamination between carcases.
3.6 Correlation
3.6.1 Requirement for correlation
The carcase and all body parts of the sampled animal must be identifiable and retained under the control of the FSA until a test result has been obtained, unless they are immediately disposed of as Cat 1 ABP (SRM) by incineration.
It is the OV’s responsibility to decide what system should be used to ensure that correlation between carcase, blood, body parts, offal, fleece and sample is maintained at all times.
3.6.2 Correlation essentials
It is essential that:
- each half of the carcase and any retained body parts are correlated with the number on the red ear tag (routine sampling) or orange ear tag (CSFS)
- all body parts of the carcase are identifiable to the sample animal during retention
- each half carcase is identified using matching numbered detention tags
3.6.3 Carcase correlation
After the fleece has been removed, attach to the carcase a detention tag numbered (colour of the day) with the spray number on the fleece. This correlates the carcase with the red or orange ear tag number.
3.6.4 Animal by-products
Use matching numbered detention tags to identify retained red and green offal. The low value of these may mean that the FBO opts to dispose of all the offal as Cat 1 ABP (SRM) by incineration or, alternatively, to batch retained offal without individual correlation pending the result.
3.7 Blood collection
3.7.1 Collection options
Blood must be collected either:
- in containers used for individual animals, the dimensions of which must be such that the splashing of blood into the surrounding area is avoided
- in a blood tank or container separate to that used for collection of blood from non-test animals
- in a blood tank or container used for collection of blood from test and non-test animals
Note: Blood collected from test animals can only be mixed with blood from non-test animals if in the case of a positive test result all blood is disposed of as Cat 1 ABP (SRM) by incineration.
3.7.2 SRM allowance
If blood is disposed of as Cat 1 ABP (SRM) by incineration, an additional weight of 2.5kg must be allowed per animal in terms of overall calculation of daily SRM weights.
3.8 Sampling arrangements
3.8.1 Suitability
In considering suitability of sampling arrangements at individual establishments, the OV must take the following points into consideration:
- there must be adequate, effective separation between the sampling location and fresh meat so as to ensure that cross-contamination of fresh meat intended for human consumption does not occur
- no aspect of hygiene must be breached
- no aspect of the SRM rules must be breached
- functional wash hand basin, soap and hand drying facilities must be within easy access of the sampling table
- only the stainless steel sampling table provided by APHA must be used for sampling; the table is the property of APHA and not the FBO
3.8.2 Sampling within the slaughterhall
If the sampling location is within the slaughterhall it should not be situated beyond the point at which the fleece is removed from the carcase.
3.8.3 Sampling equipment supplies
The TSE Testing Laboratory will supply all sampling equipment directly to FSA staff at the slaughterhouse. If supplies are running short re-order direct from the stores using the TSE Testing Laboratory consumables order form. It takes on average five working days from the placement of an order to the receipt of equipment at the slaughterhouse. Place the order before supplies run out. It is recommended that plants hold sufficient supplies for at least two weeks if possible.
See Annex 5 for a sample copy of the order form.
3.9 Sampling preparation
3.9.1 Head removal
The OV must check that the FBO removes the head ensuring that the brain stem and cerebellum remain intact within the skull. Cross-contamination between heads must be prevented.
3.9.2 Who performs sampling
Removal of the brain stem and cerebellum must only be undertaken by FSA staff trained in this process by a fully trained OV/ITL or by the TSE Testing Laboratory.
Note: Only the TSE Testing Laboratory may train staff in plants which are new to the scheme.
3.9.3 Equipment
One set of disposable equipment must be used to collect both brain stem and cerebellum from one head. Sufficient equipment to undertake testing of the day’s quota is to be laid out in preparation for undertaking brain stem and cerebellum removal, and is as follows:
- sampling table
- head tray
- instrument tray
- tag cutting scissors
- heavy duty undergloves and disposable latex gloves
- bioshield box and inner frames for pots or biobottle with packing box
- benchkote dispenser and sheets
- forceps
- scissors
- sampling spoons
- sample pots
- snap lock plastic bags
- forms bag
- sharps box and clinical waste unit
- SRM label
- disposable apron
- plastic refuse bag and paper towels
3.9.4 Cross-contamination
The brain stem and cerebellum must be collected in a manner that prevents any risk of contamination between the samples. Use new instruments and latex gloves for each head sampled, keep all parts of the sampling workstation clean and neat, and keep the pots, containers and packaging clean and dry.
3.9.5 Packaging
To prevent contamination of the bioshield box by water or blood during sampling, the bioshield box and inserts should be stored separately in a dry area. Completed sample pots can first be placed on a tray on the table and then packaged in the bioshield box on completion of the sampling.
Prior to filling with completed sample pots the bioshield box or bottle should be made ready by placing a sheet of frozen Techni-Ice at the base of the box or an ice-brix in the bottom of the bottle.
3.9.6 Procedure
For each head to be sampled the FSA officer must follow the steps in the table below:
| Step | Action |
|---|---|
| 1 |
Ensure that the safety helmet, visor (or goggles / face mask supplied as an alternative) and undergloves are worn to comply with health and safety risk assessments Note: The undergloves may be household rubber gloves or latex gloves |
| 2 | Put on a pair of latex gloves over the undergloves |
| 3 | Place a new piece of benchkote on the instrument board |
| 4 | Place new forceps, scissors and sampling spoon on the benchkote |
| 5 | Open a pot and place pot and lid on benchkote |
| 6 | Open one small plastic bag, open out and insert into the open pot |
3.9.7 Transfer of heads
Heads should be transferred either individually or collectively to the sampling site in the slaughterhouse. Brain stem and cerebellum samples are easier to remove without causing damage if they are left to cool for at least five minutes.
3.9.8 Head position
Place head upside down in the head tray with foramen magnum facing the sampling officer.
See diagrams 1 and 2 below for anatomy and outline of the dissection method.
3.9.9 Brain stem removal
Diagram 1: Anatomy and sampling steps
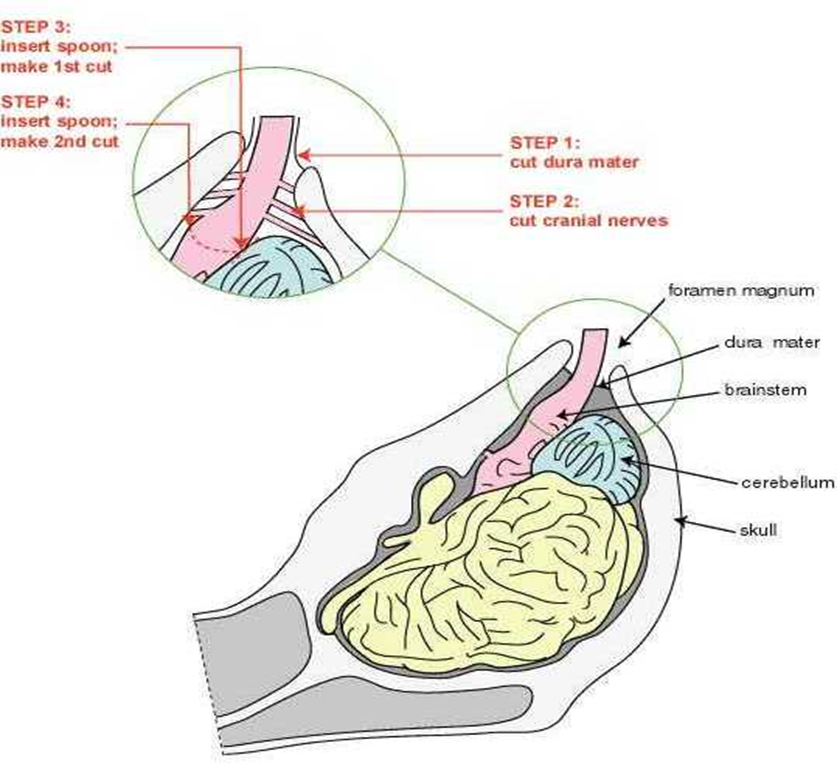
Diagram 2: the area of tissue (obex) to be targeted
3.9.10 Removal procedure
Follow the steps in the table below to remove each brain stem sample.
| Step | Action |
|---|---|
| 1 | Firstly, remove any blood clots (using scissors and forceps) obscuring the view of the brain stem and identify the dura mater. Using a fresh pair of scissors cut free the dura mater from the brain stem and its attachment to the skull. Gently hold the brain stem as close to the end as possible with the forceps. If possible hold any remaining dura mater on the surface of the brain stem as this will provide a better grip of the brain stem. |
| 2 | Identify the position of the cranial nerves within the skull. Holding the brain stem with the forceps, move the brain stem to the side and insert the scissors into the foramen magnum and cut through the cranial nerves (VII –XI) that originate from the brain stem at three and nine o'clock positions. Take care not to cut the brain stem. Do this on both sides of the brain stem. |
| 3 | Using the forceps, very gently pull the brain stem until it is straight and insert the spoon underneath it with the cutting edge of the blade facing down. Keep the blade and the spoon against the bone as it is inserted. Insert to the level of the notch of the spoon – approximately 8-10cm. When in position, point the cutting edge of the blade downward by lifting the handle upwards. Cut through the cerebellar peduncles by moving the handle from side to side. |
| 4 | After completing the cutting, carefully withdraw the spoon and insert it with the blade pointing downwards on top of the brain stem until it enters to a depth of 8-10cm. Gently move the blade side to side and downwards, and cut through the rostral medulla. Avoid rotating or excessive side to side movements of the spoon, as this will damage the obex. |
| 5 | Gently pull with the forceps, and using the spoon as a scoop or lever, remove as much brain stem as possible from the skull. If resistance is encountered, check that the cranial nerves and dura mater have been freed from the brain stem. If not, repeat step 1. However, if this area is free, reinsert the spoon and continue to cut through the brain stem with a gentle side to side and downward motion of the handle. |
| 6 |
Place the brain stem in the snap-lock plastic bag in the pot. Note: This same bag will be used to put both the brain stem and cerebellum from the animal. |
3.10 Cerebellum removal
3.10.1 Cerebellum removal procedure
After removing the brain stem, follow the procedure set out below for removing the cerebellum.
| Step | Action |
|---|---|
| 1 | With the ventral aspect of the head uppermost, look downwards through the foramen magnum and identify the cerebellum. Insert the spoon underneath the cerebellum and lever it upwards into the centre of the space vacated by the brain stem. |
| 2 | If the cerebellum is not moving freely, loosen it by moving the spoon gently around and underneath it. |
| 3 | Lift the cerebellum towards the foramen magnum using the spoon to support and guide, and gently take hold of the cerebellum with the forceps. Remove the cerebellum through the foramen magnum. |
| 4 | Place the cerebellum in the same bag with the brainstem sample. Close the bag. |
3.10.2 Equipment / hygiene procedures
After removing the cerebellum the steps in the table below must be followed.
| Step | Action |
|---|---|
| 1 | Remove the numbered section of all ear tags using the tag cutting scissors, and place them in the pot on top of the snap-lock plastic bag. Ears must not be placed with the ear tag in the pot. However, to avoid injury from sharp edges when removing metal ear tags, a small piece of ear is allowed to remain on the tag. Seal the pot. |
| 2 | Put the head and any pooled blood from the head tray in the SRM bin. Wipe down the tray with disposable paper as necessary. |
| 3 | Place the disposable scissors in the sharps box. |
| 4 |
Put the forceps, spoon, used benchkote, paper towels and apron in the clinical waste container, then remove the latex gloves and put them in the clinical waste container. See topic 3.14 on ‘Collection and disposal of sharps boxes and clinical waste units’ in this chapter. |
| 5 | Place the lid on the pot, seal and write sample number on the lid. |
| 6 | Place in the box or biobottle. |
| 7 | Repeat the process until sampling is complete and then go to Packaging and Storage of Samples on the following page. |
3.11 Packaging and storage of samples
3.11.1 Bioshield box procedure
The table below lists the steps that must be followed if using a bioshield box to despatch samples. A box can hold up to 40 samples.
| Step | Action |
|---|---|
| 1 | Ensure a pre-frozen Techni-Ice sheet is in the bottom of the box. |
| 2 | Fill lower frame with sample pots before using a second frame. |
| 3 | Place a second sheet of Techni-Ice on top of the samples. If there are no pots, still place the Techni-Ice sheet in the box. |
| 4 | Place all the TSE 6/2 / NSP 61/61A / TSE 6/3 forms in the large plastic document bag. On the outside of the bag record:
|
| 5 | Place plastic bag containing TSE 6/2 / NSP 61/61A / TSE 6/3 forms on top of Techni-Ice. |
| 6 | Apply the SRM label to the outside of the bioshield box. |
| 7 | Place the bioshield box in a plastic refuse bag to protect the surface of the box from contamination while carrying it through the slaughterhouse and during storage. |
| 8 |
Close the plastic refuse bag with a cable tie or other secure means. Ensure that all blood and any other contamination is washed off the temporary plastic refuse bag or use a new one. The plastic refuse bag must be labelled with the pre-printed temporary packaging label to prevent accidental disposal as waste. |
| 9 | Place samples in a detained chiller prior to despatch. |
3.11.2 Bioshield bottle procedure
The table below lists the steps that must be followed if using a bioshield bottle to despatch samples. A bottle can hold up to seven samples.
| Step | Action |
|---|---|
| 1 | Ensure a pre-frozen ice-brix is in the bottom of the biobottle. |
| 2 | Place a maximum of 7 pots in the biobottle. |
| 3 | Place the second ice-brix on top of the pots in the biobottle. |
| 4 | Place the bottle in its cardboard box. |
| 5 | Place all the TSE 6/2 / NSP 61/61A / TSE 6/3 forms in the large plastic document bag. On the outside of the bag record:
|
| 6 | Insert plastic bag containing the TSE 6/2 / NSP 61/61A / TSE 6/3 forms round the bottle in the biobottle box. |
| 7 | Apply the SRM label to the outside of the biobottle box. |
| 8 | Place the biobottle box in a plastic refuse bag to protect the surface of the box from contamination while carrying it through the slaughterhouse and during storage. |
| 9 |
Close the plastic refuse bag with a cable tie or other secure means. Ensure that all blood and any other contamination is washed off the temporary plastic refuse bag or use a new one. The plastic refuse bag must be labelled with the pre-printed temporary packaging label to prevent accidental disposal as waste. |
| 10 | Place samples in a detained chiller prior to despatch. |
3.12 Problems with sampling
3.12.1 Problems that may be encountered
If problems arise during sampling the following action is to be taken.
Problems that may be encountered (Sheep TSE Survey):
| Problem | Action |
|---|---|
| No ear tags present in the ear (it may have fallen out) | The sample procedure for this animal should be abandoned and the carcase processed as normal |
| No brain stem present in the head (it has been pulled out during separation of the head) | The sample procedure for this animal should be abandoned and the carcase processed as normal |
| No cerebellum collected | Do not send sample; deselect brain stem and carcase |
Problems that may be encountered (Sheep TSE Survey, CSFS):
| Problem | Action |
|---|---|
| Lost Orange Tag OR No brain stem | Do not sample; inform SSC (Worcester), record as ‘Not Sampled’ in NSP 61A and process carcase as normal |
| No cerebellum collected | Send brain stem only |
3.13 After sampling
3.13.1 Cleaning
After sampling, the sampling workstation and surrounding area must be cleaned in accordance with local protocols. Cleaning is to be supervised by FSA staff.
3.13.2 Sharps
Close, but do not seal the sharps box at the end of sampling and store it in a dry area until re-use.
See topic 3.14 on ‘Collection and disposal of sharps and clinical waste’.
3.13.3 Glove disposal
The heavy-duty gloves are to be disposed of in the clinical waste container.
See topic 3.14 on ‘collection and disposal of sharps and clinical waste’.
3.14 Collection and disposal of sharps and clinical waste
3.14.1 Service provider
SRCL provides a sharps and clinical waste collection and disposal service on behalf of FSA and Defra for slaughterhouses participating in the Sheep TSE Sampling Survey.
Sharps Box

Capacity: 12 litre
Clinical Waste Unit

Capacity: 60 litre
The exchange of sharps boxes and clinical waste units will be on an agreed frequency basis, at an agreed collection time and day.
Collection will take place regardless of whether the box is full or not.
Replacement containers will be left by SRCL after each collection.
3.14.2 Setting up or changing sharps / theatre unit arrangements
Contact the SLA and Contracts team, giving your name, the slaughterhouse approval number and the establishment’s opening hours to:
- arrange a new service
- to change the frequency of collections and numbers of sharps boxes or theatre units
SRCL then confirm by email the date of installation or acceptance of service frequency to the SLA and Contracts team and FSA staff.
SRCL will then contact the FSA staff in plant to confirm delivery arrangements. If SRCL are unable to confirm delivery details with FSA staff in plant then they will contact SLA and Contracts team to arrange alternative delivery.
FSA staff in plant must contact the SLA and Contracts team within 5 working days to confirm delivery or amendment to service.
3.14.3 Disposal of sampling equipment
All sheep TSE sampling consumables (except sharps) must be disposed in the clinical waste units. The consumable must first be put into a ‘Bio-Hazard’ polythene bag (DIF 0011).
At the end of each day the bag must be sealed and placed into the clinical waste unit. A new bag must be used each day of sampling.
Note: The unit’s sealable lid must not be ‘clicked shut’ until prepared for collection.
3.14.4 Responsibilities of the ITL
The ITL is responsible for sharps boxes and the clinical waste units, and must ensure the following are complied with:
- boxes and units must be sealed according to instructions on the box before collection
- boxes and units must be left in the agreed collection point where the replacement box will be left
- the outside of the box and unit must be clean and free of blood
- the slaughterhouse licence number must be marked on the box and unit lid in indelible ink
3.15 Storage and retention pending results
3.15.1 Retention options
Carcases and offal retained pending a test result should be held in accordance with one of the following options:
| Option 1 | In a detained chiller / area | Carcases awaiting test results must not come into contact with other detained carcases awaiting further examination by an inspector or OV |
|---|---|---|
| Option 2 | In a chiller other than the detention chiller | Carcases should be retained in a single batch that can be easily identified in a specified part of the chiller and must not come into contact with other meat |
3.15.2 Chiller control
Areas where carcases are retained must be under the control of the OV. They should be sealed, in which case the seals may not be broken except by the OV, ITL or MHI.
3.15.3 Form completion
At this point you should return to the completion of the Daily Record Sheet (TSE 6/6).
All procedures relating to chiller controls should be recorded on the TSE 6/6 including:
- date and time of sealing and person responsible
- seal number
- date and time of unsealing and person responsible
3.15.4 Body parts
Unfit material must not be stored with any other carcases or body parts that have been passed fit for human consumption. The table below details the specific requirements for various body parts of the animal.
Body parts
| Part | Action |
|---|---|
| Any offal passed fit for human consumption | Must be retained and identifiable, unless disposed of at Cat 1 ABP (SRM) by incineration |
| Fleece | Must be retained in the hide / skin room but separately from other hides / fleeces / skins; must be appropriately marked or tagged and clearly labelled ‘pending test result, retain until test result is available’ |
| Blood | Must be retained separately and identified pending a test result, unless disposed of as Cat 1 ABP (SRM) by incineration |
| SRM | Must be disposed of by incineration |
| Rumen contents and gut contents | Should be disposed of in the normal way |
| Sampled carcase or body parts of sampled carcases that are not passed fit for human consumption at post-mortem inspection | Must be held as an animal by-product until a test result is received; if a FBO does not wish to hold this material until test results are obtained it must be disposed of as Cat 1 ABP (SRM) by incineration |
3.16 Despatch of samples
3.16.1 Labelling
Both bioshield and biobottle boxes are pre-printed with a diagnostic specimen label and a black UN3373 label. In addition to these labels, an SRM label should be applied to each box.
3.16.2 Collection of samples
Predict the following weeks sampling days and times that the samples will be ready for collection.
To book a collection use the Topspeed online booking system (See Annex 4 for instruction on the despatch process).
Reference: Topspeed
Under exceptional circumstances, ad-hoc changes and collections can be made on the same day of sampling by contacting BSE Customer Care Team (0844 057 0110) before noon.
3.16.3 Transport containers
Top Speed collect the bioshield box or biobottle at a pre-notified collection time for overnight delivery to:
TSE Laboratory
Eurofins Forensic Services
Darwin House
Faraday Street
Birchwood Park
Risley
WA3 6FW
Telephone: 0844 057 0110
3.16.4 Ensure sample despatch
All samples are to be despatched to the laboratory. Any sheep samples lost prior to despatch due to an error on behalf of the FSA or an issue with the FBO will have to be made up as part of the week’s deviation.
If, for any reason, Topspeed do not collect samples, DO NOT dispose of these samples at the slaughterhouse. Samples are to be despatched to the TSE Testing Laboratory irrespective of time between harvesting and eventual despatch.
3.16.5 Notify laboratory
FSA staff should advise the TSE Testing Laboratory that samples have been taken and the courier has collected the brain stem and cerebellum samples by completing a Notification of TSE Sample Sent form and faxing (01925 248876) or emailing it to them (bse.services@lgcgroup.com). A copy of the form can be obtained from SLA and Contracts Team.
(If you require the results to be sent to a single email address rather than by fax, this should be detailed in the comments section at the bottom of the form).
See Annex 6 for a sample copy of the form.
3.16.6 Arrival time of sheep samples at laboratory
Top Speed provides a same day direct delivery service to ensure that the samples arrive at the TSE Testing Laboratory in time for the results to be provided by 4pm the following day of sampling.
3.16.7 Top Speed collection problems
In the event that samples are not collected at the correct time by Top Speed, and providing it is the fault of the courier, then the OV should contact the TSE Testing Laboratory on 0844 057 0110 to request a same day collection and delivery service to the TSE Testing Laboratory.
The TSE Testing Laboratory require samples within 24 hours of slaughter and the OV must try to ensure that samples are despatched. If a delay in the delivery occurs, the results will be issued late. The OV must ascertain whether the abattoir is willing to detain the carcases for an extended period.
Any delays in the collection or delivery of samples must be reported immediately to the SLA and Contracts Team. Delays in the provision of test results caused by the courier must not impact on the FBO. Samples not picked up from Halal abattoirs are not to be held over for collection the following day.
If the samples cannot be sent to the TSE Testing Laboratory, then an ovine animal can be deselected for testing and the samples must be disposed of as Cat 1 ABP (SRM) by incineration. Such deselection and application of the Health Mark must await confirmation from SLA and Contracts team. Samples that have been taken but then are deselected cannot be counted towards the totals on the TSE 6/1 form and these numbers must be made up.
If the TSE Testing Laboratory has started a test then an animal cannot be deselected, health marked or released until a test result has been received.
3.17 Test result and health marking
3.17.1 Test result
The test result should be received at the slaughterhouse by 4 pm on the day after sampling.
Test results will either be faxed to the plant fax number or (if requested in the comments section of the Notification of TSE Samples Sent Form) to the single email address provided.
3.17.2 Negative result
Any sheep selected for testing must not have the Health Mark applied until a negative result has been obtained by fax/email from the testing laboratory. Carcases yielding a negative result must have the Health Mark stamp applied before being released for human consumption. Any remaining material, whether or not for human consumption, may be released. The FBO must apply the identification mark to the offal.
3.17.3 Positive result
In the event of a faxed / emailed positive result from the testing laboratory, the OV should ensure that all body parts of an animal found to be positive which have been retained, including the fleece, are disposed of as Cat 1 ABP (SRM) by incineration. In addition, the OV should inform the ROD via the APHA central contact number (in England), or the DVM at the local APHA office (in Wales) of the case. A copy of the TSE 6/2, TSE 6/3 or NSP 61 and NSP 61A should be faxed to the relevant APHA office and the SLA and Contracts Team.
On occasion, retesting of a positive sample and its associated negative samples may delay release of results until 9.00 am the day following testing at the TSE Testing Laboratory. The TSE Testing Laboratory will inform FSA officers and the FBO of delays.
3.17.4 Unsuitable test result
In the event that the sample is unsuitable the OV will receive a faxed / emailed notification from the laboratory on the results form and the carcases treated in the same way as negative results.
Deselection and application of the health mark must await this faxed report.
Only APHA can give permission for sheep to be deselected from testing except when they have been selected for CSFS in which case all samples must be tested. The carcase, fleece, blood and body parts can then be released.
However, if the tests are from the annual cull animals (CSFS) all unsuitable results must be treated as positive results.
Poor quality samples reported by the lab will need to be investigated. The SLA and contracts team will notify the local team from the plant affected to request an investigation Results of the investigation and, where necessary further preventative action, need to forwarded to sla.contracts@food.gov.uk , FVC and FVL.
3.17.5 Inconclusive result
In the event of an inconclusive test result, the OV should treat all of the animal body parts in the same way as for a positive result.
3.17.6 Test result flow chart
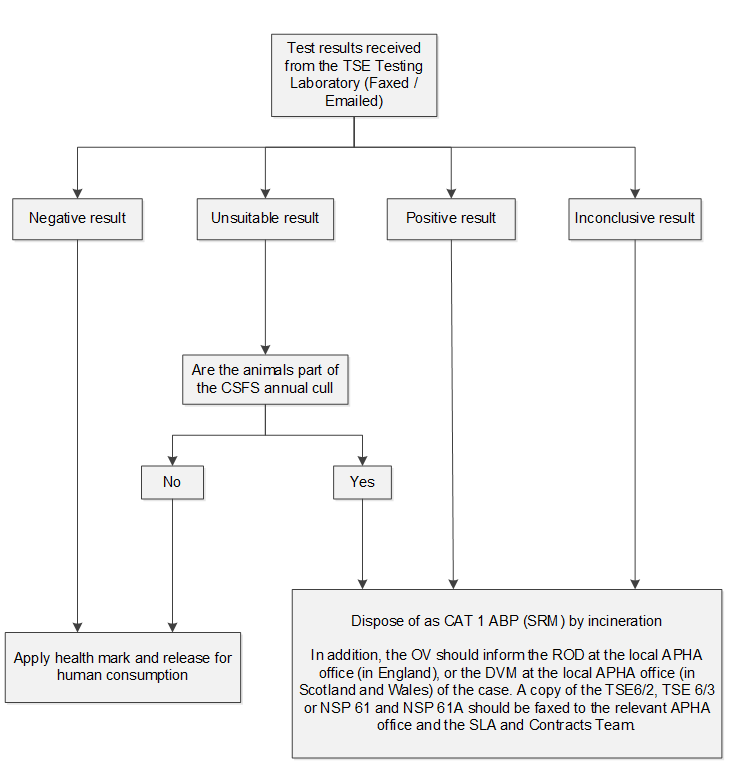
3.17.7 Non co-operation
If selected animals are not sampled and / or tested because the FBO fails to co-operate, the selected animals must not be health marked. The OV must inform SLA and Contracts Team of this action.
See chapter 7 on ‘Enforcement’ when considering any enforcement action.
4. Sheep TSE Testing: Form Completion
In this section
4.1 TSE 6/6 (Daily record sheet)
4.1 TSE 6/6: Daily record sheet (sheep and goats surveys / CSFS)
4.1.1 When and by whom?
The FSA section of the form should be filled in by an FSA AO at the dentition point. The orange tag number must be recorded in the TSE 6/6 and provides the only record that will ensure correlation between orange tag and carcase / skin.
Always protect forms against contamination (such as blood and tissue).
4.1.2 Form completion
To ensure correlation, enter the ear tag number on Daily Record Sheet (TSE 6/6) next to the spray (the kill number sprayed on the fleece) and detention (red ear tag) number with an indelible marker.
This correlates the fleece with the red tag number and ensures the ear tag number can be correctly recorded on the Rapid Testing Form (TSE 6/2) post slaughter.
Once the fleece is removed, a detention tag (colour of the day) must be applied to the carcase with the spray number written on with indelible marker.
Offal can be correlated individually or by batch, or disposed of as Cat 1 ABP SRM.
4.1.3 Filing
Retain the completed TSE 6/6 in the FSA office with a photocopy of the TSE 6/2 for matching against the test results.
4.2 TSE 6/2: Rapid testing form (sheep and goat surveys)
4.2.1 When and by whom?
A TSE 6/2 form must be completed for each sample taken from selected sheep intended for human consumption or from DoA/DiL eligible sheep in participant slaughterhouses.
The FSA section of the form should be filled in prior to slaughter (at the point of animal selection) in the case of sheep intended to slaughter and when identified in the case of DoA/DiL sheep by an FSA AO, except for the insertion of the number of permanent incisors erupted which must be done after the dentition check.
Always protect forms against contamination (such as blood and tissue).
4.2.2 Form completion
The following points should be noted when completing the TSE 6/2 form:
- it is critical that only one of the options in the TSE 6/2 form for the Scrapie surveillance is clearly marked. This allows the laboratory to assign the sample and the result in the correct sampling category
- the vendor name and address are the name of the dealer or farmer (not the slaughterhouse) that owned the animal prior to the arrival at the slaughterhouse
- Sheep intended to slaughter that do not have a flock mark or individual tag should not be sampled; any information missing must be recorded as ‘‘Not Known’’
- if the DIL/DOA sheep does not have a farmer’s mark, lot number, tattoo number or other mark the relevant data boxes should be left blank
- if breed cannot be reliably identified then the relevant data box should be annotated ‘‘Not Known’’
- it is important that boxes relating to teeth are completed as they confirm the age of the animal
- other identification present should be recorded.
4.2.3 Ear tag number
Take care when recording the number of the red ear tag as this is the link between the sample and the sample details which will be recorded on databases.
4.2.4 Form distribution and filing
Make a photocopy to be retained by the FSA in the OV office in plant for 12 months. The original TSE 6/2 should be despatched with the sample.
Retain the photocopies of each TSE 6/2 with the completed TSE 6/6 in the FSA office for matching against the test results.
4.3 NSP 61 (CSFS)
4.3.1 When and by whom?
An FSA officer must complete Section 2 of the NSP 61 form prior to despatch of CSFS samples.
Always protect forms against contamination (such as blood and tissue).
Note: The NSP 61 form is currently under review with APHA and will be added as an annex once published.
4.3.2 Form distribution and filing
Record if there is one or more DoA or DiL (details will be recorded in Section 2 of NSP 61 under the DIT/DIL samples column). If one, the pink copy of the NSP 61 will accompany the head. If more than one, a photocopy of the form will accompany the head for each DoA or DiL.
Protect forms against contamination (such as blood and tissue).
The separate copies of the form are to be sent as follows:
- yellow copy – to be retained by FSA in plant for 12 months
- blue copy – despatched with samples; this is for the TSE Testing Laboratory to send to APHA Weybridge
- white copy – despatched with samples to the TSE Testing Laboratory
- pink copy – despatched with DOA/DIL head(s) (if applicable)
- green copy – send in pre-printed envelope to ‘SSC, Membership Admin’
4.4 NSP 61A (CSFS)
4.4.1 When and by whom?
An FSA officer must complete columns 1 and 2 of the NSP 61A form prior to despatch of CSFS samples.
Always protect forms against contamination (such as blood and tissue).
Note: The NSP 61A form is currently under review with APHA and will be added as an annex once published.
4.4.2 Form completion
Form NSP 61A is not self-carbonated. Keep a photocopy of the original before sending it with the samples. There is no need to complete a TSE 6/2 for these animals.
Once the sample has been taken, record the sample number in column 1.
If any animal with an orange tag is found DoA or DiL, a tick is to be entered in column 2 (DIT/DIL) next to the animal’s details.
When the form has been completed, verify that every animal requiring testing has been sampled. If any animal is submitted for slaughter with an orange tag but is not included in the NSP 61A, record the details in the form.
4.4.3 Form distribution
Once completed make a second photocopy to be sent in the pre-printed envelope to ‘‘SSC Membership Admin’’.
4.4.4 Reporting discrepancies
Any discrepancy found between the information recorded on the NSP 61A and the animals presented must be reported to the SSC (Worcester) as follows:
SSC (Worcester) telephone: 01905 768741
OR
SSC Helpline: 0845 601 4858
OR
SSC fax number: 01905 768742
4.5 TSE 6/1: Weekly summary and deviation report (sheep and goats surveys / CSFS)
4.5.1 When and by whom?
By an FSA officer at the end of each week (Monday to Sunday).
Always protect forms against contamination (such as blood and tissue).
4.5.2 Weekly summary and deviations report: sheep survey
Complete Part 1 on the TSE 6/1, recording daily throughput and both daily and weekly surpluses and shortfalls.
Throughput information will be correlated against Defra’s statistical information.
Any weekly shortfall of samples must be carried forward to the following week and must be made up. The deviation should remain as close to zero as possible.
4.5.3 Weekly summary and deviations report: goat survey
We are currently not sampling goats so Part 2 of the form is not required to be completed.
4.5.4 Weekly summary and deviations report: CSFS
Complete Part 3 on the TSE 6/1 form, recording the number of samples collected on the relevant day(s).
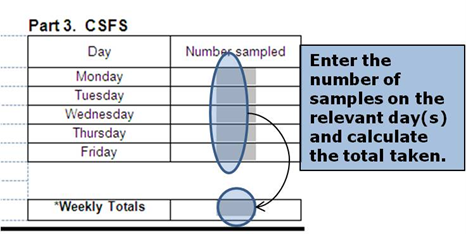
Note: CSFS samples must not be included with any normal samples recorded in Part 1 of the form.
4.5.5 Weekly summary and deviations report: general
Once completed, email a copy to the SLA and Contracts Team.
The deadline for submission is (except in exceptional circumstances agreed by SLA) 10am on the following Monday.
5. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 2: Hazard identification and control plan
Annex 3: Test categories for bovine brain stems
Annex 4: Sample despatch process
Annex 5: Sample TSE Testing Laboratory consumable order form
Annex 6: Sample notification of TSE samples sent to the TSE Testing Laboratory for testing
Sections
3. Verification and Inspection Tasks
1. Introduction
In this section
1.1 Purpose
1.1.1 Background
The correct removal and disposal of SRM in slaughterhouses and cutting plants is essential to minimise risks to public health associated with TSEs in cattle, sheep and goats.
1.1.2 Specific prohibitions
The TSE Regulations specifically prohibit the sale or supply of:
- any SRM, or food containing SRM for human consumption
- any SRM for use in the preparation of any food for human consumption
1.1.3 Duties of the FBO
It is the duty of the FBO to comply with the legislation.
1.1.4 Introduction to FSA duties
The FSA has a number of duties relating to SRM controls focused on regulating the continuous compliance of the FBO with the legislation.
These duties include:
- inspection of carcases
- verification that the FBO carries out their duties
- audit of good hygiene practice (GHP), FBOs own procedures for handling and disposal of SRM and FBO’s HACCP based procedures for ensuring the meat does not contain SRM.
1.1.5 Outside of approved establishments
Local authorities (LAs) enforce the TSE Regulations outside approved establishments.
1.2 Legislation
1.2.1 Applicable regulations
The following SRM legal controls are applicable:
- (EC) 999/2001 (as amended) specifies what SRM is and that it shall be removed in:
- a slaughterhouse, or, if appropriate, other place of slaughter
- authorised cutting plants, in the case of over thirty month (OTM) bovine vertebral column or mature ovine and caprine spinal cord
The Regulation is directly applicable throughout all Member States (MS) and lays down rules for the prevention, control and eradication of certain TSEs including, in Annex V, the definition of SRM.
- (EU) 2019/627 specifies official controls to verify continuous and effective compliance with FBOs own procedures and HACCP based procedures concerning controls on SRM:
- check the removal, separation and staining of SRM
- verify the FBO takes all necessary measures to avoid contamination of meat with SRM during slaughter (including stunning) and removal of SRM
- national legislation:
- the TSE (England) Regulations 2018
- the TSE (Wales) Regulations 2010 (as amended)
- the Animal By-Products (Enforcement) (England) Regulations 2013
- the Animal By-Products (Enforcement) (Wales) Regulations 2014
classify SRM as Category 1 Animal By-Product via the provisions of Article 8 of EC 1069/2009 and set out the rules for its disposal.
2. FSA role
In this section
2.1 Inspection duties in slaughterhouses
2.3 OV Role on supporting the authorisation of cutting plants to remove SRM VC from bovine carcases
2.1 Inspection duties in slaughterhouses
2.1.1 Legislative requirement
In accordance with specific Community rules on SRM and other animal by- products (ABP), the OV is to check the removal, separation and, where appropriate, marking of such products. The OV is to ensure that the FBO takes all necessary measures to avoid contaminating meat with SRM during slaughter (including stunning) and removal of SRM.
Reference: (EU) 2019/627, Article 29.
2.1.2 Frequency of checks
There must be frequent inspections to verify the correct application of requirements in (EC) 999/2001 (as amended) concerning SRM and slaughtering techniques. These must ensure measures are taken to avoid contamination in places where SRM is removed. There must also be a system to ensure that SRM is only used for authorised purposes and / or is disposed of in accordance with (EC) 1069/2009.
Reference: (EC) 999/2001 (as amended) Annex V, point 11
The table below lists the checks that should be carried out, the frequency and the AO responsible for performing that task.
| Task | By | Slaughterhouse or approved GHE (Recommended Minimum Frequency) | Cutting Plants (Recommended Minimum Frequency) | Additionally Authorised Cutting Plants (Recommended Minimum Frequency) |
|---|---|---|---|---|
| Removal of SRM | Any FSA team member who is appropriately authorised | Every bovine, ovine or caprine carcase | Any FSA team member who is appropriately authorised | Every day of supervision |
|
Identification and separation (ABPs are removed from food production areas as quickly as possible, avoiding cross contamination and ABPs, including SRM, are correctly identified, segregated and categorised) |
Any FSA team member who is appropriately authorised | As per Slaughter Hygiene Verification (SHV) (when applicable) or daily | Veterinary auditor (VA) during the FBO audit / unannounced inspection (UAI) | Every day of supervision |
|
Staining (ABPs, including SRM, are correctly stained where necessary) |
Any FSA team member who is appropriately authorised | As per SHV (when applicable) or daily | VA during the FBO audit / UAI | Every day of supervision |
|
Storage (ABP containers are leak proof, closable, kept in sound condition, cleaned and disinfected as often as necessary. Waste stores are pest proof) |
Any FSA team member who is appropriately authorised | At the end of processing | VA during the FBO audit / UAI | Every day of supervision |
|
Transport and disposal (ABPs, including SRM, are dispatched to approved premises with correctly completed commercial documentation) |
Any FSA team member who is appropriately authorised | Monthly | VA during the FBO audit / UAI | Monthly |
| Commercial documents (Transport records with regards to VC removal or adult sheep splitting) | Any FSA team member who is appropriately authorised | When applicable | VA during the FBO audit / UAI | When applicable |
| Blood Management | Any FSA team member who is appropriately authorised | As per SHV (when applicable) or daily | VA during the FBO audit / UAI | n/a |
| Checks on Approval Status of receiving establishments | Any FSA team member who is appropriately authorised | Monthly | VA during the FBO audit / UAI | Monthly |
| Enforcement | OV/ Any FSA team member who is appropriately authorised | As required in accordance with the hierarchy of enforcement | VA during the FBO audit / UAI | As required in accordance with the hierarchy of enforcement |
| Audit of FBOs own procedures for ABP management | OV for monthly check / VAs as part of the FBO Audit / UAI for data gathering | As determined by risk assessment | VA during the FBO audit / UAI | VA during the FBO Audit / UAI for data gathering |
2.1.3 Identification of bovine carcases or wholesale cuts containing VC-SRM
When removal of the VC is required, carcases or wholesale cuts of carcases of bovine animals containing VC shall be identified by a clearly visible red stripe on the label referred to in Article 13 of (EC) No 1760/2000.
Any other label can be used for identifying carcases or wholesale cuts of carcases of bovine animals containing VC that are not required to have the VC removed (example, under 30 months bovines from England or Wales).
Reference: (EC) No 999/2001 (as amended, Annex V point 11.3)
The FBO’s HACCP based procedures in slaughterhouses and cutting plants receiving bovine carcases or wholesale cuts should cover SRM controls, which include labelling and checks on these labelling requirements for bovine carcases and wholesale primal cuts.
Further cutting of wholesale cuts of carcases containing VC classified as SRM must only be carried out in approved cutting plants; these plants must be additionally authorised for the removal of VC from OTM carcases in accordance with the required method of operation (RMOP).
2.2 Definition of SRM
2.2.1 FSA key issue
It is imperative that all OVs and MHIs are aware of the parts of the animal that are classified as SRM by EC 999/2001 (as amended). FSA operational staff can use the following tables.
| All ages |
|
| Over 12 months | Skull excluding the mandible and including the brain and eyes, and spinal cord. |
| Over 30 months | VC including the dorsal root ganglia, but excluding:
|
| Over 12 months | Skull excluding the mandible and including the brain and eyes, and spinal cord. |
| Under 12 months | No SRM |
| Under 12 months | No SRM |
| Over 12 months (or permanent incisor erupted) |
Skull, including the brain and eyes and spinal cord. Note: Skull does not include horns. |
Additional reference can be made as to which countries are under negligible risk or controlled risk.
2.2.2 Exceptions to removal of SRM in the slaughterhouse
There are certain exceptions to the requirement to remove SRM as soon as practicable after slaughter as outlined in the table below.
| Exception | If |
|---|---|
| Older sheep or goat carcases requiring spinal cord removal | accompanied by a Transfer Permit transferred to a cutting plant authorised for removal of spinal cord, or transferred to a cutting plant in another MS where the FSA has a pre-existing agreement with the Competent Authority. |
| Bovine SRM VC from carcases and wholesale cuts from animals OTM | transferred to an approved cutting plant authorised for removal of vertebral column from animals aged OTM at slaughter, in UK, or any other MS. Should be accompanied by commercial documentation, indicating the number of carcases or wholesale cuts from which VC is required to be removed, or is not required to be removed. |
| SRM bovine VC from the carcases of animals OTM of age imported live from countries with a controlled or undetermined bovine spongiform encephalopathy (BSE) risk and slaughtered in GB | transferred to an approved cutting plant authorised for removal of VC in UK or any other MS. Should be accompanied by commercial documentation, indicating the number of carcases or wholesale cuts from which VC is required to be removed, or is not required to be removed. |
| Bovine carcases of animals OTM of age containing SRM vertebral column imported, in accordance with the community TSE Regulations | transferred to an approved cutting plant authorised for removal of VC. Should also be accompanied by commercial documentation, indicating the number of carcases or wholesale cuts from which VC is required to be removed, or is not required to be removed. |
| Bovine Heads Moved to an authorised cutting plant for head meat harvesting | transferred to an approved cutting plant authorised for Bovine Head Meat harvesting. Should also be accompanied by commercial documentation, indicating the number heads cuts from which head meat is to be harvested. |
| Material for use in education or research purposes | the specific movement is centrally approved by the FSA and supported by a valid ABP 7/1 document for the use of ABP is for diagnostic, educational and research purposes. |
2.2.3 Mechanically separated meat (MSM)
MSM means the product obtained by removing meat from flesh-bearing bones after boning, using mechanical means resulting in the loss or modification of the muscle fibre structure.
MSM derived from cattle, sheep or goat bones, including bone in cuts, must not be used in the preparation of food for human or animal consumption.
Note: This does not include meat recovered during the boning process by the use of hand-held powered knives that do not use pressure or suction.
2.3 OV Role on supporting the authorisation of cutting plants to remove SRM VC from bovine carcases, spinal cord from adult small ruminants or harvesting bovine head meat
2.3.1 Scope
The duties detailed below relate to:
- bovine carcases from animals aged OTM, imported from countries with a controlled or undetermined BSE risk
- bovine carcases from animals aged OTM, imported from countries with a negligible risk, but which have had an indigenous BSE case
- bovine carcases from animals imported alive and slaughtered when aged OTM
- bovine carcases from domestic animals aged OTM at slaughter
It will also cover:
- spinal cord removal from adult sheep
- harvesting of head meat
Only cutting plants that have been additionally authorised under the following regulations are permitted to remove VC from domestic and imported carcases from animals aged OTM, harvest head meat from bovine heads or remove spinal cord from adult ruminants:
- the Transmissible Spongiform Encephalopathies (England) Regulations 2018, Schedule 7 paragraph 8(1) and (9)
- the Transmissible Spongiform Encephalopathies (Wales) Regulations 2008, Schedule 7 paragraph 13(1)
The updated list of cutting plants authorised for the removal of VC-SRM is available.
OVs involved in the authorisation process will endeavour a better understanding of the whole process.
2.3.2 Procedure: Application packs
The OV should assist and guide the FBO in obtaining an application pack, which are available from the Approvals Team in York. The application pack contains information related to the 3 potential authorisations under the Regulations.
2.3.3 Procedure: RMOP
The removal of SRM VC must take place in accordance with the relevant protocol and the operator’s RMOP. Alternatively, the FBO may include the related procedures into their HACCP based system or as a SOP.
FBOs of stand-alone cutting plants should contact FVC in the first instance. The FVC will organise assistance through local teams; OVs or trained MHIs.
In the case of co-located cutting premises, the plant based OV should be the first point of contact to assist FBOs when completing a RMOP. In dealing with FBO queries, the OVs should play an advisory role using their professional knowledge. In case of doubt, the OV will consult on the appropriate course of action with the FVC for the region.
Once the establishments are authorised, the responsibility of keeping the procedures up to date remains with the FBO. The FSA will verify compliance during FBO audits, UAIs and any other routine visit.
2.3.4 Application for authorisation to remove SRM vertebral column from bovine carcases, spinal cord from adult small ruminants and bovine head meat harvesting
Updated [In collocated cutting plants, the OV should point the FBO to chapter 9 of the MOC where the application form (ABP 7/8) is located. The FBO must complete all relevant sections of the form and pass it to the OV for completion of section 4. Once the ABP 7/8 has been completed, the FBO must submit this together with a copy of the RMOP/SOP/HACCP based system describing how the process is to be carried out, to the FSA Approvals Team,
In standalone cutting plants, the FBO should contact approvals directly, who will send the ABP 7/8 form to the FBO. Once the form is completed, the FBO should send this together with the RMOP/SOP/HACCP based procedures to the FSA Approvals Team.
Approvals will verify that the ABP 7/8 and associated documents have been completed accordingly and will submit to the local FSA Operational team (FVL and FVC) for final verification and signature. Once cleared, Approvals will confirm in written to the FBO that the establishment has been authorised.]
2.4 Supervision
2.4.1 VC removal cutting premises
After authorisation, daily visits are required which can be carried out by the OV (collocated to a slaughterhouse) or an AO (collocated and standalone). After the initial month, the OV / AO is to review the frequency using the SRM risk tool (Annex 1). When compliance is achieved as per the SRM risk tool, the frequency can be reduced to once a week. The duration of that visit depends on the size and volume of the operation. A minimum frequency of an hour is expected.
The OA is to complete the Bovine OTM Removal Verification Checklist (Annex 3).
2.4.2 Spinal cord removal in authorised cutting plants
After authorisation, daily visits are required which can be carried out by the OV (collocated to a slaughterhouse) or by an AO (standalone and collocated).
The associated procedures submitted for the application (RMOP/SOP/ HACCP) should be available for supervisor officers during their checks and for the auditor as part of the audit process. The FBO is to review these as part of the yearly HACCP review to verify that the controls are still effective.
The visit is to verify that the spinal cord has been effectively removed from all processed carcasses. There is to be a combination of the process review and a verification of the carcasses processed before attendance.
The AO is to complete the Ovine-Caprine Removal verification Checklist (Annex 4).
2.4.3 Harvesting of bovine head meat
After authorisation, daily visit is required. That can be carried out by the OV (collocated to a slaughterhouse) or an AO (collocated and standalone). After the initial month, the OV / AO is to review the frequency using the SRM risk tool (Annex 1). When compliance is achieved as per the SRM risk tool, the frequency can be reduced to once a week. The duration of that visit depends on the size and volume of the operation
The AO is to complete the harvesting of bovine head meat verification checklist (Annex 2).
2.5 Enforcement
2.5.1 FBO fails to comply with regulations
If the FBO fails to comply with the regulations the Authorised Officer (AO) must:
- notify the FBO of all deficiencies as soon as possible
- assess the breach and take proportionate enforcement action (see below)
- record the breach in the daybook and Chronos.
2.5.2 If carcase contains SRM at inspection
Enforcement of SRM breaches should follow the proportionate and risk-based approach as detailed in the table below:
| Where | Then |
|---|---|
| The FBO has effective HACCP based systems in place, including effective training of staff | an incident in which a carcase with SRM is presented at post mortem inspection, resulting from an isolated human error, should be treated in a risk-based and proportionate manner. The incident must be brought to the FBO’s attention and recorded in Chronos. Should a contravention be repeated, the issue should be escalated through the hierarchy of enforcement in the normal way. If there are three or more SRM presentation incidents, a referral for investigation should be made for that third and any subsequent incidents. |
| HACCP based systems exist but they have not been effectively implemented or maintained | where this deficiency has led to SRM being presented for inspection, then a warning letter must be sent to the FBO, clearly setting out the deficiencies they need to rectify. The FBOs actions must include addressing the root cause of the problem, to ensure there is no recurrence. Escalate through the hierarchy of enforcement. |
| Lack of or ineffective HACCP based system leading to repetitive failures | enforcement action is to be taken against the root cause and the substantive offences using the hierarchy of enforcement (see chapter 7 on ‘Enforcement’). |
| SRM has not been removed from a carcase and has been despatched to another premises or exported (excluding VC consigned to an approved cutting plant that is authorised for VC- SRM removal) |
this SRM breach should be referred to Operations Assurance (by the OV / FVL / FVC) for internal investigation, by e-mail or phone to CSU Transactions Team York . The breach should also be referred, by the OV, for criminal investigation. A trace back exercise may be justified. See chapter 2.8 on ‘ABPs’ for further guidance. |
Effective SRM controls remain a high priority for the FSA and it continues to be an expectation of 100% FBO compliance.
2.5.3 Evidence gathering for SRM contraventions
If the FBO presents carcase(s) (or offal being harvested for human consumption) for inspection, that contain SRM or where SRM is attached, the following enforcement action should be taken by FSA operational staff:
- detain carcase(s) / offal under FSA control (if necessary, using a Detention Notice issued under Regulation 10(1) of the Food Safety and Hygiene (England) Regulations 2013 or Regulation 9 (5) of the Food Hygiene (S/W) Regulations 2006) (ENF 11/26)
- make contemporaneous detailed records in the daybook or personal notebook and sign them
- obtain photographic evidence of the SRM in situ
- obtain verification of findings from other FSA staff if possible
- notify the FBO
- retain a sample of the SRM as evidence, freeze it to preserve its condition (until the case is concluded) and store it in a secure location
- record details via the Slaughter Hygiene Verification (SHV) K2 system and Chronos.
On completion of evidence gathering, and the removal of all SRM, the carcase / offal may be health marked and enter the food chain. The matter should be referred for investigation when the third non-compliance has been identified.
2.5.4 Record of action taken
Enforcement action must be recorded. FBO non-compliances must also be recorded via the SHV K2 system in slaughterhouses. This information is used to target resources and to inform policy team on the impact of policy changes on FBO non-compliance levels.
2.5.5 Imported meat
If the OV / AO finds final product (carcases / offal) containing SRM the evidence gathering is to be undertaken in the same manner as described on 2.5.3. The incidents team and the local FVC are also to be informed via email.
3. Verification and inspection tasks
In this section
3.1 Verification tasks for pre-slaughter and pre-cutting period
3.2 At slaughter: inspection and verification duties
3.3 Verification tasks during slaughter
3.4 Verification tasks at dispatch
3.5 Verification tasks for the cutting plant
3.6 Verification tasks for the management of SRM staining, transfer and storage
3.7 Verification tasks for the disposal of SRM by incineration or co-incineration
3.1 Verification tasks for pre-slaughter and pre-cutting period
3.1.1 Authorisations and approvals
The establishment must have the appropriate authorisations / approvals in place for the operations it is carrying out, including approval for operation of the establishment.
Bovine:
- authorisation to enable cutting plants to remove SRM VC from:
- UK bovines OTM of age at slaughter
- bovines imported from countries with a controlled or undetermined BSE risk aged OTM at slaughter
- imported bovine carcases, half carcases, half carcases cut into no more than 3 wholesale cuts, and quarter carcases from animals OTM of age at slaughter
Updated [Bovine head meat harvesting:
- authorisation to enable cutting plants to harvest bovine head meat in cutting plants]
The list of establishments authorised to remove VC from bovines OTM is available.
Ovine / Caprine:
- authorisation to enable cutting plants to remove spinal cord in sheep and goats OTM, or which have a permanent incisor erupted through the gum
3.1.2 Animals found dead on arrival (DOA), dead in lairage (DIL) or stillborn
The same rules on age requirements for BSE testing (chapter 2.6 on ‘TSE testing’, section 2) apply to animals found DOA or DIL.
FBOs may contact a collector (their normal collector or the National Fallen Stock Company (NFSCo) on 0845 054 8888) within 24 hours of death to arrange delivery to an approved sampling site. If delivering the carcase themselves, they should contact an approved sampling site to agree this within 24 hours and must deliver the carcase within a further 48 hours. The barcode label must be added to a movement card from the passport and sent to the sampling site with the carcase. The passport must be returned direct to British cattle movement service (BCMS).
When the slaughterhouse is authorised for BSE testing, the OV can also authorise the FBO to take the sample and send it to a private laboratory (code FSCA2) and dispose of the body as Category 1 ABP for incineration.
Carcases of cattle awaiting a BSE test result must be sent for destruction or destroyed at Category 1 ABP premises approved to receive such carcases by either direct incineration or rendering followed by incineration of the rendered material.
The ABP commercial document for the bovine body or carcase must include, in the description of the product, the ear tag number of the bovine animal and the required method disposal by incineration after BSE testing (if it is required and if it has not been done at the slaughterhouse).
Sheep and goats that require testing must have the heads collected and despatched for testing.
3.1.3 Animals found DOA, DIL or stillborn in the Scilly Isles or Lundy Island
There is no requirement to test animals found DOA, DIL or stillborn in the Isles of Scilly or Lundy Island.
3.1.4 Hygiene
The following hygiene requirements must be met:
- premises, machinery and implements used in SRM removal are clean before operations begin and during processing to prevent cross contamination
- storage and transport bins are clean, leak free and impervious, indelibly marked / labelled with well-fitting lids which are used when the bin is used to store or transport SRM
- bins are washed and disinfected when required and not used for any other purpose
- bin liners, if used to line SRM bins, are used once only and disposed of entirely as SRM
3.1.5 Stain
The FBO must ensure that:
- there is a sufficient supply of adequate blue stain and the dye is prepared correctly using measuring equipment
- the data sheets are available
3.1.6 Specified solid waste management
All drains in areas where SRM is processed must have drain traps or gratings in place for the collection of carcase and offal solid waste. Specifications are:
- a maximum mesh size of 6mm
Reference: (EC) 142/2011, Annex IV, Chapter I, Section 2, Para 1.
- a maximum size of 4mm where the water is discharged into a public sewer drain
Note: This is required by the Environment Agency. The OV should advise the FBO and report the matter to the Environment Agency if this is not the case.
All material collected by these traps / gratings must be treated as SRM.
3.1.7 Training
The FBO must arrange or establish, in consultation with the OV, a staff training programme to ensure that all staff involved in the removal, separation, handling, staining and disposal of SRM are fully aware of the requirements of the regulations and the FBO’s own procedures, so they can operate a system that complies with the regulations.
3.2 At slaughter: inspection and verification duties
| Inspection and verification | FSA Official | Frequency |
|---|---|---|
| Check on the staining, further handling and disposal of SRM and report any cases of FBO failure*. | OV / MHI |
When establishing the frequency of checks the OV should use the ‘Risk Based Decision Tool for ABP and SRM Inspections’ (RBT) at Annex 1 as an aide memoire. This may be daily, or once every five days of processing. Checks should take no longer than 30 minutes. The frequency of checks determined by the RBT must be recorded in the plant Daybook. |
| Check on the staining, further handling and disposal of SRM and report any cases of FBO failure**. | OV / MHI | When establishing the frequency of checks, the OV should use the ‘RBT for ABP and SRM Inspections’ at Annex 1 as an aide memoire. This may be at least daily. |
|
Age checks: Bovine: See chapter 2.5 on ‘Animal identification’ for additional information. Ovine / Caprine: The FBO must establish a system that allows the FSA to be confident that carcases that require their spinal cord to be removed as SRM (See sub topic: Age checks of ovine/caprine animals) are identified. FSA must perform verification checks on FBO |
OV / MHI |
At appropriate percentage in force as per Chapter 2.5 5% of ovine / caprine presented as ‘young’. These checks should be random throughout the day, and take place in ‘real time’, (as the FBO representative performs the check). |
|
Carcase inspection: Carry out an inspection of every carcase presented to ensure that all SRM has been removed (apart from bovine VC and sheep spinal cord where applicable). |
OV/ MHI |
All carcases |
|
Application of stamps: Bovine: Apply Health Mark stamp when the meat is declared fit for human consumption and all SRM except VC has been removed. Note: Carcases with BSE test results pending must be kept secure under FSA control until a negative BSE test result is received. See chapter 2.6 on ‘TSE testing’, section 2. Ovine / Caprine: Apply Health Mark stamps when the meat is declared fit for human consumption and all SRM (except spinal cord, where carcases are to be sent with spinal cord attached for it to be removed at an authorised cutting plant) has been removed. |
Health Mark – OV / MHI | All carcases |
* Note: The RBT for ABP and SRM’ at Annex 1 is designed to assist the OV in deciding whether checks on the staining and further handling of SRM should take place on a daily basis, or less frequently. OVs should use the decision tool monthly or, in sites operating less than 3 times a week, quarterly, and record the outcome in the plant Daybook. If there are any areas of SRM handling that change, or weak areas that pose a risk of SRM entering the animal or human food chain, the OV can amend the level of checks, in consultation with the FVC. Any changes should be regularly reviewed.
The daybook and SHV system should be used to record all non-compliances found during your checks (frequency as determined by the RBT). A new record should be completed for each period of checks, for example, if the RBT determines checks should be weekly, then the checks should be completed weekly; if the RBT determines checks should be daily, then the checks should be completed daily.
** Time coding of offline SRM checks (staining and further handling) should reflect frequency of checks.
If there is any reason the OV considers that a higher level of checks is required, they should discuss this with their FVC in the first instance.
Note that every carcase will still be checked for the removal of SRM as part of routine post mortem inspection.
| Audit | By | Frequency |
|---|---|---|
| Conduct audit of FBOs own procedures and HACCP based procedures for SRM management | VA | As determined by risk assessment |
3.3 Verification tasks during slaughter
3.3.1 FBO age checks of ovine / caprine animals
FBOs must establish a system which identifies animals that are either over one year old, or have one or more permanent incisor erupted, to allow SRM to be removed from the carcase. That system should be part of the FBOs HACCP based procedures.
Options may include:
- ante-mortem checks (for example, some batches of spring lambs are clearly identifiable at ante-mortem inspection)
- post-mortem dentition checks undertaken by FBO staff (for example, at the point of head removal where this occurs manually)
- by removing SRM by default from all animals (for example, when ewes are being processed)
3.3.2 FSA verification of age checks of ovine / caprine animals
The OV must be satisfied that such systems provide the confidence required to apply the health mark after the completion of post-mortem inspection.
Checks should be undertaken to verify these systems are working.
The OV must also use other information available to him to assist in this verification task, for example, Food Chain Information (FCI) (in the form of AML1), and the previous history of that particular establishment with respect of the number of ovine / caprine carcases that require splitting to allow spinal cord removal.
Note: OVs should be aware that:
- processing patterns may change from year to year
- the number of animals presented requiring spinal cord removal varies according to the time of year
- the location of provenance of the sheep / goats can change
- the range and number of suppliers or sources from which the FBO might use in procuring animals
3.3.3 Captive bolt stunning
Where captive bolt stunning is used, the captive bolt should, if possible, be wiped clean after each use with disposable wipes that are then discarded as SRM. Ideally the bolt hole should be plugged to prevent escape of SRM during handling and dressing.
3.3.4 Bovine heads from cattle aged over 12 months of age when processed in approved slaughterhouses premises.
The following standards must be met:
- care is exercised and all hygienic precautions taken when detaching heads or removing bovine tongues
- when bovine heads are skinned, the skinning (flaying) is complete
- if head meat is to be harvested, where heads are removed from conveyor or hook systems before harvesting, the following conditions should be met:
- control measures should be in place to prevent the possible contamination of the head meat with central nervous system tissue; this includes harvesting the head meat in a dedicated area, separate from the slaughter line
- any bolt hole on the frontal bone, and the foramen magnum should be sealed with a bung, stopper or sponge; if the brainstem is required for BSE testing, the foramen magnum should be sealed immediately after sampling
- a sampling plan using an appropriate laboratory test to detect central nervous system tissue shall be in place to verify that the measures to reduce contamination are properly implemented
- head meat must not be harvested from heads where the eyes are damaged or lost prior to, or after slaughter, or from heads that have not been sealed as above
- the FBO should ensure HACCP systems, or equivalent control systems and checks are amended to reflect the measures taken to prevent contamination of the head meat with central nervous system material
- heads must not be despatched to any other premises for the purpose of head meat harvesting unless there is a specific agreement to despatch heads containing SRM to another Member State or to an authorised cutting plant in the UK. In the case of dispatching heads to another Member State, this is only after that Member State has agreed to receive the material and has approved the conditions of despatch and transport
- the head inspection point is situated close to the point of detachment from the carcase and the head is transported to this point in a manner which is both hygienic and minimises the potential for cross- contamination of meat or surroundings with SRM
- if bovine brains and eyes are removed it is only for a permitted use (for example, instructional, diagnostic or research purposes) and cannot cause contamination to meat intended for human consumption
- care should be taken during the harvesting process to ensure tonsil is treated as SRM
Note: In bovine heads from cattle aged 12 months or less, only the tonsils are SRM. This is only applicable to countries with controlled or undetermined risk of BSE (see section 2.2.1 on ‘FSA key issue’ in this chapter for full list).
3.3.5 Bovine tongues
The following standards must be met:
- tongues are harvested by a transverse cut rostral to the lingual process of the basihyoid bone at the level of the last vallate papillae
- any material behind the last vallate papillae is disposed of as SRM
- tongues are harvested after complete flaying and washing of the detached head
- tongues are trimmed to remove any residual connective tissue
- animals tested for TSE: the tongues remain correlated with the carcase pending the results
Detailed instructions around SRM removal are detailed in ANNEX 6. As for other inspection tasks, please refer to inspection card.
3.3.6 Ovine heads
Where head meat is harvested in sheep over 12 months old, the horns must be removed and the head skinned and inspected. After harvesting, the skulls must be disposed of as Category 1 ABP (SRM).
The FBO must have in place a robust system at the point of identification to ensure that heads from sheep under 12 months old are clearly separated from heads of sheep over 12 months old when the heads are separated from the carcases.
There are no derogations for adult bovine heads to be transferred to an authorised cutting plant for head meat harvesting. Hence, adult sheep meat harvesting must take place at the slaughterhouse. That can be done on a dedicated offal room.
Precautions to control cross contamination are to take place as with any other offal handling procedure.
3.3.7 Horns (bovine and ovine)
Horns of cattle, sheep and goats are not SRM, but the cornual process of the frontal bone is SRM in cattle over 12 months old.
3.3.8 Bovine spinal cord
FBO controls should include:
- spinal cord should be removed from bovine carcases using a designated tool or knife to remove the meninges, fat and debris so that no fragments of the spinal cord can remain in the spinal canal
It is recommended that operatives removing spinal cord from bovines:
- ensure spinal cord and meninges do not come into contact with the floor, or other surfaces of the slaughterhouse
- cover chain mail gloves with rubber gloves
- change protective clothing as often as necessary to minimize cross contamination
- wash hands frequently
- use clean, sterilised tools for each carcase
- wash their hands and sterilise their tools after removal of SRM from each carcase
3.3.9 Removal of spinal cord
Band saws that have a water supply should have the water ducted away from the carcase.
Drain grating must have apertures no larger than 4mm, and that the material retained in the grating is disposed of as Category 1 ABP (SRM).
Where cleavers are used the operative should examine the carcase for fragments of SRM and trim any bone spiccules and dispose of them as SRM.
3.3.10 Bone dust
Where bone dust is removed from the cut surface of the VC using a low pressure warm water wash, the washings must be prevented from contaminating the slaughter hall or other carcases.
3.3.11 Handling of SRM
The FBO must ensure that all appropriate SRM is:
- removed completely from the carcase as soon as practicable after slaughter and before the carcase is presented for post-mortem health inspection and marking
- removed by staff of the establishment who adopt the necessary hygiene measures to avoid the risk of cross-contamination, for example, avoid touching the carcase with hands or implements which have been used to remove, or come into contact with, SRM without being washed / cleaned in between
- handled in such a way that there is no contact with any other animal material
- processed under hygienic controls that are suitable and sufficient to protect public health
3.3.12 Material in contact with SRM
The following is regarded as SRM:
- any material still attached to SRM (except SRM bovine VC and sheep / goat spinal cord) after dissection of the carcase
- any animal matter that comes into contact with that material or with SRM after it has been removed from the carcase
3.3.13 Carcases presented for inspection (ovine and bovine)
Only carcases which have had all appropriate SRM removed should be presented for inspection. With the exception of bovine VC. Sheep spinal cord can still be present if there is an alternative system to re-present the carcase after spinal cord removal or a protocol is in place to transfer these carcases (unsplit) to an authorised cutting plant.
3.3.14 Live bovine animals imported into UK from countries with a controlled or undetermined risk
All imported live animals should be treated as domestic animals as far as SRM is concerned.
3.3.15 Pithing
Pithing is prohibited for bovine animals, sheep and goats, domestic or wild animals whose meat is intended for human consumption. This includes animals under 12 months in England and Wales as REUL 999/2001 Article 8 (3) makes no distinction based on age.
If pithing has been carried out in contravention of the regulations, the FBO should ensure:
- the dry landing area must be cleaned
- any brain tissue must be wiped away
- any paper towels associated with cleaning SRM contaminated equipment must be placed in the SRM bin
- the carcase must be disposed of as SRM and the disposal overseen by the FSA: includes all parts excluding hides
- the pithed carcase must be kept separate from other carcases or animal products which are not SRM
- any knives or tools which have been used must be washed and sterilised before being used on any other carcase
3.4 Verification tasks at despatch
3.4.1 Verification checks before transfer to an approved, authorised cutting premises
At a slaughterhouse that despatches carcases, the OV/AO is to make verification checks on the FBO documentation to confirm that:
- carcases containing SRM VC
- un-split adult sheep carcases containing spinal cord
- heads of bovine over 12 months old for the harvesting process
have been consigned to an approved cutting plant authorised to remove VC / sheep spinal cord (SRM) and for the harvesting process of bovine head meat.
Up to and including 30 June 2017, the commercial documentation should indicate the number of carcases and wholesale cuts in the consignment that require SRM
VC removal as well as the number of carcases and wholesale cuts where SRM VC removal is not required.
From 1 July 2017, the commercial documentation should indicate the number of carcases and wholesale cuts in the consignment that require SRM VC removal.
Reference: (EC) No 999/2001, Annex V, 11.3(b)
Although it is not a legal requirement, it is expected that FBOs agree a SOP:
- for the despatch of OTM carcases for the removal of VC (SRM)
- for the despatch of unsplit sheep carcases for the removal of spinal cord,
- for the harvesting process of bovine head
in order to ensure these are only sent to cutting plants with the appropriate authorisation.
Before being transferred to the additionally authorised cutting plant, bovine carcases requiring SRM vertebral column removal must:
- have received a negative BSE test result if BSE testing is required
- have passed a post-mortem health inspection
- carry a label bearing a red stripe
- carry a health mark
3.4.2 Loading operations: verification checks
The OV / AO must perform spot checks on loading and documentation. These spot checks should take place on a risk-based frequency, according to the effectiveness of the FBO’s controls and the frequency of the loads.
An entry in the daybook confirming that these checks have been carried out must be made, as a minimum, on a weekly basis. If it takes more than a week between loads, then an entry should be made when a transfer is completed.
Note: The carcases may be transferred as carcases, half carcases, quarters or half carcases cut into no more than three wholesale cuts.
3.5 Verification tasks for the cutting plant
3.5.1 Scope of the instructions
The duties detailed below relate to:
- bovine carcases from animals aged OTM, imported from countries with a controlled or undetermined BSE risk
- bovine carcases from animals aged OTM, imported from countries with a negligible risk, but which have had an indigenous BSE case
- bovine carcases from animals imported live and slaughtered when aged OTM
- bovine carcases from domestic animals aged OTM at slaughter
- older sheep and goats (>12months of age) where removal of spinal cord is taking place
- bovine heads that undergo the harvesting process of the head meat.
The risk status of third countries is set out in Commission Decision (EC) 2007/453.
3.5.2 FSA attendance during SRM operations
FSA presence is not required 100% of the time for the SRM control operations (see section 2.4 for details).
3.5.3 Verification requirements
FSA staff are required to verify that the cutting establishment is in possession of an authorisation for SRM control for the removal of Vertebral Column from bovine carcases or SRM spinal cord from sheep carcases or harvesting of meat of the bovine heads.
Processing should be undertaken in accordance with the FBO’s food safety management procedures and the agreed SRM removal RMOP or handling through SOPs.
3.5.4 Verification particulars
During the visit to the cutting establishment, attention should be paid to the following processes:
1. VC removal:
- Correct identification and labelling of the carcases stored in the chillers.
- FBO records on consignments received and number of carcases processed (bovine and ovine).
- Imported bovine carcases OTM.
- Adequate separation during processing between meat containing SRM and meat which does not contain SRM.
- SRM VC (see SRM chart definition at sub-topic 2.2.1 on ‘FSA key issue’ in this chapter):
- removal
- Note: an FBO may remove the VC but still leave the red label for traceability purposes. When checking red label carcases, half carcases or quarters, where an attempt to remove the VC had been made attention is to be put to verify whether that SRM removal had been effective. That is, no SRM VC remains.
- In these cases, particular attention needs to be made when assessing remaining bone fragments as whilst the vertebral body is SRM, the spinous and transverse processes of the cervical, thoracic and lumbar vertebrae, and the median sacral crest and wings of the sacrum are not SRM.
- collection
- staining and storage
2. SRM spinal cord (sheep and goats):
- FBO records on consignments received and number of carcases to be processed
- adequate stock control and segregation of the carcases
- removal of SRM spinal cord
- collection
- staining and storage
3. Harvesting of head meat must be clearly identified and supported by records.
- sampling plan to detect central nervous system tissue must be implemented
- bovine heads must be presented with front hole and foramen magnum covered and no damaged horns or eyes.
- the heads intended for transport to the cutting plant shall be suspended on a rack during the storing period and the transport from the slaughterhouse to the cutting plant.
- the heads must be stored suspended on a rack in the chiller.
- verifies that there is a procedure in place to ensure that there is no risk of cross-contamination during the processing (aide memoire).
- SRM vertebral column / spinal cord and the whole skull after the harvesting processes placed in appropriate and labelled SRM containers prior to transfer to main holding container.
- Equipment is cleansed and disinfected after completion of SRM removal.
- FBO must not apply the identification mark to meat intended for human consumption until after all SRM vertebral column / spinal cord has been removed and adequately handled for the harvesting of meat from the bovine heads
- FBO has robust systems in place to ensure that all SRM VC is removed from imported carcases (when applicable) before the meat enters the food chain.
FSA staff should also verify ABP consignment documents.
An entry in the daybook confirming operations in accordance with the approved SOPs / RMOP must be made at each visit, if applicable. Any discrepancies or findings must be communicated to the line manager ITL / FVC / AVM and be written in the daybook and Enforcement Programme with the appropriate action taken.
3.5.5 Mechanically separated meat (MSM)
MSM derived from cattle, sheep or goat bones or bone in cuts cannot be used in the preparation of food for human consumption or animal consumption.
Reference: See topic 2.2.3 on ‘Mechanically separated meat’ in this chapter.
3.5.6 VC or spinal cord removal and the harvesting of bovine head meat process
The following standards must be met:
- the plant holds the appropriate authorisation (which includes a Recommended Method of Operation (RMOP)) and/or SOPs
- effective separation of carcases containing SRM and those containing non- SRM is maintained at all times
- bovine heads from cattle aged over 12 months of age must have the bolt hole and foramen magnum covered (for example, stoppers) and no damaged or lost eyes / broken horns
- bovine heads received for the harvesting of head meat must be segregated from other products and kept on a rack during the transport and during the chilling process
- to ensure that the tonsils are treated as SRM during the harvesting meat process of bovine head (in bovine heads from over and under 12 months old)
- cleansing and disinfection is implemented before processing any non-SRM meat
- carcases/meat are held in a separate chiller or on separate dedicated rails.
3.5.7 Handling of SRM
The FBO is to ensure that SRM is:
- removed and / or controlled by staff of the establishment who adopt the necessary hygiene measures to avoid the risk of cross- contamination, for example, avoid touching the carcase with hands or implements which have been used to remove, or come into contact with, SRM without being washed / cleaned in between or the process of the harvested bovine head meat must be handled in a way that the meat is not in contact with the SRM.
- handled after removal from the carcase in such a way that there is no contact with any other animal material
3.6 Verification tasks for the management of SRM staining, transfer and storage
3.6.1 Staining of SRM
Verify all SRM is stained immediately (even if it is going for incineration on site) after removal from the carcase and the harvested meat of the head with blue agent.
Note: Where it is suspected that the correct dye is not being used, record the incident in the Daybook, preserve any relevant evidence, and report your findings to your FVC. Local Trading Standards should then be informed, so they can investigate the matter and take appropriate action.
3.6.2 Stain application
SRM is stained before it leaves the slaughter hall / authorized cutting establishments for the SRM control unless doing so risks contamination of fresh meat. If not, stain is applied as soon as it leaves the slaughter hall / authorised cutting establishments for the SRM control and undertaken in a suitable area.
The stain should be applied to each layer of SRM and a suitable tool dedicated to the task used to stir the SRM to ensure it achieves individual coverage.
3.6.3 Stain visibility
The stain is visible over 100% of the surface of all SRM, except sheep and goat heads where it needs to be clearly visible over the whole of the cut surface and majority of the head.
3.6.4 Mixing of ABPs
A system for disposal of ABPs is robust to ensure that all by-products are disposed according to their category, or if different categories are mixed with SRM, they are disposed as the higher risk category (SRM Category 1).
SRM (Category 1 ABP) is kept separate from all other ABP categories unless it is intended to dispose of all ABPs as Category 1.
If SRM is not intentionally mixed with other ABP categories, there must be established procedures and separate lines for disposal to ensure that different by- product categories cannot be mixed.
3.6.5 SRM transfer
All SRM is transferred to correctly identified storage bins without undue delay.
3.6.6 Storage of SRM
All SRM is stored entirely separately from all food material, in containers which are:
- indelibly marked
- impervious and leak proof
- lidded
3.6.7 Consignment of SRM
The FBO has:
- records of SRM consigned to an approved destination, either an:
- approved incinerator, or
- approved Category 1 processing plant, or
- approved Category 1 intermediate plant
- additional records if non-stained SRM is being consigned for BSE testing, research, educational or veterinary purpose
- arranged for adequate transportation
- a commercial document must be produced for each consignment
3.6.8 Commercial documents
The commercial document must specify:
- the name, address and approval number of despatching establishment
- the quantity, weight, volume or number of packages and a description of the SRM consigned, including, if applicable (for example, whole bovine bodies or carcases) the eartag number of the animal
- the name and address of the haulier transporting it
- the date on which the SRM was consigned from the premises
- the destination to which it was consigned including the name, address and approval or registration number
Consignment records are retained for 2 years from the date of consignment.
The OV should verify FBO controls by:
- reconciling the SRM records from the establishment with the number of animals slaughtered
- investigating discrepancies
- taking appropriate enforcement action if there is evidence of possible contraventions of the TSE or ABP Regulations
3.6.9 Verification of the SRM controls in approved, additionally authorised cutting establishments
If meat is found to contain VC (SRM) / spinal cord in adult sheep / goat after the point where it would normally be removed and head meat is contaminated from SRM after the harvesting:
- process: the meat should be detained if necessary (see chapter 7 on ‘Enforcement’)
- the FBO should be required to remove the SRM only under FSA supervision (only in VC / spinal cord removal)
- record evidence of the non-compliance
- issue verbal warning
When VC is found in packed meat the FBO may be permitted to reprocess and remove the SRM under FSA supervision, if this is practical. If this is impractical, the OV may declare the meat unfit as per the Hygiene Regulations.
For a repeated non-compliance, the OV should:
- detain the meat (if necessary) and require the FBO to remove SRM only under FSA supervision
- record non-compliance and issue written warning or consider referral for investigation, or possible suspension of the authorisation for the SRM control
- if it is a major non-compliance, then referral for investigation or suspension of the authorisation for the SRM control should be considered. The OV/AO should contact the FVC and AVM before any of these decisions are made. If suspension is the chosen option, then this will come into operation 21 days after the notice is issued (subject to appeal by the FBO). This period should give the FBO the opportunity to take corrective action otherwise the suspension will come into effect.
- a major non-compliance is regarded as:
- when an attempt to break the law is suspected, irrespective of where it is discovered
- when meat containing SRM has been despatched from the cutting plant
- there is evidence that the on-site procedures are not followed compromising food safety/ ABP/SRM controls
In the event of SRM having been despatched from the cutting plant the Incidents Team will be informed immediately.
3.6.10 Verification of the disposal of SRM in approved, authorised cutting establishments
- If the OV/AO detects Category 1 material (SRM) disposed of as Category 2 or Category 3, the OV/AO should serve a notice requiring the resultant mixture to be disposed of as Category 1 (ENF 11/12). The OV should consider enforcement action in accordance with FSA Enforcement Policy.
- If the OV/AO finds evidence that Category 1 material (SRM) has been despatched as Category 2 or Category 3 material, they should consider enforcement action in accordance with FSA Enforcement Policy and notify APHA and the relevant LA as soon as possible. APHA will carry out a risk assessment to determine (with Defra) what, if any, further action is required to safeguard animal health.
Please contact the FVC if in doubt.
3.7 Verification tasks for the disposal of SRM by incineration or co-incineration
3.7.1 Disposal of SRM by incineration
SRM from certain animals that require TSE testing must be disposed of by incineration or by rendering followed by incineration.
Reference: See chapter 2.6 on ‘TSE Testing’ for additional information.
The OV must obtain a declaration from the consignee confirming that this material is incinerated after processing.
3.7.2 Category 1 SRM by incineration
(EC) 999/2001 requires that the by-products from tested animals that have not been tested negative for a TSE must be disposed of by incineration or rendering followed by incineration:
- carcases, blood, hides / skins / fleeces and body parts from animals tested positive to a TSE
- carcases, blood, hides / skins / fleeces and body parts from ‘no test’ bovine or caprine animals
- carcases, blood, hides and all body parts of insufficient test animals
- the carcase before and two carcases after a positive bovine, their blood, hide (unless individually identified) and body parts
- the carcase before and two carcases after an insufficient test bovine, their blood and all body parts
- any body part from an animal that has been sampled for TSE that is disposed of before a test result is received
Reference: (EC) 999/2001 Annex III. Chapter A. I.6.3 and 6.4, (EC) 999/2001 Annex III. Chapter A II.7.3 and 7.4, (EC) 1069/2009 Article 12
4. Operational policy
In this section
4.1 Removal of the SRM from bovine intestines
4.1 Removal of the SRM in bovine intestines
4.1.1 SRM in bovine intestine
The following parts of the bovine intestine are designated as SRM in animals of all ages in countries with controlled risk of BSE (England, Wales, ROI, Greece and France):
- the last four metres of the small intestine
- the caecum
- mesentery
4.1.2 The bovine intestine anatomy
The small intestine starts at the pylorus and terminates at the entrance of the caecum at the ileo-caecal valve. The small intestine consists of duodenum, jejunum and ileum.
The large intestine extends from the end of the ileum to the anus. It is divided into the caecum, the colon and rectum.
The caecum is cylindrical, 50-70cm long and slightly curved with a blind end.
The mesentery is the connective tissue attached to the intestinal loops of small and large intestine including the mesenteric fat.
4.1.3 SRM removal from bovine intestine
Total separation of the intestines from other green offal must be carried out in the gut room and must include the whole length of intestines including any bag used in bunging.
SRM separation should be carried out in the gut room after post-mortem inspection in accordance with the following:
- the last four meters of the small intestine from the ileo-caecal junction, towards the small intestine, should be removed in each case; the FBO should establish an auditable and verifiable system for measuring the length of intestine to ensure that all SRM is removed
- the caecum needs to be removed
- the small intestine and large intestine need to be run off in order to remove mesentery
- the removed section of small intestine, the caecum and mesentery must be treated as SRM (Category 1 ABP)
4.1.4 SRM from bovine intestine not separated
If the FBO does not wish to separate the last four metres of small intestine, the caecum and the mesentery from the intestines of cattle they must ensure that the entire bovine intestine is treated as SRM (Category 1 ABP).
4.1.5 Mesenteric fat
Mesenteric fat is currently considered as SRM and shall be disposed of as such because it is very closely associated with the mesentery and cannot be safely separated from it.
The FBO is to ensure the mesentery, including all of the mesenteric fat, is completely removed from the harvested product.
4.1.6 FSA duties
Having due regard to hygiene and the risk of cross-contamination, FSA staff must verify FBO controls by:
- undertaking spot checks on FBOs when removing the SRM from the bovine intestine to ensure that all SRM is removed from the intestines and stained as SRM (Category 1 ABP) or
- where SRM separation is not being carried out, undertake spot checks to verify that all intestines remaining attached to the SRM are stained and disposed of as SRM (Category 1 ABP)
Note: Please refer to ‘Removal of SRM from bovine intestines’ poster for further guidance.
4.2 Non-stained SRM
4.2.1 When staining of SRM is not necessary
SRM may only be removed from approved establishments without prior staining in the following circumstances:
- whole bodies of dead cattle, sheep or goats
- consignments intended for exhibition, teaching, scientific research, special studies or analysis
- consignment to approved premises for the manufacture of products not connected with food
4.2.2 Checks on FBOs records
The AO must check the FBOs records of despatch of non-stained SRM to verify to that it is going to permitted destinations only.
4.2.3 Procedure
ABP 7/1 (Dispatch of SRM for Exhibition, Teaching, Scientific Research, Special Studies or Analysis) is used to monitor the despatch of non-stained SRM for research. The applicant must complete it in advance of non-stained SRM being removed from the establishment and receive AVL or FVL approval. The OV must complete section 3 every time SRM is despatched.
The outline procedure is as follows:
- the applicant completes section 1 of ABP 7/1 and sends the form to Operations Assurance SLA and Contract Team.
Reference: See chapter 9 on ‘Forms’
- the AVL / FVL reviews and signs the form
- copies of the signed form are then sent to the applicant, AHPA, the OV and the LA
- the OV then completes section 3 when the non-stained SRM is despatched
- copies are retained in plant.
5. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Risk based decision tool for ABP and SRM inspections
Annex 2: Harvesting of Bovine Head Meat in Authorised Cutting Plants checklist
Annex 3: Bovine OTM VC removal checklist
Annex 4: Ovine and caprine spinal cord removal checklist
Sections
1. Introduction
In this section
1.1 Purpose
1.1.1 Purpose
Slaughterhouses, wild game handling establishments, cutting plants and other meat plants produce material that is either unfit or not intended for human consumption. The purpose of this chapter is to advise FSA staff of their role in the official controls for animal by-products (ABP).
1.1.2 Reasons for ABP control
ABP are controlled to ensure:
- they do not compromise the hygienic production of meat
- they are not inadvertently or fraudulently diverted away from the disposal route back into the food chain
- human and animal health is protected and pathogens are not inadvertently spread
- they are safely and suitably handled, further used in accordance with the Regulations and safely and suitably disposed of
1.1.3 Definition
An ABP is the entire body, part of an animal or a product of animal origin which is not intended for human consumption.
1.1.4 Not intended for human consumption
Material becomes ABP when it is not intended for human consumption or is no longer intended for human consumption. For example, material may still be fit for human consumption but have no commercial value or not be intended for use on aesthetic grounds. Once material becomes ABP it cannot later revert to being a foodstuff.
1.1.5 SRM
SRM is covered in detail in chapter 2.7 on ‘Specified risk material controls’ and you should refer to it for guidance. Updated [From an Animal by-products point of view, SRM material can be classified as category 1 materials.]
1.1.6 Categories of ABP
There are three categories of ABP:
- Category 1, which includes SRM for:
- incineration
- rendering
- Category 2
- Category 3
1.2 Legislation
1.2.1 Regulations
The handling and disposal of ABPs is regulated by a number of pieces of legislation which include:
- (EC) 1069/2009
- (EC) 142/2011
- The Animal By-Products (Enforcement) (England) Regulations 2013 SI No 2952/2013
- The Animal By-Products (Enforcement)(Wales) Regulations 2014 SI No 2014/517 (W60)
- Regulation (EC) 852/2004
- Regulation (EC) 853/2004
1.2.2 Animal by-products (Enforcement) Regulations, Regulation (EC) 1069/2009 and Regulation (EC)142/2011
The Animal By-Products (Enforcement) Regulations (ABPR) apply and enforce Regulation (EC) 1069/2009 and Regulation (EC) 142/2011 and you will need to refer to both sets of legislation for guidance.
Together, they provide:
- the definition of ABP
- categories for ABP, (Categories 1, 2 and 3)
- permitted options for disposal or future use of ABP
- the staining of ABP
- the storage and labelling of ABP
- the restriction of the movement of ABP which requires staining
- the service of legal notices for the disposal of ABP or for cleaning and disinfection of vehicles, containers or establishments
1.2.3 (EC) 852/2004
Sets out the hygiene requirements with respect to the:
- storage
- handling
- disposal / elimination
of all food waste, non-edible by-products and refuse.
1.2.4 (EU) Regulation 2019/627(EU) 2019/627 requires the OV to:
- verify the FBOs continuous compliance with FBOs own procedures concerning any collection, transport, storage, handling and processing, and use or disposal of ABP (including SRM)
- during inspection, check the removal, separation, staining and labelling of ABP
- declare meat unfit that fails to comply with the decisions concerning food chain information, live animals and meat
1.2.5 Starting point in the manufacturing chain and obligations
As soon as FBOs generate animal by-products or derived products which fall within the scope of Regulation (EC) No 1069/2009, they must identify them and ensure that they are dealt with in accordance with the Regulation (‘Starting Point’).
Reference: (EC) No 1069/2009, Chapter 1, Section 2, Article 4 (1)
1.2.6 ABP control systems
FBOs are to handle their ABP in a way that they do not become a source of cross contamination for food. They are also to make sure they are segregated in a way that each category of ABP is treated as such and not disposed of or diverted to an unsuitable destination (for example, Category 3 ABP diverted to the human food chain or Category 1 being dispatched to a business not authorised for handling such products).
FBOs are required to have a HACCP based system in place to achieve the objectives listed at 1.1.2.
Legal Requirement: Article 29, of EC 1069/2009 “1. Operators carrying out one of the following activities shall put in place, implement and maintain a permanent written procedure or procedures based on the hazard analysis and critical control points (HACCP) principles for the:
(a)processing of animal by-products;
(b) transformation of animal by-products into biogas and compost
(c) handling and storage of more than one category of animal by-products or derived products in the same establishment or plant;
(d)manufacturing of pet food."
There are plants where the FBO decides to treat all ABP as a single category (for example, declaring al ABPs as category 1 for disposal). Those FBOs would be excluded from this requirement. Such simple systems can be handled without the need for a documented HACCP based system but they are not exempt from any other requirement and they are to comply with all requirements noted at 1.1.2.
1.3 Category 1 ABP
1.3.1 Definition
Category 1 ABPs are defined in Article 8 of (EC) 1069/2009.
The following are defined as Category 1 ABP. These pose the highest risk to human or animal health and include SRM:
- all SRM (see chapter 2.7 on ‘Specified risk material controls’ for further detail)
- entire bodies or parts of dead animals and carcases containing SRM at the point of disposal (unless the SRM has been removed and disposed of separately)
- all body parts, including hides and skins, of animals suspected or confirmed as being infected by a TSE
- animal material (sludge) or ABPs collected from waste water drain screenings in ruminant slaughterhouses and other premises in which SRM is removed
- animal material (sludge) or ABPs collected during the treatment of waste water
- animals killed in the context of TSE eradication measures
- wild animals when suspected of being infected with diseases communicable to humans or animals
- products derived from animals treated with substances prohibited under EC legislation or containing residues of environmental contaminants
- mixture of Category 1 material with Category 2 material
- mixture of Category 1 material with Category 3 material
Note: Since the introduction of the new TSE (England) Regulation implemented in July 2018, there is no SRM on sheep and goats under on year of age.
1.3.2 Examples of Category 1
The list below provides examples of the nature of Category 1 ABPs FSA staff encounter. The list is intended for guidance and is not exhaustive:
- SRM.
Reference: see chapter 2.7 on ‘Specified risk material controls’ for additional information.
- Carcases, blood and all parts (including hide / skin) from animals which do not prove negative for a TSE following testing.
- All parts (including hides / skins and blood) of TSE sampled carcases disposed of prior to test results being obtained.
- Products suspected of containing European Commission (EC) prohibited non-medicinal treatments or illegal substances, such as elevated dioxin or heavy metal contaminants, if such residues exceed the permitted levels (but does NOT include products containing residues of permitted veterinary drugs).
- Bodies of wild game animals suspected of being affected by disease communicable to humans or animals, such as foot and mouth disease or TB.
- Any animal material that comes into contact with SRM after it has been removed from the carcase.
- Whole bodies of cattle, water buffalo, sheep and goats over one year of age and bison either rejected at ante-mortem inspection, or found dead on arrival (DOA), or found dead in the lairage (DIL) (unless SRM has been removed at the point of disposal).
1.4 Category 2 ABP
1.4.1 Definitions
The list below provides a summary of Category 2 ABPs, as detailed in Article 9 of Regulation (EC) 1069/2009. Category 2 ABPs pose a high risk to human or animal health and comprise:
- ABPs not included in definitions for Category 1 or 3
- sludge collected from 6 mm waste water drain screenings in non-ruminant (pig and poultry) slaughterhouse or wild GHE
- products containing residues of authorised veterinary drugs and contaminants exceeding the permitted levels
- material imported from third countries or member states (MS) which does not comply with the veterinary requirements of the EU
- animals and parts of animals that die other than by being slaughtered for human consumption, including those killed for disease control purposes (unless these fall into Category 1) and foetuses
- products of animal origin that have been declared unfit for human consumption due to the presence of foreign bodies in those products
- manure and digestive tract contents
- blood from any animal which has not passed ante-mortem inspection
- mixtures of Category 2 material with Category 3 material
Any material that does not fall into Category 1 or 3 must be treated as Category 2 material.
Note: Many establishments upgrade all Category 2 to Category 1 material for commercial reasons. This is allowed under current legislation. Compliance with Category 1 materials is required in such cases.
1.4.2 Examples of Category 2 ABP
The list below provides some examples of the nature of Category 2 ABPs FSA staff encounter. The list is intended for guidance and is not exhaustive.
- Any carcase, part of a carcase or offal, not containing SRM, which comes from an animal or bird which was not presented for full ante-mortem inspection, or not presented with the necessary Food Chain Information (FCI).
- Post-mortem rejects containing pathological lesions indicating disease communicable to man or animal; examples include septicaemic carcases, pneumonic lungs, cysticercus bovis lesions, pericarditis, muscle abscesses, septic arthritic joints, and TB lesions.
- Material collected in drain traps or screens in non-ruminant slaughterhouses, where the material is carried in waste water which is destined for discharge from the plant.
- Whole bodies of pigs or poultry either rejected at ante-mortem inspection found DOA or found DIL.
- Any carcase, part of a carcase, offal or trim which is visibly contaminated by harmful materials or by contact with any unhygienic surface such that it is a risk to human or animal health. Examples include faeces, stomach contents, lubricants, condensation, rail debris, rust, faecal smears.
- Lagomorph intestines (where removed in an approved game handling establishment).
- Any meat or offal not handled or stored in accordance with the Hygiene Regulations, which results in the meat becoming spoiled so that it is a risk to either human or animal health.
- Any meat that is unfit for human consumption or is spoiled in any way as to present a risk to human or animal health.
- Mouldy or decomposing meat or offal including discoloured contents of blown vacuum packs that may pose a risk to human or animal health.
- Any meat found to have residues of substances which may pose a risk to animal or human health. (Note: This includes soliped carcases which test positive for the presence of phenylbutazone.)
- Blood from any animal that has not passed ante-mortem inspection (and therefore has not been slaughtered for human consumption).
- Deer carcases where the bullet has entered through the abdomen causing bruising, bone damage and extensive contamination which has warranted rejection of the entire carcase.
- Whole bodies of small wild game either rejected at intake inspection, or found grossly contaminated in the larder prior to processing.
- Lagomorph intestines (where removed in an approved game handling establishment).
- Any trim that is undertaken before post-mortem inspection. In game handling establishments and small slaughterhouses under cold inspection flexible attendance only.
Reference: See chapter 5 on ‘Residues’ for additional information.
1.4.3 Exception for pig, cattle and horse digestive tracts intended for biogas or composting
All sections of the digestive tract which are not SRM may be consigned from the slaughterhouse for biogas or composting, without removing the digestive tract contents, in the following circumstances only:
- the OV has made checks with APHA that the receiving premises are approved to carry out the appropriate process
- the gut contents do not present a risk of spreading any serious transmissible disease, Reference (EC) 1069/2009, Article 13(e)(ii)
- the FBO can demonstrate, to the satisfaction of the OV, that non SRM intestines, which are condemned or come from the carcase of an animal that has not passed ante or post mortem inspection, are not used for biogas or composting processes; this material must be disposed of as Category 2 material, unstained
To summarise, non-SRM unemptied digestive tracts that have passed ante and post-mortem inspection can be sent for biogas or composting, must be disposed of as Category 2 material, but staining is not required.
1.4.4 Digestive tract sections eligible for biogas
The table below shows the sections of the digestive tract eligible for biogas or composting provided the criteria listed on the previous page have been met.
| Species | Digestive tract sections eligible for biogas |
|---|---|
| Pigs and Horses | Entire digestive tract (stomach, small and large intestine) |
| Cattle | Entire digestive tract but not the last four metres of small intestine, the caecum or mesentery |
| Sheep | Entire digestive tract (stomach, small and large intestine) |
1.5 Category 3 ABP
1.5.1 Definition
The list below provides a summary of Category 3 ABPs, as defined in Article 10 of Regulation (EC) 1069/2009. These may be used for the production of pet food, subject to the provisions of (EC) 1069/2009, Article 35:
- Carcases and parts of animals slaughtered or, in the case of game, bodies or parts of animals killed, and which are fit for human consumption in accordance with Community legislation, but are not intended for human consumption for commercial reasons.
- Carcases and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection, or bodies and the following parts of animals from game killed for human consumption in accordance with Community legislation:
- carcases or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Community legislation at post mortem inspection, but which did not show any signs of disease communicable to humans or animals
- heads of poultry
- hides and skins (including trimmings and splitting), horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones, of:
- animals, other than ruminants requiring TSE testing, and
- ruminants which have been tested with a negative result in accordance with Article 6(1) of Regulation (EC) No. 999/2001
- pig bristles
- feathers
- ABPs from poultry and lagomorphs slaughtered on the farm as referred to in Article 1(3) (d) of Regulation (EC) No 853/2004, which did not show any signs of disease communicable to humans or animals.
- Blood of animals which did not show any signs of disease communicable through blood to humans or animals obtained from the following animals that have been slaughtered in a slaughterhouse after having been considered fit for slaughter for human consumption following an ante-mortem inspection:
- animals other than ruminants requiring TSE testing, and
- ruminants which have been tested with a negative result in accordance with Article 6(1) of Regulation (EC) No. 999/2001
- ABPs arising from the production of products intended for human consumption, including degreased bones and greaves.
- Products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise. It is important to note that these products are not to be despatched bearing the original ID mark if already wrapped / packed.
- Blood, placenta, wool, feathers, hair, horns and hoof cuts originating from live animals that did not show any signs of disease communicable through that product to humans or animals.
- Adipose tissue from animals that did not show any signs of disease communicable through that material to humans or animals, which were slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection.
1.5.2 Examples of Category 3
The list below provides some examples of the nature of Category 3 ABPs FSA staff encounter. The list is intended for guidance and is not exhaustive.
- Carcases or parts of carcases which have passed ante and post-mortem inspection, but for commercial or other reasons are not intended for human consumption.
- Examples: incised pig offal, pig spleens, stomachs and intestines from mammals or ratites empty of digestive material (except the last four metres of bovine small intestine and caecum which are Category 1), bovine head meat not intended for human consumption, bovine udders from animals that have passed ante and post mortem inspection and show no sign of any disease communicable to humans or animals, provided that the udders are not contaminated to the extent that they present a risk to human or animal health, skinned young lamb heads, sheep head meat, poultry necks, poultry intestines, testicles, pig rind, bones from a cutting plant.
- Unskinned bovine, ovine and caprine feet from animals that have passed ante mortem inspection, provided they are not contaminated to the extent that they present a risk to animal or public health.
- Unskinned bovine ears derived from carcases that have passed ante mortem and post-mortem inspection and are free from ear tags, infection and abscesses.
- Unskinned young lamb heads from animals that have passed ante and post mortem inspection, and are not suspected of suffering from any disease communicable to humans or animals. Where such a suspicion exists, the heads should be skinned and inspected prior to making a decision regarding their classification.
- Unskinned pig heads from pigs that have been skinned rather than scalded, singed or depilated, and are from animals that have passed both ante and post mortem inspection. Such heads should show no signs of disease communicable to humans or animals, and must be visibly clean and free from contamination.
- Parts of a carcase or offal that are not permitted by the Hygiene Regulations to be used for human consumption but which are nevertheless no risk to animal or human health. Examples include livers with fluke lesions, milk spot lesions, Muellerius lung lesions, melanosis, material trimmed from the sticking point.
- Any carcase, part of a carcase or offal not produced, stored or transported in accordance with the Hygiene Regulations which consequently renders the meat unfit for human consumption. Examples include traceable meat with no health mark, meat stored or found over temperature to the extent of making it unfit for human consumption.
- Meat which falls on a visibly clean floor, is picked up promptly and which is rejected as unfit for human consumption for that reason.
- Material collected in drain traps or screens in non-ruminant slaughterhouses, where it is established that water is being used to transport ABPs that are exclusively Category 3 in origin, and the water is not being discharged from the establishment as waste water (such as feathers and hairs from water flumes).
- Trimmed fat or waste carcase meat, that having passed ante and post-mortem inspection, is not intended for human consumption.
- Fat trimmed prior to post-mortem inspection, provided this originates from animals which did not show any disease communicable through that fat to humans or animals.
- Obvious lymph nodes and nervous tissue removed during cutting of fat from bovine animals.
- Meat rejected by the producer because it no longer meets specification.
- Poultry heads and feet that have passed post-mortem inspection on the line attached to the carcase.
- Poultry heads and feet separated from the carcase prior to post-mortem inspection but which have passed ante mortem inspection.
- Hides, skins, hooves / feet, horns, pig bristles and feathers derived from animals, other than ruminants requiring TSE testing or with a negative result, that have passed ante mortem inspection.
- Heads, feet and feathers of small wild game.
- Product past its “use by” date. Note: If frozen, the AO needs to take into account whether the product was hygienically frozen before its UBD.
- Venison Skin-on Pizzles and tails, velvet, from wild game handling establishments and farmed game slaughterhouses.
1.5.3 Further examples of poultry Category 3
The following rejected poultry meat can be treated as Category 3, provided it has passed an official post-mortem inspection point (inspection at whole-bird point is deemed sufficient and carcase can be removed at that point).
Post mortem rejections caused by the slaughterhouse process (for example, machine damage, overscald, uncut, badly bled).
- Product that is not intended for human consumption even though it is fit for human consumption (runts; carcases partially affected by abnormalities once the abnormalities are removed).
- Traumatic lesions such as bruises and fractures that are not infected.
- Carcases affected with ascites, when such condition is proven to be caused by cardiac insufficiency.
- Carcases contaminated by crop, upper digestive tract spillage or bile staining during the slaughter process.
- Unemptied poultry digestive track.
- Macerators: The use of macerators for cleaning digestive tract is acceptable providing it delivers a visibly clean product.
1.5.4 Downgrading of Category 3 material
Category 3 material that is showing signs of decomposition whilst in storage in approved premises does not need to be downgraded to Category 2 or Category 1, but cannot be processed further (for example, into pet food). The FBO should be required to separate such material from other Category 3 material. Unlike other category 3 material, decomposed category 3 material need to be stained.
This is an exception where the staining of category 3 material is required.
Reference: (EC) 1069/2009, Article 14(d).
The Animal By-Products (Enforcement) (England) Regulations 2013, Regulation 10 (f)
Enforcement: Where an FBO refuses to despatch such material from the premises, then a notice requiring the disposal of ABP under the ABP Regulations should be served by the OV. A copy of the document is at chapter 9 on ‘Forms’ (ENF 11/12). Updated [This notice is to be signed by the VEDM as per FSA policy.]
1.5.5 Blood intended for human consumption
When blood is intended for collection for human consumption, the following three requirements must be observed:
- It is from animals which have passed both ante and post-mortem inspection.
- A workable system is in place which allows the correlation of the blood with the carcase until post-mortem inspection has been completed.
- Blood from any carcase that has not passed post-mortem inspection, along with any other blood it has already been mixed with, is prevented from being despatched for human consumption.
Blood from a carcase that has not passed post-mortem inspection for human consumption may still be considered Category 3 material and go for approved Category 3 uses (including its use as pet food) providing the blood is derived only from:
- pigs and poultry, which have passed ante-mortem inspection, or
- ruminant animals, which have passed ante–mortem inspection and have received negative TSE test result, where appropriate
- animals that did not show any signs of disease communicable through the blood to humans or animals
1.5.6 Blood products of porcine origin intended for use in feed for non-ruminant
If porcine blood is to be used for the manufacturing of blood products for feeding to non-ruminant livestock:
- it should originate from animals which have passed ante and post mortem inspection
- or if rejected at post mortem inspection, it is not for any condition from the list below and:
- a workable system is in place which allows the correlation of the blood with the carcase such as a multiple blood tank collection system operated in accordance with agreed protocols
- blood from any carcase that has not passed post-mortem inspection for the reasons below, along with any other blood it has already been mixed with in the collection tank to which it has been identified as having been sent to, is prevented from being used for the manufacturing of blood products for feeding to livestock
1.5.7 Blood products of bovine origin intended for use in pet food
If bovine blood is to be used for the manufacturing of blood products for pet food:
- it is from animals which have passed ante and post mortem inspection
- or if rejected at post mortem inspection, it is not for any condition from the table below and:
- a workable system is in place which allows the correlation of the blood with the carcase such as a multiple blood tank collection system operated in accordance with agreed protocols
- blood from any carcase that has not passed post-mortem inspection for the reasons below, along with any other blood it has already been mixed with in the collection tank to which it has been identified as having been sent to, is prevented from being used for the manufacturing of blood products for feeding to livestock
- a workable system is in place which allows the correlation of the blood with the carcase such as a multiple blood tank collection system operated in accordance with agreed protocols
Pigs and ruminants conditions rendering the blood unsuitable for pet food:
| Pigs | Ruminants |
|---|---|
| Septic peritonitis | Septicaemia / peritonitis |
| Septic pleurisy | Septicaemia / pneumonia |
| Suspect pyaemia / multiple abscesses | Suspect pyaemia / multiple abscesses |
| Suspect fever / septicaemia | Septicaemia / other |
| Tuberculosis (generalised) | Tuberculosis (generalised) |
| Erysipelas (generalised) | - |
| Any notifiable disease | Any notifiable disease |
Blood rejected for any of the conditions above, although unsuitable for the manufacturing of blood products, can still go for other Category 3 uses including manufacture of bloodmeal (in the case of ruminants after TSE testing clearance).
In all circumstances, blood from animals with suspect levels of residues of authorised substances or contaminants above permitted levels should be disposed of as Category 2.
1.5.8 Pet food and raw pet food
Under (EC) No 183/2005 article 9 (2) any business producing ABP materials that are to be used for feeding animals (pets, zoo animals, etc) is to be registered as a feed business. The enforcement of these legislation falls under the LAs.
Updated [Pet food production in slaughterhouses, cutting plants and GHE can be permitted when FSA authorises the process and APHA has granted approval. In those cases, the FBO is to comply with the FSA Guidance for the co-location of food and pet food production.
Not all Category 3 ABP materials can be used for the production of pet food. Some restrictions apply:
1.5.8.1 Production of raw pet food
The FBO can only use Category 3 materials intended for the production of raw pet food if they comply with AEUR 1069/2009 Art 10 (a) and (b)(i) and b(ii). That is:
- product fit for human consumption not intended for human consumption for commercial reasons
- carcases or bodies or parts rejected as unfit for legal reasons, but which not show any signs of disease communicable to humans or animals
- poultry heads
1.5.8.2 Production of processed pet food
In the case of processed pet food any Category3 ABP can be used as long as it is not one of the materials referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009; i.e.
- hides and skins, hooves, feathers, wool, horns, hair and fur originating from dead animals that did not show any signs of disease communicable through that product to humans or animals, other than those covered under the point (b) of the same Article
- Fat from livestock that passed ante-mortem inspection
- Catering waste unless it comes from means of transport operating outside the British Islands (which is Category 1)]
1.5.9 Labelling
There are requirements with regards to labelling that apply to such products. They are described in Commission Regulation (EU) 142/2011
- Category 2 material intended to feed animals is to be labelled as “Category 2 animal by-products to feed...” (the specie/s or kind of animals they are intended to be used as feed)
- Category 1 material intended to feed animals is also to be labelled as “Category 1 materials to feed… (the specie/s or kind of animals the product is intended for)”. Note, very few Category 1 materials can go for pet food. The one exception is for imported pet food which comes from third countries and comprising materials, from Category 1 material referred to in Article 8(c) of 1069/2009. Essentially this means material from countries which may use hormones banned in livestock production in the EU (for example, USA) maybe imported for pet food provided they comply with all the other Category 3 conditions.
- When any ABP is intended to be used as raw material for the production of raw pet food it is to be labelled as “for pet food only”.
The OV is to verify these requirements are met. The ABP periodic checks are to verify what products are sold for the production of feed, this includes checking the commercial documentation.
1.5.10 Commercial documentation
Generally, Category 3 materials may be used for the manufacturing of pet food and raw pet food. The OV is to be satisfied that the materials are Category 3 material. For example, viscera not presented for post-mortem inspection that has visually contaminated digestive track is not classed as Category 3.
The OVs must ensure the commercial documentation provided complies with the requirements (see sub-topic 4.1.3).
2. FSA Role
In this section
2.1 Frequency of checks
Checks should be performed as required to verify FBO compliance.
| Task | By | At a slaughterhouse (Recommended minimum frequency) | At a cutting plant or GHE (Recommended minimum frequency) |
|---|---|---|---|
| Identification and separation | Any FSA team member who is appropriately authorised | As per Slaughter Hygiene Verification (SHV) system | At audit visits |
| Staining | Any FSA team member who is appropriately authorised | As per SHV system | At audit visits |
| Storage | Any FSA team member who is appropriately authorised | At the end of processing | At audit visits |
| Transport and disposal | Any FSA team member who is appropriately authorised | Monthly | At audit visits |
| Records | Any FSA team member who is appropriately authorised | Monthly | At audit visits |
| Blood Management | Any FSA team member who is appropriately authorised | Daily when carcases are rejected | At audit visits |
| Checks on Approval Status of receiving establishments | Any FSA team member who is appropriately authorised | Monthly | At audit visits |
| Enforcement | OV | As required in accordance with the hierarchy of enforcement | At audit visits |
| Audit of FBOs own procedures for ABP management | OV is responsible for the audit, but MHI may assist by collecting information | As determined by risk assessment | At audit visits |
|
Supervision and assistance in collection of samples for educational, diagnostic or research purposes Note: All requests for samples must be directed to the FBO as the owner of the product |
OV or MHI |
This work should only be completed where authorised by FVC FSA can assist in collection of samples providing this does not require additional time or cause lapses in the normal controls |
At audit visits |
Dependent on the outcome of the checks and inspections, the OV may use their discretion to increase or decrease the number of checks undertaken.
If the FBO is shown to be compliant with ABP requirements, it would be appropriate to reduce inspection frequencies to less than daily. If the FBO is shown to have weaknesses in their ABP controls, it would be appropriate to intensify the daily inspections.
The ‘Risk Based Decision Tool (RBT) for ABP and SRM’ in chapter 2.7 on SRM Controls at Annex 1 is designed to assist the OV in deciding whether checks on the staining and further handling of SRM should take place on a daily basis, or less frequently. OVs should use the decision tool monthly or, in sites operating less than 3 times a week, quarterly, and record the outcome in the plant Daybook. If there are any areas of SRM handling that change, or weak areas that pose a risk of SRM entering the animal or human food chain, the OV can amend the level of checks, in consultation with the FVC. Any changes should be regularly reviewed.
3. Verification procedures
In this section
3.2 Verification and inspection guidelines for ABP
3.1 Introduction
3.1.1 Verification
During inspection, the FSA AO must verify the FBOs compliance in relation to the legislation.
3.1.2 Verification procedure recording system
SHV K2 form in red meat slaughterhouses must be completed whenever the AO is aware of non-compliance. The hierarchy of enforcement should be used if a non-compliance is identified. Updated [As in other areas of enforcement, all forms of written enforcement taken by non-FSA employees should be send to the VEDM as decision maker, accompanied by all related documentation that would facilitate this decision. See Chapter 7 for reference.]
3.1.3 FSA Audit of FBO controls on ABPs
SHV K2 data will be used by the veterinary auditor (VA) to gather information on FBO compliance levels for ABP controls during the audited period.
3.1.4 ABP and SRM
SRM is Category 1 ABP.
Any SRM non-compliance should be reported via the SRM recording system and not the ABP system.
3.2 Verification and inspection guidelines for ABP
3.2.1 Other references
The following verification and inspection guidelines are not exhaustive. Reference should also be made to the relevant legislation.
The AO is to ensure the FBO complies with their own HACCP based system for handling ABPs when this requirement is applicable (for example, establishments handling more than one category of ABP).
Updated [The AO is to ensure the FBO complies with their own HACCP based system for handling ABPs when this requirement is applicable (for example, establishments handling more than one category of ABP or those than also produced pet food in line with the FSA collocated pet food guidance)]
Note: see section 5 of this chapter on ‘Enforcement’ regarding declaring meat unfit for human consumption.
3.2.2 Identification and separation
Verify that the FBO ensures that:
- all material which is ABP has been identified as ABP
- all ABP has been identified as the correct category
- any lower category ABP which has come into contact with a higher category ABP has been treated as the higher category material
- floor waste is managed suitably to reduce any risks
3.2.3 Staining
Verify the FBO ensures that:
- suitable quantities of the correct dye available for the staining of:
- Category 1 ABP (including SRM)
- Category 2 ABP (colouring agent)
- Updated [Category 3 only if shows signs of decomposition (see point 1.5.4 above) Colouring agent]
- the stain is properly prepared and there are suitable facilities for its application
- the stain is applied properly
- the stain is not contaminating meat intended for human consumption
Category 1 material containing SRM is stained with a dye. (Please note, the previous requirements on specific dye (Patent Blue V E 131) are no longer applicable).
Note: See chapter 2.7 on ‘Specified risk material controls’, sub-topic 3.6.1 on ‘Staining of SRM’ for action to take where it is suspected that the correct stain is not being used.
- Category 1 material which does not contain SRM is stained with a colouring agent using a solution of such a strength that the staining is clearly visible and remains visible after the ABP has been chilled or frozen, for example, wild deer carcases affected with TB.
- Category 2 material, with the exception of blood, gut contents and green offal mixed with gut content, is stained with a colouring agent using a solution of such strength that the staining is clearly visible and remains visible after the ABP has been chilled and frozen, and:
- the stain is applied to the whole surface of the ABP, whether by immersing it in the stain, spraying it with the solution or by any other equally effective means
- all pieces of Category 2 red meat and all poultry by-products comprising the entire poultry carcase (whether or not de-feathered or eviscerated) have had the solution applied after the surface has been opened by multiple and deep incisions
Exemption:
- Category 2 or Category 3 material placed in a container, the contents of which is mainly green offal, need not be stained, but this refers only to small quantities. Larger amounts would require staining and disposal as Category 2 if mixed with digestive tract. Note that this exception is granted when the use of this digestive tract mixed up with its contents deliver an equivalent effect to staining, so officers are to verify that effect is delivered (normally by slashing the intestine).
- ABP taken under the authority of a veterinary surgeon for examination
- ABP for educational, diagnostic and research purposes
3.2.4 Storage and labelling
ABPs must be stored and labelled in line with the legislation.
Verify the FBO ensures that:
- ABPs are stored in leak proof, impervious, lidded, indelibly marked and labelled bins
- bins are stored in a separate room or rooms capable of being securely locked or have closely fitting covers which are capable of being securely locked; storage of Category 3 ABPs in the same air space where meat fit for human consumption is also kept is possible provided cross-contamination is prevented
- the labels accurately reflect the ABP being held
Note: If there is a failure to implement this requirement, the FBO must be made aware of the non-compliance; although in cases where the labelling is legible it may be disproportionate to follow the hierarchy of enforcement to the level of recommendation for prosecution
- the storage of ABP does not risk the contamination of meat for human consumption
- ABPs stored frozen are kept in a dedicated building with separate boundaries entrances and reception bays that is approved as an ABP premises under (EC) 1069/2009. Staff and equipment must remain completely separate from the food premises and no food intended for human consumption can be taken into or stored in such premises
- any blood not intended for human consumption is stored in a leak proof, impervious facility and if it is disposed of before results from TSE tested animals have been received it is disposed of as Category 1 by incineration or co-incineration
Note: FSA is not the enforcement authority for the disposal of blood outside the premises.
- packaged ABPs bear the name of the producer and the address at which the ABP was packed, but not the ID mark
- meat declared unfit for human consumption which has been produced in slaughterhouses is stored in a locked facility when the site is closed; if the entire site is locked, the facilities where ABP is stored don’t need to be additionally lockable
- storage bins / facilities are cleaned and disinfected after use to minimise any risks, attraction of insects, birds and vermin
- storage bins / facilities when not in use (being filled) must be proofed against insects, birds and vermin
- storage of hides intended for disposal as ABP should have adequate separation from hides intended for human consumption (for example, the production of gelatine or collagen)
- the requirements for labelling apply to storage containers and it is best practices for transfer bins. Where containers are used for collection within the processing area and transfer to storage containers, the FBO should identify the risk of failing to handle ABPs correctly and have a procedure on the HACCP plan to control that risk, such as appropriate labels provided so that a clear system of identification is in place, or colour coding, to ensure that ABP bins are:
- used only for the correct category of ABP
- not used for material intended for human consumption
Collection containers must also be cleaned before return to production areas and maintained in a satisfactory condition.
Verify the FBO has procedures to ensure that ABPs are categorised correctly until despatch. This must be part of the HACCP based procedures for handling ABP, which are to be documented for sites handling more than one category of ABP.
Note: Current Defra guidance on hide storage is available on Defra’s website.
3.2.5 Disposal (including transport and despatch)
The following should be considered when disposing of ABPs:
- ABPs are despatched to plants approved or registered for the relevant category of ABP; this also applies to intermediate collection centres
- ABP hauliers must be registered. If they are part of an operation that is already approved (for example, approved renderer with own vehicles) the haulage part of the operation does not need a separate registration. The FBO can provide their vehicles and paperwork if they meet ABP rules. The FBO doesn’t need separate ABP registration of the haulage operation if this is done by the same business approved under 852 or 853/2004
Note: Category 3 hides and skins may be returned by producers to their own premises after an animal has been taken to a slaughterhouse. Any producers wishing to follow this route need to be approved or registered with APHA.
- anyone collecting or disposing of ABP uses adequately covered (except when loading) leak-proof containers or vehicles, or new, sealed packaging
- Category 2 ABP may be consigned to recognised kennels or packs of hounds. The owner must have approval from APHA to obtain such material from a slaughterhouse. If such ABP are being consigned for disposal as feed for animals the ABPs must be labelled as ‘For feeding to (the species of animal intended)’
- EU Regulations permit MS authorising the disposal of less than 20 kg of certain Category 3 material per week by other means. This provision is only applicable to products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise.
Reference: (EU) No 142/2011
- The complete list of derogations can be found online.
Reference: (EC) 1069/2009 Article 18
The Animal By-Products (Enforcement) (England) Regulations 2013 Sch 1
The Animal By-Products (Enforcement) (Wales) Regulations 2014 Sch 1
3.2.6 Disposal by incineration
Some types of Category 1 ABP must be disposed of by incineration only.
Reference: See chapter 2.7 on ‘Specified risk material controls’ for additional information.
3.2.7 Drain traps and gratings
Drain traps or gratings with a maximum size of 6mm are in place to collect Category 2 and 3 material. If waste water is discharged to a sewer in plants processing ruminant carcases the premises has drain traps or gratings with a maximum size of 4mm in place.
Updated [Note: The legal requirement that defines the need of a 6mm trap on drain traps is on AEUR 142/2011 Annex IV Section 2 (1)]
Note: Failure to comply with this requirement should be reported to the appropriate Water Authority.
3.2.8 Blood not for human consumption
Blood intended for use as pet food is derived only from:
- pigs and poultry which have passed ante-mortem inspection, or
- ruminant animals which have passed both ante and post-mortem inspection and have received negative TSE test result where appropriate
Note: see point 1.5.7 for list of conditions found at PM inspection that may render the blood as Category 2 and therefore unsuitable for pet food.
3.2.9 Blood for human consumption
When blood is intended for collection for human consumption, the following three requirements must be observed:
- it is from animals which have passed both ante and post-mortem inspection
- a workable system is in place which allows the correlation of the blood with the carcase until post-mortem inspection has been completed
- blood from any carcase that has not passed post-mortem inspection, along with any other blood it has already been mixed with, is prevented from being despatched for use as a blood product, including for human consumption
3.2.10 Digestive tract separation
If mechanical means are used to harvest intestine from ingesta, the washings are passed through a 4mm screen for ruminant ingesta and a 6mm screen for non-ruminant ingesta to prevent tissue contamination of the ingesta.
3.2.11 Disposal of intestine
The emptied intestine must be identified and disposed of correctly. Empty and visually clean green offal can be disposed of as Category 3, otherwise they are to be disposed of as Category 2.
3.2.12 Trade in Category 1 or 2 ABP and Processed Animal Proteins (PAP)
Operators intending to move Category 1 material, Category 2 material and meat-and bone meal or animal fat derived from Category 1 and Category 2 materials to another EU Member State (MS) must comply with the conditions set out in Article 48 of Regulation (EC) No 1069/2009.
If products are to be moved to other MS, the operator must notify the Member State of origin and apply to the competent authority of the MS of destination for authorisation before any movements occur.
The standard format for the application that operators must submit for authorisation to dispatch Category 1 and Category 2 ABPs referred to in Article 48 (1) is set out in Chapter 3, section 10 of Annex XVI of Regulation (EU) No 142/2011 (as amended). The application must be sent to Defra. Imports and EU Policy Team Area 5a 17 Smith Square London SW1P 3JR Email: Updated [ imports@apha.gov.uk ]
3.2.13 Trade in Category 3 ABP
Trade is permitted between all EU MS. Category 3 materials can move between MS provided that they are accompanied by a commercial document, but they must be produced, transported, handled, processed, stored, placed on the market, distributed, used or disposed of in accordance with the requirements laid down in Regulation (EC) No 1069/2009 and Regulation (EU) No 142/2011. Updated [The product also needs to be accompanied by an EHC. Further details in the Export or move animal bones, protein and other by-products guide.]
Further guidance including a model commercial document is available in Annex VIII, Chapter III of Regulation (EU) No 142/2011.
3.2.14 Transport of Category 3 ABP
FBOs may use their own means of transportation for their ABPs to an acceptable ABP destination. In those cases, commercial documentation, labelling and any other requirement also apply.
If the FBO decides to transport Category 3 ABP and food in the same vehicle, adequate separation is to be maintained (such as clean, clearly labelled containers) to prevent cross contamination. The FBO is to comply with the requirements in food law so these ABP do not become a food safety hazard.
3.2.15 Dispatch of Category 2 or 3 ABP for Exhibition, Teaching, Scientific Research or Special Studies
On occasions requests of ABP can be made for research or educational purposes.
Before ABP are dispatched from an establishment for educational, diagnostic and research purposes, requests must be directed to the FBO as the owner of the product and Form ABP 7 must be completed. This would not be applicable, should the material be fit for human consumption (for instance, a pig head that has passed ante mortem and post mortem inspection as fit for human consumption would only need commercial documentation, however, for a liver with Fasciola, the relevant ABP form would need to be completed).
Depending on the ABP Category, the following forms should be used:
- For Category 1 (SRM)- Form ABP 7/1 (This form can be found in Annex 4 of this chapter)
- For Category 2 or 3- Form ABP 7/3 (This form can be found in Annex 3 of this chapter)
Both ABP forms consist of three Parts:
Part 1 needs to be completed by the person responsible for the handling and disposal of ABP.
Note: On occasions the name and address of the research establishment will not be known at the time of the ABP collection, in this case, the name and address of the authorised transporter should be recorded.
Part 2 needs to be completed by the local OV, who needs to carry out the necessary checks before signing the document. Once those are satisfactory, they can approve the application, allowing the release of the ABP to the applicant.
Updated [Note: The animal by-product operating plants approved premises guidance must be used to check if the disposal establishment is approved by APHA and authorised to handle the relevant ABP category included in the application.]
Exemptions: Research/Educational facilities can handle and collect category 1/2/3 without approval and/or registration as they are exempt under the ABP derogation (D12), however, they are still required to submit the ABP 7 application.
Note: The complete list of derogations can be found online.
Part 3 needs to be completed by FSA staff at the establishment the ABP are dispatched from each time and shall include the following information:
- Date of dispatch of ABP;
- Type of ABP;
- Number/weight of ABP;
- Recipient’s name and signature;
- Authorised Officer name and signature.
The completed form should be retained by Operations, in plant folder. Copies should be sent to:
- The applicant via email
- The FBO
- ABPportfolio.FSA@food.gov.uk
- ABP team in APHA CSCOneHealthABP@apha.gov.uk
- Local FSA FVC/FVL
- the Local Authority in whose area the recipient establishment is situated.
Note: When needed the OV is to contact local FSA FVC/FVL to seek further clarification.
A copy of the completed form must be retained in the plant folder for 1 year.
Note: A sample of 1-2 random consignments is to be checked every month, by the local FVC to verify the final destination of collected ABPs. When authorised by the APHA, the transporter is required to keep full records (commercial documents) to trace the ABPs. The OV in the relevant SH will need to request that information directly from the Applicant and send a monthly sample to FVC for them to check the destination.
Enforcement
If there are concerns about the ABP destination, the AO is to contact APHA and notify these concerns using the following e-mails CSCOneHealthABP@apha.gov.uk (England) or apha.cymruwales@apha.gov.uk (Wales).
If there are concerns that animal by-products will be transported contrary to the requirements of (EC) 1069/2009 or ABPR, the AO should take the following action:
- Inform the FBO and transporter of the non-compliance
- Inform both the LA in which the approved premises are situated and also the LA in which the premises of the destination are situated of the potential breach of the legislation.
4. Record Keeping
In this section
4.1 FBO responsibility
4.1.1 ABP control systems
Under Article 29, of EC 1069/2009, FBOs handling ABP shall put in place, implement and maintain a permanent written procedure or procedures based on the HACCP principles for the handling and storage of more than one category of ABP or derived products in the same establishment or plant. This requirement also applies to business that process animal by-products or produce pet food.
In the case of single category (like an establishment declaring all their ABP as Category 1 for commercial reasons) the need of a written procedure is not legally required but is advisable. A system to handle that single category (store, identify, dispatch etc) is still needed and it is to be effective.
In the case on that single category of ABP Category 3 intended for feeding animals (pets, fur farms, zoos, etc) the establishment is by definition operating as a feed business and therefore should register itself as such.
HACCP principles apply under feed legislation, therefore operators are to ensure that they have a HACCP based system to handle ABP. The enforcement and supervision of activities related to the feed chain currently fall under the LA.
4.1.2 Record details
Verify the FBO meets the requirements for commercial documentation (as for all ABPs, the commercial documentation is to be signed and dated by the responsible person).
Additionally, there should only be one reference per consignment. A single note for a period of time (for example a year) is not acceptable.
Note: Commercial documents can be used as the operator’s records providing they contain the required information including the name and address of the carrier and the name and address of the receiver (this may not necessarily be the final destination).
4.1.3 Commercial documents
Verify the FBO has generated commercial documents in at least triplicate (3 copies):
- one copy accompanies the consignment to its final destination and must be retained by the receiver
- another copy is retained by the producer
- a further copy is retained by the carrier for audit purposes
Note: An electronic system equivalent to the paper based system described above is acceptable.
Reference : (EC) 1069/2009, Article 21
4.1.4 Commercial documents contents
The AO is to verify the commercial document specifies:
- the date on which material was taken from the premises
- description of the material including:
- the category of the material as per Articles 8 to 10 of 1069/2009
- the animal species
- if destined for feeding, the relevant point in Article 10 of 1069/2009 for Category 3 material and products derived therefrom
- the ear-tag number, if appropriate
- the quantity of material, in volume, weight or number of packages
- the place of origin of the material, from where dispatched
- the name and address of the carrier
- the name and address of the receiver and, if applicable, its approval or registration number (under food, ABP or animal feed legislation)
- if appropriate, the approval number of the plant of origin, and the nature and methods of the treatment
Verify that the commercial document is signed by the responsible person, and that the colour of the signature is different to that of the printing. The requirements are clearly detailed in Commission Regulation (EU) 142/ 2011.
4.1.5 ABP destroyed or used on the establishment
Verify the FBO keeps records of any ABP destroyed or used at the establishment, for example, incinerated or rendered.
4.1.6 ABP records retention
Verify the FBO retains records for a minimum period of 2 years.
5. Enforcement
In this section
5.3 Declaring meat unfit for human consumption
5.4 Enforcement of transport requirements
5.6 ABPs brought on to an FSA approved establishment
5.1 Introduction
5.1.1 Enforcement responsibility
The FSA is responsible for enforcement within approved premises, acting on behalf of Defra, Scottish Government and Welsh Government. The Local Authority (LA) is responsible for enforcement relating to ABPs outside of approved premises.
5.1.2 Approach
Where more than one legal provision is contravened:
- choose the specific provision that best fits the scenario
- escalate enforcement through the hierarchy
- this will be dictated by the powers available in the relevant implementing regulation
- once the enforcement has begun (verbal) under a particular Regulation, the escalation of the issue is to be undertaken always based on the same legal requirement; it is inappropriate to start enforcement under one legislative area (for example, verbal to written warning under Reg (EC) 1069/2009 and then decide to use Reg (EC) 852/2004 and to serve a RAN or HIN)
Where the non-compliance is a clear contravention of different EU regulations:
- it may be appropriate to escalate both issues in parallel, citing the separate legal references in all informal and formal enforcement
- this will always include verbal advice and if ignored, written letters of warning. Updated [Written advice must be referred to the VEDM unless the advice is provided by an FSA employee, see chapter 7 for further detail.]
- formal notices may only be served where the legislation provides such a power. Updated [Written advice must be referred to the VEDM unless the advice is provided by an FSA employee, see chapter 7 for further detail.]
- this may result in enforcement action for similar issues running in parallel but at a different pace, and referrals for investigation being put forward at different points in time where the non-compliances remain outstanding
- Updated [referrals are to be passed to Defra or Welsh Government unless they are exclusively linked to specific failures on staining.]
For TSE and other Regulations lacking of formal notices, where the power to issue a formal notice does not exist under the legislation verbal and written advice would naturally be followed by a referral for investigation where FBOs fail to correct the non-compliance.
Different issues escalated at different speeds can always be linked at a later date where they evidence similar problems.
Evidence identifies contraventions enforced by other competent authorities.
This evidence should be secured and passed on for action.
Note: Risk based enforcement also applies to ABP controls.
5.1.3 Offences outside premises
Where the OV suspects breaches of the legislation outside the premises, they must inform the LAs for both the transporter if it is a transport related problem and the receiving premises.
5.1.4 Non-compliance
For any non-compliance with (EC) 1069/2009, (EC) 142/2011, ABPR or (EC) 852/2004 within approved premises, the OV should use the hierarchy of enforcement and have regard to risk based enforcement principles.
If there is an imminent risk to public or animal health and the FBO refused to comply with verbal advice, immediate action must be taken.
Recurring offences: When the failures on ABP controls are the sign of a system failure the OV may consider taking action against the ABP handling HACCP based system. Sometimes, procedural failures are the root cause so action under Article 29, of (EC) 1069/2009 is to be considered when applicable (for example, more than one category of ABP being handled at that site).
Reference: See chapter 7 on ‘Enforcement’ for additional information.
5.2 Statutory notices
5.2.1 Statutory notices for non-compliances with ABPR
There are three statutory notices available to the OV to enforce contraventions of the ABPR:
- Notice for the Disposal of By-Products (ENF 11/12), where ABP are not being disposed of correctly.
- Cleansing and Disinfection Notice (ENF 11/13), where cleansing and disinfection of a vehicle, container or premises is necessary.
- Notice Prohibiting Animal By-Products being brought on to the Premises (ENF 11/14), where ABP are being brought into approved premises that are not approved as an intermediate plant.
Reference: See chapter 9 on ‘Forms’.
5.2.2 Statutory Notices for non-compliances with EC 852/2004
For contraventions of (EC) 852/2004, the OV should escalate the matter through the hierarchy of enforcement and where contravention persists, they may serve:
- a Hygiene Improvement Notice (ENF 11/23) under Regulation 6 of the Food Hygiene (Wales) Regulations 2006 (as amended), or Regulation 6 of the Food Safety and Hygiene (England) Regulations 2013
If an imminent risk to public health occurs, the OV may serve:
- a Remedial Action Notice (ENF 11/24) under Regulation 9 of the Food Hygiene (Wales) Regulations 2006 (as amended), or Regulation 9 of the Food Safety and Hygiene (England) Regulations 2013 where verbal advice is ignored
Reference: For additional information see chapter 7 on ‘Enforcement’, section 4.5 ‘Remedial Action Notices’ and section 4.6 ‘Hygiene Improvement Notices’.
5.3 Declaring meat unfit for human consumption
5.3.1 FSA role and responsibility
As part of FSA verification duties, Regulation (EU) 2019/627 places certain obligations upon the OV to declare meat unfit for human consumption in specific circumstances. These include situations where any of the requirements contained in the provisions listed below are met:
- Articles 40 and 41, decisions concerning food chain information
- Article 43, decisions concerning live animals
- Article 45, decisions concerning meat
The OV must advise the FBO that the material in question can no longer be considered food, despite any previous intentions the FBO had with respect to that material. The OV must put in writing that the material is being formally ‘declared unfit for human consumption’ and cite the requirements of the European Regulations.
The material must be stained and disposed of as an ABP in accordance with the both the European and domestic ABP legislation.
Where the FBO fails to stain, dispose and consign the material in the correct manner, the OV must issue a formal ‘Notice for the disposal of animal by-products’ (ENF 11/12) requiring the disposal of the material within 48 hours. That 48 hour deadline can be altered if there are justifiable reasons for it. The Authorised Officer is to manually alter the date on the form.
Where the OV has served a notice which has not been complied with, they may arrange for it to be complied with at the expense of the person on whom the notice was served (Regulation 25(3) of the ABPRs (England), Regulation 25 (3) of the ABPRs (Wales)).
If the FBO refuses to comply with the formal notice, evidence must be gathered to support the breach of the notice and the matter must be referred for investigation (see chapter 7 on ‘Enforcement’).
Reference: See chapter 7 on ‘Enforcement’, annex 5.
5.4 Enforcement of transport requirements
5.4.1 AO action
If it is obvious that animal by-products will be transported contrary to the requirements of (EC) 1069/2009 or ABPR, the AO should take action as outlined in the table below.
| Stage | Description |
|---|---|
| 1 | The AO informs the FBO and transporter of the non-compliance and that the matter will be reported to the LA who has enforcement responsibility for ABP during transportation. |
| 2 | The AO informs both the LA in which the approved premises is situated and also the LA in which the premises of destination is situated of the potential breach of the legislation. |
| 3 | The AO gathers evidence and records in the FSA daybook:
|
5.5 ABP destination concerns
There are scenarios where the FSA officer may be concerned about the final destination of a batch of animal by-products. That could be linked to the immediate disposal of large quantities of product after being rejected as unfit or found during a routine review of FBO records
In these cases, the AO is to contact APHA and notify these concerns using the following e-mails CSCOneHealthABP@apha.gov.uk (England) or apha.cymruwales@apha.gov.uk (Wales).
5.6 ABPs brought on to an FSA approved establishment
There are scenarios where an FSA Officer may find evidence of Animal By- products brought onto an FSA approved establishment under Assimilated Regulations (EC) No 852/2004 and 853/2004.
Even in cases where dual approval has been granted (for example, production of pet food), those premises had been authorised to have dual approval under the condition that they only process ABPs generated on site by activities that they are approved for.
Therefore, when the AO finds evidence of ABPs brought onto an FSA approved establishment, the FSA Officer is:
- to request this practice to be stopped with immediate effect
- to verify that ABPs already on site are categorised appropriately, and
- that the establishment has the means to dispose all the ABPs through permitted disposable routes
When the FSA Officer is not satisfied with the correct categorisation of these ABPs, they may ask for this to be re-categorised, and in cases where the FBO is reluctant to comply, the following notice can be used:
- for England- Notice for the Disposal of Animal By-Products- ENF 11/12(E)
- for Wales- Notice for the Disposal of Animal By-Products- ENF 11/12(W)
In cases where the Food Business Operator fails to comply with the request of not bringing more ABPs, a Statutory Notice for Contraventions of (EC) 1069/2009 might be served under The Animal By-Products (Enforcement) (England) Regulations 2013, where verbal advice is ignored:
- for England- Notice Prohibiting Animal By-Products Being Brought onto the Premises - ENF 11/14(E)
- for Wales- Notice Prohibiting Animal By-Products Being Brought onto the Premises - ENF 11/14(W).
6. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 1: AB31 Form - REMOVED
Annex 2: Risk based decision tool for ABP and SRM inspections
Annex 3: Updated [Form: ABP 7-3 Dispatch of Animal By-products (Category 2 / Category 3) for Exhibition, Teaching, Scientific Research, Special Studies]
Annex 4: Updated [Form: ABP 7-1 Dispatch of SRM for Exhibition, Teaching, Scientific Research, Special Studies of Analysis]
Sections
1. Introduction
In this section
1.1 Overview
The FSA is responsible for the inspection and enforcement of hygiene regulations on registered cow’s milk production holdings in England and Wales and for inspections at registered milk production holdings for other species.
1.2 Legislation
1.2.1 Regulations
The following list is not intended to be exhaustive, but details the main legislation under which official hygiene controls are conducted on dairy production holdings:
- (EC) 852/2004
- (EC) 853/2004
- (EU) 2017/625
- (EU) 2019/627
- The Food Safety and Hygiene (England) Regulations 2013 (as amended)
- The Food Hygiene (Wales) Regulations 2006 (as amended)
- (EC) 178/2002
- (EC) 2073/2005
- (EC) 2074/2005
1.3 Background
1.3.1 Registration requirement
Food Business Operators (FBOs) are required to register details of their milk production business and premises.
Reference: The Food Safety and Hygiene (England) Regulations 2013 (as amended)
The Food Hygiene (Wales) Regulations 2006 (as amended)
Regulation (EC) No 852/2004, Article 6
1.3.2 Advisory visits
Any FBO who intends to start milking for the first time or who intends to put in new milking equipment should contact the Approvals and Registrations Team, where the FBO can be put in touch with their local Dairy Hygiene Inspector (DHI) for an advisory visit to take place at the planning stage. This should prevent any issues developing with poor structural design or inappropriate equipment being purchased. These visits are voluntary and are at the discretion of the FBO.
1.3.3 Registration contact details
The FBO should complete an ‘Application for registration of a milk production holding’. Application forms are available from the FSA website at Food Standards Agency - Dairy hygiene inspections or contact the Approvals and Registrations Team.
1.3.4 Registered Milk production premises database
Following receipt and processing of a completed Application for registration, details of the premises will be entered on to the database of registered milk production premises.
The Approvals and Registrations Team will issue a letter to the FBO, confirming registration of the premises, and will confirm the registration details to other interested parties (Rural Payments Agency (RPA)), (local authorities (LA)).
Following confirmed registration of the premises they will be inspected within three months and thereafter according to the frequency as detailed in sub-topic 1.4.1 on ‘Inspection frequency’.
Important Note: If the application for registration indicates an intention to sell raw drinking milk (RDM) direct to the consumer or a business that is already registered for wholesale production, the same letter is sent to the producer and an immediate inspection visit will be scheduled. This visit should be completed as a priority and undertaken as soon as possible (but definitely no later than 2 weeks after the notification has been given). The intention of the inspection will be to ensure that all legal obligations are being met and to collect a verification sample to be sent for examination.
1.3.5 Who conducts dairy hygiene inspections?
Dairy hygiene inspections on registered milk production holdings in England and Wales will be conducted by FSA Operations Group Dairy Hygiene Inspectors (DHIs), following a schedule of visits provided to them. The majority of inspections are carried out without an appointment being arranged (unannounced).
Reference: See section 2 on ‘Inspection Procedures’ in this chapter.
1.4 Inspection frequency
1.4.1 Frequency of inspection
The routine inspection frequency for registered milk production premises is based upon:
- the type of product
- species producing the milk
- the date of the last inspection
- membership of the third-party accreditation scheme (Red Tractor Farm Assurance Dairy Scheme (RTFADS)
- the compliance history of the farm
- any other local intelligence, complaints or investigations that might grant an inspection
Frequency of inspection
| Production status | Species | Inspection interval | Inspection by |
|---|---|---|---|
| Raw milk sales (for drinking) | Cows | 6 months | FSA |
| Raw milk sales (for drinking) | All other species | 6 months | FSA (LA remains the enforcement authority) |
| Raw milk production | Sheep, goats, buffalo | 2 years | FSA (LA remains the enforcement authority) |
| Raw milk production non-assured dairy farm | Cows | 2 years | FSA |
| Raw milk production- assured dairy farm | Cows | 10 years | FSA |
| Any establishment following enforcement action (for example, warning letter, HIN, court action) | All | According to specified deadline, until issue resolved. Following satisfactory resolution, an inspection after 6 months. If satisfactory at that point, return to default intervals as specified above. | FSA |
2. Inspection procedures
In this section
2.3 Preparing for an inspection
2.1 Types of inspection
2.1.1 Pre-production inspection
This type of visit will be an advisory visit undertaken before production commences, when the FBO of a newly registered premises is proposing to sell either untreated (raw) milk direct for human consumption or raw milk for pasteurisation. These visits are not compulsory and are at the discretion of the FBO.
2.1.2 Primary inspections
This is a routine, scheduled inspection, determined through a risk based process. The primary inspection will identify the level of FBO compliance with legislative requirements. The result of the primary inspection will dictate any follow up action required. Primary inspections are conducted without prior notice to the FBO.
Other circumstances which will lead to a primary inspection being conducted are detailed in the following paragraphs.
2.1.3 Inspection in response to receipt of a complaint
In the event that a complaint is received about a milk production premises, an inspection should be carried out within five working days to investigate the issue. The only exceptions to this are if the complaint is in relation to RDM (in which case the visit should take priority and be arranged for as soon as possible), or if enforcement action is in progress and a follow up inspection is already planned.
2.1.4 Inspection in response to a report from a third party
If information is received from a third party body (such as RTFADS, RPA, a laboratory or a milk buyer) which suggests that an inspection is required, an inspection will be scheduled to investigate the issue within five working days of receiving and understanding the complaint, unless enforcement action is in progress and a follow up inspection is already planned.
2.1.5 Follow up inspections
Follow up inspection visits are carried out to check that improvements identified at a previous inspection have been carried out. It may be necessary to undertake more than one follow-up visit, with appropriate enforcement action being taken as necessary, until compliance is achieved.
2.2 Scheduling of inspections
2.2.1 Scheduling of inspections
Visits are to be scheduled in an order of priority based on risk and premises location.
The DHI should create a schedule of visits using the following order of priority:
- inspection of premises that have indicated an intention to sell RDM direct to the final consumer either as part of an initial registration or as an additional activity for a business already registered (visit as soon as possible)
- inspections in response to a report of a raw drinking milk (RDM) failed sample (within 5 working days)
- inspections in response to a complaint (visited within 5 working days)
- inspections in response to information received from a third party (visited within 5 working days)
- inspections in response to a report of an antibiotic failure (within 10 working days)
- follow up visits / inspections (as appropriate, depending on the issue)
- primary inspections generated under routine inspection frequency arrangements
The DHI will be provided with details of the type of inspection to be carried out and the compliance history of the establishment to be inspected.
2.3 Preparing for an inspection
2.3.1 Equipment
DHIs have been provided with, and must carry the following equipment when undertaking dairy hygiene inspections:
- mobile phone (including access to GPS software)
- FSA ID card and warrants
- official notebook
- hard copies of enforcement notices
- tablet for recording inspection details
- torch
- FSA lairage coat & spares
- GPS equipment
- green safety wellingtons
- waterproof leggings and jacket
- FSA lairage coat
- hard hat
- infra-red calibrated thermometer
- disposable nitrile gloves
- bucket and brush
- 5 litre water container filled with tap water or disinfectant already mixed to recommended dilution
- approved disinfectant
- hand sanitiser
- hand wipes
- first aid kit
- storage box to contain all Personal Protective Equipment (hard case suitable for cleansing and disinfection)
- tamper-proof evidence bags
2.3.2 Preliminary actions on arrival
The DHI must:
- identify themselves, showing their FSA Authorisation card; the DHI must always carry the FSA warrants with them during inspections
- establish to whom they are talking and their position in the business
- if that person is not the FBO, determine whether the FBO is available; if not, consider whether the person has been designated as a duly authorised representative by the FBO and is an appropriate individual to accompany the inspector and to speak on behalf of the business
- if the DHI is satisfied that they are dealing with the FBO or their appropriate representative, they should conduct a pre-inspection meeting
Note: If there is no appropriate person available (FBO or their representative) the inspection should not proceed and must be rescheduled.
2.3.3 Pre-inspection meeting
At the pre-inspection meeting, the DHI must:
- explain the purpose of the inspection and the approach being taken
- check that the business details which FSA hold are current, namely:
- holding number (county parish holding (CPH) number)
- legal name of the business
- names of business partners, if relevant
- contact telephone / mobile numbers / email address
- LA area in which the business is located
- check farm assurance status if appropriate
- herd size
- types of milk use, whether milk is sold untreated direct for human consumption or whether the FBO has knowledge that the milk is being used to produce unpasteurised products such as cheese or cream
- milking times
- water supply details and testing results
- milk purchaser details (destination for pasteurisation)
- current TB status
- change into PPE and disinfect boots/leggings as soon as is practical to limit the chance of contamination
2.4 Primary inspections
2.4.1 Overview
The inspection should cover the entire process of milk production, including the housing of the animals, storage and dispatch of the raw milk, with particular emphasis on the milking process, but not to extend to any bottling, wrapping and packaging processes.
Ancillary processes such as cleaning and maintenance schedules, waste management or laboratory results should be inspected.
During the course of the inspection, the DHI should discuss issues relating to the effectiveness of control systems in ensuring safe food production with the FBO, management representative and other relevant personnel.
2.4.2 Key areas for review
The inspection will concentrate on the key areas as listed below, together with an overall assessment of the hygiene conditions and management practices at the premises:
- animal cleanliness and health
- veterinary medicines, usage/records
- milking operations
- operator hygiene and cleaning routine
- general hygiene and management
- equipment cleanliness and cleaning methods
Each of these areas is covered on the digital enabled ‘Hygiene inspection report: Dairy’ form (DH2) on the tablet, and guidance on what to review in each of these key areas is provided in the following paragraphs.
2.4.3 Animal cleanliness and health
Depending on the time the inspection is carried out, the milking process will either be observed by the DHI or will be discussed with the FBO. If inspections are carried out during milking operations, the DHI should ensure that any disruption to milking is minimised.
Animals presented for milking must have clean teats, udders and adjacent parts before milking takes place.
The DHI must establish:
- that the FBO is aware and complies with the requirement that milk from heavily soiled animals should not be sold for human consumption, due to the high risk of contamination
- whether the FBO is aware and complies with the requirement that milk must only come from animals that present no sign of disease that might result in the contamination of milk and colostrum; this includes:
- animals suffering from any infection of the genital tract with discharge
- those suffering from enteritis with diarrhoea
- animals with a recognisable inflammation of the udder or udder wound likely to affect the milk or colostrum
- the tuberculosis (TB) and brucellosis (BR) status of the individual animal and herd; raw cow’s milk for direct human consumption or for use in the production of unpasteurised dairy products must only come from animals and herds that are free from TB and BR.
The DHI should ask questions about procedures for milking animals that are observed to have problems of this type and how milk from these animals is isolated and discarded as Animal By-Product (ABP).
The details of any non-compliance must be recorded on the inspection form and the DHI must ensure that the FBO is made aware of the non-compliances.
2.4.4 Veterinary medicines
The DHI should inspect the FBOs veterinary records. Medicine usage records should be kept up to date with treatments (within 72 hours of drug administration). There is no set form or medium in which the records must be kept, but there should be an overview of the medicine storage undertaken and the records should show the following as a minimum:
- the name of the veterinary medicine used
- the date of administration
- the name of the person who administered the medication
- the quantity of veterinary medicine used
- the identity of the animal / group of animals treated
- the date on which any withdrawal period for milk, meat or any other product ended / ends
- any necessary batch numbers for medicines
2.4.5 Milking operations: teat preparation
Teats, udders and adjacent parts must be clean before milking. The DHI should check that:
- appropriate facilities are available to enable the washing and drying of soiled teats and udders
- that foremilk is taken from each animal at each milking and examined for abnormality or that an equivalent method is used (conductivity tests)
- the milking routine demonstrates that adequate procedures to avoid contamination of the milk are applied
- teat cup liners are free from faecal contamination
- teat dips and sprays are being used in accordance with manufacturer’s instructions.
2.4.6 Milking operations: detection and rejection of abnormal milk and milking of TB reactors
The DHI should establish what procedures are in place for isolating TB reactors and animals being treated with veterinary medicines and/or unhealthy animals.
The DHI should check that the FBO is aware and is taking appropriate actions to detect and ensure that milk for human consumption derives only from individual animals that:
- do not show symptoms of disease communicable to humans through milk, or any signs of disease that may contaminate the milk, are free from enteritis with diarrhoea, inflammation of the udder or infectious discharge from the genital tract
- are free from udder wounds likely to affect the milk
- do not show a positive reaction to tests for TB or BR or are not deemed to show a positive reaction
- have not been subjected to unauthorised or illegal treatments and that the correct withdrawal periods have been observed for legal treatments
- that foremilk is taken from each animal at each milking and examined for abnormality or that an equivalent method is used (conductivity tests)
- that milk unfit for human consumption is rejected at the time of detection
- that milk obtained from animals undergoing medical treatment, likely to transfer residues to the milk, or from animals with infections which can be passed on through milk, is kept out of the human food chain
- that treated animals are effectively identified and that there is a readily available way of verifying which animals must have their milk kept out of the food chain and when it can be put back into the food chain; what procedures are acceptable will depend on the size and nature of the business
- that the FBO is aware and takes appropriate action to ensure that milk from TB reactors, direct contacts or inconclusive reactors (IRs) must be kept out of the food chain
- what method is, or would be, used to keep milk from TB or BR reactors out of the human food chain
- that the FBO is aware that if a herd loses its TB free status, milk produced on the holding from animals in the herd which are not reactors must be heat treated and the milk buyer must be notified.
2.4.7 Operator hygiene and cleaning routines
To judge compliance in this area, the DHI should determine:
- the clothing worn during milking
- that protective clothing is clean and is kept clean or changed as needed
- the availability of facilities for washing hands and arms and, if at an observed milking, that they are kept clean
- the availability of facilities for cleaning the structure of the milking area
- how the milking area is kept clean during milking
- how the milking area is cleaned at the end of milking
- that scheduled cleansing and disinfection procedures are carried out
2.4.8 General hygiene and management
The DHI should assess the milking area, food storage room and dairy wash-room for cleanliness, construction and location:
- walls, floors, roof windows and doors and any fixtures should be inspected, they should be tight fitting and discourage any vermin entry
- floors should be free draining
- all surfaces should be in a sufficiently good state of repair to enable them to be kept clean by the methods being used and should not themselves represent a hazard
- there is adequate protection against vermin; the DHI should question the FBO on their control procedures and may request the FBO to provide evidence if this is considered necessary
- discuss milk hygiene test results from milk buyers such as Total Viable Count (TVC), bactoscans and somatic cell counts with FBO and note them on the Dairy Hygiene Inspection report (DH2 – remember that the results need to be assessed in rolling geometrical average); also record if test results were not seen
Note: If not seen due to the FBO not being able to find them, ask the FBO to send them to you before the visit report (DH2) is completed and sent (a follow up phone conversation with the FBO could be had if needed). If the FBO has not got the sample results or they are not acting on them, this needs to be enforced (please see Section 4- Enforcement).
- there is effective separation from areas used to house animals
- it is constructed and maintained to limit the risk of contamination
- if there are separate food storage rooms and wash-rooms, both of these areas need to be inspected and assessed to the same standards.
Any areas of non-compliance should be discussed with the FBO and recorded on the tablet.
2.4.9 Equipment and cleaning methods
The location and condition of the milking and cooling equipment, the method of cleaning and cleanliness of the equipment must ensure that the milk is not subjected to avoidable contamination. These aspects should be assessed in accordance with the following guidelines, and any non-compliance recorded on the tablet.
2.4.10 Cleanliness of milk tanks and equipment
The DHIs assessment of the cleanliness of the milk tanks and equipment should cover:
- all surfaces intended to come into contact with milk sold for human consumption
- any other internal surfaces where air movement within the plant could contaminate the milk
- external surfaces close to inlets where air entering the equipment could carry contamination into the milk
- condition of milk tanks and equipment
If the tank contains milk, the DHI should check the temperature shown on its thermometer and record this on the tablet, this temperature can also be compared to previous recorded temperatures for comparisons. When this is not possible, milk collection tickets should be checked for the temperature at the time of collection. The cooling process must begin immediately after milking. The DHI must ascertain the FBOs method for taking milk temperatures and make sure they are in compliance with legislation. If any doubt exists over effective operation, the DHI needs to request servicing / calibration of FBOs equipment to confirm whether or not it is in good working condition and reliable readings are being obtained from it.
Prior to collection, the milk temperature must not exceed 8°C in the case of daily collection or 6°C if collection is less frequent than daily.
If the tank is fitted with serviceable filters, the servicing of these should be checked.
2.4.11 Inappropriate articles and processes
The milk storage area and any separate dairy wash-rooms should be assessed for the presence of any inappropriate or hazardous articles and processes. Only items directly related to milking and cleaning of milking equipment should be stored in these rooms. Check that:
- the milk storage area and rooms connected with milk storage areas are free from any articles that should not be there or any poisons and items likely to cause contamination
- clutter is not preventing effective cleaning
- that the use of rooms connected to milk storage areas does not result in potential contamination of the milk storage area.
2.4.12 Cleaning process and records
This covers the processes for the milking machine and the milk cooling/storage equipment. Depending on the time of the inspection, the cleaning and disinfection routines should be observed, monitored and/or discussed. Consideration should be given to the suitability of the cleaning process, including:
- frequency of application
- method of application
- detergents / disinfectants used, concentration, contact time
- water temperature
- evidence of residues / contamination within the milking equipment
- FBOs validation and verification of the method
The FBO or their representative may be requested to provide evidence that the temperature of the water and concentration of the chemical is regularly monitored.
2.4.13 Water supply
Check that the FBO has arranged with the LA for the monitoring of all private water supplies used for cleaning and disinfection of milking equipment and milk storage tanks, hand washing and washing of teats and udders. The FBO should, on request, provide evidence of the last test date and results. The FBO would need to communicate the frequency of this test.
The type of water supply (mains, or private supply) should be noted.
Where a private supply is in use, the DHI must check:
- the purposes it is used for
- the treatment is applied to the private water supply for each form of use
If a private water supply is being used and not tested by the LA, the inspector should inform the FBO or their representative, that FSA will inform the LA of the situation.
The DHI should seek immediate advice from the LA regarding testing, and from LDHI / FVL (Dairy) to determine what further action should be taken.
Where a private water supply is in use but the LA have reported that it is unsatisfactory due to contamination (for example, bacterial), the FBO should be informed that they must change to mains water or ensure that all water used for dairy purposes is treated. This requirement should be recorded on the DH2.
2.5 RDM premises only
Where a premise is producing RDM for direct supply to the final consumer, they are required to have a Food Safety Management System (FSMS). They must also demonstrate that they can verify the controls they have in place through this system is effective and being applied correctly, there is no other way to demonstrate this than by having a programme of sampling, the results of which can be used as evidence. For these premises at least one of the 6 monthly inspections each year will require the DHI to undertake an audit of the FSMS checking that it covers the elements required and that records are accurate and up to date as required, this audit will take place alongside the usual routine hygiene inspection. They will also be required to audit the FBO’s own sampling results, this will be aimed at gaining assurance that the controls applied are effective whilst also checking to see that all results have been satisfactory. Any unsatisfactory results should have been reported the FSA at the time that they were received. If this is not the case, then enforcement action may be appropriate. The audit checklist on the tablet will assist the DHI with prompts regarding the areas that should be covered as part of the audit. It is important to ensure that checks are made to verify that activities recorded within the FSMS are actually being applied correctly in practice. Checks of the operations of the processes recorded in the FSMS should be undertaken as far as practical. Where possible, reviewing previous records can be used to provide an overall picture of historic activity.
2.6 Follow up inspections
2.6.1 Follow up inspections
A follow up inspection should concentrate on the items that contravened the regulations at the previous inspection.
The DHI must assess each contravention as
- satisfactorily corrected
- not rectified
2.6.2 Follow up inspection: steps
The DHI must:
- record findings on the DH2 and dependent upon the action taken at the previous inspection, follow up with a warning letter, a HIN or in Wales only, a Remedial Action Notice (RAN)
- record those contraventions that are now satisfactory on DH2 and if there are no further outstanding contraventions update the DH1 with the date of compliance achieved, copies of all completed reports will be emailed to the FBO when they are submitted
- record any contraventions that are outstanding on the DH1, remedial actions required and update DH2
- record any significant new contraventions that were observed on the DH1 and remedial actions required
- record any recommendations of good practice you have suggested that may be appropriate
- summarise the contraventions with the FBO, giving sufficient technical advice on how to comply with the Regulations
- where a further secondary inspection is required, specify that a clear timescale for completion of the required works and must be recorded on the DH1
- when a HIN has been complied with to the satisfaction of the DHI, send a letter to the FBO informing them that the formal notice has been complied with
- when a HIN has not been complied with the next stage of enforcement is a Referral for Investigation.
2.7 Guidance on applying final compliance ratings
The below guidance provides a framework for DHIs to reference when deciding on the final compliance rating to be applied following their inspections. These are just meant for guidance and it will be up to the DHI to have the final decision and this can be based on factors not mentioned in the guidance below. Explanation on the decision for the final rating applied should always be provided to the FBO as part of the closing meeting.
| Rating | Guidelines |
|---|---|
| Good |
|
| Generally Satisfactory |
|
| Improvement Necessary |
|
| Urgent Improvement Necessary |
|
2.8 Post inspection procedures
2.8.1 Concluding the inspection
Before leaving the farm the DHI should ensure that all required information has been obtained and conclude the inspection visit with a post-inspection meeting. They should ensure all PPE is cleaned and disinfected thoroughly.
2.8.2 Post inspection meeting
The FBO of the establishment or management representative, together with other relevant managers / supervisors should be present at this meeting.
- The DHI should summarise the inspection, highlighting significant findings and ensuring that a clear distinction is made between contraventions of the legislation and recommendations of good practice.
- The DHI should explain what enforcement action (if any) will be taken.
- Solutions to problems arising and timescales for required actions should be discussed at the post-inspection meeting.
- Whether virtual evidence of compliance would be acceptable.
- Any issues raised at this meeting, which have not already been recorded, should be noted.
2.8.3 Notification of Inspection form
At the conclusion of the post-inspection meeting, and following successful completion of digital forms, the FBO should be informed that copies of all completed forms will be sent to their nominated email address.
2.8.4 Procedures after leaving the farm: primary inspection
Following a primary inspection, DHI should ensure that any completed forms are submitted via the tablet, they should allow any time needed to undertake recording of any enforcement of any non-compliances found during the inspection. Where remedial actions are required and a secondary inspection will take place, the time allowed for completion of the required works should be clearly stated in any correspondence.
2.8.5 Enforcement action
Any warning letter, HIN or RAN (Wales only) resulting from the inspection, should be drafted and sent electronically to the Dairy Operations mailbox on the same day that the inspection took place. It is important that inspectors allow time at the end of each dairy day in case of this. Following the appropriate quality checks, the Dairy Hygiene Data Team will post warning letters to the FBO. If a HIN or RAN is issued these must be either handed to the FBO directly or the DHI should post them recorded delivery. The cost of the postage can be claimed as part of routine expense claims. Copies of the HINs/RANs should be emailed to the dairy hygiene data team for storage.
3. Raw cows’ drinking milk sampling
In this section
3.1 Overview
The FSA’s DHIs have the responsibility for the collection and dispatch of Raw Cow’s Drinking Milk (RCDM) samples from registered milk production holdings in England and Wales.
3.2 Introduction
3.2.1 Legislation
Regulation 34, Schedule 6 of The Food Safety and Hygiene (England) Regulations 2013 (as amended) / Regulation 32, Schedule 6 of the Food Hygiene (Wales) Regulations 2006 (as amended) places restriction on the sale of raw milk intended for human consumption.
3.2.2 Standards applicable to RCDM
RCDM must meet the following standards:
- plate count at 300C (cfu/ml) ≤ 20,000
- coliforms (cfu/ml) < 100
3.2.3 Background
Under the consolidated EU hygiene rules, which took effect from 1 January 2006, Member States are able to introduce or maintain national rules prohibiting or restricting the placing on the market, within its territory, of raw milk or raw cream intended for direct human consumption.
RCDM must be labelled on the container or on a notice which must be displayed prominently at a farm catering establishment stating,
In England 'This milk has not been heat treated and may therefore contain organisms harmful to health’.
In Wales “This milk has not been heat treated and may therefore contain organisms harmful to health. The Food Standards Agency strongly advises that it should not be consumed by children, pregnant women, older people or those who are unwell or have chronic illness”.
A sample of raw milk will usually be procured for microbiological analysis at least twice per year. Whenever possible this sample will be taken in its final product container (FPC), if this is not possible then a sample will be collected from the bulk tank.
Samples must be delivered to the designated laboratory within 24 hours of the time at which the sample was collected (not 24 hours from the time at which the courier collected the sample from the DHI) to maintain the integrity of the sample.
3.3 Sampling frequency
3.3.1 Sampling frequency
Establishments wishing to sell RCDM must have the milk sampled and tested at least twice per year by FSA, to verify compliance with microbiological standards for total bacterial count, coliforms and pathogens that can commonly be found in raw milk
3.3.2 Routine sample
Routine sampling (RT) is conducted twice yearly. To take account of resource deployment, the next routine sample can be taken 6 months after the previous compliant sample.
3.3.3 Sample failure: FBO ceases sale of RCDM for direct human consumption
Should a routine sample of untreated milk fail to achieve the standards required by the regulations:
- the milk producer will be advised by telephone to cease selling raw milk directly to the public this will be recorded on the DH1 as verbal advice provided the failure occurred due to unsatisfactory results for indicator bacteria (TVC/Coliforms)
- If the failure is a result of a pathogen failure then sales MUST stop and the DHI should inform the FBO of this and record on the DH1 as above, if it is felt necessary enforcement at this stage could move straight to a warning letter, advice on this can be sought from the LDHI
- The producer should be advised to undertake an investigation into the root cause of the problem and consider their own sampling regime to test the effectiveness of any corrective actions taken
- follow up visits and further sampling will be undertaken where necessary but not before the FBO has provided some evidence that they have applied corrective action that has been effective
- this will continue until such time that satisfactory samples have been achieved and the DHI is content with the improvement in hygienic conditions at the premises; these follow up samples are taken by the DHI as per normal procedures
3.3.4 Sample failure: FBO continues to sell RCDM for direct human consumption
It is an offence to place raw milk on the market that has failed to meet the requirements of the regulations. Should a routine sample of untreated milk fail to achieve the standards required by the Regulations and the milk producer continues to sell untreated milk after having been advised to cease sales:
- escalation of enforcement should be applied. The enforcement approach and timescales applied could vary depending on whether the failure was due to indicator bacteria or pathogens, advice should be sought from the LDHI
- first failure: verbal advice
- second failure: inspection followed by formal warning in writing (Warning Letter)
- third failure: issue of a HIN or RAN (Wales)
- fourth or subsequent failure: referral to the Lead DHI for consideration of further action including issuing of a HEPN/HEPO in England and referral for investigation.
- in all cases it will be important to get advice from the LDHI on the correct course of action to take.
3.4 Sampling follow up
Upon receipt of a non-compliant test result, either for indicator bacteria or detection of a pathogen, the establishment will move to follow up (FU). The DHI must arrange to conduct an inspection of the establishment once they have been notified by the FBO that an investigation into the root cause of the issue has been done, that corrective action has been taken and their own sampling is indicating that this has been effective.
If the DHI is satisfied conditions upon the farm (such as cleanliness, maintenance, animal cleanliness and hygienic operations) are favourable, the FSMS is being implemented effectively and the FBO has sampling evidence suggesting this then a verification sample will be taken by the DHI and delivered to the nearest nominated laboratory.
If the DHI is dissatisfied with conditions on the farm (such as unsatisfactory cleanliness and maintenance, dirty animals and unhygienic operations) and the FSMS is not being implemented effectively, appropriate action must be taken to achieve compliance (see section 3) and recorded on the Digital Corrective Action Report form (DH1). A further sample will be taken only when the DHI is satisfied that the FBO has achieved compliance and conditions at the farm and its operations are satisfactory and evidence as such is provided.
If there is any doubt in the inspector’s mind which tests should be completed at the follow up stage then advice should be sought from the LDHI.
Should there be any dispute or disagreement on sampling results between FSA and FBO advice should be sought from the LDHI on the steps to be taken.
3.5 Sampling procedure
3.5.1 General considerations
This procedure describes how work is to be planned, lists the materials required to undertake sampling and inspection duties and details how records are to be kept.
In the interest of economy and efficiency, sampling should be planned as far as possible to farms / processing establishments in close proximity. The DHI must ensure that they have all the relevant details of the sampling schedule including the names, addresses and registration numbers of RCDM production holdings and laboratory reference (sampling point reference) for each premises.
All records MUST be completed at the time of sampling and / or inspection. Under no circumstances should records be completed after sampling / inspection nor should records be made in a notebook and then be transcribed onto the official record after sampling / inspection.
Where records are to be despatched in the secure insulated box these must always be placed in a sealed plastic bag to prevent damage.
Where the sampling DHI observes irregularities or instances where hygiene regulations are being contravened, these should be noted on the DH1 on the tablet in accordance with usual inspection procedures.
3.5.2 Pre-sampling checks
Before commencing routine or follow up RCDM sampling, the DHI must ensure that they have considered the following:
- courier service (if used) should be booked at least 24 hours in advance of collection using the Topspeed website
- sampling times should be considered with regard to which courier service is being used
- for same day service samples must be collected before 9am
- for overnight service samples must not be collected until after 10pm
They should also ensure that they have the following equipment:
- an adequate supply of pre-prepared disinfectant solution prepared according to the manufacturer’s instructions; FAM 30 should be mixed in the following ratio:
- 22ml of FAM30 to every 4 litres of clean water
- clean protective garment either:
- FSA green lairage coat
- white disposable lab coat
- clean wellington boots
- hairnet or other suitable covering for the head
- single use nitrile gloves
- container and brush for the disinfection of wellington boots
- disinfectant hand wipes
- hand sanitising gel
- data logger (supplied by PHE)
- sterile sampling container(s)
- tamper evident sample bags
- pens (always ensure that you have at least 2)
- supply of RCDM sampling sheets for dispatch to the lab
- calibrated temperature probe / thermometer
- sample dispatch cool box (supplied by PHE and containing all supplies needed as indicated by the packing instructions sheet PHE have supplied to the DHI’s)
- sufficient supply of refrigerant material (supplied by PHE). These should be frozen for at least 24 hours at <-18 degrees celsius before using
- security seals to seal cool box (supplied by PHE)
- courier contact details to arrange collection
- fully charged tablet to record any findings.
3.5.3 On farm procedure
When entering the farm, the sampling DHI must clean their boots using the pre-prepared FAM30 solution, using the provided container and brush. If gloves are used whilst cleaning boots, these must be removed and discarded before entering the milk storage area.
Before entering the milk storage area, the sampling DHI must put on their protective outer garment, hair covering and clean single use nitrile gloves. The external surface of the gloves should be sanitised using the hand gel provided.
3.5.4 Milk sampling
Whenever possible, routine samples should be taken from the FPC. If an FPC is not available, then the sample should be collected using a sterile single use sample pot. The source of any sample should be clearly recorded on the RCDM sampling sheet.
The PHE sampling record documentation must be completed and placed in a plastic bag to be sent with the sample.
3.5.5 Bulk storage sampling
A definition of a bulk storage container will vary depending on the scale of operation and could be:
- bulk tank
- milk churns
- storage jug in refrigerated store
The type of bulk storage vessel must be clearly indicated on the RCDM sampling sheet.
If a bulk tank is sampled the temperature reading must be checked. If the milk is <6°C then the milk agitator must be activated for at least 2 minutes prior to sampling. If a mobile bulk storage vessel is used then the contents must be dipped at least 15 times to achieve a sufficient agitation.
If the milk is >6°C (because milking has recently taken place) then time should be allowed for it to cool to <6°C before sampling should take place.
Remove the sterile sample pot / dipper from the packaging. Do not handle the inner surfaces of lids or necks of sampling vessels and dippers. You must discard any equipment or samples which come in contact with non-sterile surfaces. Dip the sample pot below the surface level of the bulk storage vessel and collect at least 30ml of liquid milk. This milk should then be decanted into another sterile pot taking care not to spill any milk onto the screw mechanism of the pot, the lid should be added and tightened adequately enough to prevent any leakage.
Place the collected milk sample into a tamper evident bag and seal. Ensure that the details of the establishment are clearly recorded on the space provided on the tamper evident bag and ask the FBO to sign the bag to confirm the details are correct.
3.5.6 Finished product container sampling
If the temperature is to be taken from the FPC, extra care should be taken not to cross contaminate the sample. The supplied non-invasive laser probe must be used to take temperature. The milk temperature should be no more than 6°C in order to be able to take the sample.
Clearly record the details of the establishment in the space provided on the tamper evident bag. Place the FPC into the bag and seal. Record the serial number of the tamper evident bag in the space provided on the RCDM sampling sheet.
3.5.7 Sample storage during collection
All samples must be transported in the PHE supplied cool storage box containing coolant materials. The container will ensure that the ambient internal temperature is maintained, provided that the lid is securely fitted.
The sampling DHI must take care to ensure that all samples are transferred to the cool storage box as soon as is practicable to ensure that the sample is cooled to and maintained at a temperature of no greater than 6°C.
When the first sample is transferred to the storage box the temperature logger provided must be activated according to the instructions provided by PHE.
3.6 Sample despatch
3.6.1 Sample delivery options
The method used to deliver the samples to the nominated laboratory will largely depend upon location. However, each DHI will have the option to use the nominated courier service if required.
It is essential that whenever possible the most efficient means of sample delivery are employed.
All milk samples must be delivered to the nominated laboratory within 24 hours of sample collection by the DHI. Please note the clock starts from the time that the DHI collects the sample and not from the time the courier picks the sample up.
No samples are to be delivered to the labs on a Friday each week. The only exception to this would be if sampling was being undertaken as part of the incident/outbreak protocol, if this is the case the DHI must contact the lab prior to sending the samples on a Friday.
3.6.2 Direct DHI drop off
In instances where the nominated laboratory is close to the home address of the DHI, close to the RCDM establishment or along the route of the sampling DHIs journey, it is acceptable for the DHI to drop the samples off at the laboratory directly.
When all samples are collected, contact must be made with the nominated laboratory to inform them that the samples are to be delivered. The laboratory must be made aware of how many samples are to be consigned.
3.6.3 Courier delivery option
Any samples that require a courier to facilitate delivery to the nominated laboratory must be notified before 4pm on the previous day. Book a collection at Topspeed. See Annex 10 for further information on the booking process.
The collected samples must be packaged into the PHE cool box following the packaging instructions supplied by PHE and making sure there is sufficient supply of refrigerant material to maintain the samples refrigerated throughout transportation. The temperature logger must be activated and hung /clipped into the sample holding frame taking care that it does not come into contact with the refrigerant blocks.
RCDM samples taken and booked for collection via Topspeed before 9am in areas north of Birmingham will go on a same day run to PHE - FERA York.
RCDM samples taken and booked for collection after 10am will go on the over-night run to PHE – FERA York.
RCDM samples taken and booked for collection before 9am in areas south of Birmingham will go on a same day run to PHE Porton Down.
There is no over-night run to PHE Porton Down.
3.7 Raw drinking milk from horses
Specific legislation on horse identification considers the fact that, horses are food producing animals and understands the specific situation of horses which are born as animals of a food producing species, but which are not in all cases primarily bred for that purpose and are in most cases not kept throughout their lives by food business operators.
Horse passports contain certain pages where, if the horse is eligible for slaughter for human consumption, veterinarians are required to record the administration of vaccines and any of the “essential substances”.
Where the passport has been signed to indicated that the horse is non-eligible for slaughter for human consumption, the horse loses the status as food producing animal and veterinarians can prescribe active substances from either the allowed or the prohibited substances lists.
Horses live long lives compared to more traditional food producing animals and can change ownership frequently during their life which makes tracing the use of medicinal products extremely difficult. Veterinary records are not always found in the passport, but records must be kept for at least five years even if the animal has been sold or slaughtered during that time and the FBO no longer has the passport.
The identification document (passport) is the target of significant fraud. The main risk represents the illegal reintroduction into the food chain of horses previously excluded from slaughter for human consumption and treated with medicinal products not authorised for food producing animals.
Regulation (EU) No. 37/2010 regulates the pharmacologically active substances that can be administered to food producing animals and also those which are prohibited because no MRL (maximum residue limits of veterinary medicinal products in foodstuffs of animal origin) have been established.
Food producing animals can only be treated with substances from the “Allowed Substances” and should never be treated with any of the “Prohibited Substances”. Phenylbutazone (BUTE) is not included in the list of prohibited substances, but it is not included in the list of allowed substances either. BUTE cannot be administered to horses unless their passports are marked as non-eligible for slaughter for human consumption.
Because of the peculiarities around the veterinary medicines administered, horses signed as non-eligible, should be considered as horses removed from the human food chain altogether and that their milk should not be allowed into the human food chain.
4. Enforcement
In this section
4.2 Relevant references and definitions
4.3 Legislation and enforcement provisions
4.5 Division of enforcement responsibilities
4.7 Recording and monitoring enforcement action
4.8 Guidance on completion of the corrective action log (CAL)
4.9 Gathering and preserving evidence
4.12 Detention under the Food Safety Act 1990
4.13 Certification procedure for non-compliant food
4.15 Hierarchy of enforcement: Introduction
4.16 Informal enforcement action: Verbal
4.17 Informal enforcement action: Written
4.18 Formal enforcement action: Statutory notice
4.19 Statutory notices for hygiene contraventions
4.20 Remedial action notice (RAN): Wales only
4.21 Hygiene improvement notice (HIN)
4.22 Hygiene emergency prohibition notices (HEPN)
4.23 Hygiene emergency prohibition orders (HEPO)
4.24 Referral for investigation
4.25 Change of FBO during enforcement action
4.26 Warrant to enter premises
4.27 Process for obtaining a warrant to enter premises, in England and Wales
4.1 Purpose
4.1.1 FSA enforcement role
These enforcement arrangements apply to all primary production dairy establishments registered in England and Wales and under supervision by FSA DHIs.
Enforcement action is taken in accordance with the FSA Dairy Hygiene procedures
4.2 Relevant references and definitions
4.2.1 Authorised Officers (AOs)
AOs involved in enforcement activities must bear in mind the definitions contained within the various pieces of legislation.
4.2.2 Food business operator (FBO)
FBO means the natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control.
Reference: (EC) No 178/2002, Article 3, Paragraph 6.
4.2.3 Court
References to the ‘Court’ in England and Wales should be taken to mean the Magistrates’ Court.
4.2.4 Justice of the Peace
References to the ‘Justice of the Peace’ (JP) should be taken to mean the Magistrate.
4.2.5 Duly authorised representative
Duly authorised representative is a responsible person who has the authority to act on behalf of the FBO.
4.2.6 Legal definitions
Most legislation includes a definition section that provides guidance on many of the phrases contained within it.
The table below identifies where this guidance can be found in the main pieces of legislation that we enforce.
Legal definitions
| Legislation | Location of definition |
|---|---|
| (EC) 178/2002 | Articles 2 and 3 |
| (EU) 2019/627 | Article 2 |
| (EC) 852/2004 | Article 2 |
| (EC) 853/2004 | Article 2 and Annexes I, II, III |
| The Food Safety Act 1990 (as amended) | Sections 1,2 and 53 |
| The Food Safety and Hygiene (England) Regulations 2013, as amended/ The Food Hygiene (Wales) Regulations 2006, as amended | Regulation 2 |
4.2.7 Guidance documents
European Commission Guidance document on the implementation of certain provisions of (EC) No 852/2004
European Commission Guidance document on the implementation of certain provisions of Regulation (EC) No 853/2004
Guidance Notes for FBOs on Food Safety, Traceability, Product Withdrawal and Recall – A guide to compliance with Articles 14, 16, 18 and 19 of General Food Law Regulation (EC) 178/2002
Reference: These documents can be viewed using the web links quoted above, and are also reproduced in Volume 2 of the MOC.
4.3 Legislation and enforcement provisions
4.3.1 Requirement to enforce
Each Member State (MS) must enforce food law by monitoring and verifying that relevant legislative requirements are met through a system of official controls and other activities. It is for each member state to lay down the rules on measures and penalties to be applied when infringements of food law are detected.
Reference: (EC) 178/2002, Article 17, Paragraph 2.
Food law includes all statutes, regulations and administrative provisions governing food in general, and food safety in particular. It covers all stages of production, processing and distribution of food, and also of feed produced for, or fed to, food-producing animals.
4.3.2 Enforcement provisions
EC Regulations are directly applicable in all MS. They detail both the legal requirements the FBO must comply with, as well as official controls that must be applied by the competent authority.
They do not, however, specify the powers of AOs, powers of entry, time limits to bring a prosecution, or the split in enforcement responsibilities between different agencies that enforce the same legislation.
Each MS is required to separately introduce national implementing legislation providing enforcement powers, setting out offences for failure to comply with the European Regulations and to establish the administrative system under which non-compliances by FBOs can be brought before the courts.
4.3.3 General principles
(EC) No. 178/2002 sets out the general principles and requirements of food law, establishes the European Food Safety Authority (EFSA) and lays down procedures in matters of food safety. It contains:
- definitions (such as food, food business operator)
- basic principles: FBO responsibility for food safety
- traceability requirements
4.3.4 EC Hygiene Regulations
The expression ‘Hygiene Regulations’ is defined in Regulation 2 of the domestic hygiene regulations to include:
(EC) 852/2004 dealing with the hygiene of foodstuffs. It applies to all food businesses, encourages good hygiene practices and introduces the concept of industry guides.
(EC) 853/2004 laying down specific hygiene rules for food of animal origin. It sets out additional requirements beyond 852/2004 for specific food of animal origin; such as milk, eggs, meat, fish.
(EU) 2019/627 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. It covers the nature of official controls, inspection, verification, auditing and the role of AOs.
4.3.5 Domestic Regulations
In addition to the EU legislation listed above, ‘Hygiene Regulations’ also includes the domestic national hygiene regulations introduced in the respective devolved countries to allow for the enforcement and administration of EU hygiene legislation.
4.3.6 Amendments
Periodically EU and domestic legislation is amended and such changes must be read in conjunction with the original published versions. Whilst the MOC is updated with consolidated versions of the EU Regulations, it is suggested that if you are unsure whether the most up to date version of the legislation exists, you should check the latest version of the EC Regulations.
4.3.7 Domestic regulations
The domestic regulations that apply to dairy establishments include:
- the Food Safety and Hygiene (England) Regulations 2013 (as amended) / The Food Hygiene (Wales) Regulations 2006 (as amended), which provide enforcement powers in respect of the obligations that apply in Regulations (EC) No. 852/2004, (EC) No. 853/2004 and (EU) No. 2019/627
- the General Food Regulations 2004 (Wales only); these provide enforcement powers in respect of the obligations that apply in (EC) 178/2002, for example:
- Article 14 ‘the food safety requirements’
- Article 19 ‘recall, withdrawal and notification requirements’
Note: Specific provisions contained in the Food Safety Act 1990 and its amendments still apply to these Regulations; for example, powers and definitions.
4.3.8 Enforcement Concordat
In addition to the legal requirements imposed by the EC legislation, FSA Operations Group has been a signatory to the Enforcement Concordat since June 2001 and is required to adhere to its main principles, which include proportionality and consistency of enforcement.
The DTI Enforcement Concordat: Good Practice Guide for England and Wales states of proportionality that apart from taking a progressive approach, enforcement will mean applying the principles of risk assessment to enforcement activity and enforcement bodies should focus their attention on the most serious risks, or where potential hazards are least well controlled. Compliance in lower risk business activities should be encouraged by being open and helpful (paragraph 44).
In respect of consistency, the Enforcement Concordat states that ‘it is important to ensure, and demonstrate, that enforcement activities are consistent both within a single enforcement body and between enforcers regionally and nationally. Whilst consistency of approach does not mean uniformity, it does mean taking a similar approach in similar circumstances to achieve similar ends’ (paragraph 50).
4.4 Premises database
4.4.1 Database contents
An electronic record will be maintained for all dairy establishments inspected by the FSA. This will be in the form of a database which will include details of the farm registration, the FBO responsible for potential offences, all correspondence in chronological order, copies of all formal notices and inspection reports.
Prior to an inspection, DHIs will extract the relevant information required to inform their inspection.
4.4.2 Security
The inspection reports and all enforcement literature must be kept securely at all times and will be incorporated on to the database upon completion of the visit. Contents of the database may contain evidence that is required for formal court action at a later date, together with additional unused material that the FSA may have to disclose should a case go to trial.
4.5 Division of enforcement responsibilities
4.5.1 FSA enforcement responsibilities
- Inspection and enforcement of Regulation (EC) No. 852 and (EC) No. 853/2004 at an establishment where one or more farmed animals are kept to produce milk with a view to placing it on the market as food.
- Sampling of raw cow’s milk supplied direct for human consumption for compliance with the microbiological requirements of Schedule 6 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) / Food Safety and Hygiene (Wales) Regulations 2006 (as amended).
- Enforcement of the national marketing requirements for the sale of raw cow’s milk intended for direct human consumption under Schedule 6 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) / Food Hygiene (Wales) Regulations 2006 (as amended).
- Inspection and Enforcement of the labelling requirements in schedule 6 of the domestic hygiene regulations with respect to the health warnings required for RCDM sales.
- Enforcement of Regulation (EC) No. 178/2002 with respect to Articles 14, 18 and 19 at primary production establishments producing raw cow’s milk for direct supply for human consumption.
4.5.2 LA enforcement responsibilities
- Inspection and enforcement of Regulation (EC) No. 852 and Regulation (EC) No. 853/2004 at establishments undertaking heat treatment of raw milk and further processing of milk and milk products at milk establishments subject to approval under Regulation (EC) No. 853/2004.
- Sampling of raw milk (other than cow’s milk) supplied direct for human consumption for compliance with the microbiological requirements of Schedule 6 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) / Food Hygiene (Wales) Regulations 2006 (as amended).
- Inspection and enforcement of the safety labelling requirements in schedule 6 of the domestic hygiene regulations with respect to the health warning required for all raw milk sales (except cows and buffalo).
- Inspection and enforcement of Regulations (EC) No. 852 and 853/2004 at establishments undertaking bottling operations.
- Enforcement of Regulation (EC) No. 178/2002 with respect to Articles 14, 16, 18 and 19 at primary production establishments producing raw milk other than cow’s milk for direct supply for human consumption.
4.6 Communication with FBOs
4.6.1 Communication channels
The key to a successful working relationship is communication. There is nowhere that this is more important than in relation to guiding the FBO on compliance with legal requirements, as well as best practice.
The majority of day-to-day compliance can be achieved through verbal discussion.
It is important that contingency arrangements exist to avoid difficulties when the FBOs normal contact person is unavailable.
Note: Establish details of any duly authorised representative that has authority to act on behalf of the FBO. This is especially important for the service of RAN (Wales only).
4.6.2 FBO contact details
The AO must have available all registration details for the FBO, including:
- full name(s) of owner / partners
- address(es)
- telephone number(s)
- limited company name and registered office address
- any duly authorised representative
Regulation (EC) No. 852/2004, Article 6, Paragraph 2, requires FBOs to ensure that the Competent Authority always has up to date information on establishments, including the notification of any significant change in activities and any closure of an existing establishment.
However, this requirement is rarely complied with and therefore DHIs should always check that the registration details of the establishment have not changed to ensure information held by the FSA is accurate.
Updates on registration details must be provided to the Approvals and Registrations team. This will ensure that the AO is always aware of the legal entity responsible for any potential offences within the establishment, whether they are a sole trader, partnership or limited company and whether the FBO has started to sell raw drinking milk for direct consumption without notifying the FSA.
4.6.3 Key communication functions
The AO is responsible for:
- advising the FBO on compliance with legal requirements
- advising the FBO when infringements of legal requirements have been detected
4.7 Recording and monitoring enforcement action
4.7.1 FSA corrective action log
Enforcement action taken must be recorded accurately on the corrective action log of the Hygiene Inspection Report.
The purpose of this document is to help the AO in their:
- assessment and prioritisation of enforcement action
- communication of enforcement action to other AOs
- tracking or monitoring of enforcement action through to compliance or a referral for investigation
The document:
- acts as an aide memoire and provides a record of enforcement action taken in the establishment
- enables the FSA to assess the FBOs past record as regards compliance with food law
- contributes to any changes in the inspection frequency as a result of formal enforcement action that has been taken
- provides an outline of the non-compliances to ITLs, FVCs, management and internal audit
4.7.2 Ongoing enforcement action
Prior to visiting any milk production holding, the AO must:
- familiarise themselves with all ongoing enforcement action
- maintain the momentum of existing enforcement action; only where they are able to support this enforcement, should they escalate it
4.7.3 Completing the corrective action report
The corrective action report should be a ‘live’ form, updated as necessary every time enforcement action is taken.
Non-compliances of a recurring nature (that are not solved when corrective action is requested) should be entered under the same reference number in the corrective action log, in order to demonstrate continuity of enforcement action and if necessary, prove repeated non-compliance in cases referred for investigation. In electronic forms, the additional entries can be made on subsequent lines of the same box, underneath the original entry.
Where a non-compliance is corrected but reoccurs some time later, details may be entered under a different reference number, which you may cross-reference to the previous entry where details of the offence were recorded.
4.8 Guidance on completion of the corrective action log (CAL)
4.8.1 Reference number
The AO should enter the milk production holding’s CPH number, followed by the month, then the last two digits of the year, and lastly a sequential number for each deficiency (for example, HN/07/12/001, HN/07/12/002/, HN/07/12/003/) on the CAL.
These numbers should correlate with the reference number for any written enforcement. In letters, the reference number should be provided in the format of, producer ID, Letter type, DHI initials, date of issue, (for example 1234-WL-CB-01.04.19-01). This reference number should also be entered into the subject bar of any email communication linked to it; this will aid with storing of these documents.
4.8.2 Regulation reference and deficiency
The AO should:
- state the year and Regulation reference number
- give a short description of the deficiency, for example, failure to clean equipment
More than one line may be used if required.
4.8.3 Action required
The AO must detail any action the FBO must take to comply with the requirements of the legislation.
4.8.4 Agreed completion dates
The AO must insert the date agreed with the FBO for the correction of the deficiency, or the date for compliance specified in any formal notice.
If the FBO does not agree to a completion date, the AO must still insert the date they consider appropriate and indicate that it was ‘not agreed’. Any letters should also include this date.
A revisit should be made to the milk production holding after the date on which compliance was required to be achieved. Where compliance has not been achieved by the due date, the FBO should be reminded of the issue and enforcement action should be escalated to the next stage of the hierarchy.
When agreeing or setting completion dates, a reasonable deadline for the rectification of each deficiency should be agreed. The deadline should be realistic to allow the FBO to rectify the deficiency, whilst still considering the risk to public health.
4.8.5 Detention notice served and withdrawn
The AO should specify the date on which a formal detention notice (DH ENF 11/1) has been served on the FBO. It is essential that any investigation to determine the fitness of the milk for human consumption is undertaken in a timely manner, paying particular attention to the 21 day limit.
Where the AO is satisfied that the detained milk can enter the food chain, they should insert the date on which the detention notice is withdrawn, below the date on which it was served.
If, as a result of investigation, the AO decides not to release the milk for human consumption, they must insert ‘Not Released’ under the date the notice was served. If the milk is not voluntarily surrendered, it must be formally seized prior to the expiry of the 21 day period.
4.8.6 Date compliance achieved
Record the actual date on which compliance is achieved, even if it is the same day that enforcement action was taken.
4.8.7 Structural work
Where structural work must be undertaken, the ‘corrective action’ section of an advisory letter or HIN should be specific enough to explain the legal requirement and the outcome to be achieved, without being too prescriptive about the exact way in which this must be achieved.
There may be many ways that the FBO can achieve compliance, but provided they comply with the legal requirement, they have the option to do the work in the way they see fit, or carry out works to an equivalent effect.
4.8.8 Monitoring progress
The AO should regularly monitor progress towards compliance to identify whether the deficiency is likely to be rectified within the agreed time scale. If necessary, they should ask to see evidence of how corrective action is progressing, such as planning permission application / copies of quotes for work / structural plans.
Where the work does not progress at the agreed rate, the AO should consider escalating the issue, such as serving a HIN, which will formalise the agreed time scale and thereby maintain the momentum in enforcement.
It is important that agreed action plans are set out at the start and that the AO takes a reasonable approach where certain issues arise that are outside the FBOs control, provided that the risk to public health permits this approach.
4.9 Gathering and preserving evidence
4.9.1 Introduction
The AO must gather evidence at the time the offence is witnessed, making detailed contemporaneous notes, in their pocketbook, which at a later stage could be relied upon in Court. (See guidance below or further guidance in this section). It may be impossible to gather evidence retrospectively as it may no longer exist. Evidence may come in a variety of forms and must supplement a witness statement as an exhibit in order that it may be admissible in court. Where possible, or if problems are envisaged, it is always useful to obtain corroboration and assistance from other colleagues.
Detailed evidence gathering at the time of the offence will provide the AO with as much material as possible to support their witness statement and prove the elements of the offence.
Note: look after evidence - keep it secure. It is fundamental to proving the offence should formal action be pursued.
4.9.2 Best evidence rule
The AO should also have regard to the ‘best evidence’ rule. Whenever possible, any original items of evidence should be preserved, (the original form of a document, rather than a photocopy). If the evidence is a milk sample, this should be submitted for testing as soon as possible and a Certificate of Analysis retained. Where practical, the FBO should also be given the opportunity to have the evidence examined by an expert before destruction.
The AO may also wish to consider taking photographs and / or sample evidence before perishable goods are destroyed. If there is doubt about what evidence should be retained, the AO can obtain further advice from FSA Legal.
4.9.3 Note taking
When gathering evidence, remember to record the details of any other persons present. This will enable the FSA Investigation Officers (IO) to identify all potential witnesses in the case and will enable witness statements to be taken.
The AO must make full use of their pocketbook to make factual contemporaneous notes. These may be referred to in court to help recollect facts and figures that it is impossible to recall in detail after the event.
In court, a witness is able to refer to contemporaneous notes recorded in their pocketbook, that were made either at the time of the incident or straight afterwards. Where an officer refers to their notebook when giving evidence in court, the defence is entitled to see that notebook.
Note: However, witnesses are not permitted to read from their witness statement when giving evidence, except in certain limited circumstances.
4.9.4 Important points
Pocketbooks may be inspected in court, therefore the following guidance must be followed to maintain validity:
- record name on front cover, designation and date started
- make all entries with ink or ballpoint pen
- include only original entries and do not copy notes from elsewhere
- record the date and time at commencement, and upon completion
- enter the notes at the time ‘the offence’ is witnessed or as soon as possible afterwards (contemporaneously), whilst the facts are fresh in the memory
- to make alterations, strike a pen through the error and write the correction; then initial in the left hand column
- notes must not be erased
- do not remove pages from the notebook
- sign and date each entry at the bottom of each page
Entries must be relevant, factual, legible, concise and written in plain English.
If accompanied by a colleague whilst witnessing a contravention, one AO may record the details in their pocketbook. The other may read through the notes made and where they agree with what has been recorded, they may countersign at the end of the entry to acknowledge that it is a true and accurate account of events
4.9.5 Security
The AO is responsible for ensuring the security of their notebook and for producing it in court. Further notebooks are available from CSU York on return of the completed notebook.
4.9.6 Return of all notebooks
Notebooks remain the property of the FSA and must be returned to York prior to leaving the FSA.
4.9.7 Disclosure of unused material
The Criminal Procedures and Investigations Act 1996 (CPIA) places an obligation on the prosecuting authority to retain and record all relevant information relating to any formal action.
The prosecuting authority, a term which includes the AO, the IO and the FSA itself, has a duty to disclose to the defence all relevant unused material which:
- might undermine the case for the prosecution, or
- might reasonably be expected to assist the defence case
This material may include:
- informal and formal memos
- email traffic
- previously unreported offences and / or warnings recorded on operational paperwork
- daybook entries
- contemporaneous notebook entries
- minutes of meetings
- draft witness statements
- photographs as part of unused material
- instructions to expert witnesses or analysts
4.9.8 Storage and availability
Anything that is relevant to the case and which is not used by the prosecution is unused material and can be potentially disclosed. This fact makes it important that when notes are taken, emails written or drafts prepared, they should be made on the understanding that the defence may be entitled to see them and refer to them in open court. Even if there are good reasons for arguing that they are so sensitive that the defence should not see them, it is for the court to decide.
The AO and FSA team should ensure that:
- all material should be recorded and retained
- all material should be safely stored
The IO must be made aware of its existence as soon as possible after a recommendation is made.
4.9.9 Photographic evidence
Taking photographs for the purposes of evidence gathering will often be a fundamental part of the evidence gathering process.
The AO may inform the FBO of their intention to take photographs as a matter of courtesy, however, the FBO cannot stop an AO from taking photographs for the purposes of evidence gathering and it could be an offence of obstruction for them to prevent the AO carrying out their duties.
- when photographs are taken, details should be recorded in a contemporaneous notebook, including the photograph number, the subject, location and date / time. Colleagues should assist one another in this process, if available.
- photographs should be taken with a suitable digital camera; however, a record must be kept of how the digital information was downloaded and on to what medium it was stored, together with the ‘Supporting evidence photographic report’ for recording full details of digital images taken (DH ENF 11/14).
Reference: See sub-topic 4.9.11 on ‘Digital camera protocol’ in this section for additional information.
- always add details to the reverse of the photograph, clearly indicating the subject matter, location and other relevant details.
- record details of when and where any films are processed (if relevant).
- video filming may be very useful to demonstrate a particular operation. However, it is advisable that where the AO is not familiar with the equipment, that they receive some instruction and / or practice with the equipment prior to gathering the evidence that may be required for court.
Note: Any verbal comment recorded whilst any filming is being undertaken must later be transcribed word for word and will constitute part of the evidence.
Tip: Give the camera lens time to adjust to the temperature / humidity before taking pictures in order to prevent fogging.
4.9.10 Digital camera protocol
When the AO captures images using a digital camera, they must ensure the following:
- the memory card is clear of previous images
- any poor quality images must not be deleted
- full particulars of images are recorded using the ‘Supporting evidence photographic report’ available at Annex 6
- images, along with the corresponding photographic evidence report, are downloaded onto the hard-drive of a computer
- the images and supporting photographic evidence report are copied onto two separate non-reusable CD-ROMs
- one CD is marked as the ‘Master copy’; this must be bagged / tagged / its details recorded in the AOs contemporaneous note book and stored somewhere secure
- the other CD is marked as the ‘Working copy’; it should also be tagged and its details recorded in the AOs contemporaneous note book and stored in a secure place for the IO.
4.9.11 Supporting evidence photographic report
The Supporting Evidence Photographic Report provides a contemporaneous record of images taken whilst gathering evidence.
In ideal circumstances, the report should be completed at the time the evidence is gathered. However, when this is not feasible, it should be completed as soon as possible thereafter.
The report should be stored electronically in the same file as the images to which it relates.
A new report should be prepared to accompany images of each separate incident.
The Supporting Evidence Photographic Report is available at Annex 6 to this chapter. (This will open as a separate document when accessed using the chapter bookmark on the left hand side of this page.)
4.9.12 Retention of unused photographic images
All unused photographs, images and negatives must be retained. These may be disclosed to the defence as ‘unused material’ in line with the provisions of the Criminal Procedures and Investigations Act 1996 in England and Wales.
4.9.13 Powers to photograph
Although all AOs have powers to take photographs for the purpose of evidence gathering, they must always seek the permission of the FBO, if they are taking photographs for any other reason than evidence gathering.
4.9.14 Samples: physical confirmation of the failure
A variety of different types of sample may be used as evidence, for example:
- dirt from soiled equipment
- caked milk residues on equipment
- samples of milk
The AO should inform the operator of their intentions. Enlist the services of a colleague (if available) to witness the collection of the sample and also to record details of what, when, where and how, it was obtained, putting the date and time in their pocket notebook. The samples should be effectively bagged and labelled with all relevant details. It should then be sealed with a talisman security tag.
All samples must be kept under secure conditions in an environment where they will not deteriorate. Details of storage location and transportation should also be recorded to maintain continuity of evidence. Temperature logs and relevant calibration records of chillers and freezers, where evidence samples are stored, should be accurately maintained, as they may be required as legal evidence in court.
4.9.15 Temperature readings: factual figures
The AO should ensure that where thermometers are used for evidential purposes: the thermometer used is correctly calibrated, and prior to a court hearing, ensure it is recalibrated. The calibration certificate will be required as an exhibit. All relevant temperatures must be recorded where necessary (ambient, surface).
4.10 Information obtained from unauthorised sources (RIPA)
4.10.1 Introduction
This topic covers instruction on dealing with information which may be provided under the Regulation of Investigatory Powers Act 2000 (known as RIPA).
4.10.2 Information received
Under the law, AOs should take extreme care when dealing with a case where farm staff, consumers, residents, or other contacts have provided information about possible offences or misconduct.
Where this sort of information is provided, the AO must always inform their line manager, who must in turn notify FSA Legal Services and the Investigations Branch.
4.10.3 Questioning contacts
Any person passing on information must never be tasked to obtain or pass on information about possible offences or misconduct. If they are asked to pass on information, it almost certainly will not be possible to conduct a successful investigation into the allegations since it will not be possible to use the evidence obtained.
4.10.4 Use of informers
It is essential that AOs remember not to ask contacts to obtain or pass on information about possible offences or misconduct even where they have first come forward of their own free will and given information about such matters.
AOs must not try to get someone to act as an informer or obtain information in an undercover way.
4.10.5 Example
A disgruntled employee contacts you to inform you that the FBO of a farm is secretly selling raw cow’s milk direct for human consumption at a local fitness club, without FSA sampling and contrary to the marketing provisions. He is in a position to know when this is happening and to contact you at the time it is taking place.
4.11 Surrender, detention, seizure and condemnation voluntary surrender
4.11.1 Means of voluntary surrender
Where milk has not been produced / processed or distributed in accordance with the hygiene regulations or fails to meet the food safety requirements, the FSA should seek voluntary surrender of the milk.
An ‘Agreement to Destroy Food’ (DH ENF 11/7) notice should be completed where any dispute arises, or where issues are more complex. For example:
- there are large quantities of milk
- the FBO is selling raw milk to third parties for further processing to satisfy contracts
This agreement should be completed before the milk is consigned to the slurry tank.
Reference: See chapter 9 on ‘Forms’ for DH ENF 11/7.
4.11.2 Legal powers
The authorised AO has powers to detain food under:
- Section 9 of The Food Safety Act 1990 (as amended), via Regulation 23 of the Food Hygiene (Wales) Regulations 2006 / Regulation 25 of the Food Safety and Hygiene (England) Regulations 2013 (as amended).
4.11.3 Labelling detained milk
Milk detained for further inspection should be stored securely in a container that allows for its individual identification. Containers can be tagged using individually numbered talisman seal(s). The individual seal numbers should be recorded with any other relevant details on the Detention of Food Notice.
The seals must remain in place until the milk has been sampled, or a decision made on whether or not the milk complies with the food safety requirements.
4.11.4 Detention tape
Detention tape may be used to help identify large quantities of raw milk in conjunction with a Detention of Food Notice – for example, the contents of a bulk tank.
4.11.5 When to formally detain
There may be occasions where milk cannot be dealt with immediately because an investigation into:
- the fitness and suitability of any milk under the FBOs control is required
- the suitability of milk coming from a specific animal due to its TB /BR or animal welfare status
- any antibiotic residues contained in the milk or the administration of any banned substances to the food producing animals under the FBOs control
For example, in such circumstances, the AO will require the FBO to store the milk in an appropriate container. The product and / or animal must be sampled / tested and any non-compliant milk must be disposed of as an ABP. In the event that the FBO refuses to dispose of non-compliant milk, further action will be required through the LA or APHA, who have relevant enforcement powers to require disposal under the respective legislation.
4.12 Detention of food under the Food Safety Act 1990
4.12.1 Relevant legislation
Regulation 23 of The Food Hygiene (Wales) Regulations 2006 and / or Regulation 25 of The Food Safety and Hygiene (England) Regulations 2013 (as amended) allow the AO to detain suspect food for further investigation. This is achieved via the detention provisions contained in Section 9(3)(a) of the Food Safety Act 1990, which provides powers for the AO to detain, inspect and seize any food that is thought may not comply with the food safety requirements and is intended for human consumption. The Detention of Food Notice to use in these circumstances is the DH ENF 11/1.
4.12.2 When to serve a Detention of Food Notice (DH ENF 11/1)
When FBOs are unwilling to either surrender milk that the AO has judged unfit, or to detain milk for further sampling to enable its fitness or compliance with food safety requirements to be properly assessed, the AO must detain or seize (as appropriate) the food in accordance with Food Safety Act Section 9.
Note: The AO shall as soon as is reasonably practicable, and in any event within 21 days, determine whether or not he is satisfied that the food complies with the food safety requirement.
4.12.3 Reasons for service
Milk which fails to comply with food safety requirements under Article 14, (EC) No. 178/2002, includes:
- milk that is unsafe
- milk that is unfit for human consumption
- milk that is injurious to health
4.12.4 Service of notice
Prior to serving a notice, the AO must have in their possession all the evidence to justify its service. The Detention of Food Notice should be served by hand on the person in possession of the milk who is deemed to be ‘the owner’.
4.12.5 Content of notice
The notice must specify:
- description
- quantity
- identification marks if any (detained tags, numbers or labels)
- any location the food may be moved to (if applicable)
4.12.6 Number of notices
Where a quantity of milk of different types or batches is being detained, the AO should issue a separate Detention of Food Notice for each type or batch.
Where the milk that fails to comply with the hygiene requirements is part of a batch of the same class or description, it shall be presumed unless the contrary is shown that the whole batch fails to comply and the AO should detain all of it. Part of the food may subsequently be seized if necessary and an Order for Condemnation of Food applied for. The Detention Notice must be withdrawn in respect of the remainder if the AO is satisfied that the problem affects only part of the batch.
Reference: The Food Hygiene (Wales) Regulations 2006 (as amended), Regulation 27 (3) / Food Safety and Hygiene (England) Regulations 2013 (as amended), Regulation 29 (3) and Food Safety Act 1990 Section 8 (3).
4.12.7 Right of appeal
No right of appeal exists for a Detention of Food Notice under the Food Safety Act 1990. However, if not voluntarily surrendered the food must be seized and taken before a Justice of the Peace for them to decide whether it should be condemned or not.
4.12.8 Time limit
The AO shall, as soon as is reasonably practicable, and in any event within 21 days, determine whether or not they are satisfied that the milk complies with the food safety requirement.
If they are satisfied that the food complies with food safety requirements, the AO must immediately withdraw the notice.
Or, if the AO is not satisfied that the food complies, they must seize the food and have it dealt with by a Justice of the Peace.
4.12.9 Withdrawal
If the notice is to be withdrawn, the AO must immediately serve a Withdrawal of Detention of Food Notice upon the recipient of the original Detention of Food Notice.
If a Detention of Food Notice is withdrawn, or condemnation order is refused, compensation is payable to the owner of the food for any depreciation in its value which can be shown to result from the AOs actions.
The AO must ensure that all detained food is suitably and securely stored to minimise any deterioration.
4.12.10 AO checklist
Where the detained food is not released, specify in the AO checklist on the reverse of the Detention of Food Notice:
- the nature of disposal
- whether an Agreement to Destroy Food Notice was signed by the FBO and the Notice reference number
- whether the detention led to the food being certified, seized and taken before a court to have it condemned
4.13 Certification procedure for non-compliant food
4.13.1 Legislation
Regulation 27 of the Food Hygiene (Wales) Regulations 2006 / Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013 (as amended) specifies that where food has not been ‘produced, processed, or distributed’ in accordance with the ‘Hygiene Regulations’ it shall be treated for the purposes of Section 9 of the Food Safety Act, as failing to comply with the food safety requirements.
Art 14 (EC) No. 178/2002 defines the expression ‘food safety requirements’ to include:
- food which is unsafe, injurious to health or unfit for human consumption, having regard to the normal conditions of use of the food by the consumer and to the information provided to the consumer, including information on the label, or other information generally available to the consumer concerning the avoidance of specific adverse health effects from a particular food or category of foods.
- in determining whether any food is injurious to health, regard shall be had to the probable immediate short and long term effects of that food on the health of a person consuming it, but also on subsequent generations; to the probable cumulative toxic effects; to the particular health sensitivities of a specific category of consumers where the food is intended for that category of consumers.
- in determining whether any food is unfit for human consumption, regard shall be had to whether the food is unacceptable for human consumption according to its intended use, for reasons of contamination, whether by extraneous matter or otherwise, or through putrefaction, deterioration or decay.
Where evidence can be tendered to prove that the milk has not been produced, processed or distributed in accordance with the Hygiene Regulations, certification of the non- compliant milk will deem the milk to fail to comply with the food safety requirements of Regulation (EC) No. 178/2002 through the provisions of Regulation 27(2) (Wales) or Regulation 29(2) (England), and the Magistrate will be required to condemn the milk.
4.14 Condemnation procedure
4.14.1 When to apply for a condemnation order from the court
Only where milk has failed to be produced, processed or distributed in accordance with the hygiene regulations, or breaches the ‘food safety requirements’ should the AO:
- formally detain the food (DH ENF 11/1)
- certify the food as non-compliant (DH ENF 11/25)
- formally seize the food (DH ENF 11/ 27)
and apply to a Magistrate to issue a Condemnation Order.
Note: It is not a legal requirement to formally detain the milk prior to its formal seizure.
4.14.2 England and Wales
In England and Wales a Condemnation Order may be obtained from a Justice of the Peace at the Magistrates’ Court.
4.14.3 Action to take
The AO is to follow the steps in the table below.
Regulation: The Food Safety Act 1990 Section 9 (3) (b), Section 9(4) (b).
Reference: See Code of Practice.
Action to take
| Action | Detail |
|---|---|
| Detain the food (ENF 11/1) |
Where the milk you suspect does not comply with the food safety requirements and needs to be secured to prevent it from being used for human consumption, is should be formally detained using a Food Safety Act Detention of Food Notice (DH ENF 11/1). Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/1. |
| Advise FSA Legal | FSA York will arrange legal representation. A summary of events and copy of all legal notices must be sent to FSA Legal. |
| Contact local police | Establish which court covers the area for the establishment where the detained milk is held. |
| Contact the Court |
Speak to the Clerk of the Court to establish local procedures. Explain:
In England and Wales, establish with the clerk a date, time and location for the court hearing. The location can be either the local courtroom or the holding depending upon circumstances. Note: the court date must coincide with the availability of the FSA legal representative, so liaise with FSA York prior to serving the Food Condemnation Warning Notice (DH ENF 11-3). |
| Complete and serve Certification of Food Notice (DH ENF 11/25) |
Once the AO has determined that the food has not been produced, processed or distributed in accordance with the provisions of the Hygiene Regulations they must serve notice on the FBO with the reasons why it fails to comply. Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/25. |
| Complete and serve a Seizure of Food Notice (DH ENF 11/27) |
If after certifying the food, the FBO refuses to voluntarily surrender it, complete a Seizure of Food Notice (DH ENF 11/27) and serve it on the FBO and a copy on the owner of the food where relevant. Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/27. |
| Complete and serve a Food Condemnation Warning Notice (DH ENF 11/3) |
DH ENF 11/3 should be served on the owner / person in charge of the food (FBO). Ensure that the notice is served by the most appropriate method available in the circumstances to ensure that all relevant parties are informed of the time and place of the hearing in good time. Document and retain records of service to show the court. Retain copies of the Food Condemnation Warning Notice, the Certification of Food Notice and the Seizure of Food Notice to produce to the Justice of the Peace, the Clerk to the Court and the FSA legal representative. Always have a copy of the Food Law Code of Practice and Practice Guidance. Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/3. |
| Attend the hearing |
Prepare three copies of the Complaint for Condemnation of Food Order (DH ENF 11/15) and of the Order for Condemnation of Food (DH ENF 11/16) itself for the Justice of the Peace to sign. Read the papers again before going to court. Attend court early to meet the FSA advocate. On attending the hearing, the AO should take:
Reference: See chapter 9 on ‘Forms’ for the DH ENF 11/15 and 11/16. Explain clearly when presenting the evidence in court:
|
| If successful: maintain supervision to ensure milk is disposed of | If the Justice of the Peace issues an ‘Order for Condemnation of Food’, upon receipt of the Order ensure that the person in charge of the food (and the owner if notified) receives a copy. Ensure that the details of disposal have been recorded and that a copy of the waste transfer note has been scanned into the dairy hygiene database. |
| If unsuccessful: milk must be restored to owner | Where any issue of compensation arises, the AO must not discuss or negotiate any compensation for depreciation in value of the food. The AO should ask the FBO / owner of the food to put any complaint in writing to the HOD. |
4.15 Hierarchy of enforcement: Introduction
4.15.1 The hierarchy of enforcement
Updated [The flow diagram below outlines the stages that comprise the hierarchy of enforcement and explains that the process begins with informal action (verbal advice or letter) and progresses to formal action (formal statutory notice or referral for investigation).]
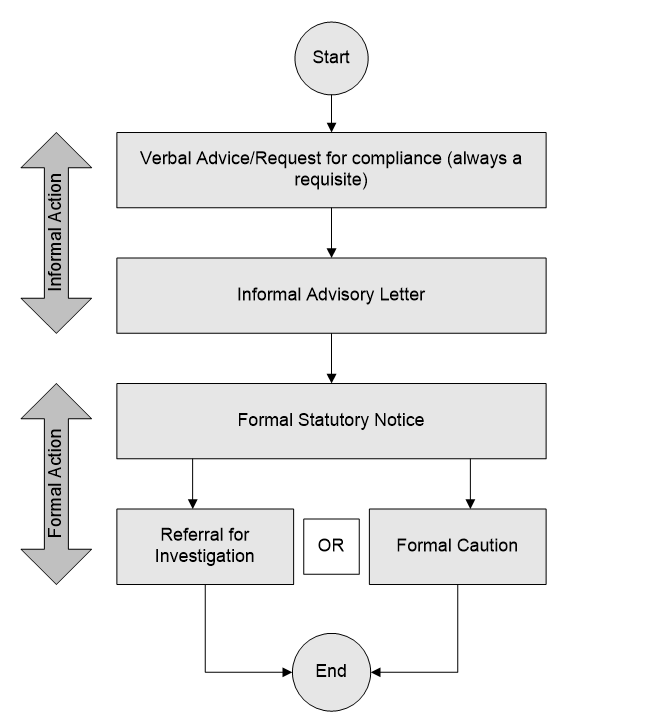
4.15.2 Approach to the hierarchy
The approach to the hierarchy of enforcement and level at which the AO commences enforcement action will be dependent upon:
- the urgency / severity of the situation
- the most appropriate course of action that will control the risk
- the enforcement tools available under that piece of legislation
- the history of the FBO and their willingness to comply
4.15.3 Enforcement
The term ‘Enforcement’ includes advisory visits, assisting the FBO with compliance and formal enforcement action (see Enforcement Concordat: Good Practice Guide for England and Wales Paragraph 88).
Verbal advice, written advice and written warnings all constitutes informal enforcement action.
Formal enforcement action includes official detention of food, the service of formal notices, and referrals for investigation and prosecutions.
4.15.4 Subject of enforcement action
Any FBO who is the subject of enforcement action should be kept fully informed of any intended or actual enforcement action by the AO.
4.16 Informal enforcement action: verbal
4.16.1 When to give verbal advice
The first stage of enforcement action should be education and advice. Whilst it is the FBOs job to know the legal provisions relating to their business, the AO should ensure that, where necessary, they clarify and update the FBO on any relevant legal requirements, so that they understand any all legal outcomes that must be achieved.
Verbal advice should go hand in hand with all stages in the enforcement process to help the FBO achieve compliance and understand the enforcement action being taken. For example, AOs must always try to explain to the FBO why immediate action may be required, why a statutory notice is being served, or why the matter is being referred for investigation, if appropriate.
Where verbal advice relates to a structural issue or is of a technical nature, it is helpful to follow up such discussions with a letter confirming the issues.
It is important that the AO does not continue to give verbal advice where this is being ignored, without escalating enforcement action in the appropriate way.
Note: Where immediate action is required on public health grounds, verbal advice should be given, but if ignored it would be appropriate to move straight to formal enforcement action to secure compliance as soon as possible (for example, Public Health – RAN (in Wales) or hygiene emergency prohibition notice (HEPN) for matters where there is an imminent risk of injury to health).
4.16.2 Records
If it appears likely that the enforcement may be escalated through the enforcement hierarchy or the FBO has a history of non-compliance, verbal advice should be recorded on the CAL.
4.17 Informal enforcement action: written
4.17.1 Advisory letters
Advisory letters are considered ‘informal’ enforcement action and failure by the FBO to comply with a letter of advice will not necessarily constitute an offence. However, an advisory letter produced later in court will help to demonstrate fairness and proportionality in the enforcement approach and that the FBO may have ignored previous advice.
Advisory letters should be sent by the AO to the FBO when:
- the FBO or a staff member has failed to take appropriate corrective action following verbal advice
- there is a contravention of the Regulations which does not have an immediate impact on public health
The AO should inform the FBO of their intention to write an advisory letter. Ideally, the AO should meet with the FBO or their representative before issuing an advisory letter to discuss all the issues including the timescale for completion. It is good practice to ask the FBO to confirm in writing their agreement to any timescale. Accurate minutes of any meetings in respect of compliance should be taken.
In advisory letters, the AO must not warn of prosecution action in the event of future contraventions, as this could prejudice any future formal investigation.
All advisory letters must be sent by the AO on official letterhead paper and be typed. In the case of advisory letters sent to limited companies, these should be addressed to the Company Secretary at the Registered Office address and a copy handed to the person appearing to be in charge at the farm.
4.17.2 Checklist for warning letters
The table below lists the points that an AO should follow when drafting a warning letter. Updated [It explains what does and does not need to be included within the warning letter, how the letter should be structured and who needs to be informed that the letter has been drafted.]
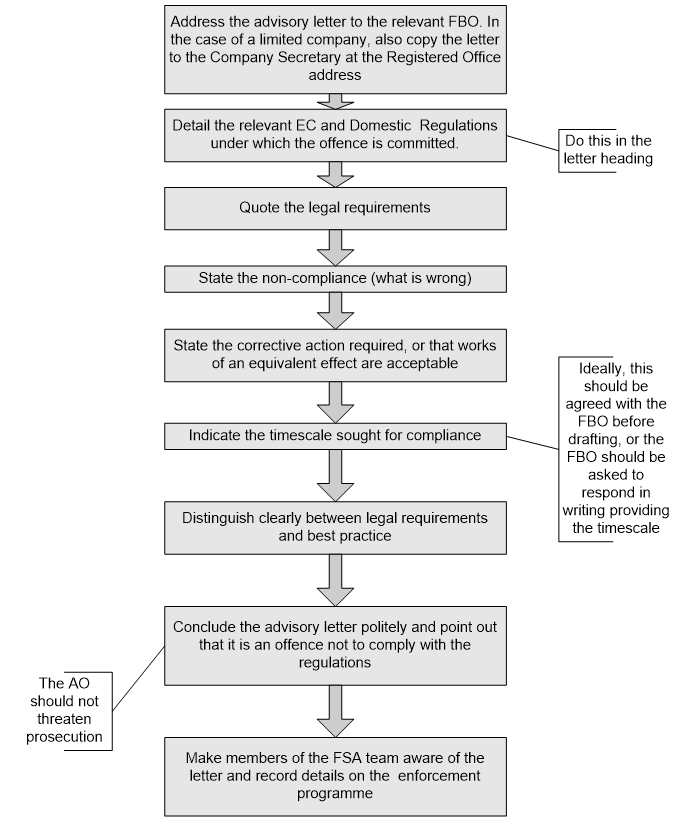
4.18 Formal enforcement action: Statutory Notice
4.18.1 Preparation for formal action
Before taking formal enforcement action, the AO should:
- aim to advise the FBO verbally of this intention
- be fully aware of any ongoing enforcement action by reviewing the corrective action report
- ensure that evidence has been secured to demonstrate that the contravention still exists that will warrant the escalation of enforcement action
4.18.2 Statutory Notices
Statutory Notices are legal documents and care must be taken to ensure they are completed correctly and used appropriately. They should only be served by FSA AOs authorised to do so.
4.18.3 Checklist prior to serving Statutory Notices
The diagram below lists the points that an AO should follow before serving a Statutory Notice. Updated [it includes a decision tree that can be followed once verbal advice has been issued.]
The AO should:

4.18.4 Checklist when serving Statutory Notices
At the time of serving a formal Statutory Notice, the AO should ensure that all the following checks are complied with:
- the formal notice is addressed to and served on the correct person / legal entity
- the local manager or duly authorised representative has received a copy of the formal notice where the original was served on the limited company and sent c/o ‘The Company Secretary’ to the Registered Office address
- the action required is clearly stated with details of any time limits within which compliance must be achieved
- the notice is clearly worded, concise and easily understood; it is typed, dated and signed by the AO
- the notice accurately reflects the non-compliance and refers to the relevant legislation breached
- an official hard copy notice should be used and not a photocopied, sample or draft
- all sections have been completed correctly and any irrelevant areas deleted as necessary
- the notice includes any relevant information on rights of appeal and on the applicable procedure and time limits
- a copy has been retained for scanning into the premises file
If any of the above checks are not complied with then the AO must ensure that action is taken to secure compliance before proceeding to serve a formal Statutory Notice.
4.19 Statutory Notices for hygiene contraventions
4.19.1 Food Hygiene Regulations
The Food Hygiene (Wales) Regulations 2006 / Food Safety and Hygiene (England) Regulations 2013 (as amended), provide 3 types of notice for hygiene non-compliances:
- Remedial Action Notice (Regulation 9(1)) (Wales only)
- Hygiene Improvement Notice (Regulation 6)
- Hygiene Emergency Prohibition Notice and Order (Regulation 8)
Reference: See section 4.11 on ‘Surrender, detention, seizure and condemnation’ in this chapter for details of detention for further investigation under Section 9 of the Food Safety Act 1990, via the provisions of Regulation 23 (Wales)/Regulation 25 (England) of these Regulations.
4.19.2 Service details
The Food Safety Act Detention Notice should be served on the person in charge of the food.
4.19.3 Formal service and delivery of notices
When drafting formal notices, it is very important to ensure they are directed at the correct legal entity responsible for any potential offences that can be committed.
4.19.4 Finding company addresses
Checks on a company’s registered office details may be done through Companies House website. Click on to the free company details link under the ‘find company information’ heading.
The organisation can also be contacted on 0870 33 33 636, or by email at 1HH enquiries@companies-house.gov.uk between 08:30 and 18:00, Monday to Friday.
4.19.5 Statutory Notices for hygiene contraventions
| Notice Type | Legislation | Purpose | Serve upon |
|---|---|---|---|
| Hygiene Improvement Notice | Regulation 6 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 6 Food Safety and Hygiene (England) Regulations 2013 (as amended) | To seek compliance with non-urgent hygiene deficiencies | FBO |
| Detention of Food Notice | Section 9 Food Safety Act 1990 via [Regulation 23 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 25 Food Safety and Hygiene (England) Regulations 2013 (as amended)] | To detain food while further investigation is carried out. | The person in charge of the food (the FBO) |
| Certification of Food Notice | Regulation 27 of The Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 29 Food Safety and Hygiene (England) Regulations 2013 (as amended) | To certify food that has not been produced, processed or distributed in accordance with the Hygiene Regulations to deem it to fail to comply with the Food Safety Requirements and facilitate condemnation. | The person in charge of the food (the FBO) |
| Seizure of Food Notice | Section 9 Food Safety Act 1990 via [Regulation 23 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 25 Food Safety and Hygiene (England) Regulations 2013 (as amended)] | To formally seize food in order that it may be taken before the court to be condemned | The person in charge of the food (the FBO) |
| Remedial Action Notices [Wales only] | Regulation 9 Food Hygiene (Wales) Regulations 2006 (as amended) | To seek compliance with hygiene matters that require immediate rectification | FBO or duly authorised represent-ative |
| Hygiene Prohibition Order | Regulation 7 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 7 Food Safety and Hygiene (England) Regulations 2013 (as amended) | Prohibition of a food business proprietor or manager from participating in the management of any food business | FBO |
| Hygiene Emergency Prohibition Notices and Orders | Regulation 8 Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 8 Food Safety and Hygiene (England) Regulations 2013 (as amended) | To obtain the backing of the court to deal with circumstances that pose an imminent risk of injury to health | FBO |
4.20 Remedial Action Notice (Wales only)
4.20.1 When to use a Remedial Action Notice (DH ENF 11-24 (W))
The RAN may be used:
- when any of the requirements of the Hygiene Regulations are being breached
- when inspection under the Hygiene Regulations is being hampered
They are a versatile tool that may be used to ensure the FBO takes immediate action to achieve compliance, for example, poor operational practices, person hygiene, failure to adequately clean.
The AO must verbally request that the FBO rectifies the situation and only serve the notice if compliance is not met. It is essential to gather the necessary evidence at the time the contravention is identified to justify its service in case an appeal is lodged by the FBO.
The AO must verbally inform the FBO of the intention to serve the notice and record the information in the corrective action report.
Note: Currently RANs are only available for use in registered premises in Wales. See Annex 7 to this chapter for examples of situations when it may be appropriate to serve a RAN.
4.20.2 Purpose of a RAN
RAN’s place a legal requirement on a FBO to take immediate action to achieve compliance with the hygiene regulations. This may require:
- prohibiting the use of any equipment or any part of the establishment specified in the notice
- imposing conditions upon or stopping a process
- requiring the rate of operation to be reduced to such extent as specified in the notice, or to be stopped completely
The notice must clearly specify the non-compliance identified by the AO and the remedy the FBO must achieve.
RAN’s can be used to direct the FBO to rectify both hygiene and structural / maintenance deficiencies, which fall under Regulation (EC) 852 and 853/2004 that require immediate action.
In the case of maintenance and structural problems, that do not pose an immediate risk and can be rectified in the longer term, a HIN served under Regulation 6 of The Food Hygiene (Wales) Regulations 2006 should be used.
4.20.3 Service and withdrawal
A separate RAN should be served on the FBO in respect of each specific legal deficiency. In limited circumstances, the effect of serving the notice may be to stop the entire operation.
RANs will not normally be served on the premises as a whole, but may be in certain circumstances, for example a pest infestation, e-coli in the water supply or inadequate cleaning.
Note: Where a notice has the effect of stopping the operation completely, the AO must inform their line manager to ensure the action is proportionate to the risk.
4.20.4 Tagging
Any equipment that has been the subject of a RAN must be identified and tagged using a numbered security seal, and accurately cross-referenced to a RAN.
4.20.5 Alternative service methods
Where it is not possible to identify the name and address of the person on whom the notice should be served, it can be served by addressing it to the FBO in their capacity as ‘occupier’ of establishment at which corrective action is required (name the establishment). The notice may then either be handed to someone else at the establishment who appears to be in charge, or by attaching the notice or a copy of it to some conspicuous part of the establishment.
The provisions relating to the service of notices are Regulation 28 of The Food Hygiene (Wales) Regulations. They correspond with the provisions of Section 50 of the Food Safety Act 1990.
4.20.6 Information for notices
The following information is to be included on the reverse of the AO copy:
- the name of the farm representative to whom any copy notices have been handed, where the original has been served at the registered office address of a limited company
- any comments made by the farm representative when handed the notice
- details of any food detained at the same time as the service of the RAN
- the reference number of the Detention Notice served
- details of any appeal that is lodged by the FBO in respect of the service of the RAN
4.20.7 Rights of appeal
The FBO has the right of appeal (Regulation 20) to a Justice of the Peace regarding the decision of the AO to serve a RAN.
If the FBO appeals, the AO must notify the Lead DHI and the FSA Legal team to arrange legal representation for the appeal hearing.
The provisions of RAN remain in force until such time as the appeal is upheld.
4.20.8 If removed or defaced or destroyed
The notice is the property of the FSA. If the AO discovers that any notice affixed to an establishment has been removed, defaced, or destroyed, the notice should be replaced as soon as possible and the events recorded in the AOs contemporaneous notebook.
4.20.9 Failure to comply
Failure to comply with a RAN is an offence (Food Hygiene (Wales) Regulations 2006, Regulation 9(7)). If the FBO has failed to comply with such a notice, complete a Referral for Investigation report – DH ENF 11/6.
4.20.10 Corroborative evidence rules
Where possible, service of a notice should be evidenced or corroborated in some way. If a notice is served by hand, then a witness should corroborate this fact. Both, the AO who served the original notice and any corroborating witness should sign a copy of the notice and indicate the date and time of service.
When posting a notice, the AO should obtain a proof of postage certificate and retain this as evidence. Where this is not possible, they should record the details of where the notice is posted and the postage address in their contemporaneous pocket book and if possible have a colleague corroborate the postage and countersign the entry.
4.20.11 Multiple offences
Where different offences have been identified, a different notice must be served for each and every contravention. These must never be recorded on the same notice.
This will prevent situations where an FBO complies with one of the issues detailed on the notice, but not the others.
In these circumstances, a notice containing multiple offences:
- cannot be withdrawn as there will be certain issues still outstanding
- cannot be referred for investigation as certain aspects of the notice have been complied with
- if appealed, will result in all of the issues being the subject of the appeal, even where the FBO may have been happy to comply with some of the issues
4.21 Hygiene Improvement Notices
4.21.1 When to use a HIN (DH ENF 11-23 (E/W))
The HIN should be used:
- where there is a record of non-compliance with breaches of the regulations
- where the history of compliance by the FBO is such that the AO has reason to believe that an informal approach will not be successful
- where formal action is proportionate to the risk to public health
Both verbal and written advice should be given to a FBO prior to a HIN being served. However, there may be circumstances where the AO believes this informal approach will be unsuccessful. If these informal stages are to be bypassed, the AO must have suitable evidence to demonstrate that the FBO has ignored previous informal advice in this area, prior to circumventing the informal approach.
4.21.2 Purpose of a HIN
They place a legal requirement on a FBO to take action to achieve compliance with the EU Food Hygiene Regulations. They can be used to require the FBO to:
- address any hygiene deficiency that does not require immediate action
- repair a structural defect with the building
- to build or construct additional facilities to cope with an increased throughput
The HIN must clearly specify the non-compliance identified by the AO and set out the remedy the FBO must achieve.
Note: As RANs are not available in England, HINs are used to achieve compliance with most breaches of the Regulations. Where a matter is clearly causing an imminent risk of injury to health, a Hygiene Emergency Prohibition Notice should be considered.
4.21.3 When not to issue a HIN
A HIN cannot be used to impose a continuing burden, and should not be used in the following circumstances:
- where breaches exist which require immediate rectification; in these circumstances it is more appropriate to use a RAN (in England, a HIN will have to be used)
- where the contravention might be continuing, for example, personal cleanliness of staff, and a HIN would only secure an improvement at one point in time; in these circumstances it is more appropriate to use a RAN (in England, a HIN will have to be used).
- where there is a breach of good hygiene practice but no failure to comply with an appropriate regulation
- where there is evidence of an imminent risk of injury to health; in these cases it is more appropriate to use an Hygiene Emergency Prohibition Notice (subject to HOD / FSA legal approval)
Note: A HIN cannot be issued unless a contravention of the appropriate regulations is identified.
4.21.4 Service
HINs must be served on the FBO.
Note: Where the FBO is a limited company, the envelope but not the notice itself should be addressed to the Company Secretary at the registered office address.
Details of how the notice was served should be recorded on the back of the HIN and in the AOs contemporaneous pocket book.
4.21.5 Service checklist
When serving a HIN the AO must:
- have in their possession all the evidence to justify its service
- verbally inform the FBO of the intention to serve the notice
- state why it is served and the action needed to remedy the breach
- sign, date and type the notice
4.21.6 Drafting and serving a notice to a sole trader
Ensure that the name on the formal notice indicating the individual, who acts as the FBO, is sufficient to identify that individual beyond doubt, and will include both their forename(s) and surname.
Where family members have the same names, try to include any additional names that the person may have, to avoid confusion. The notice may be served by hand on the sole trader at the farm, or served by mail addressed to them personally at the farm address.
4.21.7 Drafting and serving a notice to a partnership
Where a number of individuals act as the FBO under a partnership type arrangement, a copy of the notice must be served on each and every partner. The box identifying the FBO must include the full name of all partners.
The notices may be served by hand on each partner at the farm, if they are present, or served by mail addressed to each of them personally at the farm address. A covering letter should be included explaining that the same notice has been served on all partners in the business.
4.21.8 Drafting a notice to an FBO with limited liability status
Where the FBO has limited liability status, the name of the FBO will be the full name of the limited company, for example, ‘ABC Dairy Ltd’. The notice must be sent to the registered office or principal address of the company, with a copy of the notice handed to the relevant person in charge at the farm. The envelope and any covering letter must be addressed to ‘The Company Secretary’.
Note: The Company Secretary is the person responsible within a limited company structure, who receives such notices. They are not the FBO and therefore should not be referred to on the formal notice.
4.21.9 Content of notice
The notice must specify the:
- grounds for believing the FBO is failing to comply with the regulations
- precise nature of the alleged breach
- measures needed to be taken to secure compliance
- timescale for compliance
- appeal provisions, including the name and address of the relevant local court
Note: Alternative works of equivalent effect may be acceptable.
4.21.10 Time limits
HINs place the FBO under a legal obligation to take specified action within a set time period, during which operations may continue.
The time period given as the date of compliance must not be less than 14 clear days. When calculating this, the AO must not include the day on which the notice is served. They should begin counting from the day after the day on which they intend to serve the notice, count 14 clear days and then put the date for compliance as the day after this.
Example:
- Offence is identified on 1 January
- Notice to be served on 1 January
- The AO must count 14 days starting from 2 January; this will be the close of operations on 15 January
- Mark the date for compliance as 16 January
Note: The period specified for compliance by the AO must be reasonable, given the measures required, and should, if possible, be agreed with the recipient.
Where the AO is unsure what may constitute a reasonable timeframe to specify in a HIN, it is important that they seek advice from FSA Legal to avoid appeals being lodged for unrealistic timescales.
4.21.11 Posting
Ideally, all HINs should be posted at a Post Office and proof of posting obtained. Where it is impossible to gain access to a Post Office the notice should be posted in a post box, with corroboration obtained if possible and a record made in the AOs pocketbook which should be countersigned.
4.21.12 Right of appeal
Recipients have a right of appeal against Hygiene Improvement Notices to the magistrates’ court. During the appeal period, the requirements of the notice are suspended.
In the event of an appeal by someone who is aggrieved by the service of the HIN, the AO is to inform the Lead DHI and the FSA Legal team to arrange legal representation for the appeal hearing.
4.21.13 Requests for notice extension
If the FBO were to request an extension to a HIN, this must be in writing prior to the expiry of the HIN.
Where there is a genuine reason for such an extension, the AO should withdraw the existing notice. A template letter is available in Annex 4 of this chapter.
The AO must issue a new HIN with a revised time frame that:
- provides a minimum 14 clear days to comply
- concludes on an agreed date that the FBO / AO believes that compliance may be achieved
The AO must consider again whether the conditions prevailing at the plant still warrant the issuing of a new notice. Where this is the case, new evidence must be gathered to justify its service.
The AO must retain the written request for the extension as well as the original notice. This will ensure that where any complaints or appeals are lodged against the timescales in the second notice, the AO can demonstrate the overall timeframe provided in both notices and the proportionality of their actions.
4.21.14 Failure to comply
Failure to comply with an HIN is an offence. If the FBO has failed to comply with a notice, complete a Referral for Investigation report for the breach of the formal notice as well as a breach of the substantive offence that led to the notice being served in the first place.
Reference: See the topic 4.24 on ‘Referral for investigation’ in this section for additional information.
4.21.15 Compliance and withdrawal
After the service of a HIN, the AO must check that it is complied with by the stated date.
Where compliance is achieved, the AO must confirm formally in writing that they are satisfied with the works carried out.
Measures that achieve the same outcome as those specified in the notice must be accepted as achieving compliance.
Where the AO is satisfied that the action required in the HIN has been carried out and compliance has been achieved to their satisfaction, a template is available in Annex 2 to this chapter that can be used as the basis of a letter to the FBO.
Where the AO has served the HIN in error and / or it has to be withdrawn due to a technicality, a template is available in Annex 3 to this chapter which can be used as the basis of a letter to the FBO.
4.22 Hygiene Emergency Prohibition Notices (HEPN)
4.22.1 Caution
HEPN can only be issued after authorisation from FSA Legal.
4.22.2 When to use
Issuing a HEPN should only be considered after discussion with the FVC, and where there is an imminent risk of injury to health that requires the backing of the court, for example, contamination of the potable water supply.
Reference: Specific examples and further guidance are given in the Code of Practice made under Regulation 24 (Wales) / Regulation 26 (England) of the Regulations.
The HEPN must be served on the FBO or using the same procedures as outlined in the topic ‘Hygiene Improvement Notices’.
Note: The limited timescales are set out in the subsequent topics.
4.23 Hygiene Emergency Prohibition Orders (HEPO)
4.23.1 Application process
The section below provides an overview of the application process for a HEPO.
| Stage | Description |
|---|---|
| 1 |
The AO must give the proprietor at least U1 day’s notice of their intention to apply for a HEPO by serving a HEPN on the FBO. A HEPN has an immediate prohibitory effect and once served the AO should contact the local court to immediately arrange for a hearing. Note: A copy of the HEPN must be affixed in a conspicuous position to the premises at which the notice relates. |
| 2 | The AO applies for an HEPO from the magistrates’ court within 3 days of the service of the notice. The day of the service of the notice being day one. There is no legal requirement for the application to be heard in 3 days, although the court should be asked to list the hearing at the earliest opportunity. Once made the HEPO supersedes the HEPN. The AO must also affix a copy of the HEPO in a conspicuous position to the premises at which the Order relates. |
| 3 |
Once the FBO applies, in writing, for the HEPO to be lifted, the application must be determined as soon as practicable and within 14 days. Once the AO is satisfied that the proprietor has taken significant steps to remove the health risk(s) specified in the notice, the AO should sign the withdrawal certificate at part 5 of the HEPN. Reference: The Food Hygiene (Wales) Regulations 2006 (as amended)/ Food Safety and Hygiene (England) Regulations 2013 (as amended), Regulation 8. |
4.23.2 Sources of advice
Advice should be sought from FSA Legal, who will, assist in the preparation of the case prior to the court's hearing of a HEPO.
4.23.3 Evidence
Monitoring of the prohibition and any action taken by the proprietor must be recorded. Suitable evidence should be gathered prior to serving the HEPN for production in court.
4.23.4 Procedure
The table below shows the steps for an AO to follow when applying for a HEPO.
| Stage | Description |
|---|---|
| Contact local court to arrange hearing | The application of the HEPO must be lodged with the court within 3 days of service of the HEPN. On establishing dates and times, the AO must notify the FBO by serving a Notice of Intention to Apply for a HEPO. |
| Prepare for hearing | Prior to the hearing the AO should:
|
| At the hearing | It is crucial that the AO has gathered significant evidence at the time the HEPN was served and that this evidence is presented to the court. |
| Court will decide whether to issue the HEPO or not |
If the order is made the AO should produce the completed order for signing by the magistrate. The order must then be served on the FBO as soon as possible and a copy affixed to the premises in a conspicuous place. Any breaches of the order whist in force should be recorded and evidence collected. The matter should then be referred for investigation. |
| Risk is removed | The AO must then formally cancel the HEPO by writing to the FBO. The withdrawal of such a HEPO must not be unreasonably withheld. Once the order has been complied with, the business can recommence its operation. |
4.24 Referral for investigation
4.24.1 Appropriate uses
A referral for investigation is required in the following circumstances:
- breaches of the European Regulations and / or the Food Safety Act leading to an imminent risk to public health
- continual failure to observe requirements of Regulations
- obstruction of FSA personnel engaged in official duties
- failure to comply with a HIN or RAN (RAN - Wales only)
- breaches of an official detention notice
4.24.2 Evidence
The AO must collect adequate evidence at the time of the offence before referring the matter for investigation. The AO must identify the contravention and complete the corrective action report.
4.24.3 Referral to FSA Legal
Where the AO considers that an incident requires investigation, the matter will be referred to FSA Legal for an investigation to be undertaken.
Note: The process to follow when making a referral for investigation is detailed in the table in sub-topic 4.24.8 on ‘Protocol for referral for investigation’.
4.24.4 Decision to prosecute
In England and Wales, the decision whether or not to prosecute for contraventions of hygiene legislation is made by FSA Legal, after being investigated by an FSA Investigation Officer.
4.24.5 Rules of evidence
The AOs main task will be in the gathering of facts and evidence at the time of the offence, which may be used in court at a later stage. An AO must not attempt to conduct a full investigation as specific training is needed to ensure that the investigation is carried out in compliance with the Police and Criminal Evidence Act 1984 (PACE) (or equivalent) requirements. Only specially trained FSA Investigation Officers conduct investigations. AOs must not attempt to caution suspects or take statements.
4.24.6 Statements
Statements will be taken by an FSA Investigation Officer. They are a record of specific events an individual witnessed in a chronological order. They must refer to all relevant evidence and produce these as exhibits for the case. For example:
- photographs
- samples
- copies of notices
- copies of notebook entries
Exhibits are usually identified by the initials of the AO and then consecutively numbered. The IO will assist with numbering when preparing the final statement.
Note: Where the AO is satisfied that the action required or work specified in a formal notice has been completed, the date that it was completed should be specified in a witness statement and on the back of the copy notice.
4.24.7 Protocol for a referral for investigation
The table below outlines the process and rationale for a referral by the AO for formal investigation.
| Process | Rationale |
|---|---|
| The AO is to discuss the issue with the HOD for Dairy Hygiene when the contravention occurs, and prior to completing the DH ENF 11/6 ‘Referral For Investigation’. | This will ensure that the HOD is aware of all formal enforcement action taking place at the farm. |
| If advice is needed on the correct enforcement approach, the AO should consult with the Lead DHI in the first instance and further advice can be obtained from FSA Legal if necessary. | Early advice will provide the necessary support to quickly address any queries regarding the enforcement approach. |
| The AO is to collect all relevant evidence relating to the referral. | All relevant evidence must be supplied to prove the elements of the offence. Photographs must be taken to assist the court, especially where the nature of the offence may be difficult to visualise. |
| The AO must send all evidence together with the DH ENF 11/6 to CSU at FSA York within 10 working days of the offence being identified and the AOs decision to refer for investigation. | Hygiene offences carry a 12-month time limit within which charges should be laid at the court. The time limit between identifying obstruction contraventions and laying charges at the court is 6 months. The IO must be afforded enough time to investigate the offences identified. |
| Once received, CSU will acknowledge receipt of the recommendation, paperwork and allocate a unique number to the referral. This number will be notified to the AO in the confirmation email. (If confirmation is not received within 5 working days the AO should contact CSU. | Confirmation will be sent to provide assurance that the documentation has been received. |
| If compliance is achieved after a referral for investigation has been made, the AO must record this. | This will demonstrate the effectiveness of operators ‘Due diligence’ systems and identify any defences or mitigation that the operator may wish to put forward. |
| Where additional information is required, FSA Legal will request further details, to gain a better understanding of the issues involved. | Where; evidence is lacking, the issue is complex, the approach taken by the AO requires further explanation. FSA Legal may contact relevant colleagues so that a comprehensive and informed picture can be gained of the issues surrounding the referral. |
| This may include checking that: | |
|
To prove the elements of the offence beyond all reasonable doubt. |
|
To stand up to legal scrutiny. |
|
To make sure that all the procedural requirements relating to enforcement have been followed. |
|
To ensure that the notice is legally compliant and defendable in case of an appeal. |
|
To ensure that all policies and procedures have been complied with. |
|
When the investigation is complete FSA solicitors will review all case files relating to hygiene contraventions and make a decision on the appropriate course of action. This could be:
|
When a decision is made NOT to take the case forward, the AO and Lead DHI will be advised of the reason by FSA legal. |
| OR | - |
|
When a decision is made to take the case forward to court, the IO must inform the AO and HOD before informing the farmer of this intention. |
| Where the FBO pleads not guilty and the case goes forward to trial, all witnesses must be made aware of where and when their presence will be required at court. | Any AO who is unfamiliar with court procedure may benefit from some discussion with FSA Legal before any court appearance. Witnesses will have the opportunity to review their statements before appearing at court. |
| If a case goes to court and the FBO pleads guilty. Once the sentence has been determined, this information will be cascaded back to the AO, Lead DHI and the HOD. | If the witnesses are not required to attend a guilty plea, the outcome of the case will be cascaded to all witnesses. |
4.25 Change of FBO during ongoing enforcement action
4.25.1 New FBO
From the moment a new FBO takes over a food establishment, they are responsible for its condition and operation. Any enforcement action initiated prior to the change of ownership should be reassessed. Where the new FBO fails to immediately address any outstanding enforcement issues, these should be pursued by the AO, through the hierarchy in the normal way.
4.25.2 Re-issue of notices
In the event of the premises changing ownership when a notice is still in force, the existing notice should be withdrawn. If the new FBO fails to immediately address the outstanding issues a similar notice should be issued on the new FBO with an explanation of why the notice is being issued. Evidence must be gathered again to justify the service of the notice.
The situation should always be reconsidered prior to re-issuing the notice. The AO may have to justify to a court on some future occasion why they (re-)issued the notice.
Note: Where the FBO has changed, a new registration must be completed by the FBO and submitted to Approvals and Registrations Team so that the establishment’s registration details and ownership records can be updated.
4.26 Warrant to enter premises
4.26.1 Access refused
An AO who has been refused entry to premises should contact the Lead DHI and seek further advice from FSA Legal.
In the event that access to farm premises is refused, it may be necessary for an AO to apply to a Justice of the Peace for a Warrant to Enter Premises, to gain access to carry out their duties.
FSA Legal should be contacted for advice on any refusal by the FBO to grant entry to an AO. Where there is a threat of violence, reference should be made to the Bullying and Harassment Policy UH for guidance. A report must also be made to the local police force.
Examples of when it is necessary to apply for a Warrant to Enter Premises include:
- circumstances where the AO needs to enter to find out if there has been a contravention of the Hygiene Regulations
- entry is required to find out if there is evidence of any such contravention and
- reasonable access has been refused or the AO believes it will be refused and the AO has given the occupier notice of intention to apply for a warrant
- the premises are unoccupied
- asking for permission, or giving notice of asking for permission would defeat the object of the entry
- where urgent access is needed
4.26.2 Execution of the warrant
The warrant must be executed within one month and is no longer valid once the AO has used it to gain access. It cannot be used twice. When executing a Warrant to Enter Premises in England or Wales, Code B of the Codes of Practice, made under the Police and Criminal Evidence Act 1984 (PACE), should be complied with. Legal advice on this and other aspects of the Warrant should be obtained before entry is attempted. FSA Legal will advise further.
4.26.3 Access
Advise the local police of the intention to execute the Warrant at a certain time and date. The establishment must be visited as soon as possible and, on production of the Warrant to Enter Premises, the occupier should grant access. If the occupier fails to grant access, he or she will be in breach of the warrant. Record the events in the contemporaneous notebook and inform the Lead DHI and FSA Legal.
4.26.4 Forced entry
The Warrant to Enter Premises allows the use of force to gain entry when necessary. However, the AOs should never attempt a forced entry themselves, but contact the Police for assistance.
4.27 Process for obtaining a warrant to enter premises, in England and Wales
Updated [The diagram below explains how warrants of entry can be obtained and deals with two scenarios, entry with warning and entry without warning. It explains the steps that would need to be taken and the process to follow.]

4.27.1 Hampered or obstructed inspection
Regulation 14 of The Food Hygiene (Wales) Regulations 2006 (as amended) / Regulation 16 of The Food Safety and Hygiene (England) Regulations 2013 (as amended), give authorised inspectors a right of entry in both England and Wales and the inspector is entitled to receive any assistance that they may require from the FBO. If the inspection is refused, obstructed or is hampered the inspector should consider if any grounds given for hampering the inspection are reasonable (for example, a farm or family emergency such as a bereavement, TB testing or RPA inspection taking place). If so, agree to come back after at a later date.
If the grounds are not reasonable and it is considered safe to do so, the inspector should inform the FBO that they are hampering the inspection. If cooperation is not obtained a letter will be sent to the FBO and further legal action will be considered.
The DHI should report any instance of obstruction to the ITL for further action. The DHI should make a comprehensive note of the circumstances surrounding the visit, who they spoke to and the reasons they were not allowed to carry out the inspection. This note should be made in the DHIs note book and in the Dairy Hygiene Inspection Report (DH2).
The DHI should send the standard letter ‘Hampered / Obstructed Inspection’ DH ENF 11/12 (E&W)) indicating to the FBO their legal powers of entry and potential offences for obstructing the authorised officer. The DHI will undertake a further visit accompanied by a colleague.
Until a visit is completed the premises will remain on the outstanding visits schedule.
5. Milk safety incidents
In this section
5.1 Introduction
5.1.1 Background
Within England and Wales FSA, DHIs have responsibility for controls up to and including any bulk storage vessel, after which enforcement responsibilities pass to the LA Environmental Health Team. FSA Northern Ireland subcontracts dairy official controls across the food chain to DAERA.
A key part of the FSA’s work is investigating and managing incidents to ensure that food safety is protected. The procedures to be followed when managing an incident are included in the FSA’s Incident Response Protocol (Revised May 2012).
5.1.2 Section objective
Describe the DHIs role in the event of a milk incident including how this relates to the FSA’s Incident Response Protocol.
Describe the procedures to be followed in the event of a reported milk incident to ensure that it is managed consistently and promptly in order to minimise the risk to public health.
Clearly identify the roles and responsibilities of those involved during a milk incident.
5.1.3 Regulations
The procedures in this section apply when dealing with reported milk incidents in accordance with:
- (EU) 2019/627
- (EC) 852/2004
- (EC) 853/2004
- (EU) 2017/625
- The Food Hygiene (Wales) Regulations 2006 (as amended) / the Food Safety and Hygiene (England) Regulations 2013 (as amended)
- (EC) 178/2002
- The General Food Regulations 2004
5.2 Incidents
5.2.1 Types of milk incident
Milk incidents may include:
- milk from TB / BR reactor animals entering the food chain / milk storage vessels
- incidents of physical or chemical contamination including taints, the presence of unauthorized substances or substances present above legal limits
- food poisoning outbreaks
- sabotage of product (malicious tampering)
5.2.2 Notification
A DHI may be alerted to a potential milk incident in a number of ways including:
- outcome of an inspection
- notification by FBO
- routine laboratory analysis
- complaint / laboratory analytical results from LA Environmental Health Department
- notification by other government departments (Defra, AHPA, RPA)
- notification by a member of the public or media
As notifications may be received from different sources, it is vital that this information be passed to the relevant DHI and the Lead DHI immediately. If the DHI is unavailable or cannot be contacted within 1 hour of receipt, another DHI within close proximity must be informed.
The DHI receiving the notification will fulfil the role of preliminary investigating officer and must carry out an assessment of the potential incident.
The DHI is required to notify FSA Incidents Unit and relevant LAs upon identification of a potential milk safety incident. Login details for the electronic FSA Incidents report have been issued to DHIs.
Notifications must be recorded on the summary section of the Dairy Hygiene Report form (DH2).
5.2.3 Assessment of potential incident
Within the FSA Incident Response Protocol, an incident is defined as:
‘Any event where, based on the information available, there are concerns about actual or suspected threats to the safety or quality of food and /or feed that could require intervention to protect consumers’ interests’.
To determine whether an actual or suspected threat to the safety of milk exists, the DHI must immediately assess the information available to determine the probability that an incident has occurred.
5.2.4 Classification of incident
All potential incidents will be classified as high, medium or low as follows:
- High probability – evidence is available suggesting a systematic breakdown of controls within the milk supply and / or multiple potential incidents have been reported / identified.
- Medium probability – inconclusive evidence available to determine if probability is ‘High’ or ‘Low’, more information needed to determine action.
- Low probability – no evidence of breakdown of control systems, only an isolated potential incident identified.
5.3 Action in case of Incident
5.3.1 Key actions
As soon as a potential incident has been classified, the DHI must complete the actions for each classification as outlined below.
Upon receipt of a potential milk incident notification an assessment must be initiated in accordance with the protocols of this section.
5.3.2 High probability
High probability action must be completed on the same day as receipt of notification of the incident.
- Report incident to Lead DHI / FVL to agree classification.
- Report incident to FSA Incidents Unit by completing the on-line incidents notification.
(Login details have been issued to DHIs). Confirmation of receipt of the report will be sent by Incidents Unit to the dairy hygiene inbox.
- Complete a preliminary investigation at affected milk production holding and record all details on the DH2 on the tablet.
- Arrange for samples to be tested (if required).
- Re-assess risk following investigation and / or upon receipt of testing results, liaising with the Lead DHI.
- Lead DHI will provide a preliminary report to the Head of Operational Delivery.
5.3.3 Medium probability
Action to be completed on the same day as receipt of notification of the incident.
Please note medium probability classification is to be used only where there is insufficient information to classify the incident as either high or low. All incidents must eventually be classified as either high or low once sufficient evidence is available.
- Report incident to Lead DHI to agree classification.
- Report incident to FSA incidents team by completing the on-line incidents notification.
(Login details have been issued to DHIs). Confirmation of receipt of the report will be sent by the Incidents Unit to the dairy hygiene inbox.
- Complete a preliminary investigation at affected milk production holding and record all details on the DH2 on the tablet.
- Arrange for samples to be tested (if required).
- Re-assess risk following investigation and / or upon receipt of testing.
- Once probability is changed to either high or low, then actions outlined in those classifications must be taken.
Lead DHI will provide a preliminary report to the Head of Operational Delivery.
5.3.4 Low probability
First 3 points to be completed on the same day as receipt of notification of the incident. Remaining action to be completed within 3 days of receipt of the notification of the incident.
- Report incident to Lead DHI to agree classification.
- Report incident to FSA Incidents Unit by completing the on-line incidents notification. (login details have been issued to DHIs). Confirmation of the report will be sent by the Incidents Unit to the dairy hygiene inbox.
- Complete a routine inspection following the guidance found at section 2 on ‘Inspection procedures’ of this chapter.
5.3.5 Escalation
Once the FSA Incidents Unit have been notified of a milk incident, they will decide if they should invoke their incident response protocol and establish an Operational Incident Management Team (OIMT) or a Strategic Operational Incident Management Team if the incident was of a sufficiently high level.
Where the FSA OIMT has been established, the DHI will take direction from the OIMT for the remainder of the investigation, continuing to provide technical support and participate in the OIMT as requested.
5.3.6 Enforcement
In the event that the DHI is required to undertake enforcement duties, the relevant enforcement procedure in Section 4 of this chapter must be followed.
Enforcement actions may include detention, seizure, Warning Letters, HIN,RAN - Wales only and HEPN.
If the DHI is made aware that milk from animals that are not officially TB free has been consigned for wholesale sales, or that milk from herds that have lost their official TB free status has been sold as RCDM then appropriate action must be taken to ensure that the FBO is aware of the seriousness of the offence.
In such incidents, enforcement starting no lower down the enforcement hierarchy than a Warning Letter must be issued, indicating clearly the legislative requirements relating to the breach.
5.3.7 Interpretation of laboratory results and guidance on follow up action on RDM incidents
The Dairy Hygiene team, in collaboration with Food Hygiene Policy and Food Incidents teams, has developed guidance in case of incidents in case of incidents related to RDM. This guidance can be found at Annex 9.
The guidance outlines how to interpret laboratory results and what action, investigations and sampling should be taken by DHIs regarding unsatisfactory results from samples of milk to be sold as RDM.
The guidance covers:
- interpretation of results for both pathogenic and indicator criteria
- actions and further investigations
- resuming RDM sales
- questions to consider for information gathering purposes
- additional action subject to the outcome of on-farm investigations
5.4 Roles and responsibilities
5.4.1 The Dairy Operational Lead
The Dairy Operational Lead (DOL) is responsible for providing strategic support in the management of the incident including updates to Senior FSA management.
5.4.2 Lead DHI / FVC
The lead DHI / FVC:
- has responsibility for the co-ordination of the incident, if necessary identify and assign DHI
- provides support to the investigating officer and guidance where enforcement action is taken
- provides DOL and FVL with regular updates.
5.4.3 DHI
Up to and including bulk storage vessel:
- carries out preliminary assessments and take action as necessary to protect public health
- report incident to LA, LDHI, ITL and FSA Incidents team as required
- implements formal / informal enforcement actions as necessary
- identifies the source of the incident (in liaison with the FBO) and ensure that the appropriate action is taken to rectify the problem
- completes DH2/DH1 as appropriate.
5.4.4 Local Authority
The LA is responsible for all controls after the bulk storage vessel, including following up where necessary with milk purchasers, processors and consumers.
6. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Dairy hygiene enforcement policy This document has been withdrawn and is currently subject to review
Annex 2: HIN withdrawal letter: Improvements complete
Annex 3: HIN withdrawal letter: Issued in error
Annex 4: HIN withdrawal letter: Period extension
Annex 5: Hampered / Obstruction inspection letter- This document has been removed and can be found within chapter 9 ‘Forms’ as DH ENF 11/12 (E&W)
Annex 6: Supporting evidence photographic report template- This document has been removed and can be found within chapter 9 ‘Forms’ as DH ENF 11/14
Annex 7: Use of RANs in Wales: examples
Annex 8: New RCDM labelling enforcement provisions / responsibilities
Annex 9: Guidance on RDM incidents
Annex 10: Sample despatch process
1. OV Flexible Attendance
2. PIA System
3. Annexes
Sections
1. OV Flexible attendance
In this section
1.2 Flexible attendance general issues
1.3 Implementation of flexible attendance
1.4 Specific requirements: Red meat slaughterhouses
1.5 Specific requirements: Game handling establishments
1.6 Specific requirements: Poultry establishments with MHI inspection
1.7 Monitoring of establishments with flexible attendance
1.1 Introduction
1.1.1 Objective
This document sets out guidance for Field Veterinary Co-ordinators (FVCs) to enable them to identify slaughterhouses and game handling establishments (GHEs) that do not require the full time presence of an Official Veterinarian (OV) during post-mortem inspection (PMI) and to assess the OV hours required. The objective of the procedure is to provide a risk base framework to aid consistent decision making in respect of OV attendance.
1.1.2 Legislation
Regulation (EU) 2017/625 requires the competent authority (CA) to ensure that an OV is present at slaughterhouses throughout ante-mortem and PMI and at GHEs throughout PMI but has allowed the Commission to make exceptions to this general requirement in delegated acts.
(Delegated Regulation (EU) 2019/624 allows for some exceptions to this requirement in in low-capacity slaughterhouse or game-handling establishments.
Post-mortem inspections may be performed by an official auxiliary under the responsibility of the official veterinarian (OV is not present on the premises, see definition below in 1.2.1), when the following criteria and conditions are met:
(a) the slaughter or game-handling activities are carried out in a low-capacity slaughterhouse or game-handling establishment which slaughters or handles:
(i) less than 1 000 livestock units per year; or
(ii) less than 150 000 poultry, lagomorphs and small wild game per year;
(b) the competent authority may increase the thresholds laid down in point (a) ensuring that the derogation is applied in the smallest slaughterhouses and game handling establishments complying with the definition of low-capacity slaughterhouse or game-handling establishment (further details in Legislation)
(c) the establishment concerned has sufficient facilities to store meat with abnormalities separately from other meat until the official veterinarian can inspect the meat with abnormalities in person;
(d) the official veterinarian is present in the establishment at least once a day, including regularly during slaughter activities;
(e) the competent authority has put in place a procedure to assess on a regular basis the performance of official auxiliaries in these establishments, including:
(i) monitoring individual performance;
(ii) verifying documentation on inspection findings and comparing it with the corresponding carcasses;
(iii) checks of carcasses in the storage room;
(f) a risk analysis has been carried out by the competent authority, taking at least account of the following elements:
(i) the number of animals slaughtered or handled per hour or per day;
(ii) the species and class of animals slaughtered or handled;
(iii) the throughput of the establishment;
(iv) the historical performance of slaughter or handling activities;
(v) the effectiveness of any additional measures in the food chain taken to guarantee the food safety of animals intended for slaughter;
(vi) the effectiveness of the hazard analysis and critical control point (HACCP)-based procedures;
(vii) audit records;
(viii) the competent authority's historical records of ante-mortem and post-mortem inspections
Reference: Regulation (EU) 2019/624 Article 7
1.2 Flexible attendance general issues
1.2.1 Definitions
‘Under the responsibility of the official veterinarian’ means that the official veterinarian assigns the performance of an action to an official auxiliary.
‘Under the supervision of the official veterinarian’ means that an action is performed by an official auxiliary under the responsibility of the official veterinarian and the official veterinarian is present on the premises during the time necessary to perform that action.
‘Low-capacity slaughterhouse’ means a slaughterhouse designated by the competent authorities on the basis of a risk analysis and in which slaughtering takes place only during part of the working day or takes place during the whole working day but not on each working day of the week.
‘Low-capacity game-handling establishment’ means a game-handling establishment designated by the competent authorities on the basis of a risk analysis and in which game-handling takes place only during part of the working day or takes place during the whole working day but not on each working day of the week.
Flexible attendance – a risk assessment shows that the OV’s continuous presence during post-mortem inspection is not required.
Full time attendance – the OV is required to be continuously present at the premises throughout ante and post-mortem inspection.
For the purposes of applying OV flexibilities at all qualifying premises, EU regulations permit reduced OV presence on the basis of a risk assessment which shall cover public health, animal health and welfare assessments.
100% OV attendance (throughout post-mortem inspection) is not required when the Meat Hygiene Inspectors (MHIs) carry out post-mortem inspection at establishments meeting the flexible attendance risk assessment criteria.
1.2.2 Statement of resources
The Statement of Resources (SOR) meetings between the FSA and FBOs capture the service requirements for official controls. The Charges for Controls in Meat Premises Guidance will help assess these service requirements and best options for delivery.
SORs must capture the flexibility arrangements agreed with the FBO in the other business information section of the SOR template. Inspection Team Leaders (ITLs) should capture the OV Flexibility requirement on the SOR as follows:
- Outline the FVC recommendation of OV Flexibilities
- Capture broadly any flexible start/ finish time of the OV as recommended by the FVC liaising with the FBO and OV contract supplier.
- System for OV/FBO communications in adopting the flexibility recommendations, for example, a 1 hour reduction of OV attendance on a particular day for ante-mortem activities may be agreed on a daily basis between OV and FBO with a note made in the daybook by OV.
ITLs must make business decisions for the SOR to establish the most cost efficient service which may result from OV Flexibilities at individual premises. The OV may be retained on site carrying out other inspection duties thereby reducing other elements inspection team costs such as premium rate working of employed inspectors.
The OV may continue to provide a versatile resource in the team carrying out meat inspection, CCIR activities, detention line work and other monitoring and verification which must be considered by ITLs to establish the most cost efficient service at individual establishments.
1.2.3 Inspection tasks
The OV must be allowed sufficient time to carry out the inspection tasks required by Regulation (EU) 2017/625 and detailed in Regulation (EU) 2019/627:
- checks on Food Chain Information (FCI)
- ante-mortem inspection; which can be carried out up to 24 hours before slaughter
- verification of compliance with welfare regulations
- post-mortem inspection
- checks on removal, separation and marking of SRM and ABP
- ensuring sampling takes place, for example, residues, trichinella, TB, TSE.
OAs may assist the OV with all the above tasks.
Note: For details, please check sub-topic 1.3.7 on ‘Daily OV tasks’ and sub-topic 1.3.8 on ‘Team tasks’ in topic 1.3 on ‘Implementation of flexible attendance’.
In relation to flexible attendance, it is important to note that OVs, when present, will constantly be checking on compliance with the Hygiene Regulations and, where necessary, carry out enforcement. However, the Regulations do not require OVs to remain at an establishment once they have completed their responsibilities as above unless they have identified public, animal health and / or welfare issues that require their continued presence.
1.2.4 Cold inspection
‘Cold inspection’ occurs when there is no official presence during dressing of carcases. The OV leaves after ante-mortem inspection and returns later to carry out post-mortem inspection. Please see Annex 11 of this chapter.
1.2.5 Risk analysis
The Regulations require a risk analysis to be carried out to determine whether flexible attendance can be implemented at specific premises. The risk analysis is primarily carried out to assess the risk that unfit meat might be placed on the market if the OV is not present to supervise post-mortem inspection. The following points are to be taken into account when carrying out the risk assessment:
- current capacity / number of animals slaughtered / handled daily including procedures and facilities to detain uncommon abnormalities
- species and class of animals slaughtered (for example, older animals are likely to have a greater number of abnormalities requiring OV attention)
- the history of the quality of animals and the need for carcases to be detained
- the history of the performance of slaughter / handling activities
- the effectiveness of the HACCP-based system in place
- audit records and history of official AM and PM records.
Reference: (EC) 2019/624, Article 7
1.2.6 Comparison between full time and flexible attendance
The following chart shows the comparison between full time and flexible attendance requirements:
Comparison between full time and flexible attendance
| Full time Attendance | AM | PM |
|---|---|---|
| Slaughterhouses | OV | OV / MHI / PIA |
| GHEs | N/A | OV / MHI |
Comparison between full time and flexible attendance
| Flexible Attendance | AM | PM |
|---|---|---|
| Slaughterhouses | OV | MHI / OV part time |
| GHEs | N/A | MHI / OV |
| Premises with Cold Inspection | OV | OV / MHI (end of operations) |
1.3 Implementation of flexible attendance
1.3.1 FBO role
FBOs wishing to apply for flexibility in OV attendance need to discuss their eligibility with their FSA ITL in the first instance during SOR meetings. The ITL should then contact the local FVC and inform the FVC of the FBO’s request. Any risks identified to the OV not being on site during production must be fully considered by the FBO before agreeing OV flexibility on in their establishment.
1.3.2 FVC role
All establishments within the FVC’s span of control must undergo an OV flexibility assessment utilising the outcome of the last completed FBO full audit. For establishments where a flexible attendance arrangement was implemented, this assessment will be used for monitoring purposes (see topic 1.7 on ‘Monitoring of establishments with flexible attendance’).
FVC should actively look for opportunities to implement flexibility liaising with contractors and local inspection teams to identify opportunities available.
The FVC is responsible for the co-ordination of tasks necessary to implement flexible attendance.
Once informed about FBO’s interest in flexible attendance the FVC should make contact with the FBO and explain the process and requirements.
The FVC should agree a date with the FBO, OV and the contract Area Veterinary Manager (AVM) to visit the premises and to carry out the assessment necessary to evaluate suitability of the premises for implementation of the flexible arrangement.
1.3.3 Examples where flexible attendance should be considered
Each establishment is different and opportunities for flexible attendance will vary. There are, however, common aspects that should be considered when looking at the operational pattern, process details and options for reduced level of official controls.
The following operational examples could indicate to the local FSA team and FBOs that there are opportunities for flexible attendance:
- scheduled arrivals of animals allowing for planning of operations and OAs carry out post-mortem inspection
- inspection, for example, the OV can leave early or arrive late depending on the plant production and FBO requirements
- establishments in a local geographical area which may be served by one roving OV (serving multiple premises), with OAs carrying out post-mortem inspection.
1.3.4 General requirements for implementation of flexible attendance
All establishments wishing to implement flexible attendance arrangement must meet the following general requirements:
Compliance history (full audit period utilised)
Implementation of flexible attendance should only be considered in establishments with good compliance history utilising the outcomes from the last completed FBO audit that means:
- ‘Good’ or ‘Generally satisfactory’ outcome of the last FSA audit
- any non-compliances raised by the FSA team are promptly resolved by the FBO (no need to routinely escalate to formal enforcement, hygiene improvement notice (HINs), remedial action notice (RANs)
- good welfare standards – no ‘critical’ welfare non-compliances
- implemented and maintained food safety management system based on HACCP principles (including adequate controls over removal and disposal of SRM)
Throughput / animals slaughtered
The daily species / type of animals slaughtered, the throughput of the establishment and the line speed should not compromise food safety, specifically:
OA should have enough time to inspect all animals and put aside meat with uncommon conditions for OV inspection
FBO should ensure that only young and healthy animals are slaughtered, or adequate arrangement should be in place for slaughter of older animals
FBO should not accept animals originating from farms under movement restrictions due to diseases
FBO should not accept animals from emergency on farm slaughter unless a provision is made for the post-mortem inspection to be carried out by the OV.
Ante-mortem arrangement
Positive ante-mortem release system has to be implemented and maintained to ensure that only animals that have undergone ante-mortem inspection have been slaughtered for human consumption.
Facilities for storage of meat
Adequate facilities have to be in place for storage of meat with uncommon conditions for OV inspection for all species slaughtered.
Ante and post-mortem inspection records (last 3 month period)
FSA ante and post-mortem records for the establishment wishing to implement a flexible attendance arrangement should not indicate frequent and regular findings that would require OV consultation / inspection.
1.3.5 Specific requirements
In addition to the general criteria, the specific requirements applicable to certain types of establishments must be taken into consideration. (See topic 1.4 on ‘Specific requirements: Red meat slaughterhouses’, topic 1.5 on ‘Specific requirements: Game handling establishments’ and topic 1.6 ‘Flexible attendance: Poultry establishments with MHI inspection’.)
1.3.6 Assessment of premises
The FVC should carry out an assessment of each premise in conjunction with the OV and FBO, taking into consideration the operating practices and agreed operating hours, using ‘Assessment for OV Flexible Attendance’ at Annex 1.
| - | Assessment Process Steps |
|---|---|
| 1 | FVC agrees a date with the FBO, OV and contractor AVM and visits the premises to carry out the assessment. |
| 2 | FVC discusses the outcome of the assessment with a relevant FVL. |
| 3 | FVC uploads Annex 1 ‘Assessment for OV Flexible Attendance’ form to the area SharePoint site |
| 4 | FVC informs the ITL / Operations Manager (OM) / Head of Operational Delivery (HOD) about the recommendation, in writing. |
| 5 | ITL notifies OV, contractor AVM and FBO about the recommendation and agrees SOR. |
| 6 | FVC populates the OV Flexibility information specific to the establishment on the K2 system once this is available. Whilst the K2 system is not available this could be recorded in the OV Flexible Attendance Post Implementation assessment document (Annex II) OV feedback comments. |
1.3.7 OV tasks
When assessing the premises, the FVC must be satisfied that the OV will have enough time to carry out the daily OV tasks, which include:
Food chain information – all FCI records provided by the FBO must be assessed by the OV prior to slaughter.
Ante-mortem inspection – all animals slaughtered for human consumption must undergo ante-mortem inspection carried out by the OV less than 24 hours before slaughter.
Welfare checks – the OV should assess the welfare of animals at intake or in the lairage and ensure a system is established to verify welfare up to and including the stun / stick / bleed process. Daily welfare checks can be carried out by the OA as per the instruction in chapter 2.3 on ‘Animal welfare’.
Post-mortem inspection – the OV should carry out the PMI verification check as per the detailed instruction in chapter 2.4 on ‘Post-mortem, health and identification marking’. In the case of emergency slaughtered animals, the OV must personally carry out the PMI.
Enforcement – the OV should spend as much time as needed to take an enforcement action (or further investigate) when their daily checks, or checks carried out by other members of the FSA team, indicate that the FBO is not in compliance with relevant requirements of food hygiene law.
Paperwork / records – sufficient time should be provided to the OV to complete all daily paperwork and records, as required by FSA policies and procedures.
Other OV routine checks as deemed necessary.
1.3.8 Team tasks
Before flexible arrangement is implemented, the FVC and OV must be satisfied that there are adequate and suitably trained staff and FSA procedures that cover:
- PMI – all animals slaughtered for human consumption must be subject to PMI
- SRM removal and handling – the OV is expected to use the ‘Risk Based Decision Tool (RBT) for SRM Inspections’ on a monthly basis to set the frequency of checks as per chapter 2.7 on ‘Specified risk material controls’. Depending on the frequency that was set (daily / every 5 days), the FSA team must carry out all SRM removal, handling and disposal verification checks as per details in chapter 2.7 on ‘Specified risk material controls’
- ABPs – FSA team must verify that all ABPs are correctly identified, stained and stored until collection as per the instruction in chapter 2.8 on ‘Animal by-products’
- animal identification – daily FSA cattle ID checks must be carried out to the frequency set by the OV (based on FBO’s compliance history) as per chapter 2.5 on ‘Animal identification’, section 4
- sampling – all required sampling takes place and samples are appropriately identified and handled and sent to the appropriate laboratory for testing
- cleaning and disinfection (C and D) checks – team must ensure that C and D checks are carried out as per the frequency set for the plant
- records keeping and data input – a system must be in place to ensure that all records are kept and maintained and all data is correctly and timely inputted into relevant systems.
1.3.9 Hours of attendance by OV
Due to the reactive nature of the OV role, the times set out by the FVC in the flexibility assessment are only indicative. The OV must have the time available to respond to issues as they arise. Certain issues may take considerable time to address, for example, gathering evidence, enforcement, working with other enforcement agencies, food complaints.
1.3.10 Appeals
Appeals by the FBO against the decision of the FVC should be made through the Statement of Resources appeal system.
1.4 Specific requirements: red meat slaughterhouses
1.4.1 Flexible OV attendance at red meat slaughterhouses
Abnormal meat is product with pathological changes not routinely seen at PMI. Where the changes may indicate a notifiable disease, veterinary advice must be sought immediately. Arrangements must be in place for the OV to examine uncommon abnormal meat identified at PMI, including where slaughter does not take place on sequential days. More about abnormal common/uncommon meat can be found in chapter 2.4 on ‘PM, health and identification marking’.
1.4.2 OV attendance at post-mortem inspection in red meat premises
The activities and circumstances which require OV attendance at post-mortem inspection in red meat premises are:
- where facilities are insufficient to hold carcases and offal with uncommon conditions for OV inspection
- for post-mortem inspection of:
- animals that have undergone emergency slaughter
- animals that are suspected of having a disease or condition that may adversely affect human health or animal health
- cattle from herds not declared officially free of TB
- cattle, sheep and goats from herds that have not been declared officially free of brucellosis (BR)
- in the case of an outbreak of a notifiable animal disease to which the animals concerned are susceptible and which come from the affected region
- to confirm identity and verify correct disposal of carcases when a non-negative BSE test result is received
Reference: (EU) 2019/624, Article 8.
1.4.3 Red meat premises with cold inspection
The OV should make scheduled PMI visits to check on the accuracy of the PMI by the MHI at a frequency outlined in the instructions for PMI in chapter 2.4 on ‘Post-mortem, health and identification marking’.
1.5 Specific requirements game handling establishments
1.5.1 Flexible OV attendance at game handling establishments
Game Handling Establishments are eligible to be considered for flexible OV attendance during PMI. Refer to previous topic 1.2 on ‘Flexible attendance: General issues’ and the previous sub-topic 1.3.4 on ‘General requirements for implementation of flexible attendance’.
Where inspections are carried out by a MHI, the OV should visit the premises at least once every month. If the establishment is conditionally approved the OV will be required to visit the plant at least once every 5 operational days until full approval is granted.
1.5.2 OV attendance at post-mortem inspection in game handling establishments
The activities and circumstances which require OV attendance at PMI in game handling establishments are:
- where facilities are insufficient to hold all carcases and offal with uncommon abnormalities and OV inspection (only uncommon abnormal findings need be held for OV inspection not common PM findings)
- for PMI of animals suspected of having a disease or condition that may adversely affect human health or animal health
- if the hunter’s declaration makes reference to TB the OV must make a professional judgement and inform APHA using TB5.
- in the case of an outbreak of a notifiable animal disease to which the animals concerned are susceptible and which come from the affected region.
- in cases when the flexible attendance is no longer applicable.
1.6 Flexible attendance: Poultry establishments with MHI inspection
1.6.1 Flexible OV attendance at Poultry establishments with MHI PM inspection
Reference: (EU) 2017/625 Article 18(3)
Poultry slaughterhouses are eligible to be considered for flexible OV attendance during post-mortem inspection. Refer to previous topic on ‘Flexible attendance: General issues’ and the previous sub-topic 1.3.4 on ‘General requirements for implementation of flexible attendance’.
The MHI may discard abnormal poultry meat. Uncommon abnormal meat does not need to be systematically inspected by the OV; however:
- the OV must have time to complete their specific inspection duties to inspect the viscera and body cavities of a representative sample of birds each day (statistically a minimum of 300 birds per day)
- the OV must have time to undertake a detailed inspection of a random sample of rejected carcases / parts of carcases from each batch of birds
1.6.2 OV attendance at PMI in poultry slaughterhouses
The activities and circumstances which require OV attendance at PMI in poultry slaughterhouses are:
- for PMI of animals suspected of having a disease or condition that may adversely affect human health or animal health
- in the case of an outbreak of a notifiable animal disease to which the animals concerned are susceptible and which come from the affected region
1.6.3 Poultry premises with delayed evisceration
The OV / MHI / plant inspection assistant (PIA) shall inspect all carcases and viscera following delayed evisceration. Where PIAs are utilised the OV must attend at all times during the process.
Where the MHIs are carrying out PMI the FVC shall establish an OV site visit routine to verify operations dependant on throughput and general assessment.
The FBO must contact the service delivery manager / OV at least 24 hours in advance so that the FSA can arrange for adequate supervision levels.
1.7 Monitoring establishments with flexible attendance
1.7.1 Monitoring
FBOs in establishments with flexible attendance arrangement in place should have their performance regularly monitored. For that purpose the FSA will use as indicators the information gathered during the official control tasks (for example, audit outcomes and enforcement action records).
FBOs must be able to demonstrate that all public health, animal health and welfare risks are controlled and that the flexible attendance does not create any additional risks associated with their process.
As part of FSA monitoring, the FVC is required to carry out two types of assessments:
- Post implementation assessment – to be carried out four weeks after implementation of flexible attendance; following this assessment, the FVC must complete the form ‘Post implementation assessment’ at Annex 2 and upload it to the local SharePoint site.
- Triggered assessment – to be carried out by the FVC, if there is evidence that an establishment no longer fulfils the criteria to maintain a flexible attendance arrangement (based on audit outcome, compliance history or changes to operational procedures); the FVC should complete ‘Assessment for OV Flexible Attendance’ at Annex 1 and upload it to the local SharePoint site. K2 should be updated for central record purposes.
Monitoring and triggers for review are as follows:
- Audit outcome (Improvement Necessary / Urgent Improvement Necessary) – The FVC should monitor FSA audit data to assess performance of establishments. Plants that have previously been assessed as suitable for OV flexibilities falling into Improvement Necessary / Urgent Improvement Necessary FBO audit outcome categories must have OV flexibilities reviewed by the FVC. Where Urgent Improvement Necessary / Improvement Necessary audit score is a result of ongoing enforcement and open non-compliances, the OV flexibility should be removed. However, if the non-compliances are historic and closed, the local FSA team (FVC / FVL) should decide whether the OV flexibility should be removed / reduced or not.
- Compliance of FBO – Establishments with flexible attendance in place but where hygiene standards have dropped (formal notices) or critical welfare NCs have been found should have the flexibility assessment arrangement reviewed/removed. FVC and contract OV/AVM communications should be established locally to ensure prompt action in these cases.
- Changes to operational procedures – level of flexibility might need to be assessed if FBO or OV notifies the FVC of changes to operational procedures at the establishment (for example, changes in pattern, animal delivery, type of animals processed). FVC and contract OV/AVM communications should be established locally to ensure prompt action in these cases.
1.7.2 Outcome of FVC assessments
Following the completion of any assessment, the FVC should confirm to the ITL if the levels of attendance can be maintained (or further decreased).
In cases where the FVC finds sufficient evidence that criteria for flexible attendance are no longer met, they can recommend an increase in the level of attendance or remove the flexible attendance arrangement as detailed in the topic 1.8 on ‘Review / Removal of flexible attendance’.
1.7.3 Assessment of performance of official auxiliaries
The performance of OA deployed in establishments with a flexible attendance arrangement in place should be regularly assessed by the OV as follows:
- PMI verification checks which will allow OVs to monitor PM performance and accuracy of judgement
- OV should verify on a monthly basis that PM records are accurate and all procedures are followed. Records of that verification should be kept in the FSA Day Book
1.8 Review / removal of flexible attendance
1.8.1 Review
The flexible attendance arrangement is not permanent and can be reviewed / removed. If the FVC finds during monitoring sufficient evidence that requirements for flexible attendance are no longer met, the FVC can increase the level of attendance (including complete removal of flexible attendance arrangements). In those cases, the FVC should follow the process steps below.
Note: The OV hours will be reviewed at each Statement of Resources meeting.
1.8.2 Process steps
The table below details process steps that should be followed during the review of flexible attendance arrangement by the FVC:
| - | Process Steps |
|---|---|
| 1 | Outcome of FVC assessment indicates that the requirements for flexible attendance are not met. |
| 2 | FVC discusses the outcome of the visit and evidence gathered with a relevant FVL. |
| 3 | FVC informs the ITL / OM / HOD about the recommendation to increase attendance, in writing. |
| 4 | ITL notifies FBO about the recommendation and agrees SOR. |
| 5 |
FVC populates the OV flexibility information specific to the establishment on the K2 system when this feature is available. Whilst the K2 system is not available this could be recorded in the OV Flexible Attendance Post Implementation assessment document (Annex II) OV feedback comments. |
1.8.3 Appeals
Appeals by the FBO against the decision of the FVC should be made through the Statement of Resources appeal system.
1.9 FSA role
1.9.1 Field Veterinary Co-ordinator
FVCs shall assess premises for OV flexibility communicating findings formally via required documentation and liaise appropriately with plant OVs, FSA ITLs and contractors to implement changes to the operational requirements. FVCs are responsible for ensuring that team members know how to contact them by having local procedures / arrangements in place to deal with routine and non-routine issues in the OVs absence.
1.9.2 Field Veterinary Leader / Heads of Operational Delivery
FVLs will consider the decisions made at premises within the different clusters in their area for consistency of application.
1.9.3 Service Delivery Managers
The ITL must assess the risks to the delivery of official controls and ensure that non-veterinary staff are capable of fulfilling the supervisory role in the absence of the OV at individual premises. ITLs shall amend SORs following OV flexibility decisions. The business comments section of the SOR must capture the agreed flexibility applied.
1.9.4 Regulatory Delivery and Operational Transformation (RDOT) Division
Drawing on information recorded centrally, the RDOT Division at FSA will maintain an accurate record of premises where flexible arrangements are in place.
1.9.5 OV contract supplier role
OV contract suppliers will identify OV flexibility opportunities and liaise with the FSA FVC to implement opportunities ensuring the OV attendance is flexible to meet the needs at individual premises.
2. PIA system
In this section
2.4 Roles and responsibilities
2.5 Establishment permit assessment
2.6 Establishment monitoring assessment
2.7 Withdrawal of establishment permit
2.10 Assessment process for poultry establishments wishing to move to PIA system
2.11 Assessment process for poultry establishments already using PIAs
2.1 Introduction
This section outlines a standardised process to assess suitability of poultry slaughterhouses to use Poultry Inspection Assistants (PIAs) to carry out official control duties.
2.2 Legislation
Regulation 2017/625 Article 18(3) permits the use of slaughterhouse staff in establishments slaughtering poultry or lagomorphs to assist in the performance of tasks relating to official controls on the basis of a risk analysis and on condition that staff:
- act independently from the production staff of the slaughterhouse;
- have undergone appropriate training to carry out these tasks; and
- carry out these tasks in the presence and following the instructions of the official veterinarian or of the official auxiliary.
Slaughterhouse staff shall comply with the minimum training requirements set out in Chapter II of Annex II Regulation (EU) 2019/624 to the extent relevant for their assistance tasks.
2.3 Assessment arrangements
The FSA is responsible for carrying out a risk assessment on those premises wishing to implement a PIA system or to move from a MHI to a PIA system to confirm that they have a robust food safety management system in place. This is done through the “Establishment Permit Assessment” which is separate to the approval process.
The “Establishment Permit Assessment” will be carried out at each specific plant requesting the use of PIAs. There are three different scenarios detailed below:
Scenario 1:
1. For plants using MHIs moving to a PIA system, the risk analysis is based in past FBO performance, so provided there is evidence of a sustained and effective food safety management system based on HACCP principles in place for at least six months, a PIA system can be implemented after the assessment as per MOC instructions has been completed.
If an FBO has been compliant for the previous 6 months and has sufficient number of PIAs trained and ready to operate, the implementation of the PIA system can be done without any delay (see point 2.9).
Scenario 2:
2. For an approved establishment already using PIAs moving into new premises or changing ownership, the FSA can decide, on a case by case basis, if the timings to permit the introduction of the PIA system in the new establishment can be reduced. That can be done for instance by carrying out the assessment of the PIA system in the previous establishment, by temporarily supervising the PIA procedures in the new site with a small team of inspectors, or a mixture of measures appropriate to each case.
In this second scenario, the PIA implementation process can take just a few days, if the FBO had a history of compliance in the previous site and the PIA team is ready to move in.
Scenario 3:
3. For a newly approved establishment with no previous history of PIA usage, the risk analysis considers that the FBO shall provide evidence of a sustained and effective food safety management system based on HACCP principles which in this case will be assessed throughout the approval process, which in normal circumstances will last between 3 and 6 months. Once a full approval is granted, the FBO can implement a PIA system, provided it meets the MOC assessment criteria.
In this last scenario, the PIA implementation process can take between 3 and 6 months, which should allow the FBO Food Safety Management System to be fully implemented, and to train and develop the PIA pool.
Following the implementation of the PIA system, in order to ensure a consistent approach, assessments must also be completed regularly on the establishment’s suitability to continue with PIA systems. This is known as the “Establishment Monitoring Assessment”. This assessment should be based on the effectiveness of the implementation of the FBO food safety management systems, PIA performance and capability of the PIAs to address hygienic and process issues. This interim monitoring assessment will take place at least once between full audits and/or when the competent authority considers necessary.
2.4 Roles and responsibilities
2.4.1 Head of Field Operations / Operations Head Veterinarian
The Head of Field Operations is the owner of this process with the Operational Head Veterinarian having the ultimate responsibility for all technical aspects.
2.4.2 ITL
The decision making process will take place at a cluster / business area level. ITLs, with the ultimate support of their HOD, will manage operational implications and will determine timescales for introduction of any changes, in consultation with the FBO and FVC / FVL. Human resources colleagues will provide support on staffing issues.
2.4.3 FVC
FVCs will be required to carry out necessary technical assessments in their clusters, on behalf of the HOD.
The FVC will use information provided by the OV and local FSA Team on the day-to-day running of the business by the FBO when making their assessment. They should discuss any resource implications with the ITL.
2.4.4 FVL
Where further assurance or guidance is required (for example, where the FBO does not agree with the FVC decision), an FVL may provide additional technical advice.
The FVL may also carry out the establishment assessments or provide advice to the ITL / FVC / HOD on the best course of action if technical issues arise.
2.4.5 Approvals and registrations team
The Approvals and Registrations Team will be responsible for the administration of the establishment permit process. They will maintain copies of the permit visit reports and keep records of all assessed establishments centrally. Following a successful establishment assessment, a letter will be sent from the Approvals and Registrations Team to the FBO confirming the establishment’s PIA permit.
They will also be responsible for coordinating the withdrawal of the establishment’s PIA permit if required.
2.4.6 Central Support Unit in York (CSU)
CSU will be responsible for keeping records of the establishment monitoring assessment visits and linking these to the audit frequency. A monitoring visit will be carried out between full audits or at any other time if the FVL/FVC considers it is necessary.
In addition, CSU will be responsible for the administration of the PIA authorisation and withdrawal processes.
2.4.7 OV
The OV is responsible for PIA assessments and constant monitoring of their performance. With regards to practical arrangements for post-mortem inspection in plants with PIA systems, the OV (or the official auxiliary (OA), if applicable) shall personally carry out a daily inspection of the viscera and body cavities of a representative sample of birds of each flock.
- The number of birds included in the flock’s representative sample shall be decided by the OV (or the OA) following a risk assessment based on data obtained from the Food Chain Information, AMI of the flock, daily post-mortem inspection findings, and any other relevant data.
- Article 2(3)(b) of retained Regulation (EC) No 2160/2003 defines “flock” as all poultry of the same health status kept on the same premises or in the same enclosure and constituting a single epidemiological unit; in the case of housed poultry, this includes all birds sharing the same airspace.
- The number of birds checked per flock and the outcomes, should be recorded in the daybook.
Reference: Retained Regulation (EU) 2019/627, Article 25(1)(a)
Retained Regulation (EC) 2160/2003, Article 2(3)(b)
2.5 Establishment permit assessment
2.5.1 Notify ITL
An FBO should make a request to transfer to a PIA system to the ITL, who should inform the FVC / FVL at the earliest opportunity. The ITL will need to consider staffing implications and impact on existing FSA staffing at the premises.
2.5.2 FVC / FVL action
The FVC must visit the establishment and complete relevant parts of the ‘Assessment of PIA systems in poultry slaughterhouses’ PIA 4 form (Annex 10). A technical decision is required on whether the necessary systems are in place and PIAs are trained as required (see Chapter 10 Section 3 on PIA training). This assessment should be completed in accordance with deadlines established by the FVC (FVL) and ITL (in consultation with the FBO).
2.5.3 Suitable outcome
In this instance the ITL and FVC / FVL will discuss time-scales and operational management of the process with the FBO.
The FVC / FVL should email a copy of the completed PIA 4 form to the Approvals and Registrations Team. The team should update the central record of assessed establishments, send an authorisation letter to the FBO and notify CSU of the outcome.
2.5.4 Unsuitable outcome
The FVC / FVL should share their findings with the FBO and ITL and include the reasons behind their decision in writing. An action plan should be provided by the FVC / FVL of the areas that need improvement with a proposed timescale. The FVC / FVL should monitor progress towards addressing the necessary requirements. Once corrective actions are implemented the FVC / FVL must carry out a further assessment within the proposed timescales, or earlier upon the request of the FBO.
After the further assessment has been completed, the FVC / FVL should notify the FBO and ITL of the outcome and email a copy of the completed PIA 4 form to the Approvals and Registrations Team for information and filing. The Approvals and Registrations Team should update the central record of assessed establishments and send a copy of the report to the FBO.
2.5.5 Appealing the outcome of a refused establishment permit assessment
Where the FBO does not agree with the FVC / FVL decision, they may appeal to the Operations Head Veterinarian. The Operations Head Veterinarian is responsible for appointing an FVL / FVC from a different area as an Investigating Officer.
The Investigating Officer (IO) will have 14 days to gather the required evidence, conduct the investigation and submit a report with findings and conclusions to the Operations Head Veterinarian.
The Investigating Officer might consider visiting the premises before concluding the report.
Upon completion of the investigation the Operations Head Veterinarian will advise the FBO of the outcome of the appeal in writing.
2.6 Establishment monitoring assessment
2.6.1 Monitoring
All establishments permitted to use PIA system shall undergo a regular monitoring assessment to determine if the food safety management systems continue to be effectively managed by the FBO.
The frequency of the monitoring assessment will be risk based and correlated with the audit frequency of the establishment.
At least one plant assessment should be carried out by FVC / FVL between full FBO audits. The frequency of the monitoring assessments is based on the current audit system outcome; establishments with the lowest audit score should be assessed at least once every two months and the best performing plants once every 18 months.
Monitoring
| Audit outcome | Full audit frequency |
|---|---|
| Good | 18 months |
| Generally satisfactory | 12 months |
| Improvement necessary | 3 months |
| Urgent Improvement necessary | 2 months |
An additional establishment monitoring assessment can be triggered, regardless of the audit frequency, if serious concerns are raised by FSA field team regarding poor level of compliance (for example, sudden decline in hygiene standards, insufficient staffing levels, serious HACCP failures).
For establishments awarded poor audit scores (‘Improvement Necessary’ or ‘Urgent Improvement Necessary’) an assessment should be carried out as soon as possible from the date of the audit report being sent to FBO.
During the assessment the FVC / FVL should complete relevant parts of the establishment assessment PIA 4 form.
Where the FVC / FVL already has a good knowledge of the establishment, it may be possible to complete the monitoring assessment as a desk-based exercise, in consultation with the establishment OV. Establishments falling within the Improvement Necessary / Urgent Improvement Necessary categories should be visited.
2.6.2 Suitable outcome
The establishment is considered suitable to continue with its PIA system. In this instance, the FVC / FVL will complete the establishment assessment PIA 4 form and discuss their findings and decision with the FBO, also informing the ITL of the outcome. A copy of the PIA 4 form should be sent to CSU.
2.6.3 Minor deficiencies outcome
The establishment has minor deficiencies that must be addressed to allow the FBO to continue using PIA systems. The FVC / FVL should advise the FBO in writing on corrective actions that are considered necessary to ensure that the PIA inspection system can continue. The FVC / FVL should also provide a reasonable time-scale for the completion of such actions.
In conjunction with the establishment OV, the FVC / FVL will monitor progress to ensure that the identified deficiencies are addressed. The FVC / FVL should use their professional judgement to decide if a further establishment visit is necessary. A copy of the PIA 4 form should be sent to the CSU.
2.6.4 Major deficiencies outcome
The establishment has major deficiencies that must be corrected to allow the FBO to continue using PIA systems. Where there are major deficiencies – such as serious or multiple hygiene breaches, poorly implemented / maintained food safety management system, PIAs failing to perform their duties to the required standard and / or allowing unhygienic / unfit product to enter into the food chain - the FVC / FVL should discuss findings with the FBO and ITL.
A support MHI may be introduced onsite as an interim measure until the necessary deficiencies are addressed. This will need to be within a short timescale, depending on the nature of the risks.
The FVC / FVL should provide the FBO with a written summary of identified deficiencies and a clear timeframe to rectify them.
In conjunction with the slaughterhouse OV, the FVC / FVL should monitor the establishment to ensure that the identified deficiencies are addressed.
The FVC / FVL should carry out an additional monitoring assessment within an agreed timeframe. In this assessment, the FVC / FVL must consider whether:
- the FBO has remedied the deficiencies;
- an extension to the MHI support role is needed; or
- a full reversion to a MHI system is necessary.
The ITL will need to consider operational implications and should liaise with their HOD and FVC / FVL as appropriate. A copy of the PIA 4 form should be emailed to the CSU.
Note: Reverting back to a MHI system should only happen as a last resort, where it is clear that arrangements are unsatisfactory and that the FBO is not taking appropriate responsibility to implement corrective actions and ensure that public health is safeguarded.
2.7 Withdrawal of establishment PIA permit
Where very serious deficiencies are identified during the routine monitoring assessment visit the FSA local management might consider increasing the level of official controls in the premises and deployment of additional FSA staff.
- FVC / FVL communicates to the FBO the deficiencies identified during the monitoring assessment and provides a timeframe for rectification. All identified issues and non-compliances have to be communicated to the local FSA management (ITL, HOD) at the same time.
- FVC / FVL is required to reassess the establishment within the agreed timeframe to evaluate improvement.
- FVC / FVL must communicate the outcome of the second assessment to the FBO and confirm the suitability of the PIA system or recommend to the Operations Head Veterinarian a withdrawal of establishment PIA authorisation if the observed improvement was not satisfactory.
- Findings of the FVC / FVL reassessment and recommendation made by FVC / FVL must be discussed within the local FSA team. Sufficient evidence supporting the recommendation should be presented to the Operations Head Veterinarian.
- Operations Head Veterinarian assesses the presented evidence and advises the FBO and the local FSA team in writing of his decision.
2.8 Hybrid PIA / FSA systems
Hybrid PIA / FSA systems may be acceptable under exceptional circumstances, for example, in larger industrial slaughterhouses where MHIs carry out online inspection duties at certain inspection points, and others are manned by PIAs. Typically, though, an FBO would be expected to have an OV only; an OV plus MHI team or OV plus PIA(s) model in place.
As described above, use of support MHIs may also be accepted as an interim measure at slaughterhouses using PIAs where it is judged that premises have major measurable deficiencies which must be corrected to allow the FBO to continue using PIA systems.
2.9 TUPE considerations
The Transfer of Undertakings (Protection of Employment) (TUPE) 2006 Regulations preserve employees' terms and conditions when a business or undertaking, or part of one, is transferred to a new employer. The FSA has received legal advice that the transfer from FSA MHI to PIA systems (or reverse) could be challenged under the TUPE 2006 Regulations.
ITLs must be aware of possible implications of TUPE when discussing staffing options with FBOs of poultry slaughterhouses and should consult with Human Resources colleagues in this event. Opportunities for redeployment within the FSA will still need to be considered, in the normal way.
It remains a commercial decision for the FBO in determining whether to move to a PIA system. FBOs wishing to implement a PIA system must seek their own legal advice on the impact of TUPE.
2.10 Assessment process for poultry establishments wishing to move to PIA system
The table below summarises the steps that need to be taken when assessing establishments that wish to move to the PIA system – as detailed in the chapter.

2.11 Assessment process for poultry establishments already using PIAs
The table below summarises the steps that need to be taken when assessing establishments already using the PIA system – as detailed in the chapter.

3. Inspection in co-located cutting plants
3.1 Introduction
3.2 OV inspection in co-located cutting plants
3.1 Introduction
3.1.1 Objective
This document sets outs the FSA Operational Policy for the inspection of co-located cutting plants (Co-CPs).
3.1.2 Legislation
Articles 18 (1) and (2) (d) of the retained Official Controls Regulation (EU) 2017/625 (OCR) lay down the requirements on official controls and the action to be taken by the competent authority (CA) in relation to the production of products of animal origin intended for human consumption, among others, in cutting plants.
3.2 OV inspection in co-located cutting plants
3.2.1 Cutting plants co-located with slaughterhouses and/or Game Handling Establishments (GHEs)
Many slaughterhouses and/or GHEs have a co-located cutting plant. Approved cutting plants co-located to a slaughterhouse and/or an GHE are also subject to official controls.
Retained Regulation (EU) 2019/627 sets down specific requirements for auditing activities performed by the Competent Authority (CA). The audits at FSA meat approved establishments are performed by the Veterinary Auditors or Audit Veterinary Leaders. How the FSA carries out audits in meat approved establishments can be found in Chapter 4.1 (Audit).
In order to verify food business operator's compliance, as per Article 14 of the OCR, audits have to be supplemented with regular inspections. In co-located cutting plants, these inspections are usually carried out by the OV or by Official Auxiliaries (OAs), working under the supervision of the OV. The frequency of these audits and inspections are determined on a risk-based approach.
In stand-alone cutting plants, the FSA has a programme of Unannounced Inspections (UAI) linked to the audit outcome defining the frequency of these UAI. The procedures for UAIs are detailed in Chapter 4.7
3.2.2 OV attendance
The level of OV attendance and frequency of the audits and inspections are outlined in Chapter 4.1 (Audit).
In particular, Chapter 4.1, Sections 1.1.1 (OV presence) and 1.3 (Relationship between audit visits and OV attendance) cover the attendance by OVs and/or OAs at cutting plants depending on whether they are co-located with a slaughterhouse or GHE or are stand-alone cutting plants. In both cases, cutting plants need to be inspected between audits.
3.2.2.1 OV attendance at co-located CP (Co-CP)
At cutting plants co-located with slaughterhouses and/or GHEs, the OV is to determine the frequency at which to carry out inspections of the co-located establishment, so that compliance in all approved activities is verified.
The frequency of inspections is risk based and may vary depending on several factors, for example, approved activities, weekly throughput, export activities… To determine the frequency of those inspections, the OV should use the Co-CP risk-based decision tool (Annex 5).
This tool will help the OV determine if the inspections of the Co-CP should take place weekly, monthly or bi-monthly. Those inspections need to be recorded using the Co-CP inspection report. The duration of those visits will depend on the size and volume of the operation. The time spent undertaking those inspections and completing the report needs to be recorded in the timesheet against the appropriate code for the co-located establishment.
In addition to this, OVs are also expected to walk around the co-located establishment regularly, but there is no need to record this time against the Co-CP or to produce a full report of these extra visits.
OVs should use the decision tool quarterly or, in sites where the Co-CPs operates less than 3 days per week, annually, and record the outcome in the plant daybook.
If there are any operational changes in the Co-CP or any concerns, the OV can amend the level of checks in consultation with the FVC accordingly. Any changes need to be reviewed regularly.
Co-CPs may need to be visited more regularly than the frequency obtained using the risk-based decision tool, for activities including SRM removal supervision, verification of export requirements; For example, where the OV is required to provide support health attestations (SHA) to facilitate exports from the co-located establishment, the number of inspections needs to allow for the OV to be able to verify compliance with the requirements for which veterinary certification is being provided. These visits can be used to support the formal inspection of the Co-CP, however there would still be a need to complete the formal inspection/report in addition to those. If the number of visits is more than the number required by the risk tool, there is no need to produce a report every time. Any deficiency however needs to be brought to the attention of the FBO and recorded in the daybook and Chronos as per the MOC instructions.
3.2.2.2. OV attendance on co-located cutting plants operating outside the normal operating hours.
At Co-CPs where the operations take place outside the normal operating hours of the slaughterhouse and the OV is not in attendance, the unannounced inspections (UAIs) are arranged centrally by the FSA and are carried out by other OVs or by OAs.
In establishments approved for ready to eat products (RTE), the inspections can only be undertaken by authorised and RTE trained OVs or authorised and RTE trained OAs. An increased frequency of visits is expected in RTE establishments reflecting the higher risk of the products being produced.
3.2.3 Record of the inspections
OVs and/or OAs need to record the findings of the inspections. The reports to be used are available via the K2 system.
In the case of Co-CPs, findings of the inspections need to be included in the daybook as well as in the specific report. Guidance on completion and storage of the co-located CP inspection report is available in Annex 6.
4. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: OV Flexible Attendance – Assessment
Annex 2: OV Flexible Attendance – Post implementation assessment
Annex 3: PIA – Assessment of PIA systems in poultry slaughterhouses (PIA 4)
Annex 4: Cold Inspection Guidance
Annex 5: Risk based decision tool for inspections to co-located cutting plants
Annex 6: Guidance for the completion of the co-located cutting plant inspection report
Sections
1. Introduction
In this section
1.1 Background
(EC) No 853/2004 requires meat to attain a specific core temperature (7ºC for carcase meat and 3°C for offal), arrived at by a continuous decrease in temperature following the slaughter and dressing process in slaughterhouses. This temperature must be reached before the meat leaves the slaughterhouse and must also be maintained during storage and transport.
(EC) No 853/2004 also requires that during cutting, boning, et cetera at cutting plants meat is maintained at or below the temperatures above (with some flexibility in cutting plants co-located with a slaughterhouse).
The Regulation provided a derogation for meat to be transported before it has attained that temperature if meat is intended for the production of specific products and if the competent authority (CA) authorises it. The Regulations also state the conditions that must be met for such authorisation to be provided. This derogation has been applied in the UK.
(EU) No 2017/1981 came into force on 21 November 2017 and introduced changes to the derogation in three key aspects:
- firstly, the derogation for the transport of meat intended to be used for the production of specific products can only be used if such transport is justified for technological reasons
- secondly, it introduced more flexible approaches to the temperature conditions during transport of carcases or part carcases (half carcases, quarters, or half carcases cut into three wholesale cuts)
- finally, carcases or part carcases which have been transported taking advantage of the derogation immediately above may be boned and cut prior to reaching a core temperature of 7°C at the establishment of destination, provided the air temperature ensures a continuous decrease of the temperature of the meat and it is chilled subsequently until it reaches 7°C, if not already below
The use of the revised derogations in bullet point 2 above are dependent authorisation by the CA. The derogations specify requirements for the meat core and surface temperatures, maximum times for reaching required surface temperatures, transport air temperatures, and are subject to FBOs demonstrating acceptable levels of carcase surface microbiological contamination. These all vary depending on the intended duration of the journey to the establishment where the meat is to be chilled to a core temperature of 7°, and the species of meat.
The temperature conditions during the transport of fresh meat must not deviate from the principle that the meat should reach 7°C by a continuous decrease of temperature before being placed on the market.
1.2 Legal requirements
1.2.1 Legislation
(EC) No 853/2004, Annex III, Section I, Chapter VII, 3. Amended by:
(EU) No 2017/1981, Article 1
Meat must attain a core temperature of 7°C (carcase) or 3°C (offal) or less before transport and remain at that temperature during transport. However, slaughterhouse FBOs may be authorised for:
a) the transport of meat at temperatures higher than the above for the production of specific products for which such transport is justified for technological reasons, or
b) the transport of carcases, half carcases, quarters or half carcases cut into no more than three wholesale cuts that have been partially chilled but have not yet reached a core temperature of 7°C, subject to certain conditions being met.
In addition, FBOs at businesses receiving the carcases or part carcases in point b above may bone and cut those prior to reaching a core temperature of 7°C if meat is subjected to temperatures that ensure a continuous decrease of the temperature of the meat and the meat is chilled to 7°C as soon as it is cut.
Updated [Transport of over temperature meat to the EU and Northern Ireland
Delegated Regulation (EU) 2024/1141 came into force on 9 May 2024 which amended Regulation (EC) 853/2004. This included a number of changes to Annex III, Section I, Chapter VII (3) in particular laying down requirements for recording and monitoring surface temperatures of meat.
Food business operators should be aware that they need to comply with these requirements if they wish to despatch over temperature meat to the EU and to Northern Ireland].
1.2.2 Authorisation for the transport of meat intended for production of specific products (‘warm’ meat)
Transport of meat that has not been chilled to a core temperature of 7°C (carcase) or 3°C (offal) may be authorised to produce specific products provided that:
- the transport of that meat from one establishment to another takes place in accordance with the requirements specified by the CAs of both origin and destination
- the meat leaves the slaughterhouse, or a cutting room on the same site as the slaughterhouse, immediately (interpreted as up to 3 hours from the completion of the post-mortem inspection of the first animal slaughtered to be transported warm to the departure of the vehicle)
- transport takes no more than two hours
- the need for meat to be transported before it is chilled is justified for technological reasons
Note: ‘specific products’ can be any product for which the CA grants an authorisation and specifies the requirements to be respected. However, this derogation is only allowed when the chilling may not contribute to the technically most appropriate processing of the product and where it is better the product is not chilled before starting or carrying out transport. It may be used for the transport of meat, whether carcase or offal.
FBOs wishing to transport over temperature meat to produce specific products must justify the technological reasons for which the product must remain above the temperature described in Annex III, Section I chapter VII 1(a) of 853/2004 as amended prior to obtaining authorisation.
1.2.3 Authorisation for the transport of partially chilled carcases, half carcases, quarters or half carcases cut into three wholesale cuts
The transport of partially chilled carcases or part carcases of bovine, ovine, caprine and porcine animals may start before a core temperature of 7°C is attained providing that:
- the slaughterhouse FBO has received written authorisation from the CA (the FSA) to dispatch partially refrigerated carcases or part carcases
- the slaughterhouse FBO food safety management system based on the HACCP principles caters for the transport of partially chilled carcases or part carcases and temperature is monitored and recorded
- the vehicle transporting the partially chilled carcases or part carcases is fitted with an instrument that appropriately monitors and records the air temperatures
- the transport vehicle must only collect the partially chilled carcases or part carcases from one slaughterhouse (but multiple drops are permitted)
- if the transport vehicle has both carcases chilled to a core temperature of 7°C or below and partially chilled carcases or part carcases, then the partially chilled carcases or part carcases must have a maximum core temperature of 15°C
- the slaughterhouse FBO must provide a declaration that accompanies the consignment stating:
- the duration of the chilling before loading
- the time at which the loading of the carcases or part carcases started
- the surface temperature of the meat at the time loading started
- the maximum air temperature at which the carcases or part carcases may be subjected during transport
- the maximum transport time permitted
- the date of authorisation to use the derogation and the name of the CA (for example the FSA)
- the FBO of destination has notified the CA (which in the UK may be the FSA or the local authority (LA) before receiving for the first time partially chilled carcases or part carcases, and
- the slaughterhouse FBO can demonstrate compliance with the temperature and microbiological requirements that apply to the specific duration(s) of transport for which they have been authorised. These are outlined in the table below:
For a maximum transport time of 6 hours:
| Species | Surface temperature (2) | Maximum time to chill to surface temperature (3) | Maximum transportation air temperature (4) | Maximum daily mean carcase aerobic colony count (5) |
|---|---|---|---|---|
| Ovine and caprine | 7°C | 8 hours | 6°C | log10 3.5 cfu/cm² (2.8) |
| Bovine | 7°C | 20 hours | 6°C | log10 3.5 cfu/cm² (2.8) |
| Porcine | 7°C | 16 hours | 6°C | log10 4 cfu/cm² (3.3) |
For a maximum transport time (1) of 30 hours:
| Species | Surface temperature (2) | Maximum time to chill to surface temperature (3) | Core temperature (6) | Maximum transportation air temperature (4) | Maximum daily mean carcase aerobic colony count (5) |
|---|---|---|---|---|---|
| Porcine | 7°C | 16 hours | 15°C | 6°C | log10 4 cfu/cm2 (3.3) |
For a maximum transport time of 60 hours:
| Species | Surface temperature (2) | Maximum time to chill to surface temperature (3) | Core temperature (6) | Maximum transportation air temperature (4) | Maximum daily mean carcase aerobic colony count (5) |
|---|---|---|---|---|---|
| Ovine and caprine | 4°C | 12 hours | 15°C | 3°C | log10 3 cfu/cm² (2.3) |
| Bovine | 4°C | 24 hours | 15°C | 3°C | log10 3 cfu/cm² (2.3) |
(1) The maximum transport time is the maximum time allowed from the start of loading the meat until the moment it reaches its final destination. In the event of any delays, this maximum transport time must be shortened by the duration of the delay, ie a delay of 2 hours in loading for a 6-hour journey will mean that the maximum transport time will be 4 hours.
(2) Maximum surface temperature at loading.
(3) The surface temperature is accompanied by a maximum time to chill which is the maximum time allowed from the moment of killing until the product reaches the maximum surface temperature.
(4) The maximum transportation air temperature varies depending on the length of transport and the species and is the maximum temperature to which the meat should be subjected at loading and for the duration of the transport.
Multiple drop-offs are allowed but all must be completed within the maximum time of transport.
(5) Maximum daily mean carcase aerobic colony count (ACC), using a rolling window of 10 weeks. This is the sampling required under (EC) No 2073/2005. In the UK weekly sampling is required collecting samples from five carcases and changing the day of sampling each week so that each day of the week is covered (where this is feasible, as not all slaughterhouses process all species every day).
Note: the daily mean carcase ACC is to be calculated by first taking a log value of each individual test result and then calculating the mean of these log values.
In a rolling window approach a sufficient number of sampling units (n) is collected for a defined period of time (the ‘window’). The results of the latest ‘n’ sample units are compared with the microbiological limits (m, M) using the acceptance number ‘c’. As a new result from the sampling period is available, it is added to the window while the oldest result is removed.
For the purposes of Regulation (EU) 2017/1981 read in conjunction with Regulation (EC) No 2073/2005:
- m = log10 3.5 cfu/cm² or log10 4 cfu/cm²2 or log10 3 cfu/cm²2 (adapted as required to the non-destructive method)
- M = m
- n = 10 (taken as the mean of 5 samples in a day, for 10 weeks)
- c = 0 (the daily mean ACC must be at or below ‘m’)
The figures in the legislation refer to the results using the excision method. Those between brackets denote the equivalent maximum daily mean when using the swabbing method for sampling
(6) Core temperature: this measurement is applicable for transport times of 30 and 60 hours. This temperature is to be taken at the time of loading and thereafter at the thickest part of the carcase.
1.2.4 Cutting and boning of meat transported under the derogation for partially chilled carcases or part carcases
Carcases, half carcases, quarters, or half carcases cut into no more than three wholesale cuts may be boned and cut prior to reaching a core temperature of 7°C when they have been transported under the derogation set out in point 1.2.3 above.
In this case, throughout cutting or boning, the meat must be subjected to air temperatures that ensure a continuous decrease of the temperature of the meat. As soon as it is cut and, where appropriate, packaged, the meat must be chilled to at least 7°C if it is not already below this temperature.
Meat must remain in the establishment of destination until the required core temperatures has been reached. In the UK, this is 7°C at approved establishments under EU legislation, and 8°C at any other establishment as required by UK domestic legislation.
2. Authorisation of premises
In this section
2.1 Transport of over temperature meat intended for the production of specific products
2.1 Transport of over temperature meat intended for the production of specific products
2.1.1 Authorisation of slaughterhouses
Slaughterhouse FBOs wishing to make use of the derogation must complete the application form ‘Application for the transport of meat intended for the production of specific products’ (annex 1) which will be reviewed by the OV. Where the OV is not satisfied the conditions can be met then the OV will inform the FBO that the authorisation cannot be recommended and the reason for this.
Providing the OV is satisfied that the necessary requirements can be complied with the OV will complete section 2 of the application and submit this to the Approvals and Registrations Team. It will then be reviewed centrally by the FSA, in particular to consider the technological reasons that apply, before an authorisation can be granted.
The application will need to record:
- the names and addresses of the establishments which will receive the meat and the estimated duration of transport to the destination (considering whether they will usually be sent directly or be part of a multiple drop delivery)
- where applicable, the name and address of any FBO acting as intermediary between the slaughterhouse and the establishment of destination, especially where the slaughterhouse operator does not know where intermediaries (dealers) are transporting the meat and this is deemed to be commercially sensitive information
- the species of animals and the type of meat intended for the production of specific products to be transported to each receiving establishment
- a general description of the technological reasons for which the meat must be transported over temperature
The authorisation documents will be issued by the Approvals and Registrations Team. All authorisations, where granted, will be limited to the types of product and destinations in the application documents(s).
2.1.2 Establishments of destination
Conditions may be imposed in certain circumstances on the establishments of destination. For example, the CA for the establishment of destination may impose conditions upon the processing of the specific product.
In some slaughterhouses the FBO may not know the establishment of destination of the meat, for example when this is purchased by an intermediary who will distribute the meat to other customers. As the list of customers supplied by the intermediary may be commercially sensitive information, then the slaughterhouse operator may be allowed to supply over temperature meat to intermediaries on condition that the latter has provided the OV with a list of their customers with their name and address, their CA and the estimated duration of transport. Updated [In these cases, the OV must include these details as part of the application for authorisation]. This information will be held by the FSA and will not be shared with the slaughterhouse FBO.
If an intermediary (or any other customer) who intends to collect meat intended for the production of specific products does not provide the information required above, then the slaughterhouse FBO will not be authorised to supply meat that has not reached the required core temperature to that customer. This customer may however be supplied with meat that has been chilled to the required core temperature (7°C for carcases and 3°C for offal) at the slaughterhouse.
Authorisations for the transport of meat for the production of specific products consist of two documents:
- The actual authorisation, with an indication of the maximum transport times that apply
- An annex listing the destinations to which deliveries can be made, as detailed in the application document
An authorisation may be issued that does not include one or several of the destinations in the application document. This will apply when the CA of the establishment of destination has advised the latter cannot comply with the requirements to bring the temperature down to the required level.
All establishments are required to keep adequate traceability records and make these available to the FSA, on request, without undue delay. Records should be kept for a minimum of three months.
2.2 Transport of partially chilled carcases, half carcases, quarters and half carcases cut into no more than three wholesale cuts
2.2.1 Authorisation of slaughterhouses
The slaughterhouse FBO must complete the application form ‘Application for the transport of partially chilled carcases, half carcases, quarters and half carcases cut into no more than three wholesale cuts’ (annex 2) which will be reviewed by the OV.
The application will need to record:
- the maximum duration of travel for which the authorisation is sought
- the species of animals
Note: this derogation only applies to red meat carcases or part carcases. Offal must be chilled to a core temperature of 3°C unless intended for the production of specific products as per sub-section 2.1 above.
In order to be authorised slaughterhouse operators will need to demonstrate that they are able to comply with the required conditions as listed in sub-topic 1.2.3 on Authorisation for the transport of partially chilled carcases, half carcases, quarters or half carcases cut into three wholesale cuts. This includes verification that third-party transport vehicles are equipped and fitted with an instrument that appropriately monitors and records air temperatures to which the carcases and part carcases will be subjected and the receiving FBO has provided notification to the competent authority (this may be the FSA or the LA).
Where the OV is satisfied that the conditions for the transport of partially chilled carcases or part carcases can be met then the OV will make a recommendation for the authorisation to be granted.
The completed applications will be submitted by the OV to the Approvals and Registrations Team. The authorisation documents will be issued by this team.
All authorisations, where granted, will be limited to the types of carcases or part carcases and maximum transport times applied for.
2.2.2 Establishments of destination
Conditions may be imposed in certain circumstances on the establishments of destination. For example, where product is to be transported to another EU country their CA may specify the conditions under which establishments may receive this.
The businesses receiving partially chilled carcases or part carcases from authorised slaughterhouses must inform their CA before they receive these for the first time. It is recommended that these notifications are made in writing via email.
Since the transport to these destinations for the first time may only commence after the notification has been made, the FBO at the authorised slaughterhouse must have a documented procedure to verify that this notification has been issued. The slaughterhouse FBO should keep records of how this has been done. It is recommended that business operators at establishments of destination copy the authorised slaughterhouse in their notification to the relevant CA when they do this via email.
Note: the authorisation may be issued before the initial dispatch of partially chilled carcases or part carcases to the receiving establishment has started. It is not necessary, for authorisation purposes, for the slaughterhouse FBO to demonstrate that the receiving establishment has notified the CA of the arrival of the first consignment of partially chilled carcases of part carcases.
However, this notification must be made before the first delivery of partially chilled carcases or part carcases. Failure to verify that this notification has been made before that first delivery may affect the ability of the slaughterhouse to continue supplying over temperature meat.
Contact details for LAs can be found online.
Where the establishment of destination is FSA approved (for example: another slaughterhouse or a cutting plant) then the FBO of the destination establishment must contact the Approvals and Registrations Team via email.
In some slaughterhouses the FBO may not know the establishment of destination of the meat, for example when this is purchased by an intermediary who will distribute the meat to other customers. In that case slaughterhouse operators must ensure that the intermediaries are aware that each of their customers must notify the relevant CA before the first delivery takes place, and that this has been confirmed. This should be recorded in the slaughterhouse operator’s record referred to above.
Updated [Intermediaries must provide the OV with a list of their customers including their name and address, their CA and the estimated duration of transport. In these cases, the OV must include these details as part of the application for authorisation. This information will be held by the FSA and will not be shared with the slaughterhouse FBO].
If an intermediary (or any other customer) who intends to collect over temperature meat does not provide the information required above, then the slaughterhouse FBO must not supply partially chilled carcases or part carcases to that customer since it cannot be confirmed that the required notification has been made by the FBO at the establishment of destination. This customer may however be supplied with meat that has been chilled to the required core temperature (7°C for carcases and 3°C for offal) at the slaughterhouse.
Intermediaries collecting meat from authorised slaughterhouses who do not disclose the name and address of their customers to the slaughterhouse food business operator and/or the FSA must accept responsibility for ensuring that their customers have notified the CA before the first consignment of partially chilled carcases or part carcases are received. They should also keep records to demonstrate how this has been done.
The authorisations for the transport of partially chilled carcases or part carcases will be a single document with no reference to the destinations to which meat can be transported but limiting the maximum transport time. However, slaughterhouse FBOs should be able to demonstrate that they have carried out checks to verify whether the establishment(s) of destination can be reached within the maximum transport time permitted.
In order to allow verification that partially chilled carcases or part carcases can be delivered to the establishments of destination within the transport time applied for, slaughterhouse operators must have a documented procedure and records in place to verify that meat can be delivered to each of the customers receiving this meat within the maximum transport time that applies (for example 6, 30 or 60 hours), also considering where there are several drops in the same consignment.
The OV must verify that slaughterhouse operators keep a list with the names and addresses of the establishments which will receive the partially chilled carcases or part carcases and the estimated duration of transport to these. This list needs to be updated when new customers are added and will need to be reviewed regularly to ensure it is kept up to date.
Alternative systems may also be acceptable if they have the same effect, and their suitability will be assessed on a case by case basis.
A copy of these lists will be used by FSA to issue notifications to other CAs for the establishments of destination (see sub-section 2.3 below).
All establishments are required to keep adequate traceability records and make these available to the FSA, on request, without undue delay. Records should be kept for a minimum of three months.
2.3 Authorisation procedure
Slaughterhouse FBOs seeking an authorisation to transport over temperature meat, whether warm (to produce specific products) or partially chilled, should contact the OV to discuss their proposal.
Copies of application documents are available as annexes at the end of this chapter. Annex 1 applies to the transport of meat for specific products and annex 2 to the transport of partially chilled carcases or part carcases.
Once the FBO has completed section 1 of the application form(s) and the OV is satisfied that the information is correct and the FBO can in principle comply with the conditions of authorisation, the OV will complete section 2 of the application document.
The OV will then send a copy of the application and supporting documents to the Approvals and Registrations Team preferably via email.
The Approvals and Registrations Team will notify the CA for the establishments of destination that the establishment will be receiving over temperature meat for the production of specific products and/or partially chilled meat.
The notification part of the process is:
a) to inform the relevant authority of the intention by a business to receive meat that has not been chilled, or fully chilled, so that they can verify the ability of the receiving establishment to handle over temperature meat in compliance with the requirements of Regulation (EC) No 852/2004 and/or (EC) No 853/2004 as amended.
b) for meat intended for the production of specific products, agree the conditions under which transport of over temperature meat will be authorised.
c) for partially chilled carcases or part carcases, to make the CA aware that they should receive or have received (depending on when the FSA issued the notification) a notification from the establishment(s) of destination advising of the delivery of the first consignment of over temperature meat.
Where the establishment of destination is FSA approved, the FBO should contact the Approvals and Registrations Team. The Approvals and Registrations Team will then notify the FVL / FVC of the cluster where the receiving plant is.
Authorisations for the transport of meat for the production of specific products will be limited to the establishments listed in the application document, and the authorisation will only be issued once confirmation has been received from the CA of the establishment of destination that there is no impediment for over temperature meat to be received at that establishment. The establishments eligible for receiving this meat will be listed in an annex to the authorisation.
The Approvals and Registrations Team will keep a central record of all establishments authorised to transport over temperature meat. Authorisations will be issued centrally by the Approvals and Registrations Team on receipt of correctly completed applications and any supporting documents.
The OV should ensure that the FBO can comply with the requirements in order make use of the derogation. This includes (the following is not an exhaustive list):
2.3.1 Over temperature meat intended to produce specific products
- the transport must start immediately (no more than a 3-hour period from the completion of the post-mortem inspection of the first animal slaughtered to the departure of the vehicle) and must not take more than 2 hours
2.3.2 Partially chilled meat (carcases or part carcases)
- the FBO must demonstrate the ability to monitor and record meat temperatures. As surface temperature is one of the parameters specified, the FBO must have calibrated thermometers capable of measuring both surface and, where applicable (for example more than 6 hours of transport time) core temperatures, both measured at the thickest part of the carcase
- the slaughterhouse FBO must have a system in place to verify that vehicles to be used for the transport of the carcases or part carcases are fitted with an instrument that appropriately monitors and records air temperatures during transport. Where the vehicles are not under the direct control of the slaughterhouse operator (for example when customers collect the meat in their own vehicles) the slaughterhouse operator must verify that lorries are adequately equipped before loading of carcases or part carcases starts
- the slaughterhouse FBO must have a system in place to provide a declaration to accompany each consignment that has the required information (for example, duration of chilling before loading, time at which the loading started, surface temperature at start of loading, the maximum temperature at which the meat may be subjected during transport)
- the slaughterhouse FBO has adequate facilities that can achieve the surface temperatures (7 or 4 °C) that apply to the expected transport time within the specified time and, where required, the core temperatures
Note: the surface temperature refers to the external layer of the carcase up to approximately 5mm.
- the slaughterhouse FBO is conducting weekly (or fortnightly, see bullet point below) tests for carcase ACC as required by Regulation (EC) No 2073/2005, and the results of these were satisfactory in a 10-week rolling window
- Regulation (EC) No 2073/2005 allows that testing frequency may be reduced to fortnightly if satisfactory test results are obtained for six consecutive weeks. In the event of an unsatisfactory result, the weekly sampling should re-start and be maintained until there are another six weeks of satisfactory results
- the FSA will collate the results of the sampling to carry out a risk assessment of the sampling frequency at small businesses
- Note 1: while Regulation (EC) No 2073/2005 requires satisfactory test results each week during the rolling 10-week window, the FSA will allow FBOs to continue supplying partially chilled meat if:
- there are no more than two non-satisfactory test results during the 10-week window, and
- the average of the weekly test results in the rolling window does not exceed the value of maximum daily mean carcase ACC as defined per category of time temperature combination, and
- actions have been taken to investigate the cause of the unsatisfactory results and corrective action taken, in line with HACCP principles
- Note 2: establishments that because of their throughput are currently exempt from testing or do testing at frequencies lower than those above may be authorised in some cases.
Where establishments of destination are outside the UK the FSA must be notified. The FSA will liaise with the CA of the country of destination to agree the conditions under which this transport will be permitted.
The customer base may change over time. Establishments authorised for the transport of over temperature meat for the production of specific products who require a new destination needs to be added will need to make a separate application for that particular destination. Likewise, some destinations may need to be removed from the authorisation (for example, when the CA for the premises of destination requests this due to inability to ensure appropriate chilling of meat).
The changes required should be requested by the FBO and communicated by the OV to the Approvals and Registrations Team so that an amendment can be made on the annex that accompanies the authorisation and that lists the establishments to which transport of over temperature meat has been authorised.
Establishments authorised for the transport of partially chilled carcases or part carcases will need to ensure that the list of customers to whom they supply this meat is reviewed and updated as necessary. Before any new customer is added the FBO must verify that delivery can be made within the maximum transport time authorised.
Authorisations should be the subject of regular reviews to ensure they remain up to date.
A copy of the application form will be sent by the OV to the Approvals and Registrations Team and may be done via email.
3. Enforcement
In this section
3.2 Amendments, suspensions and revocations of authorisations
3.1 Failure to comply
Where an FBO fails to comply with the conditions of the authorisation, the OV should follow the normal hierarchy of enforcement. This may include, when required, the service of notices, both Hygiene Improvement Notices (HINs) and Remedial Action Notices (RANs).
Consideration must be made of what the issue is and what the best enforcement route would be. Where there are several breaches, enforcement for each may need to be escalated at a different pace. For example: where a FBO is not taking samples for microbiological testing or is not taking effective corrective actions it may be appropriate to follow the hierarchy of enforcement and escalate to serving HINs, while the fact that there are no adequate microbiological test results resulting from the above demonstrating the dressing procedures are hygienic may prevent the dispatch of partially chilled carcases and require serving a RAN to stop it.
3.2 Amendments, suspensions and revocations of authorisations
Once an authorisation to transport over temperature meat has been granted it may be amended, suspended or revoked if the FSA is satisfied the conditions under which it was granted are no longer being met.
3.2.1 Amendments
Amendments may be requested for changes such as different species or additional/alternative maximum travel times, for meat intended for the production of specific products or, for changes to the establishments of destination. Request for amendments should be made in writing to the Approvals and Registrations Team and confirmed in a revised authorisation.
3.2.2 Suspension
Where the FBOs controls have not been sufficiently robust at the point of despatch, the FSA may suspend the authorisation for the transport of over temperature meat.
Updated [The OV should make a recommendation for suspension to the Approvals Team using the template letter (Annex 3). The recommendation should include sufficient supporting evidence to enable a decision to be made by the Field Veterinary Leader (FVL). The Approvals Team will notify the food business operator of the FVL decision in writing].
The suspension will remain in place until the FBO provides adequate guarantees that the transport of over temperature meat can resume in line with the legislation.
3.2.3 Revocation
Where the conditions for the transport of over temperature meat are seriously breached, or where no acceptable guarantees of remedial action have been offered by the FBO after a suspension, the OV may consider the revocation of the authorisation to transport over temperature meat from the slaughterhouse.
This would include, but is not limited to, breaches such as:
- in relation to specific products
- non-authorised types of meat have been dispatched
- meat has been dispatched to non-authorised destinations
- meat has been dispatched to establishments in breach of the 2-hour driving requirement
- in relation to carcases, half carcases, quarters and half carcases cut into no more than three wholesale cuts
- meat has been dispatched to establishments outside the maximum transport time
- meat has not been chilled to the required maximum surface and/or core temperatures before dispatch
- failure to comply with RANs and/or HINs served in relation to the transport of over temperature meat
Updated [The OV should make a recommendation for revocation to the Approvals Team using the template letter (Annex 3). The recommendation should include sufficient supporting evidence to enable a decision to be made by the Field Veterinary Leader (FVL). The Approvals Team will notify the food business operator of the FVL decision in writing].
3.3 Appeals process
Where a suspension or revocation is issued, the FBO will have the right to appeal this decision.
The FBO should make their representation in writing to the Approvals and Registrations Team within 20 working days of the date of the notification. Updated [The appeal will be determined by a Senior FSA Veterinary Manager].
During the process of the appeal, the effect of suspension or revocation will remain in place, so the FBO must cease transportation of over temperature meat to establishments no longer contained in their authorisation.
The outcome of the appeal will be communicated to the OV and to the FBO within 20 working days from receipt of the FBO’s appeal representation.
4. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Application for the transport of meat intended for the production of specific products
Annex 3: Suspension of over temperature meat transport authorisation
Sections
1. Introduction
In this section
1.1 Definition
An Edible Co-Product is a part of a slaughtered animal or a product of an animal that is unsuitable for human consumption at the time of production in the slaughterhouse, but which can later be processed for use in human food.
Examples of these include hides and skins later processed into gelatine and collagen, cattle feet, sheep heads and feet, bile, sheep intestines processed into sausage casings, omental fat processed into lard, etc.
Edible co-products have often been called “by-products” at the slaughterhouse, leading to the mistaken belief that these can be handled like animal by-products.
The table below includes a non-exhaustive list of items under each category.
Examples
| Edible Product (Meat, offal) | Edible Co-product | Animal By-products Category 3 | Animal By-products Category 2 | Animal By-products Category 1 | Pharmaceutical use |
|---|---|---|---|---|---|
|
Wholesale and retail meat Carcase material used for meat recovery (e.g. MSM) Blood, livers and kidneys used to make edible products |
Raw fatty tissues used for edible fat and greaves (“rendered animal fats and greaves”). Raw fit bones and hide splits for edible gelatine and collagen. Intestines used for edible casings. Sheep heads and feet, cattle feet and masks (e.g. scalded or depilated). |
Parts of animals slaughtered and found fit, but not intended, for human consumption |
Poultry dead on arrival. Many Post-mortem failures, soiled or contain medicine residues. |
TSE positives. SRM. |
Intestine mucosa intended for heparin production. Bile to be used on the perfume industry or like salts. Blood used for production of serum or immunoglobulins |
1.2 Products covered in this chapter
- Processing of intestines, stomachs and bladders: Stomachs, bladders and intestines that have been submitted to a treatment such as salting, heating or drying after they have been obtained and after cleaning.
- Ruminant feet, heads and cattle ears (scalded, depilated). The processing of heads after skinning is not included in this Chapter.
- Cattle and sheep masks and lips (muzzles): Including cattle and sheep masks and muzzles scalded / depilated intended for human consumption.
- Bile and gallbladders, harvested for human consumption. The chapter does not cover harvesting of bile as an ABP or under the exception for pharmaceutical use.
- Gelatine and collagen:
- Gelatine means natural, soluble protein, gelling or non-gelling, obtained by the partial hydrolysis of collagen produced from bones, hides and skins, tendons and sinews of animals.
- Collagen is the protein-based product derived from animal bones, hides, skins and tendons manufactured in accordance with the relevant legal requirements.
- Rendered Animal Fats and greaves:
- Greaves means the protein-containing residue of rendering, after partial separation of fat and water.
- Rendered animal fats derived from rendering meat, including bones, and intended for human consumption.
- Immature eggs: harvesting of unformed eggs from spent lying hens to be used for human consumption. This does not include fertilised eggs (animal by-products, as they are embryos).
Note: This chapter does not include any reference to edible co-products from dairy industry.
1.3 Contact and useful links
- Queries regarding this chapter can be directed to the Other Products Of Animal Origin (OPOAO) portfolio group: OPOAO@food.gov.uk
- Export Health Certificates (EHCs) for the different products described in this chapter can be found by following the government guidance online.
2. Legislative References
In this section
2.1 Regulation (EC) No 178/2002
2.2 Regulation (EC) No 852/2004
2.1 Regulation (EC) No 178/2002
Regulation (EC) No 178/2002 lays down general food safety requirements, according to which food must not be placed on the market if it is unsafe.
2.2 Regulation (EC) No 852/2004
Regulation (EC) No 852/2004 lays down the principles of food hygiene. In particular, article 5, requires FBOs to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles.
2.3 Regulation (EC) No 853/2004
Regulation (EC) No 853/2004 lays down specific requirements for the manufacture of some edible co-products, e.g. gelatine, collagen, rendered animal fats and greaves, treated stomachs, bladders and intestines.
2.4 Regulation (EU) 2017/625, Regulation (EU) 2019/624, Regulation (EU) 2019/625 and Regulation (EU) 2019/627
These Regulations set out the official control requirements for products of animal origin for enforcement authorities. All establishments that handle edible co-products will therefore be subject to audit and inspection by the competent enforcement authority.
2.5 Food hygiene regulations
The Food Hygiene (S/NI/W) Regulations 2006 (as amended) / The Food Safety and Hygiene (England) Regulations 2013 (as amended) make it an offence for any person to contravene or fail to comply with the specified community provisions.
2.6 TSE related regulations
(EC) 999/2001 (as amended) lays down rules for the prevention, control and eradication of certain TSEs.
The Transmissible Spongiform Encephalopathies (England) Regulations 2018 and The Transmissible Spongiform Encephalopathies (Wales) Regulations 2018 implement Regulation (EC) 999/2001 in England and Wales.
2.7 Animal by-products (Enforcement) (England) Regulations 2013, Animal by-products (Enforcement) (Wales) Regulations 2014, Regulation (EC) 1069/2009 and Regulation (EC)142/2011
The Animal By-Products (Enforcement) Regulations (ABPR) apply and enforce Regulation (EC) 1069/2009 and Regulation (EC) 142/2011.
Note: References to EU legislation refer to Retained EU Law in England and Wales.
3. Products
In this section
3.1 Processing of intestines, stomachs and bladders
3.2 Ruminant heads, feet, and cattle ears (scalded or depilated)
3.3 Cattle masks and lips (muzzles)
3.1 Processing of intestines, stomachs and bladders
3.1.1 Introduction
This section covers the processing of intestines, stomachs and bladders for human consumption, i.e. stomachs, bladders and intestines that have been subjected to a treatment such as salting, heating or drying after they have been harvested and after cleaning. These processes all require approval under the regulations below.
Food borne pathogens such as Salmonella, Campylobacter and E.coli commonly reside in the gut flora of animals, including those that are clinically healthy. It is therefore very important that hygiene requirements are followed by FBOs when stomachs, bladders and intestines are treated for human consumption, in order to prevent these pathogens passing into the human food chain.
3.1.2 Legislation
Regulation (EC) 853/2004, Annex III, Section I, Chapter IV, Paragraph 18 (domestic ungulates). The Food Business Operators need to make sure that when destined for further handling, stomachs must be scalded or cleaned (in the case of stomachs of young ruminants intended for rennet production the stomachs need only be emptied) and intestines must be emptied and cleaned.
Regulation (EC) 853/2004, Annex III, Section XIII. Animal intestines, bladders and stomachs may be placed on the market only if:
a) they derive from animals which have been slaughtered in a slaughterhouse, and which have been found fit for human consumption following ante-mortem and post-mortem inspection;
b) they are salted, heated or dried;
c) after the treatment referred to in (b), effective measures are taken to prevent re-contamination.
Untreated stomachs, bladders and intestines are considered offal and therefore must be kept at 3°C. However, once they are treated, this requirement may no longer apply. (i.e. Salting, curing drying etc)
3.1.3 Stomachs
Harvesting and initial cleaning – Stomachs are separated from the intestines and other tissues before being opened and the contents removed. This is followed by washing: stomachs must be cleaned (visibly clean) before they leave the slaughterhouse where they were harvested.
Stomachs that have been emptied and cleaned must be kept in hygienic conditions and cooled down following a chilling curve that ensures a continuous decrease of the temperature to +3°C or below, unless they are processed without undue delay at the same establishment to obtain the final product.
After initial cleaning, stomachs may either be ‘treated’ at the slaughterhouse if approved for this purpose or transported from the slaughterhouse directly to another establishment approved for that purpose. They may also be transported frozen.
Thorough Washing - Alternatively or in addition to the previous process, stomachs can be washed at +65-70°C (sometimes lower temperatures) for between 6 and 15 minutes in order to wash the inner surface of the stomach; they are then rinsed and hung on racks to drain. Full racks are moved to the chiller for chilling to +3°C or below. They may be frozen for delivery to an approved tripe processing plant.
Scalding - involves treatment at higher temperatures, reaching normally +85-90°C for between 6 and 15 minutes to degrease and refine the outside surface of the stomach.
Note: Time/temperature parameters of washing and scalding are the most commonly used. FBOs need to identify their optimal conditions for their process based on the HACCP principles.
Note: Requirements for butcher shops selling green tripe to the final consumer
- ‘Green tripe’ or ‘tripe’ is offal; it originates from the forestomach of ruminants.
- Green tripe must only be sourced from an approved slaughterhouse with packaging bearing the identification mark of that establishment. The mark denotes that the product has been produced in accordance with the regulations following ante-mortem inspection of the animal from which it derived and satisfactory post-mortem inspection. The product should arrive visibly clean and should have been kept at no more than +3°C during transport.
- As with all foods, butchers selling green tripe directly to the final consumer need to comply with the relevant requirements of Regulation (EC) No 852/2004. Appropriate food safety management procedures based on HACCP principles should be followed to minimise the potential risk of other foods stored in the shop becoming contaminated with foodborne pathogens which may be associated with this type of product.
- A ‘one step back’ traceability trail needs to be maintained. When retailing to final consumers, customer records are not required.
- The product should be labelled correctly with the name of the food appearing on either a label attached to the food or on a ticket or notice at the point of sale.
Food business operators must ensure that food satisfies the requirements of food law which are relevant to their activities (Article 17 of Regulation (EC) 178/2002 refers).
In addition, green tripe (untreated) can also be sold directly from the slaughterhouse to the final consumer.
Storage in cold stores is also permitted, only when intended for further processing and if the stomachs are emptied and visibly clean from extraneous contamination. It is advisable to label the product with clear information to make customers aware of the need for the product to be further processed.
3.1.4 Intestines
Harvesting - In the gut room, the small intestine is separated from the stomach; it is then separated from the mesentery and the large intestine, either mechanically or by ‘pulling’.
Manure Stripping – Intestines must be emptied and cleaned (i.e. stripped) in the slaughterhouse of harvesting. They are passed through a manure stripper (a set of rollers) to squeeze out the intestinal contents without damaging the sub-mucosa. Once the intestines are emptied of intestinal content, these are called ‘runners’. In some cases, the equipment can also act as a crusher and begin the process of removing the mucosa. After stripping, the runners are tied into hanks and put into containers of cold water, with or without salt, to await further processing – either in the same premises or in another establishment approved for that purpose.
Note: the term ‘cleaning’ is used for the process of removing the layers of the intestine to leave the sub-mucosa i.e. the casing, which will then be salted to become a ‘treated’ product. Treatment must take place in an establishment approved for this activity. Intestines, runners and unsalted casings are considered to be untreated and unprocessed products.
As offal, runners must be kept in hygienic conditions and cooled on a chilling curve that ensures a continuous decrease of the temperature to +3°C or below. After manure stripping and washing, runners may either be treated at the slaughterhouse, if approved for this activity, or transported directly to another establishment approved for that purpose. They may also be transported frozen.
3.1.5 Treatment
Stomachs, bladders and intestines become ‘treated’ only when they are salted, heated or dried.
Heating, salting and drying are used as a treatment/preservative method, because most bacteria, fungi and other potentially pathogenic organisms cannot survive high temperatures, a highly salty environment due to the hypertonic nature of salt, or low water activity. Any living cell in such an environment will become dehydrated and die or become temporarily inactivated. The processes of salting and drying must be included in the documented HACCP based procedures and the FBOs should have a validation system in place on the process to ensure food safety.
3.1.6 Identification marking
Stomachs, bladders and intestines despatched treated or destined for treatment should bear an appropriate Identification Mark (Annex II of Regulation (EC) 853/2004, refers).
3.1.7 Export
Untreated stomachs, bladders and intestines can be exported for further handling destined for human consumption, provided these are cleaned and fully emptied of digestive tract content. It is advised that these products are exported with clear information in the labelling, for example: “untreated stomachs, for further processing”.
For further details for export requirements refer to the export chapter.
3.2 Ruminant heads, feet, and cattle ears (scalded or depilated)
3.2.1 Introduction
This section covers the processing of ruminant heads, feet, and cattle ears for human consumption.
Note: In relation to heads, SRM requirements apply and only heads with no SRM can be destined for human consumption.
3.2.2 Legislation
Carcases and other parts of the body intended for human consumption must be completely skinned, except in the case of updated [porcine animals] the heads of ovine and caprine animals and calves, the muzzle and lips of bovine animals and the feet of bovine, ovine and caprine animals. Heads, including muzzle and lips, and feet must be handled in such a way as to avoid contamination. Regulation (EC) 853/2004, Annex III, Section I, Chapter IV, Paragraph 8.
When destined for further handling, heads and feet must be skinned or scalded and depilated. Regulation (EC) 853/2004, Annex III, Section I, Chapter IV, Paragraph 18.
3.2.3 Harvesting & processing
Ruminant heads, feet, and cattle ears destined for human consumption (edible co-products), must be skinned and or scalded and depilated, and they must be subjected to PMI.
In relation to PMI, ruminants’ heads, must be presented in such a way so that PM inspection can be carried out effectively in compliance with the requirements of Regulation 853/2004 and to the satisfaction of the OV.
Unprocessed ruminant feet could also be despatched to another red meat abattoir or red meat cutting plant, to be scalded and depilated or skinned there. In this case, feet must be visibly clean before being dispatched.
Heads of cattle destined for human consumption can also be despatched to a cutting plant authorised for this specific activity, but these must be skinned before they leave the establishment of slaughter
Note: (Heads of cattle 8 months old or younger can be either skinned or scalded and depilated).
If the intention is to harvest sheep heads for human consumption (either whole or part for example tongues and brains) after PM inspection and after chilling then the heads must be fully flayed before presentation for PM inspection.
To note that there are instances when the requirement to fully flay the head may not apply.
Where parts of the head are removed before PM inspection, partial skinning can take place provided the food business has procedures in place to ensure that the parts are harvested hygienically, and that effective SOPs are in place to the satisfaction of the OV.
Where parts of the head are removed after PM inspection but before chilled storage, partial skinning can take place provided the food business has procedures in place to ensure that the tongues are harvested hygienically, and that effective SOPs are in place to the satisfaction of the OV.
i. Feet processed on site:
Post-mortem inspection can be done before or after further treatment (such as dehairing) on an individual basis or in batches at the abattoir.
If post-mortem inspection takes place before treatment, a further spot check will be needed to ensure that these feet are free from any pathology.
Food grade caustic soda (sodium hydroxide) may be used as a processing aid to help in the removal of the hair of the feet. It should be thoroughly rinsed off afterwards and its use must be included in the HACCP plan for the processing operation. Caustic soda must not be used in any part of the slaughter operation or for carcase washing.
ii. Feet processed at a different approved site:
Post-mortem inspection must be done on an individual basis or in batches at the abattoir.
After treatment a further spot check will be needed to ensure that these feet are visibly clean and free from visible pathological lesions before shipping for further processing.
When authorised by the competent authority, visibly clean feet may be transported to and skinned or scalded and depilated in an approved establishment further handling the feet for processing into food (paragraph 18, Chapter IV, Section I, Annex III of Regulation (EC) No. 853/2004).
Applicable to all cases
Processed or unprocessed, ruminant heads, feet, and cattle ears, a full correlation system must be implemented by the FBO to ensure that if a carcase is detained and subsequently condemned, the entire correlated batch is disposed of as unfit for human consumption. FBOs may assist the inspection process and set aside feet with identified abnormalities.
The final product or the edible co-product destined for further handling is to be dispatched with the ID mark clearly applied according to legislative requirements to the container and/or packaging used.
There is no specific requirement to additionally authorise or approve the processing of ruminant feet, cattle ears and heads, as these activities can be considered part of the slaughter process, the exception being the processing of ruminant feet at a different establishment as detailed above.
3.3 Cattle and sheep masks and lips (muzzles)
3.3.1 Introduction
This section covers the processing of cattle and sheep masks and muzzles for human consumption.
Cattle and sheep masks refers to the skin of the head after flaying. It does not include the ears or the eyelids but may include lips and the muzzle of cattle and sheep. It should not include the area of skin that surrounds the bolt hole (where this method of stunning is used) as there is a risk that it will be contaminated with specified risk material (SRM). For practical purposes and to minimise the risk of cross-contamination it is advisable to remove this as soon as possible.
There is no specific requirement to additionally authorise or approve the harvesting of cattle and sheep masks and/or muzzles, as this activity can be considered as part of the slaughter process.
The cattle masks guidance can be found in Annex 1.
3.3.2 Harvesting & processing
Cattle and sheep masks and muzzle can only be harvested in approved slaughterhouses from animals that have passed both ante mortem and post-mortem inspection.
Where the masks are intended for human consumption, they must also be scalded and depilated. Only masks that have been harvested at the slaughterhouse may be processed at that slaughterhouse. Masks cannot be taken to another slaughterhouse for cleaning/processing (i.e. scalding and depilating), neither can they be brought in from another slaughterhouse or other premises – even if these other premises are in the same ownership or are very close to the slaughterhouse of harvesting. Therefore, masks for human consumption must not leave the site of harvesting until they have been adequately processed and all hair has been removed (paragraph 18, Chapter IV, Section I, Annex III of Regulation (EC) No. 853/2004).
Food grade caustic soda (sodium hydroxide) may be used as a processing aid to help in the removal of the hair of the masks. It should be thoroughly rinsed off afterwards and its use must be included in the HACCP plan for the processing operation. Caustic soda must not be used in any other part of the slaughter operation or for carcase washing.
The food hygiene legislation does not permit burning the hair from the head’s skin of any animal other than for small amounts of hair that have not been removed by the scalding operation.
‘Raw’ (or just harvested) cattle masks waiting to be processed for human consumption are offal. As offal, the masks should be stored at no more than +3°C.
3.3.3 HACCP/SOPs
All stages of the process must be included in the food business operator’s HACCP based food safety management system. The food business operator must develop and agree with the incumbent OV the relevant SOPs that ensure hygienic production. The risk of SRM contamination from eyes that are damaged during slaughter and dressing needs to be taken into consideration and masks and muzzles from heads with damaged eyes must not be harvested. Also, if a captive bolt system of stunning was used, the SOP will include trimming the area around the bolt hole.
The food business operator must also have a system to ensure that unfit product, e.g. masks and muzzles from carcases which have been considered unfit for human consumption, do not enter the food chain. If the material has been batched before inspection and cannot be correlated to the condemned carcase, the whole batch must be treated as Animal By-Products.
If the mask (or head) is separated from the rest of the carcase before completion of the post-mortem inspection, the food business operator must have a system/procedure in place to link the mask with the carcase.
3.3.4 Identification marking
Processed cattle masks and muzzles (final product) intended for human consumption leaving the slaughterhouse must be clearly marked on the container or packaging with an oval identification mark bearing the information laid down in Section I, Annex II of Regulation (EC) 853/2004.
3.4 Bile and gallbladders
3.4.1 Introduction
This section covers the harvesting, handling and despatch of bile or gallbladders for human consumption as food or as an ingredient for the pharmaceutical industry. This section also covers the process of concentrating bile for export to third countries.
There is no specific requirement to additionally authorise or approve the harvesting of bile and/or gall bladders as this activity can be considered part of the slaughter process.
To note that bile and gallbladders may also be harvested as Category 3 animal by-product, in this case all stages of this process must be kept separated from the production of bile and gallbladders for human consumption.
Where bile is being supplied as a pharmaceutical and is not intended to be, or cannot be, used as food, then the EU food hygiene provisions will not apply but separate authorisation under ABP/Medicinal Products legislation will most probably be required in these instances. These cases will need to be discussed with APHA and/or VMD individually.
3.4.2 Legislation
Bile is considered to have no significant use as food and as such, would require authorisation under the Novel Food Regulation 2009 before it may be placed on the market within the UK.
3.4.3 Harvesting of bile and gallbladders
Bile and gallbladders can only be harvested in approved slaughterhouses from animals that have passed both ante and post-mortem inspection. Once harvested for human consumption, bile and gallbladders must be treated as edible co-product by being stored and despatched under temperature-controlled conditions same as offal.
3.4.4 Export
Bile and gallbladder can be harvested/pre-processed for export for either food or for pharmaceutical use.
Where bile and gallbladders are intended for export for food use, it should be treated as an edible co-product with the FBO treating the harvesting, collection, storage and transport of bile and/or gallbladders in-line with the same food hygiene requirements as fresh meat/offal.
It will be necessary for the FBO responsible for exporting the product to demonstrate that the product they produce complies with any legal requirements that might exist for this product in the country of destination. The OVs will want to satisfy themselves that the products to be exported do comply with the requirements in the country of destination in the same way as for any product exported to a third country.
In addition to the requirements of the importing country, the product must not be injurious/harmful to human health. The UK competent authority responsible must request evidence from the food business operator that the product is safe, e.g. does not contain excessive levels of heavy metals or other contaminants. It has also been reported that gall bladders can also act as a reservoir for Salmonella. Therefore, the FBO should consider all these factors during the hazard analysis.
Where activities include concentrating the raw material for export, there is an expectation that the food business operator should be able to provide evidence on how any food safety risks associated with the final product have been assessed under the HACCP based principles. In addition, verification by the relevant UK competent authority is required prior to the operations commence.
There might be instances where the FBO will also need an Export Health Certificate for this product. If more advice is required, then contact should be made with APHA.
3.5 Gelatine and Collagen
3.5.1 Introduction
This section covers the manufacture of gelatine and collagen for human consumption as well as the collection and storage of raw materials intended to be used for the manufacture of gelatine and collagen for human consumption.
Gelatine can be used for pharmaceutical or photographic processes whilst Collagen may be produced also for medical and cosmetic purposes. Those are not included in the scope of this chapter.
Establishments that generate or process raw material for the production of gelatine or collagen for human consumption must be registered, approved or specifically authorised by the competent authority.
Approval will not be required however if the premises carry out only transport operations or storage of products not requiring temperature-controlled storage conditions (Regulation (EC) 853/2004, Article 4.2).
3.5.2 Legislation
Regulation (EC) No 853/2004:
Annex I, point 7.7 - definition for gelatine: ‘Gelatine’ means natural, soluble protein, gelling or non-gelling, obtained by the partial hydrolysis of collagen produced from bones, hides and skins, tendons and sinews of animals.
Annex III, Section XIV, Chapter I, Paragraph 3 – raw materials for the production of gelatine.
Annex I, point 7.8 - definition for collagen: ‘Collagen’ means the protein-based product derived from animal bones, hides, skins and tendons manufactured in accordance with the relevant requirements of this Regulation.
Annex III, Section XV, Chapter I, Paragraph 3 – raw materials for the production of collagen.
Regulation (EC) No 2073/2005
Annex I, Chapter 1, point 1.10 - Food Safety Criteria (Salmonella), for edible gelatine/collagen.
International trade:
- Regulation (EU) 2019/625, Article 7 (e), quotes that in order to ensure compliance with Union food hygiene rules, or with rules recognised to be at least equivalent, products from establishments manufacturing raw materials intended for the production of gelatine and collagen, should only be allowed entry into the Union if these establishments appear on lists drawn-up and updated in accordance with Article 127(3)(e) of Regulation (EU) 2017/625 and which are published by the Commission.
- Import of Gelatine and Collagen for Human Consumption from Third Countries (February 2020)
3.5.3 Raw material
Raw materials for the manufacture of gelatine or collagen for human consumption may include:
- Bones, other than specified risk materials as defined in Article 3(1)(g) of Regulation (EC) No. 999/2001.
- Hides and skins of farmed ruminant animals.
- Pig skins.
- Poultry skin.
- Tendons and sinews.
- Wild game hides and skins.
- Fish skin and bones (not covered in this MOC Chapter)
Such raw material can originate from approved (e.g. slaughterhouses, cutting plants), registered (e.g. butcher shops, establishments handling fishery products) or specially authorised sites (e.g. collection centres and tanneries) complying with Reg. (EC) 852/2004 and fulfilling the requirements laid down in Reg.853/2004 (see subchapter 3.5.2).
The registration/approval/special authorisation number of those sites will be recorded in the document accompanying the raw material (see Annex 2 of this MOC chapter).
Raw materials must derive from animals that have been slaughtered in a slaughterhouse and whose carcases have been found fit for human consumption following ante-mortem and post-mortem inspection (including a negative result from the BSE test where this is required by Community legislation) or, in the case of hides and skins from wild game, found fit for human consumption.
The use of hides and skins is prohibited if they have undergone any tanning process, regardless of whether this process was completed. For the purpose of this section, ‘tanning’ means the hardening of hides, using vegetable tanning agents, chromium salts or other substances such as aluminium salts, ferric salts, silicic salts, aldehydes and quinones, or other synthetic hardening agents.
When hides and skins are not intended for the production of edible gelatine or collagen, then they become animal by-products. They must be handled and stored separately from hides and skins destined for the production of edible gelatine/collagen.
If the raw materials (e.g. hides) are separated from the carcase before completion of the post-mortem inspection, the operator must establish a system to ensure that if a carcase is declared unfit for human consumption or it is detained, any body parts from that carcase are also declared unfit or are detained.
If it is impossible to identify the hide or other body part from a particular carcase declared unfit, the entire batch of hides or other body parts derived from that carcase must be declared unfit.
3.5.4 Treatment
Examples of treated raw materials for the production of edible gelatine and details on the applicable treatments are listed in Regulation (EC) No 853/2004, Annex III, Section XIV, Chapter I, Paragraph 4.
Treatment requirements for the manufacture of gelatine (production process for gelatine) are quoted in Regulation (EC) No 853/2004, Annex III, Section XIV, Chapter III, Paragraph 1.
On a similar note, examples of treated raw materials for the production of edible collagen and details on the applicable treatments are listed in Regulation (EC) No 853/2004, Annex III, Section XV, Chapter I, Paragraph 4.
Treatment requirements for the manufacture of collagen (production process for collagen) are quoted in Regulation (EC) No 853/2004, Annex III, Section XV, Chapter III, Paragraph 1.
A food business operator may produce and store both gelatine/collagen intended for human consumption and gelatine/collagen not intended for human consumption in the same establishment, provided that the raw materials and the production process comply with the requirements applying to gelatine/collagen intended for human consumption.
3.5.5 Temperature controls
Raw material for the production of gelatine/collagen for human consumption must be transported and stored chilled (to below +3°C) or frozen, unless they are dispatched and processed within 24 hours after their production.
However, degreased and dried bones or ossein, salted, dried and limed hides, and hides and skins treated with alkali or acid may be transported and stored at ambient temperature.
3.5.6 Identification mark
When delivered to a collection centre or tannery, the raw material used in the manufacture of gelatine or collagen must, instead of carrying an identification mark, be accompanied by a document containing the information set out in the Appendix to Annex III of Regulation (EC) No. 853/2004. This document can be found in Annex 2 to this MOC chapter.
Collection centres and tanneries supplying raw material to a gelatine or collagen processing establishment must ensure that the raw materials are accompanied by the document described above.
Once produced, the gelatine/collagen must bear an identification mark containing the approval number of the gelatine/collagen plant where they were produced.
3.5.7 Labelling
Wrapping and packaging containing gelatine must bear the words “gelatine fit for human consumption” and must indicate the date of minimum durability (Regulation (EC) 853/2004, Annex III, Section XIV, Chapter V).
Wrapping and packaging containing collagen must bear the words “collagen fit for human consumption” and must indicate the date of preparation (Regulation (EC) 853/2004, Annex III, Section XV, Chapter V).
3.6 Rendered animal fats and greaves
3.6.1 Introduction
Rendering is the process of extracting fat for human consumption from meat by melting (heat treatment), with the greaves being the protein containing the residues of the rendering process.
3.6.2 Legislation/Approval of premises
Food business operators must ensure that establishments collecting or processing raw materials for the production of rendered animal fats and greaves and preparing rendered animal fats and greaves, comply with the requirements laid down in Regulation (EC) No. 853/2004, Annex III, Section XII.
Establishments that collect or process raw material to produce rendered animal fats and greaves must be approved by the competent authority. Approval will not be required if the premises carries out only transport operations, or the storage of products not requiring temperature-controlled storage conditions.
3.6.3 Raw materials
Raw materials must:
- derive from animals which have been slaughtered in a slaughterhouse, and which have been found fit for human consumption following ante-mortem and post-mortem inspection
- consist of adipose tissues or bones which are reasonably free from blood and impurities; and
- come from establishments registered or approved under Regulation (EC) No. 852/2004 or Regulation (EC) No. 853/2004.
3.6.4 Processing and temperature controls
Raw material for the production of rendered animal fats and greaves (unless the fat is rendered within 12 hours after the day on which they were obtained) must be transported and stored at an internal temperature no greater than +7°C.
Centres for the collection of raw materials and further transport to processing establishments must be equipped with facilities for the storage of raw materials at a temperature of not more than +7°C.
However, the above refrigeration facilities are not necessary if the arrangements for the supply of raw materials ensure that they are either (a) never stored or transported without active refrigeration, or (b) rendered within 12 hours after the day on which they were obtained.
Greaves intended for human consumption must be stored in accordance with the following temperature requirements:
a) When greaves are rendered at a temperature of not more than +70°C, they must be stored: at a temperature of not more than +7°C for a period not exceeding 24 hours; or at a temperature of not more than –18°C.
b) When greaves are rendered at a temperature of more than +70°C and have a moisture content of 10% (m/m) or more, they must be stored: at a temperature of not more than +7°C for a period not exceeding 48 hours or a time/temperature ratio giving an equivalent guarantee; or at a temperature of not more than –18°C.
c) When greaves are rendered at a temperature of more than +70°C and have a moisture content of less than 10% (m/m), there are no specific requirements.
During the rendering of raw materials, the use of solvents is prohibited. Rendered animal fat, depending on the type, must meet the standards laid down in the table in point 4 of Chapter II of regulation (EC) 853/2004, Annex III Section XII.
Please be aware that temperature requirements are now different for Northern Ireland.
As per Regulation (EU) 2021/1374 (which amended Reg 853/2004) There are no longer any temperature requirements for the storage of greaves. Technological developments have allowed certain packaging techniques, such as vacuum-packaging for which the specific temperature requirements are not needed to ensure the safety of food derived from greaves. Food business operators should ensure the safety of food derived from the greaves by good hygiene practices and procedures based on Hazard Analysis and Critical Control Point (HACCP) principles.
3.6.5 Identification mark
Both the raw material (i.e. the fat and/or bones) and the final product (i.e. the rendered animal fats and greaves) must bear an identification mark showing the approval number of the establishment where they were produced.
3.7 Immature eggs
3.7.1 Introduction
OVs have observed FBOs harvesting and selling “eggs” at various stages of their development for human consumption, when “spent hens” are consigned to slaughter. This includes:
- fully formed eggs laid naturally following their consignment from a production site to a poultry slaughterhouse and during lairaging and hang on,
- what appear to be fully formed eggs “pulled” from dead birds after killing and prior to evisceration,
- what appears to be fully formed eggs with cracked shells found either in the lairage or on the evisceration line,
- nearly fully formed eggs that are harvested from the evisceration line,
- immature ova / yolks, harvested from the dead bird after PMI for sale to the Kosher market, for what are described as “traditional products”.
3.7.2 Definition
“Eggs” are defined as eggs in shell — other than broken, incubated or cooked eggs — that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products.
3.7.3 Legislation
Regulation (EC) 853/2004, Annex I, Point 5.1
Regulation (EC) 589/2008, Article 1(k)
For further reference please see annex 3
3.7.4 Collection of eggs
3.7.4.1 Collection at different steps in the slaughterhouse
Slaughterhouses do not satisfy the relevant definitions of “producer”, “registered production site”, “collection centre” or “a packing centre” and therefore FBOs cannot sell shell eggs. As such, those eggs will not comply with the definition of fresh eggs.
FBO will not be able to give eggs away to plant staff for free.
The only exception refers to FBO harvesting fully formed eggs for their own personal consumption.
Eggs that have not been laid prior to the animal’s death, cannot be “eggs” by definition, but would fall under the definition of “offal” - fresh meat, other than that of the carcase, including viscera and blood.
As offal, the “egg” could be harvested as an edible co-product, ID marked and sold for human consumption,
3.7.4.2 Harvest of ova/yolk
The unformed proto eggs or ova would fall under the definition of “offal”. However, harvesting such products should only be done after the completion of a successful ante and post mortem inspection at which there are no grounds to declare the carcase / offal unfit for human consumption.
The harvesting of offal is a legally permissible activity, provided that a hazard analysis has been undertaken, risks are being managed, the product is treated hygienically, appropriately chilled to 3 degrees C, ID marked and labelled correctly as a food product, with appropriate allergen labelling.
3.7.4.3 Disposal
Eggs must be disposed of as Category 3 Animal By-products.
If eggs originate from an animal rejected with a condition communicable through the egg itself to humans or animals, it would be a Category 2 ABP.
See Annex 3 for more information related to the harvesting of eggs in poultry abattoirs.
4. FBO Role
4.1 HACCP
Edible co-products can only be harvested in approved slaughterhouses from animals that have passed both ante and post-mortem inspection.
All stages of the process must be included in the food business operator’s HACCP based food safety management system, as per any other food process. The FBO has to consider hazards associated with the relevant steps and processes and subsequently implement controls to ensure food safety.
As unprocessed edible co-products might be handled in approved slaughterhouses and cutting plants, the risk of cross-contamination between these products and meat or other products must be taken into consideration.
The food business operator must also have a system to ensure that unfit product (for example co-products from carcases that have been considered unfit for human consumption), does not enter the food chain and that a clear separation is kept between products intended for human consumption and animal by-products.
If edible co-products are further processed, the process should be validated and verified under the HACCP principles.
4.2 Batching system
The FBO shall have in place a batching system when harvesting certain edible co-products (for example feet, heads, ears) in case a carcase has to be disposed of as an animal by-product.
This means that co-products must be correlated with carcases and offal. A batching system is acceptable provided that in the case of a post-mortem rejection of a carcase, the entire batch of related co-products is rejected. Any edible co-product batching system must provide full correlation with the carcasses and offal.
5. FSA Role – Inspection, Verification and Audit
5.1 Inspection duties in slaughterhouses
5.1.1 OV duties
In addition to the normal controls, the OV should carry out verification checks to ensure that the meat complies with the required regulatory regime applicable to edible co-products.
Those checks should be made to ensure:
- The overall hygiene performance around edible co-products generated and/or processed on site.
- The OV includes these as part of the off-line duties for the Official Auxiliaries
- The supervision of the hygienic processing, separation, storage, temperature requirements, packaging, wrapping, ID marking and commercial documents are included
- Production takes place in line with the on-site HACCP and all relevant legal requirements
- Edible co-products are consigned to appropriate premises (i.e. approved, registered) and that accompanying documentation is correct and has been completed accurately (for example ABP commercial documents are not to be used for the transport of edible co-products)
- With regards to segregation, ABP and co-products are clearly separated (for example hides intended for gelatine/collagen production from ABP hides).
- The product bears an identification mark in accordance with Article 5 and Annex II of Regulation (EC)853/2004. Identification marks are applied, even when the product is destined for further processing before being placed on the market for human consumption.
5.1.2 Frequency of checks
The OV is to ensure that the production of edible co-products is part of the routine hygiene verification checks. The frequency of these checks is proportionate to:
- Size of the establishment
- Volume and type of co-products
- Layout
- History of compliance (including audit score)
For further guidance, see chapter 2.4 section 12 and 13: Slaughter hygiene Verification in Red Meat and Poultry.
Note: The FSA is no longer required to carry out 100% checks on compliance with SRM removal requirements. However, the OV must verify that the FBO has robust systems in place to ensure that meat entering the food chain is free from SRM. For example, Sheep or cattle heads containing SRM).
However, 100% verification of the removal of spinal cord SRM from relevant cattle and sheep carcases is still required.
5.2 Inspection duties in cutting plants
Some cutting plants may process edible co-products as part of their normal operation.
These operations are to be covered during the unannounced inspection (UAI) visits and under the audit scope. There is not a dedicated section in the UAI report for edible co-products, so they are to be included within the general supervision sections.
Authorised Officers inspecting this kind of plants are to be familiar with this section of the MOC and the relevant legal requirements when assessing these activities
5.3 Identification marking
Regulation (EC) 853/2004, Chapter II, Article 5, paragraph 1 states that “Food business operators shall not place on the market a product of animal origin handled in an establishment subject to approval in accordance with Article 4(2) unless it has an identification mark applied in accordance with Annex II, Section I, of this Regulation.”
Edible co-products are therefore to be dispatched bearing an ID mark even when going for further processing. The exception being the dispatch of hides for collagen and gelatine as per section 3.5.6.
AO supervising edible co-product related issues are to verify application of ID mark takes place before dispatch.
When an establishment receives raw materials for further processing as edible co-products, they are to be ID marked as per Regulations.
Note: It is important to bear in mind the ID marking requirements for liquids (i.e. bile, melted fat, etc). In particular Regulation (EC) 853/2004, Annex II, Section I, point C, paragraph 12, where it is quoted that "In the case of liquid, granulate and powdered products of animal origin carried in bulk, and fishery products carried in bulk, an identification mark is not necessary if accompanying documentation contains an indication of the country of origin (GB), the approval number and be on oval shape”. That would be for instance applicable to the transport of bile if in containers.
If the edible co-products are no longer intended for human consumption or they are handled and/or stored unhygienically, they become unsuitable for human consumption and should be treated as ABPs and dealt with as such. Once they have been downgraded to ABPs, they cannot revert into the human food chain.
5.4 Audit duties
The FBO’s food safety management procedures based on HACCP principles, associated records and processes will be assessed during audit both at slaughterhouses and at cutting plants involved in the supply chain.
Information about the auditing process can be found in Chapter 4.1 There is a specific section in the FBO Audit Aide Memoire (section 3.4), that covers the handling of edible co-products.
Reference: Chapter 4.1 Audit, Annex 1 ‘Audit Aide Memoire’.
6. Enforcement
Edible co-products are food so the same hierarchy of enforcement that applies to fresh meat should be used, see Chapter 7 “Enforcement”.
Deficiencies found by the AO are to be recorded in CHRONOS and the daybook when available (i.e. slaughterhouses and VC removal authorised cutting plants).
7. Annexes
Annex 1 Guidance on the harvesting and processing of cattle masks for human consumption

Note: This Guidance is provided on the basis of current science and knowledge. It will be reviewed if new science or evidence emerges.
Introduction
- A number of approved slaughterhouses in the UK are interested in harvesting/ processing cattle masks for human consumption. The following guidance will help those food business operators to undertake this activity hygienically. Regulations (EC) Nos. 852/2004 and 853/2004 will apply to this activity. This guidance may also be used for harvesting/processing the masks of other animals for human consumption should food business operators wish to consider doing this.
- For ease of reference, throughout this document ‘cattle masks’ refers to the skin of the head after flaying. It does not include the ears or the eyelids but may include lips and the muzzle of cattle. It should not include the area of skin that surrounds the bolt hole (where invasive stunning is used) as there is a risk that the skin in the proximity of the bolt hole will be contaminated with specified risk material (SRM). Please refer to paragraphs 12 and 18 for further details.
- Cattle masks for human consumption must only be harvested from heads originating from animals that have been slaughtered in an approved slaughterhouse and whose carcases have been found fit for human consumption following ante mortem and post-mortem inspection. Full traceability is required, therefore if the mask is separated from the rest of the hide, or the head is separated from the carcase before completion of the post-mortem inspection, the food business operator must have a system/procedure in place to correlate the mask and/or the head with the carcase.
- This must ensure that if a carcase is declared unfit for human consumption the mask and/or the head is also declared unfit and is removed from the slaughter/processing area as an animal by-product (ABP). When it is impossible to identify the head and/or the mask from a particular carcase that has been declared unfit, the entire batch of heads/masks must be declared unfit and consigned as ABP. The category of ABP will depend on the reason for rejection. Any batching system must provide full traceability with the carcass and offal of origin.
- At no point should skins, including masks, for human consumption - or other material for human consumption - be classified as, or come into contact with, material that is not fit for human consumption or that has been labelled as ABP. Slaughterhouse procedures should be arranged so that there is clear separation and cross contamination is, at all times, avoided. If the cattle masks do come into contact with ABPs then they too should be treated as ABP and immediately removed from the food area.
- Unprocessed cattle masks intended for human consumption are offal. As offal, the masks should be stored at no more than 3°C. The finished product is an edible co-product that should also be stored and refrigerated at no more than 3°C (or frozen) including during transport and despatch to another food premises.
- Only cattle masks that have been harvested at the slaughterhouse may be processed at that slaughterhouse. Unprocessed masks cannot be taken to another slaughterhouse for cleaning/processing (i.e. scalding and/or depilating), neither can they be brought in from another slaughterhouse or other premises – even if these other premises are in the same ownership or are very close to the slaughterhouse of harvesting. It is not permissible for the cattle masks to be stored at an off-site cold/chill store until the slaughterhouse is ready to process them.
- Whilst masks can be produced from cattle of any age, heads of cattle 8 months old or younger if destined for further handling for human consumption must be either skinned or scalded and depilated; this must be undertaken before they leave the establishment of slaughter. The scalding and depilation of heads of cattle over 8 months is not permitted.
Processing cattle masks
9. Bovine heads intended for human consumption must be completely skinned, except for the muzzle and lips (paragraph 8, Chapter IV, Section I, Annex III of Regulation (EC) No. 853/2004, refers).
10. In the case of calves, if the heads are not skinned, they must be scalded and depilated before leaving the premises of harvesting (paragraph 18, Chapter IV, Section I, Annex III of Regulation (EC) No. 853/2004).
11. Normal practice at most slaughterhouses in the UK is to remove the head from the carcase before skinning the head in preparation for post-mortem inspection; alternatively, the head can be left attached to the carcase until after the skin has been completely removed (where using a hide puller to remove skin from carcase and head) then removing the head for inspection. The mask is then removed from the rest of the hide.
12. If an invasive system of stunning was used, an area of approximately 5-10cm radius around the bolt hole should be removed to mitigate the risk of cross contamination from leakage of brain tissue and/or CNS fluid. This is better achieved if done before scalding and depilation.
13. Cattle masks should be processed in a separate part of the premises and not in the same area in which slaughter takes place. During and after processing there should be clear separation to avoid the risk of cross contamination of the processed mask from other processed or unprocessed masks/foods, the slaughter hall and other parts of the premises or actions undertaken in or around the premises. After processing, cattle masks should be visibly clean and the skin should be free of hairs, extraneous detritus and other contamination.
14. The processing of cattle masks must form part of the food business operator’s HACCP system and must take into account potential hazards, including the potential for cross contamination with other material or ABPs and contamination with SRM. At all times, before and after processing, masks must be stored and despatched at no more than 3oC.
15. Food grade caustic soda (sodium hydroxide) may be used as a processing aid[1] to help in the removal of the hair of the masks. It should be thoroughly rinsed off afterwards and its use must be included in the HACCP plan for the processing operation. The food hygiene legislation does not permit burning/singeing the hair from the head skin of any animal other than for small amounts of hair that have not been removed by the scalding operation.
SRM controls
16. There is no legal requirement to plug the bolt hole where the invasive system of stunning is used or the foramen magnum, when part of the head is intended for human consumption except when head meat is harvested offline (i.e. without removing the bovine head from the hook) or when the head is transferred to a registered cutting plant to be processed, in which case, it is required to plug the bolt hole.
17. However, to achieve best practice, sealing the bolt hole with an impermeable and durable stopper would help to reduce the risk of cross contamination resulting from the leakage of brain tissue and CNS fluid. Additional precautions include to avoid the harvesting of any head meat where the eyes are damaged and to handle the mask carefully for avoiding accidental contamination from the foramen magnum dripping during the harvesting and handling.
18. To reduce the risk of contamination with SRM from around the captive bolt hole a piece between 5 and 10cms in diameter around the hole should be removed. The exact size should be decided on a case-by-case basis, in consultation with the relevant veterinary staff at the plant and considering the way the carcases and heads are handled in those premises and the method of collection of the head skin. This practice should be built into the operating procedure for removing the mask. There is no legal requirement to sample masks for CNS contamination when they are harvested in the slaughterhall line without removing the bovine heads from the hook or directly from the pulled hide.
Approval
19. Harvesting and processing cattle masks does not require a separate approval, but this activity should be recorded as part of the operations undertaken at a slaughterhouse when consideration of approval is sought or when a food business operator starts this process.
20. Processed (i.e. scalded, depilated and cleaned) cattle masks for human consumption are considered to be an edible co-product and should leave the slaughterhouse with the appropriate commercial documentation and bearing the ID mark of the slaughterhouse.
Traceability
21. Article 18 of Regulation (EC) No. 178/2002 requires food business operators to be able to identify the person or persons from whom they have received food (including food producing animals) for human consumption and to whom they have supplied food for human consumption.
22. To achieve this, food business operators must have in place systems and procedures that allow for the full traceability of food, including food-producing animals, entering their premises. This traceability must be maintained throughout all stages of production, processing and distribution. This information must be made available to the competent authorities on demand.
Compulsory Beef Labelling Scheme
23. Cattle masks fall outside the scope of the Compulsory Beef Labelling Scheme.
[1] ‘processing aid’ is defined in Regulation (EC) No 1333/2008, as meaning any substance which:
i. is not consumed as a food by itself.
ii. is intentionally used in the processing of raw materials, foods or their ingredients, to fulfil a certain technological purpose during treatment or processing; and
iii. may result in the unintentional but technically unavoidable presence in the final product of residues of the substance or its derivatives provided they do not present any health risk and do not have any technological effect on the final product.
Annex 2 Model documentation to accompany raw material destined for the production of collagen / gelatine

Annex 3 Q&A Harvesting of eggs at poultry slaughterhouses for human consumption
Q1. What are the Issues?
OVs have observed FBOs harvesting and selling “eggs” at various stages of their development for human consumption, when “spent hens” are consigned to slaughter. This includes:
- fully formed eggs laid naturally following their consignment from a production site to a poultry slaughterhouse and during lairaging and hang on,
- what appear to be fully formed eggs “pulled” from dead birds after killing and prior to evisceration,
- what appears to be fully formed eggs with cracked shells found either in the lairage or on the evisceration line,
- nearly fully formed eggs that are harvested from the evisceration line,
- immature ova / yolks, harvested from the dead bird after PMI for sale to the Jewish community, for what are described as “traditional products”.

Q2. What is the legal definition of an “egg”?
“Eggs” are defined in:
Regulation (EC) 853/2004, Annex I, Point 5.1 “eggs in shell — other than broken, incubated or cooked eggs — that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products.
Regulation (EC) 589/2008, Article 1(k) ‘eggs’ means eggs in shell — other than broken, incubated or cooked eggs — that are produced by hens of the species Gallus gallus and are fit for direct human consumption or for the preparation of egg products;
Q3. Can shell eggs that have been naturally laid and found; in transport crates following transport from a production site to a slaughterhouse, or in the hang on section of the lairage prior to the bird being killed, be marketed and sold for human consumption, either:
- as Class A / B eggs,
- sold at a local public market,
- door to door, or
- at the factory gate.
No. Whilst fully formed eggs in shell, laid naturally prior to the death of the bird, comply with the definition of “eggs” in both the egg marketing and food hygiene legislation, the eggs were not laid:
- whilst at a “production site” [defined in Regulation (EC) 589/2008, Article 1(p) as an “establishment keeping laying hens, registered in accordance with Commission Directive 2002/4/EC”], and subsequently
- sent to a “packing centre” [defined in Regulation (EC) 589/2008, Article 1(q) to mean a packing centre within the meaning of Regulation (EC) No 853/2004 that is authorised according to Article 5(2) of this Regulation and where eggs are graded by quality and weight. Regulation (EC) 853, Annex I, Point 5.4 defines a packing centre as an establishment where eggs are graded by quality and weight], or
- to a “collector” [defined in Regulation (EC) 589/2008, Article 1(c) as an establishment registered in accordance with Article 6 of Regulation (EC) No 852/2004 to collect eggs from a producer for delivery to a “packing centre”, to a market selling exclusively to wholesalers whose undertakings are “approved” as packing centres, or to the food or non-food industry], which the slaughterhouses does not do.
To be sold to retail or catering establishments or sold at markets direct to consumers, shell eggs have to comply with the “Class A” definition of fresh eggs under the marketing requirements and be weighed, graded or supplied with the appropriate marketing information from a packing centre.
To be sold as “Class B” eggs, they must only be delivered to the food industry or to the non-food industry, but only if they bear a label with the dispatching packing centre details, packing centre code and marked with “Class B” or “B” and display a packing date.
The FBO of the slaughterhouse could not be “registered” under Article 6 of Regulation (EC) 852/2004 as a “collection centre”, as collectors by definition transport ungraded eggs from registered producers to packing centres, the food industry or non-food industry, which they do not!
Slaughterhouses do not satisfy the relevant definitions of “a registered production site” or “a packing centre” and therefore FBOs cannot sell eggs direct to the final consumer in a retail transaction, to the food industry, from the “farm gate” or “door to door” and could not comply with the labelling and traceability requirements required in the marketing legislation.
Even though FBOs can be exempt from the requirements of the EU Hygiene Regulation (EC) where there is a “direct supply, by the producer, of small quantities of primary products to the final consumer or to local retail establishments directly supplying the final consumer”, this does not exempt them from the EU marketing regulations and the slaughterhouse FBO is not a producer of eggs as a primary product or a production site as defined above [Regulation (EC) 852/2004, Article 1(2)(d) and Regulation (EC) 853/2004, Article 1(3)(c)].
Q4. Can an FBO give ungraded shell eggs away to plant staff for free?
No. Giving or selling eggs to plant staff will fall under the definition of “marketing” [Regulation (EC) 589/2008, Article 1(n) means holding eggs for the purpose of sale, including offering for sale, storage, packing, labelling, delivery, or any other form of transfer, whether free of charge or not] and would be unlawful under the egg marketing legislation.
Member States may exempt FBOs from the requirements of Regulation (EU) 1308/2013, where eggs are sold directly to the final consumer by the producer:
(a) on the production site, or
(b) in a local public market or by door-to-door selling in the region of production of the Member State concerned (Regulation (EU) 1308/2013, Annex VII, Part VI, Point I. 2).
Slaughterhouse FBOs are not “producers” and cannot take advantage of this exemption.
Eggs sold by “producers” to final consumers at local public markets in the region of production of the Member State, must still be marked with quality and weight grading stating either - Class A or "fresh" or Class B. Member States may exempt producers from this requirement who have no more than 50 laying hens, provided that the name and address of the “producer” is indicated at the point of sale.
Q5. Can an FBO harvest fully formed eggs for their own personal consumption?
Yes. The domestic preparation, handling or storage of food for private domestic consumption is exempt from the scope of Regulations (EC) 853/2004, Article 1(3)(c) / Regulation (EC) 178/2002, Article 1(3) and would fall outside the definition of marketing under Regulation (EC) 589/2008, Article 1(n).
Q6. Can eggs recovered from the lairage or from transport crates prior to the birds death that are cracked, be sold to the food industry as eggs, or for the manufacture of “egg products” or “liquid eggs”?
No. The use of eggs with cracked shells is only permitted for use as a raw material, if they have been consigned directly from a “production” site or “packing centre” to an approved establishment for the manufacturer of liquid eggs or to a processing establishment where they must be broken as soon as possible [Regulation (EC) 853/2004, Annex III, Section X, Chapter II, Part II, point 1].
Q7. Can “eggs” retrieved from a dead bird on the processing line during evisceration or pulled from a dead bird prior to evisceration, which have not been physically laid naturally by the bird prior to its death and the shells of which are not fully developed, be sold for human consumption?
Raw materials used for the manufacture of egg products must come from eggs, the shells of which are fully developed and which must have been supplied from the establishment of “production” or from a “packing centre”.
Eggs that have not been laid prior to the animal’s death, cannot be “eggs” by definition, but would fall under the definition of “offal” in Regulation (EC) 853/2004, Annex I, Point 1.11 and 1.12:
- Offal - fresh meat, other than that of the carcase, including viscera and blood,
- Viscera - includes organs of the thoracic, abdominal and pelvic cavities as well as the trachea and oesophagus and in birds the crop.
As offal, the “egg” could be harvested as an edible co-product, ID marked and sold for human consumption, however, there does not appear to be a market for such a product and FBOs are consigning such products as Category 3 ABP.
Q8. Is it lawful for FBOs to harvest for human consumption the ova / yolk (in the early stages of its development before the point at which the albumen / shell membrane is added) to be used in soups for the Jewish and other communities?
Yes, the unformed proto eggs or ova would fall under the definition of “offal” in Regulation (EC) 853/2004 (see above). However, harvesting such products should only be done after the completion of a successful ante and post-mortem inspection at which there are no grounds to declare the carcase / offal unfit for human consumption.
Whilst an argument had been raised that a flexibility could be applied under the Hygiene Regulations for the harvesting of such proto eggs as a traditional method or product demanded by the Jewish and other communities as an ingredient for traditional soups etc. this is not the case, given that the harvesting of offal is a legally permissible activity, provided that a hazard analysis has been undertaken, risks are being managed, the product is treated hygienically, appropriately chilled to 3°C, ID marked and labelled correctly as a food product, with appropriate allergen labelling.
Q9. How are proto eggs currently being harvested?
When harvesting yolks for the kosher Market ova have been:
- collected carefully from the oviduct at the evisceration point after PMI, to ensure correlation to rejections is possible,
- repeatedly salted, soaked and washed before wrapping, ID marking and refrigeration or freezing,
- sold to the final consumer directly from the plant or to kosher shops that sell to the final consumer without modification to the product, variation in the ID marking or packaging used by the slaughterhouse,
- sold without the appropriate labelling required when selling direct to the final consumer or to mass caterers under Regulation (EU) 1169/2011, namely:
- durability marking, Article 24 and Annex X,
- instructions for use, Article 27,
- storage conditions or conditions for use, Article 25,
- labelling for certain substances or products causing allergies or intolerances, Article 21 and Annex II.
Q10. Can this harvesting method mitigate against potential pathogens and the risk that the unprotected egg yolk can become contaminated during extraction and further handling?
Science colleagues provided the following advice:
- Concerns over the risk of salmonella, mycobacterium avium (M. avium) etc. in laying hen flocks should be evident in the FCI. UK flocks are either vaccinated or state negative results after intensive testing (e.g. every 15 weeks) as such the health risk is minimal.
- It is highly unlikely that laying hens will have had antimicrobials administered that require a withdrawal period, but this is monitored by VMD who sample regularly.
- When assessing the microbiological risk relating to pathogenic survival in partially formed eggs after a salting and cleansing procedure, risks may be mitigated where the flocks from which the eggs were laid are Salmonella (SE and ST) negative and this is reported in the FCI.
- Based on historical CCIR, the presence of M. avium in slaughtered flocks is unlikely, but that should be determined by PMI findings in every batch (i.e. - presence of tubercular nodules in intestine, liver, spleen, ovaries and bone marrow but the pulmonary lesions, which are a striking feature of tuberculosis in other species, are rarely observed in birds).
- M. avium can cause infections in people who are immunocompromised, and evidence indicates that M. avium can survive the acidic conditions in the human stomach (pH <3) and infection could occur via the gastrointestinal tract.
- The yolk should be heat treated for the appropriate time / temperature (70°C for 2 minutes or equivalent), which should destroy microbial vegetative cells. It would be advisable to have a label indicating that adequate heat treatment is necessary, in the same way other potential pathogen associated with fresh meat or offal can be eliminated or reduced to an acceptable level if cooked correctly. Yolks inserted into soup raw, may result in an insufficient heat treatment depending on the stage at which the egg is added to the soup and its temperature.
- The procedure of salting yolks before being added to the soup will not in itself mitigate against M. avium, as it is salt tolerant and research undertaken on casings, found M. avium survival in salted guts.
Q11. What is the appropriate disposal route for fully formed shell eggs laid naturally or partially formed eggs harvested from the dead animal?
If the FBO cannot legally harvest eggs for human consumption / to plant staff or to consume them personally, eggs must be disposed of as Category 3 Animal By-products (Article 10, K (ii) of Regulation (EC) 1069/2009). Failure to do so can be enforced through the service of a notice under Regulation 25 (2) of the domestic Animal By-products (Enforcement) England Regulations 2013 to require their disposal. If eggs originate from an animal rejected with a condition communicable through the egg itself to humans or animals, it would be a Category 2 ABP. In practical terms this is unlikely but would include entire carcases rejected prior to extracting the egg from the hen, and there are very few conditions communicable through eggs to human or animals linked to a full rejection.
Likewise, it is impossible to correlate the bird from which a fully formed shell egg has been derived when they are being collected in the lairage or from transport crates. This loss of correlation may have implications for eggs from birds subsequently rejected later in the process.
Definitions
Regulation (EC) 853/2004, Annex I, defines:
- ‘Eggs’ means eggs in shell, other than broken, incubated or cooked eggs — that are produced by farmed birds and are fit for direct human consumption or for the preparation of egg products.
- ‘Liquid egg’ means unprocessed egg contents after removal of the shell.
- ‘Cracked eggs’ means eggs with damaged shell and intact membranes.
- ‘Packing centre’ means an establishment where eggs are graded by quality and weight.
- ‘Egg products’ means processed products resulting from the processing of eggs, or of various components or mixtures of eggs, or from the further processing of such processed products.
- ‘Offal’ means fresh meat other than that of the carcase, including viscera and blood.
- ‘Viscera’ means the organs of the thoracic, abdominal and pelvic cavities, as well as the trachea and oesophagus and, in birds, the crop.
Regulation (EC) 589/2008, Article 1 defines:
- ‘Collector’ means any establishment registered in accordance with Article 6 of Regulation (EC) No 852/2004 to collect eggs from a producer for delivery to a packing centre, to a market selling exclusively to wholesalers whose undertakings are approved as packing centres, or to the food or non-food industry.
- ‘Eggs’ means eggs in shell — other than broken, incubated or cooked eggs — that are produced by hens of the species Gallus gallus and are fit for direct human consumption or for the preparation of egg products.
- ‘Broken eggs’ means eggs showing breaks of both the shell and the membranes, resulting in the exposure of their contents.
- ‘Marketing’ means holding eggs for the purpose of sale, including offering for sale, storage, packing, labelling, delivery, or any other form of transfer, whether free of charge or not.
- ‘Operator’ means a producer and any other natural or legal person involved in the marketing of eggs.
- ‘Production site’ means an establishment keeping laying hens, registered in accordance with Commission Directive 2002/4/EC (1).
Regulation (EC) 178/2002, Article 2 defines:
- ‘Primary products’ means products of primary production including products of the soil, of stock farming, of hunting and fishing.
Sections
1. Introduction
In this section
1.1 Legislation
1.1.1 Applicable regulations
- Assimilated Regulation (EC) 852/2004
- Assimilated Regulation (EC) 853/2004
- Assimilated Regulation (EC) 2073/2005
- The Hygiene (Wales) Regulations 2006 (as amended)
- The Food Safety and Hygiene (England) Regulations 2013 (as amended)
- Assimilated Regulation (EU) 2017/625
- Assimilated Regulation (EU) 2019/625
- Assimilated Regulation (EU) 2019/626
- Assimilated Regulation (EU) 2019/628
- Assimilated Regulation (EC) 999/2001
- The TSE (England) Regulations 2018
- The TSE (Wales) Regulations 2018
- Regulation (EC) 99/2002
- Regulation (EC) 1760/2000
- Regulation (EC) 178/2002
- The General Food Regulations 2004
- The Official Controls (Animals, Feed and Food) (England) Regulations 2006
- The Official Controls (Animals, Feed and Food) (Wales) Regulations 2007
- The Trade in Animals and Related Products Regulations 2011
- The Trade in Animals and Related Products (Wales) Regulations 2011
- Assimilated Regulation (EC) 1069/2009
- Assimilated Regulation (EC) 142/2011
- The Animal By-Products (Enforcement) (Wales) Regulations 2014
- The Animal By-Products (Enforcement) (England) Regulations 2013
- The Cattle Identification Regulations (England) / (Wales) 2007 (as amended)
- The Disease Control (England) / (Wales) Order 2003 (as amended).
1.2 FBO responsibilities
1.2.1 Duties of the FBO
It is the Food Business Operator’s (FBO) duty to ensure that imported meat and meat products, and meat and meat products for export entering the establishment comply with all relevant legislation.
FBOs must have appropriate food safety management systems in place to ensure that imported carcases and carcases for export meet requirements for removal of specified risk material (SRM).
1.2.2 Notification of imported beef
The FBO must contact their Inspection Team Leader (ITL) 72 hours in advance of an imported beef delivery from countries with a controlled or undetermined BSE risk containing vertebral column (VC).
The ITL will arrange appropriate FSA verification of the process and controls in place, as required.
2. Imports
In this section
2.2 Consignments returned from MS
2.4 Additional duties for imported beef
2.1 Imports from MS
2.1.1 Background
Within the Single Market, trade in animal products and live animals between Member States (MS) is subject to harmonised EU rules.
All MS must operate a system of intensified checks at the point of origin, to ensure that only those consignments complying with EU rules may enter intra-EU trade.
Single market rules dictate that there are no routine animal or public health checks at ports of entry from other EU MS, but random and non-discriminatory spot checks at the place of destination are permitted.
Most products of animal origin consigned to the UK from other MS must originate in an approved establishment and be accompanied by an official health certificate or commercial document (depending on the product). The certificate or document would contain information on the origin and destination of the products and may include public or animal health attestations.
2.1.2 Reason for checks
The OV carries out random verification that the meat complies with the EU rules. These checks are to ensure that fresh meat, poultry meat and other animal products comply with animal and veterinary public health conditions relating to trade. For example, checks are made on health marking or identification marking and accompanying documentation.
Regulation: Regulation (EC) No. 853/2004, Article 5
Random checks should be made to ensure:
- SRM controls have been fully complied with
- the meat is properly health marked or identification marked
- accompanying documentation is correct and has been completed accurately
- hygiene rules have not been breached
- any seals on packaging are intact
- the consignment has not come from a restricted region subject to specific animal health controls
Note: The FSA is no longer required to carry out 100% checks on compliance with SRM removal requirements. However, the OV must verify that the FBO has robust systems in place to ensure that meat entering the food chain is free from SRM.
2.1.3 Action post check
If the initial checks on health marking or identification marking and documentation raise suspicion that rules have been breached, the OV is to use professional judgement on what further action is appropriate.
2.2 Consignments returned from MS
2.2.1 Meat returned from MS
Consignments of meat and other animal products which originated in GB can be rejected by a competent authority of another MS for failure to comply with the regulations. For example, meat incorrectly or inadequately health marked or identification marked.
These consignments may only be returned to GB if authorisation is granted by the following authorities:
England:
- Defra Imports and EU Trade Team
Nobel House
Area 46
17 Smith Square
London
SW1P 3JR
020 7238 6000
ITAP@defra.gsi.gov.uk
Wales:
-
James Gibbs
Exotic Animal Diseases Branch
Office of the Chief Veterinary Officer
Department for Environment and Sustainable Development
Welsh Government
Cathays Park
Cardiff
CF10 3NQ
02920 823831
james.gibbs@wales.gsi.gov.uk
2.2.2 FSA OV action
The licence from the competent authority of the MS will provide the reason for return. Upon receipt at the GB plant, the OV should establish whether the meat:
- poses a risk to human or animal health
- fails to comply with the relevant regulations, or
- needs to be placed under restrictions, for example pending further decisions for salmonella cases
If the OV suspects that the returned meat or animal products are unsatisfactory, then action should be taken.
2.3 Third country imports
2.3.1 Background
Each import consignment must:
- come from a country approved to export that type of product to the EU
- be accompanied by animal health and public health certification
- come from EU-approved premises
- enter the EU through a Border Control Post (BCP) where veterinary checks must be carried out
Remember that general EU regulations will also apply. Link to lists of third country Product Establishments:
2.3.2 Border Control Posts (BCPs)
BCPs operate under the responsibility of a Portal OV. The designation for a Portal OV is different to the designation of OV by the FSA. Portal OVs are appointed by a local authority (LA), or Port Health Authority and designated by Defra after completion of a specific Defra led training course.
2.3.3 Checks on third country imports
The table below outlines the checks on third country imports.
| Step | Details |
|---|---|
| 1 | Meat, and other products of animal origin, from third countries outside the EU are checked at an authorised BCP on the EU’s external border. This could be a BCP in GB or elsewhere within the EU. |
| 2 | After satisfactory inspection at the BCP, the Portal OV issues a certificate confirming that veterinary checks have been carried out. The certificate is known as a Common Health Entry Document (CHED). Reference: See Annex 1 in this chapter. |
| 3 | The original health certificates issued by the originating country are retained at the BCP and an authenticated copy is given to the transporter. |
| 4 | CHED travels with the consignment to the first approved establishment whilst under customs bond. There is no need for the authenticated copy of the CHED to accompany the load. |
| 5 | From the first approved establishment, the meat then travels with commercial documents only. |
| 6 |
The FSA OV at any subsequent approved establishment will make random checks on consignments and accompanying paperwork. These checks include: EU rules on the origin of the product (authorised country and establishment)
|
2.3.4 Legislation relating to inspection and checking of imports
Regulations:
- Regulation (EC) 999/2001 Annex IX, Chapter C, (as amended)
- Regulation (EU) 2017/625, Articles 43 to 57.3, Article 65, Article 66.6
- The Trade in Animals and Related Products Regulations 2011, Regulations 15, 19 and 20
- The Trade in Animals and Related Products (Wales) Regulations 2011, Regulations 15, 19 and 20
2.3.5 Documentation missing
All third country meat, regardless of the point of entry, should arrive at the first point of destination accompanied by a CHED.
If documentation for a consignment selected for checking is missing, contact Operations at York.
2.3.6 UK meat returned from third country
UK meat and animal products returned from outside the EU are subject to the conditions laid down in:
- the Trade in Animals and Related Products Regulations 2011, Regulation 27 (in England)
- the Trade in Animals and Related Products (Wales) Regulations 2011, Regulation 27
Re-import will only be permitted if there is evidence that the product has not lost its EU status. The normal requirements are:
- the consignment is returned with the original export health certificate
- a statement giving the reasons why the consignment is being returned
- a guarantee that the conditions governing storage and transport have been observed and that the product has not been handled
- in the case of products in a sealed container, a certificate from the carrier stating that the contents have not been handled or unloaded
- in the case of products not in a sealed container, a declaration that it has not undergone any handling other than, in the case only of packaged products, loading and unloading of unopened packages
The OV should check to see that all such consignments are accompanied by a CHED and the necessary third country guarantees, and that EU requirements concerning marking are still met.
2.4 Additional duties for imported beef
2.4.1 Overview of OV responsibilities
The OV must carry out random inspection of consignments of imported meat in the cutting premises to verify FBO compliance with SRM controls to ensure that imported beef is free from SRM (spinal cord).
2.4.2 Verification checks
The FSA is no longer required to carry out 100% checks on compliance with SRM removal requirements. However, the OV must verify that the FBO has robust systems in place to ensure that meat entering the food chain is free from SRM.
The level of checks will depend on the meat being imported. Random unannounced verification inspections should be carried out by an authorised officer (AO), with further intelligence based inspections as appropriate (taking into account status of the country of origin).
Full checks on the FBO’s procedures must be carried out as part of the audit process (see chapter 4 on ‘Audit, HACCP and verifying operator’s own checks’). As part of the audit, the OV must verify that the FBO has robust systems in place to ensure that meat entering the food chain is free from SRM.
2.4.3 TSE Regulations
Imported animals and animal products must meet the requirements of Regulation (EC) 999/2001 (as amended) which lays down rules designed to prevent, control and eradicate certain Transmissible Spongiform Encephalopathies (TSEs).
Regulation (EC) 999/2001 applies to production and placing on the market of live animals and products of animal origin, and in certain specific cases to exports.
2.4.4 Definition of SRM in imported beef
SRM in beef imported from countries with a controlled or undetermined BSE risk is defined as:
| All ages |
|
|---|---|
| Over 12 months | Skull excluding the mandible and including the brain and eyes, and spinal cord |
| Over 30 months | VC including the dorsal root ganglia, but excluding:
|
Note: Before reporting SRM, remember to check the age of the animals on the documentation. Only the intestines, tonsils and the mesentery in imported beef from animals under twelve months of age are designated as SRM.
2.4.5 Permitted cuts containing SRM vertebral column
It is permitted to import from other MS:
- whole carcases
- half carcases
- half carcases cut into no more than 3 wholesale cuts
- quarter carcases
with the VC remaining, providing that they are sent directly to a licensed cutting plant which holds an additional approval to remove bovine VC.
Reference: See chapter 2.7 on ‘SRM’ for further details on additional approvals to remove bovine VC.
2.4.6 Non-permitted cuts containing SRM vertebral column
Smaller cuts of beef containing VC are not permitted to be imported into GB from countries with a controlled or undetermined BSE risk and are an illegal import of SRM, unless accompanied by a declaration stating they are derived from animals which are under 30 months at the time of slaughter.
Reference: See topic 2.5.16 on ‘Unsatisfactory consignments: SRM’ in this chapter for additional information.
2.4.7 Operator responsibility for beef from countries with a controlled or undetermined BSE risk containing vertebral column
The FBO must contact their ITL 72 hours in advance of an imported beef delivery from countries with a controlled or undetermined BSE risk containing VC.
The ITL will arrange appropriate FSA verification of the process and controls in place.
2.4.8 Commercial document
A commercial document must accompany the load specifically indicating the number of bovine carcases or cuts:
- from which removal of the VC is required
- from which removal of the VC is NOT required
Reference: (EC) 999/2001 (as amended) Annex V, 11 3 (b).
2.4.9 Labelling requirements
Bovine carcases, or parts of carcases, containing VC from countries with a controlled or undetermined BSE risk if over 30 months of age will have a red striped label attached as described in Regulation 1760/2000 and the VC must be removed at a cutting plant which holds an approval for its removal.
EU legislation requires the label to indicate:
- the ID number for the animal (or relevant group of animals)
- the approval number for the slaughterhouse / cutting establishment and / or
- the MS of slaughter, cutting or export
Reference: (EC) 1760/2000, Article 12 requires the label to be attached to the meat, pieces of meat or to the packaging material.
2.5 Unsatisfactory consignments of imported meat
2.5.1 Categories
Unsatisfactory consignments may be classified into the categories listed below:
- Public health:
- wrapping and packaging
- contamination / fitness for consumption
- health marking
- identification marking and labelling
- health certificates / commercial documents
- temperature
- disease / animal health
- SRM presence
- unsatisfactory sampling results.
- Unchecked consignments:
- that have not been brought into GB through an approved BCP
- that have been removed from a BCP without a CHED or the authority of the OV at the BCP
- that were transferred from the BCP to a destination not specified in the CHED.
- Animal health:
- notifiable disease detected
- identified as likely to cause a serious hazard to humans or animals
- an uncertified product comes from an area infected by an epizootic disease
- documentation fails to certify the consignment is free from disease.
2.5.2 Action
The action taken for the unsatisfactory consignments will depend upon the severity of the problem and instruction given by the FSA.
It is necessary that any unsatisfactory consignments are reported in accordance with this chapter.
2.5.3 Provisions applicable to the wrapping and packaging of foodstuffs
Wrapping and packaging must conform to the requirements of Regulation (EC) 852/2004 Annex 2, Chapter X.
Checks should be made to ensure the integrity of the packaging and that the packaging fully protects the meat from the risk of contamination.
2.5.4 Health marking / identification marking
Imported consignments received in approved establishments must bear the appropriate health mark / identification mark.
Reference: Health marking requirements of Regulation (EC) 853/2004, Articles 5 and 6.1(c)(i), Regulation (EU) 2017/625, Article 18.4, and Commission Implementing Regulation (EU) 2019/627, Article 48 and Annex II. Identification marking requirements of Regulation (EC) 853/2004, Articles 5 and 6.1(c)(i) and Annex II.
2.5.5 Documentation
Consignments received in approved establishments must be accompanied by the appropriate documentation.
Reference: See ‘FSA role: checks on imported fresh meat’ in this chapter for additional information.
2.5.6 Temperature limits
Fresh meat must not be exported from the country of origin, including EU MS, unless it has been chilled to a specified internal temperature, and maintained at that temperature throughout the period of transport. The internal temperature that the meat must not exceed is listed in the tables below:
| Type of meat | Part of meat | Maximum temperature (°C) |
|---|---|---|
| Fresh meat (red) | Carcases and cuts | +7 |
| Fresh meat (red) | Offal | +3 |
| White meat | Poultry carcase | +4 |
| White meat | Poultry offal | +4 |
| Wild game | Large | +7 |
| Wild game | Small | +4 |
| Type of meat | Maximum temperature for storage and transport (°C) |
|---|---|
| Mince meat (Fresh) | +2 |
| Mince meat (Frozen) | -18 |
| Meat preparations (Fresh) | +4 |
| Meat preparations (Frozen) | -18 |
| Mechanically separated meat (Fresh) | +2 |
| Mechanically separated meat (Frozen) | -18 |
2.5.7 Unsatisfactory consignment from MS: public health, legislation
Action may be taken for any import which does not meet animal or public health conditions relating to the legislation.
Regulations:
- Regulations (EC) 852/2004, 853/2004
- Regulation (EU) 2017/625
- Regulation (EC) 2073/2005 (as amended)
- The Food Hygiene (Wales) Regulations 2006
- The Food Safety and Hygiene (England) Regulations 2013
- Regulation (EC) 178/2002, Article 14
- The General Food Regulations 2004
- Regulations (EC) 1069/2009 and 142/2011
- The Animal By-Products (Enforcement) (No 2) (Wales) Regulations 2011
- The Animal By-Products (Enforcement) (England) Regulations 2013
2.5.8 Unsatisfactory consignment from MS: public health, OV action
The OV is to take the action detailed in the following table in the event of an unsatisfactory consignment from a MS due to a public health issue.
Note: Operations will advise the OV in writing, by fax or e-mail, of the action required for any unsatisfactory meat.
Reference: See topic 2.5.16 on ‘Unsatisfactory consignments: SRM’ for additional guidance.
Unsatisfactory consignment from Member States: OV action
| Step | Action |
|---|---|
| 1 |
Serve either:
to detain unsatisfactory meat, until an FSA decision is provided. The costs incurred to comply with any Notices issued are borne by the owner or his agent. |
| 2 | Copy completed notice to the Corporate Support Unit (CSU) York Transactions Team by email. |
| 3 |
Complete a form IMP 8/1 Imported meat: Unsatisfactory consignments from MS / third countries, as fully as possible. Email the completed form to the Operations team, then send it by post to Operations, Food Standards Agency, York. Reference: See chapter 9 on ‘Forms’ for form IMP 8/1. Operations will inform the relevant technical and policy team. |
| 4 | Take photographs of the non-compliant consignment and email or post these to CSU York Transactions Team. |
| 5 | Email copies of the commercial documentation, where available, to CSU York Transactions Team. |
| 6 | Operations will contact the OV when a decision is made on the action to take, so the OV can require the consignment to be:
Where the product must be destroyed as an ABP, request voluntary surrender and oversee staining (where required) and disposal. Where the FBO refuses to dispose of a consignment, the OV should require its disposal in accordance with the ABP (Enforcement) (England) / (Wales) Regulations. A Notice for the Disposal of ABP (ENF 11/12) should be served for its disposal, if required. |
| 7 |
Confirm to the CSU York Transactions Team when the consignment has been disposed of, released or returned. Reference: See point 2.5.17 ‘Disposal of unsatisfactory consignments’ in this section for additional information. |
2.5.9 Communication with exporting country
It may be necessary for the FSA or Defra to raise the matter with the exporting country. This will be done by the Chief Veterinary Officer (CVO).
Caution: The OV must not take the matter up directly with the exporting country.
2.5.10 Unsatisfactory consignment from third countries: public health, legislation
Where meat imported from a third country is suspected to be unfit for human consumption, the OV has powers to require destruction or re-export.
Note: The OV should not exercise these powers, but should follow the process below. Operations will advise the OV (in writing, or email) of the action for any unsatisfactory meat.
Reference:
- Regulation (EU) 2017/625, Articles 65 to 72
- The Trade in Animals and Related Products Regulations 2011, Regulation 20
- The Trade in Animals and Related Products (Wales) Regulations 2011, Regulation 20
- Regulation (EU) 2017/625
- Regulations (EC) 1069/2009 and 142/2011
- The Animal By-Products (Enforcement) (No 2) (Wales) Regulations 2011
- The Animal By-Products (Enforcement) (England) Regulations 2013.
2.5.11 Unsatisfactory consignment from third country: public health, OV action
The OV is to take the following action in the event of an unsatisfactory consignment from a third country:
Unsatisfactory consignment from third country: Official veterinarian action
| Step | Action |
|---|---|
| 1 | Serve a detention notice under Regulation (EU) 2017/625, Article 66(6) (Form ENF 11/32). |
| 2 | Copy completed notice to the CSU York Transactions Team by email (access contact details in chapter 1 on ‘Introduction’). |
| 3 |
Complete a form IMP 8/1 Imported meat: Unsatisfactory consignments from MS / third countries, as fully as possible. Email the completed form to the CSU York Transactions Team, then send it by post to Operations, Food Standards Agency, York. Reference: See chapter 9 on ‘Forms’ for form IMP 8/1. Operations will inform the relevant technical and policy team(s). |
| 4 | Operations will notify the OV if further information is required. For example, photographs, copies of commercial documentation. |
| 5 | Take relevant photographs wherever possible and email or post these to the CSU York Transactions Team. |
| 6 | Operations will contact the OV when a decision is made on the future of the consignment. Operations cascades information to the OV, so that they:
|
| 7 |
Details of any non-conforming products or illegally imported products from third countries must be entered on an IIT1 form. Contact Operations (contact details as above) for a blank copy of form IIT1 for completion. Completed IIT1 forms should be sent to: England: iit@defra.gov.uk Wales: james.gibbs@wales.gov.uk Also send a copy of the completed IIT1 to CSU York Transactions Team. |
| 8 |
Confirm to Operations when the consignment has been disposed of, released or returned. Reference: See 2.5.17 on ‘Disposal of unsatisfactory consignments’ in this section for additional information. |
2.5.12 Communication with exporting country
It may be necessary for the FSA or Defra to raise the matter with the exporting country. This will be done by the Chief Veterinary Officer (CVO).
Caution: The OV must not take the matter up directly with the exporting country.
2.5.13 UK BCP imports
In the case of meat imported through a UK BCP, the OV should immediately notify Operations who will inform the appropriate body.
Unsatisfactory consignment from third country: Official veterinarian action
| Meat imported | Operations contact |
|---|---|
| Through a UK BCP | APHA official responsible for the BCP |
| Through another MS BCP | FSA to contact MS |
2.5.14 Unchecked consignments
Where evidence exists that consignments may have been:
- imported into GB other than through an authorised BCP
- removed from a BCP without a CHED or the authority of the BCP OV
- transported from a BCP to a destination other than the one specified in the CHED, the OV should follow the steps in the following table:
Unchecked consignments
| Step | Action |
|---|---|
| 1 |
Seize and detain the consignment under The Trade in Animals and Related Products Regulations 2011, to conduct further checks. Regulation:
|
| 2 | Copy completed notice to the CSU York Transactions Team by email (access contact details, Chapter 1, page 1-5). |
| 3 |
Complete form IMP 8/1 as fully as possible. Email the completed form to Operations (contact details as above), then send it by post to Operations, Food Standards Agency, York. Reference: See chapter 9 on ‘Forms’ for form IMP 8/1. Operations will inform the relevant technical and policy team(s). |
| 4 | Operations will notify the OV if further information is required. For example, photographs, copies of commercial documentation. |
| 5 | Take relevant photographs wherever possible and email or post these to Operations. |
| 6 |
Once the OV has clarified whether the product constitutes an unchecked consignment, they should await an FSA policy decision via Operations. Operations cascades the information to the OV, so that the OV may:
Where the FBO refuses to deal with the consignment in accordance with the OV’s instructions, the OV may formally seize the consignment in accordance with the Trade in Animals and Related Products Regulations 2011 (and equivalent legislation in Wales), and require it to be disposed of, consigned outside the EU, or destroyed under the same conditions as above. An importer whose products are liable for seizure may also have breached Regulation 13 and 16 of TARP 2011 in England and Wales. Reference: See chapter 7 on ‘Enforcement’ for additional information. |
| 7 | Details of any non-conforming products or illegally imported products from third countries must be sent to Defra, Scottish Government or Welsh Government, as appropriate. Use the IIT1 form- see page 4-10 of this Chapter for details of where to obtain the form and where to send it once completed. |
| 8 |
Confirm with Operations when the consignment has been disposed of, released or returned. Reference: See 2.5.17 on ‘Disposal of unsatisfactory consignments’ in this section for additional information. |
2.5.15 Unsatisfactory consignments: animal health
A consignment may be considered unsatisfactory:
- if the presence of an agent responsible for a notifiable disease is detected
- if any cause likely to constitute a serious hazard to humans or animals is present
- that uncertified product comes from an area infected by an epizootic disease.
Examples:
- consignments imported from countries or regions that are not permitted due to disease outbreaks
- meat from FMD vaccinating countries.
Defra and APHA take responsibility for all animal health aspects of imports.
OV action:
The OV is to take the following action in the event of an unsatisfactory consignment due to an animal health issue.
Note: Operations will advise the OV in writing, by e-mail, of the action required for any unsatisfactory meat.
Official veterinarian action
| Step | Action |
|---|---|
| 1 |
Detain the meat until further advice is given by APHA via Operations. Meat from other MS: Detain under the Food Hygiene (Wales) Regulations 2006, Regulation 23 or 9(5) – form ENF 11/1 or ENF 11/26 / Food Safety and Hygiene (England) Regulations 2013, Regulation 25 or 10(1) – form ENF 11/1 or ENF 11/26 respectively. Meat from a third country: Where the meat has entered the UK through the appropriate BCP and following appropriate checks has been released onto the market, non-compliant meat should be detained using domestic powers as set out above. |
| 2 | Copy completed notice to the CSU York Transactions Team by email (access contact details in chapter 1 on ‘Introduction’). |
| 3 |
Complete form IMP 8/1 as fully as possible. Email the completed form to CSU York Transactions Team (contact details as above), then send it by post to Operations, Food Standards Agency, York. Reference: See chapter 9 on ‘Forms’ for form IMP 8/1. |
| 4 |
APHA contacts Operations when a decision is made on the future of the consignment. Operations cascades the information to the OV, so that they may release it for human consumption or: From MS: require destruction of the consignment as an ABP. If the FBO refuses, serve an ENF 11/12 to require its disposal. From third countries: require destruction of the consignment as an ABP, or any other appropriate measures necessary to protect human or animal health. Reference: See chapter 7 on ‘Enforcement’ for additional information and chapter 9 on ‘Forms’ for the ENF notices. |
| 5 | Details of any non-conforming products or illegally imported products from third countries must be sent to Defra, Scottish Government or Welsh Government, as appropriate. Use the IIT1 form: see topic 4.3.2 on ‘Unsatisfactory consignment from third country: OV action’ in this section for details of where to obtain the form and where to send it once completed. |
| 6 |
Confirm to Operations when the consignment has been disposed of, released, or re-despatched. Reference: See 2.5.17 on ‘Disposal of unsatisfactory consignments’ in this section for additional information. |
2.5.16 Unsatisfactory consignments: SRM
The steps below explain the OV role when SRM is identified.
Note: Operations will advise the OV in writing, by fax or e-mail, of the action required for any unsatisfactory meat.
Reference: See section 2.4 on ‘Additional duties for imported beef’ in this chapter for additional information.
| Step | Action |
|---|---|
| 1 | Inform the FBO of the situation and ensure the entire consignment is unloaded and inspected. |
| 2 |
Consignments from other MS: Detain the product under the Food Hygiene (Wales) Regulations 2006, Regulation 23 or 9(5) / Food Safety and Hygiene (England) Regulations 2013, Regulation 25 or 10(1) – form ENF 11/1 or ENF 11/26 respectively – until the FSA decision is given. Consignments from third countries: Detain the product under Regulation 2017/625, Article 66.1 - form ENF 11/32. |
| 3 | Copy completed notice to Food Incidents Team and Imported Food Team by email or fax (access contact details in chapter 1 on ‘Introduction’). |
| 4 |
Complete form IMP 8/1 as fully as possible. Email or fax the completed form to the Food Incidents and Imported Food teams (contact details as above) then send by post to the Food Incidents and Imported Food teams. Reference: See chapter 9 on ‘Forms’ for form IMP 8/1. |
| 5 | Email or fax copies of commercial documentation, where available, to the Food Incidents and Imported Food teams. |
| 6 | Take relevant photographs and email or post to the Food Incidents and Imported Food teams. |
| 7 |
The Food Incidents Team and / or the Imported Food Team will contact the OV when a decision is made on the future of the consignment. The Food Incidents and Imported Food teams will cascade information to the OV, so that they can: Consignments from other MS:
(See chapter 2.8 on ‘Animal by-products’ for additional information) Consignments from third countries:
|
| 8 | Details of any non-conforming products or illegally imported products from third countries must be sent to Defra, Scottish Government or Welsh Government, as appropriate. Use the IIT1 form: see topic 4.3.2 on ‘Unsatisfactory consignment from third country: OV action’ in this section for details of where to obtain the form and where to send it once completed. |
| 9 |
Confirm to the Food Incidents and Imported Food teams when the consignment has been disposed of or released. Reference: See topic 2.5.17 on ‘Disposal of unsatisfactory consignments’ in this section for additional information. |
2.5.17 Disposal of unsatisfactory consignments
Permitted disposal routes:
APHA will be able to advise on the approval status of plants receiving all categories of by-products from approved establishments, or see: Guidance for the ABP industry on GOV.UK
Reference: See chapter 2.8 on ‘Animal by-products’ for additional information.
Despatch
To prevent diversion of unfit consignments back into the human food chain, the OV must supervise the despatch of such consignments to approved rendering sites. The product must be removed from the establishment as detailed in this section, depending on the category of ABP.
Category 3 animal by-product:
It may be possible for disposal at an approved petfood plant as a Category 3 ABP. In such a case the OV is to:
- check the consignment is sent to an approved (registered) plant
- obtain an estimate of the weight of by-product despatched
- obtain confirmation of receipt at the pet food plant
Note: this may require liaison with a LA; contact Operations for assistance in making arrangements
- forward a copy of the receipt by email or post to Operations
2.6 Checks on imported live animals
2.6.1 General conditions and checks applicable to live animals entering GB from EU MS
Importers are required to give at least 24 hours’ notice in writing to the APHA office responsible for the place of destination of the animals of their intention to import from another MS.
The Notice should state:
- name, full postal address and telephone number of the importer
- name, full postal address and telephone number of the place destination
- date and time of arrival at place of destination
- details of the animal(s) being imported including quantity, breed, sex, passport number (if applicable), name (if applicable)
- name and full postal address of the premises of origin where the animal(s) are being imported from
- signature and date
Reference: The Trade in Animals and Related Products Regulations 2011- Part 2 (England and Wales).
The animals must be taken directly to the place of destination which must be the place of destination given on the export health certificate. The appropriate health certificate must accompany the consignment to its place of destination where it must be retained by the consignee for a minimum period of 12 months. The route plan or animal transport certificate must also accompany the consignment.
All consignees must, before the consignment is divided up or subsequently marketed:
- check either that each animal is identified and that they are accompanied by certification in accordance with community or national rules, or
- notify APHA of any irregularity or anomaly in either identification or in certification of the animals, or
- where an irregularity or anomaly in the certification is found, isolate the animals or products in question until a veterinary inspector has authorised their release in writing
2.6.2 General conditions and checks applicable to live animals entering Great Britain from a third country
Note: For background information only – this relates to the work of Portal OVs.
The following conditions apply in respect of animals originating in a third country which are either imported directly into Great Britain or are imported via another MS:
- live animals may only be imported into Great Britain through an approved BCP, which must be approved to handle the category of animal being imported; further information is supplied on Defra’s website
- importers must provide one working day's advance notice in writing ( email is acceptable) to the APHA official responsible for the BCP, of their intention to import; they must include the information contained in Part 1 of the CHED
- under Regulation (EC) 1/2005 and the Welfare of Animals (Transport) (England) Order 2006 and the Welfare of Animals (Transport) (Wales) Order 2007, importers must comply with the rules of animal welfare during transport
- on arrival, the animal(s) must be conveyed directly to the BCP where they will be subject to documentary and identity checks and, in most cases, to a physical examination
- importers must notify the APHA official responsible for the BCP if, for any reason, the arrival of a consignment is cancelled, postponed or delayed
- the animals will not be permitted to leave the BCP or the HMRC clearance area, except with a CHED provided by the Portal OV, confirming that all the veterinary checks have been carried out; the animals must be taken directly to the place of destination which must be the destination given on the CHED
- on arrival at the destination after leaving the BCP, animals for breeding and production may not be moved from the establishment unless authorised in writing by APHA
2.6.3 General conditions and checks applicable to live animals entering GB from a third country via other MS
The following conditions apply in respect of animals originating in a third country which are imported into Great Britain via another MS and where the veterinary checks at a BCP have been carried out in the MS of entry.
- The animal must, on arrival in Great Britain, be accompanied by a CHED and an authenticated copy of the original health certificate, issued by the Portal OV of the BCP. These documents must be retained by the consignee for at least 12 months and be made available, on request, to an officer of Defra, Scottish Government, Welsh Government or LA.
- Importers must notify APHA, in writing (email is acceptable), at least 24 hours in advance of the expected date of arrival in Great Britain. Details provided must include the nature of the consignment and the anticipated arrival date.
2.6.4 Imported cattle identification
All cattle born or imported (not direct to slaughter) into GB since 1 July 1996 must be registered with British Cattle Movement Service (BCMS) within 15 days of arrival at the holding. They must be moved to the licensed slaughterhouse accompanied by an official GB passport.
For imported animals with BCMS-issued passports, the passport will provide details of the country from which the animal was imported.
Direct to slaughter: MS
All cattle imported from EU MS or from Northern Ireland, Isle of Man or the Channel Islands and sent direct for slaughter must be accompanied by:
- a passport issued by the MS or island authority
- an export health certificate
- a Permit Authorising Movement of Cattle (BT1) issued by DAERA (for cattle from Northern Ireland only)
Direct to slaughter: third countries
Animals imported since 1 July 1996 from third countries, will be accompanied by a GB passport unless they are presented for slaughter within 15 days of import, in which case they will be accompanied by an export health certificate.
FSA action
Chapter 2.5 on ‘Animal identification’ sets out all FSA action required including:
- FSA responsibilities in relation to checking cattle ID
- action to take when cattle are not properly identified
2.6.5 Cattle from EU MS and countries with a controlled or undetermined BSE risk
Cattle imported live from all EU MS and countries with a controlled or undetermined BSE risk are subject to SRM controls when slaughtered in GB. These controls may vary from those for cattle born, reared and slaughtered in the UK and involve the removal of additional SRM from the carcase.
Example: Vertebral column in an approved cutting plant.
Reference:
(EC) 999/2001 (as amended), Annex IX, Chapter B on Imports of bovine animals.
Commission Decision 2007/453/EC for lists of countries or regions by BSE risk category.
2.6.6 Cattle from the Isle of Man and the Channel Islands
Cattle imported live from the Isle of Man and the Channel Islands are subject to the same SRM controls as the UK.
2.6.7 Welfare issues for imported animals
Resting
The animals may be rested prior to slaughter, provided that the health certificate is valid at the date of slaughter.
Legislation
- Regulation (EC) 1/2005 and
- The Welfare of Animals (Transport) (England) Order 2006, or
- The Welfare of Animals (Transport) (Wales) Order 2007 (WATO).
Documentation required during transportation
The person transporting animals must carry with them documentation stating:
- the origin of the animals and their ownership
- the place, date and time of departure
- the intended destination
- the expected duration of the intended journey
which must be made available to the CA on request.
Reference: (EC) 1/2005, Article 4, Paragraph 2.
OV duties
The OV should check that consignments have been transported in accordance with the legislation quoted above.
The OV at the slaughterhouse should carry out checks as part of their animal welfare inspections under Regulation (EU) 2017/625 to ensure that water and feeding intervals, journey times and resting periods comply with Regulation (EC) 1/2005, Annex I, Chapter V.
A model document setting out the journey details that must be recorded is contained in Regulation (EC) 1/2005, Annex II.
Note: Journey time begins when the first arrival is moved. A ‘long journey’ will be any journey exceeding 8 hours.
Additional provisions relating to long journeys are contained in Regulation (EC) 1/2005, Annex I, Chapter VI. Certain derogations from these provisions exist for journeys less than 12 hours.
Reference: WATO, Part 3.
Where the haulier breaches these provisions, the OV should refer such matters to APHA / LA.
Reference: See chapter 2.3 on ‘Animal welfare’ for additional information.
2.6.8 Incorrect certification or identification of imported animals
Confirmation to APHA
All the animals that are imported must be accounted for.
The OV should confirm to APHA that all the animals certified have arrived and have been slaughtered.
Unidentified animals
If the OV identifies that imported animals are accompanied by incorrect certification or cannot be readily identified, the animals must not be slaughtered, and they must immediately notify APHA, who will arrange for the animals to be examined by a VO.
Reference:
(EC) 853/2004, Annex II, Section II, Para 2(a) and Annex III, Section I, Chapter IV, Para 3
The Trade in Animals and Related Products Regulations 2011
The Trade in Animals and Related Products (Wales) Regulations 2011
APHA action
After examination, the APHA VO will either certify that the animals are:
- fit to be slaughtered and used for their intended purpose, or
- by notice in writing served on the person in charge of the animals, require the animals to be slaughtered and destroyed or re-exported (in exceptional circumstances), in each case at the expense of the importer.
Reference:
The Trade in Animals and Related Products Regulations 2011, Regulations 5 and 15
The Trade in Animals and Related Products (Wales) Regulations 2011, regulations 5 and 15
Disease Control (England) (Wales) Order 2003.
2.6.9 Detained animal arrangements
The various methods of detention available to the OV are detailed in chapter 7 on ‘Enforcement’. To summarise, detention is possible under:
- the Food Hygiene (Wales) Regulations 2006, Regulation 9 (5) / Food Safety and Hygiene (England) Regulations 2013, Regulation 10 (1) for consignments from other MS (form ENF 11/26 – Detention Notice)
- The Trade in Animals and Related Products Regulations 2011 and the equivalent in Wales, Regulation 20(6) for unchecked consignments from third countries (form ENF 11/33 Seizure and Detention Notice)
Protocol
As best practice, the OV and the FBO should agree a detention procedure within the establishment.
3. Exports
In this section
3.3 FBO and OV duties in third country approved establishments
3.1 Introduction
3.1.1 Purpose
For commercial purposes, FBOs may decide to trade their products outside of the UK.
It is the duty of the FBO to ensure compliance with all relevant legislation and/or other requirements regarding production of meat and meat products for export.
Depending on the country of destination and the type of product, the FBO may be required to put additional measures and controls in place regarding production of meat eligible for export.
3.1.2 EU Exports
EU trade is the movement of products to EU countries and Northern Ireland (NI). Meat and other products of animal origin (POAO) can be placed on the EU market provided they:
- have been produced in an approved meat establishment that is also listed as approved to export POAO to the EU (see Businesses approved to export to the EU)
- have been produced in compliance with the requirements of the appropriate EU Export Health Certificate
- have been subject to the official controls in retained Regulation (EU) 2017/625; and
- comply with any exceptional requirements and are not subject to specific restrictions.
Movement of meat and meat products to the EU and NI must be accompanied by an official Export Health Certificate (EHC)
Internal movement of meat and meat products within Great Britain (GB), to be exported to the EU, is generally accompanied by a Support Health Attestation (SHA).
3.1.3 Non EU exports
Meat and products of animal origin intended for export to Non-EU countries must comply with assimilated EU legislation, unless otherwise requested by the authorities of the importing country or established by the laws, regulations, standards, codes of practice and other legal and administrative procedures as may be in force in the importing country.
Any additional specific requirements of the importing country, as agreed between Defra and the importing competent authority, must also be adhered to. The specific requirements differ between countries and type of product. It is the exporter’s responsibility to be aware of and comply with any extra requirements.
Such additional requirements may include the specific approval of the establishment (‘site-specific approval’), continuous compliance with the requirements of the approval, and certification issued by the CA.
3.1.4 Competent Authorities
In the UK, competent authorities work together to safeguard public, animal and plant health, to promote animal welfare and protect consumers. Their activities are coordinated and they co-operate with each other in order to ensure that there are no gaps in delivery. The UK’s system of official controls is described in the UK’s Multi Annual National Control Plan (MANCP).
1. Defra is the Central Competent Authority for policy development and policy delivery in relation to exports and is responsible for the negotiations and agreements with the Competent Authorities of the importing countries regarding requirements for UK exporters.
Defra is also responsible for liaising with individual FBOs which are due to be inspected by the importing country representatives and liaising with them to identify any requirements following an inspection. This includes communication to FBOs of any remedial actions required to secure approval and notifying establishments if their approval to export to any country is to be suspended or revoked.
On behalf of Defra, FSA (FSS in Scotland and DAERA in Northern Ireland) assess compliance of establishments with the requirements of the importing country and make formal recommendation to Defra on whether an establishment should be recommended as eligible to export the relevant meat commodity to the specific country. Defra reserves the right to put forward an establishment for listing by the importing country or to de-list establishments for export. There is a Roles and Responsibilities document setting out the roles and responsibilities for the export of products of animal origin amongst Defra, APHA, FSA, FSS and DAERA. FSA has a memorandum of understanding (MOU) setting out the day-to-day working relationship with FSS in Scotland and a Service Level Agreement (SLA) for the provision of official controls services in slaughterhouses and cutting plants with DAERA in Northern Ireland.
2. APHA is an executive agency of Defra and the first point of contact for FBOs in Great Britain wishing to apply for listing as an exporter for a particular country. The APHA Centre for International Trade (CIT) is also responsible for the delivery of Export Health Certification. which must accompany exports of POAO to other countries, issuing export health certificates (EHC) to exporting FBOs for individual consignments.
They are also responsible for appointing qualified certifying OVs (in England, Wales and Scotland) who have completed the required training modules (Official Controls Qualification (Veterinary) OCQ(V)) to inspect export consignments and ensure that the EHC is completed correctly.
APHA contact details can be found online.
A list of practices with qualified OVs to inspect consignments and complete EHCs can be found online.
3. FSA is the central competent authority for food, feed safety and hygiene policy in England, Wales and Northern Ireland, and with regards to exports, is responsible for:
- Verifying that the plant intending to export is compliant with domestic and assimilated legislation.
- Establishing and maintaining a transparent and ongoing programme of export approval inspections and audits of meat establishments and standalone cold stores that require competent authority approval for specific non-EU export requirements.
- Providing conclusions and recommendations on Non-EU export audits to assess continuous compliance with the importing country requirements.
- Notifying Defra when the outcome of a FBO audit (see Chapter 4) provides evidence of unsatisfactory compliance in exporting premises (for example, ‘Improvement Necessary’ or ‘Urgent Improvement Necessary’).
- Notifying Defra when the outcome of an audit against Non-EU export requirements provides evidence of unsatisfactory compliance.
- Notifying Defra of progress on FBOs corrective actions and confirmation when such actions have been completed to a satisfactory level.
- Promoting/providing training to OVs working on behalf of FSA on compliance with export requirements.
- Verifying that OVs working in exporting plants are fully familiar, competent and effective in implementing FSA procedures for verification of export compliance in accordance with the FSA export guidance set out in the MOC.
3.1.5 Inward inspections from Non-EU countries
Importing countries visit the UK to conduct inspections at establishments which export, or wish to export to that country, to assess the controls in place.
In preparation for an inward inspection, Defra will issue a Core Script advisory document to assist both FBOs and competent authority officials in approved establishments. Defra holds this document and modifies it depending on the country and commodity concerned. OVs should consult this document and make it available to the FBO when first advised of a potential inward inspection. These visits fall into the following categories:
Systems audit
This involves assessment of UK wide national controls in place for meat hygiene, animal health, disease control, welfare, animal by-products, TSEs, import controls. It involves an in-depth assessment of the competent authority, structures, controls, legislation, and its implementation. It will include visits to competent authority offices, farms, laboratories, border control posts, as well as approved meat establishments. The choice of establishments to be inspected may be pre -selected by the visiting inspection team rather than the Competent Authority.
A successful outcome will result in the competent authority being granted permission to approve establishments for export to that country.
Site-specific premises approval by a Non-EU country
Updated [This involves some/all of the above assessments, but the meat establishments/standalone cold stores visited will be those wishing to obtain approval to export to that country. A successful outcome will result in only those establishments inspected by the importing country being approved for export.]
Study Visit
Updated [This occurs when the UK is seeking to access a new market and the importing country decides to conduct a study tour to focus in detail on certain aspects of the UK control systems. No approvals are given at this stage.]
3.2 Non-EU country approval
3.2.1 Site specific approval
Some non-EU countries require compliance with specific requirements, therefore, a site-specific approval is required.
Updated [It is the exporter’s responsibility to obtain any site-specific approval required by the importing country before export takes place. Approval inspections will vary depending on the importing country and may be carried out by officials from the importing country, or by the FSA Officials, who will recommend the site for approval to Defra.]
The FBOs should register their interest in specific country approval through APHA Centre for International Trade (CIT) in Carlisle (exports@apha.gov.uk or 03000 200 301).
The following countries and commodities require establishments to obtain a site-specific approval prior to export:
Country Site-Specific Approval
| Pork | Beef/ Lamb | Poultry |
|---|---|---|
| USA | Singapore (beef) | Eurasian Customs Union |
| South Korea | Japan (beef/lamb) | South Korea |
| Japan | China (beef) | Japan (heat processed poultry meat) |
| China | USA (beef /lamb) | - |
| Australia | - | - |
| Mexico | - | - |
Some of these countries require approval of all sites in the food chain, including stand-alone cold stores, whereas others require approval of the slaughter / cutting sites only (for example, Singapore). Specific APHA issued Notes for Guidance (NFG) should be consulted in each case before certification is issued. These can be found online.
Updated [Standard Operating Procedures (SOPs) are essential where specific approval is required for export. SOPs should meet the requirements of the particular country for the required products to be exported from intake to dispatch and should be reviewed when there is a change or, at least, once a year. SOPs should be authorised, signed and dated by the FBO before production for export commences. FBOs should train their staff on the SOPs and monitor and verify that they are effectively implemented. SOPs should be regularly verified by the FSA.
Required Methods of Operation Procedures (RMOPs) are also required for certain countries such as USA and Canada (in the case of Precursor Material of bovine origin or Finished Raw Ground Beef Product), where specific operational conditions must be met. The RMOP is a formal version of an SOP and needs to be signed by the FBO, the site OV and the FSA representative, which will be either the FVL or FVC.]
Note: All the establishments receiving new export approval visit must first be fully approved by the competent authority (FSA or LAs)
3.2.2 Listing of establishments
Updated [Some countries, such as the Republic of South Africa and Canada, only require premises and commodities to be formally listed on the website of the competent authority in those countries before exports can commence. These countries accept domestic equivalence and do not require site-specific approval; however, in order to demonstrate compliance with domestic requirements, their last FBO audit outcome must be either ‘Good’ or ‘Generally
Satisfactory’ in FSA approved establishments, or compliant in their latest food hygiene inspection in establishments under the Local Authorities (e.g., stand-alone cold stores). The FSA website can be consulted for the most up to date audit categories. A list of the current audit outcomes is available at the following link: Meat Establishment Audits (food.gov.uk)]
It is essential that in all of the above cases, any change to FBO trading name and details is notified to APHA CIT to avoid rejection of consignments on arrival. If FSA becomes aware of a change that has not been notified, the FSA official can notify APHA CIT making sure that the FSA Exports Team is also informed.
The requirement for an establishment to be listed on a country website will be detailed in the associated APHA issued Notes for Guidance for that country and should be consulted in all cases prior to export.
3.2.3 Summary of process for Non-EU country exports approval
| Step | Action |
|---|---|
| 1 | The FBO contacts APHA Centre for International Trade (CIT) in Carlisle to apply for approval to export to a specific country (exports@apha.gov.uk) |
| 2 | APHA CIT Product Exports Team sends the application form to FSA Exports Team, copied to Defra Market Access Team, who log this on their ‘Approval Tracker’ spreadsheet. |
| 3 | The FSA Exports Team checks the most recent FBO audit report (AUD 9/3); establishments with audit outcomes of “Improvement Necessary” and “Urgent Improvement Necessary” are not eligible for approval to export to another country. In case of stand-alone cold stores, the FSA Exports Team checks the levels of compliance in the most recent food hygiene inspection carried out by the Local Authorities. |
| 4 | FSA Exports Team writes to the applicant setting out the specific export approval process, requirements and costs, seeking written confirmation that they understand these and wish to continue (which forms the basis of their contract with the FSA to carry out this non-regulatory work). |
| 5 | If the establishment confirms they are content to continue, FSA Exports Team forwards this confirmation to FSA Approvals Team asking for them to process the application. FSA Approvals Team will allocate the application to the FVL who will contact the FBO for them to get ready for an export approval assessment. |
| 6 |
The export approval inspection visit is carried out by the FVL. FVLs and FVCs must be confident with the export requirements as well as colleagues from the SDP (OVs and AMs/TMs working in the plant wishing to export). The approval in slaughterhouses, cutting plants and cold stores will follow the steps described below: 6.1 Slaughterhouses
6.2 Cutting plants / Cold stores
|
| 7 | Once the FVL is confident that the establishment complies with the domestic, assimilated and export specific requirements, the FVL will send an approval checklist with the recommendation for full approval to the Export Veterinary Leader (EVL). |
| 8 | If the establishment does not comply with the requirements, the FVL will recommend refusal of approval informing the FBO, the Exports Team, the Approvals Team and the EVL of this decision via email. The deficiencies found during the visit will be detailed on this email. |
| 9 | The EVL will assess the information and, if satisfactory, will forward it along with any other supporting documentation to Defra’s Market Access Team copying the FSA Exports Team and the FSA Approvals Team. |
| 10 | The EVL will advise Defra that they make the appropriate recommendation to the authorities in the destination country. |
| 11 | Defra will send their recommendation to the relevant British Embassy or direct to the relevant authorities in the destination country. |
| 12 | Destination country authorities will make their decision on whether to approve the establishment to export, will notify Defra Market Access Team of their decision and will update the list of approved exporting establishments on their website. |
| 13 | Defra will notify the establishment and the FSA Exports Team that the destination authorities have agreed to their application for approval to export and they are now listed. FSA Exports Team will inform Approvals Team when they receive this notification |
| 14 | The plant will enter the export audit cycle at the frequency required by the importing country. |
A flowchart detailing the steps for the export's approval process can be found in Annex 3.
Note: The approvals checklists are based on the domestic and assimilated legal requirements, country specific requirements and a Defra country agreed protocol. These can be provided by the Exports Team. Copies of the export approval checklists can be found in Section 4 - Annexes.
3.2.4 Maintenance of Approval – Audit and Verification Checks
FSA OVs with permanent presence in export approved premises must complete daily and weekly checklists to verify ongoing compliance with the importing country requirements (see Section 4 - Annexes). They should also be able to sign any internal movement documentation.
In addition, these premises are required to be audited against the specific country requirements, regardless of commercial activity, in order to maintain the export approval. The audit frequency is annually, semi-annually or quarterly depending on the non-EU importing country.
3.3 FBO and FSA duties in Non-EU country approved establishments
3.3.1 FBO duties
FBOs that have been approved / listed to export to Non-EU countries need to:
- Comply with the requirements of domestic and assimilated law that apply to approved meat establishments and implement food safety management systems through HACCP-based procedures.
- Comply with the specific requirements of the importing country (these will be different for different countries and commodities) and obtain specific approval and/or listing (as required).
- Notify any changes in trading name or any other relevant details to the FSA Approvals Team and to APHA CIT.
- Complete and implement Standard Operating Procedures (SOPs) and, if required, Sanitary Standard Operating Procedures (SSPOs) for the specific requirements of the importing country.
- Comply with an RMOP (Required Method of Operation) when required by the importing country (for example USA, and meat for grinding to Canada) detailing specific operational conditions that must be met by the establishment (formal version of an SOP).
- When required by the country (for example, USA) carry out a pre-shipment review; prior to shipping the product review the records associated with its production including CCPs, deviations, corrective actions, associated pre-requisites, disposal of defective products, etc. This is a ‘double check’ of the records. The reviewer must sign and date the review form.
3.3.2 FSA duties
In slaughterhouses, where there is a permanent OV presence, the OV must comply with the following requirements:
- complete specific training on the requirements for exports to the specific country
- ensure access to the updated copies of the FBO’s RMOP, SOPs and SSOPs and verify them regularly (as required by the specific country)
- verify the plant HACCP-based procedures
- complete the verification checklists as required for each particular country at the required frequency (see table below and Section 4 - Annexes)
- document issues identified, and corrective actions taken using the appropriate checklists for each particular country
- verify the pre-shipment review (when required by the importing country, for example USA)
- checks on microbiological tests carried out by the FBO including confirmation that they are using an approved lab and the required accredited testing method
- hold and have blank copies of relevant internal movement documentation, export health certificates and notes for guidance of the relevant country export requirements
- sign and retain copies of internal movement documentation.
| Forms to be completed by the OV | Countries |
|---|---|
|
Daily verification of FBO controls Form 10 A - Pork checklist Daily/Weekly Form 11 A – Pork checklist Monthly/Quarterly/Annually Form 10 B – Beef & Lamb checklist Daily/Weekly Form 11 B – Beef & Lamb checklist Monthly/Quarterly/Annually
|
USA, China, South Korea, Singapore, Australia, Japan, Mexico |
|
HACCP- Routine Random Verification Checklist Forms 1, 1A, 2, 3, 4, 9 - Randomly 1 Direct Observation (DO) & 1 Record Keeping (RK) for each CCP at least once a week |
USA |
|
Preoperational Sanitation SOP checks Form 8 - 1 DO* & 1 RK** at least once a week |
USA |
|
Daily Operational Sanitation SOP checks Form 7- Every exporting day |
USA |
|
Random Re-inspection Checks Form 5 - Every commodity, every exporting day |
USA |
|
Monthly Random Verification Checklist- Micro testing Form 6 (Form 6A - Pork and Form 6B - Beef & Lamb) - Every time micro testing is carried out by FBO |
USA, China, South Korea, Canada (beef plants with N60 sampling) |
|
Pre-shipment Review Every consignment |
USA |
| Exports of meat and offal to Non-EU countries: monitoring Cold Store checklist |
USA, China, South Korea, Singapore, Australia, Japan, Mexico Cold Store only |
| Frequency | Verification Form |
|---|---|
| DAILY | Form 1A Form 5 Form 7* Form 8** Form 10A – Pork Form 10B – Beef & Lamb |
| WEEKLY | Form 1 Form 7* Form 8** Form 10 A – Pork Form 10 B – Beef & Lamb Cold store monitoring checklist |
| MONTHLY | Form 6 A – Pork Form 6 B – Beef & Lamb Form 11 A – Pork Form 11 B – Beef & Lamb Cold store monitoring checklist |
| QUARTERLY | Form 9 Form 11 A – Pork Form 11 B – Beef & Lamb Cold store monitoring checklist |
| ANNUALLY | Form 11 A – Pork Form 11 B – Beef and lamb Form 3 |
| 1. After every CCP critical limit breach 2. Corrective Actions in Response to a Deviation Not Covered by a Specific Corrective Action, or an Unforeseen Hazard |
Form 2 |
| After each change in HACCP | Form 3 |
| When conducting a pre-operational inspection to support the OVs recording their findings. | Form 7A |
| When routine HACCP verification tasks/events or results suggest the plant is not controlling its food safety system | Form 9 |
Note 1: Form 7 to be completed daily for Operational SSOPs and weekly for Pre-Operational SSOPs when Form 8 is no longer required.
Note 2: Form 8 to be completed every day/once per week depending on FBO performance for initial post approval period only until OV has confidence in FBO compliance or when OV has concerns that satisfactory FBO performance is not being maintained.
3.4 Non-EU export audits in approved establishments
3.4.1 Audits by the competent authority
On behalf of Defra, the FSA (FSS/DAERA) ensures an establishment approved for export continues to maintain the terms and conditions of the export approval.
Updated [Audits will be carried out by Export Veterinary Auditors (EVAs) or Veterinary Auditors (VAs), specifically trained on Non-EU exports. The audit report will be submitted to the Approvals Team, who will store it securely and distribute it to the FBO, FSA/LAs and Veterinary Delivery Partners (VDPS) by e-mail.]
A Non-EU exports audit report template can be found in Annex 5.
If the Auditor / OV is informed by the FBO that they would like to surrender their export approval, they should ask the FBO to notify APHA CIT in Carlisle and the FSA Approvals and Exports Teams.
The audit includes operational and systems compliance (as documented in MOC Chapter 4) and includes auditing the OV performance (in plants with permanent OV presence) to ensure that they are carrying out the required checks and completing records at the established frequency required.
Exporting stand-alone cold stores, for which compliance with domestic legislation is under the jurisdiction of LAs, are also subject to FSA export audits at the required frequency agreed by Defra with the importing country.
The length of the audit will depend on the type of establishment. Audit work includes preparatory work, on-site audit and post-audit work. During the on-site visit, the Auditor’s approach involves reality checks and documentation checks of FBO Food Safety Management Systems, including, when applicable, Animal Health and Identification, Animal Welfare, Hygiene Production, Environmental Hygiene, HACCP based procedures, SRM controls, handling of ABP, etc. It also involves interviews with the relevant FSA officials and observation of official control activities. The evidence gathered is used to assess the effectiveness of the control systems.
The export audit is independent from the FBO audit although both can be carried out together if coincidental in time.
Auditors can access the scheduler through the K2 system to organise the export audits.
For FSA approved establishments newly approved to export to the USA, monthly audits are required during the first 3 months and, once satisfactory standards have been demonstrated, the frequency reduces to quarterly audits.
China and Eurasian Customs Union require quarterly audits whilst South Korea, Singapore, Australia, Japan and Mexico require annual audits, except heat processed poultry meat to Japan, where the audit frequency is twice a year. This frequency must be kept in order to maintain the export approval regardless of commercial activity.
Audit frequency
| Monthly | Quarterly | Yearly | Semi-annually |
|---|---|---|---|
| USA (first three months) | USA (after first three audits) | Singapore | Japan (heat processed poultry meat) |
| - | Eurasian Customs Union | South Korea | - |
| - | China | Japan | - |
| - | - | Australia | - |
| - | - | Mexico | - |
3.4.2 Non-compliances
Deficiencies found during the audit should be reported by the FSA auditor as ‘lapse’ or as ‘non-compliance’.
Definition of compliant, non-compliance and lapses:
Compliant - food business is operating in accordance with its food safety management systems, food safety standards and has met the requirements of the EU and importing country regulations.
Lapse - not likely to compromise public health (including food safety) or animal health or welfare or lead to the handling of unsafe or unsuitable food. A lapse is an isolated low risk situation and does not compromise achieving control measures of the food safety program.
Non-compliance - likely to compromise export trade, public health (including food safety) or animal health or welfare or may lead to the production and handling of unsafe or unsuitable food if no remedial action is taken.
Lapses and non-compliances identified during the audit are discussed between the auditor and the FBO during the closing meeting at the end of the audit. The audit report contains a Corrective Action Report (CAR) listing the deficiencies identified.
The FBO must complete the CAR and submit it to the site OV and the auditor within 10 working days of receiving the report. The CAR must address the following for each deficiency:
- Immediate action taken to resolve the issue, including if applicable, adequate disposition of product that may be contaminated.
- Root cause of the deficiency.
- Corrective action: measures taken to address the root cause including monitoring and verification procedures put in place to ensure that those measures are effective
- Preventative action
Where deficiencies cannot be corrected immediately:
- Long term corrective action planned.
- Control measures and monitoring procedures in place to deal with hazards introduced in the meantime.
- Time scales for long term corrective actions.
If the FBO does not provide a corrective action report within the agreed 10 days, the auditor will have to contact the FBO in writing, to remind them of the requirement to provide evidence of the corrective actions they intend to implement.
In plants with an on-site FSA OV, the OV is responsible for verifying the corrective actions taken by the FBO and completing the "CA verification" column of the CAR.
In stand-alone cutting plants and cold stores, the responsibility falls with the auditor.

The diagram above defines the submission of the Corrective Action Report (CAR). Steps:
- Non-EU Export (NEUE) Audit visit
- EVA sends the audit report with CAR (within 12 working days)
- FBO completes the proposed CAR and sends it to the OV
- OV review proposed CAR (if needed, the OV ask for more clarification)
- OV review proposed CAR and if adequate signs the document and sends it back to the FBO
- The FBO sends the completed and signed CAR together with the evidence to the EVA (max. 10 working days)
- EVA reviews CAR (if needed, ask for more information and sends the CAR back to the FBO)
- After all parties (FBO, OV and EVA) agree on correctly completed CAR, the FBO sends the document to Approvals@food.gov.uk for secure storage
Breaches of the domestic legislation will lead to the hierarchy of enforcement being followed as per FSA procedures. Since compliance with domestic and assimilated law is a minimum requirement to export, failing to address non-compliances in a timely manner could result in a recommendation to Defra to suspend the export activity and / or de-listing from the importing country approved establishments list (for example when the FBO audit has an outcome of ‘Improvement Necessary or ‘Urgent Improvement Necessary).
Breaches of the importing country specific requirements will follow a similar enforcement hierarchy and export will cease until compliance can be guaranteed. The ultimate sanction for non-compliance with export requirements would be the recommendation to Defra to remove / suspend the establishment’s ability to export (de-listing). It is ultimately Defra’s decision to remove/suspend an establishment to export. In some cases (for example USA exports) this will be preceded by a Notice of Intention to De-list (NOID) and an increase in the frequency of audits and inspections.
Approval and audit documents for each establishment will be stored securely by FSA Approvals. Audit reports can be found on the plant files in SharePoint.
3.5 Training for Authorised Officers on non-EU exports
3.5.1 Introduction
This section details the process and the requirements to be fulfilled in order for a veterinarian or inspector to work and / or supervise as an Authorised Officer (AO) in an exporting plant.
Authorised Officers (AOs) working and / or supervising exports in an establishment approved to export to Non-EU countries need to be specifically trained on the country's requirements.
3.5.2 Background
The overall FSA lead in exports to Non-EU countries reside in the Exports Team based in the International and UK Affairs Directorate, and in the Export Veterinary Auditors team (EVA) based in the Operational Delivery Directorate.
The Export and EVA teams lead on the delivery of Non-EU export related matters on behalf of the FSA.
OVs appointed in establishments exporting to Non-EU countries, in addition to the general NOV training programme and OV inductions, receive specific training on export requirements of the specific Non-EU countries their establishment is approved for.
All UK veterinarians are required to complete Continuing Professional Development (CPD) related and suitable to their work.
3.5.3 Specific training on Non-EU exports
New Authorised Officer (AO) training: when an AO starts working at an exporting plant, for example on USA approved premises, their line manager is responsible for ensuring that they are fully trained before they are deployed to work at this establishment. This training is provided by their line manager with the support of the EVA team and experienced AOs. This training will be also required for relief AOs covering exporting establishments in advance of deployment to the site. The training consists of:
- Theoretical training: ahead of attending the plant, the AO is provided with links to all relevant legislation, instructions and updates. Also, relevant available presentations are shown and discussed with the AO.
- Practical training: the AO shadows the resident AO on hygiene inspection and auditing (pre-ops, CCPs, SPSs and SSOPs), Pre-shipment Checks Supervision and Certification Procedures, and Antemortem and Post-mortem Procedures.
Self-learning: AOs involved in export work are encouraged to utilise the available resources to keep up to date with their knowledge:
- Regulations for that specific country (e.g. FSIS directives for exports to the USA), Export Health Certification requirements set out in the Notes for Guidance (NFG) issued by the Animal and Plant Health Agency (APHA) for each specific country, export approval check-lists detailing the most relevant requirements, audit check-lists, etc.
- MOC Chapter 3 which covers all the relevant and updated information regarding exports, including Inward Missions, Approval process, Audit procedures and completion of specific forms
- FSA SharePoint where the most recently updated information on export requirements is available (for example, FSIS requirements and directives, audit reports, USDA forms).
FSA training: this is provided to AOs through training material, updates and cascading of information. The aim of this training is to ensure that all AOs working in exporting plants are trained on the particular requirements of the specific country.
Training and updates are also delivered at the quarterly FSA VA and FVL meetings, where there is a standing item of the agenda dedicated to exports.
The EVAs and the Exports Team work closely together coordinating the dissemination of briefings, updates, instructions, legislation, etc to relevant staff.
AOs working in plants exporting to the USA can receive USDA FSIS updates / digest bulletins by email directly. Relevant communications from FSIS, the US Embassy in London, Defra, APHA, the FSA Exports team and the UK Export Certification Partnership (UKEC) are communicated and cascaded to all the relevant AOs.
The knowledge of the AOs working at the exporting plants is assessed during Non-EU export audits and recorded in the ‘OV performance’ section of the audit report.
This assessment identifies particular training needs, that will result in regular training updates and the indication of any specific technical training required.
3.6 APHA authorisation for certification
3.6.1 Authorisation of OVs
Export health certification is carried out by appointed OVs on behalf of APHA.
The authorisation of OVs to sign Export Health Certificates is different from the authorisation of OVs under The Food Hygiene (Wales) Regulations 2006 / The Food Safety and Hygiene (England) Regulations 2013 to enforce assimilated Regulation (EU) 2017/625.
Training and authorisation of OVs for export purposes must be arranged with APHA and is provided in the UK by a contracted training provider (Improve International).
Note: OVs must not complete export health certificates unless they have completed the required APHA training modules and are authorised to do so. If in doubt, the OV should seek technical guidance from their line manager or APHA.
3.6.2 Internal movement documents
When the export health certification takes place at an establishment at the end of a chain of movements through different establishments, a certifying OV needs support certification issued by the OVs from previous establishments that have handled the product to ensure that full food chain information is checked.
There are three types of internal movement documents: the Internal Movement Certificate (IMC), the Support Health Attestation (SHA) and the Veterinary declaration. The OV signing internal documents does not need to be appointed by APHA.
Internal Movement Certificate: is a veterinary declaration confirming the meat referred to in the consignment complies with the import conditions of the country of destination. IMCs are agreed by Defra and the importing country for movement of product within the UK in order to facilitate the eventual export health certification [e.g. China – pig meat (7006EHC/IMC); Australia – fresh pig meat intended for further heat treatment (6838EHC/IMC); Canada – fresh beef (7833EHC/IMC)].
Support Health Attestation: a veterinary declaration confirming the meat referred to in the consignment comply with the import conditions of the country of destination, used for any country where IMCs are not applicable (e.g. exports to the EU), for movement of product within the UK in order to facilitate the eventual export health certification.
Veterinary declaration: a veterinary written statement confirming that the meat complies with the export requirements. These declarations typically cover a longer period than IMCs and SHAs and can include more than one consignment. Veterinary declarations can be issued to support the certification process when there is no specific requirement for an IMC or an SHA [e.g. Republic of Congo – meat/meat products (7211EHC)].
3.6.3 Certification
Export Health Certificates (EHC) are issued by APHA. There are specific certificates for different products and destinations.
It is the exporter’s responsibility to apply for certificates and ensure that they are available at the time of certification.
Specimen copies of Export Health Certificates and relevant Notes for Guidance (NFG) can be downloaded online. The EHCs are issued directly to the certifying OVs via APHA’s online platform.
Alternatively, the exporter can contact APHA.
Follow the link to find a practice with OVs to sign a certificate.
The original certificate shall be completed by the OV who then has to create a copy of each EHC issued. Each copy shall be signed, stamped and the words “Certified Copy” added by the OV.
The original EHC should be presented to the importing authorities for inspection and clearance by the exporter (either electronically or physically), while the Certified Copy must be retained by the OV for their records.
Additional instructions may be found in the Notes For Guidance of each EHC.
Please be aware that there may be frequent changes to NFGs and internal movement documents, so it is of paramount importance that OVs keep their knowledge up to date and check the latest OV Briefing Notes in APHA Vet Gateway website.
4. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: Mode common veterinary document (CHED)
Annex 2: Sample document: IIP1 form
Annex 3: Third country exports approval process
Annex 4a: Cold store third country checklist
Annex 4b: Form 1 HACCP system – verification checklist – FBO monitoring and verification
Annex 4c: Form 1a HACCP system – verification checklist – FBO monitoring and verification
Annex 4d: Form 2 HACCP system – verification checklist – FBO corrective action
Annex 4e: Form 3 HACCP system – verification checklist – FBO reassessment
Annex 4f: Form 4 HACCP system – verification checklist – FBO records keeping
Annex 4g: Form 5 - random re-inspection checks
Annex 4h1: Form 6A - FBO microbiological testing verification checklist (pork and poultry)
Annex 4h2: Form 6B – FBO microbiological testing verification checklist (beef and lamb)
Annex 4i1: Form 7 – daily sanitation SOP checks (SSOPs)
Annex 4i2: Form 7A – verification of the FBO pre-operational checks
Annex 4j: Form 8 – pre-operational sanitation checks (SSOPs)
Annex 4k: Form 9 HACCP system – directed verification checks
Annex 4l1: Form 10A – daily verification of FBO controls for export
Annex 4l2: Form 10B -verification of FBO controls for export (beef and lamb)
Annex 4m: Form 11 – weekly verification of FBO controls for export - REMOVED
Annex 5: Third country audit report template
Annex 6: Surrender of third country approval - REMOVED
Annex 7a: Third country approval checklist (Australia - Pork)
Annex 7b: Third country approval checklist (Japan – Pork, Beef and lamb)
Annex 7c: Third country approval checklist (Singapore - Beef)
Annex 7d1: Third country approval checklist (USA - Pork)
Annex 7d2: Third country approval checklist (USA – Beef)
Annex 7e: Third country approval checklist (South Korea – Pork & Poultry)
Annex 7f1: Third country approval checklist (South Korea - Pork)
Annex 7f2: Third country approval checklist (South Korea – Poultry)
Annex 7g: Third country approval guidance (South Korea)
Annex 8: UK CCA Verification procedures USDA
Annex 9: RMOP for USDA FSIF certified establishments
Annex 10: RMOP for establishment exporting beef to Canada
Annex 11: E.coli Official Sampling
Annex 12: US intact beef letter
Annex 13: RMOP for USDA FSIS certified establishments (lamb)
1. Introduction
2. Legislation
3. FBO Responsibility
4. FSA Role
5. Risk Assessment
6. Annexes
Sections
1. Introduction
In this section
1.3 Relationship between audit visits and OV attendance
1.4 Commencement of FBO audits following approval or periods of closure
1.1 Definitions
The following definitions apply for the purpose of this chapter.
1.1.1 OV presence
OVs are present in slaughterhouses and at the Smithfield Market to carry out inspection tasks every operational day.
Daily OV presence is not required for co-located cutting establishments and other establishments such as for standalone cutting plants and game handling establishments (GHE). However, co-located establishments operating at times coinciding with the slaughterhouse operational hours are under the supervision of the resident OV. Issues identified during the visits to the co-located cutting plant should be entered in the Chronos system and enforced under the standard enforcement principles. Deficiencies identified during these visits will be taken into account during the overall site audit.
Co-located establishments operating at times different from the slaughterhouse operational hours should be subjected to unannounced inspections (UAIs) same as stand-alone cutting plants. These establishments have already been included in the K2 system at the request of the FVC and a UAI visit request is automatically generated as if these were stand-alone cutting plants. If considered necessary, the inspector carrying out a UAI can also request the production of a UAI report for any co-located establishment to the K2 manager through the FVC.
1.1.2 Official visit
Official visits to any establishment (regardless of OV presence in slaughterhouses for carrying out inspection tasks), may be conducted for the purpose of carrying out a full audit, partial audit and/or a UAI.
1.1.3 Full audit
A full audit is an assessment of the FBO Food Safety Management Systems (FSMS). All listed approved FBO activities must be audited (within one day, or several days depending of complexity of the establishments considering several processes and operations).
1.1.4 Partial audit
Following a full audit, a partial audit will focus on specific themes identified as being non-compliant during the full audit.
Partial audits may be carried out on-site or remotely. See more details on remote audits in section 4.9.
1.1.5 Unannounced inspection
In addition to partial audits, and as part of the scheduled audit programme (see audit outcome and frequency of visits), UAI can take place to follow up specific issues identified during the audits or to verify continued compliance between audits.
1.2 Purpose of audits
1.2.1 Relevant premises
These audit arrangements apply to all meat establishments approved in England and Wales and under veterinary control.
These are:
- red meat / farmed game slaughterhouses
- poultry meat slaughterhouses
- cutting plants
- game establishments
- minced meat, meat preparations and mechanically separated meat establishments co-located with slaughterhouses or cutting plants
- meat product plants and ‘ready to eat’ establishments co-located with slaughterhouses and cutting plants
- co-located cold stores
1.2.2 Risk assessment scheme
The audit risk assessment scheme applies the requirement of retained EU laws (REUL) 2019/627 Article 4 to determine the frequency of audit using the risk criteria set out in that Regulation:
- public health risks
- animal health risks (where appropriate)
- animal welfare risks (where appropriate)
- type of process carried out
- throughput
- FBOs past record of compliance with food law
Note: Risks associated with the throughput and type of process are not specifically listed in the AUD 9-3, but have been incorporated in the body of the audit report document.
1.2.3 Aim of audits
The aim of the FBO audit is to verify compliance with the legal requirements and to ensure adequate FBOs standards in relation to public health, animal health and welfare.
The audit sections in the audit report are based on the priorities set for the FSA that have been agreed between the FSA, Defra and industry stakeholders.
Audit findings should provide individual FBOs as well as the relevant competent authority (FSA and Local Authorities) with information on Non-Compliances (NCs) identified against regulatory requirements, and/or areas in need of correction or improvement. For the competent authority (CA), this may result in the review of the MOC or the development of new guidance, procedures and training.
1.2.4 ‘Effective’ audit
An effective audit of FBOs obligations in respect of public health, animal health and welfare is defined as follows:
- complies with the requirements of REUL 2019/627 to determine the frequency of audit on the basis of risk
- applies appropriate standards in determining the level of assurance that can be given to the CA about the FBO management procedures and identification of risk
- accurately assesses the FBOs level of compliance with legal requirements and identifies necessary enforcement actions
- recognises the FBOs good practices and identifies opportunities for improvement
- communicates audit findings to the FBO and the CA
- is consistent in its approach
1.2.5 Compliance audit and systems based audit
An effective audit of FBO controls will require the use of both ‘compliance audit’ and ‘systems based audit’ techniques, which are described below:
| Audit technique | Description |
|---|---|
| Compliance audit |
This is a review and examination of FBO records and activities to assess compliance with legislative requirements and the FBOs established policies and operational procedures. Much of the audit work to support compliance assessment will take place in the operational environment. In establishments where there is frequent OV presence, this assessment work will be ongoing as part of the FSA team’s normal duties between the production of audit reports. |
| Systems based audit |
The auditor should seek to establish that the FBOs controls are fit for purpose and that the FBO has effective systems and processes in place to implement them on a continuous basis. Weaknesses and strengths in the FBOs control system should be recorded. Much of the audit work to support the systems assessment is likely to take place outside the operational environment. |
1.2.6 Publication of FBO’s audit report
The Freedom of Information Act 2000 gave individuals a general right to information held by public authorities (subject to certain exemptions) and to have this information communicated to them. The Environmental Information Regulations 2004 also provides a right of public access to a range of environmental information held by public authorities.
Important note: Audit reports will be published for FSA approved meat establishments in England and Wales on the FSA website after the period for appeals has expired.
1.3 Relationship between audit visits and OV attendance
1.3.1 Overview
All audits of FSA approved establishments are to be carried out by Veterinary Auditors (VAs) or Audit Veterinary Leaders (AVLs), who are independent and separate from operations and routine inspection duties.
The audit frequency represents the minimum number of times in a period that a completed audit report will be produced by a VA / AVL. This approach applies to slaughterhouses with or without a co-located cutting plant, game handling establishments, standalone cutting plants and cold stores under FSA supervision (for example, Smithfield Market).
Note: for simplification, further references to VAs / AVLs will be referred to as auditors unless specifically stated as VA or AVL.
1.3.2 Premises with frequent OV presence
OVs who work in a slaughterhouse approved for co-located operations may enter the production areas of other operations regardless of the audit timetable. However, the OV should consider the reasons for entry and ensure that it is part of their official control role. Daily checks in co-located operations are not required and the frequency of inspections should be determined based on risk assessment and third country export requirements.
Reference: The Food Hygiene (Wales) Regulations 2006 (as amended), Regulation 14, 2 / The Food Safety and Hygiene (England) Regulations 2013 (as amended), Regulation 16, 2.
Co-located operations will be audited at the same time as the slaughterhouse, as part of the same process, with a single audit report being produced.
1.3.3 Premises with infrequent OV presence
Stand-alone cutting plants and any co-located operations will also be audited at the same time. In between audits or partial audits there may be UAIs.
1.4 Commencement of FBO audits following approval or periods of closure
1.4.1 Premises with specific requirements
The table below summarises the circumstances under which specific types of establishments operate under a different audit regime.
| Establishment | Audit regime |
|---|---|
| All conditionally approved establishments (slaughterhouses, cutting plants and GHEs) |
FBO audit by an auditor will not commence until full approval has been granted to the establishment following the FVL approval assessment(s). The OV / FVC may be requested to conduct monitoring and enforcement visits during the period of conditional approval; this will be at the specific request of the FVL. Where full approval has been granted, the first audit will take place in 3 months, from the date of full approval. The first UAI will take place during the first 3 months, from the date of full approval. |
| Existing premises: on change of FBO |
A change of FBO marks the end of an existing establishment’s approval. The new FBO is required to make an application for a new approval. FBO audit by auditors will not commence until full approval has been granted following the FVL approval assessment(s). If during an audit it is identified that the legal entity has changed and a new approval is required, the audit must be stopped and the approvals team informed. The OV / FVC may be requested to conduct monitoring and enforcement visits during the period of conditional approval; this will be at the specific request of the FVC / FVL respectively. Where full approval has been granted, the first audit will take place in 3 months, from the date of full approval. |
| Existing premises with full approval- on application to extend or vary activities | In these circumstances, the FBO audit should continue as already scheduled for the fully approved activity. The additional activity will only need to be audited once full approval for that activity has been granted and following the FVL’s approval assessment. Any revision to the audit frequency, made necessary by the additional activity, will be established at the next regular scheduled audit after full approval is granted. For example:
|
|
Seasonal closure* and temporary or long-term closures *Seasonal closures are pre-notified routine breaks in operation, to a seasonal pattern |
Following a period of closure, the FBO is required to notify FSA at least 2 weeks prior to re-commencing operations. The FBO must not re-commence operations until a pre-opening FSA visit has been conducted. Note: Periods of closure are defined at paragraph 112 in the ‘Operational policy for the approval of meat establishments undertaken by the FSA’. Where the outcome of the pre-opening visit confirms that the establishment meets all legislative requirements, the next FBO audit by the auditor should be completed no later than 2 months from operations re-commencing. |
| Premises under recommendation to suspend/withdraw approval | Audit activity is to be discontinued after a recommendation has been submitted by the FVL. Once the outcome has been decided, the audit cycle will be reinitiated with a full audit after 3 months. This audit will still take into account any minor non-compliance that remained open in the last audit, and that has not been part of the formal approval review. Note: The auditor would need to check with the FVL / AVL / Approvals team the relevant information from the review process as part of the audit preparation. |
2. Legislation
In this section
2.1 Requirement for audit
2.1.1 General requirements for official controls
It is a principle of REUL 2017/625 and 2019/627 that official controls will verify the FBOs compliance with REUL 852/2004, 853/2004 and other REUL and national regulations that apply to approved meat establishments.
Part of that verification process is the audit of good hygiene practices and HACCP-based procedures as required by REUL 852/2004 Article 5 and REUL 853/2004 Annex II, Section II, the FBOs food safety management system.
In addition to the audit of good hygiene practice, the auditor must verify the FBOs continuous compliance with their own procedures for, amongst others, all aspects of animal by-product (ABP) handling (including SRM control), animal identification and animal health and welfare.
In addition to the audit of HACCP-based procedures the auditor must check that the operator’s procedures guarantee, to the extent possible, that meat is free from patho-physiological abnormalities, faecal or other contamination and SRM (subject to Community rules).
Reference: REUL 2017/625, Article 18 and REUL 2019/627, Article 3
2.1.2 Food fraud
The recommendation of the Food Fraud Task Report 2007 is that auditors and other officials visiting food premises should bear in mind the possibility of fraudulent activities.
2.1.3 GHP audit
Audits of good hygiene practices shall verify that FBOs apply procedures continuously and properly. A detailed list of pre-requisites to consider can be found in sub topic 3.2.2 on ‘HACCP and pre-requisites’ in Part 1.
Reference: REUL 2019/627, Article 3,1
2.1.4 HACCP audit
Audits of HACCP-based procedures are to verify that FBOs are applying procedures continuously and properly. The auditor must determine whether the procedures guarantee, to the extent possible, that products of animal origin:
- comply with microbiological criteria laid down under EU legislation
- comply with Retained EU legislation on residues, contaminants and prohibited substances
- do not contain physical hazards, such as foreign bodies
Reference: REUL 2019/627, Article 3, 2 and 3
Where a food business operator takes additional measures to guarantee food safety by implementing integrated systems, private control systems or independent third-party certification, or by other means, and where these measures are documented and animals covered by such schemes are clearly identifiable, the competent authorities may take such measures into account when carrying out audits to review good hygiene practices and the HACCP-based procedures.
Reference: REUL 2019/627, Article 4,2.
3. FBO Responsibility
In this section
3.1 Compliance with the legislation
3.1.1 FBO standards
The FBO is required to comply with the requirements of REUL 852/2004, 853/2004 other REUL and national regulations that apply to approved meat establishments. These are the standards against which the auditor will assess the FBO performance at audit.
Food safety management systems must be implemented and must be sufficient to achieve the objectives of the Regulations.
3.1.2 Access, records and assistance
The FBO is required to offer all assistance needed to ensure that official controls carried out by the Competent Authority can be performed effectively, and in particular to:
- give access to all buildings, premises, installations or other infrastructures
- make available any documentation and records required under the Regulations or considered necessary for judging the situation.
Reference: Retained (EU) legislation 2017/625, Article 15, The Food Hygiene (Wales) Regulations 2006 (as amended) / The Food Safety and Hygiene (England) Regulations 2013 (as amended).
3.2 HACCP based systems
3.2.1 Obligation to implement
The FBO, considering the nature and size of the business, has a duty to implement a permanent procedure based on the 7 HACCP principles of:
- identifying any hazards that must be prevented, eliminated or reduced to acceptable levels
- identifying the critical control points (CCPs) / control points required by regulations at the step or steps at which control is essential to prevent or eliminate a hazard or to reduce it to acceptable levels
- establishing critical limits / legal limits at CCPs / control points required by regulations which separate acceptability from unacceptability for the prevention, elimination or reduction of identified hazards
- establishing and implementing effective monitoring procedures at CCPs / control points required by regulations
- establishing corrective actions when monitoring indicates that a CCP / control point required by regulation is not under control
- establishing procedures, which shall be carried out regularly, to verify that the measures outlined above are working effectively
- establishing documents and records commensurate with the nature and size of the food business to demonstrate the effective application of the measures outlined above
When any modification is made in the product, process, or any step, FBOs shall review the procedure and make the necessary changes to it.
The FBO must also provide the CA with evidence of their compliance and ensure that any documents describing the procedures are up-to-date at all times.
Reference: Retained (EC) legislation 852/2004, Article 5
Reference: See MOC Volume 2, 14f on EU guidance document on the implementation of procedures based on HACCP principles, and on the facilitation of the implementation of the HACCP principles in certain food businesses;
3.2.2 HACCP and pre-requisites
HACCP systems are not a replacement for other food hygiene requirements, but a part of a package of food hygiene measures that must ensure safe food. It must be borne in mind that ‘prerequisite’ food hygiene requirements must be in place prior to establishing HACCP procedures, including in particular:
- checks on food chain information (FCI)
- the design, layout and maintenance of premises and equipment
- pre-operational, operational and post-operational hygiene
- personal hygiene
- training in hygiene and in work procedures
- pest control
- water quality
- temperature control
- controls on food entering and leaving the establishment, any accompanying documentation
These requirements are designed to control hazards in a general way and they are clearly prescribed in Community law. They may be supplemented with guides to good practices established by the different food sectors.
Reference: EU guidance document on the implementation of procedures based on HACCP principles, and on the facilitation of the implementation of the HACCP principles in certain food businesses.
Note: Other requirements of Community law, such as traceability, the withdrawal of food and the duty of informing the CAs should, although not covered under the food hygiene rules, also be considered as prerequisite requirements.
Reference: Retained (EC) legislation 178/2002, Articles 18 and19.
4. FSA Role
In this section
4.4 Completing the audit report
4.1 Responsibilities
4.1.1 Who conducts the audit?
Specially trained and experienced veterinary auditors will conduct audits at all approved meat establishments under FSA responsibility.
Note: OVs and novice OVs (NOV) do not undertake audit work but will provide supporting evidence for the audit. All relevant evidence gathered by them during the audit period must be available for the auditor (including the up to date ‘Enforcement Programme’ available in Chronos).
4.1.2 Audit tasks
The following table identifies the different tasks and responsibility for completion.
| Task | Timescale | Responsibility |
|---|---|---|
| Arrange audit visit date with FBO or their representative | Based on risk rate frequency for the month the audit is due; best practice is a minimum of 2 weeks before audit is due | Auditor |
| Confirm audit visit date in writing/ e-mail | Via K2, shortly after arranging visit | Auditor |
| Audit preparation gathering information on FBOs food safety management systems | - | Auditor |
| Gather information on food safety management systems | - | MHI / OV / NOV |
Carry out audit visit:
|
Depending on the complexity of the establishment, the auditor should consider allocating one or more audit days. | Auditor |
| Compile audit report and submit in K2 | Within 5 working days after the visit | Auditor |
| Audit report authorisation in K2 | Within 10 days of the audit visit | - |
| Distribute completed audit report to FBO, with copies provided to relevant FSA officials as required | - | Auditor or AVL Generated automatically by K2 |
4.1.3 Auditor’s code of ethics
The following four principles are the standards of conduct that are expected from auditor carrying out FBO audits:
- Integrity
- Auditors shall demonstrate integrity in all aspects of their work. The relationship with OVs, MHIs and with FBOs should be one of honesty and fairness. This establishes an environment of trust which provides the basis for all activities carried out by the auditor.
- Objectivity
- Auditors shall display professional objectivity when providing their opinions, assessments and recommendations. The auditor should not be unduly influenced by the views of others or by personal interest.
- Competency
- The auditor shall not carry out audits if they feel they do not have the base auditor competency or if they lack technical competency in the area being assessed. All auditors are to hold Food Safety Lead Auditor and Intermediate level HACCP qualifications.
- Confidentiality
- Auditors shall safeguard the information they obtain while carrying out their duties. There should not be any unauthorised disclosure of information unless there is a legal or professional requirement to do so.
4.1.4 Auditor duties
The auditor is responsible for:
- arranging the audit visit with the FBO
- completing the audit within the calendar month of the designated audit frequency
- auditing the FBOs FSMS and FBOs compliance with animal health and welfare Regulations
- completing the Audit report (AUD 9-3)
- determining an audit outcome and audit frequency
- advising the FBO on compliance with legal requirements in relation to the audit
- (in stand-alone establishments) agreeing any necessary remedial action and timescales with the FBO, ensuring deficiencies are effectively addressed liaising with the UAI team as required, and escalation of any necessary enforcement activity as a result of the visit.
4.1.5 Auditor exclusions
The auditor should not:
- assume accountability for FBO compliance
- take over tasks that are for the FBO to perform
- act as a quality assurance manager
- act as an advocate between industry and the FSA
- write company procedures or HACCP plans, although advice may be given
- provide the FBO with a copy of the un-checked audit report
4.1.6 Assurance measures: AVL duties
As an assurance measure, AVLs will carry out quality checks on a representative sample of issued audits within their areas (initially 10%). Those checks should include audits of poor performing plants (assessed as Improvement necessary and Urgent Improvement Necessary).
The AVL will also be responsible for profiling the audits in their area and ensuring targets are met.
4.1.7 Field staff duties
Field staff working regularly in an establishment must ensure that they are familiar with the procedures and systems put in place by the FBO, in particular for the processes for which they have an inspection role.
Note: The OV must ensure that MHIs working under their technical responsibility maintain a current understanding of the FBOs procedures and systems.
4.1.8 Automated system actions
The K2 system will:
- monitor the scheduling of the audit visits in accordance with the minimum audit frequency determined by the audit category
- monitor the timely production of audit reports
- distribute the completed report to the FBO
- maintain audit records
4.2 Audit schedule
4.2.1 Arranging visits
The auditor will contact the FBO, where possible, one month in advance of the audit being due (two weeks’ notice is acceptable but not best practice) to agree a date for the audit visit.
FBO audits should be arranged whilst the establishment is in operation and product being processed. If necessary, an audit may take place over a number of days of a week in order that as many processes as possible are audited. Where the establishment is not operational the audit may be delayed until the establishment is in operation with the agreement of the auditor.
The scheduling of the audit visits will be monitored in order to ensure that audit targets and frequencies are met.
The agreed date of the audit visit must be confirmed in writing to the FBO. This letter will provide the FBO with prior warning of an audit; outlining the scope of the audit and the access and information that will be required.
Notification of the audit will allow the FBO to make themselves, or the relevant members of their management team, available. In addition, it allows the FBO to have any necessary documentation available for audit.
Note: Where applicable (for example, seasonal operations), in order to confirm that the establishment is truly not operational, a regular programme of unannounced inspections should be set up until the audit takes place.
Reference: See sub-topic 4.7.6 on ‘Unannounced inspection’ in part 1 for additional information.
4.2.2 Target for subsequent audit completion
Subsequent audit visits will be within the month determined by the last audit category.
4.2.3 Alternative arrangements
Where an audit date has been scheduled with the FBO and the FBO needs to cancel / rearrange, the auditor shall reschedule the audit working collaboratively with the FBO to agree a mutually agreeable date and time, updating K2 accordingly with the current agreed date and the reason for the cancellation.
However, cancelling audits at short notice creates a considerable problem to the FSA in terms of wasted hours and a knock-on effect to the number of FSA audits and workload of FSA auditors accumulating into proceeding months. This situation can incur a cost to the agency due to auditors wasted time preparing and travelling to audits which are subsequently cancelled.
The VA should notify the AVL of the problems in arranging the audit. At the discretion of the AVL, a letter can be sent to make the FBO aware of the impact and the potential implication cancelations at short notice have on the audit system. The FBO audit cancellation letter template (Annex 5) can be tailored to the different scenarios that may occur.
4.3 Audit protocol
4.3.1 Collecting evidence as to the compliance of the FBO
In slaughterhouses: FSA staff are present every day the plant operates. As part of day to day business they should record objective evidence as to the level of compliance by the FBO with both his own procedures and with legislative requirements.
In cutting plants: FSA staff will normally only be present to conduct the audit, although the premises should have been the subject of UAIs in the period since the last audit. Prior to a scheduled audit taking place, the auditor should establish whether any UAIs have taken place and if so, what enforcement activity arose as a result.
Both the OV and MHI have an important role to play in identifying and recording NCs. Objective evidence of NC issues may be recorded:
- on the relevant operational form
- in the daybook
- in the enforcement programme (Chronos)
Note: ‘Major’ or ‘critical’ NCs should trigger an immediate action.
4.3.2 Assessment of operational records
Prior to the audit, the auditor must review enforcement records for the period since the last audit and use this information when assessing the effectiveness of the FBOs food safety management procedures and HACCP based system, taking account of corrective actions. For the purpose of the assessment, the auditor might request and review other records they find relevant, including hygiene, welfare, ABPs forms and UAI reports.
Auditors can obtain additional information about the level of FBO’s compliance in an establishment through contact with the local FSA team (MHIs, OVs, FVC and FVLs).
Reference: See sub-topic 5.2.1 on ‘FBO compliance history’ in part 1 for additional information.
4.3.3 The opening meeting
Start each audit with an opening meeting with the FBO (or appropriate representative) and outline the:
- reason for and scope of the audit, anticipated length of the audit and the day programme
- information and access that will be required
- purpose of the subsequent closing meeting
- publication of audit categories
The opening meeting should also be used to:
- confirm that there are no changes to FBO, structures, equipment or activities since the last audit and that all necessary approvals are in place
- highlight that if during an audit it is identified that there has been a change of legal entity, the audit will be stopped, and the approvals team informed; a new approval is required
- review of the Non-compliance Report (NCR) from the last audit
- highlight any issue identified from the audit preparation review of operational forms.
4.3.4 When carrying out the audit
During the audit, the auditor will:
- collect and record objective evidence of the FBOs compliance with legislative requirements for food safety management systems based on HACCP principles, including ABP and where appropriate, SRM, animal health and welfare procedures
- inspect the establishment (‘reality checks’) to observe whether the FBO’s procedures in practice reflect the policies and procedures as documented
Note: In slaughterhouses some of this information will be gathered on a daily basis by MHIs / OVs.
- score individual questions and sections as compliant or non-compliant (minor, major, critical)
- determine the overall audit outcome as Good, Generally Satisfactory, Improvement Necessary and Urgent Improvement Necessary
4.3.5 Serious issues identified during audit
If an issue of serious public health, animal health or welfare arises during an audit (for example, considered ‘critical’), the auditor should:
- inform the FBO, the OV (where appropriate) immediately, and the FVL / FVC as soon as possible
- take / instruct the OV for any necessary enforcement action to be taken
- consider curtailing the current audit
4.3.6 Reference to previous audit reports
During subsequent audits, the auditor should refer to the previous Audit Report to direct priorities during audit in a risk based manner. The auditor should put special attention on areas where major or critical NCs were identified. Those will always have to be reassessed in the next audit.
4.3.7 Audit notes
It is important that audit notes are taken during the audit as they constitute an essential element to support the auditor audit findings and justify the audit assessments.
Auditors can use the audit checklist (Annex 3) to record evidence.
Each page should include:
- have the audit number which comprises the four-digit approval number, site type and audit date (month/year), that is xxxx-SH-mm/yy
- contain contemporaneous, detailed and legible notes which are cross-referenced to the aide memoire reference notes of the AUD 9/3 form
- date and signature of the auditor
Audit notes do not need to be submitted with the audit report but they should be retained and made readily available for next audit or as and when requested.
Audit notes must be retained for a minimum of 2 years (more than 2 years if there are ongoing outstanding enforcement actions).
Updated [Audit notes should not substitute the use of contemporaneous notebook for recording enforcement evidence admissible to court if the occasion arises].
4.3.8 FBO involvement in audit
The auditor should expect to be accompanied by the FBO (or a nominated representative) during the visit.
4.3.9 The closing meeting
The audit must be concluded with a closing meeting with the FBO (or appropriate representative) which will:
- summarise the audit findings (positive and negative)
- outline any NCs
- discuss the corrective action required, including any proposed timescales and possible enforcement action for Stand Alone Cutting Plants (SACPs)
- give an indication of the expected audit category
- give details of report procedure
- give details of publication of the audit categories
- outline subsequent action and right of appeal
The closing meeting provides an opportunity for the FBO to respond to audit findings, to discuss his proposed actions and to provide any further supporting evidence if he disagrees with any audit findings.
The resident OV in slaughterhouses, co-located cutting plants and GHE shall attend the closing meeting, whenever possible.
4.3.10 Further information provided by the FBO
The FBO may provide additional evidence following discussions at the closing meeting. Provided this evidence is received by the auditor within 5 working days of the audit, it may be taken into consideration.
4.3.11 Audit report
The Audit report (form AUD 9/3) must be compiled from the audit findings and should not be materially different from the findings presented verbally during the closing meeting.
The completed report should be submitted by the auditor within 5 working days of the audit visit.
Reference: See topic 4.4 on ‘Completing the Audit Report’ in part 1 for additional information.
4.3.12 Submission of Audit report (AUD 9/3)
The following table details the process which should be followed after completion of the audit report.
| Step | Action |
|---|---|
| 1 | The auditor completes and submits audit report within 10 working days. |
| 2 | K2 automatically records the audit report |
| 3 | K2 distributes the completed audit report to the FBO, Service Delivery Partner (SDP) and to other parties if required for assurance checks. |
4.3.13 Auditor’s feedback to the FSA team
The SDP receives a copy of the completed audit report sent to FBO. The resident OV is responsible for making all members of the team aware of the audit results, including NCs, the corrective action and timescales.
Note: Any FSA performance issues identified during an audit must be reported using the K2 system.
4.4 Completing the Audit Report
4.4.1 Use of objective evidence
As the formal record of the audit findings, the audit report must contain objective evidence to support the overall findings of the audit and the results given to the FBO during the closing meeting of the audit visit.
Although it was agreed with industry stakeholders that the audit report will mostly contain exception reporting, good audit practice dictates that reports should include both positive and negative reporting. The trigger for the auditor to make narrative entries in the supporting evidence box will be based on the score in the assessment box. Assessment boxes which have not been marked as ‘compliant’, or changing scores from the previous baseline audit will require an entry in the supporting evidence box.
Note: The audit writing guidance document (Annex 2) has been developed to assist auditors with aspects of report writing. It includes tips on style, accuracy, consistency and objectivity.
4.4.2 Use of positive language
The auditor should use positive language during the closing meeting and in the audit report.
This will help to promote constructive communication of audit findings between the auditor and the FBO, better participation and resolution of NCs through joint identification of action and opportunities for improvement, which is the main aim of the audit.
4.5 Audit assessment
4.5.1 Recording compliance
Each question of the audit report requires the auditor to gather evidence regarding the level of compliance with the stated outcomes and record it as compliant minor, major, or critical NC.
Note: Only one NC is to be recorded for each question; this is to be especially considered when using the link tool explained in 4.6.5.
4.6 Actions following the audit
Note: For the purposes of this section, the following definitions apply:
- deficiency – an individual and very specific failure to comply with the legislative requirements (for example, in-rolling, dirty surface, uncut bird(s)) which are entered individually in the enforcement programme and are used as supporting evidence to justify audit NCs
- NC – a failure to comply with legislative requirements against a question and which is supported by one or several related deficiencies
- question – each sentence intended to elicit information in the audit report and which is assessed depending on the level of compliance
- section – a group of questions in the audit report under the same general heading
4.6.1 Audit outcome
The approach following the audit will depend on the outcome of the audit and the number of identified minor, major and critical NCs.
In slaughterhouses, co-located cutting plants and wild game establishments the resident OV owns and is responsible for the amendment, completion and update in Chronos. When the incumbent OV is not present at any stage of the audit, the auditor will ensure that the deficiencies are effectively communicated to the plant lead OV so that they can update the Chronos system and follow up on the enforcement.
For stand-alone cutting establishments, the responsibility is shared; this means the auditor will take any necessary enforcement action and record it in Chronos, but then the responsibility will be transferred to the field team.
4.6.2 Request to change the auditing frequency / early audit
Audit frequencies can be re-assessed at the request of FSA and/or the FBO.
On FBO formal request, the date of the audit may be brought forward under certain specific circumstances (for example, during busy periods, for commercial reasons or after a bad audit outcome).
However, an early audit should not be requested immediately after an unsatisfactory audit. In these circumstances scheduled audit frequency can be only changed if all major and critical NCs were signed off as complete and in the case of stand-alone cutting plants, an UAI has been completed as specified in the requirements. This should be assessed on a case by case basis.
The FSA may also decide to carry out a full audit of an establishment prior to its scheduled date if serious deficiencies are identified. This can be requested by either the field or assurance FSA teams.
Field teams request: if falling standards on a particular establishment leads to the last audit outcome not reflecting the actual situation of the site, despite the progression of the enforcement and the approach through the Intervention Protocol. For example, establishment on extended audit frequency with sudden continuous increase in the level of enforcement.
Assurance team request; if after a partial audit the number of major or critical NCs increases to the extent of these exceeding the permitted numbers in the previous audit outcome.
In order to keep the separation between the audit and enforcement functions, an audit cannot be brought forward from its frequency unless the Auditor is satisfied that all appropriate enforcement is in place, as it is a basic principle of auditing that an audit should not be another enforcement tool.
Each proposal will be discussed on a case by case basis with the AVL and the Approvals and Veterinary Audit Lead with a decision being made on the evidence available to ensure a consistent approach.
Audits may only be postponed in exceptional circumstances, for example, if the establishment is not operational when the audit is due or other unforeseeable circumstances.
4.6.3 Minor NCs
Minor NCs are followed up by the resident OV in the case of slaughterhouses, co-located cutting plants and GHE or during UAIs in the case of stand-alone cutting plants. FVC / OV / MHI involved in the UAIs can assess the corrective action taken by the FBO on the deficiencies identified during the visits.
The minor NCs will be reassessed in the following partial/full audit by the auditor, based on the information provided by the field teams, and then the auditor will decide to either close it or maintain it open.
4.6.4 Critical and major NCs
Auditors will carry out partial audits of any establishment with critical and/or major NCs to assess progress towards compliance. These visits will be chargeable to the FBO and will be treated separately to the UAI programme.
- Critical NCs can only be closed off by the auditor following an on-site partial audit where compliance could be verified.
- Major NCs can be assessed without the need of a visit if the VA considers that sufficient evidence of compliance can be obtained remotely (from the FSA local team or FBO) to close this off where:
- The auditor is satisfied that a major NC identified at the full audit (from the Chronos report) has already been effectively rectified by the FBO during the audit period, that major NC can be closed off at the time of audit reporting. No visit or partial audit report will be required.
- The audit outcome is ‘generally satisfactory’, the auditor has the option to accept evidence provided by the FBO and corroborated by the resident OV or the UAI to close off a major NC. A visit is not essential, but a partial audit report is required. The auditors have the discretion to visit plant if they consider it necessary.
4.6.5 NC closed count to vs do not count to outcome
When a NC is closed, either at a full or partial audit, the auditor should decide if the closed NC will count towards the outcome of the audit or not:
- If the NC raised at a full audit is closed at the next full or partial audit and the deficiencies have been resolved within the agreed timescale and without the need to escalate enforcement, the auditor should mark it as closed – do not count to outcome. The NC will not appear in the next full audit report.
- If the NC is closed at the next full or partial audit but the agreed timescales to resolve the deficiencies have not been met and/or enforcement has required escalation, the auditor should mark it as closed – count to outcome. This will not appear automatically in the next full audit report and should be manually added in the following audit report. The auditor should decide the final assessment based on the evidence available during the audit period.
- If a NC raised at the full audit is closed at the full audit, it should always count to the outcome. This may be for matters that happened during the audited period (for example, raised by the OV on site or by the inspector during a UAI visit) but that had been correct at the time of the audit.
4.6.6 Use of the link tool
Linking of NCs should be done
- to prevent the same deficiency from being raised as different NCs in more than one question in the audit report.
- If the auditor considers that there is a deficiency that constitutes a NC that applies to several questions, the auditor should use the link tool so that the same deficiency is recorded in all the applicable questions. This will count as a single NC for audit outcome purposes and all linked questions will have the same NC with the same score recorded against them in the NC report.
Examples:
- NCs relating to contamination / cross-contamination (section 3) might be linked to the FBOs food safety management system failure so consideration should be given to linking these to the relevant question in the HACCP section (section 5).
- NCs relating to inadequate welfare practices might be linked to the FBOs welfare management system failure so consideration should be given to linking these to other questions in section 2.
4.6.7 Contribution of minor NCs to the severity of Major NCs
If the use of the linking tool is not justified due to the same deficiency not affecting two different questions, the auditor can justify the increase in the severity of a question -scoring that question as a Major NC- based on the fact that deficiencies considered in other questions contribute to this assessment.
The other questions to which the Major NC relates will be individually assessed/scored, through the auditor’s risk assessment, and the auditor can make a reference in the description of the relevant question, for example ‘this deficiency contributes to the assessment of Major in the NC raised in question X’ but without using the link facility. The contributing questions can have a different score from the one they are contributing to.
4.7 Unannounced inspections (UAIs)
4.7.1 Background
Unannounced inspections (UAIs) support the audit process and ensure the FSA’s obligations are met through the verification of FBOs compliance with relevant legal requirements in FSA approved meat establishments where there is no regular official presence or in the case of co-located cutting plants, when the activities take place outside the statement of resources (SOR) hours.
Assimilated EU regulations require official controls to be carried out without prior notice to the FBOs, except where such notice is necessary and duly justified for the official control to be carried out, for example in the case of audits. The programme of unannounced inspections is an essential part of the delivery of official controls in support of the FSA audit programme.
Official Veterinarians (OVs) and Official Auxiliaries (OAs) may undertake UAIs on stand-alone/co-located cutting plants under the direction of the relevant Field Veterinary Coordinator (FVC) for the area.
Aim of UAIs
The aim of the UAIs is to verify the FBO’s compliance with the legal requirements in relation to food safety and to ensure adequate FBOs standards in relation to public health.
UAIs verify FBO compliance between full audits and assess the FBO’s continued and effective application of legal requirements, food safety management systems and HACCP based procedures. UAI findings should provide individual FBOs as well as the relevant competent authorities (FSA and Local Authorities) with information on contraventions identified against regulatory requirements, and/or areas in need of correction or improvement. If contraventions are identified during an UAI, proportionate and risk-based enforcement action may need to be taken.
UAI tasks include, amongst others, the follow up on findings from previous audits and/or previous UAI visits. The contraventions identified during UAIs are considered part of the overall picture of FBO performance/compliance between full audits which have an impact on the final audit outcome for the audited period.
Programme of UAIs
The Field Veterinary Leaders (FVLs) are accountable for the UAI programme within their region. However, this is organised by the FVCs, with the support of the regional Lead Unannounced Inspector (LUAI).
While standing alone cutting plants are always subject to UAIs, not all co-located cutting plants are.
Approved cutting plants that are co-located to and operating at the same time as approved slaughterhouses where there is permanent OV presence will not be subject to UAI visits. However, if the co-located cutting premises operate only at different times to the slaughter operations when there is no OV presence, UAI visits must be scheduled accordingly.
If the co-located cutting plant operates both on slaughter and non-slaughter days, it will not be subject to UAIs as long as all the approved activities can be observed on the days when the OV is in attendance. However, if there are specific issues or concerns, UAI visits can be scheduled by the FVC on non-slaughter days.
The same principle is also applicable to approved cutting plants that are co-located to game handling establishments (GHE). When there is permanent OV presence at the GHE, the co-located cutting plant will not be subject to UAI visits. However, in the cases where the co-located cutting premises operates only at different times from the GHE, it will be subject to UAIs. Other specific scenarios will be considered by the relevant FVC. When there are issues or concerns, UAIs can be organised at the discretion of the FVC.
Please see Chapter 2.10, section 3, for details of inspections in co-located cutting plants that do not fall under the UAI regime.
For the cutting plants that are to be subjected to UAIs, the FVC allocates the minimum number of UAIs per establishment as indicated by the UAI scheduler in the UAI application, which is in line with the frequency of UAIs shown in the table below. Please refer to section 4.7.2 for details. In addition, the FVC carries out a risk assessment and evaluates the outcome of previous audits, unannounced inspections, any food incidents and/or complaints, and the enforcement activity at the premises. Following the review of this information and after carrying out a risk assessment, FVCs may decide to increase the number of UAIs at the establishment.
The UAIs are assigned by the FVC using the UAI application in the K2 system, taking into account the establishment location and staff resources available. The UAI application can be found at the following link: UAI application.
The relevant Authorised Officers and their line managers are automatically informed by email when the UAIs have been scheduled to them. The schedule includes the date by which the UAIs are to be undertaken, allowing time for resources to be allocated.
4.7.2 Frequency of UAI visits
All eligible cutting plants must receive at least one UAI during the period between full audits. After an UAI, the need for further UAIs may be identified and FVCs should utilise a risk-based approach when scheduling such inspections.
The following table shows the minimum frequency of UAIs required.
| Establishment | Frequency |
|---|---|
| Conditionally approved establishments (stand-alone and eligible co-located cutting plants) | UAIs will not commence until full approval has been granted to the establishment. Where full approval has been granted, the first UAI will take place during the first 3 months, from the date of full approval, and should occur before the first full audit. |
| Approved establishments (stand-alone and eligible co-located cutting plants) |
A minimum of one UAI between full audits. Please note that approved establishments receiving unannounced inspections can be subject to extended audit frequencies if two consecutive “good” audit outcomes are achieved, such premises will require additional UAI visits (minimum of 2 UAIs in the audit cycle) as described in section 5.3.3 (extended audit frequency). |
| Stand-alone and eligible co-located cutting plants that are approved for ready-to-eat (RTE). |
A minimum of two UAIs between full audits. Please note that RTE approved establishments subjected to extended audit frequencies will require a minimum of 3 UAIs in the audit cycle. |
| Stand-alone and eligible co-located cutting plants where serious deficiencies have been identified during an audit or at a previous UAI. |
It is appropriate to schedule additional UAIs as the FVC deems necessary. An UAI may also be scheduled prior to a partial audit to verify compliance on an unannounced basis. |
| Stand-alone and eligible co-located cutting plants where intelligence and/or food complaints are received relating to the approved premises. | It is appropriate to schedule a visit by an unannounced inspector or FVC to investigate such occurrences. These visits would not normally be classed as UAIs, but if new contraventions are identified during the visit, a UAI report should be completed, and the visit will be classed as a UAI. |
Where UAIs identify serious contraventions related to food safety, the Authorised Officer conducting the UAI should inform the relevant FVC, who in turn will discuss it with the FVL, and together will assess if the meat establishment needs to be placed under the intervention protocol. A review of approval may be triggered, too. FBO audits may also be brought forward in certain circumstances and at the request of the field operations team. Each case is to be considered on its own merit.
The routine programme of inspections does not supersede review of approval protocols or emergency inspections following receipt of intelligence / food complaints.
Responsibilities
4.7.2.1 Who conducts the UAI?
Only OAs who have completed the UAI training and have passed a practical assessment by a FVC, and OVs, who are suitably trained/familiarised with the process, are assigned to undertake UAIs.
FSA employed unannounced inspectors are supported by a local FVC, a regional Lead Unannounced Inspector (LUAI), and their relevant Inspection Team Leaders (ITLs). Unannounced inspectors working for the Service Delivery Partner (SDP) shall be trained and supported through their normal managerial structure.
4.7.2.2 Tasks
4.7.2.2.1 Unannounced inspector duties
The unannounced inspector is responsible for:
- arranging the UAI visit with the ITL
- completing the UAI within the timescales indicated by the FVC
- inspecting the establishment to verify FBO’s compliance with legal requirements
- advising the FBO on compliance with legal requirements and agreeing any necessary corrective action and timescales with the FBO
- ensuring deficiencies are effectively addressed; liaising with the auditors’ team or the plant OV as required, and taking any necessary enforcement activity as a result of the visit
- completing the UAI report in the UAI app
The table below summarises the unannounced inspectors’ tasks and provide details of actions required and timescales.
| Tasks | Responsibilities and actions | Timescales |
|---|---|---|
| Organising visits | Liaise with ITL and FVC to ensure assigned visits are completed on time and in line with the scheduled request. Sufficient time must be planned to include preparation, visit, updating of enforcement, report writing time and other visit related admin tasks. | UAIs must be organised shortly after they have been assigned. |
| Preparation for the UAI visit |
Use the UAI Application to access the information required to prepare the visit. Liaise with Veterinary Auditors (VAs), FVCs, LUAIs and plant OVs when applicable. Complete any formal notices that may need to be served and send them to FVC for accuracy check. |
Prior to the UAI visit. |
| UAI visit |
All UAI visits should be carried out in accordance with the FSA Health and Safety at Work Policy and Lone Worker Provisions.
Take proportionate enforcement if contraventions are identified. Collect evidence of contraventions identified (photos, videos, seize evidence…) and make notes in the contemporaneous notebook when needed. |
To notify your manager of onsite arrival and departing during the UAI visit. |
| Report Submission |
To use the UAI Application to write the report and update the enforcement programme. To ensure all reports are submitted timely, plant profiles are updated, enforcement programme is updated, and any photos/ evidence are stored in the plant folder of the Share-point. Contraventions identified during UAIs need to be recorded in Chronos and enforced following the hierarchy of enforcement and in accordance with the FSA enforcement policy. |
Five working days to complete reports and any other task required. Reports are to be submitted to FBOs within ten working days of the UAI, allowing the extra five working Written advice is to be completed, |
| Formal Notices |
Complete formal notices and send them to FVC for accuracy check. This could be done before the visit, as part of preparation, if the unannounced inspector considers an issue is likely to escalate, or during the visit if necessary. Whenever possible, to discuss the serving of a notice with the relevant FVC whilst at the premises. |
Notices must be sent to the FVC for approval before serving, or if not achievable, scans of notices must be sent to the FVC on the same day they were handed and before posting. |
| Feedback | Reporting significant findings of UAIs back to the FVC. |
After the visit. |
4.7.2.2.2 FVC duties
| Tasks | Responsibilities and actions | Timescales |
|---|---|---|
| Schedule UAIs | Schedule UAIs, in line with the requirements described in section 4.7.3 using the UAI Application. | Frequency to be in line with the requirements described in 4.7.4 or as indicated by the scheduler in the UAI app. |
| UAI visits to establishments approved for RTE and/or other products of animal origin (OPAO) |
Carry out UAIs at RTE and/or OPAO establishments or ensure they are timely assigned to:
|
Frequency to be in line with the requirements explained in section 4.7.3 or as indicated by the scheduler in the UAI App. |
| Technical advice |
Support FSA employed unannounced inspectors with technical advice and knowledge where possible during the allocated UAIs from preparation of the visit to submission of the report. If the FVC is not available during the visit times, it should provide details of someone within the technical management chain available to take calls from the unannounced inspector should the need arise. |
Before the visit, during or after if requested by the unannounced inspector. |
| Written advice | Check all written advice produced by Unannounced inspectors before postage to FBO | Written advice is to be checked within three working days from receipt of the letter. Submission to FBOs must be within five working days of the UAI visit. |
| Formal notices |
Review all formal notices produced by unannounced inspectors before service. This work needs to be prioritised and completed without any delay. On the exceptional occasions when this is not achievable, for example when legal advice is required before serving a notice or liaison with the Veterinary Enforcement Delivery Managers (VEDMs) for opinion/ consistency is needed, the FVC will review the notice as soon as possible after it is handed out to the FBO and before posting. |
During preparation, before the visit, if indicated by escalation in Chronos. Final check should be completed on the same day as the visit and before postage. |
| Accuracy and consistency checks. |
Conduct checks on a representative sample of reports to improve UAI standards. Record the findings using the UAI development review form in line with the established internal framework. |
In accordance with the internal framework. Minimum, 1 report check per inspector per quarter, if inspector completes up to 10 UAIs, and 2 reports’ checks, if more than 10 UAIs are carried out per quarter. |
| Performance |
Liaise with LUAI to obtain/pass feedback on the unannounced inspector's performance. Undertake one shadow visit per year with each FSA employed unannounced inspector working in the region, including the LUAI. Assess the inspector performance during the UAI visit and record it using the UAI development review form in line with the established internal framework. |
Annually for each Inspector. |
4.7.2.2.3 LUAI duties
| Tasks | Responsibilities and actions | Timescales |
|---|---|---|
| UAI visits | Carry out some UAIs in challenging establishments as requested by the FVC. Including UAIs at RTE and/or OPAO establishments if the relevant training/qualification has been obtained. | When requested by FVCs. |
| Delivery of the UAI programme |
Work with FVCs to support co-ordination of UAIs across the region and explore improved ways of delivering them more effectively and efficiently. Promote and improve the delivery and quality of UAIs. |
Regularly. |
| Support and guidance |
Support and develop the skill sets of unannounced inspectors in their area of control, assessing the capability of UAI resource in the region including competence levels and identifying development needs. Organise regional meetings to provide guidance and ensure consistency within the unannounced inspectors team. |
Ongoing.
Quarterly. |
| Performance |
Liaise with FVC and ITLs to obtain/pass feedback on the unannounced inspector's performance. Undertake one shadow visit per year with each inspector working in the region. Record the findings using the development review form in line with the established internal framework. Where appropriate LUAIs are to contribute to performance management meetings. |
Annually for each inspector. |
| Review UAIs documentation/ software | Regularly review and where necessary update all documentation / software such as the UAI aide memoire and the UAI application, ensuring all are up to date and continual improvement. | Annually as a minimum. |
4.7.2.3 Unannounced inspectors support and development
The LUAI acts as a coach and mentor to unannounced inspectors looking to continually improve the delivery and quality of UAI across the agency.
The LUAI will develop and review training materials, arrange practical and theoretical training and assess the competency of existing and new staff being deployed into the UAI role.
The LUAI will regularly undertake visits with all unannounced inspectors within his region to provide advice and support. They also act as a safety element in difficult UAIs, when more than one unannounced inspector is needed due to safety concerns.
4.7.2.4 Assurance measures
As an assurance measure, FVCs will conduct quality checks on a representative sample of UAI reports within their areas.
The FVC and LUAI will also be responsible for assessing the performance of the FSA employed unannounced inspectors by shadowing them during a visit to an establishment and completing a development review form each on an annual basis.
4.7.2.5 Automated system actions
The UAI app will:
- suggest the possible scheduling dates of the UAIs in accordance with the minimum UAI frequency.
- displays the current status of scheduled UAIs.
- provide all the links to the materials required to fully prepare a visit.
- store guidance documentation for unannounced inspectors.
- allow the timely production of UAI reports.
- automatically distribute the completed reports to the FBOs when submitted.
- produce data on UAIs completed.
4.7.3 Conducting the UAI
4.7.3.1 Pre-inspection preparation:
Prior to carrying out any UAI to a cutting plant, the unannounced inspector must ensure that:
- They are clear on the scope of activities to be reviewed during the UAI.
- Where appropriate, they have discussions with the Veterinary Auditor (VA), who conducted the last audit, and the OV responsible for the plant (when the cutting plant is co-located to an abattoir or GHE) prior to the UAI. This is to discuss areas of operation to be reviewed following the last audit findings, the enforcement programme, local requirements, and capture areas the auditing VA would like more focused attention.
- They also liaise with their FVCs should they need to discuss technical issues relating to the inspection visit. This includes when the preparation of enforcement notices could be required. The SDP staff conducting UAIs will follow their own processes under the arrangements of the fully managed service.
- The FVC, ITL, LUAI or SDP management, following their own internal arrangements, are aware of the day and time of the inspection and have contact details for any assistance required from those persons.
- They have all the required equipment and documentation with them, such as authorisations and FSA ID card, contemporaneous notebook, printed version of the UAI report form for the inspection (note: this can be accessed and printed from the UAI app), calibrated thermometer, camera or work phone, torch, blank notices, FSA detained tape, evidence bags, seals and any other equipment and information required for that visit.
Please note this is not an exhaustive list.
Guidance on how to prepare and complete an UAI is available on the UAI application under the “completing assigned UAI” tab.
During the preparation the following documents, which can be accessed through the UAI application, should be reviewed:
- Previous UAIs
- Previous FBO audits and the FBO audit calendar
- View open and/or pending deficiencies in the enforcement programme accessing Chronos
- Establishment information on E&P
- Companies house information for legal entity and company status
- Plant details in the SharePoint folder
- Map of establishment location
4.7.3.2 The UAI
The unannounced inspector should carry out the inspection following the protocols established in the guidance and following any instructions provided by the FVC/LUAI, specific to the plant in question.
If, when undertaking an UAI, the unannounced inspector is refused access to the premises, they should contact the FVC (or the LUAI in their absence) immediately, to seek guidance and note the details in their contemporaneous notebook. The SDP staff should also notify their line management in these circumstances (see Chapter 7, Annex 21 Q&A on Obstruction, for further information).
The UAI should include the following stages:
1. Opening meeting
The unannounced inspector should have a brief meeting with the FBO, or FBO’s representative, at the beginning of the UAI, ensuring this does not cause unnecessary delays before commencing the inspection. The following details should be covered:
- inform of the purpose of the UAI
- explain charging arrangements
- confirm that there are no changes in the legal entity, process, or layout since the previous UAI/audit
- discuss pending deficiencies from the last audit/UAI(s) at the establishment
If the unannounced inspector has any concerns in relation to the establishment, they should prioritise the visit to the production areas and discuss the above details at a later stage.
2. Inspection
The inspection is divided in two parts:
- Visit to the production areas
This is based on “reality checks”. The unannounced inspector observes all the operations being carried out in the production areas, assessing the hygiene of the operations and staff practices, temperature control, maintenance/cleaning of equipment and premises and handling and storage of animal by products. Please note this is not an exhaustive list.
Pending contraventions in Chronos are also assessed and verified. New findings (if identified) are further discussed with the FBO/FBO’s representative.
If an issue of serious public health arises during the course of the UAI, regardless of whether it is outside of the pre-defined scope of the inspection, immediate enforcement action, including gathering of evidence, must take place. The FVC / LUAI or the SPD internal management structure may provide advice to the unannounced inspector on the necessary enforcement action to be taken and appropriate evidence to be gathered. It is always good practice to obtain corroboration whenever possible. Please note that on these circumstances It may be appropriate to consider curtailing the inspection.
- Review of documentation
The unannounced inspector should review operational records, CCP monitoring records, and ABP commercial documents. Other records such as water results and pest controls records can also be reviewed. Any documentation related to pending deficiencies included in Chronos needs to be checked.
If there are new contraventions identified during the UAI, the unannounced inspector may consider what further documentation needs to be requested/assessed.
3. Closing meeting
The UAI must be concluded with a closing meeting with the FBO (or appropriate representative) in which the unannounced inspector will:
- summarise the findings (positive and negative)
- discuss the corrective actions required, including any proposed timescales and enforcement action
- outline subsequent actions and the possibility for the FBO to provide supporting evidence after the inspection
At the end of the UAI, particularly in the cases when significant contraventions have been identified, the unannounced inspector will inform the FVC. The FVC will assess the findings, using a risk-based approach, to decide if any further visits may be required.
4.7.3.3 Completion and submission of the report
Please follow the guidance on “completing an assigned UAI” available in the UAI application.
The UAI report is to be completed by exception reporting, which means, everything that has been checked during the UAI is compliant, if not highlighted in Chronos. The information entered in Chronos, is the only information that will appear in the body of the report, which is why it is important to describe any contraventions correctly, including the risks posed, any mitigating circumstances and if the risk was high, the corrective actions taken by the FBO to resolve the issue.
In the cases where no contraventions are identified, the unannounced inspector will need to provide a brief explanation on the comments section of the report as per the guidance.
The report includes a Non-Compliance Report (NCR) identifying areas requiring corrective action and any corrective actions completed (in grey) from previous audits / inspections at the establishment. New corrective actions identified on the day of the inspection are shown in bold and marked as !NEW.
The UAI report must be compiled from the inspection findings and should not differ from the findings presented verbally to the FBO, or FBO representative, during the closing meeting.
The completed report should be submitted by the unannounced inspector within five working days of the visit, and under no circumstances over ten working days from the UAI.
4.7.3.4 Enforcement
When completing the UAI report with the contraventions identified during the UAI visit, such contraventions will appear by default in Chronos with the deadlines agreed during the closing meeting with FBO/FBO’s representative. If contraventions are due to be escalated to the next step within the hierarchy of enforcement, the unannounced inspector must undertake the required enforcement action (for example, drafting a notice such a HIN or a RAN).
Enforcement undertaken needs to be done in a timely manner. Any formal enforcement completed by FSA employed staff, including written advice, needs to be submitted to the FVC for checking prior to serving. In the case of the SDP, enforcement decisions will be taken by the VEDMs following their established process.
Where during the UAI, the inspector has identified a non-compliant product and evidence indicates that the cause of the non-compliance has originated at a slaughterhouse or other plant under FSA supervision, the unannounced inspector must complete the Internal Communication of Non-Compliance Report (ENF 11/22) and gather evidence /deal with the affected products as per the instructions on the guidance section of the form.
Non-compliant products in which the cause of the non-compliance has originated in establishments approved under the Local Authorities and issues with imported products imported need to be reported to the FVC. Photos relevant to the contraventions identified and copies of notices and/or letters should be uploaded to the premises folder on SharePoint.
4.7.3.5 Timesheets
Authorised Officers (AOs) that carry out UAIs at FSA meat establishments are required to complete a timesheet to record certain activities related to those official controls.
Charging for UAIs needs to be done in accordance with the “Time Recording Coding – Additional Guidance for Authorised Officers conducting Unannounced Inspections”. This document should be read in conjunction with the “e-Timesheet User Guide-Coding Guidance”. Both documents are available in the UAI application.
4.7.4 Complaints
There is no statutory right of appeal against the outcome of an UAI or decision(s). However, if an FBO is aggrieved and seeks to challenge that outcome/decision(s), they may do so by promptly applying to the court for permission to issue a Judicial Review claim.
Where an FBO is dissatisfied with how the UAI was undertaken and wish to complain, they need to follow the FSA’s complaint process. Details of the FSA Complaints Policy can be noted here.
4.8 Enforcement
4.8.1 Slaughterhouses, game handling and co-located cutting plants
At Full Audit Auditor identifies NC (new & from the enforcement programme):
1. Urgent Improvement Necessary. Next full audit in 2 calendar months
Resident OV enforces/updates Enforcement Programme and feeds back to auditor on NCs (New and from Enforcement Programme)
Partial audit within 1 calendar month unless Full audit scheduled in that calendar month:
- If all Majors/Criticals are not closed another partial audit takes place within 1 calendar month.
- If all Majors/Criticals are closed, the auditor closes the non-compliances and no partial audits will take place until the next Full Audit.
2. Improvement Necessary. Next full audit in 3 calendar months
Resident OV enforces/updates Enforcement Programme and feeds back to auditor on NCs (New and from Enforcement Programme)
Partial audit within 1 calendar month unless Full audit scheduled in that calendar month
- If all Majors are not closed another partial audit takes place within 1 calendar month.
- If all Majors are closed, the auditor closes the non-compliances and no partial audits will take place until the next Full Audit.
3. Generally Satisfactory. Next full audit in 12 calendar months
Resident OV enforces/updates Enforcement Programme and feeds back to auditor on NCs (New and from Enforcement Programme)
Partial audit within 3 calendar months unless Full audit scheduled in that calendar month
- If all Majors are not closed another partial audit takes place within 3 calendar months.
- If all Majors are closed, the auditor closes the non-compliances and no partial audits will take place until the next Full Audit.
4. Good. Next audit in 18/12 calendar months
Resident OV enforces/updates Enforcement Programme and feeds back to auditor on NCs (New and from Enforcement Programme)
No partial audit takes place until the next Full Audit.
These courses of actions are summarised in the chart below.
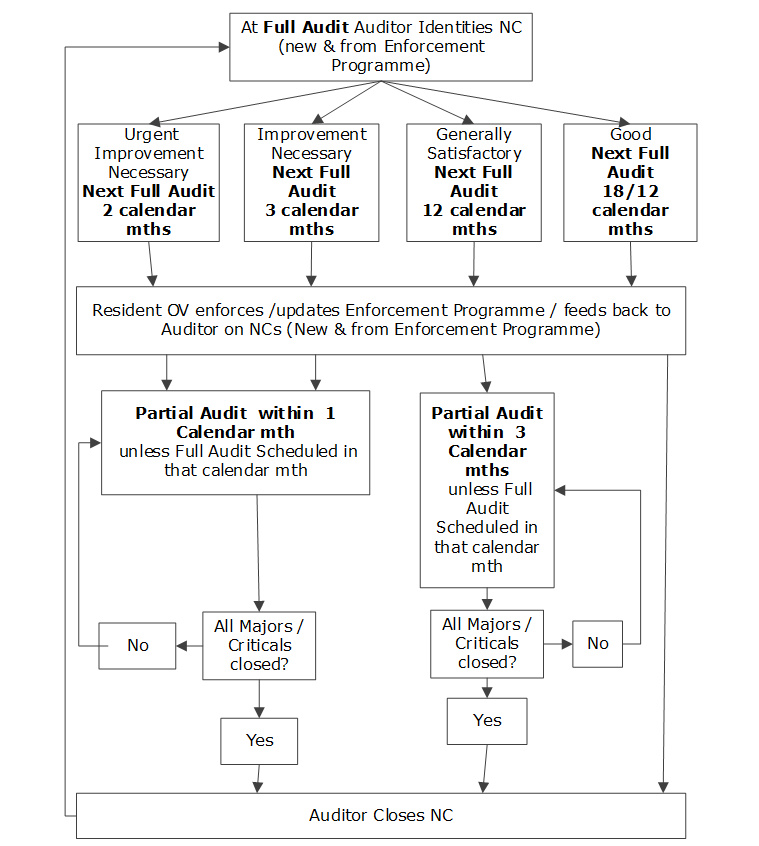
Note: This chart does not include the extended audit frequency for establishments with Good / Good outcomes in their last two audits.
4.8.2 Stand-alone cutting plants
At Full Audit Auditor identifies NC (new & from the enforcement programme):
1. Urgent Improvement Necessary. Next full audit in 2 calendar months
New minor Non-compliances. Auditor serves enforcement / updates ENF passes ownership to UAI process.
New Critical/Major non-compliances identified at Full Audit. Auditor serves enforcement / updates Enforcement Programme and follows the hierarchy of enforcement until closed.
Partial audit within 1 calendar month unless Full audit scheduled in that calendar month:
- If all Majors/Criticals are not closed another partial audit takes place within 1 calendar month.
- If all Majors/Criticals are closed, the auditor closes the non-compliances and no partial audits will take place until the next Full Audit.
2. Improvement Necessary. Next full audit in 3 calendar months
New minor Non-compliances. Auditor serves enforcement / updates ENF passes ownership to UAI process.
New Major non-compliances identified at Full Audit. Auditor serves enforcement / updates Enforcement Programme and follows the hierarchy of enforcement until closed.
Partial audit within 1 calendar month unless Full audit scheduled in that calendar month
- If all Majors are not closed another partial audit takes place within 1 calendar month.
- If all Majors are closed, the auditor closes the non-compliances and no partial audits will take place until the next Full Audit.
3. Generally Satisfactory. Next full audit in 12 calendar months
New minor Non-compliances. Auditor serves enforcement / updates ENF passes ownership to UAI process.
Partial audit within 3 calendar months unless Full audit scheduled in that calendar month
- If all Majors are not closed another partial audit takes place within 3 calendar months.
- If all Majors are closed, the auditor closes the non-compliances and no partial audits will take place until the next Full Audit.
4. Good. Next audit in 12 calendar months
New minor Non-compliances. Auditor serves enforcement / updates ENF passes ownership to UAI process.
No partial audit takes place until the next Full Audit.
In all 4 Full Audit Categories the UAI Process monitors all Minor, Major and Critical Non-compliances and feeds back to the Auditor.
These courses of actions are summarised in the chart below.
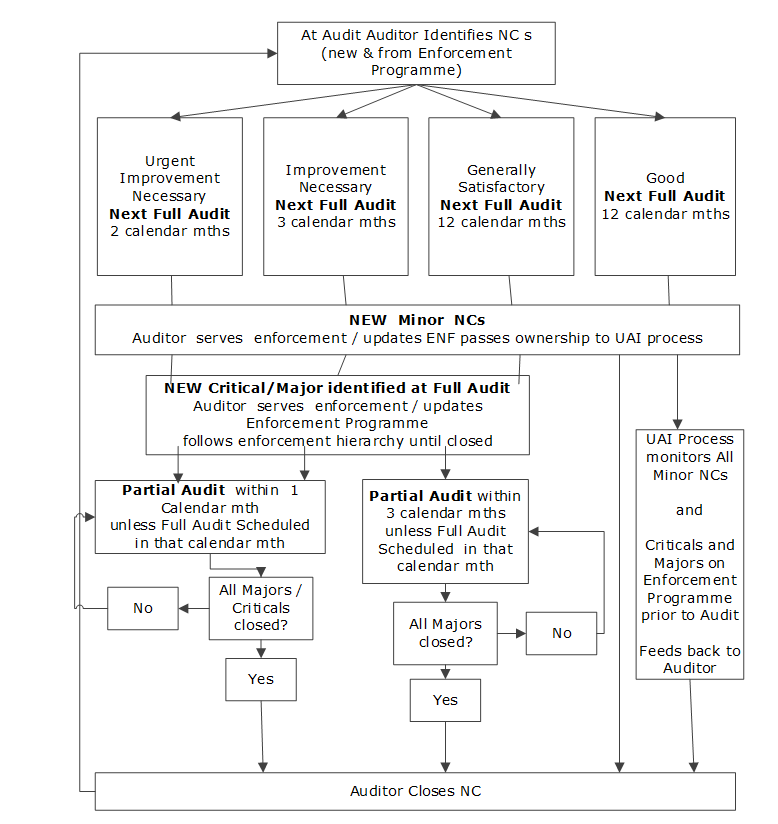
Note: This chart does not include the extended audit frequency for establishments with Good / Good outcomes in their last two audits.
4.9 Remote Auditing
4.9.1 Background and purpose
Auditors utilise an approved software to conduct Remote Audits, which is in compliance with the FSA security policies available at Privacy notice for audits of slaughterhouses and GHEs and a specific document for the security of the software used for Remote Audits is available at TEXO_Data_Protection_(GDPR)_Procedure.pdf.
4.9.2 Definitions
In accordance to how the audit is carried out, there are 3 main types of audits:
-
On-site audit: An FSA audit involving on-site visit and auditing of documents/records at the premises.
- Remote audit: An FSA audit carried out by the auditor without visiting the site but supported by the FSA team (input from all Authorised Officers -AOs visiting the plant) and/or the evidence provided electronically by the Food Business Operator (FBO).
- Semi-remote audit: An FSA audit with a reduced on-site component in which part of the audit is carried out on-site (for example targeted physical checks) and part completed remotely (for example documentation/records, meetings).
4.9.3 When to use Remote Audits?
The Auditor will have to assess the suitability of this technology depending on the outcome of the previous Full Audit, the FBO’s availability and the possibility to use it due to coverage in the establishment.
The decision tree below can help deciding when a Partial Audit is eligible for remote auditing using remote technology.
Full Audit:
Always requires On-site visit.
Partial Audit:
There are some circumstances where partial audits can take place remotely.
a. Previous outcome IN or UIN requires on-site visit
b. Previous outcome Generally Satisfactory. Eligible for Remote Partial Audit until all Major Non-compliances are closed.
1. The nature of the Non-compliance allow for remote assessment:
- The FBO is capable / willing to use the technology.
- The technology can be used in this establishment. Partial audit to be done remotely.
- The technology cannot be used in this establishment. On-site visit. - The FBO is not capable /willing to use the technology. On-site visit required.
2. The nature of the Non-compliance doesn’t allow for remote assessment. On-site visit required.
c. Previous outcome Good. No partial audit required.
In cases where the use of the FSA application (Fuse) is not possible, alternative technology/tools can be used for a remote partial audit with only documentary non-compliance, as a virtual tour of the premises is not required. Virtual tours can only be done using the FSA application.
These courses of actions are summarised in the chart below.
Updated [

]
5. Risk Assessment
In this section
5.2 Audit compliance assessment
5.1 Audit report
5.1.1 Audit report form
The Audit report form (AUD 9/3) is available via the K2 system.
5.1.2 Summary of findings
The report contains an area to summarise the audit findings. The summary of findings should include positive findings (good practice), negative findings (NCs) and a brief description of any variations from the previous audit enabling the FBO and other interested parties to review the audit without needing to read the full detail contained within the report.
5.1.3 Non-Compliance Report (NCR)
At the end of the audit report there is a section containing the NCR.
The NCR summarises and provides a short description of the NCs identified.
Once the FBO receives the report with the NCR, the FBO is responsible for rectifying the NC identified during the audit.
5.1.4 Correction of NC
During the next audit, the auditor must verify whether the FBO has taken corrective actions and indicate those which have been completed.
5.2 Audit compliance assessment
5.2.1 FBO compliance history
The history of compliance relates to the deficiencies identified against legislative requirements or the FBOs own procedures and requiring OV intervention during the audit interval or the ongoing NCs from the previous full audit.
Note: FBO initiating corrective actions where the FBO has identified a breakdown in controls is a sign of a healthy food safety management system.
During the audit, the auditor will record evidence of the FBO compliance history, which will result in a risk score under each category based on the following criteria and type of NC:
| Title | Description |
|---|---|
| Compliant | Compliance with a food safety programme, food regulatory requirements and animal health and welfare regulations (in the case of slaughterhouses) is achieved if the food business is operating in accordance with its food safety management systems, food safety standards and has met the requirements of the regulations. |
| Minor |
A NC that is not likely to compromise public health (including food safety), animal health and welfare or lead to the handling of unsafe or unsuitable food. An isolated low-risk situation and does not compromise achieving control measures of the food safety program; that is, overall the food safety program is still effective in controlling the food safety hazards. When viewed collectively a number of related minor NCs may represent a major NC. Examples (not exhaustive):
|
| Major |
A major NC is a one that is likely to compromise public health (including food safety), animal health and welfare or may lead to the production and handling of unsafe or unsuitable food if no remedial action is taken. When viewed collectively a number of related major NCs may represent critical NC. Examples (not exhaustive):
|
| Critical |
A critical NC is one where the contravention poses an imminent and serious risk to public health (including food safety), animal health and welfare. Examples (not exhaustive):
|
5.2.2 Audit categories
Using objective evidence, the type of NCs identified during an audit reflects the extent and effectiveness of compliance. The following grading system is outlined in the table below:
| Compliance rating | Description | Tolerance for audit outcome |
|---|---|---|
| Good | No issues of significance for public health, animal health or animal welfare during the entire audit period. | No majors or critical on day of audit or during audit period |
| Generally Satisfactory | No immediate issues of significance for public health, animal health or animal welfare identified on the day of the audit. Any NCs identified during the audit period corrected promptly. |
No more than 2 majors during audit or during audit period rectified promptly No critical during audit period |
| Improvement Necessary | Major NCs identified at audit and/or NCs during the audit period not always responded to and corrected promptly. |
3-6 majors during audit or during audit period No critical during audit period |
| Urgent Improvement Necessary | Multiple major NCs or critical NC identified during audit visit or interim audit period. Official intervention required to ensure public health safeguards. | 1 critical or >6 majors during audit or during audit period |
5.3 Audit outcome and frequency of inspections
5.3.1 Determination of frequency
The frequency of audit reporting is determined on a risk basis; assessed, in part, on the outcome of previous audits as outlined in this chapter.
The scheme differentiates between slaughterhouses with or without co-located cutting plants, approved GHE and standalone cutting plants. Audit frequency for slaughterhouses / co-located cutting plants / approved GHE ranges from 2 to 18 months and for standalone cutting plants ranges from 2 to 12 months (due to an absence of routine official presence in standalone cutting plants 12 months remains the maximum frequency).
In addition to a scheduled full audit, a follow up partial audit is to be carried out in some establishments which is dependent on the full audit outcome.
5.3.2 Audit frequency
Please also see sub topic 5.3.3 Extended audit frequency.
The tables below list the minimum audit frequencies applicable to specific types of food establishment. They also include the number of necessary partial audits and UAIs that have to take place.
Updated [Audit frequencies for slaughterhouse / co-located cutting plants and approved game handling establishments
| Audit outcome | Follow up partial audit | Full audit frequency |
|---|---|---|
| Good | 0 | 18 months* |
| Generally satisfactory | Within 3 months* | 12 months |
| Improvement necessary | Within 1 month | 3 months |
| Urgent Improvement necessary | Within 1 month | 2 months |
* Where there is sufficient evidence provided to the auditor by the FBO, and verified by the OV where possible, that the NC has been rectified, this can be closed off without the need for an establishment visit (it is at the discretion of auditor to decide if a visit is required). This is only possible if the audit outcome is ‘generally satisfactory’.]
Audit frequencies for standalone cutting plants and cold stores (for example, Smithfield Market)
| Audit outcome | Follow up partial audit | Minimum number of UAIs during interim audit period* | Full audit frequency |
|---|---|---|---|
| Good | 0 | 1 | 12 months |
| Generally Satisfactory | Within 3 months | 1 | 12 months |
| Improvement necessary | Within 1 month | 1 | 3 months |
| Urgent Improvement necessary | Within 1 month | 1 | 2 months |
*RTE establishments will receive one additional unannounced inspection by a trained OV.
Additional visits based on the audit outcome
| Audit Outcome | Revisits |
|---|---|
| Good |
N/A |
|
Generally Satisfactory or Improvement Necessary |
Follow up partial audits (where required) to be carried out by the auditor Unannounced inspections to be carried out by a MHI or an OV (for example, in RTE establishments or co-located cutting plants) Major NCs in all instances shall be closed off by the auditor either following a site visit or upon acceptance of corroborated evidence of compliance Minor NCs can be signed off by the auditor upon information received by the field team |
| Urgent Improvement Necessary |
Follow up partial audits (where required) to be carried out by the auditor Unannounced inspections to be carried out by a MHI or an OV (for example, in RTE establishments or co-located cutting plants) Critical NCs can only be closed off by the auditor following an on-site partial audit where compliance could be verified Major NCs in all instances shall be closed off by the auditor either following a site visit or upon acceptance of corroborated evidence of compliance Minor NCs can be signed off by the auditor upon information received by the field team |
5.3.3 Extended audit frequency
Extending audit frequency aims to provide recognition for FBOs who have sustained a high level of compliance over two consecutive audit outcomes with an aim to ultimately reducing footfall resulting from official control activities without increasing the risk to consumer protection or confidence.
The tables below list the minimum audit frequencies applicable to specific types of food establishment. They also include the number of necessary partial audits and UAIs that have to take place.
The FSA reserves the right to re-audit meat premises at any time and will act on intelligence and evidence in line with existing intervention protocols. Taking compliance history into consideration encourages businesses to maintain high standards at all times.
Extended audit frequencies for slaughterhouses / co-located cutting plants and approved game handling establishments
| Audit outcome | Standard frequency | Follow up partial audit | Extended frequency |
|---|---|---|---|
| Good / Good | 18 months | 0 | 36 months |
Extended audit frequencies for standalone cutting plants and cold stores
| Audit outcome | Follow up partial audit | Minimum number of UAIs during interim audit period | Current full audit frequency | Extended frequency | Minimum number of UAIs during interim audit period |
|---|---|---|---|---|---|
| Good/ Good | 0 | 1 | 12 months | 24 months | 2 |
RTE establishments will receive one additional (3) UAIs during the interim audit period by a trained OV.
Any plant that qualified for extended audit frequencies and subsequent audit outcomes drop to Generally Satisfactory, Improvement Necessary or Urgent Improvement Necessary is automatically disqualified from the extended audit frequency system. They can requalify for extended audit frequencies by achieving two consecutive Good / Good outcomes, but, in the meantime, will revert back to standard audit frequencies.
However, if during the full audit of an establishment that is under the extended audit frequency, the auditor raises a Critical/Major NC for deficiencies that have occurred during the extended period but for which the FBO implemented remedial/preventive actions promptly and effectively with no recurrence observed (or reported by the OV/UAI) afterwards, the NC can be “closed do not count to outcome”. This decision is at the auditor's professional judgment for isolated issues fully resolved at the time of the audit, in consultation with the AVL.
In these cases, the audit's outcome will be ‘Good’, but there will be an impact on the Extended Audit Frequency (EAF) status, as this will be removed until the next audit. The auditor will have to communicate to the K2 team the need to change the EAF status manually; otherwise, the K2 system will automatically include the establishment in the EAF. The status can be re-gained if a Good outcome, with no other Critical/Major NCs within the second audit cycle, is achieved.
5.4 Review and right of appeal
5.4.1 Regulators code
The appeals route for FBO audits follows the regulators code.
5.4.2 FBO right to seek review
If an FBO is dissatisfied with the outcome of discussions with the auditor after the closing meeting, or the audit report once received from the Updated [FSA Veterinary Audit Team (VAT)], the FBO has the right of appeal in line with the following procedures:
| Stage 1 Appeal | Action |
|---|---|
| Try to resolve informally | All efforts should be made to resolve any misunderstanding or dissatisfaction informally on a local basis between the auditor, AVL and FBO. |
| Direct FBO to Updated [the approvals team] to request an audit appeal form | If a FBO, or their representative, still wishes to appeal an audit report they should be directed to the Updated [approvals team] to request the audit appeal form ‘Request for a review of the audit of the FBOs food safety management system’. |
| Updated [Approvals] receives request for audit appeal form | On receipt of the FBO’s request for an appeal request form, the Updated [approvals team] will send the form to the FBO, ensuring that the auditor is notified of the request, to ensure that all possible efforts have been made to resolve the matter informally. |
| FBO submits formal appeal, with supporting evidence |
The FBO, or their representative, should complete their part of the form, stating which sections of the audit report the FBO is appealing against and giving objective evidence to support the claim that the auditor’s assessment is incorrect. Any supporting evidence should be copied and sent with the form to the Audit Coordinator within 14 calendar days of receiving the initial audit report. Appeals which are not supported with objective evidence may be rejected. |
| Investigating Officer (IO) appointed |
Updated [On receipt of the completed appeal form, the approvals team will provide the Head of Veterinary Audit with a copy of the appeal, including any supporting evidence. The Head of the Veterinary Audit will be responsible for appointing an AVL from a different area as the Investigating Officer (IO), and confirming the details. |
| IO reviews the supporting evidence supplied by the FBO |
The IO will consider if the appeal has sufficient evidence to continue, if not the FBO will be notified that the appeal will not progress any further. IOs will focus on scores challenged and the submission of evidence to carry out the investigation. The IO is not obliged to examine other aspects of the audit to which the appeal is related; however, as findings are sometimes interrelated the IO will take these into account where it is appropriate to do so. The IO will not overlook other relevant information which may be used to inform any decision made. |
| IO conducts an investigation |
The IO conducts an investigation and completes a report before the last date for completion (stated in part 1 of the appeal request form). The IO will determine which considerations should be made when making the assessment. Examples as follows:
Note: IOs should always consider visits to premises where serious concerns are arising, such as critical or multiple major NCs. |
| Investigation outcome |
Updated [On conclusion, the IO distributes their completed report to the approvals team, who will take the necessary actions, depending upon the outcome of the IO’s investigation. The approvals team will email the IO’s report to the FBO, (including any amended audit report if applicable) and copy the correspondence to the AVL and the Head of the Veterinary Audit Team]. The IO is responsible for discussing the investigation findings with the AVL, auditor and the FBO (or their representative) regardless of whether the investigation report resulted in an amendment or the score was upheld. |
5.4.3 Stage 2 appeals
FBOs can request a Stage 2 appeal when they are not satisfied with the outcome of the stage 1 appeal.
A £250 fee is payable by the FBO for a stage 2 appeal process as a contribution to the FSA’s costs. Stage 2 appeals will not commence until the fee has been paid. If the review/appeal rules in the FBO’s favour and the audit frequency has been changed the £250 will be refunded. If the appeal changes the outcome of some sections, but this does not lead to a change in the overall audit outcome, the fee will not be refunded.
| Stage 2 Appeal | Action |
|---|---|
| FBO exercises their right to appeal at stage 2 | FBO notifies the Updated [Approvals Team] in writing (for example, via email or post) within 7 calendar days of receiving the stage 1 outcome notification of his intention to appeal the stage 1 outcome. The required £250 payment should also be enclosed. |
| Updated [Approvals] receives FBO written confirmation and payment |
On clearance of payment Updated [the Head of the Veterinary Audit Team] will contact an independent IO appointed by the FSA to carry out the investigation. Stage 1 appeals pack is sent to Independent IO for review.
any additional document reviewed during the stage 1 appeal] |
| Independent IO |
The appeal will be determined within 14 calendar days by the independent person nominated by the FSA. The nominated person:
|
5.4.4 Updated [Continuation of the audit process
Whilst the appeal process is taking place, the audit schedule continues as normal. If the outcome of the appeal supports the FBO claims and this impacts on the audit outcome, the audit frequency will be adjusted to the new outcome in line with the MOC instructions.
During the appeal process the audit outcome will not be published on the FSA website].
6. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices.
Annex 2: Audit writing guidance
1. Introduction
2. Common Issues of HACCP Auditing
3. Audit and Enforcement
Sections
1. Introduction
In this section
1.1 Legislation
1.1.1 HACCP legislative framework
The following table summarises the different pieces of legislation that cover FBO and OV responsibilities in relation to HACCP based procedures.
HACCP legislative framework
| Regulation | Issue | Who is responsible? | Other documents |
|---|---|---|---|
| Reg (EC) 852/2004 Ch II, Article 5 | Put in place, implement and maintain a permanent procedure based on HACCP principles | FBO |
|
| Reg (EC) 852/2004 Annex II, Ch XII | Train staff responsible for the development and maintenance of HACCP based procedures in the application of HACCP principles | FBO |
Commission Guidance |
| Reg (EC) 853/2004 Annex II, Section II | List of HACCP based objectives for incoming animals accepted for slaughter | FBO |
Commission Guidance |
| Regulation (EU) 2017/625 Article 14, Article 18 and Annex II Ch I | Audit and verification that FBOs implement and apply HACCP-based principles continuously and properly | OV |
Food Safety Management Diary for meat producers |
| Commission Delegated Regulation (EU) 2019/624 Article 7, Article 9, Annex II, Ch I, Ch II and Ch III |
Audit and verification that FBOs implement and apply HACCP-based principles continuously and properly Training requirements for OVs Training requirements for OAs Training requirements for ‘other staff designated by the competent authority’ |
OV |
Food Safety Management Diary for meat producers |
| Commission Implementing Regulation (EU) 2019/627 Article 3, Article 4, Article 7 and Article 25 |
Audit and verification that FBOs implement and apply HACCP-based principles continuously and properly | OV |
Food Safety Management Diary for meat producers |
1.1.2 (EC) 852/2004 evidence
The FBO shall provide the OV with evidence of their compliance with the HACCP legal requirements, taking into account the nature and size of the business, and ensure that any documents describing the procedures are up to date at all times.
The instructions in this chapter reflect the minimum requirements expected to consider an FBO plan of HACCP-based procedures adequate and in compliance with the Regulations.
Reference: (EC) 852/2004, Chapter II, Article 5.
1.1.3 Regulation (EU) 2017/625 OV verification of FBO HACCP based procedures
The OV is required to conduct audits to verify that food business operators apply HACCP based procedures continuously and properly. In particular, audits must take account of:
- identified risks
- information which may mislead the consumer
- FBO past records
- Results of FBO own verification
- Results of third party or private quality assurance schemes (1)
- Any other information which might indicate non-compliance
(1) Only for the purpose of ascertaining compliance with food safety, animal health and welfare rules
1.1.4 Delegated Regulation (EU) 2019/624
Official veterinarians, official auxiliaries and other staff designated by the competent authority must be suitably trained and authorised by the competent authority to gather evidence on FBO HACCP based procedures.
OAs and other staff designated by the competent authority working under the responsibility of the OV may collect information on GHP and HACCP based procedures to contribute to the audit process.
1.1.5 Implementing Regulation (EU) 2019/627 on the specific requirements of auditing HACCP based procedures
Determine to the extent possible that FBO procedures continuously and properly guarantee that fresh meat:
- complies with microbiological criteria;
- complies with legislation regarding chemical and physical hazards; and
- complies with legislation regarding faecal contamination and contamination with specified risk material.
And that the food business:
- Uses guidance and third-party assurance data correctly, where applicable.
- Has sufficiently competent staff to comply with the requirements.
1.1.6 Key reference documents
The European Commission has produced a guidance document for the implementation of procedures based on HACCP principles and to facilitate the implementation of HACCP principles in certain food businesses.
Reference: MOC, Volume 2 Legislation for additional information.
‘The Diary’ has been produced by the FSA for smaller operators and can be found within the MOC titled as ‘Food Safety Management Diary’.
The Diary is specifically designed to facilitate FBOs to keep records relating to the hygienic operation of their businesses. It also includes draft documentation on prerequisites and HACCP.
The use of the Diary by FBOs is voluntary.
Reference: See the topic 2.10 on ‘Principle 7: documentation’ in part 2 for additional information.
1.2 Characteristics of HACCP based procedures
1.2.1 Purpose
HACCP principles are a tool for FBOs to use to control hazards that may occur in food.
HACCP is a set of 7 principles used to assess hazards and establish control systems that focus on prevention of problems rather than relying solely on end-product testing.
1.2.2 Implementation requirements
The successful application of HACCP based procedures requires the following:
- the FBO must already have implemented the hygiene controls that are required by legislation (prerequisites / good hygiene practice)
- requires the full commitment of management and the involvement of the work force
1.2.3 ‘Traditional’ HACCP vs. HACCP based procedures
‘Traditional’, ‘classic’ or ‘technical’ HACCP is not the same as ‘HACCP based procedures’.
Traditional HACCP evolved from spacecraft manufacture to guarantee the safety of astronauts’ food. It remains appropriate for industrial production of processed foodstuffs involving for example, sterilisation or pasteurisation steps.
It is however acknowledged in (EC) 852/2004 and particularly in the Commission’s guidance on HACCP that such a technical approach may not be appropriate for all types and sizes of food businesses. In the case of meat plants, for example, it can be sufficient to apply the principles in a more flexible way following guides to practice.
1.2.4 ‘Flexibility’: Nature and size of the operations
Flexibility regarding the application of HACCP principles may be applied, taking into account:
- the nature of the operations
- the size of the business
‘Flexibility’: Nature and size of the operations
| Flexibility taking into account | Comments |
|---|---|
| Nature of the operations |
In businesses handling food with no significant food safety hazards (for example, greengrocers) a hazard analysis confirming that is the case can be sufficient. In businesses handling many foods (for example, restaurants) a simplified approach using a diary can be sufficient. In businesses involving simple processing (for example, slaughterhouses and cutting plants) a generic plan with a diary for record keeping can be sufficient as long as they are adapted to reflect company conditions. In food manufacturing businesses, particularly with procedures that will eliminate hazards (for example, canning plants) full technical HACCP is more appropriate. OV auditors should consider whether the HACCP based procedures are appropriate for the type of business. |
| Size of the business / documentation |
The size of business and resources available will have a bearing on the complexity of the HACCP based system; however a simple, easily managed system can achieve the safe production of food as well as a more complex system. A traditional HACCP system relies heavily on recording that all the procedures are being followed correctly, probably by the Quality Control, Quality Assurance or HACCP team. Small and medium sized businesses rarely require the same level of documentation. They may choose to record when things go wrong, called ‘exception reporting’. Reference: See the topic 2.10 on ‘Principle 7: Documentation’ in Part 2 for additional information. OV auditors should note that there is no value in FBO documentation being disproportionate to the level of risk and the recording of HACCP based monitoring procedures being a burden to small-medium businesses. |
1.2.5 Flexible application of HACCP principles
FBO application of HACCP principles should meet the following criteria:
- identify the main hazards associated with the type of product produced and the operations carried out
flexibility: hazards - generic descriptions of hazards may be sufficient
- identify those Critical Control Points (CCPs) / Control Points (CPs) necessary to control those hazards; the FBO may choose to have in the plan only CPs which are legal requirements
flexibility: CCPs - generic guidance may include pre-determined CCPs in the preparation, manufacturing and processing of food
- establish critical (or legal) limits against which to monitor the effectiveness of control measures at CCPs / CPs
flexibility: critical limits - it is not always necessary to fix a numerical value, especially where monitoring procedures are based on visual observation (for example, the faecal contamination of carcases in a slaughterhouse)
- monitor CCPs / CPs
flexibility: monitoring - may be a simple procedure, for example, a visual observation to monitor whether the correct de-hiding procedure is being applied during slaughter where this part of the slaughter process has been identified as a CCP for preventing carcase contamination
- take the necessary corrective actions based on the results of the monitoring activities
- record the observations and corrective actions taken; the requirement of retaining documents needs to be flexible in order to avoid undue burdens for small / medium businesses
flexibility: recording – in the case of visual monitoring procedures it can be acceptable to record results only when there is a problem and the corrective action that has been taken; that is, ‘exception reporting’; a diary can be a suitable method of record keeping
- verify the HACCP-based procedures
flexibility: verification – checking all aspects of the HACCP plan can be spread throughout the year so that all aspects are verified at least once a year to meet the requirement for ‘regular’ verification
1.2.6 Review of HACCP based procedures
The HACCP procedures should be reviewed and necessary changes made by the FBO when any modification is made in the product, process or any step.
1.2.7 OV role
OVs, through auditing, need to determine the level of FBO compliance with HACCP principles always taking into consideration the possibility of implementing simplified HACCP based procedures particularly in small / medium sized businesses.
2. Common issues of HACCP Auditing
In this section
2.3 Implementing and maintaining HACCP based procedures
2.4 Principle 1: Hazard analysis
2.5 Principle 2: Determine Critical Control Points (CCPs) / Control Points (CPs)
2.6 Principle 3: Establish critical limits (CLs) / legal limits (LLs)
2.7 Principle 4: Monitoring of CCPs / CPs
2.8 Principle 5: Corrective action procedures
2.1 Introduction
This section covers common issues for OVs to consider when auditing a food safety management system based on HACCP principles in compliance with the regulation.
2.2 Training
2.2.1 Staff responsible for HACCP based procedures
Those responsible for the development and maintenance of HACCP-based procedures have received adequate training in the application of HACCP principles.
Reference: (EC) 852/2004, Annex II, Chapter XII, 2.
2.2.2 Training: common issues
The following table contains examples of common issues that the OV and OV auditors could find when auditing HACCP based procedures and guidance on how the OV should make the assessment to determine FBO compliance:
Training: common issues
| Common Issues | OV advice / guidance |
|---|---|
| No member of staff with formal training |
Formal training is not a legal requirement; the FBO however, should show that they have received ‘supervision, instruction and / or training’. This can be achieved in a number of ways including (list not exhaustive):
These may or may not be accredited courses; however, there should be evidence of training. Examples include: certificates, completed test papers, questionnaires, personal assessment papers and individual training records showing instruction or training received. |
| The FBO believes they do not require any training at all as the HACCP based system has been written by an external adviser / consultant |
If external advisers / consultants are used, they should do so as part of a HACCP team, providing instruction and guidance rather than working independently and writing the system for the FBO. It may mean that the FBO is unable to answer questions or make amendments without reference to the adviser. This raises the question of whether the staff can be maintaining their HACCP-based procedures and has adequate training to do so. Instruction given by the external adviser / consultant to the FBO should be recorded on individual training records. Primary responsibility for food safety rests with the FBO, so ownership of the food safety system should be that of the FBO. Reference: (EC) 852/2004, Chapter I, Article 1, 1(a). |
2.3 Implementing and maintaining HACCP based procedures
2.3.1 HACCP implementation and maintenance
FBOs shall put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles taking into account the nature and size of the business.
Reference: See Section 10.6 of Volume 2 of the MOC and Regulation (EC) 852/2004, paragraph 15 of the recital as well as Article 5, 1.
2.4 Principle 1: Hazard analysis
2.4.1 Hazard identification
The FBO is responsible for identifying any significant hazards that must be prevented, eliminated or reduced to acceptable levels.
Reference: (EC) 852/2004 Article 5, 2(a).
2.4.2 Hazard identification: common issues
The following table contains examples of common issues that the OV and OV auditors could find when auditing HACCP based procedures and guidance on how the OV and OV auditors should make the assessment to determine FBO compliance:
Hazard identification: common issues
| Common Issues | OV / OV auditor advice / guidance |
|---|---|
| The product description does not include technical information | Flexibility as to what is included should relate to the technical nature of the production process. For example, a meat plant producing a meat preparation and / or meat product is likely to require a greater amount of data such as microbiological criteria, moisture content. than a meat plant that simply cuts and packs a raw product. Large meat plants that have qualified technical teams / advisers may have the necessary skills to write a very detailed and validated technical description of the process; this may not be the case in small – medium businesses with fewer resources. |
| Flow diagram does not show all steps in a process | A flow diagram (CODEX HACCP guideline) used in a traditional HACCP system will describe all inputs into the food business (such as packaging and ingredients), the different stages of process, the outputs (including food and waste) as well as how different foods are stored and despatched, where applicable. Generic systems based on HACCP principles may use a ‘simplified’ flow diagram; this is an identification (rather than description) of each process step. Certain process steps may be grouped together when the risks are the same, for example, removing bones from a carcase and cutting the boneless meat into cubes. Although these are two different procedures, the hazards will be the same, therefore the process step may be written and simplified as follows:
|
| Hazards identified do not specify individual contaminants such as salmonella, rust, chemicals, peanuts |
A technical HACCP study completed by a multi-disciplinary team will be based on extensive research to ensure that all potential hazards, biological, physical, chemical and allergenic are identified for example, the effect of competition from spoilage bacteria on the survival of food-borne pathogens. This level of detail is unlikely to be achieved by small – medium businesses with limited resources, who may address individual hazards by groups, for example, Biological contamination: The naming of each type of pathogenic bacteria that may be a contamination / cross-contamination hazard would be appropriate for larger plants but not for businesses following a generic plan. At the chilling step, a generic hazard will be ‘Growth of bacteria due to inadequate temperature control’. It is unnecessary for the FBO to have an in-depth understanding of microbiology. It is sufficient that the plan recognises the dangers of poor temperature control in relationship to bacterial growth. Importantly, FBOs should recognise the need to minimise the level of micro-organisms at each stage of the supply chain as there is a risk of cross contamination of ready-to-eat products by raw meat before it is itself cooked. |
|
Hazards identified do not specify individual hazards such as salmonella, rust, chemicals, peanuts |
Physical contamination: Individual hazards, such as parts from machinery, contamination from building fabric, may be combined and identified as ‘contamination due to foreign objects’. Chemical contamination: The plan may not identify a significant chemical hazard. Cleaning chemical hazards should be controlled by the application of hygiene controls, such as appropriately adhering to cleaning procedures (as documented in Cleaning Schedules) which should include a list of chemicals used. In respect of allergenic reactions, few people display allergic reactions to meat, so in raw meat slaughter / processing it is unlikely to be a significant hazard, however this risk would need to be considered when processing a meat preparation or product, which may contain relevant products such as soya, egg, sesame. OVs and OV auditors should note the above differences applying flexibility. |
| Inaccurate control measures identified |
‘Control measures’ are necessary to control significant hazards from contaminating a food, for example, the chilling of meat down to a desired temperature and the implementation of maintenance procedures. The plan of HACCP-based procedures may not distinguish control measures from monitoring procedures and may include visual inspections / observations as control measures. Even though visual inspection (observation) is technically a monitoring procedure rather than a control measure, it can still be accepted as a control, provided it is accurately documented and that action is taken where and when non-compliance is observed |
2.5 Principle 2: determine CCPs / CPs
2.5.1 CCP / CP Identification
Identifying the CCPs or control points at the step or steps at which control is essential to prevent or eliminate a hazard or reduce it to acceptable levels.
Reference: (EC) 852/2004 Article 5, 2(b).
2.5.2 Difference between CCP and CP
In the processing of fresh meat and offal, it may not be possible to prevent or eliminate hazards and reduction steps may not be measurable in the same way as, for example, when food is canned.
Therefore, FBOs may consider that for their product and / or operations there are no ‘traditional’ CCPs. There are process steps, however, where controls are necessary to meet legal objectives. If these process steps are not chosen as CCPs they should nevertheless be included in the plan of HACCP-based procedures as control points (CPs) required by legislation and records of monitoring and corrective actions should be kept.
Examples of those control points are:
- acceptance of animals for slaughter, to ensure animals are identified, clean and healthy
- evisceration and dressing, to ensure absence of visible contamination
- temperature controls to limit growth of micro-organisms
- receipt / pre-cut inspection of raw meat, to ensure raw materials are free from contamination
2.5.3 CCPs / CPs common issues
The following table contains examples of common issues that the OV and OV auditor could find when auditing and/or verifying HACCP based procedures. The table also provides advice and guidance on how the OV and OV auditors should make the assessment to determine FBO compliance:
CCPs / CPs common issues
| Common Issues | OV advice / guidance |
|---|---|
| CCPs either not present or not identified correctly |
In certain food businesses, there will be steps in the process that are critical to the safe production of food, for example, cooking a raw food to a specified core temperature. A decision tree may be used to determine CCPs. On the other hand, a small-medium slaughterhouse or cutting plant handling raw meat may follow a generic approach where CCPs / CPs are pre-determined and so, whilst decision trees can be very useful in certain circumstances, there are times when a decision tree may not be needed. |
2.6 Principle 3: Establish critical limits / legal limits
2.6.1 Establishing limits
Establishing critical limits (or legal limits) at CCPs (or control points) which separate acceptability from unacceptability for the prevention, elimination or reduction of identified hazards.
Reference: (EC) 852/2004 Article 5, 2(c).
Limits do not need to be a fixed numerical value that requires measurement. Limits can be monitored through visual observation, for example, faecal contamination of carcases.
2.6.2 Difference between critical limits and legal limits
Critical limits separate acceptability from unacceptability or safe from unsafe food at CCPs. Critical limits must be at least as strict as legal requirements that apply at that process step for example, temperatures for raw meat.
LLs are values set out in the legislation, for example, storage temperatures for meat, regardless of whether or not the FBO identifies a CCP based on a risk assessment.
2.6.3 Critical limits / legal limits – common issues
The following table contains examples of common issues that the OV and OV auditors could find when auditing and/or verifying HACCP based procedures. The table also provides advice and guidance on how the OV and OV auditors should make the assessment to determine FBO compliance:
CLs / LLs - common issues
| Common Issues | OV advice / guidance |
|---|---|
| Hygiene controls set as Critical Limits | In a technical HACCP system CLs may include:
|
| Hygiene controls set as Critical Limits, |
In some cases, the plan of HACCP-based procedures may not distinguish critical limits from the application of hygiene controls for example, cleaning procedures, maintenance procedures and pest control. Where FBOs have decided to have CPs (instead of CCPs) which are legal requirements, the LLs may include strict adherence to a hygiene control. |
| Understanding the difference between compliance (through LLs) and risk assessment (through CLs) |
Ensure the FBO is fully aware of the need for compliance with legal limits first and foremost. Compliance should not be confused with the FBO risk assessment. |
2.7 Principle 4: monitoring of CCPs / control points
2.7.1 Monitoring procedures
Establishing and implementing effective monitoring procedures at CCPs (or CPs).
Reference: (EC) 852/2004 Article 5, 2(d).
2.7.2 Monitoring procedures: common issues
The following table contains examples of common issues that the OV and OV auditors could find when auditing and/or verifying HACCP based procedures. The table also provides advice and guidance on how the OV and OV auditors should make the assessment to determine FBO compliance:
Monitoring procedures: common issues
| Common Issues | OV advice / guidance |
|---|---|
| Monitoring procedures not recorded |
Monitoring procedures are an important part of a HACCP based system, in some cases monitoring may not be recorded, or recorded just to pass an audit or a verification check. FBO monitoring procedures should be meaningful, easy to understand and should relate directly to SOPs. Reference: See topic 2.10 on ‘Principle 7: Documentation’ in part 2. |
| Plan not a true reflection of reality |
The monitoring procedures described in the plan should reflect what is actually happening at each process step and with each product. Where SOPs are documented, these should accurately reflect the activity they are related to. |
| Disproportionate monitoring procedures | Extensive record keeping may prove to be burdensome for an FBO to maintain (for instance when documentation / records have been produced by a third party (consultant) who does not understand the food business operations, for example,
Monitoring is ‘the act of conducting a planned sequence of observations or measurements of control parameters to assess whether a CCP (or CP) is under control’ therefore monitoring may or may not include written records of any checks carried out. Information recorded will be dependent on the risk of the operations; that is, the type of food and size of the business. Documentation should not cause an unnecessary burden to small – medium businesses. The FBO may choose to record by exception (using a diary such as the Food Safety Management Diary for Meat Producers) in which case the amount and type of records will not be the same as those used in a traditional HACCP system. The Diary may also be the preferred choice of the FBO to record occasional checks, for example, product temperatures taken on a daily basis, rather than recording on separate sheets of paper. Reference: See the topic 2.10 on ‘Principle 7: documentation’ in part 2 for additional information. |
2.8 Principle 5: corrective action procedures
2.8.1 Establishing corrective actions
The food business needs to establish the corrective actions to be taken when monitoring procedures indicate that either a CCP or a CP is not under control.
Reference: (EC) 852/2004 Article 5, 2(e).
2.8.2 Corrective actions: common issues
The following table contains examples of common issues that the OV and OV auditors could find when auditing and/or verifying HACCP based procedures. The table also provides advice and guidance on how the OV and OV auditors should make the assessment to determine FBO compliance:
Corrective actions: common issues
| Common Issues | OV advice / guidance |
|---|---|
| Plan not a true reflection of reality | The corrective action procedures described in the plan should be reflected in what actually happens when the FBO loses control. |
| Corrective actions not recorded |
Corrective actions are an important part of a plan of HACCP-based procedures to help the FBO regain control of the process. In some cases, the actions taken may not be recorded as the FBO does not want to admit to failures. The impression given is that the FBOs never have any problems with their hygiene control procedures. In fact, the record of corrective actions shows that the plan based on HACCP principles is a ‘healthy’ plan that works effectively. Corrective actions should ensure that the risk to consumers are eliminated, prevented or reduced for example, immediate trimming of faecal contamination followed by a root cause analysis to prevent recurrence |
| Corrective actions not recorded |
Problems always occur and records should be made when they do. These records are important, not only for the FBO to validate and verify their own systems but also to enable verification of the HACCP based system by the OV and auditors. Examples on how to record corrective actions may include:
|
2.9 Principle 6: validation, verification and review
2.9.1 Validation
Validation in HACCP terms refers to obtaining evidence that a control measure or combination of control measures, if properly implemented, is capable of controlling the hazard to a specified outcome.
Validation is performed at the time a control measure or a food safety control system is designed, or when changes indicate the need for re-validation. Validation of control measures is, whenever possible, performed before their full implementation.
2.9.2 Verification
Verification is the application of methods, procedures, tests and other evaluations, in addition to monitoring to determine compliance with the HACCP plan and aims to respond to the question: is it working?
FBOs need to establish verification procedures which shall be carried out regularly to verify that what is written in the HACCP plan is actually being carried out in the workplace.
Verification and auditing methods, procedures and tests, including random sampling and analysis, can be used to determine if the HACCP system is working and is effective.
Reference: (EC) 852/2004 Article 5, 2(f).
2.9.3 Review
When any modification is made in the product process, or any step, the food business operators shall carry out a review of the HACCP based procedure plan(s) to ensure that the plan(s) and associated documentation are up to date.
Reference: (EC) 852/2004 Article 5, 2.
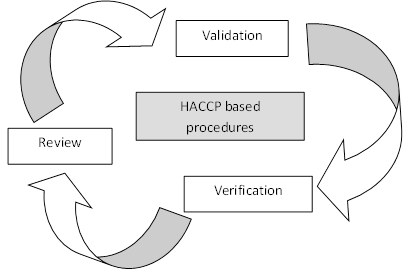
2.9.4 Validation / verification / review: common issues
The following table contains examples of common issues that the OV and OV auditors could find when auditing and/or verifying HACCP based procedures. The table also provides advice and guidance on how the OV and OV auditors should make the assessment to determine FBO compliance:
Validation / verification / review: common issues
| Common Issues | OV advice / guidance |
|---|---|
| Plan not a true reflection of reality | The validation, verification and review procedures described in the plan should be reflected in what actually happens on the ground. The FBO should comply with the requirements set up in their food safety management programme or amend those if appropriate. |
| No records of Verification / Validation / Review of the HACCP plan(s) |
Plans based on HACCP principles allow for flexibility in the application. The FBO may combine validation (of the HACCP plan), verification and review (of the system); as it may be difficult for the FBO to distinguish between them. Absence of separate validation / verification / review checks does not necessarily mean these have not been carried out. Verification of these procedures may be completed by an internal audit / or external audit(s) carried out by the competent authority or third party auditors. Examples of separate validation, verification and HACCP plan review forms are provided in the Food Safety Management Diary for Meat Producers, which the FBO may choose to use. If the Diary is used the 4-weekly reviews also accomplish verification of the FBOs hygiene controls. |
| Procedures not updated following modification of a product or process |
Encourage the FBO to always consider, at the earliest opportunity, how modifications will impact on their HACCP based procedures. Although the modification may initially impact on a single product or process, it is just as important to review all products and processes to ensure any collateral impact is addressed. Ensure validation of new equipment is in accordance with technical specifications where appropriate. |
2.9.5 Microbiology
Microbiological testing is another way for the OV to verify (and the OV auditor to audit) the microbiological aspect of FBO HACCP based procedures.
Minimum microbiological requirements applicable to the FBO are contained in Regulation (EC) 2073/2005.
Surface microbiological testing is normally not a legal requirement but the FBO may decide to do so as a way of verification of the effectiveness of their cleaning procedures. However, FBOs producing ready to eat foods which pose a Listeria monocytogenes risk to public health must sample processing areas and equipment as part of their sampling scheme.
It is most important that if the OV or OV auditor wishes to verify HACCP based procedures using microbiological testing, that they use the same sampling sites, methods and techniques as the FBO.
Reference: See part 3 section 3 on ‘Verification of microbiological criteria’ for additional information.
2.10 Principle 7: documentation
2.10.1 Establish documents and records
Establishing documents and records commensurate with the nature and size of the business to demonstrate the effective application of the measures outlined in subparagraphs (a) to (f):
a. identifying hazards
b. identifying CCPs (or control points)
c. establishing critical limits (or legal limits) at CCPs (or control points)
d. establishing and implementing monitoring procedures
e. establishing corrective actions
f. establishing verification procedures (including validation and review)
Reference: (EC) 852/2004 Article 5, 2(g)
2.10.2 Types of documents and records
Three types of paperwork are necessary:
- HACCP plan(s) documenting application of HACCP principles (may be a generic plan – amended to reflect the company procedures including prerequisites that are control measures)
- the company’s HACCP-based procedures, policies, staff instructions, SOPs etc (should include prerequisites as control measures)
- records of monitoring, corrective action, validation, verification and review (the Food Safety Management Diary may be used where appropriate)
2.10.3 Documentation: common issues
Documentation: common issues
| Common Issues | OV advice / guidance |
|---|---|
| Plan not a true reflection of reality | The documentation referred to in the plan of HACCP-based procedures should be reflected in those actually used on the ground by the FBO. |
| Disproportionate documentation |
Documentation, especially if it is produced by an external adviser, may be disproportionate to the size, type of business and type of food produced. It may be too technical for the FBO or plant staff to understand or follow; it may duplicate existing records or seek to introduce a far more complex system of recording than is appropriate. Staff instructions and SOPs should be written in such a way that they are easily understood by users and verifiers (including OVs and auditors). Version control is of paramount importance to ensure that all FBO staff, OVs and auditors have the most up-to-date instructions. In these cases, it may be appropriate to encourage the FBO to consult with their adviser / consultant and work together to produce a workable, more easily managed HACCP based system, reminding the FBO that the HACCP based system is their control system and they should retain ownership. |
| Inadequate documents/records | While documentation/records need not be complex and onerous it should provide sufficient information about the FBO procedures and controls |
2.10.4 Food safety management diary for meat producers (‘The Diary’)
The use of the FSA’s Food safety management diary for meat producers (the ‘Diary’) is an acceptable method of record keeping.
When using the diary, the FBO or any other responsible person should sign the Diary every day to confirm in a meaningful way that:
- opening, operational and closing checks have been carried out
- hygienic production has been followed
- what (if any) corrective actions have been taken
These should not be just a tick in a box for the sake of keeping a record: if these have been ticked the workplace must accurately reflect the check carried out. For example, areas are clean /, equipment is clean, knife sterilisers are working correctly.
The daily Diary pages are not intended to replace all existing documentation. They will need to be supported by additional record forms and procedures / staff instructions such as:
- individual staff training records
- cleaning schedules
- maintenance plans
The Diary provides examples of such documents that FBOs may adapt for their own use. FBOs may choose to keep such prerequisite records in the Diary binder.
The use of the Diary by FBOs is voluntary. It will not be appropriate in businesses that already have good existing records, and may not be entirely sufficient where, for example, the business is accredited to a Quality Assurance scheme or customers require more extensive documentation.
Reference: An electronic version of the Diary can be found online.
2.10.5 Exception recording
FBOs may choose to do exception recording, only to make record when a problem or something out of the ordinary is identified and the corrective actions to regain control. This applies particularly to checks that are more or less continuous for example, visual monitoring of each carcase, or where separate checklists are kept for example, cleaning checks.
Examples of exceptional recording:
- record when temperatures exceed the critical limit / legal limit and the action taken to regain control instead of having to tick / cross a separate list
- instead of making ticks and crosses in a cleaning checklist every day, an alternative could be recording only when cleaning problems are identified including the corrective action
- trimming contamination from a carcase
- recording problems that occur during a process for example, gut spillage during evisceration
- action taken when there are signs of pest infestation
- action taken if refrigeration equipment requires repair
- problems with faulty equipment and what was done to put it right
- staff not adhering to pre-requisite or other procedures and what corrective actions were required for example, supplementary or refresher training, cleaning of a piece of equipment
- knife sterilisers that are not working at 82°C or above and what corrective actions were required for example, repair / renew equipment
FBOs should nevertheless be encouraged to record the results of occasional checks to demonstrate that their procedures are working effectively, for example,
- periodic checks of knife sterilisers
- chiller temperatures
Note: The recording of corrective actions taken to regain control can be used to demonstrate that the HACCP plan is working effectively.
2.10.6 Management checks
Management checks are an integral part of FBO food safety management to ensure that management are fully engaged in the implementation of the HACCP plan and that prerequisite controls are working effectively.
Four weekly checklists are provided in the Diary to encourage FBOs to undertake a regular review of all aspects of their hygiene controls. There is space to record any persistent problems (which may include concerns raised by OV inspections or audits) or any significant changes that have been made and how they are being dealt with, including any consequences for their HACCP-plans.
Reference: See the topic 2.9 on ‘Principle 6: validation, verification and review’ in part 2 for additional information.
3. Audit and enforcement
In this section
3.1 OV audit of HACCP principles and microbiological testing
3.1 OV audit of HACCP principles and microbiological testing
3.1.1 Audit 9/3 form
OV auditors should use the audit report form AUD 9/3 on K2 to audit FBO compliance in the application of HACCP based procedures.
Reference: see K2 for the AUD 9/3 audit report form.
When one establishment has several HACCP based procedures plans, the OV auditor only needs to complete one ‘HACCP based procedures’ section of the form AUD 9/3 which will cover the audit findings for all the HACCP based procedures plans of one establishment.
3.1.2 Confidence in FBO’s food safety management systems AUD 9/3
The results of the audit of the FBO compliance in the application of HACCP based procedures is one of the main audit components to be used by the OV auditors to determine the ‘Food safety systems based on HACCP principles’ (confidence in FBOs food management systems) score in part 2 of the form AUD 9/3.
3.1.3 HACCP audit objective
The objective of the OV audit should be to establish whether the FBO can show that they have implemented and are maintaining a system based on HACCP based procedures in compliance with the regulations and to the satisfaction of the competent authority.
Note: HACCP based procedures will not work without sufficient / adequate / appropriate prerequisites (good hygiene practices) being in place (as required by (EC) 852/2004 in particular).
3.1.4 Technical deficiencies
The plan based on HACCP principles may not be technically correct, but this does not make it invalid (or require formal enforcement action) as it may still be able to achieve the main purpose of controlling those hazards identified by the FBO.
Example:
A flow diagram may not correctly reflect the operations carried out; however, it is possible that there is no risk for public health as the risks have been correctly identified.
Reference: See sub-topic 3.2.1 on ‘OV advisory role’ in part 2 for additional information.
3.1.5 OV HACCP audit
The OV auditor should determine the FBO level of compliance through Section 5, (HACCP based procedures section) of the AUD 9/3.
The assessment in each section of the AUD 9/3 describes the compliance criteria as either ‘compliant’, or in the case of non-compliance, categorises that non-compliance as minor, major, or critical. The auditor uses their professional judgement to reach a decision on each criterion based on the evidence and also on the guidance provided in this chapter.
3.1.6 Microbiological testing audit
The OV auditor should verify that the FBO complies with the microbiological sampling requirements, laid down in Regulation (EC) 2073/2005, in accordance with Regulation (EU) 2017/625 and Regulation (EU) 2019/627.
OV auditor verification and reporting through question 3.9 (for slaughterhouses) or 3.13 (for cutting plants) of AUD 9/3 includes FBO responsibility for:
- sampling at the required frequency
- following the sampling rules
- interpretation of the sampling results (do these look manufactured or unrealistic?)
- identification of patterns and trends in test results
- identification of failures in the processing techniques that should have been identified and addressed
- corrective action, where necessitated by the results obtained
- a product recall, where necessitated as a result of unsatisfactory food safety criteria results
3.2 Enforcement: HACCP
3.2.1 OV advisory role
Where the OV finds that the FBO has HACCP based procedures but there are deficiencies that do not pose a public health risk, the OV should not serve a formal notice, but advise, educate and encourage rectification of the HACCP based procedures.
Reference: For guidance on HACCP implementation.
The electronic version of the Diary can be found online.
The OV advisory role does not extend to personally writing any part of the FBOs food safety system for example, HACCP plans and monitoring documentation.
3.2.2 Objective evidence
It is essential to gather evidence of legal contraventions for example,
- the slaughter for human consumption of animals whose identity cannot be reasonably ascertained
- carcases presented with faecal contamination at post mortem inspection, when these are related to the inadequacy (or non-existence) of the FBOs HACCP-based food safety management procedures
3.2.3 Notification to the FBO of deficiencies
If after verbal advice and an advisory letter the FBO has made:
- no effort to implement a food safety management system based on HACCP based procedures, or
- negligible effort to implement a food safety management system based on HACCP based procedures, or
- once implemented, the FBO has failed to maintain a system based on HACCP based procedures
The OV is to serve a Hygiene Improvement Notice (HIN) for each of the HACCP principles that are not being complied with, in line with the principles of enforcement in Chapter 7.
Reference: (EC) 852/2004, Chapter II, Article 5 and (EC) 853/2004, Annex II, Section II.
3.2.4 Establishment functions
Separate HIN’s are to be served on each of the establishment’s approved functions, in line with the principles of enforcement in Chapter 7, such as slaughtering and cutting. Separate notices avoid:
- having to withdraw an entire notice that has only be partially complied with
- the suspension of entire notices because of appeals over one issue
- the service of more notices on those areas still outstanding
3.2.5 Time scales for compliance with formal notices
The timescale for compliance with the HIN will depend upon the size of the establishment, the nature and complexity of the operations and the history of compliance of the FBO. The OV is responsible for making an assessment of the specific circumstances for the plant to provide a reasonable timescale in line with the enforcement concordat and risk based procedures (it is proportionate).
3.2.6 Failure to comply with the notice
If the FBO fails to comply with a formal notice, the OV should consult with their regional manager before deciding on the next step.
Reference: See chapter 9 on ‘Forms’.
The OV must keep a record of the FBOs progress on HACCP implementation made after a recommendation for prosecution has been made. This will help identify actions that should have been taken earlier and will help to counter any mitigating factors that the FBO puts forward if the case goes to court.
3.2.7 OV records of FBO compliance
The OV must keep records of any advice given to the FBO in the establishment daybook.
1. Introduction
2. Lactic acid to reduce microbiological surface contamination in bovine carcases
3. Verification of Microbiological Criteria
4. Traceability
5. Procedures for the verification of the manufacture of beef patties and burgers intended to be consumed less than thoroughly cooked at
retail level
6. Annexes
Sections
2. Lactic acid to reduce microbiological surface contamination in bovine carcases
3. Verification of Microbiological Criteria
6. Verification of Water Testing Procedures
1. Introduction
In this section
1.1 Background
1.1.1 General obligations regarding the organisation of official controls
Regulation (EU) 2017/625 requires official controls to be undertaken regularly, on a risk basis and with appropriate frequency to achieve the objectives of the regulations, taking account of:
- identified risks associated with animals and goods; the activities under the control of operators; the location of the activities, and the use of products, processes, materials or substances that may influence food safety, integrity and wholesomeness, or feed safety, animal health or animal welfare;
- any information indicating the likelihood that consumers might be misled, in particular as to the nature, identity, property, composition, quantity, durability, country of origin or place of provenance, method of manufacture or production of food;
- feed or food business operators' (FBOs) past record as regards compliance with feed or food law or with animal health and animal welfare rules
- the reliability and results of own checks controls performed by the operators or by a third party at their request;
- any information that might indicate non-compliance with the food and feed safety, animal health or animal welfare requirements set out in these regulations.
Reference: (EU) 2017/625, Article 9(1).
2. Lactic acid to reduce microbiological surface contamination in bovine carcases
2.3 Concentration and applications of solution
2.4 Exceptions to the use of lactic acid
2.1 Background
2.1.1 Substances to remove surface contamination
EU hygiene legislation provides for the use of potable water to remove surface contamination from products of animal origin. However, it does also provide for other substances to be used for this purpose, provided that they have been approved under a procedure laid down in Regulation 853/2004.
The first substance approved for this purpose is lactic acid used to reduce microbiological surface contamination on bovine carcases. It was adopted by the European Commission as Commission Regulation 101/2013 on 4 February 2013 and entered into force on 25 February 2013.
The measure was preceded by a thorough risk assessment by the European Food Safety Authority (EFSA), which resulted in a favourable opinion published on 26 July 2011 on the safety and efficacy of lactic acid.
2.2 Legislative references
2.2.1 Relevant legislation
- Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs
- Regulation (EC) No 1333/2008 on food additives
- Commission Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008
- Commission Regulation (EU) No 380/2012 amending Annex II to Regulation (EC) No 1333/2008 as regards the conditions of use and the use levels for aluminium-containing food additives
- Commission Regulation (EU) No 101/2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcases
2.2.2 Commission Regulation (EU) No 101/2013
Commission Regulation (EU) No 101/2013 allows FBOs to choose to use lactic acid to reduce microbiological surface contamination on bovine carcases, half carcases or quarters at the slaughterhouse, in compliance with the conditions set out in the Annex to the Regulation.
2.3 Concentration and application of solutions
2.3.1 Requirements for lactic acid solutions
Solutions which may be used must be prepared from lactic acid that meets the specifications for use as a food additive, set out in Regulation (EU) No 231/2012.
Note: The specifications set out in Regulation (EU) No 231/2012 are reproduced in Annex 1 at the end of this chapter.
2.3.2 Concentration of prepared lactic acid solution
The prepared solution must be between 2% to 5% lactic acid solution in potable water.
2.3.3 Application of prepared lactic acid solution
The prepared solution must be:
- applied and used at temperatures of up to a maximum of 55°C
- applied either by spraying or misting
- applied under controlled and verifiable conditions integrated into a HACCP-based management system including, at least, the criteria set out below under HACCP
2.3.4 To what may the prepared lactic acid solution be applied?
The prepared solution must only be applied to entire carcases, half-carcases or quarters of meat from domestic bovine animals (including buffalo, water buffalo and bison), at the slaughterhouse.
2.4 Exceptions to the use of lactic acid
2.4.1 Visible faecal contamination
Lactic acid solutions must not be applied to carcases with visible faecal contamination.
2.4.2 Irreversible physical changes
The application of lactic acid solutions must not result in any irreversible physical changes to the meat.
2.5 Minimum HACCP requirements
2.5.1 HACCP
The FBO’s HACCP plan should, as a minimum, incorporate the following elements:
- Sampling of carcases for the purposes of assessing compliance with microbiological criteria within the meaning of Regulation (EC) No 2073/2005 must be carried out before the application of lactic acid solutions to the carcases, half-carcases or quarters.
- Lactic acid concentration during treatment must be monitored as part of the HACCP plan, verified by periodic monitoring, documented and recorded.
- The temperature of the solution during treatment must, as part of the HACCP plan, be documented and recorded and continuously monitored using measuring instruments.
2.6 FBO duties
2.6.1 Use of lactic acid
The FBO must ensure that lactic acid is only used at the dilution specified in the legislation.
The FBO should, where possible, notify the FSA OV of their intention to use lactic acid as a decontamination agent and ensure that the OV is familiar with the relevant sections of the HACCP plan.
2.6.2 Update to HACCP plans
The FBO must ensure that their HACCP plan includes a section detailing the conditions for the use of, controls and verification of the procedures for the use of lactic acid.
2.6.3 Communication of information
Slaughterhouse FBOs using lactic acid solutions to reduce microbial surface contamination of entire carcases, half-carcases or quarters, must inform the FBO receiving the treated carcases or half-carcases or quarters of such use.
This information should be documented- for example, included in the commercial documents which accompany treated meat.
2.7 FSA role
2.7.1 Check suitable HACCP plan in place
The OV and FSA team must ensure that where the FBO intends to use lactic acid as a decontamination agent, there is a suitable HACCP plan in place as detailed in the legislation.
The FBO HACCP plan and associated records should be verified during audit with particular reference to the records required by the legislation.
2.7.2 Monitor use
The use of lactic acid should be monitored to ensure that it is not applied to carcases that have faecal contamination and is used at the correct dilution and within the specified temperature range.
2.7.3 Frequency of verification at audit
Until further instructions are provided, should the FBO choose to use lactic acid as a decontaminant, the FVC should contact the Field Operations helpline on 01904 232083 to discuss the frequency at which the verification at audit as detailed in the following paragraph should take place.
2.7.4 Verification at audit
When carrying out an audit of FBO controls where lactic acid is being used, OVs should verify the controls the FBO has in place to ensure that the requirements of EU 231/2012 have been met, namely:
- The lactic acid meets the requirements of Regulation (EU) No 231/2012.
- The lactic acid is made up in a solution of between 2% and 5% in potable water.
- The lactic acid solution is applied at a temperature below 55°C.
- The lactic acid solution is only applied to carcases free from visual faecal contamination.
- Microbiological testing is carried out before the use of lactic acid solution.
- The FBO is notifying customers receiving treated carcases of the treatment applied with lactic acid.
These checks should be recorded on the audit report form in Part 2 on ‘HACCP based procedures’.
2.7.5 Health mark legibility
If the application of the lactic acid solution interferes with the legibility of the health mark, this should be resolved between the FBO and the OV, in full consultation with the FVC and Area Veterinary Manager (AVM).
3. Verification of Microbiological Criteria
In this section
3.2 Legislation and guidance documents
3.3 Testing requirements: slaughter operations
3.4 Testing requirements: other operations
3.5 Testing requirements: ready to eat products
3.8 OV role: all establishments
3.1 Background
3.1.1 Purpose of Microbiological Testing
Assimilated Regulation (EC) 2073/2005 (as amended) lays down the microbiological criteria for certain microorganisms and the implementing rules to be complied with by Food Business Operators (FBOs) when implementing the general and specific hygiene measures referred to in Article 4 of assimilated Regulation (EC) 852/2004. The competent authority shall verify compliance with the rules and criteria laid down in this regulation under Article 18 of assimilated Regulation (EU) 2017/625.
The purpose of microbiological testing is to ensure that:
- results support validation or verification of the correct functioning of FBO’s procedures based on HACCP principles and good hygiene practice
- the supply, handling and processing of meat under the FBO control are carried out in such a way that the process hygiene criteria are met
- the food safety criteria are met throughout the shelf life of the product under reasonable conditions of distribution, storage and intended use as described in the food safety management system; and
- process controls are reviewed when microbiological results indicate that these processes are out of control
- corrective actions are taken to protect the health of consumers when test results are unsatisfactory under the process hygiene criteria (for example, improve food safety systems) or the food safety criteria (for example, by withdrawing or recalling non-compliant products). More details on corrective actions are provided in point 3.7.2 below.
3.1.2 Microbiological criteria
Two different types of criteria are established in assimilated Regulation (EC) 2073/2005, Food Safety Criteria and Process Hygiene Criteria. Food safety criteria assess the safety of a product; process hygiene criteria assess the hygienic functioning of production processes. The main difference between them is the additional action required when the results are not within limits.
When food safety criteria are not met, the batch of food affected should be removed from (recalled) or not placed on (withdrawn) the market.
Failure to meet either type of criteria should always result in an investigation to find the root cause of the contamination and regain control to prevent contamination of future production. The FBO must take action under assimilated Regulation (EC) 2073/2005, Article 7, paragraphs 1 to 4, as well as the appropriate corrective action defined in their HACCP plans and any additional action to protect public health.
Depending on which microbiological limits have been exceeded, to fully comply with the criteria, the FBO is required to take different actions in accordance with point 3.7 below.
3.1.2.1 Food safety criteria
Food safety criteria have been set for fresh poultry meat, minced meat, meat preparations, meat products, mechanically separated meat, gelatine and collagen and ready-to-eat foods. Other than meat, these criteria are also set for milk and dairy products, eggs products and fishery products. For the full list of food safety criteria, microbiological requirements refer to Annex I, Chapter 1 of Regulation (EC) 2073/2005 EUR-Lex – 02005R2073-20200308 – EN – EUR-Lex (europa.eu).
Demonstration of compliance with food safety criteria for meat and processed meat is required as follows:
- Absence of Salmonella spp in:
- minced meat and meat preparations intended to be eaten raw
- minced meat and meat preparations intended to be eaten cooked
mechanically separated meat (MSM)
meat products intended to be eaten raw
meat products made from poultry meat intended to be eaten cooked
fresh poultry meat (this is applicable only if Salmonella Typhimurium or Salmonella Enteritidis are identified)
gelatine and collagen
- Listeria monocytogenes less than 100 cfu/g in ready-to-eat meats that either do not support the growth of Listeria or have evidence that Listeria will not reach levels greater than 100 cfu/g during their shelf-life.
- Absence of Listeria monocytogenes before the food is placed on the market for foods that support growth and do not have shelf-life assessment data.
3.1.2.2 Process Hygiene Criteria
The purpose of testing against the process hygiene criteria set for carcases and certain processed meats (and other products of animal origin) is not to assess the fitness of individual carcases or processed meats for human consumption. The results provide an indication of performance and hygienic control during slaughter, dressing and/or production process at the time of sampling and therefore will allow to confirm if the food safety management systems at all stages of production are effective.
For the full list of process hygiene criteria, microbiological requirements refer to Annex I, Chapter 2 of Regulation (EC) 2073/2005 EUR-Lex – 02005R2073-20200308 – EN – EUR-Lex (europa.eu).
Demonstration of compliance with process hygiene criteria for meat and processed meat is required as follows:
- Aerobic Colony Count (ACC) and Enterobacteriaceae – on cattle, sheep, goats, horses and pig carcases (below specified limits).
- Salmonella spp – on cattle, sheep, goats, horses, pig, broiler and turkey carcases (absence from a specified number of samples per 50 samples examined).
- Campylobacter spp – on broiler carcasses (below specific limits from a specified number of samples per 50 samples examined).
- Aerobic Colony Count (ACC) and E. coli – in minced meat and mechanically separated meat (below specified limits).
- E. coli – in meat preparations (below specified limits).
3.2 Legislation and guidance documents
3.2.1 Assimilated Regulation (EC) 2073/2005
Assimilated Regulation (EC) 2073/2005 (as amended) sets out the microbiological criteria for certain micro-organisms and the implementing rules to be complied with by FBOs, when implementing the general and specific hygiene measures referred to in Article 4 of Assimilated Regulation (EC) No 852/2004.
3.2.2 Assimilated Regulation (EC) 2160/2003
Assimilated Regulation (EC) 2160/2003 (as amended) on the control of Salmonella and other specified food-borne zoonotic agents applies in relation to Salmonella testing.
3.2.3 Assimilated Regulation (EC) 178/2002
Assimilated Regulation (EC) 178/2002 lays down general food safety requirements, according to which food must not be placed on the market if it is unsafe. Article 19, point 4 of this regulation states that FBOs shall collaborate with the competent authorities on action taken to avoid or reduce risks posed by a food which they supply or have supplied. As a result of this provision, the FSA expects FBOs to inform the FSA Food Incidents branch at foodincidents@food.gov.uk if they consider that a food placed on the market may be injurious to health. FBOs have an obligation to withdraw or recall any unsafe food from the market.
3.2.4 Assimilated Regulation (EC) 852/2004
FBOs are required to comply with microbiological criteria under Article 4 of assimilated Regulation (EC) 852/2004.
3.2.5 Food Hygiene Regulations
The Food Hygiene (S/W) Regulations 2006 (as amended) / The Food Safety and Hygiene (England) Regulations 2013 make it an offence for any person to contravene or fail to comply with the specified community provisions.
Schedule 2 of these Regulations lays out the requirement in respect of assimilated Regulation (EC) 2073/2005, in that the FBO will have to take the appropriate measures laid down in Article 7, Points 1 to 4 when test results prove unsatisfactory.
3.2.6 Assimilated Regulation (EU) 2017/625
Article 15 establishes that FBOs shall give staff of the competent authorities access to their computerised information management systems, their documents and any other relevant information when required for the completion of official controls.
3.2.7 Guidance for Veterinary Auditors: FBO Audit Aide Memoire Appendix 1
OVs will find it useful to refer to the Audit Aide Memoire in Annex 1 of Chapter 4.1, in particular:
- Section 3.9 (micro criteria in slaughterhouses)
- Section 3.13 (micro criteria in cutting plants)
- Section 5.13 (using results to verify HACCP based procedures)
and to the tables provided in Appendix 1 of the Audit Aide-Memoire where full details of the microbiological criteria that are laid down by Assimilated Regulation (EC) 2073/2005 under both Food Safety Criteria and Process Hygiene Criteria, and sampling frequencies are reproduced.
Reference: Chapter 4.1 Audit, Annex 1 ‘FBO Audit Aide Memoire’, Appendix 1.
3.3 Laboratory requirements
3.3.1 Analytical reference method
The laboratory undertaking testing for the food business operator should use the organism-specific method:
- for Salmonella EN/ISO 6759
- for Listeria monocytogenes EN/ISO 11290 -1 and 2
- for Enterobacteriaceae ISO 21528-2
- for E. coli ISO 16649-1
- for Aerobic Colony Count (ACC) ISO 4833
- for Campylobacter ISO10272-2
It is best practice (not a legal requirement) that the laboratory undertaking testing for the food business operator (FBO) is accredited by UKAS for the examinations required in meat samples https://www.ukas.com/find-an-organisation/.
It is advisable (not a legal requirement) that the laboratory takes part in a recognised proficiency testing scheme for the examinations required, for example, FAPAS proficiency testing: https://fapas.com.
The use of alternative analytical methods is acceptable when the methods are validated against the reference method in Annex I of Assimilated Regulation (EC) 2073/2005 and if a proprietary method, certified by a third party in accordance with the protocol set out in EN/ISO standard 16140 or other internationally accepted similar protocols, is used. Links to internationally accredited bodies that provide a list of their alternative methods can be found below:
The testing laboratory will be able to supply the equipment and consumables necessary for sampling.
3.3.2 Laboratory test portions - processed meat
The test portion size for minced meat, mechanically separated meat, meat preparations and meat products is specified in the Regulation for Salmonella as either 25g or 10g and for Listeria Monocytogenes in ready-to-eat meat as 25g. SOPs should state the sample size. If FBOs decide to use a larger portion than required by the regulations, this should be to the satisfaction of the competent authority and, if results are unsatisfactory in a larger portion, the same corrective action is to be taken as for a smaller portion.
The laboratory test portion weight for minced meat, mechanically separated meat or meat preparations for ACC and E. coli examination is not specified in the Regulation so the ISO standard (6887-2) that establishes specific rules for the preparation of meat and meat products samples should be followed which specifies a 25g sample.
The laboratory must be able to obtain both test portions (for ACC and for E. coli testing) from each sample it receives. Test portions should be taken from throughout the sample including the surface and the interior.
3.3.3 Pooling of samples for Salmonella testing
The pooling of samples for Salmonella testing is permitted only if it takes place at the testing laboratory and where evidence is available to show that the sensitivity of the method is not reduced. A note explaining how to undertake pooling is included in the reference method for Salmonella EN ISO 6579: 2002.
3.3.4 Sample information
Information about the batch sent to the lab must be recorded on a sample form. This should include:
- name and species of the product (e.g., beef burger, turkey mince)
- pack description (for example, retail 500g pack)
- physical state (for example, fresh or frozen)
- type of packaging (for example, MAP)
- date of production
- traceability code and source (slaughterhouse, cutting plant)
3.4 Testing requirements: Slaughter operations
3.4.1 Testing requirements - Red meat slaughterhouses
Testing in red meat slaughterhouses is to verify process hygiene criteria only; there are currently no requirements for food safety microbiological criteria. Process hygiene criteria set indicative microbiological values above which corrective actions are required to maintain the hygiene of the process. For details on corrective actions see point 3.7.2 below.
Note: There are some exceptions on microbiological testing for small slaughterhouses according to their annual throughput. Details on testing frequency and exemptions are in the table in point 3.4.3.1. Seasonal throughput increases are to be taken into consideration when calculating the annual throughput. Updated [Throughput for specific slaughterhouses can be gathered from the IRIS application, E&P – View or requested to the FSA CBI team at CBI@food.gov.uk.]
Testing at red meat slaughterhouses shall include carcases of cattle, sheep, goats, horses and pigs. See point 3.10 below for details on sampling methods.
Carcases of cattle, sheep, goats and horses:
Five carcases of each species are required to be sampled per sampling session. One sample is from one carcase.
- for Aerobic Colony Count (ACC) and Enterobacteriaceae (ENT), use the specified mean log level below for the five samples. The limits given in the regulation are for an excision method; the limits for the swab or sponge method are lower and are given in (brackets) in the table below under the figures for excision.
- for Salmonella (Sal), the criterion is equal to or below 2 positives in 10 consecutive sampling sessions (that is 50 samples) using a sponge method.
| Result | ACC | Ent | Sal |
|---|---|---|---|
| Unacceptable - mean log /number of positives is above |
5.0 (4.3) |
2.5 (1.8) |
>2/50 |
| Acceptable - mean log below |
5.0 (4.3) |
2.5 (1.8) |
- |
| Satisfactory - mean log /number of positives is equal to or below |
3.5 (2.8) |
1.5 (0.8) |
≤2/50 |
Carcases of pigs:
Five carcases are required to be sampled per sampling session. One sample is from one carcase.
- For Aerobic Colony Count (ACC) and Enterobacteriaceae (ENT), use the specified mean log level below for the five samples. The figures given are for the excision method, the figures for the swab or sponge method are lower and are given in the table below in (brackets) under the figures for excision.
- For Salmonella (Sal), the criterion is equal to or below 3 positives in 10 consecutive sampling sessions (that is 50 samples) using a sponge method.
| Result | ACC | ENT | Sal |
|---|---|---|---|
| Unacceptable - mean log /number of positives is above |
5.0 (4.3) |
3.0 (2.3) |
>3/50 |
| Acceptable - mean log below |
5.0 (4.3) |
3.0 (2.3) |
- |
| Satisfactory - mean log /number of positives is equal to or below |
4.0 (3.3) |
2.0 (1.3) |
≤3/50 |
Note: The mean log value for ACC and ENT of the five carcases tested per sampling session can be calculated by adding the 5 individual log results together and dividing by 5. Results shall be reported as cfu/cm2. The lab can give the results as cfu/swab; in this case, the FBO needs to know how many cm2 are covered in the swab and divide the result to provide the results as cfu/cm2. Results for Salmonella will be presence/absence in the tested area.
3.4.2 Testing requirements - Poultry slaughterhouses
Broilers and turkeys are tested for Salmonella to check food process hygiene criteria.
The samples taken to check process hygiene criteria can also be used to verify compliance with food safety criteria requirements. To this effect, FBOs must carry out further tests where Salmonella spp results have been positive to identify whether Salmonella enteritidis or Salmonella typhimurium are present.
Broilers are also tested for Campylobacter to check the process hygiene criteria in the slaughterhouse.
FBO Campylobacter results should be entered into CaPTa (Campylobacter Process Hygiene Criteria Testing Application) at the required frequency. An internal guide on how to enter the data in the system has been produced by the FSA and can be found in the following link CaPTa User Guidance v1.
Note: Slaughterhouses with an annual throughput below 1,000,000 broilers or turkeys (less than 20,000 broilers/turkeys per week) are exempt from Salmonella testing requirements. No exception is currently considered for Campylobacter testing.
Note: For details on testing frequencies and exemptions see table in point 3.4.3.2. Seasonal throughput increases are to be taken into consideration when calculating the annual throughput. Updated [Throughput for specific slaughterhouses can be gathered from the IRIS application, E&P – View or requested to the FSA CBI team at CBI@food.gov.uk.]
Testing at poultry slaughterhouses should include carcases of broilers (for Salmonella and Campylobacter) and turkeys (for Salmonella only):
Neck skins from at least 15 carcases are required to be sampled per sampling session. One sample is composed of three pooled neck skins collected after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase; the neck skins from 3 carcases from the same flock or origin shall be pooled to form 5 x 25g final samples. The same samples can be used for testing Salmonella and Campylobacter in broilers if tested in the same laboratory (see note below if not processed at the same laboratory).
- For Salmonella (Sal), in broilers and turkeys, the criterion is equal to or below 5 positives (presence) in 10 consecutive sampling sessions (that is 50 samples). If a sample tests positive for Salmonella spp. in any sampling session it must be serotyped for S. enteritidis and S. typhimurium to check compliance with the food safety criteria.
- For Campylobacter (Campy), only in broilers, the criterion is equal or below a specified number of positives (more than 1000 cfu/g) in 10 consecutive sampling sessions (that is 50 samples). The current criterion accepts up to 15/50 positive samples to comply. The number of positives accepted will decrease to 10/50 from 1 January 2025.
| Result | Sal | Campy |
|---|---|---|
| Unacceptable - presence /number of positives is above | >5/50 | >15/50 |
| Satisfactory - absence /number of positives is equal to or below | ≤5/50 | ≤15/50 |
Note: when testing for Salmonella and Campylobacter in broilers is carried out in two different laboratories, neck skins from a minimum of 20 carcases shall be sampled at random after chilling during each sampling session. One sample is composed of four pooled neck skins. Each sample will be then split in two to be tested for Salmonella and Campylobacter separately. See point 3.10 below for sampling methods and Annex 2 for Campylobacter testing requirements.
3.4.3 Sampling frequency at slaughterhouses (red meat and poultry)
Carcases of red and poultry meat are to be tested weekly initially. The day of sampling shall be changed each week to ensure that each day of the week is covered. Reduced sampling frequency can be applied if results are satisfactory after a number of weeks; this will be different according to the annual throughput of the slaughterhouse. For details on sampling frequency and reduced sampling frequency refer to tables 3.4.3.1 for red meat and 3.4.3.2 for poultry.
3.4.3.1 Sampling frequency in red meat slaughterhouses
| Category | Annual throughput per species | Initial sampling frequency | Reduced sampling frequency if results are satisfactory |
|---|---|---|---|
| Standard 1 |
Over:
(>400 or 2,000/week) |
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 2 |
|
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 3 |
(>30 or 150/week) |
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 4 |
|
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
| Small 5 |
|
Enteros and ACC: Salmonella: |
Enteros and ACC: Salmonella: |
3.4.3.2 Sampling frequencies in poultry meat carcases
| Category | Annual throughput of turkeys or broilers | Initial sampling frequency (one sample is three neck skins) | Reduced frequency if results are satisfactory |
|---|---|---|---|
| Standard 1 | Over 7,500,000 (>150,000/week) |
Salmonella: Campylobacter: |
Salmonella: Campylobacter: |
| Small 2 | Below 7,500,000 but over 1,000,000 (>20,000week) |
Salmonella: Campylobacter: |
Salmonella: Campylobacter: |
| Small 3 | Below 1,000,000 (<20,000/week) |
Salmonella: Campylobacter: |
Salmonella: Campylobacter: |
3.4.4 OV to monitor FBO sampling results at the slaughterhouse
The OV should regularly monitor the sampling technique, the procedures for transporting the samples to the laboratory, the laboratory reference method used and the provision of results at slaughterhouses where sampling and testing are required.
The requirement for the OV to verify the sampling arrangements is part of the Slaughter Hygiene Verification (SHV) system for both red and poultry slaughterhouses. The verification must be completed at least monthly or aligned with the FBO testing frequency if this is less than monthly. (see Chapter 2.4, Sections 12.7 for red meat and 13.7 for poultry).
The microbiological testing arrangements and actions taken following receipt of the test results will also be verified by Field Veterinary Leaders (FVLs) and Field Veterinary Coordinators (FVCs) during the approval process and by Veterinary Auditors during full audits.
Verification aims to ensure the FBO has complied with the sampling requirements and to ensure non-compliant results are acted upon. Assimilated Regulation (EU) 2019/627 also requires the competent authority to supervise the FBO action plan where there are repeated failures, and that may require the OV to apply the hierarchy of enforcement (see Enforcement in point 3.9 below).
Note: Species for which criteria are not specified, for example, game, rabbits, ducks and geese carcases, are not required to be sampled. Turkey carcases are not required to be sampled for Campylobacter.
3.5 Testing requirements: Other activities
3.5.1 Criteria requirements: other activities
Testing is required for minced meat, meat preparations, meat products, gelatine, collagen and mechanically separated meat (MSM). Also required for milk and dairy products, egg products and fishery products. For details see Annex I, Chapters 1 and 2 of Regulation 2073/2005.
Sampling is on a batch basis.
Note: A batch is defined as a group or set of identifiable products obtained from a given process under practically identical circumstances, produced in a given place and within one defined production period. FBO’s documentation should explain how a batch, for the purposes of microbiological sampling, has been established.
3.5.2 Testing for Food Safety Criteria - Other activities
Minced meat and meat preparations intended to be eaten raw: Salmonella should be absent on 5 x 25g samples from a batch of minced meat or meat preparations intended to be eaten raw made from any species of meat, for example, steak tartare or burgers intended to be consumed less than thoroughly cooked (LTTC). Guidance for LTTC is available in Section 5 of this chapter.
Minced meat and meat preparations from poultry meat intended to be eaten cooked: Salmonella should be absent on 5 x 25g samples from a batch of minced meat or meat preparations made from poultry meat intended to be eaten cooked. This applies to poultry meat of all species including ducks, geese, turkeys spent hens and broilers. For example, minced chicken, turkey burgers, chicken sausages, chicken and turkey escalopes.
Minced meat and meat preparations from red meat intended to be eaten cooked: Salmonella should be absent on 5 x 10g samples from a batch of minced meat or meat preparations made from other species than poultry intended to be eaten cooked. This applies to all species of red meat including game, for example, minced meat for bolognese sauce or shepherd’s pie, sausages, and burgers.
Mechanically separated meat (MSM): Salmonella should be absent on 5 x 10g samples from a batch of MSM when produced with the techniques referred to in paragraph 3 of Chapter III of Section V of Annex III to Regulation (EC) 853/2004.
Meat products intended to be eaten raw: Salmonella should be absent on 5 x 25g samples from a batch of meat products intended to be eaten raw, for example, air-dried smoked duck, partially fermented sausages or biltong. This does not apply to products where the manufacturing process or the composition of the product will eliminate the Salmonella risk such as certain types of salami, nor does it apply to fully cooked ready-to-eat meat products such as cooked ham.
Meat products from poultry meat intended to be eaten cooked: Salmonella should be absent on 5 x 25g samples from a batch of meat products made from poultry meat intended to be eaten cooked, for example, turkey bacon and chicken nuggets.
Gelatine and collagen: Salmonella should be absent on 5 x 25g samples from a batch of products placed on the market during their shelf-life.
Fresh poultry meat (other than poultry carcases): Absence of Salmonella enteritidis and Salmonella typhimurium from 5 x 25g fresh poultry meat samples from a batch including meat from hens, broilers and turkeys (including breeders).
3.5.3 Testing for Process Hygiene Criteria – Other activities
Minced meat and mechanically separated meat (MSM)– five samples must be taken from one batch per sampling session and tested:
- for Aerobic Colony Count (ACC) – all five samples must be less than 5 x 106 cfu/g of which at least three samples must be less than 5 x 105 cfu/g.
- for E. coli (EC) – all five samples must be less than 500 cfu/g of which at least three samples must be less than 50 cfu/g.
Meat preparations – five samples must be taken from one batch per sampling session and tested:
- for E. coli (EC) – all five samples must be less than 5000 cfu/g of which at least three samples must be less than 500 cfu/g.
If a process hygiene criterion is not met, the meat can be placed or remain on the market, but the FBO must review the production processes and improve process hygiene to ensure future production will meet the criteria. The actions should be included in the food safety management procedures, which should also include relevant actions specified in Annex I (Chapter 2) of the Regulation. Enforcement authorities will require sufficient evidence that the food business operator has taken the appropriate corrective action. See point 3.7.2 below for corrective actions.
3.5.4 Sampling frequency for other activities
Weekly samples are required from one batch of minced meat, meat preparations or MSM. The day of sampling shall be changed each week (if possible) to ensure that each day of the week is covered. The weekly frequency can be reduced to once every two weeks after 6 consecutive weeks of satisfactory results for E. Coli or ACC and after 30 consecutive weeks of satisfactory results for Salmonella.
| Product | Test | Initial frequency | Reduced frequency if results are satisfactory |
|---|---|---|---|
| Minced meat and meat preparations | E. Coli and Aerobic Colony Count | Weekly | After 6 consecutive weeks of satisfactory results Once every 2 weeks |
| Minced meat and meat preparations and fresh poultry meat | Salmonella | Weekly | After 30 consecutive weeks of satisfactory results Once every 2 weeks |
3.5.5 Exception to testing - Other activities
Establishments producing an average of less than 2 metric tonnes per week of combined minced meat and meat preparations intended to be eaten cooked are currently not required to take any samples. This exception is based on of a risk analysis carried out by FSA as the competent authority.
Note: This exception does not apply to Mechanically Separated Meat (MSM) or minced meat / meat preparations intended to be eaten raw or undercooked (for example, burgers intended to be eaten less than thoroughly cooked) or if production increases during short periods (for example, summer or Christmas seasonal production peaks), where the FBO shall test during the period exceeding the 2 tonnes per week.
3.5.6 Labelling requirements
Article 6 of assimilated Regulation (EC) 2073/2005 requires that minced meat and meat preparations (made from species other than poultry) which are intended to be eaten cooked, must be clearly labelled when placed on the market to inform the consumer of the need for thorough cooking before consumption.
Note: This labelling requirement does not apply to minced meat or meat preparations made from poultry meat.
3.6 Testing requirements: Ready-to-Eat (RTE) products
3.6.1 Listeria monocytogenes (foodstuff)
Ready-to-eat (RTE) products are defined as food intended by the producer or the manufacturer to be consumed directly without the need for cooking or other processing. RTE products are to be tested for food safety criteria in accordance with the following:
a) For RTE foods that can support the growth of L. monocytogenes (for example cooked meats, soft cheeses or smoked fish), 5 x 25 g samples from a batch should be taken and there are two criteria:
- not exceeding 100 cfu/g for the duration of the shelf-life of the product, or
- absence in 25g before the food has left the immediate control of the FBO if the FBO cannot demonstrate that the product will not exceed 100 cfu/g during its shelf -life.
b) For RTE foods unable to support the growth of Listeria monocytogenes: less than 100 cfu/g throughout their shelf-life.
The following are considered to fall into this category
- meat products which have received heat treatment or other processing effective to eliminate L. monocytogenes, when recontamination is not possible after this treatment (for example, products heat treated in their final package)
- products with pH ≤ 4,4
- products with aw ≤ 0.92
- products with pH ≤ 5 and aw ≤ 0,94
- products with a shelf-life of less than 5 days
Note: Regular testing under normal circumstances is not required for products that have received heat treatment or other processing effective to eliminate L. monocytogenes when recontamination is not possible after this treatment (for example, products heat treated in their final package such as hams or other cooked meats).
3.6.2 Processing areas and equipment (environmental testing for RTE processing plants)
Article 5 of Regulation (EC) 2073/2005 requires that FBOs manufacturing RTE products sample the processing areas and equipment for Listeria spp. as part of their sampling scheme. If Listeria spp. are detected, serotyping to identify Listeria monocytogenes will be required.
Environmental testing programmes should include food contact and non-food contact surfaces.
Food contact surfaces may include conveyor belts, working tables, knives and utensils, gloves and aprons, slicers, dicers, filling/packaging machines, transport racks, trays, scales, hoppers, etc.
Non-food contact surfaces may include the exterior of food contact equipment, control panels, sides of weigh scales, floors, walls, refrigeration units, drains, etc.
All samples should show negative results as the presence of Listeria monocytogenes in production areas, particularly in high-risk areas, can re-contaminate RTE products. If found, it is important to investigate the possible sources and establish measures to prevent the introduction of Listeria monocytogenes in the products.
3.6.3 Frequency of testing for RTE products
The legislation does not set a testing frequency for Listeria monocytogenes in RTE foods or the environment.
FBOs shall decide the appropriate sampling frequencies based on their HACCP principles and take into account the intended use of the foodstuff. It is for the FBO to demonstrate that the testing shows satisfactory results and based on this, determine the sampling interval.
Initially, it may be best to test weekly, or at whatever frequency the FBO produces RTE foods if less than weekly. Information on testing for Listeria is available online as well as FSA advice.
Once the FBO has results over a period of time and there are no failures, then the FBO may increase the testing interval based on the evidence of testing and their food safety programme. Changes in the factors affecting the process and product such as change in suppliers or batches or changes in the processes will determine the need for further testing.
If the OV has any concerns surrounding the frequency of testing, they should escalate the matter to the FVC in their area.
3.7 Unsatisfactory results
3.7.1 Unsatisfactory results
In the event of unsatisfactory results as regards both food safety and process hygiene criteria, the actions to be taken by the FBO laid down in Article 7 of assimilated Regulation (EC) 2073/2005 shall be taken together with other corrective actions defined in their HACCP-based procedures.
3.7.2 Corrective actions
Trend analysis of the results should be undertaken, and corrective actions might include:
- improvements in slaughter hygiene
- review of process controls
- review of the origin of animals
- review of biosecurity measures in the farms of origin
- improvements in production hygiene and cleaning procedures
- improvements in the selection and/or origin of raw materials
In addition, FBOs shall take measures to find the cause of the unsatisfactory results to prevent the recurrence of the unacceptable microbiological contamination. Those measures may include modifications to the HACCP plan(s) or other food hygiene control measures in place.
The FBO should ensure test results are retained for inspection by the OV / FVL / FVC / Veterinary Auditor. As a minimum, results should be retained for at least 1 audit period or 52 samples, whichever is greater.
If food safety criteria are exceeded, it indicates that the batch tested is unsatisfactory and should be removed from (recalled) or not placed on (withdrawn) the market. However, products which are not yet at the retail level may be submitted for further processing by a treatment that eliminates the hazard in question. This treatment may only be carried out by FBOs other than those at the retail level. The product may be treated at the same establishment or at another approved establishment under Regulation (EC) 853/2004. The treatment has to be effective in destroying the microorganism and evidence of corrective action taken and traceability must be kept. The FBO shall notify the competent authority (FSA Incidents team). Guidance on how to undertake the notification and a link to the incident report form can be found here.
A batch of mechanically separated meat (MSM) with unsatisfactory results with respect to the Salmonella criterion may be used in the food chain only to manufacture heat-treated meat products in establishments approved under Regulation (EC) 853/2004.
In some circumstances, withdrawal or recall of the affected product will not be possible due to the product having been consumed by the final consumer because of the length of time that it takes for some testing to be completed (for example, Salmonella serotyping). In these circumstances, the FBO should review their procedures to ensure the root cause is identified and process controls streamlined to prevent any re-occurrence.
The OV / FVL / FVC / Veterinary Auditor shall verify that the FBO has taken action and has reported the unsatisfactory results for food safety criteria to the FSA Incidents Team.
Reference: Regulation (EC) 2073/2005, Annex I, Chapters 1 and 2 and Article 7 Regulation (EC) 853/2004, Annex III, Section V, Chapter III, Point 3(e)
3.8 OV and other Authorised Officers' role: all establishments
3.8.1 OV / FVL / FVC /Veterinary Auditor responsibility
FVLs will verify FBO procedures and initial results during the approval process of both, slaughterhouses and cutting plants.
FVCs undertaking unannounced inspections (UAI) in standalone cutting plants and OVs undertaking UAIs in RTE cutting plants shall undertake verification checks on sampling and testing in every visit.
Veterinary Auditors will verify procedures, sampling frequency, sampling reference method and results at every full audit in all establishments.
The role of the OV in slaughterhouses and co-located cutting plants is to:
- monitor the FBO’s compliance with microbiological criteria testing as required by the established SHV Procedures (see Chapter 2.4, Sections 12.7 and 13.7 for details) including regular verification of the sampling methods;
- verify that testing has been carried out following the requirements of the appropriate legislation
- verify the method of despatch to the testing laboratory
- verify that the laboratory methods used are the reference method or an alternative in accordance with Article 5 of Regulation 2073/2005
- verify that the results fall within the required limits and are produced at the required frequency in accordance with the establishment's throughput
- verify that where any further action by the FBO is required, this action is taken promptly and is documented within their HACCP-based procedures and;
- take appropriate enforcement action if this is necessary.
Unsatisfactory results shall be followed up in further visits / partial audits as appropriate.
3.8.2 Verify testing requirements and sampling frequencies
The testing requirements and sampling frequencies which the FBO must follow are detailed in Annex I to Regulation (EC) No 2073/2005. Refer to the tables provided in points 3.4.3.1 and 3.4.3.2 above for a summary of requirements for slaughterhouses and to points 3.5 and 3.6 for requirements in cutting plants.
The Authorised Officer should refer to these resources as required, and ensure that they are familiar with the requirements and testing frequencies for the establishments at which they are based or approving /inspecting /auditing.
3.8.3 Verify that the results fall within the required limits
Assimilated Regulation (EC) 2073/2005 Article 9, requires the food business operator to analyse the trend of results and if the trend is towards unsatisfactory results, take action to prevent microbiological risks.
The OV must periodically review the test results at least monthly, or as required by the established SHV criteria, at slaughterhouses and co-located establishments and during audits in non-co-located establishments, or UAIs in RTE standalone cutting plants. The OV / Veterinary Auditor / FVC must follow up on unsatisfactory results closely and verify that the FBO takes adequate corrective actions until controls are re-gained.
Microbiological sampling results
| Hazard | Process hygiene criteria |
|---|---|
| Salmonella spp. (carcases) |
Results are reported as ‘detected’ or ‘absent’. Results from a number of samples throughout the specified sampling period must be returned as ‘absent’. |
| Campylobacter spp. (broiler carcases) | The limit is 1,000 cfu/g. Satisfactory results if a maximum of 15 samples out of 50 (10 consecutive sampling sessions) are below this limit and unsatisfactory if more than 15 samples out of 50 are above this limit. The established maximum number of samples above the limit will decrease to 10 in 2025. |
| Enterobacteria- ceae (Enteros) (red meat carcases) | For the process hygiene criteria in slaughterhouses processing cattle, sheep, goats, horses or pig carcases. Use the specified mean log level established for the five samples. See point 3.4.1 |
| Aerobic colony count (ACC) |
For minced meat and MSM, all five samples must return results of less than 5 x 106 cfu/g and of those five samples, at least three must return results of less than 5 x 105 cfu/g. |
|
E coli (minced meat /meat preparations) |
For minced meat and MSM, all five samples must return results of less than 500 cfu/g and of those five samples, at least three must return results of less than 50 cfu/g. For meat preparations, all five samples must return results of less than 5,000 cfu/g and of those five samples, at least three must return results of less than 500 cfu/g. |
| Salmonella (minced meat /meat preparations) |
If any of the test results from samples of minced meat, MSM or meat products is positive for Salmonella spp, then the batch must be removed from the market. Please refer to instructions later in this chapter in point 3.9 on ‘Enforcement: microbiological criteria’. If the product is at retail and is intended to be cooked before eating, it must be withdrawn as a minimum. The FBO may decide to instigate a recall. If the product is RTE, then a recall is required. |
|---|---|
| Listeria monocytogenes (RTE foods) |
In foods that support the growth of Listeria monocytogenes: Absence in 25g before the food is placed on the market if the FBO is not able to demonstrate that the product will not exceed the limit of 100 cfu/g throughout the shelf-life or less than 100 cfu/g where the FBO can satisfactorily demonstrate that the product will not exceed the limit of 100 cfu/g at the end of the shelf-life. The operator may fix intermediate limits during the process that must be low enough to guarantee that the limit of 100 cfu/g is not exceeded at the end of shelf-life. In foods that do not support the growth of Listeria monocytogenes: less than 100 cfu/g throughout shelf life. The following are considered to fall into this category:
|
3.9 Enforcement: microbiological criteria
3.9.1 OV / Veterinary Auditor advisory role
Where the FBO is not following the sampling, testing and corrective action requirements contained in Assimilated Regulation (EC) 2073/2005, the OV / Veterinary Auditor, as a first step on the hierarchy of enforcement, should consider informal action to achieve compliance. Where this includes failure to comply with the process hygiene criteria for either Salmonella or Campylobacter on several occasions this will include requiring the FBO to submit to them an action plan to achieve compliance which shall be supervised by the OV. In other cases, this can include educating the FBO and encouraging rectification and providing advice.
Reference: Regulation (EU) 2019/627, Articles 35 and 36.
3.9.2 OV / FVC / Veterinary Auditor Actions
The following table contains examples of FBO non-compliance and the possible enforcement actions that the OV / FVC or the Auditor may take.
In addition, where the FBO has exceeded a reduced testing interval, the OV / FVC or the Auditor should inform the FBO that they must commence testing at the shortest interval and demonstrate that they meet the requirements of testing before moving to a reduced testing frequency again.
Before taking formal action the OV or the Auditor must ensure that enforcement actions are in line with the MOC chapter 7 on ‘Enforcement’.
| FBO fails to | OV / FVC /Auditor informal action | OV /FVC / Auditor formal action |
|---|---|---|
| Comply with the size, number of samples and frequency of testing for the required microorganisms, use the reference method or an alternative that complies with Article 5 of Regulation 2073/2005 | Verbal advice / Written advice | Remedial Action Notice (RAN) |
| Perform removal from the market or not place on the market (for unsatisfactory food safety criteria) | Verbal advice / Written advice (for inadequate procedures) |
|
| Undertake trend analysis of results and take adequate corrective actions | Verbal advice / Written advice | HIN – if FBO is asked to provide trend analysis for results already received. RAN – If FBO is asked for a trend analysis for microbiological results from the point of serving the notice. |
| Take adequate corrective actions (for unsatisfactory process hygiene criteria) | Verbal advice / Written advice |
RAN - if there is evidence of the process resulting in unacceptable levels of contamination. |
| Heat treat MSM produced in accordance with 853/2004, Annex III, Section V, Chapter III, Point 3 with unsatisfactory Salmonella results if it is to go into the food chain | Verbal advice / Written advice | Detain, seek voluntary surrender or seizure in accordance with procedures in MOC chapter 7 on 'Enforcement', Section 3. |
3.10 Sampling Methods
3.10.1 Sampling Methods for red meat carcases
The destructive and non-destructive sampling methods, the selection of the sample sites and the rules for storage and transport of samples are described in standard BS EN ISO 17604.
Five carcases shall be sampled at random during each sampling session. Sample sites must be selected taking into account the slaughter technology used in each plant. The purpose is to sample the sites with the highest levels of contamination.
When sampling for analysis of Enterobacteriaceae and ACC, four sites of each carcase shall be sampled. Four tissue samples representing a total of 20 cm2 shall be obtained by the destructive method (excision). When the samples are taken from different sample sites on the carcase, they shall be pooled before examination. When using the non-destructive method (sponge swab), the sampling area shall cover a minimum of 100 cm2 (50 cm2 for small ruminant carcases) per sampling site.
When sampling for Salmonella analysis, an abrasive sponge sampling method shall be used selecting the areas most likely to be contaminated. The total sampling area shall cover a minimum of 400 cm2 (200 cm2 for small ruminant carcases). The surface is calculated by multiplying the width of the sponge by the length of the swabbing on the carcase. Excision cannot be used for Salmonella testing.
A single sponge sampling method can be used for the three tests (Enterobacteriaceae, ACC and Salmonella).
Use sterile dry abrasive sponge swabs (10x10 cm or 5x10 cm, folded in half) in sterile plastic sample bags (waffle-style cellulose sponge dishcloths and stomacher bags).
Diluent sterile 0.9% unbuffered sodium chloride solution. The sponge should be rehydrated in the sample bag with approximately 10ml diluent. The sponges should be damp without excess diluent in the bag. Alternatively, sponges can be rehydrated, stored frozen and defrosted before use.


Trained operatives should grasp the sponge through the bag folding the bag back over the hand avoiding that the sponge, the diluent, or the internal surface of the bag come into contact with other surfaces.


A randomly chosen side of a randomly chosen carcase after inspection and before chilling is to be tested. The wipe with the sponge is to be applied with firm pressure and a slight side-to-side movement down one side of the carcase starting at the back leg and moving across the carcase. A firm consistent pressure is to be used. The length of the wipe should be approximately 100 cm for adult sheep, goats and pigs and 150 cm for adult cattle and horses.


Once finished, the operative should refold the bag over the sponge and secure the bag with a closure.
The bag should be labelled and the following information recorded:
- date of sampling
- species
- origin of animal (farm postcode, slaughtering reference)
- length of wipe for red meat (an estimate is sufficient)
Sponge samples should be kept cool and delivered to the laboratory within two hours. If longer than two hours the samples should be placed into an insulated cool box containing frozen freezer blocks or crushed ice. The samples should be kept cold but not allowed to freeze.
Sample testing in the lab should commence within 24 hours of sampling.
Reference: Regulation (EC) 2073/2005, Annex I, Chapter 3
3.10.2 Sampling Methods for poultry carcases
For the Salmonella analyses, a minimum of 15 carcases shall be sampled at random during each sampling session and after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase. On each occasion, the neck skin samples from three carcases shall be pooled before examination to form 5 x 25g final samples.
For the Salmonella and Campylobacter analyses, if carried out in the same laboratory, a minimum of 15 carcases shall be sampled at random during each sampling session after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase. On each occasion, the neck skin samples from the three carcases shall be pooled before examination to form 5 x 26g final samples.
If Salmonella and Campylobacter analyses are carried out in different laboratories, a minimum of 20 carcases shall be sampled at random during each sampling session and after chilling. A piece of approximately 10g from neck skin shall be obtained from each carcase. On each occasion the neck skin samples from four carcases shall be pooled before examination in order to form 5 x 35g final samples which will then be split to form 5 x 25g final samples to be tested for Salmonella and 5 x 10g final samples to be tested for Campylobacter.
If the slaughterhouse is exempt from carrying out Salmonella testing and only
Campylobacter testing is required, a minimum of 15 carcases shall be sampled at random during each sampling session and after chilling. On each occasion the neck skin samples from three carcases shall be pooled before examination to form 5 x 10g final samples.
A poster summarising the different options can be found in Annex 2.
Operatives taking the sample should use gloves and scissors wiped with an alcohol wipe. Grip the plastic bag at the bottom and fold it back over the gloved hand. Avoid the internal surface of the bag or the scissors contacting other surfaces.

Grasp the neck skin through the bag and cut off approximately 10g with the clean scissors, repeat with two further neck skins to make a total of three (four in the case of broilers when Salmonella and Campylobacter are going to be tested in separate laboratories) in one bag. Fold the bag back over the sample and tie it to secure the neck skin samples inside. Clean gloves and scissors with alcohol wipes and repeat.


For pieces of poultry meat (other than carcases): aseptically remove 25g taking any skin as a priority and supplementing with surface muscle slices. Whole pieces can be taken and sent to the laboratory for skin and muscle slice removal.
Bags should be labelled and the following information recorded:
- date of sampling
- species
- origin of birds (farm postcode, slaughtering reference)
Salmonella and Campylobacter samples shall be transported to the laboratory at a temperature not lower than 1°C and not higher than 8°C. The time between the sampling and the testing for Campylobacter shall be less than 48 hours to ensure the integrity of the sample is maintained. Samples that have reached a temperature of 0°C shall not be used to verify compliance with the Campylobacter criterion. Testing should commence within 24 hours of sampling.
Reference: Regulation (EC) 2073/2005, Annex I, Chapter 3
3.10.3 Environmental testing
FBOs manufacturing RTE foods, which may pose a Listeria monocytogenes risk for public health, are required to sample the processing areas and equipment for Listeria monocytogenes as part of their sampling scheme. Regulation (EC) 2073/2005 does not establish a sampling frequency, but this should be based on risk and trends analysis should be followed to decide if the frequency can be reduced.
For other establishments, FBOs may choose to sample the process environment to validate and verify the cleaning procedures as part of their HACCP plan(s). Rapid methods can be useful.
When the criteria for carcases or processed meat are not met, sampling of the processing environment should be considered as part of the investigatory action.
FBOs should determine what sites to sample throughout their establishment (from raw material intake to final dispatch) taking into account previous microbiological results from environmental monitoring. Sampling should be focused at sites where contamination is most likely to occur and include both, food contact and non-food contact surfaces.
The BS EN ISO standard 18593 provides useful information and can be used as the reference method.
4. Traceability
In this section
4.5 FBO responsibilities: provision of information on frozen food of animal origin
4.1 Introduction
4.1.1 Definition and scope
Traceability, as defined by article 3, paragraph 15 of Regulation (EC) No. 178/2002, means ‘the ability to trace and follow a food, feed, food-producing animal or substance intended to be, or expected to be incorporated into a food or feed, through all stages of production, processing and distribution.’
The following pages provide further background, a summary of the FBO’s responsibilities and guidance on the role of the OV in monitoring and verifying FBO compliance with the traceability requirements.
4.2 Legislative references
4.2.1 Traceability legislation
(EC) No. 178/2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority (EFSA) and laying down procedures in matters of food safety, as read with …
Commission Implementing Regulation (EU) No. 931/2011 on the traceability requirements set by Regulation (EC) No 178/2002 of the European Parliament and of the Council for food of animal origin.
Commission Regulation (EC) No. 853/2004, Annex II, Section I, Part A, Paragraph 4, laying down specific hygiene rules for food of animal origin.
Commission Regulation (EU) No. 16/2012 amending Annex II to Regulation (EC) No. 853/2004 of the European Parliament and of the Council as regards the requirements concerning frozen food of animal origin intended for human consumption.
4.3 Background
4.3.1 Comprehensive system of traceability to be established
The aim is to ensure that unsafe food is not placed on the market and that the systems in place to identify and respond to food safety problems allow for the proper functioning of the internal market and the protection of public and / or animal health.
This level of protection can be jeopardised where it is impossible to trace food and feed. It is therefore necessary for FBOs to ‘establish a comprehensive system of traceability within their businesses so that targeted and accurate withdrawals can be undertaken or information can be easily provided to consumers or control officials when required, thereby avoiding the potential for unnecessary wider disruption in the event of food safety problems.’
Reference: (EC) No. 178/2002, Recital 28
To achieve the traceability of food as set out in Article 18 of Regulation (EC) No 178/2002, the names and addresses of both the FBO supplying the food and the FBO to whom the food was supplied are needed (except when they are final consumers).
In the sector of food of animal origin additional information is required such as the volume or quantity of the food, a reference identifying the lot, batch or consignment, as appropriate, a detailed description of the food and the date of dispatch.
There is however no legal requirement for the origin of food to remain identifiable during production at an establishment.
4.3.2 Insufficient documentary records
Food or feed business operators must ensure that traceability of food, feed, animals or substances which may be incorporated into a further product can be assured at all stages.
Food crises in the past have revealed that documentary records were not always sufficient to allow full traceability of suspect foods. Furthermore, recent experience has shown that FBOs do not generally possess the information needed to ensure that their systems identifying the handling or storage of foods is adequate, in particular in the sector of food of animal origin.
Reference: Commission Implementing Regulation (EU) No. 931/2011
4.3.3 One step back, one step forward
To achieve the traceability of food as set out in Article 18 of Regulation (EC) No. 178/2002, the names and addresses of both the FBO supplying the food and the FBO to whom the food was supplied are needed. The requirement relies on the ‘one step back – one step forward’ approach which requires that FBOs have in place a system enabling them to identify their immediate supplier(s) and customer(s), except when they are the final consumer.
With regards to food, the implementation of a traceability system is an essential element in ensuring food safety and the reliability of information provided to consumers.
Traceability does not itself make food safe, but it is an essential way of providing assurance and assisting in containing food safety problems.
4.4 FBO responsibilities
4.4.1 FBO to identify suppliers and direct recipients
Traceability is a requirement to be complied with in addition to the food bearing a health mark or an identification mark.
FBOs are required to identify the suppliers and direct recipients of their food / feed.
The responsibility to devise such traceability systems rests with FBOs that place such food or feed on the market as they are best placed to identify and manage their suppliers and customers.
4.4.2 Format of relevant information
Without prejudice to specific requirements, industry is allowed some flexibility concerning the format in which relevant information is made available. However, it requires both food businesses and the control authorities to take an active role in ensuring effective implementation.
It is the need to maintain and provide traceability information that is of primary importance, rather than the format in which it is kept.
However, the information needs to be sufficiently organised to enable availability ‘on demand’, without undue delay.
4.4.3 Traceability to be established at all stages
The traceability of food, feed, food-producing animals, and any other substance intended to be, or expected to be, incorporated into a food or feed must be established at all stages of production, processing and distribution along the food / feed chain.
4.4.4 Identify suppliers
FBOs must be able to identify any person from whom they have been supplied with a food, a feed, a food-producing animal, or any substance intended to be, or expected to be, incorporated into a food or feed.
To this end, such operators must have in place systems and procedures which allow for this information to be made available to the competent authorities on demand.
4.4.5 Identify businesses supplied
Food and feed business operators must have in place systems and procedures to identify the other businesses to which their products have been supplied. This information must be made available to the competent authorities on demand.
4.4.6 Food adequately labelled or identified
Food or feed which is placed on the market or is likely to be placed on the market in the Community must be adequately labelled or identified to facilitate its traceability, through relevant documentation or information in accordance with the relevant requirements.
Reference: (EC) 178/2002, Article 18.
Labelling legislation is generally enforced by Local Authorities (LAs) or by the Rural Payments Agency (Beef Labelling Scheme).
4.4.7 Information to be made available by the FBO
Commission Implementing Regulation (EU) No. 931/2011, Article 3, states that:
- FBOs shall ensure that the following information concerning consignments of food of animal origin is made available to the FBO to whom the food is supplied and, upon request, to the CA:
- an accurate description of the food
- the volume or quantity of the food
- the name and address of the FBO from which the food has been dispatched
- the name and address of the consignor (owner) if different from the FBO from which the food has been dispatched
- the name and address of the FBO to whom the food is dispatched
- the name and address of the consignee (owner), if different from the FBO to whom the food is dispatched
- a reference identifying the lot, batch or consignment, as appropriate
- the date of despatch
- The information referred to in paragraph 1 is to be made available in addition to any information required under relevant provisions of EU legislation concerning the traceability of food of animal origin.
4.4.8 Updated on a daily basis
The information referred to in paragraph 1 (as quoted above) is to be updated on a daily basis and kept at least available until it can be reasonably assumed that the food has been consumed. The period during which this information must be available depends on the shelf life of product and guidance is available (see later in this chapter).
Reference: Commission Implementing Regulation (EU) No. 931/2011, Article 3, 3.
4.4.9 Provision of information without undue delay
When requested by the CA, such information is to be provided without undue delay. The appropriate form in which the information must be made available is up to the choice of the supplier of the food, as long as the information requested in paragraph 1 is clearly and unequivocally available to and retrievable by the business operator to whom the food is supplied.
4.4.10 Internal traceability
The regulations do not require a link between incoming and outgoing products, (so called ‘internal traceability’), nor is there any requirement for records to be kept identifying how batches are split and combined within a business to create particular products or new batches.
The decision on whether to implement an internal traceability system, and when implemented the level of detail of such an internal system, is a commercial decision left to the FBO and may be commensurate with the size and nature of the food business.
Nevertheless, an internal traceability system would contribute to more targeted and accurate withdrawals. FBOs are likely to save costs in terms of time of a withdrawal and in avoiding unnecessary wider disruption which in turn would help maintain consumer confidence. Traceability systems can also provide information within food businesses to assist in process control and stock management.
4.4.11 Always applicable
The traceability requirements of Article 18 of Regulation 178/2002 are general requirements and are always applicable to all food / feed.
FBOs should determine whether specific sectorial traceability provisions applicable to their sector or specific regulations laying down marketing and quality standards for certain products (for example, Beef Labelling Scheme, Poultry Meat Marketing Standards) already meet the requirements of the regulations.
4.4.12 Retention period for traceability records
The minimum period of time for keeping traceability records is not specified in the Regulations and it is for the business to decide. However, failure to produce adequate records would constitute a breach of the requirements.
Current European Commission guidance suggests that a general rule of a 5 year period from the date of manufacturing or delivery to destination would meet the objective of the regulations.
Reference: Guidance on the Implementation of Articles 11, 12, 14, 17, 18, 19 and 20 of Regulation (EC) No. 178/2002 on General Food Law.
4.4.13 Specific examples of suggested record retention periods
The common rule above can be adapted for products with a short shelf life:
- for highly perishable products with a ‘use by’ date less than 3 months or without a specified date, destined directly to final consumer, records could be kept for 6 months after date of manufacturing or delivery
- for products with a ‘best before’ date, records could be kept for the period of the shelf life plus 6 months
- for products without a specified durability date, the 5 years period could apply
4.5 FBO responsibilities: provision of information on frozen food of animal origin
4.5.1 Information requirements for frozen food of animal origin
For frozen food of animal origin, Regulation (EC) No. 853/2004 (as amended by Regulation (EU) No. 16/2012) requires FBOs to make available to the FBOs they supply information concerning the date of production and, if different, also the date of freezing.
4.5.2 Date of production
In this context, ‘date of production’ means:
- the date of slaughter in the case of carcases, half carcases or quarter carcases
- the date of killing in the case of bodies of wild game
- the date of harvesting or catching, in the case of fishery products
- the date of processing, cutting, mincing or preparation, as appropriate, for any other food of animal origin
Reference: (EC) No. 853/2004 (as amended by (EU) No. 16/2012), Annex II, Section IV.
4.5.3 Information to be made available
Until the stage at which frozen food of animal origin is labelled for the consumer in accordance with Directive 2000/13/EC (the EU Food Labelling Directive) or used for further processing, FBOs must ensure that they make the following information available to the FBOs they supply and, upon request, to the CA:
- the date of production; and
- the date of freezing, if different from the date of production
Where a frozen food of animal origin is made from a batch of raw materials with different dates of production and of freezing, the oldest dates of production and / or of freezing, as appropriate, must be made available.
Reference: (EC) No. 853/2004 (as amended by (EU) No. 16/2012), Annex II, Section IV.
4.5.4 Format of the information
The appropriate format in which the information must be made available is for the FBO supplying the frozen food of animal origin to decide, but they must ensure that the required information is clearly and unequivocally available to, and retrievable by, the FBO to whom the food is supplied.
Reference: (EC) No. 853/2004 (as amended by (EU) No. 16/2012), Annex II, Section IV.
4.6 FSA role
4.6.1 AO responsibility
As part of the official controls carried out by the CA for food, the AO has responsibility for ensuring that the traceability requirements are complied with.
4.6.2 OV to monitor traceability system
The AO should monitor the FBO’s traceability system in place.
This will be achieved by learning about how the FBO created it, uses it and how the system works in practice. Each FBO will have their own traceability system(s) and the OV should familiarise themselves with it in order to understand and monitor it.
The AO should ensure that any other relevant legislation with an impact on traceability data is also implemented by relevant FBOs in addition to the general traceability requirements. For example, the requirement for labelling bovine carcases with red stripe label when the removal of the vertebral column is required or the need for commercial documents to contain this information as required by Regulation (EC) No. 999/2001 (as amended), Annex V, 11.3, b; or the requirement for internal traceability to be maintained for beef under the Beef Labelling Scheme (the latter is not enforced by the FSA).
Reference: See chapter 2.7 on ‘SRM’, section 2 for details relating to the example quoted above.
4.6.3 AO to verify traceability system
The AO should verify that the traceability system in place is being carried out in accordance with the requirements of the relevant legislation. This should include a traceability check ‘in situ’ in addition to a check on the historical traceability records.
The traceability check ‘in situ’ should take the form of selecting a product from the intake or dispatch bays where finished products or ingredients are found, identifying the information available on the products and seeking the relevant traceability records: both intake and despatch documents should have all the required information.
This check ‘in situ’ may be performed in the event of finding raw materials, ingredients and / or products with poor or unclear traceability data, when there is suspicion that product may have been mislabelled (for example, meat substitutions) and / or as often as the OV considers necessary to ensure that the FBO satisfies the requirements of the regulations with regards to traceability.
4.6.4 AO to verify FBO takes further action
The AO should verify that where further action by the FBO is required, this action is taken promptly and efficiently.
Where traceability details on the product and / or records are not available and / or are proven to be wrong, the FBO will need to demonstrate what action is taken to correct it.
4.6.5 AO to take enforcement action where appropriate
The AO should take appropriate and proportionate enforcement action when necessary, as described in MOC chapter 7 on ‘Enforcement’. Some specific examples are given on the following pages.
4.7 Enforcement action: examples
4.7.1 Health marked product
Where health marked products fail to comply with the traceability requirements of Article 18, Regulation (EC) No. 178/2002 as read with Commission Implementing Regulation (EU) No. 931/2011, this will constitute an offence under Regulation 4(c) of the General Food Regulations 2004.
4.7.2 Health marked product: enforcement action in cases of non-compliance
Health marked carcases and primal cuts which have not left the slaughterhouse or been further cut or processed in a cutting plant, may not be certified under Regulation 27 of the Food Hygiene (Wales) Regulations 2006 / Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013 as failing to comply with the ‘Hygiene Regulations’. This is because Regulation (EC) No. 178/2002, Article 18, as read with Commission Implementing Regulation (EU) No. 931/2011 are excluded from the definition of ‘Hygiene Regulations’ and the requirements for traceability of ID marked products contained in Regulation (EC) No. 853/2004, Annex II, Section I, will not apply in these circumstances.
Where health marked products which have not left the approved slaughterhouse or been cut or further processed in a cutting plant have associated commercial documentation that fails to comply with the traceability requirements, they should be formally detained until the commercial documentation has been altered to accurately detail the products being consigned from the establishment in accordance with the legal requirements.
Where products bearing a health mark have been despatched without adequate traceability information, the FBO must be advised in the first instance in order that they take corrective action. Enforcement should be escalated to ensure that commercial documents reflect the information required under Article 18, Regulation (EC) 178/2002 as read with Commission Implementing Regulation (EU) No. 931/2011. If serious / repetitive breaches have been identified, the FBO should be referred for investigation.
4.7.3 Health mark legibility
Where the traceability deficiency identifies a failure to comply with the food safety requirements, the FBO shall initiate procedures to withdraw or recall the food in accordance with Article 19 of Regulation (EC) 178/2002. Where health marked products have been consigned to another establishment, the FBO of the recipient plant(s) should be informed, as their ability to comply with the traceability requirements may be hampered as a result of the inaccurate information they receive, which may cause them to inadvertently mislabel products they subsequently supply.
The AO / enforcement authority responsible for enforcement action at subsequent establishments must be informed so that all appropriate action is taken. This may include formally detaining product until commercial documentation has been provided that accurately details the products consigned by the supplier.
4.7.4 ID marked product
Where ID marked products fail to comply with the traceability requirements of Article 18, Regulation (EC) No. 178/2002 as read with Commission Implementing Regulation (EU) No. 931/2011, they will also breach the provisions of Regulation (EC) No. 853/2004, Annex II, Section I, Part A, paragraph 4.
This will constitute an offence under Regulation 17 of the Food Hygiene Wales) Regulations 2006 / Regulation 19 of the Food Safety and Hygiene (England) Regulations 2013, as well as Regulation 4 (c) of the General Food Regulations 2004 (Wales).
Failure to comply with Regulation (EC) No. 853/2004, Annex II traceability requirements will apply only to products that have been further cut or processed and have received an Identification Mark.
4.7.5 ID marked product: enforcement action in cases of non-compliance
Where ID marked products fail to comply with the traceability requirements of Regulation (EC) No. 853/2004, enforcement should be escalated in accordance with MOC chapter 7 on ‘Enforcement’.
An assessment should also be made with respect to any potential fraud, the FBO’s ability to trace all meat to comply with any product recall or withdrawal requirements and to determine whether the raw materials for the product were processed lawfully in approved establishments.
Where appropriate, non-compliant ID marked products may be formally certified under Regulation 27 of the Food Hygiene (Wales) Regulations 2006 / Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013, as not having been produced, processed or distributed in accordance with the ‘Hygiene Regulations’ due to its failure to comply with Regulation (EC) No. 853/2004. Where voluntary surrender is not forthcoming, non-compliant products may be formally seized and a Condemnation Order applied for at the Magistrates / Sheriffs Court. Non-compliant product that has been so certified will be deemed to fail to comply with the food safety requirements and the FBO must initiate withdrawal or recall of the product in line with Article 19 of Regulation (EC) 178/2002. A referral for investigation may also be appropriate for serious or repetitive breaches or where public health protection is being compromised.
4.8 Summary
4.8.1 Summary
Checks on compliance with traceability requirements will be achieved initially over the duration of the approval process, followed by audits at the appropriate frequency and during UAI to approved establishments.
Reference: The FBO Audit Aide Memoire located at Chapter 4.1 Audit, Annex 1, contains pointers for the auditing AO to consider in relation to traceability. A traceability system can be considered acceptable when it delivers accurate information in a timely manner. Assurance of this may be attained by checking product and records data against the system in place.
It is essential that the FBO’s traceability system is designed to follow the physical flow of the product and helps to identify its location at a given moment in time.
This means that the FBO must provide evidence of the traceability for animals, raw materials and/or ingredients received at the premises, allowing for the identification of their location. The same applies to any products manufactured on site that are to be despatched.
5. Procedures for the verification of the manufacture of beef patties and burgers intended to be consumed less than thoroughly cooked at retail level
In this section
5.1 Background
5.1.1 History and key issues
The sale and consumption of beef patties and burgers served less than fully cooked is a trend that has been steadily increasing in the UK. A number of catering chains and outlets now offer this option to customers. The FSA has produced guidance for FBOs and LAs with advice on safe systems for serving less than thoroughly cooked patties and burgers and on the enforcement approach.
This section is intended to complement the LA document, by giving FBOs and FSA officials information about how to approach, audit and assess approved manufacturers that supply catering establishments (for example, restaurants and pubs) that intend to serve less than thoroughly cooked beef patties and burgers to the final consumer.
5.1.2 Identification of retail establishments
New and existing FBOs who intend to serve less than thoroughly cooked patties and burgers are required to notify their LA before they commence this activity in line with requirements in food hygiene legislation. This will allow LAs to assess the FBO’s proposed HACCP based procedures and discuss as appropriate.
It will also allow the identification of establishments involved in the supply chain, particularly those that provide minced meat or meat preparations to the caterer.
5.2 Legislative references
5.2.1 Regulation (EC) No 178/2002
(EC) No 178/2002 lays down general food safety requirements, according to which food must not be placed on the market if it is unsafe.
5.2.2 Regulation (EC) No 852/2004
Regulation (EC) No 852/2004 lays down the principles of food hygiene. In particular article 4 requires FBOs to put in place, implement and maintain a permanent procedure or procedures based on the HACCP principles.
5.2.3 Regulation (EC) No 853/2004
Regulation (EC) No 853/2004 lays down specific requirements for the manufacture of minced meat and meat preparations. In particular Section V, Chapter III of Annex III concerns the age and / or temperature of meat used for minced meat and meat preparations.
5.2.4 Regulation (EC) No 2073/2005
Regulation (EC) No 2073/2005 (as amended) sets out the microbiological criteria for certain micro-organisms and the implementing rules to be complied with by FBOs when implementing the general and specific hygiene measures referred to in Regulation (EC) No 852/2004.
5.2.5 Food hygiene regulations
The Food Hygiene (S/W) Regulations 2006 (as amended) / The Food Safety and Hygiene (England) Regulations 2013 make it an offence for any person to contravene or fail to comply with the specified community provisions.
5.3 Understanding the hazards
5.3.1 E. coli O157 and other STEC (Shiga-toxin producing E. coli) and Salmonella
The key microbiological hazards that can be associated with raw beef are E. coli O157 and other STEC (Shiga-toxin producing E. coli) and Salmonella.
E. coli O157 and other STEC are of particular concern because although uncommon they can have a low infectious dose and can cause serious illness and lead to death in some cases.
The main source of E. coli is the intestines of cattle and sheep. When cattle and sheep are slaughtered there is the potential for E.coli O157 from the animal’s gut and hide to contaminate the carcases during the slaughter and preparation of the meat.
Salmonella can also be present in the gut of animals, particularly poultry. While the number of cases of disease associated with
Salmonella is decreasing it can also cause death.
Contamination of whole cuts of beef and lamb therefore tends to be only on the outside of meat.
5.4 FBO role – minimum HACCP requirements
5.4.1 HACCP
The law requires FBOs to have food safety management procedures in place, based on HACCP principles, which effectively control the risks at all stages of the food chain. Consequently, the FBO’s documentation has to consider the key SOPs / CP / CCPs in the process at all stages.
Operators in the supply chain of meat that is intended for the production of burgers and patties intended to be consumed less that thoroughly cooked, must be able to demonstrate that their own procedures are effective in reducing the risk to an acceptable level and adapt their food safety management systems accordingly.
5.4.2 At slaughterhouse level
The FBO has to consider at least the following steps to assess the hazards and subsequently implement controls to ensure food safety:
- intake of livestock (for example clean livestock policy)
- removal of the hide
- evisceration process
- chilling of meat
- intervention techniques (for example, use of lactic acid or steam vacuum)
Further guidance on food safety management for businesses can be found on the FSA website.
Further guidance on acceptable standards for clean cattle and sheep can be found online.
5.4.3 At cutting plant level
Likewise, the FBO must assess the hazards associated to the cutting plant processes, including the mincing, mixing and the forming of patties and burgers. To that effect, the FSA has produced a model HACCP plan that can be used to guide FBOs when developing controls in their production.
5.4.4 Application of microbiological criteria
The Microbiological Criteria Regulation contains two food safety criteria applicable to the manufacture of minced meat and meat preparations:
- minced meat and meat preparations intended to be eaten raw (absence of Salmonella in 25g)
- minced meat and meat preparations made from species other than poultry intended to be eaten cooked (absence of Salmonella in 10g)
Burgers that are not thoroughly cooked will contain some meat that is not cooked all the way through. The FSA therefore considers that the more stringent of the two criteria (absence in 25g, for mince or meat preparations intended to be eaten raw) should be applied.
Note: Current UK exemption under the microbiological criteria for small establishments producing less than 2 tonnes of minced meat / meat preparations per week is not applicable to those establishments producing patties and burgers intended to be eaten less than thoroughly cooked. Therefore, even if only a small amount of such products is produced, microbiological testing is required.
5.4.5 Ageing of meat
Establishments supplying or manufacturing raw materials for retail establishments serving beef patties or burgers less than thoroughly cooked shall comply either with the requirements set in regulation 853/2004 in relation to the age of the meat used for mincing:
- no more than 6 days from slaughter unless boned and vacuum packed where the age limit is 15 days
- for frozen meat there is not a specific requirement other than it must be boned before freezing and stored only for a limited period
or comply with the FSA guidance when exceeding the established age for the manufacture of minced meat (patties).
Note: This requirement is only applicable to the manufacture of patties / minced meat only products. Meat preparations (for example, burgers) are not obliged to comply with age limit requirements.
5.4.6 Raw materials
For the manufacture of burger patties (minced meat shaped into burger patties but meeting the definition of minced meat), the raw material used must:
- comply with the requirements for fresh meat and derive from skeletal muscle
- not derive from:
- scrap cuts or scrap trimmings
- MSM
- the region of the carpus or tarsus
- meat containing bones or skins
- meat of the head (except masseters)
- bone scrapings
- the diaphragm (unless the serosa has been removed)
- the non-muscular part of the linea alba
For the manufacture of burgers (also raw patties, but due to the addition of seasonings or other ingredients they meet the definition of meat preparations), the raw materials used must meet the requirements of fresh meat or the above requirements. However, if the final product is intended to be heat treated (as in the case of burgers, even those that will not be thoroughly cooked), scrap cuts or scrap trimmings and MSM complying with the microbiological requirements for minced meat can be used (see sub-topic 5.4.3 At cutting plant level).
5.5 FSA role – verification and audit
5.5.1 OV role
The OV in slaughterhouses or cutting plants sourcing establishments involved in the serving of beef patties and burgers less than thoroughly cooked must ensure that the FBO has developed a suitable HACCP plan and carry out spot checks to ensure this is implemented accordingly.
5.5.2 Verification at audit
The FBO’s food safety management procedures based on HACCP principles, associated records and processes will be verified during audit both at slaughterhouses and at cutting plants involved in the supply chain.
Attention should be paid to the validation procedures, particularly where the minced meat or meat preparations are supplied ready for use by the caterer with only minimal cooking required before it is served to the final consumer.
In addition, some of the treatments described in the guidance for caterers and LAs may be applied at the cutting plant; officials must therefore familiarise themselves with its content.
5.5.3 Other guidance
In addition to the guidance for caterers and LAs, and the FSA guides above, the FSA guidance on the control of cross-contamination may be of assistance.
6. Verification of Water Testing Procedures
6.1 Introduction
6.2 ‘Potable’ water
6.3 Legal requirements for water usage
6.4 Monitoring of Water Supply (private and/or public)– FBO Obligations
6.5 Private Water Supplies
6.6 Public Mains Supply
6.7 Water Testing Programme
6.8 Links to the Water Supply (Water Quality) Regulations
6.1 Introduction
Water can be a potential source of microbiological and chemical hazards. Micro-organisms that cause food poisoning can survive for days or even months in water.
Procedures are needed to minimise the risk of such hazards causing contamination and therefore illness to consumers.
Examples demonstrating the importance of monitoring the water supply:
| Problem | Effect | Outcome |
|---|---|---|
| Water supplies can become polluted with human sewage or agricultural waste containing faecal contamination from animals | Contaminated water supply (biological contaminants) | A source of microbiological contamination |
| Water supplies can be a source of chemical contaminants, such as heavy metals, pesticides, nitrates, and industrial pollutants, which can be transferred from water used in processing or cleaning onto food | Contaminated water supply (chemical contaminants) | A source of chemical contamination |
| Water distribution system not kept clean, adequately maintained, or infrequently used water storage tanks and/or pipes | Bacteria can, in specific circumstances, multiply or create biofilms in water distribution systems and chemical residues can be present and spread to other parts of the food production system and transferred to food | A source of microbiological and/or chemical contamination |
6.2 'Potable' water
There are multiple provisions within the retained EU Food Hygiene Regulations that require an FBO to use ‘potable’ water in food production.
6.2.1 The definition of 'potable' water
‘Potable water’ is defined in Article 2 of Retained Regulation (EC) 852/2004 (as amended following EU exit):
(i) as regards England, water meeting the requirements laid down in the Private Water Supplies (England) Regulations 2016 (as amended);
(ii) as regards Wales, water meeting the requirements laid down in the Private Water Supplies (Wales) Regulations 2017.
Whilst Article 2 refers to the ‘Private’ Water Supplies Regulations to define ‘potable’ water, the water requirements referred to in these regulations (see point 2.2 below) are applicable whether the FBO is getting their water from a private or a public (mains) water supply.
6.2.2 The Private Water Supplies Regulations
The requirements / standards for water that FBOs must achieve where potable water is required, are as follows:
PART 1 of the Private Water Supplies regulation defines ‘water intended for human consumption’ as all water:
(a) either in its original state or after treatment, intended for drinking, cooking, food preparation or other domestic purposes, regardless of its origin and whether it is supplied from any distribution network, from a tanker, or in bottles or containers;
(b) used in any food production undertaking for the manufacture, processing, preservation or marketing of products or substances intended for human consumption unless, in accordance with retained Regulation (EC) 852/2004, the competent authority, is satisfied that the quality of the water cannot affect the wholesomeness of the foodstuff in its finished form.
PART 2 states that for water to be regarded as wholesome the following conditions are to be met:
(a) it does not contain any micro-organism, parasite, or substance, alone or in conjunction with any other substance, at a concentration or value that would constitute a potential danger to human health,
(b) it complies with the concentrations or values prescribed in Part 1 of Schedule 1 for each parameter, and
(c) the water satisfies the formula “[nitrate]/50 + [nitrite]/3 ≤ 1”, where the square brackets signify the concentrations in mg/l for nitrate (NO3) and nitrite (NO2).
6.3 Legal requirements for water usage
6.3.1 Retained Regulation (EC) 852/2004
Annex II, Chapter II (2) “Requirements in rooms where foodstuffs are prepared, treated or processed” require adequate facilities to be provided, where necessary, for the cleaning, disinfecting and storage of working utensils and equipment. These facilities are to be constructed of corrosion-resistant materials, be easy to clean and have an adequate supply of hot and cold water.
Annex II, Chapter II (3) requires that adequate provision is to be made, where necessary, for washing foods. Every sink or other such facility provided for the washing of food is to have an adequate supply of hot and/or cold potable water consistent with the requirements of Chapter VII and be kept clean and, where necessary, disinfected.
Annex II, Chapter VII, refers to hygiene requirements for FBOs with regards to Water Supply:
- There is to be an adequate supply of potable water, which is to be used whenever necessary to ensure that foodstuffs are not contaminated.
- Where non-potable water is used, for example for fire control, steam production, refrigeration, and other similar purposes, it is to circulate in a separate duly identified system. Non-potable water is not to connect with or allow reflux into, potable water systems.
- Recycled water used in processing or as an ingredient is not to present a risk of contamination. It is to be of the same standard as potable water, unless the competent authority is satisfied that the quality of the water cannot affect the wholesomeness of the foodstuff in its finished form.
- Ice which comes into contact with food, or which may contaminate food is to be made from potable water and handled and stored under conditions that protect it from contamination.
- Steam used directly in contact with food is not to contain any substance that presents a hazard to health or is likely to contaminate the food.
- Where heat treatment is applied to foodstuffs in hermetically sealed containers it is to be ensured that water used to cool the containers after heat treatment is not a source of contamination for the foodstuff.
6.3.2 Retained Regulation (EC) 853/2004
Article 3(2) of Chapter II refers to Food Business Operators obligations: FBOs shall not use any substance other than potable water to remove surface contamination from products of animal origin, unless use of the substance has been prescribed by the appropriate authority.
Annex III, Section I, Chapter IV (9): when not skinned, porcine animals must have their bristles removed immediately. The risk of contamination of the meat with scalding water must be minimised. Only approved additives may be used for this operation. Porcine animals must be thoroughly rinsed afterwards with potable water.
6.3.3 Water Fittings Regulations
In order to protect the water supply from contamination, it is important that fixtures and fittings are correctly installed and in a good state of repair. The Water Supply (Water Fittings) Regulations 1999 set out requirements for the design, materials, installation and maintenance of plumbing systems, water fittings and water-using appliances. Businesses have the legal duty to ensure that their systems satisfy these requirements. The regulations apply in England and Wales to all plumbing systems, water fittings and equipment supplied, or to be supplied, with water from the public water supply. This applies to systems in all types of premises. The FSA is not the enforcement authority for these regulations but should be aware of the possibility of contamination from this source.
6.4 Monitoring of Water Supply (private and/or public)– FBO Obligations
Supply – FBOs will have to consider the need for adequate water supplies for food processing, cleaning and other requirements in the design and construction of premises or when buildings are rebuilt, altered, or refurbished.
Capacity – FBOs are to make sure that the water distribution system has sufficient capacity to meet demand at peak times (for example, during cleaning).
Water storage tanks – when used, these should be made of inert material to avoid chemical contamination of water and corrosion. They must be kept covered and secured to prevent contamination. Tanks should be cleaned regularly to prevent any build-up of organic or mineral material that could act as a source of microbial growth and contamination. Disinfection systems, if used, should follow ‘specialists' advice. Examples of disinfection systems can include chlorination, ozonation or UV light.
Pipes – the composition and condition of the pipes can influence what chemicals may be released into the system and the types of bacteria that colonise water distribution systems and pipe surfaces. Some pipe materials may break down and release bio source compounds, such as iron, hydrogen, and phosphate, that can encourage biofilm growth and bacteria to multiply. Biofilms coating the interior surfaces of water distribution pipes usually develop slowly and can take years to reach maturity. Older pipes are therefore more prone to developing large amounts of scale and rust. Water distribution systems should be kept in good condition through regular inspections for signs of damage, corrosion and leaks and records of these checks should be kept by the FBO as part of their pre-requisites controls.
Plans – water distribution systems can be complex, especially in larger premises. Detailed water distribution plans will help to identify any redundant pipe work that could act as a reservoir of microbiological contamination and to define an area to be isolated if contamination occurs. FBOs should keep an accurate and dated plan of the potable water system (and any non-potable water system if applicable), including pipe work, point of entry of water into the premises and numbered outlets. The plan should be submitted with applications of approvals of new premises and should be kept updated if alterations are made.
Non-potable water – may be used in food premises for certain purposes such as fire control, non-food contact steam generation or refrigeration. Potable and non-potable water systems are to be clearly identified, particularly water outlets to avoid misuse of non-potable water.
Ice used in contact with food – FBOs need to make sure ice is made from potable water and is handled and stored under conditions that protect it from contamination (for example containers are kept covered and are cleaned and disinfected periodically).
Water testing – regular testing of water samples from cold or mixed hot/cold water outlets where the water could come into direct contact with food, food processing equipment, or food handlers, will indicate whether contamination is occurring and if water is potable. Water testing programmes, as described in point 7 below, should be established by FBOs at their premises as part of their pre-requisite programmes and at a frequency based on a risk assessment.
6.5 Private Water Supplies
Water that does not originate from public mains is described as private water supply. It can be from ground waters (for example, boreholes, wells, and springs) or from surface waters (for example, streams, rivers, lakes, and lochs). A Local Authority (LA) will carry out a risk assessment for every private water supply in their area and review and update that risk assessment at least every 5 years (or earlier if considered that the existing risk assessment is inadequate) in accordance with the requirements of the Private Water Supplies Regulations. FBOs can and should request a copy of the risk assessment to their LA where a private water supply is feeding their business.
Private water supplies are not treated and therefore are more likely to be contaminated with micro-organisms and/or chemicals. Private water supplies may become contaminated with harmful pathogens if the borehole headworks are not sealed properly, there is ingress of surface water or access to spring sources allowing water sources to be contaminated by faecal matter.
Where water is drawn from a private supply it may require disinfection treatment (for example, filtration, ultra-violet light, chlorination). FBOs using private water supplies must consult a water treatment specialist to help identify the most effective method and requirements for regular maintenance.
6.6 Public Mains Supply
Water may be drawn from the public ‘mains supply’ network operated by a water company (a water undertaker or licensed water supplier). Water suppliers are required to monitor the microbiological and physical-chemical quality of mains water entering the premises to demonstrate that it meets the standards required in the Water Supply Regulations. A copy of their test results can be obtained but usually with a delay.
FBOs must demonstrate that the water used at their premises is potable so will need to carry out their own independent verification tests as described in point 7 below.
Water tanks are common habitats for water microbes; systems with water tanks that do not turn over frequently can encourage microbiological activity. If mains water is stored in tanks before use and/or if the water distribution system is complex and/or old, the water can become contaminated after entering the premises. This is to be taken into consideration as part of the FBO’s risk assessment.
Regular testing of water samples from cold or mixed hot/cold water outlets where the water could come into direct contact with food, food processing equipment or food handlers will indicate whether contamination is occurring on site. FBOs will need to carry out a risk assessment at their premises to decide their own water testing programme as part of their pre-requisite programme.
6.7 Water Testing Programme
6.7.1 Microbiological Parameters
The testing programme, for both public and private water supplies, shall include testing for the following microbiological parameters to demonstrate that the water is potable:
- E. coli and Enterococci as per Part 1 of Schedule 1 of the Private Water Supplies Regulations Presence of these organisms in the water is an indication of faecal contamination and is considered high risk.
- Indicator Parameters listed in Part 2 of Schedule 1 of the Private Water Supplies Regulations, including Coliforms, TVC at 22°C, and, in the case of surface water or groundwater that has been influenced by surface water, Clostridium Perfringens. Routine sampling of TVC at 22°C will help to understand baseline levels and therefore identify any significant deviation from that baseline (when there are abnormal changes).
- The frequency of testing shall be decided by the FBO based on a risk assessment taking into consideration the particularities at their premises and the type of production. As an example; when the risk is considered low (e.g. small cutting plant where water is from mains supply, pipe systems are well maintained, there is no storage tank, water is not used as an ingredient and where previous test results have been satisfactory), yearly testing for all the microbiological parameters detailed in Parts 1 and 2 of Schedule 1 (as above) might be sufficient. In any case, a risk assessment would be necessary to ascertain the sampling frequency.
- FBOs with a private water supply should liaise with their local authority to establish any additional parameters they should be testing for and the frequency of testing, taking into account any risk factors specific to their area and to their establishment (including type of production). FSA officials should ensure they are aware of any additional information provided by the local authority when assessing whether a water testing programme is adequate.
- FBOs who use a public mains supply, although they will likely need to test less frequently, their water testing programme will have to take into consideration any particular risks in the area (for example through information provided by their water supplier) and any particular risks linked to their establishment and type of production (for example if they use water storage tanks or if the water is used as an ingredient).
6.7.2 Physical-chemical Parameters
- Private water supplies should be tested by local authorities for residues of pesticides that may come from crop spray runoff after heavy rain and heavy metals that may be present in the rock strata where the water originates. FBOs should request a copy of the test of the private water supply undertaken by the local authority in their area and this information should be considered when determining how often to test and what to test for.
- Public mains water suppliers should provide an annual summary of the physical-chemical analysis of the water in the area. A copy of these results can be obtained from the supplier for the previous year.
6.7.3 Water sampling procedures
Water samples need to be taken carefully so that no contamination is introduced when the sample is taken. Staff taking the samples should receive specific training. All samples are to be identified with the specific sample point location (all sample points should be identified in the water distribution plan).
FBOs should use laboratories that are accredited by a recognised body for the relevant test methods used in water sampling such as UKAS or at least participate in proficiency testing schemes.
6.7.4 FBO follow-up actions
Presence of E. coli or Enterococci: These faecal indicators are high risk and must not be present in potable water (the limit is 0/100ml).
If the water supply has become contaminated before entering the premises, it is the water supplier’s or local authority’s responsibility to communicate the issue to the FBO and restore potable water quality.
Regardless of whether the contamination occurred before or after entering the FBO premises, if the water supply is contaminated at the establishment, the FBO must take urgent corrective actions to ensure food safety. Corrective actions may include (this list is not exhaustive):
- isolating affected water outlets / tanks until satisfactory microbiological test results are obtained
- retesting at the point of entry, at the outlet from which the contaminated sample was obtained and at any other associated outlets
- stopping production where no potable supply can be provided
- dealing with any product that has potentially been contaminated
- investigating the potential source of the contamination and, if necessary, contact the water supplier or the local authority
- establishing and, where necessary, eliminating the underlying cause to prevent similar contamination incidents in the future, such as, for example the installation of either a water filtration and chlorination system or a water filter and ultra-violet sterilisation system
- reviewing sampling and testing procedures and improve staff instructions and training as required
Presence of Indicator Parameters such as:
- Coliforms: 0/100ml
- Abnormal changes (deviations from the trend identified during routine testing or considerable deviations from results provided by the water supplier or the local authority) to TVC at 22°C: number/ml
- Clostridium perfringens: 0/100ml (only in the case of surface water or groundwater that has been influenced by surface water)
Coliform bacteria are microbes found in the digestive systems of warm-blooded animals, in soil, on plants, and in surface water. These microbes typically do not make people sick; however, because microbes that do cause disease are hard to test for in the water, “total coliforms” are tested instead. If the total coliform count is high, then it is very possible that harmful germs like viruses, bacteria, and parasites might also be found in the water.
Incidents where the maximum concentration of indicator parameters has been exceeded or where abnormal changes in TVC numbers are observed, require a similar approach to investigating failures of E. coli and/or Enterococci, although the risk to human health is likely to be lower, which may allow the company to take different investigation paths.
When deviations occur, this may trigger further investigation and follow-up actions should include, at the very least, re-sampling of the outlet from which the non-compliant sample was taken as well as from any other sampling point considered relevant. Re-sampling should include testing for E. coli and Enterococci. If the test is again positive for the indicators but there is no evidence of faecal or other contamination, FBOs should investigate the source of the problem and keep re-testing until a compliant result is received.
| Microbiological Parameter | Satisfactory level (number/100 ml) | Satisfactory level (number/ml) |
|---|---|---|
| E.coli | 0 | N/A |
| Enterococci | 0 | N/A |
| Clostridium Perfringens (In the case of surface water or groundwater that has been influenced by surface water) | 0 | N/A |
| Coliforms | 0 | N/A |
| TVCs at 22°C | N/A | No abnormal change |
6.7.5 FSA oversight and follow-up actions
FSA officials should be satisfied that the risk assessment undertaken by the FBO to establish the water testing programme is appropriate for their establishment and has taken into consideration relevant factors, for example (this list is not exhaustive):
- the size of the establishment
- the origin of the water supply (private or mains)
- the use/non-use of water storage tanks
- the type of production (water/ice used as an ingredient)
and that the programme is documented, includes follow up actions in the event of unsatisfactory results and is effectively implemented. Field Veterinary Leaders will undertake an initial assessment of the documented procedures during the Approval Visits. Copies of all relevant documents should be retained. Not presenting evidence of water testing results, might lead to the approval being refused.
If an FBO does not implement an adequate water testing programme, enforcement action may be appropriate based on failure to ensure that the water they use at their premises is potable as required by Article 2 of retained (EC) 852/2004 Regulation, and failure to adopt appropriate hygiene measures.
When there is evidence of E. coli and/or Enterococci being present in the water and the FBO does not take immediate action, enforcement action should be considered by FSA Officials. Enforcement action may include the use of FSA powers to make FBOs take the steps outlined in section 7.4 above.
If enforcement is proposed based on the presence of indicator parameters or suspected contamination other than E. coli or Enterococci, expert advice will be needed to determine if the water in question falls outside the definition of potable water set out at 2.2 above. FSA Officials should contact the Meat Hygiene Policy Team at meathygiene@food.gov.uk to facilitate this.
At all stages when considering enforcement actions, FSA officials should ensure that the strength and reliability of any evidence of contamination is considered. If there is high risk and strong evidence, then enforcement action is more likely to be appropriate than if there is low risk with weak evidence. If the evidence is poor then further investigation may be needed before taking enforcement, this will always depend on the facts and risk of the individual case.
6.8 Links to the Water Supply (Water Quality) Regulations
It should be noted that the FSA is not the enforcement authority for these regulations. They are included here for reference.
6.8.1 Public water supply
England
The Water Supply (Water Quality) Regulations 2016 (legislation.gov.uk)
The Water Supply (Water Quality) (Amendment) Regulations 2018 (legislation.gov.uk)
Wales
The Water Supply (Water Quality) Regulations 2018 (legislation.gov.uk)
Northern Ireland
The Water Supply (Water Quality) Regulations (Northern Ireland) 2017
Scotland
The Public Water Supplies (Scotland) Regulations 2014
6.8.2 Private water supplies
England
The Private Water Supplies (England) Regulations 2016 (legislation.gov.uk)
The Private Water Supplies (England) (Amendment) Regulations 2018 (legislation.gov.uk)
Wales
The Private Water Supplies (Wales) Regulations 2017 (legislation.gov.uk)
Northern Ireland
The Private Water Supplies (Northern Ireland) Regulations 2017
Scotland
The Water Intended for Human Consumption (Private Supplies) (Scotland) Regulations 2017
7. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Annex 1: The specification for lactic acid
Annex 2: Poster for Campylobacter sampling in broiler carcases under the Process Hygiene Criteria
1. Overview
2. Sampling
3. Suspect Substances, Animals and Carcases
updated [4. Follow up samples]
5. Annexes
Sections
3. Suspect Substances, Animals and Carcases
1. Overview
In this section
1.1 Introduction
1.1.1 Statutory requirements
The UK has in place a statutory veterinary residue surveillance scheme in fulfilment of its obligations retained under the Official Controls Regulation 2017/625 and, Council Directive 96/22/EC.
This programme helps to ensure that consumers are protected against potentially harmful residues of veterinary medicines.
1.1.2 Co-ordination and collection
The Veterinary Medicines Directorate (VMD) is responsible for the co-ordination and management of the UK programme and for the management and operation of the National Surveillance Scheme (NSS) in GB.
The total number of samples required to fulfil GB’s obligation is determined annually by the VMD, who will then request samples from individual slaughterhouses.
The FSA undertakes the collection of samples from licensed slaughterhouses under contract to the VMD.
1.2 Legislation
1.2.1 Applicable legislation
The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 and The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) Regulations 2019 SI No. 569 (W 125) implement the requirements of Council Directives 96/22/EC.
The legislation requires targeted sampling for veterinary residues by trading partners. They lay down the frequency of sampling required for substances.
1.2.2 Sampling of suspect animals
The Directives also require sampling to be undertaken where the Official Veterinarian (OV) suspects or has evidence that animals have been treated with unauthorised substances or may contain residues of authorised substances above the Maximum Residue Limits (MRL). Casualty animals without any reference of treatments (and compliance with its withdrawals periods) in the food chain information (FCI) should be considered for testing if their condition is likely to have required treatment.
1.2.3 Authorisation
The authorisation certificate brings FSA staff within the definition of “authorised officer”. FSA staff must not undertake work for which they have not been authorised. If in doubt, please consult your ITL for advice.
1.2.4 Powers of the Authorised Officer (AO)
The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (England and Scotland) Regulations 2015 and Regulation 2019 (Wales) give FSA authorised officers:
- the power to detain and inspect animals prior to slaughter
- the power to detain animals for a further examination to be carried out and if necessary samples of tissues / fluids to be taken for analysis
- the power to detain the animal / carcase or group of animals / carcases until the results of the analysis is available
An AO has the power to take a sample from any animal, whether or not intended for human consumption.
1.3 FBO responsibility
1.3.1 Information on origin
Only animals for which full information of the farm submitting for slaughter or source (for example, market or collection centre) is available can be sampled. This information will be essential in tracing the owner, should further action requiring definite identification be necessary.
Slaughterhouse operators are required to keep such records on all animals and it is an offence not to do so.
Reference: The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 Regulation 31 (1) & The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) SI No. 569 (W 125) Regulations 2019 32 (1).
1.4 FSA role
1.4.1 OV responsibility
The OV must:
- ensure that only authorised FSA staff carry out sampling under their supervision
- ensure continuity of evidence when samples are collected, prepared, labelled, stored and despatched
- always obtain indisputable evidence for the origin of the animals sampled
- where the farm submitting for slaughter is unknown, determine the most recent origin by giving the name and status of the person supplying the animal to the slaughterhouse
- file a hardcopy or an electronic copy of the FCI accompanying the animal/batch, signed and dated by the OV, along the local copy of the RIM 1 form
- record in the daybook a confirmation that the above has been completed for the RIM samples (including RIM ref. numbers) taken on that day
- agree with the FBO a method for informing their representative of the collection of the samples after selecting the animals to be sampled and the residue it will be tested for. Some FBO may need to know this information for complying with customer’s requirements (for example, certain export markets)
- encourage the FBO to request from VMD and check the results for the RIM testing and to incorporate them as part of verification records of the HACCP for chemical hazards.
- agree witnessing of sampling by FBO representative
1.4.2 FSA duties
FSA staff must check that the FBO keeps source records according to the requirements of the regulations.
Reference: The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 Regulation 31 (1) & The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) SI No. 569 (W 125) Regulations 2019 32 (1).
1.4.3 Action if no or inadequate records
The AO must bring to the attention of plant management and the OV if no records of the farm submitting for slaughter are kept, or if the records are deficient.
The OV is to follow the hierarchy of enforcement, and:
- record any discussion with the FBO in the daybook
- confirm the deficiency in writing
- Note: a specimen letter (see annex 1) suitable for this purpose is included in this chapter
- keep a copy in the plant file
- enter details onto the ENF 11/5 (Enforcement Programme)
- make a further check of records within 28 days of delivery of the above letter
- if records are still inadequate, make a referral for investigation
Reference: See chapter 7 on ‘Enforcement’ for additional information.
Reference: See chapter 9 on ‘Forms.
1.4.4 Examples of inadequate records
Here are two examples of inadequate FBO records:
- name and address of producer / last owner not recorded
- FSA records indicate 20 animals were presented for ante-mortem inspection – FBO records only show 18 animals have been delivered
1.5 Cross contamination of samples
1.5.1 Purpose of National Surveillance Scheme (NSS)
The aim of the NSS is to detect whether authorised, unauthorised Veterinary Medicinal Products (VMPs), contaminants and prohibited substances are being used in food producing animals and that the conditions attached to authorised VMPs are being observed.
1.5.2 Follow-up action
The Directives and the Animals and Animal Products (Examination of Residues and Maximum Residue Limits) (England and Scotland) Regulations 2015 / (Wales) 2019 require follow-up action to be taken where:
- samples are found to contain residues of VMPs above the permitted maximum residue limit, or
- where residues of unauthorised substances have been detected
This could involve legal proceedings and consequently it is important that the instructions given in this chapter are followed.
1.5.3 On-farm investigation where a positive test result is recorded
When a sample tests non-compliant for a VMP, a Veterinary Officer (VO) from Animal and Plant Health Agency (APHA) visits the farm of origin of the sample to carry out an investigation as to how the residue in the sample may have occurred.
1.5.4 When FSA staff should not act as sampling officers
Laboratory analytical methods are extremely sensitive in identifying and measuring banned substances, down to less than 1 part per billion (the equivalent of a grain of sand in an area the size of an Olympic swimming pool). It is because of this sensitivity that sampling officers, who may have been exposed to certain medicinal products taken by them, by members of their family or by pets, should not take samples during the course of the treatment.
A list of those compounds that are, potentially, the most likely to cause problems is shown in the following table; some of these substances can also be prescribed to companion animals.
Sampling officers should not carry out any sampling during the treatment period.
| Type of Medication | Active Ingredients | May be used in the treatment of: |
|---|---|---|
| Inhaler (containing beta-agonists) |
|
Asthma |
| Skin creams (containing steroids) |
|
Skin conditions, such as dermatitis |
| Non-steroidal anti-inflammatory gels |
|
Pain relief, headaches, arthritis, fever |
| Other topical preparations |
|
Bacterial eye infections |
| Tablets |
|
Joint disease, auto-immune disease |
2. Sampling
In this section
2.3 Red meat: Collecting samples
2.4 Red meat: Collecting blood
2.5 Red meat: Collecting blood for serum analysis
2.6 Red meat: Packing blood samples for despatch
2.8 Poultry meat: Collecting blood for serum analysis
2.9 Poultry meat: Packing blood samples for despatch
2.1 Sampling programme
2.1.1 Sampling requests
Establishments will receive requests each quarter from VMD to collect samples from cattle, sheep, goats, pigs, horses, poultry and game for residue analysis. The residues in meat (RIM) 1 form is the Primary Sample Request form and contains pre-printed information on animals to be selected for sampling.
VMD will send RIM 1 forms to individual plants, unless a base plant has been designated by prior agreement.
Note: Samples must be collected exactly as described in the month specified on the RIM 1.
The animal(s) selected for sampling must fit the information on the RIM 1.
Reference: See annex 2 for a sample RIM 1 form.
2.1.2 When to collect
Samples required for a specified month must be collected during the month stated, spread as evenly as possible throughout the month and not collected on the same day.
Only one sample for a specified residue from animals from the same farm submitted for slaughter must be collected on the same day. More than one sample from the same producer can only be submitted in cases when the species required are not available from another producer.
2.1.3 RIM 1 reference number
Each RIM 1 form has a unique Sample Reference Number (RIM No), which must not be altered. The number must be quoted in any correspondence about the sample and recorded in the daybook on the day of sampling and, for cross-reference, in the copy of the associated FCI to be filed.
2.1.4 RIM labels
Each RIM 1 form is accompanied by an adhesive label printed with:
- the Sample Description
- Sample Reference Number (RIM No) and bar-code.
2.1.5 Samples from animals intended for human consumption
Samples must only be taken from animals, poultry or game intended for human consumption, and not from cull schemes.
2.1.6 Selection criteria
Animals must be selected taking into account the criteria that appear on the RIM 1 form and the criteria below:
- species, sex, age and farming system
- information about the producer
- indication of the use of pharmacologically active substances
- normal use of pharmacologically active substances in the particular production system
- other factors which may make it appropriate to ‘target’ certain animals for sampling; for example:
- animals selected for hormonal growth promoters should be well muscled and a good size for their age
- animals that are small for their age may be appropriate for sampling for antimicrobials since illness could affect their growth, and therefore they are more likely to have been treated
Instructions on the sampling of ‘suspect’ animals can be found in section 3 on ‘Suspect substances, animals and carcases’ in part 1.
2.1.7 Identification of animals
Animals suitable for sampling are to be individually identified and/or clearly marked before slaughter. FBO electronic ID systems are also suitable for the tracing of the movement of an animal through the process In the absence of an electronic system, the identification of the animal must be preserved at flaying or robustly correlated with the kill number by using one of the following methods:
- attach a talisman tag
- apply a cut mark
- attach a detained tag
- note the slap mark / tattoo
- using a record correlating kill number and animal identification number (for example, ear tag). Using a gap in the line may help in keeping the correlation through the processing line.
- Both kill number and animal ID should be recorded on the RIM 1 form
Note: In the case of poultry, it is sufficient to identify the batch from which the sample(s) are to be taken.
2.1.8 Exception to identification of animals pre-slaughter
Where an animal selected in the lairage from the specific group of sheep fails to produce the required quantity of urine, the sampling officer may select another animal which has a full bladder from the same group of animals.
The sampling officer must ensure that the animal can be traced back to the farm or market of origin.
2.1.9 Sample security and continuity of evidence
The results of analyses for all substances could lead to legal proceedings. It is important that there is continuity of evidence; therefore, samples must be accurately identified and secured in a FSA freezer.
Where there is no FSA freezer available in the establishment, alternative arrangements for the storage of samples will need to be made in agreement with the FSA local management team. FSA SLA and Contracts team should be notified of this agreement.
The names of all AOs involved in collecting or handling samples must be recorded in the daybook. The name and signature of the sampling officer must be the same as that on the RIM 1 form and the tamperproof bag.
2.1.10 Completing the summary worksheet
Record the following information on the Summary Worksheet:
- date of collection
- date of despatch
- consignment note number
2.1.11 Sampling not possible
Where a sample collection fails due to insufficient material or where sampling is not possible (for example, due to plant closure, killing pattern or availability of species requested), the OV is to:
- If a sample is temporarily unavailable, this can be taken in the next month provided it is in the same sample quarter (for example a July sample can be taken in September)
- complete the RIM 1 form remarks box, giving the reasons why the sample cannot be taken and
- return RIM 1 form to:
Veterinary Medicines Directorate
Residue Section
Woodham Lane
New Haw
Surrey
KT15 3LS
Send an email to the SLA and Contracts Team (sla.contracts@food.gov.uk), explaining why the sample was not collected.
Note: Due to health and safety considerations, poultry serum samples are not to be taken from unstunned birds in Halal establishments. The remarks box of the RIM 1 should be completed accordingly in the event that any such sampling requests are received by a plant.
2.2 Sampling equipment
2.2.1 Use of containers
It is important that only the specified sampling containers are used, as failure to do so may result in the sample being rejected by the laboratory as unassailable.
2.2.2 Supplies
VMD will supply:
- RIM 1 forms
- adhesive labels
- summary worksheets
- sampling equipment
- tamperproof bags
Maintain sufficient supplies of polystyrene boxes, outer cartons and Freezella packs at the slaughterhouse.
Note: The laboratory will return RIM boxes after use to Topspeed for one to one exchange on the next collection.
If a replacement box is not left at the point of collection, please email rim@topspeedcouriers.co.uk immediately.
2.2.3 Sampling equipment orders
Sampling equipment can be re-ordered by emailing:
2.3 Red meat: Collecting samples
2.3.1 Samples to collect
Kidney, kidney fat, liver, muscle, blood and urine must be collected from the identified, marked carcases that have been inspected and passed as fit for human consumption. The quantity of material collected from each species must be that specified as in the table below.
Samples to collect
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Kidney | Sheep / goats | At the post-mortem inspection point | A pair of kidneys | Sealable plastic bag |
| Kidney | Pigs | At the post-mortem inspection point | One whole kidney | Sealable plastic bag |
| Kidney | Cattle / horses | At the post-mortem inspection point | A portion of kidney; at least 100g taken from one pole so as to exclude pelvic tissue | Sealable plastic bag |
| Kidney | Calves | At the post-mortem inspection point | A pair of kidneys | Sealable plastic bag |
| Kidney fat | Cattle / sheep / goats / pigs | At the post-mortem inspection point | At least 50g of kidney fat | Sealable plastic bag |
| Liver | Cattle / sheep / goats / pigs / horses | At the post-mortem inspection point | At least 100g of liver | Sealable plastic bag |
| Muscle | Cattle / sheep / goats / pigs / horses | At the carcase inspection point | At least 200g of muscle from the diaphragm region of the animal | Sealable plastic bag |
| Urine | Cattle / sheep / pigs | After removal from carcase by incision into the bladder | At least 50ml | 80ml pot then sealable plastic bag |
2.3.2 Special surveys
For specific surveys, sample requests may require muscle and kidney samples to be taken from the same animal. Details will be printed on the RIM 1 form which will be marked ‘Special Survey’.
2.3.3 After sampling
Immediately after collection, the container or bag must be correctly sealed to avoid leakage, and placed into a tamperproof bag with the absorbent pad.
Reference: See the topic ‘Tamperproof bags’ in this section for additional information.
2.4 Red meat: Collecting blood
2.4.1 When and where to collect
Collect blood for serum samples and plasma analysis from the identified, marked carcase. This should be done directly at the sticking point, into the plastic vending cup provided and after the initial flow of blood has slowed.
2.4.2 Alternative collection site
Where collection at the sticking point poses a potential risk to the AO, for example, from carcase kicking, blood should be taken from the heart on the pluck line into the plastic vending cup provided.
A small incision can be made into one of the four chambers of the heart and blood carefully poured into the cup.
2.4.3 Sample handling
These samples must be:
- packaged according to the instructions in this topic
- despatched separately from other samples
- despatched on the same day of collection for bovine animals not requiring a bovine spongiform encephalopathy (BSE) test
- despatched on the day following collection for bovine animals requiring a BSE test, after receipt of a negative test result
Reference: See topic 2.14 on ‘Packaging and despatch of samples’ in part 1 for additional information.
Note: This will require that the courier is booked prior to taking the sample.
Caution:
- Samples can be refrigerated or kept in a cool dark place until collected by Topspeed.
- Samples must not be frozen.
- Please ensure that you place 2 unfrozen Freezella packs in the box. The polystyrene casing may be chilled before use.
- Keep box out of direct sunlight.
- Despatch Monday to Thursday only.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Blood (serum) | Cattle / horses |
At the sticking point or pluck point (heart) Reference: See topic 2.5 on ‘Collecting blood for serum analysis’ in part 1 for additional information |
At least 30ml | 3 x Sarstedt blood tubes then into absorbent wallet, keeping tubes upright |
| Blood (plasma) | Cattle / horses |
at the sticking point or pluck point (heart) Reference: See topic 2.5 on ‘Collecting blood for plasma analysis’ in part 1 for additional information |
At least 75ml | 2 x Li-heparin LH / 25ml monovette then absorbent wallet, keeping tubes upright |
2.5 Red meat: Collecting blood for serum and plasma analysis
2.5.1 Serum analysis
You must follow the correct procedure for collection of blood for serum analysis as described in the table below:
| Step | Action |
|---|---|
| 1 | Collect at least 50ml of blood into the plastic vending cup provided for immediate transfer into 3 x 10ml serum tubes. |
| 2 | Remove the screw cap on the top of the serum tube ensuring that the beads are in the bottom of the tube. |
| 3 |
Pour the blood into the tubes, filling to the line below the threaded top. Caution: Do not overfill or some beads may float to the top and be lost. The beads are coated in a substance that acts as a clotting activator to ensure that the blood clots and the serum becomes separated. |
| 4 | Replace the screw cap on each tube. |
| 5 |
Invert each tube gently 4-5 times to ensure the blood is mixed with the beads. Note: THE TUBE SHOULD NOT BE VIOLENTLY SHAKEN; doing so may cause haemolysis and the sample would therefore be deemed unassailable by the laboratory. |
| 6 | Write the RIM numbers on each tube in the space marked ‘Ref No’. Keep the test tubes stored upright in the four bay absorbent wallet and in a cool place (preferably in a refrigerator) prior to despatch. Each wallet can accommodate one sample of three tubes. |
| 7 | When ready for despatch, place the wallet inside the tamperproof bag. |
| 8 |
Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. Place the tamperproof bag securely inside the polystyrene box. Note: Do not tape the tamperproof bag to the inside of the polystyrene box. |
2.5.2 Plasma analysis
You must follow the correct procedure for collection of blood for plasma analysis as described in the table below:
| Step | Action |
|---|---|
| 1 | The blood should be immediately transferred from the plastic vending cup into 2 x 25ml heparin syringes. |
| 2 | Remove the small orange cap on the top of the syringe and pull the plunger fully back ensuring that the heparin coated beads fall to the bottom. |
| 3 |
Unscrew the lid and pour the blood into the syringe, filling to the line below the threaded top. Caution: Do not overfill or some beads may float to the top and be lost. The beads are coated with the anti-coagulant heparin and are essential to ensure the blood does not clot. |
| 4 | Replace the screw cap and the small orange cap. Break off the plunger at the base of the tube. |
| 5 |
Invert the syringe gently 4-5 times to ensure the blood is mixed with the anti-coagulant. Note: THE TUBE SHOULD NOT BE VIOLENTLY SHAKEN; doing so may cause haemolysis and the sample would therefore be deemed unassailable by the laboratory. |
| 6 | Carefully place the syringes into the tamperproof bag. |
| 7 | Label the tamperproof bag and follow instructions on the bag to seal. |
| 8 |
Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. Place the tamperproof bag securely inside the polystyrene box. Note: Do not tape the tamperproof bag to the inside of the polystyrene box. |
2.6 Red meat: Packing blood samples for despatch
2.6.1 Packing serum and plasma samples for despatch
Samples are to be packed for despatch as follows:
| Step | Action |
|---|---|
| 1 | Place the tamperproof bag containing the samples securely into the polystyrene box. |
| 2 | Seal the polystyrene box. |
| 3 | Place the top two copies of RIM 1 form on top of the polystyrene lid. |
| 4 | Place polystyrene box in cardboard outer carton. |
| 5 | Apply the adhesive address label provided by the carrier to the outer carton across the box flaps. Ensure all other labels on the carton are removed. |
| 6 | Mark the box with ‘This Way Up’ to ensure careful handling. |
Caution: RIM 1 forms must not be sent separately from the samples to which they relate.
2.7 Poultry meat samples
2.7.1 Samples to collect
Samples of liver and muscle must be taken from identified birds that have been inspected and passed as fit for human consumption. The following table gives details of the types of samples and the quantity required.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Liver | Chickens and ducks | Evisceration inspection point | 50g pooled from at least 6 birds | Sealable plastic bag |
| Liver | Turkeys | Evisceration inspection point | 50g pooled from at least 2 birds | Sealable plastic bag |
| Breast muscle | Chickens, ducks and geese | Taken off line to enable muscle to be cut off | 200g from 1 bird | Sealable plastic bag |
| Breast muscle | Turkeys | Taken off line to enable muscle to be cut off | 200g from 1 bird. Sample can be taken from more than one bird in a co-located cutting plant where there is sufficient traceability | Sealable plastic bag |
2.7.2 Special surveys
For specific surveys, sample requests may require muscle and liver samples to be taken from the same group of birds. Details will be printed on the RIM 1 form which will be marked ‘Special Survey’. Tissues should be pooled from the required number of birds as indicated in the table below:
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Liver | Chickens (Broilers) | Evisceration inspection point | 50g liver pooled from at least 6 birds | Sealable plastic bag |
| Muscle | Chickens (Broilers) | Taken off line to enable muscle to be cut off | 100g muscle pooled from at least 6 birds | Sealable plastic bag |
2.7.3 After sampling
Tissue samples must be placed immediately into the sealable plastic bag provided, then into a tamperproof bag.
2.8 Poultry meat: Collecting blood for serum analysis
2.8.1 When and where to collect
Collect blood for serum analysis from at least six birds from the same flock. Only sample birds from single sheds, do not sample birds from mixed sheds. This should be done shortly after neck cutting, into the plastic vending cup provided.
2.8.2 Sample handling
These samples must be:
- packaged according to the instructions in this topic
- despatched separately from other samples
- despatched on the same day of collection
Reference: See topic 2.14 on ‘Packaging and despatch of samples’ in part 1 for additional information.
Note: This will require that the courier is booked prior to taking the sample.
Caution:
- Samples should be refrigerated but must not be frozen.
- Please ensure that you place 2 unfrozen Freezella packs in the box.
- The polystyrene box may be chilled before use.
- Keep box out of direct sunlight.
- Despatch Monday to Thursday only.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Blood (serum) | Chickens, ducks and turkeys |
Shortly after cutting point Reference: see topic 2.8 on ‘Collecting blood for serum analysis’ in part 1 for additional information |
30ml from at least 6 birds | 3 x Sarstedt blood tubes |
2.8.3 Serum analysis
Follow the procedure for the collection of blood for serum analysis as described in the table below.
Blood can be safely collected once birds have ceased swinging after cutting.
| Step | Action |
|---|---|
| 1 |
Using the plastic vending cup provided, collect at least 30ml of blood from at least six birds in the same group. Note: Blood can coagulate quickly so collect enough for one tube at a time. |
| 2 |
Remove the screw caps from the tops of the three Sarstedt serum tubes ensuring that the beads are in the bottom of the tube. Note: These beads are coated in a substance that acts as a clotting activator to ensure the blood clots and the serum becomes separated. |
| 3 |
Pour the blood into each tube, filling to the line below the threaded top. Caution: Do not overfill or some beads may float to the top and be lost. |
| 4 | Replace the screw cap on each tube. |
| 5 |
Invert each tube gently 4-5 times to ensure the blood is mixed with the beads. Note: THE TUBE SHOULD NOT BE VIOLENTLY SHAKEN; doing so may cause haemolysis making the sample unassailable. |
| 6 | Write the RIM number on each tube in the space marked ‘Ref No’. Keep the tubes stored upright in the four-bay absorbent wallet and in a cool place (a dark place or refrigerator) prior to despatch. Each wallet should contain one sample only. One sample = 3 tubes from 6 birds from the same batch. |
| 7 | When ready for despatch, place the wallet inside the tamperproof bag. |
| 8 | Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. Place the tamperproof bag securely inside the polystyrene box. |
2.9 Poultry meat: Packing blood samples for despatch
2.9.1 Packing serum samples for despatch
Samples are to be packed for despatch following the steps detailed below:
| Step | Action |
|---|---|
| 1 |
Place the tamperproof bag securely inside of the polystyrene box ensuring the tubes are not free to move around. Note: Fold the tamperproof bag over so that the signatures and barcode label are folded in on themselves. |
| 2 | Seal the polystyrene box. |
| 3 |
Place the two copies of the RIM 1 form on top of the polystyrene lid. Caution: RIM 1 forms must not be sent separately from the samples to which they relate. |
| 4 | Place polystyrene box in cardboard outer carton. |
| 5 | Apply the adhesive address label provided by the carrier to the outer carton across the box flaps. Ensure all other labels on the carton are removed. |
| 6 | Mark the box with ‘This Way Up’ to ensure careful handling. |
2.10 Game samples
2.10.1 Definition of farmed game
Farmed game is animals which are not domestic but have been reared within a restricted area.
2.10.2 Farmed game samples
Samples will be requested from deer, partridge, pheasant, red grouse, quail and breeding boar.
2.10.3 Definition of wild game
Wild game is animals that are hunted and shot in the wild for human consumption.
2.10.4 Wild game samples
Samples will be requested from deer, pheasant and partridge.
2.10.5 Large game samples to collect
Samples of kidney, kidney fat, liver and muscle must be taken from deer carcases which have been passed fit for human consumption and for which the origin or source can be identified.
2.10.6 Small game samples to collect
Samples of muscle consist of an entire oven ready carcase of a bird and must be taken from a batch of birds which have been passed as fit for human consumption and for which the origin or source can be identified.
| Sample | Species | Where to collect | Amount | Container |
|---|---|---|---|---|
| Kidney | Deer | After post-mortem inspection | A whole kidney | Sealable plastic bag |
| Kidney Fat | Deer | After post-mortem inspection | At least 50g of kidney fat | Sealable plastic bag |
| Liver | Deer, partridge, pheasant, red grouse | After post-mortem inspection | At least 100g liver | Sealable plastic bag |
| Muscle | Deer | After post-mortem inspection | At least 50g of diaphragm muscle | Sealable plastic bag |
| Muscle | Partridge, pheasant, quail | Inspection point at the end of the line | An oven ready carcase | Sealable plastic bag |
2.10.7 After sampling
Tissue samples must be placed immediately into the sealable plastic bag provided, and then into a completed tamperproof bag.
2.11 Completing the RIM form
2.11.1 Details to record
The following details must be fully recorded on the RIM 1 form:
- sex and age of animal sampled
- identification of the animal sampled; this enables the AO to cross check with the slaughter records to establish the source of the animal – types of identification:
- ear tag number for cattle, sheep and goats
- slap mark, ear tag or tattoo for pigs
- farm address for poultry
- for cattle the breed of animal sampled (including cross breeds)
- whether the animal is from organic production
- obtain from the slaughterhouse or game handling
- establishment records:
- the farm submitting for slaughter, or if unavailable, the source of animals sampled such as market and lot number, and
- the name and status of the person supplying the animal to the slaughterhouse
- any extra information, for example, kill numbers, which may help in any subsequent tracing
- the date of collection of the sample
- the date of despatch of the sample
- name and designation of collecting officer; this must be the same as on the tamperproof bag
- carrier consignment reference number
Note: If you make an error when recording any of the above data on the RIM 1 form, or anything is unclear that might need going over again, cross through the entry and enter the correct details, then initial the change. Any necessary amendments must be made before the copies of the RIM 1 form are separated. Do not use correction fluid. The original ‘incorrect’ entry must be legible.
2.12 Tamperproof bags
2.12.1 Use of tamperproof bags
Tamperproof bags are an important stage in maintaining continuity of evidence, since the detection of residues in a sample may result in an investigation and potential legal proceedings.
2.12.2 Sealing
Tamperproof bags should be sealed:
- remove the blue strip
- press the orange strip down over the glue firmly
- check the bag is sealed properly before labelling
- check the bag has been signed by the sampling officer and witnessed by the FBO representative
Note: The sampling officer must be the same person that signed the RIM 1 form.
Wherever possible this should be done in the presence of the FBO or person responsible for the source of the sample.
2.12.3 How to label tamperproof bags
Labelling must be carried out immediately after each sample is taken. As far as reasonably possible, completion of labelling should be done in the continued presence of the FBO or person responsible for the source of the sample.
| Step | Action |
|---|---|
| 1 |
Attach the white bar-coded sample label to the front of the bag in the marked space before putting the sample in the tamperproof bag. Caution: Ensure that the bar code label is not creased or otherwise damaged whilst sticking it to the bag. |
| 2 |
Sign and date in the space provided (must be the same person that signed the RIM 1 form). Note: Use only ballpoint to write on the bag. |
| 3 |
The owner or person responsible should also sign and date the tamperproof bag, confirming that the information recorded on it is correct. Note: Refusal to sign should be noted on the front of the bag. |
| 4 | Place the sample in the tamperproof bag and seal by removing the blue strip. |
| 5 | Once sealed, the bag must not be opened until the sample has reached the laboratory. |
| 6 | Record the names of all authorised staff involved in collecting samples in the daybook. |
2.13 Storage of samples
2.13.1 Chilling and freezing
Once the sample has been sealed in the tamperproof bag and the bag has been labelled, samples must be kept chilled from the time of collection and during preparation. With the exception of blood collected for serum and plasma analysis, samples should then be hard frozen on the day of collection.
Note: If necessary, samples must be kept cool by means of insulated containers containing frozen Freezella packs / Biotherm dry ice shippers.
Samples must be frozen for a minimum of 48 hours. Maintain the samples hard frozen until despatch.
Note: The freezer compartment of a domestic refrigerator is not adequate for hard freezing samples.
2.13.2 Freezing of samples prior to despatch
When freezing samples:
- in large chest freezers:
- place samples in the polystyrene box
- leave the lid off the polystyrene box and freeze the whole box containing samples
OR
- in small freezers:
- leave samples in freezer until ready for despatch, then place in polystyrene box
To avoid samples defrosting prior to testing do not over fill the box, and send two boxes if necessary.
2.13.3 Storage
With exception of serum and plasma, all prepared samples must be stored prior to despatch:
- in secure, dedicated FSA freezers
- at a temperature between -15°C and -20°C
Samples must not be allowed to thaw once frozen.
2.14 Packing despatch of samples
2.14.1 Packing samples for despatch
Note: These instructions apply to all surveillance samples except serum and plasma.
Reference: See topics 2.5 and 2.8 on ‘Collecting blood for serum analysis’ and 2.6 and 2.9 on ‘Collecting blood for plasma analysis’ in part 1 for additional information.
Samples are to be packed for despatch as follows:
| Step | Action |
|---|---|
| 1 | Place a frozen Freezella pack / Biotherm dry ice shipper at the base of the polystyrene box. |
| 2 | Place the frozen samples in the box. |
| 3 | Place the second Freezella pack / Biotherm dry ice shippers on top of samples. |
| 4 | Seal the polystyrene box. |
| 5 | Place the top two copies of RIM 1 form on top of the polystyrene lid. |
| 6 | Place polystyrene box in cardboard outer carton. |
Note: To prevent movement, small samples should be wrapped with the Freezella pack / Biotherm dry ice shipper in insulating material before being placed into a polystyrene box.
In periods of hot weather, add an extra Freezella pack / Biotherm dry ice shipper to avoid thawing.
2.14.2 Labelling cardboard outer cartons
Boxes are to be labelled as follows:
| Step | Action |
|---|---|
| 1 | Apply the adhesive address label provided by the carrier to the outer carton across the box flaps. |
| 2 | Mark the box with ‘this way up’ to ensure careful handling. |
2.14.3 Despatching samples
Samples are to be despatched to the laboratory after a minimum of 48 hours hard freezing. Despatch must be no more than five working days after collection, including the day of collection, as this can lead to sample deterioration, and delay on-farm investigation of positives that may result. Where an FBO only operates one day a week, this can be extended to six working days.
The despatch process is detailed in Annex 17.
Note: Serum and plasma samples from bovine animals not requiring a BSE test must be despatched on the same day as collection.
Note: Serum and plasma samples from bovine animals requiring a BSE test must be despatched on the day following receipt of a negative test result.
Note: Samples must not be sent on Fridays or on days preceding public holidays.
2.14.4 Despatch of all residue samples
FSA officers at slaughterhouses must send all red meat, poultry meat, game meat and suspect samples to:
Residues Statutory Programme
Fera Science Ltd
Room 50G30
Sand Hutton
York
YO41 1LZ
2.14.5 Despatch failure
Should despatch fail, you must make an attempt to rearrange despatch:
- ensure the samples have not thawed
- follow points 1 to 3 in sub-topic 2.14.3 on ‘Despatching samples’ in part 1, explaining the reasons behind the failure
Then telephone VMD
(01932 338329) to explain the failure and what follow up action has been taken.
VMD will contact the laboratory to advise them to expect samples arriving with incorrectly dated paperwork.
2.14.6 Retention of documents
After completion of each month’s sampling, the completed Summary Worksheet and RIM 1 form should be retained in plant for 1 year.
2.14.7 Complaints procedure
Should Topspeed fail to collect samples within the agreed timeframe, contact the SLA and Contracts Team by phone (access contact details in chapter 1 on ‘Introduction’), who will escalate the failure to Topspeed headquarters.
2.15 Actions on positive samples
In case of positive results for routine RIM samples, the action to be taken by the OV will depend on the Risk Management Advice provided by the Incidents team and shared subsequently with Field Operations. Before a decision is taken regarding the affected carcase/product, the OV should liaise with the relevant Field Veterinary Coordinator (FVC)/Field Veterinary Leader (FVL).
3. Suspect Substances, Animals and Carcases
3.4 Sampling and despatch procedures for suspect live animals and suspect carcases
3.1 Suspicion of unauthorised substances: Suspected use of authorised veterinary medicines above the MRL and contaminants
3.1.1 Sampling of suspect animals
The legislation requires sampling to be undertaken where the OV suspects or has evidence that animals have been treated with unauthorised substances or may contain residues of authorised substances above the MRL.
3.1.2 Procedures
This topic covers the action to be taken when there are grounds to suspect that a carcase or live animal contains:
- prohibited substances
- unauthorised substances
- residues of an authorised substance at concentrations above the MRL
- a contaminant above the threshold level (see annexes for signs that would give rise to suspicion)
- contaminants in compounds of concern
| Term used | Meaning |
|---|---|
| Prohibited substance | Means any beta-agonist, hormonal or thyrostatic substance, and those specified in Table 2 GB MRL www.gov.uk/guidance/maximum-residues-limits-mrl. |
| Unauthorised substance | Means any substance not included in Table 1 GB MRL. |
| Authorised substance | Means a substance specified in Table 1 GB MRL. |
The following table contains a list of those substances contained in Table 2 of Commission Regulation (EU) 37/2010.
| Annex IV Substances |
|---|
| Aristolochia ssp and preparations thereof |
| Chloramphenicol |
| Chloroform |
| Chlorpromazine |
| Colchicine |
| Dapsone |
| Dimetridazole |
| Metronidazole |
| Nitrofurans (including Furazolidone) |
| Ronidazole |
3.2 Suspect live animals
3.2.1 Inspection of animals under Regulation 20
Under the Residues Regulations, AOs have the power to detain an animal or group of animals for inspection to ascertain whether they have been treated with an unauthorised substance.
Reference: The Animals and Animal Products (Examinations for Residues and Maximum Limits) (England and Scotland) Regulations 2015 SI No. 787 Regulation 20 and The Animals and Animal Products (Examination for Residues and Maximum Residue Limits) (Wales) Regulations 2019 SI No. 569.
3.2.2 Suspicion of illegal substances
If the AO suspects that an animal has been illegally treated with an unauthorised substance you must notify the OV immediately of your suspicions.
The OV should serve a Form E notice if the FBO or slaughterhouse staff do not co-operate in allowing the inspection to take place.
Reference: See annex 9 for a sample Form E notice.
3.2.3 Signs of hormone growth promoters: live animal
The following signs in a live animal may indicate the illegal use of hormone growth promoters:
- secondary sexual characteristics:
- crest development
- teat development
- restlessness; animals do not settle in the lairage, mill around
- behavioural changes:
- mounting
- aggression
- an even level of finish in a group of cattle of different breed / types
3.2.4 Signs of beta-agonist: live animal
The following signs in a live animal may indicate the illegal use of beta–agonist growth promoters:
- good conformation with little fat
- hyperaesthesia and tachycardia may be present
3.2.5 Result of inspection
If after carrying out the inspection, the OV is satisfied that the animal has not been treated with an unauthorised substance, you should lift the Form E notice by serving a Form F notice on the owner.
Reference: See annex 10 for a sample Form F notice.
3.2.6 Examination of animals under Regulation 21
If as a result of the inspection referred to above, the OV still suspects that the animal or group of animals may contain an unauthorised substance, a Form G notice should be served on the owner of the animal(s) to detain them for further examination. This notice will remain in place until the results of the examination, including analysis of samples, are known.
Reference: See annex 11 for a sample Form G notice.
The OV should make a detailed examination of the animals. This must include checking for evidence of implants and other signs which could indicate the use of unauthorised substances.
3.2.7 Samples to take
Where an implant is not found but the OV is suspicious of the illegal use of other prohibited substances, you should take the following samples:
- hormones - take blood and either urine or faeces
- beta-agonists - take urine
If other unauthorised substances are suspected then advice should be sought from the VMD or veterinary auditor (VA) (Residues) on the appropriate samples to be collected.
3.2.8 Slaughter of detained animals
Animals must not be held in the lairage for more than 48 hours. As it is unlikely that the results of analysis on the sample will be available, the animal should be slaughtered and the carcase and offals detained under Regulation 34(2).
Reference: See topic 3.3 on ‘Suspect carcases’ in part 1 for additional information.
3.2.9 Signs of a suspect substance in live animals
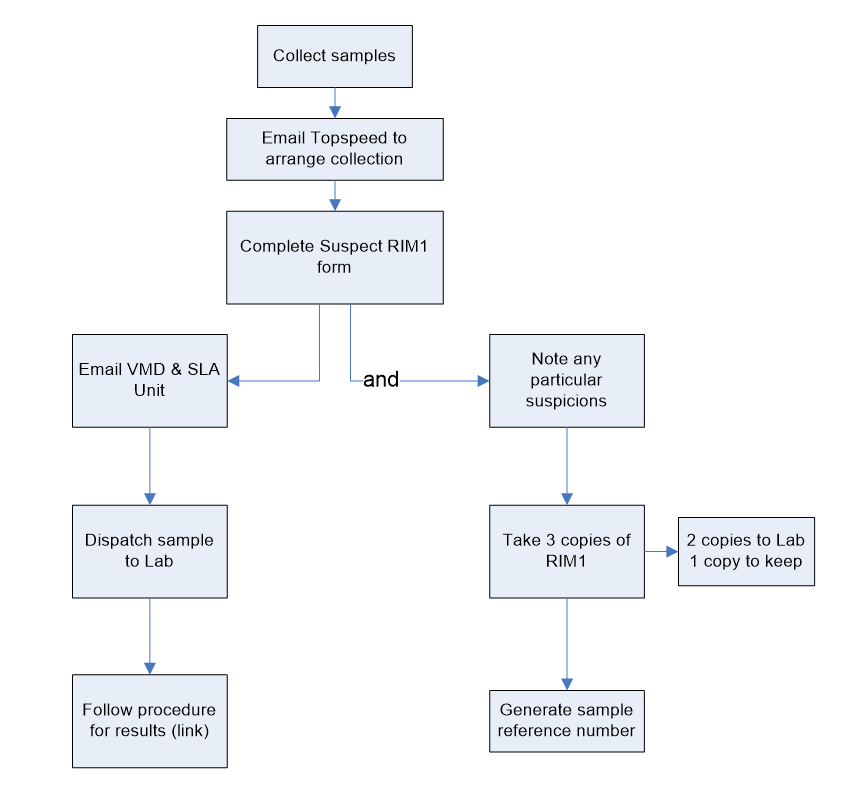
After collection of samples send an e-mail to Topspeed to arrange Collection and complete Suspect RIM1 Form. Upon completion:
Action 1: Notify by email to VMD and SLA Unit, then dispatch sample to the lab, and follow procedure for results.
Action 2: Note any particular suspicions observed in the RIM forms. Send 2 Copies of the completed RIM forms to the lab, keeping the third one on site. Finally, generate a sample reference number.
3.3 Suspect carcases
3.3.1 Detention under Regulation 34(2)
The OV has the power under Regulation 20(2)a and 21(2)b of the Residues Regulations to detain and sample any carcase if they suspect the illegal use of unauthorised substances, or if they suspect that an authorised substance in excess of the MRL may be present in the animal concerned.
The OV must serve Form C on the owner or person in charge of carcase(s). This will remain in force until investigations are completed.
Reference: See annex 7 for a sample Form C notice.
3.3.2 Signs of authorised substances above the MRL
The following signs may raise concerns that a carcase contains authorised substances, such as veterinary medicines, above the MRL:
- signs of recent illness, particularly:
- mastitis (signs may be seen prior to removal of udder)
- lameness / arthritis
- pleurisy / pneumonia
- poor condition
- metritis (signs may be seen prior to evisceration or during inspection of the offal)
- emergency slaughter animals
- injection sites, particularly:
- bruising / discoloration
- smell (especially with tetracyclines)
- swellings
Note: For sites with an oily adjuvant, consider illegal hormone treatment.
The conditions listed above should prompt evaluation regarding whether animals have received recent treatment and whether the withdrawal period has been properly observed by the farmer before sending them for slaughter. See summary table in section 3.4.5.
OVs working at establishments that handle Emergency Slaughter (ES) on a routine basis should prioritize sampling those animals using the table ”Summary of conditions to be considered for suspect sampling” in section 3.4.5. OVs should aim to test two carcasses from ES per year for the presence of non-steroidal anti-inflammatory drugs (NSAIDs) as a priority and/or antimicrobials where relevant. Additional residue samples can be taken for non-ES if deemed appropriate.
3.3.3 Signs of hormone abuse: carcases
The following signs may indicate the illegal use of hormones in a carcase:
- presence of implants or pellets
- injection site
- if detected and an oily adjuvant is present, or when the site is in an unusual place, the possibility of the presence of injectable hormones should be considered
3.3.4 Signs of beta-agonists: carcases
The following signs may indicate the illegal use of beta-agonists in a carcase:
- good conformation with little fat
- flaccidity of the trachea
3.3.5 Evidence of implants
If there is evidence of an implant in the ear, you must detain the carcase and submit the whole ear containing the implant for analysis.
If the implant is discovered in any other part of the carcase, then the surrounding tissue should be excised with the implant and submitted for analysis. Do not attempt to dissect the implant out before despatch.
3.3.6 Types of implant
The table below lists the types of hormonal growth promoter implants which may be found:
| Name of Product | Type of Implant | Active Ingredients | Withdrawal period | Sex of animal used in |
|---|---|---|---|---|
| Compudose 200 | Cylinder |
17 beta-oestradiol 24mg |
0 | Steers |
| Compudose 365 | Cylinder |
17 beta-oestradiol 45mg |
0 | Steers |
| Finaplix | 15 yellow pellets | Trenbolone 140mg | 60 days | All |
| Forplix* | No description available |
Trenbolone 140mg Zeranol 36mg |
Never licensed in the UK | * |
| Implixa BF | 10 white pellets |
Testosterone 200mg oestradiol 20mg |
90 days | Females |
| Implixa BM | 10 white pellets |
Progesterone 200mg oestradiol 20mg |
90 days | Males |
| Ralgro | 3 white pellets | Zeranol 36mg | 70 days | All |
| Revlor | 8 yellow pellets |
Trenbolone 140mg oestradiol 20mg |
60 days | Steers, male and female veal calves |
| Synovex C | 4 yellow pellets |
Progesterone 100mg oestradiol benzoate 10mg |
0 | Males |
| Synovex H | 8 white pellets |
Testosterone 200mg oestradiol 20mg |
0 | Females |
| Synovex S | 8 yellow pellets |
Progesterone 200mg oestradiol 20mg |
0 | Males |
3.4 Sampling and despatch procedures for suspect live animals and suspect carcases
3.4.1 Sampling equipment
If an animal needs to be tested as a suspect, use the Suspect sampling kit provided by VMD.In the case that more than on sample is required, use the sampling kit provided for routine requests and replenish by emailing the SLA and Contracts Team (sla.contracts@food.gov.uk).
A consolidated order will be sent to VMD each Friday and the kit will be despatched to the specified plant.
3.4.2 Suspect RIM1 form
Complete the RIM 1 form marked ‘SUSPECT’ (see example at annex 15). A copy of the ‘Suspect’ RIM 1 form can be obtained by contacting the SLA and Contracts Team or a sample form can be found at annex 15.
If a particular hormone or unauthorised substance is suspected note it on the form. Take three copies of the completed form:
- two for despatch to the laboratory
- one to be retained in the plant file for 12 months from the date of sampling
3.4.3 Sample reference number to use
The OV should generate their own sample reference number using the following:
- slaughterhouse approval number
- the last two digits of the year
- a sequential number (approval / year / number)
One sample number per sample sent must be generated.
Note: Record the numbers used in the daybook.
3.4.4 Reporting suspicious cases
When animals or carcases are detained and sampled under the Residues Regulations, an on-farm investigation may be required. As a result, the OV must inform:
- the VMD via email using the following address: residues@vmd.gov.uk
- the SLA and Contracts Team via email (access contact details in chapter 1 on ‘Introduction’)
| Step | Action |
|---|---|
| 1 |
Detain animal / carcase for examination. Note: All samples MUST be collected, prepared and despatched in accordance with the procedures covered previously in this chapter. |
| 2 | Collect samples as detailed in section 2 on ‘Sampling’ of part 1 with the exception of hard freezing. |
| 3 | Arrange collection by Topspeed using the process at Annex 18. |
| 4 | Complete SUSPECT RIM 1 documentation. |
| 5 | Email VMD and the SLA and Contracts Team. They will alert the laboratory and ensure that the sample is analysed as soon as possible after arrival. |
| 6 | Despatch sample to the laboratory – Residues Statutory Programme, Fera Science Ltd. |
Process:
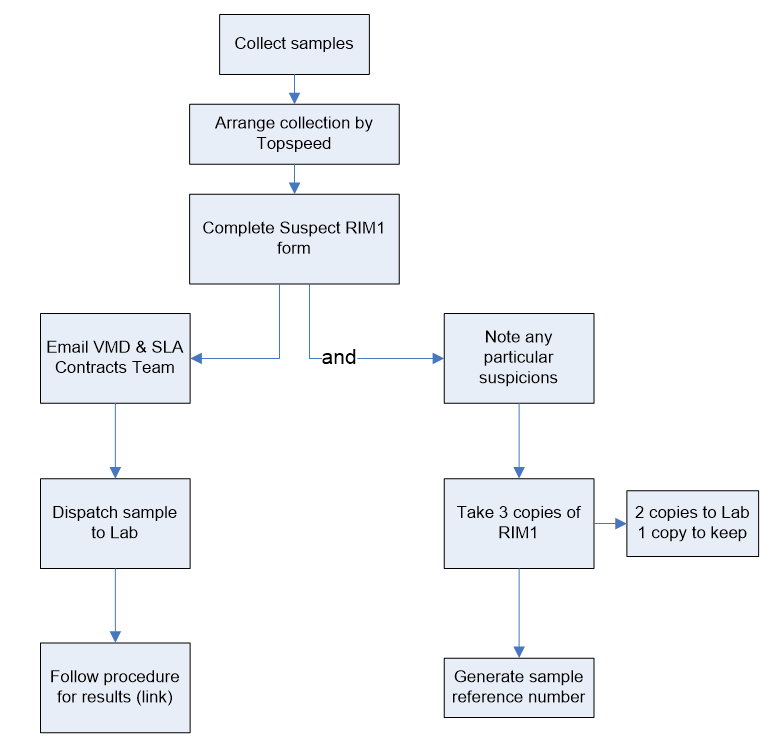
After collection of samples send an e-mail to Topspeed to arrange Collection and complete Suspect RIM1 Form. Upon completion:
Action 1: Notify by email to VMD and SLA Unit , then dispatch sample to the lab and follow procedure for results.
Action 2: Note any particular suspicions observed in the RIM forms. Send 2 Copies of the completed RIM forms to the lab, keeping the third one on site. Finally, generate a sample reference number.
3.4.5 Samples required
A list of the types of analyses and the samples required is given in the following table. For advice on the type of sample to collect for authorised substances not listed, you should contact the VA (Residues).
Example: For antimicrobial or sulphonamide analysis, a kidney sample should be collected.
Samples from carcases
| Analyses | Species | Sample Type |
|---|---|---|
| Antimicrobials | Cattle, sheep, pigs, horses, deer | Kidney |
| Antimicrobials | Poultry | Muscle |
| Table 2** | Cattle, sheep, pigs, deer, horses | Kidney |
| Table 2** | Pheasant, partridge, poultry, deer | Muscle |
| Sulphonamides | Cattle, sheep, pigs, horses | Kidney |
| Sulphonamides | Poultry | Muscle |
| Quinolones / fluoroquinolones | Poultry | Muscle |
| Tetracyclines | Poultry | Muscle |
| Thiamphenicol | Poultry | Muscle |
| Altrenogest | Pigs | Kidney fat |
| Metals | Cattle, sheep, pigs, horses | Kidney |
| Metals | Poultry | Liver |
| Metals | Pheasant, partridge, deer | Muscle |
| Anti-endoparasitic substances | Cattle, sheep, pigs, poultry, deer | Liver |
| Nicarbazin, lasalocid and ionophores | Poultry, deer, Cattle, sheep | Liver |
| Sedatives / beta-blockers | Cattle, sheep, pigs, horses | Liver |
| NSAIDS | Cattle, sheep, pigs, horses | Kidney |
| NSAIDS | Poultry | Liver |
| Paracetamol | Poultry | Liver |
| Pyrethroids | Cattle, sheep, pigs, poultry, deer | Liver |
| Carbamates | Poultry, deer | Liver |
| Beta-agonists | Cattle, sheep, pigs, poultry, deer | Liver |
| Synthetic hormones | Cattle, sheep, pigs | Urine |
| Synthetic hormones | Poultry | Liver |
| Thyrostats | Cattle, sheep, pigs | Urine |
| Thyrostats | Poultry | Liver |
| OCs, PCBs and OPs | Cattle, sheep, pigs | Kidney fat |
| OCs, PCBs and OPs | Poultry, deer | Liver |
| Dexamethazone / methazone | Pigs | Liver |
| Carbadox | Pigs | Kidney |
| Gestagens | Cattle, sheep, pigs | Kidney fat |
| Natural hormones | Cattle | Serum |
| Natural hormones | Poultry | Liver |
| Methyl-testosterone | Pigs, sheep | Urine |
| Nortestosterone | Cattle, sheep, pigs | Urine |
| Synthetic hormones | Cattle, sheep, pigs | Urine |
| Zeranol | Cattle, sheep, pigs | Urine |
| Nortestosterone | Cattle, sheep, pigs | Urine |
| Natural hormones | Cattle, sheep, pigs | Serum |
| Thyrostats | Cattle, sheep, pigs | Urine |
| Beta-agonists | Cattle, sheep, pigs | Urine |
| Gestagenic substances | Cattle, sheep, pigs | Urine |
Note for antimicrobial samples: if a suspect sample is to be tested for antibiotics, the OV is required to select a “panel” for testing at the time of submission. These can be broadly categorised into four ”AMS panels”: AMS1 (penicillins, sulphonamides and tetracyclines), AMS2 (quinolones), AMS4 (aminoglycosides) and AMS5 (macrolides).
Summary of conditions to be considered for suspect sampling.
| - | Condition | Substance to test |
|---|---|---|
| AMI |
Secondary sexual characteristics: crest development teat development, restlessness; animals do not settle in the lairage, mill around Behavioural changes like mounting, aggression An even level of finish in a group of cattle of different breed/types |
Hormones |
| AMI | Good conformation with little fat, hyperaesthesia and tachycardia. | Beta-agonist |
| PMI | Signs of recent illness: mastitis, lameness/arthritis, pleurisy/pneumonia poor condition, metritis | For OV to decide |
| PMI | Emergency slaughter animals | For OV to decide |
| PMI | Injection sites, particularly: bruising / discolouration smell (especially with tetracyclines), swelling | Tetracyclines if bad odour, Hormone if oily adjuvant Others: For OV to decide |
| PMI | Good conformation with little fat, flaccidity of the trachea | Beta-agonists |
| PMI | Evidence of implants | Hormone Growth promoter |
3.5 Results: live animals
3.5.1 Notification of results
The VMD will inform the OV of the results by written confirmation as soon as results are available.
3.5.2 Compliant results
In the event of compliant results, the OV must serve a Form H notice cancelling Form G. Send a copy of the completed Form H to the VMD.
Reference: See annex 12 for a sample Form H notice.
3.5.3 Non-compliant results
In the event of non-compliant results, further action depends on the type of substance found; the VMD will issue specific instructions for each case.
3.5.4 Prohibited substances found
If prohibited substances are found the VMD will request that the OV serve a Form I notice on the owner or person in charge of the animal(s). This notice gives conditions and the time within which the animal(s) must be disposed of as a Category 1 Animal By-Product.
Reference: See annex 13 for a sample Form I notice.
3.5.5 Failure to comply
If the owner or person in charge of the animal(s) fails to comply with Form I you should serve a Form J notice and make arrangements for the disposal of the animal(s). The costs of such action will be recovered from the owner or person in charge of the animals.
Reference: See annex 14 for a sample Form J notice.
3.5.6 Investigation
The detection of residues of unauthorised substances will be immediately investigated by APHA. The OV responsibility is to support the investigation
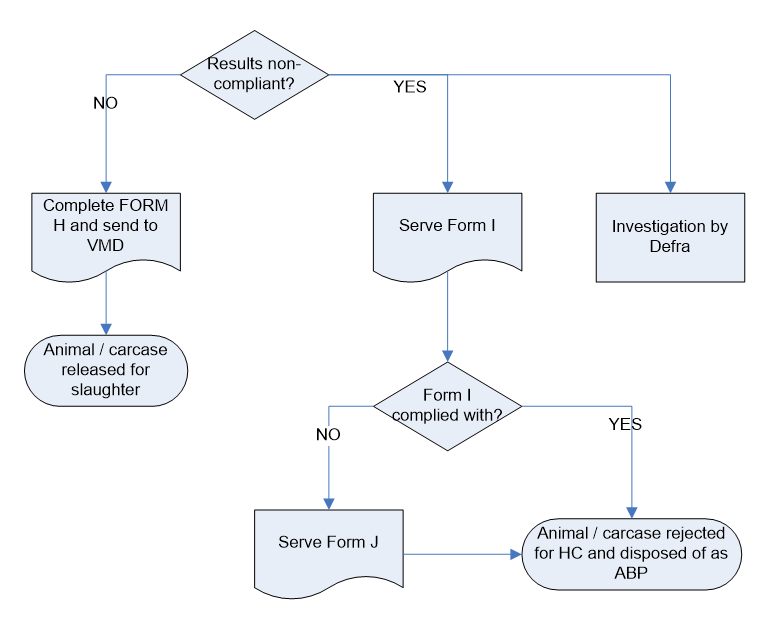
Sampling results
Compliant:
Complete Form H, Send to VMD and release Animal/carcase for slaughter.
Non-Compliant:
Branch 1: Serve Form I
- If not complied: serve Form J, reject Animal/carcase for HC and dispose of as ABP.
- If complied: reject animal/carcase for HC and dispose of as ABP.
Branch 2: on farm Investigation by APHA/Defra.
3.6 Results: Suspect carcases
3.6.1 Results
The OV will be the result is available by written confirmation.
3.6.2 OV action on receipt of positive results
If the results are positive, the OV who was responsible for sending the sample(s) will be sent Form A and Form B and a copy of the original RIM 1 form by the laboratory.
Reference: See annex 5 on ‘Form A’ and annex 6 on ‘Form B’ for samples.
The OV is to:
- give the forms to the owner or person in charge of the carcase
- declare the meat unfit for human consumption
- request voluntary surrender of the carcase
If the FBO refuses to surrender the carcase, you must put in writing the reason why the meat is being formally declared as unfit for human consumption in accordance with retained Regulation (EU) 2019/627, Article 45(f).
Note: Where the FBO continues to refuse to dispose of meat that has been declared unfit, follow the ABP provisions relating to the treatment of meat declared unfit for human consumption in chapter 2.8 on ‘Animal by-products’.
Reference: (EU) 2019/627, Article 43(i)
See chapter 7 on ‘Enforcement’ for additional information.
Caution: If the result is non-compliant for an unauthorised substance, the OV will be contacted by VMD and given further specific instructions.
3.6.3 Compliant results
If the result is compliant, complete Form D and release the carcase for slaughter.
Reference: See annex 8 for a sample Form D.
3.6.4 Follow-up investigation
A follow-up investigation will be carried out and may also be considered for further action.

Results Non-Compliant
If No:
Complete Form D and release carcase for slaughter.
If Yes:
Branch 1: Lab sends Form A and B and RIM1 to the OV who gives those to the owner or person in charge. OV to declare the meat for unfit for human consumption rejecting the animal/carcase for HC and to be disposed of as ABP.
Branch 2: On farm Investigation by APHA/DEFRA
updated [4. Follow up samples]
There may be occasions where Veterinary Medicines Directorate (VMD) will ask FSA staff to attempt to take, target or ‘follow-up’ a sample for a particular producer or County Parish Holding (CPH) number, based on the positive result of a sample previously processed.
To increase chances of obtaining a sample, given that a producer can choose to send animals to a number of Food Business Operators (FBOs) for slaughter (or not send any further animals to slaughter at all), VMD will email the SLA and Contracts team a central spreadsheet with the details of the original sample. The spreadsheet will show the original sample in this kind of format:
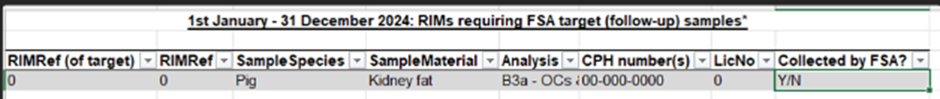
The SLA and Contracts team will send information about the required sample to:
- the FSA ITL of the plant for awareness that additional samples to be taken
- the FSA Field Veterinary Coordinator for the area where the plant is located for awareness that additional samples need to be taken
- the Service Delivery Partner mailbox for appropriate cascade through their local management channels. They should send the information to the OV at local plants and/or the relevant FSA OAs/MHIs responsible for in-plant sampling, so they can be on the lookout for an opportunity to collect such a sample. Samples should be taken the next time that animals from that farmer are received, although there is not a cut out date for the submission of each sample
- RIM form for the relevant follow up sample will be sent with the forms for routine testing on the same month that the follow up sample has been triggered
The next time that animals from the relevant farms are received in the slaughterhouse, the follow up sample must be taken. Regular RIM sample processes shall be followed.
In the case that animals requiring follow up sampling are received in a different plant and identified by the OV/FSA team, the follow up sample must be taken at this abattoir. Suspect sample kit and annex 2. (RIM sample) should be used.]
5. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 3: How to complete a RIM 1 form
Annex 4: How to complete a tamperproof bag
Annex 5: Form A: Primary Analysis Certificate
Annex 6: Form B: Reference Analysis Certificate
Sections
1. Overview
In this section
1.1 Introduction
1.1.1 Survey overview
The Veterinary Medicines Directorate (VMD) oversees the monitoring programme of AMR in indicator and zoonotic bacterial species from fattening pigs, broilers and fattening turkeys at slaughter.
1.1.2 Co-ordination and collection
The Antimicrobial Resistance Team at the VMD is responsible for the co- ordination of the AMR monitoring programme:
Emails: amr@vmd.gov.uk
The contractors for the monitoring programme are Animal Plant and Health Agency (APHA). APHA will be responsible for the testing of samples submitted and determining the total number of samples required from selected abattoirs. APHA will send sampling schedules and kits to participating abattoirs.
FSA AO (Authorised Officer) will undertake the collection of samples from approved abattoirs participating in the monitoring programme.
The survey requires the collection of samples from a number of different slaughtered batches / producers for each species.
APHA send the sampling kits to participating abattoirs. Sampling schedules are sent from APHA to FSA, which are in turn forwarded on to the relevant contact at participating abattoirs.
Samples will be collected on the following annual basis:
| Species | Year |
|---|---|
| Broiler/Turkeys | even years |
| Pigs | odd years |
1.2 FSA role
1.2.1 Target population
Broilers, fattening turkeys and fattening pigs.
1.2.2 AO requirements
The AO must:
- ensure continuity of evidence when samples are collected, prepared, labelled, stored and despatched, and
- always obtain evidence for the origin of the samples collected
- ensure the data collection forms, AMR1 (for Turkeys), AMR2 (for Pigs) and AMR3 (for Broilers) are fully completed, and three copies are taken (see annexes 1, 2 and 3 for examples of the forms)
- ensure one copy of the AMR form is sent with the samples, the second copy is given to the named FBO contact (which will be supplied by SLA and Contracts Team) and the third copy is retained by the FSA team.
1.2.3 Relevant establishments
These instructions apply to FSA staff at plants participating in the monitoring programme on AMR in indicator and zoonotic bacterial species in pigs, broilers and turkeys.
1.2.4 Time coding
All work undertaken as part of this survey in the collection, storage, packaging and despatch of samples is to be coded to GFSA.
2. Sampling
In this section
2.3 Broilers and Turkeys: collecting samples
2.1 Sampling programme
2.1.1 Sampling requests
FSA AOs in plant will receive a sampling schedule prepared by APHA, from the SLA and Contracts Team, which will list the number of batches that need to be sampled during the sampling period (the schedule will be sent in advance either monthly or quarterly, as appropriate).
The schedule will provide details on the date of sampling, the number of batches that need to be sampled on a given day and the ID of the batch to sample (with first and second reserves).
Please note that as the sampling schedule is weighted according to plant throughput, larger processing plants will sample more regularly than smaller processing plants.
Note: The ID batch number refers to:
- the sequence of slaughter batches going through the abattoir on the day of sampling (for broilers and turkeys)
- the sequence of slaughtered animals (for pigs)
For example, ID batch 2 would be the second batch of turkeys slaughtered on the given sampling day or the second pig killed on the slaughter line on the given sampling day.
2.1.2 Monitoring definitions
A ‘slaughter batch’ is defined as, a quantity of broilers/turkeys which have been raised on the same farm premises, in the same house, and delivered to the abattoir in the same vehicle.
Pig Kill Number is the actual pig kill number of the sampled pig at the start of the slaughter line on that particular day of sampling.
2.1.3 Exclusion criteria
Slaughter batches / loads from more than one house or from more than one farm (mixed batches) are to be excluded from the monitoring programme.
2.1.4 Selection of slaughter batches
To avoid bias, slaughter batches must be randomly selected for sampling.
When collecting broiler or turkey samples, beside each allocated sampling day on the schedule there are three numbers per sampling batch labelled ‘ID of batch to sample’, ‘ID batch (1st reserve)’ and ‘ID batch (2nd reserve)’. These are random numbers generated using the average number of batches processed during the abattoir’s working day and represent the particular batch that must be identified and sampled.
Batches of broilers or turkeys from mixed houses, or from more than one farm, must be excluded. Therefore, if the selected batch is from a mixed house or from more than one farm, then the reserve batch should be sampled if that is not a mixed batch. The ID of the batch sampled should be marked clearly on the data collection form, AMR1 or AMR3.
Example:
| Allocated sampling days | ID of batch to sample | ID batch (1st reserve) | ID batch (2nd reserve) |
|---|---|---|---|
| 08/11/18 | 5 | 7 | 15 |
| 13/11/18 | 2 | 6 | 24 |
| 13/11/18 | 3 | 9 | 17 |
| 05/12/18 | 7 | 2 | 5 |
When collecting pig samples, if the selected animal on the schedule is from a mixed batch / load or from more than one farm, select a pig from a single producer and sample. The ID (kill number) of the pig sampled should be marked clearly on the data collection form AMR2.
Sampling for the surveillance programme will only be carried out Monday to Friday. If broilers, turkeys or pigs are not slaughtered on the specified sampling day, please sample the same ID batch number allocated but on the next processing day.
The revised sampling date and the ID of the batch sampled should be marked clearly on the data collection forms, AMR1, AMR2 or AMR3.
Reference: See annex 1 for a sample copy of form AMR1, annex 2 for a sample copy of form AMR2, annex 3 for a sample copy of form AMR 3 and annex 5 for guidance notes on arranging the delivery to APHA laboratories.
Note: If the samples cannot be despatched on the same day as collection, or if there are any questions on the sampling schedule, contact the SLA and Contracts Team at sla.contracts@food.gov.uk.
2.1.5 Repeat sampling
When preparing to take a sample, please ensure the ‘previously sampled report’ from APHA is reviewed to prevent repeated sampling of the same flocks or holdings. This report is usually sent on a monthly basis.
2.1.6 Selection process
The following list outlines the slaughter batch selection process:
- If the batch identified for sampling is not eligible (it is not from a single house or cannot be sampled) sample the 1st reserve batch for broilers and turkeys or select a suitable animal to sample from for pigs.
- If the 1st reserve batch is not eligible or cannot be sampled, sample the 2nd reserve batch (Note: for broiler or turkey sampling only).
- If the 2nd reserve batch is not eligible or cannot be sampled, sample the next available eligible batch on the same processing day (Note: for broilers and turkeys only).
- Mark the sampled ID batch number on the AMR1, AMR2 or AMR3 forms.
- If there are no more eligible batches processed on the same day, sample the first available eligible batch on the next processing day (and mark the date and batch number clearly on the AMR1, AMR2 or AMR3 forms). Please notify APHA Weybridge by email to advise them of the despatch delay: AMRSurvey@apha.gov.uk.
2.2 Sampling equipment
2.2.1 Introduction
APHA will provide the relevant establishments with sampling kits and the data collection forms (AMR1, AMR2 or AMR3). The SLA and Contracts Team will contact FSA staff at the establishments to inform them of delivery arrangements for the sampling kits.
2.2.2 Non-delivery of sample kits
Sampling kits and forms should be received at least four days before sampling begins. If the kit and form are not received, or if any of the equipment listed below is missing, contact the SLA and Contracts Team at sla.contracts@food.gov.uk.
2.2.3 Sampling kit contents Broiler and Turkey kit:
- 1 x Biotherm shipping box
- Broilers – Biotherm 7
- Turkeys – Biotherm 5 and 7
- Sample pots
- Broilers – 2 x 300ml screw cap sampling pot
- Turkeys – 300 ml screw cap sampling pot
- A4 pathoseal absorbent bags for sample pots
- sterile gloves
- grip-seal bags
- AMR1 and AMR3 forms
- 2x Biochills (frozen) - these must be kept away from direct contact with the samples using the bubble wrap (for broiler samples) or the polystyrene spacers (for turkey samples)
- 1 or 2x Biochills (chilled)
- bubble wrap (Broilers)
- polystyrene spacer (Turkeys)
- security seal
- UN3373 label
Note: Larger boxes for multiple samples may contain four Biochills. Two Biochill packs must be completely frozen when packed in the sampling box, ensure that they are placed in a freezer at least 48 hours before sampling. In addition, one (or two, depending on the size of the box) Biochill packs should be chilled in the refrigerator.
The frozen Biochills are placed on top of the polystyrene separator (which comes in the biotherm 5 and 7 boxes). The chilled Biochill pack(s) can be placed alongside the sample. See details for packaging in section 2.7.3.
Updated [Pig kit: for sampling 1 caeca per fattening pig:
- 1 x Biotherm shipping box
1 x 90ml screw cap sampling pot
disposable scalpel
A4 pathoseal absorbent bag (with absorbent lining)
sterile gloves
grip-seal bags
AMR2 form
2x Biochills (frozen) - these biochills will be labelled “freeze”. These frozen biochills must be kept away from direct contact with the samples using the polystyrene spacer
1x Biochill (chilled) - supplied for larger biotherm boxes and/or in summer when weather is hot, to aid in maintaining a cool temperature during transport). These biochills will be labelled “chill (2-8°C) only”. When used these must be placed in contact with the samples
polystyrene spacer (to separate samples from frozen Biochill packs)
bubble wrap (to stop pot moving around in the bottom of box)
security seal
UN3373 label
Note: Two Biochill packs must be completely frozen when packed in the sampling box, so ensure that they are placed in a freezer at least 48 hours before sampling. Any additional Biochills supplied (one or two, depending on the size of the box or time of year for example in summer) must be chilled in the refrigerator for 48 hours before sampling. Each biochill will be labelled with either ‘freeze’ or ‘chill’ pending its intended use.]
2.3 Broiler and Turkey: Collecting samples
2.3.1 Caeca samples
For turkeys:
One pair of full and intact caeca will be sampled at the evisceration point from one bird per slaughter batch. The pair of caeca will be put into the appropriate sampling pot.
Turkey sampling is to be carried out at the time of evisceration. Birds are to be sampled at random during the selected batch avoiding the first part of the batch.
Depending on the line speed, and facilities available in each establishment, the paired caeca taken from each bird can be separated from the eviscerated intestines either on the slaughter line, or alternatively the whole offal can be removed and carried in a tray or similar receptacle to a separate area before removing the caeca.
Note: It is important that full and intact caeca are collected.
For broilers:
10 pairs of full and intact caeca from 10 birds within the same slaughter batch will be sampled and each pair of caeca put into the appropriate sampling pots (two pots provided per sample).
Sampling is to be carried out at the time of evisceration. Birds are to be sampled at random during the processing of the selected batch but avoiding sampling the first birds slaughtered in the batch. Consecutive birds must not be sampled but a random interval between the 10 birds sampled is the aim. The birds need not be collected from the entire batch; sampling 10 birds at random from approximately 50 or 100 birds slaughtered within the batch is acceptable.
Depending on the line speed, and facilities available in each premise, the paired caeca taken from each bird can be separated from the eviscerated intestines either on the slaughter line, or alternatively the whole offal can be removed and carried in a tray or similar receptacle to a separate area before removing the caeca.
Note: It is important that full and intact caeca are collected.
2.3.2 Sample handling
Samples must:
- be allowed to cool down in the pots prior to packing
- be packaged according to the instructions in this topic (an A4 guidance sheet will be provided in each sampling kit)
- be despatched, on the same day of collection (where possible)
- samples should aim to arrive at APHA Weybridge within 36hrs of sampling and 48 hrs at the latest (if the sample is collected on a Thursday or Friday and arrives over the weekend, the sample can be suitably stored and then tested on the Monday, i.e. within 96 hours).
Reference: See topic 2.7 on ‘Storage, packaging and despatch of samples’ in for additional information.
Caution:
- Samples must be kept in a cool dark place until collected by Topspeed.
- Samples must not be frozen.
- Keep box out of direct sunlight.
- Despatch Monday to Friday only.
2.4 Pig: Collecting samples
2.4.1 Caeca samples
20g of caecal content will be sampled at the green offal inspection point from one fattening pig. The caeca will be put into a sampling pot.
Pig sampling is to be carried out at the Green Offal Inspection Point. Pigs are to be sampled from the selected kill number identified at the evisceration point. Depending on the facilities available in each establishment, the caecum taken from each pig can be separated from the eviscerated intestines either at the inspection point on the slaughter line, or alternatively the whole offal can be removed and carried in a tray or similar receptacle to separate area before removing the caecum.
Note: It is important that 20g of caecal content are collected.
2.4.2 Sample handling
Samples must:
- be allowed to cool down in the pots prior to packing
- be packaged according to the instructions in this topic (an A4 guidance sheet will be provided in each sampling kit)
- be despatched, on the same day of collection (where possible)
- samples should aim to arrive at APHA Weybridge within 36hrs of sampling and 48hrs at the latest (if the sample is collected on a Thursday or Friday and arrive over the weekend, the samples can be suitably stored and then tested on the Monday, i.e. within 96 hours).
Reference: See topic 2.7 on ‘Storage, packaging and despatch of samples’ in part 2 for additional information.
Caution:
- Samples should be allowed to cool down before packing in the shipping box
- Samples must be kept in a cool dark place until collected by Topspeed.
- Samples must not be frozen
- Keep box out of direct sunlight
- Despatch Monday to Friday only
2.5 Minimising the risk of sampling contamination
2.5.1 Caeca sample contamination
The main objective is to collect the caeca whilst minimising any external contamination.
For turkeys, one pair of full and intact caeca will be sampled at the evisceration point from one bird per slaughter batch. This is best achieved by careful manual traction to the portion of intestine either side of the caeca so that both caeca are removed intact with a short length of intestine.
For broilers, ten pairs of full and intact caeca will be sampled at the evisceration point from ten randomly selected birds per slaughter batch.
The sampler needs to verify that the caeca are intact and full. If they are not, the paired caeca should be disregarded and a new bird selected instead.
The caeca will be put into the appropriate sampling pot. Each sampling pot should then be sealed securely and placed into a small pathoseal absorbent bag (one pot per bag.
Note: For turkeys (and pigs), caeca from different slaughter batches (or carcasses) should not be placed in the same pot. For broilers, ten full and intact caeca can be placed in the same pot (or shared between the two pots provided depending on volume).
For pig samples, this is best achieved by careful piercing of the caecum so that caecal contents can be collected in the sampling pots.
Caecal content should be collected per fattening pig and put into a labelled pot. Each pot should then be sealed securely and placed into a small pathoseal absorbent bag.
Note: Caecal content from different fattening pigs should not be placed in the same pot.
2.6 Completing the AMR forms
2.6.1 Details to record
The following details must be fully recorded on the AMR1, 2 and 3 forms:
- abattoir details
- sampling details including the name of the sampler and the date and time of collection
- confirmation of the type of animal slaughtered, for example, fattening turkey or fattening pig
On the AMR1 and AMR3 forms, for turkey and broiler surveys, respectively, the following details must also be fully recorded:
- producer details, for example, farm name, address, county parish holding (CPH) number
- batch details including the number of birds in the batch slaughtered, the number of birds in the house, the shed / house number, age of the birds and the average weight of the birds
On the AMR2 form, for the pig survey the following details must also be fully recorded:
- producer details, for example, farm name, address, CPH number
- animal details including the slapmark number and the weight of the carcase
Note: If an error is made when recording any of the above data on the AMR form, or if anything is unclear that might need going over again, cross through the entry and enter the correct details then initial the change. Any necessary amendments must be made before the copies of the AMR form are despatched with the sample.
2.7 Storage, packaging and despatch of samples
2.7.1 Chilling
Samples must be kept chilled (not frozen) from the time of sampling until delivery to APHA. Please allow time for the samples to cool down in the pots before packing the kit box. Once packed, place the closed sampling kit in a cool area and away from direct heat until the courier arrives. If a cool room is available, the entire sampling kit can be stored there until despatched to APHA Weybridge.
2.7.2 Specimen collection and handling
Analysis can be affected by the growth of other bacteria. Therefore, care must be taken to ensure that samples are taken appropriately, chilled as described above and transported to APHA Weybridge as quickly as possible.
Extreme temperatures must be avoided.
2.7.3 Packing
Packing in line with the following procedures:
- Ensure that the APHA reference number at the top of the data collection form AMR1 / AMR2/ AMR3 matches the number on the sample sampling pot.
- Once the sample has been placed in the pot, allow time for the sample pots to cool down to room temperature before placing it into the kit box.
- Remove 2x Biochills from the freezer (and 1 or 2x Biochills from the chiller).
Broilers: samples to be placed at the bottom of the biotherm 7 box, then place the polystyrene divider on top with the frozen Biochill packs on top of the polystyrene divider. Chilled Biochill pack to be placed alongside the sample.
Turkeys: samples to be placed at the bottom of the biotherm 5 or 7 box, then place the polystyrene divider on top with the frozen Biochill packs on top of the polystyrene divider. Chilled Biochill pack to be placed alongside the sample.
Pigs: wrap the sample in bubble wrap to avoid contact with frozen Biochills. Place the polystyrene divider on top of the sample/s, then place the two frozen Biochills on top of this. Chilled Biochill pack to be placed alongside the sample.
- Slide the completed form into the plastic document pouch to protect from any leakages that may occur and place into the sampling kit.
- The sample box must be closed securely without delay. It is important that the pack is not left open (or closed without freezer packs) for any length of time as this may damage the samples.
Note: All frozen and chilled Biochill packs provided in the sampling kit should be used. Care must be taken not to place the frozen packs in direct contact with the specimen pots (however, the chilled Biochill packs can be placed alongside the sample). Please refer to Annex 4 for details on packing instructions.
2.7.4 Labelling cardboard outer cartons
Apply the adhesive address label provided by the carrier to the outer carton across the box flaps.
Apply the UN3373 diamond label and Biological substance category B labels to the outer carton.
Apply the security seal to the carton lid.
2.7.5 Despatching samples
Samples are to be despatched to APHA using the Topspeed next day service:
| Step | Action |
|---|---|
| 1 | Arrange collection by Topspeed using the process at annex 5. |
| 2 | Provide Topspeed with the following information:
|
| 3 | Write the barcode nos. as reference for the collection in the plant day book. Topspeed to collect as arranged. |
Note: Any problems collecting samples can also be notified to the Lab by emailing the AMR Survey Team at AMRSurvey@apha.gov.uk as well as calling the SLA and Contract Team at sla.contracts@food.gov.uk.
2.7.6 Despatch of all samples
Samples are to be sent to:
Bacteriology, Building 17 Animal
Plant Health Agency
Woodham Lane
New Haw,
Addlestone Surrey
KT15 3NB
2.7.7 Despatch failure
Should despatch fail, you must contact Topspeed and make an attempt to rearrange despatch, then notify APHA Weybridge by email to advise them of the despatch failure: AMRSurvey@apha.gov.uk
2.7.8 Complaints procedure
Should Topspeed fail to collect samples within the agreed timeframe, contact the SLA and Contracts Team at sla.contracts@food.gov.uk, who will escalate the failure to Topspeed headquarters.
3. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices.
Sections
2. Action on suspicion of notifiable diseases
5. Enzootic Bovine Leukosis (EBL)
6. Transmissible spongiform encephalopathies (TSE)
8. Outbreak of Avian Influenza
9. Outbreak of Foot and Mouth Disease
10. Outbreak of Bluetongue Virus Disease (BTV)
1. Introduction
In this section
1.1 Purpose
1.1.1 Background
The prompt identification and notification of certain animal diseases allows the FSA, APHA, Defra and Welsh Government to take action to prevent the spread of the disease. This chapter covers day to day procedures in notifiable disease monitoring and passive surveillance (for example, cases when the disease is suspected) at FSA approved establishments.
Active surveillance procedures for notifiable diseases are included in other chapters of the MOC (for example, chapter 2.4 ‘Post-mortem inspection’ and chapter 2.6 ‘TSE testing’).
When an outbreak is declared, emergency instructions will be issued at the time, since different rules may apply depending on the specifics of the case. Outbreak instructions for Avian Influenza and Foot and Mouth Disease, African Swine Fever and Bluetongue are included within this chapter to facilitate the familiarisation of FSA staff with the expected procedures in place in case of outbreak in Great Britain and the pre-designation of slaughterhouses in “peace-time”.
1.1.2 Legislation
Powers to control notifiable diseases are derived from the Animal Health Act 1981 (as amended) and specific Orders made under the Act or Regulations made under the European Communities Act 1972.
1.1.3 Enforcement
The legislative powers are usually enacted by APHA staff or Local Authority (LA) inspectors. Some FSA staff are authorised under the legislation to undertake certain functions. The legislation is enforced by LAs.
1.1.4 Introduction to FSA duties
FSA staff have a duty to notify the Secretary of State or APHA Vet (Defra Rural Services Helpline on 0300 020 0301 in England or Wales Field Services on 0300 303 8268 in Wales) of any suspect case of a notifiable disease that they may encounter during the course of their work. In practice, they will deal with the APHA Vet.
The decision whether to take further action or not rests with the Vet and it is the responsibility of the Official Veterinarian (OV) to report suspect cases for the decision to be made by APHA.
Also, the FSA participates in monitoring and surveillance schemes aimed at the detection of certain notifiable diseases.
Note: ‘Suspect animal’ includes any animal in which disease is suspected and any animal which came from the same premises of origin.
2. Action on suspicion of notifiable diseases
In this section
2.1 Current notifiable diseases
2.1 Current notifiable diseases
2.1.1 Reporting notifiable diseases
Any person who suspects a notifiable disease has a duty to report it to the APHA Vet.
Official information of notifiable diseases in the UK and further guidance.
The list of notifiable diseases in England and Wales includes:
African Horse Sickness, African Swine Fever, Anthrax, Aujeszky’s Disease, Avian Influenza, BSE, Bluetongue, Brucellosis, Chronic wasting disease, Classical Swine Fever, Contagious Agalactia, Contagious Bovine/Caprine Pleuro-pneumonia, Contagious Epididymitis, Contagious Equine Metritis, Dourine, Echinococcus multilocularis, Enzootic Bovine Leukosis, Epizootic Haemorrhagic Virus Disease, Epizootic Lymphangitis, Equine Viral Arteritis, Equine Viral Encephalomyelitis, Equine Infectious Anaemia, Foot and Mouth Disease, Glanders and Farcy, Goat Pox, Lumpy Skin Disease, Newcastle Disease, Paramyxovirus in pigeons, Peste des Petits Ruminants, Porcine Epidemic Diarrhoea, Rabies, Rift Valley Fever, Rinderpest, Scrapie, Sheep Pox, Surra, Swine Vesicular Disease, Teschen Disease, Tuberculosis (Bovine), Vesicular Stomatitis, West Nile Virus.
Notifiable diseases can be:
- endemic – already present in the UK (for example, bovine TB)
- exotic – not normally present in the UK (for example, FMD, ASF, BT, AI).
2.1.2 Clinical signs of notifiable diseases
Foot and Mouth Disease in ruminants
Bluetongue in cattle and sheep
Updated [A collection of guides to notifiable diseases in animals can be found in the following link: Notifiable diseases in animals.]
2.2 FSA responsibilities and action
2.2.1 When to report
The OV must immediately report to APHA suspicious signs of notifiable disease in:
- live animals or birds
- carcases and offal
If the OV is not present the MHI must consult an OV before reporting a notifiable disease, provided that such consultation will not cause undue delay.
Reports of notifiable disease are to APHA (Defra Rural Services Helpline on 03000 200 301 in England or Wales Field Services on 03000 303 8268 in Wales) and to the FVL for onward reporting to the Portfolio Lead for notifiable diseases.
The OV (or MHI where applicable) MUST keep a written record in the daybook of the time when the suspect cases were reported and the name of the person making the report.
The OV (or MHI where applicable) must follow precisely the instructions given by the APHA Vet. The period between when the OV (or MHI where applicable) reports suspicion of disease and arrival of the VO into the establishment may be critical in controlling the spread of disease.
2.2.2 Reporting details
Provide the following information to the APHA Vet:
- the plant name, address and contact telephone number
- the animal’s breed, age, sex and identification mark(s) (eartag number or slapmark)
- details of any clinical signs and history in the suspect cases such as, time of arrival, size of the consignment, origin of the animals in the consignment and any in-contact animal from the same establishment
- details of the lesions found during meat inspection
- the name, address and the holding County Parish Holding (CPH) number of the establishment where the suspect animal or carcase(s) came from and details about when the animal arrived in the lairage and what other animals arrived in the same consignment. This will allow APHA to arrange an investigation at this establishment if needed
2.2.3 Instructions from APHA
Instructions given by the APHA Vet could include:
- isolating the animal until an investigation has been completed
- restricting movement of all animals, birds, products, vehicles or people into or out of the slaughterhouse until an investigation has been completed
- stopping slaughter
2.2.4 Record keeping
The OV must keep a contemporaneous record in the daybook of all instructions received from the APHA Vet and confirm that they have been followed.
The OV must ensure that the ante-mortem and post-mortem inspection records input in IRIS are consistent with the findings and the suspicion of the notifiable disease.
When an exotic notifiable disease is suspected, and reported to APHA, regardless of the outcome or response from APHA, the OV must send an e-mail to the FSA Incident Team describing the situation and including relevant information (for example, animal identification and origin, disease suspected, response received from APHA). The FVL for the area in which the slaughterhouse is located must be copied into this e-mail.
2.2.5 C and D
No disinfectant should be used on or near animals, birds or carcases suspected of disease, while waiting for the APHA Vet to attend, as this may adversely affect the likelihood of correct laboratory diagnosis.
2.2.6 Consultation cases
Providing that the OV is in the establishment and remains there, APHA may decide to deal with the investigation as a ‘consultation case’.
A consultation case takes place between two or more veterinary surgeons when one of them considers that a notifiable disease may be included in the differential diagnosis for a specific case, but the probability of it being that disease is very low.
The OV should discuss the report of disease with the APHA Vet on arrival at the establishment.
The APHA Vet will place restrictions only if the result of the consultation is that a notifiable disease is suspected.
2.2.7 Report case
In other cases, APHA may call the case a ‘Report Case’ and place specific restrictions on the establishment pending veterinary enquiry. These restrictions may affect the movement of animals, products, people and vehicles from the establishment.
2.2.8 Legislative responsibilities
The OV remains responsible for:
- ensuring that all public health legislation is complied with while the establishment is under APHA restrictions
- monitoring hygiene and animal welfare
- following APHA instructions and informing them immediately if any of them cannot be implemented
2.2.9 Procedure for suspect notifiable disease
If the APHA Vet agrees the possibility of a Notifiable Disease, the premises should be treated as contaminated, until proven otherwise. The Food Business Operator (FBO) should:
- not bring more susceptible animals on to the premises
- not slaughter live suspect animals (so the VO can sample them)
- isolate suspect / potentially contaminated carcases
The chart below outlines the procedure to follow if the OV suspects a notifiable disease.
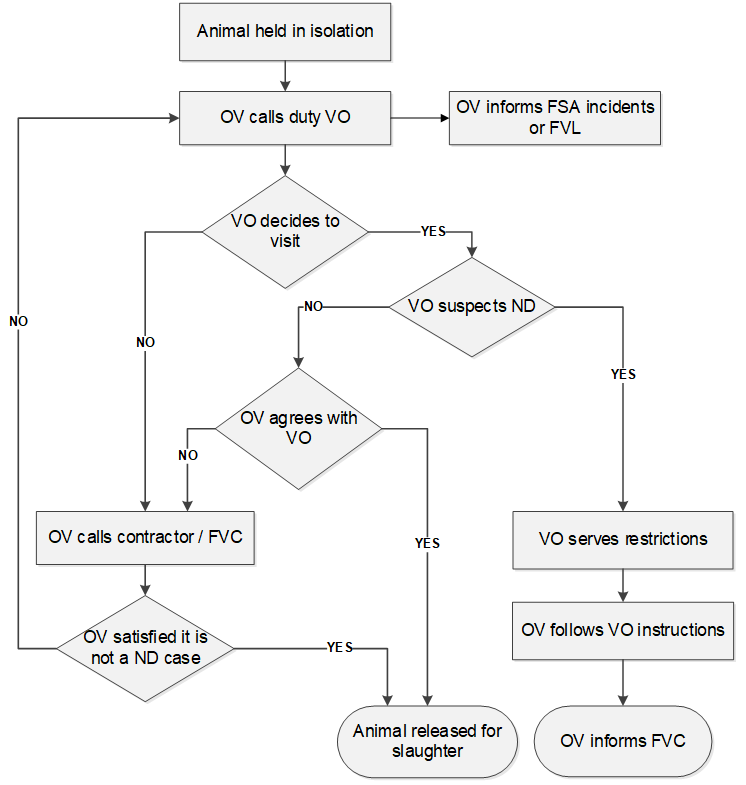
2.3 Responsibilities of APHA
2.3.1 Main duties
APHA has responsibility for:
- applying animal health disease control measures to minimise the spread of notifiable disease
- fully investigating the OV (or other FSA AO) report
2.3.2 APHA Vet investigation
An APHA Vet will visit the slaughterhouse to carry out an investigation. Other VOs may be sent to the farm of origin to undertake a simultaneous veterinary enquiry.
Once at the establishment, the APHA Vet will discuss the report with the OV / MHI / FBO and examine the suspect animals / carcases / offal. The APHA Vet may also consult with other APHA Vets who may have gone to the farm of origin to gain a full clinical picture, and with APHA Veterinary Exotic Notifiable Diseases Unit (VENDU).
2.3.3 After investigations
If the presence of a notifiable disease is ruled out, restrictions will be lifted. The OV should follow ante-mortem procedures as per 2.1.16 Ante-mortem inspection summary in MOC Chapter 2.2.
If the presence of notifiable disease cannot be ruled out, the APHA Vet will:
- serve a restriction notice closing establishments (or parts), or
- amend any restriction notice that has already been served, and / or
- collect whatever samples are necessary for diagnostic purposes
If the initial investigation began as a consultation case, it will now become a report case.
2.3.4 Restrictions
APHA will regularly review the extent of the restrictions bearing in mind quick recommence of the operations but it will be subjected to some conditions such as thorough C and D. The APHA Vet will undertake this assessment taking advice from the OV and FBO.
2.4 Other responsibilities
2.4.1 Compliance
All persons at the establishment, including FSA staff, must comply with any restrictions in any notices served on the establishment.
2.4.2 Local authority
The LA is responsible for taking enforcement action under disease control legislation.
2.5 Detained meat storage
2.5.1 Storage sites
Any meat detained at the slaughterhouse will be kept locked in a ‘storage site’ under control of the OV and APHA. Access to this storage site will be facilitated through the OV or APHA Vet. The FBO is responsible for the way the meat is stored, in compliance with (EC) 852/2004 and 853/2004.
The storage site is likely to be kept under restrictions until the final results are known and disease is confirmed or not.
2.5.2 Preparation for storage
The FBO may discuss procedures for preparing the meat for storage with APHA and FSA.
2.5.3 Test results
Negative results take longer to reach completion. APHA will provide information on how long it could take before the results are known.
2.5.4 Public health
FSA are fully responsible for ensuring that public health legislation is complied with at all times the meat is at the establishment.
Meat is to be declared unfit for human consumption if it derives from animals affected by animal diseases for which animal health rules are laid down in Directive 2002/99/EC except if it is obtained in conformity with the specific requirements provided for in that Directive. This exemption does not apply if otherwise provided for in the requirements on the official controls of tuberculosis and brucellosis provided for in Articles 33 and 34 of Regulation (EU) 2019/627.
The diseases listed in the Directive are: Classical and African Swine Fever, Foot and Mouth Disease, Avian Influenza, Newcastle Disease, Rinderpest, Sheep and Goat Plague (Peste des Petits Ruminants) and Swine Vesicular Disease.
Reference: Regulation (EU) 2019/627, Article 45 (e)
Meat shall also be declared unfit for human consumption if in the opinion of the OV, after examination of all the relevant information, may constitute a risk to human or animal health or is for any other reason not suitable for human consumption.
Reference: Regulation 2019/627 Article 45(t)
2.5.5 Clearance
Meat detained on suspicion of disease will usually be released once all the tests are negative. The OV must seek clearance from APHA and keep a written record before opening any sealed container.
2.6 C and D of the establishment
2.6.1 Requirement to Cleanse and Disinfect
When certain diseases cannot be ruled out, APHA may require the FBO to cleanse and disinfect specified parts of their establishment. FBOs are responsible for doing this at their own expense. APHA may request FSA assistance in supervising the C and D of the establishment.
When carrying out C and D activities in the event of an outbreak (or during the investigation of a suspected outbreak) of a Notifiable Disease, FBOs are requested to use the relevant disinfectant as listed on the Defra website.
These C and D activities need to be documented by protocols where the FBO should describe how to C and D the relevant equipment, utensils and vehicles. This should at least be in line with the manufacturers’ instructions for the chemical in use.
2.6.2 After C and D
The APHA Vet will be able to confirm when the operations can re-commence after the C and D - in some cases the establishment may have to be rested for a specified period. The aim will always be to allow resumption of operations as soon as possible.
3. Anthrax
In this section
3.1 Introduction
3.1.1 Background
The OV (or MHI where applicable) may consider the possibility of anthrax in the course of normal duties. In reaching a decision, the OV must take into account factors such as history or clinical signs.
Official information about Anthrax in UK can be found online.
3.1.2 Anthrax: clinical and pathological signs
Suspicion of anthrax should be considered:
- if the cause of death is unexplained, particularly sudden death, in apparently healthy animals
- when potential signs of anthrax are observed in the dead animal (for example, dark, tarry uncoagulated bloody discharges from natural orifices, rapid bloating of the carcass, incomplete rigor mortis)
- if indications in the Food Chain Information (FCI) or any other information indicate higher risk of the farm / area of origin
- if clinical signs at ante-mortem inspection indicate that the disease might be present, for example, high temperature, bloody diarrhoea or a discharge of dark tarry uncoagulated blood from the nose, mouth and anus
- if post-mortem evidence suggests that the animal might have been suffering from anthrax (for example, swollen spleen with bloodstained fluid in all body cavities).
Note: If the OV suspects anthrax, the carcase should not be opened as this can result in the formation of highly resistant Anthrax spores.
3.1.3 Suspect live animals
Suspect animals and animals in direct contact must be detained, isolated and reported to the APHA Duty Vet immediately.
The APHA Vet will place restrictions upon the animal, but it will not be slaughtered. It may be treated in situ, but for as long as the animal shows signs of disease the restrictions will remain in place.
3.1.4 Suspect carcases
In some cases, suspicion of disease will not be raised until the carcase has been opened. The whole of the suspect carcase, offal, hide and blood must be detained (including any parts already removed) and people kept away from the carcase, its parts and the area where the carcase is held.
All other carcases and offal at the establishment should be detained pending completion of enquiries. No other animals should be allowed to enter the slaughterhall until the results of the enquiry are known.
Holding pens should not be cleaned, and no other product or waste should be allowed to leave the site until authorised by APHA staff.
3.1.5 Details to report
The OV (or MHI where applicable) must report suspect cases to the APHA Vet immediately, giving details as instructed in section 2 of this chapter. The decision whether to take further action or not rests with the APHA Vet and it is the duty of the OV to report suspect cases for the decision to be made by APHA.
3.1.6 APHA action
The APHA Vet will inform that restrictions apply and will also arrange for an immediate enquiry to be carried out by APHA Vet. OVs authorised through the Official Controls Qualification (Veterinary) – Statutory Surveillance (OCQ(V)-SS) can carry out an enquiry into anthrax.
If the OV is a designated OV with an OCQ(V)-SS, APHA Vet may instruct the OV to undertake the enquiry providing suitable facilities are available for testing.
OVs cannot carry out enquiries in anticipation of authorisation from APHA.
3.1.7 C and D
Holding pens should not be cleaned and no other product or waste allowed to leave the site until authorised by APHA staff.
It is likely that APHA requires the FBO to carry out the C and D of any place associated with any animal notified as a suspect case pending the veterinary inquiry. If the results of the veterinary inquiry are positive or inconclusive, the FBO will be required to carry out a more thorough C and D procedure.
3.2 Investigation and diagnostic sampling
3.2.1 Anthrax bacilli suspected: initial investigation
Under no circumstances must the OV attempt to collect and examine samples for anthrax without having informed the APHA Vet and being authorised to do so.
If the OV is a designated OV with an OCQ(V)-SS and facilities are available, APHA Vet may request them to make the initial investigation.
3.2.2 TSE testing
If a bovine or ovine animal is found dead in the lairage or dead on arrival and the OV suspects anthrax, then the animal must be tested for anthrax and this disease rules out before being subjected to Transmissible Spongiform Encephalopathies (TSE) testing in eligible cattle or, in the case of adult sheep in selected slaughterhouses.
3.2.3 Suspect anthrax out of hours
If it is necessary for an examination for suspected anthrax to be carried out at a slaughterhouse outside normal OV hours of attendance, the APHA Vet will request an APHA vet or authorised veterinarian designated as OV with an OCQ(V)-SS to attend the establishment to conduct such an examination. If the OV is a designated OV with an OCQ(V)-SS, the APHA Vet may ask them to do this.
3.2.4 Anthrax suspected
If disease is suspected, the attending vet with an OCQ(V)-SS will report this to the APHA Vet who will make arrangements for the submission of further samples for testing.
3.2.5 Detention of suspect carcases
Where anthrax is suspected, the carcase should be detained until the results are received.
The FBO may dispose of the carcase as Category 2 Animal by-products (ABP) only if suspicion of anthrax has been ruled out.
3.2.6 Anthrax ruled out
Where the APHA vet with an OCQ(V)-SS is satisfied that anthrax does not exist in the live animal, they will notify the APHA Vet and FBO by completing form AN2 (Certificate – Non-existence of Disease in a Carcase).
Reference: See Annex 3 on ‘AN2 – Certificate’ for a sample.
If the animal has died and requires TSE testing, the procedure for testing fallen stock must be followed once the presence of anthrax has been ruled out.
If an owner requests an investigation into the cause of death, this is a private matter which must be arranged between the owner and private veterinary surgeon.
4. Bovine Brucellosis
In this section
4.1 Overview
4.1.1 Introduction
The UK achieved official brucellosis free status in 1985.
Official information about Brucellosis in the UK is available online.
There are measures in place to prevent the disease being re-introduced and subsequently spreading, such as:
- post import testing of imported cattle
- compulsory reporting of all bovine abortions and premature calvings with investigation of all outside a specified low risk category
- quarterly testing of bulk milk samples from all dairy herds, including those of producer retailers
4.1.2 Responsibilities
APHA will inform the FSA about proposed slaughter of reactors. Collection and packaging of samples from brucellosis cases consigned for slaughter is the FSA responsibility, and will include:
- reactors and inconclusive reactors to the brucellosis tests, and
- contacts with confirmed cases
The despatch of the samples to the laboratory is the responsibility of APHA who will collect the samples from the slaughterhouse.
Note: The OV must report any abortions and premature births to APHA and follow any additional instructions. All FSA staff should be aware of the potential danger of infection primarily from the uterus and udder.
4.1.3 Movement licences
Cattle from restricted premises will be consigned directly to slaughterhouses accompanied by a BS112 (Licence authorising the movement of cattle on to or off premises under restriction or authorising the movement of specified cattle which are under restriction awaiting the completion of tests for brucellosis).
APHA will send a copy of the BS112 licence, to the OV as advanced warning.
Reference: See Annex 4 on ‘BS112 – Licence’ for a sample of the form.
In addition, where the owner has opted to slaughter the animal at their own expense (private slaughter) the animal will be accompanied by form BS15B. These are handed to the FBO on arrival.
Reference: See Annex 5 on ‘BS15B – Notice’ for a sample of the form.
4.2 Slaughter and sampling
4.2.1 Slaughter procedure
The OV / MHI must collect the following samples from the carcase:
| All animals |
|---|
Paired lymph nodes
|
| In addition for bulls |
|---|
|
4.2.2 Sampling packaging
Samples must be taken as cleanly as possible using sterilised knives, and placed in a labelled polythene bag (each pair of nodes or organs should be placed in a separate bag), which is then sealed.
All specimens from each animal sampled should then be placed together in a further single outer polythene bag and this bag then sealed and labelled.
Polythene bags should be self-sealable or tightly knotted and of sufficient strength to prevent leakage and potential cross-contamination.
4.2.3 Labelling
Label all sample bags with the ear tag number plus the details of any reactor tag.
4.2.4 Storage
All samples should be placed in a refrigerator (not freezer) until collected by APHA staff. FSA staff should inform APHA when the samples are ready for collection.
5. Enzootic Bovine Leukosis (EBL)
In this section
5.2 Investigation of tumours in cattle carcases or offal
5.1 Introduction
5.1.1 Enzootic Bovine Leukosis (EBL)
The OV must notify the APHA Vet of:
- any live animal affected with, or suspected of being affected with, EBL, and
- any carcase or offal showing certain tumorous changes
Detain any suspect live animal or any suspect carcase with its offal until the APHA Vet issues instructions. Retain the passport and FCI until any investigations have been carried out.
Official information about EBL in UK can be found online.
5.1.2 Signs to report
The OV should report suspect cases in live animals or carcases when there is evidence of tumours (other than papillomata or haemangiomata) or of swollen lymph nodes (LN). Tumours in young animals normally arise from sporadic leukosis and not EBL; the latter being associated with tumours in animals aged three years or more.
Note: Swollen lymph glands identified in a live animal suffering from EBL will be painless.
5.1.3 Documentation
Animals from establishments under movement restrictions because of EBL may be moved to slaughter under licence from APHA (Form EBL9).
Reference: See Annex 6 on ‘EBL9 – Licence’ for a sample of the form.
Other animals licensed for slaughter from restricted establishments will not usually need to be inspected by an APHA Vet and the FSA should subject such carcases and their offal to normal meat inspection procedures, paying particular attention for evidence of tumorous change.
5.1.4 Dentition check
Whenever suspect disease is reported in a live animal, the APHA Vet will ask for the date of birth of the animal recorded in the cattle passport and whether either of the animal's second pair of permanent incisors has erupted – that is, whether there are more than two ‘broad teeth’.
If the answer is no, then in most cases no further action will be required other than the provision of outline data (APHA is required to keep a record of such cases for reporting to the EU), and the animal can be slaughtered and subjected to normal post-mortem inspection procedures and judgement.
5.1.5 Three or more permanent incisors
If either of the second pair of permanent incisors has erupted (there are three or more ‘broad teeth’), then APHA will instruct an APHA Vet to carry out an investigation, and the OV must ensure the animal is detained in the lairage pending this investigation.
5.1.6 After the investigation
Following the completion of the APHA Vet investigation, the animal may be slaughtered and subjected to normal post-mortem inspection procedures and judgement.
Appropriate samples of tumorous swollen lymph nodes should be taken from the carcase or offals at the request of the APHA Vet, where EBL has not been ruled out. As per instructions in section 5.2.2
The carcase and offal need not be detained pending the results of the tests on any collected samples.
5.2 Investigation of tumours in cattle carcases or offal
5.2.1 Tumours in cattle
All cattle tumours seen at post-mortem inspection are notifiable, with the exception of papillomata or haemangiomata and should therefore be reported IMMEDIATELY to the APHA Vet, who will note the details of all cases and instruct when sampling by the FSA is to be carried out.
A large proportion of tumour notifications concern animals aged less than two years. Although collection of tumour specimens from cattle with fewer than three permanent incisors is not normally required, APHA retains discretion to require sampling or to instruct an APHA Vet to carry out an investigation.
5.2.2 Sampling of tumours
When asked to do so, the FSA is responsible for collecting the appropriate samples from carcases and / or offal and retaining these along with details of the tumour site and the FCI. Cattle passports and FCI should always be retained by the FSA to assist APHA in the process of tracing.
The FSA will arrange for collection of the samples and complete all relevant details on the EBL7 submission form. Details to be included in the from include the WAS number provided by APHA. The FSA will prepare, pack and send the samples along with the completed submission forms to the laboratory.
FSA staff must positively differentiate between lesions which are tumorous (EBL) and those which are tuberculosis (TB) as different sampling and diagnostic testing is required.
The FSA will sample a tumorous carcase and / or its offal, the following 2 sets of samples should be collected:
- tissue samples for Polymerase Chain Reaction Test (PCRT)
- tissue samples for histology
5.3 Sampling of tumour carcases
5.3.1 Samples of PCRT
A PCRT has been developed to detect the presence of Bovine Leukosis Virus (BLV – the agent responsible for EBL infection) in cattle tissues and LN.
The PCRT requires fresh refrigerated samples.
5.3.2 Samples of histology
Samples for histological analysis are also needed as a backup should the fresh samples prove unsatisfactory for PCRT.
These samples should consist of a specimen from each of the grossly affected organs and representative enlarged LNs.
5.3.3 Collection of samples
Follow the steps in the tables below to collect the samples.
Note: Remove samples within 24 hours of slaughter.
Sample for PCR test:
| Step | Action |
|---|---|
| 1 | Use sterilised knives and gloves for each carcase |
| 2 | Take tissue sample from undisturbed part of tumour and from one accessible non-lesioned lymph node of 5-10g |
| 3 | Transfer sample to individual sterile 60 ml pot |
| 4 | Write ‘PCR Test’, ear tag number, Work Schedule Activity (WSA) and organ tissue sampled on label and stick on pot |
| 5 | Store chilled until dispatch by courier |
Sample for histology:
| Step | Action |
|---|---|
| 1 | Take sample from affected organs and representative enlarged LNs |
| 2 | Cut specimens about 1cm thick; a slice of organ should show both normal and diseased tissue |
| 3 | LNs should be transverse across the long axis of the node and should include the capsule |
| 4 | Transfer sample to individual sterile 60ml pot |
| 5 | Write ‘Histo Test’, ear tag number, case reference number and organ tissue sampled on label and stick on pot |
| 6 | Store chilled until dispatch by courier |
5.3.4 Post-mortem inspection
Once the required samples have been removed, the carcase may be subjected to normal post-mortem inspection procedures and judgement – it need not be detained pending the results of the tests for EBL.
5.3.5 Recording of post-mortem findings
Details of the tumour site should be recorded on the form EBL7, together with all available identification information. Complete only those parts of the form for which you have information; the remainder will be completed by APHA staff.
Reference: See Annex 7 on ‘EBL7 – Submission form’ for a sample of the form.
5.3.6 Notifying FSA
The OV should notify the Service Level Agreement (SLA) and Contracts team by email of the following details of the sample:
- Plant number
- Plant name
- Date case found
- passport number of the sampled animal, date of birth and breed
- name of owner and premises of origin or market lot number if applicable
- CPH number
- name of APHA office contacted
- case reference number assigned by APHA after initial notification
- date despatched via Topspeed
5.4 Packaging and despatch
5.4.1 Packing
- All samples must be submitted in a 60ml pot.
- Outside of pot must be kept clean.
- Remember to tighten lids. Give an extra turn before packing.
- Avoid cross threading the lids as they will cause the pots to leak.
- Place each individual pot in a plastic bag which is knotted tightly. Trim off excess bag.
- Place all bagged pots into a biobox / biobottle along with the absorbent pad / material and seal the box. The process for sending forms is as follows:
- Signed original EBL7 forms must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the original forms internally to the relevant APHA regional office
- Copies of the EBL7 forms should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. Copies of these forms should be faxed or emailed to the relevant APHA office. The OV should retain a further copy in the plant files for future reference (retention period 12 months).
- Place biobottle into the outer box.
- Attach address label.
- Attach security seal
- Store the package in the chiller until the time of collection. Ideally place in a waterproof bag / container to avoid contamination.
5.4.2 Despatch
The current courier for the new sampling process is Topspeed Couriers. The courier process is as follows:
As soon as you receive the sampling request information from APHA, email: ebl@topspeedcouriers.co.uk with the following information:
- establishment name and approval number
- slaughter date of the samples (this information will allow Topspeed Couriers to plan the collections to include multiple pickups where possible)
- destination laboratory:
BLV – PCR Virology Department
APHA Weybridge
New Haw
Addlestone
Surrey
KT15 3NB
- name and telephone number for the FSA contact at the plant
On detection of a tumour that needs samples submitting, notify the courier that samples are required to be collected. The courier will organise a collection which meets the two working days delivery requirement (for example, a tumour found on Monday; samples are required to be with APHA by 5pm Wednesday. However, collection could take place on Monday, Tuesday or Wednesday, as the couriers are required to consolidate their delivery runs to be cost effective.)
5.4.3 Ordering consumables
The OV at each abattoir is responsible for ensuring that there are sufficient supplies of consumables for packing samples. It is important that only the specified packaging materials (such as pots and labels) are used as failure to do so may result in the sample being un-assayable at the lab.
The consumables must be ordered directly from APHA Weybridge by using the following procedure:
- Fill in the requisition form (Annex 8a/b) specifying the type of materials required and the number of units.
- Make sure that you complete all the boxes (establishment name, address, FSA contact name and telephone number, and any others).
- The requisition form should be emailed to: StoresStockOrders@apha.gov.uk or faxed to APHA Weybridge: 01932 357497.
APHA will endeavour to complete delivery of consumables orders within 7 working days of receipt. If you have any queries regarding an order that you have placed you should telephone the APHA stores in Weybridge on 01932 359451.
6. Transmissible spongiform encephalopathies (TSE)
In this section
6.1 TSE overview
6.1.1 Introduction
This section outlines action to be taken when a TSE is suspected in an animal.
Instructions regarding sampling of animals when TSEs are not suspected can be found in the chapter 2.6 on ‘Transmissible Spongiform Encephalopathy’.
6.1.2 Information about TSEs
Information about TSEs is carried on Defra’s website.
Information about the clinical signs of the most relevant TSEs:
6.1.3 Reporting requirements
TSEs are notifiable diseases and their suspicion must be reported immediately to APHA.
6.1.4 Records
For all reported cases, the OV should ensure accurate details are recorded in the daybook.
6.2 Reporting suspicions
6.2.1 Suspect live animals
If FSA or plant staff suspect that live cattle, sheep, goats or deer are affected with Bovine Spongiform Encephalopathy (BSE), Scrapie or other TSE, they must take action as detailed in this topic.
The requirement of an OV to carry out the ante-mortem inspection of every animal is key for the identification of clinical suspects of TSE. OVs working in ruminant slaughterhouses must be aware of the clinical signs of TSE and take them into consideration during the ante-mortem inspection. Links with information about clinical signs of TSE including videos are available at point 6.1.2 of this section.
Caution: The OV, especially in the case of BSE, should be aware that an affected animal may, because of behavioural changes associated with the disease, be likely to cause injury to itself, other livestock or staff.
| Step | Action |
|---|---|
| 1 | Suspect animal is held in isolation in the lairage. On no account should a suspect animal be allowed to enter the main slaughterhall unless and until the OV is satisfied that it should no longer be considered a suspect. |
| 2 |
The OV telephones the APHA Vet to notify the suspicion of a TSE. There are two possible outcomes to the telephone conversation:
If 1 occurs then the OV should follow Option 1 below. If 2 occurs the OV should follow steps at Option 2 below. |
Option 1 The table below details the action to take if the APHA Vet cannot rule out the disease over the phone.
| Step | Action |
|---|---|
| 1 |
The APHA Vet answering the call makes arrangements for a APHA Vet to visit the slaughterhouse as soon as possible to carry out an investigation. APHA may request the following details:
|
| 2 | The OV obtains FCI and cattle passport before the APHA vet arrives |
| 3 | The FBO informs the owner of the animal |
| 4 | The APHA vet examines the animal and determines whether the disease can be ruled out or not on clinical grounds |
| 5 | The OV report by e-mail to the FSA incidents team and the FVL for the establishment, the suspected case including details of the animal (including eartag number, date of birth), scanned copies of relevant documents (for example, cattle passport, ARAMS document, FCI) and an update about the referral to APHA. |
Option 2 The table below details the action to take if the VO considers they are able to negate the disease over the phone.
| Step | Action |
|---|---|
| 1 | Updated [Once the case is negated, the OV shall carry out the ante-mortem inspection following the procedures as per 2.1.16 (Ante-mortem inspection summary in MOC Chapter 2.2)] |
| 2 | The OV report by e-mail to the FSA incidents team and the FVL for the establishment the suspected case including details of the animal (including eartag number, date of birth), scanned copies of relevant documents (for example, cattle passport, FCI) and an update about the referral to APHA |
6.3 At visit: APHA Vet does not suspect TSE
The APHA Vet considers that the suspect is not affected by BSE, Scrapie or other TSE.
Updated [Notifiable disease would then be ruled out and the OV should follow ante-mortem procedures as per 2.1.16 Ante-mortem inspection summary in MOC Chapter 2.2.]
Note: Certain bovine animals which are not considered to be BSE suspects require TSE testing (see chapter 2.6 ‘TSE testing’).
6.4 At visit: APHA Vet suspects TSE
6.4.1 Restrictions on animals
If the APHA Vet cannot rule out the suspicion of the disease on clinical grounds, they will serve restrictions on the animal. Once restricted, the FBO must not allow the animal to be slaughtered.
6.4.2 Slaughter and destruction
The APHA Vet will euthanize the animal by injection of barbiturate and arrange for the dead animal to be transported either to an incineration plant or a veterinary laboratory where the head will be sampled.
In the case of sheep or goats, if the suspect animal is considered fit to travel, the APHA Vet may make arrangements to transport it live under licence to the nearest available veterinary laboratory.
6.4.3 Restrictions on premises
No restrictions will be imposed on the slaughterhouse premises in the case of a TSE suspect, provided the animal was not slaughtered, although the APHA Vet may give advice on cleaning and disinfection in clinically positive cases.
6.4.4 Informing the FVL and the FSA Incident Team
The OV should inform their FVL and the FSA Incidents Team that a TSE suspect animal has been killed at or removed from an approved establishment by APHA staff.
7. Tuberculosis (TB)
In this section
7.4 Reactor animals: notifications and responsibilities
7.5 Reactor animals: inspection requirements
7.6 Reactor animals: actions when rejected at ante-mortem due to being dirty
7.7 Reactor animals: post-mortem decision
7.11 The slaughterhouse case: additional detailed inspection
7.12 The slaughterhouse case: sampling
7.13 Packing and despatch of samples
7.14 Private Slaughter of TB Reactors (Rs), Direct Contacts (DCs) and Inconclusive Reactors (IRs)
7.1 Introduction
7.1.1 Introduction
Bovine TB is an infectious and contagious disease of cattle and one of the biggest challenges for the cattle farming industry. It is caused by the bacterium Mycobacterium bovis (M. bovis), which can also infect and cause TB in many other mammals.
APHA is responsible for the control of TB in farms. The FSA, through an SLA, deals with sampling of tuberculin tested animals at APHA’s request and suspect TB lesions identified at slaughterhouses.
If TB is suspected in the carcase of any bovine, deer or farmed mammal, APHA must be notified immediately.
Reference: The Tuberculosis in Animals (England) Order 2021 and the Tuberculosis (Wales) Order 2010 (as amended).
Note: Health and safety procedures must be adhered to when handling suspect TB lesions. See FSA’s Health and safety manual.
7.1.2 Definitions
TB reactor plants are red meat slaughterhouses where reactor (and Direct Contacts (DC)) animals that have undergone a tuberculin test are sent for slaughter. Slaughterhouses access this status through a contract with APHA.
In some instances, where an agreement has been reached between APHA/FBO/ OV, the keepers can send Reactors, Direct Contacts, Inconclusive Reactors for a private slaughter at a slaughterhouse which is not a TB reactor plant. These are defined, in this context, as Rs / DCs/ IRs animals sent for a private slaughter.
Reference: See topic 7.14 on: Private Slaughter of TB Reactors (Rs), Direct Contacts (DCs) and Inconclusive Reactors (IRs)
Depending on the result of the tuberculin test, animals can be classed as reactors (R), inconclusive reactors (IR) and direct contacts (DCs). These animals can be compulsorily (R and DC) or voluntarily (IR) slaughtered.
Restricted premises are those farms where APHA has established cattle movement restrictions.
A full list of the movement licences for these animals and the relevant TB forms is given in the Annex list.
7.1.3 Timesheet coding
All work undertaken by the FSA on behalf of APHA (such as additional inspection requirements, Reactor tag checking, collection and submission of samples and record keeping) must be coded to GNTB.
7.1.4 Scope of the instructions
This section details instructions to FSA staff for dealing with reactors and other cattle from restricted premises, including:
- forms accompanying animals from restricted premises
- inspection of R, IRs and DCs
- death of R/IRs/DCs before reaching the slaughterhouse
- collection and submission of samples
- form completion
- carcases and offal from cattle with suspicious lesions encountered in the course of normal production, also known as ‘The Slaughterhouse Case’
- carcases and offal from other species with suspicious TB lesions
The instructions apply to:
- R and DCs compulsorily slaughtered by APHA
- IRs voluntarily slaughtered but for which APHA require samples, that is stock accompanied by a TB24 and where advance warning has been given by APHA by means of entering information on TB110 (reactor abattoirs) or via SLA and Contract team (elsewhere), whether alive or dead
- cattle and any other mammals that have been slaughtered in the course of normal production, where lesions consistent with TB are found during post-mortem inspection, also known as slaughterhouse cases.
They do not apply to other cattle from TB restricted herds.
Note: The OV must be aware that animals with clinical TB must not be slaughtered for human consumption.
Reference: Regulation (EU) 2019/627, Article 45(f).
7.2 Slaughter
7.2.1 Where or when to slaughter
Where animals have reacted positively or inconclusively to the tuberculin test, or there are other grounds for suspecting infection, they are to be slaughtered separately, taking precautions to avoid the risk of contamination of other carcases, the slaughter line and staff present in the slaughterhouse.
This applies to:
- cattle that require a TB24 movement licence and have been entered on a TB110 by APHA
- cattle that have a TB24 marked ‘Inconclusive Reactor’
- deer that require a TB24a movement licence and APHA has advised of intended slaughter by means of a TB55a form
- sheep or other mammals that were tuberculin tested
It does not apply to animals moved under any other licences, or with a TB24 where the animal is not included on a TB110.
To reduce cross-contamination, the slaughter line must be cleansed and disinfected after processing reactor cattle, IRs and DCs. All such cattle should either be slaughtered:
- last in the day, before full C and D of the slaughter line
- at any other time provided that the slaughter line is cleaned and disinfected before the slaughter of non-suspect animals resumes
- in a separate slaughterhall used for diseased animals or those suspected of being diseased
Reference: Regulation (EU) 2019/627, Article 33.
Any species with TB suspect lesions found during the course of post mortem inspection, particularly where there are no suitable facilities for detailed inspection and sampling in the dressing line, should immediately be placed in the detained area.
7.2.2 Transfer of carcases and offal to the detained facilities
When transferring offal / carcases to a detained area for further inspection or sampling, care must be taken to prevent cross-contamination of other meat / equipment / fittings in the slaughterhall. In the event of suspected contamination, C and D of the affected area / equipment must take place before production recommences.
Note: Failure by the plant operator to co-operate with this procedure would constitute a contravention of the operator’s responsibility to prevent cross-contamination and must be dealt with accordingly.
Reference: Regulation (EU) 2019/627, Article 33
7.3 Reactor animals
7.3.1 Types of animals
The table below shows the animals that may be despatched from TB-restricted premises.
| Consigned to slaughter | By | Examples |
|---|---|---|
| Compulsorily | APHA | Test reactors, DCs |
| Voluntarily | Herd owner | Fat stock, surplus calves, culled cows/which the herd owner chooses to slaughter |
7.3.2 Forms
In addition to the official identification documents and the FCI, animals from TB-restricted establishments may also be accompanied by one or more of the following forms:
- Emergency Slaughter Certificate
- TB24, TB24b, TB24c, TB24g, TB16b, TB24a, TB55a
- electronic notification by APHA via a TB110 sent to the OV by noon the day before the kill
Reference: See Annexes 9 to 14 for sample movement licences and FCI forms.
7.3.3 C and D of transport vehicles
All the cattle from bovine Tuberculosis restricted farms moved to slaughter, including animals with negative test results, are covered by a general of specific movement licence requiring the transport vehicle to be cleansed and disinfected with a disinfectant and concentration approved under Tuberculosis Orders. The OV must verify availability of the approved disinfectant during the routine attendance and verify its adequate use during the established C and D verification checks (see MOC chapter 2,2. Section 5). The list of approved disinfectants and concentrations.
7.3.4 Food chain information
All animals sent for slaughter must be provided with FCI.
Since some TB restricted animals are compulsorily slaughtered, the OV should verify that withdrawal periods have been observed for veterinary medicines and other treatments administered to the animals, this includes substances used for diagnosis purposes such as tuberculin.
Keepers submitting cattle from a farm with movement restrictions due to TB must declare this as part of the FCI. APHA requires all cattle moving for slaughter from TB-restricted herds to be marked with an orange stripe along the back. This is irrespective of test results so applies to animals moving under general licence as well as with movement licences.
The OV must be present on site during the processing of animals from a TB restricted farm.
Reference: Regulation 853/2004, Annex II, Section II and Regulation (EU) 2019/627, Article 10, 1
7.3.5 TB110 electronic TB sampling and submission form
APHA will submit electronically a TB110 form providing details of the reactor and DC cattle sent for compulsory slaughter and the sampling code that applies to each herd. This code determines the level of sampling that is required.
Note: In most of the cases, these animals will only be sent to selected slaughterhouses contracted by APHA for processing TB suspect cattle, excepting the animals subject to private slaughter. Contact the SLA and Contract Team for the current list of those slaughterhouses and the associated APHA TB diagnostic laboratory.
A number of Reactors/ DCs and IRs may be privately slaughtered by the owner. The owner can choose any non-APHA contracted cattle plant to slaughter them (as long as there are adequate inspection facilities and capability of the plant to process TB animals), but similar arrangements to those above apply.
APHA will e-mail a TB110 to the OV and other agreed FSA officers by noon the day before the kill date.
The TB110 must be completed after post-mortem inspection, recording the findings. The process for sending the forms is as follows:
- signed hard copy TB110 must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle; APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office
- copies of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox; a copy of the forms should be faxed or emailed to the relevant APHA office; the OV should retain a further copy in the plant files for future reference (retention period 12 months)
Reference: See Annex 15 on ‘Sample: TB110 Reactor Sampling and submission form’ for a sample of the form.
7.3.6 TB55a movement licences
Form TB55a is the proposal to slaughter deer. It will inform the OV of the arrival of deer from a restricted TB premises.
A copy of the TB55a will be sent by fax to the OV in advance.
Reference: See Annex 19 on ‘Sample: TB55a’ for a sample copy of the form. See The Tuberculosis in Animals (England) Order 2021.
Note: Reactor deer moved for slaughter under movement licence must have a broad arrow 15 cm long clipped on the left hind quarter.
7.3.7 TB24 movement licences
Form TB24 is a movement licence issued by APHA authorising transport of cattle (reactors, IRs, DCs and any cattle from TB restricted herds that have not been tested for TB) to a slaughterhouse. It must accompany animals during transport. Most animals accompanied by a TB24 need to be slaughtered separately, and if they appear on the TB110, inspected in detail.
Some cattle that are not reactors, IRs or DCs may travel to slaughter under a TB24. These cattle do not in principle have a higher risk of infection with TB than other cattle from restricted herds.
Since the EC regulations require that animals that have reacted inconclusively to the tuberculin are to be slaughtered separately, APHA will mark the TB24 of these animals with the words ‘Inconclusive Reactor’.
When animals that should have arrived with a TB24 are found not to have one, this should be reported to APHA and the relevant Trading Standards department.
Reference: See topic 7.2.1 on ‘Where or when to slaughter’ onwards in section 7.
Reference: See Annex 9 on ‘Sample: TB24’.
7.3.8 TB24a movement licences
Form 24a is a licence issued by APHA authorising movement of deer to a slaughterhouse. It must be given to the FSA representative on arrival to the slaughterhouse.
A copy of the TB24a will be sent by fax to the OV in advance.
Note: For welfare reasons the deer should be slaughtered within 3 hours of arrival at the slaughterhouse and shall not be removed from there alive.
Reference: See Annex 18 on ‘Sample: TB24a’ for a sample copy of the form.
7.3.9 TB24b/g/h movement licences
Form TB24b is a movement licence issued by APHA authorising transport of cattle, listed by ear tag, from TB restricted herds to a slaughterhouse via an approved TB slaughter gathering (collection centre / market).
Form TB24g is a licence authorising movement of cattle from approved finishing units under restrictions to a licensed slaughterhouse via an approved TB slaughter gathering (collection centre / market).
Form TB24h is a licence authorising movement of cattle from approved finishing units or licencing finishing units under restrictions to a licensed slaughterhouse via an approved TB slaughter gathering (collection centre / market.)
Animals eligible for a TB24b/g/h are not considered reactors, IRs or DCs. They need only be subject to normal inspection procedures.
Reference: See Annex 12 on ‘TB24b’ for a sample copy of the form and Annex 13 on ‘Sample TB24g’ for a sample copy of the form and Annex 32 for sample copy of TB24h form.
Note: Bovine animals moved to slaughter under the authority of TB24b are permitted to travel on the same vehicles with bovine animals from other restricted premises transported under a similar licence, as stated in TB24b.
7.3.10 TB24c movement licences
Most clear testing cattle and calves under 8 weeks of age travelling direct to slaughter from holdings under TB restrictions, no longer require a specific TB24/TB24b licence. These animals can be consigned to slaughter by their owners under the terms of a general movement licence (TB24c), issued by the APHA at the time the herd is placed under restrictions.
Herd owners who are granted a general TB24c licence will not be required to forward a copy to the slaughterhouse, nor will it be necessary for a copy of the general TB24c licence to travel with the animals.
These animals, as with all cattle from a TB restricted herd, should be identified by means of an orange stripe along the back and FCI should indicate the herd is under restriction, but they will be subject to the normal inspection procedures.
General TB24c licences will automatically expire on lifting of TB restrictions. APHA retains the power to rescind a general movement licence at any time.
Reference: See Annex 10 on ‘Sample: TB24c’ for a sample copy of the form.
7.3.11 Exclusions from general licence (TB24c)
Reactors, IRs, DCs and any untested cattle aged 8 weeks or more are explicitly excluded from the general licence and will continue to be licensed to slaughter by APHA, under a specific TB24 travelling with the animal.
Animals may arrive at the slaughterhouse accompanied by TB24s prior to the OV receiving notification from APHA. In these circumstances, FSA staff should inform APHA of the arrival of such animals and wait for instructions.
7.3.12 TB16b movement licence
TB16b movement licences are issued to authorise movement of ear tag listed cattle from restricted premises to Approved Finishing Units, Approved Quarantine Unit or to a slaughterhouse through a Dedicated Sale for TB Restricted Cattle. These animals have passed a tuberculin test in the 90 days before movement and are not reactors, IR or DC. The licences should accompany the animals to the abattoir but, as with animals moved under a TB24b/g, they need only be subject to normal post-mortem inspection procedures.
Reference: see Annex 11 on ‘Sample: TB16b’ for a sample copy of the form.
7.3.13 FSA copy of licences
The person transporting the animals, on arrival at the slaughterhouse, must give a copy of the TB24, TB24b, the TB24g, TB24h TB16b, TB24a or the TB55a licences to the FSA representative.
The table below shows which forms, licences and certificates accompany which animals to the slaughterhouse.
| Form / licence | Reactors | DCs | IRs | Cattle not tested for TB | Clear-testing cattle and calves under 8 weeks | On-farm slaughter |
|---|---|---|---|---|---|---|
| FCI | Yes | Yes | Yes | Yes | Yes | Yes |
| TB110 | Yes | Yes | Yes | No | No | Yes |
| TB24 | Yes | Yes | Yes | Yes | May happen | No |
| TB24b | No | No | No | No | Yes | No |
| TB24c | No | No | No | No | Yes | No |
| TB24g/h | No | No | No | Yes | No | No |
| TB16b | No | No | No | No | Yes | No |
| TB24a (deer) | Yes | Yes | Yes | No | No | No |
| TB55a (deer only) | Yes | No | No | No | No | No |
7.3.14 Irregularities
APHA will contact the OV if, after submission of the TB110, there is any change to the number of cattle sent for slaughter or to the sampling code.
Note: in some cases fewer cattle may be delivered than expected, but never more than pre-arranged.
If the OV believes that animals from a TB restricted establishment have been presented for slaughter without all the necessary documentation, they should inform APHA and the LA.
APHA should also be contacted if, due to missing paperwork, conflicting information, or any other circumstances, the OV is not sure if an animal from a TB restricted establishment requires detailed post-mortem examination and sampling.
7.4 Reactor animals: notification and responsibilities
7.4.1 Overview of responsibilities
Type: Reactors, IRs and DCs
| Responsibility | Duty |
|---|---|
| APHA |
|
| FSA |
|
7.5 Reactor animals, inconclusive reactors and direct contact cattle: inspection requirements
7.5.1 Additional detailed inspection
A detailed inspection must be carried out on animals included in the following categories:
- Reactor or direct contact cattle compulsorily purchased and slaughtered by APHA at contracted slaughterhouses. (These animals must arrive at the slaughterhouse with FCI advising they originate from a restricted herd, a movement licence (TB24), and be listed on the TB110.)
- Reactors, DCs or IR cattle privately slaughtered (these will be accompanied by the same documents as above but they may be sent to any slaughterhouse). When samples are required for animals in this category, APHA will submit the TB110 form to the FSA staff at the selected slaughterhouse.
- Deer compulsorily purchased and slaughtered by APHA.
- Other farmed non-bovine animals (pigs, sheep or goats) compulsorily purchased and slaughtered by APHA.
In the case of reactor animals, IRs and DCs the following LNs and organs must be examined in detail (visual inspection, palpation and incision) if they have not been examined already:
- Retropharyngeal LN*
- Parotid LN
- Submandibular / Submaxillary LN
- Bronchial* and Mediastinal* LN
- Lungs*
- Pleura
- Hepatic LN
- Liver
- Mesenteric LN (representative sample)
- Supramammary LN
- Udder**
- Prescapular LN
- Superficial inguinal LN
* Tissues where tuberculosis lesions are most commonly found
** See subtopic below
Note: Additional examinations of any other lymph nodes, such as those enlarged and / or haemorrhagic, may take place whenever considered necessary.
Reference: Regulation (EU) 2019/627, Article 14.
7.5.2 Udder inspection
The inspection of udders from reactor cattle is particularly important as they are not routinely incised unless they are for human consumption. In addition to the visual inspection and incision of the supra-mammary LNs, the udder of cows must be visually inspected and palpated. If abnormalities are found during these, or when the udder is intended for human consumption, then deep incisions must be done into each quarter of the udder as far as the lactiferous sinuses.
Reference: Regulation (EU) 2019/627, Article 19, 2 (g).
7.5.3 Incision method
Cuts into the LNs should be made across the node in at least two directions (criss-cross pattern) to reveal as much as possible of the core of the node. Care should be taken to examine the tips of the node. This method will reveal most TB lesions or reveal an area which appears abnormal which can be further incised.
Lesions in the lungs, liver and udder are most commonly found on inspection or palpation. Where abnormalities are felt on palpation the abnormal areas should be incised for further investigation. Careful small incisions at the border of the lesions should be made to reduce exposure to infective material. If the lesion is found to be typical of TB, no further incision is required into that lesion.
7.5.4 Hygiene precautions
Any equipment used to incise or examine the LNs must be cleansed and sterilised before undertaking post-mortem procedures on subsequent carcases, including changing of gloves in between different carcases/ sets of offal, when lesions are identified.
7.6 Reactor animals: actions when rejected at ante-mortem due to being dirty
Whenever a TB Reactor animal is rejected at ante-mortem inspection because it was dirty and it could not be processed hygienically, the OV must inform APHA by reporting through the ‘LA notification form: welfare breaches’ found in Chapter 2.3 on Animal Welfare, Annex 4, as an animal welfare concern, including a picture(s) of the rejected animal(s) and its ear tag.
The LA notification form is to be submitted to the CSC one health welfare mailbox CSCOneHealthWelfare@apha.gov.uk like other animal welfare referrals.
The OV will also complete the relevant details of this event in the comments box of the TB110 form, describing the reason why the animal was rejected, for example: when presented for slaughter the animal was not clean and it could not be processed hygienically, adding details of the nature of the contamination.
7.7 Reactor animals: post-mortem decision
7.7.1 Judgement of meat
Decision on whether meat is fit for human consumption is based on the findings during post-mortem inspection.
Where there are indications of generalised TB or TB lesions with emaciation the entire carcase and all the blood and offal should be rejected as unfit for human consumption.
All meat from animals in which post-mortem inspection has revealed localised TB in a number of organs or a number of areas of the carcase are to be declared unfit for human consumption. However, when a TB lesion has been found in the LNs of only one organ or part of the carcase, only the affected organ or part of the carcase and the associated LNs need to be declared unfit for human consumption.
Reference: Regulation (EU) 2019/627, Article 33
7.8 Reactor animals: sampling
7.8.1 Relevant animals
In general, the collection of diagnostic samples by the FSA is limited to reactors and DCs compulsorily slaughtered and some reactors, DCs or IRs which have been privately slaughtered (cattle entered on a TB110 as requiring detailed post-mortem inspection).
When reactors, DCs or IRs arrive to a non-contracted plant (considering that farmers do have the option of private slaughter), APHA will issue a TB110 and advice on the sampling protocol. These animals cannot be considered / treated as slaughterhouse cases.
7.8.2 Responsibility for collecting samples
APHA, before sending animals to the abattoir, will provide the OV with the details of likely numbers and sampling protocol 48 hours in advance and will then submit electronically to the OV a copy of the TB110 (see Annex 15) by noon the day before the kill date. The form will include:
- the number of animals to be sent from each holding
- the reason for submission (reactor, IR, DC)
- the sampling code for each batch
Once the required samples have been collected the carcases and offal can be released if they have been found fit for human consumption.
7.8.3 Death of reactors / DC / IR on arrival or in lairage
In the event of a Reactor being found dead on arrival (DOA), or dead in the lairage (DIL), the OV must contact APHA and explain the circumstances. APHA will inform the OV if any diagnostic samples for TB are to be collected.
Reference: The OV must be aware of the requirement to test for TSEs in O48M/O24M DOA or DIL bovines as per instructions in chapter 2.6 on ‘TSE Testing’ and also consider the possibility of anthrax.
7.8.4 Sampling codes
APHA will request a sampling protocol for suspect animals from each farm using three sampling codes (SC1, SC2 and SC3). The sampling codes are allocated by APHA depending on the herd history and its current status. In addition, APHA will indicate whether additional or exceptional sampling is required.
| Sampling code 1 | Sampling code 2 | Sampling code 3 |
|---|---|---|
| Visible lesions (VL) | Visible lesions (VL) | Visible lesions (VL) |
|
For animals from herds in England or Scotland, collect samples from maximum of 3 VL animals per herd. No NVL samples required For animals from herds in Wales (CPH starts 52-60), collect samples from maximum of 3 VL animals per herd. No NVL samples required |
Do not collect samples unless APHA request |
Collect samples from maximum of 3 VL animals per herd. Do not collect samples unless APHA request |
| No visible lesions (NVL) | No visible lesions (NVL) | |
|
For animals from herds in England or Scotland, submit samples from 10 animals per herd (or from all if less than 10 animals) * For animals from herds in Wales (CPH starts 52-60), submit samples from 3 animals per herd (or from all if less than 3 animals) ** |
Do not collect samples unless APHA request |
*APHA will indicate which 10 need to be sampled where all are NVL and more than 10 cattle are submitted from each farm
**APHA will indicate in the Specific Info box of TB110 which 3 animals need to be sampled where all from the same farm are NVL.
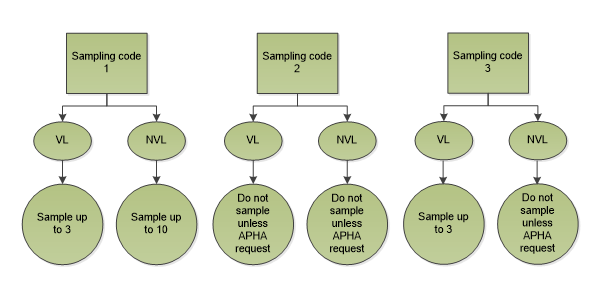
7.8.5 Sampling code 1: typical lesions identified (VL)
All lesions typical of TB should be collected when required (sampling code 1 and 3 or sampling code 2 with specific request from APHA).
A typical lesion is where infection with M bovis is suspected and common colours (cream / yellow) and common consistency (caseous / calcified / purulent) are identified.
TB lesions in pigs are generally whitish-yellow granulomatous lesions which may contain areas of calcification. When collecting these suspected TB lesions, those with the most characteristic TB lesions should be chosen and only tissue samples from a maximum of three VL from pigs from the same herd should be collected.
APHA has defined a VL as a lesion that is visible to the naked eye and typical of infection with M bovis.
Lesions due to skin TB should not be collected and will not be classed as VL.
All the lesions from each carcase should be pooled and placed in a single sealed 60 ml plastic pot to give one submission per animal. The samples should be two-thirds of the pot and should include the lesion plus some normal tissue from the border of the lesion, where possible. However, this may result in a large amount of tissue if a carcase presents multiple TB lesions. In this situation, sample only the two most characteristic lesions; however, if the lesion in its entirety does not fill two-thirds of the pot, please include comments to that effect in the relevant comments box of the form.
Note: Unaffected LNs must never be submitted when typical TB lesions have already been found in the same carcase.
7.8.6 Sampling code 1: typical lesions not identified (NVL)
NVL are those where no lesions typical of infection with M bovis are visible to the naked eye.
While this is not part of the APHA definition of NVL, for practical purposes this includes both where no lesions are found and where there are lesions that can be seen but infection with M bovis has been ruled out.
Where no lesions are found it is necessary to collect samples from all the following LNs:
- all bronchial and mediastinal LNs
- paired retropharyngeal LNs
- any other LN if enlarged, abnormal and / or haemorrhagic
Section 7.8.6 will also apply to non-bovines if a skin testing regime is in place.
Note: As of 1st September 2023, the MHI and OVs would no longer be required to collect samples from NVL TB reactors from Welsh holdings (CPH 52-60) in two separate pots. These samples can be collected in a single pot by pooling broncho-mediastinal and retropharyngeal LN, as per the procedure for samples from NVL TB reactors originating from English or Scottish holdings.
7.8.7 Sampling code 1: atypical lesions identified
An atypical lesion is a lesion where infection cannot be definitely attributed to M bovis and where common colours (cream / yellow) or common consistency (caseous / calcified / purulent) are not identified, but where infection with M bovis cannot be ruled out.
Please note that an atypical lesion is neither a VL nor NVL for reporting purposes.
In terms of case management, APHA treats such lesions as NVL. Therefore, an atypical lesion should be recorded as NVL in the TB110, and ‘atypical lesions’ note entered in the TB110 comments box. The lesion descriptions should clearly reflect that it is atypical having ‘A’ at the end (e.g. M3YMxA).
If both typical and atypical lesions are found on the same carcase, submit samples from the typical lesion only. The only exception to this is when suspect udder / supra-mammary lesions are found; these should be submitted in addition to the typical lesion and in a separate pot (one per holding).
Where only atypical lesions are found, sample a pool of LNs and record as NVL but also collect and send the atypical lesion in a separate pot.
This should only be used where a decision cannot be made and the possibility of infection with TB cannot be ruled out.
When lesions are found in mesenteric lymph nodes in reactors from herds in Scotland, these should be treated as atypical, recorded as NVL in the TB110, and ‘atypical lesions’ note entered in the TB110 comments box.
7.8.8 Sampling code 2
Where APHA has allocated a sampling code 2 to a batch of animals there is no need to collect any samples, with only two exceptions:
- APHA may specifically request samples in certain cases.
- Where atypical lesions are found and there are no typical lesions in any animal from the same herd, sample the atypical lesion only and send for polymerase chain reaction (PCR) testing, making remarks to that effect on the ‘specific information’ section of the TB110.
7.8.9 Method
Each animal from which samples are needed must be individually sampled. Samples from more than one animal must never be pooled in the same pot. Care must be taken to prevent cross-contamination.
The following method should be used to collect samples for TB diagnosis.
| Stage | Description |
|---|---|
| 1 | Collect samples cleanly to limit contamination. Ensure the equipment used for inspection and sampling of carcases is disinfected between carcases to prevent the possibility of cross-contamination. |
| 2 | Dissect samples free of surrounding tissues to limit the volume of tissue submitted. Samples should be as fat and muscle free as possible. |
| 3 |
Where the carcase had VL or NVL samples are to be treated as follows: VL: Remove suspicious node or lesion in its entirety if small or a sample the size of 2/3 of a pot if large and pool up to two of the lesions from the same area of the carcase in a pot. If the lesion in its entirety does not fill 2/3 of the pot please include comments to that effect in the relevant comments box of the form. NVL: Pool LNs collected from the same carcase and place in a pot. The 60ml pot should be 2/3 full. If there are any atypical lesions, collect separately from pool. |
| 4a | Mesenteric chain LNs should only be collected when no other lesions are present. They must not be included in the pooled sample and must be collected separately from other LNs from the same carcase. This is to minimise contamination of the pooled sample with bacteria that could inhibit the growth of M. bovis in the laboratory. |
| 4b | Suspicious lesions in the supramammary nodes should always be submitted from any carcase (max. 1 per CPH). As for mesenteric nodes they should not be included in any pool of samples they need to be submitted in a separate pot. |
| 5 | The OV must be present in the slaughterhall during the post-mortem inspection to ensure that the correlation is maintained and that findings are accurately recorded for each carcase. The OV must also ensure that the samples are secured prior to despatch. |
| 6 |
APHA requires complete and accurate records of all findings from each animal, including those from which no samples have been taken, in the electronic form (TB110). This information will be used in deciding the future management of the herd. The completed form must be e-mailed to APHA (at the email address from which the TB110 originated) before despatch of samples (by 3pm if samples sent to the lab on the same day, or by noon next day when the samples are despatched the following day). If samples are collected, the TB110 must also be emailed to the APHA laboratory (TBDiagnosticTeam@apha.gov.uk). A hard copy of the TB110 must be signed by the OV and should be faxed without delay to the relevant APHA office. The signed hard copy must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office. A copy of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. The OV should retain a further copy in the plant files for future reference. |
| 7 | Each sample pot must have a unique traceability label stuck on the outside of the pot. The outside of the pot must be kept clean and the lids must be tightly closed to prevent leakage. In the event of the pot getting wet, it must be dried to ensure that the traceability label can be affixed when the sample is placed inside the pot. To maintain traceability, pots must be labelled before being moved from the slaughterhall. Each pot must then be placed inside a bag which is knotted tightly and excess bag trimmed off. |
| 8 | If more than one pot is submitted for a single animal (i.e. pool in one pot and atypical lesion in a separate pot) place all the individual sample pots, each in its own bag. |
| 9 | All bagged pots must then be placed in a biobox or biobottle (depending on number of pots) which is sealed. A copy of the completed forms must then be placed in a ziplock bag which is taped to the outside of the biobox. |
| 10 | Further packaging (box / bag) is then applied in line with courier instructions (see topic 7.12 on ‘Packing and despatch of samples’). |
| 11 | Retain chilled, pending their collection by a courier for transfer to the APHA laboratory. They must not be frozen unless instructed to do so by APHA. If frozen the sample and the packaging must be marked: ‘frozen sample’. |
7.8.10 Sampling code 3
Where APHA has allocated a sampling code 3 to a batch of animals, only VL samples need to be taken up to a maximum of 3 animals per specific holding.
Check the ‘specific information’ column of the TB 110 form because in some cases only 1 or 2 samples per holding may be required by APHA.
Please ensure that at least 2/3 of the pot is full when collecting the sample.
NVL lesions do not need to be submitted with this sampling code.
7.8.11 Completion of sampling and submission from (TB110)
The TB110 has two parts.
- The first will be completed by APHA with details of the holding, CPH number, ear tags, any other relevant information and the sampling code that applies to each batch.
- The second part must be completed by the FSA and be signed by the OV. The findings in each carcase, including those for which samples are not required, must be recorded using codes to identify the LNs / tissues and the description of the lesions where applicable (see below).
Where lesions are found in the lungs and / or udder suggestive of possible discharge of bacilli to the exterior (open tuberculosis) this has epidemiological importance and should be recorded in the comments box of the TB110.
The form must be sent electronically on completion to the originating email address. A hard copy of the TB110 must be signed by the OV and should be faxed without delay to the relevant APHA office. The signed hard copy must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office.
A copy of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. The OV should retain a further copy in the plant files for future reference
The TB110 must also be sent electronically to the APHA laboratory (TBDiagnosticTeam@apha.gov.uk) and a signed hard copy must accompany the samples.
7.8.12 Completion of TB50/TN50/TN60
The TB50 form is used to record post-mortem findings on suspect TB carcases of bovine animals (cattle, buffalo and bison).
The TN50 form is used to record post-mortem findings in all farmed non-bovine species such as deer, pigs, goats and sheep.
The TN60 form is used to record post-mortem findings in wild deer carcases only.
These three forms are to be used for ‘The slaughterhouse case’ only (see section 7.10).
Note: There is no need to complete TB50/ TN50 forms for bovine/ non-bovine reactors slaughtered at APHA contracted abattoirs, as the post-mortem findings are collated on the TB110.
Reference: For copies of these three forms see Annex 16a ‘Sample: TB50’, Annex 16c ‘Sample: TN50’ and Annex 16d ‘Sample: TN60’.
Bovine species:
The details of each case need to be recorded on a TB50 form signed by the OV and emailed to the APHA mailbox specified on the form, so that TB Officers can make a decision as to whether samples must be sent to the laboratory. Please note, each slaughterhouse case detected requires a separate sample submission form (TB50) to be completed.
Non-bovine species:
In England and Wales, for cases reported in non-bovines, APHA will not issue a WSA ID number, therefore this does not need to be recorded at the top of the TN50 form. In Scotland, a WSA number will be supplied by Scottish Government to the OV once the suspicion has been reported. Please note, each slaughterhouse case detected requires a separate sample submission form (TN50) to be completed.
A copy of the TN50 form is to be emailed ASAP to the relevant email address: see 7.10.5 for details for the different countries. In addition, a copy of the TN50 should be emailed to sla.contracts@food.gov.uk. No samples should be submitted or discarded without authorisation from APHA.
Wild deer:
For wild deer carcasses brought to FSA establishments for post-mortem inspection, the detection of suspect TB lesions must be reported to APHA. The completed TN60 form must be emailed to the APHA mailbox specified on the form, so that TB Officers can make a decision as to whether samples must be sent to the laboratory.
7.8.13 Codes used to complete the TB forms
Codes will be used to describe the lesions, with six criteria used: location, number, size, colour, consistency / texture and presentation.
- Location: Retropharyngeal (RP); Parotid (PA); Submandibular / Submaxilary (SM); Bronchial and Mediastinal (BM); Lungs (Lu); Pleura (Pl); Hepatic (HEP); Liver (Li); Prescapular (PSc); Superficial Inguinal (SI); Mesenteric (MES); Supramammary (SMA); Udder (U); Other (O)
- Number:
- Single (S) – a distinct single lesion in the LN / organ
- Multiple (M) – up to 6 distinct lesions in the LN / organ
- Diffuse (D) – multiple lesions throughout the LN / organ that may or may not coalesce / lesion spreading over a large area, not concentrated
- Size:<2mm – (1); 2-10mm – (2); 11-50mm-(3); >50mm- (4)
- Colour: Cream (C); Yellow (Y); White (W); Other (O)
- Consistency / texture: Caseous (Ca); Calcified (Cf); Purulent (P); Granulomatous (Gr); Mixed [Ca and CF] or [Ca and P] (Mx)
- Presentation: Typical (T); Atypical (A)
For atypical lesions if the description cannot be provided from the above options a description can be entered in the comments box.
Reference: a template for recording findings on the line during post-mortem inspection is available at Annex 17 on ‘Description of lesion template’.
Note: For packing and despatch of samples, please see topic 7.12 on ‘Packing and despatch of samples’ later in this section.
7.9 Reactor tag sampling
7.9.1 Overview
The aim of this programme is to compare the ears collected from TB reactors in order to audit fraudulent procedures in relation to reactor removal. This will be audited by cross matching 2 tissue samples:
- tissue collected in the DNA capsule when tagging TB reactors at the time of the TB test
- tissue taken from the ear of TB reactors at the point of slaughter
The Reactor Ear testing programme will comprise of 3 elements:
- targeted collection where FSA have identified at point of slaughter possible tampering with tags, either official or reactor tags, or missing reactor tags
- targeted collection where APHA identify a risk and request FSA to collect both whole ears (which do not have to be connected), from specifically identified animals
- random collection of the required number of ears selected by FSA at each slaughterhouse on a monthly basis
The OV at contracted TB Reactor slaughterhouses will prepare a protocol (already known also as authorised method of operation) to summarise and ensure that the previously described instructions for the processing of TB Reactor animals are followed. Guidance for the preparation of such protocol can be found in Annex 15b.
7.9.2 Notification to slaughterhouse / FSA of reactor details
Animals submitted for slaughter for TB control will either be R or DCs and will be sent for slaughter in one of the following ways:
- submitted as part of haulage and salvage to one of the slaughterhouses contracted by APHA to process TB reactors
- private slaughter organised by the owner but moved under licence issued by APHA
DCs will not have reactor tags and are excluded from this programme, however any other suspicion of fraud should be investigated as described in the MOC.
Most TB reactors will have a reactor tag applied. However, there are a few exceptions to that rule where reactors may not be tagged and are considered ineligible categories:
- reactors identified following skin test re-interpretation (standard to severe) after PM/ PCR (or culture) results
- animals have not been tagged at Tuberculin Test day 2 (test reading day) for operational reasons
- gamma positive reactors
The assumption is therefore that apart from those ineligible for this programme all reactors disclosed at a skin test and entering the slaughterhouse will be marked with a reactor tag. In the comments box of the TB110, the following reasons will be given to indicate that an animal will not have a reactor tag and is ineligible:
- ‘tag not applied’ where APHA are aware that an animal has not been tagged for any reason
- ‘re-interpretation’ where an animal became a reactor after the skin test due to re-interpretation of the skin measurements
- ‘gamma’ where an animal has failed the gamma interferon test
7.9.3 Action when animal arrives at slaughterhouse
Apart from those specifically requested by APHA, the level of reactor animal identity checking by FSA should be as per existing instructions in the MOC.
Where FSA undertake an identity check, the following details should be compared with the information submitted to them by APHA:
- ear tags match the cattle passport
- reactor tag present if not reported as ‘tag not applied’ or one of the categories not eligible for tagging (re-interpretations or gammas)
The following action should be taken:
- record findings, on ID checklist or FBO sheets where applicable
- check if any evidence of tampering or other fraud
- if evidence found, notify LA Trading Standards as per existing processes and retain relevant part of the animal
7.9.4 When is an ear sample required?
The reactor tag scheme requires a sample (comprising both whole ears and all tags present in those ears) to be collected from any animal which comply with one of the criteria described below.
A sample will be required in the following circumstances unless otherwise instructed. The FSA Targeted and the APHA Targeted may be required in slaughterhouses in England and Wales whilst the FSA Random samples are required in slaughterhouses in England and Wales:
- FSA targeted – Whenever a FSA Operations Group officer finds evidence of fraud, the tag has been tampered with or other ID non-compliance (NC).
- For example, reactor tag missing when expected to be present (TB110 will state if ‘not applied’ or one of the other ineligible categories), ear tags tampered with, indecipherable documentation, animal does not appear to match that expected (age, breed, sex). Guidance is being produced, that gives details of what constitutes ear tag tampering.
- APHA targeted - When requested by APHA, Intelligence led targeted examination of animal ID and sampling.
- APHA will state ‘COLLECT EARS’ in the TB110 comments box when ears are required to be collected.
In exceptional cases APHA may contact the FSA representative at a slaughterhouse (by phone) to request an urgent identify check and request ear samples to be taken.
- FSA random – random testing process.
- Slaughterhouses in England, 3 samples should be collected every month at each of the slaughterhouses which regularly receive reactors. The random samples should only be taken from reactors which originate from England and which have reactor tags.
- Slaughterhouses in Wales, 3 samples should be collected every month at each of the slaughterhouses which regularly receive reactors. The samples should be taken at different times in the month and not together and only from animals with reactor tags.
- Random samples should not be collected on a Friday although targeted samples may have to be taken.
For all other animals, that is TB reactors that have not had a reactor tag applied or Direct Contacts, any suspicion of fraud should be investigated as described in the chapter 6 on ‘notifiable diseases’, section 7.
7.9.5 Collection of sample, packing and despatch of ear samples from FSA
For continuity of evidence all processes should be completed by the same person (removal of the ears, completion of sample submission form, labelling and bagging in tamperproof / evidence bag and packaging of samples packed for dispatch).
The following protocol should be followed:
A Preparation of packing systems:
| Step | Action |
|---|---|
| 1 | The packing materials consist of the following:
|
| 2 | Biotherm 5 boxes have been issued for routine sampling and only one pair of ears should be packed in this system. In the event multiple sample collection is required (targeted sampling) the Biotherm 10 and 15 systems should be used and will be supplied by APHA. |
| 3 |
All Biotherm systems will be supplied by APHA and need to be prepared for first initial use; once preparation has been completed, using the protocol below, the systems can be re-used and will be returned by APHA. Nett Qty: One sample Dry Ice: less than 1 kilogram. Name and telephone number of responsible person: FSA contact name and number Ice Brix must be ‘hard’ frozen before use, x2 Ice Brix should be sufficient for the Biotherm 5 system. |
| 4 |
On the lid of the box complete legibly and accurately. 
a) Consignee details with: APHA TB DNA Testing b) Consignor details with: Full Address of the abattoir |
| 5 | Open the box and remove the labels supplied, place to one side. |
| 6 |
On the front panel stick the UN3373 label in one of the pre-marked diamonds and place the Biological Substance Category B label adjacent to the UN3373 diamond (see photographs). 

|
| 7 |
Discard the Infectious Substance label; this must not be used. |
| 8 |
Complete legibly and accurately the front panel: Proper shipping name: Biological Substance Category B UN Number: UN3373 Nett Qty: One sample Dry Ice: less than 1 kilogram. Name and telephone number of responsible person: FSA contact name and number Ice Brix must be ‘hard’ frozen before use, x2 Ice Brix should be sufficient for the Biotherm 5 system. |
B Notify APHA that ear samples have been taken:
| Step | Action |
|---|---|
| 1 | Whenever ear samples are taken, FSA abattoir staff must notify APHA Central Tagging Team that a sample has been taken and submitted to APHA Lab at Weybridge. |
| 2 |
A copy of the signed sample submission form (Annex 22 on ‘Material for DNA analysis’) should be scanned and emailed to the APHA central tagging team at: CSC.TBDNATagging@apha.gov.uk |
C Collection and preparation of ears (x1 pair):
| Step | Action |
|---|---|
| 1 | Place the pair of ears (from the same animal) into a grip seal bag (8” x 11”); remove any excess air from the bag and seal. |
| 2 | Place the bagged sample (x1 pair of ears) inside another grip seal bag (8” x 11”), add an absorbent pad, remove excess air and seal. |
| 3 | Place the ‘double bagged’ sample (x1 pair of ears) into the tamperproof / evidence bag and seal to meet continuity of evidence requirements. |
| 4 | Complete legibly and accurately the tamperproof / evidence bag in the section marked ‘FSA Use Only’. |
| 5 | Put in the refrigerator or freezer for chilling. This will reduce excessive moisture collecting in the bag. |
| 6 | Complete the sample submission form legibly and accurately. If samples have been taken due to evidence of tampering, ensure the tampering suspected box is ticked on the sample submissions form. |
| 7 | Send a copy by fax to APHA Central Tagging Team (as above at B step 2) and place in a grip seal bag (8” x 11”) remove excess air and seal. |
| 8 | Add the hard frozen Ice Brix to the Biotherm system and place the sample next to the Ice Brix (x2 Ice Brix per biotherm system). |
| 9 | Place the sample submission form on top of the sample (inside a plastic bag), close the polystyrene lid (expanded polystyrene), close outer flaps and seal with security label or brown tape. Where samples from more than one animal are in the box, ensure the bag containing the sample submission form is attached to the corresponding tamperproof bag. |
| 10 | As soon as you receive the sampling request information from APHA, email the APHA Preferred Courier (currently Topspeed Couriers at tb@topspeedcouriers.co.uk) with the following information:
|
D Preparation of biotherm replacement of outer box:
| Step | Action |
|---|---|
| 1 |
If the outer carton becomes damaged a replacement carton should be obtained and prepared for use, using the protocol below: N.B. A replacement outer carton is not supplied with UN3373 label and this will need to be obtained when ordering replacement carton
‘BIOLOGICAL SUBSTANCE CATEGORY B’ |
| 2 |
Complete legibly and accurately the front panel: Proper shipping name: Biological Substance Category B UN Number: UN3373 Nett Qty: One Sample Dry Ice: less than 1 kilogram. Name and telephone number of responsible person: FSA contact name and number |
| 3 | Insert the polystyrene box |
| 4 | Ice Brix must be ‘hard’ frozen before use, x2 Ice Brix should be sufficient for the Biotherm 5 system. |
| 5 | Follow Collection and Preparation of Ears (x1 pair) protocol |
| 6 | Resupply of packaging and dispatch equipment should be ordered by completing and submitting the CS115 form (Annex 21) |
7.10 The slaughterhouse case
7.10.1 Definition
Carcases and offal with suspicious TB lesions found during routine meat inspection are called ‘slaughterhouse cases’. The animals may or may not have come from a TB restricted premises.
7.10.2 Responsibilities
The table below outlines the responsibilities.
| Responsibility | Duty |
|---|---|
| APHA |
|
| FSA |
|
7.10.3 Skin tuberculosis
Animals presenting skin lesions only should not be treated as a slaughterhouse case, surveillance is not required and samples do not need to be collected. M bovis is rarely isolated from skin lesions, as usually these lesions are caused by environmental mycobacteria.
7.10.4 Differentiate between lesions
Because different sampling and diagnostic testing is required in each situation, FSA staff must positively differentiate between lesions which are:
- tuberculous (TB) Action – Inform APHA and collect samples for analysis
- tumorous (EBL) Action – Reference: See section 5 on ‘Enzootic Bovine Leukosis’ for additional information
7.10.5 Notifying APHA
Where the OV cannot positively rule out TB as the possible cause of the lesion(s) the suspect case must be reported to APHA without delay. The OV must inform APHA by telephone, to allow trace back to the farm of origin, giving details of the case such as:
- the nature of lesions found with their location
- the name and address of the person submitting the animal with ear tag number, lot number, CPH number and kill number in the additional remarks box of the TB50
- a description of the animal
- when the sample can be despatched
7.10.5.1 Contact details for slaughterhouse cases in bovine animals:
If the animal is detected in a slaughterhouse in England:
- Contact the administrative team dealing with bovine slaughterhouse cases at 0208 026 0178 or,
- E-mail the administrative team dealing with bovine slaughterhouse cases at: CSC.TBSlaughterhouseCases@apha.gov.uk.
If the animal is detected in a slaughterhouse in Wales:
- E-mail APHA Wales administrative team dealing with slaughterhouse cases at: APHAWalesTBSlaughterNon-Bovine@apha.gov.uk or,
- Contact APHA Wales at 03000 303 8268 and ask to be put through the administrative team dealing with slaughterhouse cases.
In all cases, following to the initial notification of a suspect slaughterhouse case to APHA, email the completed TB50 form to the respective team mailbox as prescribed above.
7.10.5.2 Contact details for slaughterhouse cases in non-bovine species:
If the animal originates from England:
- E-mail the non-bovine administrative team at CSC.TBOS@apha.gov.uk or,
- contact the non-bovine administrative team at 0208 720 0992.
If the animal originates from Scotland:
- E-mail ScotlandDutyVet@apha.gov.uk and copy in ScotlandEndemics@apha.gov.uk or,
- contact APHA Scotland on 03000 600708 and ask to be put through to the Duty Vet.
If the animal originates from Wales:
In both cases the email should include in the subject the species (for example “PIG SLAUGHTERHOUSE CASE”) and marked as ‘High importance’. The following information should be included in the email:
- Completed TN50/TN60
- CPH of originating farm
- Name of the owner
- Name of the farm
- Slaughter Date
- Slaughterhouse name and approval number
- Number of animals
- Contact mobile number OV
The OV will be contacted as soon as possible, to discuss if samples need to be submitted for PCR testing. Where an APHA Vet is not available to review the slaughterhouse case notification immediately, samples should not be sent, nor discarded (advice from APHA can be sought the next day). It may be that samples will need to be submitted from more than three carcasses with Visible Lesions, from the same holding.
More about the completion of TB50/TN50/TN60 can be found in sections 7.8.12 and 7.12.2. Details on the codes to be used for the completion of the forms are described in section 7.8.13.
The OV must retain legible copies of the animal’s identification (for example, cattle passport), kill sheet, FCI and other records that can be necessary for future investigations.
Reference: The Tuberculosis in Animals (England) Order 2021 and the Tuberculosis (Wales) Order 2010 (as amended).
7.10.6 APHA action
On notification from the FSA of the finding of suspect TB lesions, APHA must provide the sample WSA ID number that must be recorded in the box at the top of the TB50 form.
As of 1st September 2023, APHA will no longer apply triage to the reported bovine slaughterhouse cases for animals originating from English and Scottish holdings, i.e. from this date onwards all bovine SLH cases would be subject to laboratory investigation. All actions taken by the OVs regarding SLH cases would remain unchanged apart from the need to await APHA’s decision on sampling requirement. This would allow OVs to proceed directly to sample packing/labelling and dispatch once they have obtained the required WSA number from APHA.
For non-bovine cases, APHA will continue to triage and advise whether samples should be submitted for laboratory investigation. In England and Wales, the WSA ID number is not required.
7.10.7 Movement to detained area
After dressing, carcases and offal suspected of being affected with tuberculosis should be placed immediately in the detained area before additional detailed inspection is carried out and before being sampled, if required by APHA.
7.10.8 Transfer of carcases and offal
When transferring offal / carcases to a detained area for further inspection or sampling, care must be taken to prevent cross-contamination of other meat / equipment / fittings in the slaughterhall. In the event of suspected contamination, C and D of the affected area / equipment must take place before production recommences.
The PCR will detect DNA. Immersing the knives in water at 82°C or above will not inactivate the DNA. Adequate cleaning of the knives used to take the samples prior to disinfection will be essential to remove residues and to avoid potential false positive results.
Note: Failure by the plant operator to co-operate with this procedure would constitute a contravention of the operator’s responsibility to prevent cross-contamination and must be dealt with accordingly.
Reference: Regulation (EU) 2019/627, Article 33
7.11 The slaughterhouse case: additional detailed inspection
7.11.1 Detailed inspection
In the case of animals in which there are grounds for suspecting TB the following LNs and organs must be examined in detail (visual inspection, palpation and incision) if they have not been examined already:
- Retropharyngeal LN*
- Parotid LN
- Submandibular / Submaxillary LN
- Bronchial* and Mediastinal* LN
- Lungs*
- Pleura
- Hepatic LN
- Liver
- Mesenteric LN (representative sample)
- Supramammary LN
- Udder**
- Prescapular LN
- Superficial inguinal LN
* Tissues where tuberculosis lesions are most commonly found
** See subtopic below
Note: Additional examinations of any other LNs, such as those enlarged and / or haemorrhagic, may take place whenever considered necessary.
Reference: Regulation (EU) 2019/627, Article 14
7.11.2 Udder inspection
The inspection of udders in ‘slaughterhouse case’ is particularly important as they are not routinely incised unless they are for human consumption. In addition to the visual inspection and incision of the supra-mammary LNs, the udder of cows must be visually inspected and palpated. If abnormalities are found during these, or when the udder is intended for human consumption, then deep incisions must be done into each quarter of the udder as far as the lactiferous sinuses.
Reference: Regulation (EU) 2019/627, Article 19, 2(g)
7.11.3 Incision method
Cuts into the LNs should be made across the node in at least two directions (criss-cross pattern) to reveal as much as possible of the core of the node. Care should be taken to examine the tips of the node. This method will reveal most TB lesions or reveal an area which appears abnormal which can be further incised.
Lesions in the lungs, liver and udder are most commonly found on inspection or palpation. Where abnormalities are felt on palpation the abnormal areas should be incised for further investigation. Careful small incisions at the border of the lesions should be made to reduce exposure to infective material. If the lesion is found to be typical of TB, no further incision is required into that lesion.
7.11.4 Hygiene precautions
Any equipment used to incise or examine the LNs must be cleansed and sterilised before undertaking post-mortem procedures on subsequent carcases.
7.11.5 Correlation of TB suspect carcases and offal
The OV at any red meat slaughterhouse, where a TB suspect carcase and offal might be identified, will prepare a protocol to ensure the proper identification and correlation of TB suspect carcasses and offal. The protocol will be tailored to each plant so that any issues related to identifying and correlating the TB suspect carcase and offal are addressed. It must state that ‘each TB suspect carcase and offal are identified by a detained grey tag’. Guidance for the preparation of such protocol can be found in Annex 16b.
The detained grey tags will be ordered by the Inspection Team Leader (ITL) from CSU@food.gov.uk to ensure that each red meat slaughterhouse holds a stock of these tags on the premises.
7.11.6 Judgement of meat
Decision on whether meat is fit for human consumption is based on the findings during post-mortem inspection.
Where there are indications of generalised TB or TB lesions with emaciation. the entire carcase and all the blood and offal should be rejected as unfit for human consumption.
All meat from animals in which post-mortem inspection has revealed localised TB in a number of organs or a number of areas of the carcase are to be declared unfit for human consumption. However, when a TB lesion has been found in the LNs of only one organ or part of the carcase, only the affected organ or part of the carcase and the associated LNs need to be declared unfit for human consumption.
Reference: Regulation 2019/627, Article 33.
7.12 The slaughterhouse case: sampling
7.12.1 Collection of samples
When VLs found during post-mortem inspection cause suspicion of TB, samples need to be collected and sent for analysis, when authorised by APHA. The sampling procedures are the same as previously described for reactors, where VL are found and Sampling Code 1 applies. Please note that NVL samples are NOT to be sent for slaughterhouse cases.
Remove suspicious node or lesion in its entirety if small or a sample the size of two-thirds of the pot if large and pool up to two of the suspected lesion tissues from the same carcase.
If the size of the affected tissue and / or lesions from slaughterhouse cases is too small to make up two-thirds of the pot, then comments must be included on the TB50/ TN50/TN60 form to that effect. If the lesion identified is small, but there are multiple lesions, the multiple lesions must be included to make up the maximum required volume. However, mesenteric LN and supramammary / udder tissue are exceptions and should be submitted separately, as they are generally more heavily contaminated with other bacteria and could interfere with the TB culture process conducted on PCR positive samples to enable Whole Genome Sequencing (WGS) analysis.
Please note that fat interferes with the mycobacterial detection diagnostic methods for TB (both PCR and culture) and there is a specific requirement from APHA to trim the sample of fat tissue as much as possible.
Samples are not required from clear testing cattle/ farmed non-bovine species from TB restricted establishments (arriving at the slaughterhouse without a TB24), unless lesions suggestive of TB are found during post-mortem inspection. In this case, the ‘slaughterhouse case’ procedures apply.
7.12.2 Completion of TB50 / TN50 / TN60 form
In addition to the telephone report, fill in a separate sample submission form (TB50/ TN50/TN60) for each slaughterhouse case detected. The OV must give a detailed description of the location and nature of the suspect lesions on the TB50 / TN50 / TN60, including comments where the sample is smaller than the required volume. A properly completed TB50/ TN50/TN60 form (including the WSA number, unless specifically this number is not required) will enable APHA to quickly trace back the slaughterhouse case to its herd of origin. Based on this information APHA will put in place the appropriate TB control measures.
In this type of scenario, the OV is expected to either confirm the lesion as being characteristic of TB or, alternatively, be able to rule it out. If the OV has any doubts and / or difficulties are found when completing the TB50/ TN50/TN60 form, the OV can contact APHA and discuss any concerns with the APHA Vet to obtain the necessary advice.
Reference: See Annex 16a for sample of TB50 form, Annex 16c for sample of TN50 form an Annex 16d for a sample of TN60.
7.12.3 Distribution of the TB50/ TN50/ TN60 form
- The properly completed and signed TB50/ TN50/TN60 form must initially be emailed to the local relevant APHA administrative team (see 7.10.5.1 and 7.10.5.2) as soon as possible. Subsequently: signed hard copy of original TB50/ TN50/TN60 form must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle; APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office
- a copy of the form should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox; copy of the forms should be emailed to the relevant APHA administrative team
OV should retain a further copy in the plant files for future reference (retention period 12 months).
Where a non-bovine is being sampled, the OV must also send a copy of the completed TN50 / TN60 to SLA and Contracts.
7.12.4 Packing and despatch of all TB samples
Samples must be sent to the APHA laboratory with the forms. They should be sent as soon as possible and by the next working day at the latest.
If APHA advises that the samples do not need to be sent to the laboratory then they must be disposed of as ABP. These discarded samples are classed as category 2 ABP and can also be disposed of as category 1 ABP.
Reference: See topic 7.12 on ‘Packing and despatch of samples’ at the end of this section.
7.13 Packing and despatch of samples
7.13.1 Packing
- All samples must be submitted in a 60ml pot.
- Outside of pot must be kept clean.
- Remember to tighten lids. Give an extra turn before packing.
- Avoid cross threading the lids as they will cause the pots to leak.
- Stick label on outside of pot: ear tag / CPH printed on label.
- Place each individual pot in a plastic bag which is knotted tightly. Trim off excess bag.
- If submitting more than one pot for a single animal (pool in one pot) atypical lesion in a separate pot:
- Label each pot and write on label what is in each pot, for example, pool / mesenteric.
- Place each pot in a separate bag and tie as previously.
- Place both bagged pots in a third bag and tie the bag.
- Make note in comments section on the TB110 or TB50/ TN50 / TN60 detailing how many pots submitted and what is in each pot.
- Place all bagged pots into a biobox / biobottle along with the absorbent pad / material and seal the box. The person introducing samples inside the biobox / biobottle must wipe their hands with 70% ethanol wipes before introducing the samples. The outside of the biobox / biobottle must also be wiped. The process for sending forms is as follows:
- Signed hard copy TB110 and original TB50/ TN50/ TN60 forms must be placed in an envelope, this envelope should be marked ‘Originals’ and placed between the outer box and the biobox / biobottle. APHA laboratory staff will forward the signed hard copies internally to the relevant APHA regional office.
- Copies of those forms should be placed in a ziplock bag and taped to the outside of the biobottle / placed in biobox. Copies of these forms should be faxed or emailed to the relevant APHA office. The OV should retain a further copy in the plant files for future reference. (Retention period 12 months)
- Place biobottle into the outer box. Before use the biobox / biobottle must be stored in a separate clean area to avoid possible cross contamination.
- Attach address label.
- Attach security seal.
Store the package in the chiller until the time of collection. Ideally, place in a waterproof bag / container to avoid contamination. The outer box needs to clearly read: ‘TB samples open only in CL3’. If the box has not been pre-stamped, please write or use the sticker provided.
Despatch: The current courier for the sampling process is Topspeed Couriers. The courier process is as follows:
| Step | Action |
|---|---|
| 1 | As soon as you receive the required information from APHA, book a collection with the following information:
|
| 2 | The APHA preferred courier will confirm the date that the samples will be collected. If samples need to be kept at the establishment overnight, please ensure that they are sealed in the packaging requested from APHA and store in a chiller or cold room. |
| 3 | The APHA preferred courier is required to deliver the samples within 2 working days. For example, if samples are taken on Tuesday, samples are required to be with APHA by 5pm on Thursday. Samples can be delivered up to 3pm only on a Friday. |
| 4 | On detection of a slaughterhouse case, notify the courier that samples are required to be collected and they will organise a collection which meets the 2 working days delivery requirement; for example, a SH case found on a Monday, samples are required to be with APHA laboratory by 5pm Wednesday but collection could either take place on Monday, Tuesday or Wednesday, as the couriers are required to consolidate their delivery runs to be cost effective. |
7.13.2 Ordering consumables
The OV at each abattoir is responsible for ensuring that there are sufficient supplies of consumables for packing samples. It is important that only the specified packaging materials (such as pots and labels) are used as failure to do so may result in the sample being un-assayable at the lab.
The consumables must be ordered directly from APHA Weybridge by using the following procedure:
- Fill in the requisition form (Annex 8a and 8b)
- Make sure that you complete all the boxes (establishment name, address, FSA contact name and telephone number, and any others).
- The requisition form should be emailed to: StoresStockOrders@apha.gov.uk.
APHA will endeavour to complete delivery of consumables orders within 7 working days of receipt. If you have any queries regarding an order that you have placed you should telephone the APHA stores in Weybridge on 01932 359451.
7.14 Private Slaughter of TB Reactors (Rs), Direct Contacts (DCs) and Inconclusive Reactors (IRs)
7.14.1 Background
On 1st of November 2018, with the implementation of The Cattle Compensation (England) (Amendment) Order 2018 (SI 2018/754), Defra introduced a change to the existing private slaughter arrangements for cattle compulsorily removed for TB control purposes in England.
Note: more details about compensation payments could be found at the TBHub website.
As a result of this change, APHA and FSA agreed a process to follow for the private slaughter of Rs, DCs and IRs at non APHA contracted slaughterhouses. The aim is that FSA staff at the non-contracted abattoirs are given enough notice to ensure the required enhanced post-mortem and sampling procedures can be followed according to the agreed instructions.
7.14.2 Notification process
It involves the completion of an agreement form (TR558) which will display the proposed kill date and the last date by which a R/DC can be slaughtered as a private kill.
Reference: See Annex 31b for sample TR558 - Private Slaughter of TB Reactors/Direct Contacts/Inconclusive Reactors Arrangements Agreement Form.
APHA will provide the TR558 form to cattle keepers interested in the private slaughter of Rs, DCs and IRs on their request.
For the private slaughter of TB Rs, DCs or IRs from herds in England, the cattle keepers must initially discuss their intentions with the FBO of the slaughterhouse where the cattle will be processed, signing the TR558 form. Where the FBO has shown an interest in processing these private Rs, DCs or IRs, the agreement will be updated with the proposed kill dates and passed to the OV. The OV will assess the feasibility of the request and completion of form (see 7.14.3). The agreement form will be then sent back to APHA with no less than 24 hours before the scheduled/agreed kill date.
For the private slaughter of Rs, DCs or IRs from herds in Wales, APHA will complete the keeper/FBO sections of the agreement form on their behalf and return it to both the FBO and FSA for the confirmation of the kill date. If both the FBO and FSA agreed (see 7.14.3), the OV must return the form to APHA by 5.00pm the day after receiving the form from APHA.
Note: the notification process depends on the location where the TB Rs, IRs or DCs have been disclosed.
If the deadline date cannot be met or will be missed, then the R/DCs may need to be removed and killed at another abattoir privately or through the APHA haulage and salvage contract.
Once the completed TR558 agreement form is received, APHA will produce the relevant movement licence for the animal/s and TB110 form, identifying any samples required.
Note: The same process will be followed for the private slaughter of IRs with the difference that there will not be a latest date by which they will need to be slaughtered by.
7.14.3 OV assessment
Where the FBO intends to process private Rs, DCs, or IRs cattle, the OV should carry out a technical assessment to decide whether the premises are/are not adequate for processing Rs, DCs or IRs prior to signing the TR558 form.
Note: Annex 31a- FSA TB Ready Check list can be used for this purpose.
Any concerns that the OV might have about the lack of adequate inspection facilities and capability of the plant to process these cattle (e.g. deficiencies related to animal identification, correlation, provision of relevant PPE, segregation, availability of sampling kit and most importantly sample correlation with the animal’s identification, cleaning and disinfection) should be discussed with the FVC/FVL of the area. APHA should also be informed of the outcome as soon as possible.
Note: If a TB R, DC or IR animal is over 48 months old and there are not agreed procedures in place for BSE testing (e.g. RMOP) in the proposed slaughterhouse, the OV should refuse a private kill.
8. Outbreak of Avian Influenza
In this section
8.3 Movement licences for poultry to slaughter
8.4 Movement of meat during outbreak in domestic poultry
8.7 FBO duties within AI free zones
8.8 FSA duties in establishments within the protection, surveillance, restricted and free zones
8.1 Introduction
8.1.1 Avian Influenza outbreak
This section describes the actions when an Avian Influenza outbreak is declared.
For suspected cases of Avian Influenza found at slaughterhouses, section 2 (Action on suspicion of notifiable diseases) of this chapter must be followed.
In preparation for a potential outbreak of Avian Influenza (AI), the FBO and the OV should have contingency plans in place, including, if the FBO considers it necessary, the pre-designation of the slaughterhouse for processing birds from controlled zones.
Upon confirmation that AI has been found in poultry, captive and/or wild birds in England and Wales, animal health protection measures are imposed to prevent the spread of disease. Scotland and Northern Ireland might follow similar approach.
The latest information about the AI situation in UK, including the “Rules on meat produced from poultry and farmed game birds originating in Protection Zone(s)” and applicable general licences for meat can be found in the above link.
Pictures of clinical signs of avian influenza are available in this link.
8.1.2 Domestic legislation
Domestic legislation that applies in the case of a domestic poultry outbreak:
- the Avian Influenza and Influenza of Avian Origin in Mammals (England) / (Wales) (No. 2) Order 2006
- the Avian Influenza and Influenza of Avian Origin in Mammals (Scotland) / (Wales) Order 2006
- the Avian Influenza (Vaccination) (England / Wales) Regulations 2006
- the Avian Influenza (Slaughter and Vaccination) (Scotland) Regulations 2006
- the Avian Influenza (H5N1 in Poultry) (England / Wales) Order 2006
Domestic legislation that applies in the case of a wild bird outbreak:
- the Avian Influenza (H5N1 in Wild Birds) (England / Wales) Order 2006
- the Avian Influenza (H5N1 in Wild Birds) (Scotland) Order 2006 as amended
- the Avian Influenza and Influenza of Avian Origin in Mammals (England) / (Wales) (No.2) Order 2006
8.2 Controlled zones
8.2.1 Controlled zones and AI free zones
Controlled zones may be declared around the place(s) where birds have been found to be infected with notifiable AI virus. Those zones are classified as follows:
- Low Pathogenic Restricted Zone (RZ): minimum size of 1km around the premises where low pathogenic notifiable AI has been confirmed.
- Protection Zone (PZ) (or wild bird control area or wild bird protection zone, under the Wild Bird Order): a ring with a radius of at least 3 km around the point where high pathogenic AI virus has been confirmed.
- Surveillance Zone (SZ) (or wild bird monitoring area or wild bird surveillance zone, under the wild bird Order): a ring with a radius of at least 10 km around the point where high pathogenic AI virus has been confirmed and including the PZ.
- Restricted Zone (RZ): an area beyond the SZ that separates the SZ from the Free Zone normally when H5N1 is present, but not exclusively. To note that RZ are not always declared, but when they are they must be adjacent to SZ or other RZ and centred around the IP, and there may be more than one declared.
- Temporary movement restriction zones (TMRZ) and temporary control zones (TCZ): temporary zone with a radius of at least 10 km around the point where AI of the subtype H5 or H7 has been confirmed but not the virus N-type and pathogenicity. These zones are normally used prior to confirmation of pathogenicity. TCZ can also have two separate areas within – TCZ A and TCZ B – which replicate PZ and SZ in terms of size and control measures applicable within.
- Avian influenza prevention zone (AIPZ): zone declared by the secretary of state in England or the Welsh Ministers in Wales when considered necessary to reduce the risk of transmission of NAD from wild birds to poultry (including game birds and poultry kept as pets) or other captive birds (e.g., requirement for poultry and other captive birds to be housed within a define area; ban on hunting, etc).
- Controlled Zones (CZ): means a protection zone, a surveillance zone, a restricted zone, a low pathogenic avian influenza restricted zone, a temporary movement restriction zone, a temporary control zone, an avian influenza prevention zone, or an avian influenza (restrictions on mammals) zone.
An APHA Interactive Avian Influenza Disease Map is available here.
Moreover, there are a few other zones of importance in an outbreak of Avian influenza:
- Free Zone (FZ): area outside the PZ, SZ and RZ that is free of notifiable AI. The transport of live birds and meat within this area is allowed without restriction.
- Vaccination Zone (VZ) (or emergency vaccination zone or preventive vaccination zone): The Secretary of State or the Welsh Ministers considers that poultry or other captive birds in this zone should be vaccinated under a preventive vaccination plan or an emergency vaccination notice.
8.3 Movement licences for poultry to slaughter
Biosecurity and veterinary surveillance measures are imposed within the controlled zones to prevent the introduction of the virus into healthy poultry flocks. These include licensing birds to slaughter, under a specific movement license issued by a Veterinary Inspector (VI) or by an inspector under the direction of a VI.
Whether these licences are available or not will depend on an assessment by APHA/Defra/Welsh Government. These may not be available for premises located in certain zones or for certain species of birds. The requirement for a movement licence applies even where poultry sheds are co-located on the same premises as the abattoir.
Part of the movement licence requires completion by the OV/MHI confirming the slaughtering of all the birds and requires that the FBO returns the completed licence to the APHA licencing team. The OV should monitor that FBO returns the movement licences to APHA without delay.
It is to be noted that there is the potential that different movement licences operate in England and Wales, respectively, and the correct licence must be applied for.
8.3.1 Types of movement licences
Movements involving poultry for slaughter from and/or to premises situated within a PZ, SZ or RZ are only allowed under a movement licence issued by an APHA VI or Inspector under the direction of a VI (check licence requirements for details):
There are three types of movement licence:
- Specific Movement Licence (LS)
- Multiple Movement Licence (LM)
- General Movement Licence (LG)
Full details of movement licences can be found online APHA licensing team Bird flu (avian influenza) movement licences - GOV.UK (www.gov.uk).
Note: Movements of poultry to an abattoir located within the same farm complex also need to be licenced.
Note: Welsh Government may also issue movement licences for premises located in Wales
8.3.2 Specific movement licences (LS)
The movement of poultry from a PZ or SZ to any abattoir, or the movement of any poultry to an abattoir located within a control zone, is subject to LS.
A VI or Inspector under the direction of a VI must examine the birds within 24 hours before they leave the premises of origin. This licence is issued as a single document for each flock (not lorry load) of birds and can only be used once.
The person transporting the birds must comply with the conditions attached to the licence; carry the original with them and produce it on request.
Conditions for movement of live poultry are detailed in the licence and are specific to that individual move.
8.3.3 Multiple movement licences (LM)
For regular movement of poultry from a PZ or SZ to any abattoir, or the movement of any poultry to an abattoir located within a control zone a Multiple Movement Licence (LM) may be used. This licence is issued as a unique document that can be used between the same premises of origin and destination for several times and for a limited period as instructed by APHA when issuing the licence.
The person transporting the birds must comply with the conditions attached to the licence; carry the original document with them and produce it on request. The licence and the consignment note must be produced on request to an inspector or other officer of the Secretary of State / Welsh Ministers on demand and allow a copy or extract to be taken; and on such demand, furnish his name and address.
Conditions for movement of live poultry are detailed in the licence and are specific to the individual licence.
8.3.4 General licence (LG)
These licenses allow on certain instances for the movement of poultry to take place without applying for a specific or multiple licence. The person moving live birds and poultry products under this licence must at all times carry a consignment note.
This licence is not issued as a document, but is published on the Defra or on the Welsh Government website. It is for the person responsible for the transport of the poultry to ensure this movement is covered by the LG and that its conditions are met.
8.3.5 Conditions of the LG
Conditions of the LG are listed in the licence.
8.3.6 No licence required
The movement of live poultry between premises situated in the FZ to a slaughterhouse in the FZ does not require a licence.
8.3.7 Summary
The tables below summarise the movement licences required for poultry to slaughter during AI outbreaks:
Movement licences for live poultry to slaughter in a highly pathogenic AI outbreak:
|
Birds from |
To slaughterhouse situated in |
|||
|
PZ (3km) |
SZ (10km) |
RZ |
FZ |
|
|
PZ (3km) |
Specific Licence (LS) |
|||
|
SZ (10km) |
||||
|
RZ |
General Licence (LG)*/Specific License (LS) [* A specific or multiple licence will be required if a LG is not issued by Defra and/or Welsh Government] |
|||
|
FZ |
|
No licence required |
||
Movement Licences for live poultry to slaughter in a low pathogenic AI outbreak:
|
Birds from |
To slaughterhouse situated in |
|
|
LPRZ (1km) |
FZ |
|
|
LPRZ (1km) |
Direct movement under a General Licence (LG) by VI / AHI to designated slaughterhouse |
|
|
FZ |
|
No licence required |
Movement Licences for live poultry to slaughter in an H5N1 in a wild bird AI outbreak:
|
Birds from |
To slaughterhouses situated in |
||
|
PZ (3km) |
SZ (10km) |
FZ |
|
|
PZ (3km) |
Specific Licence (LS) |
||
|
SZ (10km) |
|||
|
FZ |
*General Licence (LG) Specific License (LS) [* A specific or multiple licence will be required if a LG is not issued by Defra and/or Welsh Government] |
No licence required |
|
8.4 Movement of meat during outbreak in domestic poultry
The movement of poultry and wild bird meat, minced meat, meat preparations and meat products from poultry or wild bird meat originating from within a PZ will be subjected to restrictions as follows:
- meat from poultry originating in a PZ must be restricted meat; the restricted meat or its packaging must bear a special mark which will replace the ID mark and be produced in accordance with the domestic legislation
- the categorisation as restricted meat and special mark may also apply to poultry meat from a PZ produced within the 21 days prior to the estimated earliest infection date
- When applied to retail packaging, the size of the mark may vary according to the size of the packaging; however, it must be legible to the naked eye

Only designated slaughterhouses can slaughter poultry meat from birds originating in the PZ, SZ and RZ.
Also, cutting plants, cold stores and establishments preparing minced meat, meat products and meat preparations situated within the PZ can dispatch their products into GB providing the applicable general licence/s are complied with, the raw material has been obtained in accordance with the domestic legislation and is not intended for supply to international trade, including NI.
8.4.1 Hunting ban
Where there is a confirmed outbreak in wild birds the hunting of wild birds may be prohibited in the wild bird PZ or wild bird control area and the wild bird SZ or wild bird monitoring area.
Where the confirmed outbreak is in domestic poultry no person shall release game birds in the PZ or SZ.
A shooting ban might also be introduced.
8.5 FBO responsibilities
8.5.1 Designation of slaughterhouses under a domestic poultry outbreak
Slaughterhouses will need to be designated by the FSA on behalf of Defra or Welsh Government before receiving and processing poultry from premises within a:
- highly pathogenic AI protection zone
- highly pathogenic AI surveillance zone
- highly pathogenic AI restricted zone
- low pathogenic AI restricted zone
- vaccination zone
In addition, slaughterhouses situated within a zone subject to movement restrictions must also be designated by Defra in case of slaughterhouses located in England or by the Welsh Government, in the case of being located in Wales, to receive and process poultry including where this originates from an area free from disease restrictions.
In both England and Wales, the applications will be assessed and pre-designated by FSA with recommendation given to Defra or Welsh Government, who will then sign off/grant the designation.
Designations are only valid during the outbreak period. Once the outbreak officially ends, the designations for the slaughterhouses will be removed. In the case of a new outbreak, the establishment will have to apply for the activation of the pre-designation. This can be done by approaching the approvals team on approvals@food.gov.uk
There are two types of designation for slaughterhouses:
- Level 1. To receive and process poultry from premises within:
- a highly pathogenic AI surveillance zone,
- a highly pathogenic AI restricted zone,
- a low pathogenic AI restricted zone (or vaccination zone in Scotland)
- or being a slaughterhouse within a zone subject to movement restrictions, to receive and process poultry from premises within an area free from disease control restrictions.
- Level 2. To receive and process poultry from premises within a highly pathogenic AI protection zone (Protection Zone) and, therefore, producing restricted meat.
Only approved establishments can be designated. Establishments, registered with the local authorities and slaughtering on farm fewer than 10,000 birds per year do not follow this designation process, although, if they are located in the protection or surveillance zone, will have to obtain a licence from APHA and meet some specific criteria if they want to operate during an outbreak.
Designations only apply to slaughterhouses processing farmed poultry, including farmed game birds. There are no controls on meat produced from wild game birds, including those shot within PZ or SZ, except for when H5N1 High Path AI is isolated. In that case, controls on wild game meat from Approved Game Handling Establishments (AGHE) might be implemented.
Activation of a designation does not mean the establishment will be able to receive poultry for slaughter automatically as the movements of poultry from controlled zones will still need to be accompanied by a specific or multiple licence (LS or LM).
8.5.2 Designation and activation process
Local teams (OVs, TMs, FVCs and FVLs) play a key role in the designation process, but the overall responsibility rests with the FVL. Support can be sought from notifiable-disease.portfolio@food.gov.uk at any time during the process.
The FBO must contact approvals@food.gov.uk to request an application form (Annex 25). Upon completion by the FBO of part 1 of the “Application for Designation of a Slaughterhouse” (Annex 25) and once the OV is satisfied that the abattoir has the necessary SOPs in place and meets the criteria for the level required, the OV should complete and sign Part 2 of the aforementioned document. The FBO can then submit the application form to the approvals team approvals@food.gov.uk.
The approvals team will subsequently assign a FVL to each application. An on-site visit to the establishment by the AVM, FVC or FVL to assess the procedures and SOPs needs to be considered on a case-by-case basis. For Lv1 designations with normally compliant FBOs and experienced OVs, a specific visit might not be necessary, and the recommendation for the establishment to be designated can be made as a desktop exercise. Lv 2 designation, or a new application, might require a more in-depth assessment, and an on-site visit can be more appropriate. It is for the FVL to decide what the best approach is on each situation.
Once the FVL is satisfied that the SOPs and the establishment meets the requirements, part 3 should be signed and sent back to the approvals team, that in turn will liaise with a Defra representative for the signature of part 4 if the premises is in England, or to a Welsh Government representative if the abattoir is located in Wales.
Once the designation is activated by Defra/Welsh government, the approvals team will then send to the FBO, copy in the ND lead, FVL, FVC, AM, TM and OV via email the designation documents and will update the information in Establishments and People database.
8.5.2.1 Designations of slaughterhouses located nearby to active poultry farms.
Abattoirs, adjacent to an active commercial poultry premises, for example a broiler or laying farm, should not be designated, unless enhanced biosecurity measures are implemented as below to prevent the spread of Avian Influenza to nearby farms. Where they achieve designation, these abattoirs will remain designated until the NAD outbreak finishes. At that point their pre-designated status will be removed from Establishments and People Data base.
To evaluate the specific circumstances of each case, it is recommended that a joint visit is carried out by an FSA and an APHA veterinarian to assess the risks on site and provide advice to the FBO on the key areas where biosecurity needs to be enhanced.
The FBO is then responsible for preparing an SOP to manage these risks. Once the FSA is satisfied with the proposed measures, the designation can be signed off in accordance with the set of conditions/restrictions imposed and detailed in Part 4 of the designation approval document (Annex 25).
In essence, this means that there is scope for each situation to be assessed throughout the duration of the outbreak and treated accordingly on a case-by-case basis, in consultation with the Notifiable Disease Portfolio Team.
Associated risk factors to be taken into consideration during the assessment of abattoirs with active nearby farms can include (not exclusively):
- Physical separation between the farm and the abattoirs (for example fence, different entries, use of different tools and equipment)
- Location/extent/species of the on-farm poultry populations
- Outdoor/free-range/indoor units
- Staff movements
- Pest control (e.g. birds, feral cats, rodents, insects...)
- Geographical factors (e.g. nearby water reservoirs or streams)
- Animal welfare implications on farms associated to the abattoir (e.g. integrated systems)
- Airborne cross-contamination
- C&D procedures
- Handling and Storage of ABPs.
Please note that the risks associated to Level 2 designations will be higher and a more thorough assessment and SOP might be required.
8.5.3 Pre-designations and annual review
FBOs can apply at any time for their establishment to be pre-designated. The process is the same as detailed in 8.5.1, except for the completion of part 4 of Annex 25 (activation of the pre-designation), which will not be required at this stage. The same procedure as above should be followed.
Establishments with a pre-designated status can ask for their procedures to be reviewed on a yearly basis. This review will be carried out by the local FSA veterinary team before the start of the AI season (end of September each year). For that purpose, Annex 28 has been created, which is a simplified version of the initial pre-designation document.
The FSA approvals team in liaison with the Notifiable Disease Portfolio will send a reminder to each poultry slaughterhouse OV, FVC and FVL of the reminding them of the importance to review the AI pre-designated status prior to the AI season starts. The OV, in liaison with the FSA local team will review the FBO SOPs and procedures on site to ensure these are still valid and up to date using the Annex 28 check list.
Once the AI annual pre-designation checklist (Annex 28) is completed and signed by the OV/FVC/FVL, and upon submission to approvals@food.gov.uk, the approvals team will send to the FBO, cc'ed to ND lead, FVL, FVC, AM, TM and OV via email confirmation of the pre-designation being renewed and will update the information in Establishments and People database.
Establishments that do not review their pre-designations every year, will have to go through the review of their pre-designation before they can apply for activation of designation to operate during an outbreak.
8.5.4 Production of poultry meat from a PZ
Meat from birds sourced from the Infected Premises (IP) and slaughtered within the 21 days from the earliest date of infection must be traced and detained. Defra/Welsh Government may decide the disposal of that meat.
Meat produced from birds originating in the PZ and slaughtered within the 21 days from the earliest date of infection at the IP of that zone might become restricted meat once the PZ is declared. Defra/WG will instruct FSA on the actions to be taken in terms of tracing and re-labelling of this meat.
Production of poultry meat, minced meat, meat products and meat preparations from healthy birds originating in the PZ can take place in slaughterhouses, cutting plants and other establishments within the PZ, SZ, RZ and / or FZ if:
- the slaughterhouse is approved and designated and complies with all the designation requirements
- the birds have been transported under a Licence
- the meat is produced in accordance with EU Regulations 852/2004, 853/2004 and the subject to official controls under Regulation (EU) 2017/625 and the associated Commission Delegated and Implementing Regulations
- the special mark is applied to the final product, indicating that the meat is restricted
- the conditions of the applicable general licence(s) are complied with
Restricted poultry meat and products from healthy birds originating from the PZ can be marketed within GB with no further treatment.
Meat from the PZ can only be exported (including NI) if ALL of the following conditions are complied with:
- the product undergoes heat treatment to inactivate any AI virus present (e.g. minimum of 70C reached through the entire meat or product)
- the oval ID Mark is applied after the treatment has been completed
- the establishment applying the heat treatment and the oval ID mark follows the conditions as detailed in general licences
- requirements of the Export Health Certificate are met
Where birds from a PZ are slaughtered and processed, the parts of the slaughterhouse and the equipment used for the slaughter and processing of those birds must be cleansed and disinfected before other poultry is slaughtered or processed.
See 8.10.2 below for further details
8.5.5 Production of poultry meat from SZ and RZ
These restrictions only apply for a highly pathogenic outbreak in domestic poultry.
Production of poultry meat, minced meat, meat products and meat preparations from healthy birds originating in the SZ (or RZ when applicable) can take place in slaughterhouses, cutting plants and other establishments within the PZ, SZ, RZ or FZ provided
- the birds have been transported under a licence, and
- the slaughterhouse is approved, designated and complies with all the designation requirements
- The meat is handled and transported in compliance with the applicable general licence(s)
It is important to note that meat from poultry originating from the SZ or from abattoirs located within the PZ, SZ and RZ, despite being “unrestricted” might still be subject to international trading restrictions (see 8.5.1 for details). In most instances this meat will be only suitable for trade within GB.
8.5.6 Production of wild feathered game meat from PZ and SZ
Defra/Welsh Government may establish restrictions and controls on wild feathered game meat depending on the epidemiology and risk of the outbreak.
8.6 Traceability and Commercial documents
8.6.1 Commercial documents for poultry meat and wild feathered game meat
For details of commercial and export documentation see the Defra website. It is important to note that during AI outbreaks, regionalisation measures around the Infected Premises (i.e. PZ, SZ, RZ) are normally applied, limiting the export capacity of the premises in the area (for example farms and abattoirs). Each receiving country might apply different criteria and any individual requirement must be assessed before signing any EHC. To facilitate traceability and the completion of the EHC for the EU and NI, Support Health Attestations (SHA) have been made available.
8.6.2 Traceability of poultry meat before the PZ
Traceability of the meat is a legal requirement in all circumstances. The FBO should be aware that robust internal traceability systems will help to minimise the costs of the required tracing of the meat produced from the PZ before the declaration of the PZ.
8.6.3 Reporting requirements for the traceability of restricted meat
For FBOs of slaughterhouses which have been designated as Level 2, every time they process poultry originating from the PZ, must complete and submit approvals@food.gov.uk the traceability information for this meat using the form provided at the time of the designation (annex 27). This information will be used to verify the compliance with the general licence for restricted meat, through risk-based unannounced inspections at cutting plants receiving that meat.
The OV of a slaughterhouse which has been designated as Level 2 must verify that FBO completes and provides the traceability information requested in the form for all the dispatched restricted meat to the FSA Approvals team without delay.
8.7 FBO duties within the AI free zone in not designated slaughterhouses
8.7.1 Restriction applicable to establishments
Slaughterhouses within the FZ must be designated by Defra in England/by the Welsh Government in Wales to receive birds that originate within the PZ, SZ, RZ or VZ.
Reference: See sub-topic: ‘Designation of slaughterhouses under a domestic poultry outbreak’.
Any slaughterhouse situated within the FZ can receive birds that originate within the FZ without any designation
8.7.2 Production of poultry meat from the FZ
There are no restrictions to the production of poultry meat from birds originating in the AI FZ in establishments situated within the AI FZ, providing it is produced in accordance with Commission Regulations 852/2004, 853/2004 and subjected to official controls and inspection in accordance with Regulations (EU) 2017/625, 2019/624, 2019/625 and particularly 2019/627, and that adequate biosecurity measures are implemented.
The oval ID Mark is to be applied.
8.7.3 Production of wild feathered game meat from the FZ
There are no restrictions to the production of wild feathered game meat from birds originating in the AI FZ in establishments situated within the AI FZ, providing it is produced in accordance with Commission Regulations 852/2004, 853/2004 and subjected to official controls and inspection in accordance with Regulations (EU) 2017/625, 2019/624, 2019/625 and particularly 2019/627.
Where the establishment is approved, the oval ID Mark is to be applied.
8.8 FSA duties in establishments within the PZ, SZ, RZ and in designated slaughterhouses in the FZ
8.8.1 FSA presence
The FSA must be present at all times when slaughtering takes place until all birds have passed post-mortem inspection in designated slaughterhouses.
If there is an outbreak of a notifiable disease in poultry (such as Avian Influenza or Newcastle Disease), batch AMI is not permitted when poultry is moved to the slaughterhouse under a movement licence (e.g. from a restricted zone). In those circumstances, the OV must undertake immediate full veterinary Ante-Mortem Inspection (AMI) upon unloading from the vehicle.
Poultry which have come from premises in the PZ are deemed to be under official control, and must be lairaged, slaughtered, chilled and stored separately from product which is not under official control until such time as it is wrapped and packaged.
The OV must verify that the FBO complies with the requirements to keep those birds and meat separated and the required Cleaning and Disinfection takes place before slaughter of other birds commences.
8.8.2 Confirmation of designation
OVs must obtain confirmation from the FSA that the slaughterhouse is designated before receiving birds from PZ, SZ or RZ for slaughter. Up to date designation status can be found in Establishments and People, under slaughterhouse activities, The latest designation application, including specific plant procedures to meet the designation requirements, can be found in the plant folder. The OV must verify that the FBO is in compliance with the procedures set out in the designation. A summary of the main OV/FSA team checks required during an AI outbreak can be found in Annex 33
OVs are to encourage FBOs to apply for pre-designation to the FSA so they can minimise disruption should they require designation at a later stage.
8.8.3 Movement of live birds
Live birds originating from the FZ can be accepted by a slaughterhouse situated within the FZ without a movement licence.
The OV or MHI must obtain from the FBO a list of all expected farms delivering live birds from PZ, SZ and / or RZ for slaughter 24 hours in advance.
On arrival to the slaughterhouse the OV or MHI must inspect the movement licence or consignment note to verify:
- the origin of the birds
- that the consignment is intended for that slaughterhouse
- that where the birds originate from a PZ, they are kept separated from other birds, by means agreed with in the application for designation
Reference: See topic ‘Movement licences for poultry to slaughter’.
8.8.4 Cleansing and Disinfection
Additional FSA checks are required to verify compliance with the Cleansing and Disinfection (C&D) conditions attached to the licences:
After unloading at the premises of destination, the parts of the vehicle used to transport (including crates and equipment) anything which might be contaminated with mud, slurry, animal faeces, excretions, feathers or any other similar matter must be cleansed and disinfected on site.
FSA staff must carry out 100% checks of C&D of crates, modules and vehicles used to:
- transport birds from a PZ or SZ
- transport birds to a slaughterhouse located within a PZ or SZ.
Note: AI C&D related checks, including any breaches/actions encountered by the OV, should be recorded daily using the Cleansing and Disinfection of Livestock Vehicles K2 form.
Additional FSA staff may be required to perform those checks.
A reminder of some important areas to be considered:
- Birds in the sheds / crates / modules must have no access to the abattoir area. There should be no poultry freely roaming within the curtilage of the abattoir. Welfare standards must be maintained.
- The hard-standing area used for the Cleansing and Disinfection of the livestock transport must be maintained clean and free of animals / vermin / pets. This area must be cleaned and disinfected before commencing operations if necessary.
- All transport vehicles and crates must be thoroughly cleansed and disinfected after unloading the birds. Special care must be taken to avoid any recontamination of vehicles, modules and crates after C&D, particularly through soil and dirt adhering to the wheel arches and surrounding parts (as this is not controlled by the wheel mats at the exit). This may necessitate spraying the exterior of the vehicle at the boundary of the site.
- The abattoir must be clean prior to commencing killing. Naturally vermin, wildlife (including wild birds) and poultry should not have access to the abattoir to avoid transmission of undetected disease.
No additional FSA C&D checks are required for the transport of birds within FZ. However, the FBO must maintain high standards of Cleansing and Disinfection of all crates, modules and vehicles.
8.8.5 Confirmation of slaughter
The OV or an MHI under the OV supervision must confirm that the birds arriving under a Specific Licence have been slaughtered by endorsing the licence presented by the FBO.
Where any problems arise relating to live bird movements the OV must contact the local Trading Standards Office and the local APHA office.
The OV should verify that the FBO is returning the completed Specific Licence to the issuing APHA office.
8.8.6 Use of Plant Inspection Assistants (PIAs)
PIAs are not authorised to carry out Animal Health Official Controls. Only OVs or MHIs are authorised to carry out animal health tasks (e.g. C&D verification procedures). However, PIA can still carry out enhanced post-mortem, under the supervision of the OV.
8.8.7 Application of special mark
The special mark must be applied to poultry meat from birds originating from the Protection Zone or its packaging under the direction and control of the FSA.
8.8.8 Restricted meat
The term “restricted meat” is used to mean:
- meat from poultry originating from a Protection Zone (PZ)
- when instructed by Defra/Welsh Government, meat from poultry originating from an area that subsequently became a protection zone and was slaughtered within 21 days of the estimated earliest date of infection in the relevant zone,
- meat that has not been kept separate from the previous 2 categories
- any meat, processed meat or meat products derived from any of the above that has not been heat treated to a temperature of at least 70°C reached through the entire meat or product
The term “unrestricted meat” is used to mean:
- meat from poultry originating outside of a PZ
- meat from poultry originating in an area that subsequently became a PZ but was slaughtered at least 21 days before the date estimated by a veterinary inspector as being the earliest date of infection at a premises in the relevant PZ
- any meat falling into the restricted meat category that has been heat treated to at least 70°C throughout by an approved establishment (in accordance with Article 4 of Regulations (EC) 853/2004)
Restricted meat bearing the special mark can be dispatched from designated slaughterhouses to approved establishments under a general licence issued by Defra or the Welsh Government and marketed within GB.
When dispatching any restricted meat, the FBO of level 2 designated slaughterhouses are required to notify their clients to make sure they are aware of the requirement of maintaining the special mark after the meat is cut or processed in the cutting plants and the need of keeping the segregation of the restricted meat from other meat (annex 26).
The primary purpose of this special mark is to prevent the meat or resultant products from being exported.
Establishments that intend to further process meat bearing the special mark don’t need to be designated. Further process in this context means any activity that removes the special mark from the meat / wrapping / packing. When removed, the special mark will need to be replaced by the new establishment’s own special (“beer mat”) mark all the way down to retail level.
FSA staff will carry out risk-based unannounced inspections (UAIs) in establishments receiving meat bearing the special mark to ensure that it is processed separate from meat bearing the oval ID mark and that the special mark is maintained.
FSA officials are to check, in particular:
- correct marking (special mark) of the meat obtained from birds originating from the PZ
- separation of special marked meat from oval ID mark meat in cutting establishments
- special marked meat is processed separately from oval ID marked meat
- if special marked meat is mixed with oval ID mark meat, the resulting meat / products must bear the special mark
- special marked meat is wrapped / packed with the special mark of the new establishment where it has been unwrapped / unpacked; the requirements for the correct application of the special mark are equivalent to those for the oval ID mark
When restricted meat is heat treated to at least 70°C throughout in an approved establishment (in accordance with Article 4 of Regulations (EC) 853/2004) to produce unrestricted meat, the HACCP based procedures, document and records – particularly the monitoring records for the heat treatment process and the prevention of cross-contamination must be strictly adhered to.
8.9 Waste disposal
8.9.1 Waste disposal
Lv2 Designated slaughterhouses slaughtering birds from the PZ:
Specific ABP categorisation and disposal rules apply for ABPs originating from birds from the PZ as follows:
- No category 3 ABP is allowed to go into raw pet food production.
- FBO will need a written confirmation from the rendering company that Category 3 ABP material will be subjected to a minimum heat treatment of 70°C.
- If the FBO does not have this confirmation, Category 3 ABPs from birds originating from a PZ must be disposed as Category 2 ABP or above.
- If Category 3 ABPs from birds originating in the PZ get mixed with Category 3 ABP from birds outside the PZ, the above controls apply to the entirety.
Meat processing establishments:
Meat processing establishments handling meat from birds from PZ must dispose Cat 3 ABP through a route that involves heat treatment to a minimum of 70°C.
8.10 Disinfection procedures (level 2 designations only)
8.10.1 Disinfection of contaminated litter, manure and slurry
Manure and used bedding must be treated by one of the following methods:
- be steam treated at a temperature of at least 70°C
- be destroyed by burning
- be buried deep enough to prevent access by wild birds and animals
- be stacked to heat, sprayed with disinfectant and left for at least 42 days
Slurry must be stored for at least 60 days after the last addition of infectious material unless (in the case of slurry which has been treated in accordance with a VIs instructions) a VI authorises a shorter storage period.
Manure, litter and bedding which may be contaminated may, if licenced by a VI, be moved to:
- a treatment plant carrying out procedures for the destruction of AI virus
- storage prior to destruction
- such other place as the VI may license.
The transport of such manure, litter or bedding must be in closed, leak-proof vehicles or containers and in accordance with an APHA’s VIs instructions. Standard land spreading is not permitted from material that originates from poultry in the PZ
8.10.2 General procedures for cleansing, disinfection and treatment
Defra/Welsh Government approved disinfectants must be used in live animal areas in accordance with this list.
FBOs using a detergent and/or disinfectant agent must ensure that they are used as effectively as possible, to the correct concentration as stipulated in the approved disinfectants list and must, in particular, give consideration to the following in deciding which products to use and how to use them:
- the nature of the premises to be cleansed or disinfected
- the type of vehicle or other thing to be cleansed or disinfected
- any instructions from the manufacturer of the product (or of a veterinary inspector) as to pressure, concentration, minimum temperature and required contact time
FBOs must ensure during the Cleansing and Disinfection processes that:
- bedding, litter and faecal matter from transport vehicles are thoroughly soaked with disinfectant if not disposed as required in 8.10.1
- equipment and installations are effectively Cleansed and Disinfected
- the ground, any floors, ramps and walls are washed and Cleansed and Disinfected by thorough brushing and scrubbing
When washing with liquids applied under pressure, recontamination of areas or parts previously cleansed must be avoided.
FBOs carrying out a Cleansing and Disinfection procedure must ensure that a written record of that procedure is made, showing the date and time the procedure took place. Such record must be kept at the premises for a period of 12 months.
8.11 Enforcement
8.11.1 Enforcement of licence requirements
The LA is the enforcing authority for movement controls.
FSA staff are authorised to verify compliance with the conditions of the licence. Any suspected NC must be reported to the LA Trading Standards Department and the local APHA office.
8.11.2 Enforcement of Cleansing and Disinfection requirements
Additional FSA checks are required to verify compliance with the Cleansing and Disinfection conditions attached to the licences.
In accordance with the Transport of Animals (Cleansing and Disinfection) (No 3) (England) (Wales) (2003) Order, Defra/WG can ask FSA staff to carry out 100% checks of C and D of crates, modules and vehicles used to:
- transport birds from a PZ or SZ
- transport birds to a slaughterhouse situated within a PZ or SZ
Where Cleansing and Disinfection is unsatisfactory, FSA officers must serve a notice on the person in charge of the vehicle and report the incident to the LA.
Reference: See Chapter 2.2 Section 5 on ‘Cleansing and Disinfection’.
8.11.3 Designated slaughterhouses - suspension
Updated [If the OV or AO finds that the FBO is not meeting the conditions of the designation, they should ask the FBO to address the issue promptly, if the issue is not resolved within the agreed deadline another request to take action can be sent in writing, noting this advice in the daybook.
If this approach does not resolve the issue, the OV should then write to the Approvals team and FVL/FVC suggesting in writing that the designation should be suspended or revoked, along with evidence of the breaches and actions taken.]
8.12 Timesheet codes
8.12.1 GAVI code
Any time spent by FSA officials on additional controls related to AI must be coded as GAVI in the timesheet.
In particular, FSA Veterinarians and OVs who undertake work to support the designation process should code any additional time spent to GAVI.
The additional official controls required due to the slaughterhouse being within the PZ, SZ or RZ, or receiving birds from a PZ, SZ or RZ, including additional Cleansing & Disinfection checks, potentially increased PM inspection and paperwork verification checks, should be coded to GAVI. The scheduled C&D checks should continue to be coded to GAWG (please refer to C&D section under chapter 2.2).
9. Outbreak of Foot and Mouth Disease
In this section
9.1 Introduction
9.1.1 Purpose of section
This section describes how an outbreak of Foot and Mouth Disease (FMD) would be managed in Great Britain focussing on the controls applicable in slaughterhouses, cutting plants and meat processing plants.
For suspected cases of FMD found at slaughterhouses, section 2 (Action on suspicion of notifiable diseases) of this chapter must be followed.
Responsibility for managing outbreaks in the different countries of Great Britain falls to the respective Governments; Defra, Welsh Government and Scottish Government. Northern Ireland is recognised as a separate epidemiological unit and would expect to operate separate but similar controls in the event of an outbreak in accordance with EU and national law.
FSA Operations is a key operational partner of Defra and the Welsh Government in managing the outbreak in relation to slaughterhouses, approved meat establishments and registered dairy production holdings. Food Standards Scotland (FSS) is the key operational partner of Scottish Government for the same issues.
These instructions reflect the FMD Control Strategy for Great Britain 2011.
9.1.2 Background
FMD is a highly infectious, notifiable vesicular disease of domestic ruminants (cattle, sheep, goats), pigs, other farmed or wild cloven-hoofed mammals.
The economic significance of this disease is very high due to its ability to spread very rapidly and its profound effect on productivity. A very small quantity of the virus is capable of infecting an animal, and the disease could spread rapidly throughout the country if it is not controlled quickly.
FMD is not considered a public health threat. The FSA’s advice is that FMD is not transmitted to humans through the food chain.
9.1.3 Clinical signs and effects
Seven distinct serotypes of the FMD virus have been identified. The clinical signs of FMD are similar to other vesicular diseases and confirmation of diagnosis can only currently be made following laboratory tests. Affected animals have a high fever, which is followed by the development of blisters mainly in the mouth and on the feet. In some species however (notably sheep and goats), the disease is less severe or occurs as a sub-clinical infection.
Some strains can give rise to high levels of mortality in young animals. In adult animals the disease is not usually fatal, however it causes severe pain and distress, especially in cattle, and animals may be left permanently lame with reduced productivity following recovery.
Further information about the clinical signs of FMD is available here:
The virus is present in great quantity in the fluid from the vesicles, and it can also occur in the saliva, milk and dung. Contamination of any objects with any of these secretions or excretions is a danger to other susceptible animals. Heat and some disinfectants will destroy the virus, whereas cold and darkness tend to keep it alive. Survival of the virus in the environment depends on a range of factors and is highly variable. Under field conditions, this can range from days to months.
The virus can be transmitted on fomites (an inanimate object capable of transmitting infectious organisms from one individual to another, for example, vehicles and farm equipment), as well as mechanically by animals and other living vectors. Susceptible animals can pick up the virus either by direct contact with an infected animal, or by contact with foodstuffs or other things which have been contaminated by an infected animal, or by eating or coming into contact with some part of an infected carcase.
Airborne spread of the virus can also occur and, under favourable climatic conditions, the disease could spread several miles by this route.
9.1.4 Legislation
Primary domestic legislation:
- The Animal Health Act 1981 and the European Communities Act 1972.
- The Animal Health Act 2002 (England and Wales) amending the AHA 1981.
- Animal Health and Welfare (Scotland) Act 2006.
Council Directive 2003/85/EC implemented by the following domestic legislation:
- The Animal Health Act 1981 (Amendment) Regulations 2005
- The Foot and Mouth Disease (England) Order 2006,
- The Foot and Mouth Disease (Control of Vaccination) (England) Regulations 2006
- The Foot and Mouth Disease (Wales) Order 2006
- The Foot and Mouth Disease (Control of Vaccination) (Wales) Regulations 2006
- The Foot and Mouth Disease (Scotland) Order 2006
- The Foot and Mouth Disease (Slaughter and Vaccination) (Scotland) Regulations 2006
- The Foot-and-Mouth Disease (Scotland) Amendment Order 2007
- The Foot-and-Mouth Disease (Scotland) Amendment (No. 2) Order 2007
Further information regarding the legislation is available on the Defra website.
During an outbreak additional legislation may come into force imposing new or varying existing measures.
9.1.5 Controlled zones
According to the OIE, “zone” means a part of a country defined by the Veterinary Authority, containing an animal population or subpopulation with a specific animal health status with respect to an infection or infestation for the purposes of international trade or disease prevention or control.
Zones will be put in place on suspicion or confirmation of disease at an IP to limit spread of disease. The zones have associated restrictions on the movement of animals, animal products and anything else which can spread disease. The restrictions are stricter close to IP.
A Protection Zone (PZ) - mandatory on confirmation of disease and will cover a minimum of 3km radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
A Surveillance Zone (SZ) - mandatory on confirmation of disease and will cover a minimum of 10km in radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
A Restricted Zone (RZ) - will be declared to implement a national movement ban across GB by each Administration at the beginning of any FMD outbreak.
Other zones may be established at the time of suspicion or if vaccination is implemented.
9.1.6 Designation
There are two types of FMD designation for establishments:
- Level 1. Establishments producing unregulated meat during an outbreak of FMD.
- Level 2. Establishments producing regulated meat during an outbreak of FMD.
‘Regulated meat’ means, for the purposes of this document, fresh meat etc. referred to in article 21(1) and article 28(1) of Schedule 5 of the Foot-and-Mouth Disease (England) Order 2006 or Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006.
‘Regulated meat’ is meat from susceptible animals originated in PZ or SZ produced within the 21 days prior to the earliest infection date and any other meat which may not have been stored and transported separately from it.
Regulated meat does require specific treatment for inactivating the virus and must be ‘regulated meat’ at all times until it is subjected to a specific treatment in a designated treatment establishment.
Approved meat establishments will need to be designated by the FSA on behalf of Defra or by the Welsh Government (FSS will do so on behalf of Scottish Ministers) before receiving and processing susceptible animals and meat from premises within a PZ or SZ.
Establishments operating and situated within a PZ or SZ must be designated to be able to operate during the outbreak regardless of the origin of the animals or meat.
Additionally, all the slaughterhouses operating during an FMD outbreak within the Restricted Zone (RZ) (likely the whole of Great Britain) need to be designated even if they are not receiving animals from a PZ or SZ.
In order to facilitate preparedness for a potential outbreak, FBOs may apply for a pre-designation anytime. On request of the FBO, the pre-designation may be subsequently activated during an outbreak of FMD. Regardless of pre-designation, plants are not designated until the FSA or Welsh Government activates the designation during an outbreak. Movement of animals or product to the plant may additionally require a movement licence issued by APHA.
Designations are only valid during the outbreak period and to process products produced during the outbreak period. Once the outbreak officially ends, the establishment will remain pre-designated providing that there are no relevant changes. If there are any changes affecting the legal entity, the management, the biosecurity facilities or the control procedures required for the designation, the FBO must inform both the OV and FSA Approvals and, if applicable, re-apply for a pre-designation. In the case of a new outbreak, the establishments will have to apply for the activation of the pre-designation.
Only approved establishments demonstrating robust compliance with all the designation requirements will be designated to operate during an FMD outbreak.
For any establishment handling ‘regulated meat’ (level 2 designation) they must have satisfactory chiller capacity for maintaining the separation between different categories of meat. This will imply separate chillers in the case of exposed meat or clear physical separation in case of fully wrapped and packed meat and satisfactory handling and disposal of ABP generated from ‘regulated meat’.
For slaughterhouses applying for any designation (i.e. level 1 or level 2), the following requirements must be met:
- Satisfactory C and D facilities for ensuring the 100% C and D of livestock lorries on site. This may be achieved by limiting throughput.
- Satisfactory capacity and arrangement for the handling, treatment and disposal of manure.
- Satisfactory presentation of heads and feet for post-mortem inspection.
- Satisfactory biosecurity measures in place covering all visitors, vehicles and laundry.
- Satisfactory arrangements with the FSA for ensuring full OV attendance and the post-mortem inspection of all the heads and feet of susceptible species.
For treatment establishments, satisfactory implementation of HACCP-based procedures guaranteeing and demonstrating the effective treatment of the ‘regulated meat’.
Activation of a designation does not mean the establishment will be able to receive animals for slaughter or meat as such movements will additionally require a licence.
9.1.7 FMD suspected in a slaughterhouse
The actions to be taken by the OV in cases of suspected vesicular diseases found at the ante-mortem or post-mortem inspection are explained in Section 2 (Action on notifiable diseases) of this chapter.
Where the APHA veterinary inspector is unable to rule out disease during the investigation of a suspected slaughterhouse case, all animals present will be slaughtered quickly and the meat isolated whilst investigations are undertaken.
No meat is allowed to be removed from the premises until the VI is satisfied that meat to be moved is not at risk of spreading FMD virus. Meat, other products and by-products of animal origin that have come from suspected animals, or may have come into contact with such products or by-products, will be isolated within the slaughterhouse pending the outcome of the investigation.
The place of origin of all animals suspected of being affected by FMD will be investigated. The FBO is advised to detain the meat in suitable conditions to ensure that the meat remains fit for human consumption if disease is negated and the meat is released for sale.
If FMD is confirmed, this meat and any other product or by-product from the animal will be disposed of.
9.2 FMD Outbreak
9.2.1 Outbreak – Early Stages
On confirmation of FMD, a total nationwide ban on the movement of animals is likely to be implemented (National Movement Ban). During this time, the animals already present in the lairages of the slaughterhouses must be slaughtered as soon as possible and without delay.
Only animals completing journeys that started before the national movement ban should arrive at slaughterhouses after the national movement ban is put in force. No other animals should arrive at the slaughterhouse during the full movement ban.
9.2.2 Outbreak – FSA Action
Any consignment of livestock breaching the national movement ban must be reported to Trading Standards immediately and the FVL must be informed as soon as possible.
Any undue delay in the slaughtering beyond 48 hours of arrivals must be reported to APHA and the FVL.
FSA staff must carry out a thorough ante-mortem and post-mortem inspection of those animals to ensure that they are not showing any sign of FMD. In addition to the inspection requirements, this will imply the post-mortem inspection of all the heads and feet of susceptible animals even if they are not harvested for human consumption.
Flexibilities in the deployment and completion of the official inspections are cancelled during the outbreak so cold inspection and OV-flexibility arrangements are cancelled.
9.2.3 Outbreak – Designation
During this stage, the OV should discuss with the FBO the potential FMD designation of the slaughterhouse in preparation for the national movement ban being relaxed. For this, the FBO must provide assurance to the OV about the compliance with all designation requirements. FBOs wishing to be designated will need to submit the application fully completed and countersigned by the OV to the FSA approvals team.
FBOs should prepare the slaughterhouse for the potential FMD designation by cleansing and disinfecting the lairage leaving it ready for the required daily cleansing and disinfection required by the designation.
Slaughterhouses located outside of the SZ or PZ can dispatch the meat without restrictions providing that the meat is not coming from animals originated in those areas.
Slaughterhouses, cutting plants and meat processing establishments located in a PZ or SZ would require FMD designation for allowing the marketing of meat, regardless of the course of the animal. The OV must inform the FBO of this requirement and record that in the daybook. Any failure to comply with this requirement must be reported to APHA, Defra and / or Welsh Government.
Any ‘regulated meat’ found in the establishment must be detained using a Detention Notice (ENF 11-26) waiting for a Defra or Welsh Government decision for that meat.
9.2.4 Controlled zones during the suspicion phase
When an APHA VI is unable to rule out disease during the investigation of a suspected case, samples will be taken. The APHA veterinary inspector will also serve a notice on the occupier of the premises designating it a Suspect Premises. No movements of any person or thing is permitted on or off the premises unless licensed by a VI.
If samples are submitted because FMD cannot be ruled out, a Temporary Control Zone (TCZ) may be put in place around the suspect premises with a default size of 10km in radius. The zone can be larger or smaller if considered more appropriate for controlling the spread of disease. Within the TCZ, movements of susceptible animals to and from premises (including into or out of the zone) are not allowed except under licence.
A Supplementary Movement Control Zone (SMCZ) may also be established at suspicion stage, restricting the movement of animals in a wider area.
If laboratory tests and veterinary investigations do not indicate the presence of FMD any longer (or the virus of any other notifiable vesicular disease), restrictions on the premises will be lifted.
9.2.5 Controlled zones after confirmation of FMD
The following zones will be put in place on confirmation of disease at an IP to limit spread of disease. The restrictions are stricter close to IP:
- A Protection Zone (PZ) - mandatory on confirmation of disease and will cover a minimum of 3km radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
- A Surveillance Zone (SZ) - mandatory on confirmation of disease and will cover a minimum of 10km in radius from the IP. The Authority may decide not to put in place a PZ where the premises are a slaughterhouse or a place where animals have been sent by the Authority following suspicion of disease in an animal in transit.
- A Restricted Zone (RZ) will be declared to implement a national movement ban across GB by each Administration at the beginning of any FMD outbreak.
9.2.6 Controlled zones if vaccination is implemented
Routine, preventative vaccination is banned under EU law, allowing the EU to maintain the highest FMD status under international trade rules of “countries free from FMD without vaccination”. However, from the outset of an outbreak the Government is legally obliged to consider whether vaccination would assist disease control and as appropriate activate arrangements to implement vaccination.
If vaccination is implemented, it will normally be carried out within Vaccination Zone(s) (VZ).
A Vaccination Surveillance Zone (VSZ) extending at least 10 km beyond the edge of the vaccination zone will be put in place. This zone and its restrictions remain until FMD-free status is achieved.
Once vaccinated, live animals cannot be traded either within the EU or Internationally. EU safeguard measures (for example, special certification or special marking) will be in place restricting non-heat-treated meat and meat products to the domestic market for most of the duration of an outbreak. The nature of the restrictions may depend on the slaughter date of the animal.
9.2.7 Movement of susceptible animals
At the start of any outbreak, there will be a high degree of uncertainty about where in the country FMD may exist. The position will start to become clearer as tracings, surveillance and the epidemiological investigation progress. Decisions onto change control measures will only be taken when the epidemiological position for any particular outbreak indicates that the risk of spread can be adequately mitigated by biosecurity conditions. It is essential that restrictions remain in place as long as necessary to ensure the disease can be controlled and eradicated as quickly as possible.
Changes in movement restrictions can be expected to be phased. The first phase will be limited to those activities which need to happen at the beginning of any outbreak to address immediate animal welfare needs, for example, movement of dairy cows for milking, transport of feed to animals within zones or very low risk activities, collection and processing of milk.
Restrictions can be expected to be eased incrementally as certainty about the outbreak increases. Low risk movements will be considered, for example, movements direct to slaughter to a designated slaughterhouse within a short distance, before higher risk movements to live.
Government will address issues relating to ensuring what operations industry can reasonably continue to carry out during an outbreak through discussion with the FMD core group in England and industry stakeholders in Scotland and Wales.
9.3 Controls on meat
9.3.1 Meat controls in the Temporary Control Zone (TCZ) and Supplementary Movement Control Zone (SMCZ)
There is no specific control requirement for meat and milk from TCZ and SMCZ, unless premises are also within another zone, in which case the conditions for that zone apply.
However, the controls for PZ and SZ will be applied retrospectively and therefore, some of the meat will subsequently need to be traced, marked and treated if FMD is confirmed (see below sub topics).
9.3.2 Tracing of potentially infected material from an IP
Meat and meat products, carcases, milk and milk products, hides and skins derived from susceptible animals from the IP will need to be traced. Once traced the owner will be required to either dispose of them, or treat them as directed to kill any virus that may be present. This includes meat, milk or other products at the IP that were produced from susceptible animals originating from the IP or in some cases originating from other farms where the IP product has been in contact with such products. Compensation is not paid.
The FSA Incidents team will coordinate the tracings of meat and other products of animal origin intended for human consumption from animals originated in the IP. Once found, FSA staff should detain them for ensuring their adequate disposal.
9.3.3 Tracing of ‘regulated meat’ produced before the confirmation of the IP and the establishing of the PZ and SZ
Meat produced from animals originating from an area that subsequently became a PZ which was produced in the 21 days before the earliest infection date in that PZ and any other meat which was not stored and transported separately from it becomes ‘regulated meat’. Such must be traced for ensuring its marking and treatment.
If Defra / Welsh Government require the tracing, the FSA Incidents team will coordinate the tracings of meat and other products of animal origin intended for human consumption. Once found, FSA staff should detain them for ensuring their adequate disposal or over-marking followed by treatment.
9.3.4 Meat controls in a Protection Zone (PZ)
Fresh meat from animals originating from a PZ can be marketed if either:
- it was produced more than 21 days before the earliest infection date and stored and transported separately from meat produced 21 days or fewer before the earliest infection date; or
- a treatment is applied before being marketed. This meat is ‘regulated meat’ until it is treated for inactivating the FMD virus.
The production of ‘regulated meat’ requires:
- separation of animals and product in abattoirs, transport and storage and subsequent plants until treatment complete,
- meat to be health marked or identification marked and that mark to be over stamped until treated, and
- meat to be treated for inactivating the FMD virus in an FMD designated establishment. The main treatment allowed for meat and offal is heat treatment (cooking). (See 9.3.9)
Slaughterhouses handling animals originating from farms in the PZ must be designated for FMD.
Any commercial premises located in the PZ which handles meat must be designated for operating under the FMD outbreak.
9.3.5 Requirements for fresh meat, minced meat, mechanically separated meat and meat preparations
Where this meat is from susceptible animals and produced on approved establishments in a protection zone, the establishment must be designated by the FSA for operating during the FMD outbreak.
The establishment must process only meat which was either:
- produced in the protection zone more than 21 days before the earliest infection date there, or
- produced from animals reared and slaughtered outside a protection zone, or
- produced from animals transported to the establishment under the authority of a licence granted under paragraph 12(2)(e) of Schedule 5 of The Foot and Mouth Disease (England) Order 2006 or paragraph 12(2)(e) of Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006 and slaughtered there.
The establishment must at all times during the production process stores, identifies and transports restricted meat separately from other meat.
9.3.6 Meat controls in a Surveillance Zone (SZ)
Fresh meat from animals from a SZ can be marketed if either:
- the animals were on the same premises for at least 21 days before slaughter and were identified so as to allow tracing of the premises; and the meat has been detained under supervision for at least 7 days and until any suspicion of infection on the premises of origin has been ruled out; or
- the animals were on the same premises for at least 21 days before slaughter during which no susceptible animals were brought onto the premises; samples taken within the 48 hours before loading have tested negative; and meat has been detained under supervision for 24 hours and not released until after a repeat inspection of animals on the premises of origin has ruled out on clinical grounds the presence of infected or suspect animals.
Treatments required for meat before being marketed:
- Separation required in abattoirs, transport and storage and subsequent premises until treatment complete.
- Beef and sheep carcases (excluding heads, viscera and offal) must be health marked or identification marked, those marks over-stamped and subsequently heat treated (cooked) or matured and deboned to specific standards (see 9.3.9 and 9.3.10).
- Pig meat (excluding heads, viscera and offal) must be health marked or identification marked, those marks over-stamped and subsequently heat treated (cooked) to specific standards (see 9.3.10).
- Offal must be trimmed to specific standards (see 9.3.9), packaged, identified by an over-stamped ID Mark and subsequently treated by specific heat treatment or by specific fermentation and maturation for destroying the FMD virus (see 9.3.9).
9.3.7 Requirements for fresh meat, minced meat, mechanically separated meat and meat preparations from susceptible animals and produced on approved establishments in a SZ.
The establishment must be designated for operating during the FMD outbreak.
The establishment must process only meat which was either:
- produced from animals transported to the slaughterhouse from the surveillance zone and it falls within paragraph 28(4), 28(5) or 28(6) of Schedule 5 of The Foot and Mouth Disease (England) Order 2006 or Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006
- produced from animals reared and slaughtered outside the surveillance zone and its associated protection zone, or
- produced from animals transported to the slaughterhouse from the protection zone under the authority of a licence granted under paragraph 12(2)(e) of Schedule 5 of The Foot and Mouth Disease (England) Order 2006 or Schedule 4 of the Foot and Mouth Disease (Wales) Order 2006.
The establishment must at all times during the production process; store, identify and transport products intended to be eligible for despatch outside the protection zone separately from those which are not eligible for that movement, and in accordance with the conditions of the authorisation.
9.3.8 Meat controls from a VZ
If vaccination is used for the control of the disease, Defra and the Welsh Government will issue guidance for the meat controls from a VZ.
9.3.9 Meat treatments
These procedures are required for meat and offal from susceptible animals from a PZ and for offal and certain meat from a SZ.
Meat requiring treatment to ensure the destruction of FMD virus must have undergone any of the following treatments:
- heat treatment in a hermetically sealed container at a level of at least FO3;
- heat treatment at a minimum temperature of 70°C, reached throughout the meat;
- heat treatment in a hermetically sealed container to at least 60°C for a minimum of 4 hours, during which the core temperature must be at least 70°C for 30 minutes;
- natural fermentation and maturation of not less than nine months, resulting in the following characteristics
- Aw value of not more than 0.93, or
- pH value of not more than 6.0;
- heat treatment ensuring a core temperature of at least 65°C is reached for the time necessary to achieve a pasteurisation value equal to or more than 40.
9.3.10 Trimming-offal standards
The following procedures are required for meat from susceptible animals from a SZ:
- heart from which lymphatic glands, connective tissue and adhering fat has been completely removed,
- liver from which lymphatic glands, adhering connective tissue and fat has been completely removed,
- whole masseter muscles,
- tongues with epithelium and without bone, cartilage and tonsils,
- lungs from which the trachea and main bronchi and the mediastinal and bronchial lymphatic gland have been removed,
- other offal without bone or cartilage from which lymphatic glands, connective tissue, adhering fat and mucous membrane have been removed.
9.3.11 De-boning standards
Meat (together with diaphragms but excluding offal) is deboned if the bone and main accessible lymphatic glands have been removed.
9.3.12 Maturation
Carcases are matured if they:
- have been matured at a temperature of more than 2°C for at least 24 hours; and
- have a pH value in the middle of the Longissimus dorsi recorded at less than 6.0.
9.4 FBO responsibilities
9.4.1 Application for FMD designation
The FBO should apply to be pre-designated or designated to the FSA Approvals Team (Approvals@food.gov.uk), who will provide the latest version of the application form containing all the requirements and instructions necessary for the designation.
9.4.2 Biosecurity
Good biosecurity standards in slaughterhouses must be implemented at all times but, during an FMD outbreak, they must be heightened.
Guidance on biosecurity is available on the Defra pages of Gov.uk.
9.4.3 Marking of meat
A special mark (for example, Round Mark with the letters GB instead of the oval mark with the letters EC) may be required for all the meat produced in the Restricted Zone (i.e. either the whole GB or any regionalisation which may be allowed) by international trade safeguard measures. In previous outbreaks, the round mark required by the Commission Decision 2001/304/EC included size specification for that special mark (GB = 7 mm, Establishment No = 10 mm, Circle outer diameter = 50 mm, Line thickness of circle = 3 mm).

All the ‘regulated meat’ (meat for PZ or SZ) must have an ‘regulated meat’ heath mark or ID mark clearly applied to it. Every single health mark and ID mark applied on “restricted meat” must be clearly ‘regulated meat’.
‘regulated meat’ means, in relation to a health marked or ID marked item, bearing an additional diagonal cross consisting of two straight lines intersecting at the centre of the health mark or ID mark and allowing the information there to remain legible (whether or not that additional cross is applied by the same stamp as the mark).
All meat with an ‘over-stamped’ heath mark or identification mark is ‘regulated meat’ must be treated in a designated establishment using specific treatments for destroying the FMD virus.
Regulated meat can be transported to a designated treatment centre for an approved treatment to ensure any undetected FMD virus is destroyed. After treatment, the restricted markings can be removed and the normal oval (or round mark if the EU Decision requires this) health mark be applied.
9.4.4 Traceability & record keeping
Traceability of the meat is a legal requirement in all circumstances. The FBO should be aware that robust internal traceability systems will help to minimize the costs of the required tracing of the meat produced from the PZ before the declaration of the PZ.
The occupier of every premises in a PZ or SZ where susceptible animals are kept shall create and maintain the records regarding the number of each species of animal kept and the stock of meat, meat products, carcases, hides and skins, manure, fodder and used litter. The occupier shall maintain these records updating them within 24 hours of any change.
9.5 FSA responsibilities
9.5.1 FSA presence
During an FMD outbreak, OV flexibilities at slaughterhouses and Game Handling Establishments are suspended. At least one OV must be present at all times when slaughtering until all animals have passed post-mortem inspection.
Additional FSA attendance may be required to provide the controls and verification required for the control of the outbreak.
Strict biosecurity practices must be implemented by FSA staff at all times. Particular attention must be paid to the use, handling and disposal of protective clothing and the C and D of footwear and equipment.
9.5.2 Confirmation of FMD designation of the slaughterhouse
OVs must obtain confirmation from the FSA that the slaughterhouse is designated by the FSA in England or by Welsh Government in Wales for handling regulated meat and / or operating within a PZ / SZ before releasing animals from PZ or SZ for slaughter.
OVs must obtain confirmation from the FSA that the cutting plants or treatment establishments to where ‘regulated meat’ is dispatched are designated for handling and treating ‘regulated meat’.
FSA staff should encourage FBOs to apply for pre-authorisation to the FSA even if they do not need it, so they can operate without disruption should they require it at a later stage.
9.5.3 Movement of animals
On arrival to the slaughterhouse the OV or MHI must inspect the movement licences and accompanying documents for every animal or batch of animals to verify:
- the origin of the animals
- that the consignment is intended for that slaughterhouse
- that where the animals originate from a PZ / SZ, they are kept separated from other animals.
9.5.4 C and D
Additional FSA checks are required to verify compliance with the C and D conditions attached to the licences.
After unloading at the premises of the destination, the parts of the vehicle used to transport anything which might be contaminated with mud, slurry, animal faeces, excretions or any other similar matter including the wheels and wheel arches must be C and D on site.
The provisions for signing the driving declaration and leaving the establishment without the vehicle being C and D are not applicable in control zones during an FMD outbreak.
FSA staff must carry out 100% checks of C and D of vehicles used to transport animals from a PZ / SZ and of all vehicles if the slaughterhouse is located in a PZ / SZ. Additional FSA staff may be required to perform those checks.
The hard standing area used for the C and D of the livestock transport must be maintained clean and free of animals / vermin / pets. This area must be C and D after finishing operations. Other vehicles should not have access to this loading area for the duration of operation as a designated establishment.
C and D must include the wheels and wheel arches.
All transport vehicles must be thoroughly C and D after unloading the animals and before leaving the slaughterhouse. Special care must be taken to avoid any recontamination of vehicles after C and D, particularly through soil and dirt adhering to the wheel arches and surrounding parts (as this is not controlled by the wheel mats at the exit). This may necessitate spraying the exterior of the vehicle at the boundary of the site.
The abattoir must be clean prior to commencing killing. Naturally vermin and poultry should not have access to the abattoir to avoid transmission of undetected disease.
No additional FSA C and D checks are required in a slaughterhouse located outside of the PZ / SZ where the transport of animals originated from outside the PZ / SZ. However, the FBO must maintain high standards of C and D of all vehicles and no transport vehicle must leave any designated slaughterhouse without being C and D.
9.5.5 Confirmation of slaughter
The OV or an MHI under the OV supervision, must confirm that the animals arriving under a Specific Licence have been slaughtered by endorsing the licence presented by the FBO.
Where any problems arise relating to animal movements the OV must contact the local Trading Standards Office and APHA.
The OV should verify that the FBO is returning the completed Specific Licence to the issuing APHA office.
9.5.6 Enhanced post-mortem inspection
The designation of slaughterhouses includes the requirement of the presentation of all the heads and feet from all the susceptible animals to the official post-mortem inspection.
The FSA is committed to provide additional resources when necessary for allowing the post-mortem inspection of heads and feet but in certain circumstances the speed of the line may need to be reduced for allowing that inspection.
9.5.7 FSA verification of FBO controls in slaughterhouses
The OV must verify that the FBO complies with the objectives of the FMD legislation, movement licences and, where applicable the conditions of the FMD authorisation. In particular:
- FMD designation status of the slaughterhouse.
- Movement licences of animals admitted for slaughter.
- C and D of ALL the livestock transport vehicles before leaving the establishment.
- Slaughtering of animals no later than 24 hours after unloading.
- ‘Regulated meat’ is meat from animals within the designated protection or SZ. Such meat must:
- be marked as ‘restricted meat’ by ‘over-stamping’ of the Health Mark or Inspection Mark.
- be kept separately from other meat at all times
- be transported separately and only to designated premises
- not be traded or sold in the UK
- not be traded with other EU states
- not be exported from the EU
- Full traceability of ‘regulated meat’
- Verification of the destination of all the dispatched ‘regulated meat’.
- Application of Special Mark to ‘unregulated’ meat.
- Adequate handling, storage and disposal of ABP in compliance with designation conditions and FMD Order.
- Adequate handling, storage and disposal of manure in compliance with designation conditions and FMD Order.
9.5.8 FSA verification of FBO controls in cutting plants
UAIs must be organised for verifying that FBO complies with the objective of the FMD legislation, movement licences and, where applicable, the conditions of the FMD designation. In particular:
- FMD designation status of the cutting plant.
- Traceability documentation of meat received at the establishment.
- ‘Regulated meat’ is meat from animals within the designated protection or SZs. Such meat must:
- be marked as ‘regulated meat’ by ‘over-stamping’ of the Health Mark or Inspection Mark.
- be kept separately from other meat at all times
- be transported separately and only to designated premises
- not be traded or sold in the UK
- not be traded with other EU states
- not be exported from the EU
- Full traceability of ‘regulated meat’.
- Verification of the destination of all the dispatched ‘regulated meat’.
- Application of Special Mark to “unregulated” meat.
- Adequate handling, storage and disposal of ABP in compliance with designation conditions and FMD Order.
9.5.9 FSA verification of FBO controls in treating establishments
UAIs must be organised for verifying that the FBO complies with the objective of the FMD legislation, movement licences and, where applicable, the conditions of the FMD designation. In particular:
- FMD designation status of the treating establishment.
- Traceability documentation of meat received at the establishment.
- ‘regulated meat’ is meat from animals within the designated protection or SZs before applying a specific treatment as per FMD legislation. Such meat must:
- be kept marked as ‘regulated meat’ by and ‘over-stamped’ Health Mark or Inspection Mark.
- be kept separately from other meat at all times
- be transported separately and only to designated premises
- not be traded or sold in the UK
- not be traded with other EU states
- not be exported from the EU
- Full traceability of ‘regulated meat’.
- HACCP-based procedures demonstrating that the treatment comply with the specific legal requirement and, therefore, would ensure that the FMD is destroyed. (See 9.6.9)
- After treatment, the meat is considered unrestricted and the ‘over-stamped’ markings can be removed.
- Application of Special Mark to ‘unregulated’ meat when required.
- Adequate handling, storage and disposal of ABPs.
9.6 Animal by-products and co-products
9.6.1 ABPs and co-products produced in a PZ or a SZ or from animals originating in such a zone.
ABPs and co-products other than hides, skins, wool, ruminant hair or pig bristles must not been sold or consigned for sale unless they satisfy one of the following requirements:
- They were produced more than 21 days before the earliest infection date in the PZ, or in the case of a SZ, the associated PZ and at all times stored and transported separately from animal products not so produced.
- They have undergone one of the following treatments:
- heat treatment in a hermetically sealed container at a level of at least FO3;
- heat treatment in which the centre temperature is raised to at least 70°C for at least 60 minutes.
- Blood and blood products used for technical purposes have undergone any of the treatments referred to in point B(3)(e)(ii) of Chapter IV of Annex VIII to Regulation (EC) No. 1774/2002.
- Lard and rendered fats have undergone the heat treatment referred to in point B(2)(d)(iv) of Chapter IV of Annex VII to Regulation (EC) No. 1774/2002.
- Petfood and dog chews complying with the requirements of points B(2), (3) or (4) of Chapter II of Annex VIII to Regulation (EC) No. 1774/2002.
- Game trophies of ungulates complying with the requirements of points A(1), (3) or (4) of Chapter VII of Annex VIII to Regulation (EC) No. 1774/2002.
- Animal casings have been cleaned, scraped and either salted with sodium chloride for 30 days or bleached or dried after scraping and were protected from recontamination after treatment.
- It forms part of a composite product (that is, a manufactured or processed product containing more than one ingredient at least one of which is an animal product) and each ingredient which is an animal product has been treated as above or was not produced from susceptible animals originating on IP, suspect premises or contact premises or in a temporary control zone, protection zone, surveillance zone or vaccination zone.
9.6.2 Hides, skins, wool, ruminant hair and pig bristles produced in a PZ or a SZ or from animals originating in such a zone
Hides, skins, wool, ruminant hair and pig bristles of susceptible animals originating in a PZ or SZ must not been sold or consigned for sale unless either:
- they were produced more than 21 days before the earliest infection date in the PZ, or in the case of a SZ, the associated PZ, and at all times stored separately from hides and skins which were not so produced; or
- it has been treated for complying with the requirements in article 20 of Regulation (EC) No. 1774/2002 and points:
- In the case of hides and skins: A(2)(c) or (d) of Chapter VI of Annex VIII to Regulation (EC) No.1774/2002
- In the case of wool, ruminant hair and pig bristles: A(1) of Chapter VIII to Regulation (EC) No. 1774/2002
9.6.3 Manure produced in a PZ
Particular controls apply to manure from premises in a PZ where susceptible animals are kept; or collected from vehicles carrying susceptible animals from or within a PZ.
It must only be dispatched under a licence granted by an APHA inspector.
9.7 Enforcement
9.7.1 Enforcement of licence requirements
The LA is the enforcing authority for movement controls.
FSA staff are authorised to verify compliance with the conditions of the licence. Any suspected NC must be reported to the LA Trading Standards Department and the local APHA office. The FVL must be informed.
9.7.2 Enforcement of C and D requirements
Additional FSA checks are required to verify compliance with the C and D conditions of the licences and authorisations.
Where C and D is unsatisfactory, FSA officers must serve a notice on the person in charge of the vehicle under the Transport of Animals (Cleansing and Disinfection) (No 3) (England) (Wales) (2003) Order, Scotland (2005) Order, and report the incident to the LA.
Additionally, the breach of the terms of their licence under the FMD Order should be enforced.
Reference: See Chapter 2.2 Section 5 on ‘Cleansing and disinfection’.
9.7.3 Designated establishments
Where the OV or AO is not satisfied that the FBO is complying with the conditions to be designated, they must advise the FBO to correct the deficiency immediately reflecting this as verbal advice in the enforcement programme.
Where this informal enforcement approach is not successful, the OV must contact the FSA Approvals Team immediately and recommend that the designation is suspended.
9.8 Timesheet code
Any time spent by FSA officials on additional controls related to FMD must be coded as GFMD in the timesheet.
10. Outbreak of Bluetongue Virus Disease (BTV)
In this section
10.2 Ante-Mortem Requirements for BTV
10.1 Introduction
10.1.1 Background
Bluetongue Virus Disease (BTV) is a notifiable disease of ruminants, including sheep, cattle, deer, goats and camelids. It is generally accepted that BTV does not cause disease in other animals or humans.
The severity of the infection depends upon the strain of the virus and may be affected by serotype. There are currently 29 different BTV serotypes.
The serotype involved in the outbreak of BTV in Northern Europe was identified as serotype 8 (BTV 8). Other serotypes common in mainland Europe include BTV 1 and BTV 4.
Spread between animals is almost exclusively via vector transmission - infected midges moving between and biting animals susceptible to the disease. It is not spread through carcases or fomites, such as contaminated vehicles, boots or clothing. However, infected midges trapped inside animal transport vehicles could potentially spread disease over large distances.
BTV is mainly spread by adult infected midges biting an animal susceptible to the disease. Although susceptible animals are vulnerable throughout the warmer months of the year, the peak populations of the vector midge (various Culicoides species) occur in the late summer and autumn, particularly at dawn and dusk. The midge however can also overwinter in buildings.
Updated [The virus can also be transmitted via infected germinal products (semen, ova, and embryos) as well as passed on maternally from mother to unborn offspring].
The latest information about BTV can be found online.
These instructions are applicable in case of outbreak of BTV. However, part of these instructions may be applicable to certain scenarios (situations) where there is a risk of introduction of the disease through the import of animals.
10.1.2 Zones for BTV
Once circulation of disease is officially confirmed, appropriate areas are declared as a Control Zone (CZ) or a Temporary Control Zone (TCZ) (which must include the infected premises) and a Restricted Zone (RZ) (made up of a Protection Zone (PZ) and Surveillance Zone (SZ)).
- CZ or TCZ - at least 20km around infected premises.
- PZ - at least 100km around infected premises (with flexibility to adjust according to epidemiological circumstances).
- SZ - at least 150km around the infected premises (with flexibility to adjust according to epidemiological circumstances).
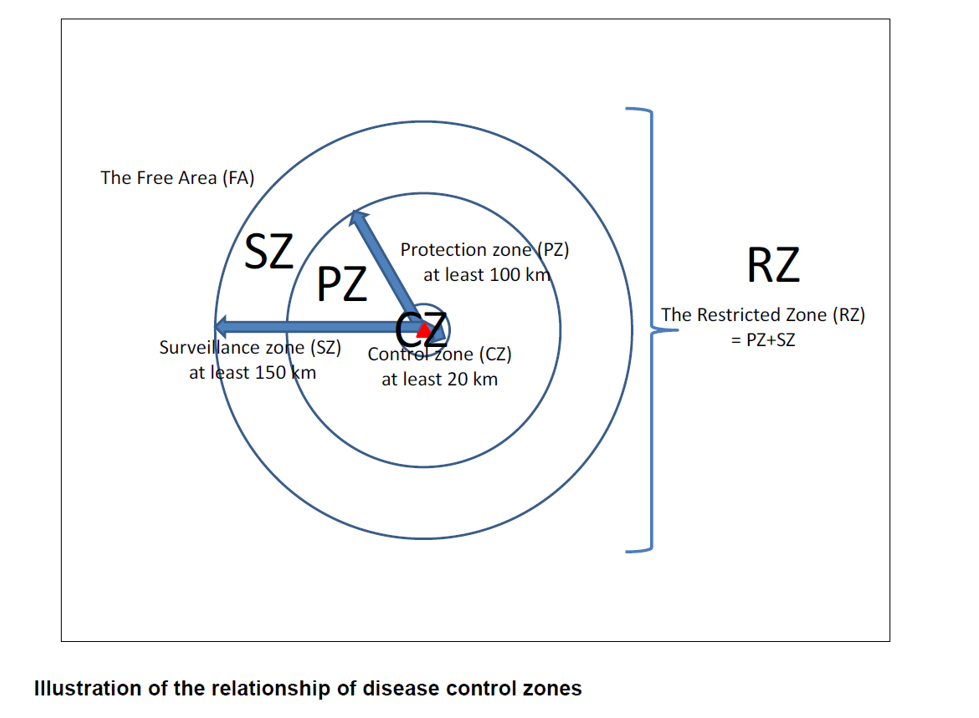
Updated [Illustration of the relationship of disease control zones from GB Bluetongue Virus Disease Control Strategy]
The rest of England, Scotland and Wales that are not under movement restrictions are known as the ‘free area’ (FA).
A map with the current zones will be available on the relevant devolved government policy websites. Updated [The size of the zones, which are centred around the infected premises, will take account of geographical barriers, whether or not individual or multiple serotypes are circulating, and the circumstances of the outbreak. Where they overlap and could be combined a practical and proportionate approach will be taken.]
An outbreak is only confirmed once disease is found to be circulating in the midge vector populations. A single infected animal alone would NOT therefore constitute an outbreak, unless it can be proven to have become infected by resident midge vectors via another animal in the vicinity.
During a BTV outbreak, restrictions may apply to red meat slaughterhouses approved for processing ruminants and game handling establishments (GHE) operating within RZ.
Only specifically designated slaughterhouses outside the PZ are permitted to receive ruminants from the BTV zones under a General Licence.
There are no restrictions on the movement of non BTV susceptible species such as pigs and poultry within the BTV RZ. Slaughterhouses processing such species alone (for example, pigs) will operate as normal.
FSA duties (for example, audits) in operating cutting establishments will continue as normal.
Each movement is also subject to special conditions as stipulated on the license which has implications for both the transporter and the slaughterhouse operator on arrival.
Updated [The conditions of the movement licence for animals from any BTV zone to a slaughterhouse in a free area will require that the slaughterhouse processing them is designated.
On some occasions, movements within and into the zones will also require a movement licence, e.g., movements of animals to a slaughterhouse in the TCZ.]
The operator of the slaughterhouse in an SZ also needs to have a licence to operate if they receive animals from a PZ.
10.1.3 Legislation
England
Bluetongue Regulations 2008 as amended. These regulations implement Council Directive 2000/75 and enforce Commission Regulation 1266/2007.
Wales
The Bluetongue (Wales) Regulations 2008 as amended. These Regulations implement Council Directive 2000/75/EC and enforce Commission Regulation (EC) No1266/2007
Scotland
The Bluetongue (Scotland) Order 2012 No. 199. This Order implement Council Directive 2000/75 and enforces Commission Regulation 1266/2007
You can view these at www.legislation.gov.uk.
10.2 Ante-Mortem Requirements for BTV
10.2.1 Importance of official ante-mortem inspection
Although BTV has no public health implications, it has major significance for animal health and substantial economic consequences.
It is important for the OV to be vigilant for BTV clinical signs at ante-mortem and post-mortem inspections on all ruminants.
Updated [Photographic images of clinical signs could be found online.]
If you suspect the presence of the disease, contact APHA immediately following the procedure of reporting suspect cases of Notifiable Diseases established in the Section 2 of this MOC Chapter.
The Food Chain Information (FCI) should reflect any treatment applied to the animal such as insecticides or repellents and the OV must verify compliance with the withdrawal periods. Action in line with MOC instructions for suspected residues (Chapter 5) should be considered.
Licensed insecticides or repellents which must be applied to lorries prior to loading or buildings, in accordance with the manufacturer instruction do not need to be declared in the FCI.
Flexibilities on OV attendance are not applicable in slaughterhouses receiving animals covered by BTV movement licences.
10.2.2 ‘Dusk’ rule
Animals moved under a General Licence from a RZ to a slaughterhouse within the PZ have to be moved and slaughtered at least two hours before sunset at the latest (the ‘dusk’ rule).
The OV is to report any non-compliance with the requirements of the ‘dusk rule’ to their Local Authority Trading Standards Office and to the Field Veterinary Leader who may consider the recommendation of the suspension of the designation of the slaughterhouse.
10.2.3 Identification of a suspect case
On identification of a suspect case of the disease (or if in doubt) the OV must notify the APHA immediately, inform the FBO of the slaughterhouse and report it to the Field Veterinary Leader and the Incidents team.
After notification, the OV must follow the instructions issued by the APHA Vet.
FBO’s should be aware that if a BTV positive case is identified in a slaughterhouse, APHA will serve a Notice declaring the slaughterhouse as an infected premises and certain conditions will then be imposed.
Note: See Section 2 in particular 2.2.9 ‘Procedure for suspect notifiable disease’ in this chapter.
10.2.4 Residues
BTV movement controls may involve the use of insecticides and repellents at farm level and during transport. This should be taken into consideration when carrying out the ante-mortem inspection and, particularly, during the assessment of the FCI.
Withdrawal periods prior to slaughter must be adhered to as stipulated by the manufacturers’ instructions for any pour on insecticides or residual sprays that are applied to animals.
The insecticides required for premises and vehicles are not usually licenced for being used directly on the animals. Those environmental insecticides or repellents must be licensed by the HSE and used as per manufacturer’s instructions. As they are not applied directly on the animals, they are not required to be recorded in the FCI.
Insecticides or repellents used on the animals must be licenced by the VMD, used as per manufacturer’s instructions, the withdrawal periods observed and declared in the FCI.
10.3 Movements Rules for BTV
10.3.1 FBO duties
FBO should be aware that for animals moved under a General Licence the movements must be timely reported to the British Cattle Movement Service (BCMS) for cattle and Livestock Information Service (LIS) for sheep and goat in England and EIDCymru in Wales.
The FBO should endorse the movement licence by signing it to confirm that the animals have arrived and been slaughtered immediately.
The FBO must send the completed movement licence (including the FBO and OV / MHI parts as per point 10.3.4) to the issuing APHA licencing office.
10.3.2 Movement of live animals
The movement of susceptible live animals (for example, ruminants) will be subject to restriction.
The direct movement to slaughter of ruminants is likely to be allowed soon after the start of an outbreak providing the movement takes place under a general licence or a specific licence issued by APHA.
A sample of the specific movement licence is shown below:




For movement licences and most up to date licensing requirements FSA staff / FBOs may consult the DEFRA website/Welsh Government website or contact their local APHA office in the first instance.
10.3.3 Movement of Fallen Stock & Deer Carcases
There are no movement restrictions on fallen stock from slaughterhouses and deer carcases to GHE.
10.3.4 FSA duties
The OV is to report any non-compliance with the requirements of the movement licences to their Local Authority Trading Standards Office.
The OV / MHI should endorse the movement licence by completing the part dedicated to the OV / MHI for confirming that all ruminant animals covered by the licence have been slaughtered in compliance with the movement licence conditions. Once this part has been completed, the licence must be returned to the FBO.
On arrival at the slaughterhouse the animals must be slaughtered no later than two hours prior to sunset on the same day as arrival and the journey must be planned accordingly in conjunction with the slaughterhouse operator.
The OV should verify that the FBO is complying with the licence requirement of returning the completed movement licence to the issuing APHA licencing office.
10.4 Designation of Slaughterhouses for BTV
10.4.1 Designation requirement for slaughterhouses in relation to BTV
The following establishments will require designation by Defra / by the Welsh Ministers based on FSA recommendation before receiving and processing animals:
- Slaughterhouses located in a Bluetongue free zone receiving animals from Protection, Surveillance, or Restricted zones.
- Slaughterhouses in surveillance zones receiving animals from protection zones.
- Slaughterhouses processing imported animals representing a higher risk of introduction of the disease.
- Slaughterhouses receiving animals that require designation as a condition of the movement license, usually including movements of animals originating in Temporary Control Zones, Control Zones and from premises under Bluetongue restriction notices.
- Any slaughterhouse as required as a condition from an animal movement licence
For a designation to be issued the following conditions shall also be met:
- animals must not be resident within the confines of the slaughterhouse, including its lairage
- Slaughterhouses with nearby farming activity shall have facilities and procedures in place to fully manage biosecurity risks presented by the farming operations
- Field lairages not to be used for receiving animals moving under a licence to a designated slaughterhouse
- Updated [Specific midge control measures, controls on the times of arrival and killing of animals, biosecurity controls, controls for the manure and measures for cleaning of the lairages and associated areas
The conditions to obtain designation are intended to minimise potential disease risk presented by biting midges, the vector for this disease. They are specified in Part 1 of the designation application form. Annex 29.]
10.4.2 FBO responsibility
The slaughterhouse operators can apply for a pre-designation anytime. On request of the FBO, the pre-designation may be subsequently activated during an outbreak or any of the circumstances named in 10.4.1.
FBO requiring pre-designation / designation should contact the FSA Approvals team for requesting the required application. A sample of the pre-designation/designation form which includes the requirements can be found in Annex 29.
FBO of pre-designated slaughterhouse will require the activation of the designation after verifying that all the information included in the pre-designation form remains accurate and the requirements will be complied.
If the slaughterhouse is located within a SZ, the FBO would require to contact APHA for obtaining a general licence to slaughter animals in the BTV SZ which have moved direct from premises in the BTV PZ.
10.4.3 FSA duties
The OVs are to encourage the FBOs to apply for pre-designation to the FSA as part of their contingency plans and to facilitate preparedness. This would help them to operate without disruption during the outbreak.
The OV will have to verify compliance with the designation rules and, once satisfied with that, recommend the pre-designation/designation of the slaughterhouse if all the rules are complied with.
The specific pre-designation and designation conditions are described in the pre-designation/designation application form. They include:
- Controls related to the compliance of the “dusk rule”. The rules are only applicable for animals moved under a BTV licence. The procedures in place should be documented for facilitating staff training and implementation when the designation is activated.
- Controls of slaughtering other animals without undue delay. Movement rules require that animals should be slaughtered no later than 48 hours after arrival (for animals moved under BTV movement licences, the “dusk rule” apply). This applies at both pre-designation and designation stages. If the FBO is not complying with this condition, the OV should not recommend the pre-designation.
- Requirements in relation to biosecurity. In particular the slaughterhouse has to keep yards and surrounds clean and minimise areas which could harbour vectors. This applies at both pre-designation and designation stages. If the FBO is not complying with this condition, the OV should not recommend the pre-designation.
- Requirements in relation to the management of manure. When the designation is activated, Manure and other material likely to attract midges must be removed from in and around the animal areas of the abattoir. It shall be removed completely from the site at least once in the 7 days preceding and at least once in the 7 days after the slaughter of any animals that are required to be slaughtered at a designated slaughterhouse, to minimise sites likely to harbour the vector.
- Pre-designated slaughterhouses must have documented procedures reflecting the capability of applying that requirement when the designation is activated.
- Updated [Requirement in relation to the cleaning and disinfection of lairage and associated areas. Although the lairage should be cleansed and disinfected as part of the abattoir’s normal operating protocol, there are no additional requirements for disinfection under BTV designation. For BTV controls, the cleaning procedures for the lairage, unloading areas and walkways must be clearly documented including frequency. Those procedures must be implemented as per established procedures in both pre-designation and designation stages and these can mirror regular abattoir cleaning SOPs. The procedures may establish different frequencies during outbreaks, to meet the requirements of the designation, in particular:
o Manure and other material likely to attract midges must be removed from in and around the animal areas of the abattoir at least once in the 7 days preceding and at least once in the 7 days after the slaughter of any animals that are required to be slaughtered at a designated slaughterhouse.
o Unloading facilities for vehicles and any walkways likely to become soiled with dung must be fully cleansed and disinfected on at least a daily basis.] - Requirement in relation to control of vector The slaughterhouse must have controls in place to minimise risks of vector attack. These may include:
- Adequate cleaning of yards and other areas within slaughterhouse curtilage to minimise areas which could harbour vectors.
- Appropriate use of insecticides, ensuring they are dry before animals enter the treated area.
- As an alternative or in addition to the use of insecticides, the use of electronic or other means to control flying insects.
- Adequate storage away from animal areas for and disposal of the manure and other material likely to attract midges.
On granting the pre-designation / designation, the OV should keep a copy in the plant file and verify compliance.
FSA auditors should verify compliance with the conditions established in the pre-designation / designation document and that the information on it remains accurate.
Failure to comply with the conditions should trigger the consideration of the suspension or revocation of the pre-designation / designation. The OV should contact the FVL in those cases.
10.4.4 Designation and activation process
Local teams (OVs, TMs, FVCs and FVLs) play a key role in the designation process, but the overall responsibility rests with the FVL. Support can be sought from notifiable-disease.portfolio@food.gov.uk at any time during the process.
The FBO must contact approvals@food.gov.uk to request an application form (Annex 29). Upon completion by the FBO of part 1 of the “Application for Designation of a Slaughterhouse” and once the OV is satisfied that the abattoir has the necessary SOPs in place and meets the criteria for the level required, the OV should complete and sign Part 2 of the aforementioned document. The FBO can then submit the application form and standard operating procedures to the approvals team (approvals@food.gov.uk)
The approvals team will subsequently assign a FVL to each application. An on-site visit to the establishment by the TM, FVC or FVL to assess the procedures and SOPs needs to be considered on a case-by-case basis. It is for the FVL to decide if a visit is required.
Once the FVL is satisfied that the SOPs and the establishment meets the requirements, part 3 should be signed and sent back to the approvals team, that in turn will liaise with a Defra representative for the signature of part 4 if the premises is in England, or with a Welsh Government representative if the abattoir is located in Wales.
Once the designation is activated by Defra/Welsh Government, the approvals team will send the designation documents to the FBO, with copies to the ND lead, FVL, FVC, AM, TM and OV via email, and will update the information in Establishments and People database. A copy of the Designation will be kept in the plant file in SharePoint.
10.5 Meat controls in BTV
There are no specific meat restrictions (for example, special mark or treatment) related to BTV.
10.6 Enforcement
10.6.1 Enforcement of licence requirements including “dusk rule”
The LA are the enforcing authorities for movement controls.
FSA staff are authorised to verify compliance with the conditions of the licence. Any suspected breach of the movement controls or licences must be reported to the LA Trading Standards Department and the local APHA office. The FVL must be informed.
10.6.2 Designated and licenced establishments
Updated [If the OV or AO finds that the FBO is not meeting the conditions of the designation, they should ask the FBO to address the issue promptly, if the issue is not resolved within the agreed deadline another request to take action can be sent in writing, noting this advice in the daybook.
If this approach does not resolve the issue, the OV should then write to the Approvals team and FVL/FVC suggesting in writing that the designation should be suspended or revoked, along with evidence of the breaches and actions taken.]
10.7 Timesheet code
Work undertaken by the OV to verify compliance with the designation rules should be coded to GDIS.
11. Outbreak of African Swine Fever (ASF)
In this section
11.3 ASF case in a slaughterhouse/GHE (Slaughterhouse case)
11.4 ASF case in a farm (Infected Premises)
11.5 ASF controls related to Protection Zone and Surveillance Zone
11.6 Pre-designation & Designations of meat establishments
11.1 Introduction
11.1.1 Background
This section describes how an outbreak of African Swine Fever (ASF) would be managed at FSA Field Operations level, particularly the controls applicable in FSA approved slaughterhouses, wild game establishments, cutting plants and treatment centres when an ASF outbreak is confirmed and control zones are declared.
ASF is a notifiable disease of animals. The domestic legislation requires that any person who suspects that a domestic or feral pig or carcase is infected with the disease must immediately notify the appropriate authority through Animal and Plant Health Agency (APHA). Animals of the Suidae family including pigs and wild boar are susceptible to ASF.
The ASF virus can remain active for months or years in pig meat products and can be a significant source of spread and dispersal of ASF in pigs.
For suspected cases of ASF found in slaughterhouses or in wild game establishments, section 2 (Action on suspicion of notifiable diseases) of this chapter must be followed but further detailed guidance specific for ASF is included in this section (point 11.3).
Before the confirmation of any outbreak of ASF, particularly when the international epidemiological situation indicates higher risk of introduction of the disease in GB, the FBO and the OV should establish contingency plans which may include the application for pre-designation of the slaughterhouse for processing pigs from controlled zones. Further guidance is provided in this section (point 11.2).
The latest information about ASF in UK can be found online.
Pictures of clinical signs and lesions of AFS are available online.
Industry guidance on cleansing and disinfection of vehicles transporting pigs are available online.
Upon confirmation that ASF has been found in domestic pigs or wild boar in GB, animal health protection measures are imposed to prevent the spread of disease.
In relation to ASF controls, the applicable legislation defines:
- “Restricted animal” is an animal of a species susceptible to ASF which is at, in or from:
- suspect premises
- an establishment where a disease is suspected
- infected premises
- an establishment where a disease is confirmed
- an infected area
- a protection zone, or
- a surveillance zone.
- “Restricted meat” is meat, including meat that has come into contact with meat produced on or after the date that a protection zone or a surveillance zone is declared, or an earlier date where the Secretary of State specifies such a date for the purpose of disease control from a restricted animal. Where restricted meat has been treated in accordance with the relevant legislation at a treatment centre it ceases to be regarded as restricted meat.
- “Designation of premises, slaughterhouses and game handling establishments”: The Secretary of State may designate any establishment or premises for the purposes of slaughtering animals, or cutting, preparing, processing, packing, wrapping, storage or treatment of meat.
- During an outbreak, the animals and other potentially infectious material (for example, manure, restricted meat, animal by-products) would be moved under specific or general licences.
- A person moving any pig under the authority of a specific licence must: carry the licence or a copy of it at all times during the movement; and on demand by an inspector or other officer of the appropriate authority, produce the licence or a copy and allow a copy or extract to be taken.
- A person moving any pig under the authority of a general licence must at all times during the movement, carry a document containing details of what is being transported, including the quantity; the date of the movement; the names of the persons responsible for the pig or thing being moved at the place of departure and the place of destination; the addresses of the place of departure and the place of destination; on demand by an inspector or other officer of the appropriate authority, produce the document and allow a copy or extract to be taken; and keep the document for at least six months.
Where disinfection of vehicles transporting pigs and areas where live animals are handled is carried out during the outbreak, the disinfectants must be approved by the appropriate authority and shown on the list of approved disinfectants published under the “General Orders” and used at the authorised dilution rate and in accordance with the manufacturer’s instructions.
The disinfectant that is used needs to be approved, a list of approved disinfectants can be found online.
11.1.2 Legislation and disease control strategy
The main legislation applicable during an ASF outbreak is:
- The Diseases of Swine Regulations 2014
- Animal Health Act 1981 as amended by the Animal Health Act 2002
- The Products of Animal Origin (Disease Control) (England) Regulations 2008 (SI 2008/465) (as amended)
- The Products of Animal Origin (Disease Control) (Wales) Regulations 2008 (SI 2008/1275) (as amended)
- The Diseases of Animals (Approved Disinfectants) (England) Order 2007
- The Diseases of Animals (Approved Disinfectants) (Wales) Order 2007
The Disease Control Strategy for African and Classical Swine Fever in Great Britain describes how government and others would manage an outbreak of either ASF or Classical swine fever (CSF) in Great Britain (GB) and is available online.
11.2 Preparedness for ASF
11.2.1 FBO preparedness for a potential outbreak of ASF
FBO handling or processing pigs, wild boars or their meat should be prepared for the possibility of an ASF outbreak affecting GB. This preparation should include the implementation of good biosecurity standards in the premises, the establishing of procedures for minimizing the impact of a potential withdraw/recall and the establishing of procedures for the adequate handling of restricted meat.
FBO must provide the required facilities and equipment for the cleansing and disinfecting of vehicles transporting pigs on site. FBO should promote and supervise the adequate implementation by drivers of the sequence of stages for dry-cleaning, washing and disinfection using disinfectant authorised under the general order as required by the legislation and the good practices promoted by the industry which can be found online.
FBO should establish a cleaning schedule and a documented procedure for the frequent and effective cleansing and disinfection of lairages, animal by-product areas and associated areas and implements.
FBO should clearly define the batches, establish robust internal traceability of meat at slaughterhouse and cutting plant and implement an adequate cleansing and disinfection of the food contact surfaces between batches (for example, by using cleaning with detergent followed by use of effective food-grade disinfectant effective against the virus following their manufacturer instructions and using adequate concentration and contact time).
Further guidance for traceability and preparedness for recalls/withdrawals is available at the FSA’s Guidance on Food Traceability, withdrawals and recalls within the UK Food Industry.
FBO should consider applying for a pre-designation for ASF which would improve their preparedness and speed up the designation process in case of ASF outbreak (see point 11.5 for further details).
FBO should schedule the arrival of animals for allowing their slaughter, in peacetime, within 48 hours of arrival and, when possible, the day of arrival. The schedule for arrival of animals and slaughtering should allow the adequate cleaning and disinfecting of lairages ensuring the adequate contact time for the disinfectants to act and their rising and/or drying as per manufactures instructions for preventing the risk of residues from disinfectants in the meat.
Slaughterhouses are animal holdings and therefore, the FBO’s slaughterhouses have a responsibility to prevent animal diseases. General guidance for animal diseases prevention including biosecurity measures, staff and visitors’ controls, buildings, equipment and vehicles controls are available online.
The industry has produced further guidance and materials.
11.2.2 FSA team preparedness
The OV at pig slaughterhouses and Game Handling Establishments processing wild boars should discuss regularly with the FBO their preparedness for ASF outbreaks using the guidance provided in this section and particularly the points raised under the heading 11.2.1.
When the establishment has been pre-designated for ASF, the OV should review the pre-designation at least once a year, using the opportunity for providing advice for improving the FBO preparedness and reviewing FSA team preparedness including awareness and procedures in place.
The FSA team can improve the preparedness for an ASF outbreak by:
- Being able to identify the signs of ASF infection at the ante-mortem and port-mortem inspection and the reporting procedures to APHA (Points 11.1.1 and section 2 of this MOC chapter) on suspicion of notifiable diseases.
- Applying strict biosecurity practices such as:
- Comply with FBO’s biosecurity procedures.
- Ensure a good separation of personal clothing and protective clothing using designated changing areas.
- Keep the FSA room tidy and clean.
- Eat only in designated areas separated from protective clothing and inspection equipment.
- Use dedicated protective clothing in the lairage and yards.
- Comply with the FSA procedures in relation to PPE
- HSW3 – Personal Protective Equipment (PPE) found on Share Point, particularly:
- Staff must not take protective clothing home for laundering. If overalls need to be transported to another site for laundering, they should be placed in special bags for transport and then placed directly into laundry bin. Those special bags are available from procurement.
- When carrying out ante-mortem inspection, non-white coats may be worn with waterproof over trouser or leggings. Coats must not be taken outside the establishment and must be sent to laundry as per other protective clothing. Waterproof over trouser/leggings must be disinfected with an approved disinfectant.
- Arrange systems for the frequent change or/and disinfection of the protective clothing used in the lairage, yards and animal by-product areas.
- The use of disposable PPE may be considered particularly during an outbreak when dealing with restricted animals or meat. When used, the disposal must be established observing good biosecurity practices.
- Use of designated parking.
- Ensure robust cleaning and disinfection practices for the PPE and tools.
- Ensure a safe handling, storage and dispatching of dirty protective clothing.
- Plan a suitable system for overcrossing the Health Mark in case of being necessary during the outbreak.
11.3 ASF case in a slaughterhouse/GHE (Slaughterhouse case)
11.3.1 Definition
Live animals, carcases and offal with suspicious ASF clinical signs or lesions found during routine ante-mortem or post-mortem inspection, are called ‘slaughterhouse cases’. The animals may or may not have come from premises included in ASF Surveillance Zone/Protection Zone.
11.3.2 FBO duties
The FBO should assist to OV and APHA Vet during the investigation of the suspected case and, as per legislation, must comply with
- not to move, or permit to be moved
- the pig or carcase which is the subject of the notification from the premises where it is located;
- any other pig or carcase to or from those premises;
- any other animal from those premises if the veterinary inspector is of the opinion that it is likely to spread disease;
- anything off those premises unless the veterinary inspector is of the opinion that it is not likely to spread disease;
- to ensure that any person who has been in contact with any pig or carcase, or who has been on any part of the premises that may be contaminated with disease, takes all necessary biosecurity precautions to reduce the risk of spreading disease before leaving the premises; and
- not to permit any pig to be slaughtered unless authorised by a veterinary inspector; and
- to identify and isolate any carcase in which ASF has been suspected, any carcase originating from the same premises (and any carcase that has been in contact with any such carcase) so that such carcases do not come into contact with any other pig or carcase at the slaughterhouse.
11.3.3 FSA duties
On suspicion of ASF during the ante-mortem or post-mortem inspection, the OV must follow the instruction laid out in Section 2 in particular point 2.2.9 ‘Procedure for suspect notifiable disease’ in Chapter 6 (Notifiable Diseases) of MOC Volume 1.
If ASF is identified/suspected at the slaughterhouse, the OV must report to APHA who will provide further instructions and inform the FBO of the suspicion requesting to stop the production as established by the legislation.
When APHA is notified of suspicion of disease in pigs at an establishment, the establishment will be placed under restrictions and further movements of animals onto the premises prohibited whilst investigations take place. The killing of pigs will be halted by the FBO until the APHA Vet arrives and assesses the situation. Suspect animal(s) must be kept isolated and no product of animal origin (for example, meat, animal by-products) or potentially contaminated items/vehicles/person must leave the establishment.
If instructed by the APHA Vet, any remaining pigs will be killed without delay and the meat detained and kept separate from other meat.
Meat and any product (for example, animal by-products, edible co-products, manure, digestive tract contain) that has come from the suspect pig/s or may have come into contact with such meat/products, will be detained pending the outcome of the investigation. If swine fever is confirmed these meat/products will be handled and disposed of as category 2 material (category 1 material if originated from wild animals) in leakproof and closed containers. All meat at the premises will temporarily be detained until the APHA Vet has assessed the risk of the meat/products being infected or contaminated with swine fever virus. Where there is no risk of ASF infection or contamination, meat/products may be released otherwise it will be detained pending test results.
If ASF can be negated by the APHA Vet on clinical grounds, restrictions can be lifted and normal business resumed. All meat that had been detained will be released for sale subject to it continuing to comply with food hygiene requirements.
The establishment is not treated as an Infected Premises (IP) as it is likely pigs will have arrived already infected with ASF, although this may have been transmitted to others in the lairage or carcasses contaminated with ASF virus.
If samples need to be taken to confirm or negate the presence of ASF then restrictions will remain in force for 24-48 hours; this will prevent further animals being brought into the abattoir for slaughter. The APHA Vet will assess which pigs in the lairage may be infected and take the necessary samples.
Cross-contamination is a risk at both production areas (between meat/products) and lairages (between animals). The APHA Vet, working with the OV and FBO, will determine which animals and products may be at risk and thus detained and potentially disposed of. Withdrawal/recall of meat/products exposed to the risk of contamination may be necessary.
All animal waste (including manure, digestive tract content, hair, bones) and animal-by products have to be handled and transported preventing the risk of spread of the virus and disposed in a way assuring inactivation of virus.
In case of requiring a recall/withdrawal, the quantities/batches affected will depend on the batch size, segregation during processing & traceability. It will also depend on the quality and frequency of staff hand washing and sterilisation of tools and the cleansing and disinfection procedures and their robust implementation particularly for food contact surfaces, scalding tank/steam chambers and depilation machinery.
The potential need of recall/withdrawal would likely be for animal health purposes only as there would not be public health concerns. Depending of the risk assessment, the product may be subjected to a withdrawal rather than a recall. In that case, the withdrawal may be applied up to retail or retail distribution but not consumers and probably not from shop shelves. In order to minimize the size of the recall/withdraw, small batches, cleaning and disinfection between batches and robust traceability systems are recommended.
Similar procedures would be applied in Game Handling Establishments where ASF is suspected.
The OV must verify the compliance with the FBO’s duties established in the legislation, reminding the FBO of their obligations and reporting any breach to the APHA Vet.
11.4 ASF case in a farm (Infected Premises)
11.4.1 Background
Animals at a farm where ASF has been confirmed will be disposed of at the farm and will never be sent to a slaughterhouse. However, animals from that farm may have been sent to slaughter before disease was confirmed, whilst the disease was incubating.
Therefore, the meat from these pigs may be affected by ASF and will need to be traced, withdrawn, and disposed of. Animal products potentially infected with ASF will be disposed of as category 2 animal by-products.
11.4.2 FBO duties
The FBO and OV will be notified by APHA that the slaughterhouse has received pigs from an IP and the products from these pigs must be withdrawn and disposed of.
The FBO is responsible for disposing of the carcase/meat and derived products including animal by-products, coproducts. If the product has already left the establishment the FBO is responsible for notifying the recipient that they have similar responsibilities to dispose of the meat or notify other premises if the meat has been moved. Records must be retained for inspection.
Meat must be withdrawn by processors, manufacturers, distributors and retailers as far as retail shelves but not from end consumers.
Any person who is in possession of meat from a restricted animal originating from the “relevant date” from suspect premises, or meat that has come into contact with such meat, must detain that meat until those premises are no longer suspect premises. “Relevant date” means the date the suspect premises or infected premises became subject to disease restrictions, or any earlier date where the Secretary of State specifies such a date for disease control purposes.
Any person in possession of meat produced from a restricted animal originating from infected premises from the relevant date, or meat that has come into contact with such meat, must destroy that meat without delay.
Any person who has owned or been in possession of meat referred above must endeavour to trace that meat and inform the recipient of that meat, other than a consumer, that it is from infected premises.
11.4.3 FSA duties
FSA must follow APHA instructions. APHA will determine the earliest estimated date of introduction of the ASF virus on the IP farm following the epidemiological investigation and will provide instructions about the requirements for tracing, withdrawal and disposal of any meat and animal-by-products.
Pigs moved from the IP to slaughter in the period after ASF may have been introduced at the farm, but before disease restrictions were imposed may have been infected with ASF. Therefore, will be subjected to APHA epidemiological investigation and risk analysis. The meat from these pigs may be infected with ASF and will need to be traced, withdrawn and disposed of.
The FBO and OV will be notified by APHA that the slaughterhouse has received pigs from an IP and the products from these pigs must be withdrawn and disposed of. The FBO is responsible for disposing of the carcasses/meat and derived products including animal by-products and co-products). If the product has already left the establishment, the FBO is responsible for notifying the recipient and they have similar responsibilities to dispose of the meat or notify other premises if the meat has been moved. Records must be retained for inspection. Meat must be withdrawn by processors, manufacturers, distributors and retailers as far as retail shelves but not from end consumers.
The OV should verify:
- the FBOs batching procedures in place for assessing the risk of other meat being contaminated. This would require the consideration of the FBO’s internal traceability procedures, cleaning schedules, frequencies and procedures applied in the lairage, scalding tank/depilation machine and food contact surface (for example, evisceration/cutting equipment, cutting board).
- the FBO’s traceability records for the affected meat liaising with the FSA Incidents team for the wider supervision of the any withdraw needed.
- The FBOs traceability records for any category 3 material and manure/digestive tract contain generated or potentially contaminated with the virus. This would require the assessment of the batching of animal by-products and the frequency of collection and liaison with APHA.
11.5 ASF controls related to Protection Zone and Surveillance Zone
11.5.1 Background
After the confirmation of ASF, control zones will be established around the Infected Premises (IP):
- The Protection Zone (PZ) will centre around the IP with a radius of at least 3 km
- The Surveillance Zone (SZ) will centre around the IP with a radius of at least 10 km. The PZ will be found within the SZ.
11.5.2 Movement restrictions
An automatic ban on movements of pigs across GB is not proposed for ASF. Therefore, the movement restrictions are likely to affect mainly premises and animals in the PZ/SZ.
Pigs cannot be moved off or onto premises in the PZ or SZ. Pigs may be moved within a premise as long as they do not cross a public or private road. Derogations are unlikely to be available in the period following declaration of the zones. However, after a few weeks have passed since the last confirmed case in the area, government may start to consider the case to allow limited movement of pigs off premises in the PZ or SZ:
- for immediate slaughter in a designated slaughterhouse
- to other premises within the same zone due to welfare problems
- for culling and movement of the carcase to a rendering plant for processing
- pigs may be licensed from outside the control zones onto premises within zones.
APHA may license for the movement of a pig from outside the surveillance zone (SZ) to a designated slaughterhouse within the zone (SZ) for immediate slaughter provided that the vehicle transporting the pig is thoroughly cleansed and disinfected at the slaughterhouse after the pig has been unloaded. The licence may be granted by a veterinary inspector to allow movement of a pig after the expiry of the relevant period specified in the legislation, if the pig is transported directly to a designated slaughterhouse. The APHA Vet has individually inspected the pigs on the premises of origin, samples have been taken and the pigs are transported in a vehicle sealed by an APHA Vet.
APHA may license the movement of a pig from outside the protection zone (PZ) to a designated slaughterhouse inside the zone for immediate slaughter provided that the vehicle transporting the pig is thoroughly cleansed and disinfected at the slaughterhouse after the pig has been unloaded. The licence may be granted by APHA Vet only after the expiry of the relevant period specified in the legislation.
Trucks and vehicles that have carried live pigs or other livestock or material which may be contaminated with ASF virus are prohibited from leaving premises in the PZ/SZ unless they have undergone cleansing and disinfection (C&D). In the PZ, C&D of such vehicles must be inspected and authorised by a Veterinary Inspector.
11.5.3 Operation of meat establishments within the PZ/SZ
Pig slaughterhouses and GHE located in the PZ/SZ would need to be designated for being able to operate during the outbreak. Pre-designation of the slaughterhouses before the outbreak would facilitate the designation when required.
The movement of pigs from outside the PZ/SZ to a slaughterhouse located within the zones may be licensed from early in the outbreak as the movement is from a low disease risk area to a slaughterhouse for immediate slaughter. Slaughterhouses operating within a control zone must be designated. There are no specific controls on the meat produced from pigs originating from outside the zones. The practice of allowing C&D of vehicles away from the slaughterhouse will be suspended in these circumstances and they must fully C&D prior to leaving the slaughterhouse.
Cutting plants located in within the PZ/SZ do not need to be designated for ASF unless that they are accepting restricted meat.
11.5.4 Operation of establishments outside the PZ/SZ
Pigs originating outside the PZ/SZ and slaughtered at a slaughterhouse outside the PZ/SZ will not be subject to any additional controls, save any imposed in wider movement restriction or other control zones. There is no requirement for the slaughterhouse to be designated or for the meat to be controlled or (heat) treated.
The practice of allowing C&D of vehicles away from the slaughterhouse may be suspended if the disease situation requires.
Slaughterhouse located outside the PZ/SZ may be designated for processing pigs from the PS/SZ. Once pigs originating from within the PZ/SZ can be licenced to slaughter they must go to a slaughterhouse designated to slaughter animals from the PZ/SZ. Ideally the slaughterhouse will be located within the PZ/SZ, but regardless of location it must be designated.
Cutting plants and treatment establishments accepting restricted meat must be designated for ASF.
11.5.5 Operation of GHE
On confirmation of disease in feral pigs, the appropriate authority may declare a feral pig control zone.
Meat from a feral pig hunted in any feral pig control zone must not be placed on the market by any person unless the carcase has tested negative for ASF and an APHA Vet considers there is no risk of the spread of disease.
GHE accepting feral pigs located in the feral pig control zone or receiving bodies from that area have to be designated.
11.5.6 FBO duties
The legislation requires that meat establishments may only receive restricted meat/animals if it is a designated establishment. Therefore, an FBO at a pig slaughterhouse must prevent the acceptance of restricted animals unless the slaughterhouse has been granted with a designation for ASF.
Upon confirmation of an ASF outbreak in Great Britain, the FBOs should enhance their Food Chain Information (FCI) controls and establish procedures for ensuring that movement rules are observed by the pig suppliers and lorries transporting pigs to the slaughterhouse. FBO should be mindful that the potential slaughtering of restricted pigs from control zones in non-designated slaughterhouse is a breach of the legislation and will involve withdrawals/recalls and production disruptions.
Specific movement licences accompanying the pigs may require countersigning by the FBO slaughtering the pigs and/or the OV of the establishment. FBO should read and act upon the instructions provided in the movement licences.
When the vehicle transporting the pigs has been sealed by APHA, the FBO must request the presence of the OV for verifying the unsealing of the vehicle.
If a pig slaughterhouse is located in a PZ/SZ, the FBO would not be able to operate during the outbreak unless it is designated for ASF.
Manure or slurry, including digestive tract contain, from pig origin must not be transported or spread unless that it is done under a licence granted by an APHA Vet.
11.5.7 FSA duties
The OV must ensure that all the pigs accepted into the slaughterhouse:
- In the case of pigs from outside the PZ/SZ, the conditions of the general licence have been complied with.
- In the case of pigs from PZ/SZ, the conditions of the specific licences have been complied and they have been accepted into a slaughterhouse designated for ASF.
- In case of slaughterhouses located within or receiving pigs from PZ/SZ, the slaughterhouse is designated for that outbreak of ASF. A pre-designation does not qualify the slaughterhouse for receiving animals from PS/SZ.
When the vehicle transporting the pigs has been sealed by APHA, the OV must verify the unsealing of the vehicle.
In the case of the movement licence requiring an endorsement from FSA staff at the establishment, the OV should complete it as appropriate and ensure the instructions of the movement licence are followed.
When any breach in the movement licence conditions or standards is identified, the OV must report it to the relevant Local Authority (LA) recording the details in the daybook and inform to the Field Veterinary Leader (FVL)/Field Veterinary Coordinator (FVC) providing details of the breach and the response from the LA.
When any breach in the movement licence conditions has been committed by the FBO of a designated slaughterhouse, the request of a suspension of the designation should be considered by the OV in liaison with the FVL.
Pig slaughterhouse located within a PZ/SZ would require a designation for ASF for operating during the outbreak. When a pig slaughterhouse falls within a PZ/SZ while it has not been pre-designated for ASF yet, the OV should discuss with the FBO the need of pre-designation for being able to operate and provide advice for applying for a designation if needed (the FBO may consider to stop operations as well).
On confirmation of an ASF outbreak in GB, when a pig slaughterhouse is pre-designated, the OV should review and verify the compliance with the designation conditions for being able to recommend or not its designation and advice the FBO of any identified shortcoming which would need to be corrected before the FBO can apply for the designation.
When inspecting cutting plants during an outbreak, the inspector must ensure that pig carcases and pig meat bare a valid health mark or identification mark. Meat bearing crossed health marks or crossed identification marks must not be in cutting plants which have not been designated for ASF.
11.6 Pre-designation & Designations of meat establishments
11.6.1 Background
During an outbreak of ASF, the following designation requirements apply:
- Slaughterhouses/GHEs accepting susceptible animals from PZ/SZ (or Feral Pig Investigation or Control Zones) would need to be designated.
- Slaughterhouses located in PZ/SZ would need to be designated.
- Cutting plants accepting restricted meat must be designated for that.
- Treatment establishments receiving restricted meat for ASF treatment must be designated.
The pre-designation process allows the FBO to be prepared for applying for a designation in the case of an outbreak and will make the designation process quicker.
Animal By-Products from restricted animals must be categorised as Category 2 material (category 1 material if originated from wild animals). Any other animal by-product contacting directly or indirectly those products or meat from restricted animals must be categorised as Category 2 material as well.
11.6.2 Pre-designation process in peace-time
The aim of the pre-designation process is to allow the FBO and the resident FSA officials to be prepared in advance for applying for a designation if needed during an outbreak and for speeding up the designation process during an outbreak.
FBOs of slaughterhouses, GHEs, cutting plants and treatment establishments may apply for a pre-designation for ASF anytime. If the pre-designation is granted, on request of the FBO, the pre-designation may be subsequently activated during an outbreak.
A site approved for several co-located activities (slaughter, cutting, processing) under the same approval number would require a separate pre-designation /designation for each activity.
FBO requiring pre-designation should contact the FSA Approvals team and request an application. A sample of the pre-designation/designation form for slaughterhouses and GHEs which includes the requirements can be found in Annex 30.
11.6.3 Designation process during an ASF outbreak
FBO of pre-designated establishments will require the activation of the designation after verifying that all the information included in the pre-designation form remains accurate and the requirements will be complied with.
Designations cannot be granted unless there is an official confirmed an outbreak and ASF restrictions are in place. The pre-designation does not guarantee the designation.
Establishments which are not pre-designated at the time of the outbreak can apply for a designation. However, the designation process may take longer than in the case of pre-designated establishments.
During an ASF outbreak, FBO of pre-designated establishment requiring designation should contact the FSA Approvals team for requesting the activation of the designation. FBO of other establishment requiring designation should contact the FSA Approvals team for requesting the designation. A sample of the pre-designation/designation form for slaughterhouses and GHEs which includes the requirements can be found in Annex 30.
Designated slaughterhouses located within a Protection Zone may require additional approval from DEFRA – Welsh Government or Scottish Government for operating.
11.7 Meat and animal by-product controls in ASF designated establishments
11.7.1 Background about meat controls
Meat produced from pigs originating from the PZ/SZ (regardless of where they were slaughtered) is termed “restricted meat”. Such meat receives a special mark (a crossed through oval health mark) and cannot be sold fresh. It must be treated at a designated treatment centre and prior to treatment only handled at designated premises.
In some circumstances Third Countries may require the implementation of additional safeguard measures that apply to pigs, pork and pork products produced within the UK or a region of the UK. Should this happen, additional measures, such as special marking and trade restrictions may be imposed. Where a special stamp is proposed to indicate meat is restricted to the domestic market, it is likely a round stamp will be adopted.
It is paramount that “restricted meat” is continuously and robustly identified, separated and clearly marked with crossed identification mark or crossed health mark and that its traceability is thorough and consistent at all the stages.
Any risk of direct or indirect cross-contamination from “restricted meat” to other meat must be prevented.
The legislation requires that records related to “restricted meat” must be kept for 3 years from the date of slaughter, movement or treatment.
The FBO must not place on the market or export any “restricted meat”.
11.7.2 Background about animal by-products controls
All the animal by-products generated from “restricted animals” or “restricted meat” and any by-product contacting directly or indirectly with them must be categorised as category 2 material (category 1 material if originated from wild animals).
Category 2 material must be stored and dispatched in closed leak-proof containers, observing adequate biosecurity practices.
Containers of category 2 material must be cleansed and disinfected immediately after its use. When the establishment is located in a restricted area or has been handling restricted meat, the disinfection of the containers should be carried out using an approved disinfectant at the appropriate concentration.
Manure, litter and slurry including digestive tract contain from designated slaughterhouses can only be dispatched under a special licence issue by APHA.
The yards and the animal by-products handling and storage areas must be kept clean and, when necessary, disinfected.
11.7.3 Marking of restricted meat
Restricted meat must be marked with a specific Health Mark/Identification Mark which is Health Mark or Identification Mark over-stamped with a diagonal cross. This can be:
- a diagonal cross, superimposed on the health mark or identification mark applied under article 5 of Regulation (EC) No 853/2004 laying down specific hygiene rules for food of animal origin, or;
- a health mark oval stamp, 6.5 cm wide and 4.5 cm high with two straight lines crossing at the centre of the stamp in such a way that the information of the Health Mark is not obscured.
11.7.4 Movement of restricted meat
Restricted meat can only be moved or dispatched:
- From designated slaughterhouses to designated cutting plants. When the cutting plant is collocated, it requires a separate designation and the movement records between the slaughterhouse and the cutting plant must be kept.
- From designated slaughterhouses or designated cutting plants to designated treatment establishments.
Movements of restricted meat must be reduced to the minimum and re-dispatching of restricted meat is not allowed.
Freezing of restricted meat should not be carried out. The restricted meat must be treated without delay. Once it has been treated in a designated treatment centre, the meat product may be frozen as it will not be restricted any more.
The movement of restricted meat must be carried out preventing any risk of contamination from the restricted meat to other products or the environment during the handling, loading/unloading and transport.
The commercial document accompanying the meat must clearly state that the meat is “restricted meat”.
Any person who is in possession of restricted meat must make records of the following:
- the quantity of meat handled;
- the disease which caused the meat to be subject to restrictions under the disease legislation;
- the quantity of meat placed into and removed from cold storage;
- the date of such movement into or out of cold storage;
- the quantity of such meat that is no longer intended for human consumption.
Records must be kept for 3 years from the date of slaughter, movement or treatment.
11.7.5 Treatment of restricted meat
One the following treatments must be applied to “restricted meat” in designated treatment establishments:
- Heat treatment in a hermetically sealed container with an F value of 3 or more (where F is the killing effect on bacterial spores: an F value of 3 means that the coldest point in the product has been heated sufficiently to achieve the same killing effect as 121°C in three minutes with instantaneous heating and chilling).
- Heat treatment at a minimum temperature of 80°C which must be reached throughout the meat.
- Heat treatment in a hermetically sealed container to at least 60°C for a minimum of 4 hours during which time the core temperature must be at least 70°C for 30 minutes.
- Natural fermentation and maturation of not less than nine months for boneless meat resulting in the following characteristics: a Water Activity (Aw) value of not more than 0.93 or a pH value of not more than 6.
- Treatment of hams and loins involving natural fermentation and maturation for at least 190 days for hams and 140 days for loins.
In addition to the HACCP records, the occupier of a treatment centre where restricted meat is treated must keep records of the following:
- the date of the treatment;
- the species of animal from which the meat came;
- the quantity of meat treated
- the treatment applied.
Records must be kept for 3 years from the date of slaughter, movement or treatment.
11.7.6 FBO duties
The occupier of an establishment must ensure that restricted meat is marked with the special mark (over crossed health mark or identification mark) required by the legislation.
A person must not be in possession or control of restricted meat unless it is marked with the required special mark and the establishment is designated for ASF.
A person must not remove the special mark except to enable cutting, preparing, processing, packing or treatment of the restricted meat. Any person who removes the special mark, other than a person treating meat at a designated treatment centre, must reapply the special mark, with the appropriate plant approval number, after cutting, preparing, processing or packing the meat. The reapplication of the special mark should be immediate to avoid keeping the meat unattended at any time without being properly identified with the special mark.
Any person who is in possession of restricted meat must make records of the following:
- the quantity of meat handled;
- the disease which caused the meat to be subject to restrictions under the disease legislation;
- the quantity of meat placed into and removed from cold storage;
- the date of such movement into or out of cold storage;
- the quantity of such meat that is no longer intended for human consumption.
The occupier of a treatment centre where restricted meat is treated must keep records of the following:
- the date of the treatment;
- the species of animal from which the meat came;
- the quantity of meat treated;
- the treatment applied.
Records must be kept for 3 years from the date of slaughter, movement or treatment.
11.7.7 FSA duties
FSA staff delivering official controls in approved establishments during an ASF must verify compliance with the rules for “restricted meat” in particular:
- Restricted animals and restricted meat can only be present in establishment specifically designated for ASF. The OV/MHI must request a copy of the designation to the FBO for verification purposes.
- Requirements established in the designation for ASF.
- Complete separation from other meat / animals / animal by-products.
- Continuous and adequate application of the required marking at all the times.
- Dispatching of restricted meat to exclusively ASF designated premises
- Records regarding traceability of restricted meat and animal by-products
- Treatment parameters and practices.
FSA staff must detain any meat when in doubt and seek clarification.
FSA staff inspecting designated establishments must record in the daybook details of the verification of “restricted meat”.
FSA must report any incident related to “restricted meat” to FSA Incidents, inform immediately the FVL and refer it to the relevant Local authority.
11.8 Regionalisation
In addition to the controls related to Protection and Surveillance Zones, regionalisation may be implemented as part of the ASF controls.
Regionalisation is allowed at international level for the handling of outbreaks of ASF while reducing the impact of ASF outbreaks in international trade. The legal basis for regionalisation or zoning is in the World Trade Organisation (WTO) – World Organisation for Animal Health (OIE) is in the Article 6 of the Sanitary and Phytosanitary (SPS) Agreement and the chapter 4.3 of the OIE Terrestrial Animal Health Code.
The OIE defines zone/region as a clearly defined part of a territory containing an animal subpopulation which a distinct health status with respect to specific disease for which required surveillance, control and biosecurity measures have been applied for the purpose of international trade.
Regionalisation is applied in the European Union for the management of the ASF outbreaks in its member states. The Implementing Decision 2014/709/EU establishes the criteria for demarcating Parts I, II, III and IV subjected to specific restrictions in relation to animals, meat and by-products:
- Part IV: occurrence of ASF in both domestic pigs and wild boar. The disease control presents specific challenges due to the systemic and high-level non-compliance by stakeholders with the relevant EU requirements, in particular in relation to identification, registration and traceability of pigs and there are certain difficulties for the veterinary authorities to ensure the conformity with those requirements.
- Part III: occurrence of ASF in both domestic pigs and wild boar.
- Part II: occurrence of ASF only in wild boar.
- Part I: higher risk area with no cases, nor outbreaks, of ASF and where higher surveillance (in particular passive) is applied adjacent to a Part II, III or IV.
The restrictions relevant for meat controls applied to the parts can be summarised as:
- Live pigs cannot be dispatched from Parts II, III or IV.
- Pig meat, pig meat preparations and pig meat products cannot be dispatched from Parts III or IV.
- Animal by-products from porcine animals cannot be dispatched from Parts III or IV.
A map summarising the current regionalisation applied in the EU member states is available online.
11.9 Enforcement related to ASF controls
The Diseases of Swine Regulations 2014 are enforced by the relevant local authority.
A person must not intentionally obstruct or impede anyone acting in the execution or enforcement of The Diseases of Swine Regulations; without reasonable cause, proof of which lies on the person charged, fail to give to any person acting in the execution or enforcement of these Regulations any assistance or information that is reasonably required; provide to anyone acting in the execution or enforcement of these Regulations any information knowing it to be false or misleading or not believing it to be true; or fail to produce a record when required to do so by any person acting in the execution or enforcement of these Regulations.
The Product of Animal Origin (Disease Control) Regulations are enforced by the Secretary of State or by local authority in England, by the Welsh Ministers or by local authority in Wales.
FSA staff are authorised to act on matters arising under the following legislation:
- The Diseases of Swine Regulations 2014 (to be confirmed)
- The Product of Animal Origin (Disease Control) Regulations 2008 (to be confirmed)
Therefore, FSA staff can verify compliance with those Regulations in an FSA approved establishment while breaches would need to be reported to the relevant Local Authority for its enforcement.
The FSA can hold the granting of pre-designations/designations and recommend the suspension or withdrawal of them to Defra and Welsh Government at any time when their conditions are not complied with.
11.9.1 Enforcement of licence requirements
The Local Authorities (LA) are the enforcing authorities for movement controls.
FSA staff are authorised to verify compliance with the conditions of the licence. Any suspected breach of the movement controls or licences must be reported to the LA Trading Standards Department and the local APHA office. The Field Veterinary Lead (FVL) must be informed.
11.9.2 Designated establishments
At pre-designation stage, if the OV or Authorised Office (AO) identify concerns about the compliance with the pre-designation conditions, the issue must be discussed with the FBO in the first place. When this informal approach is not successful, the OV should recommend the suspension of the pre-designation of the slaughterhouse to the Field Veterinary Leader or Field Veterinary Coordinator.
Where the OV or AO is not satisfied that the FBO is complying with the designation conditions, they must advise the FBO to correct the deficiency immediately reflecting this as verbal advice in the enforcement programme. When this informal enforcement approach is not successful, the OV must contact immediately the FSA Approvals Team and Field Veterinary Lead / Field Veterinary Coordinator and recommend that the designation is suspended.
11.9.3 Restricted meat
Whenever any need for clarification or suspected breach affecting the meat in an FSA approved establishment is identified in relation to ASF controls, the OV/AO should initially detain the meat using a Detention Notice (ENF 11/26) and consider the derived animal by-products detaining them if applicable. This will allow the gathering of all the relevant information and its sharing with FSA Incidents and the relevant enforcement authority.
11.10 Timesheet code
Work undertaken by the OV to verify compliance with the designation rules and the additional tasks carried out during the ASF outbreaks should be coded in the timesheets as GDIS.
12. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 1: AN24 - Form A: Notice
Annex 7: EBL7 – Submission form
Annex 8a: CS117 – TB/EBL FSA consumables for other red meat abattoirs form
Annex 8b: CS118 – TB/EBL FSA consumables for APHA contracted abattoirs
Annex 15a: Sample: TB110 Reactor sampling and submission form
Annex 15b: Guidance for preparing protocols in contracted TB reactor slaughterhouses
Annex 17: Description of lesion template
Annex 20: FSA consumables requisition form - REMOVED
Annex 21: CS115 – DNA equipment form
Annex 22: Material for DNA analysis
Annex 23: Sample Despatch Process
Annex 24: Aujeszky’s Disease Training Note
Annex 25: Application for designation of slaughterhouses for Avian influenza or Newcastle Disease
Annex 26: Avian Influenza restricted meat: Communication lines for FBOs
Annex 27: Avian Influenza: Traceability of restricted meat
Annex 28: Specific licence – movement of poultry to slaughter from premises in a PZ or SZ
Annex 29: Application for designation of a slaughterhouse for Bluetongue
Annex 30: Application for designation of a slaughterhouse for African Swine Fever
Annex 31a: FSA TB Ready check list
Sections
2. Legislation, Enforcement Roles and Provisions
1. Introduction
In this section
1.1 Purpose
1.1.1 FSA enforcement role
These enforcement arrangements apply to all meat establishments approved in England and Wales and under veterinary control.
Enforcement action should also be taken in accordance with the FSA Enforcement Policy.
1.2 Relevant references
1.2.1 Authorised Officers (AOs), Inspectors and Authorised Persons
Operational staff must have a delegated authority to act as an “Authorised Officer” under food hygiene and safety legislation, “Authorised Person” under animal by-product legislation or an “Inspector” under TSE and animal welfare legislation. This will provide officers with the appropriate powers of entry and authority to undertake enforcement under the respective legislation.
For ease of reference, the MOC will refer to all officers as AOs, however, it is important to be aware of the designation that applies under respective pieces of legislation.
1.2.2 Food Business Operator (FBO)
FBO means the natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control (Assimilated Regulation (EC) 178/2002, Article 3, Point 6).
The Food Safety Act 1990, Section 53 (1) uses the expression “Proprietor” to refer to the person who carries out the business.
The Animal By-Products (ABP) legislation identifies the “Operator” who must comply with the requirements of assimilated law and domestic ABP legislation. Operator is defined as the natural or legal person having an animal by-product or derived product under their control, including carriers, traders and users [Assimilated Regulation (EC) 1069/2009, Article 3].
The assimilated and domestic welfare at slaughter legislation refer to the “Business Operator” as the natural or legal person having under its control an undertaking carrying out the killing of animals or any related operations falling within the scope of the Regulations [Assimilated Council Regulation (EC) 1099/2009, Article 2 (l)].
1.2.3 Court
References to ‘court’ should be taken to mean, in England and Wales, a Magistrate’ court or Crown Court.
1.2.4 Justice of the Peace
References to a ‘Justice of the Peace’ should be taken to mean, in England and Wales, a Magistrate.
1.2.5 Duly authorised representative
Duly authorised representative is a responsible person who has the authority to act on behalf of the FBO. This person should be identified either on the approval application document or through some other form of written correspondence.
1.2.6 Legal definitions
Most legislation includes a definition section that provides guidance on many of the phrases contained within it. The table below identifies where this guidance can be found in the main pieces of legislation that we enforce.
Note: At the point of the UK’s exit from the EU, most operative EU Regulations and Decisions were incorporated into domestic law as retained direct EU legislation under the European Union (Withdrawal) Act 2018. From 1 January 2024, under the Retained EU Law (Revocation and Reform) Act 2023, retained EU law became ‘assimilated law’.1
Legislation that was, prior to 1 January 2024, referred to as “retained direct EU legislation” (which may often have ‘EU’ or ‘EC’ included in its title) should now be identified as ‘assimilated law’ where applicable to GB. However, references to relevant EU legislation in respect of Northern Ireland remain unaffected. Where appropriate, previously published references to ‘Retained’ or ‘REUL’ should be updated to “assimilated”.
| Legislation | Location of definition |
|---|---|
| Assimilated Regulation (EC) 178/2002 | Articles 2 and 3 |
| Assimilated Regulation (EC) 852/2004 | Article 2 |
| Assimilated Regulation (EC) 853/2004 | Article 2 and Annexes I, II, III |
| Assimilated Regulation (EU) 2017/625 | Article 3 |
| Assimilated Regulation (EU) 2019/625 | Article 2 |
| Assimilated Regulation (EU) 2019/624 | Article 2 |
| Assimilated Regulation (EU) 2019/627 | Article 2 |
| Assimilated Regulation (EU) 2019/628 | Article 2 |
| Assimilated Regulation (EC) 999/2001 | Article 3, Annex I |
| Assimilated Regulation (EC) 1069/2009 | Article 3 |
| Assimilated Regulation (EU) 142/2011 | Annex I |
| Assimilated Regulation (EC) 2073/2005 | Article 2 |
| Assimilated Regulation (EC) 1099/2009 | Article 2 |
| The Food Safety Act 1990 (as amended) | Sections 1,2 and 53 |
| All domestic regulations, for example, the Food Safety and Hygiene (England) Regulations, the TSE Regulations, Animal By-Product (ABP) (Enforcement) Regulations, the Welfare of Animals at the Time of Killing Regulations | Regulation 2 ‘Interpretation’ |
1.2.7 Guidance documents
- EU Commission Guidance on Implementation of HACCP.
- EU Commission Guidance on Implementation of assimilated Regulation EC 852/2004.
- EU Commission Guidance on Implementation of assimilated Regulation EC 853/2004.
- EU Guidance on Key questions relating to import requirements.
- EU Commission Staff Working Document on the understanding of certain provisions on flexibility provided in the Hygiene Package.
- Assimilated Regulation (EC) 2073/2005 Microbiological Criteria for Foodstuffs.
- Assimilated Regulation (EC) 178/2002 Guidance Notes for Food Business Operators on Food Safety, Traceability, Product Withdrawal and Recall.
- Food Law Code of Practice and Practice Guidance.
- Industry Guide on Edible Co-products and Animal By-products.
- Food Safety Management Diary for Meat Producers.
- The Wild Game Guide and Photo Annex.
- European Commission Notice on the implementation of food safety management systems covering prerequisite programs (PRPs) and procedures based on the HACCP principles, including the facilitation/flexibility of the implementation in certain food businesses (2016/C 278/01).
- Chronos User Guide.
1.2.8 Judicial Review
Where a statutory right of appeal does not exist, you may challenge a decision by a public body, such as the FSA, through Judicial Review. A claim for Judicial Review must be made to the High Court promptly and in any event within 3 months after the grounds for claim arise. You may wish to take legal advice on your rights to challenge the decision. You can find more information online in the ‘Administrative Court Judicial Review Guide’.
2. Legislation, Enforcement Roles and Provisions
In this section
2.1 Legislation and enforcement provisions
2.2 Division of enforcement responsibilities
2.4 Recording and monitoring enforcement action
2.1 Legislation and enforcement provisions
2.1.1 Code of Practice
The ‘Food Law Code of Practice and Practice Guidance’ have been issued under:
- Section 40 of the Food Safety Act 1990 (as amended)
- Regulation 26 of The Food Safety and Hygiene (England) Regulations 2013 / Regulation 24 of The Food Hygiene (Wales) Regulations 2006, and
- Regulation 6 of The Official Feed and Food Controls (England / Wales) Regulations 2009
To provide guidance for food authorities on enforcement issues under the legislation.
Note: Whilst the FSA is not a food authority, it is an enforcement authority and the principles set out in the Code have been mirrored in this chapter.
2.1.2 Requirement to enforce
Competent Authorities (CAs) must ensure that food law is complied with by monitoring and verifying that relevant legislative requirement are met through a system of official controls and other activities.
In case of suspicion of non-compliance, the CA shall perform an investigation to confirm or to eliminate that suspicion. Where the non-compliance is established and if appropriate, the CA shall take enforcement action when they find the law has not been complied with (Title VII, Articles 137 to 142 of assimilated Regulation (EU) 2017/625).
Where non-compliance is established, the competent authorities shall take:
- any action necessary to determine the origin and extent of the non-compliance and to establish the operator’s responsibilities; and
- appropriate measures to ensure that the operator concerned remedies the non-compliance and prevents further occurrences of such non-compliance. When deciding which measures to take, the competent authorities shall take account of the nature of that non-compliance and the operator’s past record with regard to compliance.
References: Assimilated Regulation (EU) 2017/625 Article 138 and assimilated Regulation (EC) 178/2002, Article 17, Paragraph 2.
Food law includes all statutes, regulations and administrative provisions governing food in general, and food safety in particular, whether at Community or national level. It covers all stages of production, processing and distribution of food, and also of feed produced for, or fed to, food-producing animals.
2.1.3 General principles
Assimilated regulation (EC) 178/2002 sets out the general principles and requirements of food law and lays down procedures in matters of food safety. It contains:
- definitions (of food, food business operator, and other terms)
- basic principles – FBO responsibility for food safety
- traceability requirements
2.1.4 Official Controls
Assimilated Regulation (EU) 2017/625 and the Regulation 2017/625 package insofar as it and they apply to food; set out the official controls that MUST BE performed to ensure the verification of compliance with feed and food law, animal health and animal welfare.
Key points covered are:
- organisation of official controls
- crisis management
- imports from third countries
- financing / charges
- national enforcement measures
2.1.5 Assimilated “Hygiene Regulations”
The “hygiene regulations” are defined and include:
- Assimilated Regulation (EC) 852/2004 dealing with the hygiene of foodstuffs. Key points:
- applies to all food businesses
- looks for good hygiene practice and HACCP based procedures
- concept of industry guides
- Assimilated Regulation (EC) 853/2004 laying down specific hygiene rules for food of animal origin. Key points:
- requirements beyond 852/2004 for food of animal origin
- approval of meat premises
- identification marking
- objectives of the HACCP based procedures
- food chain information
- Assimilated Regulation (EC) 2073/2005 on microbiological criteria for foodstuffs
- Assimilated Regulation (EU) 2019/624 concerning specific rules for the performance of official controls on the production of meat. It contains:
- nature of official controls – for example, inspection, verification, auditing
- role of OV and MHI and trained, qualified operatives, and
- control on imports
- Assimilated Regulation (EU) 2019/627 concerning uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption
- Assimilated Regulation (EU) 2015/1375 laying down specific rules on official controls for Trichinella in meat and
- The Food Safety and Hygiene (England) Regulations 2013 and The Food Hygiene (Wales) Regulations 2006.
2.1.6 Legislative Amendments
Legislation is amended periodically, and it is important that the original published versions are read in conjunction with any amendments.
Note: Users must ensure that they access the latest “consolidated” version of assimilated Law and its respective implementing enforcement legislation.
Volume 2, Chapter 14 of the MOC, provides links to all relevant assimilated law and UK statutory instruments on legislation.gov.uk.
2.1.7 Implementing enforcement regulations
The main domestic regulations include:
- The Food Safety and Hygiene (England) Regulations 2013 and The Food Hygiene (Wales) Regulations 2006.
Note: These provide enforcement powers in respect of the obligations that apply in assimilated Regulations (EC) 852/2004, 853/2004, 2073/2005 and 2015/1375, together with assimilated Regulation (EU) 2017/625, and the OCR package in so far as it and they relate to food. In practice the main OCR regulations that will be utilised will be assimilated Regulations (EU) 2017/625, (EU) 2019/624 and (EU) 2019/627.
- The Official Feed and Food Controls (England / Wales) Regulations 2009.
Note: These provide enforcement powers in respect of the obligations that apply in assimilated Regulation (EU) 2017/625.
- The General Food Regulations 2004 (Wales) and The Food Safety and Hygiene (England) Regulations 2013 (England):
- Provide enforcement powers in respect of the obligations that apply in assimilated Regulation (EC) 178/2002. For example:
- Article 14 ‘the food safety requirements’
- Article 19 ‘recall, withdrawal and notification requirements’
- Provide enforcement powers in respect of the obligations that apply in assimilated Regulation (EC) 178/2002. For example:
Articles 15 and 16 are the enforcement remit of the local “Food Authority”.
The Agency has a duty to verify FBO compliance with traceability requirements under Article 18 as read with assimilated Commission Implementing Regulation (EU) 931/2011). Failure by FBOs to provide traceability information is a contravention of assimilated law and may also constitute an obstruction of the AO.
Whilst responsibility to prosecute contraventions of Article 18 of assimilated Regulation (EC) 178/2002 is the remit of the local “Food Authority”, [see Regulation 5(6) Food Safety and Hygiene (England) Regulations 2013], failure to comply with the traceability requirements of ID marked products of animal origin is a contravention of assimilated Regulation (EC) 853/2004, Annex II, Section 1, Part A, 4 and the FSA has responsibility for its enforcement and powers to prosecute.
Likewise, any obstruction of FSA personnel seeking traceability information during the performance of their functions under assimilated Regulations (EC) 178/2002 and assimilated regulation (EU) 931/2011 or the hygiene package can be enforced by the FSA.
Note: Implementing enforcement regulations are also amended periodically, and reference should be made to the consolidated text on “legislation.gov.uk” or the original legislation as drafted in conjunction with the respective amendments. Unfortunately, legislation.gov.uk website is not always up to date so consider seeking guidance from legal when in doubt.
2.1.8 Regulators’ Code
In addition to the legal requirements imposed by the EU legislation, the FSA must have regard to the statutory Regulators’ Code (made under the Legislative and Regulatory Reform Act 2006) when setting standards, determining policies and procedures with respect to guidance provided and in the application of its regulatory functions. However, this will be subject to any legal requirements affecting the exercise of all regulatory obligations.
The principles of the Regulators’ Codes are set out in the FSA Enforcement Policy [MOC Volume 1, Chapter 7, Annex 2].
2.1.9 Premises file and contents
A premise file must be maintained by the OV at all slaughter establishments supervised by the FSA. This should include details of the plant approval, the correct legal entity responsible for potential offences, all correspondence in date order, copies of all letters, formal notices, minutes of meetings, accounts of telephone conversations, audit reports and informal notes taken. Documents relating to the escalation of enforcement in slaughterhouses must also be uploaded onto SharePoint.
Audit reports, letters, formal notices and other correspondence served on FBOs of non-slaughter establishments must also be retained and sent to CSU York to be scanned and retained in electronic premises folders on SharePoint.
2.1.10 Security
The premises file and all enforcement documents must always be kept secure. When not being referenced or updated, the premises file should be kept in a locked filing cabinet within the FSA office in slaughterhouse establishments and in the appropriate electronic premises file for all other establishments. It will contain evidence that may be required at a later date, together with additional unused material that the prosecution (the FSA) may have to disclose should a case go to trial [see the Criminal Procedure and Investigations Act 1996 in Section 2.5.8]. All enforcement documents must be kept for 6 years in line with the FSAs retention policy.
2.2 Division of enforcement responsibilities
Some enforcement responsibilities are prescribed in legislation, e.g. Regulation 5 of The Food Safety and Hygiene (England) Regulations 2013
2.2.1 FSA enforcement responsibilities
- Red meat slaughterhouses (cattle, pigs, sheep and goats, domestic solipeds, large farmed game, ratites).
- White meat slaughterhouse (poultry, lagomorphs, farmed game birds).
- Game handling establishments (wild game dressing and cutting).
- Cutting plant.
- Establishments approved as ‘slaughterhouses’ for activities limited to the dressing of carcases.
- Any of the following activities, where co-located with a slaughterhouse, cutting plant or game handling establishment:
- minced meat
- meat preparation
- mechanically separated meat (MSM)
- meat products
- processing plant (for meat products, rendered animal fats and greaves, treated stomachs, bladders and intestines, gelatine and collagen)
- cold storage
2.2.2 Official Controls
OVs are appointed by the Competent Authority to perform those official control activities set out in Article 18 of assimilated Regulation (EU) 2017/625 (the Official Control Regulation), e.g., ante and post-mortem inspection, general verification duties
Article 18,5 of assimilated Regulation (EU) 2017/625 also requires OVs to remain responsible for the “decisions” taken following those official controls provided for in Article 18, 2 and 18,4, even where the performance of an action is assigned by the OV to an official auxiliary. These “decisions” include, verifying compliance with Articles 40 and 41 (measures in case of non-compliance with requirements for food chain information), Article 43 (measures in case of non-compliance with requirements for live animals) and Article 45 (measures in case of non-compliance with fresh meat) of assimilated Regulation (EU) 2019/627 and where appropriate, declaring meat unfit for human consumption and verifying its disposal in accordance with all relevant animal by-product controls.
2.2.3 Other Official Activities
All OVs will be responsible for taking any action necessary to determine the origin and extent of a non-compliance and to establish an FBOs responsibilities, as set out in Article 138,1(a) of assimilated Regulation (EU) 2017/625. This may involve officially detaining food or animals to verify their compliance before a decision can be made on whether any other official activities may need to be undertaken.
Decisions concerning those tasks provided for in Article 138(1)(b), 138(2) and 138(3) will be made by directly employed AO of the Competent Authority, who are suitably qualified, competent, and trained in enforcement, such as Field Veterinary Leads, Veterinary Co-ordinators, Veterinary Auditors and Unannounced Inspectors.
Decisions concerning the escalation of enforcement by contracted OVs (cOVs) in slaughterhouses or cOVs undertaking unannounced inspections, will be made by an FSA Veterinary Enforcement Delivery Manager (VEDM). This includes the decisions made after the provision of written advice, through to formal enforcement action and referrals for investigation.
See Section 4 for further details and Annex 15 – Quick Start Guide - Procedure for enforcement escalation OV to Enforcement Delivery Managers.
2.2.4 Local authority (LA) enforcement responsibilities
- Hunters supplying small quantities of wild game or wild game meat directly to the final consumer / local retailers.
- Primary production of wild game carcases by hunters, including game larders they operate.
- Producers supplying small quantities of meat from poultry and lagomorphs slaughtered on farm directly to the final consumer / local retailers.
- Butchers’ shops (retailing meat to the final consumer or exempt under marginal, localised and restricted).
- Any of the following not co-located with slaughterhouses, cutting plants or game handling establishments:
- meat preparations establishment
- minced meat establishment
- mechanically separated meat establishment
- processing plant (for meat products, rendered animal fats and greaves, treated stomachs, bladders and intestines, gelatine and collagen)
- cold stores where storage is the only activity
- Premises manufacturing composite products containing meat and other edible co products.
- Collection centres and tanneries that handle raw material for the production of collagen and gelatine.
2.3 Communication with FBOs
2.3.1 Communication channels
Effective communication is essential when guiding an FBO on compliance with legal requirements as well as best practice.
The majority of day-to-day compliance can be achieved through verbal discussion.
AOs should work to establish agreed lines of communication with the FBO and their staff. It is also important that contingency arrangements exist to avoid difficulties when the FBOs normal contact person is unavailable.
Where a duly authorised representative exists, it is essential to have some written confirmation that the person may act on behalf of the business and/or as a central point of contact.
2.3.2 FBO contact details
The AO must have available at the establishment the contact details for the FBO. For example:
- full name(s) and address(es), including full limited company name and registered office address
- e-mail address and telephone number(s)
Where any ownership or approval details change at an establishment, the FBO is obliged to inform the competent authority of that change:
The FBO shall ensure that the competent authority always has up to date information on establishments, including the notification of any significant change in activities and any closure of an existing establishment.
Reference: Assimilated Regulation (EC) 852/2004, Article 6, Paragraph 2.
This information should subsequently be provided to:
- FSA York Finance
- Approvals and Registrations Team
- Inspection team at a slaughterhouse/auditor
This will ensure that the AO is always aware of the legal entity responsible for any potential offences within the establishment, whether they are a sole trader, partnership or limited company.
2.3.3 Key communication functions
The AO is responsible for:
- advising the FBO on compliance with legal requirements
- advising the FBO on corrective actions/measures when non-compliance with legal requirements have been detected
- ensuring FBOs undertake preventative measures and discuss where they are not effective
2.4 Recording and monitoring enforcement actions
2.4.1 Chronos / FSA enforcement programme
Enforcement action taken by AOs must be recorded accurately in Chronos.
The purpose of the system is to generate records of live and historic enforcement interventions and to help AOs in their:
- assessment and prioritisation of enforcement action
- communication of enforcement action in real time to other members of the inspection team and wider FSA audit and Unannounced Inspection colleagues
- tracking or monitoring of enforcement action through to compliance or a referral for investigation
The system:
- acts as an aide memoire, providing a comprehensive record of enforcement action taken in the establishment
- enables the FSA to assess the FBOs record as regards compliance with regulatory food, welfare, TSE and animal by-products legislation
- contributes to the risk assessment process and will help set the frequency of future audits
- provides an outline of the non-compliances to both (VAs) and internal audit staff
2.4.2 Ongoing enforcement action
When attending any establishment as an OV, auditor or UAI, the AO must:
- familiarise themselves with all ongoing enforcement action, and
- where repeated contraventions on the same subject are identified, maintain the momentum of enforcement and continue to escalate the issue through the appropriate hierarchy of enforcement.
2.4.3 Completing Chronos
For Guidance on the completion of Chronos, see Annex 12 of Chapter 7 for the Chronos User Guide. Chronos is a ‘live’ system, updated as necessary every time enforcement action is taken.
2.4.4 Monitoring progress
The AO should regularly monitor progress towards compliance to identify whether the deficiency is likely to be rectified within the agreed time scale. If necessary, they should ask to see evidence of how corrective action is progressing, for example, planning permission application / copies of quotes for work / structural plans.
Where the work does not progress at the agreed rate, the AO should escalate the matter and consider serving a HIN to formalise a suitable time scale, thereby maintaining the momentum in enforcement.
However, it is important that an agreed action plan is set out at the start and that the AO takes a reasonable approach where certain issues arise that are outside the FBOs control.
2.4.5 Structural work
Where structural work must be undertaken, the ‘corrective action’ section of a Written Advice Letter or Hygiene Improvement Notice (HIN) should be specific enough to explain the legal requirement and the outcome to be achieved, without being too prescriptive about the exact way in which this must be achieved.
There may be many ways that the FBO can achieve compliance, but provided they comply with the legal requirement, they have the option to carry out work to an equivalent effect.
2.5 Gathering and preserving evidence
2.5.1 Introduction
The AO must gather evidence at the time the offence is witnessed, making detailed contemporaneous notes, which at a later stage can be relied upon in Court. It is often impossible to gather evidence retrospectively as it may no longer exist.
Evidence may come in a variety of forms and must supplement a witness statement as an exhibit in order that it may be admissible in court. It is also useful to obtain corroboration and assistance from other colleagues where possible.
Detailed evidence gathering at the time of the offence will provide the AO with as much material as possible to support their witness statement and prove the elements of the offence or justify any other enforcement action in court, such as:
- appeals against the service of formal notices,
- appeals against the suspension or revocation of a Certificate of Competence or Temporary Certificate of Competence,
- appeals against the suspension or withdrawal of an establishment approval.
Note: Keep all evidence secure. It is fundamental to proving the offence should formal action be pursued.
2.5.2 Best evidence rule
The AO should also have regard to the ‘best evidence’ rule. Whenever possible, all original items of evidence should be preserved, for example, the original form of a document, rather than a photocopy. If the evidence is a part of a carcase, for example, SRM, a broken limb or the body of an animal, it should be preserved to maintain its integrity and stop any deterioration that would limit its evidential value.
If it is not practical or not possible to preserve the evidence, at the plant, for example, if perishable goods are involved and no facilities are available to freeze the product or keep it secure, the AO should contact FSA management to organise alternative facilities.
FBOs should be given the opportunity to view physical evidence or oversee its examination or analysis by an expert before any potential court case.
The AO should take photographs wherever necessary and/or sample evidence where perishable goods are destroyed on testing. If there is doubt about what evidence should be retained, the AO should obtain further advice from FSA Legal.
2.5.3 Note taking
When gathering evidence, remember to record the details of any other persons present, to identify all potential witnesses in the case. This will enable corroborative witness statements to be taken; or for the investigating officer to test the strength of the evidence overall.
The AO should make full use of their pocketbook to make factual contemporaneous notes. These may be referred to in court to help recollect facts and figures that are impossible to recall in detail after the event.
Note: In court, a witness is able to refer to contemporaneous notes recorded in their pocketbook that were made either at the time of the incident or shortly afterwards, whilst events are still fresh in their memory.
However, witnesses are not permitted to read from their witness statement when giving evidence, except in certain limited circumstances.
Note: Where an officer refers to their pocketbook when giving evidence in court, the defence is entitled to see that notebook.
2.5.4 Use of FSA official pocketbook
The pocketbook is essential for recording details of incidents at times when a plant daybook is not readily available in the slaughterhouse or generally to record events when in other further processing plants.
In slaughterhouses, the use of the pocketbook is not to replace the plant daybook for recording day-to-day activities but should supplement completion of the daybook.
Only official pocketbooks are to be used when conducting FSA activities.
Note: Auditors’ notes should not substitute the use of contemporaneous notebook for recording enforcement evidence admissible to court if the occasion arises. This was advised by the Agency’s Senior Enforcement Advisor. The AOs performing FBO’s FSMS audits shall use their contemporaneous notebook on occasions that enforcement may be escalated to referral for investigation and potential prosecution of the FBO.
2.5.5 Important points
Pocketbooks may be inspected in court; therefore, the following guidance must be followed to maintain validity:
- Record name on front cover, designation and date started.
- Make all entries in pen and not pencil.
- Include only original entries and do not copy notes from elsewhere.
- Record the date and time at commencement, and upon completion.
- Enter the notes at the time ‘the offence’ is witnessed or as soon as possible afterwards (contemporaneously), whilst the facts are fresh in the memory.
- To make alterations, AOs must strike a pen through the error, with a single line to make the correction and place their initials in the left-hand column to verify that they made the changes. Notes must not be erased or obliterated with tip-ex.
- Do not remove numbered pages from the notebook.
- Sign and date each entry at the base of each page.
Entries must be relevant, factual, legible, concise and written in English.
If accompanied by a colleague whilst witnessing a contravention, one AO may record the details in their pocketbook, whilst the other may read through the notes made and where they agree with what has been recorded, they may countersign at the end of the entry to acknowledge that it is a true and accurate account of events.
Where the AO and FBO have had a conversation regarding action to be taken to achieve compliance, it may be beneficial to ask the FBO to sign the notes taken by the AO as an accurate account of what was agreed.
2.5.6 Security
The AO is responsible for ensuring the security of their notebook and for producing it in court. Further notebooks are available from York – contact corporate support unit (CSU) by email or from SDP’s.
2.5.7 Return of all notebooks
Notebooks remain the property of the FSA and must be returned to CSU York when an AO leaves FSA employment or retained by the SDP.
2.5.8 Disclosure of unused material
The Criminal Procedure and Investigations Act 1996 (CPIA) places an obligation on the prosecuting authority to retain and record all relevant information relating to any enforcement action.
The prosecuting authority – a term which includes all AOs, the wider FSA team, the Investigating Officer (IO), the prosecuting lawyers and the enforcement agency itself – has a duty to investigate all reasonable lines of enquiry and disclose to the defence all relevant unused material which:
- might undermine the case for the prosecution, or
- might reasonably be expected to assist the defence case
This material may include:
- informal and formal memos
- email traffic
- previously unreported offences and/or warnings recorded on operational paperwork
- daybook entries
- contemporaneous notebook entries
- minutes or recordings of meetings
- draft witness statements
- photographs used as exhibits, together with unused photographs. If photographs are downloaded onto a CD-R/ DVD-R, retain both “Master” and “Working Copies” and where they are stored on an encrypted memory stick, retain the stick
- instructions to expert witnesses or analysts
- Completed EDP form Annex 17
VEDM checklist
ED policies, procedures and guidelines: structure, responsibilities, members, etc
Risk assessments,
Evidence of communication and meetings FBO-cOV, cOV-VEDM, TM-VEDM, etc
Disclosure may also be ordered in civil appeals against FSA enforcement action, such as appeals against the service of formal enforcement notices, the suspension / revocation of Certificates and Temporary Certificates of Competence, and suspension / withdrawal of an establishment’s approval. Judicial Review cases are also subject to a similar ‘duty of candour’ requirement. In all cases it is important to bring all relevant material available, even though this may undermine the FSA position or assist the other side, to the attention of the legal team.
2.5.9 Storage and availability
Anything that is relevant to the case, and which is not used by the prosecution is unused material and can be potentially disclosable. It is therefore important that when notes are taken, emails written or drafts prepared, AOs should be mindful that the defence may be entitled to examine them and refer to them in open court. Even where there are good reasons for arguing that some material is so sensitive that the defence should not see them, there is a high threshold which needs to be met to satisfy the court that this is the case.
The AO and FSA team should therefore ensure that:
- all material relevant to an enforcement intervention is recorded and retained
- all material is safely stored
The IO must be made aware of the existence of all relevant material as soon as possible after a referral for investigation is made.
2.5.10 Photographic evidence
Taking photographs in approved establishments for the purposes of evidence gathering is a fundamental part of the evidence gathering process.
The AO may inform the FBO of what is intended as a matter of courtesy, however, the FBO cannot stop an AO from taking photographs for the purposes of evidence gathering. Any attempt to obstruct the AO carrying out their duties is an obstruction and the person involved may be committing an offence. Further guidance around dealing with obstruction can be found in Annex 21, Chapter 7.
- When photographs are taken, details should be recorded in a contemporaneous notebook, including the photograph number, the subject, location, date and time. Colleagues should assist one another in this process where they are available.
- Photographs should be taken with an FSA/SDP provided mobile phone using the approved application and a record must be kept of how the digital information was downloaded and on to what medium it was stored.
- Where the subject in the photograph is not clear, it may assist the court to have a colleague appear in the photograph to point to the item that needs identifying.
- Video filming is often essential to demonstrate particular high-speed operations / operational practices or animal welfare incidents.
- If printed, it is useful to add details to the reverse of the photograph, clearly indicating the subject matter, location and other relevant details.
Although all AOs have powers to take photographs or videos for the purpose of evidence gathering, they must always seek the permission of the FBO if they are taking photographs for any other reason than evidence gathering.
Where FBOs prevent AOs from taking photographs, the FBO should be reminded that they may be committing an obstruction offence. If the FBO states the establishment has a no camera policy, the FBO should be advised that such a policy or notice has no legal effect on AOs who are authorised to carry out official controls and the policy / notice should be reported to FSA Management. FBOs cannot prevent AOs undertaking enforcement or from using evidence gathering equipment in a food establishment.
Note: Any verbal comment recorded whilst any filming is being undertaken must later be transcribed verbatim and will constitute part of the evidence if the case goes to trial.
Tip: In high humidity areas, give the camera lens time to adjust to the temperature / humidity before taking pictures or videos in order to prevent fogging.
Further guidance for FSA/SDP personnel can be found within the Operational Instructions on the Taking, Handling and Storing Photographic and Video Evidence document. [See Annex 19, Chapter 7]
2.5.11 Capture and download of images using the mobile phone camera or video
Prior to using your works mobile phone, download and use the “Timestamp Camera App” for taking photographs and videos to be used for evidential purposes. This app will time-date stamp all photographs and videos.
The pictures/videos taken using the Timestamp Camera App can be automatically uploaded to the Plant Folder in SharePoint to be accessible wherever the AO accesses their work profile (for example, Laptop or Thin Client).
Please see Annex 13, Timestamp Camera App Guide for further information.
Note: The location function must be turned off within the TimeStamp app.
Avoid using non-work issued camera phones to take evidential images. Personal devices will contain personal photographs and mistakes made when selecting and separating personal images from evidential images may risk evidential photographs being missed, thereby failing to comply with evidential disclosure rules. Likewise, private images may be selected and downloaded by mistake, which could result in personal images being inadvertently disclosed to the defence.
2.5.12 Capture and download of images using a digital camera
When the AO intends to capture images using a digital camera, they should ensure the following:
- Ensure the memory card is clear of previous images, unless you have come from another visit and not had the opportunity to download the images.
- Capture photographic images or video footage of everything that can evidence the contravention of the legislation.
- If poor quality images are captured, photographs must not be deleted as they will be classed as “unused material” under the CPIA 1996 [see 2.5.8]. If the case is referred for investigation, the Investigating Officer must be made aware of the existence of such images.
- Full details of all images captured by an AO must be recorded on the “Supporting Evidence Photographic Report” [see Annex 6, Chapter 7]. Each witness must submit their own Supporting Evidence Photographic Report with a referral for investigation to avoid any confusion of which witness is exhibiting which photograph.
- All images taken that relate to the case, together with the corresponding photographic evidence report, are uploaded into the plant folder within SharePoint.
- Send all evidence that is part of a referral for investigation to CSU York.
2.5.13 Supporting evidence photographic report
The ‘Supporting evidence photographic report’ has been introduced to provide a contemporaneous record of images taken whilst gathering evidence.
In ideal circumstances, the report should be completed at the time the evidence is gathered. However, when this is not feasible, it should be completed as soon as possible thereafter. Where a referral for investigation is being submitted, one report should be generated from each individual who gathers photographic evidence.
The report should be stored electronically in the same file as the images to which it relates.
A new report should be prepared to accompany images of each separate incident.
This is available at Annex 6 on ‘Supporting evidence photographic report’ of this chapter.
2.5.14 Samples: physical confirmation of the failure
Various different types of samples may be gathered as evidence, for example:
- rust / dirt scrapings
- samples of meat / offal / SRM
- trimmings of faecal or other contamination
- heads, mandibles, ears or limbs of animals
- whole carcases or joints
- bodies of dead animals
The AO should always inform the FBO of their intentions to sample a product. They should enlist the services of a colleague to witness the collection of the sample (if available) and record details of what the sample was, where the sampling took place and how it was sampled; recording the date and time the sample was procured in their pocket notebook.
Samples should always be bagged and labelled with all relevant details and sealed with a tamper evident seal.
All samples must be kept under secure conditions in an environment where they will not deteriorate. Details must be maintained of all locations where samples are stored, all transportation between such locations and the temperature at which the samples have been maintained to ensure continuity of evidence. A temperature log for chillers, freezers, transport etc. must be maintained and relevant calibration records for thermometers as they may be required as evidence in court.
2.5.15 Post-mortem evidence
There may be circumstances where an animal has died in transit or in the lairage, and a post-mortem examination would be required, for example to support a case for a breach of animal welfare legislation.
Before undertaking any post-mortem examination, the AO must have regard to the requirements in chapter 6 on ‘Notifiable diseases’.
Where the AO is to perform a post-mortem examination on site consideration must be given to the following:
- there should be suitable isolation facilities in the lairage to carry out the examination
- hygiene procedures must be followed and Cleansing and Disinfecting (C and D) carried out following examination and disposal
- the AO should have the appropriate protective clothing and equipment required for the procedure
- a detailed report of the findings must be prepared at the time
- photographic evidence should be gathered having regard to the guidance contained in this chapter
- appropriate specimens should be retained, for example, fracture site, limbs or bodies of animals / birds and stored as outlined below to maintain continuity of evidence
Note: Once examined, the specimen should be retained in a secure location in case the FBO requires their own appointed representative to view the evidence.
Where an on-site post-mortem examination is not considered appropriate, the carcase can be sent to the nearest APHA laboratory for examination. Continuity of evidence must be maintained as outlined below.
The HOD / FVC should be consulted before initiating an off-site post-mortem examination or advising the laboratory that the carcase is being sent.
The HOD / FVC will advise on any financial implications involved in the cost of the APHA post-mortem and report.
Note: Body parts that are required as evidence, but are, by definition, ABP must be retained until the conclusion of the court case. Afterwards, they must be disposed of appropriately.
2.5.16 Temperature readings: factual figures
The AO should ensure that where thermometers are used for evidential purposes, the thermometer used is calibrated annually, and where required for evidence in court is recalibrated prior to the case file being submitted. The calibration certificate must be checked to ensure it matches the thermometer being used and must be kept safe as it will be required as an exhibit in prosecution cases.
All relevant temperatures must be recorded where necessary. Check the legislation to identify any legal requirements or the elements of the offence that need to be proved, e.g. ambient temperatures for animal welfare cases and cutting rooms, surface temperatures and internal temperature for meat transportation etc. It is useful to ask a colleague to help record temperature details at the time the readings are taken.
Tip: The AO should ensure that when asked, they can explain what temperature related to which carcase / animal etc. together with its location.
2.5.17 Light meter readings: factual figures
When gathering evidence of poor lighting conditions, light meters contained on reliable mobile phone applications may be used, but where formal action is being undertaken, a calibrated light meter must be sourced with an up-to-date calibration certificate to ensure it is of known accuracy on the date of use. Ensure that the light meter corresponds with the calibration certificate and is within calibration before taking the reading.
Tip: Take a light meter reading when normal processing conditions exist and not when the sunshine is streaming in and no processing is being carried out.
2.5.18 Humidity readings: factual figures
When gathering evidence of poor humidity conditions/ventilation etc., ensure that a recently calibrated hygrometer is sourced and used to ensure it is of known accuracy on the date of use.
2.5.19 Internal Communication of Non-Compliance (ENF 11/22)
Where contraventions are discovered at cutting or further processing plants during audits or unannounced inspection and evidence indicates that the root cause originated at a slaughterhouse or other plant under FSA supervision, the AO who identifies such issues should record all relevant details on the ENF 11-22 form.
The form and any accompanying evidence should be communicated to the AO with responsibility at the establishment where the contravention is suspected to have originated. It is important that sufficient supporting evidence is gathered to demonstrate the contravention occurred at the dispatching plant and enable the respective AO to undertake a thorough investigation and take any appropriate enforcement action.
There may be scenarios where evidence suggests the contravention occurred during transport, in which case it is vital that the AO identifies who is responsible for the transportation. If it is owned by the cutting plant / slaughterhouse, it will be considered part of the approved establishment and any failures with its chilling facilities or hygiene etc. will remain the jurisdiction of the FSA and colleagues can gather evidence from data loggers and take appropriate action.
If the transport is owned by a third party, it will likely be considered part of a registered business and jurisdiction for enforcement of hygiene deficiencies or faulty chilling equipment will rest with the Local Authority. Likewise, the appropriate LA will have to be contacted to obtain relevant temperature records.
Further details on the completion of the ENF 11-22 can be found on the reverse of the document.
2.6 Information obtained from unauthorised sources – Regulation of Investigatory Powers Act (RIPA)
2.6.1 Introduction
This topic covers instruction on dealing with information which may be provided under RIPA.
2.6.2 Information received
When plant staff or a member of the public wishes to supply information about potential offences or wrongdoing, care must be taken to protect their anonymity. You should take the following action:
- Obtain their details, including a contact number along with a safe a convenient time for a member of staff to speak to them. These details must not be recorded in the plant Daybook / in the AOs contemporaneous pocketbook or somewhere which could be seen by others. These original notes should be kept in a safe place as they may be required at a later date.
- Contact the National Food Crime Unit (NFCU) via email foodcrime@food.gov.uk or submit a webform with the details. This can be found on the FSA website report a food crime.
- If you wish to speak to someone from the NFCU in person for advice contact the Confidential hot line.
- You should not task an individual to obtain more information for you, because this may put the persons health and safety at risk. Tasking individuals in this way also requires an authority under RIPA legislation and failure to comply with these requirements will mean that it is almost impossible to conduct a successful investigation into the allegations, as the evidence obtained will be considered inadmissible.
- If the individual does not want you to take their personal details, direct them to the FSA website and the confidential hotline advising them that they can leave information anonymously.
If you believe that the individual is unlikely to want to speak directly with another member of staff from the FSA, obtain as much information as possible by asking the following questions:
- Who is involved in the wrongdoing?
- What/How has it happened?
- Where did it happen?
- When did it happen?
- Who else knows about the wrongdoing?
AOs must not approach anyone to act as an informer or obtain information in an undercover way.
2.6.3 Example 1
A disgruntled employee contacts you to inform you that the operator of an approved slaughterhouse and cutting plant is using the establishment at night, without FSA supervision to slaughter and process cattle which have no passports or missing ear tags. They are in a position to know when this is happening next and to contact you at the time it is taking place.
2.6.4 Example 2
A delivery driver from an approved establishment has delivered several consignments of over temperature sheep carcases to a large city market. They are concerned that they may be prosecuted, together with the originating plant operator if a load is intercepted at the market. They are willing to provide information relating to dates times and consignment details of deliveries which they believe have not been chilled to the correct temperature before transportation.
3. Surrender, Detention, Seizure and Condemnation
In this section
3.2 Online temporary detention
3.3 Detention under the Food Hygiene / Food Safety and Hygiene Regulations
3.4 Detention under the Food Safety Act 1990
3.6 Seizure and Detention under Animal Welfare Legislation
3.7 Seizure under the Mandatory Use of CCTV in Slaughterhouses (England) Regulations 2018
3.1 Voluntary surrender
3.1.1 Meanings of voluntary surrender
Where meat has not been produced in accordance with the hygiene regulations or is unfit for human consumption, the AO should seek voluntary surrender of the meat.
Voluntary surrender is an everyday occurrence within a slaughterhouse and should always be evidenced by completing a ‘Rejected Meat Receipt’ (PMI 4/8). This will identify the carcase, part carcase, and offal and should be issued for all routine matters and signed by the AO and a responsible member of the plant management.
The FBO should be encouraged to sign an ‘Agreement to Destroy Meat’ (ENF 11/7) notice should be completed where any dispute arises, or where issues are more complex. For example, where:
- there are large quantities of meat
- an animal’s identification is being questioned
- the farmer retains ownership of the carcase after processing and the FBO feels that their consent is required
This Notice should be completed before the meat is disposed of as an ABP and is in addition to the ‘Rejected Meat Receipt’.
Reference: See chapter 9 on ‘Forms for PMI 4/8 and ENF 11/7.
3.1.2 Detention - Legal powers
Official detention is defined as “the procedure by which the competent authorities ensure that animals and goods subject to official controls are not moved or tampered with pending a decision on their destination; it includes storage by operators in accordance with the instructions and under the control of the competent authorities” – Article 3, assimilated Regulation (EU) 2017/625.
All AOs have powers to detain:
- “food” or “live animals” under the Food Safety and Hygiene (England) Regulations 2013 and the Food Hygiene (Wales) Regulations 2006 (as amended), for further examination of the product or to sample the product,
- “food” under the Food Safety Act 1990 (as amended), via the above Regulations, for further investigation
Formal detention provides the ability to quarantine suspected non-compliant product and verify its compliance with food law. There will be various occasions where an AO is conducting official controls and the status of the product is uncertain. Detention will permit further checks / investigation to be undertaken as necessary to determine the origin and extent of the non-compliance and to establish the operator’s responsibilities.
The CA is also under an obligation, in the case of suspicion of non-compliance, to perform an investigation to confirm or to eliminate that suspicion.
See Article 137,3(b) and 138,1(a) of assimilated Regulation (EU) 2017/625
3.2 Online temporary detention
3.2.1 Holding carcases identified for rectification
In many slaughterhouses, the majority of detained carcases are rectified on the detained rail, under the supervision of an MHI dedicated to that task.
Colour-coded plastic hook tags can be used to identify carcases for detention. The colour-coded tags are used to signify specific conditions and serve to alert the MHI to the action required. Make the FBO aware of the system. The colour-coded tags should be used to represent the following:
| Colour | Use for |
|---|---|
| Red | Pathology |
| Yellow | SRM |
| Green | Contamination |
| Blue | Sample identification tag |
| Grey | TB carcases |
3.2.2 Labelling detained carcases
Carcases and offal that have been detained for further examination and that require more secure individual identification can be tagged using individually numbered talisman seal(s).
To maintain correlation between the carcase and offal, several talisman seals must be used. The individual seal numbers should be recorded with any other relevant details for the carcase and cross-referenced on the formal detention notice.
The seals must remain in place until the carcase and offal have been re-inspected and a decision made on the fitness of the carcase and offal for human consumption.
3.2.3 Detention tape
Detention tape should be used to help identify any boxed meat or shrink-wrapped pallets of boxed meat and should be used in conjunction with the formal detention notice.
3.2.4 When to formally detain
There may be occasions where meat cannot be dealt with immediately on the detained rail because:
- the AO may wish to undertake a further examination of the carcase to identify any signs of oedema / emaciation, fever or other pathological conditions that may not be evident when the carcase is still warm
- the AO is investigating the traceability of an ID marked product in accordance with Annex II, Section I, A4 of assimilated Regulation (EC) 853/2004
- the AO may wish to sample the product for the presence of any undesirable or illegal substance or veterinary residue
In such circumstances, the AO will require the FBO to store the suspect meat in a detained chiller and should ensure its security.
3.2.5 Assessment of the detention facilities and history and confidence in management
Detention facilities vary in type, size and security, the OV must assess how satisfactory the facilities are and how the FBO intends to detain meat that has to be stored for further examination / investigation.
The assessment should identify:
- how secure the facilities are, including number of people who are in possession of a key
- the level of confidence in management and their staff
- whether previously detained meat has ever been placed on the market, gone missing or been moved contrary to the OVs instructions
- whether the size of the detained facility is sufficient to accommodate all the suspect meat
- whether the meat has already received a health mark or identification mark
The decision whether to formally detain meat with a Detention of Food Notice (ENF 11/1) or a Detention Notice (ENF 11/26), when legally available as an option will depend on all the above factors.
A Q&A on Formal Detention can be found in Annex 14 of Chapter 7, which provides further guidance on when to use detention powers and which formal notice can be used.
Reference: See chapter 9 on ‘Forms’ for ENF 11/1 and ENF 11/26.
It may not be necessary to formal detain product in routine non-contentious day to day situations, for example:
- where meat is stored over night for routine rework and has not been health marked, and
- is secured in lockable detained facilities on the premises,
- where the FBO has always been compliant and has a good relationship with the FSA, or
- where carcases have been tested for BSE / trichinella and are awaiting a negative test result prior to being health marked
However, where formal detention is legally available and:
- where there are contentious issues,
- a history of non-compliance at the plant,
- the FBO has no detention facilities, detention facilities that are too small / not secure enough,
detention may be required.
The AO must as a matter of good practice, formally detain the animal / food using the relevant formal detention notice (see below), to ensure that all non-compliant product is effectively secured, to demonstrate that the AO has formally detained the product and to allow formal action to be taken if the FBO breaches the requirements specified in a formal detention notice.
Note: Assimilated Regulation (EC) 853/2004, Annex III, Section I, Chapter IV, Paragraph 12 also requires the FBO to follow the instructions of the OV to facilitate post-mortem of all meat and offal. Where they fail to do so, this may constitute an offence of obstruction.
Where no formal detention notice is served, movement of the product by the FBO will not result in a breach of a detention notice but may constitute an obstruction of the AO.
3.3 Detention under the Food Safety and Hygiene (England) Regulations 2013 and Food Hygiene (Wales) Regulations 2006
3.3.1 Relevant legislation
Powers to serve a Detention Notice (ENF 11/26) derive from Regulation 10(1) of The Food Safety and Hygiene (England) Regulations 2013 or Regulation 9(5) of The Food Hygiene (Wales) Regulations 2006.
3.3.2 General principle
The Detention Notice ENF 11/26 provides powers to an AO to detain any animal or food (specified in the notice), in any establishment subject to assimilated Regulation (EC) 853/2004, either on the premises, or at another location (specified in the notice).
Detention under the provisions of Regulation 10(1) or 9(5) is only possible in circumstances where further examination of the animal or food is required, or sampling of the product is undertaken (for example, when an animal does not match the details on its passport or where the presence of a potential residue is suspected).
3.3.3 Declaring unfit
Formal detention is inappropriate where an AO has evidence that animals or meat are required to be declared unfit for human consumption under assimilated Commission Implementing assimilated Regulation (EU) 2019/627, Articles 40 to 45. Once meat has been declared unfit, it will become an ABP, is no longer food for human consumption and disposal must be in line with all relevant provisions of assimilated law and domestic ABP and TSE Regulations. See Annex 5 ‘Flow diagram’ and chapter 2.8 ‘Animal by-products’, section 5.
Note: where the FBO does not voluntarily surrender non-compliant product and the meat has to be declared unfit, this must be done in writing, setting out the rationale for the action [see Annex 16]. If the FBO refuses, a Disposal Notice under the domestic ABP Regulations should be served requiring the disposal of the product [see ENF 11/12 England and ENF 11/13 Wales].
3.3.4 AO duties
The AO should:
- discuss the reason for service of the detention notice with the FBO
- ensure detained animals or meat are accurately identified, e.g. for meat using an individually numbered talisman seal, the details of which must be recorded on the detention notice
- use FSA detention tape where product is stored alongside other products with which it could be confused
- once identified, ensure that the detained meat is secured so that it cannot be tampered with
- record details of the date and time of service of the notice on the back of the form, in a pocketbook, or in the plant daybook
- ensure that the FBO can easily identify what has been detained at the time of service
- advise the FBO of the likely timescale for the examination / sampling, so that they can take steps to prevent deterioration of the product; for example, boning under FSA supervision and freezing to preserve the value of the meat
3.3.5 Service of a Detention Notice
The detention notice:
- should be served by hand on the FBO or their duly authorised representative
- must also be served on the FBO at the registered address of the business (for limited companies)
- may be handwritten
- must be served as soon as practicable
Note: the AO must always retain a copy of the notice served!
3.3.6 Time period
No time period exists within which the examination / sampling must take place, however, this must be completed as soon as practicable.
3.3.7 Right of Appeal
A statutory right of appeal does not exist under Regulation 10 for the service of a Regulation 10(1) detention notice under The Food Safety and Hygiene (England) Regulations 2013 or Regulation 9 of The Food Hygiene (Wales) Regulations 2006. However, the notice could be challenged via Judicial Review in the High Court. [Further guidance on a Judicial Review can be found in section 1.2.8]
3.3.8 Withdrawal
Where the product is found to be compliant, the notice may be withdrawn by completing the withdrawal section at the base of the detention notice, once the AO is satisfied that the animal / meat complies with the requirements of the hygiene legislation and there are no grounds for declaring it unfit for human consumption. The meat may then be ID Marked / Health Marked or released for human consumption.
Decision Not to Withdraw
If the AO is not satisfied that the meat is fit for human consumption, they should seek voluntary surrender and disposal as an ABP.
Where voluntary surrender is not forthcoming, prior to the meat having been health / ID marked, the AO should first send a letter to the FBO explaining why they are declaring the animal / meat unfit (see Annex 16). Where the FBO continues in their refusal to surrender the product, the AO must serve an ABP notice requiring the disposal of the meat under the ABP enforcement regulations.
Where meat has already been Health / ID marked and due to a breach of the Hygiene Regulations, it has not been produced, processed or distributed in accordance with this legislation, the AO may “Certify” the product under Regulation 29 of the Food Safety and Hygiene (England) Regulations 2013 or Regulation 27 of the Food Hygiene (Wales) Regulations 2006. If the FBO refuses to surrender the product, it may be formally seized and taken before a Magistrate to seek a Condemnation Order.
Where the FBO refuses to surrender the food, it may be possible for to be formally seized under the provisions of Section 9 (3) (b) of the Food Safety Act 1990 (as amended) and taken before a Magistrate (Justice of the Peace) to apply for a Condemnation Order. More guidance can be found at 3.4 below and at Annex 14.
Note: Detention under the provisions of Regulation 10(1) or 9(5) of the domestic hygiene regulations is intended to be used for short term issues to allow for the further examination / sampling of the product by the FSA.
Note; any detention for further examination or sampling, must involve examination or sampling of the product being detained. The AO of the FSA should take responsibility for any sampling or examination and not leave this to the FBO or another competent authority.
3.3.9 AO checklist
Where the detained food is not released, specify the following information on the reverse of the Detention Notice:
- the nature of disposal and the category of ABP that the food was consigned under
- whether an agreement to destroy food Notice was signed by the FBO and the notice reference number
- whether the detention led to the food being certified, seized and taken before a court to seek condemnation
3.4 Detention of Food under the Food Safety Act 1990
3.4.1 Relevant legislation
Regulation 25 of The Food Safety and Hygiene (England) Regulations 2013 and Regulation 23 of The Food Hygiene (Wales) Regulations 2006 also allow the AO to detain suspect food for further investigation, but not to detain live animals.
Section 9 of the Food Safety Act 1990 will only apply to food that has been placed on the market and as such, the detention, seizure and condemnation provisions cannot be invoked until product has reached the point at which a Health or ID mark has been applied.
Section 9(3)(a) of the Food Safety Act 1990, provides powers for the AO to detain, inspect and seize any food that is thought may not comply with the “food safety requirements” in Article 14 of assimilated Regulation (EC) 178/2002 and is intended for human consumption. The “Detention of Food Notice” (ENF 11/1) can be used to formally detain product in such circumstances.
Note: Service of a formal “Food Detention Notice” (ENF 11/1) is inappropriate when the AO is required to declare material unfit for human consumption. This is because it has already been determined that the meat is unfit for human consumption and is therefore an animal by-product. Disposal as an ABP should therefore be in line with the requirements of the ABPs Regulations. See Annex 5 ‘Flow diagram’ in this chapter and chapter 2.8 on ‘Animal by-products’, section 5.
To formally detain a product that is not food with a food detention notice and require the same product to be disposed of as an ABP is contradictory.
A detention Notice (ENF 11/26) can also be used after a Heath Mark or ID Mark is applied provided that further examination or sampling of the product is to be carried out.
3.4.2 When to serve a Food Detention Notice (ENF11/1)
When FBOs are
- unwilling to surrender food that the AO has judged unfit, or
- un-cooperative with respect to the voluntary detention of meat after the ID Mark / Health Mark has been applied and is part of a further investigation into its fitness / compliance with the food safety requirements
the AO must formally detain and or seize (as appropriate) the food in accordance with Food Safety Act, Section 9.
Note: The AO shall as soon as is reasonably practicable, and in any event within 21 days, determine whether or not they are satisfied that the food complies with the food safety requirement.
Certification of Food
Legislation:
- Regulation 29 (3) of The Food Safety and Hygiene (England) Regulations 2013 and Regulation 27(3) of The Food Hygiene (Wales) Regulations 2006 allows for the Certification of food where it has not been ‘produced, processed, or distributed’ in accordance with the “Hygiene Regulations”, it shall be treated for the purposes of Section 9 of the Food Safety Act, as failing to comply with the food safety requirements.
- Article 14 of assimilated Regulation (EC) 178/2002 identifies the food safety requirements.
- Assimilated Regulation (EU) 2019/627, Articles 40, 41, 43 and 45 identify the circumstances where meat is required to be declared unfit for human consumption.
3.4.3 Reasons for service
Meat which fails to comply with food safety requirements under Article 14, assimilated Regulation (EC) 178/2002 includes:
- meat that is unsafe
- meat that is unfit for human consumption
- meat that is injurious to health.
3.4.4 Service of notice
Prior to serving a notice, the AO must have in their possession all the evidence to justify its service. The Detention of Food Notice should be served by hand on the person in possession of the meat who is deemed to be ‘the owner’. A copy of the notice can be forwarded to the monetary owner, if different.
The AO must ensure that all detained food is suitably stored to minimise any deterioration; and securely stored in a lockable room where a security talisman tag has been applied to the chiller door to prevent its distribution.
Note: The owner of the meat could be the owner of the animal from which the meat was produced, for example, the farmer.
3.4.5 Content of notice
The notice must specify:
- a unique reference number to prevent it being confused with any other detention notice, see reference format in section 4.3.4
- description (carcase / box type, lot mark, colour, markings)
- quantity
- identification marks if any (detained tags, numbers or labels)
- the location where the product is being detained and any alternative location to where it may be moved (if applicable)
- why, in the officer’s opinion, the food does not comply with the food safety requirements, linking the matter to Article 14 of assimilated Regulation (EC) 178/2002
3.4.6 Number of notices
Where a quantity of meat of different types or batches is being detained, the AO can issue a separate Detention of Food Notice for each type or batch.
In more complex cases, to avoid the need to issue multiple Notices, the AO may also create a separate schedule or appendix to a notice listing all detained products. The schedule may be referenced in the box identifying the product in the body of the actual detention notice itself. This will allow food that has subsequently been inspected and found to be compliant to be identified and positively released without the need to re issue notices each time the details of a detained product changes. The officer should sign and date next to the product that was examined / re-examined and is able to be released.
Where the meat that fails to comply with the hygiene requirements is part of a batch of the same class or description, it shall be presumed unless the contrary is shown, that the whole batch fails to comply and the AO should detain all of it. Part of the food may subsequently be seized if necessary and an Order for Condemnation of Food applied for. The Detention Notice must be withdrawn in respect of the remainder of product if the AO is satisfied that the problem affects only part of the batch.
Reference: 29 (3) of The Food Safety and Hygiene (England) Regulations 2013, Regulation 27 (3) The Food Hygiene (Wales) Regulations 2006 and the Food Safety Act 1990 Section 8 (3).
3.4.7 Right of appeal
No statutory right of appeal exists for a Detention of Food Notice under the Food Safety Act 1990. However, where voluntarily surrender is not forthcoming, the meat must be formally seized and taken before a Justice of the Peace to seek a Condemnation Order. The JP / Magistrate will be the arbiter of whether the product must be condemned. [See section 3.5 for the Condemnation procedure]
3.4.8 Time limit
The AO shall, as soon as is reasonably practicable, and in any event within 21 days, determine whether they are satisfied that the meat complies with the food safety requirement.
If they are satisfied that the food complies with food safety requirements, the AO must immediately withdraw the notice and if not, certify and seize the food to seek a Condemnation Order.
3.4.9 Withdrawal
If the notice is to be withdrawn, the AO must immediately serve a Withdrawal of Detention of Food Notice upon the recipient of the original Detention Notice - ENF 11/2.
If a Detention of Food Notice is withdrawn, or Condemnation Order is refused by the Court, compensation is payable to the owner of the food for any depreciation in its value which can be shown to result from the AOs actions
3.4.10 AO checklist
Where the detained food is not released, specify in the AO checklist on the reverse of the Detention Notice:
- the nature of disposal and the category of ABP that the food was consigned under
- whether an Agreement to Destroy Meat Notice (ENF 11/7) was signed by the FBO and the Notice reference number
- whether the detention led to the food being certified, seized and taken before a court to have it condemned.
3.5 Condemnation procedure
3.5.1 When to apply for a condemnation order from the court
Where meat has been Health Marked / ID marked and it has not been produced, processed or distributed in accordance with the hygiene regulations, or breaches the ‘food safety requirements’ the OV should:
- formally detain the food (ENF 11/26 or 11/1)
- certify the food as non-compliant (ENF 11/25)
- formally seize the food (ENF 11/ 27) within 21 days of the issue of any “Food Safety Act Detention of Food Notice” (ENF 11/1)
- apply to a Magistrate for a Condemnation Order
3.5.3 Obtaining a condemnation order
In England and Wales, a Condemnation Order may be obtained from a Justice of the Peace at the Magistrates’ court.
3.5.4 Action to take
The AO is to follow the steps in the table below to apply for a Condemnation Order, making sure that all formal documents are served on the FBO in line with Regulation 30 of The Food Safety and Hygiene (England) Regulations 2013 or Regulation 28 of The Food Hygiene (Wales) Regulations 2006 and copies are handed to local management.
Reference: Food Safety Act 1990 Section 9 (3) (b), Section 9(4) (b).
| Step | Action |
|---|---|
| 1 |
Ensure that any food that you suspect does not comply with the food safety requirements is formally detained using a Food Safety Act Detention of Food Notice (ENF 11/1) or Detention Notice (ENF 11/26). Reference: See chapter 9 on ‘Forms’ for ENF 11/1. |
| 2 |
If the AO has determined that the food has not been produced, processed or distributed in accordance with the provisions of the “Hygiene Regulations” they must complete and serve a Certification Notice (ENF 11/25) on the FBO, with the reasons why it fails to comply. They must also inform FSA Legal to make them aware that legal representation may be required. Note the definition of Hygiene Regulations does not include assimilated Regulation (EC) 178/2002, the assimilated ABP or TSE legislation. Reference: See chapter 9 on ‘Forms’ for ENF 11/25. |
| 3 |
If after certifying the meat, the FBO refuses to voluntarily surrender the food, complete a Seizure of Food Notice (ENF 11/27) and serve on the FBO and a copy on the owner of the food where relevant. Reference: See chapter 9 on ‘Forms’ for ENF 11/27. |
| 4 | Advise FSA legal on the intention to apply for a condemnation order at the court. They will arrange legal representation. A summary of events in the form of a witness statement and copy of all legal notices and relevant documents must be sent to FSA Legal. |
| 5 | FSA Legal will establish which court covers the area for the establishment where the detained food is held and speak to the Clerk of the Court to establish local procedures. Explain:
|
| 6 |
Complete and serve the Food Condemnation warning Notice (ENF 11/3). Ensure that the notice is served by the most appropriate method available in the circumstances to ensure that all relevant parties are informed of the time and place of the hearing in good time. Document and retain records of service to show the court. Retain copies of the Condemnation Warning Notice, Certification of Meat Notice and Seizure of Food Notice to produce to the Justice of the Peace, the Clerk to the Court and the FSA legal representative. Reference: See chapter 9 ‘Forms’ for ENF 11/3. |
| 7 | Attend court hearing |
| 8 |
Prepare three copies of the Complaint for Condemnation of Food Order (ENF 11/15) and of the Order for Condemnation of Food (ENF 11/16) itself for the Justice of the Peace to sign. Read the papers again before going to court. Attend court early to meet the FSA advocate. At the hearing, the AO should take:
Reference: See chapter 9 ‘Forms’ for ENF 11/15 and ENF 11/16. Explain clearly when presenting the evidence in court:
|
| 9 | If successful and the Justice of the Peace issues an ‘Order for Condemnation of Food’, upon receipt of the Order, ensure that the person in charge of the meat (and the owner if notified) receives a copy. Ensure that the disposal of the meat is supervised, and details of disposal have been recoded and a copy of the waste transfer note has been kept on file. |
| 10 | If unsuccessful, where any issue of compensation arises, the AO must not discuss or negotiate any compensation for depreciation in value of the meat or food. The AO should ask the FBO / Owner of the food to put any complaint in writing to the HOD. |
3.6 Seizure and Detention under Animal Welfare Legislation
Inspectors authorised to execute and enforce assimilated (Regulation (EC) 1099/2009) and the devolved Welfare of Animals at the Time of Killing Regulations have powers under Regulation 37 to:
- seize and detain any carcase or part of a carcase for further examining, investigating or testing,
- seize and detain any equipment or instrument for further examining, investigating or testing,
- seize any computers and associated equipment for the purpose of copying data, but only if the inspector has a reasonable suspicion that an offence under these Regulations has been committed and provided that they are returned as soon as practicable.
An inspector must, as soon as reasonably practicable:
- provide the person responsible for items seized with a written receipt identifying those items; and
- after deciding the items are no longer required, return them, apart from those to be used as evidence in court proceedings.
Where items have been seized for use in evidence in court proceedings and it is subsequently decided:
- no court proceedings are to be brought; or
- those items are no longer needed as evidence in court proceedings; or
- the court proceedings are completed and no order in relation to those items has been made by the court,
the inspector must return the items as soon as is reasonably practicable.
3.7 Seizure under the Mandatory Use of CCTV in Slaughterhouses (England) Regulations 2018
Updated [Inspectors who have entered premises for the purposes of executing and enforcing the Welfare of Animals at the Time of Killing Regulations or assimilated Regulation (EC) 1099/2009, may for those purposes, or the purposes of executing and enforcing the devolved CCTV in Slaughterhouses Regulations:]
- seize or take a copy of any images or information obtained by such a CCTV system,
- seize any CCTV equipment, including computers and associated equipment, installed as part of such a CCTV system which does not comply with regulation 3(2)(a), for the purposes of copying images or information.
The Inspector must as soon as reasonably practicable, provide the person appearing to be responsible for any items seizes with a written receipt identifying those items, using the WEL 11-41 form;
and
As soon as reasonably practicable after deciding that those items are no longer required, return them to that person, apart from those to be used as evidence in court proceedings.
Where items have been seized for use in evidence in court proceedings and it is subsequently decided:
- that no court proceedings are to be brought; or
- that those items are no longer needed as evidence in court proceedings; or
- the court proceedings are completed and no order in relation to those items has been made by the court,
an inspector must, as soon as is reasonably practicable return the items to the person appearing to be responsible for them.
4. Hierarchy of Enforcement
In this section
4.4 Statutory Notices for Hygiene Contraventions
4.5 Remedial Action Notices (RAN)
4.6 Hygiene Improvement Notices (HIN)
4.7 Hygiene Emergency Prohibition Notices (HEPN)
4.8 Hygiene Emergency Prohibition Orders (HEPO)
4.9 Referral for investigation
4.10 Protocol for referral for investigation
4.11 Referral for investigation: FSA Legal
4.12 Change of FBO during enforcement action
4.13 Warrant to enter premises
4.14 Process for obtaining warrant to enter premises in England and Wales
4.1 Introduction
4.1.1 The hierarchy of enforcement
The hierarchy that an AO follows will be dependent on the legislation contravened, the enforcement powers available and the risk associated with the non-compliance.
Matters Requiring Immediate Rectification
Where contraventions need to be remedied immediately based on public health / animal health or animal welfare risk, AOs must first verbally request the FBO rectifies the issue, but where they fail to respond, the advice may be followed immediately with the service of a formal notice requiring immediate rectification, such as a Remedial Action Notice for breaches of the Hygiene Regulations, a Welfare Enforcement Notice for breaches of animal welfare legislation or Notice requiring the Disposal of an ABP (see later).
The officer may wish to formally detain or quarantine product suspected to be unfit and conduct further examination / investigation or sampling at the same time as the service of a RAN.
In less urgent cases, the AO must follow the hierarchy available to them under the respective legislation that they are enforcing.
| Legislative Area Contravention | Verbal | Written | Formal Notice Available | Referral for Investigation |
|---|---|---|---|---|
Assimilated Hygiene Regulations- (EC) 852/2004, (EC) 853/2004, (EU) 2017/625 and the OCR package in relation to food (EC) 2073/2005 (EU) 2015/1375 The Food Safety and Hygiene (England) Regulations 2013 and The Food Hygiene (Wales) Regulations 2006 |
Yes | Not required |
RAN’s are available to require immediate rectification for breaches of the “Hygiene Regulations”. See Regulation 9(1) of The Food Safety and Hygiene (England) Regulations 2013 and devolved equivalents. |
Yes |
Assimilated Hygiene Regulations- (EC) 852/2004, (EC) 853/2004, (EU) 2017/625 and the OCR package in relation to food (EC) 2073/2005 (EU) 2015/1375 The Food Safety and Hygiene (England) Regulations 2013 and The Food Hygiene (Wales) Regulations 2006 |
Yes | Yes |
See Regulation 6(1) of The Food Safety and Hygiene (England) Regulations 2013 and devolved equivalents. |
Yes |
| Assimilated Food Safety Regulation (EC) 178/2002 and (EU) 931/2011 The Food Safety and Hygiene (England) Regulations 2013 and the General Food Regulations 2004 |
Yes | Yes | No formal notice is available because assimilated Regulation (EC) 178/2002 is the EU Food Safety Regulation and is not part of the “Hygiene Regulations” | Yes |
| The assimilated TSE Regulations (EC) 999/2001, The TSE (England) Regulations 2018 and The TSE (Wales) Regulations 2018 |
Yes | Yes | No formal notice is available under the domestic TSE Regulations | Yes |
| The assimilated Animal By-product Regulations (EC) 1069/2009, Assimilated regulation (EU) 142/2011, The Animal By-products (Enforcement) (England) Regulations 2013 and The Animal By-products (Enforcement) (Wales) Regulations 2014 |
- | - | Formal notices are available under The Animal By-Products (Enforcement) (England) Regulations 2013, however, often such notices may not be relevant to the issue observed and a referral may follow verbal and written advice. | - |
| The assimilated Animal By-product Regulations (EC) 1069/2009, Assimilated regulation (EU) 142/2011, The Animal By-products (Enforcement) (England) Regulations 2013 and The Animal By-products (Enforcement) (Wales) Regulations 2014 |
Yes | Yes | ABP Notice for the Disposal and where applicable storage pending disposal of ABP Regulation (25(2)(a) of The Animal By-products (Enforcement) (England) Regulations 2013 and the Wales equivalent. | Yes |
| The assimilated Animal By-product Regulations (EC) 1069/2009, Assimilated regulation (EU) 142/2011, The Animal By-products (Enforcement) (England) Regulations 2013 and The Animal By-products (Enforcement) (Wales) Regulations 2014 |
Yes | May or may not be required depending on the imminent risk | ABP Notice requiring the cleansing and disinfection of premises and where applicable the method of such cleansing and disinfection. Regulation (25(2)(b) of The Animal By-products (Enforcement) (England) Regulations 2013 and the Wales equivalent. | Yes |
| The assimilated Animal By-product Regulations (EC) 1069/2009, Assimilated regulation (EU) 142/2011, The Animal By-products (Enforcement) (England) Regulations 2013 and The Animal By-products (Enforcement) (Wales) Regulations 2014 |
Yes | May or may not be required depending on the imminent risk | The prohibition of ABPs being moved or brought into food premises. The Animal By-products (Enforcement) (England) Regulations 2013 and the Wales equivalent. |
Yes |
The assimilated Animal Welfare Regulations assimilated Regulation (EC) 1099/2009, The WATOK (England) Regulations 2015 and The WATOK (Wales) Regulations 2014 |
- | - | Welfare Enforcement Notice are available under Regulation 38 of the WATOK (England) Regulations 2015 and their Wales equivalent. | - |
| The assimilated Animal Welfare Regulations, assimilated Regulation (EC) 1099/2009, The WATOK (England) Regulations 2015 and The WATOK (Wales) Regulations 2014 |
Yes | Not required | WEN’s are available to require immediate rectification for breaches of the welfare at slaughter legislation where any avoidable pain, distress or suffering is evident | Yes |
| The assimilated Animal Welfare Regulations, assimilated Regulation (EC) 1099/2009, The WATOK (England) Regulations 2015 and The WATOK (Wales) Regulations 2014 |
Yes | Yes | WEN’s are available to require rectification for more systemic breaches of the welfare at slaughter legislation for example SOP's | Yes |
4.1.2.1 Enforcement Decision Making Function
Where AOs identify contraventions with the legislation, they provide verbal advice to the FBO. Where the FBO fails to implement corrective measures; enforcement is escalated. All AOs trained in enforcement will provide verbal advice, however, the decision to escalate enforcement action beyond verbal advice and the actions / measures required by the FBO will be determined by directly employed staff of the Competent Authority.
FSA Unannounced Inspectors / Veterinary Auditors / Field Veterinary Coordinators or Field Veterinary Leads will make decisions to escalate enforcement themselves and will act as decision makers in their own right.
They will also provide the rationale to escalate enforcement matters that fall under the “established non-compliance” provisions in Article 138,1(b) and 138,2 of assimilated Regulation (EU) 2017/625 and will communicate any relevant right of appeal, where one exists in accordance with Article 138,3.
4.1.2.2 Enforcement Decisions in slaughterhouses and further processing plants
Contracted OV Responsibilities
Contracted OVs (cOVs) in slaughterhouses and any cOVs conducting unannounced inspections (cUAI) are also responsible for verifying FBO compliance through a variety of official controls. Where they identify contraventions of the legislation, they will provide verbal advice and request that the FBO rectifies the issue.
COVs are responsible for the decisions regarding official control delivery and remain responsible for the decisions that flow directly from such official controls (Article 18(5) of assimilated Regulation (EU) 2017/625).
Where the FBO has been provided with verbal advice by a cOV and has failed to take appropriate corrective action, the decision to escalate enforcement action following on from verbal advice, will be referred to an FSA Veterinary Enforcement Decision Maker (VEDM).
To allow the VEDM to make a decision on the appropriate course of action, the cOV will:
- complete an EDP Form [Annex 17],
- set out the history of enforcement with regards to the specific non-compliance,
- attach all relevant supporting evidence, and
- draft appropriate enforcement documents,
4.1.2.3 VEDM Responsibilities
To arrive at an appropriate decision, the VEDM will review all relevant evidence, consider whether they agree with the proposed next steps, the actions / measures the FBO should take to bring them back into compliance and set out the rationale for that course of action.
The VEDM will also take account of the nature of the non-compliance and the operator’s past record to determine the actions / measures they deem appropriate to ensure compliance. The action will include but will not be limited to those matters contained in Article 138(1)(b) and 138(2) of assimilated Regulation (EU) 2017/625.
VEDMs will maintain regular contact with contracted OVs and Technical Managers in their area to keep up to date with any new or emerging enforcement issues.
After careful examination of the evidence presented by the cOV, the VEDM will review all letters or formal notices etc. and where they agree, make any annotations necessary and send all completed and signed documents back the cOV, together with any signed enforcement documents.
The cOV must:
- serve the signed letters / formal notices etc. in accordance with the guidance on service within this Chapter,
- retain a copy of the letter / formal notice etc. for the plant file for reference and as evidence,
- record all enforcement interventions in the plant Day Book and in Chronos.
Referrals for investigation should be discussed with the VEDM prior to submitting the ENF 11-6. If the VEDM agrees with the course of action, they will communicate their final decision to the cOV and submit the referral and all relevant documents to CSU to be logged and referred onto FSA Legal Services.
Note: welfare referrals must be submitted to the Welfare Triage Panel for consideration. The VEDM will be part of the panel considering the appropriateness of the referral for formal investigation.
The table below identifies the enforcement documents requiring a decision by the VEDM.
| Form Reference | Title | Decision to be approved by the VEDM | Comments |
|---|---|---|---|
| ENF 11/1 | Detention of Food Notice – Food Safety Act | No | Formal Detention is an action flowing directly from an OC. The decisions for which remain with the OV. |
| ENF 11/2 | Withdrawal of Detention of Food Notice | No | See above |
| ENF 11/3 | Food Condemnation Warning Notice | Yes | - |
| ENF 11/6 | Referral for Investigation | Yes | - |
| ENF 11/7 | Agreement to destroy meat | No | - |
| ENF 11/11 | Notice of intention to apply for a Hygiene Emergency Prohibition Order | Yes | - |
| ENF 11/12 | Notice for the disposal of Animal By-Products | Yes | - |
| ENF 11/13 | ABPR Cleaning & Disinfection Notice | Yes | - |
| ENF 11/14 | ABPR Notice Prohibiting By-Products being brought on to the Premise | Yes | - |
| ENF 11/15 | Complaint for Condemnation of Food Order | Yes | - |
| ENF 11/16 | Order for Condemnation of Food | Yes | - |
| ENF 11/17 | Complaint for Hygiene Emergency Prohibition Order | Yes | - |
| ENF 11/18 | Hygiene Emergency Prohibition Order | N/A | Issued by the Court |
| ENF 11/19 | Notice of Intention to Apply for a Warrant of Entry | Yes | - |
| ENF 11/20 | Application for Warrant to Enter Premises | Yes | - |
| ENF 11/21 | Warrant to Enter Premises | N/A | Issued by the Court |
| ENF 11/23 | Hygiene Improvement Notice | Yes | - |
| ENF 11/24 | Remedial Action Notice | Yes | - |
| ENF 11/25 | Certification of Meat Failing to Comply with the Requirements of the Hygiene Regulations | Yes | - |
| ENF 11/26 | Detention Notice – Food Hygiene Regs | No | - |
| ENF 11/27 | Seizure of Food Notice – Food Hygiene Regs | Yes | - |
| ENF 11/28 | Hygiene Emergency Prohibition Notice | Yes | - |
| ENF 11/29 | Risk Assessment Form | Yes | - |
| WEL 11/34 | WATOK Enforcement Notice | Yes | - |
| WEL 11/35 | WATOK Completion Notice | Yes | - |
| WEL 11/36 | WATOK Refusal to Issue a Completion Notice | Yes | - |
| WEL 11/37 | WATOK Seizure and Detention Receipt | Yes | - |
| WEL 11/38 | CCTV Enforcement Notice | No | CCTV legislation is not made under assimilated Law and the requirements for CCTV do not fall under Article 1(2) of 2017/625 |
| WEL 11/39 | CCTV Completion Notice | No | See above |
| WEL 11/40 | CCTV Refusal to Issue a Completion Notice | No | See above |
| WEL 11/41 | CCTV Seizure Receipt | Yes | Seizure of equipment is a matter to be determined by the CA |
| Chapter 2.3 Annex 2b | CoC suspension letter | Yes | - |
| Chapter 2.3 Annex 2c | CoC revocation letter | Yes | - |
| Chapter 2.3 Annex 2d | Return of CoC after suspension letter | Yes | - |
| Chapter 2.3 Annex 2e | Failure to re-train revocation letter | Yes | - |
| Chapter 2.3 Annex 2f | Return of CoC after review letter | Yes | - |
| Chapter 2.3 Annex 2g | Return of CoC after FTT decision letter | Yes | - |
4.1.2.4 Approach to the hierarchy
The approach to the hierarchy of enforcement and level at which the AO commences enforcement action will be dependent upon:
- the urgency / severity of the situation
- the most appropriate course of action that will control the risk
- the enforcement tools available under that piece of legislation
- the history of the FBO and their willingness to comply
- the FSA Operations Enforcement Policy
4.1.3 Enforcement; informal and formal action
The term ‘Enforcement’ is not defined in legislation, and neither is there any legal definition of informal and formal enforcement action. The OCR however, uses the expression “Other Official Activities” to define certain actions taken by the CA other than Official Controls, which includes actions where established non-compliance has been identified.
4.1.4 Subject of enforcement action
Any FBO or person who is the subject of enforcement action should be kept fully informed of any intended or actual enforcement intervention by the AO.
4.1.5 The health mark and enforcement hierarchy
The application of the health mark is not part of the hierarchy of enforcement. However, the AO is not permitted to apply the health mark in red meat plants where:
- the animal and meat have not undergone ante-mortem and post-mortem inspection respectively in accordance with assimilated regulation (EU) 2017/625 Article 18, paragraph 4 and paragraph 2 (a) and 2 (c)
- there are grounds for declaring the meat unfit for human consumption, or in the OVs opinion, after examination of all relevant information, the meat constitutes a risk to public or animal health or is not suitable for human consumption in accordance with assimilated Regulation (EU) 2019/627, Articles 40, 41, 43, 45.
- where the meat fails to comply with the provisions of Article 14.5 of assimilated Regulation (EC) 178/2002 in that the food is unacceptable for human consumption according to its intended use, for reasons of contamination (whether by extraneous matter or otherwise), or through putrefaction, deterioration or decay
Similarly, the Identification Mark (assimilated Regulation (EC) 853/2004, Article 5,2) should only be applied by the FBO to products in poultry slaughterhouses and all cutting plants if the product has been manufactured in accordance with the requirements of assimilated Regulation (EC) 853/2004, in establishments meeting the requirements of Article 4 of assimilated Regulation (EC) 853/2004.
This breach will:
- constitute an offence under Regulation 19 of The Food Safety and Hygiene (England) Regulations 2013 and Regulation 17 of The Food Hygiene (Wales) Regulations 2006
- potentially warrant the service of a Remedial Action Notice under Regulation 9(1) of the same Regulations, immediately prohibiting the use of the mark
4.1.6 When to give verbal advice
The first stage of enforcement action considered by the AO should always be education and advice. Whilst it is the FBOs responsibility to know which legal provisions are applicable to their business, the AO should ensure that, where necessary, they clarify and update the FBO on any relevant legal requirements. This is to ensure that the FBO understands the outcome to be achieved.
Verbal advice should go hand in hand with all stages in the enforcement process to help the FBO achieve compliance and understand why enforcement action is being taken. For example, AOs must always try to explain to the FBO why immediate action may be required, why a statutory notice is being served, or why the matter is being referred for investigation, if appropriate.
Where verbal advice is of a technical nature, it may be helpful for this to be followed up with a letter in writing confirming the discussion / meeting.
It is important that the AO does not continue to give verbal advice where this is being ignored, without escalating enforcement action in the appropriate way.
Note: Where immediate action is required on public health or animal welfare grounds, verbal advice should be given, but if ignored it may be appropriate to move straight to - enforcement action to secure compliance as soon as possible (for example, Public Health - RAN, Animal Welfare – WATOK Enforcement Notice).
4.1.7 Records
Unless the AO witnesses a one-off low risk issue; if it appears likely that enforcement may be escalated, or the FBO has a history of non-compliance, verbal advice should be recorded on Chronos, the FSA enforcement system.
4.2 Written Advice
4.2.1 Written Advice
Letters of advice when produced later in court will help to demonstrate fairness and proportionality in the enforcement approach and that the FBO may have ignored previous advice.
Advisory letters should be sent, where:
- the FBO or a staff member has failed to take appropriate corrective action following verbal advice and/or
- where there is a contravention of the Regulations which does not have an immediate impact on public health or animal welfare
The AO should inform the FBO of the intention to write an advisory letter. Ideally, the AO should meet with the FBO or their representative before drafting such an advisory letter to discuss all the issues including the timescale for completion. It is good practice to ask the FBO to confirm in writing their agreement to any timescale.
Accurate minutes of any meeting with the FBO should be taken in respect of achieving compliance.
Letters of advice must be typed and sent on FSA official letterhead paper. In the case of advisory letters sent to limited companies, these must be addressed to the FBO c/o The Company Secretary and sent to the Company’s Registered Office address. A copy must also be handed to plant management. A copy of the letter may be sent as an email attachment provided that it is also sent by post.
4.2.2 Composing Letters of Advice
The guidance below lists the points that an AO should follow when drafting letters.
Letters of advice must be typed and sent on FSA letterhead paper. They should also include the date of the letter, the establishment reference number (or CPH number for diary holdings) and the FSA reference. [Details of the correct format for reference numbers can be found in MOC Chapter 7, Section 4.3.4]
The letter should be addressed to either an individual or to the Food Business Operator. Where being addressed to an individual, avoid using titles such as Mr, Mrs, Ms etc and use the individuals full name or the initial from their first name followed by their surname. For example: Dear J. Smith / John Smith. In the event that the AO does not know the name of a particular individual to whom the letter should be addressed to, Dear Food Business Operator should be used.
It is advisable to separate the letter out into sections for ease of reading and clarity around the different parts of the letter. The suggested sections are:
Title: If the letter is in direct response to an inspection / audit / UAI etc. a reference to that can be used to provide context to the letter. If the letter is in response to an identified non-compliance, then this could be cited along with the date of the alleged contravention.
Legislation: The relevant assimilated law and Domestic Implementing regulations should be cited. Where more than one needs to be cited, they should be grouped together by theme. For example, where the letter is going to refer to hygiene and ABP issues, the EU and domestic hygiene legislation should be cited first followed by the EU and domestic ABP regulations.
Following the provision of the relevant legislation, a statement should be provided explaining that “All references to EU legislation are references to assimilated EU law”.
Introduction: A brief explanation of why the letter is being written, together with dates and times of any visit / audit / inspection / incident and details of the individual(s) who conducted the visit if they are not the signatory of the letter.
Contraventions: The contraventions that have been identified should be listed, including the date and time they were identified and the full legislative provision that the FBO is failing to comply with in both assimilated law and/or domestic implementing regulations. Where verbal advice has been provided previously for the same contravention, dates of such advice should be included to demonstrate that the hierarchy of enforcement has been followed. The AO should identify and describe the risk posed by the contravention.
Where the letter is being used to advise of multiple contraventions, they should repeat the previous step and group the contraventions by theme. For example, all hygiene contraventions should be listed first, followed by ABP contraventions etc.
Corrective Actions / Measures Required: The corrective actions that would achieve compliance should be listed. It should be explained that works to an equivalent affect may be undertaken providing compliance with the regulations is achieved.
Root Cause Analysis: If the AO identifies that existing corrective measures are incapable of effectively dealing with the problem, it is not necessary for the AO to identify the root cause of the problem in the letter. It is acceptable, however, to add a standard statement requiring the FBO to review and update their corrective measures, to ensure they are effective at curing the root cause of the problem and dealing with any non-compliant product.
Time Limits for Compliance: A date should be supplied by which time compliance should be achieved. Where possible, this date should be pre-agreed with the FBO to ensure that it is an appropriate time frame. Where it has not been pre-agreed, the FBO should be requested to confirm agreement in writing.
General Obligations on the FBO / BO / Occupier: The FBO should be reminded of their general duty under assimilated law and the specific provision cited.
Offences: The letter should state to the FBO that a contravention of the legislation stated within the letter is an offence and specify where. For example: for hygiene offences Regulation 19 of the Food Safety and Hygiene (England) Regulations 2013 creates an offence not to comply with the specified EU provisions.
Best Practice / Good Manufacturing Practice / Good Hygienic Practice: General advice can also be provided on good manufacturing / industry practice.
Add a link to relevant guidance documents that may exist on FSA / Defra / HMRC / WSTA websites. This may include guidance from respected institutions and EU Guidance documents / Commission Notices etc.
Appeals: There is not a statutory right of appeal against written enforcement advice, however, a Judicial Review may still provide a challenge to the way in which a decision has been taken.
The letter should be signed off by thanking the FBO for working in conjunction with the FSA. The AO should ensure that their name and contact details are included at the end of the letter in case the FBO wishes to discuss any of the details of the letter.
When drafting written advice, the AO must not warn of prosecution action in the event of future contraventions, as this could prejudice any future formal investigation.
A template letter can be found in Annex 20 and further guidance around Accessibility can be found on the FSA Intranet.
4.3 Statutory Notices
4.3.1 Preparation for formal action
Before taking enforcement action, the AO should:
- advise the FBO verbally of this intention
- be aware of all ongoing enforcement action by reviewing the enforcement history on Chronos
- have regard to the FSA Operational Enforcement Policy
- ensure that evidence has been secured to demonstrate that the contravention still exists that will warrant the escalation of enforcement action
- gather evidence to justify the service of the formal notice and to support any potential appeal against the service of the notice.
4.3.2 Statutory notices
Statutory Notices are legal documents and care must be taken to ensure they are completed correctly and used appropriately. They should only be signed by AOs.
4.3.3 Process to follow prior to serving a formal enforcement notice
The flow chart below explains the process to follow when an AO is considering the serve of a formal Statutory Notice. The AO should:
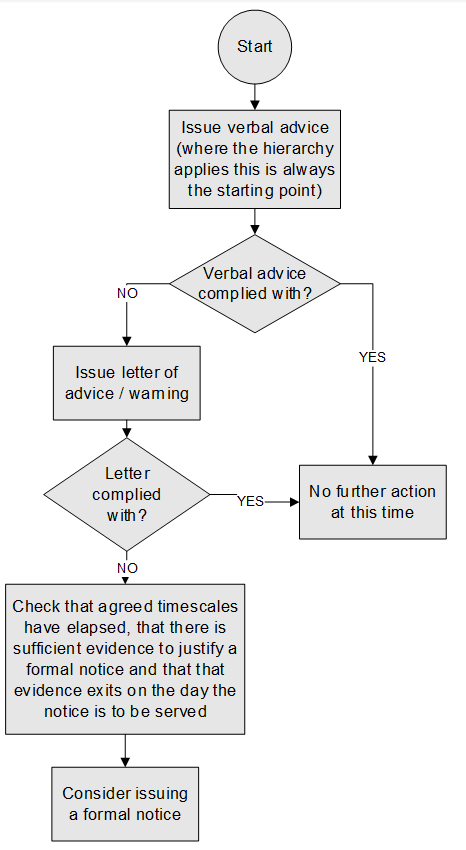
The table below contains a checklist to determine if a formal notice has been drafted and served correctly
| Step | Action |
|---|---|
| 1 | Ensure the notice has a unique reference number to prevent it being confused with any other formal notice, see suggested format below |
| 2 | Ensure the formal notice is addressed to and served on the correct person / partners or legal entity, |
| 3 | Ensure local plant management have received a copy of any formal notice where the original was served on the limited company and sent c/o ‘The Company Secretary’ to the Registered Office address, |
| 4 | Check the notice is clearly worded, concise and easily understood; it is typed (unless drafted by hand and served immediately), dated and signed by the AO |
| 5 | Ensure the notice accurately describes the noncompliance and associated risk and is not just copying the wording of the legislation |
| 6 | Ensure the notice clearly describes the action required to remedy the breach of the legislation and cure the contravention identified in the contravention box, |
| 7 | Check the notice provides a suitable time frame within which compliance should be achieved, |
| 8 | Ensure an official up to date version of the notice is used (taken from the MOC) |
| 9 | Make sure all sections of the Notice have been completed correctly and where options exist in the notice, any references that are not relevant Have been deleted as necessary |
| 10 | Ensure the notice includes all required information on rights of appeal, and |
| 11 | A copy of the notice served has been retained and copied / scanned as a permanent record |
If any of the above checks are not complied with, the AO must ensure action is taken to secure compliance before proceeding to serve the Notice.
4.3.4 Unique reference numbers on enforcement documents
All enforcement notices require the officer to insert a unique reference number, which allows them to be referenced accurately in all correspondence with FBOs and in any legal proceedings. Likewise, other enforcement documents also benefit from some form of referencing, to prevent them from being mixed up with any other similar documents.
There is no legal format for the way in which a reference number is created, however, for consistency it may be useful to follow the example format below, for all enforcement documents:
- Plant number – 4-digit approval number (e.g. 0000)
- Initials of the Document Type (e.g. HIN)
- The Year (e.g. YYYY)
- A sequential number, indicating the number of that type of document served that calendar year (e.g. 01)
E.g. “0000 – HIN – 2021 – 01”
The table below provides examples of written correspondence / formal notices and the unique reference code that should be used to identify them
| Written Correspondence / Formal Notice | Unique Reference Number Used to Identify the Document |
|---|---|
| Plant Profile | 0000 - PP - 2022 - 01 |
| Remedial Action Notice | 0000 - RAN - 2022 – 01 |
| Agreement to Destroy Meat Notice | 0000 - ATDM - 2022 – 01 |
| Detention of Food Notice | 0000 - DOFN – 2022 – 01 |
| Detention Notice | 0000 - DN - 2022 – 01 |
| Written Advice | 0000 - WA - 2022 – 01 |
| Hygiene Improvement Notice | 0000 - HIN - 2022 – 01 |
| Hygiene Improvement Notice Compliance Letter | 0000 – HINCL – 2022 - 01 |
| WATOK Enforcement Notice | 0000 – WEN – 2022 - 01 |
| WATOK Completion Notice | 0000 – WCN – 2022 - 01 |
| Refusal To Issue a WATOK Completion Notice | 0000 – RTIWCN – 2022 - 01 |
| CCTV Enforcement Notice | 0000 – CCTVEN – 2022 - 01 |
| CCTV Completion Notice | 0000 – CCTVCN – 2022 - 01 |
| Refusal To Issue a CCTV Completion Notice | 0000 – RTICCTVCN – 2022 - 01 |
| ABP - Disposal Notice | 0000 – ABP-DN – 2022 - 01 |
| ABP C&D Notice | 0000 – ABP-C&DN – 2022 - 01 |
| ABP Prohibiting ABP Being Brought Onto Premises Notice | 0000 – ABP-PABPBBOPN – 2022 - 01 |
| ENF 11/22 Internal Communication of non-compliance | 0000 - ICONC - 2022 – 01 |
| Written Advice Declaring Animals or Meat Unfit for Human Consumption | 0000 - WADUHC - 2022 - 01 |
| Letter of extension of a Written Advice | 0000-WA-2024-01- EXT |
| Letter of extension of a HIN | 0000-HIN-2024-01- EXT |
| Letter of cancelation of HIN (due to an error/legal reasons) |
0000-HIN-2024-01- Cancelation |
| Letter to withdraw a HIN (after FBO complied with the notice) |
0000-HIN-2024-01- Withdrawal |
| Letter of cancelation of RAN (due to an error/legal reasons) |
0000-RAN-2024-01- Cancelation |
| Withdrawal of RAN notice (when FBO complies with the Notice) |
The RAN has a section for withdrawing the notice once compliance has been achieved, therefore the reference number does not change as we are withdrawing the same notice we served originally. |
| Withdrawal of ABP notice (when FBO complies with the Notice) |
The ABP Notice has a section for withdrawing the notice once compliance has been achieved, therefore the reference number does not change as we are withdrawing the same notice we served originally. |
| Letter to withdraw a WEN due to an error/legal reasons (as when we cancel a HIN or RAN) | 0000-WEN-2024-01- Withdrawal |
| Warning Letter to CoC Holder | 0000-WW- 1234 -2024-01 |
| CoC Suspension Letter | 0000- CoC Susp - 1234 -2024-01 |
| CoC Revocation Letter | 0000- CoC Rev - 1234 -2024-01 |
| Letter to Return a CoC after Suspension | 0000-CoC Return- 1234- 2024-01] |
A sequential number should be used for each successive document of a particular type, starting at the beginning of each calendar year.
4.4 Statutory notices for hygiene contraventions
4.4.1 The Food Safety and Hygiene (England) Regulations 2013 and the Food Hygiene (Wales) Regulations 2006
The Food Safety and Hygiene (England) Regulations 2013 and The Food Hygiene (Wales) Regulations 2006, provide powers to serve 3 different types of notices for hygiene non-compliances:
- Remedial Action Notice (Regulation 9(1))
- Hygiene Improvement Notice (Regulation 6)
- Hygiene Emergency Prohibition Notice and Order (Regulation 8)
Reference: See section 3 on ‘Surrender, detention, seizure and condemnation’ in this chapter for details of detention of animals or food for further examination and sampling under Regulation 10(1) of the England Regulations and 9(5) of the Welsh Regulations and detention for further investigation under Section 9 of the Food Safety Act 1990, via the provisions of Regulation 25 of the domestic England Regulations and Regulation 23 of the domestic Welsh Regulations.
4.4.2 Service details
Regulation 10(1) and 9(5) Detention Notices served under The Food Safety and Hygiene (England) Regulations 2013 and The Food Hygiene (Wales) Regulations 2006, should be served on the FBO, or their duly authorised representative.
The Food Safety Act Detention Notice should be served on the person in charge of the food.
4.4.3 Formal service and delivery of notices
Formal notices must be served on the correct legal entity responsible for any potential offences and to the correct address for that entity.
4.4.4 Finding company addresses
Checks on a company’s registered office details may be done by logging on to Companies House website and clicking on to the free company details link under the ‘find company information’ heading.
The organisation can also be contacted on 03031234500, or by email at enquiries@companies-house.gov.uk between 08:30 and 18:00, Monday to Friday.
The table below provides examples of the range of enforcement notices that are available to an authorised officer / person / inspector to seek compliance under the respective legislative area
| Type of Notice | Legislation | Purpose | Should be served upon |
|---|---|---|---|
| Detention Notice | Regulation 10(1) of The Food Safety and Hygiene (England) Regulations 2013 / Regulation 23 Food Hygiene (Wales) Regulations 2006 | To detain any live animal or food for the purpose of further examination / sampling of the animal or food by the CA | The FBO |
| Detention of Food Notice | Section 9 Food Safety Act 1990 [via Regulation 25 of The Food Safety and Hygiene (England) Regulations 2013/ Regulation 23 Food Hygiene (Wales) Regulations 2006] | To detain food while further investigation is carried out | The person in charge of the food (the FBO) |
| Certification of Meat Notice | Regulation 29 of The Food Safety and Hygiene (England) Regulations 2013/ Regulation 27 of The Food Hygiene (Wales) Regulations 2006 | To certify that food has not been produced, processed or distributed in accordance with the Hygiene Regulations and fails to comply with the food safety requirements | The FBO or person in charge of the food. |
| Seizure of Food Notice | Section 9 Food Safety Act 1990 [via Regulation 25 of The Food Safety and Hygiene (England) Regulations 2013/ Regulation 23 of The Food Hygiene (Wales) Regulations 2006] | To seize food in order that it may be taken before the court to be condemned | The person in charge of the food (the FBO) |
| Remedial Action Notices | Regulation 9 Food Safety and Hygiene (England) Regulations 2013 / Regulation 9 Food Hygiene (Wales) Regulations 2006 | To seek compliance with hygiene matters that require immediate rectification | FBO or Duly Authorised Representative |
| Hygiene Improvement Notice | Regulation 6 of The Food Safety and Hygiene (England) Regulations 2013 / Regulation 9 of The Food Hygiene (Wales) Regulations 2006 | To seek compliance with hygiene matters that do not require immediate rectification or it is not practical for the FBO to comply immediately | FBO |
| Hygiene Emergency Prohibition Notice (and Order) | Regulation 8 of The Food Safety and Hygiene (England) Regulations 2013 / Regulation 9 of The Food Hygiene (Wales) Regulations 2006 | To impose a prohibition on the:
|
FBO |
| Hygiene Prohibition Order | Regulation 7 of The Food Safety and Hygiene (England) Regulations 2013/ Regulation 7 of The Food Hygiene (Wales) Regulations 2006 | Prohibition of a food business proprietor or manager from participating in the management of any food business | FBO |
| Welfare Enforcement Notice | Regulation 38 of The WATOK (England) Regulations 2015 and The WATOK (Wales) Regulations 2014 | To:
|
The Business Operator / person |
| CCTV Enforcement Notices | The Mandatory Use of CCTV in Slaughterhouses (England) Regulations 2018 |
|
The Business Operator / person in charge |
| Animal By-products notices | Regulation 25 of The Animal By-Products (Enforcement) (England) Regulations 2013 and The Animal By-Products (Enforcement)(Wales) Regulations 2014 |
|
The occupier or person in charge of or responsible for the premises |
4.5 Remedial Action Notices
4.5.1 When to use a Remedial Action Notice (ENF 11/24 (E and W))
RAN may only be used:
- when any of the requirements of the Hygiene Regulations* are being breached, or
- when inspection under the Hygiene Regulations is being hampered
*“The Hygiene Regulations” in this context means either the provisions of the assimilated EU Hygiene Regulations (852/2004, 853/2004, 2073/2005 and 2015/1375), assimilated Regulations 2017/625 and the Regulation (EU) 2017/625 package in so far as it and they relate to food and The Food Safety and Hygiene (England) Regulations (2013) / The Food Hygiene (Wales) Regulations 2006.
Note: Assimilated Regulation (EC) 178/2002 is not included within the definition of the “Hygiene Regulations” and as a result RANs may not be used to address breaches of this regulation.
RANs should be used specifically where the AO considers that the FBO should take immediate action to achieve compliance, where the rate of operation of the plant is detrimental to its ability to comply with the Hygiene Regulations and may be used to direct the FBO to maintain sampling at the frequencies determined within assimilated Regulation (EC) 2073/2005.
The AO must verbally request that the FBO rectifies the situation and instigate service of the notice if compliance is not met. It is essential to gather the necessary evidence at the time the contravention is identified to justify service of the notice, in case an appeal against the RAN is lodged by the FBO.
The AO must verbally inform the FBO of the intention to serve the notice and record the information in Chronos.
4.5.2 Purpose of a RAN
A RAN places a legal requirement on a FBO to take immediate action to achieve compliance with the Hygiene Regulations. The AO must specify on the notice whether the RAN is intended to:
- prohibit the use of any equipment or any part of the establishment specified in the notice, or
- impose conditions upon or stop a process, or
- require the rate of operation to be reduced to such extent as specified in the notice, or to be stopped completely
RANs can be used to direct a FBO to immediately rectify any of the deficiencies, which fall under assimilated Regulations (EC) 852 and 853/2004, (EC) 2073, (EC) 2015/1375, (EU) 2017/625 and the assimilated Regulation (EU) 2017/625 package in so far as it and they relate to food, together with the domestic hygiene regulations themselves.
In the case of maintenance and structural problems, that do not pose any imminent risk to public health and can only be rectified in the longer term, a Hygiene Improvement Notice should be used. This would be served under Regulation 6 of The Food Safety and Hygiene (England) Regulations 2013 / Regulation 6 of The Food Hygiene (Wales) Regulations 2006.
4.5.3 Identification of the non-compliance
Where the RAN is being served under section 9(1)(a), because a requirement of the Hygiene Regulations is being breached, the AO is required to:
- specify how the requirement(s) of the Hygiene Regulations have been breached in the “contravention” box of the RAN. It is not sufficient to merely repeat the legal requirement set out in the legislation, as this does not specify the precise nature of the breach.
- describe the specific contravention observed, referencing its location in the establishment and enough information to differentiate it from other areas with which it could be confused. The description of the issue should specify how it fails to meet the legal requirements specified in the notice and identify any relevant risks to the product,
- ensure that each notice only cites one breach / contravention, 4.5.13
- cite the relevant legal reference(s) of the Hygiene Regulations that are being breached, ensuring this identifies the exact provision(s) that place an obligation on the FBO. This should include the Articles setting out the general obligations to comply with the relevant provisions in the Annexes to the EU Regulation, and the specific requirement contained in that Annex, for example, assimilated Regulation (EC) 853/2004, Article 3 and Annex III, Section I, Chapter IV, Paragraph 7(b) (i),
- if the non-compliance is covered by more than one legal provision, cite the most relevant provision that applies. Where there are no specific requirements, use the more generic references which apply to the scenario in question.
- describe the measure(s) / action(s) which, in your opinion, the FBO must take to remedy the breach identified in the “contraventions” box of the notice. Sometimes, it may not be possible to identify the exact solution to a problem, for example, an engineering solution to a ventilation / humidity issue. In such cases, it is acceptable to set out the requirements of the regulations that must be complied with and any objectives of that regulation to avoid contamination. The FBO may also be directed to contact an engineer to try and find a solution to such problems.
- ensure that the contravention(s), legal reference(s) and action(s) all link to one another. The measure(s) to be taken must be relevant to the issues specified in the contravention box of the notice. The AO must not require the FBO to undertake actions to remedy failures which they have not identified as contraventions in the earlier part of the notice
- if a RAN is served under Regulation 9(1)(d), allowing conditions to be imposed on a process in the establishment, ensure that the process in question is specified in the notice; examples of a process might be “evisceration”, “dressing” and “skinning” or “wrapping”, “packaging”, "microbiological sampling" etc.
FBOs have a responsibility to monitor all significant hazards in a process, determine where a process is out of control through effective monitoring of a critical limit, identify the root cause of such non-compliances and rectify them through effective control measures / corrective actions, as part of their HACCP based procedures. However, in many cases FBOs will have failed to monitor and failed to undertake the corrective actions identified in their HACCP plan.
Whilst HINs can be used to require compliance with the systemic HACCP deficiencies, RAN can also be used to address any immediate hygiene risks evident as a result of the FBOs failure to have taken any appropriate corrective actions.
In some cases – for example, where a slaughter process is clearly out of control, where the root cause of a serious problem is unknown, or where an AO has already served a RAN which has been breached by the FBO – the AO should consider serving a RAN which prohibits the carrying out of a process (under Regulation 9(1)(d)) or requiring the FBO to stop operations completely (under Regulation 9(1)(e)).
RANs to Stop a Process
In cases where:
- the process is clearly out of control and the FBO is not taking responsibility to address the root cause(s),
- where the root cause(s) is/are varied, or
- where an AO has already served a RAN, which has been breached by the FBO and already referred for investigation
The AO should consider serving a RAN which prohibits the carrying out of a process (under Regulation 9(1)(d)) or requiring the FBO to stop operations completely (under Regulation 9(1)(e)). This may be done in conjunction with a refusal to apply a health mark or the detention of meat if applicable.
A RAN may be used to stop the operation completely in circumstances such as pest infestation, failure of sterilisers, inadequate overnight cleaning, failure of the hot water supply, lack of potable water supply or where the behaviour of the FBO is hampering adequate health inspection.
Note: Where the notice has the effect of stopping the operation completely, the AO must ensure that the action requested of the FBO is proportionate to the risk.
4.5.4 Service and withdrawal
A separate RAN should normally be served on an FBO in respect of each separate deficiency (there may be rare cases where there are multiple breaches that are clearly of the same theme, see below in 4.5.13).
Notices should be served on the correct legal entity and at the correct address. If this is a limited company, it should be posted to the registered office address, c/o the Company Secretary. The notice must also be handed to someone in charge at the plant and a copy retained.
If the business is a limited company, service is not deemed to have occurred unless the notice has been served on the company at their registered office address of that company by post – See Regulation 30, The Food Safety and Hygiene (England) Regulations 2013 and devolved equivalents.
4.5.5 Tagging
All equipment that has been the subject of a RAN must be clearly identified and cross referenced. Tagging the equipment using a numbered security seal, may be used to clearly identify it for the FBO to action any deficiency.
4.5.6 Who to serve the notice on
RANs must be served on the FBO or a “duly authorised representative” of the business. Information on the identity of any duly authorised representative will be contained in the approval document for the business. If this person is not defined by the business or that person is not present / has left the business; the notice must always be directed by default to the FBO.
The notice may be handed to an FBO if they are a sole trader or to all partners in the business, if they are present at the plant.
4.5.7 Alternative service methods
Where it is not possible to identify the name and address of the FBO on whom the notice should be served, it can be served by addressing it to the FBO in their capacity as “occupier” of the establishment at which corrective action is required (naming the establishment). The notice may then either be handed to someone else at the establishment who appears to be in charge, or by attaching the notice or a copy of it to some conspicuous part of the establishment.
The provisions relating to the service of hygiene related notices are contained within Regulation 30 of The Food Safety and Hygiene (England) Regulations 2013/Regulation 28 of The Food Hygiene (Wales) Regulations. They correspond with the provisions of Section 50 of the Food Safety Act 1990.
4.5.8 Information for notices
A copy of the Notice served must be retained and the following information is to be recorded on the reverse of the Notice:
- the name of the plant representative to whom any copy notices have been handed (in circumstances where the original has been posted to the FBO at the plant or served at the registered office address of a limited company)
- any comments made by the FBO or plant representative when handed the notice
- details of any food detained at the same time as the service of the RAN
- the reference number of any Detention Notice served
- details of any appeal that is lodged by the FBO in respect of the service of the RAN
4.5.9 Rights of appeal
The FBO has the right of appeal in accordance with (Regulation 22 (England), Regulation 20 (Wales) to a Justice of the Peace regarding the decision of the AO to serve a Remedial Action Notice. If the FBO appeals, the AO / VEDM must notify CSU York Transactions Team immediately.
The provisions of the Remedial Action Notice remain in force until such time as the appeal is upheld.
4.5.10 If removed or defaced or destroyed
The notice is the property of the FSA. If the AO discovers that any notice affixed to an establishment has been removed, defaced, or destroyed, the notice should be replaced as soon as possible, and the events recorded in the officer’s pocketbook and/or plant daybook if at a slaughterhouse.
4.5.11 Failure to comply
Failure to comply with a RAN is an offence (Regulation 9(5) of The Food Safety and Hygiene (England) Regulations 2013 / Regulation 9 (7) of The Food Hygiene (Wales) Regulations 2006). If the operator has failed to comply with such a notice, the AO should complete a Referral for Investigation report - ENF 11/6.
4.5.12 Corroborative evidence rules
The service of a notice should be evidenced or corroborated in some way wherever possible. If a notice is served by hand, either secure a second AO to corroborate this fact or ask the FBO to sign your pocketbook to acknowledge receipt. Both, the AO who served the original notice and the corroborating officer should sign a copy of the notice and indicate the date and time of service and should also make a note of the details of service in the Plant Daybook and/or their pocketbook.
A witness is required to observe any AO fixing a notice to the premises wherever possible, or a photograph is required to evidence it has been attached.
When posting a notice, the OV should obtain a proof of postage certificate and retain this as evidence. Where this is not possible, the AO should record the details of where the notice is posted and the postage address in their pocketbook and have a colleague corroborate the postage and countersign the entry. Where no colleague is available to corroborate postage, record details of posting in the same way as above and photograph the envelope.
It is also possible to send notices via recorded delivery, however, if the FBO has a history of refusing to accept such correspondence, the notice should always be posted at a post office and a certificate of posting obtained as above.
4.5.13 Multiple contraventions
Where different contraventions have been identified, a different notice should be served for each and every separate contravention.
A notice containing multiple contraventions:
- will be more complicated to draft and it is more likely that an FBO may be confused by what the AO is trying to convey; this may affect the validity of the Notice as it is important that enforcement requirements placed upon an FBO are clear,
- will require actions that must be capable of curing all the issues cited in the contravention section,
- cannot be withdrawn if there are certain issues still outstanding, even where some issues have been complied with,
- cannot be referred for investigation as certain aspects of the notice may have been complied with and some not,
- if appealed, will result in all of the issues being the subject of the appeal, even where some may have been actioned.
In limited circumstances, it may be acceptable to cite more than one issue and legal reference on a RAN or HIN (see section on HINs), provided that:
- the legal references and contraventions relate to the same theme, for example, maintenance, cleanliness of the premises
- the actions the AO requires the FBO to take are capable of curing all the contraventions identified earlier in the notice,
- where multiple issues of the same theme are identified, it may be useful to list them on a schedule that is annexed to the Notice.
4.5.14 Withdrawal of a RAN
A RAN is often used to correct problems with operational practices that pose a potential risk to the safe production of food. They may be left in place until the AO is satisfied that the FBO has complied with the legal requirement. There may often be occasions where the non-compliance is intermittent, and the AO wishes to be satisfied that the FBO / their staff have changed their behaviour before withdrawing the notice.
However, the AO must monitor the situation and come to a determination within a reasonable time frame given the non-compliance they are requiring the FBO to correct. The time frame for removing the notice may vary depending on the nature of the non-compliance; however, if the Notice is breached, it must be referred for investigation.
If the officer is satisfied that the actions required by the Notice have been complied with, it must be withdrawn and it is not appropriate to leave the notice in place for long periods after this point as it does not offer certainty for the FBO as to whether they will face any future legal proceedings, since they are now complying with the Hygiene Regulations.
However, in situations where compliance with the requirements are intermittent, it is important to remember that there is no maximum timeframe to leave a RAN in place, and no requirement about when a RAN has to be withdrawn, except that it should be withdrawn once the AO is satisfied that it has been complied with.
Notification of withdrawal of a RAN must be achieved in the same way that the notice was served. If the FBO is a limited company, and the Notice was served at the company’s registered office address (with a copy of the Notice having been handed to a member of staff in charge at the production plant), the withdrawal notice must also be sent in the post to the registered office address, and a second copy should be handed to someone appearing to be in charge / duly authorised representative at the plant.
4.6 Hygiene Improvement Notices
4.6.1 When to use a Hygiene Improvement Notice (ENF 11/23 (E and W))
HIN should be used:
- where there is a record of non-compliance with breaches of the Hygiene Regulations
- where the history of compliance by the FBO is such that the AO has reason to believe that an informal approach will not be successful
- where formal action is proportionate to the risk to public health
A HIN should not be used for non-hygiene related matters, for example, failure to comply with the provisions of the Animal By-Product Regulations, WATOK or TSE Regulations.
Both verbal and written advice should be given to a FBO prior to a HIN being served. However, there may be circumstances where the AO believes this informal approach will be unsuccessful or the issue is a repetitive one. If these informal stages are to be bypassed, the AO must have suitable evidence to demonstrate that the FBO has ignored previous informal advice in this area, prior to circumventing these requirements.
4.6.2 Purpose of a HIN
The purpose of a HIN is to place a legal requirement on a FBO to take action to achieve compliance with the assimilated Food Hygiene Regulations.
A HIN may require the FBO to:
- address any hygiene deficiency that does not require immediate action
- repair a structural defect with the building
- to build or construct additional facilities to cope with an increased throughput
- address failures to implement and maintain a sound HACCP based system
The identified action must be stated on the HIN.
4.6.3 When not to issue a HIN
A HIN cannot be used to impose a continuing burden, and should not be used in the following circumstances:
- where the contravention might be a continuing one, for example, wooden pallets stored in the presence of unprotected fresh meat and the Notice would only secure an improvement at that point in time
- where breaches exist that pose a potential and imminent risk to health and urgent action is needed; in these cases, it is more appropriate to use a RAN and in more serious situations (subject to agreement from the relevant Head of Operational Delivery) a Hygiene Emergency Prohibition Notice
- for the failure by an operative to implement good operational hygiene
An HIN cannot be issued unless a contravention of the Hygiene Regulations is identified.
4.6.4 Service
HINs can only be served on the FBO (i.e. a sole trader / on each partner / a limited company). Only the AO who observes the deficiency should serve the formal notice.
Note: Where the FBO is a limited company, the envelope is to be addressed to the limited company c/o The Company Secretary at the Registered Office. However, details of the Company Secretary must never be used on the formal notice itself, as that must only be addressed to the FBO.
If the Notice has been served by post on the FBO, a copy of the Notice should be handed to someone at the plant address by the AO that observed the deficiency. Details of how the notice was served should be recorded on the back of the HIN.
4.6.5 Service checklist
When serving a HIN the AO must:
- have in their possession all the evidence to justify its service
- verbally inform the FBO of the intention to serve the notice
- state why it is served, and the action needed to remedy the breach
- sign, date and if possible, type the HIN
4.6.6 Drafting and serving a notice to a sole trader
Ensure that the name of the individual FBO on the formal Notice clearly identifies that individual beyond doubt and will need to include both their forename(s) and surname.
Where family members have the same first names, try to include any additional forenames that the person may have, to avoid confusion. The notice may be served by hand on the sole trader at the plant or addressed to them personally at the plant address. A copy of the Notice should be posted to the FBO at the plant address and proof of posting obtained.
4.6.7 Drafting and serving a notice to a partnership
Where a number of individuals act as the FBO under a partnership arrangement, a copy of the Notice must be served on each and every partner. The box identifying the FBO must include each and every partner’s full name.
The notices may be served by hand on each partner at the plant or addressed to each of them personally at the plant address, with a covering letter explaining that the same notice has been served on the other partners in the business. Proof of posting should be obtained.
4.6.8 Drafting a notice to a FBO with limited liability status
Where the FBO has limited liability status, the name of the FBO will be the full name of the limited company, as registered at Companies House, for example, ‘ABC Meat Ltd’. The Notice must be sent by post to the registered office or principal address of the company, with a copy of the Notice handed to the relevant person in charge at the plant. The envelope must be addressed to the limited company c/o ‘The Company Secretary’, where one exists.
Note: Whilst a company secretary is no longer a legal requirement within a limited company structure, where they exist, they are generally the person responsible within a limited company structure, who is responsible for receiving formal documentation. They are not, however, the FBO or proprietor, and therefore the company secretary details should not be referred to on the formal notice itself.
4.6.9 Content of notice
The notice must specify the:
- grounds for believing the FBO is failing to comply with the Hygiene Regulations
- precise nature of the alleged breach
- measures needed to be taken to secure compliance
- timescale (date) for compliance (minimum 14 days)
- appeal provisions, including the name and address of the relevant local court
Note: Where an FBO undertakes alternative works of equivalent effect, this may be acceptable.
4.6.10 Drafting the notice
The AO is required to:
- describe the contravention that has been observed that constitutes a breach of the Hygiene Regulations. It is not sufficient to merely repeat the legal requirement set out in the legislation, as this does not specify the precise nature of the breach
- cite the relevant legal reference(s) within the Hygiene Regulations, ensuring that this identifies the exact point or paragraph that places an obligation on the FBO, including the general obligation for the FBO to comply with the relevant provisions within the Annex(es) of the legislation where applicable; for example, Article 3 and Annex II, Chapter X, Point 1 of assimilated Regulation (EC) 852/2004
- where the contravention breaches various legal requirements, use the most relevant and specific provision where this exists; however, where there are no specific requirements, use a more generic references which applies to the contravention in question
- describe the measure(s) / action(s) in the AOs opinion the FBO must take to secure compliance with the contravention(s) identified earlier in the notice
- ensure the contravention(s), legal reference(s) and action(s) all link to one another. The AO must not require the FBO to undertake actions or measures that are not relevant to the identified contravention detailed earlier in the notice or are not directly able to cure that breach
- set out a timescale which is a minimum of 14 clear days from the date the notice is served
- in rare cases where the AO identifies more than one legal reference and contravention on the same notice, it is important that these are clearly of the same theme and that the time frame for compliance is suitable for all issues
4.6.11 Drafting notices with more than one legal breach identified
A notice will generally only deal with one contravention. This will avoid potential problems if the Notice is appealed, where all of the issues cited on the notice will be held in abeyance until the court makes a determination on the validity of the Notice.
Where different contraventions need to be remedied within different time frames; for logistical and operational reasons, you cannot place separate time scales for different issues on the same notice and therefore the contraventions should be the subject of separate notices.
The more contraventions that are cited in a Notice, the more complicated the Notice will be to draft, and it is more likely that an FBO may be confused by what the AO is trying to convey. This may also affect the validity of the Notice as it is important that enforcement requirements placed upon an FBO are clear.
The actions / measures the FBO must take that are specified by the AO in the notice, must be capable of curing all the issues cited in the contravention section. Failure to do so will make it problematic to ensure that the actions the FBO must take, marry up with all the relevant contraventions identified by the Notice and will secure compliance.
It may be acceptable to cite more than one legal reference or issue on a notice, provided that:
- the legal references link to all the contraventions described by the AO
- they relate to the same theme
- the actions / measures the AO requires the FBO to take are capable of curing all the contraventions identified in the notice and ensure all legal obligations are adequately dealt with.
4.6.12 Posting
Ideally, all HINs should be posted at a Post Office and a certificate of posting obtained. Where it is impractical to gain access to a Post Office the notice should be posted in a post box, corroboration obtained by a colleague where they are available, and a record made in the AOs pocketbook which should be countersigned.
It is possible to send notices via recorded delivery, however, if the FBO has a history of refusing to accept such correspondence, the notice should always be posted at a post office and a certificate of posting obtained as above.
4.6.13 Right of appeal
Recipients have a right of appeal against the service of a Hygiene Improvement Notice to the Magistrates’ Court. During the appeal period, the requirements of the notice are suspended.
In the event of an appeal by someone who is aggrieved by the service of the HIN, the AO is to inform CSU York Transactions Team immediately, who will arrange legal representation through FSA Legal for the appeal hearing.
4.6.14 Requests for notice extension
If the FBO were to request an extension to a HIN, this request must be in writing and requested prior to the expiry of the notice. This will be an informal arrangement between the AO and FBO as there is no legal basis for the AO to extend the notice. It will constitute an informal undertaking by the AO not to refer the matter for investigation unless the FBO continues to be non-compliant after the agreed extension date.
Where there is a genuine reason for such an extension, the AO should discuss with the FBO the length of time required to comply and confirm their agreement to the extension in writing.
The AO must review the works carried out by the FBO after the agreed extension date specified in the letter has expired. They should either withdraw the Notice or refer the breach of the Notice for investigation; see below.
4.6.15 Failure to comply
Failure to comply with an HIN is an offence.
If the FBO has failed to comply with a notice, complete a Referral for Investigation report for the breach of the formal notice as well as a breach of the substantive offence that led to the notice being served in the first place.
Reference: See the topic 4.9 on ‘Referral for investigation’ for additional information.
4.6.16 Compliance and withdrawal
After the service of a HIN, an AO must check that it is complied with by the stated date.
Where compliance is achieved, an AO must confirm formally in writing that they are satisfied with the works carried out.
Measures that achieve the same outcome as those specified in the notice must be accepted as achieving compliance.
A template letter is available in Annex 4 to this chapter that can be used as the basis of a letter to the FBO where:
- the AO is satisfied that the action required in the HIN has been carried out and compliance has been achieved to their satisfaction, or
- the AO has served the HIN in error and/or it has to be withdrawn due to a technicality or is legally defective in some way.
Reference: See Annex 4 (a) and (b) for letter templates on ‘Hygiene Improvement Notice and defective notice withdrawal.
4.7 Hygiene Emergency Prohibition Notices
4.7.1 Caution
Hygiene Emergency Prohibition Notices (HEPN) should only be issued by AOs after consultation with FSA Legal.
4.7.2 When to use
Issuing a HEPN should only be considered after discussion with the FVC, and where there is an imminent risk of injury to health that requires the backing of the court, for example, contamination of the potable water supply.
Reference: Specific examples and further guidance are given in the Code of Practice made under Regulation 26/Regulation 24 of the Regulations.
The HEPN must be served on the FBO by using the same procedures as outlined in the topic ‘Hygiene Improvement Notices’.
Notify the FVL in case a review of the FBOs approval is required.
Note: Limited timescales are imposed for the service of the HEPN on the FBO and the subsequent Notices required before the application to the court for the Order (see below).
A table to identify the procedure for the service of a NEPN and application for a HEPO is set out in the next section
4.8 Hygiene Emergency Prohibition Orders
4.8.1 Application process
The table below provides an overview of the application process for a HEPO.
| Steps | Description of Actions to Draft and Serve a Hygiene Emergency Prohibition Notice and apply to the court for a HEPO |
|---|---|
| 1 |
A HEPN must first be served on the FBO. It has an immediate prohibition effect on a process / equipment or the premises. Once served the AO should contact FSA legal to immediately arrange for a hearing at the local Magistrates Court. The AO must give the proprietor at least 1 full day’s notice of their intention to apply to the court for a HEPO by serving a HEPN on the FBO. Once served the AO should contact the local court to immediately arrange for a hearing. Note: A copy of the HEPN must be affixed in a conspicuous position to the premises at which the notice relates. It is crucial that the AO gathers appropriate evidence at the time the HEPN was served and that this evidence is presented to the court. |
| 2 | The AO must apply for a HEPO from a magistrates’ court with jurisdiction in the area where the plant is located. The application to the Court must be made within three days of the service of the HEPN. The day of the service of the notice is regarded as day one. There is no legal requirement for the application to be heard in three days, although the court should be asked to list the hearing at the earliest opportunity. Once made the HEPO supersedes the HEPN. The AO must also affix a copy of the HEPO in a conspicuous position to the premises at which the HEPO relates. |
| 3 | Inform FSA Legal of the need to make an application. FSA Legal will contact the local court to arrange a hearing. The hearing must take place within three days of service of the HEPN. On establishing dates and times, the AO must notify the FBO by serving a Notice of Intention to Apply for a HEPO. |
| 4 |
Prior to the hearing the AO should:
The AO should also prepare three copies of:
The AO must monitor the premises whilst awaiting the hearing and record any breaches of the notice or changes in circumstances at the plant. |
| 5 | The AO must serve on the FBO a Notice of Intention to Apply for a HEPO at least one day prior to making an application to the Court for a HEPO. |
| 6 |
If the order is granted the AO should have produced a draft HEPO for signature by the Magistrate. The Order must then be served on the FBO as soon as possible and a copy affixed to the premises in a conspicuous place. Any breaches of the order whist in force should be recorded and evidence collected. The matter should then be referred for investigation. |
| 7 | The AO must then formally cancel the HEPO by writing to the FBO. The withdrawal of such a HEPO must not be unreasonably withheld. Once the order has been complied with, the business can recommence the process / operation or use of equipment / the premises connected with their business. |
| 8 |
If the FBO applies, in writing, for the HEPO to be lifted, the application must be determined as soon as practicable and within 14 days. Once the AO is satisfied that the proprietor has taken significant steps to remove the health risk(s) specified in the notice, the AO should sign the withdrawal certificate at part 5 of the HEPN. Regulation: The Food Safety and Hygiene (England) Regulations 2013 / The Food Hygiene (Wales) Regulations 2006, Regulation 8. |
4.8.2 Sources of advice
Advice should be sought from FSA Legal, who will assist in vetting the HEPN and preparation of the case prior to the court's hearing of a HEPO.
4.8.3 Evidence
Monitoring of the prohibition and any action taken by the proprietor must be recorded. Suitable evidence should be gathered prior to serving the HEPN for production in court. A statement should be produced setting out the incident and evidence that supports the application to the Court.
4.8.4 Procedure
The table below shows the steps for an AO to follow when making an application to the court for a HEPO.
4.9 Referral for investigation
4.9.1 Appropriate uses
Referring an incident for investigation is not, in itself, enforcement. Its purpose is not to remedy a breach but to allow the relevant legal team to determine if there is a case for prosecution. However, the hierarchy of enforcement should be followed where necessary to ensure prosecutions are only brought in appropriate cases.
An immediate referral for investigation is required in the following circumstances:
- Presentation of SRM on three or more occasions.
- SRM being consigned from the premises still attached to the meat (except in the case of VC being consigned to approved cutting premises)
- Where contraventions of the legislation have been escalated through their natural hierarchy and the FBO continues to breach the requirements.
- Where FBOs have failed to test bovine animal’s, which require BSE testing
- contraventions of assimilated Regulation (EC) 1099/2009 and / WATOK, where avoidable pain, distress or suffering has occurred during handling or slaughter
- breaches of assimilated law or domestic food safety or hygiene legislation leading to an imminent risk to public health
- obstruction of FSA personnel engaged in official duties
- failure to comply with any formal enforcement notice
“Note, for animal welfare cases a RFI triage proforma should be completed and the case submitted to the Welfare Triage Panel before initiating the referral for investigation (see Guidance at Annex 14)”
- AO decision made to refer the case for formal investigation. Unless the suggested referral comes from a cOV, in which case, the VEDM must make the decision to refer the case for formal investigation. Remember that at this stage it is just a recommendation - the FBO is innocent until proven guilty in a court of law.
- The AO must send all evidence, together with the ENF 11/6 and the latest FBO audit report, to CSU at FSA York within 10 days of the offence being identified and the AO’s decision being made to refer investigation.
- COVs must send this information to the VEDMs who will pass it onto CSU in York if they support the decision to formally refer the matter for investigation. The information should reach CSU within 10 working days from the AOs / VEDMs decision to refer the matter after the offence was identified.
- Hygiene offences carry a 12-month time limit from when they are discovered by the AO; within which charges should be laid at the court. The time-limit between identifying obstruction contraventions and laying charges at the court is 6 months. The time-limit between identifying animal welfare cases and laying charges is 6 months, beginning with the date on which evidence which the prosecution thinks is sufficient to justify the proceedings comes to the prosecutor’s knowledge. The IO must be afforded enough time to investigate the offences identified
4.9.2 Evidence
The AO must collect adequate evidence at the time of the offence before referring the matter for investigation.
Where a formal notice is being served, the AO must gather evidence at the time, to justify its service in case of an appeal and to evidence the original contravention of the assimilated law requirements.
Evidence must be gathered again if the notice is breached, because breach of the notice is a separate offence.
The AO must identify the contravention(s) and update Chronos.
Regard should be given to the Enforcement Policy and relevant guidance prior to any referral for investigation being put forward.
4.9.3 Referral to FSA Legal
Where the AO considers that an incident requires investigation, the matter will be referred to FSA Legal for an investigation to be undertaken.
Note: See the table in Section 4.11 on ‘Protocol for a referral for investigation’.
4.9.4 Decision to prosecute
In England and Wales, the decision whether or not to prosecute for contraventions of food safety, hygiene and SRM controls is made by an experienced prosecutor in FSA Legal, having been investigated fully by an FSA Investigating Officer.
The decision to prosecute for contraventions in England of the animal welfare, TSE (RMOP and BSE testing) and animal by-product legislation will be made by a lawyer at the Crown Prosecution Service (CPS), on the basis of an investigation carried out by an FSA Investigating Officer. The decision to prosecute for contraventions of the equivalent legislation in Wales is the responsibility of the Welsh Government, again on the basis of an FSA investigation.
4.9.5 Rules of evidence
The AOs main task will be to gather facts and evidence at the time of the offence, which may be used in court at a later stage. An AO must not attempt to conduct a full investigation as specific training is needed to ensure that the investigation is carried out in compliance with the Police and Criminal Evidence Act 1984 (PACE) (or equivalent) requirements. Only specially trained FSA Investigation Officers conduct investigations. AOs must not attempt to caution or interview suspects or to take statements, unless specifically trained to do so.
4.9.6 Statements
Statements will be taken by an FSA Investigation Officer. They are a record of specific events an individual witnessed in a chronological order. They must refer to all relevant evidence and produce these as exhibits for the case, e.g.:
- photographs / videos
- physical samples
- copies of notices
- copies of daybook entries
- copies of relevant parts of the FBOs HACCP plan and records of corrective actions undertaken
Exhibits are usually identified by the initials of the AO and then consecutively numbered. The IO will assist with numbering when preparing the final statement.
Note: Where the AO is satisfied that the action required or work specified in a formal notice has been completed, the date that it was completed should be specified in a witness statement and on the back of the copy notice.
4.10 Protocol for a referral for investigation
4.10.1 Protocol for a referral for investigation
The table below outlines the process and rationale for a referral by the AO for formal investigation.
| Process | Rationale |
|---|---|
|
If an AO is referring the matter, they should discuss the issue with the rest of the inspection team (in slaughterhouses), where relevant when the contravention occurs, and prior to completing Chronos and the ‘Referral for Investigation’. “Note, for animal welfare cases an RFI triage proforma should be completed and the case should be triaged before initiating the referral for investigation (see Guidance at Annex 15)” |
This will ensure that the entire inspection team is aware of all formal enforcement action taking place at the plant. All members of the Inspection Team are Authorised Officers and must assist with any enforcement intervention as and when required, including acting as a witness in court if necessary. Remember that at this stage, the referral is a recommendation for investigation. The FBO is innocent until proven guilty. |
| The AO is to ensure the inspection team is aware of any proposed enforcement action before the FBO is advised of referral. | This will forewarn and forearm colleagues that a contravention has been referred for investigation. |
| A summary of all enforcement should be shared at team meetings, detailing the stage to which any investigation has progressed. | The team as a whole will be more effective in identifying similar breaches, and no individual AOs will unknowingly condone activities that another AO has attempted to stop. |
| If advice is needed on the correct enforcement approach, the AO should consult with FSA Legal at an early stage if necessary. | Early advice will provide the necessary support to quickly address any queries regarding an enforcement intervention. Likewise, it will avoid unnecessary mistakes, lead to a more consistent approach, reduce legal challenges, improve evidence gathering and ultimately improve the success of cases in court. |
| AO / VEDM decision made to refer the case for formal investigation. | Remember that at this stage it is just a recommendation – the FBO is innocent until proven guilty in a court of law. |
| The AO is to collect all evidence relating to the referral | All relevant evidence must be identified, secured and supplied with the prosecution file to enable the referral for investigation to be considered by the lawyer assessing the case. In particular, photographs must be taken to assist the court, especially where the nature of the offence may be difficult to visualise or where photographs / videos are essential to prove the elements of the offence. |
| The AO must send all evidence together with the ENF 11/6 and the latest FBO audit report, to the CSU at FSA York within 10 working days of the offence being identified and the AOs decision being made to refer the case for investigation must also be included, together with the outcome of the Triage Panel in the case of animal welfare referrals. | Hygiene offences carry a 12-month time limit from when they are discovered by the AO; within which charges should be laid at the court. The time limit between identifying obstruction contraventions and laying charges at the court is 6 months. The time limit between identifying animal welfare cases and laying charges is 6 months, beginning with the date on which evidence which the prosecution thinks is sufficient to justify the proceedings comes to the prosecutor’s knowledge. The IO must be afforded enough time to investigate the offences identified. |
| Once received, CSU will acknowledge receipt of the recommendation and associated paperwork and allocate a unique number to the referral. This number will be notified to the AO in the confirmation email. (If confirmation is not received within 5 working days the AO should contact CSU.) | Confirmation will be sent to provide assurance that the documentation has been received. |
| Inform the FBO as a matter of courtesy | After a recommendation for investigation has been passed forward, it is inappropriate for Officers to pass comment on the potential outcome of any investigation under consideration. |
4.10.2 Process to be followed
| Process | Rationale |
|---|---|
| If compliance is achieved after a referral for investigation has been made, the OV must record this in Chronos, including compliance with any formal notice and inform the Investigating Officer (IO) in the case. | This will demonstrate the effectiveness or not of the operator’s ‘Due Diligence’ systems and identify any defences or mitigation that the operator may wish to put forward. |
| Where additional information is required, FSA Legal will request further details, to gain a better understanding of the issues involved. | Where evidence is lacking, the issue is complex, the approach taken by the AO requires further explanation. FSA Legal may contact relevant colleagues to ensure that a comprehensive and informed picture can be gained of the issues surrounding the referral. |
This may include checking that:
|
To prove the elements of the offence beyond all reasonable doubt. |
|
To stand up to legal scrutiny. |
|
To make sure that all the procedural requirements relating to enforcement have been followed. |
|
To ensure that the notice is legally compliant and defensible in case of an appeal. |
|
To ensure that long delays are not holding up the recommendation process and the FSA does not have to defend any ‘abuse of process arguments’ by the defence alleging delays in investigating offences |
|
To ensure that all operational instructions in the MOC and the FSA Operations Enforcement Policy have been complied with. |
|
To make sure that all the procedural requirements relating to enforcement have been followed. |
| Where an AO identifies further contravention(s) of a case that has already been referred for investigation, the AO must discuss the issue with the FBO in the usual way, gather additional evidence and record the issue on the relevant systems (e.g. Chronos, Slaughter Hygiene Verification -SHV, and in the Day Book, their Pocket Book, etc.). The AO must also contact the relevant IO of this further contravention. Where the IO has already submitted the prosecution file to lawyers, a new referral will need to be made of the contravention. If the prosecution case file is still open, the IO will take a further statement from the AO.
Please follow the Chronos User Guidance on Managing further contraventions after an RFI to understand how to add further contraventions into Chronos. |
Although RFI is the highest level of the FSA enforcement hierarchy and no additional enforcement may need to be undertaken by the AO, it is important for the IO and the FSA Legal team to build an overall view of the extent of a contravention and whether or not the FBO is repeatedly committing the same offence. The collection of the evidence on further contraventions and its communication to the IO will further strengthen an existing case or be the start point for a further referral. |
4.11 Referral for investigation: FSA Legal
4.11.1 FSA protocol
The table below outlines the process and rationale for formal investigation by the FSA.
| Process | Rationale |
|---|---|
| FSA Legal Services will review the referral and appoint an IO to formally interview the alleged defendant(s), take statements from all relevant AOs and any other potential witnesses. | FSA Legal will inform the AO and CSU, which IO has been appointed to each case. |
|
When the investigation is complete:
Potential actions could be:
|
When a decision is made NOT to take the case forward FSA Legal will advise the AO / ITL and CSU. |
|
When a decision is made to take the case forward to court, the IO must inform the AO / FSA / ITL and CSU prior to informing the FBO of this intention. This will ensure the Inspection Team is aware of the fact that the FBO will be facing formal action, so that they are aware of any potential conflict. |
|
Any AO who is unfamiliar with court procedure may benefit from some discussion with their FVC or the IO before any court appearance. Arrangements can also be made for a visit to the court before the trial takes place. Witnesses will be sent a copy of their statement to review before appearing at court. |
|
Witnesses are not required to attend court when the FBO offers a guilty plea, however, the outcome of the case will be cascaded to all witnesses. |
4.12 Change of FBO during enforcement action
4.12.1 New FBO
From the moment a new FBO takes over an establishment, they are responsible for its condition and operation. Any enforcement action initiated prior to the change of ownership should be reassessed. Where the new FBO fails to immediately address any outstanding issues, these should be pursued by the AO, through the hierarchy in the normal way by starting the enforcement from verbal advice. Where there are a number of issues, it may be beneficial to set these out in an advisory letter, with any appropriate time scales. If matters need immediate rectification, formal notices may be served on the new FBO and any historical notices will no longer have any legal effect.
4.12.2 Re-issue of notices
In the event of the premises changing ownership whilst a formal notice is still in force, the existing Notice should be cancelled, because it will not be enforceable against the new FBO.
If the new FBO fails to address the outstanding issues the issue should be escalated in line with the usual hierarchy. If ultimately a new notice is necessary, evidence must be gathered at the time the new notice is served to justify it’s service and to support any potential challenge if the notice is appealed.
Note: Where the FBO has changed, the Approvals and Registrations Team should be informed so that an FVC can assess the new FBOs operating practices.
4.13 Warrant to enter premises
4.13.1 Access refused
An AO who has been refused entry to an establishment should always point out the legal powers that the officer has, and which legislation provides that power. If the FBO continues to refuse access, the AO should notify the FBO of the obstruction provisions within the legislation and advise they contact their legal adviser if they need to check such details.
If access is still refused, the AO should contact their FVC/HOD immediately for further advice. In the event that access to an establishment is refused, it may be necessary for an AO to apply to a Justice of the Peace, for a Warrant to Enter Premises, to gain access to carry out their duties.
FSA Legal should be contacted for advice on any refusal by the FBO to grant entry to an AO.
Where there is a threat of violence, reference should be made to the bullying and harassment policy for guidance. A report must also be made to the local police force.
Examples of when it is necessary to apply for a Warrant to Enter Premises include:
- circumstances where the AO needs to enter to find out if there has been a contravention of any relevant legislation, or
- entry is required to find out if there is evidence of any such contravention
- reasonable access has been refused or the AO believes it will be refused,
- the AO has given the occupier notice of intention to apply for a warrant
- the premises are unoccupied
- asking for permission, or giving notice of asking for permission would defeat the object of the entry
- where urgent access is needed
4.13.2 Execution of the warrant
The warrant must be executed within one month and is no longer valid once the AO has used it to gain access. It cannot be used twice. When executing a Warrant to Enter Premises in England or Wales, Code B of the Codes of Practice, made under the Police and Criminal Evidence Act 1984 (PACE), should be complied with. Legal advice on this and other aspects of the Warrant should be obtained before entry is attempted. FSA Legal will advise further.
4.13.3 Access
Advise the local police of the intention to execute the Warrant at a certain time and date. The establishment must be visited as soon as possible and, on production of the Warrant to Enter Premises, the occupier should grant access. If the occupier fails to grant access, he or she will be in breach of the warrant. Record the events in the contemporaneous notebook and inform the FVC/HOD.
4.13.4 Forced entry
The Warrant to Enter Premises allows the use of force to gain entry when necessary. However, the AOs should never attempt a forced entry themselves, but should contact the Police for assistance.
The process flow below sets out the process for applying for a warrant at the court
4.14 Process for obtaining a warrant to enter premises in England and Wales
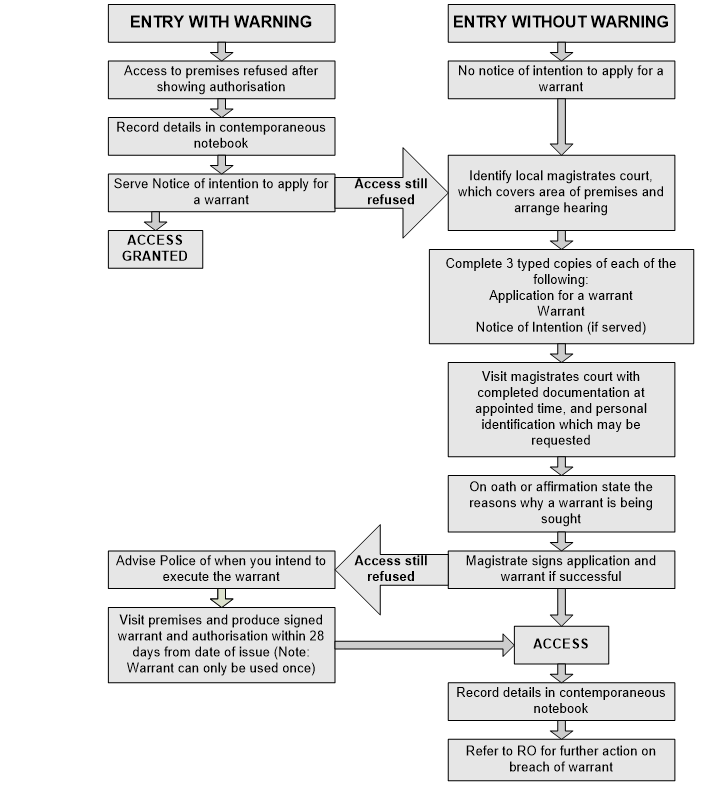
5. Risk based enforcement
In this section
5.1 Why a ‘risk Based’ approach: Legal references
5.1 Why a ‘risk based’ approach: Legal references
5.1.1 Introduction
The FSA adopts a risk based system of assessing public health and animal welfare risk in line with both legal requirements and codes of enforcement practice.
5.1.2 Risk analysis and risk assessment
Food safety and hygiene legislation makes various references to the competent authority applying a risk-based approach to the delivery of official controls.
Assimilated Regulation (EC) 178/2002, Article 6, Paragraphs 1 and 2 state that:
‘in order to achieve the general objective of a high level of protection of human health and life, food law shall be based on risk analysis, except where it is not appropriate to the circumstances or the nature of the measure’, and that ‘Risk assessment shall be based on the available scientific evidence and undertaken in an independent, objective and transparent manner’.
5.1.3 General rules on official controls
Assimilated Regulation (EU) 2017/625, Article 9, 1 requires Competent Authorities to perform official controls on all operators regularly, on a risk basis and with appropriate frequency, taking account of identified risks associated with animals and goods, the activities under the control of operators, the location of the activities or operations of operators, the use of products, processes, materials or substances that may influence food safety, integrity and wholesomeness, or feed safety, animal health or animal welfare, plant health or, in the case of GMOs and plant protection products, that may also have an adverse impact on the environment;
Any information indicating the likelihood that consumers might be misled, in particular as to the nature, identity, properties, composition, quantity, durability, country of origin or place of provenance, method of manufacture or production of food;
The operators’ past record as regards the outcome of official controls performed on them and their compliance with the rules referred to in Article 1(2);
Article 9,2 - Competent authorities shall perform official controls regularly, with appropriate frequencies determined on a risk basis, to identify possible intentional violations of the rules referred to in Article 1(2), perpetrated through fraudulent or deceptive practices.
5.1.4 Risk based enforcement
Assimilated Regulation (EU) 2017/625, Article 138,1 states:
When deciding which measures to take, the competent authorities shall take account of the nature of that non-compliance and the operator’s past record with regard to compliance.
5.1.5 Regulators Code
In accordance with the Regulators Code, the FSA bases its regulatory activities on risk. Paragraph 3.2 requires Regulators to “…consider risk at every stage of their decision-making processes, including choosing the most appropriate type of intervention or way of working with those regulated; targeting checks on compliance; and when taking enforcement action.
5.1.6 Suspected breaches
Where breaches have been identified:
- persistent offenders should be identified quickly and face proportionate and meaningful sanctions
- regulators must act in a way that is proportionate to the risks as they understand them, except where immediate action is required
5.1.7 Risk based enforcement, not risk based compliance
FBOs have a duty to comply with the general hygiene requirements laid down in Annex II to assimilated Regulation (EC) 852/2004, (see Article 4) as well as specific requirements contained in Annex II and III of assimilated Regulation (EC) 853/2004, (see Article 3).
All legal references applicable to taking a risk-based approach apply to the competent authority and not the FBO. Therefore, whilst the FSA must take a risk based and proportionate approach to its enforcement interventions, the FBO must comply with all relevant legal requirements. The FBO cannot conduct a risk assessment and decide to comply only with certain areas that they consider to be medium to high risk.
5.2 Risk management
5.2.1 Purpose of a risk management system
The purpose of a risk management system is to communicate effectively between colleagues when describing and comparing risks and to ensure that the different components of the risk assessment process have been defined.
In this way, we can objectively compare both food hygiene and animal welfare risks at different premises, where FBOs employ different food safety management systems and have different attitudes towards compliance.
5.2.2 Defining risk
The risk assessment consists of two independent components:
- likelihood – how likely is it, that the risk is realised
- impact – how bad the outcome could be if it were realised
When describing risk, it is helpful to use a consistent approach to help avoid ambiguity:
5.2.3 Examples of how to describe risk
The following examples are typical high risk issues, that would be escalated immediately and for which a risk assessment should not have to be recorded:
- the risk that carcases with faecal contamination are produced, because the FBO has not implemented or maintained their HACCP system to prevent dirty livestock from being slaughtered, resulting in consumers getting food poisoning – high public health risk
- ‘the risk that animals arriving at the slaughterhouse cannot lie down, stand up or turnaround without difficulty, because the FBO has used untrained staff without the necessary competence/CoC to lairage animals, resulting in animals being placed in a race and being overcrowded thereby experiencing avoidable pain/distress/suffering – high animal welfare risk
Low risk
- ‘the risk that wrapped and packaged meat will become cross contaminated from contact with a wall with a cracked tile, because the FBO has not implemented an adequate maintenance programme to replace broken tiles which means they cannot be effectively cleaned, resulting in the potential for consumers getting food poisoning- This is a technical breach of structural maintenance requirement. There is no risk to the product if it is wrapped and packaged and cannot come into contact with the walls – low public health risk
- If unwrapped product were to be stored in the same area in direct contact with cracked wall tiles, the risk would increase.
- ‘the risk that wrapped vacuum packed meat will become contaminated from contact with used packaging and from other environmental contamination, because the wrapped meat is removed from a non-waxed boxes, that are not easy to clean, and replaced in similar boxes, resulting in the potential for consumers getting food poisoning – Whilst the unwaxed packaging material is not capable of being effectively cleaned, the product is already wrapped in modified atmosphere packaging. This will prove low risk to the vac packed product in question. However, the situation would need to be logged and monitored, to ensure the risk of cross contaminating by the food handler to other un-wrapped products from the dirty packaging did not arise. Low public health risk, but the risk could increase depending on the likelihood factors. Also if the labelling of the new boxes did not have the correct labelling or ID marks applied, this would be a separate contravention.
5.3 Risk assessment: Defining impact and likelihood
5.3.1 Categorising impact
Impact can be categorised as:
1 = minor impact, technical breach with minimal or no implications
2 = moderate impact
3 = major impact
4 = catastrophic impact
5.3.2 Rating the impact
If completing the risk assessment documentation, the AO must:
- Assess and describe the ‘Reasonably Foreseeable Worst-Case Impact’ (RFWCI) for the event. This is not intended to capture the worst possible scenario that has far-fetched or improbable consequences, but those reasonably foreseeable outcomes of consequences flowing from the scenario.
- Rate the impact: 1 for a minor impact, up to 4 for a catastrophic impact.
- It is not a requirement to envisage bizarre events or acts of God.
- It is, however, a requirement to understand that many risks are ‘reasonably foreseeable’ through a pro-active approach to risk management.
5.3.3 Scoring the impact
The impact rating will be determined by:
- the species of meat being processed
- the bacteria associated with that type of meat
- the intended customers of the FBO
- whether the customers are part of a vulnerable group
5.3.4 Categorising likelihood
Likelihood can be defined and categorised as:
1 = unlikely – not expected to happen
2 = possible – may occur occasionally
3 = likely – will probably occur
4 = has happened or is almost certain to occur at any moment
5.3.5 Likelihood factors
- Has the event already occurred, or could it occur at any moment?
- Intensity (speed of the line, pressure by management for operatives to do the job, operative being paid per carcase and not by time).
- Personnel (number of staff to train, competencies, turnover).
- Duration (how long does the activity take, does it require a long concentration span?).
- Accident, incidents, near misses (past history of the FBO).
- Supervision of staff.
- Environment, age of equipment, ventilation, maintenance.
- Complexity of operation – multi species.
5.3.6 Recording likelihood data
When describing the likelihood factors, the account must be backed up with objective evidence. The likelihood should describe an unambiguous data driven account.
Gather and retain suitable evidence to demonstrate that the likelihood factors have been accurately considered.
5.3.7 Assessing likelihood
Impact and likelihood are treated as independent variables when undertaking a risk assessment.
Care should be taken to ensure that once the RFWC impact has been considered, you do not assess the likelihood of the RFWC factor for example, food poisoning and death occurring in consumers.
It is the likelihood of the risk being realised that must be assessed, for example, assess the likelihood of carcases becoming contaminated within the plant that could potentially lead to the RFWC factor.
5.3.8 Rating likelihood
- Look at the likelihood data for the risk.
- Check that the data is related to the concern and not the impact.
- Rate the likelihood.
5.3.9 Risk matrix
The risk score is a multiple of the reasonably foreseeable worst case impact and likelihood factors that prevail at the specific plant in question resulting from the food safety management systems the FBO has in place.
5.3.10 Scoring
A Risk Matrix score is achieved once the impact and likelihood scores have been identified using the guidance in 5.3.1 and 5.3.4 respectively. Those two scores are multiplied against each other to give a Risk Matrix score between 1 and 16. A higher risk issue is going to have a higher score whilst a lower risk issue will have a lower score. Scores between 1 and 3 are classed as low risk. Guidance on the recording procedures for low risk and medium to high risk contraventions are detailed in Section 5.4 below.
5.3.11 Trend
Assigning a trend allows you to indicate whether the risk is increasing, unchanged or decreasing, even where the overall score on the matrix remains the same.
For example, where an overall score is 4x4=16, the FBO may have taken some corrective action to improve the process and initial indications suggest that this has started to work. In this case the trend could change to demonstrate an improving status, even though the overall risk score may still remain unchanged.
All risks in the red (R) and amber red (AR) zone should have appropriate countermeasures by the competent authority to manage both the likelihood and impact with actions by the FBO to address both.
| Trend | Colour rating |
|---|---|
| Unknown; baseline to be established | - |
| Situation Worsening; risk increasing | R |
| Situation Stable; risk unchanged | A |
| Situation Improving; risk decreasing | G |
5.4 Recording procedure
5.4.1 Recording requirements for risks scoring 4 to 16 (ENF 11/5)
All contraventions must be recorded in the Chronos Enforcement System (ENF 11/5).
Reference: See chapter 7, Section 2.4 - Recording and Monitoring Enforcement Actions.
Where the assessed risk scores medium to high, between 4 and 16, the AO should progress the matter through the hierarchy in the normal manner, gathering evidence at the time the offence is identified.
Reference: See topic 2.6 on ‘Gathering and preserving evidence’ for additional information.
5.4.2 Exception reporting requirements: low risks scoring 1 to 3 (ENF 11/29)
Where it has been determined that the risk posed is low, with a score between 1 and 3, the:
- non-compliance must be recorded in Chronos, evidence of the low likelihood factors must be retained, to justify the low-risk assessment
- the rationale for not escalating the non-compliance, perceived to be low risk, must also be recorded on the ‘Risk Assessment for Enforcement Form’ (ENF 11/29)
The assessment by the AO must detail the appropriate evidence and back up the likelihood factors. This will act as ‘tangible’ evidence of the decision-making process that:
- justifies the reason for not progressing the non-compliance
- provides a rationale for colleagues to ensure a consistency of approach
Non-compliance should always be brought to the attention of the FBO. However, where there is no justification in escalating the issue, an entry should also be made in the ‘Contravention Update’ column of the Chronos record.
Note: All issues identified as a low-risk (1 – 3) must be assessed by the FVCs and re-assessed at successive audit frequencies by Veterinary Auditors to identify any changes to the likelihood factors and at monthly frequencies in slaughterhouses or where likelihood factors change.
Where the likelihood factors remain the same, the AO must record this in their contemporaneous pocketbook or in the plant Day Book at a slaughterhouse.
Note: Where the likelihood factors have changed and the risk has increased, evidence must be gathered and the issue progressed through the hierarchy in the usual manner, copying in the VEDM as required. The existing Chronos record should be updated with new Competent Authority Actions to reflect this.
For example, an isolated maintenance issue that has no major impact on public health should be pointed out to the FBO and recorded in Chronos. Where the risk assessment results in a low-risk score, the matter can be recorded and closed off by cross referencing the ENF 11/29 with the Status column in Chronos.
Where the unresolved maintenance issue becomes more serious or where other minor maintenance issues emerge that individually may not pose a major risk to public health, but cumulatively may lead to the deterioration in the fabric of the building, this will be indicative of a failure by the FBO to have in place and implement a suitable maintenance programme.
In such circumstances, it would be reasonable for all these matters to update the existing Chronos entry and escalated through to compliance.
Reference: Assimilated Regulation (EC) 852/2004, Annex II, Chapter I, Para 1.
Other non-compliances that may constitute a low risk might include:
- minor cleaning issues in non-production areas
- operatives not wearing the appropriate protective clothing (for example, snoods) in boxed meat areas
- other low risk issues the FBO has identified themselves through effective monitoring systems, that have not been rectified immediately, but for which the risk is being managed and a plan exists for the matter to be resolved and the appropriate improvements to their process is being implemented
Reference: See chapter 9 on ‘Forms’ for ENF 11/29.
5.4.3 Audit
An audit trail must be established to demonstrate that the FSA is managing risk appropriately. Veterinary Auditors will review the risk assessment to satisfy themselves it provides the appropriate assurance and ensure the quality of documentation by monitoring the effective recording of evidence for low risk issues.
5.4.4 Risk assessment process
The flowchart below outlines the steps in the risk assessment process.
5.4.5 Reporting considerations 1: proactive risk assessment
An initial risk assessment should be undertaken when the potential risk is first identified. Where the risk is then realised because the event has occurred, a further assessment will need to be undertaken.
It is important that initial risk assessments are not undertaken in hindsight and that all potential risks are identified.
5.4.6 Reporting considerations 2: frequency v likelihood
The likelihood rating for non-compliances should not be scored low after the risk has already been realised.
Do not confuse frequency with likelihood and score the risk low because the event has only occurred infrequently.
If an event could happen at any time, or has already occurred, the likelihood score must be high.
The frequency with which an event occurs is academic, once it has happened.
5.4.7 Reporting considerations 3: FSA controls not a likelihood factor!
The likelihood of an event occurring should not result in a low score, when the FSA has an inspection point at a specific stage in the process that is able to identify a problem.
The presence of an FSA inspection point should not be used to mitigate the risk score.
The likelihood of an event occurring will not be affected by the presence of the FSA Operations Group. It is the FBOs systems and controls that are being assessed to determine the likelihood factor [Y], not the presence of the FSA carrying out an inspection checks at a particular point in the process.
5.4.8 Other factors to be considered
The FSA has identified certain high-level outcomes that are to be achieved:
- to limit food borne illness caused by meat
- to detect and control animal diseases
- to achieve high standards of animal welfare in approved establishments
- to facilitate the international trade of animal products
- meat entering the food chain is free from SRM
- animals intended for the food chain are tested for BSE / TSE where BSE testing is required
- meat from all animals tested for BSE / TSE does not enter the food chain unless tested negative
- meat from over age animals does not enter the food chain
- evidence of deliberate fraud
Where such contraventions are identified, that compromise these outcomes, the overall risk (reputational / business / public health / animal welfare) to the organisation will be high. As such these matters should always be recorded on Chronos and progressed through the hierarchy.
5.4.9 ENF 11/29 risk assessment form
It is important that the FSA can objectively assess the consistency of risk assessments. To ensure consistency of approach, the Risk Assessment Form (ENF 11/29):
- must contain valid data
- must demonstrate the data can be tested and is true
- must be consistent and appropriate (for example, the impact is reasonably foreseeable, the likelihood is of the risk being realised)
- must demonstrate competent authority controls are proportionate to the risks
6. Intervention Protocol
In this section
6.6 Identification for review of approval
6.9 Medium risk establishments: Improvement necessary
6.10 Serious risk establishments: Urgent improvement necessary or Successive Improvement Necessary
6.11 Approach to FBOs identified as Serious risk establishments and support to frontline teams
6.1 Introduction
This document provides guidance to HODs, OMs, FVLs, FVCs, VEDM, Service Delivery Managers, and frontline teams on:
- Monitoring performance of approved meat establishments; and
- Action that should be taken in the event that an FBO does not put in place measures to raise levels of compliance with legal requirements.
FBOs can access this protocol.
6.2 Strategic aims
The goal of the Intervention Protocol is to safeguard consumers and improve public health by improving overall business compliance through:
- Targeting high impact intervention where risks to public health exist
- Seeking prompt compliance in high-risk areas of non-compliance and targeting intervention.
- Provide a graduated and proportionate response to legislative non-compliance ensuring advisory and deterrent elements, along with the escalation of sanctions, where necessary, based on the level of non-compliance risk at individual establishments.
6.3 Background
As part of the intervention protocol, we want to ensure that all FBOs of approved meat establishments are complying with legal requirements and are taking responsibility for the production of safe meat. FSA resources and attention will be directed to non-compliant FBO establishments utilising non-compliances identified during official control activities outlined below:
- results of FBO audits
- findings from unannounced inspections (for example, routine or investigating complaints)
- establishment level inspection and audit findings (serious deficiencies or where evidence of repeated stoppage exists)
The protocol also brings in a process for recommending the appropriate withdrawal of approval as the ultimate sanction for poor performance by FBOs, whilst taking an open and transparent approach to informing FBOs about what we are doing and why, in accordance with risk-based assessment methodology.
It will provide operational staff with clarity on when to act and what action to take. However, the FSA must take action quickly in the event of serious FBO non-compliances / or persistently contravening the regulations. Where a FBO fails to put in place the necessary measures leading to significant public health, animal health and welfare improvement, FSA officials will review their approval status where evidence exists indicating the presence of major or critical non-compliances. This could lead to their approval being withdrawn or suspended.
By gathering high quality evidence at the earliest stage via audits, unannounced inspections and regular official control activities, prompt intervention will be taken with the right enforcement actions.
Openness is one of the core principles of the FSA and underpins our strategic outcome that consumers and customers should have the information and understanding they need to make informed choices. Evidence suggests that consumer confidence and purchasing choice can also be a powerful incentive to drive up the standards of businesses.
Advice and education that can be applied will often secure sustained compliance as well as delivering a more cost-effective enforcement regime. Voluntary compliance is likely to be more sustainable in the long term than formal enforcement action as outlined in the following illustration:
6.4 Summary of risk rating
Actions taken by official staff will be driven by findings from audits, unannounced inspections and other official control activities. The FSA will use results from inspections and audit of FBOs to support informed tactical actions. Using these, we will
- escalate where necessary quickly the enforcement activity for high risk and/or persistent non‐compliances; and
- Identify and prioritise criteria to assess risk‐based planning and delivery of official controls.
Educational approaches should be considered at low and medium risk establishments and FSA training materials are available.
The table below presents a summary of tactical information on required actions, using the audit outcomes as a guide to plant level characteristics of compliance.
| Compliance Category | FBO status | Intervention |
|---|---|---|
|
Serious Risk Urgent Improvement Necessary (2 month audit category) Successive outcomes of Improvement Necessary |
Approval will be formally reviewed if the outcome of Official Controls indicate the major or critical non-compliances are open 1 critical or >6 major non-compliances during audit or during audit period 3-6 majors during successive audits |
The FVL will conduct a preliminary examination of the results of official controls/local intelligence and identify the major and or critical non-compliances which have been evidenced and cross referenced with the Corrective Actions Report to see if they remain open. Where the critical/major non-compliances remain open The HOD will issue a letter to the FBO setting out the serious deficiencies which have been identified and providing the FBO with an opportunity to provide adequate guarantees over future production. The time provided for response to be commensurate with the major/critical non-compliances. FBO to be removed from audit cycle pending the response. FVL conducts an assessment visit once response is received/timescale for response elapses and makes recommendation whether to withdraw or suspend the approval or to allow the approval to remain if they are content the deficiencies which gave rise to the major and or critical non-compliances are largely resolved. |
|
Medium risk Improvement Necessary (3 month audit category) |
3-6 Major non compliances identified for which improvement is required by the time of the next full audit |
Monitor via unannounced inspections (UAIs) in cutting plants and routine attendance in slaughterhouses and follow up audit visits. Advice FBO on educational programmes aimed at improving compliance (FSA training package). Establishment will move into the serious risk category if they are unable to improve sufficiently by the time of the next audit. |
|
Low Risk Good / Generally Satisfactory |
Compliant or broadly compliant (no more than 2 major non-compliances which are to be resolved quickly |
Monitor via UAIs in Cutting Plants and routine attendance in slaughterhouses and follow up audit visits for any remaining major non-compliances. Advise FBO on improving compliance where conditions are deteriorating during interim audit period. |
6.5 Approvals
Approval of establishments must be kept under review by the competent authority whilst carrying out official controls (Article 148 of assimilated Regulation (EU) No 2017/625). This regulation also includes the requirement to include initiating action to withdraw or suspend the approval in certain circumstances (Article 138).
The process for a review of approval leading to a suspension or withdrawal is detailed in Chapter 1 of the MOC.
In this intervention protocol the FSA is strengthening the links between official control activities, enforcement, and review of approvals. Audit is a useful tool for risk-profiling premises. Having good quality audits / UAIs, and good quality enforcement action, will ensure that the right evidence is available to review approval, where there are concerns around non-compliance, repeated stoppages and/or deficiencies.
The diagram below shows the cycle of assurance provided by periodic audit, supplemented by unannounced inspection and where conditions deteriorate the implementation of risk-based enforcement.
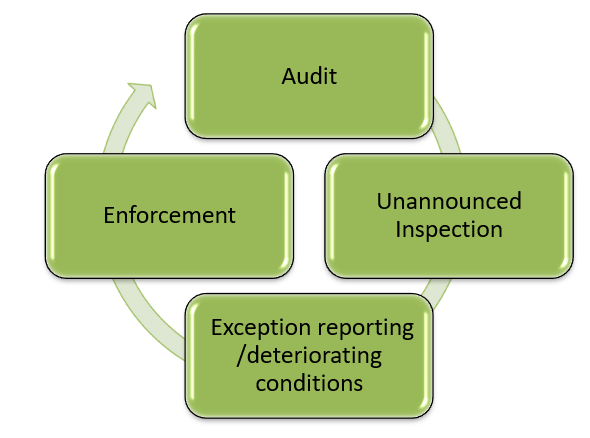
6.6 Identification for review of approval
Drawing on findings from the carrying out of official controls, or as a result of local intelligence, FVLs will have responsibility for initially assessing whether to undertake the process to initiate a review of approval in accordance with this protocol. Other matters which trigger a review of approvals are outlined in the approvals policy, for example, fire. HODs have an overarching responsibility to make sure that appropriate action is being taken.
6.7 Additional controls
Past experience has demonstrated that introducing additional controls may provide an effective incentive to the FBO and deliver improvements in compliance. The HOD should consider whether additional official controls are required (up until satisfactory compliance is achieved), taking advice from the FVL / Coordinator. For example, an additional AO may be brought in to focus upon enforcement and hygienic production and practice, allowing the resident AO to continue to carry out daily duties. It will also ensure that public health risks are safeguarded ahead of any such review.
Charges for the cost of these additional controls may be made towards the FBO.
The HOD has ultimate responsibility for determining where additional controls should be put in place. The HOD should inform the FBO in writing prior to additional controls being introduced, explaining reasons for this action and that charges for these additional controls will be passed onto the FBO. Any changes to resourcing (for example, requirement for a second OV) should be communicated to the contract Technical Manager (TM) and to the Head Office for the contract supplier in advance, in the normal way.
The Statement of Resources must also be amended by FSA Service Delivery Managers to reflect changes to resourcing.
When reviewing corrective actions taken by the FBO the following considerations must be taken into account:
- confirm what actions were taken and why, the appropriateness of the actions
- review any records that demonstrate the effectiveness of the corrective actions
- observation the changes; a follow-up inspection may be needed to confirm that the corrective action has been completed and is effective
Once appropriate action has been taken to address non-compliances, the additional resource should be removed and this made clear to the FBO, backed up by evidence from the FSA enforcement programme demonstrating improving conditions, Adjustments can then be made to the Statement of Resources.
The HOD should inform the FBO where normal resourcing is being re-established, drawing on advice from the FVL / Coordinator, with formal confirmation provided in writing. The contract supplier should be advised on any changes.
6.8 Low risk establishments
Low risk establishments will have audit outcomes of Good and Generally Satisfactory, with audit frequencies of 12 and 18 months (18 months for slaughterhouses only).
All cutting plants not co-located to a slaughterhouse will have at least one interim UAI. Field Veterinary Coordinators should monitor the results of all UAIs and ensure enforcement action and/or official control activities are escalated accordingly and as per the Intervention Protocol.
The FSA reserves the right to carry out an early audit if the results of UAI or other enforcement action indicates compliance has significantly deteriorated since the last ’Good’ or ‘Generally Satisfactory’ audit outcome.
| Compliance Category | FBO status | Intervention |
|---|---|---|
|
Low Risk Good / Generally Satisfactory (12/36 months audit frequencies) |
Compliant or broadly compliant (no more than 2 major non-compliances which are to be resolved quickly |
Monitor via UAIs and follow up audit visit for any remaining major non compliances. |
6.9 Medium risk establishments: Improvement necessary
Establishments entering into the ‘Improvement Necessary’ audit outcome will be subject to a further full audit within 3 months. In addition to this they will also receive a partial audit during that three-month period. This action is designed to drive up improvement in lower compliance premises by linking audit outcomes to follow-up action.
A letter will be sent to the FBO accompanying the audit outcomes report explaining the need for improvement and encouraging the FBO to look at their most recent audit report and/or UAI report (where applicable) and in particular the Corrective Action Report and Enforcement Programme. These should identify key areas where the FBO needs to take action or make improvements. The FBO will be advised that a failure to exit the Improvement Necessary outcome by the time of their next full audit will result in a review of approval.
Improvement Necessary establishments will be those which are exhibiting major non-compliances that are likely to compromise public health (including food safety), animal health and welfare, or which may lead to the production and handling of unsafe food if remedial action is not taken.
There is a role for the FSA as a regulator to work with FBOs to facilitate compliance. The key to a successful working relationship is communication. There is nowhere that this is more important than in relation to guiding the FBO on compliance with legal requirements.
Improvement Necessary premises have the following audit outcome profile:
| Audit outcome | Definition | Non-compliance threshold |
|---|---|---|
| Improvement necessary | Major non-compliances identified at audit and/or non-compliances during the audit period not always responded to and corrected promptly. |
3-6 majors during audit or during audit period No critical during audit period |
The FBO will be required to tactically address non-compliance concerns over the next audit period.
| Compliance Category | FBO status | Intervention |
|---|---|---|
| Medium risk Improvement Necessary |
3-6 Major non-compliances identified for which improvement is required by the time of the next full audit |
Monitor via UAIs and follow up visits. Advise FBO on educational programs aimed at improving compliance (FSA training package) Establishment will move into the serious risk category if they are unable to improve sufficiently to exit the ‘Improvement Necessary’ status by the time of the next audit |
Medium risk establishments should be identified utilising official control activities and dealt with in order of non-compliance, for example, by prioritising premises which are demonstrating significant enforcement.
6.10 Serious risk establishments: Urgent improvement necessary
In line with audit outcomes, establishments can be identified as Urgent Improvement Necessary based on the severity and quantity of non-compliances.
Urgent Improvement establishments may have a critical non-compliance where the contravention poses an imminent and serious risk to public health (including food safety), animal health and welfare and/or multiple major non-compliances (as per MOC guidance) which are likely to compromise public health (including food safety), animal health and welfare or may lead to the production and handling of unsafe or unsuitable food if no remedial action is taken.
| Audit outcome | Definition | Non-compliance threshold |
|---|---|---|
|
Urgent Improvement necessary Successive outcomes of Improvement Necessary |
Multiple major non-compliances or a critical non-compliance identified during audit visit, interim audit period or from the results of other official controls such as Unannounced Inspections. Official intervention required to ensure public health safeguards. |
1 critical or >6 major non-compliances during audit or during audit period 3-6 majors during successive audits |
Urgent Improvement Necessary interventions and procedures are of paramount importance and the FSA needs to escalate enforcement activity quickly to influence food business perceptions around risk and consequences of non-compliance.
6.11 Approach to FBOs identified as Serious Risk Establishments and support to frontline teams
Following an audit which places an establishment in Urgent Improvement Necessary or keeps them in Improvement Necessary, notification will be sent to the FBO by the Head of Delivery, this is to emphasis the seriousness of the FBOs current position following audit. This letter will inform the FBO that they will now be subject to a formal review of approval if the Agency is not content the major/critical non-compliances identified in the audit are still resolved.
As a starting point, the FBO has a responsibility to operate in compliance with the regulations and should be encouraged to look at their most recent audit report and/or UAI report (where applicable) and in particular the Corrective Action Report and Enforcement Programme. An FBO may determine other ways of achieving compliance with the law as these may be equally valid.
The FVL will now have the responsibility to conduct a formal review of approval. The Corrective Action report and Enforcement Programme will be key in identifying the outstanding major/critical non-compliances.
Where the trigger point is identified from the outcome of an audit the review will initially be a desk top exercise considering the results of official controls to identify which major/critical non-compliances appear to be unresolved.
Where the trigger point is identified by the outcome of other intelligence the FVL will either conduct a similar desk top exercise or arrange an unannounced assessment to establish if major/critical non-compliances are present.
A letter identifying the issues found during the desk top exercise or the FVL visit will be drafted by the FVL on behalf of the HOD. This letter will explain to the FBO that they now have an opportunity to provide adequate guarantees over future production and will provide the timescale for which they need to provide these guarantees. The letter will also explain the audit cycle has been paused whilst the review of approval is conducted.
The HOD will then review the letter and if in agreement that a review of approval is now appropriate will send the letter to the Approval and Registration Team to issue the letter in the HOD’s name and to update Establishment & People to show the establishment is now to be removed from the audit scheduler.
HODs should also consider and authorise any additional controls recommended by the FVL, and/or UAIs within the interim audit period.
Any interim unannounced inspection should check the FBO is making progress against any agreed timescales. Any issues or concern over action being taken should be raised with the contract TM in the first instance, as appropriate, or with the Field Veterinarian in the case of standalone cutting plants.
Following receipt of the FBOs guarantees the FVL should then visit the premises to verify the adequacy of the guarantees provided by the FBO.
The guarantee will be a clear undertaking of action by an FBO to permanently remedy the major/critical non-compliances which have made that FBO subject to this review. There must be clear evidence of the intended action, the timescale for implementation and the expected outcome.
Where the FVL is not content the deficiencies leading to the major/critical non-compliances have been sufficiently resolved or the guarantees have not yet taken full effect a recommendation to withdraw or suspend the approval respectively can be made to the Head of Operational Assurance and Excellence.
If the FVL finds evidence of major or critical non compliances which were not included in the original letter seeking guarantees, then a further letter should be issued asking the FBO to provide further assurances on these non-compliances and a further verification visit should be arranged once a response is received. The FVL cannot use evidence of major non-compliances as rationale for recommending withdrawal or suspension of approval unless the FBO has been formally notified of these non compliances and provided an opportunity to resolve them.
If there is still evidence of current deficiencies the FVL should consider if the FBO has at least decreased the level of risk to that of a Low Risk establishment. If the FVL is satisfied the risk has been reduced and has confidence any remaining non compliances are going to be resolved quickly they can recommend ending the review of approval and the audit cycle recommencing with a partial or full audit.
If no guarantees are provided within the timeframe given to the FBO approval will be withdrawn.
6.12 Dealing with adverse behaviour by the FBO
It is appreciated that, whilst many FBOs will respond positively and will want to put in place measures for improvement, others may react in a negative way. There are a wealth of resources available on Digital Workplace on avoiding confrontation or aggression in the workplace, including a code of conduct, and what to do when an incident happens.
6.13 Routine monitoring
HODs should review action taken at establishments within their areas at their Operations Management Team meetings, drawing on advice from their FVLs.
Trends of compliance are monitored at a national level at the Field Management Group meeting. This includes a review of latest audit scores and changes to establishments that are identified as Serious Risk Establishments.
6.14 Support available
FVLs / FVCs will ensure that support is in place for frontline teams and will liaise with the contract TM working at establishments identified as Serious Risk Establishments to ensure a consistent approach is taken.
The Head of Field Operations will offer guidance and support to the HOD and staff, as will legal and veterinary and technical colleagues.
The Operational Division's Approvals and Registrations Team will receive the review of approvals from the FVL and compile a submission to the Head of Operational Assurance and Excellence. This submission will provide a background to the case, referencing the FVL report and accompanying evidence.
6.15 Review
These guidelines will be kept under review yearly and will be updated as required.
7. Annexes
Updated [Note: These pages can only be accessed by FSA staff on FSA devices. Local Authorities should check in the Food Law Code of Practice and available on FSA LINK or within your local Food Liaison Group or on the Knowledge Hub to see if there are other LAs that are willing to share their template forms]
Annex 1: Examples of enforcement of the EC Hygiene Regulations
Annex 3: Enforcement Concordat
Annex 4a: Template for HIN compliance letter
Annex 4b: Template for HIN/RAN cancel letter
Annex 5: Flow diagram: The treatment of animals, meat and food unsuitable for the human food chain
Annex 6: Supporting evidence photographic report template
Annex 7: Removed
Annex 8: Removed
Annex 10: Intervention Protocol - FVL Review of Approval Report
Annex 11: Intervention Protocol – Process flowchart
Annex 13: Timestamp Camera App Guide
Annex 14: Q&A on Formal Detention
Annex 16: A proforma letter for OVs to Declare product Unfit for Human Consumption
Annex 17: EDP Form cOV to VEDM
Annex 18: Q&A on the Enforcement Prerequisites
Annex 19: Operational Instructions for Taking, Handling and Storing Photographic and Video Evidence
1. Introduction
Sections
1. Introduction
In this section
1.1 Reason for completion and importance
1.1 Reason for completion and importance
1.1.1 Reasons for use
Time codes are used by the FSA so we can:
- make payment to the employee or contractor
- correctly charge the FBO and Government customers for the work we have, and
- allow our managers to monitor resources and forecast budgets.
1.1.2 Accuracy
Using the correct activity code, rate and accurately reflecting the work done is essential for the business of the FSA.
Incorrect timesheet completion can lead to incorrect charges levied against FBOs, incorrect amounts recovered from other Government Departments and incorrect monthly payments to employees and contractors.
If anybody that is required to complete a timesheet is unsure how to complete a particular section, or how to record a particular activity, guidance must be sought before submission by contacting your line manager in the first instance.
1.2 Types of codes used
1.2.1 Site codes
Site codes are used by FSA employees and contract staff. These codes describe the type of establishment in which work duties have been carried out by the individual. Site codes also determine the type of charge that should be levied and the customer that should receive the charge.
Note: Site codes are specified by the Finance Department.
| Sift Code | Premises description |
|---|---|
| RSL | Red meat slaughterhouse |
| PSL | Poultry and rabbit slaughterhouse |
| RCP | Red meat cutting plant |
| PCP | Poultry cutting plant |
| GHE | Game handling establishment |
| OFS | On farm slaughter |
| GCP | Cutting of wild game meat |
| RCS | Co-located cold store premises specifically identified on the approval as red meat cold store |
| PCS | Co-located cold store premises specifically identified on the approval as a poultry meat cold store |
1.2.2 Rate codes
The table below shows the rate codes that are to be applied by FSA Operations Group employees only, depending on the time of day and how many hours have been worked.
Note: Rate codes are specified by the Finance Department.
| Rate code | Used for | Details |
|---|---|---|
| 00 | Normal time | Normal working hours included in monthly salary. No additional payments made. |
| 01 | Single time overtime |
Overtime worked outside normal hours paid at single time. Example: After normal/contractual hours for an employed OV. |
| Time and a half | Overtime paid at time and a half. Examples:
|
|
| 03 | Double time | Overtime paid at double time, worked outside normal working hours. Example: Sundays. |
| 04 | Double time Bank Holidays and time off in lieu |
Used where double time is paid. Example: Bank Holiday work. Basic pay plus single time is paid in the month’s salary, and the time off in lieu is taken. |
| 05 | Triple time Bank Holidays No time off in lieu |
Used where triple time is paid. Example: Bank Holiday work. Salary plus double time is paid. NO time off in lieu. |
1.2.3 Contractual overtime
Contractual overtime counts as superannuable pay; it is therefore important to differentiate between ordinary and contractual overtime. Superannuable overtime forms part of an employee’s salary for pension purposes.
| Rate Code | Overtime paid at |
|---|---|
| 06 | Single time |
| 07 | Time and a half |
| 08 | Double time |
1.2.4 Contractors
Contractors should use rate code 00 unless otherwise advised.
1.2.5 Activity codes
Activity codes are used by FSA employees and contract staff in completing their timesheets. These codes describe the type of work that has been carried out by the individual, the type of charge that should be levied and the customer that should receive the charge.
Activity codes are also used to provide information to FSA management and are a data source on which many high level decisions are based. It is essential that activity codes are used accurately. Activity codes are specified by:
- Finance department
- The SLA and Contracts Team
1.2.6 Time code charts
Charts detailing industry, Government and non-chargeable activity codes, and who should use them, are in the e- Timesheet User Guides produced by FSA Finance. The user guides are available on the FSA Operations Group Core and can be accessed when you log into the time recording system to update your e-Timesheet. You can also access through Yammer.
1.2.7 Additional codes
In addition to those codes listed others are available to cover unusual eventualities. Where additional codes are to be used for a short period of time, specific instructions will be issued as appropriate by:
- Operations and Performance Team
- Finance Department
- Operations Business Assurance
1.3 Activity code prefixes and how codes are charged
1.3.1 Activity code prefixes
Activity codes are prefixed to indicate who the work is charged to.
| Prefix | Charged to | Purpose |
|---|---|---|
| G |
Government customers, Example: Defra Note: Charges are based on the full cost of the work carried out |
Records time spent on activities for Government customers covered by SLAs. Note: G Codes are specified by SLA and Contracts Team |
| H |
The FBO Note: Charges are based on the full time cost of the work carried out |
Records time spent on activities not covered by the charges regulations (Example: activities which are not official controls). Note: H Codes are specified by Operations Data and Performance. |
| I |
The FBO Note: Charges are calculated on the full time cost of the work carried out, then discounted on a plant / site specific basis |
Records time spent on activities that are covered by the charges Reference: (Example: official controls). Note: I Codes are specified by Operations Data and Performance. |
| N |
Non-chargeable Note: Charges are not passed on but may be included in the overhead calculation |
Records time spent on FSA business or management purposes that cannot be charged on to anyone else. |
1.3.2 How to determine which code to use
| Rate code | Overtime paid at |
|---|---|
| 1 |
Choose the appropriate Establishment Site in which the work is being undertaken. Examples: RSL, PSL Reference: See site codes in section 1. |
| 2 |
Choose the appropriate Rate code to use. Examples: 00, 02. Reference: See rate code in section 1. |
| 3 |
Choose the activity code that best describes the work being undertaken. Reference: See the e-Timesheet User Guides, held on the front page of the e-Timesheet system. |
| 4 | Log in and complete all boxes on the electronic time sheet. |
1.3.3 Who to contact for code enquiries
If you are uncertain of which code to use, in the first instance contact your line manager. Line managers are able to obtain further assistance as specified in the table below if required:
| Code | Contact |
|---|---|
| G | SLA and Contracts Team |
| N | Finance |
| I, H | Operations Data and Performance |
1.3.4 Short term or project codes
Some activity codes will be activated for a limited period to carry out specific surveys or projects (for example GFMD). These codes will not always be listed in the user guides due to the limited time they may be in use. Where these codes are to be used, a specific instruction will be issued to participating establishments.
1.3.5 Seeking approval to use a locked code
Certain government activity codes are not available for general use, for example GMLS, GFSA or GDIS. In order to prevent accidental use, these types of codes are locked down by the Finance Department.
If you believe that you need to use a locked code, your line manager should contact the SLA and Contracts Team.
1.4 Auditing time recording
1.4.1 Reasons for audit
The FSA Chief Executive is the Accounting Officer and is responsible to Parliament for the proper use of public funds. The Chief Executive must assure themselves that adequate internal controls exist in order to manage, at a reasonable level, the risks to the achievement of FSA aims and objectives.
Internal controls are designed to ensure that risks such as fraud, and the inaccurate billing of customers, for example, are minimised.
1.4.2 Who undertakes the audits?
Audits on time recording are carried out by the Verification and Audit Unit (VAU), the National Audit Office (NAO) and some Government customers to ensure that work is recorded accurately and has been correctly charged to our customers.
1.4.3 What will be audited?
The VAU audit team will undertake visits to establishments to gather relevant information, including checking that:
- The Statement of Resource is appropriate
- The daybook entries and other operational documents tally with the times and codes recorded on timesheets
- Finance procedures and policy is being followed
All audit teams (VAU, NAO and Government departments) will undertake visits to FSA at York to inspect timesheet records and associated transactions.
1.4.4 What happens after audit?
The findings will provide assurance that funds are being used properly. Any corrective action will be initiated where necessary.
1. Introduction
2. Forms
Sections
1. Introduction
In this section
1.1 Background
1.1.1 Requests for forms
The forms in this chapter can be completed on screen, sent by email or, if a signature is required, printed and completed in hardcopy.
Note: Hard copies of the forms can be printed from the digital MOC download or from Digital Workplace. Should you not have access to a printer at your plant, seek help from other FSA offices via your ITL. It is not necessary to print the forms in colour.
1.1.2 Version control
It is essential that the correct version of each form is used. Check the revision date of hard copy forms against those in this chapter. These can be identified by the revision date (for example Rev 04/19) and the form number (for example ABP 7/1).
Caution: Out of date forms should not be used and any stocks should be destroyed.
1.1.3 New / amended forms
Requests for the introduction of new forms or changes to existing forms can be made by emailing MOC@food.gov.uk.
1.1.4 Official forms
Only official MOC forms should be used for reporting purposes. These must follow the MOC forms style guide and can only be issued by the MOC Team. If an unofficial form is being used locally by an FSA operational team, it must contain, as a minimum, the same information contained in an equivalent official version.
1.1.5 Secure storage and disposal of forms
In line with government policy, official forms have been protectively marked in accordance with the classification system introduced on 1 April 2014. Further guidance on the protective marking system can be found on Digital Workplace.
Completed forms must be stored in locked cabinets and securely disposed of by approved methods.
1.2 Form numbering and retention
1.2.1 Form suites
The forms provided in this chapter have been numbered in individual suites relating to the relevant chapter. The breakdown of suites is as follows:
| Abbreviation | Title |
|---|---|
| ABP | Animal By-Products |
| AID | Animal Identification |
| AMI | Ante-Mortem Inspection |
| AUD | Audit |
| CIR | Collection and Communication of Inspection Results (FCI / CCIR) |
| DH | Dairy Hygiene |
| ENF | Enforcement |
| IMP | Imports / Exports |
| PMI | Post-Mortem Inspection |
| TSE | Transmissible Spongiform Encephalopathy |
| WS | Wine Standards |
| WEL | Welfare |
| HM DEL | Health Mark Delegation |
1.2.2 Retention periods
For audit and records management purposes, copies of complete forms are to be retained in plants for the period shown on each form and then destroyed. The retention periods have been set to ensure that copies of forms in plants are kept for as long as required for legal and business purposes and are then disposed of to minimise paper storage in plants.
2. Forms
In this section
2.1 ABP
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| ABP 7/1 | 02/23 | Dispatch of SRM for Exhibition, Teaching, Scientific Research, Special Studies or Analysis | Ops | 1 year |
| ABP 7/2 | 04/14 | Transfer Permit: Sheep or Goat Carcases | With carcase | 1 year |
| ABP 7/3 | 02/23 | Dispatch of Animal by-products (Category 2/Category 3 for Exhibition, Teaching, Scientific Research, Special Studies | Ops | 1 year |
| ABP 7/8 | 06/24 | Application for authorisation of SRM | Ops Approvals | 1 year |
2.2 AID
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| AID 5/1 | 04/18 | Eligibility Record – Bovine | BCMS | 2 years |
| AID 5/4 | 04/14 | Cattle Identification Non-Compliance Report | BCMS | 6 years |
| AID 5/7 | 06/19 | Food Standards Agency Referrals to Local Authority | Local Authority | 1 year |
2.3 AMI
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| AMI 2/1 | 04/12 | Ante-Mortem Health Inspection Pen Card | N/A | Do not retain once used |
| AMI 2/2 | 12/19 | Ante-Mortem Health Inspection Suspect Animal | N/A | 1 year |
| AMI 2/3 | 12/19 | Ante-Mortem Inspection Record for Red Meat | N/A | 1 year |
| AMI 2/4 | 12/20 | Ante-Mortem Inspection Record - Avian | N/A | 1 year |
| AMI 2/5 | 10/16 | Daily Ante-Mortem Inspection Record – Red Meat (excluding Cattle, Calves and Pigs) | N/A | 1 month from completion |
| AMI 2/6 | 04/19 | Weekly Ante-Mortem Inspection Report – Red Meat (excluding Cattle, Calves and Pigs) | Ops | 1 year |
2.4 AUD
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| AUD 9/7 | 04/14 | Daily Inspection Report – Wholesale Markets | Ops | 1 year |
2.5 CIR
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| CIR 12/1 | 02/17 | Record of Rejection Conditions - Avian | N/A | 1 year |
| CIR 12/2 | 02/17 | Collection and Communication of Inspection Results (CCIR) – Pigs | N/A | N/A |
| CIR 12/3 | 02/17 | Collection and Communication of Inspection Results (CCIR) – Cattle | N/A | N/A |
2.6 DH
2.7 ENF
2.8 IMP
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| IMP 8/1 | 12/19 | Import Failures: Public Health | Ops | Do not retain at plant |
| IMP 8/2 | 08/18 | Inspection Report: Imported Beef | Ops | 1 year |
2.9 PMI
2.10 TSE
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| TSE 6/1 | 08/19 | Sheep TSE Brain Stem Sampling Weekly Summary and Deviations Report | SLA Unit | 1 year |
| TSE 6/2 | 08/23 | Rapid Testing of Brainstem Scrapie Survey: Sampling and Testing of Sheep Submitted at the Abattoir | N/A | 1 year |
| TSE 6/4 | 08/18 | Bovine Animal Requiring BSE Testing for Human Consumption | N/A | 1 year |
| TSE 6/5 | 08/18 | Bovine Requiring BSE Testing NOT for Human Consumption | Ops | 1 year |
| TSE 6/6 | 04/14 | Sheep Brain Stem Sampling - Daily Record sheet | N/A | See form |
| TSE 6/7 | 12/19 | Results and Release. Brainstem BSE Testing | Ops | 1 year |
| TSE 6/9 | 12/19 | FSA Check at Brain Stem Sampling Point | N/A | See form |
| TSE 6/10 | 12/19 | Security Seal Form | N/A | See form |
| TSE 6/11 | 12/19 | BSE Sampling Serial Number Report | See form | See form |
2.11 WSB
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| WSB 1 | 08/15 | 5X5X5 – Information Intelligence Report | N/A | 1 year |
| WSB 7 | 08/18 | Certificate of sampling | Ops | 1 year |
| WSB 7a | 08/18 | Certificate of sampling EU | Ops | 1 year |
| WS ENF 1-1 | 04/19 | Enforcement Notice | Ops | 1 year |
| WS ENF 1-2 | 04/19 | Prohibition Notice | Ops | 1 year |
| WS ENF 1-3 | 04/19 | Warning Notice | Ops | 1 year |
2.12 WEL
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| WEL 3/1 | 06/22 | Daily Welfare Assessment Report Red Meat Slaughterhouse | N/A | 1 year |
| WEL 3/2 | 06/22 | Daily Welfare Assessment Report Poultry Meat Slaughterhouse | N/A | 1 year |
| WEL 11/34 | 01/22 (E) | WATOK Enforcement Notice (England) | CSU | 6 years |
| 01/22 (W) | WATOK Enforcement Notice (Wales) | CSU | 6 years | |
| WEL 11/35 | 02/23 (E) | WATOK Completion Notice (England) | CSU | 6 years |
| 02/23 (W) | WATOK Completion Notice (Wales) | CSU | 6 years | |
| WEL 11/36 | 01/22 (E) | WATOK Refusal Notice (England) | N/A | 6 years |
| 01/22 (W) | WATOK Refusal Notice (Wales) | N/A | 6 years | |
| WEL 11/37 | 01/22 (E) | WATOK Seizure and Detention Receipt (England) | N/A | 6 years |
| 01/22 (W) | WATOK Seizure and Detention Receipt (Wales) | N/A | 6 years | |
| WEL 11/38 | 01/22 (E) | CCTV Enforcement Notice (England) | CSU | 6 years |
| 09/24 (W) | CCTV Enforcement Notice (Wales) | CSU | 6 years | |
| WEL 11/39 | 01/22 (E) | CCTV Completion Notice (England) | CSU | 6 years |
| 09/24 (W) | CCTV Completion Notice (Wales) | CSU | 6 years | |
| WEL 11/40 | 01/22 (E) | CCTV Refusal to Issue a Completion Notice (England) | CSU | 6 years |
| 09/24 (W) | CCTV Refusal to Issue a Completion Notice (Wales) | CSU | 6 years | |
| WEL 11/41 | 06/24 (E) | CCTV Seizure Receipts (England) | CSU | 6 years |
| 09/24 (W) | CCTV Seizure Receipts (Wales) | CSU | 6 years |
Applications for a Certificate of Competence.
2.13 HM DEL
| Form no. | Rev date | Form title | Submit to | Retain for |
|---|---|---|---|---|
| HM DEL | 10/20 | Delegation of Application of the Health Mark to FBO Staff Supervision and Performance Monitoring | N/A | 1 year |
1. OV Training
2. UAI Training
3. PIA Training
4. Wild Game Training
5. Annexes
Sections
1. OV Training
In this section
1.1 Introduction
1.1.1 Process and legal requirements
This guidance document details the process and the requirements to be fulfilled in order for a veterinarian to be appointed as an OV under (EU) 2017/625 and (EU) 2019/624.
1.1.2 Eligibility for appointment as an OV
To be eligible for appointment as an OV, the candidate must:
- hold a veterinary degree (see topic 1.5 on ‘OVs from other countries’ regarding equivalent qualifications from other countries)
- be a member of the Royal College of Veterinary Surgeons (RCVS)
- have successfully passed the theory examination at the end of a university OV course
- have successfully completed their practical probationary period and passed the assessment which is conducted at the end of the probationary period
1.1.3 OV role
The following summarises the main duties and responsibilities of an OV according to legal requirements. Reference: (EU) 2017/625 Article 18
- food chain information (FCI)
- ante-mortem inspection
- animal welfare
- post mortem inspection
- specified risk material and other animal by products (ABPs)
- laboratory testing
- health marking of carcases of domestic ungulates, farmed game mammals other than lagomorphs, and large wild game, as well as half carcases, quarters and cuts produced by cutting half-carcases into three wholesale cuts.
1.1.4 OV auditing tasks
Currently OVs do not carry out auditing tasks as these are performed by designated FSA VAs, however OVs will assist in collecting evidence on compliance of the systems below, which is used during audits. They must also gain auditing experience as part of their Probationary Period.
Audit of good hygiene practices and hazard analysis and HACCP based procedures:
- checks on FCI
- design and maintenance of premises
- pre-operational, operational and post-operational hygiene
- personal hygiene
- training in hygiene and in work procedures
- pest control
- water quality
- temperature controls
- controls on food entering and leaving the establishment and any accompanying documentation
- verification that FBO applies HACCP based procedures continuously and properly
- verification of compliance with microbiological criteria
- verify application of the identification mark.
1.1.5 OVs other tasks
- communication of inspection results
- decisions concerning FCI
- decisions concerning live animals
- decisions concerning animal welfare
- decisions concerning meat
- enforcement
- supervision of MHIs carrying out official controls.
1.2 The University course
1.2.1 Legal requirements
The competent authority may appoint veterinarians who have passed a test meeting the requirements contained in (EU) 2019/624 Annex II.
1.2.2 Theoretical syllabus
An OV must have an adequate knowledge of the following areas:
- National and Union legislation on human health, food safety, animal health, animal welfare and pharmaceutical substances
- principles of the common agricultural policy, market measures, export refunds and fraud detection (including the global context: World Trade Organisation sanitary and phytosanitary agreement, Codex Alimentarius, the World Organisation for Animal Health
- essentials of food processing and food technology
- principles, concepts and methods of good manufacturing practice and quality management
- pre-harvest quality management (good farming practices)
- promotion and use of food hygiene, food related safety (good hygiene practices)
- principles, concepts and methods of risk-analysis
- principles, concepts and methods of HACCP, use of HACCP throughout the food production food chain
- prevention and control of food-borne hazards to human health
- population dynamics of infection and intoxication
- diagnostic epidemiology
- monitoring and surveillance systems
- principles and diagnostic applications of modern testing methods
- information and communication technology when relevant as working tools
- data-handling and applications of biostatistics
- investigations of outbreaks of food-borne diseases in humans
- relevant aspects concerning transmissible spongiform encephalopathies TSEs
- animal welfare at the level of production, transport and slaughter
- environmental issues related to food production (including waste management)
- precautionary principle and consumer concerns
- principles of training personnel working in the production chain
- health rules as regards animal by-products and derived products
- fraud aspects.
1.2.3 Practical training subsequent to the university course
Once the candidate has passed the theoretical examination at the end of the OV university course, they must carry out practical training, for a minimum of 200 hours. During this period, the NOV works under the supervision of an existing OV.
1.2.4 Universities
The contact details for universities delivering OV courses are as follows:
Langford Continuing Education Unit
University of Bristol School of Veterinary Science
Langford House
Langford
Bristol
BS18 7DU
Tel: 0117 3941649
Website: bristol.ac.uk/vet-school/
E-mail: langford-ce@bristol.ac.uk
1.2.5 University examination
After undertaking the university training course, the veterinarian must pass a test to confirm knowledge of the subjects contained in the OV theoretical syllabus.
The Competent Authority (CA) may not require the candidate to complete the OV course, or certain parts of it, if it is satisfied that the candidate has acquired the required knowledge as part of:
- a veterinary degree
- though continuous professional development
- as a result of a post graduate qualification
- past experience, for instance candidates with sufficient experience as an OV in other Member States
In any of the circumstances detailed above, and subject to agreement from the CA, the veterinarian may be permitted to complete only part of the modules, or none of them, but they MUST sit and pass all aspects of the exam. The candidate should contact the University for further information in relation to the specific arrangements for sitting the examination.
1.3 The probationary period
1.3.1 Appointment as a Novice OV
Once the veterinarian has passed the university test and becomes a NOV, they should complete the form at annex 2 on ‘Application for OV appointment and authorisation’ and submit to CSU, along with a copy of the University certificate.
CSU York Transactions Team
Room 112
Food Standards Agency
Kings Pool, Peasholme Green
York
YO1 7PR
Telephone: 01904 232177
E-mail: CSU@food.gov.uk
CSU will provide the NOV with:
- a letter of appointment from FSA, providing information regarding the probationary period and incorporating a schedule detailing OV practical experience requirements for the different types of establishments
- FSA OV authorisation
1.3.2 Practical training
The legislation requires the OV to carry out practical training for a probationary period for at least 200 hours under the supervision of an existing OV before starting to work independently.
During their probationary period, the NOV needs to gain experience in all areas covered in the university training syllabus.
Additionally, they should develop knowledge and experience of:
- FSA working practices
- FSA IT systems and procedures (e.g. Innova)
- FSA audit of FBO food safety management systems
The NOV must not personally conduct audits but should observe and assist with the audit process to develop their auditing skills and gain an understanding of the auditing procedures.
1.3.3 Supervision
The NOV must work under supervision of an OV (Supervisory OV (SOV)) during the probationary period. The veterinary contractor employing the NOV will nominate a supervisor.
Regular contact must be maintained between SOV and NOV. Both SOV and NOV must demonstrate that they have followed up the issues discussed between them (e.g. by way of entries in the ‘Learning log and portfolio of experience’).
1.3.4 OV practical experience requirements
The NOV is responsible for ensuring that the visits and a minimum of 200 practical training hours in specific types of establishments have been met. Although the FSA takes a flexible approach with regards to the minimum number of training hours in specific establishments, it is mandatory that the hours in establishments listed on the ‘OV practical experience requirements’ (Appendix A of the letter of appointment) are met.
The NOV must keep a record of the total number of hours completed in each type of establishment as part of their ‘Learning log and portfolio experience’.
The table below contains details of the establishments, species and minimum total hours that every NOV must complete during their probationary period.
It also provides details and suggestions on how the NOV will be able to achieve satisfactory learning processes on the specific requirements for these establishments, and how they will be able to gain the necessary knowledge in auditing FSMS.
For other types of establishment and the hours that the FSA strongly recommends, refer to annex 5 on ‘OV practical experience requirements’ in this chapter.
| Establishment | How NOV to gain knowledge |
|---|---|
| Red meat slaughterhouse (covering at least cattle, sheep and pigs slaughter) | Work as slaughterhouse OV (if possible with co-located cutting plant) under supervision of the SOV |
| Red meat cutting plant | Conduct unannounced inspections (UAIs) and shadow audits in cutting plant with an appointed VA |
| White meat slaughterhouse (covering at least poultry slaughter) | Work as slaughterhouse OV (if possible with co-located cutting plant) under supervision of the SOV |
| White meat cutting plant | Conduct UAIs and shadow audits in cutting plant with an appointed VA |
| Minced meat establishment | Conduct UAIs and shadow audits in cutting plant / processing plants with an appointed VA |
| Meat preparations establishment | Conduct UAIs and shadow audits in cutting plant / processing plants with an appointed VA |
| Meat products establishment | Conduct UAIs and shadow audits in cutting plant / processing plants with an appointed VA |
| Total minimum hours | 200 |
Visits to a wild game establishment are not compulsory; however, if the NOV wishes to gain OV appointment in relation to wild game, they must complete the following activities and allocated compulsory hours (*):
| Establishment | Activity | Minimum hours |
|---|---|---|
| Approved Game Handling Establishment |
Dressing of wild game: large: deer |
14(*) |
| Approved Game Handling Establishment | small: game birds, lagomorphs | 7(*) |
| Approved Game Handling Establishment | Cutting of wild game | 7(*) |
Part of the statutory requirements regarding OV training and probationary period is that this training must include the auditing of FSMS.
In order to gain this experience, the NOV must accompany (shadow) a VA in at least 1 full FBO Audit of FSMS. This audit must cover as many activities as possible, and both slaughterhouse and cutting plant activities must be included.
The training must include the understanding of the audit process and familiarization with audit outcomes and reporting systems.
1.3.5 NOV tasks
The NOV may conduct the following tasks in approved establishments:
- inspection tasks (ante mortem and post mortem)
- other checks (welfare, ABP, SRM, etc)
The NOV may charge when carrying out the above tasks by completing the relevant timesheet and code. However, when both the SOV and NOV are in attendance, the contractor may only charge for one person.
The NOV should accompany a VA auditing FBO procedures to ensure the NOV becomes familiar with the auditing systems and procedures, including writing the audit report.
The NOV must not personally conduct audits but may assist with the audit process (e.g. by providing evidence collected by ongoing supervision of FBO’s own procedures in slaughterhouses) to develop their auditing skills and gain an understanding of the auditing procedures.
1.3.6 Learning log and portfolio of experience
The NOV must ensure that their ‘Learning log and portfolio of experience’ is completed before the assessment of competence takes place.
Note: The ‘Learning log and portfolio of experience’ is provided to the NOV by their employing Contractor practice.
1.3.7 Essay
The NOV is required to choose a relevant topic and carry out a critical evaluation in relation to public health, animal health and / or animal welfare. The essay / report must contain a minimum of 800 words and a maximum of 1000 words.
In agreement with their Contractor, the NOV must send their proposed report / essay topic title, along with a brief description (no more than 300 words) of its contents and structure to CSU, for agreement as to the relevance and suitability of the chosen topic.
The topic of the essay / report is to be approved by the Training and Development Portfolio Leader and by the relevant subject Portfolio Leader (either an FVL or an AVL) within 10 working days. If the suggested topic is not deemed to be appropriate, the FVL will give advice and guidance as to potential alternatives.
1.4 Assessment of NOVs
1.4.1 Assessment request
The NOV, in consultation with his employing contractor practice, is responsible for requesting the assessment of competence. This request should be made to CSU.
The following documents should be sent when requesting the assessment:
- completed ‘Assessment request’ form (see annex 6)
- copies of ‘Certificates of practical experience’ (see annex 5)
- a copy of the essay / report, with a copy of the notification from the Portfolio Leaders confirming that the topic is acceptable
These should be sent at least 15 working days before the date on which the NOV expects to complete their OV Assessment. Do not send the original documents with the ‘Assessment request’ form.
1.4.2 Assessment procedure
Once the ‘Assessment request’ form has been received by CSU and the practical experience requirements verified, CSU will notify the employing Contractor practice, who will allocate an assessor and arrange a date and venue for assessment.
As soon as the NOV has been offered a date for the assessment, they must submit a copy of their ‘Learning log and portfolio of experience’ and a copy of the completed essay / report to the appointed assessor, to arrive at least 5 working days before the date of the assessment.
On the assessment day, the NOV needs to present the original completed ‘Learning log and portfolio of experience’ to the assessor.
The assessor will conduct the assessment through observation, questioning, exploration of actions taken, evidence of liaison with establishment teams(s) and discussions regarding the practical experiences that the NOV has undertaken during the probationary period.
The assessor may also ask questions in relation to the contents of the completed ‘Learning log and portfolio of experience’, the essay / report and establishments other than the type at which the assessment is taking place.
The assessor will complete an ‘Assessment of practical application experience’ form, giving feedback regarding the evidence gathered during the assessment day. The outcome of the assessment will be stated; the individual will either have been successful or will require further experience. In the case of the latter, guidance will be given as to the amount and type of further experience required.
Note: All NOVs must successfully complete their practical training and assessment within 12 months of completion of the OV course.
The completed ‘Assessment of practical application experience’ must be sent by the assessor to CSU, who will notify the NOV of the outcome within 10 working days of receipt.
1.4.3 Successful NOV
After the completion and successful achievement of the assessment, the NOV will receive a letter and a copy of the ‘Assessment of Practical Application Experience’ from the CSU, confirming that they may begin working independently as an OV, including the conduct of audits.
This letter will be copied to the employing contractor.
Reference: see annex 5 on ‘Assessment of practical application experience’ and annex 8 on ‘Official Veterinarian confirmation of appointment letter’ in this chapter.
1.4.4 Unsuccessful NOV
If after the completion of the assessment, the NOV has not shown the level of competence / experience required, CSU will forward the completed ‘Assessment of Practical Application Experience’ to the NOV, within 5 working days of receipt.
The ‘Assessment of practical application experience’ will define the areas requiring further attention, and the suggested additional hours of experience required.
Arrangements to cover any skills gap(s) need to be organised between the NOV and their employing contractor.
The NOV should continue to complete the ‘Learning log and portfolio of experience’ during the additional hours required.
Note: Authorisation to work as an NOV will be withdrawn by the FSA for any NOV who does not successfully complete the practical training and assessment within 12 months of completion of the OV course.
When the NOV and SOV are satisfied that the further experience required from the initial assessment has been gained, a re-assessment should be requested following the process as defined in sub-top 1.4.1on ‘Assessment request’ as defined above.
Notwithstanding the over-riding 12 month period as mentioned above, where a NOV has reached 400 hours within their probationary period and has not successfully passed the assessment, their future as a NOV with the FSA will be considered.
1.4.5 Appeal procedure
If a NOV disagrees with the outcome of an assessment, they should put their appeal in writing, outlining their grounds for appeal, to CSU, within 10 working days of the receipt of the assessment decision. If the appeal is considered to have merit, a re-assessment will be arranged by CSU, to be carried out by a different assessor. The outcome of this re-assessment will be final.
1.5 OVs from other countries
1.5.1 RCVS membership
OVs are required to be members of the RCVS to complete their practical in plant training.
The RCVS will be able to confirm the equivalency of veterinary qualifications obtained in other member states.
1.5.2 OVs from other Member States
In accordance with Directive 89/48/EEC (Mutual Recognition of Professional Qualifications) and Section 1.2.2 of the Food Law Code of Practice (made under the Food Hygiene (E/S/W) Regulations 2006), an OV from another member state may feel it unnecessary to undertake the entire OV designation course and / or undertake the 200 hours practical NOV training, as they consider they have sufficient knowledge and experience of the requirements detailed in (EU) 2019/624, Annex II, Chapter II including domestic legislation and enforcement procedures.
1.5.3 OVs from Third Countries
Where the OV comes from a third country (outside the EU) they must confirm that their veterinary degree and OV designation are acceptable and equivalent to the similar qualifications issued in the EU. The RCVS can confirm its status.
1.5.4 Required knowledge
Regardless of when the OV qualified and depending on whether they have had a gap in their employment, the OV must be acquainted with:
- post 2006 EU hygiene legislation,
- domestic hygiene, animal health and welfare legislation
- associated EU, FSA and Defra guidance documents
- knowledge of veterinary risk assessment and enforcement
- drafting and service of formal notices
- evidence gathering techniques
1.5.5 OV University course exam
All prospective GB OVs must sit and pass all aspects of the OV course university exam and will be required to provide the following documentary evidence:
- their veterinary degree
- post graduate qualification in veterinary public health
- documentary evidence of past experience
The candidate should contact a university providing OV courses if they wish to submit themselves direct for the examination.
If the OV fails the university examination, they may be required to sit the entire OV course before reassessment.
1.5.6 Assessment of Competence
On successful completion of the University examination, OVs are required to successfully undertake an in-plant assessment of competence, irrespective of whether they have been required to undertake the minimum 200 hours practical experience.
The OV must demonstrate experience of FSA documentary procedures and certification (for example audit of FBOs, wild game, meat products, meat preparations, ready to eat etc). These documents will form part of the assessment.
Whilst experience of working as an OV in a country outside GB may be counted towards / in lieu of the 200 hour practical experience period, such hours must be certified (by letters of reference or endorsement from the CA or governing body) and documented in the portfolio of practical experience.
Letters of reference should specify relevant information such as length of time working as an OV, type of premises, etc.
The OV must follow the procedure outlined in topic 1.4 on ‘Assessment of NOVs’ by submitting their portfolio of practical OV experience and essay to FSA, in order that an in-plant assessment may be arranged.
The OV must be able to demonstrate competence in line with the ‘Day one competencies’ detailed in annex 9.
If the OV passes the FSA assessment, they may be appointed as a fully qualified OV.
If the OV fails the FSA assessment, they will be required to undertake plant based practical experience in the areas where they failed to achieve the necessary standard as outlined by the assessor, then be re assessed.
2. UAI training
In this section
2.1 Introduction
To enable MHIs to undertake enforcement action whilst conducting UAIs, the FSA requires the MHI to carry out theoretical and practical training for a probationary period as required.
During this period, the MHI will undertake at least 10 UAIs, under the supervision of the FVC.
EC law indicates Official Auxiliaries (OAs) ‘may only collect information regarding good hygienic practices and HACCP-based procedures’ in their role of assisting the OV in the specified audit and inspection tasks.
2.2 Classroom training and examination
An MHI must have adequate knowledge of the following areas when carrying out enforcement activity:
- knowledge of legislative requirements, for example, minced meat / meat preparations temperature control limits
- essentials of food processing and food technology
- principles, concepts and methods of good manufacturing practice and quality management
- promotion and use of food hygiene, food related safety (good hygiene practices)
- principles, concepts and methods of risk-analysis
- principles, concepts and methods of HACCP, use of HACCP in food production and processing
- prevention and control of food-borne hazards related to human health
- assessment of food safety management systems
- investigations of outbreaks of food-borne diseases in humans
- relevant aspects concerning TSEs and ABP
- enforcement procedures
After undertaking the classroom training course, the MHI will undertake tests to confirm knowledge of the subjects detailed above via a range of enforcement scenarios during the classwork. Once the MHI has passed the classroom enforcement scenarios they must carry out practical training and be assessed by an FVC.
Note: MHIs must pass each classroom enforcement scenario in addition to the FVC assessment before they can carry out enforcement during UAIs.
2.3 Probationary period: Practical experience
During their probationary period, the MHI should gain experience in all areas covered in the training.
Additionally, they should develop knowledge of:
- FSA working practices
- FSA audit of FBO food safety management systems
The MHI must work under supervision of a FVC during the probationary period. Regular contact must be maintained between FVC and MHI.
Both FVC and MHI must demonstrate that they have followed up the issues discussed between them (e.g. by way of entries in action plans and portfolios of evidence created by the MHI - see topic 2.4 on ‘Portfolio experience’)
The FVC is responsible for ensuring that the required 10 UAIs, under their supervision, have been conducted by the MHI. This should include a minimum of 2 accompanied inspections.
2.4 Portfolio of experience
The MHI must maintain a portfolio which demonstrates appropriate experience.
An assessment of competence will take place at the end of the probationary period and the portfolio will be one of the main components of the assessment. It should therefore be completed as fully as possible, making sure that entries are made for areas where experience has been gained.
The MHI should demonstrate through their portfolio that all of the learning areas listed below are covered, including details of what has been found, discussed, researched (for example, phone calls, liaison with other enforcement bodies, FBOs, other colleagues)
- environmental issues related to food production (including waste management)
- precautionary principle and consumer concerns
- principles of training of personnel working in the production chain
- an aptitude for multidisciplinary co-operation
These areas are incorporated in the MHI theoretical classroom training programme.
Performance evidence can include records of ‘live’ observations carried out by the MHI. These observations can be collated on reports and will be assessed after the event.
Variety is also important. The evidence should include a range of conversations or meetings with different people and about different topics. It is acceptable for the MHI to use the same evidence more than once.
The MHI may obtain witness testimonies from supervisors or colleagues to show evidence that they may not have been able to demonstrate to the assessor through observation.
There may be times when an MHI will produce several samples of evidence, but if the assessor is still not confident that they clearly show the candidate’s competence, they will ask for additional evidence to be provided
Quality of evidence is considered more important than quantity to avoid the assessors time be taken up with sifting through large volume of evidence.
| Evidence requirement | Criteria |
|---|---|
| Appropriate | Content is appropriate to the level required |
| Reliable | Is produced unaided and represents the normal standard of the MHIs work and work of the same quality can be reproduced |
| Valid | Meets the performance criteria in the syllabus, for the range of knowledge and skills |
| Current | Meets the performance criteria, for the range of knowledge and skills set out in the syllabus; an assessor would ask the MHI to provide new evidence to ensure that their skills and knowledge are still up to date |
Portfolio experience evidence requirements
| Evidence requirement | Criteria |
|---|---|
| Current | The FVC must be satisfied that evidence of prior achievement is good enough to show that the candidate is currently competent |
| Sufficient |
There are enough samples of evidence to show competence That the samples show consistency in competent performance |
| Consistent | The FVC is satisfied that the candidate will be able to perform at this standard in future |
2.5 Assessment procedure
Once the MHI has completed the required training programme, passed the enforcement scenario exercises and completed the required amount of practical visits they should request an assessment from the FVC and liaise with the ITL to allocate an appropriate time at an appropriate venue.
On the assessment day, the MHI needs to present their portfolio of experience to the assessor. The FVC will conduct the assessment through observation, questioning, exploration of actions taken, evidence of liaison with establishment teams(s) and discussions regarding the practical experiences that the MHI has undertaken during the probationary period.
The FVC may also ask questions in relation to the contents of the completed portfolio of experience. After the assessment, the FVC will complete an ‘FVC assessment of MHI’ report, giving feedback regarding the evidence gathered during the assessment day.
The outcome of the assessment will be stated; the individual will either have been successful or will require further experience. In the case of the latter, the FVC should advise as to the amount and type of further experience required.
MHIs will be notified of the outcome by the FVC at the earliest opportunity following assessment.
CSU will forward the completed ‘FVC assessment of MHI’ report to the MHI within 5 working days of receipt.
Where the MHI is unsuccessful the ‘FVC assessment of MHI’ report will define the areas requiring further attention, and the suggested additional hours of experience required. Arrangements to cover any skills gap(s) need to be organised between the MHI and the FVC.
The MHI should continue to complete their ‘Portfolio of evidence’ during the additional hours required.
Note: Authorisation to carry out enforcement work will not be granted by FSA to any MHI who does not successfully complete the practical training and FVC Assessment within three assessment attempts.
When the FVC is satisfied that the further experience required following the initial assessment has been gained, they should inform the MHI to request a re-assessment, following the process as defined in sub-topic 1.4.2 on ‘Assessment procedure’ above.
If, after their third unsuccessful assessment, an MHI disagrees with the outcome of the assessment, they may appeal in writing, outlining their grounds for appeal, to CSU, within 10 working days of the receipt of the assessment decision.
If the appeal is considered to have merit, a re-assessment will be arranged by CSU, to be carried out by a different FVC. The outcome of this re-assessment will be final.
2.6 Internal verification
An internal verifier (FVL) from within FSA will check the quality of the FVC work.
They may wish to review the MHI’s portfolio of evidence to sample evidence assessed, or may wish to speak to MHIs to check how well they feel they are being supported to achieve the authorisation to carry out UAIs.
There will also be a verification check by FVLs of MHI’s activities and overall quality of UAIs and resulting enforcement activities taken. Furthermore, verification visits will be conducted for live field-based checks twice per year.
2.7 CPD requirement
A CPD requirement of two days per year must be completed by MHIs who carry out enforcement activity. The criteria for training will be established via the Technical Development Programme and publicised appropriately.
3. PIA Training
In this section
3.4 Assessment of PIA competency
3.1 Introduction
3.1.1 Introduction
This document contains information in relation to the training and authorisation of slaughterhouse staff to perform official control duties in white meat slaughterhouses as Plant Inspection Assistants (PIAs).
The FSA is no longer responsible for training PIAs, but is the CA to authorise PIAs to form part of the independent inspection team under the supervision, direction and responsibility of the OV.
There are three different groups of slaughterhouse staff qualified to work as part of the independent inspection team:
- those trained and assessed by OVs before 2006 (pre 2006 PIAs)
- those trained and qualified under the award scheme operating between 2006 – March 2009
- those trained and qualified under the new award scheme since March 2009
Note: PIAs already authorised under previous arrangements will remain authorised, subject to ongoing satisfactory performance of official control duties.
The training course will be provided by training centres that have been approved by awarding bodies. FBOs interested in training PIAs should contact the approved awarding bodies for further information on approved training centres.
Once an individual has successfully passed the examination to become a PIA, the FSA may authorise them to work as part of the inspection team.
3.1.2 Legislation
EU 2017/625 Article 18 allows Member States to permit slaughterhouse staff to take over the activities of OAs in controlling the production of poultry and rabbits provided that they have undergone training and passed a test in accordance with the requirements set out in point 5 of Chapter III of Annex II of EU 2019/624 in so far as they apply to the tasks they are authorised to undertake.
3.2 Overview and process map
3.2.1 Use of slaughterhouse staff
The Regulations permit the use of slaughterhouse staff to carry out tasks of the OAs and form part of the CA's independent inspection team only at establishments producing poultry and rabbit meat that have a proven record of a sustained and effective food safety management system based on HACCP principles.
3.2.2 FBO role
FBOs wishing to use PIAs for the first time should notify the ITL at the earliest opportunity.
Where a PIA system is already in place and new staff are being trained to be PIAs, the FBO should advise the OV so that arrangements can be put in place for an assessment to be made once the qualification has been obtained.
It is recommended that the FBO, the PIA candidate, the slaughterhouse OV and the training provider consult each other during the process.
3.2.3 PIA Role
PIAs can work in place of OAs with regard to the specific tasks that they are authorised to perform. PIA authorisations are now species specific (broilers and hens; ducks and geese; turkeys; farmed wild game) and not plant specific. Any previous authorisation will need to be amended to reflect this change. All PIAs must be subject to regular checks by the OV to assess their ongoing competency to carry out official duties.
3.2.4 OV role
PIAs work under the supervision, direction and responsibility of the OV. They are subject to regular checks by the OV to assess their ongoing competency to carry out official duties.
3.2.5 Process map
Updated [Once the establishment has been authorised by the FSA to establish a PIA system, the following steps need to be followed:

1. The FBO will select (or recruit) the PIA candidate(s)
2. The FBO will contract an FDQ/RSPH accredited training body to access the PIA training course
3. The training will be delivered to the selected candidate(s)
4. The PIA candidate(s) will have to pass the required written examinations
5. Once passed the relevant examinations, the candidate(s) will receive the qualification from FDQ/RSPH
6. The PIA candidate(s) will contact the plant OV to request authorisation
7. The OV will conduct a competency assessment
8. If the candidate(s) pass the OV assessment, the OV will complete the competency verification form (Annex 11) and will send it to the PIA candidate(s); if they do not pass the OV assessment, the OV will provide feedback on the reasons why and will agree an action plan (once the action plan is completed the candidate will request a new authorisation to the OV)
9. Once they have received the competency verification form from the OV, PIAs will send the following documents to Corporate Support Unit csu@food.gov.uk:
- Qualification certificate
- Competency verification form (Annex 11)
- Application for PIA appointment (Annex 12)
10. Business Support will issue the authorisation
11. PIA(s) can commence work under the supervision of the OV.
Further details of the process are provided in the sections below.]
3.3 PIA Qualification
3.3.1 Level 2 award for proficiency in poultry meat inspection
The FSA worked with Improve (the Sector Skills Council covering the meat sector) as well as the British Poultry Council, awarding bodies and sector employers to develop a new qualification for slaughterhouse staff (PIAs).
The Level 2 Award for Proficiency in Poultry Meat Inspection has been designed to equip individuals with the knowledge and understanding to enable them to work as members of teams that carry out official controls in poultry slaughterhouses.
3.3.2 PIA syllabus
The Level 2 award is divided into three units:
- introduction to food safety management;
- regulations and responsibilities; and
- post mortem inspection of poultry with a choice of either:
- broilers and hens
- turkeys
- ducks and geese
- non-hunted game birds
Each unit is assessed by an examination.
Holders of the qualification will have a broad knowledge and understanding of food safety and food hygiene and understand the regulatory framework within which the meat industry operates. They will also have a good appreciation of the anatomy, physiology, pathology, production methods and inspection procedures for the specific species chosen for unit 3.
3.3.3 Provision of training
Training is provided by trainers that have been approved by either of two awarding bodies, the Royal Society for Public Health (RSPH) and the Food and Drinks Qualifications (FDQ).
Anyone seeking information about the training and qualification should contact:
Note: The FSA is no longer involved in the training of PIA candidates and is not the competent authority to approve the qualification.
3.4 Assessment of PIA competency by the OV
3.4.1 OV evaluation of candidate
Once a PIA candidate has obtained a Level 2 Award for Proficiency in Poultry Meat Inspection, the OV will carry out an initial evaluation to verify the candidate’s competence to perform their duties to a satisfactory standard as part of the inspection team. This is particularly important at establishments where the OV has no previous knowledge of the candidate.
3.4.2 Authorisation request
The FBO or PIA candidate is responsible for requesting authorisation. This request will normally be made once the candidate has obtained the PIA qualification. This request should be made to the slaughterhouse OV, allowing enough time for all the necessary arrangements to be made.
The OV must make a note of such a request in the Day book and inform the FVC.
3.4.3 OV requirements
The assessment of competency can only be carried out by OVs that:
- have successfully finalised their probationary period as a NOV) and are a fully appointed OV
- have appropriate experience (not less than 6 months) in the relevant species that the PIA candidate is to handle
The OV may at their own discretion seek assistance from OAs in conducting the assessment of PIA candidates.
3.4.4 Coding and charges
Assessment and supervision of PIA candidates is considered to be a standard requirement of the CA within the current regulatory framework.
For the day to day supervision of PIAs within normal processing hours, the INSP code must be used by all FSA personnel involved.
However, for assessment of competency prior to PIA authorisation, the HASL code must be used.
3.4.5 Assessment
The OV will conduct the assessment of the candidate through questioning and observation, including on-line post-mortem inspection for a period under the direct supervision of the OV. The OV may at their discretion seek information from poultry meat hygiene inspectors (PMHIs) for this assessment.
Candidates must demonstrate that they:
- understand the responsibilities of the FSA particularly in relation to their duties
- recognise the different conditions found at post-mortem inspection as described in all the ‘poultry condition cards’
- are able to record the above conditions in a satisfactory manner
- are able to cope with the speed of the line in which the carcases are presented
- follow good hygiene practices
- understand and are able to complete any paperwork associated with the performance of their official duties
PIAs have already demonstrated competency to obtain their qualification certificate. The OV should nevertheless be satisfied that they can carry out their duties effectively at the establishment where they will be working. The duration of the assessment will therefore depend on the particular circumstances of the establishment, including whether the OV has previous knowledge of the candidate or whether training was carried out at the plant or elsewhere.
The OV will complete a ‘Verification of Competency’ form recording the outcome of the assessment.
The completed form should be retained at the establishment filing system for 6 years.
Reference: See annex 11 on ‘Verification of Competency’.
If unsuccessful see subtopic 3.5.2 on ‘Unsuccessful PIA candidate’.
Note: There will be no PIA rebate until the PIA has been authorised by the OV to work as part of the CA independent inspection team and appears as such on the FSA central database. The OV must be under no pressure to authorise a PIA until confident that the required criteria have been fulfilled.
3.4.6 Transferring of PIAs between establishments
PIAs may move between establishments, providing these are processing the same species for which the PIA is authorised. No additional authorisation is necessary.
PIAs can carry out post-mortem inspection duties only in establishments that have been authorised to operate a PIA system. PIAs may be used to carry out these duties provided the OV is notified in advance and OVs at the receiving establishment are provided with a proof of qualification and an evidence of their fitness for purpose (e.g. report from the OV at the PIA’s base establishment).
Note: Establishments operating a PIA system require full time OV supervision and the transferring PIAs are subject to standard performance monitoring checks of the resident OV.
3.5 Appointment of successful PIA
3.5.1 Appointment procedure
The successful candidate should request official authorisation as a PIA for the relevant species and send the following information to the FSA:
- application for PIA authorisation form Annex 12
- copy of Level 2 Award certificate issued by RSPH or FDQ
- the ‘Verification of Competency’ form signed by the OV (Annex 11)
E-mail: csu@food.gov.uk
The PIA will receive an authorisation from FSA at York, with information on their duties and responsibilities.
3.5.2 Unsuccessful PIA candidate
If the OV is not satisfied that the PIA candidate has demonstrated the knowledge and skills required to become authorised, the OV will document the areas requiring further training or development in the last section of the ‘Verification of Competency’ form.
When the PIA candidate is satisfied that the further training required from the initial assessment has been undertaken, a re-assessment may be requested – see previous subtopic 3.4.2 on ‘Authorisation request’.
3.5.3 Appeal procedure
If a PIA disagrees with the outcome of an assessment, they may appeal, in writing, within 10 working days of the receipt of the assessment decision. The appeal should outline their grounds for appeal and be sent to CSU.
The CSU York Transactions Team will pass the appeal to the relevant FVC. The FVC, after considering the case and available evidence, may organise a re-assessment by a different assessor. The outcome of this re-assessment will be final.
3.6 Monitoring PIA performance
3.6.1 Ongoing review and assessment of PIA performance
Where an FBO has a PIA system in place, OVs must monitor PIA performance on an ongoing basis and identify issues where their performance affects the level of hygiene at the premises, with a special emphasis on the product being processed.
The OV must supervise the PIA’s work and carry out regular performance tests to ensure their suitable performance.
The OV must supervise the PIAs daily. In addition to the daily supervision the OV is to conduct PIA performance checks weekly and record the results of the these in the table of the form PIA PM-1. The OV should initial the relevant box in the bottom section of ‘PIA supervision and monitoring form (PIA PM-1) to record that they have supervised the PIA.
The OV can ask a PMHI to assist in monitoring PIA performance on each shift for sites where PMHIs assist with the official controls. The PMHI should record their monitoring on the PIA PM-1 form and report any deficiency to the OV. Completed PIA PM-1 forms should be kept in the establishment file for six years.
Where the OV considers the performance of the PIAs to be unsatisfactory then the PIAs shall be replaced by OAs.
At an individual level, OVs must monitor the effectiveness of post-mortem inspection carried out by PIAs, using the same measures as for assessing MHIs. Please refer to MOC Chapter 2.4 on ‘Post-mortem, health and identification marking’ for details.
3.6.2 Withdrawal of PIA authorisation
In cases where the performance of a PIA is found unsatisfactory during an establishment assessment visit or daily OV supervision and/or performance monitoring of the PIA, the FSA can suspend or revoke the PIA authorisation following the required protocol.
All records and letters regarding PIA performance and supervision/monitoring should be kept by the OV in the plant.
See below process summary:
| Step | Process summary |
| 1 | The OV informs the PIA about all observed deficiencies and areas of poor PIA performance. A clear timeframe for improvement is agreed, if necessary. All discussed deficiencies and actions are summarized in a letter to the PIA, with a copy of the letter given to the FBO. |
| 2 | The OV monitors the PIA performance and records evidence of good and poor practices (Day Book entries, PMI PM-1 form, AUD 9-2). |
| 3 | OV writes to inform the PIA about the observed improvement of the performance, closing the case. |
| 4 | Should the PIA performance fail to improve, the OV recommends the revocation of the PIA authorisation to the Operations Head Veterinarian and informs the FBO / PIA about the recommendation in writing. |
| 5 | Operations Head Veterinarian gathers required evidence and makes a decision, following consultation with the local FSA team. The Operations Head Veterinarian should inform the FBO and the PIA of his decision in writing. If the PIA authorisation is withdrawn the Operations Head Veterinarian should notify CSU. |
| 6 | The FBO / PIA returns the authorisation by recorded / registered post to PIA Authorisation Return CSU York Transactions Team |
In cases where serious concerns are raised regarding the performance of a PIA their authorisation can be immediately suspended by an OV following consultation with an FVC / FVL. In these instances, the FBO has to be informed about the reason of suspension and requested to remove the person from the PIA duty until a decision is made on further steps.
4. Wild Game Training
In this section
4.1 Introduction
4.1.1 Purpose
This document contains information on the training available for OVs and OAs that will carry out official duties at approved Game Handling Establishments (GHEs) and that gained their authorisation prior to 2006.
This training will enable them to become appointed in wild game under (EU) 2019/624. OVs appointed after 2006 should follow the NOV guidance. OAs who qualified prior to 2006 should undergo this training and be appointed by the FSA prior to carrying out official control duties in an approved GHE.
4.1.2 Prerequisites
To be eligible to become appointed for wild game the candidate should:
- hold a veterinary degree
- be a member of the RCVS
4.1.3 Working arrangements
Authorisation will only be issued after both parts of the training have been completed and the individual assessed, therefore, all wild game candidates should complete both theoretical and practical training prior to carrying out inspection duties in approved GHEs.
4.1.4 OV and OA wild game inspection role
The table below summarises the main inspection duties and responsibilities of FSA staff specific to wild game.
| Tasks | OV/OA Role |
| Inspection tasks |
|
| Other tasks |
|
4.2 The wild game course
4.2.1 Legislation
This training enables the OV / OA to have adequate knowledge of wild game topics, to maintain up to date knowledge and to keep abreast of new developments up to the training date.
4.2.2 Theoretical training
The OV / OA should have an adequate knowledge of the following areas:
- background knowledge of the wild game meat industry including processing and inspection of wild game
- small and large wild game animal identification
- hunting seasons
- post-mortem inspection of small and large wild game
- storage and transport of small and large wild game
- notifiable diseases of small and large wild game
- HACCP principles applied to wild game
- wild game traceability
- health marking of large wild game
- ID marking of small wild game
- handling of ABPs at approved GHEs
- record keeping at approved GHEs
- export of wild game
The OV theoretical training and assessment has to be delivered by a wild game appointed FVL / FVC.
The OA theoretical training can also be delivered by a wild game appointed OV (but not an NOV). However, this needs to be cleared with the Business Manager prior to the training taking place.
4.2.3 Practical training
Once the OV or OA has been given the theoretical training and been assessed and signed off by the trainer as being competent they will have to carry out practical training for a minimum time of:
- 2 hours observing the dressing in large game
- 2 hours observing the dressing in small game
- 1 hour observing the cutting of small and / or large wild game
Practical training and assessment of practical training must be carried out by a wild game appointed OV (but not an NOV) or FVL / FVC.
Ideally, practical training should occur after the candidate has undergone the theory part; however, this is not compulsory and practical training may be carried out before the theory takes place. Please discuss this with your trainer prior to commencing the training.
4.2.4 Assessment
The delegate is responsible for ensuring that the ‘Certificate of wild game experience’ is signed by the trainer.
The assessment may be done at the end of each training period.
Note: Where candidates feel they would like further training before being assessed, they should inform the trainer and agree with them subsequent training sessions and the date when the assessment will take place.
The assessor will conduct the assessment through observation, questioning, exploration of actions taken, and discussions regarding the practical experiences that the candidate has had during the training period.
The candidate should bring the ‘Certificate of wild game experience’ with them to all training sessions so that it can be signed off by the trainer. The assessor will complete the ‘Certificate of wild game experience’ giving feedback as regards the evidence gathered during the assessment day and the outcome in relation to the individual either being successful or requiring further experience.
In the case of the latter, the assessor will have to provide an estimate of the amount and type of further experience required.
When the candidate and trainer are satisfied that the further experience required from the initial assessment has been gained, a re-assessment should be requested at the end of the final training session.
It should be noted that where an individual has reached 35 hours within their training period and has not successfully passed the assessment, the assessor will make a decision with regards to their future as a wild game appointed OV/OA with the FSA.
4.2.5 Appointment
Once the OV / OA has completed the theoretical and practical training and each aspect of the training has been signed off by the trainer they should send a copy of the ‘Certificate of wild game experience’ to their HRA.
The OV / OA will receive an amended authorisation covering wild game.
4.2.6 Appeal procedure
If a candidate disagrees with the outcome of an assessment, they should put their appeal in writing outlining their grounds for appeal to CSU 10 working days of the receipt of the assessment decision.
If FSA considers the appeal to have merit, a re-assessment will be arranged to be carried out by a different assessor. The outcome of this re-assessment will be final.
5. Annexes
Please note these pages can only be accessed by FSA staff on FSA devices.
Annex 1: OV Training – Theoretical syllabus
Annex 2: OV Training – Application for OV appointment and authorisation
Annex 3: OV Training – Sample OV appointment letter
Annex 4: OV Training – Learning log and portfolio of experience
Annex 5: OV Training – Certificate of practical application experience
Annex 6: OV Training – Assessment request form
Annex 7: OV Training – Assessment of practical application experience
Annex 8: OV Training – Sample OV confirmation letter
Annex 9: OV Training – Day one competencies
Annex 10: UAI Training – FVC assessment of MHIs
Annex 11: PIA Training – Verification of Competency
Annex 12: PIA Training – Application for PIA Appointment
Annex 13: PIA Training – PIA Performance Monitoring
Annex 14: Wild Game Training – Certificate of wild game experience
Sections
1. Introduction
In this section
1.1 Overview
The FSA are responsible for the authenticity, traceability and labelling of wine sector products in the wholesale market and wine sector products produced in the UK.
1.2 Legislation
The following list is not intended to be exhaustive but details the main legislation under which authenticity, traceability and labelling checks are conducted in wine businesses.
- EU No 1308/2013 - Common organisation of agricultural markets including wine
- EU No 1306/2013 - Financing, management and monitoring of the common agricultural policy
- EU No 251/2014 - Definition and labelling of aromatized wine products
- EU No 2017/670 - Authorised production processes for aromatised wines
- EU No 33/2019 - Detailed wine labelling rules
- EU No 34/2019 - PDO/PGI applications and protections
- EU No 934 – Authorised oenological practices and restrictions
- EU No 935 – Alcohol analysis methods and additional enrichment applications
- EU No 273/2018 Accompanying documentation – VI1s. Vineyard registers
- EU No 274/2018 - Vine plantings, production declarations and iso-topic ratio analysis
- The Wine Regulations 2011 – SI and UK wine sector legislation
2. Visit guidelines
In this section
2.4 Additional requirements for specific categories of trader
2.1 Introduction
2.1.1 Visit types
Visits can be divided into four main types:
- initial registration or deregistration, physical or remote
- routine
- investigation or complaint
- additional (for example, sampling visits, consultation visits)
2.1.2 Visit purpose
The key purposes for making visits are for Inspectors to:
- check that the FBO is acting honestly and that adequate systems are in place to verify the authenticity, traceability and legally compliant labelling of wine products
- identify any new wine traders in the supply chain or any new unregistered vineyards
- gather intelligence about any new products or suspect products in circulation
- offer advice to the FBO on changes to legal requirements.
Further guidance is given later in this chapter in respect of visits to specific types of premises.
2.1.3 Visit format
The key purposes for making visits are for Inspectors to:
- check that the FBO is acting honestly and that adequate systems are in place to verify the quality authenticity and correct labelling of wine products
- identify any new wine traders in the supply chain or any new unregistered vineyards
- gather intelligence about any new products or suspect products in circulation
- offer advice to the FBO on changes to legal requirements or best practice
Further guidance is given later in this chapter in respect of visits to specific types of premises.
The format for a visit will vary depending on whether it is:
- the first visit (for example, registration visit)
- a formal investigation
- carried out under an official warrant
- requested as part of a multi-agency operation
- a routine visit and the trader or vineyard owner is known to the inspector
- requested by the FBO
- by appointment or unannounced.
In the case of visits requested by other agencies the Inspector must clarify that the agency concerned has conducted a risk assessment and that the Inspector will be safe. Ideally, written confirmation of this should be obtained prior to the visit.
2.2 Considerations
2.2.1 Announced or unannounced visits
One of the key considerations in deciding whether to visit by appointment or unannounced is whether the FBO is regarded as an honest and legitimate trader.
If it is likely that suspicious activities or suspect products may be found and prior warning will provide the FBO with an opportunity to hide such activity or remove such products, then unannounced visits may be more appropriate.
2.2.2 Announced visits
In many cases it is preferable to make routine visits by prior agreement. For example, many traders and vineyard holders work from their own homes where immediate powers of entry are not available.
In addition, with the extensive travelling involved, it makes both environmental and common sense to ensure that the person who needs to be seen is available.
Prior warning of a visit should not be given if it is seriously suspected that it would defeat the purposes of the visit.
2.2.3 Unannounced visits
On-the-spot checks shall be unannounced. However, provided that the purpose of the check is not compromised, advance notice limited to the strict minimum necessary may be given. Such notice shall not exceed 48 hours.
Unannounced visits are likely to be made to new traders or new vineyard sites where it has not been possible or it is impracticable to make contact with the FBO beforehand.
Unannounced visits should also be considered where a visit is being made following intelligence that suggests the trader might be a supplier of a suspect wine.
Unannounced visits may also be considered appropriate when making other announced visits in a geographical area where perhaps time becomes available to carry out additional visits in the same area.
2.2.4 Pre-visit planning
Prior to any inspection, the Inspector should check:
- previous visit history
- previous infringement reports
- advice history, especially in respect of any derogations that may have been given
2.2.5 Pre-visit risk assessment
Wine Inspectors will routinely visit a wide variety of premises in connection with their work. They will, therefore, be familiar with the likely risks to be encountered when visiting new traders.
The two main risks associated with carrying out inspections are:
- the nature of the premises to be visited
- the personnel involved in the business operation
The nature of the premises includes taking into account factors such as whether fork lift trucks are used.
Most new traders will be identified either:
- as a result of a visit to an established trader (for example by being advised directly, by checking orders and invoices or because an infringement has been detected) or,
- as a result of receiving a direct enquiry from a new trader.
So long as the existing trader can provide adequate information about the new trader, and provided any infringement is not of a suspicious nature, it is likely that a visit can be arranged to the new trader in the normal way.
Similarly, visits following requests for advice from new traders are not likely to pose problems.
In most cases, on arrival Inspectors will be able to assess whether the new premises to be visited appear to be similar to other categories of premises they have visited and they will be able to apply the criteria from the Health and Safety Manual.
In addition to physical hazards, Inspectors will normally be able to assess the nature and attitude of the person running the business and rely on their training and interpersonal skills to reduce the risks of any confrontational incident.
2.2.6 Suspicious traders
In view of the increasing number of incidents of alleged smuggled or counterfeit wine, it is possible that some new traders will be identified (via intelligence from other authorities and/or the National Food Crime Unit (NFCU)). It is advisable for additional precautions to be taken before visiting them.
The fact that some of these traders may have operated undetected for a number of years could in itself be regarded as suspicious. In addition, they may operate from remote or isolated premises and/or with unscrupulous staff.
Whilst it is possible that many of these “new” traders will turn out to be legitimate, from a wine standards’ point of view it is important that Inspectors consider the possible risks to their own health and safety prior to visiting. In particular, if there are suspicions as to the legitimacy of the operation, it is likely that an initial visit will need to be made unannounced.
Usually, unless Inspectors have strong reasons for doing otherwise and are confident that their Health and Safety will not be at risk, they should only conduct such visits if accompanied.
2.2.7 Points to consider
What information about the trader is readily available?
- Does the NFCU already have information on the trader?
- Check by emailing the source of any NFCU intelligence
- What information appears on Companies’ House website?
- Has the company been dissolved and re-established with a (slightly) different name on several occasions?
- Have the key staff changed repeatedly?
- Have they been associated with the dissolved companies?
- Are they up to date with accounts?
- What information is available from the Internet?
- Do they have a functioning website with contact details and product information?
- Do general searches reveal adverse information regarding the person running the business? Is there any suggestion of criminal activity or violent incidents?
- Carry out a postcode search and/or check the location of the premises
- Is it practical to carry out an initial “drive by” visit?
- Are the premises isolated and away from general view?
- Do they form part of a normal wider industrial park?
- Does the park look well run with plenty of other people coming and going?
- Is the address a private residence? (Must be an announced visit)
- Do the premises look like a drinks business?
- Is the company trading name displayed?
- Are there any sign written vehicles outside?
- Do company personnel have uniforms?
- Can you get a mobile phone signal in case you later need to raise an alarm?
- Is there somewhere safe to leave your vehicle where it will not be identified or potentially damaged?
- Are the Local Authorities (LAs) (Environmental Health, Trading Standards or HMRC) aware of the trader concerned? Would they wish to be involved in a joint inspection?
- Is it possible to find out if the person has a criminal record (particularly for incidents involving violence)?
2.2.8 Joint visits with other authorities
Do not assume that it is automatically safe to visit a new or existing trader at the request of another authority. It is important to check that the authority has conducted its own pre-visit risk assessment
Any joint visit at the request of HMRC officers will have been subjected to an HMRC risk assessment. Usually, this will involve them doing as much research as possible on the place and individuals involved.
If HMRC officers are planning a major operation they will normally request criminal records searches and try to establish if there are fire arms licences etc. They will also check their own systems to see if officers have had trouble in the past. On occasions, they will deploy body armour in criminal investigations where an arrest is likely. The Police will normally be made aware of such activity and will attend as required.
If the perceived threat is so severe that HMRC feel that body armour should be deployed then it is unlikely HMRC will ask Wine Standards to attend until after the premises have been entered and it has been established that it is safe for Wine Standards to enter.
Our own pre-visit risk assessment prior to agreeing to attend a joint visit should be to ask for email confirmation that:
- HMRC has conducted its own risk assessment
- that you will not be asked to enter until you have been notified that is safe for you to do so
Ideally, more than one Wine Inspector should attend to corroborate any evidence needed for any subsequent action by the FSA and to assist in collecting samples.
On the day in question Wine Inspectors should introduce themselves to the responsible HMRC Lead Officer and confirm the above points verbally. Wine Inspectors should only enter the premises if satisfied that it is safe to do so and they should leave immediately if the situation changes and personal safety is compromised.
2.2.9 General precautions
- Take a balanced view based on all the information available and only visit if it is safe to do so
- Try to visit with a colleague
- If carrying out joint visits discuss the potential risks in advance with all other parties
- Advise Wine Team Leader in advance of the intended visit
- If going by car, park in a safe location and ensure a safe exit is available
- Ensure you have your identity card and warrant readily available
- Ensure your mobile phone is fully charged in case you need it to call assistance
- Wear appropriate clothing – for example, safety shoes may be good idea even if they would not otherwise be worn to an office situation
- Advise Wine Team Leader after the visit has been completed.
2.3 During the visit
2.3.1 New businesses
In respect of new registrations, unannounced visits or formal investigations, Inspectors must:
- show their official identification
- explain the FSA remit and the general purpose of the visit
- explain the immediate implications for the trader or vineyard owner
- enquire if there are any specific health and safety requirements and/or concerns related to the site.
2.3.2 Desktop registration or deregistration
Open source research based on third party information or direct correspondence from an unregistered business in order to capture information about the business model and advice given for infringements before a physical visit can be carried out.
2.3.3 Existing businesses
In general, routine visits or those requested by a trader or vineyard owner do not need the same degree of formal ‘introduction’ by the Inspector. However, Inspectors should have a structure for the visit and should use the Inspection Rating Scheme and trader or vineyard questionnaires as the basis for the visit, even if they do not precisely follow each section.
2.3.4 Visit progress
It is not possible to examine every single bottle of wine and every single accompanying document. Inspectors must use their discretion and act on any current information / intelligence regarding current topics, infringements or suspect brands of wine.
2.3.5 Physical examination of stock
Checking of stock and accompanying documentation should be based on:
- stock for new traders
- current trader infringement history
- new products including products from new or rarely seen areas
- changes in legal requirements for labelling or documentation
- intelligence led concerns regarding potentially suspect wine
- stock from previous corrections
- wines on voluntary or formal movement controls
- random checks
As the inspection progresses, Inspectors must draw the FBO’s attention to any infringements that are discovered.
At the end of the visit, the Inspector should explain what action the FBO needs to take and this should be confirmed by use of the Visit Advice Document and/or by email.
For more serious infringements it may be necessary to issue formal notices in addition to or instead of the Visit Advice Document.
Formal notices should be served in accordance with section 3 on ‘Enforcement’.
2.3.6 Intended outcomes
At the end of any visit, the FBO should understand why they have been visited and the reasons for any action that they will need to take.
The Inspector should have identified:
- what activities are taking place
- the types of products being stored and/or traded
- what, if anything, has changed since the previous visit
- what key contacts exist
- what due diligence procedures are followed
- the adequacy of records
- whether previous infringements have been corrected
- what new infringements exist and which of those are serious
- what corrective action needs to be taken and whether any formal intervention is needed
- what further communication might be needed with other agencies, other Inspectors or other FSA teams
In respect of infringements likely to result in enforcement action inspectors should collate details in a way that will enable them to be correctly recorded on the database and, if necessary, referred to other Inspectors for follow up action.
Inspectors shall take photographs and obtain copies of any relevant documentation to support written notes that they make. In the case of serious infringements, all photographs should be taken and recorded in accordance with the evidence gathering procedure (chapter 7 on ‘Enforcement’, section 2). All photographs should be logged with a description of the wine and which label is photographed.
2.3.7 Follow up actions
Following the visit, the Inspector will:
- advise the FBO of any action they need to take; for minor infringements this may be verbally, supported by the use of a Visit Advice Document, and/or by email. For more serious infringements it may be necessary to issue formal notices in addition to or instead of the Visit Advice Document. Formal notices should be served in accordance with section 3 on ‘Enforcement’.
- write a visit report:
summarising who they met and their status
summarising what was discussed (see trader or vineyard discussion
documents as a guide)
explaining any significant changes to operations
noting any future proposals the FBO may have
recording any infringements found - Updated [notify other Inspectors of any infringements found that relate to their traders or vineyards or of any new traders or vineyards that need visiting in their region
- update the wine app re contact details, infringements, changes of vineyard and parcels status
- advise other agencies and/or other FSA teams]
2.3.8 Inspectors’ discretion
The above guidance should not prevent the Inspector using discretion and adopting a different style or approach should the nature of the visit change as it is being carried out; for example, if a registration visit or a routine visit reveals criminal activity or serious infringements or if the Inspector’s safety becomes compromised.
2.3.9 Trader visits
Visits should ideally take place in the presence of the FBO or another member of the management team or a designated contact. If this is not possible it is permissible for the Inspector to proceed, especially in respect of stock or record checks.
In many cases, the FBO may suggest that the Inspector inspects stock or records unaccompanied. This is acceptable provided the Inspector feels this is appropriate and that they will both be safe and receive any necessary co-operation from other staff at the premises. However, if problems are identified during the inspection, they must be drawn to the attention of the FBO (see section 3 on ‘Enforcement’).
Inspectors must:
- ascertain the range and scale of activities, such as volumes, shipping, importing and wholesaling, using annex 1 on ‘Trader questionnaire’
- discuss relevant items from annex 2 on ‘Trader discussion document’
- inspect the premises, stock arrangements, records and other documentation
- ascertain if the trader is conversant with the responsibilities for importing wine and associated due diligence
- ascertain the corrective action taken over any previous infringements that were identified
- if possible, ascertain the extent of the trader’s product knowledge
- examine labels for compliance with legislative requirements
- ascertain the product supply chain, (where the trader obtains their wine and who, in turn, they supply) in case other ‘new’ traders can be identified
- try to form a view as to the overall integrity of the trader and the likelihood for future compliance – does the trader seem open, honest and co-operative or vague / obstructive / unhelpful?
- finally, assess an overall risk rating for the trader using sub-section 1.7 on ‘Inspection rating scheme’
- Inspectors must use discretion in gathering sensitive information regarding business activities and turnover, regarding such information as confidential.
Updated [At the end of the visit, the Inspector should explain what action the FBO needs to take and this should be confirmed by email. For more serious infringements it may be necessary to issue formal notices in accordance with section 3 on ‘Enforcement’.]
2.3.10 Routine visits
A routine visit is determined by the allocated visit frequency for the purpose of:
- verifying the trader’s current situation
- to update information
- to ensure conformity with regulations
- to establish compliance with any corrective actions previously prescribed
In the main, this follows the same procedure as for a registration visit. Inspectors should cover the following:
- check the data contract information
- check for any significant changes in operation
- check that any outstanding actions or requirements have been resolved
- Updated [advise of any changes in Regulations]
- examine invoices and other accompanying documents
- check labelling of stock
- advise trader of any infringements, corrective actions and timescales required
- Updated [Infringements and follow up actions to be notified by email]
- advise if formal notices are to be issued in accordance with Section 3 – Enforcement
- check relevance of risk rating and advise trader of any likely change to visit frequency
Updated [On completion of the visit, the Inspector will record the details on the wine app
- note changes to Wine app (such as classification, trading name and/or address, visit frequency, contact information)
- record advice and/or instructions given to the trader requiring corrective action]
- inform other Inspectors, as appropriate, and maintain records of the trader's business position
A report is logged on the database. The identification of infringements concerning a trader (or notification of a new trader) not registered on the system requires copying the report to the relevant Inspector(s).
Updated [Inspectors may consider a follow up email is sufficient for the purpose of recording corrective actions. However, if the inspector feels more formal action is necessary, they should communicate this to the FBO and follow the enforcement procedure in section 3 on ‘Enforcement’.]
2.3.11 Additional visits
Additional visits may occur:
- at the discretion of the inspector concerned
- in response to information provided directly by other Inspectors or third parties such as Trading Standards
- in response to a request or an instruction from the Wine Team Leader
- as part of a routine or specific sampling exercise
- as part of a monitoring programme of products subject to prohibition notices
The format for additional visits will be determined by the reason for the visit. For example, visits for investigations or sampling should be conducted in accordance with relevant procedures for those activities.
Visits are recorded and reported in the same way as for any other visit.
2.4 Additional requirements for specific categories of trader
2.4.1 Bottling Plants
- On a first visit to a bottling plant Inspectors must do a ‘walk through / talk through’ inspection of the plant to ascertain the range and scale of activities and, in particular, covering all the points shown in Tax / bonded warehouses section.
- Ascertain the extent of the trader’s knowledge of wine legislation.
- Ascertain the responsible person(s) for various activities such as intake analysis, documentation checks, label checks and derogation requests.
- Ascertain the details of the wine traders using the bottling plant as a contract bottler and the range of wines and brands being bottled.
- Check procedures for pre-intake checks including analysis.
- Check products against shipping / importation documents.
- Establish production run checks and due diligence procedures on systems and products.
- Check procedures for change of product run to ensure no incorrect labelling of products, including misuse of historic labels.
- Use accompanying documentation to see if the operating system has a full audit trail and can readily and quickly follow through to the final label on a bottle.
- Examine accompanying documents against intake analysis results.
- Examine labels for compliance with wine legislation – these can be inspected either from ‘production files’ or inspected in the trader’s ‘sample store / library’.
- Inspect ‘production files / sample store’ for each customer and inspect label for infringements, including traceability of importer / bottler and, if codes used, details of the ‘Distributor’. Check the validity of ‘Importer / bottler codes’.
- If the company requests an importer or bottler code, follow the guidance given in 1.4.2 on ‘Issuing importer and bottler codes’.
- Ascertain what corrective actions have been taken over any previous infringements that were identified.
- As all the bottling plants are bonded facilities, follow the check list shown at the Tax / bonded warehouses topic when inspecting that part of the business.
- Try to form a view as to the overall integrity of the trader and the likelihood for future compliance.
- On repeat visits, check to see if there are any changes to operational systems, database systems or installation of new plant equipment. Inspectors must again perform a ‘walk through / talk through’ of the system to ensure full traceability of wines.
- Finally, assess an overall risk rating for the trader using sub-section 1.7on ‘Inspection rating scheme’.
2.4.2 Issuing importer and bottler codes
General
All requests for a code to be issued must be made in writing or via email indicating the type of code required, official name of the company, its trading name and its head office address.
In principle, the number to be allocated will be the trader or vineyard WSB registration number as allocated by the Wine Standards database unless this conflicts with any previously allocated number.
Procedure
On receipt of an application:
- ascertain the WSB registration number of the business from the Wine Standards database
- open the Importer / bottler code spread sheet on Wisdom (within the Traders and Vineyards subclass)
- check that the number has not previously been used under the original allocation system
- if not previously used, allocate the WSB registration number and update the spreadsheet
- update the bottler / importer field on the WSB database indicating for our purposes:
- just W followed by the number for a bottler
- WBI for a bottler and importer
- WI for just an importer
- notify the applicant in writing or via email that they may use the allocated code (omitting the sub-code) so it is just “W” followed by their allocated number
- if their WSB registration number has previously been used, create a unique four-digit reference number starting with 01 and not already in use.
- place copies of the application and the approval in the relevant trader or vineyard file on Wisdom
Note: Ensure the Excel file is correctly saved and that you have updated the database before authorising the applicant to use the code.
2.4.3 Tax / bonded warehouses
HMRC rules apply and warehouse managers are required to conform precisely to these rules.
For an Inspector’s initial visit, the format should be similar to a normal routine trader visit in respect of introductions and Inspector remit. However, in particular, the Inspector should establish:
- the key points of contact and responsibilities at the warehouse
- health and safety and/or access procedures
- documentation and recording systems including databases for stock control
- stock identification system, for example, rotation numbers and location codes (it is usually necessary to record the rotation number and location details before the documentation related to a particular product can be found)
- the number of wine traders holding stock and approximate throughput
- list of traders
- the nature of any due diligence checks carried out on behalf of clients
- other services provided, such as slip labelling
- HMRC contact
- any new traders and advise the relevant inspector of their contact details and any infringements found relating to that trader
2.4.4 Physical examination of stock
Checking of stock and accompanying documentation should be based on:
- stock for new traders
- current trader infringement history
- new products, including products from new or rarely seen areas
- changes in legal requirements for labelling or documentation
- other intelligence led concerns regarding potentially suspect wine products
- stock from previous corrections
- wines on voluntary or formal movement controls
- random checks
2.4.5 Document checks
Accompanying documentation must be checked to ensure it supports the authenticity and traceability of the product it relates to. The main documents are:
- EADS / EMCS
- VI1
- VI2
- simplified VI Certificates (Australia, USA and Chile)
- commercial documents, invoices, bill of loading and other documents
Inspectors should check that:
- the description of the product and the quantities are accurate
- the correct CN code has been used for the status of the EU product
- the analysis statement on the VI1 or VI2 matches the label of the products and are within permitted legal parameters.
2.4.6 Cash and carry premises
Cash and carry businesses are regarded as wholesale premises as usually only trade customers are permitted to purchase stock. They will also deliver to independently owned trade outlets. Inspections follow the format for normal routine trader visits unless a specific investigation or sampling exercise is being conducted.
Inspectors should check:
- for any direct imports or shipments that might alter the risk status
- the supply chain to and from other wholesalers
- the presence of unknown brands or suspect brands
- documentation; Inspectors should view with concern the absence of documentation particularly with unreasonable explanations such as ‘It’s at the accountants’ and be prepared to demand its availability and revisit; repeated excuses and the lack of documentation should be escalated via the Enforcement Procedure and in connection with HMRC and the LA
- price-lists and ‘shelf talkers’ to see if these show significant variance from general trade levels
- labelling, particularly for lesser known brands
Inspectors should adjust the risk rating to reflect the visit findings.
2.4.7 The major multiples
It is common practice for the head offices of large multiples, where control is exercised and documentation kept, to be remote from the distribution / storage depots where stock is held.
In some cases, the depots and head offices will be located in different regions. In most instances, large multiples are importers as well as retailers and some form of dual control is required.
It is policy for the Inspector in whose region the head office is located to assume the role of 'lead' Inspector and to deal direct with the company concerned. The appropriate regional Inspector carries out visits to the depots, when required, but any problems that may arise are referred to the lead Inspector. Good co-operation between Inspectors and head offices is essential.
Visits to the head offices of traders should follow the normal format:
- updating our records using the trader questionnaire
- updating the trader using the trader discussion document
- discussing previous infringements and corrective action
- identifying any proposed changes to activities
- updating contacts as staff changes frequently occur at these traders
2.4.8 Agents and brokers
Although agents and brokers are rarely the legal owners of the wine, they play an important role between wineries and wine purchasers. In the event of issues surrounding authenticity and traceability, they may be in a position to supply important additional information. They may also be the agent / broker sourcing wines for several traders. In this instance, if future corrective actions were required, it could be easier to communicate this through the agent / broker as opposed to several separate traders.
2.5 Investigations
2.5.1 Overview
Investigations may stem from:
- routine visits
- information obtained from other Inspectors or other sources
- liaison with other enforcement agencies
- requests from other countries
Inspectors must carry out a pre-visit risk assessment, particularly if the trader is unknown.
Ideally, two Inspectors should be involved in potentially serious investigations to assist in the collection and corroboration of evidence and to help ensure the safety of the officers involved.
If at any point of the visit the inspector(s) consider their safety is compromised, the visit should be aborted and the Wine Team Leader informed.
In any event, Inspectors must be aware that investigations can result in formal action being taken either against the trader concerned or against other traders in the supply chain.
In the event that action is required, inspectors should follow the enforcement procedures set out in section 3 on ‘Enforcement’.
In the event that no action is required, the inspector should consider if any information obtained may be of value to other enforcement agencies.
2.6 Vineyard visits
2.6.1 Vineyard visits
Visits to vineyards fall into the following categories:
- initial registration or deregistration (physical or remote)
- routine
- winery record checks
- investigation
2.6.2 Registration
All vineyards above 0.1ha (or smaller size if used commercially) must legally be registered with the FSA. It is an offence for a vineyard holder not to register their vines within 6 months of them being planted.
The need for an Inspector to register a vineyard on the database will stem from:
- a direct notification from the vineyard holder
- information received from other Inspectors
- information regarding the existence of the vineyard received from other parties (for example, vine suppliers or contract winemakers)
It is generally preferable for Inspectors to visit the vineyard to carry out the registration process using form ‘WSB 13’ Vineyard Registration.
Registration may be done remotely by telephone and email, especially if the Inspector will not be visiting the location of the vineyard for some time.
For newly planted vineyards there may be little to see, but a registration visit does help to explain the FSA remit and to foster a good working relationship with the vineyard holder.
As part of the registration process Inspectors must ensure that they gather details of:
- the holder’s name and contact details
- the site location
- the nature of the vineyard parcels and their intended use (PDO or PGI)
- the types of vines planted, the dates of planting and the planting density
- the current and proposed activities, and in particular:
- will grapes be sold and, if so, to whom?
- will wine be made on site?
- will wine be made on site for other growers?
- will grapes be sent to other contract winemakers and, if so, to whom?
- will wine be sold?
Inspectors must explain:
- the FSAs Wine Standards roles and responsibilities
- the need for the holder to complete annual wine production declaration if production takes place onsite
- the need to maintain accurate records
- the use of Commercial Accompanying Documents
- the background to the UK wine schemes and the role of the Wine GB
- the labelling requirements
- the need to notify changes to plantings
- the need to consult HMRC and the LA if wine is to be made on site
- if a winemaker, the need for certain notifications to be made.
Inspectors must ensure that the vineyard details are recorded on the database.
Note: The UKQWS labelling guides are available as an electronic document via the Wine GB website and should be brought to the attention of the holder.
2.6.3 Routine visits
The general objectives are broadly similar to those applying to traders and annex 4 on ‘Vineyard questionnaire’ can be used as an aide memoir:
- checking contact details
- checking changes or proposed changes to activities or status
- checking accuracy of parcels information against the information stored on the WSB database
- ascertaining the likely size and quality of the harvest – such as frost damage, budburst, flowering, mildew
- establishing who grapes will be sold to or purchased from
- establishing where wine will be made
- for contract winemakers, establishing for whom wine will be made and whether there are any ‘new’ vineyards not on the register
- establishing the types and quantities of wine that will be produced
- checking commercial accompanying document, winery records and labels
- discuss relevant items from annex 2c on ‘Vineyard discussion document’
Inspectors must ensure compliance with the production rules and they should ensure that the need to submit annual production declarations and production declarations – other uses are properly understood.
Inspectors must also explain the use of commercial accompanying documents.
2.6.4 Quality Wine Schemes - UKQWS
Updated [Protected Designation of Origin (PDO) and Protected Geographical Indication (PGI) applications are made available to Inspectors. A verification check of the winery records is an integral part of the process and any wines where the records do not adequately support the application should not be sold].
Where Inspectors find wines that do not meet the requirements, they should advise the holder as to the appropriate remedial action such as:
- re submission to a different scheme
- re-labelling
- use for other non-wine products
- destruction
Inspectors should note that the use of the winery record form is not compulsory; indeed, many winemakers are now using electronic records. Any alternative system of record keeping must include the same information as shown on the winery record form and the Inspector must be satisfied that it is fit for purpose.
Note: WSIs have an Excel spreadsheet version that can be utilised as a Winery Handout.
2.6.5 Harvest activities
Leading up to and during the annual harvest, Inspectors are expected to devote a considerable part of their time visiting larger growers and winemakers. Inspectors should:
- check likely size and quality of harvest – frost damage, budburst, flowering, mildew, pest damage and whether emergency enrichment might be requested
- check sugar levels of grapes using a refractometer (see annex 6 on ‘Refractometer guidance’) a reference chart for calculating natural potential alcohol (see sub-section 2.6.8 on ‘Oechsle Readings’) in the grapes and for calculating permitted sugar additions for enrichment is shown in on ‘Enrichment conversion table’
- establish who grapes will be sold to or purchased from
- check where wine will be made or, for contract winemakers, who wine will be made for – whether there are any ‘new’ vineyards not on the register
- check what types and quantities of wine will be produced
- check winery records, especially in respect of checking enrichment levels, de-acidification and check commercial accompanying document receipts
- remind growers and producers of the Production Declaration process
- remind growers and producers of the Commercial Accompanying Document (CAD) requirements where required.
Inspectors should cross check the database for any new vineyards or vineyards coming into production. They must register the vineyards and/or amend the parcels data or advise other Inspectors if such vineyards are in a different region.
2.6.6 Additional vineyard visits
The general concept of special visits to vineyards is similar to that outlined for traders. In particular, additional visits may be made to:
- check harvest and/or production progress
- carry out random checks of enrichment, blending, dosage
- carry out winery record verification checks in connection with UK wine schemes
- investigate discrepancies or make follow up checks on annual declarations
- discuss new proposals by the holder to make significant changes
- attend the vineyard via direct request from the FBO for a meeting.
2.6.7 Oechsle readings
This table may be used in conjunction with a suitable calibrated refractometer when carrying out enrichment operations to calculate the natural strength of the wine before enrichment and the actual alcoholic strength after enrichment. The figures in the Oechsle columns indicate the approximate amount of sugar in the must or fermenting wine in degrees Oechsle. The figure in the alcohol column indicates the approximate alcoholic strength. The table is not definitive and should be used together with the EU approved method of analysis for alcoholic strength laid down by Commission Implementing Regulation (EU) 2019/935.
| Oechsle | % Alcohol | Oechsle | % Alcohol | Oechsle | % Alcohol |
|---|---|---|---|---|---|
| 40 | 4.4 | 78 | 10.5 | 116 | 16.3 |
| 41 | 4.5 | 79 | 10.6 | 117 | 16.4 |
| 42 | 4.7 | 80 | 10.8 | 118 | 16.6 |
| 43 | 4.8 | 81 | 10.9 | 119 | 16.7 |
| 44 | 5.1 | 82 | 11.1 | 120 | 16.9 |
| 45 | 5.3 | 83 | 11.3 | 121 | 17.0 |
| 46 | 5.5 | 84 | 11.4 | 122 | 17.2 |
| 47 | 5.6 | 85 | 11.6 | 123 | 17.3 |
| 48 | 5.8 | 86 | 11.7 | 124 | 17.5 |
| 49 | 5.9 | 87 | 11.9 | 125 | 17.5 |
| 50 | 6.0 | 88 | 12.0 | 126 | 17.8 |
| 51 | 6.2 | 89 | 12.2 | 127 | 18.0 |
| 52 | 6.4 | 90 | 12.4 | 128 | 18.1 |
| 53 | 6.6 | 91 | 12.5 | 129 | 18.3 |
| 54 | 6.7 | 92 | 12.7 | 130 | 18.4 |
| 55 | 6.9 | 93 | 12.8 | 131 | 18.6 |
| 56 | 7.0 | 94 | 13.0 | 132 | 18.8 |
| 57 | 7.2 | 95 | 13.0 | 133 | 18.9 |
| 58 | 7.3 | 96 | 13.1 | 134 | 19.1 |
| 59 | 7.5 | 97 | 13.3 | 135 | 19.2 |
| 60 | 7.7 | 98 | 13.4 | 136 | 19.4 |
| 61 | 7.8 | 99 | 13.6 | 137 | 19.5 |
| 62 | 8.0 | 100 | 13.8 | 138 | 19.7 |
| 63 | 8.1 | 101 | 13.9 | 139 | 19.8 |
| 64 | 8.3 | 102 | 14.1 | 140 |
20.0 |
| 65 | 8.4 | 103 | 14.2 | 141 | 20.2 |
| 66 | 8.6 | 104 | 14.4 | 142 | 20.3 |
| 67 | 8.7 | 105 | 14.5 | 143 | 20.5 |
| 68 | 8.9 | 106 | 14.7 | 144 | 20.6 |
| 69 | 9.1 | 107 | 14.8 | 145 | 20.8 |
| 70 | 9.2 | 108 | 15.0 | 146 | 20.9 |
| 71 | 9.4 | 109 | 15.2 | 147 | 21.1 |
| 72 | 9.5 | 110 | 15.3 | 148 | 21.3 |
| 73 | 9.7 | 111 | 15.5 | 149 | 21.4 |
| 74 | 9.8 | 112 | 15.6 | 150 | 21.5 |
| 75 | 10.0 | 113 | 15.8 | ||
| 76 | 10.2 | 114 | 15.9 | ||
| 77 | 10.3 | 115 | 16.1 |
2.7 Inspection rating scheme
2.7.1 Background
The scheme was last revised in April 2020. The scheme takes account of:
- nature of business
- point in supply chain
- turnover/throughput
- history of compliance
- quality of management
A points score is allocated dependent on the type of activity and the perceived risk. In determining the final score, compliance history and management capability are factored in. The score determines the inspection frequency.
The inspection frequency may be varied at the discretion of the inspector dependent on circumstances at the time, such as allegations of fraud or risk to.
2.7.2 Scoring system
Traders
| Activity | Score |
|---|---|
|
30 |
|
20 |
|
10 |
|
5 |
|
2 |
|
0 |
Additional points
| Activity- additional points | Score |
|---|---|
|
3 |
|
3 |
Vineyards
| Activity | Score |
|---|---|
|
20 |
|
10 |
|
3 |
|
3 |
|
0 |
*Commercial vineyards who grown and sell grapes as a commercial crop would be contacted every 2 years to see if anything has changed and visit every 4 years.
**Hobby vineyards are those whose grapes are vinified onsite or by a contract winemaker for their personal consumption only. Production volume should be less than 10 hectolitres (1330x75cl bottles).
History of compliance
| Assessment | Score |
|---|---|
| Poor – general failure to meet statutory requirements; standards consistently low | 10 |
| Satisfactory – a typical business with some minor non-compliance | 3 |
| Good – a high standard of compliance | 1 |
Quality of management
| Assessment | Score |
|---|---|
| Poor – little technical knowledge or appreciation of risks and quality control; significant numbers of infringements | 10 |
| Moderate – good knowledge of regulations and control procedures | 3 |
| Good – good management systems, documented records and supplier checks; few significant complaints | 1 |
2.7.4 Inspection frequency
Traders
| Score | Visit frequency | Category |
|---|---|---|
| 30+ | 2 visits per year | 2 |
| 20+ | 1 visit per year | 1 |
| 10+ | 1 visit every 2 years | 0.5 |
| 5+ | 1 visit every 3 years | 0.33 |
| 0 | Low risk; contact every 4 years; visit if no response or change of category | 0 |
Vineyards
| Score | Visit frequency | Category |
|---|---|---|
| 20+ | 2 visits per year | 2 |
| 10+ | 1 visit per year | 1 |
| 5+ | 1 visit every 2 years | 0.5 |
| 0 | Low risk; contact every 4 years; visit if no response or change of category | 0 |
3. Sampling
In this section
3.1 Wine Sampling
During the course of routine visits, sampling of wine products may be required. The sampling could be to ascertain the legality of a wine, any potentially harmful implications for consumer health or as part of an agreed Wine Standards sampling programme. Vineyards which do not participate in the UK PDO/PGI Wine Schemes should be a particular focus for sampling.
Sampling of all wine sector products should be carried out in accordance with the instructions detailed below and also shown in annex 3 on ‘Wine sampling aide memoir’.
- At a new trader’s premises introduce yourself, show your warrant if required and explain the purpose of the visit and the reason for sampling. At an existing trader explain the purpose of sampling.
- For products in standard bottle formats, the samples must be representative of the entire lot. Ideally, the samples should not all be taken from same case. Inspectors should also have regard to possible lot number variations.
- For products in containers of more than 60 litres, the samples are representative of the contents of the container. Samples of bulk products (from containers of more than 60 litres) shall be collected in containers with a capacity of not less than 0.75 litres).
- Samples required:
- for an investigation – collect 3 samples and secure in tamperproof bags (1 left with trader / representative, 1 held by Inspector, 1 sent for analysis)
- at random based on Inspector’s suspicion of legality of wine – collect 3 samples and secure in tamperproof bags (1 left with trader / representative, 1 held by Inspector, 1 sent for analysis)
- Note: On the occasions listed above, the sampling Inspector shall record details in personal FSA evidence notebook containing the basic details of the sampling (for example, date, place, time, container / lot / rotation number of product, description, quantity of consignment) and any special observations of importance (for example, comments by the owner).
- Results will be emailed to Wine Standards Inspector who will inform the trader of results and any action required. Results to be updated in analysis folder on Wisdom by the Inspector.
- for FSA WSB agreed sampling programme – collect 2 samples and secure in tamperproof bags (1 held by Inspector, 1 sent for analysis); for example, allergen sampling programme, no movement control in place on wine or no suspicion relating to authenticity of wine
- Where samples are to be left with the trader, the Inspector should invite the trader to choose a sample for retention and make a note of the sample number retained. If the trader or their representative is not present, the Inspector shall choose the sample to be left at the premises and record the details accordingly.
- Clearly complete the exterior of the tamperproof bag sections with:
- Authority – FSA
- Identification ref no. – ‘S’ followed by WSB number / sample date / sample number / Inspector (for example, S5091/071114/01AJW); for unregistered traders replace WSB number with 9008
- Description – description of the wine
- Time and date seized / produced – time and date
- Where seized / produced – traders name; if an unregistered trader state the address
- Seized / produced by – Inspector’s name in block capitals
- Signed – Inspector’s signature
- Insert the sample into the tamperproof bag and seal, removing as much air as possible.
- Complete the ‘WSB 7 sampling certificate’. If the trader permits, make 2 photocopies and distribute as below:
- original to accompany wine to the chosen laboratory
- copy 1 to be left with trader as a receipt
- copy 2 to be retained by the Inspector
- If no photocopying facility is available, retain the original and on returning to the office scan the document and distribute as below:
- original to accompany wine to the chosen laboratory
- scanned copy to be emailed to trader as a receipt; hard copy sent by post with proof of posting if requested by trader
- scanned copy to be electronically retained by Inspector on Wisdom
- Samples should be sent for analysis on a routine basis. Inspectors should try to co-ordinate deliveries to make use of the batch price discounts or as appropriate depending upon the urgency of the required results.
- The Inspector should select which laboratory (FERA or Campden BRI) the samples should be despatched to, based upon financial and turnaround time considerations. The up to date costs and turnaround times are available on the sampling spreadsheet stored on Wisdom.
- Details of the sample, analysis requested and times of submission to FERA or Campden BRI are then updated on to the sampling spreadsheet stored on Wisdom.
- Delivery to the chosen laboratory:
- Inspectors should arrange samples to be collected by Topspeed and sent to the requested laboratory on a next day delivery service.
- Updated [Booking should be made at Topspeed - sensitive & dangerous goods courier services – stating wine collection, number of bottles, requirement to be packaged, collection address, delivery address, date of collection, a 2-hour time window for collection and contact details of the originator.]
- Contact details for Topspeed –Freephone 0800 8562464 (See annex 17).
- The Topspeed Operations office will confirm the collection and/or renegotiate a suitable collection window if needed.
- The original WSB 7 sampling certificate should be handed to the Topspeed driver with the unpackaged samples. The driver will leave written confirmation of collection which must be retained by the WSI to ensure the chain of evidence is maintained. The bottle(s) will be placed in a box and/or case to prevent them moving around the vehicle and will be delivered to Topspeed’s hub in Knutsford.
- All samples should be despatched via Topspeed from the Inspector’s home location to ensure an auditable chain of custody. On rare occasions, it may be financially beneficial to the FSA for the Inspector to personally deliver samples to either laboratory. A receipt must be obtained from the laboratory at the time of sample drop off.
- Updated [Contact the laboratory by email to inform them of the samples being despatched:
- Campden BRI – Rachel Rees:
- Rachel.Rees@campdenbri.co.uk
- winesandspirits@campdenbri.co.uk
- OR
Attention of the Wine Department
Campden BRI
Centenary Hall
Cooper's Hill Road
Redhill
Surrey
RH1 4HY
Tel switchboard: 01737 822272; direct line: 01737 824272; mobile 07775 781132
Rachel.Rees@campdenbri.co.uk
winesandspirits@campdenbri.co.uk]
4. Enforcement
In this section
4.2 Powers of an Authorised Officer
4.1 Introduction
4.1.1 General approach
The primary role of the Wine Standards team is to ensure the safety, authenticity, traceability and correct labelling of wine sector products.
The principle objective is to seek compliance by offering guidance and advice and by only taking formal enforcement action in appropriate cases where regulatory intervention is necessary in protection of the consumer.
The FSA follows the principles contained in Food Law Code of Practice (2017) and the principles of hierarchical enforcement. The Wine Regulations 2011 (as amended) provide extensive powers to Inspectors and these must be used proportionately if the general support and goodwill of the trade is to be maintained. However, it is essential that businesses acting dishonestly are dealt with in a more robust way.
Inspectors should use enforcement powers where it is appropriate to do so based upon the principles of the infringement matrices (See annexes 10 and 11). Other than in emergency situations Inspectors should consult the Wine Team Leader or FSA Legal prior to serving any formal notices.
Powers of entry, execution of warrants and/or powers to serve notices apply at all importers, bottlers, bonded warehouses, wholesale traders and vineyard premises, irrespective of whether or not the business is actually registered with the FSA. However, Inspectors must note that powers of entry without a warrant are limited in respect of private dwellings.
4.1.2 Priorities
Many infringements are ‘technical’ in nature, such as wrong heights or incorrect formats for certain information on labels, whereas others are more substantive and serious in nature.
Inspectors should primarily concentrate on the more substantive matters and should only consider more formal action in respect of minor issues if they are linked to more serious infringements relating to the same product or are as a result of repeated non-compliance by the FBO.
Serious or substantive infringements in this context include:
- something affecting consumer safety, such as a breach of winemaking rules or missing allergens information
- loss of traceability, such as incorrect bottler or importer details and/or lack of supporting documentation
- serious mis-description or incorrect classification of the wine, including misuse of protected terms or incorrect provenance statements
In considering what type of action to take Inspectors should consider the following in conjunction with annex 10 on ‘Infringement matrix still wine and annex 11 on ‘Infringement matrix sparkling wine’.
- Is the offence serious?
- Is the offence deliberate?
- Is it a repeated offence after previous warnings have been given?
- Has the FBO exercised due diligence?
- Is the FBO aware of the infringement and already trying to take corrective action?
- Has the FBO got a good track record regarding compliance and seeking advice?
- Is the FBO co-operative or obstructive?
- What is the volume of the product, the intended market and the likely sell through period?
- Inspectors must record in the visit report, the justification for wishing to take formal action and should explain this to the Wine Team Leader and/or FSA Legal when drafting formal notices. If the offense is deliberate is there any criminal intent? - if so consideration to involve the NFCU via the Wine Team Leader must be made.
Inspectors must record in the visit report, the justification for wishing to take formal action and should explain this to the Technical Inspector and/or Legal when drafting formal notices.
4.1.3 Use of official powers
Generally, the Wine Standards enforcement responsibility starts at the moment a wine sector product enters into or is intended to enter into ‘free circulation’.
In practice, this covers wine sector products stored or distributed at bonded warehouses, bottling plants, importers, shippers, wholesalers or cash and carry premises and goods at UK vineyards and wineries.
Wine Inspectors are authorised by the FSA to enforce the provisions of the UK Wine Regulations 2011, as amended. These regulations in turn specify the relevant EU provisions that can be enforced within the UK.
4.2 Powers of an Authorised Officer
4.2.1 Powers of entry
The FSA authorises Inspectors to exercise the powers listed in Regulation 8 of the UK Wine Regulations 2011 including powers to serve formal notices in their own name on behalf of the FSA.
Inspectors may enter premises, including land or a vehicle, other than those used as a domestic premises, at any reasonable time:
- for the purposes of ascertaining whether an offence has been or is being committed
- to search for evidence, to inspect any material or article, examine any register, record or document
- to seize or secure for examination samples and/or documentary evidence
4.2.2 Refusal to allow entry
Whilst Inspectors have a general power of entry and can resort to obtaining a warrant if needed (see below), they should use their professional judgment to decide whether the grounds given for refusal of entry are reasonable in the circumstances and try to negotiate entry at another time if they consider it appropriate.
If at any time the FBO or their representatives become abusive and or threatening, Inspectors must withdraw immediately and notify the Wine Team Leader.
4.3 Procedures to enter by Justice’s Warrant
4.3.1 Overview
Under Regulation 9 of the UK Wine Regulations, Inspectors may seek to obtain a Justice’s Warrant to enter premises. One or more of the following situations must exist:
- admission has been refused or refusal is expected
- giving notice of intended entry would defeat the object of entry
- entry is required urgently
- the premises are unoccupied or the occupier is temporarily absent
Inspectors must therefore ensure they have sufficient evidence to support an application for a warrant. However, as indicated above, Inspectors should not automatically seek to obtain a warrant on every case where entry is refused.
Inspectors must seek approval from the Wine Team Leader to obtain a warrant to enter premises, particularly to enter domestic premises. They must then contact FSA Legal Enforcement Advisers (Richard Withers 01904 232063) who will make the necessary arrangements for the Inspector to obtain a warrant.
If a warrant is granted, Inspectors must ensure that they are accompanied by another Inspector or such other persons as considered necessary to ensure the visit can take place safely. If the Inspector considers that a breach of the peace may occur on execution of the warrant, then the police must be notified and requested to attend before entry is affected.
The warrant must be executed within three months or such other time as allowed for by the Justice of the Peace and it may only be used once.
4.3.2 Forced entry
Although the Warrant to Enter Premises allows for the use of force to gain entry when necessary, Inspectors should never attempt a forced entry themselves.
Furthermore, even if arrangements have been made to enter premises by force, the Inspector must still try to notify the FBO of the intention to execute the warrant and to try to seek their attendance, unless so doing would negate the purpose of entry.
Inspectors must ensure that they are accompanied by another Inspector or such other persons as considered necessary to ensure the visit can take place safely.
Ideally, if the premises are on a farm or light industrial site or similar, where there are security staff and/or landlord representatives available, try to obtain access using their keys to avoid damage to property.
If no other options exist, the services of an approved locksmith and alarm engineer must be obtained to ensure that the premises can be entered with as little damage as possible and that they can be left properly secured following the visit.
The Inspector must show the warrant to all parties attending. The Inspector may be asked to sign a disclaimer for those individuals who affect entry.
In addition, the police should be invited to attend to reassure them that the forced entry is officially sanctioned and to prevent any possible breach of the peace should the FBO or his representatives return whilst entry is being made.
A copy of the warrant and any subsequent notices must be left securely in a prominent position within the premises.
Photographs should be taken at each stage of the execution of the warrant to identify any damage, the re-securing of the premises and to prove that documentation was left on site.
4.4 Types of action
4.4.1 Overview
Inspectors should use their judgement in determining what action they or the FBO will need to take following the discovery of an infringement. Inspectors must always consider the seriousness of the infringement itself and the likely level of co-operation from the FBO.
4.4.2 Future corrective action
For less serious infringements that can be corrected by changing labels or the information to be shown on future documentation, Inspectors should normally offer advice to the FBO backed up by the use of a ‘Visit advice document’ at annex 12 or follow up email.
Inspectors must consider whether the FBO will, based on previous history or their attitude at the time of the visit, be likely to follow the advice and take the corrective action requested.
If the Inspector is of the opinion that this is unlikely (for example, if previous requests have not been dealt with in a timely manner), then the Inspector may feel it is appropriate to consider the use of the hierarchy of enforcement.
4.4.3 Warning letter
An Inspector may choose to send a warning letter to an FBO if it is felt that the matter will be taken more seriously or if the Inspector feels that more formal action may follow at a later date. Such a letter must be polite and explain the nature of the problem as well as the reasons why the matter is being drawn to the FBO’s attention in writing.
The letter should indicate whether infringements are being repeated or if the same sorts of problems are being encountered with various wine products. The letter should also suggest ways in which the FBO can resolve any current problems and prevent similar problems from occurring in future.
The letter should draw the FBO’s attention to the fact that more formal action could be taken in future. The FBO should be asked to respond in writing setting out any representations they wish to make, or any assurances they may wish to make in respect of corrective action and preventative action to prevent future non-compliance.
Note: Ensure official FSA letter template is used and checked by FSA Legal if required.
4.4.4 Warning notice
Where there is documented history of an FBO failing to comply with previous advice or warning letters and/or where serious problems are found, Inspectors may choose to serve a formal Warning Notice under Regulation 10. See chapter 9 on ‘Forms’ for ‘WS ENF 1/3’.
Other than in an emergency situation, Inspectors must seek the advice of the Wine Team Leader and/or FSA Legal when drafting the notice and follow the advice regarding service of notice, including to whom any copies should be sent.
Inspectors must record all of the details in a visit report and indicate the date of service on the database. Copies of the notice and any photographs or supporting documentation must be placed within the relevant FBO file on Wisdom.
4.4.5 Enforcement notice
In cases where more serious infringements are found, or where repeated non-compliance warrants it, an Inspector may serve an Enforcement Notice under Regulation 11, requiring the FBO to take action to resolve an infringement within a specified time. See chapter 9 on ‘Forms’ for ‘WS ENF 1/1’.
Other than in an emergency situation, Inspectors must seek the advice of the Wine Team Leader and/or FSA Legal when drafting the notice and follow the advice regarding service of notice, including to whom any copies should be sent.
Inspectors must ensure that the FBO or the person in control of the wine is made aware of the appeals process detailed on the reverse of the notice.
Inspectors must record all the details in a visit report and indicate the date of service on the database. Copies of the notice and any photographs or supporting documentation must be placed within the relevant FBO file on Wisdom. Where a Regulation 12 “Prohibition Notice” has been placed on a consignment of wine that appears to fail to comply with the EU or domestic wine legislation, Inspectors should make appropriate further inquiries to authenticate its provenance (See sub-section 3.4.12 and 3.4.13 in this section on Prohibition notices for further information). Where the FBO cannot authenticate the wine, or provide satisfactory documentation for the consignment, officers should encourage the business to undertake its secure destruction at an HMRC approved facility.
Where destruction is not forthcoming, Regulation 11 of The Wine Regulations 2011, provide powers for an authorised officer to serve an “Enforcement Notice” requiring a person to take specified steps to remedy a contravention / remedy that contravention to the fullest extent possible or remedy those matters that make it likely that a contravention will arise.
Where it is impossible to remedy the lack of provenance of a consignment, Inspectors may utilise an “Enforcement Notice” to direct a person to dispose of the wine at a secure destruction facility, given that the wine cannot be released onto the market and there is no assurance of the authenticity of the product. Businesses should also be encouraged to improve their documentary procedures and address any root cause that led to the contravention if this is within their control. Inspectors should communicate any issues that led to the detention of the wine back to the authorities in the country of origin where the issue was identified to have occurred (you may need to consult with the Wine Team Leader before doing so to ascertain the appropriate contact).
When Inspectors issue an “Enforcement Notice”, they should inform the Wine Team Leader and FSA Legal.
4.4.6 Closure procedure (Warning Notice and Enforcement Notice)
On compliance with the notice the Inspector must record the details and the closure date on the database.
4.4.7 Movement controls
Any movement control of wine products can seriously damage the commercial value of the product and possibly damage the reputation of a trader that cannot fulfil orders. The removal of the wine may involve a consignment of several thousand cases and cause serious interference with the trade. Nevertheless, the lack of traceability, incorrect, misrepresented or fraudulent use of label information and/or safety concerns are potentially serious infringements that must be controlled.
Movement controls fall into two categories and both must be used with caution.
4.4.8 Temporary movement control
At certain times, Inspectors will agree with a person in charge of a batch of wine that the wine should not be moved until further investigations or certain labelling alterations have been carried out.
This approach is beneficial in bonded warehouses, in particular, to enable further time for the Inspector to make further enquiries or to carry out analysis of the wine.
The temporary movement process is considered to be a proportionate response to avoid the need for formal action against an otherwise compliant operator, for example, the bond or bottling company where goods are stored or processed for third parties.
Inspectors must bear in mind that temporary movement controls are, in effect, an informal agreement and so Inspectors must apply this approach only where they are confident that the person in charge of the consignment will honour their agreement. Where in doubt, or if there is serious cause for concern, a Prohibition Notice should be applied.
4.4.9 Procedure
- Record the relevant details of the brand, quantities, product owner.
- Take photographs, obtain copies of the relevant documents and, if necessary, take samples.
- Explain the nature of the problem to the person in charge of the wine and request that the wine is put on hold.
- Apply yellow Wine Standards Control on Movement stickers to the product to identify that it is detained.
- Confirm by using a Visit Advice Document and/or email.
- Contact the owner of the wine, as soon as practicable, to:
- explain the problem and confirm by email and/or by sending a copy of the Visit Advice Document
- ascertain the quantity of current stocks held in bond and elsewhere
- ascertain where the stock has been distributed
- ascertain if more stock is en-route and the quantities and intended arrival dates
- ascertain their intentions regarding corrective action, or if corrective action is not possible:
- their proposals for ongoing secure storage
- whether, with LA agreement, the product can be used as a non-wine sector product
- whether, subject to adequate traceability and HMRC consent, it can be exported outside the EU
- whether, with HMRC approval, it should be destroyed at the owner’s expense at an approved waste disposal facility; in this event, evidence of destruction should be obtained
- If the wine is the responsibility of an FBO in a different Inspector’s area, the Inspector responsible for the Temporary Movement Control must inform that Inspector of the situation as soon as practicable. The two Inspectors must agree a plan of action as to which of them will take ownership of the situation.
- If the brand of wine is likely to be widely distributed, all Inspectors should be advised of the problem and asked to look out for the brand when visiting other premises.
- Record details of the infringements on the database.
- If necessary, advise FSA Incidents team, HMRC and/or Trading Standards Officer.
- If a suspect wine, enter the details in the Investigations log and set up a brand folder on SharePoint.
4.4.10 Closure procedure (Temporary Movement Control)
Once an Inspector is satisfied that the matter has been resolved, they must notify the holder of the wine that it may be released and confirm this in writing or by email.
In some cases, wine may be released in batches over time as corrective action is taken. In such event, the Inspector must indicate in writing his or her agreement to the release and specify the quantities and types of wine that the approval relates to.
The Inspector must notify any other parties originally advised of the temporary movement control that the matter has been resolved.
The Inspector must complete a visit (or non-visit) report detailing the steps taken to ensure compliance and leading to the withdrawal of the control and shall record the date the TMC closed on the database.
4.4.11 Escalation procedure
In some cases, an Inspector may choose to escalate a temporary movement control to a Prohibition Notice. See Chapter 9 on ‘Forms’ for ‘WS ENF 1 / 2’.
The reasons for this might be:
- the FBO refuses to agree to a temporary movement control
- the Inspector receives information confirming that there is a serious problem with the wine
- the Inspector suspects that the owner of the wine may attempt to remove it or interfere with it before investigations are completed or before corrective action can be taken
- the person in control of the wine (the bond manager) asks for more formal action to be taken to protect their position as an intermediary
In such cases, the Inspector shall follow the procedure outline below and reflect the change of status in the visit report.
4.4.12 Prohibition notice
An Inspector is empowered to control the movement of a wine product if he has reason to believe an offence has been, is being or is likely to be committed by contravention of or a failure to comply with wine legislation.
As indicated above, this is a serious power that should only be used in appropriate situations. A consistent procedure must be followed, prior to the issue of a Prohibition Notice. See Chapter 9 on ‘Forms’ for ‘WS ENF 1 / 2’.
Other than in the case of a previously established illegal wine product, the Inspector must attempt to consult the Wine Team Leader prior to serving a Prohibition Notice. In the event of the Wine Team Leader being unavailable, the Inspector should use their professional judgement when issuing the notice and inform the Wine Team Leader at the earliest opportunity.
If practical, inspectors are strongly advised to consult with the Wine Team Leader and/or FSA Legal in respect of the wording on the Prohibition Notice to avoid potentially having to withdraw and reissue the notice.
4.4.13 Prohibition notice procedure
An Inspector must be satisfied that one (or more) of the following conditions is met before serving a Prohibition Notice:
- there is reason to believe that an offence has been committed, is being committed or is likely to be committed
- the matter is serious, as indicated earlier in this procedure document and as outlined in annex 10 on ‘Infringement matrix still wine and/or annex 11 on ‘Infringement matrix sparkling wine’
- there is a potential risk that the wine might be moved or interfered with before any investigations can be completed or any corrective action can be taken
- there is a suspicion regarding the integrity of the FBO owning the wine or on the part of others involved in the supply chain
- alternative actions are not likely to be sufficient to control the situation
The Inspector must:
- obtain the name and address of the owners of the product or those other than the warehouse keeper who have control of the product
- ascertain the nature of the infringement(s)
- obtain a full description and the quantity of the product
- where possible, obtain the details regarding the origin and movement to the current location
- ascertain whether similar stock is held elsewhere and details of any prior distribution to other wholesalers or retail outlets
- obtain / copy documentation regarding the movement of the product and product authentication
- obtain the reference number for the product, particularly the bonded warehouse rotation number
- note all of this information in their FSA evidence notebook
- obtain photographic evidence of the products subject to the Prohibition Notice, showing detention markings and the attached Prohibition Notice
- if prior approval to the service of a notice was not obtained from the Wine Team Leader, they must be notified as soon as practicable after the event
- The Prohibition Notice must state:
- a unique reference number in this format
| PN | Ref | WSB number | Date | Insp initials |
|---|---|---|---|---|
| PN | 01 | 5555 | 01/03/16 | BS |
| PN | 02 | 5555 | 01/03/16 | BS |
| PN | 03 | 5555 | 01/03/16 | BS |
-
- The first example case would have the unique reference number PN/01/5555/010316/BS
- the name of the person who appears in be in control of the product
- the address at which the wine sector product is located
- a description of the wine sector products and quantities subject to the notice
- details of the community legislation provisions the product is alleged to contravene
- The product packaging is to be clearly identified by applying red and white detention tape available from CSU and where practicable copies of the Prohibition Notices.
- where acting in lieu of another Inspector (for example, following transfer of goods), verify that the product is as described by the other Inspector and matches the description and quantities stated in the Prohibition Notice
- record and investigate any discrepancies with a view to possible legal proceedings; and also notify the Wine Team Leader and the Inspector that served the original notice
- serve the original of the Prohibition Notice on the person in immediate control of the product and/or the owner of the product, containing full details of the infringement(s) and other relevant information; a copy should also be sent via Royal Mail and a proof of postage certificate obtained
- address the notice and Prohibition Notice covering letter (see annex 13) to the Registered office and send via Royal Mail; if the notice is served after a visit, a proof of postage certificate must be obtained and kept by the Inspector along with a copy of the original notice; the Inspector must also return to the premises as soon as practical to hand deliver a copy of the notice
- fix the red / white detention tape and red Wine Standards Control on Movement stickers to the product to identify that it is detained; a copy of the Prohibition Notice must be attached as soon as practicable, and photographs of the detained stock showing the tape and attached notice must be obtained
- arrange for the warehouse keeper and owner to be made aware of the issue of the Prohibition Notice and of the appeal and complaints procedure
- ensure, as far as possible, that the warehouse keeper and the owner of the goods are informed that it is a criminal offence not to comply with the terms of the notice and that a prosecution is likely to follow if they do not comply; ideally the Inspector should try to get written confirmation of this by asking them to sign in their FSA evidence notebook or on a VAD 1 or by getting email confirmation
- follow the procedure outlined under the heading ‘Consent to Movement’ where, in certain circumstances, the FBO wishes to move stock subject to a Prohibition Notice to alternative premises
- securely retain all Prohibition Notice correspondence and other relevant documentation, particularly in circumstances where further investigations and/or formal proceedings are likely
- record all the details in a visit report and indicate the date of service on the database; copies of the notice and any photographs, supporting documentation and notes must be placed within the relevant FBO file on Wisdom
4.4.14 Monitoring
Having served a Prohibition Notice, an Inspector must instigate regular monitoring to ensure that no action is taken without the Inspector’s knowledge or approval. The stock must be physically checked in situation a minimum of once every three months for the first six months (two visits).
Additional visits after this time are at the Inspector’s discretion and must consist of physical checks and documented remote monitoring. The maximum duration between physical inspections of stock held on a Prohibition Notice must be no more than six months.
When determining the frequency of physical visits to monitor stock held on a Prohibition Notice, consideration must be given to the food safety implications of the stock held on the notice and the confidence held in the management and trader of the location at which the stock is held.
Products stored at recognised bonded warehouses where a good working relationship exists between the Inspector and the bond manager may need less monitoring than at an FBO’s own premises.
4.4.15 Consent to movement
In some circumstances, the FBO may wish to move stock subject to a Prohibition Notice to alternative premises. The reasons for this request may include the need to consolidate all affected stock in one place prior to corrective action, export or destruction.
Inspectors should not unreasonably refuse such requests but must be satisfied that the reasons for the request appear genuine and that the product will not go astray or be tampered with during the movement process.
No movement of a prohibited product may take place without written authorisation by an Inspector.
Where a request for the movement of a product from the location named in the Prohibition Notice to another location is approved a written Consent movement letter must be issued (see annex 15 on ‘Stock movement authorisation letter’).
The decision to agree to the movement is generally made at the discretion of the Inspector who served the original Prohibition Notice. However, before agreeing to such a request, the Inspector must liaise with the relevant Inspector responsible for the premises to which the wine is to be moved.
The two (or more) Inspectors involved need to agree to the issue of the Consent to Movement and to the subsequent monitoring procedures.
The original Inspector who served the Prohibition Notice will seek approval from the Wine Team Leader prior to allowing the goods to be moved.
The local Inspector responsible for the premises receiving the stock must verify the arrival of the stock at the new location. The verification process must include a check of the stock received. In particular, the Inspector must check the description, quantity and integrity of the stock.
The local Inspector is to ensure that the conditions of the Prohibition Notice are fully understood by the new keeper and/or owner of the product. Copies of the Prohibition Notice and the Consent to Movement must be supplied.
The local Inspector must instigate ongoing monitoring of the stock.
4.4.16 Refusal of a request for movement
In some circumstances an Inspector may not wish to allow the goods to be moved, particularly if there are serious concerns about whether the product will be interfered with or misappropriated during the movement process.
When a request for Consent of Movement is refused, the Inspector must provide written notice of the circumstances of the refusal, and provide guidance on appeal procedures stated on the reverse of issued notices.
4.4.17 Withdrawal or removal of a Prohibition Notice
At some point, it may be necessary to withdraw a Prohibition Notice. Reasons for this may include:
- corrective action has been taken
- the product has, with the approval of the Inspector and HMRC, been exported outside the EC or it has been destroyed
- the Inspector has received new information confirming the authenticity or legality of the product
- a decision by an assessor to uphold an appeal by the FBO
Any decision to withdraw a Prohibition Notice must be agreed by the Wine Team Leader. Provided the Wine Team Leader is satisfied that the Prohibition Notice is no longer warranted, the Inspector shall issue a Prohibition Notice withdrawal letter to the FBO and the person in control of the wine that the control is removed. See annex 14 on ‘Prohibition Notice withdrawal template’.
4.4.18 Closure procedure (Prohibition Notice)
Once an Inspector is satisfied that the matter has been resolved, they must notify the holder of the wine that it may be released and confirm this in writing or by email.
In some cases, wine may be released in batches over time as corrective action is taken. In such event, the Inspector must indicate in writing their agreement to the release and specify the quantities and types of wine that the approval relates to.
The Inspector must notify any other parties originally advised of the movement control that the matter has been resolved.
The Inspector must complete a visit (or non-visit) report detailing the steps taken to ensure compliance and leading to the withdrawal of the control and shall record on the database the date the Prohibition Notice was closed.
4.4.19 Appeals against notices
The FSA has adopted a formal process for dealing with appeals by FBOs against notices served by Wine Inspectors.
When serving notices, Inspectors must draw to the attention of the FBO, and/or the person in control of a wine product, the appeal provisions stated on the reverse of the formal notices.
Any appeal will be dealt with in accordance with the procedure. Inspectors must ensure they act appropriately and maintain adequate records to justify their actions and those of the FSA at any such appeal.
4.4.20 Prosecution
Prosecution is generally regarded as a measure of last resort. However, in some situations a prosecution should be considered at the outset of an investigation. Prosecution may also be considered in parallel with other action such as the service of a Prohibition Notice.
The following criteria may provide suitable grounds for a prosecution to be considered:
- clear evidence of a breach of winemaking rules, particularly where a risk to public safety ensues
- clear and deliberate action by an FBO to break the rules regarding PDO or PGI descriptions and/or the use of protected terms (for example, marketing counterfeit wine)
- breach of an Enforcement Notice or a Prohibition Notice
- deliberate and repeated infringements by the FBO despite previous warnings being given
Inspectors must ensure that they collect and prepare evidence in accordance with FSA MOC requirements. Ideally such evidence, if gathered during a visit, should be corroborated by other individuals such as another Inspector, a LA or HMRC officer and/or by photographic evidence.
During the evidence gathering process, Inspectors must discuss the case with the Wine Team Leader and FSA Legal.
Further investigations, including formal interviews with the FBO and/or other individuals under caution, may be undertaken by NFCU Investigators along with the Inspector.
Inspectors must follow any guidance offered by FSA Legal Enforcement Adviser and/or the NFCU Investigation team.
Ultimately, it will be for FSA legal to decide if there is sufficient evidence to warrant a prosecution.
4.4.21 Access
A warrant is only to be executed after consultation and agreement of procedure / support between FSA Wine Standards Inspector, Wine Team Leader and FSA Legal. Advise the local police of the intention to execute the warrant at a certain time and date. The establishment must be visited as soon as possible and, on production of the Warrant to Enter Premises, the occupier should grant access. If the occupier fails to grant access, he or she will be in breach of the warrant. Record the events in the FSA evidence notebook and inform the Wine Team Leader.
5. Annexes
N.B. These pages can only be accessed by FSA staff on FSA devices.
Associated Traders’ Documents
Annex 2: Trader Discussion Document
Annex 2b: Trader Discussion Topics : Inspector Answer Sheet - REMOVED
Annex 3: Wine sampling aide memoire
Associated Vineyard Documents
Annex 2c: Vineyard Discussion Document (VAD)
Annex 4: Vineyard questionnaire
Annex 5: UKVA label guidance - REMOVED
Annex 6: Refractometer guidance
Annex 7: Grape sampling request letter
Annex 8: Grape sampling questionnaire
Annex 9: Grape sampling submission document - REMOVED
Associated Enforcement Documents
Annex 10: Infringement matrix still wine
Annex 11: Infringement notice matrix sparkling wine
Annex 12: Visit Advice Document
Annex 13: Prohibition Notice covering letter
Annex 14: Prohibition Notice withdrawal template
Annex 15: Stock movement authorisation letter template
Annex 16: 5x5x5 Information intelligence report
Other
Sections
1. Introduction
In this section
1.1 Overview
This chapter sets out the procedures to be followed when escalating an incident from Field Operations to the Incidents Team.
It provides a definition of those incidents that should be escalated to the Incidents Team. It explains the difference between an incident and an issue or complaint and sets out the criteria for determining when and how you should escalate an incident to the Incidents Team for response.
There are currently multiple routes through which a complaint, issue or incident can be raised within Field Operations. Not all of these complaints, issues or incidents will require escalating to the Incidents Team; many will be handled as they are now, at the local level by Field Operations staff.
The aim of this chapter is to ensure that incidents identified as meeting the definition as described at sub-section 1.3 on Definition are escalated as early as possible to the Incidents Team so that effective and joined up response arrangements can be delivered.
1.2 Incidents Team function
The Incidents Team is responsible for 24/7/365 receipt, management and co-ordination of all food and feed safety incidents received by the FSA. The Incidents Team assures that any food / feed that is not compliant with food safety or other legislative requirements is removed from the market. The Incidents Team acts as the central hub for food and feed incidents work. It maintains the official audit trail for the investigation, co-ordinating the logging, collation and distribution of information required during the investigation. Updated [The Incidents Team arranges the issue of food alerts to local authorities, other government departments, trade organisations and other interested parties including the EC’s Rapid Alert System for Food and Feed (RASFF) notifications and mirrors FBO recalls to consumers to widen the message.]
1.3 Definition
The following definition of an incident has been taken from the FSA Incident Management Plan (IMP). The IMP defines the FSA’s response to an incident where the FSA takes responsibility, either by statutory requirement or in its role of Lead Government Department or as supporting Department, following an actual or potential threat to the safety, quality or integrity of food and / or animal feed.
The FSA defines an incident as:
“any event where, based on the information available, there are concerns about actual or suspected threats to the safety, quality or integrity of food and / or feed that could require intervention to protect consumers’ interests”.
See section 2.2.1 for escalation triggers.
1.3.1 Food hazards
Updated [A ‘food hazard’ is defined as anything present in food with the potential to harm the consumer, either by causing illness or injury; these can be a biological, chemical, and/or physical agent, or the condition of any food with the potential to cause an adverse effect on the health or safety of consumers (including outbreaks of foodborne disease and/or infectious intestinal disease). In determining whether a food hazard should be notified to the Incidents Team, Field Operations staff should consider the following criteria:
- localised food hazard – one in which food is not distributed by the FBO outside its local authority area and is not deemed to be a serious localised food hazard; should be dealt with locally by the Field Operations staff, in conjunction with other relevant agencies, if required. For example, an isolated consumer complaint of foreign body within the product.
- serious localised food hazard – one in which food is not distributed beyond the boundaries of the FBO’s local authority but which involves or may involve:
- undeclared allergens, a serious anaphylaxis reaction requiring medical intervention as a result of allergens in food, hospitalisation, or death as a result of allergens in food
- shiga toxin producing Esterichia coli, (STEC), or E. Coli O157 or other Verocytotoxin-producing Escherichia coli (VTEC)
- Clostridium botulinum
- Pathogenic Salmonella serogroups, e.g. typhimurium, Salmonella paratyphi, Salmonella enteritidis, infantis, mikawashima
- Listeria monocytogenes
- Pathogenic organisms in water systems
- Norovirus potentially present in food
- Hepatitis
- Food borne illness, (poisoning or allergic reactions) of vulnerable consumers whilst in the care of the NHS
or which the competent authority considers significant because of, for example, the vulnerability of the population likely to be affected, the numbers involved or any deaths associated with the incident. These should be notified by the Field Operations staff to the Incidents Team and other relevant agencies at the earliest opportunity and by the quickest available means and confirmed in writing using the Food / Feed Incident Report Form (Annex 1).
- non-localised food hazard – one in which food is distributed beyond the boundaries of the local authority area; should be notified by Field Operations staff to the Incidents Team and other relevant agencies at the earliest opportunity and by the quickest available means and confirmed in writing using the Food / Feed Incident Report Form (Annex 1).]
Field Operations staff should seek the advice of the FVC or Incidents Team if in doubt as to whether a food incident amounts to a food hazard.
1.3.2 Food fraud and food crime
Food crime is an umbrella term used to define the remit of the FSA National Food Crime Unit (NFCU). It is not a legal term. In this context food crime means serious dishonesty which has a detrimental impact on the safety or the authenticity of food, drink or animal feed. Food crime can be thought of as serious food fraud. Suspicions or information about food fraud or food crime should be reported to the NFCU by email or by contacting the NFCU directly.
1.3.3 Non-hazardous incidents
Non-hazardous incidents are those which may impact on the food supply chain and may include issues of quality, provenance, authenticity, composition and labelling. Significant food incidents that are not food hazards should be reported to the Incidents Team immediately. In determining significance, consideration should be given to the following factors:
- Breaches of Food Law
- Possible requirement for a co-ordinated response
- The disadvantage to consumers
- Disproportionate impact on a sector of the population
- Distribution beyond the UK
- Reputational damage to England (or the UK)
- Public concern
- Likelihood of media interest.
1.4 Lines of communication
All communication from Field Operations staff to the Incidents Team should be made through the FVC. (via normal lines of communication as summarised in the table below.)
| Advice required by | Technical advice given by |
|---|---|
| MHI | OV |
| UAI | UAI Lead / FVC |
| UAI Lead | FVC |
| cOV (contract OV) | AVM / FVC |
| eOV (employed OV) | FVC |
| FVC | FVL / Incidents Team |
| ITL | FVC |
| AM | FVC |
| OM | FVC / FVL |
Updated [In the absence of the FVC, any issues that fall within the definition of an incident (see sub-section 1.3 on Definition) or escalation triggers (see sub-section 2.2 on Escalation triggers and process) should be reported directly to the Incidents Team using the Food / Feed Incident Report Form (Annex 1).]
2. Procedures
In this section
2.1 Complaints, issues and incidents handling
2.1.1 How complaints, issues and incidents are received
Complaints, issues and incidents regarding food safety issues are received daily by the Incidents Team through a number of routes (see diagram below).
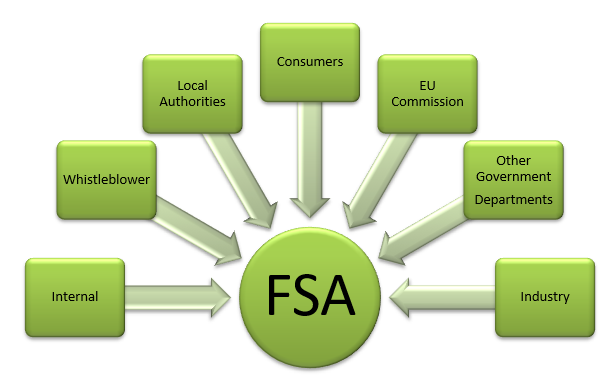
With regard to establishments approved by the FSA (under the remit of Field Operations) the majority of complaints, issues and incidents are received via Corporate Support Unit (CSU).
Whilst most of these complaints, issues and incidents can be dealt with at a local level within the routine day-to-day work of Field Operations staff, it is important to consider at an early stage whether these might highlight a wider issue that should be escalated to the Incidents Team.
Updated [The Food Law Code of Practice requires Field Operations as the Competent Authority to carry out an assessment of the food hazard and notify the FSA Incidents Team if it is assessed as serious and/or non-localised i.e. widespread.]
Early notification to the Incidents Team is crucial in responding to incidents.
For example:
’CSU received a complaint from a member of the public who had found small pieces of metal in packets of ham they had purchased from a specialist deli in their local area. The complainant purchased the ham on three separate occasions over a month and on each occasion found a tiny piece of metal was embedded within the product. Initial investigations by the Deli found that the metal contamination had not occurred on their premises.’
This example has wider implications and risk for consumers. If the product had not been contaminated at the Deli, contamination could have been caused by the way in which the ham has been produced or supplied. There is a real risk this ham has been supplied to other businesses. Therefore, the example should be escalated to the Incidents Team for further investigation.
2.1.2 Updated [Malicious Tampering or Deliberate Contamination
Malicious Tampering and Deliberate Contamination are criminal offences and occur where there is deliberate intention to contaminate and/or threaten to contaminate food or drink.
If there is suspicion of malicious tampering or deliberate contamination, the Head of Incidents should be notified immediately, and the referral confirmed in writing instead of using the incident notification form. The email should be clearly marked as ‘Official Sensitive’.
The officer that identifies or suspects this type of incident must not disclose or discuss the matter with anybody until they have discussed with the Head of Incidents, as there may be a crime in action, or covert response needed, or already underway. The Head of Incidents should advise on handling of the incident as necessary, in communication with the relevant lead authority.]
2.1.3 Responding to a food safety complaint that includes dissatisfaction about the FSA
Where a complaint also includes an expression of dissatisfaction about the FSA, the FSA’s own complaints procedure will also apply. In almost all cases, complaints against the FSA are dealt with at the local level in the first instance and advice on how to proceed can be obtained from CSU transactions. It is standard procedure that responses to complaints against the FSA include details of how the complainant can escalate their case should they be dissatisfied with the response they receive. On escalation, the FSA Complaints Co-ordinator manages the case.
2.1.4 Whistle-blowers
Where a ‘worker’ (a legal definition) contacts the FSA about matters which affect the health of any member of the public in relation to consumption of food and matters which concern the protection of consumers in England, Wales, or Northern Ireland they may be regarded as a whistle-blower and the FSA will then be acting as the ‘Prescribed Person’ within the law.
Whistle-blowing is the term used when a worker passes on information concerning wrongdoing. The wrongdoing may (although not necessarily) be something they have witnessed at work.
To be protected by whistle-blowing law, the disclosure must be a ‘qualifying disclosure’. This is any disclosure of information which, in the reasonable belief of the worker making the disclosure, is made in the public interest and tends to show that one or more of the following has occurred, is occurring or is likely to occur:
- a criminal offence
- a breach of a legal obligation
- a miscarriage of justice or
- the deliberate covering up of wrongdoing in the above categories.
It is important that whistle-blowers are managed in line with guidance that supports the relevant legislation and in these situations advice should be obtained from the NFCU by email.
2.2 Escalation triggers and process
2.2.1 When to escalate
The below can be used to assist you in determining which complaint, issue or incident should be escalated to the Incidents Team:
Hazard Factor – Harm to Individuals:
- There is a potential big impact on health - this issue can make people severely ill, cause death or lead to long term health impairment.
- There is potential for other types of consumer harm - for example, big financial loss or public distress/shock/disgust.
- in terms of Health Protection of vulnerable consumers, this is potentially a widespread issue which could affect large numbers of vulnerable customers.
Perception – Public views on detriment (public and media):
- This type of incident could lead to:
- national media reporting and/or
- large scale loss of consumer confidence
Scale – Number of products affected / number of people affected:
- Is the incident on a national, European or international scale?
- Is the issue widespread or has the potential to become widespread?
Integrity – Potential for food chain breakdown – crime/fraud:
- Are there suspected breaches in the supply chain integrity on a broad scale and/or evidence of widespread organised criminal activity?
History – Based on previous experience:
- This type of incident cannot be dealt with at a local level using field operational resources and procedures (Unannounced Meat Hygiene Inspector, Field Veterinary Coordinator, Official Veterinarian).
- Updated [There is limited previous knowledge of this issue.]
- This is an area where there is existing public concern and lack of confidence.
Other Government Department (OGD) Involvement:
- It does look like a complex issue in which OGDs are already, or are likely to be, involved in (even if FSA is not the obvious lead).
Where you identify a need to escalate you should do so in accordance with the lines of communication (see topic 1.4 on Lines of communication).
2.2.2 How to escalate a complaint, issue or incident
The FVC plays a key role in the handling procedure. CSU and other parts of the FSA pass food safety complaints, issues and incidents to the FVCs to co-ordinate a response.
The FVC will ultimately be responsible for deciding which complaints, issues or incidents should be escalated to the Incidents Team and for making that contact.
For other Field Operations staff:
- If after considering the escalation triggers (see sub-section topic 2.2.1 'When to escalate') the complaint, issue or incident meets the criteria for escalation to the Incidents Team, discuss this with the FVC as soon as possible.
- If, having reviewed the triggers above, there is still uncertainty whether the complaint, issue or incident should be escalated, contact the FVC who can provide advice.
- In the absence of the FVC, any issues that would fall within the incident’s definition (see sub-section 1.3 on Definition) or the escalation triggers (see sub-section topic 2.2.1 on When to escalate) should be reported directly to the Incidents Team.
On receipt of notification of an incident from the FVC, the Incidents Team will co-ordinate the Agency’s response.
Field Operations will have a key role to play in contributing to investigations and supporting the Incidents Team in delivering the response. The FSA Incident Management Plan outlines the Agency’s procedures for delivering its responsibilities in response to non-routine food or feed-related incidents.
Updated [The Incidents Team will seek Risk Management Advice (RMA) from the relevant Policy Team. Information required to inform the RMA includes:
- Product name;
- Batch Details;
- Durability indication (use by date, kill date etc);
- Quantity affected;
- Distribution (including international distribution);
- Food Chain Information;
- Other information.]
3. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices.
1. Introduction
2. Application Process
3. Significant Changes
4. Review of Approvals
5. Annexes
Sections
1. Introduction
1.2 Type of establishments and activities
1.3 Governance and decision making
1.1
Competent Authorities may grant approval to establishments handling, preparing or producing products of animal origin for which requirements are laid down in assimilated Regulation (EC) 853/2004.
Under the food hygiene legislation, meat plants require approval unless they benefit from specific exemptions.
1.2 Type of establishments and activities
The meat establishments listed below are subject to assimilated Regulation (EC) 852/2004 and assimilated Regulation (EC) 853/2004 and must be approved by the Agency, in order to operate, unless they meet the exemption criteria:
- Slaughterhouses
- On Farm Slaughter facilities
- Game Handling Establishments
- Cutting Plants
- Meat Wholesale Markets
These establishments are subject to official controls enforced and executed by the FSA in England and Wales.
In England and Wales where establishments are co-located with an approved Slaughterhouse, Cutting Plant or Game Handling Establishment, then the following associated meat activities are also approved by the FSA. In the case of such establishments operating in a stand-alone capacity, they are approved by the LA:
- Minced Meat Establishments
- Meat Preparations Establishments
- Mechanically Separated Meat Establishments
- Processing Plants (Meat products, Rendered animal fats & greaves, Treated stomach, bladders & intestines, Gelatine and Collagen)
- Cold Stores
- Re-wrapping / re packaging establishments
Where an approved meat establishment in England and Wales is also handling other products of animal origin (OPOAO) the Agency, in liaison and agreement with the relevant LA, may approve all operations requiring approval under Regulation 853/2004 and undertake official controls. Such approvals which remove the need for dual enforcement by the FSA and LA will be determined on a case-by-case basis.
In order to provide a recommendation for approval for the processing of other products of animal origin (such as fishery; egg; dairy; processing) the Field Veterinary Leader needs to gain agreement, from the Veterinary Audit Lead in Operations Assurance and the Head of Delivery responsible for the Approvals process, that the FSA has an appropriate level of local knowledge to be able to deliver Official Controls for the activity.
The FSA will for instance need to consider the complexity of the activity, the level of throughput and previous compliance level of the FBO. If the FSA is not content to deliver the Official Controls for this additional activity, the responsibility will default back to the LA.
Where an approved establishment undertakes cold storage of brought – in products of animal origin, the approval document should reflect the cold storage activity in addition to all other activities undertaken within the establishment.
Establishments that cut raw meat exclusively for the manufacture of meat products, minced meat, meat preparations or mechanically separated meat, require approval in respect of their manufacturing activities. They also need to comply with the requirements of Annex III of Regulation 853/2004, including those relating to cutting plants. However, because they do not place the meat they cut on the market as fresh meat they will not require approval as a ‘cutting plant’ and therefore do not require veterinary control.
The types of activities requiring approval are provided in Annex 1.
1.3 Governance and decision making
Approval assessments and recommendations in England and Wales are provided by veterinary officials in the FSA field management structure. Updated [Decisions on approval are made by a Senior Civil Servant. In the absence of a suitable Senior Civil Servant, decisions may be delegated to an AO within the Operational Assurance and Excellence Team (OAE).
The official responsible for decisions may convene a panel to assist in their deliberations. The panel will consist of, or a representative of; the Head of Operations Assurance and Excellence, FSA Legal, Head of Veterinary Audit, and the veterinary official making the recommendation. The panel will typically be convened in cases which may result in a refusal to grant approval or a withdrawal of approval. In the absence of the Head of Operations Assurance and Excellence decisions may be made by an alternative SCS in the Operations Division.]
There is a separation of functions between the officials involved in assessments, recommendations and decisions on approvals and the officials responsible for conducting the audits of approved meat establishments.
The authorised officials work in collaboration and base their decisions upon the recommendation and evidence presented by the veterinary official who conducted the approval assessment, together with other relevant information available, such as the outcomes of recent official controls.
1.4 Exemptions from approval
Regulation (EC) No 853/2004 provides certain exemptions from approval. In terms of establishments for which the FSA may need to consider for approval the exemptions fall into the following basic categories:
- Retail Establishments
- Poultry Slaughter and Cutting on Farm
- Slaughter for Private Domestic Consumption
- Wild Game
Full details of these exemptions are provided in Annex 2. For retail establishments, the flow diagram in Annex 3 can be used to determine if the operator qualifies for exemption from the need to be approved.
2. Application process
2.5 Approval assessment and visits
2.6 Allocation of approval number
2.9 Food businesses transferring from LA control
2.10 Updated [Multiple FBOs operating from one premises]
2.1 Applications for approval
Application forms are available to download from the FSA website. An FBO can only make an application for approval for an establishment under their control and only for processes and/or activities that they intend to carry out.
The applicant must provide details of:
- the premises for which approval is requested;
- the activities and species for which approval is sought; and
- the identity of the FBO including relevant contact and address details.
A food business operator means the natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control:
- A natural person is a human being, (as opposed to an artificial, legal or juristic person, i.e., an organisation that the law treats for some purposes as if it were a person distinct from its members or owner).
- A legal person has a legal name and has rights, protections, privileges, responsibilities, and liabilities under law, just as natural persons (humans) do. Legal personality allows one or more natural persons to act as a single entity (a composite person - considered under law separately from its individual members or shareholders) for legal purposes.
2.2 Past Compliance History
The FSA will take into account past compliance with the requirements of food law, animal health and animal welfare rules as a relevant factor and this may be used as an indicator of likely future compliance with the requirements of food law. This will include past compliance by the applicant and any relevant person.
In assessing compliance with the requirements of food law, the FSA will use the following criteria, as relevant:
- The potential consequences for the objective of achieving a high level of consumer protection with regards to food safety and food hygiene;
- Potential consequences for legitimate business;
- The culpability of the applicant and whether any contraventions were the result of deliberate acts;
- Whether any prior warnings, advice and/or guidance have been provided and the response to this ;
- Whether the applicant record demonstrates repeated failures of infrastructure, procedures or management controls;
- Whether the applicant has been reticent in explaining circumstances, has been uncooperative or abusive to FSA staff and those from its contractual services providers, for example, OVs provided by Service Delivery Partners or DAERA;
- Whether the applicant has previously had an application for approval refused or conditional approval not extended, full approval refused, or either conditional or full approval withdrawn, and the circumstances in each case;
- Whether the applicant has been convicted of a relevant offence;
- Whether there has been a failure to disclose any relevant offences;
- Repeated significant and / or numerous non-compliances continuing without regard to warnings or advice.
Applicants are also required to disclose in their application any relevant conviction against themselves or other relevant persons. ‘Relevant person’ is the person applying for the approval and any person connected to the applicant and includes:
For companies:
- the company itself
- the officers of the company (a director, manager, secretary or other similar officer)
- other companies, when the officers of the company were officers of that other company when they were convicted.
For partnerships and limited liability partnerships (LLP):
- the individual partners
- the individual partners in the limited liability partnership
- partners within a partnership or limited liability partnership that have either been convicted of a relevant offence themselves, or who held a position as a partner, or partner in another LLP or corporate body when it was convicted of a relevant offence
For individuals:
- the individual
- companies for which the individual is an officer (a director, manager, secretary or other similar officer)
- partnerships and limited liability partnerships that the individual is a partner of
Offences that are relevant are set out in Annex 4. The FSA must take into account the terms of the Rehabilitation of Offenders Act 1974 and the applicant does not need to disclose ‘spent convictions’ covered by that Act. The Act applies only where an individual has been convicted of an offence. However, where the person convicted is a corporate body, the FSA should have regard to whether the conviction would have been spent if it had been committed by an individual and should normally treat the corporate body in the same way.
If during checks the FSA discovers a relevant conviction against the applicant which has not been disclosed, the FSA may refuse the application or the FSA may contact the applicant which will delay the decision to approve or otherwise.
Refusal of approval would normally be appropriate for offences that demonstrate a deliberate disregard for the requirements of food law, animal health and animal welfare rules, for example where there are repeated convictions, (or deliberately making false or misleading statements).
If it thinks it right to do so, the FSA may still decide to grant conditional approval or full approval even though an applicant has demonstrated a poor record of compliance with regulatory requirements such as being convicted of a relevant offence.
2.3 Advisory visits
The FSA offers advisory visits to those food businesses that have applied for approval prior to an approval visit. The aim of an advisory visit is to help FBOs identify any problems in the areas of structure and maintenance and food safety management to avoid any potential difficulties when the establishment is assessed for approval. The FSA will apply a fully recoverable charge for all advisory visits. FBOs must be aware that there is a difference between an advisory visit and an approval visit.
The FSA reaffirms that the FBO will not be able to undertake activities, that are approved by the FSA, until an approval visit has been carried out and a decision following such visit has been formally conveyed to the FBO.
More advice on the cost and the type of guidance which is available in an advisory visit can be found on our website.
2.4 Approval Procedure
Article 148 of assimilated (EU) Regulation 2017/625 provides that competent authorities:
- shall establish procedures for food business operators to follow when applying for the approval of their establishments in accordance with Regulation 852/2004
- on receipt of an application for approval, shall make an on-site visit
- shall approve an establishment for the activities concerned only if the food business operator has demonstrated that it complies with the relevant requirements of food law
Following an on-site visit:
- the FSA may grant conditional approval if the establishment meets all the infrastructure and equipment requirements. The food business may not have a fully developed and documented HACCP based procedures, but the planned method of operation must not constitute a risk to public health and there must be adequate provision to control any such risks that have been identified. This is particularly so for high-risk food items such as ready to eat meat products and minced meat/burger intended to be eaten less than thoroughly cooked. The FBOs food safety management system needs to be available to the FSA.
- full approval shall only be granted where it appears from a new official control visit, which must be carried out within three months of conditional approval, that the establishment meets all structural and equipment requirements and other relevant requirements of food law, including the need to implement an effective food safety management system based on HACCP principles
- if clear progress has been made but the establishment and food business still does not meet all of the relevant requirements, conditional approval may be extended, but must not exceed a total of six months. In such cases the establishment must still meet all the infrastructure and equipment requirements
- the Agency shall grant full approval if the establishment and food business complies with all the relevant requirements of food law (infrastructure, equipment and operational requirements) and the establishment has been observed in operation
Full approval subsequent to conditional approval will be refused:
- if, within the three months’ conditional approval, insufficient progress has been made to meet the requirements in full and, in the judgement of the FSA, there is insufficient evidence to demonstrate that the necessary work will be completed if a further period of conditional approval is granted, conditional approval will cease to have effect
- if, at the end of the six months period there is insufficient compliance with structural, equipment or operational requirements and other relevant requirements of food law, including the implementation of an effective food safety management system based on HACCP principles
Approval will be discontinued if, following conditional approval and before consideration can be given to recommend full approval or prolong conditional approval the establishment ceases operations or a visit cannot be undertaken caused by the relevant activities not being in operation. In such cases conditional approval will cease to have effect.
In between each period of conditional/extended conditional approval the FSA may conduct unannounced visits to check the food business compliance with operational hygiene requirements. Evidence of non-compliance with these requirements may result in appropriate enforcement action and may be used as evidence in the final decision whether to grant or to refuse to grant full approval.
In the event that a decision to refuse to grant approval is made, the FBO must be given notice of the decision, the reason why the decision was made and a list of deficiencies that were noted at the time of the visit, including the requirements of the legislation in relation to hygiene, structure, HACCP or other elements relevant to the type of approval being sought and show how the FBO has failed to satisfy those requirements. The FBO of an establishment that has been refused approval has the right to appeal. From the date on which notice of the decision to refuse approval is served on the relevant person, the establishment must cease approvable activities regardless of whether an appeal is logged.
2.5 Approval assessments and visits
On-site visits undertaken with a view to the approval of premises will be undertaken by a Veterinary Official. The Agency will make an appointment with the FBO or their duly authorised representative. Following an approval visit the Agency will make an assessment of the compliance with the approval requirements for the premises and FBO controls.
Following conditional approval, measures must be taken by the FBO within the conditional approval period to remedy any operational or food safety management system deficiencies on a permanent basis. The initial conditional approval period of up to three months may only be extended for a further period if progress is made to remedy any deficiencies during the initial period and if, in the judgement of the Agency, there is evidence that the necessary work will be completed if a further period of conditional approval is granted. The total period of conditional approval cannot exceed six months; at the end of the conditional approval period, all aspects of compliance will be reassessed.
Although conditional approval may last up to three months or if extended up to a maximum of six months, assessment for full approval may be undertaken at any time after conditional approval has been granted.
Before full approval can be recommended, the food business must be observed in operation to verify that it meets all the requirements of food law, and other relevant legislation as required for the type of approval.
Where the slaughter of all species requiring approval cannot be reasonably seen on an approval visit professional judgement can be used. When the veterinary official reaches a point where they are satisfied with infrastructure, equipment and the FBO controls they should recommend approval, even if every species has not been observed in operation.
2.6 Allocation of approval number
On granting approval or conditional approval the FSA will give each approved establishment an approval number. For wholesale markets, secondary numbers indicating units or groups of units may be added to the approval number. The approval number should be unique to the establishment / wholesale market and FBO during the period they are approved.
The following numbering system for regional variations in allocating approval numbers, to establishments approved by the FSA / FSS, will apply:
- England 1000-1099, 2000-6999 & 8000-8999
- Scotland 1100-1999
- Wales 7000-7999
- Northern Ireland 9000-9999
In the case of individual units at wholesale markets, the approval number will consist of the approval number for the common parts and a secondary number that is stall specific. The secondary number allocation is applied to help overcome problems with enforcement, traceability and differing standards of compliance between the different FBOs operating within the wholesale market.
Where an establishment premise has been re-assessed for approval due to a change of FBO and approval is granted, generally a new approval number should be given. However, to have regard to issues of risk, cost and proportionality, a business may be able to retain its approval number where, other than for the change of FBO, the business is to continue to operate from the same premises and in essentially the same way, i.e. the type of food production by the business and the food safety control arrangements of the business will remain essentially the same.
If the FBO moves to a new premise the FSA may allow the FBO to retain the same approval number which was provided to them for the establishment which they are vacating. This will be subject to remaining with the same CA and the same country. The FBO will also still be required to submit a new application and be granted approval prior to operations commencing in the new location. In order to ensure that the approval number remains unique the previous approval will need to be surrendered by the FBO prior to it being granted for the approval at the new address.
In addition to the above, re-allocation of an approval number would only be permissible where the FBO of the business remains the same, and the activities remain substantially the same, when the establishment moves to a new location.
Where an establishment is approved in England or Wales by the LA and the FBO is subsequently granted an approval by the FSA, due to the establishment becoming co-located, the FSA will issue a new approval number to the establishment as a whole. In order not to penalise FBOs in this situation, upon request, a reasonable period of time (but typically not exceeding the conditional approval period) will be given for the business to use up old packaging. The request will be dealt with on a case-by-case basis in conjunction with the LA.
2.7 Seasonal Establishments
In the case of establishments operating a seasonal pattern, conditional approval may be split into two or more periods as long as the combined period does not exceed six months. Wherever possible the FSA will aim to conclude the approval process within one season even if it means that conditional approval will last for less than the allowed three or six months. However, where this is not practicable conditional approval may be split.
In these cases, the veterinary official undertaking the assessment must satisfy themselves that:
- It is practical to split the approval across one or more seasons and any potential risk to public health is managed;
- Measures will be taken by the FBO within the expected conditional approval period to remedy any operational or food safety management system deficiencies on a permanent basis; and
- Full approval is achievable within two years
2.8 Wholesale Markets
Wholesale markets are defined in Regulation (EC) No 853/2004 Annex I as food businesses that include several separate units which share common installations and sections (such as access corridors, loading bays, changing rooms, toilet facilities and water supply etc) where foodstuffs are sold to food business operators.
The common parts of wholesale meat markets must be approved as one establishment while individual stalls under the control of separate food business operators must be approved in their own right and receive an approval number consisting of the approval number for the common parts and a secondary number that is stall-specific.
Responsibility for complying with the Hygiene Regulations rests with the landlord of the market for the general areas within the market and with the individual food business operators for the unit(s) that they operate. However, FBOs for individually approved units have a duty to ensure that the common area requirements, providing the pre-requisites for hygienic operation such as waste disposal and pest control, are in place.
2.9 Food businesses transferring from LA control
Until conditionally approved by the FSA, responsibility for enforcement action remains with the LA. When assessing for approval the FSA, where possible in consultation with the relevant LA will consider whether any enforcement action for the protection of public health is needed and communicate this to the relevant LA.
With the industrial nature of the processes and likelihood of daily damage to structure and equipment, it would be unreasonable to expect any already operating premises to have all infrastructure and/or equipment fully compliant without the need for maintenance. Where the FBO can demonstrate that infrastructure and/or equipment deficiencies have been identified and scheduled for repair in a way that manages any potential risk to public health, dependent on the nature and extent of the deficiency (i.e. minor / operational wear and tear), approval or conditional approval may be recommended.
Registered establishments under retained Regulation (EC) No 852/2004 taking advantage of the exemption criteria and applying to undertake activities that are approved by the Agency, may be recommended for full approval following the initial approval visit. This can only be the case where the establishment and FBO controls have be observed in operation and complies with all the relevant requirements of food law (infrastructure, equipment and operational requirements) and any other relevant legislation as required for the type of approval.
The FBO will not be able to undertake activities, that are approved by the FSA (in this case working outside of the exemption criteria), until approval or conditional approval is granted.
2.10 Updated [Multiple FBOs operating from one premises]
Where more than one FBO wishes to use a single premise to operate separate food businesses at different times, for example FBO A operates 09:00-17:00 and FBO B operates 17:00-09:00, approval may still be permitted but these situations will be assessed on a case by case basis.
The FBO/s for the individual businesses requiring approval using one premise will need to demonstrate how they plan to manage any food safety risks adequately. When undertaking the assessment the Agency must be satisfied that infrastructure, equipment and the FBO controls are acceptable before approvals can be granted. The arrangements regarding the operating pattern and joint use of the premises will be included in the approval document as a precondition to the approval.
Where the arrangements are satisfactory, approval or conditional approval will be granted to each FBO individually with each FBO receiving their own approval number.
When carrying out official controls, if the CA needs to take enforcement action, for example due to non-hygienic operations or equipment deficiencies, this may be taken against both parties. As the terms of the joint use of the establishment are a precondition to the individual approval enforcement action must be taken against both parties regardless of which party caused the problem in the first place.
2.11 Shared facilities
In the event that an FBO requiring approval to operate an establishment can only fully meet the requirements of the regulations by sharing certain facilities with a neighbouring FBO, approval may still be possible. These situations will be treated on a case by case basis but examples would include sharing facilities such as changing rooms, toilets, loading bays and chillers.
The FBO requiring approval using shared facilities will need to demonstrate how food safety risks are managed. When undertaking the assessment, the CA must be satisfied that infrastructure, equipment and the FBO controls are acceptable before approval can be granted. Shared facilities will be identified in the approval document and marked on the site plan
An example layout of shared facilities:
Where the arrangements are satisfactory, approval or conditional approval will be granted on the basis that the facilities being shared remain available and the requirements of the regulations continue to be fulfilled.
If at a point in the future the sharing of facilities is no-longer possible, the approval will be reviewed. Also refer to Chapter 13, Section 4 Review of Meat Establishments Approval with the view to Withdraw or Suspend section and Chapter 13, Section 3.3 Changes to Curtilage.
When carrying out official controls, if the CA needs to take enforcement action, for example due to non-hygienic operations or equipment deficiencies, this will be taken against the party responsible for ensuring compliance. If both parties are responsible, enforcement may be taken against both.
The facilities which are to be shared will form part of the approved establishment but they may be shared with a registered establishment. If enforcement action is required on the shared facilities this will be undertaken by the FSA if the operator responsible for the non-compliance is carrying out operations in relation to a slaughterhouse, a cutting plant or a game handling establishment, or by the LA in other cases where so empowered and by agreement.
2.12 Mobile slaughterhouses
When considering applications from FBOs seeking to operate a mobile slaughterhouse the FSA may consider permitting the use of shared facilities. The mobile slaughter unit may utilise facilities such as toilets, changing rooms and chillers with an approved or registered food business. , These shared facilities will still be required to meet the necessary hygiene requirements for an approved slaughterhouse, and, all mobile slaughterhouses will be required to comply with the same hygiene requirements as any static slaughterhouse.
3. Significant changes
3.4 Changes of food business operator
3.5 FBO moving to a new premises
3.1 Significant Changes
The FSA must be informed of any significant change to food business operations, such as additional activities, changes to the approved curtilage, change of FBO, the closure of an establishment or surrender of approval. This is to ensure the FSA always has up-to-date information on establishments in compliance with Article 6(2) of Regulation 852/2004. Not complying with this requirement is an offence under domestic food hygiene regulations.
3.2 Additional activities
The FBO of a fully approved establishment wishing to undertake additional activities requiring approval must apply to the FSA for approval before carrying out the additional activity.
The usual approval procedures will be applied when assessing the additional activities for approval.
If a fully approved establishment’s most recent audit has the outcome ‘Improvement Necessary’ or ‘Urgent Improvement Necessary’ the FSA will not consider any applications for further activities or species until such time as the FBO has demonstrated sufficient improvement to exit the Improvement Necessary or Urgent Improvement Necessary status during a subsequent audit.
Professional judgement may be used, in the case of approving additional activities, to grant full approval in the first instance. This is only when the CA reaches a point where they are satisfied with infrastructure, equipment and the FBO controls. Examples include adding an approval (this is not an exhaustive list):
- to slaughter goats at an existing sheep approved slaughterhouse
- to cut an additional meat type at an approved cutting plant already approved to cut two or more types of meat
- for a minced meat establishment to an already approved meat preparations establishment
- for the cold storage of meat for the re-wrapping / repackaging of meat
If an establishment is conducting activities for which they are not approved appropriate enforcement should be taken and the FBO be directed to the food.gov page on which they can apply for additional activities and species.
Applying for approval of a meat or food establishment by the FSA
3.3 Changes of curtilage
The agreed curtilage is the area which has been assessed by the FSA as compliant with EU hygiene and animal welfare regulations and is delineated on the approval document by a red boundary. If a FBO changes the area in which they operate which could be in the form of an extension or utilising a room previously not included in the curtilage they need to inform the FSA of this. A failure to notify the FSA should result in appropriate enforcement action being taken.
Once the FBO notifies the FSA of changes to the area they are using, the FSA Approvals and registration team will acknowledge the change on the revised site plan provided by the FBO and will notify Field Operations. Field Operations can then asses the extent of the change and if necessary visit the premises to verify the changes have not resulted in non-compliances.
Once the FBO has notified the FSA of such changes they are entitled to use the new area as soon as they are content it complies with their regulatory requirements towards food safety and/or animal welfare.
If the FSA later find non-compliances in the new area then appropriate action will be taken following the current hierarchy of enforcement (which can vary between verbal advice, notices being issued or could ultimately lead to withdrawal of approval or prosecution).
3.4 Change of Food business operator
The approval of an “establishment” applies to both the premises and the business operating at the premises. If an approved establishment changes FBO the food business will have to be assessed and granted a new approval under the new FBO.
The approval assessment will be undertaken as soon as possible and in all cases within 20 working days of receiving an approval application from the new FBO. Therefore, in order to allow a business to carry on operating without a break in trade the FSA, upon receipt of a new application made ahead of the date of change of FBO, will conduct a new approval assessment in the immediate run up to the effective date of change. Conditionally approval may be granted to the new FBO if the premise meet the infrastructure and equipment requirements. The new FBO should therefore ensure the FSA is provided sufficient notice (at least 20 working days) of the change of FBO.
The FSA is prepared, on request by the FBO to carry out a pre change of FBO advisory visit, refer to Chapter 13 Section 2.3 Advisory Visits section.
However, any views given at such a visit will in no way provide a guarantee as to the future approval status of the business.
The different situations where a change in FBO, between different business entities, requires a new approval or where the approval can be retained are detailed in Chapter 13 Annex 5.
Article 6(2) of Regulation (EC) No 852/2004 requires the FBO to inform the FSA when there is a change of FBO. This will be by means of an application form as detailed in Application for Approval section that includes the type of business entity, name of officers and relevant address/es of the FBO wishing to apply for approval. The FBO is then obliged to keep the CA informed about significant changes to those details.
Once received by the Agency the application will be assessed in the same way as a new establishment and if approval is granted may be subject to a new approval number. Also refer to Chapter 13 Section 2.6 Allocation of Approval Number section.
Where the FSA becomes aware of a change of FBO at an establishment and the new FBO has failed to notify the change, the FSA will inform the FBO that the food business is no longer approved and must not undertake activities that require approval until a new approval has been issued. The FSA will also inform the relevant LA of this so that the LA can take appropriate enforcement action.
In the case of wholesale markets the following principles apply:
- The market overall approval (common parts) will be treated in the same way as an individual establishment FBO change but the individual units within the market do not need to be individually re-approved and can transfer over under the new market (common parts) approval.
- In the event that the common parts of a wholesale market are not granted approval, the individually approved units are not able to operate as the approval of the common parts facilities is a precondition to their approval. Where the units are able to become self-sufficient in their own right separate approval as an individual establishment can be sought.
If an individual unit of a wholesale market changes FBO, this will be treated in the same way as an individual establishment FBO change.
3.5 FBO moving to a new premise
If an approved food business relocates to a different address the FBO will need to apply for a new approval at this new address. The approval procedures will be applied when assessing the food business at the new address for approval.
3.6 Seasonal closure
An establishment may operate to a seasonal pattern with routine breaks in operation. Notification of this pattern must be provided by the FBO as part of the application process by identifying the months when the FBO intends to operate the establishment. The FBO is then obliged to keep the Agency informed about any significant changes to those details including any establishment moving to or from a seasonal pattern. When an FBO intends to re-commence operation the Agency should be notified at least two weeks before operations are intended to re-commence. This is so an inspection can be arranged to check the premises are still compliant.
3.7 Temporary closure
When an FBO needs to temporarily halt operations due to renovation / development work at an establishment or due to a temporary downturn in trade the FBO is obliged to keep the Agency informed about these significant changes to the operational pattern. In these cases the FBO should notify the Agency at least two weeks before operations re-commence.
3.8 Long term closure
When an FBO stops operations with no immediate intention to recommence for at least six months or longer the closure is classed as long-term. The FBO is obliged to keep the Agency informed about this significant change to the operational pattern and should notify the Agency at least two weeks before operations re-commence.
Following a period of closure the FBO must notify the CA before operations recommence. Keeping the CA informed of significant changes is a regulatory requirement which allows the CA to ensure it has up to date information. This notification allows the CA to plan a visit to the premises to check the establishment continues to meet all structural and equipment requirements and other relevant requirements of food law, including the existence of a food safety management system based on HACCP principles.
If there are major or critical non-compliances which indicate the establishment is a serious risk to public health or animal welfare appropriate enforcement action should be taken and the establishment could be subject to a formal review of approval.
Where the FSA becomes aware of an establishment that has re-commenced operations without first notifying the FSA appropriate enforcement action will be taken in regard to their failure to keep the FSA informed of significant changes.
The FSA will monitor establishments which have ceased operating and not informed the FSA of their future plans. If the FBO does not confirm the surrender of their approval in writing within six months of the establishment ceasing operations then the FSA will write to the FBO to confirm their approval no longer has effect and the establishment will be removed from the published list of approved meat establishments.
4. Review of approval with a view to withdraw or suspend
Where non-compliances have been established the FSA will take appropriate measures to ensure the operator concerned remedies the non-compliance and prevents further occurrences of such non-compliance. When deciding what measures to take, the FSA shall take account of the nature of that non-compliance and the operator’s past record with regard to compliance’ Article 138 (1)(b) of Regulation 2017/625.
The process the Agency will follow when reviewing approval is detailed in the enforcement chapter (Chapter 7 Section 6)
5. Annexes
Note: These pages can only be accessed by FSA staff on FSA devices.
Annex 1 List of activities subject to approval or additional authorisation
Annex 2 Exemption Criteria
Annex 3 Exemption flow chart
Annex 4 Relevant Offences
Annex 5 Change of FBO
Diseases
1.7.1 The Avian Influenza and Influenza of Avian Origin in Mammals (Wales) Order 2006 (SI No. 1762 (W184))
1.8 The Foot-and-Mouth Disease (England) Order 2006 (SI No.182)
1.9 The Foot-and-Mouth Disease (Wales) Order 2006 (SI No. 179 (W.30))
1.15 The Diseases of Swine Regulations 2014 (SI No. 1894)
1.16 The Tuberculosis (England) Order 2014 (SI No. 2383)
TSE/BSE
2.2 The Transmissible Spongiform Encephalopathies (England) Regulations 2018 (SI No. 731)
2.2.1 The Environment, Food and Rural Affairs (Miscellaneous Amendments etc.) Regulations 2019 (526)
2.3 The Transmissible Spongiform Encephalopathies (Wales) Regulations 2018 (SI No. 968 (W.195))
Food safety
3.2 The General Food Regulations 2004 (SI No. 3279)
3.3 The Food Safety Act 1990 (Amendment) Regulations 2004 (SI No. 2990)
3.4 Food Law – Code of Practice (England)
Welfare
4.1 Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing
4.2 The Welfare of Animals at the Time of Killing (England) Regulations 2015 (SI No. 1782)
4.3 The Welfare of Animals at the Time of Killing (Wales) Regulations 2014 (SI No. 951 (W. 92))
4.4 Council Regulation (EC) 1/2005 on the protection of animals during transport and related operations
4.5 The Welfare of Animals (Transport) (England) Order 2006 (SI No. 3260)
4.5.1The Welfare of Animals (Transport) (Wales) Order 2007 (SI No. 1047 (W. 105))
4.7 The Welfare of Farmed Animals (England) Regulations 2007 (SI No. 2078)
4.7.1 The Welfare of Farmed Animals (England) (Amendment) Regulations 2010 (SI No. 3033)
4.8 The Welfare of Farmed Animals (Wales) Regulations 2007 (SI No. 3070 (W264))
4.8.1 The Welfare of Farmed Animals (Wales) (Amendment) Regulations 2010 (SI No. 2713 (W229))
Animal By-Products
5.3 The Animal By-Products (Enforcement) (England) Regulations 2013 (SI No. 2952)
5.3.1 The Animal By-Products (Enforcement) (England) (Amendment) Regulations 2015 (SI No. 1980)
5.4 The Animal By-Products (Enforcement) (Wales) Regulations 2014 (SI No. 2014/517 (W. 60))
Identification
6.2 The Cattle Identification Regulations 2007 (SI No. 529)
6.2.1 The Cattle Identification (Amendment) Regulations 2007 (SI No. 1046)
6.2.2 The Cattle Identification (Amendment) Regulations 2013 (SI No. 517)
6.2.3 The Cattle Identification (Amendment) Regulations 2015 (SI No. 219)
6.3 The Cattle Identification (Wales) Regulations 2007 (SI No. 842 (W. 74))
6.3.1 The Cattle Identification (Wales) (Amendment) Regulations 2007 (SI No. 3004 (W. 260))
6.3.2 The Cattle Identification (Wales) (Amendment) Regulations 2013 (SI No. 821 (W.97))
6.6 The Sheep and Goats (Record, Identification and Movement) (England) Order 2009 (SI No. 3219)
6.7 The Sheep and Goats (Records, Identification and Movement) (Wales) Order 2009 (SI No. 3364 (W. 296))
6.8 The Equine Identification (England) Regulations 2018 (SI No. 761)
6.8.2 The Equine Identification (Wales) Regulations 2019 (No. 57 (W.20))
6.8.3 The Equine Identification (Wales) (Amendment) Regulations 2019 (No. 614 (W.128))
6.9 The Pigs (Records, Identification and Movement) Order 2011 (SI No. 2154)
6.10 The Pigs (Records, Identification and Movement) (Wales) Order 2011 (SI No. 2830 (W.303))
Animals and related products
7.1 The Trade in Animals and Related Products Regulations 2011 (SI No. 1197/2011)
7.2 The Trade in Animals and Related Products (Wales) Regulations 2011 (SI No. 2379 (W. 252))
7.6 The Products containing Meat etc. (England) Regulations 2014 (SI No. 3001)
Disease control
8.1 The Meat (Disease Control) (England) Regulations 2000 (SI No. 2215)
8.2 The Meat (Disease Control) (Wales) Regulations 2000 (SI No. 2257 (W. 150))
8.3 The Disease Control (England) Order 2003 (SI No. 1729)
8.4 The Disease Control (Wales) Order 2003 (SI No. 1966 (W. 211))
8.5 The Transport of Animals (Cleansing and Disinfection) (England) (No.3) Order 2003 (SI No. 1724)
8.8 The Diseases of Animals (Approved Disinfectants) (England) Order 2007 (SI No. 448)
8.9.1 The Diseases of Animals (Approved Disinfectants) (Wales) Order 2007 (SI No. 2803 (W.236))
8.10 The Products of Animal Origin (Disease Control) (England) Regulations 2008 (SI No. 465)
8.11 The Products of Animal Origin (Disease Control) (England) (Amendment) Regulations 2009 (SI No. 1297)
8.12 The Products of Animal Origin (Disease Control) (Wales) Regulations 2008 (SI No. 1275 (W. 132))
Pithing- removed
Feed, food and hygiene
10.3 The Official Feed and Food Controls (England) Regulations 2009 (SI No. 3255)
10.3.1 The Official Feed and Food Controls (England) (Amendment) Regulations 2011 (SI No. 136)
10.4 The Official Feed and Food Controls (Wales) Regulations 2009 (SI No. 3376 (W.298))
10.4.1 The Official Feed and Food Controls (Wales) (Amendment) Regulations 2011 (SI No. 626 (W. 90))
10.4.2 The Official Feed and Food Controls (Wales) (Amendment) regulations 2014 (SI No. 2714 (W.271))
10.5 H1 – Regulation (EC) No 852/2004 on the hygiene of foodstuffs
10.5.1 Guidance document on the implementation of certain provisions of Regulation (EC) No 852/2004
10.6 Guidance on the Implementation of procedures based on the HACCP principles
10.7 H2 – Regulation (EC) No 853/2004 laying down specific hygiene rules for food of animal origin
10.7.1 Guidance document on the implementation of certain provisions of Regulation (EC) No 853/2004
10.9 Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs
10.10 Commission Regulation (EU) No 2074/2005 (Implementing Measures)
10.13 The Food Safety and Hygiene (England) Regulations 2013 (SI No. 2996)
10.13.1 The Food Safety and Hygiene (England) (Amendment) Regulations 2014 (SI No. 2885)
10.13.2 The Food Safety and Hygiene (England) (Amendment) Regulations 2016 (SI No. 868)
10.14 The Food Hygiene (Wales) Regulations 2006 (SI No. 31 (W. 5))
10.14.1 The Food Hygiene (Wales) (Amendment) Regulations 2007 (SI No. 373 (W. 33))
10.14.2 The Food Hygiene (Wales) (Amendment) Regulations 2010 (SI No. 893 (W.92))
10.14.3 The Food Hygiene (Wales) (Amendment) Regulations 2012 (SI No. 975 (W.129))
10.14.4 The Food Hygiene (Wales) (Amendment) (No. 2) Regulations 2012 (legislation.gov.uk)
10.14.5 The Food Hygiene (Wales) (Amendment) Regulations 2014 (SI No. 1858 (W. 192))
10.14.6 The Food Hygiene (Wales) (Amendment) (No.2) Regulations 2014 (SI No. 3080 (W. 305))
10.14.7 The Food Hygiene (Wales) (Amendment) Regulations 2016 (SI No. 845 (W. 214))
10.17 Regulation (EC) No 1333/2008 on food additives (Consolidated version 12.02.2018)
Hanes diwygio
Published: 28 Hydref 2021
Diweddarwyd ddiwethaf: 6 Mehefin 2024
Hanes diwygio
Published: 28 Hydref 2021
Diweddarwyd ddiwethaf: 6 Mehefin 2024