Food allergen labelling and information requirements technical guidance
This publication aims to help food businesses follow allergen labelling requirements. It will also help authorised food officers enforce these measures.
Important
This guidance has been published as a result of a consultation which took place from March - May 2023.
Revision history
March 2025
- Updated/amended wording in paragraph: 20. Best practice box added under para. 83. Example box amended under para. 83. Best practice box amended under para. 85. Best practice box amended under para. 86 . References and Resources updated to include hyperlinks to best practice guidance on allergen information for non-prepacked food and tools at paras. 112-113 and subsequent paragraph numbering amended. Contact information amended.
August 2023
- Updated/amended wording in paragraphs: 13; 23-25; Best practice box under para. 25; 31; Best practice box under para. 38; 61; 78-80; Best practice boxes under para. 84; 88; 92; Glossary definition for coeliac disease.
- Section ‘Presentation of Voluntary Information on Prepacked Foods’ para. 64-68, plus Best Practice box added.
- NGCI statements has moved from Part 1 para. 31 to Part 2 para. 88.
Revised by: Food Hypersensitivity Policy Team
July 2023
- Review and update of formatting in line with FSA branding requirements.
Revised by: Food Hypersensitivity Policy Team
June 2023
- Review and update of best practices on Precautionary Allergen Labelling (PAL) and Non-Gluten Containing Ingredient (NGCI) statements.
- Review of legal references on Prepacked food for Direct Sale (PPDS).
- Updated paragraphs: Legal status, Review date, 1 - 4, 7 - 9, 13, 16, 23 - 24, 26, 28, 31, 38, 71 - 76, 84 and 97 – 98.
- Removed paragraphs from currently published version of guidance: 5, 17 – 19 and 78 – 82.
- All hyperlinks, page and paragraph references throughout the document have been updated in line with revisions.
Revised by: Food Hypersensitivity Policy Team
18 June 2020
- Complete document has been updated into the latest FSA guidance format. Additional text and examples have been added on prepacked for direct sale specifically paragraphs 16 to 18, 86 to 99 and the Reference and Resources page, Paragraph 32 has also been updated in light of Regulation 828/2014. References to Scotland have been removed throughout the document.
Reviewed by: Food Allergy, Intolerance and Food Hypersensitivity Team
10 April 2015
- Updated advice in paras 1, 10, 14 - 20, 31, 33, 34, 36, 39, 40, 48 – 50, 52, 55, 56, 58, 59, 65, 66 (example), 72, 73, 74 – 76, 78, 79, 82, 83, 90 – 93, 95 – 98 and References and Resources page.
Reviewed by: Food Allergy Branch
Purpose
This publication aims to help food businesses follow allergen labelling requirements. It will also help authorised food officers enforce these measures.
Legal status
This is Best Practice Guidance (for example, helpful examples of approaches you might employ but which you are not legally required to follow) and Regulatory Compliance (i.e., how to comply with regulatory requirements).
Who is this publication for?
This guidance is for:
- all food manufacturers, importers, and producers
- retailers, institutional caterers, and other food businesses
- packers
- enforcement authorities
Which UK countries does this guidance apply to?
- England
- Wales
- Northern Ireland
Review date
We will review this guidance by December 2024
Key words
- Allergy and intolerance
- Precautionary allergen labelling
- Gluten-free
- Non-gluten containing ingredients
- Prepacked food
- Non-prepacked food
- Prepacked for direct sale food
1. The legislative framework around the provision of food allergen information is largely contained in assimilated Regulation (EU) No. 1169/2011 (for England and Wales) and Regulation (EU) No. 1169/2011 (for Northern Ireland). These regulations will be referred to as the Food Information to Consumers, or FIC, throughout this document. The Food Information Regulations 2014 (FIR), the Food Information (Wales) Regulations 2014 and the Food Information Regulations (Northern Ireland) 2014 establish the enforcement measures for FIC. These regulations will be referred to as the FIR throughout this document.
2. The FIC imposes a duty on food businesses to ensure that all mandatory food allergen information (relating to 14 substances listed in the FIC that are known to cause allergies) is accurate, available, and easily accessible to the consumer. The FIC applies to all food supplied by food businesses including when food is offered complimentary or otherwise without charge. The FIC allows a distinction to be made between prepacked foods and non-prepacked foods in how mandatory allergen information is provided to consumers.
3. These guidance notes cover the interpretation and application of allergen provisions for prepacked, non-prepacked and prepacked for direct sale (PPDS) foods. The provision of voluntary information is also covered. This guidance does not cover other labelling requirements (such as other general labelling (e.g., country of origin, minced meat, quantities, additives, nutrition etc.)
4. Failure to comply with the allergen provisions may result in a food business or Food Business Operator (FBO) being served with an improvement notice or a criminal prosecution.
Intended audience
5. These guidance notes are intended to help food businesses such as producers, manufacturers, packers, importers, distributors, wholesalers, retailers, caterers, and enforcement officers responsible for enforcing relevant measures.
6. Individuals who(footnote) occasionally provide food at charity events or voluntary cake sales, for example, may also need to follow the legal requirements. If you are a charity or community food provider and unsure whether you should be registered as a food business, you should speak to your local authority’s environmental health department. Further guidance is also available in the FSA’s guidance on providing food at community and charity events which is available on the FSA’s website: Guidance on Providing Food at Community Charity Events.
Purpose of the guidance
7. These guidance notes have been produced to:
- provide best practice guidance on the practical application of FIC and FIR specific requirements on allergen labelling and information.
- develop understanding by providing regulatory guidance and interpretation in this area.
Legal status of guidance
8. Directly applicable EU legislation no longer applies in GB. EU legislation retained when the UK exited the EU became assimilated law on 1 January 2024, published on legislation.gov.uk. References to any legislation in FSA guidance with ‘EU’ or ‘EC’ in the title (e.g. Regulation (EC) 178/2002) should now be regarded as assimilated law where applicable to GB. References to ‘Retained EU Law’ or ‘REUL’ should now be regarded as references to assimilated law.
For businesses moving goods from Great Britain to Northern Ireland, information on the Windsor Framework including the NI Retail Movement Scheme (NIRMS) is available on GOV.UK.
9. These guidance notes have been produced to provide:
- guidance on legal requirements of the FIC and the FIR and
- best practice guidance on the application of the FIC and the FIR.
10. Businesses with specific queries should seek advice from their local enforcement agency, which will usually be the trading standards/environmental health department of their local authority.
11. The guidance notes on legal requirements cannot cover every situation and you may need to consider the relevant legislation itself to see how it applies in your circumstances. If you follow the guidance notes they will help you to comply with the law. You are not required by law to follow best practice guidance. All guidance on best practice is identified in bold text with a heading of Best Practice.
12. This guidance also uses practical examples to help explain the requirements. All examples are identified in bold text, with a heading of Example:
13. Around 6% of the UK adult population have a food allergy; this figure does not include those with food intolerances. In addition, it is estimated that 1 in 100 people have coeliac disease, a genetic and autoimmune disease triggered by eating gluten. This is a protein found in wheat, rye, and barley. Eating gluten triggers an abnormal immune response which results in damage to the lining of the gut and malabsorption causing nutritional deficiencies and associated complications.
14. An allergic reaction can be produced by a tiny amount of a food ingredient that a person is sensitive to (a drop of milk, a fragment of peanut or just one or two sesame seeds). Symptoms of an allergic reaction can range from mild symptoms such as itching around the mouth and rashes, and can progress to more severe symptoms such as vomiting, diarrhoea, difficulty breathing, and, on occasion, anaphylaxis (shock) and death. When people with coeliac disease consume even the smallest amount of gluten, the reaction is not the same as an allergic reaction, and they will not go into anaphylactic shock, but it will result in symptoms some of which can be serious and can result in hospital admission. These symptoms usually start a few hours after eating it and symptoms can last from a few hours to several days. Ongoing ingestion of gluten results in symptoms such as diarrhoea, constipation, nutritional deficiencies including iron, folic acid and B12 anaemias and associated complications such as osteoporosis.
15. There is no cure for food allergy or coeliac disease. The only way to manage food allergy and coeliac disease is to avoid food that triggers the abnormal immune response. Therefore, it is very important that food businesses provide consumers with clear and accurate information about allergenic ingredients in products to allow them to make safe food choices.
Main allergen labelling changes
16. From 1 October 2021, legislation to amend the FIR (footnote 1) came into force to improve the provision of information to consumers purchasing PPDS foods. These changes place a duty on food businesses to label PPDS food with the name of the food and a full list of ingredients containing emphasised allergens. These changes bring the provision of allergen information in line with labelling for prepacked food, reducing consumer confusion.
Mandatory obligations for all FBOs
17. Under Article 9(1)(c) of the FIC, all FBOs must declare the presence, whether for use as an ingredient or a processing aid, of any of the 14 major allergens listed in Annex II to the Regulation. The ways in which this mandatory information can be presented for prepacked food, non-prepacked food and prepacked for direct sale food is explained later in this guidance. However, in all cases it should be noted that in accordance with Articles 12 and 13 of the FIC the mandatory information must be easily accessible, in a conspicuous place, easily visible and clearly legible. Information must be indelible (for example on food labels where it needs to withstand handling). The information must not be hidden, obscured, detracted from or interrupted by other written or pictorial matter or any other intervening material. All information provided about allergens must be accurate, however it is provided.
The fourteen allergens (Annex II allergens)
18. The 14 allergens listed in Annex II of the FIC are recognised as the most common ingredients or processing aids that cause food allergies and intolerances. If a food contains or uses an ingredient or processing aid in the manufacture or preparation of the food derived from one of the substances or products listed in Annex II, and it is still present in the finished product, information regarding the presence or use of the allergen must be provided to the consumer.
19. The Annex II allergens are:
- Cereals containing gluten namely wheat (such as spelt and Khorasan wheat), rye, barley, oats and their hybridised strains and products thereof, except:
a) wheat based glucose syrups including dextrose
b) wheat based maltodextrins
c) glucose syrups based on barley
d) cereals used for making alcoholic distillates including ethyl alcohol of agricultural origin
- Crustaceans and products thereof (for example prawns, lobster, crabs and crayfish) Egg and products thereof
- Fish and products thereof, except:
a) fish gelatine used as carrier for vitamin or carotenoid preparations of fish gelatine or Isinglass used as a fining agent in beer and wine
- Peanuts and products thereof
- Soybeans and products thereof, except:
a) fully refined soybean oil and fat
b) natural mixed tocopherols (E306), natural D-alpha tocopherols, natural D-alpha tocopherol acetate and natural D-alpha tocopherol succinate from soybean sources
c) vegetable oils derived phytosterols and phytosterol esters from soybean sources
d) plant stanol ester produced from vegetable oil sterols from soybean sources
- Milk and products thereof (including lactose), except:
a) whey used for making alcoholic distillates including ethyl alcohol of agricultural origin
b) lactitol
- Nuts (namely almond, hazelnut, walnut, cashew, pecan nut, Brazil nut, pistachio nut and Macadamia nut (Queensland nut)) and products thereof except for nuts used for making alcoholic distillates (e.g., spirits such as vodka or whisky) including ethyl alcohol of agricultural origin
- Celery and products thereof
- Mustard and products thereof
- Sesame seeds and products thereof
- Sulphur dioxide and/ or sulphites at concentrations of more than 10 mg/kg or 10 mg/ (litre) in terms of the total SO2 which are to be calculated for products as proposed ready for consumption or as reconstituted according to the instructions of the manufacturers.
- Lupin and products thereof
- Molluscs and products thereof (for example mussels, clams, oysters, scallops, snails, and squid)
20. The use of icons or symbols to indicate the presence of allergens is permitted as long as it is accompanied by words and numbers to ensure uniform consumer understanding and to avoid misleading the consumer. Currently there is no single agreed set of icons or symbols for indicating the presence of allergens in prepacked, non-prepacked, and prepacked for direct sale food, however, we have a set of icons that food businesses can download and use on their own menus or similar.
Ingredients and processing aids excluded from the 14 allergens in Annex II
21. The FIC requires information on the presence of allergens in the final foodstuff to be provided in the manner specified by the Regulation. Some ingredients made from the Annex II foods are unlikely to cause an allergic reaction because they have been highly processed (for example fully refined soya oil or wheat glucose syrups). This is because the allergen/protein has been removed and the product has been assessed by the European Food Safety Authority (EFSA) as not possessing an allergenic risk to the consumer.
22. Substances derived from an allergenic ingredient, which have been specifically exempted from declaration under Annex II (e.g., wheat glucose syrup), do not need to be declared as allergens for example - fully refined soya oil.
Voluntary information (Article 36)
23. Any voluntary food information must comply with the requirements of Chapter V of the FIC. In particular, voluntary statements must not mislead consumers, or be ambiguous or confusing.
24. A Precautionary Allergen Label (PAL) is a statement that food businesses can choose to apply to food products where there is an unavoidable risk of allergen cross-contamination. It is commonly seen as “may contain allergen x” or “not suitable for someone with x allergy” on food products.
25. Although PAL is voluntary food information under the FIC, to avoid providing food that could be deemed unsafe, FBOs should provide consumers with accurate information about the risk of the unintended presence of allergens.
A Precautionary Allergen Labelling statement or information [such as ‘may contain’] should only be provided with prepacked or non-prepacked foods if an unavoidable risk of allergen cross-contamination has been identified following a risk assessment that cannot be sufficiently controlled through controls, such as segregation and cleaning.
Its use is not a substitute for good food hygiene and safety practices, and it could be considered misleading food information if it does not convey a real risk to the consumer. A checklist to understand when and how to apply precautionary allergen labelling to food product is provided in this FSA guidance.
-
The Food Information Regulations and equivalent legislation in Wales and Northern Ireland have been amended by The Food Information (Amendment) (England) Regulations 2022, The Food Information (Wales) (Amendment) (No. 2) Regulations 2020 and The Food Information (Amendment No. 2) Regulations (Northern Ireland) 2020.
26. The following section provides guidance and examples of compliance with the FIC provisions specific to allergen labelling for prepacked foods. This is based on the following articles:
- Article 9 on the listing of mandatory particulars
- Article 13 on the presentation of mandatory particulars
- Article 19 on foods that do not require an ingredients list
- Article 21 on labelling of certain substances or products causing allergies or intolerances
- Article 36 on applicable requirements relating to the provision of voluntary food information
List of mandatory particulars (Article 9)
27. Below, you will find guidance on the scope of each allergenic ingredient captured in Annex II of the FIC and how the allergens must be emphasised in the ingredients list (see pages 10 to 12 for exemptions).
Cereals containing gluten
28. Annex II to the FIC lists these as: wheat (such as spelt and Khorasan wheat), rye, barley and oats or their hybridised strains. Spelt and Khorasan are types of wheat, which are not suitable substitutes for people with coeliac disease and/or wheat allergy.
29. Cereals containing gluten must be declared in the ingredients list using the specific name of the cereal, i.e., wheat (such as spelt, Khorasan or Kamut), rye, barley or oats. Where ‘spelt’, ‘Khorasan’ and ‘Kamut’ have been used; the inclusion of a specific reference to wheat would be required; for example, ‘spelt (wheat)’ or ‘Khorasan wheat’ and ‘Kamut (wheat)’.
30. Ingredients which are or have been derived from cereals containing gluten will need to be emphasised within the ingredients list. This will make clear for those with an allergy to specific cereals to avoid such food; for example: ‘wheat starch’; ‘barley malt extract’. The voluntary inclusion of the word ‘gluten’ within the ingredients list following the mandatory declaration of a specific cereal (containing gluten) is permitted however the FIC requires the cereal to be emphasised, rather than the gluten; for example, ‘barley (gluten)’.
31. Where foods have been voluntarily labelled as ‘gluten-free’ they must meet the requirements set out in Regulation (EU) No. 828/2014 (footnote 1). This legislation sets out the conditions under which foods may be labelled as ‘gluten-free’ (no more than 20 mg/Kg in the food as sold to the final consumer) or ‘very-low gluten’ (no more than 100 mg/Kg gluten in the food as sold to the final consumer). When a product containing one of the cereals mentioned in Annex II (e.g., oats specially produced, prepared, or processed to reduce gluten) meets the relevant requirements of Regulation (EU) No. 828/2014, then the statement ‘gluten free’ or ‘very low gluten’ can be used on the product. However, the cereal mentioned in Annex II must still be indicated and emphasised in the list of ingredients. These rules surrounding use of the terms ‘gluten-free’ and ‘very-low gluten’ apply to all foods including non-prepacked foods such as those served in restaurants. No other statements to describe the absence or reduced presence of gluten are permitted. When gluten-free oats are used in a gluten-free product, the word ‘oats’ would still need to be emphasised and declared in accordance with Article 21 and 9 (1) (c) of the FIC.
Crustaceans
32. The rules do not name any specific species of crustaceans which means all types of crustaceans are included (for example lobster, prawns, and langoustines).
33. Labelling of crustaceans and products made from them need to have a clear reference to the Annex II food: for example, ‘prawns (crustaceans)’, ‘crayfish (crustaceans)’, ‘lobster (crustaceans)’, ‘shrimp paste (crustaceans)’.
Eggs
34. The rules do not name any species of eggs, because ‘eggs’ refers to eggs from all birds, for example from laying hens as well as eggs from ducks, quails, geese, gulls, and guinea fowl. Therefore, all eggs need to be declared when used as an ingredient or a processing aid.
Fish
35. The rules do not name any species of fish because ‘fish’ means all species of fish and fish products. The generic terms provisions allow the generic name fish to be used in an ingredient list only where there is no specific reference to a common fish species name on the label, for example fish stock.
36. Labelling of fish ingredients or products need to have a clear reference to the Annex II food; for example, ‘cod (fish)’, ‘salmon (fish)’, ‘tilapia (fish)’ unless exempt.
Peanuts
37. While peanuts may also be commonly referred to as groundnuts (which can be confused with ground/powdered nuts such as almonds or a mix of nuts and peanuts) or monkey nuts, the term peanuts must be used for products or ingredients made from them for allergen labelling purposes, as this is the term specified in Annex II of the FIC.
38. Both refined and unrefined peanut oil must be labelled with reference to peanut.
Soyabeans
39. Terms such as ‘soya’ or ‘soy’ are sufficient to indicate the soybean origin. However, less common terms such as tofu or edamame may not be recognised as originating from soya and its clear presence needs to be indicated for soya products or derivatives. e.g., tofu (soya) or edamame (soya) unless exempt.
Milk
40. The rules do not name the animal origin of milk because the word ‘milk’ includes milk from mammals such as cow, sheep, goat, and buffalo etc. It should be noted that all mammalian milk proteins have a similar structure and if someone has an allergy or intolerance to cows’ milk, they are likely to be allergic or intolerant to other mammalian milk. Therefore, all milk and milk products (including lactose) need to be declared when used as an ingredient or a processing aid unless exempt.
41. Milk products such as cheese, butter, fermented milk, and cream do not have to have an ingredients list where no other ingredients have been added other than lactic acid, food enzymes and microbiological cultures and (in the case of cheese) salt. In order to ensure that consumers still receive the information they need to clearly identify the presence of milk in such cases, the following advice may be applied. The use of sales names such as ‘cheese’, ‘butter’ ‘cream’ and ‘yoghurt’ is considered to refer clearly to the milk because legally these products can only be made from mammalian milk (Regulation (EU) No. 1308/2013 on common organisation of the markets in agricultural products including dairy designations). In such cases, further reference to milk is not necessary because the dairy designations protect such products. Therefore, cheese, and cream (footnote 2) can be emphasised within the ingredients to demonstrate the presence of a milk product.
42. However, the information must make a clear reference to milk in the case of less familiar milk products used as ingredients (e.g., fromage frais, Mascarpone, Cantal, Quark) or products being sold under a name which does not clearly refer to milk. Components derived from milk, such as lactose, casein, and whey, must be declared with a clear reference to milk e.g., ‘whey (milk)’.
Nuts
43. The rules list these as: almond, hazelnut, walnut, cashew nut, pecan nut, Brazil nut, pistachio nut, Macadamia nut or Queensland nut and products made from these nuts. The type of nut must be listed and emphasised in the ingredients panel. Other types of nuts, and other foods which are not nuts (even though they are called nuts i.e., chestnuts, pine nuts and coconut), are not named in the rules. Chestnuts, pine nuts, and coconut are also known to cause allergy in some people but must not be emphasised within the ingredients.
44. Where ingredients or processing aids derived from nuts have been used, the ingredient must be indicated with a clear reference to the nut; for example, ‘flavourings (almond)’ unless exempt.
Celery
45. This term is used generically in the FIC to refer to stick celery and celery root/tuber (also often known as celeriac). However, the term refers to any part of the celery plant and other forms that originate from it, such as celery leaf, celery root, celery seeds, celery oil, celery salt, celery spice, celery seed oil and celery seed oleoresin (an oil / resin extract from celery).
Mustard
46. This term refers to the mustard plant and other products which originate from it, such as leaves, sprouted seeds, mustard flour, table mustard, mustard oils, mustard seed oils and mustard oleoresins. The appropriate terms must be used in labelling. The rules do not name any particular species of mustards and therefore must be applied to all types of mustard.
Sesame
47. This term refers to sesame seeds, ground sesame powder and sesame oil. Products derived from sesame seeds, such as tahini, must be clearly labelled with a reference to sesame e.g., ‘tahini (sesame)’. The rules do not name any particular species of sesame seeds and therefore must be applied to all.
Sulphur dioxide and / or sulphites at levels above 10 mg/Kg or 10 mg/litre
48. The labelling rules apply to sulphur dioxide and/or sulphites that have been deliberately added for example when it has been used as a preservative or have been added to an ingredient used in a preparation of the food. The rules require sulphur dioxide and/or sulphites to be labelled when present above 10 mg/Kg or 10 mg/litre (calculated in terms of the total sulphur dioxide (SO2)) in the finished product as consumed, i.e., prepared according to the manufacturer’s instructions. The method of analysis for sulphur dioxide sulphites cannot differentiate between those naturally present in the food or added as a preservative. Where sulphur dioxide and/ or sulphite-based preservatives (even as carryover in an ingredient) have been used and the levels in the finished product are above 10 mg/Kg or 10 mg/litre, it will need to be declared on the label.
49. Under general food additives legislation, where sulphur dioxide and/or sulphites have been added and have a technological function in the finished product, the function and the name and/or e number of the additive must be included - for example: ‘Dried Apple, (Preservative: sulphur dioxide)’ – however, if only the E number is provided, a clear reference to the allergen must be provided so it is easily understood by the consumer. Under allergen labelling legislation, when sulphites are present at above 10 mg/Kg/litre in the finished product, whether or not they have a technological function, a clear declaration of sulphites and/or sulphur dioxide is always required.
Example
The term ‘sulphites’ (or ‘sulfites’) may also be used as a generic term for this ingredient. Furthermore, depending on the particular sulphite present, the chemical name may be used with the sulphite element emphasised, for example, ‘sodium metabisulphite’
50. References to sulphur dioxide and/or sulphites, which are used and found present in the finished product (ready for consumption or reconstituted according to manufacturers’ instructions) at less than 10 mg/Kg or 10 mg/litre is not required.
Lupin
51. The term lupin is used generically in the FIC to refer to both lupin seed and products from it such as lupin flour. The appropriate terms must be used in labelling. The rules do not name any particular species of lupin and therefore must be applied to all.
Molluscs
52. The rules do not name any species because the term molluscs includes all types of molluscs (for example oyster, squid, cockles, mussels, winkles, and scallops as well as land molluscs like snails).
53. Labelling of mollusc ingredients and products derived from molluscs need to have a clear reference to the Annex II food: for example, ‘mussels (mollusc)’, ‘octopus (mollusc)’, ‘oyster (mollusc)’.
Presentation of mandatory particulars (Article 13)
54. The term “mandatory particulars” refers to the information that must be provided under Article 9 (1) of the FIC. All written mandatory allergen information must be easily visible, clearly legible, and not obscured in any way. Mandatory information must not be hidden for example under a flap or across a fold or crease, detracted from or interrupted by any other written or pictorial matter or any other intervening material.
55. When considering how the required labelling information is displayed, you should take into account the following:
- Is it sufficiently visible?
- Is it readable for those with visual impairments? For example, consider individuals with colour blindness when using contrasting colours.
- Have you used the required minimum font size where the x-height (as illustrated in Annex IV of the FIC) is 1.2 mm or more must be used where labelling surface is 80 cm2 or more. Figure 1 below illustrates how the x height of the font used is measured.
Figure 1: How to measure x-Height 1 (of your font)

56. Where the food packaging or container’s largest surface area is less than 10 cm2 (e.g., a single portion sachet of sauce), the ingredients list can be omitted, provided that the ingredients information is provided by other means or made available at the consumer’s request. In such cases, the presence of Annex II ingredients in the food must be declared on the packaging. This must be done using the word ‘Contains…’ followed by the name of substance or product (e.g., Contains: celery, fish). The minimum font size rules also apply to other mandatory information as listed in Article 9 (1) of the FIC. Please refer to Article 13 of the FIC for further details.
Omission of the list of ingredients (Article 19)
57. Where the name of the product consists of a single ingredient (e.g., bag of peanuts or a box of eggs) and clearly refers to the presence of a substance or product causing allergies, further indication of the presence of the Annex II substance or product is not required. Therefore, in these examples, a bag of peanuts and a box of eggs would not need to declare the presence of peanut and egg respectively. However, where the name of the food does not clearly refer to the substance as named in Annex II, information regarding the presence in the food of an Annex II substance must be provided in the manner required for those substances. For example, gingelly oil is sesame oil and must therefore be labelled ‘Contains: sesame’.
Labelling of certain substances or products causing allergies or intolerances (Article 21)
58. Article 21 specifies that mandatory information about the presence of the Annex II ingredients which cause allergies will need to be emphasised from the other ingredients within the ingredients lists by means of contrasting font, size, style or background colour. For example, ‘INGREDIENTS: Oatmeal, sunflower oil, prawn (crustacean)’.
59. The FBO has flexibility in deciding which mode of emphasis to use to declare the presence of allergens.
Example
An allergy advice statement could be used on the product label to explain how allergens are emphasised within the ingredients list. For example: ‘Allergy advice: for allergens, see ingredients in bold’ or ‘Allergy advice: for allergens, including cereals containing gluten, see ingredients highlighted in blue.’
60. The source of allergens for each ingredient needs to be declared even if there are several ingredients from the same allergenic food.
Example
Partially Reconstituted Skimmed Milk Concentrate, Sugar, Sunflower Oil, Whey Powder (milk), Dextrose, Emulsifier (Mono- and Di-Glycerides of Fatty Acids), Flavouring, Stabilisers (Guar Gum, Sodium Alginate), Colours (Beetroot Red, Beta-Carotene).
61. If the name of an ingredient partly includes the Annex II allergen in a single word, then the name of the ingredient corresponding to the Annex II food can be emphasised. (For example: ‘sodium metabisulphite’ can be emphasised as ‘sodium metabisulphite’).
62. Where an ingredient comprises of several words (such as ‘skimmed milk powder’ and ‘egg white’) then only the Annex II food must be emphasised (in these examples, ‘skimmed milk powder’ and ‘egg white’).
63. If individual ingredients used to make a food contain added sulphur dioxide and/or sulphites, their presence must be emphasised for those ingredients separately if, when added together, the level in the overall food is >10 mg/Kg.
64. Where foods are sold under a less common name, due to appellation, trade name, foreign cuisine etc., it could be difficult to tell whether they contain any of the Annex II products/ substances (e.g., ‘monkey nuts (peanuts)’, ‘gingelly oil (sesame)’, ‘ghee (milk)’, ‘edamame beans (soya)’). In such cases there must always be a clear reference to the name of the substance as listed in Annex II.
Presentation of Voluntary Information on Prepacked Foods
65. Precautionary Allergen Labelling statements should make specific reference to one or more of the 14 regulated allergens, that may be unintentionally present in the food, so that consumer food choice is not unnecessarily limited.
66. Where an Annex II allergen is a group of foods, for example cereals and nuts, to enable consumers to have the greatest range of food choice and avoid ambiguity and confusion, it is best practice to name the specific food(s) within that allergen group, for example: not suitable for consumers with nut (hazelnut) allergy.
67. Precautionary Allergen Labelling statements should not be used in conjunction with a ‘free from’ statement for the same allergen, because a ‘free-from’ claim is a guarantee that the food is suitable for all with a food hypersensitivity to that allergen.
68. Precautionary Allergen Labelling statements can be used in combination with a ‘vegan’ label, because a ‘vegan’ label communicates different information to a ‘free from’ claim and is aimed at different consumer groups. Only free-from allergen claims are guarantees that the specified allergen is absent; to use it, a food business must have implemented strict controls to eliminate any risk of cross-contamination. The Vegan Society advise that their Vegan Trademark can be used on food products carrying a precautionary allergen label for food(s) of animal origin, provided that the labelling decision is based on an assessment of the risk of cross-contamination.
69. 'Gluten-free’ statements differ from other ‘free-from’ statements in that they are not absolute claims but stipulate that levels of gluten in the food are below 20mg/kg. A ‘gluten-free’ statement can be used in combination with a PAL statement, for example ‘gluten-free’ and ‘may contain wheat (cereal)’. An example where this would be appropriate is if there were a risk of a food being cross-contaminated with barley, but testing has shown gluten presence below 20mg/kg. This product would be safe for those with coeliac disease, but is still a risk for those with an allergy to non-gluten barley proteins. The Food and Drink Federation have produced guidance for gluten labelling on prepacked food that provides further information for businesses.
Food products without ingredients lists
70. Some foods do not require an ingredients list such as alcoholic drinks with more than 1.2% by volume of alcohol (see Article 16 (4) of the FIC). In this case the presence of any substances or products derived from the Annex II list which are present and not clear from the name of the food needs to be indicated. For example, a bottle of wine must have a statement such as: ‘Contains: sulphites, milk and egg’ if the finished product contains sulphites at more than 10 mg/litre and if milk or egg residues are detectable in the wine.
Example
Allergen(s) within a ‘Contains’ statement on products without an ingredients list do not need to be emphasised however, you can voluntarily choose to emphasise the allergens to make clear their presence in a product (e.g., ‘Contains: sulphites’),
Applicable requirements – voluntary information (Article 36)
71. Where an ingredients list is provided, the FIC does not permit the voluntary use of allergen advisory statements such as ‘Contains: wheat, egg and milk’ to repeat mandatory allergen ingredients information. Information about allergens as ingredients can only be presented in the mandatory format (i.e., emphasised within the ingredients list). This is to ensure that information is presented in a single and consistent format across food products.
Distance selling (Article 14)
72. FBOs selling prepacked foods through distance selling need to make the same level of information on allergens available to consumers, for example on their website or in their catalogue, as when the food is bought from a retail environment. (footnote 3) This is to ensure that the mandatory allergen information is available before the purchase is concluded and at the moment of delivery. Telephone numbers provided by FBOs which enable consumers to obtain oral allergen information over the telephone, must not be at an additional cost (in other words the calls must be free or non-chargeable within standard rate call plans).
73. The distance selling rule to provide information before the purchase is concluded does not apply to prepacked foods sold through vending machines.
Example
One method a business could use is to list the ingredients (with allergens emphasised in some way) on the website where the customer views the product prior to purchasing it. To make this information available to the customer at the moment of delivery, it could appear on the packaging of the food.
-
Regulation (EU) No. 828/2014 enforced in Wales by The Food Information (Wales) (Amendment) Regulations 2016 and in Northern Ireland by The Food Information (Amendment) Regulations (Northern Ireland) 2016.
-
Commission Notice on substances of products causing allergies or intolerances (2017/C 428/01).
-
For distance sales of prepacked food, other mandatory information must be provided as listed in Article 9 with exception of Article 9 (1) (f)
74. The information below provides guidance and best practice examples on the provision of allergen ingredients information for non-prepacked (such as loose fruit or vegetables or meals served in a restaurant or café) and also food packed at the consumer’s request which is covered by the same rules. Information on the use of allergens in food must be provided in a manner that is easily accessible for the consumer. The requirements are based on the following articles of FIC:
- Article 8 on responsibilities
- Article 9 on the list of mandatory particulars (see previous page for list of 14 allergens)
- Articles 12 and 13 on availability and presentation of mandatory particulars
- Article 21 on labelling of certain substances or products causing allergies or intolerances
- Article 44 on national measures for non-prepacked food
- Article 14 on distance selling
Responsibilities (Article 8)
75. Every FBO in the food supply chain is responsible for ensuring that the allergen information they provide is accurate. They must not supply food which they know or presume to be non-compliant with food information requirements.
76. Food businesses supplying food to other food businesses that is not intended for the final consumer and / or not intended for mass caterers must ensure that business to business sales of food are accompanied with sufficient information to enable subsequent food businesses to meet their responsibilities. FBOs whose activities do not affect food information must not supply food which they know or presume to be non-compliant with food information requirements. FBOs are responsible for ensuring compliance with the provisions relevant to their activities and verifying that those requirements are met. This applies to all operators including manufacturers, suppliers, and caterers.
Presentation of mandatory allergen information and National Measures
77. Allergen information for non-prepacked food can be communicated through a variety of means to suit the business format of the FBO.
78. The requirement is to provide information about the use of allergenic ingredients in a food. The provision does not require a food business to provide a full ingredients list, with the exception of prepacked for direct sale food (see Part 3 on PPDS requirements).
79. Members of staff should receive allergen training, know where to find allergen information, and know how to communicate this to customers.
80. FBOs have flexibility to provide allergen information for non-prepacked food by any means, including orally by a member of staff. No matter how information regarding allergenic ingredients is provided, it must be easily accessible and accurate.
81. Where a food business chooses to provide this information orally, the food business (including those offering drive-through sales) must use clear signposting to direct the customer to where this information can be found (e.g., by asking members of staff). In such situations there must be a statement that can be found on a notice, menu, ticket or label that is readily discernible (see Regulation 5(4) of the FIR).
82. To ensure that oral information is accurate, allergen information can be contained on a chart, in a recipe book or on ingredient information sheets, which staff can easily refer to.
83. All mandatory allergen information must be easily accessible and visible, and clearly legible to the final consumer regardless of whether they have a food hypersensitivity or not.
Food businesses should make allergen information easily available in writing for consumers and ensure staff can support this with a conversation. The information should be easy to use, clear, comprehensive, and accurate.
Example
Signposting to where allergen information will be found could be presented as a statement such as:
‘Food Allergies, Intolerances, or Coeliac Disease: Please speak to our staff about the ingredients in your meal, when making your order.’
or
'Please talk to us if you have a food allergy, intolerance or coeliac disease. We want to cater safely for everyone.' Allergen posters are available to print.
84. Allergen information must be made available for the entire dish as served; however, it can be provided in a variety of ways.
85. Where food is provided through a buffet format, the allergen information should be provided for each food item separately.
Example
Allergen information could be provided as a ‘Contains’ statement, for example ‘Chicken Tikka Masala (Contains: milk, nuts (almond)’). Another method could be the use of a chart such as the example below:
Figure 2: Allergen matrix example 1
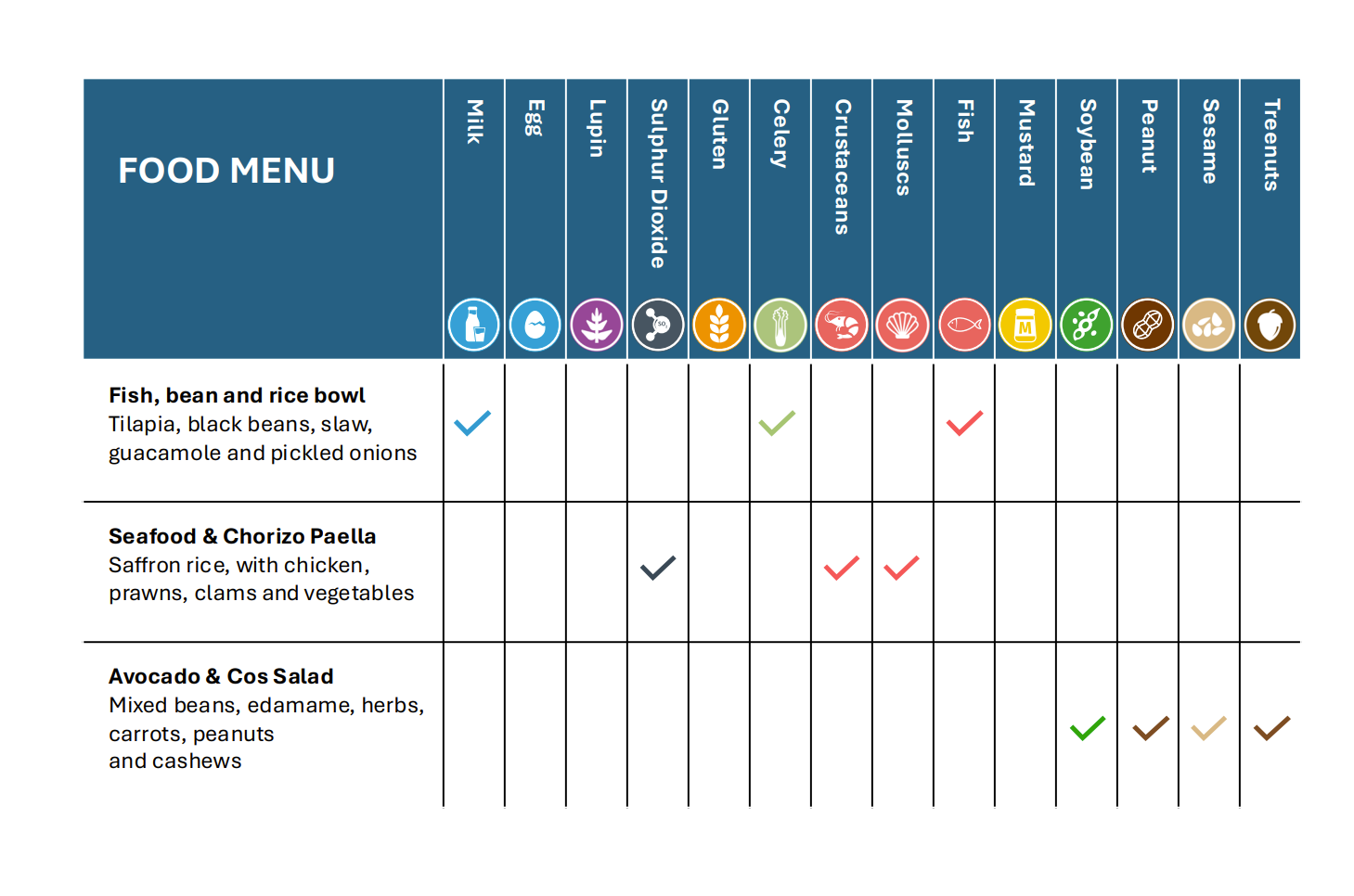
To give a better service and choice for the consumer, a food business could choose to provide a breakdown of allergenic components within a dish, rather than just providing the information on the allergens present in the entire dish.
For example, BBQ Chicken Burger and coleslaw (Chicken: wheat, fish, celery; BBQ sauce: celery, fish; Bap: wheat, eggs, and sesame; Coleslaw; egg, celery, mustard)
If a customer had an allergy to mustard for example, the dish could be served without the coleslaw.
Distance selling (Article 14)
86. FBOs selling non prepacked food through distance selling (footnote 1) (such as food businesses which offer purchase through telephone/ internet) must ensure that mandatory allergen information is available to the consumer (for free):
- before the purchase is concluded; and
- at the moment of delivery.
Allergen information is held in written form by the business and available in written form to the consumer both before the food is ordered and when it is delivered.
Our best practice guidance and tools will help you provide allergen information to consumers who order non-prepacked food for delivery or takeaway.
87. Whatever the chosen method of presentation, the FBO must always ensure that the allergen information is current and accurate.
88. The allergen information must be provided without any supplementary costs being charged to the customer by the FBO (e.g., premium line numbers).
Example
Ways of providing allergen information at the time of order include:
- the customer is signposted to where the accurate information can be obtained in writing (e.g., an online menu); or
- staff provide the allergen information orally by telephone.
To ensure that current and accurate allergen information is provided, the food business could ask the customer if allergen information is required before the order is taken on the telephone or online.
Ways of providing written allergen information at the time of delivery include:
- placing stickers on food containers to clearly identify food and allergenic ingredients used in that food (e.g., Chicken satay: ‘Contains: wheat, soy, fish, peanut’); or
- a menu is provided with the order which allows the customer to clearly identify allergenic ingredients in the food, along with clear names, or other appropriate cross references on food containers.
- written allergen information is presented to the customer, by the member of staff from the business delivering the food together with a means to clearly link the written information to each food item.
Non-Gluten Containing Ingredients
89. Non-Gluten Containing Ingredients (NGCI) statements can be misleading to consumers. ‘NGCI’ statements have been used in menus when listing a group of food items to indicate they do not have gluten-containing ingredients, when the food businesses cannot guarantee the foods are gluten-free. To help food businesses, Coeliac UK has provided guidance on Catering Gluten Free.
Avoid Non-Gluten Containing Ingredients statements, for example, “this menu has been designed for a non-gluten diet. It’s a selection of dishes that do not contain gluten in their ingredients”.
Instead, a “gluten-free” statement should be provided where strict controls ensure that food provided contain no more than 20mg/kg of gluten.
If food businesses choose to continue to use NGCI statements, they should emphasise that the foods the statement refers to are not suitable for people with coeliac disease.
90. Whether a food is prepacked for direct sale (PPDS) depends on whether, where and when it is packed in relation to the point at which it is offered for sale.
91. The specific requirements are based on the following FIC articles:
- Article 8 on responsibilities
- Article 9 on mandatory particulars
- Articles 12 and 13 on availability and presentation of mandatory particulars
- Article 21 on labelling of certain substances or products causing allergies or intolerances
- Article 14 on distance selling
92. The information below provides guidance and best practice examples on the provision of allergen information for PPDS food. The new rules for prepacked for direct sale food came into effect across all four UK nations on 1 October 2021 (footnote 1).
93. Food businesses should consider the foods they package before the process of a sale begins, in order to check if these requirements apply.
94. PPDS food is food that is packed before being offered for sale by the same food business to the final consumer:
i) on the same premises; or
ii) on the same site (footnote 2); or
iii) on other premises if the food is offered for sale from a moveable and/or temporary premises (such as marquees, market stalls, mobile sales vehicles) and the food is offered for sale by the same food business who packed it.
95. Prepacked is defined in Article 2(2)(e) of the FIC as ‘any single item for presentation as such to the final consumer and to mass caterers, consisting of a food and the packaging into which it was put before being offered for sale, whether such packaging encloses the food completely or only partially, but in any event in such a way that the contents cannot be altered without opening or changing the packaging.
96. Food is considered prepacked when it is put into packaging before being offered for sale and:
a) is either fully or partly enclosed by the packaging; and
b) cannot be altered without opening or changing the packaging; and
c) is ready for sale to the final consumer.
Figure 3: What is prepacked for direct sale (PPDS) food?
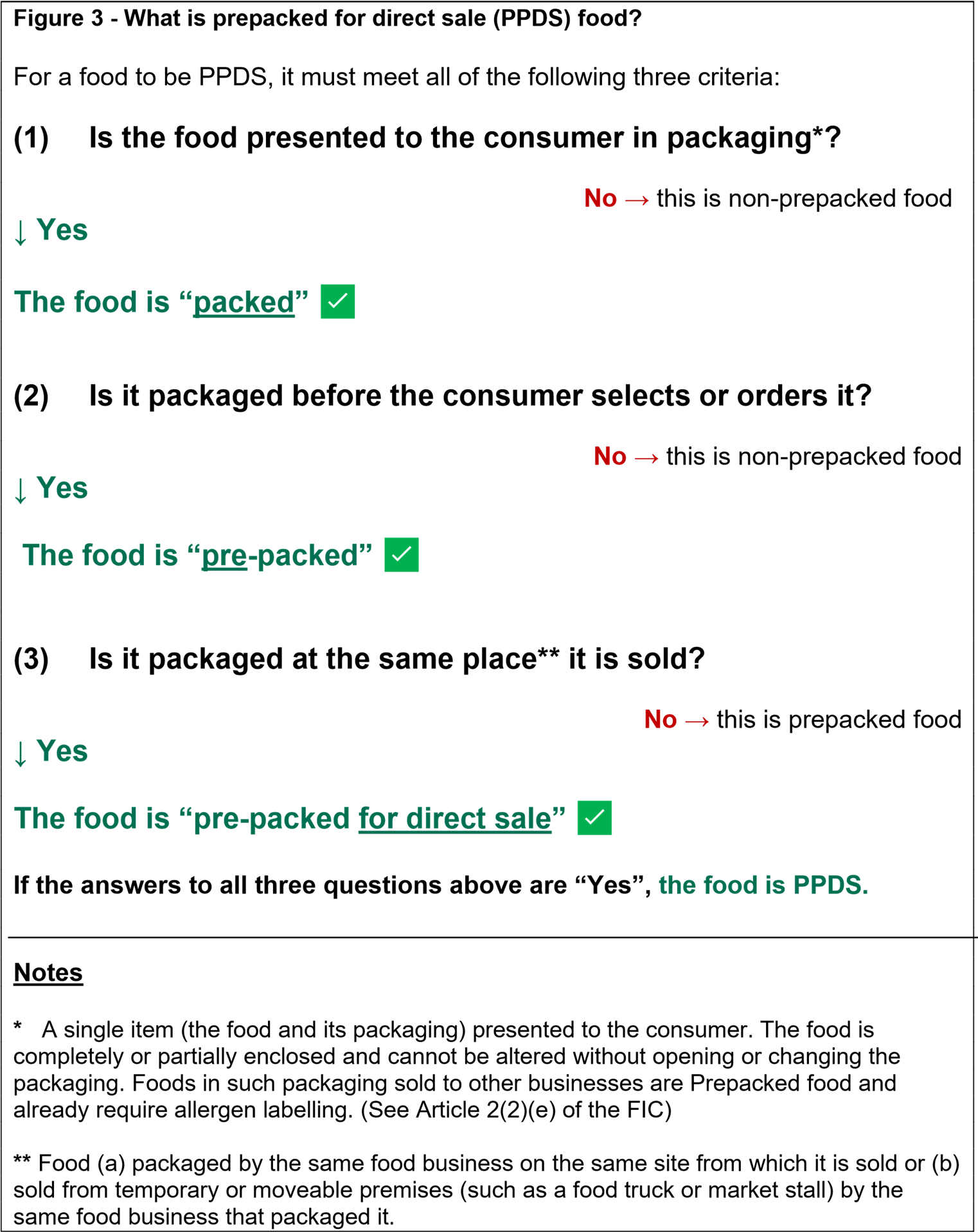
Example
PPDS food includes:
- Sandwiches placed into packaging by the food business and sold from the same premises.
- A café giving away packaged samples of a new range of cakes they have made on the same premises.
- Foods packaged and then taken by the same operator to their market stall to sell.
- A butcher who makes burgers or sausages which are prepacked to be sold on the same premises.
- Foods packed by a food business to be sold in its retail units located within the same building complex as the premises where the food was packed such as a train station, hospital, university or holiday park.
In a retail environment such as a supermarket, the following examples would also be considered to be prepacked for direct sale food, provided they are packed on the premises from which they are being sold before they are offered for sale:
- Fresh pizzas from the deli counter e.g., on a cardboard tray and wrapped in plastic; Boxed salads;
- Hot foods such as rotisserie chicken; and
- Foods that are pre-weighed and packed such as cheese or meats from a delicatessen counter or baked goods from an in-store bakery
97. Any food that is packed on the premises by the same food business in anticipation of an order, before being offered for sale, would be considered to be PPDS food. This can include food the consumer self-selects from a chiller cabinet or has to obtain from a member of staff.
98. Some fast food may be prepacked for direct sale, for example, a wrapped burger or boxed fried chicken placed under a hot lamp before being ordered in anticipation of a sale. Food placed into packaging after a consumer orders it (for example, a freshly prepared sandwich or burger that is made and wrapped after taking an order) is not PPDS food. Although these items are packed, they are not packed before being offered for sale and therefore are not prepacked for direct sale. The same rules apply to these foods as apply to other forms of non-prepacked foods such as meals served in a restaurant.
99. PPDS food does not cover food which does not have packaging, or it is packaged in a way that the food can be altered without opening or changing the packaging (for example a hot dog served on a cardboard tray.)
100. PPDS food does not cover food packed by one business and supplied to another business for sale (for example a pork pie packed by business “A” and sold by business “B” at a farmer’s market.) This is prepacked food.
List of mandatory particulars for PPDS food
101. All PPDS food must have on the package (footnote 3) or on a label attached to the package:
- the name of the food and;
- an ingredients list (footnote 4) including allergenic ingredients. The allergenic ingredients within the food must be emphasised every time they appear in the ingredients list. For example, the allergens in the food can be listed in bold, in capital letters, in contrasting colours or underlined.
102. Detailed guidance on how each allergenic ingredient captured in Annex II of the FIC must be emphasised and named in the ingredients list on PPDS food has been outlined in this guidance in paragraphs 27 to 65.
Distance selling (Article 14)
103. FBOs selling PPDS food through distance means (e.g., such as food businesses which offer purchase through telephone/ internet) will need to ensure that mandatory allergen information is available to the consumer (for free) before they buy the product and also is available at the moment of delivery. Whatever the chosen method of presentation, the FBO must always ensure that the allergen information is current and accurate.
104. The requirement for a list of ingredients does not apply to PPDS food sold online, via telephone or provided to the consumer by mail order only. This is because the national rules applicable to the provision of food information for non-prepacked food including PPDS do not apply to food sold via distance means. The applicable rules for all food sold through distance means are therefore those contained within Article 14 of the FIC.
-
The Food Information (Amendment) (England) Regulations 2022, The Food Information (Wales) (Amendment) (No. 2) Regulations 2020, The Food Information (Amendment No. 2) Regulations (Northern Ireland).
-
In this instance ‘site’ refers to a building complex such as a shopping centre or airport terminal in which the same food business operates from more than one unit within the building complex.
-
See Article 16(2) of Regulation (EU) No. 1169/2011 for the requirements applicable to packaging or containers with a surface area less than 10 cm2, so far as it relates to the particulars required by Article 9(1)(b).
-
See Food Information Regulations for more rules on the required format of the ingredients list. See Article 19 of Regulation (EU) No. 1169/2011 for foods which are not required to bear a list of ingredients.
Local authority responsibilities
105. In the UK, authorised food officers at Local Authorities have responsibility for official controls relating to allergen rules.
106. In England, there is a dual enforcement responsibility in areas where there are county councils and district councils. County councils are under a duty to enforce the FIRs. District councils have the power to enforce elements of the FIRs. Authorised food officers are encouraged to discuss and reach an understanding on how to enforce allergen requirements at a local level.
Penalties and offences
107. Failure to comply with the requirements set out in Regulation 10(1) and (2) of the FIR on the labelling of allergenic ingredients is a criminal offence and may result in a criminal prosecution being brought against an FBO. This position is the same in relation to a failure to comply with Regulation 10(1)(b) of the FIR relating to the provision of allergen information for non-prepacked foods and PPDS etc. in a manner other than one provided for in the FIC.
108. A person convicted of an offence under the FIR will be liable to a potentially unlimited fine. The level of the fine would be determined by Magistrates on a case-by-case basis.
Coeliac Disease: This is an autoimmune disease caused by an adverse reaction to eating gluten, a protein in cereals namely wheat, rye, barley, oats, spelt, Kamut or their hybridised strains. Adherence to the gluten free diet is the complete medical treatment and having coeliac disease therefore requires significant dietary modification.
Distance selling: This refers to the selling and buying of goods or services (for purposes of these guidance notes prepacked, and non-prepacked foods) without the simultaneous physical presence of the consumer and supplier to complete the contract for sale; for example, selling food by internet (internet shopping, online takeaway aggregators etc.), mail order, telephone, or television.
Final consumer: This is defined in Article 3 (18) of Regulation (EC) No.178/2002 as ‘the ultimate consumer of a foodstuff who will not use the food as part of any business operation or activity’. The final consumer will generally be the individual who will be eating or drinking the food or drink provided by the food business.
Food allergen: This is the substance in a food that can cause an allergic reaction. Allergens are normally proteins, and, in some people, the immune system thinks allergens are foreign or dangerous. The immune response to these allergenic proteins is what leads to allergic reactions. Legislation focuses on 14 specific foods of public health importance (most potent and prevalent food allergens in Europe) which are listed in Annex II to the FIC.
Food allergy: An adverse reaction to a food that involves the immune system and can be a potentially life-threatening condition. Symptoms can appear within minutes, or up to several hours after a person has eaten a food they are allergic to. There is no cure for food allergy. An allergic individual must avoid the food which makes them ill.
Food business operator (FBO): This is defined in Regulation (EC) No. 178/2002 (Article 3(3)) (General Food Law) as ‘the natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control’.
Food business: This is defined in Regulation (EC) 178/2002 (Article 3(2)) (General Food Law) as ‘any undertaking, whether for profit or not and whether public or private, carrying out any of the activities related to any stage of production, processing and distribution of food’.
Food intolerance: Most food intolerances do not involve the immune system and are generally not life-threatening. However, they can make someone feel very ill or affect their long-term health. Examples of food intolerance include lactose and gluten intolerance.
Mass caterer: This is defined in Article 2(2)(d) of the FIC as ‘any establishment (including a vehicle or a fixed or mobile stall), such as restaurants, canteens, schools, hospitals and catering enterprises in which, in the course of a business, food is prepared to be ready for consumption by the final consumer’.
Non-prepacked food: Any food presented to the final consumer or mass caterer that does not fall within the definition of ‘prepacked food’ for any reason including food not within any packaging and food packaged at the consumers’ request.
- In a physical retail environment this is likely to apply to foods which are sold loose from a delicatessen counter (e.g., cold meats, cheeses, quiches, pies, and dips), fresh pizza, salad bars, bread or pastries sold without wrapping in bakery shops or via bakery counters, meat from butchers, etc.
- In a catering environment this is likely to apply to foods which are not sold prepacked, for example food from a takeaway, or meals served in a canteen or a restaurant.
Prepacked food: This is defined in the FIC (Article 2(2)(e)) as ‘any single item for presentation as such to the final consumer and to mass caterers, consisting of a food and the packaging into which it was put before being offered for sale, whether such packaging encloses the food completely or only partially, but in any event in such a way that the contents cannot be altered without opening or changing the packaging; ‘prepacked food’ does not cover foods packed on the sales premises at the consumer’s request or prepacked for direct sale.’
Food is considered prepacked when it is put into packaging prior to before being offered for sale and:
- is either fully or partly enclosed by the packaging; and
- cannot be altered without opening or changing the packaging; and
- is ready for sale to the final consumer or to a mass caterer
Prepacked for direct sale (PPDS) food: Food that is packed before being offered for sale by the same food business to the final consumer:
i) on the same premises; or
ii) on the same site (footnote 1) ; or
iii) on other premises if the food is offered for sale from a moveable and/or temporary premises (such as marquees, market stalls, mobile sales vehicles) if the food is offered for sale by the same food business who packed it.
PPDS food does not include food packed at a consumer’s request, food not in packaging or food in packaging that can be altered without opening or changing the packaging.
109. Further advice on food allergen labelling is available on the Agency’s website: www.food.gov.uk/business-guidance/allergen-labelling-for-food-manufactu…
110. FSA allergen resources at www.food.gov.uk/allergen-resources
111. Think allergy posters and chef cards can be found here: https://www.food.gov.uk/business-guidance/allergen-guidance-for-food-bu…
112. FSA best practice guidance on allergen information for non-prepacked food can be found here: Allergen Information for Non-Prepacked Foods Best Practice: Summary
113. FSA has produced tools to support food businesses providing written allergen information to consumers, supported by a conversation, for non-prepacked food.
114. FSA has produced free online training modules to help enforcement officers and businesses understand food allergen labelling and labelling in general under the FIC. Free online allergy training can be found here: http://allergytraining.food.gov.uk/
115. The British Retail Consortium (BRC) and Food and Drink Federation (FDF) guidance on Allergen Labelling: http://www.reading.ac.uk/foodlaw/pdf/uk-12024-BRCFDF-Allergen-Labelling…
116. Food and Drink Federation Guidance on 'Allergen'-Free and Vegan Claims (February 2020): https://www.fdf.org.uk/globalassets/resources/publications/fdf-guidance…
117. Food and Drink Federation Gluten Labelling Guidance: Best Practice for Prepacked Foods which Include or Exclude Cereals Containing Gluten (June 2019): https://www.fdf.org.uk/globalassets/resources/publications/guidance/fdf…
118. British Retail Consortium & Food and Drink Federation Guidance on “Free-From” Allergen Claims (November 2015): https://www.fdf.org.uk/globalassets/resources/publications/brc-free-fro…
117. Retained Regulation (EU) No. 1169/2011 on the provision of food information to consumers (“FIC”): www.legislation.gov.uk/eur/2011/1169/contents
118. Regulation (EU) No. 1169/2011 on the provision of food information to consumers: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169
119. Retained Regulation (EU) No. 828/2014 on the requirements for the provision of information to consumers on the absence or reduced presence of gluten in food: www.legislation.gov.uk/eur/2014/828/contents
120. Regulation (EU) No 828/2014 on the requirements for the provision of information to consumers on the absence or reduced presence of gluten in food: https://eur-lex.europa.eu/eli/reg_impl/2014/828
121. Retained Regulation (EC) No. 178/2002 laying down the general principles and requirements of food law (General Food Law): www.legislation.gov.uk/eur/2002/178/contents
122. Regulation (EC) No. 178/2002 laying down the general principles and requirements of food law (General Food Law): https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002R0178&…
123. Food Safety Act 1990 and subsequent amendments: www.legislation.gov.uk/ukpga/1990/16/contents
124. Food Safety (Northern Ireland) Order 1991 and subsequent amendments www.legislation.gov.uk/nisi/1991/762/contents/made
125. The Food Information Regulations 2014 (“FIR”): www.legislation.gov.uk/uksi/2014/1855/pdfs/uksi_20141855_en.pdf
126. The Food Information (Wales) Regulations 2014: http://www.legislation.gov.uk/wsi/2014/2303/pdfs/wsi_20142303_mi.pdf
127. The Food Information Regulations (Northern Ireland) 2014: https://www.legislation.gov.uk/nisr/2014/223/contents
128. The Food Information (Amendment) (England) Regulations 2022: https://www.legislation.gov.uk/uksi/2022/481/contents/made
129. The Food Information (Wales) (Amendment) (No. 2) Regulations 2020: www.legislation.gov.uk/wsi/2020/295/pdfs/wsi_20200295_mi.pdf
130. The Food Information (Amendment No. 2) Regulations (Northern Ireland) 2020: https://www.legislation.gov.uk/uksi/2022/481/made
131. Retained Regulation (EU) No. 1308/2013 establishing a common organisation of the markets in agricultural products: www.legislation.gov.uk/eur/2013/1308/contents
132. Regulation (EU) No. 1308/2013 establishing a common organisation of the markets in agricultural products: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R1308&…
Review
133. We aim to keep all guidance up to date and regularly review it to ensure it is still relevant. The next scheduled review date for this guidance will be by December 2024.
134. We welcome your feedback on this guidance, including reports of any broken links or out-of-date content.
Contacts
- Food Standards Agency England: Kara Thomas (Food Hypersensitivity Team Leader), Email: hypersensitivitypolicy@food.gov.uk
- Food Standards Agency in Northern Ireland: Nuala Sheehy (Food Standards Lead), Email: Nuala.Sheehy@food.gov.uk
- Food Standards Agency in Wales: Steve Adie (Standards Lead), Email: steve.adie@food.gov.uk